Abstract
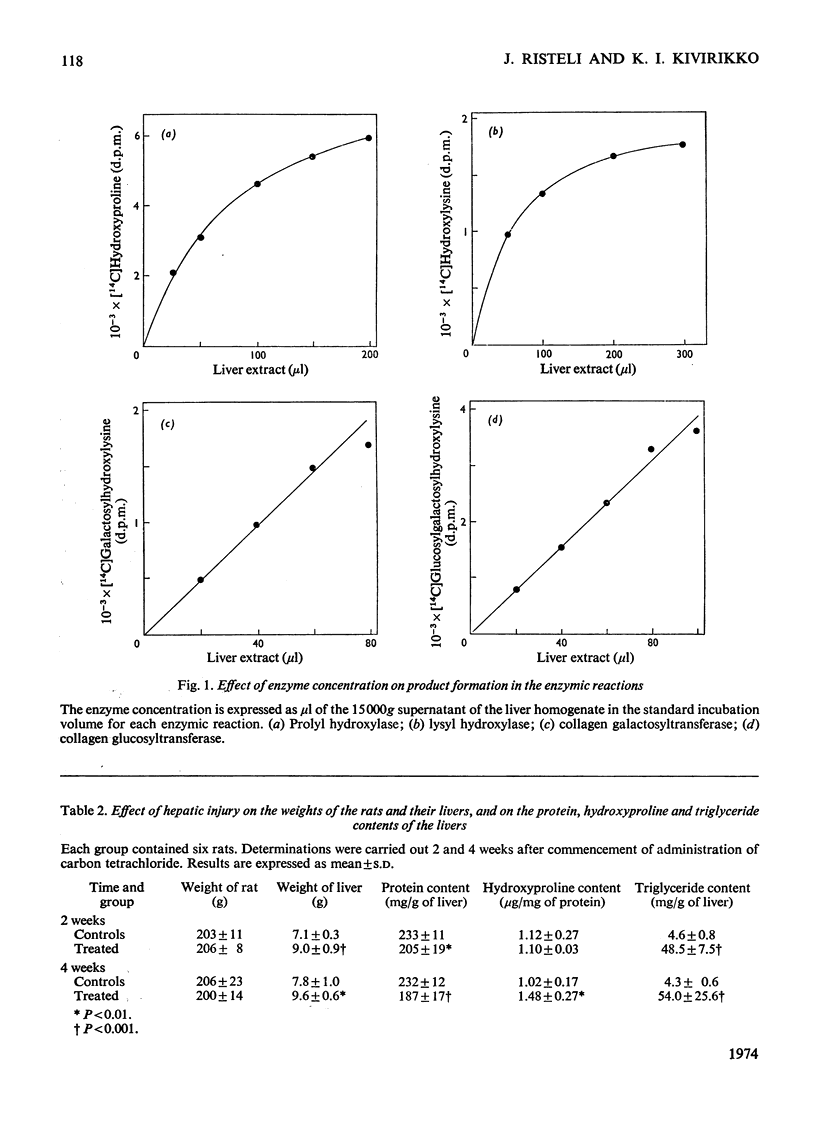
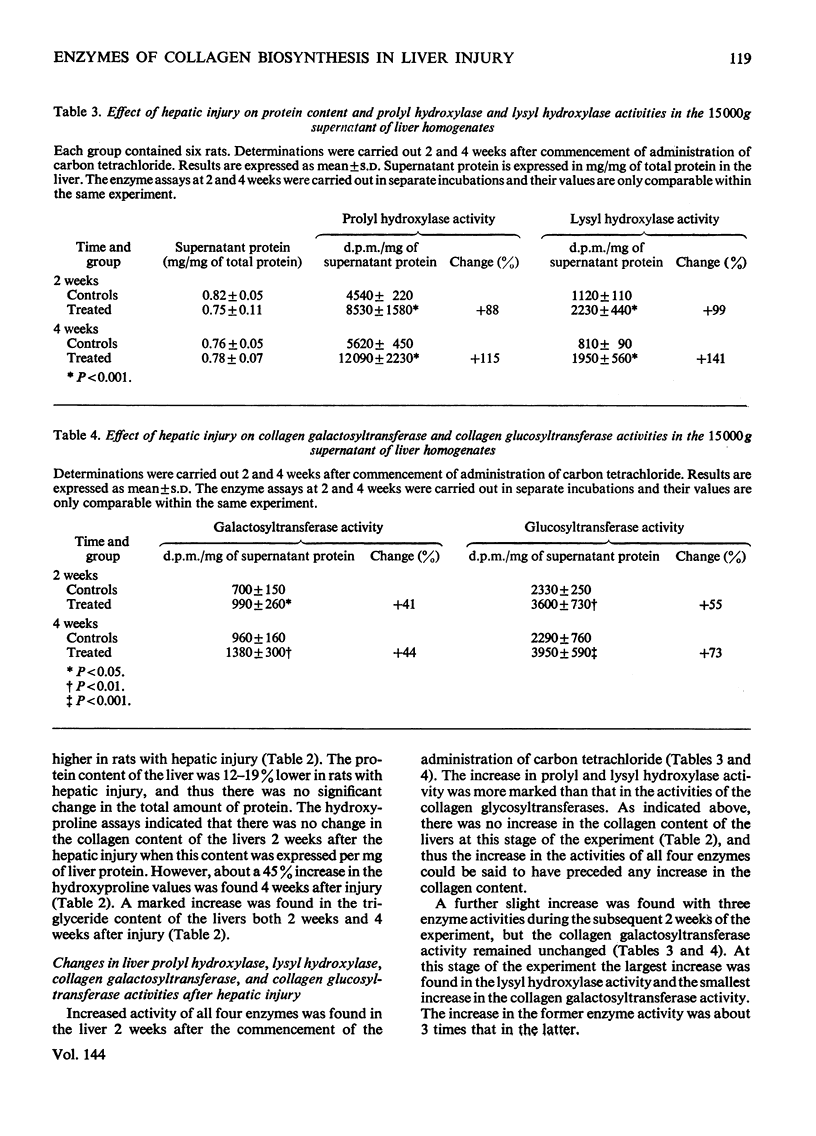
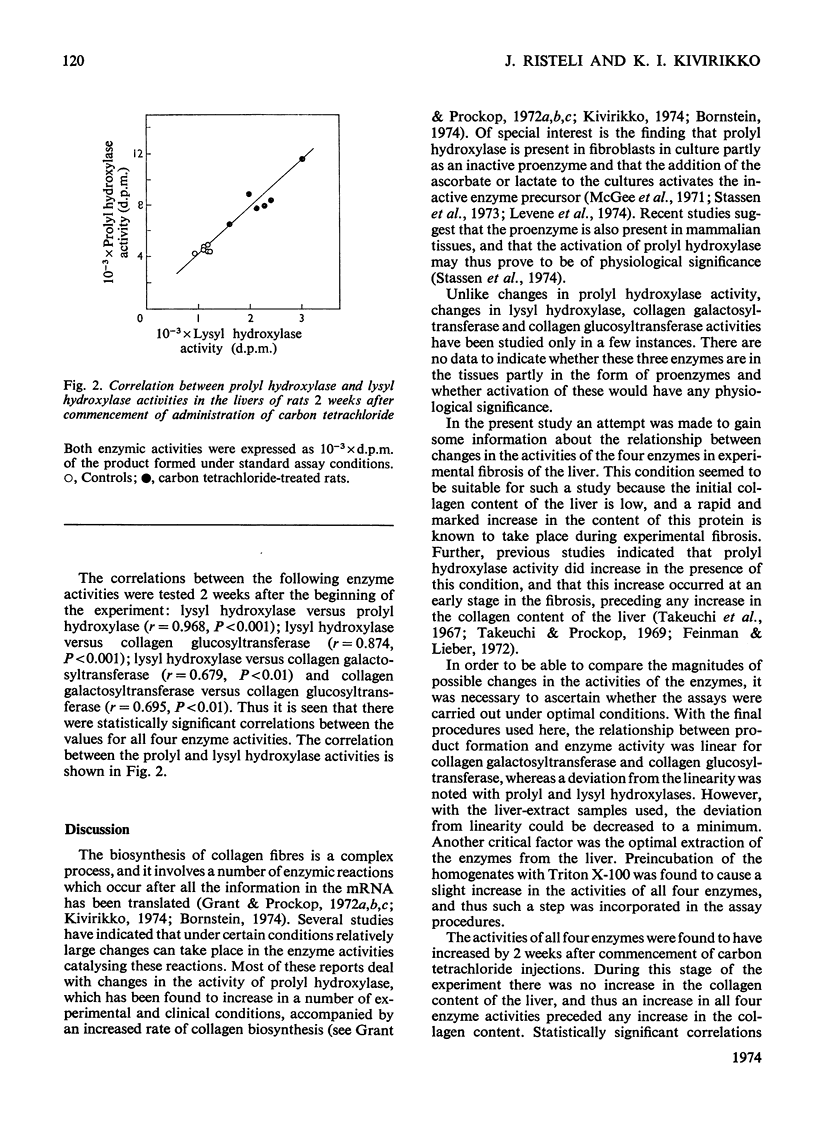
The activities of four enzymes catalysing post-translational modifications of the collagen polypeptide chains were assayed in the livers of rats with experimental hepatic injury. The liver injury was induced by injecting carbon tetrachloride twice weekly, and assays of the enzymic activities were carried out 2 and 4 weeks after commencement of administration of carbon tetrachloride. The liver homogenates were preincubated with Triton X-100 before the assays, because such treatment was found to increase the activities of all four enzymes in the supernatants of liver homogenates. The activities of all four enzymes had increased by 2 weeks after commencement of carbon tetrachloride administration. No increase was found in the collagen content of the livers at this stage and thus an increase in all four enzyme activities preceded an increase in the collagen content of the liver. A further slight increase was found in three of the enzyme activities during the subsequent 2 weeks of the experiment, whereas no further increase was found in the collagen galactosyltransferase activity. A statistically significant correlation was found between all four enzyme activities, but the magnitude of the increases varied considerably. The largest increase was found in lysyl hydroxylase activity, and at 4 weeks the magnitude of this was about three times that of the collagen galactosyltransferase activity. The results thus indicate that the increased enzyme activities cannot be explained simply by an increase in the number of collagen-producing cells having similar enzyme activity patterns to those of the cells initially present in the liver.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anttinen H., Orava S., Ryhänen L., Kivirikko K. I. Assay of protocollagen lysyl hydroxylase activity in the skin of human subjects and changes in the activity with age. Clin Chim Acta. 1973 Aug 30;47(2):289–294. doi: 10.1016/0009-8981(73)90326-4. [DOI] [PubMed] [Google Scholar]
