Abstract
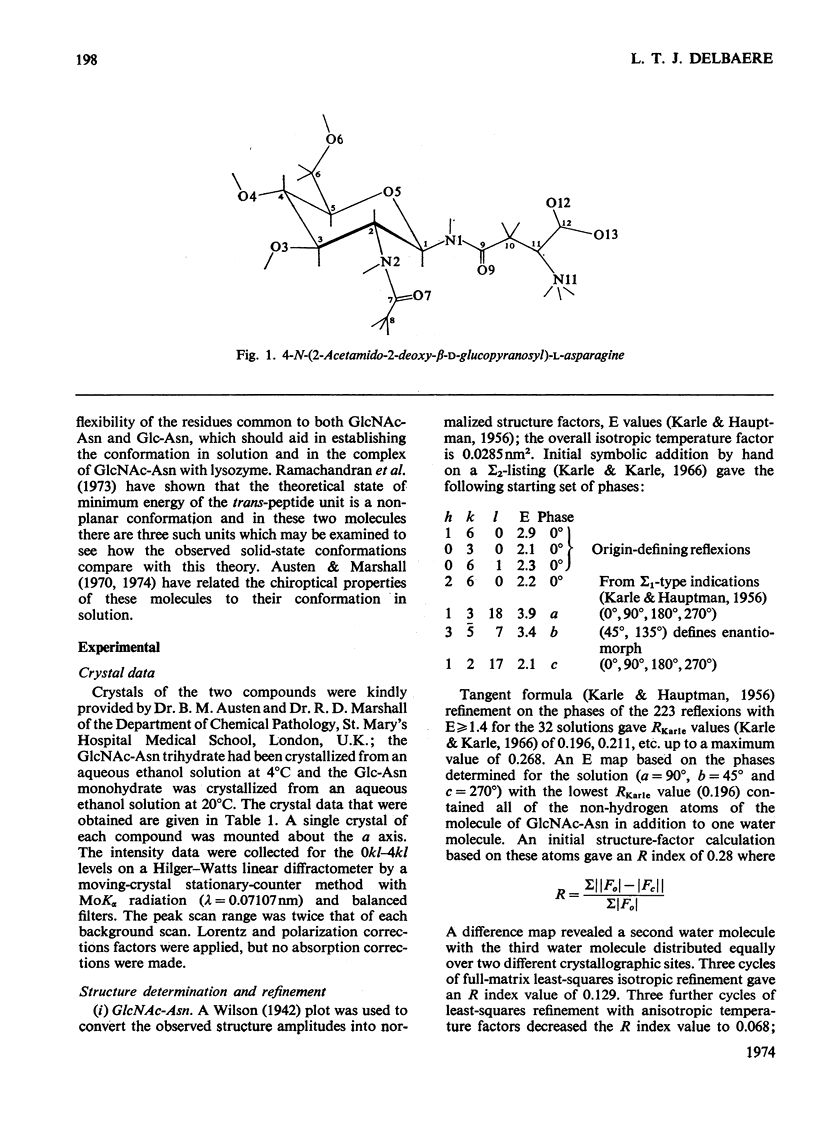
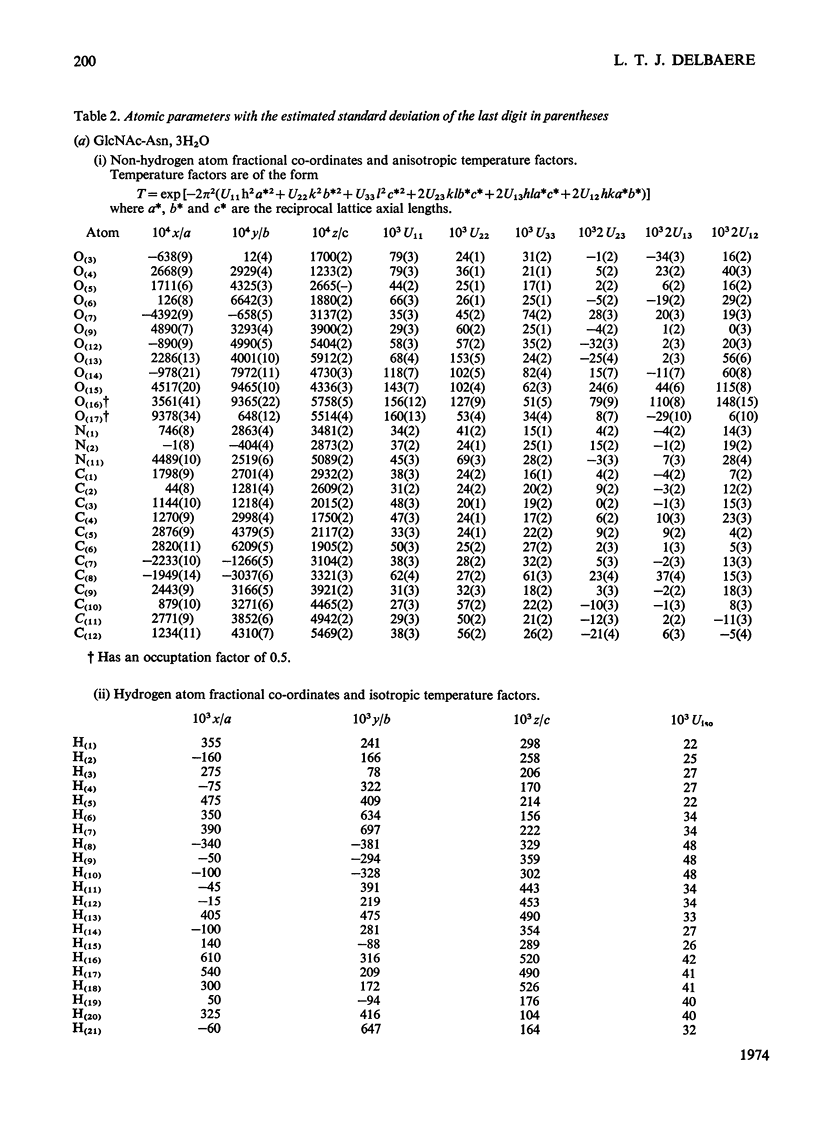
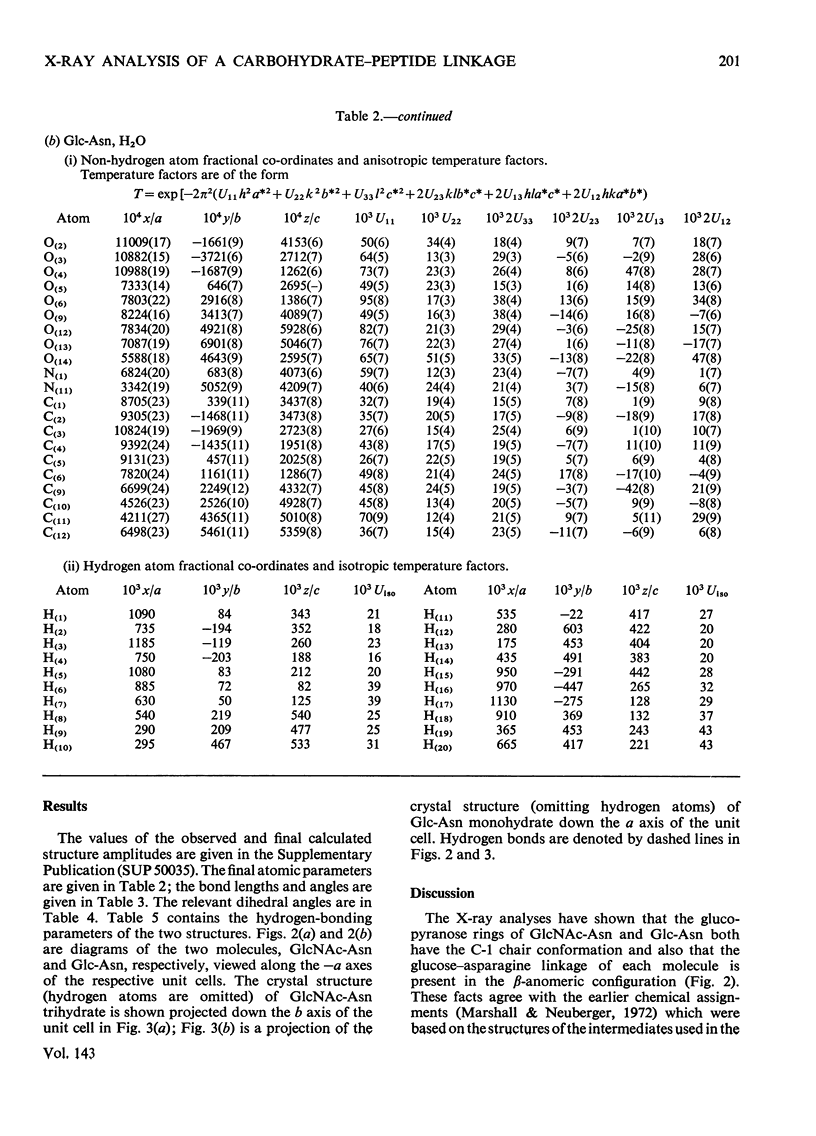
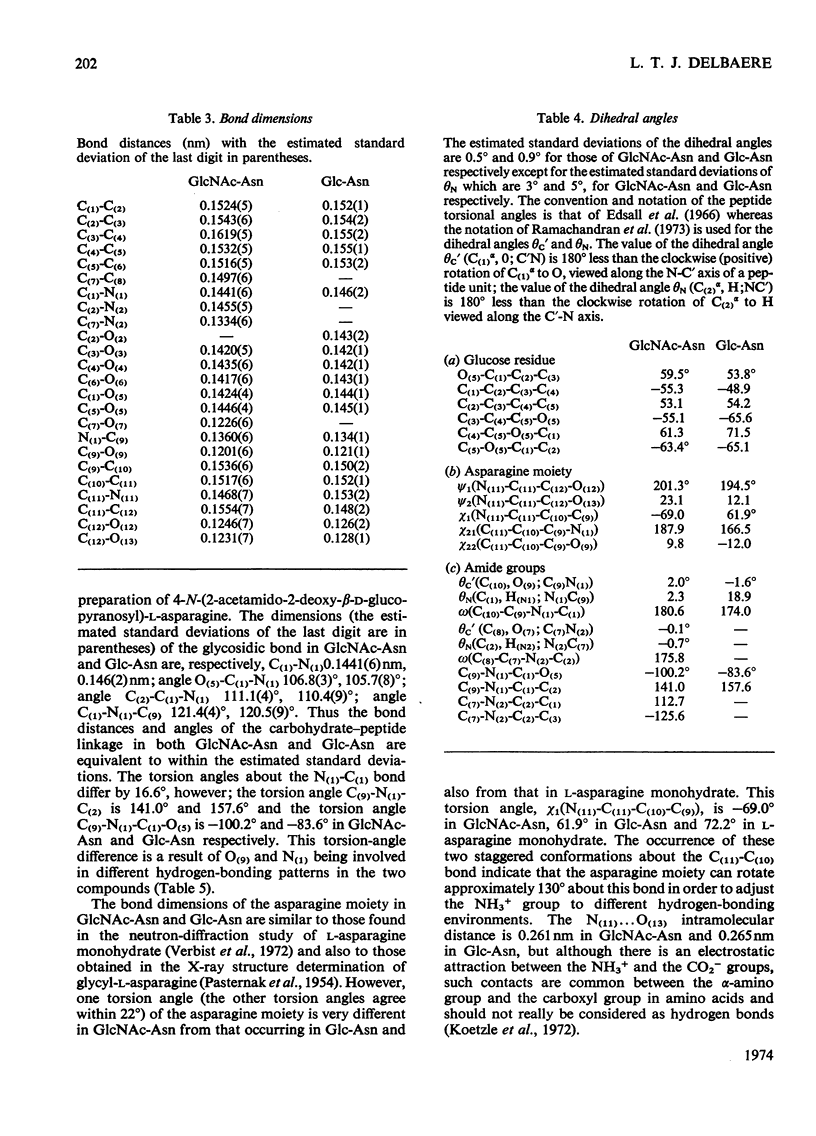
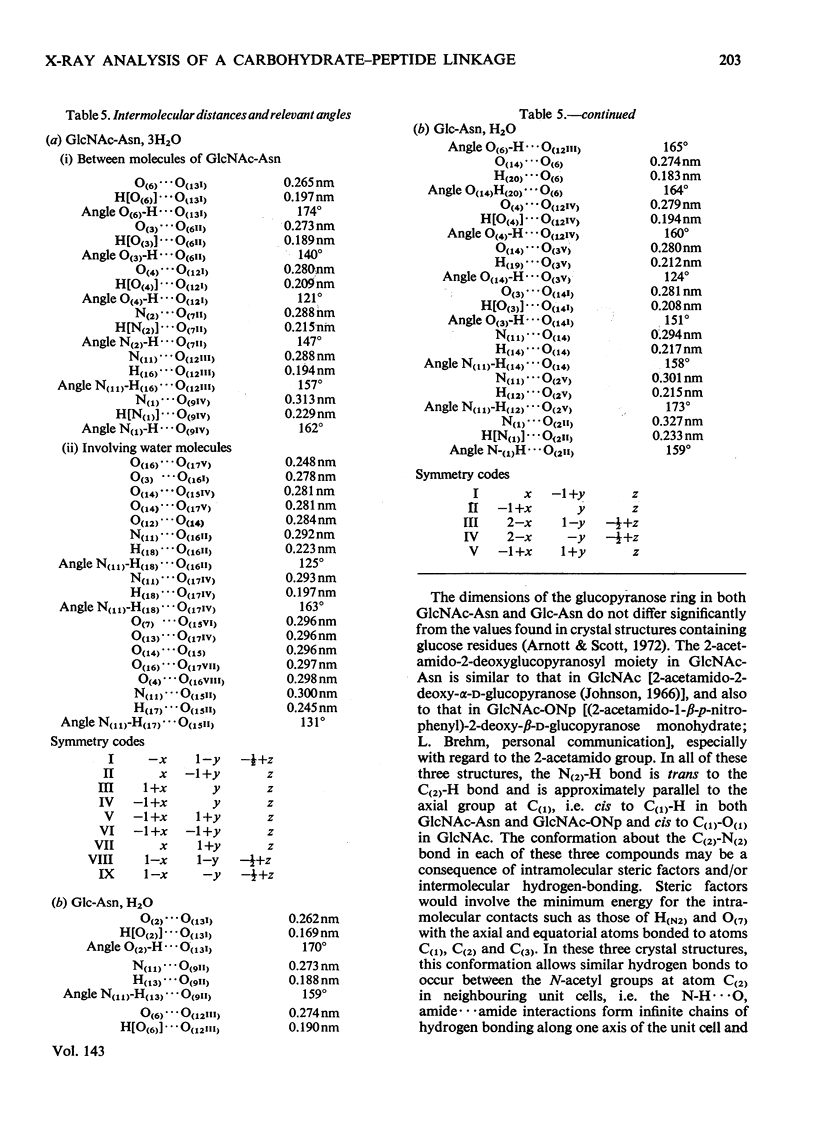
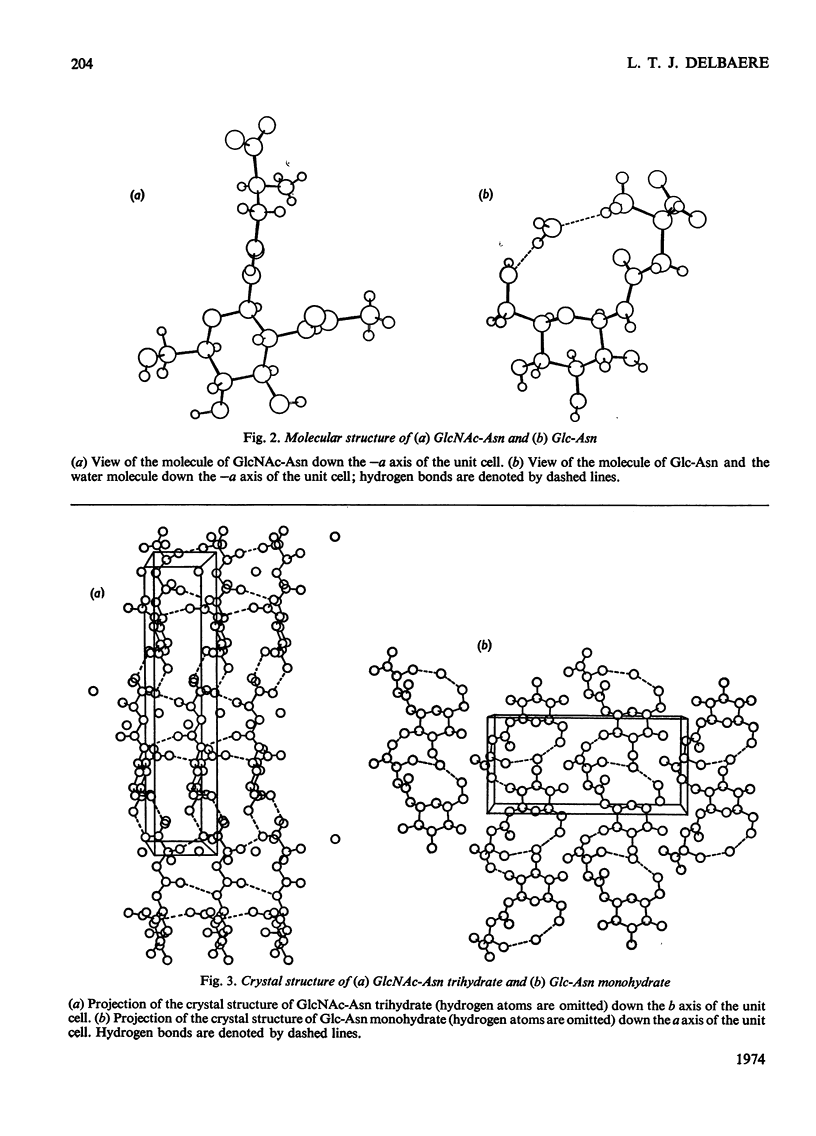
X-ray analyses have shown that the glucopyranose rings of GlcNAc-Asn [4-N-(2-acetamido-2-deoxy-β-d-glucopyranosyl)-l-asparagine] and Glc-Asn [4-N-(β-d-glucopyranosyl)-l-asparagine] both have the C-1 chair conformation and also that the glucose–asparagine linkage of each molecule is present in the β-anomeric configuration. The dimensions (the estimated standard deviations of the last digit are in parentheses) of the glycosidic bond in