Abstract
Idiopathic pulmonary fibrosis (IPF) is etiologically complex, with well-documented genetic and nongenetic origins. In this Review, we speculate that the development of IPF requires two hits: the first establishes a vulnerable bronchoalveolar epithelium, and the second triggers mechanisms that reprogram distal epithelia to initiate and perpetuate a profibrotic phenotype. While vulnerability of the bronchoalveolar epithelia is most often driven by common or rare genetic variants, subsequent injury of the bronchoalveolar epithelia results in persistent changes in cell biology that disrupt tissue homeostasis and activate fibroblasts. The dynamic biology of IPF can best be contextualized etiologically and temporally, including stages of vulnerability, early disease, and persistent and progressive lung fibrosis. These dimensions of IPF highlight critical mechanisms that adversely disrupt epithelial function, activate fibroblasts, and lead to lung remodeling. Together with better recognition of early disease, this conceptual approach should lead to the development of novel therapeutics directed at the etiologic and temporal drivers of lung fibrosis that will ultimately transform the care of patients with IPF from palliative to curative.
Idiopathic pulmonary fibrosis (IPF) is a progressive lung disease, characterized by heterogeneous subpleural patches of fibrotic remodeled lung, that follows a bronchocentric distribution (1–3). The median survival is 3–5 years after diagnosis (1). While the etiology of IPF was initially unknown (thus, the nomenclature), we now understand that IPF is etiologically complex, with well-documented genetic and nongenetic origins. Lung fibrosis genetic risk variants demonstrate an autosomal dominant pattern of inheritance with incomplete penetrance (4), and in aggregate, these genetic risk variants account for at least 30% of the etiology of IPF (5). Cigarette smoke (6) and aging (7–9) also promote the development of IPF. How these nongenetic factors interact with specific genetic variants is not clear, but cigarette smoke and aging are known to contribute to epigenetic programming of the lung. Genetic susceptibility, epigenetic programming, and maladaptive homeostatic responses likely interact in ways that are yet to be described, reprogramming cells toward a fibroproliferative phenotype in the distal lung.
Genetic studies have identified dozens of rare and common genetic risk variants for IPF within key biological pathways that primarily affect the bronchiolar and alveolar epithelia (Table 1) (10). Although the gain-of-function MUC5B promoter variant is the dominant risk factor for this disease (11), accounting for at least 50% of the genetic risk of developing IPF (5), multiple biological mechanisms involving dysregulation of host defense, cell adhesion, telomere biology, mitotic spindle assembly, surfactant protein biology, and GTPase activity are implicated in the risk of developing IPF. Importantly, all genetic variants, except possibly a rare missense mutation in SFTPC (12), demonstrate incomplete penetrance for lung fibrosis, suggesting that ectopic expression or gain/loss of function of these genes establishes a biologically vulnerable phenotype that requires subsequent insults to trigger development of IPF.
Table 1. Common and rare IPF risk variants.
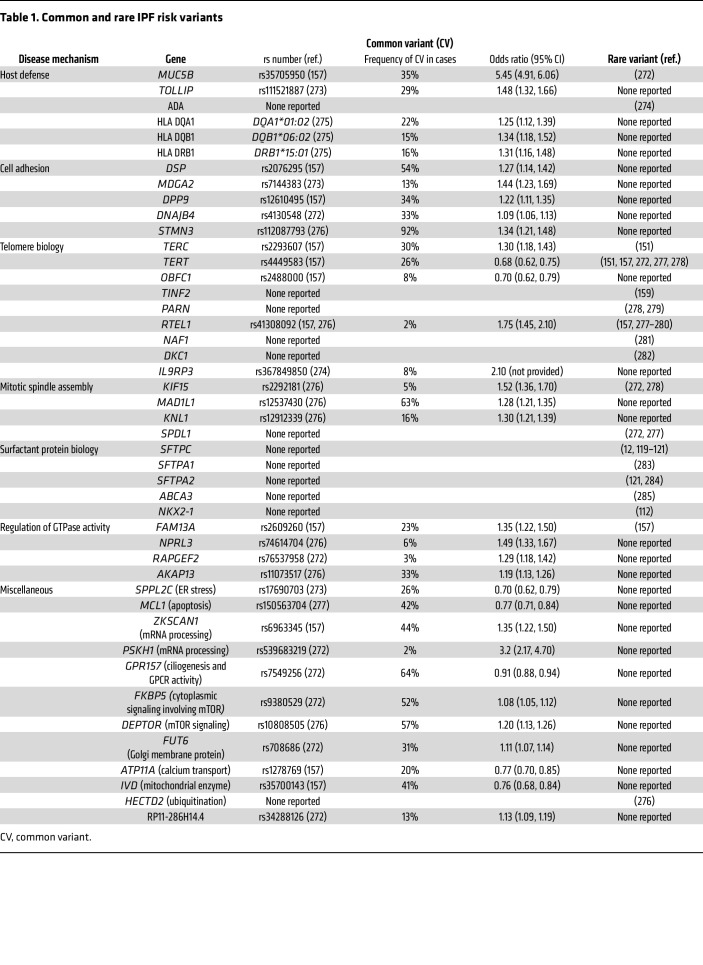
Multiple types of environmental exposures promote the development of fibrotic interstitial lung disease (ILD; IPF is a type of ILD) and are candidate second hits within the appropriate genetic context. The dominant nongenetic factors that enhance the risk of IPF are aging (1, 7, 8, 13) and cigarette smoking (6, 14, 15), with each one-year increase in age associated with an approximately 6% increase in IPF prevalence (16) and cigarette smoking associated with an approximately 3- to 5-fold increase in the risk of IPF (4, 6). Aerosolized pollutants resulting from wildfires and other combustions, ozone, particulate matter (PM2.5 and PM10), metal dust, asbestos, farming, and livestock (14, 15, 17–19) have also been associated with interstitial lung abnormalities (considered a sign of early ILD or IPF, ref. 20), IPF incidence (21), and acute exacerbations of IPF (22–25). These nongenetic IPF risk factors suggest that mechanisms involving particle deposition (20, 25, 26), mucociliary dysfunction, epithelial injury with attendant persistent inflammation (27–29), stem cell exhaustion (30–32), and cell senescence (32–35) represent key drivers of the persistent fibrotic process. These risk factors may also be influenced by genetic variants. Such observations led to the two-hit hypothesis (36); in our model, the first hit establishes a vulnerable bronchoalveolar epithelium, and the second triggers mechanisms that reprogram distal epithelia to initiate and perpetuate a profibrotic phenotype (Figure 1).
Figure 1. Two-hit model of pulmonary fibrosis.
We postulate that genetic and epigenetic etiologic drivers establish a vulnerable bronchiolar and alveolar epithelia (first hit) and that this results in homeostatic adaptation without the development of lung fibrosis. Persistent and progressive lung fibrosis can be triggered by a second hit (such as tobacco smoke, air pollution, inflammation, and/or aging) to the bronchiolar and alveolar epithelia, resulting in epithelial reprogramming, endoplasmic reticulum (ER) stress, unfolded protein response (UPR), apoptosis, and ultimately leading to fibroblast accumulation and activation, fibrosis, and abnormal lung remodeling.
In this Review, we discuss the two-hit hypothesis with an emphasis on MUC5B as the primary genetic risk factor, as it is an emerging aspect of IPF pathogenesis that has not been comprehensively addressed in prior reviews. We will also discuss how detrimental endoplasmic reticulum (ER) stress involving apoptosis, a persistent cycle of injury and repair, and activation of lung fibroblasts develop following additional damage to the terminal respiratory bronchiole. While IPF has been further characterized by dysregulation of immune cells and noncoding RNA signaling, these contributions are beyond the scope of the present discussion, and readers are directed elsewhere for comprehensive reviews of these topics (37, 38).
Mechanisms initiating epithelial vulnerability
Peripheral remodeling and loss of alveolar gas exchange surfaces in IPF highlight a need to understand how vulnerable lung epithelial cells may be reprogrammed to perpetuate a profibrotic phenotype. Early work emphasized alveolar type II (ATII) cells as the main targets of injury and drivers of fibrosis. Recently, multipotent epithelial progenitors that give rise to both terminal airway and alveolar cells have been shown to be susceptible to injury and may contribute to fibrosis (39–51). When challenged with ongoing exposures, a rodent model demonstrated that epithelial progenitors fail to return to homeostasis and instead promote persistent injury and fibrosis through maladaptive repair, which was exacerbated in the context of enhanced MUC5B expression (29).
Aberrant progenitors and regenerative epithelia.
Findings from murine and human studies demonstrate that fibrosis in IPF persists owing to sustained disruption of tissue homeostasis and recognize the central role of progenitor cells and cell populations in aberrant transitional states. The specific cell types and pathways involved in homeostatic repair and disease will likely depend on the model organism studied, owing to anatomical differences in the distal lungs among humans, nonhuman primates, and rodents (Figure 2). Yet, a subset of Wnt-responsive ATII cells proliferate in response to injury and differentiate into alveolar type I (ATI) cells to repair the alveolar epithelium following injury in mice (52, 53) and have also been shown to possess progenitor function in human organoid cultures. During fibrosis, ATII cells exhibit a transitional morphology and gene expression profile consistent with ineffectual/stalled differentiation to ATI cells (54, 55). In clustered, cystic airspaces termed honeycomb cysts in IPF, this transitional state is marked by expression of one or more keratin genes (KRT5 and KRT14) and in simple cysts, by KRT8 and KRT18 in the bleomycin mouse model (11, 56, 57). Genetic variants in the KRT8 locus are associated with IPF, and KRT8+ epithelial cells have a direct pathologic role in driving fibroblast activation, proliferation, and collagen deposition in the bleomycin model (58). Molecular pathways associated with these transitional states currently include TGF-β, p53, Notch, Sonic hedgehog (Shh), bone morphogenetic protein (BMP), and Wnt (45, 58–64).
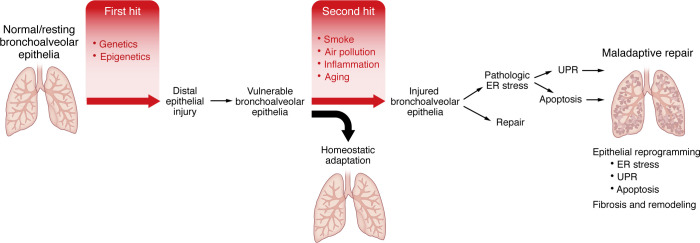
Figure 2. Model of the development of vulnerable bronchoalveolar epithelium as a contributing pathway to persistent pulmonary fibrosis.
(A) In the healthy lung, the bronchoalveolar epithelium consists of proximal epithelial cells in the terminal airways (basal cells, ciliated cells, club cells, and goblet cells) and alveolar type II (ATII) and type I (ATI) cells in the alveoli and minimal if any expression of MUC5B. Identified epithelial progenitor populations, including ITGB4β+/H2-K1hi cells in the conducting airways, BASCs at bronchoalveolar ducts in mice, and newly identified TASC, RASC, and AT0 cells in the preterminal and terminal respiratory bronchioles in humans, nonhuman primates, and ferrets are thought to be quiescent in the absence of injury. (B) In the presence of genetic variants (e.g., MUC5B), increased expression of MUC5B protein in goblet cells, and other cell types that do not typically express MUC5B protein (e.g., ATII cells), causes homeostatic ER stress, resulting in a vulnerable state that primes epithelial cell responses to subsequent injury. Repair of the bronchiolar and alveolar epithelia (B, left) is governed by epithelial cell/fibroblast/immune cell interactions near the site of injury that direct facultative epithelial progenitor cell (ATII) proliferation and differentiation into ATI cells and suppress fibroblast proliferation/activation. In addition, epithelial progenitor cells located at sites distant to the site of injury are activated and migrate to the injured alveolus (ITGB4β+/H2-K1hi cells, BASCs) to restore formation of the air/blood barrier. However, in the context of repetitive secondary injuries (below B), the persistent and enhanced ER stress induces detrimental responses in the vulnerable epithelium, causing epithelial dysfunction during injury/repair, as indicated by aberrant epithelial cell differentiation, arrested transitional cell states, and activation of aberrant basaloid cells in the alveoli. (C) This leads to profibrotic fibroblast and pericyte activation, proliferation, and excess extracellular matrix deposition. The consequence of respiratory bronchiole dropout in patients with early-stage IPF and the role of RASCs, TASCs, and AT0 progenitor populations in homeostatic repair versus a persistent fibrotic state has yet to be determined.
