Abstract
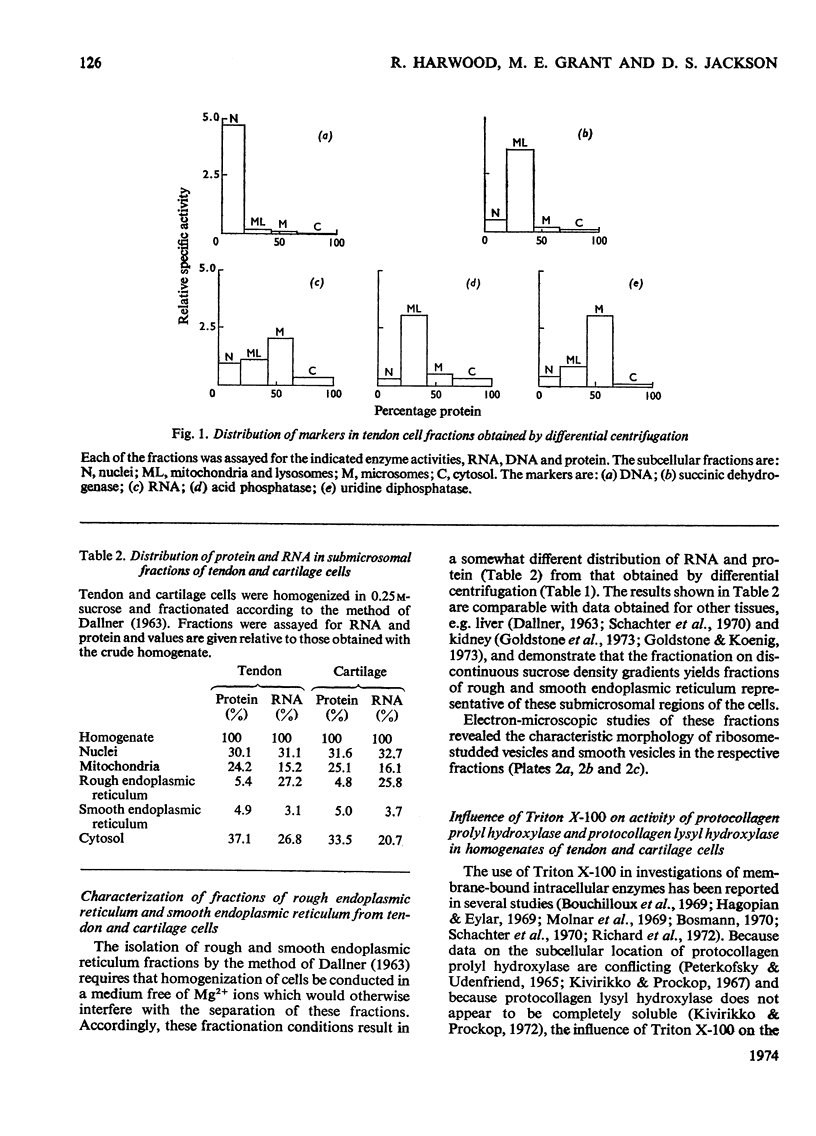
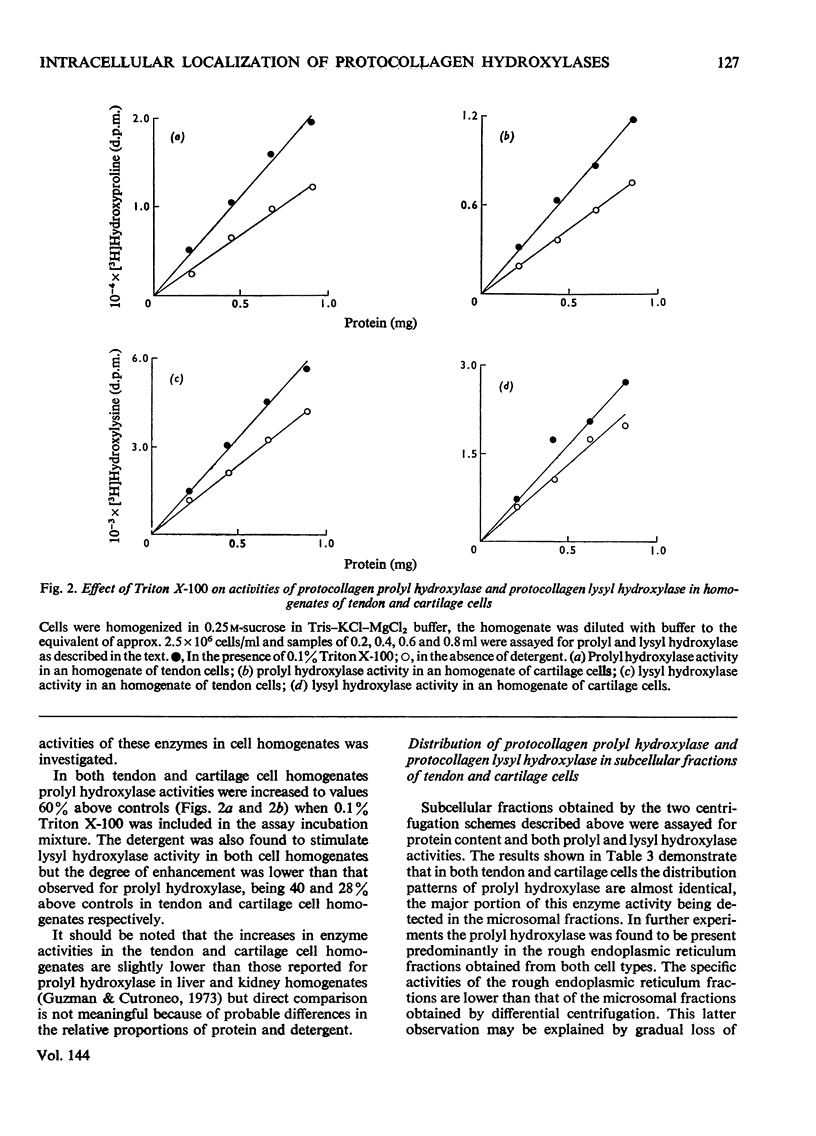
1. Subcellular fractions of freshly isolated matrix-free embryonic chick tendon and sternal cartilage cells have been characterized by chemical analysis, electron microscopy and the location of specific marker enzymes. These data indicate the fractions to be of a high degree of purity comparable with those obtained from other tissues, e.g. liver and kidney. 2. When homogenates were assayed for protocollagen prolyl hydroxylase and protocollagen lysyl hydroxylase activities, addition of Triton X-100 (0.1%, w/v) was found to stimulate enzyme activities by up to 60% suggesting that the enzymes were probably membrane-bound. 3. Assay of subcellular fractions obtained by differential centrifugation for protocollagen prolyl hydroxylase activity indicated the specific activity to be highest in the microsomal fraction. Similar results were obtained for protocollagen lysyl hydroxylase activity. 4. Submicrosomal fractions obtained by discontinuous sucrose-gradient centrifugation were assayed for the two enzymes and protocollagen prolyl hydroxylase and protocollagen lysyl hydroxylase were found to be associated almost exclusively with the rough endoplasmic reticulum fraction in both tendon and cartilage cells.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. A., Prockop D. J. Affinity column purification of protocollagen proline hydroxylase from chick embryos and further characterization of the enzyme. J Biol Chem. 1973 Feb 25;248(4):1175–1182. [PubMed] [Google Scholar]
- Bosmann H. B. Glycoprotein biosynthesis. Subcellular localization and activity in 3T3 and SV-3T3 fibroblasts of glycoprotein. N-acetylglucosaminyl transferases. FEBS Lett. 1970 May 11;8(1):29–32. doi: 10.1016/0014-5793(70)80217-4. [DOI] [PubMed] [Google Scholar]
- Bouchilloux S., Ferrand M., Grégoire J., Chabaud O. Localization in smooth microsomes from sheep thyroid of both a galactosyltransferase and an N-acetylhexosaminyltransferase. Biochem Biophys Res Commun. 1969 Oct 22;37(3):538–544. doi: 10.1016/0006-291x(69)90949-8. [DOI] [PubMed] [Google Scholar]