GlcNAc-Asn and Glc-Asn are, respectively, C(1)-N(1) 0.1441(6)nm, 0.146(2)nm; angle O(5)-C(1)-N(1) 106.8(3)°, 105.7(8)°; angle C(2)-C(1)-N(1) 111.1(4)°, 110.4(9)°; angle C(1)-N(1)-C(9) 121.4(4)°, 120.5(9)°. The glycosidic torsion angle C(9)-N(1)-C(1)-C(2) is 141.0° and 157.6° in GlcNAc-Asn and Glc-Asn respectively. Hydrogen-bonding is extensive in these two crystal structures and does affect one torsion angle in particular. Two very different values of χ1(N-Cα-Cβ-Cγ) occur for the asparagine residue of the two different molecules; the values of χ1, −69.0° in GlcNAc-Asn and 61.9° in Glc-Asn, correspond to two different staggered conformations about the Cα-Cβ bond as the NH3+ group is adjusted to different hydrogen-bonding patterns. The two trans-peptide groups in GlcNAc-Asn show small distortions in planarity whereas that in Glc-Asn is more non-planar. The mean plane through the atoms of the amide group at C(2) in GlcNAc-Asn is approximately perpendicular (69°) to the mean plane through the C(2), C(3), C(5) and O(5) atoms of the glucose ring and that at C(1) is less perpendicular (65°). The mean plane through the atoms of the amide group in Glc-Asn makes an angle of only 55° with the mean plane through these same four atoms of the glucose ring. The N(1)-H bond of the amide at C(1) is trans to the C(1)-H bond in these two compounds; the N(2)-H bond of the amide at C(2) is