Abstract
Objective
Age-related macular degeneration (AMD) is one of the leading causes of irreversible visual impairment and blindness in the elderly. As AMD is a multifactorial disease, it is critical to explore useful biomarkers and pathological pathways underlying it. The purpose of this study is to summarise current metabolic profiles and further identify potential metabolic biomarkers and therapeutic targets in AMD, which could facilitate clinical diagnosis and treatment.
Methods and analysis
Relevant metabolomics studies published before 10 December 2021 were generally reviewed from online resources by two investigators. Studies with sufficient information and data were included in this systematic review and repeatedly identified metabolites were extracted. Pathway and Kyoto Encyclopaedia of Genes and Genomes (KEGG) analyses were performed. The public Gene Expression Omnibus (GEO) database was used for coanalysis with differential metabolites to construct a pathway network via MetaboAnalyst V.5.0.
Results
16 studies were included in our analysis. 24 metabolites were repeatedly detected and regarded as potential biomarkers for AMD. Pathway analysis implied a major role of phenylalanine, tyrosine and tryptophan pathways in AMD pathology. 11 KEGG pathways were enriched, meanwhile, 11 metabolic pathway clusters were identified by coanalysing the differential metabolites and gene profiles using the GEO database.
Conclusion
In this study, we summarised 16 metabolomic studies on AMD, and 24 metabolites were identified as potential biofluid biomarkers. This provided novel insights into the pathogenic mechanisms underlying AMD. Further studies are warranted to validate and expand an effective pattern for AMD diagnosis and treatment.
Keywords: Macula, Neovascularisation, Angiogenesis
WHAT IS ALREADY KNOWN ON THIS TOPIC
Advanced metabolomics technology has been applied to reveal metabolic signatures in age-related macular degeneration (AMD). Therefore, it is meaningful to identify its candidate metabolic features.
WHAT THIS STUDY ADDS
This study adds a novel perspective on metabolic features and pathogenesis in AMD by systematically summarising 16 existing metabolomics studies and analysing their findings.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study identifies the most promising differential metabolites and pathogenic pathways in AMD, which contributes to a better understanding of AMD aetiology and also sheds light on its diagnosis and intervention.
Introduction
Age-related macular degeneration (AMD) is one of the leading causes of irreversible visual impairment and blindness in the elderly. The estimated prevalence of AMD is expected to increase to 288 million by 2040 worldwide.1 Clinically, late AMD is classified into dry (or atrophic) and wet (or neovascular) forms. The dry type features drusen deposition and subsequent geographic atrophy (GA), whereas, wet AMD is characterised by choroidal neovascularisation (CNV) and secondary intraretinal or subretinal leakage and haemorrhage. Currently, AMD diagnosis primarily relies on imaging examinations, such as fundus imaging and optical coherence tomography, which provide high-resolution images of the retina in a non-invasive manner. In addition to imaging modalities, exploring other complementary tools to further our understanding of the mechanism of AMD and provide novel targets for diagnosis and treatment is critical for clinical utility.2
AMD is a multifactorial disease with several pathological mechanisms, including ageing, inflammation and metabolic abnormality.3 Small-molecule metabolites are downstream products of genetic transcription and are influenced by environment and diet. Moreover, they can transport throughout the circulatory system and are thus recognised as practical biomarkers for various diseases. Metabolomics is used to qualitatively and quantitatively analyse and describe integrated profiles of metabolites. The analytical techniques frequently used today include nuclear magnetic resonance (NMR), gas chromatography-mass spectrometry (MS) and liquid chromatography-MS. NMR can simultaneously measure many organic compounds; however, its sensitivity is limited to micromolar levels. MS-based methods improve the sensitivity and ability to assay a diverse range of metabolites.4 With the recent development of these advanced technology platforms, metabolomics has been applied to reveal metabolic signatures in many ocular diseases. Thus, a systematic review of existing AMD metabolomics studies is meaningful to seek candidate metabolic features.
In this study, we systematically analysed 16 clinical metabolomics studies on AMD and summarised their results. By screening duplicate metabolites and pathways, we identified the most promising differential metabolites and pathogenic pathways in AMD as candidate biomarkers. These findings contributed to a better understanding of AMD aetiology and also provided novel insights for diagnosis and intervention.