- Berg R. A., Prockop D. J. Affinity column purification of protocollagen proline hydroxylase from chick embryos and further characterization of the enzyme. J Biol Chem. 1973 Feb 25;248(4):1175–1182. [PubMed] [Google Scholar]
- Blumenkrantz N., Prockop D. J. A rapid assay for 14C-labeled hydroxylysine in collagen and related materials. Anal Biochem. 1969 Sep;30(3):377–385. doi: 10.1016/0003-2697(69)90130-4. [DOI] [PubMed] [Google Scholar]
- CARLSON L. A. DETERMINATION OF SERUM TRIGLYCERIDES. J Atheroscler Res. 1963 Jul-Aug;3:334–336. doi: 10.1016/s0368-1319(63)80012-5. [DOI] [PubMed] [Google Scholar]
- Feinman L., Lieber C. S. Hepatic collagen metabolism: effect of alcohol consumption in rats and baboons. Science. 1972 May 19;176(4036):795–795. doi: 10.1126/science.176.4036.795. [DOI] [PubMed] [Google Scholar]
- Grant M. E., Prockop D. J. The biosynthesis of collagen. 1. N Engl J Med. 1972 Jan 27;286(4):194–199. doi: 10.1056/NEJM197201272860406. [DOI] [PubMed] [Google Scholar]
- Grant M. E., Prockop D. J. The biosynthesis of collagen. 2. N Engl J Med. 1972 Feb 3;286(5):242–249. doi: 10.1056/NEJM197202032860505. [DOI] [PubMed] [Google Scholar]
- Grant M. E., Prockop D. J. The biosynthesis of collagen. 3. N Engl J Med. 1972 Feb 10;286(6):291–300. doi: 10.1056/NEJM197202102860604. [DOI] [PubMed] [Google Scholar]
- Guzman N. A., Cutroneo K. R. Association of prolyl hydroxylase activity with membranes. Biochem Biophys Res Commun. 1973 Jun 19;52(4):1263–1270. doi: 10.1016/0006-291x(73)90637-2. [DOI] [PubMed] [Google Scholar]