In murine airways, bronchioles terminate directly into alveolar duct openings at the bronchioalveolar duct junction and are populated by bronchioalveolar stem cells (BASCs) that exhibit transcriptional profiles of both airway secretory and ATII cells (SCGB1A1 and SFTPC) (41). Following distal lung injury, BASCs can differentiate into airway or alveolar epithelial cells (39) or to proximal epithelial cells after airway-specific injury (40–42). Separately, rare ITGB4β+H2-K1hi progenitor cells located in proximal airways were shown to engraft into bleomycin-injured mouse lungs following intratracheal transplantation with subsequent differentiation into ATII cells (43, 44). Intralobular serous cells that coexpress SCGB3A2+SCGB1A1+ and KRT5, a marker of airway basal stem cells, were identified in an influenza acute lung injury model and may contribute to bronchiolization in IPF (45–47). These observations suggest that the plasticity of existing progenitor cells localized at the site of injury and the migration of anatomically distant epithelial cells following injury may dictate normal versus excessively fibrotic repair outcomes.
In humans, terminal respiratory bronchioles and alveolar ducts are separated by structures called respiratory bronchioles that contain airway, alveolar, and BASC-like cells. Among these, airway epithelial progenitors termed terminal airway secretory cells (TASCs, marked by SCGB3A2+SFTPB+) (48), respiratory airway secretory cells (RASCs, marked by SCGB3A1+SCGB3A2+SFTPB+CEACAM6+) (49, 50), and AT0 cells (marked by SCGB3A2+SFTPB+SFTPC+) (50) were identified as cell types of interest (Figure 2). Loss of anatomical structures in humans, such as terminal respiratory bronchioles and bronchoalveolar ducts that house the newly identified TASC/RASC populations, may play a significant role in the aberrant repair process that occurs in fibrosis (16). Recent work has demonstrated a loss of progenitor ATII cells and an increase in the number of BASCs during aging (65). It will be critical for the field to address the initial role and eventual loss of these progenitor populations in IPF. The role of genetic risk variants and/or aging in the generation of a vulnerable epithelium and potential consequences for the differentiation trajectory of these cells in vivo are incompletely understood. However, in patients with IPF, and especially those with the MUC5B promoter variant, MUC5B is ectopically expressed in the respiratory bronchiole (11, 66), a region of the lung that does not normally express MUC5B (67). This suggests that MUC5B may influence the cellular composition of these localized fibrotic regions of the lung. Furthermore, in vivo and human studies that examine the role of these newly identified progenitor populations during fibrosis development and in the context of a vulnerable lung epithelia are still needed, as much of our current knowledge stems from in vitro differentiation experiments. These should be complemented with studies in higher-order animals, including ferrets and pigs, as these species contain respiratory bronchioles with similar cellular composition and morphology found in the human lung (68).
MUC5B and host defense.
In animal models and humans, MUC5B is essential for respiratory tract host defense (69–71). This requirement is met by tissue- and cell type–specific restriction of MUC5B to the tracheobronchial airways and submucosal glands, where cells are programmed to handle its biosynthesis and secretion. In bronchioles, MUC5B is produced by surface epithelial club cells, albeit at much lower levels than in bronchial epithelia. In unaffected individuals, MUC5B is undetectable in the most distal terminal and respiratory bronchiolar airways (67, 72–74). Presumably, restricted expression of MUC5B normally limits its accumulation in the distal airspace where it could interfere with particle clearance or gas exchange (29). Ectopic expression of MUC5B in terminal and respiratory bronchiolar airways in patients with IPF, especially in those with the MUC5B promoter variant (11, 66, 75), is thought to disrupt lung homeostasis and promote fibrotic remodeling in these vulnerable distal regions of the lung.
The MUC5B gene is 39 kb in length and encodes a 5,762–amino acid protein (596 kDa) that presents intrinsic challenges to cellular proteostasis. Mucins are secretory proteins that are targeted to the ER for translation, folding, and stabilization via disulfide bond formation. MUC5B contains more than 100 disulfide internal bonds per molecule at its amino (N-) and carboxy (C-) termini (76, 77). Furthermore, its N- and C- termini are separated by an approximately 3,000–amino acid stretch of unstructured domains fated to be O-glycosylated in the Golgi. Accordingly, MUC5B synthesis evokes high levels of steady-state ER stress, and mucous cells have adapted processes to dampen activation of an unfolded protein response (UPR). Polymeric mucin production has been best studied in the context of the IRE-1 pathway. Unfolded proteins stimulate IRE-1 ribonuclease activity to remove a normally unspliced intron in XBP1, enabling translation of a transcription factor that upregulates corrective and cytotoxic ER stress responses (78, 79). Importantly, IRE-1 has both a ubiquitous isoform (IRE-1α) and a mucous cell–specific isoform (IRE-1β) (78–83). The β isoform exhibits higher thresholds for activation, lower levels of XBP1 activation, and suppresses IRE-1α–mediated UPR activation to help maintain a sustainable ER stress response during homeostasis. Importantly, mucous cells exploit this through transcription factors such as SAM pointed domain-containing ETS transcription factor (SPDEF), which coordinately regulates expression of IRE-1β (84), mucous cell chaperones (85), and mucins themselves (86–89). To minimize ER stress and restore proteostasis, cells initiate an UPR (78). The UPR provides graded responses to ER stress by decreasing ER protein levels, improving folding, degrading proteins that cannot be corrected, or ultimately shifting cells toward senescence and apoptosis (90, 91). MUC5B misexpression alone could elicit UPR signals (e.g., apoptosis) in cells lacking IRE-1β resulting in tissue damage, which has been shown to occur in distal IPF lung epithelia (11, 66, 75, 92, 93). Additional work is needed to validate the regulation of mucous cell proteostasis regulators as well as IRE-1α– versus IRE-1β–dependent UPR activation in cells ectopically expressing MUC5B in IPF.
Cell adhesion.
Cell-cell and cell-matrix contacts are critical for tissue integrity and host defense (94). Dysfunction of cell-cell and cell-matrix adhesion molecules (including desmoplakin [DSP], E-cadherin, integrins, and focal adhesion kinase [FAK]) affecting epithelial cells and myofibroblasts plays a pivotal role in the pathogenesis of IPF (95). Genetic variants of DSP are associated with IPF (Table 1) (96, 97). DSP facilitates cell adhesion in bronchial and alveolar epithelial cells, with high expression in basal cells (98), and enables cell migration, proliferation, and differentiation (99). Its dysregulation may promote progression of lung fibrosis through multiple aspects of decreased cell adhesion and disrupted tissue integrity. E-cadherin, a key component of adherens junctions, helps maintain epithelial barrier integrity through homophilic interactions between adjacent epithelial cells. In pulmonary fibrosis, decreased E-cadherin expression compromises cell-cell interactions, leading to impaired barrier function and eventual epithelial cell detachment from the basement membrane (100). Integrins are transmembrane receptors linking the extracellular matrix (ECM) to the intracellular cytoskeleton, playing a dynamic and crucial role in cell adhesion and signaling. TGF-β, a known mediator of fibrotic processes, is secreted into the microenvironment in a latent inactive form bound to latency associated protein and is activated by the binding of integrin αvβ6 (101–103). Inhibition of integrin αvβ6 in murine models of pulmonary fibrosis, including radiation- and bleomycin-induced injury, was shown to prevent lung fibrosis (104–106). Finally, FAK, a downstream effector of integrin signaling, has also been shown to regulate cell adhesion, migration, and survival of epithelial cells and differentiation and migration of myofibroblasts (107–109). In mice, small-molecule inhibition of FAK prevented bleomycin-induced lung fibrosis (109), while ATII-specific deletion of FAK following bleomycin-induced fibrosis resulted in ECM alterations, fibroblast activation, and inhibition of ATII cell apoptosis, suggesting a complex signaling dynamic between epithelial cells and fibroblasts (107). Understanding the intricate interplay between adhesion molecules and signaling pathways and if these are altered in a vulnerable host will be essential for developing targeted therapies to restore normal cell-cell and cell-matrix adhesions in fibrosis.
Alveolar homeostasis and injury.
ATII cells play a crucial role in maintaining alveolar homeostasis by producing surfactant and serving as progenitor cells that regenerate damaged alveolar epithelium. In injured and fibrotic lungs, ATII cell function is impaired, leading to disrupted surfactant production and ineffective regeneration. In addition, mutations in several genes (SFTPC, SFTPA1, SFTPA2, ABCA3, and NKX2-1) that are uniquely expressed, or highly enriched, in ATII cells have been identified in patients with IPF (110–112). These genes are critical for alveolar epithelial cell specification (NKX2-1), surfactant homeostasis and function (ABCA3, SFTPC, SFTPA1, and SFTPA2), and innate immune responses (SFTPA1 and SFTPA2), all of which work in concert to decrease surface tension within alveoli and defend against respiratory pathogens. Impaired surfactant composition and function resultant from loss-of-function mutations in surfactant-associated genes leads to alveolar instability and atelectasis (12, 113). Alveolar collapse has been reported in the unaffected parenchyma of IPF diseased lungs and has also been associated with IPF progression (114, 115). Additionally, it was shown that overexpression of the profibrotic factor TGF-β1 suppresses expression of surfactant proteins in ATII cells, leading to alveolar collapse prior to fibrosis in the bleomycin mouse model (116). Thus, alveolar collapse can contribute to early pathogenesis of IPF and may worsen upon activation of mesenchymal signaling.
N-terminal truncation mutations in surfactant protein C (SFTPC) lead to retention of SFTPC in endolysosomal compartments and aggresome formation (117, 118) and have been reported to be associated with IPF (113, 119–121). Transgenic expression of an SFTPC exon 4 truncation mutant (termed delta exon 4) in mice led to an embryonic lethal phenotype associated with high levels of transgenic protein, ER stress, and disrupted lung development (122). Expression of a different SFTPC variant (L188Q) in transgenic mice was not sufficient for the development of spontaneous fibrosis but augmented bleomycin-induced fibrosis (123). In vitro studies demonstrated that, while both the delta exon 4 mutant and the L188Q mutant induced ER stress and IL-8 secretion in A549 cells, only the delta exon 4 mutant was sufficient to activate NF-κB signaling (124). This demonstrated that expression of misfolded SFTPC protein and subsequent ER stress responses was sufficient to drive increased inflammatory signaling in ATII cells (123). To correct for the embryonic lethality and hypomorphic complications of these mutants, conditional knockin SFTPC-transgenic mice have also been created (I73T and the BRICHOS mutant C121G), which demonstrated both ER stress and spontaneous lung fibrosis (12). Confirmation of alveolar epithelial ER stress as causative for spontaneous fibrosis was demonstrated through conditional deletion of the HSPA5 gene (encoding GRP78, a molecular chaperone necessary for inhibition of ER stress signaling). Mice with ATII-specific deletion of GRP78 developed ER stress and spontaneous pulmonary fibrosis, establishing a link between ER stress and fibrotic lung disease (125). Moreover, inhibition of IRE-1α reduced ER stress and lung fibrosis in Sftpcc121g mice (93).
Telomere attrition and cell senescence.
While lung epithelial cells can have relatively long half-lives (126), epithelial cell senescence may be accelerated by a number of aging-related events, including DNA damage (127), telomere attrition (128), dysregulated proteostasis (125, 129–135), and mitochondrial stress (136). At a molecular level, senescence is a state of irreversible replicative arrest characterized by markers of DNA damage, cellular hypertrophy, upregulation of lysosomal β-galactosidase, and expression of the cyclin-dependent kinase inhibitors (CDKN1A and CDKN2A) (137). Lung epithelia in IPF express CDKN1A (138–140) and its paralog, CDKN2A (141, 142). Moreover, deletion of CDKN2A+ senescent cells was protective in murine bleomycin-induced fibrosis (32).
Additional evidence for senescent and aging-related phenotypes in IPF comes from known associations between rare mutations in genes that encode enzymes responsible for maintaining DNA integrity. Telomeres are segments of chromosomes that enable DNA repair machinery to discriminate between chromosomal ends and DNA double-strand breaks (143), and breakdown in telomere maintenance triggers cellular senescence (144–148). Telomere shortening is associated with IPF (149, 150), and it is a common finding in IPF lung epithelia (138) and peripheral blood mononuclear cells (151). Genetic variants in the telomere synthesis enzymes TERT and TERC (144, 152) have also been implicated in IPF (150, 153–158), and sporadic mutations in telomere-supporting shelterin proteins have also been found to be associated with IPF (159–161). Continued replication after telomere attrition requires telomere lengthening to prevent chromatin erosion (144, 162, 163). Haploinsufficiency of telomere maintenance complexes is sufficient to promote intergenerational telomere attrition and development of myelofibrosis and pulmonary fibrosis in dyskeratosis congenita (151, 164–166).
Epigenetic regulatory mechanisms in IPF epithelia.
Emerging transcriptomic data have defined abnormal basaloid cells as a characteristic attribute of IPF epithelia (55), and the relatively stereotypical transcriptomic features of this pathogenic basaloid cell population implicate cellular memory as a likely contributing mechanism. Transcription-based cellular memory, which arises as a consequence of autoregulated transcription factors and positive feedback circuits, is a well-described driver of lineage commitment during normal development (167), and these factors are increasingly associated with various diseases (168, 169). Cellular memory mediated by metastable transcription circuits is thus a potential contributor to both normal basal cell programming and the misprogramming of basaloid cells in IPF. As an additional, and potentially reinforcing mechanism, widespread changes in DNA methylation in whole lung tissue (170) and fibroblasts (171) have been associated with IPF. DNA methylation (172, 173); histone modifications, including methylation or acetylation; and other forms of chromatin remodeling (172, 174, 175) may play a critical role in ectopic expression of MUC5B with or without the promoter variant risk allele and in further stabilizing the aberrant basaloid cell fate. Additional understanding of how epigenetic changes and transcriptional circuits affect progenitor epithelial populations and how this cellular memory contributes both to vulnerability and disease progression is needed.