Materials and methods
Literature search
Two investigators (JW and MZ) independently performed this systematic literature review by searching PubMed and Web of Science, including articles published from inception to 31 December 2021. The search terms were formulated as (“metabolomics” or “metabonomics” or “metabolic profiling”) AND (“CNV” or “choroid neovascularization” or “PCV” or “polypoidal choroidal vasculopathy” or “AMD” or “age-related macular degeneration” or “RPE” or “retinal pigment epithelial”). Citations of each included article were examined manually. Agreements were reached on each article by both investigators after careful screening and discussion.
Selection and exclusion criteria
Only articles published in English were included. Studies were excluded for the following reasons: (1) animal or in vitro studies; (2) reviews or meta-analyses; (3) duplicate records and (4) studies without sufficient naming information for identifying metabolites.
Data extraction and processing
Sample types and sizes, populations, metabolite sequencing and analysis platforms, study design methods, and evaluation indicators were extracted and summarised from all studies. MetaboAnalyst V.5.0 (https://www.metaboanalyst.ca/) was used to analyse the pathway enrichment and topology results based on all the metabolites from included studies. Transcriptomic profiles of tissue samples from patients with AMD were obtained from the Gene Expression Omnibus database for network analysis.5 Analysis was performed using the Kyoto Encyclopaedia of Genes and Genomes (KEGG) global metabolic network. P values were calculated based on pathway cluster analysis, and the impact value was obtained from pathway topology results. Statistical significance was set at p<0.05.
Results
Characteristics of the included studies
As shown in online supplemental figure 1, 88 articles were retrieved from online resources. Among them, 16 articles with 4273 patients with AMD and 5290 healthy controls, published between 2013 and 2021, were included in the final analysis. Quality assessment of the included studies using QUADOMICS was presented in online supplemental table 1. 12 of these studies used untargeted sequencing, whereas the others used targeted sequencing. Altogether, four types of sample tissues were analysed: plasma (n=9),26,13 serum (n=3),14,16 both plasma and serum (n=1),17 urine (n=1),18 aqueous humour (n=1)19 and cultured retinal pigment epithelial (RPE) from donors (n=1).20 Three populations were included: American (n=8),26,8 10 11 18 20 Chinese (n=5)9 13 15 16 19 and European (n=5).2 11 12 14 17 Additional details are listed in online supplemental table 2.
Metabolic biomarkers and pathway analysis identified in AMD
We first examined all 16 studies to identify common metabolites in patients with AMD. In total, 322 metabolites were detected in these studies. Among them, 215 were identified using the Human Metabolome Database. Metabolites that had a frequency of two or more reports were categorised as candidate metabolites (table 1). Of these 24 metabolites, glutamine had the highest rate of repetition, which was reported in four studies. Other metabolic biomarkers included 1-palmitoyl-2-arachidonoyl-GPC (16:0/20:4n6), 1-stearoyl-2-arachidonoyl-GPC (18:0/20:4), acetate, adenosine, alanine, carnitine, citrate, creatine, cortexolone, glutamine, glycine, histidine, hypoxanthine, lysine, maltose, N-acetylasparagine, phenylalanine, proline, pyroglutamic acid, pyruvate, tyrosine, valine, oleoyl-linoleoyl-glycerol (18:1/18:2) and oleoyl-arachidonoyl-glycerol (18:1/20:4).
Table 1. Repeated metabolic biomarkers related to AMD.