- Halme J., Kivirikko K. I., Simons K. Isolation and partial characterization of highly purified protocollagen proline hydroxylase. Biochim Biophys Acta. 1970 Mar 18;198(3):460–470. doi: 10.1016/0005-2744(70)90124-5. [DOI] [PubMed] [Google Scholar]
- Juva K., Prockop D. J. Modified procedure for the assay of H-3-or C-14-labeled hydroxyproline. Anal Biochem. 1966 Apr;15(1):77–83. doi: 10.1016/0003-2697(66)90249-1. [DOI] [PubMed] [Google Scholar]
- Kirschbaum B. B., Bosmann H. B. The interaction of folic acid and glycoprotein glycosyltransferase activities of rat kidney. Biochim Biophys Acta. 1973 Sep 14;320(2):416–426. doi: 10.1016/0304-4165(73)90323-1. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Laitinen O., Prockop D. J. Modifications of a specific assay for hydroxyproline in urine. Anal Biochem. 1967 May;19(2):249–255. doi: 10.1016/0003-2697(67)90160-1. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Prockop D. J. Enzymatic hydroxylation of proline and lysine in protocollagen. Proc Natl Acad Sci U S A. 1967 Mar;57(3):782–789. doi: 10.1073/pnas.57.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivirikko K. I., Prockop D. J. Partial purification and characterization of protocollagen lysine hydroxylase from chick embryos. Biochim Biophys Acta. 1972 Feb 28;258(2):366–379. doi: 10.1016/0005-2744(72)90228-8. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Shudo K., Sakakibara S., Prockop D. J. Studies on protocollagen lysine hydroxylase. Hydroxylation of synthetic peptides and the stoichiometric decarboxylation of -ketoglutarate. Biochemistry. 1972 Jan 4;11(1):122–129. doi: 10.1021/bi00751a021. [DOI] [PubMed] [Google Scholar]
- Krane S. M., Pinnell S. R., Erbe R. W. Lysyl-protocollagen hydroxylase deficiency in fibroblasts from siblings with hydroxylysine-deficient collagen. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2899–2903. doi: 10.1073/pnas.69.10.