Bronchoalveolar epithelia, honeycomb cysts, and fibrosis
Bronchiolization of the distal airspaces and loss of small airways have been appreciated as features of the IPF lung for nearly five decades (176). Until recently, however, the mechanisms driving these cellular and structural changes and their effect on patient survival and disease progression remained unclear. Initial work determined that bronchiolization and honeycomb cysts were characterized by their remarkable similarity to the airway epithelium (11, 177) and that basal cell–related gene signatures from bronchoalveolar lavage of patients with IPF predicted significantly worse mortality (178). Recent work has begun to elucidate the cellular origins underlying bronchiolization and cyst formation, demonstrating the capacity for aberrant alveolar epithelial differentiation following injury to drive cyst formation (179, 180). Separately, it has been shown that primary human distal airway epithelial cells derived from samples from patients with IPF possess a biophysically distinct YAP-dependent collective migratory phenotype, distinguishing them from their healthy counterparts (181). Ex vivo live imaging of injured murine airways demonstrates a conservation of this YAP-dependent migratory program that is likely important in bronchiolization and cyst formation (181, 182). Additionally, YAP signaling has been shown by multiple groups as a critical regulator of ATII cell proliferation and ATI cell differentiation (182–186).
Ectopic MUC5B expression drives distal lung pathologies.
A key, currently unanswered question is whether, and if so to what extent, ectopic expression of MUC5B in bronchiolar epithelia of patients with IPF contributes to persistent, progressive fibrosis and to the formation of honeycomb cysts. Transgenic mice expressing increased levels of Muc5b in the distal airways (ectopic expression under the Scgb1a1 promoter) or alveoli (ectopic expression under the Sftpc promoter) fail to spontaneously develop fibrosis or honeycomb cysts (29). However, when transgenic Scgb1a1-Muc5b mice are injured repetitively with bleomycin, both fibrosis and microcyst formation are enhanced and prolonged (29, 187). These findings suggest that fibrosis and honeycomb cysts develop in a vulnerable lung (potentially driven by MUC5B ectopic expression) after a repetitive secondary hit that reprograms a vulnerable epithelium (Figure 2). Current findings suggest that the profibrotic effect of MUC5B ectopic expression in distal airway cells on fibroblasts may be indirect, possibly mediated by exacerbation of epithelial injury and destruction that provides an altered “substrate” or “niche” onto which lung fibroblasts migrate, gain resistance to apoptosis, persist, and continue to express and deposit fibrotic ECM (182). Thus, it will be critical to understand how excess MUC5B influences molecular drivers that can elicit a profibrotic phenotype from the underlying mesenchyme. This includes YAP signaling, which has been shown by multiple groups as a critical regulator of ATII cell proliferation and ATI cell differentiation (182–186) and well-known signaling cascades (e.g., EGFR/YAP/SRC) and novel pathways (e.g., IL-6 and IL-11) (101, 181, 182) implicated in disease initiation and progression.
Fibroblast heterogeneity during homeostasis and injury.
The alveolar walls and septa of healthy lungs contain resident PDGFRα+ alveolar fibroblasts that synthesize components of the ECM (188) and serve as niche cells that support the growth and function of ATII cells by secreting instructive factors required for ATII cell survival and proliferation (e.g., IL-6, FGF-7, Wnt) (189) and transfer phospholipid precursors from alveolar capillary endothelial cells to ATII cells (190, 191). A smaller number of PDGFRβ+ pericytes are located in alveolar walls and provide trophic support to alveolar aerocytes and general capillary endothelial cells (190, 191). In addition, PDGFRβ+ pericytes and adventitial fibroblasts surround distal airways and blood vessels. In the normal adult lung parenchyma, contractile α smooth muscle actin–expressing (α-SMA–expressing) myofibroblasts are found to extend from conducting airways out to alveolar ducts and are known as ductal myofibroblasts (192). These spatially distinct fibroblast subsets exhibit overlapping and distinct gene expression patterns that collectively contribute to their function in healthy lungs.
In response to injury and loss of ATI and ATII cells (36, 193), lung fibroblasts are rapidly mobilized and actively contribute to lung repair and regeneration. scRNA-sequencing studies in bleomycin-instilled PDGFRα-GFP and COL1A1-GFP reporter and lineage-traced mice have shown that PDGFRα+ fibroblasts and PDGFRβ+ pericytes/adventitial fibroblasts migrate, proliferate, and accumulate in bleomycin- and influenza virus–injured lungs (194–196) and become reprogrammed to express profibrotic ECM (e.g., COL1a1, SPP1, FN1, ELN) and contractile proteins (e.g., α-SMA, CNN1, TAGLN) (101, 180, 196, 197). These studies also identified novel profibrotic genes and transcription factors that differentiate newly identified profibrotic fibroblast subpopulations, including CTHRC1, THRC1, RUNX1, and SFPR1 (195, 196). A specific lung fibroblast, the alveolar fibroblast, appears to be critical to alveolar homeostasis and when stimulated with either IL-1α or TGF-β can develop into inflammatory or fibrotic fibroblasts (198). As repair continues, some of these profibrotic fibroblasts undergo apoptosis and are cleared, while those remaining (and potentially newly proliferated or migrated fibroblasts) undergo further reprogramming to express genes involved in lung development and repair (195). Together, the enrichment of these later pathways support ATII proliferation and differentiation into ATI cells, while complementary angiogenic pathways contribute to the regeneration of alveolar capillary endothelium. Finally, excess ECM is degraded, leading to restoration of lung architecture and function. We have referred to this resolution phase, which is initiated by the wave of fibroblast apoptosis, as “homeostatic fibrosis resolution” (195).
Reciprocal interactions between fibroblasts and epithelial cells within the alveolar niche are critical for homeostasis of the lung parenchyma. Seminal coculture studies have demonstrated that primary rat, mouse, and human ATII cells from nondiseased donor lungs suppress fibroblast proliferation via an autocrine signaling loop, wherein IL-1α derived from ATII cells activates COX2-dependent prostaglandin E2 (PGE2) secretion from fibroblasts that inhibits their proliferation (199–202). PGE2 synthesis is reduced in bronchoalveolar lavage fluid from patients with IPF (203), suggesting that perturbation of this ATII fibroblast signaling loop, after ATII cell injury and/or apoptosis, contributes to exuberant fibroblast proliferation in the fibrotic lung. In addition, injured ATII cells from IPF lungs show increased expression of CTGF (200), TGF-β (204–206), and Shh (207–209), all of which stimulate fibroblast proliferation and induce collagen secretion and α-SMA expression. Although critical crosstalk between fibroblast subpopulations and distal basal cells has been demonstrated in organoid cultures (50), further studies with the newly identified progenitor epithelial cell populations in the distal airways (AT0, TASC, and RASC) in vivo remain to be conducted.
Fibroblast heterogeneity during fibrosis.
A central pathologic feature of IPF and in fibrotic mice is the persistence of nonproliferating, apoptosis-resistant, and often senescent α-SMA+ and ECM-producing profibrotic fibroblasts (Figure 2) (210–214). These arise though multiple mechanisms, including increased resistance to apoptotic signals, and lead to a persistently activated profibrotic fibroblast population that promotes disease progression through unabated aberrant ECM production and eventual senescence (215) (Figure 2). Recent studies in mouse fibrosis models and human IPF tissue demonstrate an interaction between the development and persistence of senescent KRT8+ transitional basaloid epithelial cells and profibrotic fibroblasts (56, 61, 216). Often occurring at the edge of the fibroblastic foci, these cell populations are thought to represent active areas of fibrotic destruction in the lung (217). Fibroblastic foci contain discrete areas of fibroblasts, myofibroblasts, and newly formed collagen in humans. They have been shown to dissociate capillary vessels from the alveolar epithelium, disrupt normal basement membranes, and induce a transitional epithelial cell phenotype that lines the foci and results in the loss of normal alveolar septa (218). ECM in the fibrotic lung is stiffer and results in generation of greater mechanotransductive forces in fibroblasts and remodeled and aberrant epithelial cells. This is driven by the well-described positive feedback amplification loop involving expression and activation of TGF-β. This self-perpetuating circuit, which is reminiscent of the interactions described above between fibroblasts and ATII cells in healthy lungs, supports fibrotic fibroblasts and transitional basaloid cells and promotes continual disruption of epithelial barrier integrity. It also inhibits appropriate epithelial cell differentiation and aberrant fibroblast ECM production and organization, contributing to persistent and progressive disease (56, 219).
Therapeutic implications
To date, there are two approved therapies for IPF, nintedanib and pirfenidone (220, 221), which slow the progressive loss of lung function but are not curative and have side effects that limit their efficacy (222). Furthermore, patients continue to decline despite these medications and subsequently develop end-stage lung disease (223). Thus, it becomes imperative to look beyond typical antifibrotic signaling pathways for innovative therapeutic directions.
Novel targets in the clinic and on the horizon.
Genetic and nongenetic risk factors have identified several mechanisms that appear to be critical to the development of IPF and may help to identify patients at earlier disease stages. Currently, these mechanisms focus on the respiratory bronchioles and alveolar epithelia and include dysregulation of host defense, cell adhesion, telomere attrition, stem cell exhaustion, early cell senescence, and dysfunctional surfactant protein biology. This suggests that genetic variants associated with unique genes converge on specific pathways that disrupt terminal respiratory and alveolar epithelial structure and function. For example, in those with the gain-of-function MUC5B promoter variant, approaches that decrease MUC5B expression could reduce the vulnerability of the lung by improving mucociliary clearance and/or ameliorating chronic ER stress. In individuals with telomerase mutations, the accelerated rate of cell senescence could be slowed by targeting DNA repair, reducing oxidative stress, or by using senolytic agents to selectively induce death of senescent cells. A randomized phase IIa clinical trial for IPF with an anti-αvβ6 integrin monoclonal antibody has recently demonstrated a reduction of TGF-β signaling. The phase IIa INTEGRIS-IPF trial using an αvβ6 integrin small-molecule inhibitor slowed the rate of forced vital capacity decline (224, 225). Recent preclinical studies in mice have shown that therapeutic targeting of BCL-2 and its related family members, BCL-XL and BCL-W, with the BH3 mimetic drug ABT-263 (Navitoclax) reduced the severity of silica- and bleomycin-induced pulmonary fibrosis (213, 226–228) as well as scleroderma-like skin fibrosis in mice (229). Thus, the development of fibroblast resistance to apoptosis may prove relevant to persistent and progressive lung fibrosis. However, these antiapoptotic pathways may also be exploited for targeted elimination of profibrotic fibroblasts and reducing fibrosis in general. The concept of targeting therapy to specific gene variants and disease mechanisms has been successful in fields such as rheumatology and oncology and would represent an advance in the treatment of IPF.
Gene editing.
Gene editing and gene therapy technologies have developed rapidly during the last several decades (230–232). Potential treatment targets in IPF include the MUC5B promoter or telomere gene variants, which are appealing due to the elevated expression of MUC5B (173) and shortened telomeres (233–236) in all individuals with IPF, regardless of a genetic mutation. Gene transfer has been the most frequent approach used to attenuate lung fibrosis in rodent models. AAV-based delivery of telomerase (TERT) to ATII cells of bleomycin-treated TERT-deficient mice increased telomere length, proliferation, and reduced inflammation and fibrosis (236). Downregulation of the proinflammatory cytokine milieu, affecting IL-6, IL-10, IL-17A, and IL-33, also reduced lung fibrosis (237–239). Other genes, many related to vascular homeostasis, delivered to injured lungs also reduced inflammation, fibrosis, and apoptosis (240–245). In humans, both viral and nonviral delivery methods have been advanced for lung targeting (232, 246, 247). However, all current approaches are limited by the complex human lung structure with multiple barriers to delivery, such as mucous, macrophage-mediated phagocytosis, and epithelial barrier function (248, 249). Cell-based approaches with genetic reprogramming and reengraftment may be an alternative approach for IPF gene therapy (250, 251); however, these approaches may be limited by low engraftment rates, likely due to lack of appropriate niche space in lungs with established fibrosis and off-target effects of systemic gene therapy (252–254).
Epigenetic approaches to IPF therapies.
