Abstract
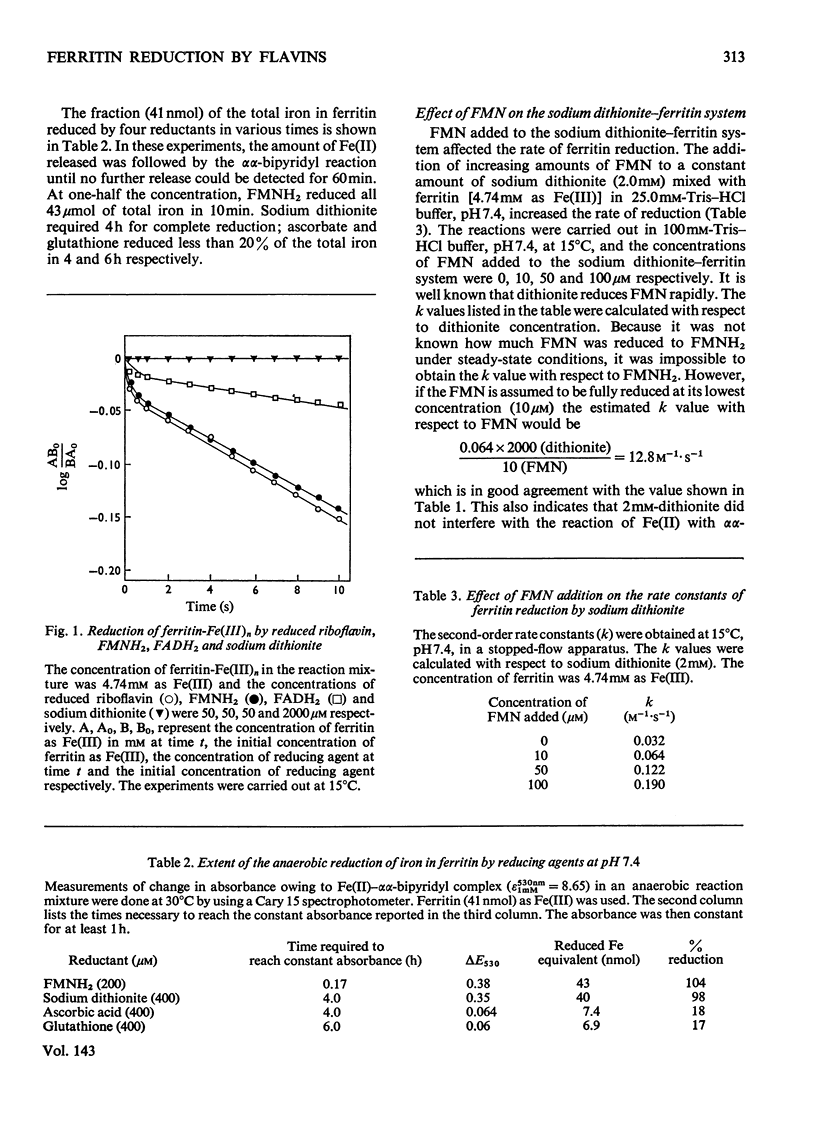
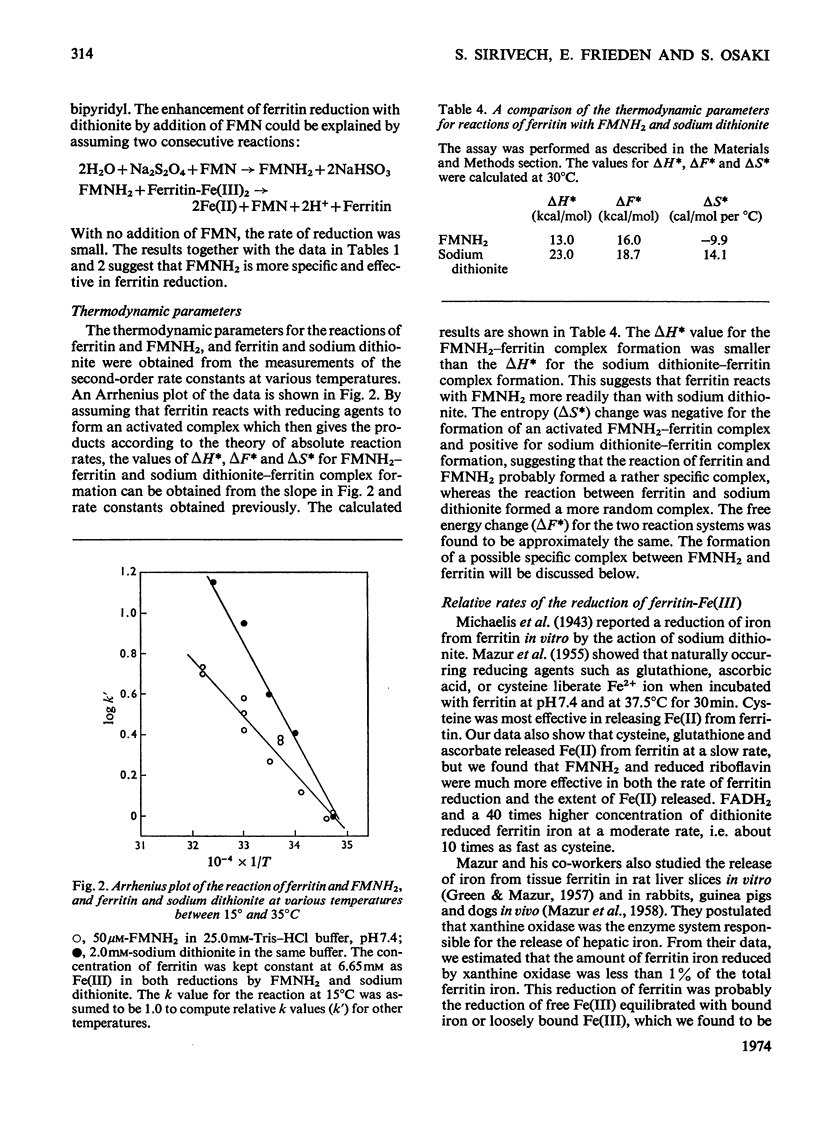
Ferritin-Fe(III) was rapidly and quantitatively reduced and liberated as Fe(II) by FMNH2, FADH2 and reduced riboflavin. Dithionite also released Fe(II) from ferritin but at less than 1% of the rate with FMNH2. Cysteine, glutathione and ascorbate gave a similar slower rate and yielded less than 20% of the total iron from ferritin within a few hours. The reduction of ferritin-Fe(III) by the three riboflavin compounds gave complex second-order kinetics with overlapping fast and slow reactions. The fast reaction appeared to be non-specific and may be due to a reduction of Fe(III) of a lower degree of polymerization, equilibrated with ferritin iron. The amount of this Fe3+ ion initially reduced was small, less than 0.3% of the total iron. Addition of FMN to the ferritin–dithionite system enhanced the reduction; this is due to the reduction of FMN by dithionite to form FMNH2 which then reduces ferritin-Fe(III). A comparison of the thermodynamic parameters of FMNH2–ferritin and dithionite–ferritin complex formation showed that FMNH2 required a lower activation energy and a negative entropy change, whereas dithionite required 50% more activation energy and showed a positive entropy change in ferritin reduction. The effectiveness of FMNH2 in ferritin–Fe(III) reduction may be due to a specific binding of the riboflavin moiety to the protein portion of the ferritin molecule.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Duggan D. E., Streeter K. B. Inhibition of ferritin reduction by pyrazolo(3,4d)pyrimidines. Arch Biochem Biophys. 1973 May;156(1):66–70. doi: 10.1016/0003-9861(73)90341-x. [DOI] [PubMed] [Google Scholar]