- Dehm P., Prockop D. J. Biosynthesis of cartilage procollagen. Eur J Biochem. 1973 May;35(1):159–166. doi: 10.1111/j.1432-1033.1973.tb02821.x. [DOI] [PubMed] [Google Scholar]
- Dehm P., Prockop D. J. Time lag in the secretion of collagen by matrix-free tendon cells and inhibition of the secretory process by colchicine and vinblastine. Biochim Biophys Acta. 1972 Apr 21;264(2):375–382. doi: 10.1016/0304-4165(72)90302-9. [DOI] [PubMed] [Google Scholar]
- Diegelmann R. F., Bernstein L., Peterkofsky B. Cell-free collagen synthesis on membrane-bound polysomes of chick embryo connective tissue and the localization of prolyl hydroxylase on the polysome-membrane complex. J Biol Chem. 1973 Sep 25;248(18):6514–6521. [PubMed] [Google Scholar]
- Fernández-Madrid F. Collagen biosynthesis. A review. Clin Orthop Relat Res. 1970 Jan-Feb;68:163–181. [PubMed] [Google Scholar]
- Goldstone A., Koenig H., Nayyar R., Hughes C., Lu C. Y. Isolation and characterization of a rough microsomal fraction from rat kidney that is enriched in lysosomal enzymes. Biochem J. 1973 Feb;132(2):259–266. doi: 10.1042/bj1320259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstone A., Koenig H. Physicochemical modifications of lysosomal hydrolases during intracellular transport. Biochem J. 1973 Feb;132(2):267–282. doi: 10.1042/bj1320267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant M. E., Prockop D. J. The biosynthesis of collagen. 1. N Engl J Med. 1972 Jan 27;286(4):194–199. doi: 10.1056/NEJM197201272860406. [DOI] [PubMed] [Google Scholar]
- Grant M. E., Prockop D. J. The biosynthesis of collagen. 2. N Engl J Med. 1972 Feb 3;286(5):242–249. doi: 10.1056/NEJM197202032860505. [DOI] [PubMed] [Google Scholar]
- Grant M. E., Prockop D. J. The biosynthesis of collagen. 3. N Engl J Med. 1972 Feb 10;286(6):291–300. doi: 10.1056/NEJM197202102860604. [DOI] [PubMed] [Google Scholar]
- Guzman N. A., Cutroneo K. R. Association of prolyl hydroxylase activity with membranes. Biochem Biophys Res Commun. 1973 Jun 19;52(4):1263–1270. doi: 10.1016/0006-291x(73)90637-2. [DOI] [PubMed] [Google Scholar]