| Metabolite name | Hits | HMDB | PubChem | Samples | KEGG | |
| PG(16:0/20:4(5Z,8Z,11Z,14Z)) | 2 | HMDB0010580 | 24779550 | Plasma | – | |
| PC(18:0/20:4(5Z,8Z,11Z,14Z)) | 2 | HMDB0008048 | 16219824 | Plasma | – | 1-stearoyl-2–2-arachidonoyl-3-sn-glycero-4–3-phosphocholine |
| Acetate | 2 | HMDB0000042 | 176 | Plasma, serum | C00033 | |
| Adenosine | 2 | HMDB0000050 | 60961 | Plasma | C00212 | |
| Alanine | 2 | HMDB0000161 | 5950 | Plasma, serum | C00041 | |
| Carnitine | 2 | HMDB0000062 | 2724480 | Plasma. aqueous humour | C00318 | |
| Citrate | 2 | HMDB0000094 | 311 | Urine, plasma, serum | C00158 | |
| Creatine | 2 | HMDB0000064 | 586 | Plasma, aqueous humour | C00300 | |
| Cortexolone | 2 | HMDB0000015 | 53477676 | Plasma | ||
| Glutamine | 4 | HMDB0000641 | 5961 | Plasma, serum, plasma | C00064 | |
| Glycine | 2 | HMDB0000123 | 750 | Urine, plasma, serum | C00037 | |
| Histidine | 3 | HMDB0000177 | 6274 | Plasma, serum | C00135 | |
| Hypoxanthine | 2 | HMDB0000157 | 790 | Plasma, serum | C00262 | |
| Lysine | 2 | HMDB0000182 | 5962 | Urine, plasma | C00047 | |
| Maltose | 2 | HMDB0037138 | 439341 | Plasma, serum | C00897 | |
| N-acetylasparagine | 2 | HMDB0006028 | 99 715 | Plasma | – | |
| Phenylalanine | 3 | HMDB0000159 | 6140 | Plasma, aqueous humour, serum | C00079 | |
| Proline | 3 | HMDB0000162 | 145742 | Plasma, aqueous humour | C00148 | |
| Pyroglutamic acid | 2 | HMDB0000267 | 7405 | Plasma | C01879 | |
| Pyruvate | 3 | HMDB0000243 | 1060 | Plasma, urine, serum | C00022 | |
| Tyrosine | 3 | HMDB0000158 | 6057 | Plasma, urine, serum | C00082 | |
| Valine | 3 | HMDB0000883 | 6287 | Urine, plasma, serum | C00183 | |
| DG(18:1(9Z)/18:2 (9Z,12Z)/0:0) | 2 | HMDB0007219 | 9543722 | Plasma | – | 1-oleoyl-2–2-linoleoyl-3-sn-glycerol |
| DG(18:1(9Z)/20:4 (5Z,8Z,11Z,14Z)/0:0) | 2 | HMDB0007228 | 9543786 | Plasma | – | 1-oleoyl-2–2-arachidonoyl-3-sn-glycerol |
AMD, age-related macular degeneration; HMDB, Human Metabolome DatabaseKEGG, Kyoto Encyclopaedia of Genes and Genomes
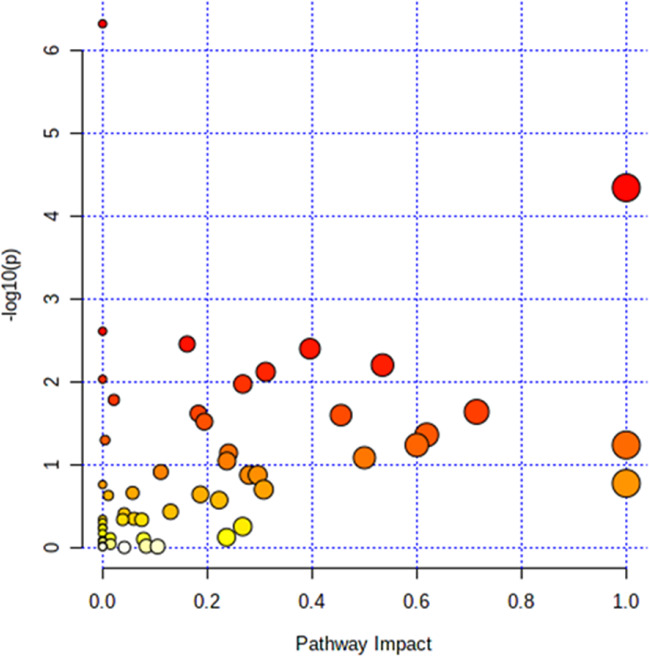
Pathway and KEGG analyses were performed for these 24 metabolites. As shown in figure 1 and online supplemental table 3, the phenylalanine, tyrosine and tryptophan pathways had hits for all four components and showed the highest impact value (impact=1.000, p=4.60E−05). Details of the other 15 enriched pathways (p<0.05) are listed in online supplemental table 3. In addition, 11 KEGG pathways (p<0.05) were enriched; of these, aminoacyl-tRNA biosynthesis ranked the highest (online supplemental table 4).