- GREEN S., MAZUR A. Relation of uric acid metabolism to release of iron from hepatic ferritin. J Biol Chem. 1957 Aug;227(2):652–668. [PubMed] [Google Scholar]
- Grace N. D., Greenwald M. A., Greenberg M. S. Effect of allopurinol on iron mobilization. Gastroenterology. 1970 Jul;59(1):103–108. [PubMed] [Google Scholar]
- Hoy T. G., Harrison P. M., Shabbir M., Macara I. G. The release of iron from horse spleen ferritin to 1,10-phenanthroline. Biochem J. 1974 Jan;137(1):67–70. doi: 10.1042/bj1370067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. M., Johnston D. O. Rate of release of iron from ferritin to 1, 10-phenanthroline. Nature. 1967 Nov 4;216(5114):509–510. doi: 10.1038/216509a0. [DOI] [PubMed] [Google Scholar]
- MAZUR A., BAEZ S., SHORR E. The mechanism of iron release from ferritin as related to its biological properties. J Biol Chem. 1955 Mar;213(1):147–160. [PubMed] [Google Scholar]
- MAZUR A., CARLETON A. HEPATIC XANTHINE OXIDASE AND FERRITIN IRON IN THE DEVELOPING RAT. Blood. 1965 Sep;26:317–322. [PubMed] [Google Scholar]
- MAZUR A., GREEN S., SAHA A., CARLETON A. Mechanism of release of ferritin iron in vivo by xanthine oxidase. J Clin Invest. 1958 Dec;37(12):1809–1817. doi: 10.1172/JCI103774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederer W. Ferritin: iron incorporation and iron release. Experientia. 1970;26(2):218–220. doi: 10.1007/BF01895596. [DOI] [PubMed] [Google Scholar]
- Osaki S., Johnson D. A., Frieden E. The mobilization of iron from the perfused mammalian liver by a serum copper enzyme, ferroxidase I. J Biol Chem. 1971 May 10;246(9):3018–3023. [PubMed] [Google Scholar]