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langness U., Udenfriend S. Collagen biosynthesis in nonfibroblastic cell lines. Proc Natl Acad Sci U S A. 1974 Jan;71(1):50–51. doi: 10.1073/pnas.71.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee J. O., Langness U., Udenfriend S. Immunological evidence for an inactive precursor of collagen proline hydroxylase in cultured fibroblasts. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1585–1589. doi: 10.1073/pnas.68.7.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee J. O., Patrick R. S. The role of perisinusoidal cells in hepatic fibrogenesis. An electron microscopic study of acute carbon tetrachloride liver injury. Lab Invest. 1972 Apr;26(4):429–440. [PubMed] [Google Scholar]
- Ohuchi K., Tsurufuji S. Protocollagen proline hydroxylase in isolated rat liver cells. Biochim Biophys Acta. 1972 Mar 8;258(3):731–740. doi: 10.1016/0005-2744(72)90174-x. [DOI] [PubMed] [Google Scholar]
- Popper H., Uenfriend S. Hepatic fibrosis. Correlation of biochemical and morphologic investigations. Am J Med. 1970 Nov;49:707–721. doi: 10.1016/s0002-9343(70)80135-8. [DOI] [PubMed] [Google Scholar]
- RUBINSTEIN H. M., PRYCE J. D. The colorimetric estimation of alpha-amino nitrogen in tissue fluids. J Clin Pathol. 1959 Jan;12(1):80–84. doi: 10.1136/jcp.12.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads R. E., Udenfriend S. Purification and properties of collagen proline hydroxylase from newborn rat skin. Arch Biochem Biophys. 1970 Aug;139(2):329–339. doi: 10.1016/0003-9861(70)90485-6. [DOI] [PubMed] [Google Scholar]
- Ryhänen L., Kivirikko K. I. Developmental changes in protocollagen lysyl hydroxylase activity in the chick embryo. Biochim Biophys Acta. 1974 Mar 20;343(1):121–128. doi: 10.1016/0304-4165(74)90243-8. [DOI] [PubMed] [Google Scholar]