Figure 1. Graph showing pathway analysis based on metabolites identified. Colour of the circles is proportional to the enrichment significance. Redder colour represents greater significance level. Size of the circles indicates the pathway impact value. Larger size represents higher impact value.
Metabolic biomarkers and pathway analysis identified in wet AMD
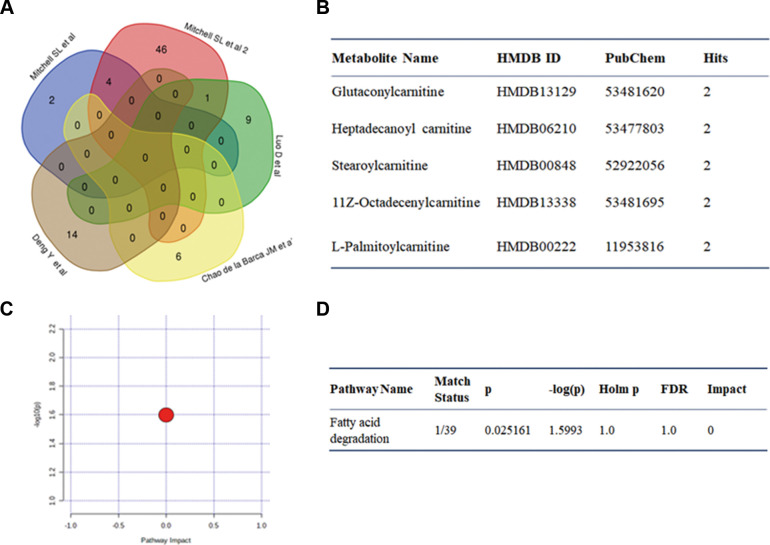
Of the 16 articles, 5 included patients with wet AMD; 5 differential metabolites were reported more than once between wet AMD and controls, including glutaconylcarnitine, heptadecanoyl carnitine, stearoylcarnitine, 11Z-octadecenylcarnitine and L-palmitoylcarnitine (figure 2A,B). Four of these were identified in two studies performed by Mitchell et al, both of which were incorporated into our analysis due to different sample sizes and analytical approaches. Fatty acid degradation was enriched for these five metabolites (figure 2C,D).
Figure 2. Graph showing pathway analysis based on metabolites identified in wet AMD. (A) Venn graph. (B) Repeated metabolites. (C, D) Pathway analysis. AMD, age-related macular degeneration; FDR, false discovery rate.
Metabolic biomarkers and pathway analysis identified in PCV
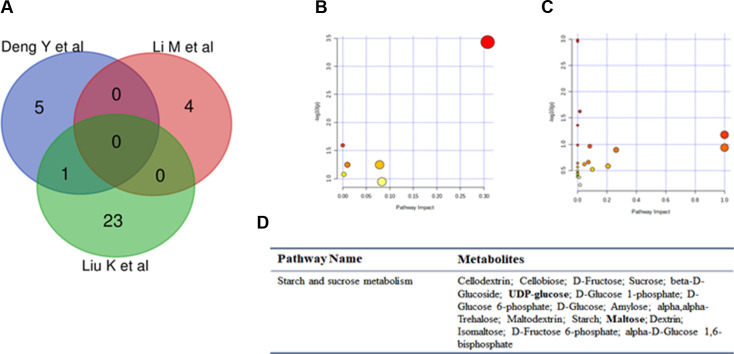
Polypoidal choroidal vasculopathy (PCV) is a subtype of wet AMD and is particularly prevalent in Asian populations.21 Its low prevalence and ethnic clustering characteristics compared with other subtypes of wet AMD resulted in limited studies. Three studies included samples from patients with PCV. We manually extracted altered metabolites and performed an advanced test (figure 3A–C). 32 KEGG pathways were identified, and only the starch and sucrose metabolism pathways were repeated in the Venn graph (figure 3D).
