Abstract
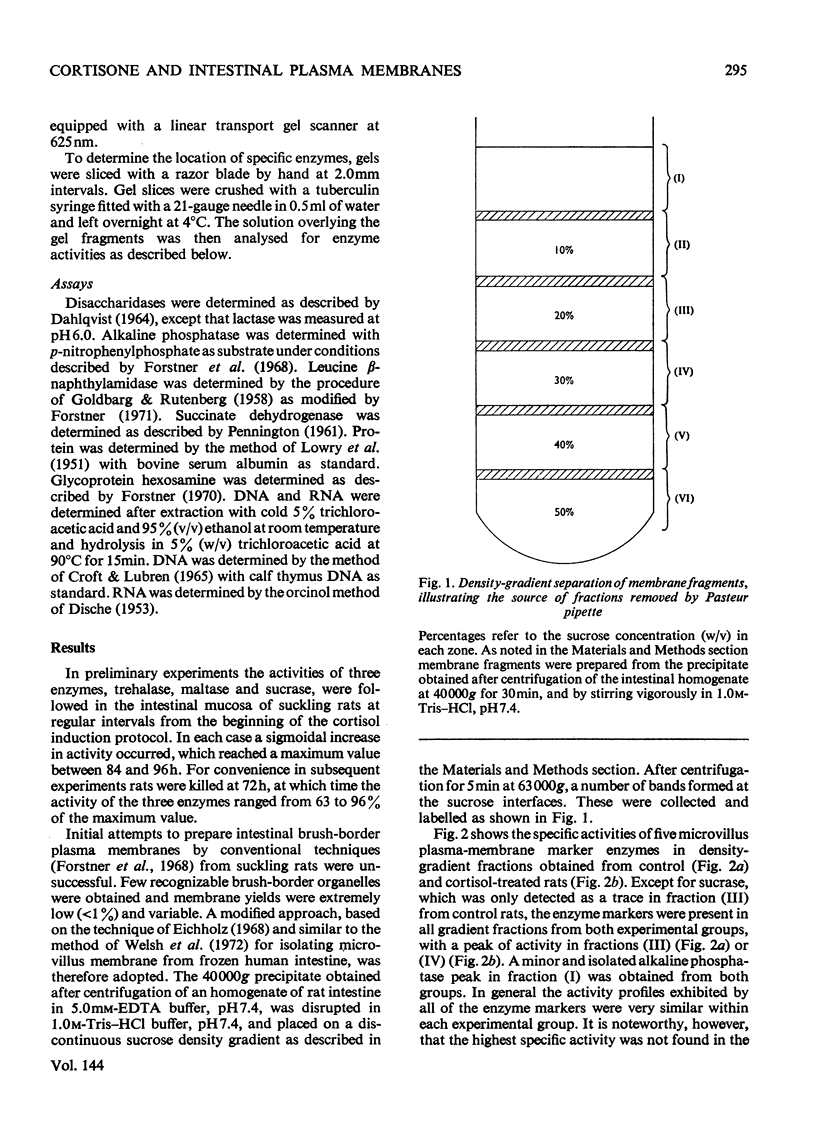
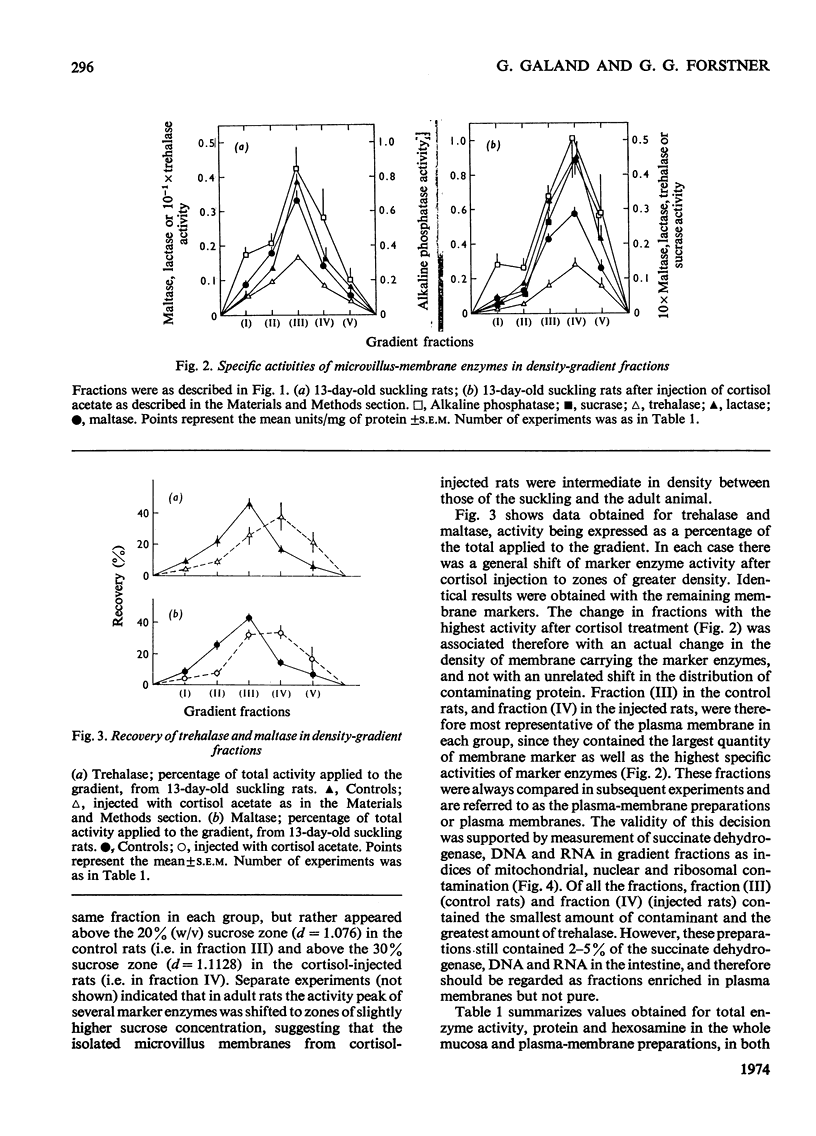
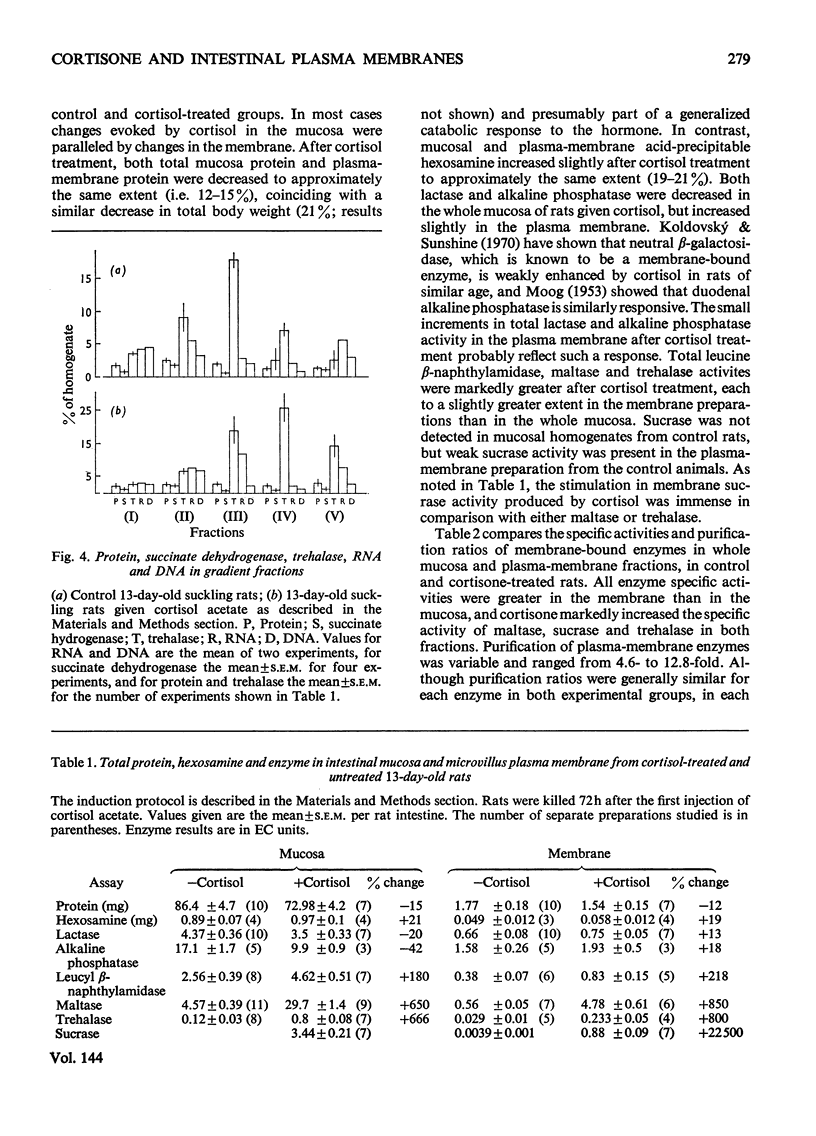
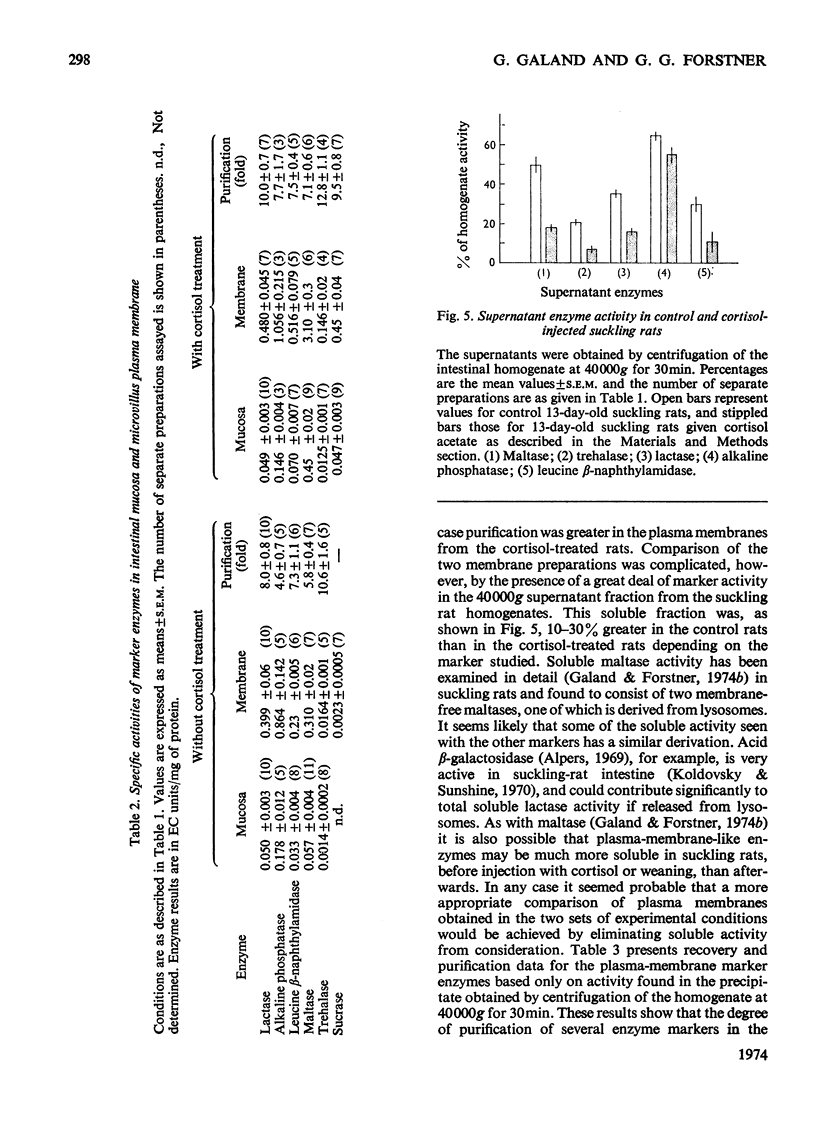
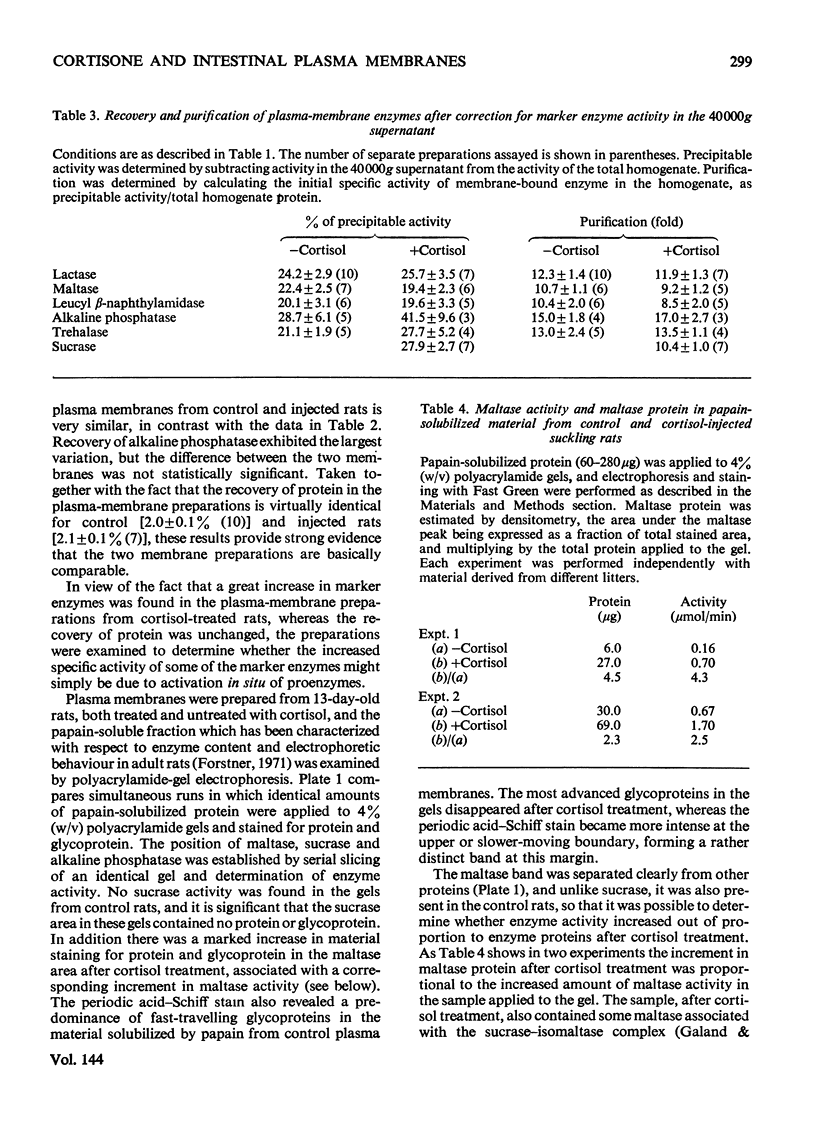
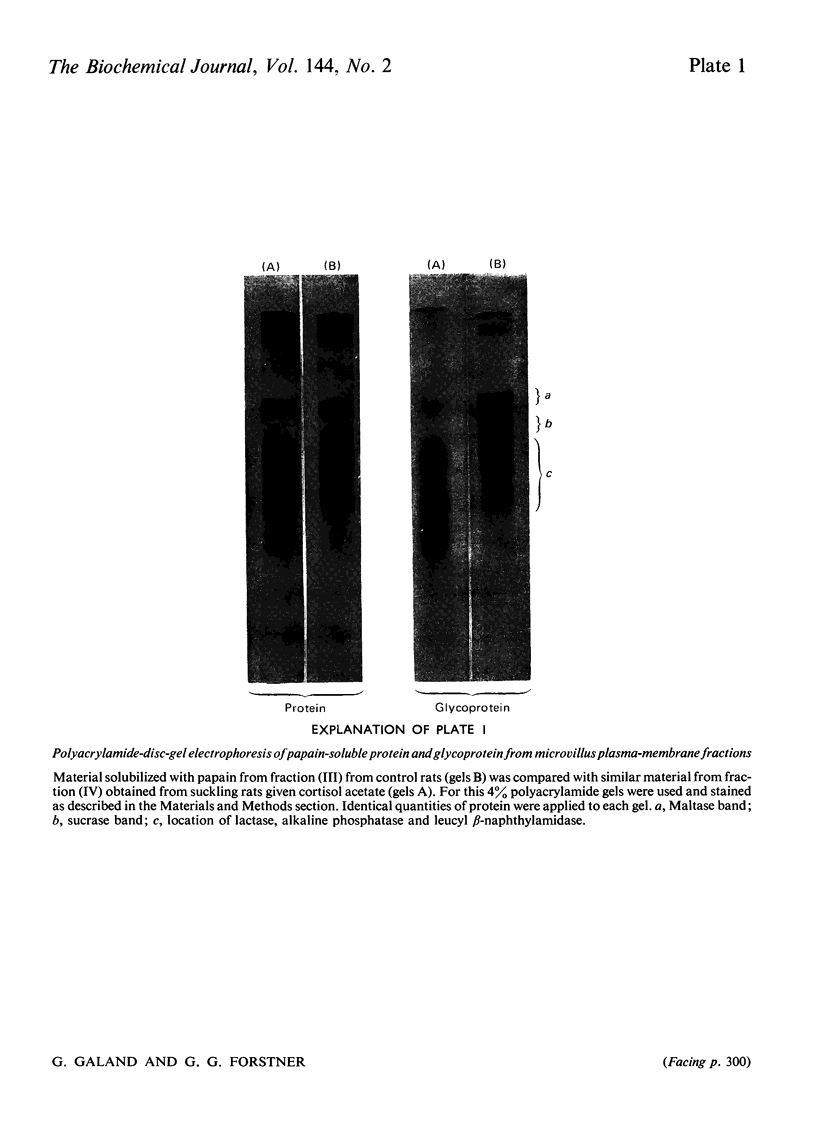
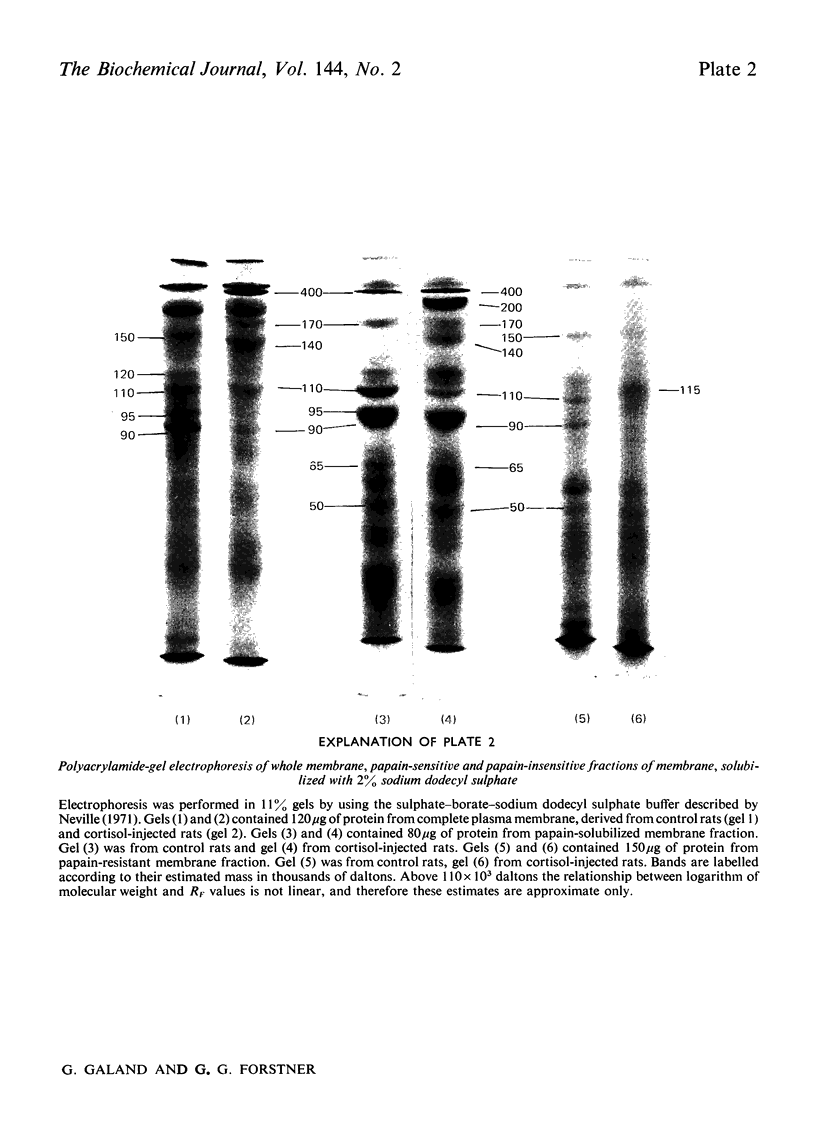
1. Cortisone administration to suckling rats leads prematurely to induction of enzymes of the intestinal microvillus plasma membrane and lengthening of the intestinal microvilli. To