- Shaba J. K., Patrick R. S., McGee J. O. Collagen synthesis by mesenchymal cells isolated from normal and acutely-damaged mouse liver. Br J Exp Pathol. 1973 Feb;54(1):110–116. [PMC free article] [PubMed] [Google Scholar]
- Spiro M. J., Spiro R. G. Studies on the biosynthesis of the hydroxylsine-linked disaccharide unit of basement membranes and collagens. II. Kidney galactosyltransferase. J Biol Chem. 1971 Aug 25;246(16):4910–4918. [PubMed] [Google Scholar]
- Spiro R. G., Spiro M. J. Studies on the biosynthesis of the hydroxylysine-liked disaccharide unit of basement membranes and collagens. I. Kidney glucosyltransferase. J Biol Chem. 1971 Aug 25;246(16):4899–4909. [PubMed] [Google Scholar]
- Spiro R. G., Spiro M. J. Studies on the biosynthesis of the hydroxylysine-linked disaccharide unit of basement membranes and collagens. 3. Tissue and subcellular distribution of glycosyltransferases and the effect of various conditions on the enzyme levels. J Biol Chem. 1971 Aug 25;246(16):4919–4925. [PubMed] [Google Scholar]
- Stassen F. L., Cardinale G. J., McGee J. O., Udenfriend S. Prolyl hydroxylase and an immunologically related protein in mammalian tissues. Arch Biochem Biophys. 1974 Jan;160(1):340–345. doi: 10.1016/s0003-9861(74)80042-1. [DOI] [PubMed] [Google Scholar]
- Stassen F. L., Cardinale G. J., Udenfriend S. Activation of prolyl hydroxylase in L-929 fibroblasts by ascorbic acid. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1090–1093. doi: 10.1073/pnas.70.4.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T., Kivirikko K. I., Prockop D. J. Increased protocollagen hydroxylase activity in the livers of rats with hepatic fibrosis. Biochem Biophys Res Commun. 1967 Sep 27;28(6):940–944. doi: 10.1016/0006-291x(67)90070-8. [DOI] [PubMed] [Google Scholar]
- Takeuchi T., Prockop D. J. Protocollagen proline hydroxylase in normal liver and in hepatic fibrosis. Gastroenterology. 1969 Apr;56(4):744–750. [PubMed] [Google Scholar]