Figure 3. Graph showing pathway analysis based on metabolites identified in PCV patients. (A) Venn graph. (B, C) Pathway analysis. (C, D) Repeated metabolites and pathway analysis. PCV, polypoidal choroidal vasculopathy.
Prediction power of the potential metabolites
Several studies evaluated the predictive potential of the identified metabolites or metabolite groups. Mitchell et al7 found 159 plasma metabolites from 100 patients with wet AMD and 192 controls. These 159 metabolites showed a 10-fold cross-validation balanced accuracy rate of 96.1% in the training set and 75.6% in the test set. Laíns et al2 found 69 metabolites in the plasma of 391 patients with AMD and 100 controls. By analysing the stage+2 eye model, the area under the curve (AUC) value was 0.815, which is better than 0.789 for the AMD/control model and 0.725 for the baseline model considering demographic covariates alone. Kersten et al14 screened four relevant metabolites (glutamine, the Glu:Gln ratio, glutaminolysis and PC.aa.C28.1). This category achieved an AUC value of 0.71. Li et al15 found 41 differential metabolites in the serum of 21 patients with PCV and 19 controls. The metabolites LPA (18:2), LPC (20:4), PC (20:1 p/19:1), SM (d16:0/22:2), PAF (35:4), PC (16:0/22:5) and PC (18:1/20:4) had an AUC of ≥0.8.
Network analysis of genes and metabolites in AMD
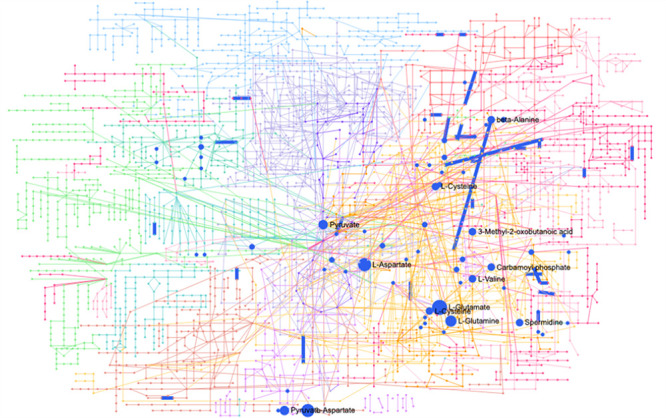
With the combination of CNV transcriptomic profiles in network analysis using MetaboAnalyst V.5.0, 11 metabolic pathways were derived. Among them, two of the pathways, biosynthesis of unsaturated fatty acids and synthesis and degradation of ketone bodies pathways, were not reported in the pathway enrichment analysis above (figure 4).
Figure 4. Network analysis of genes and metabolites in AMD. AMD, age-related macular degeneration.
Discussion
In this study, we assessed 16 studies on metabolomic profiles in patients with AMD. A total of 24 metabolites were detected to be significantly different between individuals with AMD and controls in at least two studies. Of these, glutamine was reported the most frequently. After analysing the metabolic pathways of these differential metabolites, we revealed an enrichment of 15 different pathways, including phenylalanine, tyrosine and tryptophan metabolism pathways. Accordingly, these findings identified potential biomarkers for AMD diagnosis and revealed the metabolic characteristics related to AMD.
Metabolomics is a newly emerging and promising method for revealing physiological or pathological status in a multidimensional manner. The recent advancement of NMR and MS technology helps detecting a wide range of metabolites and provides a comprehensive view of metabolic changes, which promotes the understanding of pathological mechanisms in diseases. Meanwhile, a single metabolite or metabolic panel can function as a biomarker for early diagnosis and prognosis evaluation. As the retina is a highly metabolically active tissue, metabolic studies have been widely performed in retinal diseases, such as AMD and diabetic retinopathy (DR). As a multifactorial disease, several pathological mechanisms have been identified in AMD, such as ageing, inflammation and metabolic abnormality. However, the exact pathogenesis is still unclear. Although antivascular endothelial growth factor (VEGF) injection is the first-line therapy for wet AMD, as many as 45% of patients were reported to be non-responsive to it.22 Therefore, it is critical to discover potential diagnosis biomarkers, as well as treatment approaches and prognosis prediction targets in AMD.