Epigenetic reprogramming of cells can provide reliable, long-lasting therapeutic effects in vivo (255). First-generation FDA-approved epigenetic therapeutics, such as azacitidine DNA methyltransferase inhibitors (i.e., Decitabine, Vidaza) and histone modifying drugs, have proven effective in treating diseases such as lung cancer (256, 257) but are broadly acting with profound side effects. More recently developed locus-specific epigenetic approaches to genome editing technologies hold promise in development of more effective and long-lasting epigenetic therapeutics (231). Several studies demonstrate that DNA methylation of the MUC5B promoter variant is associated with MUC5B expression (172, 173). Histone modifications, such as acetylation and chromatin remodeling, can also regulate MUC5B expression (172, 174, 175). Thus, modifications of epigenetic marks may prove beneficial in regulating MUC5B expression or other IPF risk genes, especially those associated with a gain or loss of function (258–262). Specific approaches to targeting the epigenome (255, 263–268) rely on modifying proteins to bind specific sequences in the genome by using CRISPR-deactivated Cas9 (dCas9) and related technologies. However, the main barriers for epigenetic reprogramming to become a therapeutic target for IPF remain efficiency of in vivo construct delivery into cell types of interest and the risk of side effects and nonspecific activity (252, 269, 270). Antibody drug conjugates, in which bioactive payloads can be delivered to specific cell types, have shown promise in oncology for reducing off-target effects (271). Epigenetic and other targeted cellular reprogramming efforts in IPF may be facilitated by this rapidly improving technology.
The path ahead
The complex etiology and biology of IPF creates challenges and opportunities for the path forward. Delineating pathways that lead to host vulnerability, injury, or repair will identify those responses that initiate disease versus those that result in persistent and progressive lung fibrosis.”. While defining the temporal relationship pathologically among vulnerability, early disease, and persistent and progressive lung fibrosis is critical, these pathological stages of lung fibrosis will best be understood within the context of etiologic drivers. These dimensions of IPF, etiology and stage, should highlight the key pathologic pathways and address many of the unmet needs in this complex disease, including identifying sites of lung vulnerability, defining mechanisms that adversely disrupt epithelial function and activate fibroblasts and lead to lung remodeling, and characterization of high-priority targets for intervention. For example, genetic and nongenetic drivers of IPF have identified bronchiolar and alveolar epithelia as initial targets of injury.
Mechanisms through which epithelial progenitor cell populations, especially in the distal airspace, are injured or are unable to mediate repair need further investigation. New cell types/states are continuously emerging through single-cell and spatial transcriptomics that have distinct but often overlapping identities and functions. Whether similar antiproliferative and fibroblast activation signaling pathways are operative in newly identified progenitor epithelial cell populations in the distal airways (AT0, TASC, and RASC) of human lung remains to be determined. Thus, the identity, ontogeny, transcriptional programming, and temporal-spatial relationship of epithelial progenitors to lung fibrosis represent an area of investigation with clear relevance to injury, repair, and fibroproliferation.
Understanding the consequences of genetic risk variants for cell differentiation patterns of progenitors in vivo is imperative. A key, currently unanswered question is whether, and, if so, to what extent, ectopic expression of MUC5B in bronchiolar epithelia of patients with IPF contributes to persistent, progressive fibrosis and to the formation of honeycomb cysts. Understanding the intricate interplay between adhesion molecules and signaling pathways will be essential for developing targeted therapies to restore cell-cell and cell-substrate adhesion and halt the progression of pulmonary fibrosis and prevent lung remodeling.
Finally, applying this new knowledge to early recognition of disease before the onset of irreversible and progressive lung fibrosis and developing novel therapeutics directed at etiologic and temporal drivers of lung fibrosis will ultimately transform the care of patients with IPF from palliative to curative.
Acknowledgments
This work has been supported by VAMC grants I01BX005295, I01BX003471, and I01BX005343 and NIH grants R01HL149836, R01HL158668, R01HL147860, R01HL166895, R01HL168126, R01HL147860, R01HL130938, R01HL080396, and P01HL162607.
Version 1. 01/02/2025
Electronic publication
Footnotes
Conflict of interest: DAS is the founder and chief scientific officer of Eleven P15 Inc., a company dedicated to the early diagnosis and treatment of pulmonary fibrosis.
Copyright: © 2025, Bridges et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Reference information: J Clin Invest. 2025;135(1):e183836. https://doi.org/10.1172/JCI183836.
Contributor Information
James P. Bridges, Email: BridgesJ@NJHealth.org.
Eszter K. Vladar, Email: eszter.vladar@cuanschutz.edu.
Jonathan S. Kurche, Email: jonathan.kurche@cuanschutz.edu.
Ian T. Stancil, Email: istancil@stanford.edu.
Evgenia Dobrinskikh, Email: evgenia.dobrinskikh@cuanschutz.edu.
Yan Hu, Email: yan.hu@cuanschutz.edu.
Sarah K. Sasse, Email: Sasses@njhealth.org.
Joyce S. Lee, Email: JOYCE.LEE@CUANSCHUTZ.EDU.
Rachel Z. Blumhagen, Email: blumhagenr@njhealth.org.
Ivana V. Yang, Email: ivana.yang@cuanschutz.edu.
Anthony N. Gerber, Email: gerbera@njhealth.org.
Anna L. Peljto, Email: ANNA.PELJTO@CUANSCHUTZ.EDU.
Christopher M. Evans, Email: christopher.evans@cuanschutz.edu.
Elizabeth F. Redente, Email: redentee@NJHealth.org.
David W.H. Riches, Email: richesd@NJHealth.org.
David A. Schwartz, Email: david.schwartz@cuanschutz.edu.
References
- 1.Raghu G, et al. Diagnosis of idiopathic pulmonary fibrosis. An official aTS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198(5):e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 2.Berigei SR, et al. Microscopic small airway abnormalities identified in early idiopathic pulmonary fibrosis in vivo using endobronchial optical coherence tomography. Am J Respir Crit Care Med. 2024;210(4):473–483. doi: 10.1164/rccm.202401-0249OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verleden SE, et al. Molecular approach to the classification of chronic fibrosing lung disease-there and back again. Virchows Arch. 2021;478(1):89–99. doi: 10.1007/s00428-020-02964-9. [DOI] [PubMed] [Google Scholar]
- 4.Steele MP, et al. Clinical and pathologic features of familial interstitial pneumonia. Am J Respir Crit Care Med. 2005;172(9):1146–1152. doi: 10.1164/rccm.200408-1104OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moll M, et al. A polygenic risk score for idiopathic pulmonary fibrosis and interstitial lung abnormalities. Am J Respir Crit Care Med. 2023;208(7):791–801. doi: 10.1164/rccm.202212-2257OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumgartner KB, et al. Cigarette smoking: a risk factor for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1997;155(1):242–248. doi: 10.1164/ajrccm.155.1.9001319. [DOI] [PubMed] [Google Scholar]
- 7.Hutchinson JP, et al. Increasing global mortality from idiopathic pulmonary fibrosis in the twenty-first century. Ann Am Thorac Soc. 2014;11(8):1176–1185. doi: 10.1513/AnnalsATS.201404-145OC. [DOI] [PubMed] [Google Scholar]
- 8.Olson AL, et al. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am J Respir Crit Care Med. 2007;176(3):277–284. doi: 10.1164/rccm.200701-044OC. [DOI] [PubMed] [Google Scholar]
- 9.Raghu G, et al. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174(7):810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 10.Adegunsoye A, et al. Genetics and genomics of pulmonary fibrosis: charting the molecular landscape and shaping precision medicine. Am J Respir Crit Care Med. 2024;210(4):401–423. doi: 10.1164/rccm.202401-0238SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seibold MA, et al. The idiopathic pulmonary fibrosis honeycomb cyst contains a mucocilary pseudostratified epithelium. PLoS One. 2013;8(3):e58658. doi: 10.1371/journal.pone.0058658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nureki SI, et al. Expression of mutant Sftpc in murine alveolar epithelia drives spontaneous lung fibrosis. J Clin Invest. 2018;128(9):4008–4024. doi: 10.1172/JCI99287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fell CD, et al. Clinical predictors of a diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2010;181(8):832–837. doi: 10.1164/rccm.200906-0959OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baumgartner KB, et al. Occupational and environmental risk factors for idiopathic pulmonary fibrosis: a multicenter case-control study. Collaborating Centers. Am J Epidemiol. 2000;152(4):307–315. doi: 10.1093/aje/152.4.307. [DOI] [PubMed] [Google Scholar]
- 15.Park Y, et al. Occupational and environmental risk factors of idiopathic pulmonary fibrosis: a systematic review and meta-analyses. Sci Rep. 2021;11(1):4318. doi: 10.1038/s41598-021-81591-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maher TM, et al. Global incidence and prevalence of idiopathic pulmonary fibrosis. Respir Res. 2021;22(1):197. doi: 10.1186/s12931-021-01791-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hubbard R, et al. Occupational exposure to metal or wood dust and aetiology of cryptogenic fibrosing alveolitis. Lancet. 1996;347(8997):284–289. doi: 10.1016/S0140-6736(96)90465-1. [DOI] [PubMed] [Google Scholar]
- 18.Miyake Y, et al. Occupational and environmental factors and idiopathic pulmonary fibrosis in Japan. Ann Occup Hyg. 2005;49(3):259–265. doi: 10.1093/annhyg/meh090. [DOI] [PubMed] [Google Scholar]
- 19.Tomos I, et al. Long-term personal air pollution exposure and risk for acute exacerbation of idiopathic pulmonary fibrosis. Environ Health. 2021;20(1):99. doi: 10.1186/s12940-021-00786-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rice MB, et al. Ambient air pollution exposure and risk and progression of interstitial lung abnormalities: the Framingham Heart Study. Thorax. 2019;74(11):1063–1069. doi: 10.1136/thoraxjnl-2018-212877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conti S, et al. The association between air pollution and the incidence of idiopathic pulmonary fibrosis in Northern Italy. Eur Respir J. 2018;51(1):1700397. doi: 10.1183/13993003.00397-2017. [DOI] [PubMed] [Google Scholar]
- 22.Johannson KA, et al. Acute exacerbation of idiopathic pulmonary fibrosis associated with air pollution exposure. Eur Respir J. 2014;43(4):1124–1131. doi: 10.1183/09031936.00122213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winterbottom CJ, et al. Exposure to ambient particulate matter is associated with accelerated functional decline in idiopathic pulmonary fibrosis. Chest. 2018;153(5):1221–1228. doi: 10.1016/j.chest.2017.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tahara M, et al. Exposure to PM(2.5) is a risk factor for acute exacerbation of surgically diagnosed idiopathic pulmonary fibrosis: a case-control study. Respir Res. 2021;22(1):80. doi: 10.1186/s12931-021-01671-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sese L, et al. Impact of Particulate Matter on the Natural History of IPF: A Matter of Concentrations? Chest. 2018;154(3):726–727. doi: 10.1016/j.chest.2018.05.043. [DOI] [PubMed] [Google Scholar]
- 26.Kaul B, et al. Agent orange exposure and risk of idiopathic pulmonary fibrosis among U.S. veterans. Am J Respir Crit Care Med. 2022;206(6):750–757. doi: 10.1164/rccm.202112-2724OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Svartengren M, et al. Long-term clearance from small airways decreases with age. Eur Respir J. 2005;26(4):609–615. doi: 10.1183/09031936.05.00002105. [DOI] [PubMed] [Google Scholar]
- 28.Proenca de Oliveira-Maul J, et al. Aging, diabetes, and hypertension are associated with decreased nasal mucociliary clearance. Chest. 2013;143(4):1091–1097. doi: 10.1378/chest.12-1183. [DOI] [PubMed] [Google Scholar]
- 29.Hancock LA, et al. Muc5b overexpression causes mucociliary dysfunction and enhances lung fibrosis in mice. Nat Commun. 2018;9(1):5363. doi: 10.1038/s41467-018-07768-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ortega-Martinez M, et al. Analysis of cell turnover in the bronchiolar epithelium through the normal aging process. Lung. 2016;194(4):581–587. doi: 10.1007/s00408-016-9890-3. [DOI] [PubMed] [Google Scholar]
- 31.Watson JK, et al. Distal lung epithelial progenitor cell function declines with age. Sci Rep. 2020;10(1):10490. doi: 10.1038/s41598-020-66966-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schafer MJ, et al. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun. 2017;8:14532. doi: 10.1038/ncomms14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baker DJ, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479(7372):232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Idda ML, et al. Survey of senescent cell markers with age in human tissues. Aging (Albany NY) 2020;12(5):4052–4066. doi: 10.18632/aging.102903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yousefzadeh MJ, et al. Tissue specificity of senescent cell accumulation during physiologic and accelerated aging of mice. Aging Cell. 2020;19(3):e13094. doi: 10.1111/acel.13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King TE, Jr, et al. Idiopathic pulmonary fibrosis. Lancet. 2011;378(9807):1949–1961. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- 37.Huang Y, et al. MicroRNA-21: a central regulator of fibrotic diseases via various targets. Curr Pharm Des. 2015;21(17):2236–2242. doi: 10.2174/1381612820666141226095701. [DOI] [PubMed] [Google Scholar]