- Hagopian A., Eylar E. H. Glycoprotein biosynthesis: the purification and characterization of a polypeptide. N-acetylgalactosaminyl transferase from bovine submaxillary glands. Arch Biochem Biophys. 1969 Feb;129(2):515–524. doi: 10.1016/0003-9861(69)90209-4. [DOI] [PubMed] [Google Scholar]
- Halme J., Kivirikko K. I., Simons K. Isolation and partial characterization of highly purified protocollagen proline hydroxylase. Biochim Biophys Acta. 1970 Mar 18;198(3):460–470. doi: 10.1016/0005-2744(70)90124-5. [DOI] [PubMed] [Google Scholar]
- Harwood R., Connolly A. D., Grant M. E., Jackson D. S. Presumptive mRNA for procollagen: occurrence in membrane bound ribosomes of embryonic chick tendon fibroblasts. FEBS Lett. 1974 Apr 15;41(1):85–88. doi: 10.1016/0014-5793(74)80960-9. [DOI] [PubMed] [Google Scholar]
- Harwood R., Grant M. E., Jackson D. S. The sub-cellular location of inter-chain disulfide bond formation during procollagen biosynthesis by embryonic chick tendon cells. Biochem Biophys Res Commun. 1973 Dec 19;55(4):1188–1196. doi: 10.1016/s0006-291x(73)80020-8. [DOI] [PubMed] [Google Scholar]
- Jimenez S. A., Dehm P., Olsen B. R., Prokop D. J. Intracellular collagen and protocollagen from embryonic tendon cells. J Biol Chem. 1973 Jan 25;248(2):720–729. [PubMed] [Google Scholar]
- Juva K., Prockop D. J. Modified procedure for the assay of H-3-or C-14-labeled hydroxyproline. Anal Biochem. 1966 Apr;15(1):77–83. doi: 10.1016/0003-2697(66)90249-1. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Prockop D. J. Partial purification and characterization of protocollagen lysine hydroxylase from chick embryos. Biochim Biophys Acta. 1972 Feb 28;258(2):366–379. doi: 10.1016/0005-2744(72)90228-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lansing A. I., Belkhode M. L., Lynch W. E., Lieberman I. Enzymes of plasma membranes of liver. J Biol Chem. 1967 Apr 25;242(8):1772–1775. [PubMed] [Google Scholar]
- Lazarides E. L., Lukens L. N., Infante A. A. Collagen polysomes: site of hydroxylation of proline residues. J Mol Biol. 1971 Jun 28;58(3):831–846. doi: 10.1016/0022-2836(71)90043-x. [DOI] [PubMed] [Google Scholar]
- Miller R. L. Rapid assay for lysyl-protocollagen hydroxylase activity. Anal Biochem. 1972 Jan;45(1):202–210. doi: 10.1016/0003-2697(72)90020-6. [DOI] [PubMed] [Google Scholar]