As suggested by our results, phenylalanine, tyrosine and tryptophan biosynthesis had the highest impact on pathway analysis. It belongs to glucogenic and ketogenic aromatic amino acid metabolism, and tryptophan and phenylalanine are both essential amino acids. Studies have recognised the varied content with the progression of retinal degeneration induced by ATP. Upregulated tyrosine metabolism and inflammatory pathways contributed to the pathogenesis of myopia-induced retinal degeneration.23 In the ketogenic pathway, they acted upstream of catecholamine (dopamine, norepinephrine and epinephrine) and melanin metabolism. The introduction of levodopa was reported to delay anti-VEGF treatment in patients with AMD,24 and a dopamine receptor antagonist (L745,870) was found to reduce drusen deposits and restore the RPE epithelial phenotype.25 Melanin was also recognised as an antioxidative reagent in RPE cells. Although the exact relationship between phenylalanine, tyrosine and AMD was still unclear, these findings supported their role in the pathogenesis of the disease.
Taurine and hypotaurine metabolism was the second most influential pathway, with an impact of 0.71428. Taurine is highly expressed in retina and brain. It is a metabolite with pharmacological activity and anti-inflammatory effects and is involved in energy metabolism. Taurine treatment increased the survival of photoreceptors and retinal ganglion cells. It brought out an increase in the glutathione/oxidised glutathione ratio, a decrease in oxidative stress levels and even suppression of epithelial mesenchymal transition.26 Since RPE mitochondrial dysfunction and reactive oxygen species generation were both pathological in early AMD, our findings suggested that taurine might be a potential treatment strategy.
Alanine, aspartate and glutamate metabolism was the third most important clustered pathway, in which glutamine was involved. Glutamate is a dominant amino acid neurotransmitter that mediates fast point-to-point synaptic transmission in retina and other parts of the central nervous system. Glutamate agonists cause visual dysfunction in retinal cells via the OFF pathway.27 Another metabolic study reported a similar change in aspartate and glutamate metabolism and glutamine disorder in the aqueous humour of patients with DR.28 Researchers attributed this metabolic change to the tricarboxylic acid (TCA) cycle. In DR, hyperglycaemia and hypoxia might influence the accumulation of electrons and protons, causing a decrease in the NAD/NADH ratio and enzymes, such as isocitrate dehydrogenase, which controlled the TCA cycle. As a glucogenic amino acid, glutamine was converted to CO2 and H2O to generate ATP via the TCA cycle. Similarly, hypoxia was a vital pathological mechanism of AMD. In wet AMD, hypoxia stimulated expression of angiogenic growth factors such as VEGF and promoted neovascularisation development. Animal studies have demonstrated the therapeutic effects of hypoxia-inducible factor inhibitors in laser-induced wet AMD mouse models.29 In addition to wet AMD, hypoxia was also a possible contributing factor to dry AMD. Vascular drop-out of the choriocapillaris was a hallmark of GA, which could induce hypoxia. Although the TCA cycle itself was not significantly enriched in our study (p=0.22859, false discovery rate=0.65868), cis-aconitate, citrate and pyruvate had one, two and three hits, respectively. Consequently, alanine, aspartate and glutamate metabolism, along with the TCA cycle, were promising metabolic targets.
Limitations
Our research summarised current metabolic studies on AMD and attempted to provide novel findings on the basis of previous work. In this way, we were able to analyse all the altered metabolites from different samples in a large number of participants to obtain as many descriptions of the metabolic state of AMD as possible. However, there are still several limitations. First, participants in the 16 studies were from different ethnic groups. Additionally, the inclusion criteria for each study were not unified. No consensus was reached regarding the recruited AMD stages among these studies. Technically, these studies applied various sample types and metabolic platforms; therefore, the identification and analysis procedures might be quite different. These factors might result in bias among these studies, but we attempted to combine and extract the most common metabolites, even in these circumstances. Thus, they could be recognised as the most potent biomarkers or targets. In future studies, more attention can be paid to metabolic differences of various biological samples, as well as various disease subtypes and severity, so as to gradually draw a comprehensive metabolic profile for AMD.
