Abstract
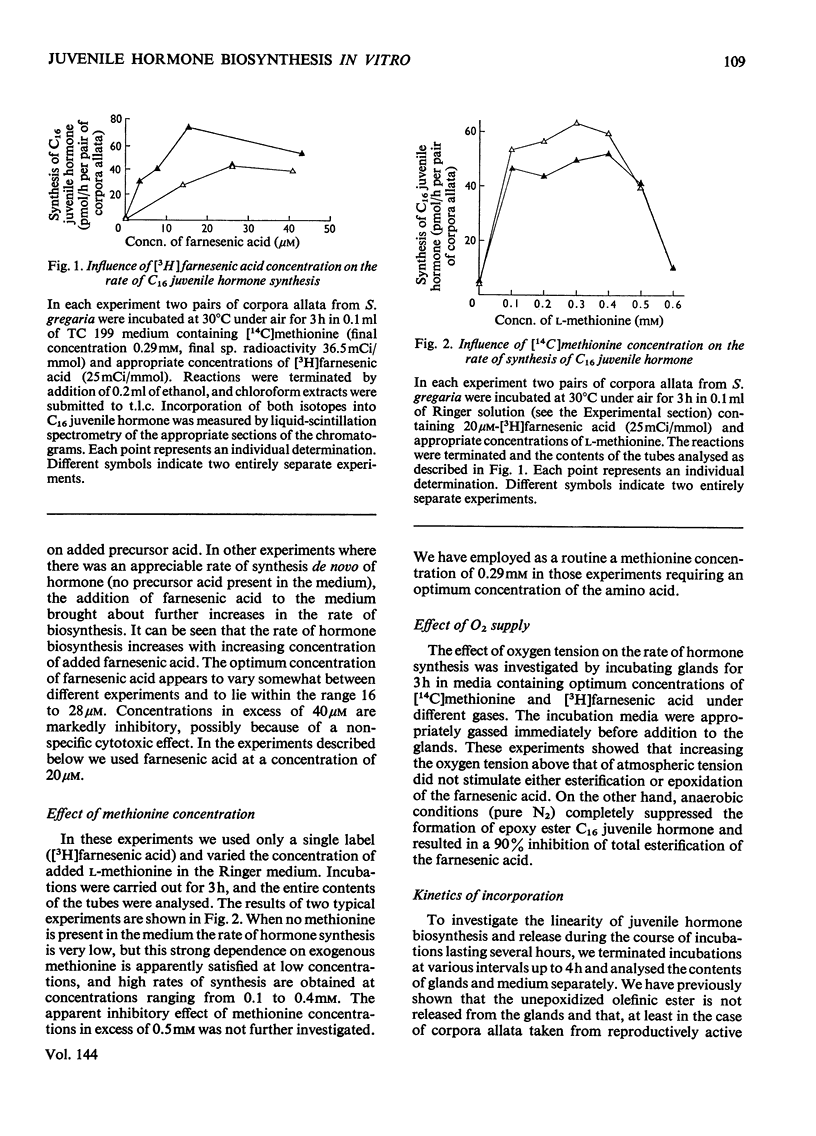
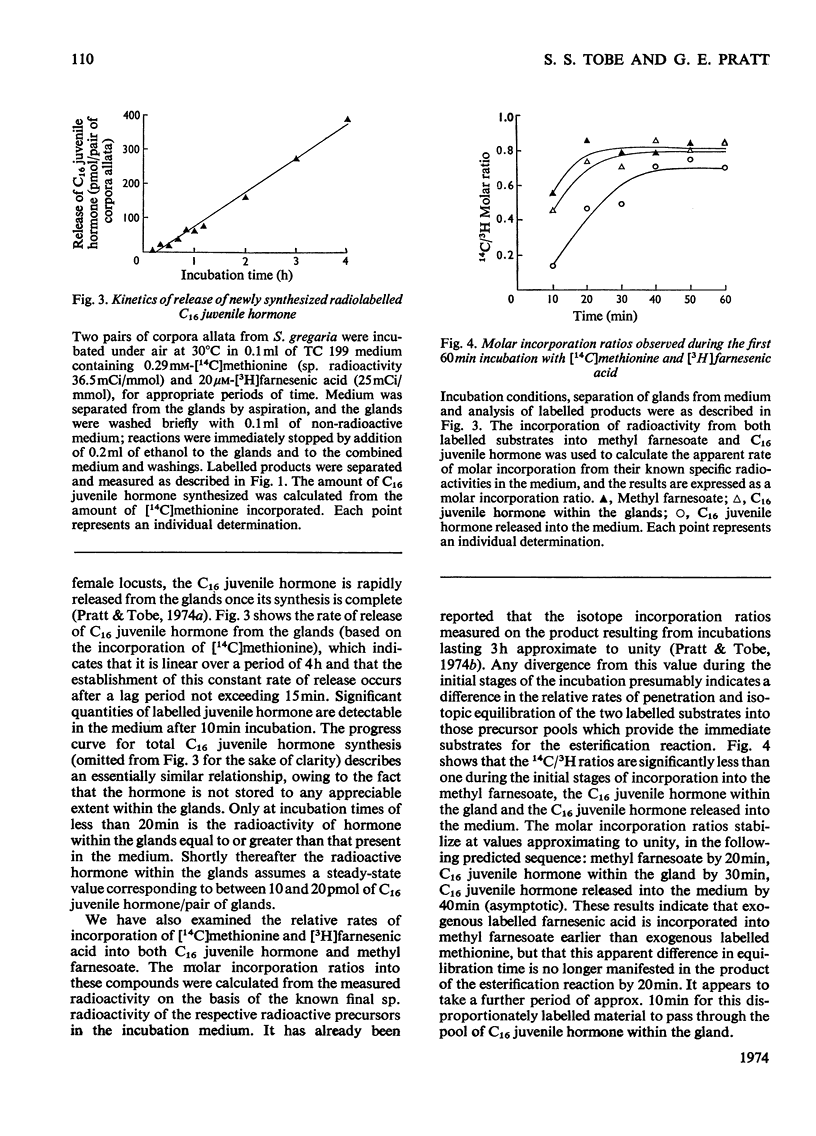
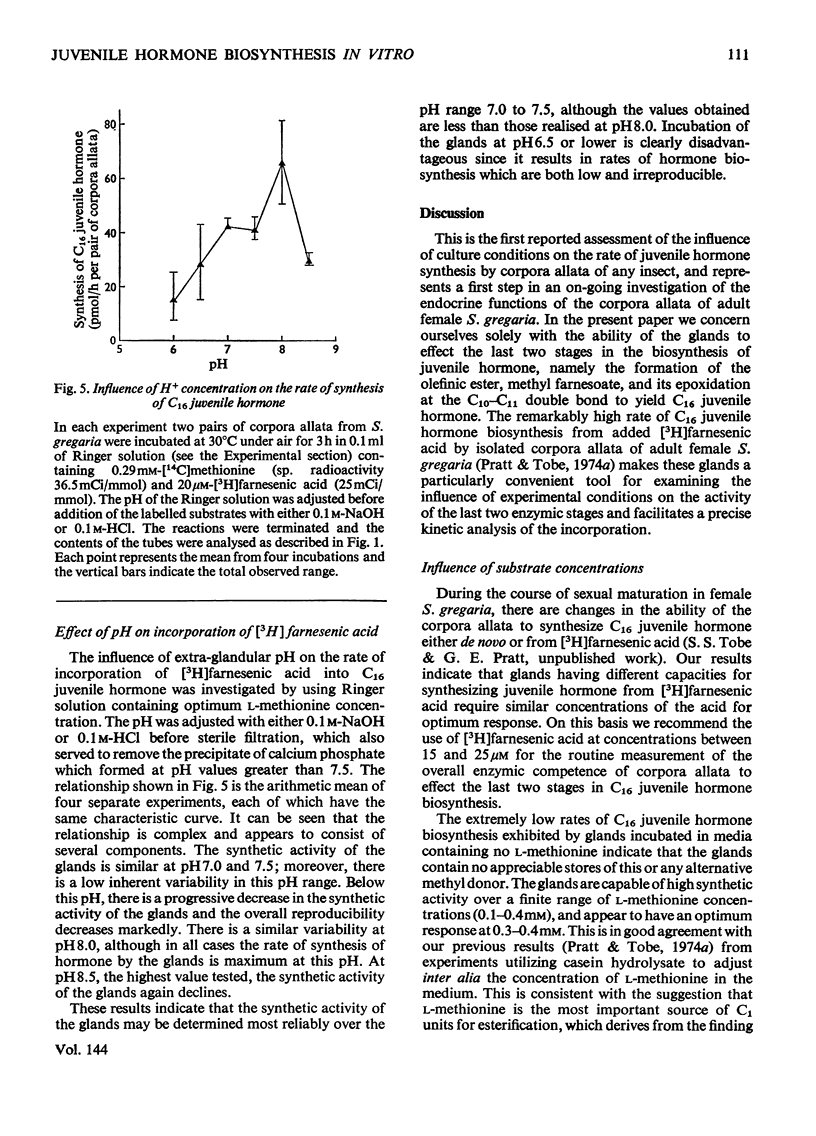
The rate at which isolated corpora allata of adult female Schistocerca gregaria incorporate [3H]farnesenic acid and [14C]methionine into C16juvenile hormone in vitro was examined at different concentrations of farnesenic acid, methionine, O2 and H+ ions. Maximum juvenile hormone biosynthesis is obtained at a farnesenic acid concentration of 20μm. The range of optimum l-methionine concentrations (0.1–0.4mm) encompasses the physiological concentration of this substrate in the haemolymph. Hormone biosynthesis is dependent on O2, but is not stimulated by hyperbaric oxygen tension. The glands had a maximum synthetic activity at pH8.0, but their activity was more reproducible in the the physiological range pH7.0–7.5. At pH6.5 and less, the synthetic ability was considerably decreased. The relative incorporations of the labelled substrates into methyl farnesoate and C16 juvenile hormone indicate that [3H]farnesenic acid comes into isotopic equilibrium