- Miller R. L., Udenfriend S. Hydroxylation of proline residues in collagen nascent chains. Arch Biochem Biophys. 1970 Jul;139(1):104–113. doi: 10.1016/0003-9861(70)90051-2. [DOI] [PubMed] [Google Scholar]
- Molnar J., Chao H., Markovic G. Subcellular site of structural glycoprotein synthesis in Ehrlich ascites tumor. Arch Biochem Biophys. 1969 Nov;134(2):533–538. doi: 10.1016/0003-9861(69)90315-4. [DOI] [PubMed] [Google Scholar]
- Munro H. N., Fleck A. Recent developments in the measurement of nucleic acids in biological materials. A supplementary review. Analyst. 1966 Feb;91(79):78–88. doi: 10.1039/an9669100078. [DOI] [PubMed] [Google Scholar]
- Olsen B. R., Berg R. A., Kishida Y., Prockop D. J. Collagen synthesis: localization of prolyl hydroxylase in tendon cells detected with ferritin-labeled antibodies. Science. 1973 Nov 23;182(4114):825–827. doi: 10.1126/science.182.4114.825. [DOI] [PubMed] [Google Scholar]
- PETERKOFSKY B., UDENFRIEND S. ENZYMATIC HYDROXYLATION OF PROLINE IN MICROSOMAL POLYPEPTIDE LEADING TO FORMATION OF COLLAGEN. Proc Natl Acad Sci U S A. 1965 Feb;53:335–342. doi: 10.1073/pnas.53.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard M., Broquet P., Louisot P. Biosynthesis of glycoproteins in the aortic wall. Study of intima microsomic mannosyl-transferase activity. J Mol Cell Cardiol. 1972 Oct;4(5):465–475. doi: 10.1016/0022-2828(72)90103-4. [DOI] [PubMed] [Google Scholar]
- Schachter H., Jabbal I., Hudgin R. L., Pinteric L., McGuire E. J., Roseman S. Intracellular localization of liver sugar nucleotide glycoprotein glycosyltransferases in a Golgi-rich fraction. J Biol Chem. 1970 Mar 10;245(5):1090–1100. [PubMed] [Google Scholar]
- Schofield J. D., Prockop D. J. Procollagen-a precursor form of collagen. Clin Orthop Relat Res. 1973 Nov-Dec;(97):175–195. doi: 10.1097/00003086-197311000-00026. [DOI] [PubMed] [Google Scholar]
- WUENSCH E., HEIDRICH H. G. ZUR QUANTITATIVEN BESTIMMUNG DER KOLLAGENASE. Hoppe Seylers Z Physiol Chem. 1963;333:149–151. doi: 10.1515/bchm2.1963.333.1.149. [DOI] [PubMed] [Google Scholar]