Conclusion
In summary, our research determined the discriminant metabolic characteristics in AMD by summarising previous studies, which was helpful in furthering our understanding of the mechanism of AMD as well as providing novel targets for clinical management. Glutamine, 1-palmitoyl-2-arachidonoyl-GPC (16:0/20:4n6), 1-stearoyl-2-arachidonoyl-GPC (18:0/20:4), acetate, adenosine, alanine, carnitine, citrate, creatine, cortexolone, glutamine, glycine, histidine, hypoxanthine, lysine, maltose, N-acetylasparagine, phenylalanine, proline, pyroglutamic acid, pyruvate, tyrosine, valine, oleoyl-linoleoyl-glycerol (18:1/18:2) and oleoyl-arachidonoyl-glycerol (18:1/20:4) were identified as main differential metabolites. Phenylalanine, tyrosine and tryptophan biosynthesis; taurine and hypotaurine metabolism and alanine, aspartate and glutamate metabolism were the top three clustered pathways in AMD development. Studies with more precise classifications, larger sampling and powerful data analysis are warranted to verify the applicability of these findings.
supplementary material
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China (81730026, 82171076), National Science and Technology Major Project (2017YFA0105301), Science and Technology Commission of Shanghai Municipality (20Z11900400).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Contributor Information
Jiali Wu, Email: wujialii7@163.com.
Min Zhang, Email: min.zhang94@qq.com.
Xiaodong Sun, Email: xdsun@sjtu.edu.cn.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
References
- 1.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–16. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 2.Laíns I, Chung W, Kelly RS, et al. Human plasma metabolomics in age-related macular degeneration: meta-analysis of two cohorts. Metabolites. 2019;9:127. doi: 10.3390/metabo9070127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fritsche LG, Fariss RN, Stambolian D, et al. Age-related macular degeneration: genetics and biology coming together. Annu Rev Genomics Hum Genet. 2014;15:151–71. doi: 10.1146/annurev-genom-090413-025610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azad RK, Shulaev V. Metabolomics technology and bioinformatics for precision medicine. Brief Bioinform . 2019;20:1957–71. doi: 10.1093/bib/bbx170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schlecht A, Boneva S, Gruber M, et al. Transcriptomic characterization of human choroidal neovascular membranes identifies calprotectin as a novel biomarker for patients with age-related macular degeneration. Am J Pathol. 2020;190:1632–42. doi: 10.1016/j.ajpath.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell SL, Ma C, Scott WK, et al. Plasma metabolomics of intermediate and neovascular age-related macular degeneration patients. Cells. 2021;10:3141. doi: 10.3390/cells10113141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitchell SL, Uppal K, Williamson SM, et al. The carnitine shuttle pathway is altered in patients with neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2018;59:4978–85. doi: 10.1167/iovs.18-25137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laíns I, Kelly RS, Miller JB, et al. Human plasma metabolomics study across all stages of age-related macular degeneration identifies potential lipid biomarkers. Ophthalmology. 2018;125:245–54. doi: 10.1016/j.ophtha.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo D, Deng T, Yuan W, et al. Plasma metabolomic study in Chinese patients with wet age-related macular degeneration. BMC Ophthalmol. 2017;17:165. doi: 10.1186/s12886-017-0555-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osborn MP, Park Y, Parks MB, et al. Metabolome-wide association study of neovascular age-related macular degeneration. PLoS One. 2013;8:e72737. doi: 10.1371/journal.pone.0072737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laíns I, Duarte D, Barros AS, et al. Human plasma metabolomics in age-related macular degeneration (AMD) using nuclear magnetic resonance spectroscopy. PLoS One. 2017;12:e0177749. doi: 10.1371/journal.pone.0177749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chao de la Barca JM, Rondet-Courbis B, Ferré M, et al. A plasma metabolomic profiling of exudative age-related macular degeneration showing carnosine and mitochondrial deficiencies. J Clin Med. 2020;9:631. doi: 10.3390/jcm9030631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng Y, Shuai P, Wang H, et al. Untargeted metabolomics for uncovering plasma biological markers of wet age-related macular degeneration. Aging . 