within the gland more rapidly than [14C]methionine. The incorporations into methyl farnesoate become stoicheiometric after 20min incubation and into C16 juvenile hormone after a further 10min. Labelled juvenile hormone is detectable after 10min incubation and the rate of incorporation is constant for up to 4h. It is proposed that the described method may be usefully employed to assess the physiological changes in the enzymic competence of the glands to effect the last two stages in C16 juvenile hormone biosynthesis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benassi C. A., Colombo G., Allegri G. Free amino acids of the haemolymph of Schistocerca gregaria Forsk. Biochem J. 1961 Aug;80(2):332–336. doi: 10.1042/bj0800332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRACE T. D. Establishment of four strains of cells from insect tissues grown in vitro. Nature. 1962 Aug 25;195:788–789. doi: 10.1038/195788a0. [DOI] [PubMed] [Google Scholar]
- Judy K. J., Schooley D. A., Dunham L. L., Hall M. S., Bergot B. J., Siddall J. B. Isolation, Structure, and Absolute Configuration of a New Natural Insect Juvenile Hormone from Manduca sexta. Proc Natl Acad Sci U S A. 1973 May;70(5):1509–1513. doi: 10.1073/pnas.70.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judy K. J., Schooley D. A., Hall M. S., Bergot B. J., Siddall J. B. Chemical structure and absolute configuration of a juvenile hormone from grasshopper corpora allata in vitro. Life Sci. 1973 Dec 1;13(11):1511–1516. doi: 10.1016/0024-3205(73)90139-2. [DOI] [PubMed] [Google Scholar]
- Pratt G. E., Tobe S. S. Juvenile hormones radiobiosynthesised by corpora allata of adult female locusts in vitro. Life Sci. 1974 Feb 1;14(3):575–586. doi: 10.1016/0024-3205(74)90372-5. [DOI] [PubMed] [Google Scholar]
- Reibstein D., Law J. H. Enzymatic synthesis of insect juvenile hormones. Biochem Biophys Res Commun. 1973 Nov 16;55(2):266–272. doi: 10.1016/0006-291x(73)91083-8. [DOI] [PubMed] [Google Scholar]
- Röller H., Dahm K. H. The identity of juvenile hormone produced by Corpora allata in vitro. Naturwissenschaften. 1970 Sep;57(9):454–455. doi: 10.1007/BF00607739. [DOI] [PubMed] [Google Scholar]