- 38.Ghonim MA, et al. Pulmonary inflammation and fibroblast immunoregulation: from bench to bedside. J Clin Invest. 2023;133(17):e170499. doi: 10.1172/JCI170499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guha A, et al. Uroplakin 3a+ cells are a distinctive population of epithelial progenitors that contribute to airway maintenance and post-injury repair. Cell Rep. 2017;19(2):246–254. doi: 10.1016/j.celrep.2017.03.051. [DOI] [PubMed] [Google Scholar]
- 40.Liu Q, et al. Lung regeneration by multipotent stem cells residing at the bronchioalveolar-duct junction. Nat Genet. 2019;51(4):728–738. doi: 10.1038/s41588-019-0346-6. [DOI] [PubMed] [Google Scholar]
- 41.Kim CF, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121(6):823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 42.Salwig I, et al. Bronchioalveolar stem cells are a main source for regeneration of distal lung epithelia in vivo. EMBO J. 2019;38(12):e102099. doi: 10.15252/embj.2019102099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strunz M, et al. Alveolar regeneration through a Krt8+ transitional stem cell state that persists in human lung fibrosis. Nat Commun. 2020;11(1):3559. doi: 10.1038/s41467-020-17358-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kathiriya JJ, et al. Distinct airway epithelial stem cells hide among club cells but mobilize to promote alveolar regeneration. Cell Stem Cell. 2020;26(3):346–358. doi: 10.1016/j.stem.2019.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaughan AE, et al. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature. 2015;517(7536):621–625. doi: 10.1038/nature14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zuo W, et al. p63(+)Krt5(+) distal airway stem cells are essential for lung regeneration. Nature. 2015;517(7536):616–620. doi: 10.1038/nature13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beppu AK, et al. Epithelial plasticity and innate immune activation promote lung tissue remodeling following respiratory viral infection. Nat Commun. 2023;14(1):5814. doi: 10.1038/s41467-023-41387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rustam S, et al. A unique cellular organization of human distal airways and its disarray in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2023;207(9):1171–1182. doi: 10.1164/rccm.202207-1384OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Basil MC, et al. Human distal airways contain a multipotent secretory cell that can regenerate alveoli. Nature. 2022;604(7904):120–126. doi: 10.1038/s41586-022-04552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kadur Lakshminarasimha Murthy P, et al. Human distal lung maps and lineage hierarchies reveal a bipotent progenitor. Nature. 2022;604(7904):111–119. doi: 10.1038/s41586-022-04541-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kurche JS, et al. MUC5B idiopathic pulmonary fibrosis risk variant promotes a mucosecretory phenotype and loss of small airway secretory cells. Am J Respir Crit Care Med. 2024;210(4):517–521. doi: 10.1164/rccm.202311-2111LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nabhan AN, et al. Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science. 2018;359(6380):1118–1123. doi: 10.1126/science.aam6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zacharias WJ, et al. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature. 2018;555(7695):251–255. doi: 10.1038/nature25786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Habermann AC, et al. Single-cell RNA sequencing reveals profibrotic roles of distinct epithelial and mesenchymal lineages in pulmonary fibrosis. Sci Adv. 2020;6(28):eaba1972. doi: 10.1126/sciadv.aba1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adams TS, et al. Single-cell RNA-seq reveals ectopic and aberrant lung-resident cell populations in idiopathic pulmonary fibrosis. Sci Adv. 2020;6(28):eaba1983. doi: 10.1126/sciadv.aba1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang P, et al. Ineffectual Type 2-to-Type 1 alveolar epithelial cell differentiation in idiopathic pulmonary fibrosis: persistence of the KRT8hi transitional state. Am J Respir Crit Care Med. 2020;201(11):1443–1447. doi: 10.1164/rccm.201909-1726LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Redente EF, et al. Persistent, progressive pulmonary fibrosis and epithelial remodeling in mice. Am J Respir Cell Mol Biol. 2021;64(6):669–676. doi: 10.1165/rcmb.2020-0542MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang F, et al. Regulation of epithelial transitional states in murine and human pulmonary fibrosis. J Clin Invest. 2023;133(22):e165612. doi: 10.1172/JCI165612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chilosi M, et al. Aberrant Wnt/beta-catenin pathway activation in idiopathic pulmonary fibrosis. Am J Pathol. 2003;162(5):1495–1502. doi: 10.1016/S0002-9440(10)64282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aharonov A, et al. ERBB2 drives YAP activation and EMT-like processes during cardiac regeneration. Nat Cell Biol. 2020;22(11):1346–1356. doi: 10.1038/s41556-020-00588-4. [DOI] [PubMed] [Google Scholar]
- 61.Huang G, et al. Basal cell-derived WNT7A promotes fibrogenesis at the fibrotic niche in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2023;68(3):302–313. doi: 10.1165/rcmb.2022-0074OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kil BJ, et al. The effect of milk protein on the biological and rheological properties of probiotic capsules. J Microbiol Biotechnol. 2020;30(12):1870–1875. doi: 10.4014/jmb.2008.08007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McKeon JL, et al. The effect of inspiratory resistive training on exercise capacity in optimally treated patients with severe chronic airflow limitation. Aust N Z J Med. 1986;16(5):648–652. doi: 10.1111/j.1445-5994.1986.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 64.Riemondy KA, et al. Single cell RNA sequencing identifies TGFβ as a key regenerative cue following LPS-induced lung injury. JCI Insight. 2019;5(8):e123637123637. doi: 10.1172/jci.insight.123637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rowbotham SP, et al. Age-associated H3K9me2 loss alters the regenerative equilibrium between murine lung alveolar and bronchiolar progenitors. Dev Cell. 2023;58(24):2974–2991. doi: 10.1016/j.devcel.2023.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nakano Y, et al. MUC5B promoter variant rs35705950 Affects MUC5B expression in the distal airways in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2016;193(4):464–466. doi: 10.1164/rccm.201509-1872LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Okuda K, et al. Localization of secretory mucins MUC5AC and MUC5B in normal/healthy human airways. Am J Respir Crit Care Med. 2019;199(6):715–727. doi: 10.1164/rccm.201804-0734OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Frohlich E. Animals in respiratory research. Int J Mol Sci. 2024;25(5):2903. doi: 10.3390/ijms25052903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roy MG, et al. Muc5b is required for airway defence. Nature. 2014;505(7483):412–416. doi: 10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alsamri MT, et al. Genetic variants of small airways and interstitial pulmonary disease in children. Sci Rep. 2021;11(1):2715. doi: 10.1038/s41598-021-81280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Costain G, et al. Hereditary mucin deficiency caused by biallelic loss of function of MUC5B. Am J Respir Crit Care Med. 2022;205(7):761–768. doi: 10.1164/rccm.202106-1456OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363(23):2233–2247. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dickey BF, Whitsett JA. Understanding Interstitial Lung Disease: It’s in the Mucus. Am J Respir Cell Mol Biol. 2017;57(1):12–14. doi: 10.1165/rcmb.2017-0116ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ogino K, et al. Involvement of PM2.5-bound protein and metals in PM2.5-induced allergic airway inflammation in mice. Inhal Toxicol. 2018;30(13-14):498–508. doi: 10.1080/08958378.2018.1561769. [DOI] [PubMed] [Google Scholar]
- 75.Seibold MA, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med. 2011;364(16):1503–1512. doi: 10.1056/NEJMoa1013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thornton DJ, et al. Structure and function of the polymeric mucins in airways mucus. Annu Rev Physiol. 2008;70:459–486. doi: 10.1146/annurev.physiol.70.113006.100702. [DOI] [PubMed] [Google Scholar]
- 77.Evans CM, et al. Idiopathic pulmonary fibrosis: a genetic disease that involves mucociliary dysfunction of the peripheral airways. Physiol Rev. 2016;96(4):1567–1591. doi: 10.1152/physrev.00004.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Korennykh A, Walter P. Structural basis of the unfolded protein response. Annu Rev Cell Dev Biol. 2012;28:251–277. doi: 10.1146/annurev-cellbio-101011-155826. [DOI] [PubMed] [Google Scholar]
- 79.Tsuru A, et al. Negative feedback by IRE1β optimizes mucin production in goblet cells. Proc Natl Acad Sci U S A. 2013;110(8):2864–2869. doi: 10.1073/pnas.1212484110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cloots E, et al. IRE1β does not affect mucus secretion during allergic asthma development in a house dust mite murine model. Allergy. 2021;76(11):3546–3549. doi: 10.1111/all.15045. [DOI] [PubMed] [Google Scholar]
- 81.Martino MB, et al. The ER stress transducer IRE1β is required for airway epithelial mucin production. Mucosal Immunol. 2013;6(3):639–654. doi: 10.1038/mi.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oikawa D, et al. Direct association of unfolded proteins with mammalian ER stress sensor, IRE1beta. PLoS One. 2012;7(12):e51290. doi: 10.1371/journal.pone.0051290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bertolotti A, et al. Increased sensitivity to dextran sodium sulfate colitis in IRE1beta-deficient mice. J Clin Invest. 2001;107(5):585–593. doi: 10.1172/JCI11476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tonelli C, et al. A mucus production programme promotes classical pancreatic ductal adenocarcinoma. Gut. 2024;73(6):941–954. doi: 10.1136/gutjnl-2023-329839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cloots E, et al. Activation of goblet-cell stress sensor IRE1β is controlled by the mucin chaperone AGR2. EMBO J. 2024;43(5):695–718. doi: 10.1038/s44318-023-00015-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rajavelu P, et al. Airway epithelial SPDEF integrates goblet cell differentiation and pulmonary Th2 inflammation. J Clin Invest. 2015;125(5):2021–2031. doi: 10.1172/JCI79422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Korfhagen TR, et al. SAM-pointed domain ETS factor mediates epithelial cell-intrinsic innate immune signaling during airway mucous metaplasia. Proc Natl Acad Sci U S A. 2012;109(41):16630–16635. doi: 10.1073/pnas.1208092109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen G, et al. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J Clin Invest. 2009;119(10):2914–2924. doi: 10.1172/JCI39731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Park KS, et al. SPDEF regulates goblet cell hyperplasia in the airway epithelium. J Clin Invest. 2007;117(4):978–988. doi: 10.1172/JCI29176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Iwawaki T, et al. Translational control by the ER transmembrane kinase/ribonuclease IRE1 under ER stress. Nat Cell Biol. 2001;3(2):158–164. doi: 10.1038/35055065. [DOI] [PubMed] [Google Scholar]
- 91.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13(3):184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Furusawa H, et al. Chronic hypersensitivity pneumonitis, an interstitial lung disease with distinct molecular signatures. Am J Respir Crit Care Med. 2020;202(10):1430–1444. doi: 10.1164/rccm.202001-0134OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Katzen J, et al. Disruption of proteostasis causes IRE1 mediated reprogramming of alveolar epithelial cells. Proc Natl Acad Sci U S A. 2022;119(43):e2123187119. doi: 10.1073/pnas.2123187119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Eisele NA, Anderson DM. Host defense and the airway epithelium: frontline responses that protect against bacterial invasion and pneumonia. J Pathog. 2011;2011:249802. doi: 10.4061/2011/249802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hu Q, et al. Cell Adhesion Molecules in Fibrotic Diseases. Biomedicines. 2023;11(7):1995. doi: 10.3390/biomedicines11071995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mathai SK, et al. Desmoplakin variants are associated with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2016;193(10):1151–1160. doi: 10.1164/rccm.201509-1863OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fingerlin TE, et al. Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat Genet. 2013;45(6):613–620. doi: 10.1038/ng.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hao Y, et al. Genome-wide association study: functional variant rs2076295 regulates desmoplakin expression in airway epithelial cells. Am J Respir Crit Care Med. 2020;202(9):1225–1236. doi: 10.1164/rccm.201910-1958OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Muller L, et al. Desmosomes as signaling hubs in the regulation of cell behavior. Front Cell Dev Biol. 2021;9:745670. doi: 10.3389/fcell.2021.745670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yuksel H, et al. E-Cadherin: an important functional molecule at respiratory barrier between defence and dysfunction. Front Physiol. 2021;12:720227. doi: 10.3389/fphys.2021.720227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stancil IT, et al. Integrin axis regulates airway biophysical dysfunction in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2022;66(2):235–237. doi: 10.1165/rcmb.2021-0224LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reed NI, et al. The αvβ1 integrin plays a critical in vivo role in tissue fibrosis. Sci Transl Med. 2015;7(288):288ra79. doi: 10.1126/scitranslmed.aaa5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Conroy KP, et al. αv integrins: key regulators of tissue fibrosis. Cell Tissue Res. 2016;365(3):511–519. doi: 10.1007/s00441-016-2407-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Puthawala K, et al. Inhibition of integrin alpha(v)beta6, an activator of latent transforming growth factor-beta, prevents radiation-induced lung fibrosis. Am J Respir Crit Care Med. 2008;177(1):82–90. doi: 10.1164/rccm.200706-806OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Horan GS, et al. Partial inhibition of integrin alpha(v)beta6 prevents pulmonary fibrosis without exacerbating inflammation. Am J Respir Crit Care Med. 2008;177(1):56–65. doi: 10.1164/rccm.200706-805OC. [DOI] [PubMed] [Google Scholar]