investigate the membrane changes that might be involved, a method for the isolation of a fraction enriched with microvillus plasma membrane was developed in suckling rats. Plasma-membrane fractions were compared from 13-day-old control rats and from 13-day-old rats given cortisol acetate by subcutaneous injection for 3 days. 2. After cortisol injection, the activity of maltase, trehalase, sucrase and leucyl β-naphthylamidase increased markedly, and to the same extent, in intestinal homogenates and plasma-membrane preparations. Purification, and recovery of five marker enzymes with respect to homogenate activity, and recovery of protein, were similar for both membrane preparations, particularly after correction for non-membrane activity, which was high in suckling rats and affected by cortisol. 3. In material released from the plasma membrane by digestion with papain, maltase protein was increased after cortisol injection at least as much as maltase activity. Sucrase activity increased at least 200-fold, and this increase was associated with the appearance of a new sucrase band on polyacrylamide-gel electrophoresis. 4. Sodium dodecyl sulphate electrophoresis of plasma-membrane proteins revealed at least four additional macromolecules after cortisol injection. Concurrently several proteins disappeared from the plasma membrane. The added proteins appeared in the main to be removed from the plasma membrane by papain, whereas the deleted proteins were in the papain-resistant fraction. 5. Enzymic stimulation induced by cortisol acetate in the suckling-rat plasma membrane therefore appears to involve the addition of new proteins, rather than activation of proteins in situ. Deletion of proteins from the membrane during induction of hydrolytic enzymes may reflect other phenomena such as protein reorganization associated with the change in microvillus shape.