trans to the C(2)-H bond in GlcNAc-Asn. The values of the observed and final calculated structure amplitudes have been deposited as Supplementary Publication SUP 50035 (26 pages) at the British Library (Lending Division), (formerly the National Lending Library for Science and Technology), Boston Spa, Yorks. LS23 7BQ, U.K., from whom copies may be obtained on the terms given in Biochem. J. (1973) 131, 5.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austen B. M., Marshall R. D. Optical rotatory dispersion of 1-N-beta-L-aspartyl-2-acetamido-2-deoxy-beta-D-glucopyranosylamine. Biochim Biophys Acta. 1970 Sep 22;215(3):559–561. doi: 10.1016/0304-4165(70)90110-8. [DOI] [PubMed] [Google Scholar]
- Blake C. C., Johnson L. N., Mair G. A., North A. C., Phillips D. C., Sarma V. R. Crystallographic studies of the activity of hen egg-white lysozyme. Proc R Soc Lond B Biol Sci. 1967 Apr 18;167(1009):378–388. doi: 10.1098/rspb.1967.0035. [DOI] [PubMed] [Google Scholar]
- Edsall J. T., Flory P. J., Kendrew J. C., Liquori A. M., Némethy G., Ramachandran G. N., Scheraga H. A. A proposal of standard conventions and nomenclature for the description of polypeptide conformations. J Mol Biol. 1966 Jan;15(1):399–407. doi: 10.1016/s0022-2836(66)80240-1. [DOI] [PubMed] [Google Scholar]
- Johnson L. N. The crystal structure of N-acetyl-alpha-D-glucosamine. Acta Crystallogr. 1966 Dec 10;21(6):885–891. doi: 10.1107/s0365110x66004146. [DOI] [PubMed] [Google Scholar]
- Ramachandran G. N., Kolaskar A. S. The non-planar peptide unit. II. Comparison of theory with crystal structure data. Biochim Biophys Acta. 1973 Apr 20;303(2):385–388. doi: 10.1016/0005-2795(73)90374-7. [DOI] [PubMed] [Google Scholar]
- Ramachandran G. N., Lakshminarayanan A. V., Kolaskar A. S. Theory of the non-planar peptide unit. Biochim Biophys Acta. 1973 Mar 23;303(1):8–13. doi: 10.1016/0005-2795(73)90142-6. [DOI] [PubMed] [Google Scholar]
- Winkler F. K., Dunitz J. D. The non-planar amide group. J Mol Biol. 1971 Jul 14;59(1):169–182. doi: 10.1016/0022-2836(71)90419-0. [DOI] [PubMed] [Google Scholar]