- 106.Henderson NC, et al. Targeting of αv integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med. 2013;19(12):1617–1624. doi: 10.1038/nm.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wheaton AK, et al. Lung epithelial cell focal adhesion kinase signaling inhibits lung injury and fibrosis. Am J Physiol Lung Cell Mol Physiol. 2017;312(5):L722–L730. doi: 10.1152/ajplung.00478.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhao XK, et al. Focal adhesion kinase regulates fibroblast migration via integrin beta-1 and plays a central role in fibrosis. Sci Rep. 2016;6:19276. doi: 10.1038/srep19276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lagares D, et al. Inhibition of focal adhesion kinase prevents experimental lung fibrosis and myofibroblast formation. Arthritis Rheum. 2012;64(5):1653–1664. doi: 10.1002/art.33482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ding D, et al. Genomic fingerprint associated with familial idiopathic pulmonary fibrosis: a review. Int J Med Sci. 2023;20(3):329–345. doi: 10.7150/ijms.80358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Michalski JE, Schwartz DA. Genetic risk factors for idiopathic pulmonary fibrosis: insights into immunopathogenesis. J Inflamm Res. 2020;13:1305–1318. doi: 10.2147/JIR.S280958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sutton RM, et al. Rare surfactant-related variants in familial and sporadic pulmonary fibrosis. Hum Mutat. 2022;43(12):2091–2101. doi: 10.1002/humu.24476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lawson WE, et al. Genetic mutations in surfactant protein C are a rare cause of sporadic cases of IPF. Thorax. 2004;59(11):977–980. doi: 10.1136/thx.2004.026336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mai C, et al. Thin-section CT features of idiopathic pulmonary fibrosis correlated with micro-CT and histologic analysis. Radiology. 2017;283(1):252–263. doi: 10.1148/radiol.2016152362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wittwer MF, et al. Signs of alveolar collapse in idiopathic pulmonary fibrosis, hypersensitivity pneumonitis and systemic sclerosis revealed by inspiration and expiration computed tomography. BioMed. 2023;3(4):471–483. doi: 10.3390/biomed3040038. [DOI] [Google Scholar]
- 116.Beike L, et al. Surfactant dysfunction and alveolar collapse are linked with fibrotic septal wall remodeling in the TGF-β1-induced mouse model of pulmonary fibrosis. Lab Invest. 2019;99(6):830–852. doi: 10.1038/s41374-019-0189-x. [DOI] [PubMed] [Google Scholar]
- 117.Kabore AF, et al. Biosynthesis of surfactant protein C: characterization of aggresome formation by EGFP chimeras containing propeptide mutants lacking conserved cysteine residues. J Cell Sci. 2001;114(pt 2):293–302. doi: 10.1242/jcs.114.2.293. [DOI] [PubMed] [Google Scholar]
- 118.Wang WJ, et al. Deletion of exon 4 from human surfactant protein C results in aggresome formation and generation of a dominant negative. J Cell Sci. 2003;116(pt 4):683–692. doi: 10.1242/jcs.00267. [DOI] [PubMed] [Google Scholar]
- 119.Nogee LM, et al. A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N Engl J Med. 2001;344(8):573–579. doi: 10.1056/NEJM200102223440805. [DOI] [PubMed] [Google Scholar]
- 120.Thomas AQ, et al. Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. Am J Respir Crit Care Med. 2002;165(9):1322–1328. doi: 10.1164/rccm.200112-123OC. [DOI] [PubMed] [Google Scholar]
- 121.van Moorsel CH, et al. Surfactant protein C mutations are the basis of a significant portion of adult familial pulmonary fibrosis in a Dutch cohort. Am J Respir Crit Care Med. 2010;182(11):1419–1425. doi: 10.1164/rccm.200906-0953OC. [DOI] [PubMed] [Google Scholar]
- 122.Bridges JP, et al. Expression of a human surfactant protein C mutation associated with interstitial lung disease disrupts lung development in transgenic mice. J Biol Chem. 2003;278(52):52739–52746. doi: 10.1074/jbc.M309599200. [DOI] [PubMed] [Google Scholar]
- 123.Lawson WE, et al. Endoplasmic reticulum stress enhances fibrotic remodeling in the lungs. Proc Natl Acad Sci U S A. 2011;108(26):10562–10567. doi: 10.1073/pnas.1107559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Maguire JA, et al. Endoplasmic reticulum stress induced by surfactant protein C BRICHOS mutants promotes proinflammatory signaling by epithelial cells. Am J Respir Cell Mol Biol. 2011;44(3):404–414. doi: 10.1165/rcmb.2009-0382OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Borok Z, et al. Grp78 loss in epithelial progenitors reveals an age-linked role for endoplasmic reticulum stress in pulmonary fibrosis. Am J Respir Crit Care Med. 2020;201(2):198–211. doi: 10.1164/rccm.201902-0451OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Blenkinsopp WK. Proliferation of respiratory tract epithelium in the rat. Exp Cell Res. 1967;46(1):144–154. doi: 10.1016/0014-4827(67)90416-8. [DOI] [PubMed] [Google Scholar]
- 127.Chen J, et al. Identification of a DNA damage-induced alternative splicing pathway that regulates p53 and cellular senescence markers. Cancer Discov. 2017;7(7):766–781. doi: 10.1158/2159-8290.CD-16-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Harley CB, et al. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345(6274):458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 129.Syntichaki P, et al. eIF4E function in somatic cells modulates ageing in Caenorhabditis elegans. Nature. 2007;445(7130):922–926. doi: 10.1038/nature05603. [DOI] [PubMed] [Google Scholar]
- 130.Dang W, et al. Histone H4 lysine 16 acetylation regulates cellular lifespan. Nature. 2009;459(7248):802–807. doi: 10.1038/nature08085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509(7501):439–446. doi: 10.1038/nature13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Polymenis M, Kennedy BK. Unbalanced growth, senescence and aging. Adv Exp Med Biol. 2017;1002:189–208. doi: 10.1007/978-3-319-57127-0_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Anisimova AS, et al. Protein synthesis and quality control in aging. Aging (Albany NY) 2018;10(12):4269–4288. doi: 10.18632/aging.101721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Martinez Corrales G, et al. Partial inhibition of RNA polymerase I promotes animal health and longevity. Cell Rep. 2020;30(6):1661–1669. doi: 10.1016/j.celrep.2020.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kim HS, et al. The p38-activated ER stress-ATF6α axis mediates cellular senescence. FASEB J. 2019;33(2):2422–2434. doi: 10.1096/fj.201800836R. [DOI] [PubMed] [Google Scholar]
- 136.Correia-Melo C, et al. Mitochondria are required for pro-ageing features of the senescent phenotype. EMBO J. 2016;35(7):724–742. doi: 10.15252/embj.201592862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kuilman T, Peeper DS. Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer. 2009;9(2):81–94. doi: 10.1038/nrc2560. [DOI] [PubMed] [Google Scholar]
- 138.Lee JS, et al. Molecular markers of telomere dysfunction and senescence are common findings in the usual interstitial pneumonia pattern of lung fibrosis. Histopathology. 2021;79(1):67–76. doi: 10.1111/his.14334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kuwano K, et al. P21Waf1/Cip1/Sdi1 and p53 expression in association with DNA strand breaks in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1996;154(2 pt 1):477–483. doi: 10.1164/ajrccm.154.2.8756825. [DOI] [PubMed] [Google Scholar]
- 140.Minagawa S, et al. Accelerated epithelial cell senescence in IPF and the inhibitory role of SIRT6 in TGF-β-induced senescence of human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2011;300(3):L391–L401. doi: 10.1152/ajplung.00097.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yao C, et al. Sin3a regulates epithelial progenitor cell fate during lung development. Development. 2017;144(14):2618–2628. doi: 10.1242/dev.149708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yao C, et al. Senescence of alveolar type 2 cells drives progressive pulmonary fibrosis. Am J Respir Crit Care Med. 2021;203(6):707–717. doi: 10.1164/rccm.202004-1274OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.McClintock B. The stability of broken ends of chromosomes in zea mays. Genetics. 1941;26(2):234–282. doi: 10.1093/genetics/26.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yu GL, et al. In vivo alteration of telomere sequences and senescence caused by mutated Tetrahymena telomerase RNAs. Nature. 1990;344(6262):126–132. doi: 10.1038/344126a0. [DOI] [PubMed] [Google Scholar]
- 145.Lundblad V, Blackburn EH. An alternative pathway for yeast telomere maintenance rescues est1- senescence. Cell. 1993;73(2):347–360. doi: 10.1016/0092-8674(93)90234-H. [DOI] [PubMed] [Google Scholar]
- 146.d’Adda di Fagagna F, et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426(6963):194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 147.Opresko PL, et al. Oxidative damage in telomeric DNA disrupts recognition by TRF1 and TRF2. Nucleic Acids Res. 2005;33(4):1230–1239. doi: 10.1093/nar/gki273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Alder JK, et al. Telomere dysfunction causes alveolar stem cell failure. Proc Natl Acad Sci U S A. 2015;112(16):5099–5104. doi: 10.1073/pnas.1504780112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.McDonough JE, et al. A role for telomere length and chromosomal damage in idiopathic pulmonary fibrosis. Respir Res. 2018;19(1):132. doi: 10.1186/s12931-018-0838-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Duckworth A, et al. Telomere length and risk of idiopathic pulmonary fibrosis and chronic obstructive pulmonary disease: a mendelian randomisation study. Lancet Respir Med. 2021;9(3):285–294. doi: 10.1016/S2213-2600(20)30364-7. [DOI] [PubMed] [Google Scholar]
- 151.Armanios MY, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356(13):1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 152.Blasco MA, et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91(1):25–34. doi: 10.1016/S0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- 153.Tsakiri KD, et al. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci U S A. 2007;104(18):7552–7557. doi: 10.1073/pnas.0701009104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Alder JK, et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci U S A. 2008;105(35):13051–13056. doi: 10.1073/pnas.0804280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gaysinskaya V, et al. Synonymous mutation in DKC1 causes telomerase RNA insufficiency manifesting as familial pulmonary fibrosis. Chest. 2020;158(6):2449–2457. doi: 10.1016/j.chest.2020.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Allen RJ, et al. Genetic variants associated with susceptibility to idiopathic pulmonary fibrosis in people of European ancestry: a genome-wide association study. Lancet Respir Med. 2017;5(11):869–880. doi: 10.1016/S2213-2600(17)30387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Moore C, et al. Resequencing study confirms that host defense and cell senescence gene variants contribute to the risk of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2019;200(2):199–208. doi: 10.1164/rccm.201810-1891OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Allen RJ, et al. Genome-wide association study of susceptibility to idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2020;201(5):564–574. doi: 10.1164/rccm.201905-1017OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Alder JK, et al. Exome sequencing identifies mutant TINF2 in a family with pulmonary fibrosis. Chest. 2015;147(5):1361–1368. doi: 10.1378/chest.14-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sfeir A, de Lange T. Removal of shelterin reveals the telomere end-protection problem. Science. 2012;336(6081):593–597. doi: 10.1126/science.1218498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Denchi EL, de Lange T. Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature. 2007;448(7157):1068–1071. doi: 10.1038/nature06065. [DOI] [PubMed] [Google Scholar]
- 162.Lundblad V, Szostak JW. A mutant with a defect in telomere elongation leads to senescence in yeast. Cell. 1989;57(4):633–643. doi: 10.1016/0092-8674(89)90132-3. [DOI] [PubMed] [Google Scholar]
- 163.Bryan TM, et al. Evidence for an alternative mechanism for maintaining telomere length in human tumors and tumor-derived cell lines. Nat Med. 1997;3(11):1271–1274. doi: 10.1038/nm1197-1271. [DOI] [PubMed] [Google Scholar]