2021;13:13968–4000. doi: 10.18632/aging.203006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kersten E, Dammeier S, Ajana S, et al. Metabolomics in serum of patients with non-advanced age-related macular degeneration reveals aberrations in the glutamine pathway. PLoS One. 2019;14:e0218457. doi: 10.1371/journal.pone.0218457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li M, Zhang X, Liao N, et al. Analysis of the serum lipid profile in polypoidal choroidal vasculopathy. Sci Rep. 2016;6:38342. doi: 10.1038/srep38342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu K, Fang J, Jin J, et al. Serum metabolomics reveals personalized metabolic patterns for macular neovascular disease patient stratification. J Proteome Res. 2020;19:699–707. doi: 10.1021/acs.jproteome.9b00574. [DOI] [PubMed] [Google Scholar]
- 17.Acar İE, Lores-Motta L, Colijn JM, et al. Integrating metabolomics, genomics, and disease pathways in age-related macular degeneration: the EYE-RISK consortium. Ophthalmology. 2020;127:1693–709. doi: 10.1016/j.ophtha.2020.06.020. [DOI] [PubMed] [Google Scholar]
- 18.Laíns I, Duarte D, Barros AS, et al. Urine Nuclear Magnetic Resonance (NMR) metabolomics in age-related macular degeneration. J Proteome Res. 2019;18:1278–88. doi: 10.1021/acs.jproteome.8b00877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han G, Wei P, He M, et al. Metabolomic profiling of the aqueous humor in patients with wet age-related macular degeneration using UHPLC-MS/MS. J Proteome Res. 2020;19:2358–66. doi: 10.1021/acs.jproteome.0c00036. [DOI] [PubMed] [Google Scholar]
- 20.Zhang M, Jiang N, Chu Y, et al. Dysregulated metabolic pathways in age-related macular degeneration. Sci Rep. 2020;10:2464. doi: 10.1038/s41598-020-59244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheung CMG, Lai TYY, Ruamviboonsuk P, et al. Polypoidal choroidal vasculopathy: definition, pathogenesis. Ophthalmology. 2018;125:708–24. doi: 10.1016/j.ophtha.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 22.Lux A, Llacer H, Heussen FMA, et al. Non-responders to bevacizumab (Avastin) therapy of choroidal neovascular lesions. Br J Ophthalmol. 2007;91:1318–22. doi: 10.1136/bjo.2006.113902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng L, Li X, Liu J, et al. RNA-Seq analysis reveals an essential role of the tyrosine metabolic pathway and inflammation in myopia-induced retinal degeneration in guinea pigs. Int J Mol Sci. 2021;22:12598. doi: 10.3390/ijms222212598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Figueroa AG, Boyd BM, Christensen CA, et al. Levodopa positively affects neovascular age-related macular degeneration. Am J Med. 2021;134:122–8. doi: 10.1016/j.amjmed.2020.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma R, George A, Nimmagadda M, et al. Epithelial phenotype restoring drugs suppress macular degeneration phenotypes in an iPSC model. Nat Commun. 2021;12:7293. doi: 10.1038/s41467-021-27488-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Homma K, Toda E, Osada H, et al. Taurine rescues mitochondria-related metabolic impairments in the patient-derived induced pluripotent stem cells and epithelial-mesenchymal transition in the retinal pigment epithelium. Redox Biology. 2021;41:101921. doi: 10.1016/j.redox.2021.101921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milla-Navarro S, Diaz-Tahoces A, Ortuño-Lizarán I, et al. Visual disfunction due to the selective effect of glutamate agonists on retinal cells. Int J Mol Sci. 2021;22:6245. doi: 10.3390/ijms22126245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin H, Zhu B, Liu X, et al. Metabolic characterization of diabetic retinopathy: an (1)H-NMR-based metabolomic approach using human aqueous humor. J Pharm Biomed Anal. 2019;174:414–21. doi: 10.1016/j.jpba.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 29.Ibuki M, Shoda C, Miwa Y, et al. Therapeutic effect of garcinia cambogia extract and hydroxycitric acid inhibiting hypoxia-inducible factor in a murine model of age-related macular degeneration. Int J Mol Sci. 2019;20:5049. doi: 10.3390/ijms20205049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.