- 164.Armanios M, et al. Haploinsufficiency of telomerase reverse transcriptase leads to anticipation in autosomal dominant dyskeratosis congenita. Proc Natl Acad Sci U S A. 2005;102(44):15960–15964. doi: 10.1073/pnas.0508124102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Parry EM, et al. Syndrome complex of bone marrow failure and pulmonary fibrosis predicts germline defects in telomerase. Blood. 2011;117(21):5607–5611. doi: 10.1182/blood-2010-11-322149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Parry EM, et al. Decreased dyskerin levels as a mechanism of telomere shortening in X-linked dyskeratosis congenita. J Med Genet. 2011;48(5):327–333. doi: 10.1136/jmg.2010.085100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Weintraub H, et al. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 1991;251(4995):761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- 168.Tsaytler P, et al. BMP4 triggers regulatory circuits specifying the cardiac mesoderm lineage. Development. 2023;150(10):dev201450. doi: 10.1242/dev.201450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cao T, et al. A CGA/EGFR/GATA2 positive feedback circuit confers chemoresistance in gastric cancer. J Clin Invest. 2022;132(6):e154074. doi: 10.1172/JCI154074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yang IV, et al. Relationship of DNA methylation and gene expression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2014;190(11):1263–1272. doi: 10.1164/rccm.201408-1452OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Huang SK, et al. Lung fibroblasts from patients with idiopathic pulmonary fibrosis exhibit genome-wide differences in DNA methylation compared to fibroblasts from nonfibrotic lung. PLoS One. 2014;9(9):e107055. doi: 10.1371/journal.pone.0107055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Vincent A, et al. Epigenetic regulation (DNA methylation, histone modifications) of the 11p15 mucin genes (MUC2, MUC5AC, MUC5B, MUC6) in epithelial cancer cells. Oncogene. 2007;26(45):6566–6576. doi: 10.1038/sj.onc.1210479. [DOI] [PubMed] [Google Scholar]
- 173.Helling BA, et al. Regulation of MUC5B expression in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2017;57(1):91–99. doi: 10.1165/rcmb.2017-0046OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gally F, et al. The MUC5B-associated variant rs35705950 resides within an enhancer subject to lineage- and disease-dependent epigenetic remodeling. JCI Insight. 2021;6(2):e144294144294. doi: 10.1172/jci.insight.144294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bergougnoux A, et al. The HDAC inhibitor SAHA does not rescue CFTR membrane expression in Cystic Fibrosis. Int J Biochem Cell Biol. 2017;88:124–132. doi: 10.1016/j.biocel.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 176.Fulmer JD, et al. Small airways in idiopathic pulmonary fibrosis. Comparison of morphologic and physiologic observations. J Clin Invest. 1977;60(3):595–610. doi: 10.1172/JCI108811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Plantier L, et al. Ectopic respiratory epithelial cell differentiation in bronchiolised distal airspaces in idiopathic pulmonary fibrosis. Thorax. 2011;66(8):651–657. doi: 10.1136/thx.2010.151555. [DOI] [PubMed] [Google Scholar]
- 178.Prasse A, et al. BAL Cell Gene Expression Is Indicative of Outcome and Airway Basal Cell Involvement in Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med. 2019;199(5):622–630. doi: 10.1164/rccm.201712-2551OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kathiriya JJ, et al. Human alveolar type 2 epithelium transdifferentiates into metaplastic KRT5+ basal cells. Nat Cell Biol. 2022;24(1):10–23. doi: 10.1038/s41556-021-00809-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cassandras M, et al. Gli1+ mesenchymal stromal cells form a pathological niche to promote airway progenitor metaplasia in the fibrotic lung. Nat Cell Biol. 2020;22(11):1295–1306. doi: 10.1038/s41556-020-00591-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Stancil IT, et al. Interleukin-6-dependent epithelial fluidization initiates fibrotic lung remodeling. Sci Transl Med. 2022;14(654):eabo5254. doi: 10.1126/scitranslmed.abo5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Stancil IT, et al. Pulmonary fibrosis distal airway epithelia are dynamically and structurally dysfunctional. Nat Commun. 2021;12(1):4566. doi: 10.1038/s41467-021-24853-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Liu Z, et al. MAPK-Mediated YAP activation controls mechanical-tension-induced pulmonary alveolar regeneration. Cell Rep. 2016;16(7):1810–1819. doi: 10.1016/j.celrep.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 184.Hicks-Berthet J, et al. Yap/Taz inhibit goblet cell fate to maintain lung epithelial homeostasis. Cell Rep. 2021;36(2):109347. doi: 10.1016/j.celrep.2021.109347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Aravamudhan A, et al. TBK1 regulates YAP/TAZ and fibrogenic fibroblast activation. Am J Physiol Lung Cell Mol Physiol. 2020;318(5):L852–L863. doi: 10.1152/ajplung.00324.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.DiGiovanni GT, et al. Epithelial Yap/Taz are required for functional alveolar regeneration following acute lung injury. JCI Insight. 2023;8(19):e173374. doi: 10.1172/jci.insight.173374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kurche JS, et al. Muc5b enhances murine honeycomb-like cyst formation. Am J Respir Cell Mol Biol. 2019;61(4):544–546. doi: 10.1165/rcmb.2019-0138LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Green J, et al. Diversity of interstitial lung fibroblasts is regulated by platelet-derived growth factor receptor α kinase activity. Am J Respir Cell Mol Biol. 2016;54(4):532–545. doi: 10.1165/rcmb.2015-0095OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Desai TJ, et al. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature. 2014;507(7491):190–194. doi: 10.1038/nature12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kato K, et al. Pulmonary pericytes regulate lung morphogenesis. Nat Commun. 2018;9(1):2448. doi: 10.1038/s41467-018-04913-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Filler SG, et al. An enzyme-linked immunosorbent assay for quantifying adherence of Candida to human vascular endothelium. J Infect Dis. 1987;156(4):561–566. doi: 10.1093/infdis/156.4.561. [DOI] [PubMed] [Google Scholar]
- 192.Narvaez Del Pilar O, et al. Three-axis classification of mouse lung mesenchymal cells reveals two populations of myofibroblasts. Development. 2022;149(6):dev200081. doi: 10.1242/dev.200081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 194.Zepp JA, et al. Distinct mesenchymal lineages and niches promote epithelial self-renewal and myofibrogenesis in the lung. Cell. 2017;170(6):1134–1148. doi: 10.1016/j.cell.2017.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Redente EF, et al. Loss of Fas signaling in fibroblasts impairs homeostatic fibrosis resolution and promotes persistent pulmonary fibrosis. JCI Insight. 2020;6(1):e141618. doi: 10.1172/jci.insight.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tsukui T, et al. Collagen-producing lung cell atlas identifies multiple subsets with distinct localization and relevance to fibrosis. Nat Commun. 2020;11(1):1920. doi: 10.1038/s41467-020-15647-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Link PA, et al. Combined control of the fibroblast contractile program by YAP and TAZ. Am J Physiol Lung Cell Mol Physiol. 2022;322(1):L23–L32. doi: 10.1152/ajplung.00210.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Tsukui T, et al. Alveolar fibroblast lineage orchestrates lung inflammation and fibrosis. Nature. 2024;631(8021):627–634. doi: 10.1038/s41586-024-07660-1. [DOI] [PubMed] [Google Scholar]
- 199.Portnoy J, et al. Alveolar type II cells inhibit fibroblast proliferation: role of IL-1alpha. Am J Physiol Lung Cell Mol Physiol. 2006;290(2):L307–L316. doi: 10.1152/ajplung.00102.2005. [DOI] [PubMed] [Google Scholar]
- 200.Pan T, et al. Rat alveolar type II cells inhibit lung fibroblast proliferation in vitro. Am J Respir Cell Mol Biol. 2001;25(3):353–361. doi: 10.1165/ajrcmb.25.3.4004. [DOI] [PubMed] [Google Scholar]
- 201.Lama V, et al. Prostaglandin E2 synthesis and suppression of fibroblast proliferation by alveolar epithelial cells is cyclooxygenase-2-dependent. Am J Respir Cell Mol Biol. 2002;27(6):752–758. doi: 10.1165/rcmb.4857. [DOI] [PubMed] [Google Scholar]
- 202.Moore BB, et al. Alveolar epithelial cell inhibition of fibroblast proliferation is regulated by MCP-1/CCR2 and mediated by PGE2. Am J Physiol Lung Cell Mol Physiol. 2003;284(2):L342–L349. doi: 10.1152/ajplung.00168.2002. [DOI] [PubMed] [Google Scholar]
- 203.Borok Z, et al. Augmentation of functional prostaglandin E levels on the respiratory epithelial surface by aerosol administration of prostaglandin E. Am Rev Respir Dis. 1991;144(5):1080–1084. doi: 10.1164/ajrccm/144.5.1080. [DOI] [PubMed] [Google Scholar]
- 204.Khalil N, et al. TGF-beta 1, but not TGF-beta 2 or TGF-beta 3, is differentially present in epithelial cells of advanced pulmonary fibrosis: an immunohistochemical study. Am J Respir Cell Mol Biol. 1996;14(2):131–138. doi: 10.1165/ajrcmb.14.2.8630262. [DOI] [PubMed] [Google Scholar]
- 205.Khalil N, et al. Increased production and immunohistochemical localization of transforming growth factor-beta in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 1991;5(2):155–162. doi: 10.1165/ajrcmb/5.2.155. [DOI] [PubMed] [Google Scholar]
- 206.Khalil N, et al. Regulation of the effects of TGF-beta 1 by activation of latent TGF-beta 1 and differential expression of TGF-beta receptors (T beta R-I and T beta R-II) in idiopathic pulmonary fibrosis. Thorax. 2001;56(12):907–915. doi: 10.1136/thorax.56.12.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Cigna N, et al. The hedgehog system machinery controls transforming growth factor-β-dependent myofibroblastic differentiation in humans: involvement in idiopathic pulmonary fibrosis. Am J Pathol. 2012;181(6):2126–2137. doi: 10.1016/j.ajpath.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 208.Bolanos AL, et al. Role of Sonic Hedgehog in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2012;303(11):L978–L990. doi: 10.1152/ajplung.00184.2012. [DOI] [PubMed] [Google Scholar]
- 209.Delgadillo H, et al. The MHC2TA gene polymorphisms are not associated with restenosis after coronary stenting in Mexican patients. Arch Cardiol Mex. 2012;82(3):208–213. doi: 10.1016/j.acmx.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 210.Bamberg A, et al. Protein tyrosine phosphatase-N13 promotes myofibroblast resistance to apoptosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2018;198(7):914–927. doi: 10.1164/rccm.201707-1497OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hou J, et al. Co-delivery of siPTPN13 and siNOX4 via (myo)fibroblast-targeting polymeric micelles for idiopathic pulmonary fibrosis therapy. Theranostics. 2021;11(7):3244–3261. doi: 10.7150/thno.54217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Golan-Gerstl R, et al. Cellular FLICE-like inhibitory protein deviates myofibroblast fas-induced apoptosis toward proliferation during lung fibrosis. Am J Respir Cell Mol Biol. 2012;47(3):271–279. doi: 10.1165/rcmb.2010-0284RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zhou Y, et al. Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J Clin Invest. 2013;123(3):1096–1108. doi: 10.1172/JCI66700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ashley SL, et al. Targeting inhibitor of apoptosis proteins protects from bleomycin-induced lung fibrosis. Am J Respir Cell Mol Biol. 2016;54(4):482–492. doi: 10.1165/rcmb.2015-0148OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Waters DW, et al. Fibroblast senescence in the pathology of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2018;315(2):L162–L172. doi: 10.1152/ajplung.00037.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kobayashi Y, et al. Persistence of a regeneration-associated, transitional alveolar epithelial cell state in pulmonary fibrosis. Nat Cell Biol. 2020;22(8):934–946. doi: 10.1038/s41556-020-0542-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Cool CD, et al. Fibroblast foci are not discrete sites of lung injury or repair: the fibroblast reticulum. Am J Respir Crit Care Med. 2006;174(6):654–658. doi: 10.1164/rccm.200602-205OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Yamaguchi M, et al. Fibroblastic foci, covered with alveolar epithelia exhibiting epithelial-mesenchymal transition, destroy alveolar septa by disrupting blood flow in idiopathic pulmonary fibrosis. Lab Invest. 2017;97(3):232–242. doi: 10.1038/labinvest.2016.135. [DOI] [PubMed] [Google Scholar]
- 219.Conforti F, et al. Paracrine SPARC signaling dysregulates alveolar epithelial barrier integrity and function in lung fibrosis. Cell Death Discov. 2020;6:54. doi: 10.1038/s41420-020-0289-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Richeldi L, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 221.King TE, Jr A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 222.Dempsey TM, et al. Adoption of the antifibrotic medications pirfenidone and nintedanib for patients with idiopathic pulmonary fibrosis. Ann Am Thorac Soc. 2021;18(7):1121–1128. doi: 10.1513/AnnalsATS.202007-901OC. [DOI] [PubMed] [Google Scholar]
- 223.Herberts MB, et al. Idiopathic pulmonary fibrosis in the United States: time to diagnosis and treatment. BMC Pulm Med. 2023;23(1):281. doi: 10.1186/s12890-023-02565-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Raghu G, et al. Randomized Phase IIa clinical study of an Anti-αvβ6 monoclonal antibody in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2022;206(9):1166–1168. doi: 10.1164/rccm.202205-0868LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Pliant. Pliant Therapeutics Announces Positive Safety and Efficacy Data from Phase 2a INTEGRIS-IPF Clinical Trial of PLN-74809. in Patients with Idiopathic Pulmonary Fibrosis. https://ir.pliantrx.com/news-releases/news-release-details/pliant-therapeutics-announces-positive-safety-and-efficacy-data Updated July 10, 2022. Accessed October 28, 2024.
- 226.Cooley JC, et al. Inhibition of antiapoptotic BCL-2 proteins with ABT-263 induces fibroblast apoptosis, reversing persistent pulmonary fibrosis. JCI Insight. 2023;8(3):e163762. doi: 10.1172/jci.insight.163762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Gu L, et al. Targeting Cpt1a-Bcl-2 interaction modulates apoptosis resistance and fibrotic remodeling. Cell Death Differ. 2022;29(1):118–132. doi: 10.1038/s41418-021-00840-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Shi YF, et al. Combining triptolide with ABT-199 is effective against acute myeloid leukemia through reciprocal regulation of Bcl-2 family proteins and activation of the intrinsic apoptotic pathway. Cell Death Dis. 2020;11(7):555. doi: 10.1038/s41419-020-02762-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lagares D, et al. Targeted apoptosis of myofibroblasts with the BH3 mimetic ABT-263 reverses established fibrosis. Sci Transl Med. 2017;9(420):eaal3765. doi: 10.1126/scitranslmed.aal3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kulkarni JA, et al. The current landscape of nucleic acid therapeutics. Nat Nanotechnol. 2021;16(6):630–643. doi: 10.1038/s41565-021-00898-0. [DOI] [PubMed] [Google Scholar]
- 231.Doudna JA. The promise and challenge of therapeutic genome editing. Nature. 2020;578(7794):229–236. doi: 10.1038/s41586-020-1978-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Li B, et al. Combinatorial design of nanoparticles for pulmonary mRNA delivery and genome editing. Nat Biotechnol. 2023;41(10):1410–1415. doi: 10.1038/s41587-023-01679-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.van Batenburg AA, et al. Telomere shortening and DNA damage in culprit cells of different types of progressive fibrosing interstitial lung disease. ERJ Open Res. 2021;7(2):00691-2020. doi: 10.1183/23120541.00691-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Snetselaar R, et al. Short telomere length in IPF lung associates with fibrotic lesions and predicts survival. PLoS One. 2017;12(12):e0189467. doi: 10.1371/journal.pone.0189467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kropski JA, et al. Extensive phenotyping of individuals at risk for familial interstitial pneumonia reveals clues to the pathogenesis of interstitial lung disease. Am J Respir Crit Care Med. 2015;191(4):417–426. doi: 10.1164/rccm.201406-1162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Povedano JM, et al. Therapeutic effects of telomerase in mice with pulmonary fibrosis induced by damage to the lungs and short telomeres. Elife. 2018;7:e31299. doi: 10.7554/eLife.31299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Gao Q, et al. Lentivirus expressing soluble ST2 alleviates bleomycin-induced pulmonary fibrosis in mice. Int Immunopharmacol. 2016;30:188–193. doi: 10.1016/j.intimp.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 238.Cipolla E, et al. IL-17A deficiency mitigates bleomycin-induced complement activation during lung fibrosis. FASEB J. 2017;31(12):5543–5556. doi: 10.1096/fj.201700289R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kurosaki F, et al. AAV6-mediated IL-10 expression in the lung ameliorates bleomycin-induced pulmonary fibrosis in mice. Hum Gene Ther. 2018;29(11):1242–1251. doi: 10.1089/hum.2018.024. [DOI] [PubMed] [Google Scholar]
- 240.Watanabe M, et al. Hepatocyte growth factor gene transfer to alveolar septa for effective suppression of lung fibrosis. Mol Ther. 2005;12(1):58–67. doi: 10.1016/j.ymthe.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 241.Kijiyama N, et al. Intratracheal gene transfer of tissue factor pathway inhibitor attenuates pulmonary fibrosis. Biochem Biophys Res Commun. 2006;339(4):1113–1119. doi: 10.1016/j.bbrc.2005.11.127. [DOI] [PubMed] [Google Scholar]
- 242.Farkas L, et al. VEGF ameliorates pulmonary hypertension through inhibition of endothelial apoptosis in experimental lung fibrosis in rats. J Clin Invest. 2009;119(5):1298–1311. doi: 10.1172/JCI36136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Wang X, et al. Vasohibin attenuates bleomycin induced pulmonary fibrosis via inhibition of angiogenesis in mice. Pathology. 2010;42(5):457–462. doi: 10.3109/00313025.2010.493864. [DOI] [PubMed] [Google Scholar]
- 244.Shenoy V, et al. The angiotensin-converting enzyme 2/angiogenesis-(1-7)/Mas axis confers cardiopulmonary protection against lung fibrosis and pulmonary hypertension. Am J Respir Crit Care Med. 2010;182(8):1065–1072. doi: 10.1164/rccm.200912-1840OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Yang J, et al. Overexpression of inhibitor of DNA-binding 2 attenuates pulmonary fibrosis through regulation of c-Abl and Twist. Am J Pathol. 2015;185(4):1001–1011. doi: 10.1016/j.ajpath.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Huang T, et al. Treating pulmonary fibrosis with non-viral gene therapy: from bench to bedside. Pharmaceutics. 2022;14(4):813. doi: 10.3390/pharmaceutics14040813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.McCarron A, et al. Effective viral-mediated lung gene therapy: is airway surface preparation necessary? Gene Ther. 2023;30(6):469–477. doi: 10.1038/s41434-022-00332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kim N, et al. Barriers to inhaled gene therapy of obstructive lung diseases: A review. J Control Release. 2016;240:465–488. doi: 10.1016/j.jconrel.2016.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Bañuls L, et al. Gene therapy in rare respiratory diseases: what have we learned so far? J Clin Med. 2020;9(8):2577. doi: 10.3390/jcm9082577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Chen X, et al. Clinical applications of mesenchymal stromal cell-based therapies for pulmonary diseases: An Update and Concise Review. Int J Med Sci. 2021;18(13):2849–2870. doi: 10.7150/ijms.59218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Vaidyanathan S, et al. High-efficiency, selection-free gene repair in airway stem cells from cystic fibrosis patients rescues CFTR function in differentiated epithelia. Cell Stem Cell. 2020;26(2):161–171. doi: 10.1016/j.stem.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Cao J, et al. An easy and efficient inducible CRISPR/Cas9 platform with improved specificity for multiple gene targeting. Nucleic Acids Res. 2016;44(19):e149. doi: 10.1093/nar/gkw660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Manghwar H, et al. CRISPR/Cas systems in genome editing: methodologies and tools for sgRNA design, off-target evaluation, and strategies to mitigate off-target effects. Adv Sci (Weinh) 2020;7(6):1902312. doi: 10.1002/advs.201902312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Schuster A, et al. RNAi/CRISPR screens: from a pool to a valid hit. Trends Biotechnol. 2019;37(1):38–55. doi: 10.1016/j.tibtech.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 255.Cappelluti MA, et al. Durable and efficient gene silencing in vivo by hit-and-run epigenome editing. Nature. 2024;627(8003):416–423. doi: 10.1038/s41586-024-07087-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Chao YL, Pecot CV. Targeting epigenetics in lung cancer. Cold Spring Harb Perspect Med. 2021;11(6):a038000. doi: 10.1101/cshperspect.a038000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Kuehl PJ, et al. 5-Azacytidine inhaled dry powder formulation profoundly improves pharmacokinetics and efficacy for lung cancer therapy through genome reprogramming. Br J Cancer. 2020;122(8):1194–1204. doi: 10.1038/s41416-020-0765-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Song J, et al. Targeted epigenetic editing of SPDEF reduces mucus production in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2017;312(3):L334–L347. doi: 10.1152/ajplung.00059.2016. [DOI] [PubMed] [Google Scholar]
- 259.Klinkhammer BM, et al. PDGF in organ fibrosis. Mol Aspects Med. 2018;62:44–62. doi: 10.1016/j.mam.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 260.Yang IV, Schwartz DA. Epigenetics of idiopathic pulmonary fibrosis. Transl Res. 2015;165(1):48–60. doi: 10.1016/j.trsl.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Epstein Shochet G, et al. TGF-β pathway activation by idiopathic pulmonary fibrosis (IPF) fibroblast derived soluble factors is mediated by IL-6 trans-signaling. Respir Res. 2020;21(1):56. doi: 10.1186/s12931-020-1319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Yang D, et al. The histone methyltransferase DOT1L is a new epigenetic regulator of pulmonary fibrosis. Cell Death Dis. 2022;13(1):60. doi: 10.1038/s41419-021-04365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Gilbert LA, et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell. 2013;154(2):442–451. doi: 10.1016/j.cell.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Mendenhall EM, et al. Locus-specific editing of histone modifications at endogenous enhancers. Nat Biotechnol. 2013;31(12):1133–1136. doi: 10.1038/nbt.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Konermann S, et al. Optical control of mammalian endogenous transcription and epigenetic states. Nature. 2013;500(7463):472–476. doi: 10.1038/nature12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Stolzenburg S, et al. Targeted silencing of the oncogenic transcription factor SOX2 in breast cancer. Nucleic Acids Res. 2012;40(14):6725–6740. doi: 10.1093/nar/gks360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Zeitler B, et al. Allele-selective transcriptional repression of mutant HTT for the treatment of Huntington’s disease. Nat Med. 2019;25(7):1131–1142. doi: 10.1038/s41591-019-0478-3. [DOI] [PubMed] [Google Scholar]
- 268.Nuñez JK, et al. Genome-wide programmable transcriptional memory by CRISPR-based epigenome editing. Cell. 2021;184(9):2503–2519. doi: 10.1016/j.cell.2021.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Chandler RJ, et al. Vector design influences hepatic genotoxicity after adeno-associated virus gene therapy. J Clin Invest. 2015;125(2):870–880. doi: 10.1172/JCI79213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Li T, et al. CRISPR/Cas9 therapeutics: progress and prospects. Signal Transduct Target Ther. 2023;8(1):36. doi: 10.1038/s41392-023-01309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Maecker H, et al. Exploration of the antibody-drug conjugate clinical landscape. MAbs. 2023;15(1):2229101. doi: 10.1080/19420862.2023.2229101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Partanen JJ, et al. Leveraging global multi-ancestry meta-analysis in the study of idiopathic pulmonary fibrosis genetics. Cell Genom. 2022;2(10):100181. doi: 10.1016/j.xgen.2022.100181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Noth I, et al. Genetic variants associated with idiopathic pulmonary fibrosis susceptibility and mortality: a genome-wide association study. Lancet Respir Med. 2013;1(4):309–317. doi: 10.1016/S2213-2600(13)70045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Donoghue LJ, et al. BPIFB1 loss alters airway mucus properties and diminishes mucociliary clearance. Am J Physiol Lung Cell Mol Physiol. 2023;325(6):L765–L775. doi: 10.1152/ajplung.00390.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Fingerlin TE, et al. Genome-wide imputation study identifies novel HLA locus for pulmonary fibrosis and potential role for auto-immunity in fibrotic idiopathic interstitial pneumonia. BMC Genet. 2016;17(1):74. doi: 10.1186/s12863-016-0377-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Allen RJ, et al. Genome-wide association study across five cohorts identifies five novel loci associated with idiopathic pulmonary fibrosis. Thorax. 2022;77(8):829–833. doi: 10.1136/thoraxjnl-2021-218577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Peljto AL, et al. Idiopathic pulmonary fibrosis is associated with common genetic variants and limited rare variants. Am J Respir Crit Care Med. 2023;207(9):1194–1202. doi: 10.1164/rccm.202207-1331OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Zhang D, et al. Utility of whole genome sequencing in assessing risk and clinically relevant outcomes for pulmonary fibrosis. Eur Respir J. 2022;60(6):2200577. doi: 10.1183/13993003.00577-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Stuart BD, et al. Exome sequencing links mutations in PARN and RTEL1 with familialpulmonary fibrosis and telomere shortening. Nat Genet. 2015;47(5):512–517. doi: 10.1038/ng.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Cogan JD, et al. Rare variants in RTEL1 are associated with familial interstitial pneumonia. Am J Respir Crit Care Med. 2015;191(6):646–655. doi: 10.1164/rccm.201408-1510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Stanley SE, et al. Loss-of-function mutations in the RNA biogenesis factor NAF1 predispose to pulmonary fibrosis-emphysema. Sci Transl Med. 2016;8(351):351ra107. doi: 10.1126/scitranslmed.aaf7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Kropski JA, et al. A novel dyskerin (DKC1) mutation is associated with familial interstitial pneumonia. Chest. 2014;146(1):e1–e7. doi: 10.1378/chest.13-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Takezaki A, et al. A homozygous SFTPA1 mutation drives necroptosis of type II alveolar epithelial cells in patients with idiopathic pulmonary fibrosis. J Exp Med. 2019;216(12):2724–2735. doi: 10.1084/jem.20182351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Wang Y, et al. Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. Am J Hum Genet. 2009;84(1):52–59. doi: 10.1016/j.ajhg.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Campo I, et al. A large kindred of pulmonary fibrosis associated with a novel ABCA3 gene variant. Respir Res. 2014;15(1):43. doi: 10.1186/1465-9921-15-43. [DOI] [PMC free article] [PubMed] [Google Scholar]