Full text
PDF




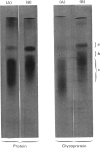
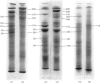
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpers D. H. Separation and isolation of rat and human intestinal beta-galactosidases. J Biol Chem. 1969 Mar 10;244(5):1238–1246. [PubMed] [Google Scholar]
- Brown K. M. Sucrase activity in the intestine of the chick: normal development and influence of hydrocortisone, actinomycin D, cycloheximide, and puromycin. J Exp Zool. 1971 Aug;177(4):493–506. doi: 10.1002/jez.1401770410. [DOI] [PubMed] [Google Scholar]
- CLARK S. L., Jr The ingestion of proteins and colloidal materials by columnar absorptive cells of the small intestine in suckling rats and mice. J Biophys Biochem Cytol. 1959 Jan 25;5(1):41–50. doi: 10.1083/jcb.5.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROFT D. N., LUBRAN M. THE ESTIMATION OF DEOXYRIBONUCLEIC ACID IN THE PRESENCE OF SIALIC ACID: APPLICATION TO ANALYSIS OF HUMAN GASTRIC WASHINGS. Biochem J. 1965 Jun;95:612–620. doi: 10.1042/bj0950612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogoli A., Eberle A., Sigrist H., Joss C., Robinson E., Mosimann H., Semenza G. Subunits of the small-intestinal sucrase-isomaltase complex and separation of its enzymatically active isomaltase moiety. Eur J Biochem. 1973 Feb 15;33(1):40–48. doi: 10.1111/j.1432-1033.1973.tb02652.x. [DOI] [PubMed] [Google Scholar]
- DAHLQVIST A. METHOD FOR ASSAY OF INTESTINAL DISACCHARIDASES. Anal Biochem. 1964 Jan;7:18–25. doi: 10.1016/0003-2697(64)90115-0. [DOI] [PubMed] [Google Scholar]
- DISCHE Z. Qualitative and quantitative colorimetric determination of heptoses. J Biol Chem. 1953 Oct;204(2):983–997. [PubMed] [Google Scholar]
- DOELL R. G., KRETCHMER N. INTESTINAL INVERTASE: PRECOCIOUS DEVELOPMENT OF ACTIVITY AFTER INJECTION OF HYDROCORTISONE. Science. 1964 Jan 3;143(3601):42–44. doi: 10.1126/science.143.3601.42. [DOI] [PubMed] [Google Scholar]
- Doell R. G., Rosen G., Kretchmer N. Immunochemical studies of intestinal disaccharidases during normal and precocious development. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1268–1273. doi: 10.1073/pnas.54.4.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichholz A. Studies on the organization of the brush border in intestinal epithelial cells. V. Subfractionation of enzymatic activities of the microvillus membrane. Biochim Biophys Acta. 1968 Aug;163(1):101–107. doi: 10.1016/0005-2736(68)90037-0. [DOI] [PubMed] [Google Scholar]
- Fish W. W., Reynolds J. A., Tanford C. Gel chromatography of proteins in denaturing solvents. Comparison between sodium dodecyl sulfate and guanidine hydrochloride as denaturants. J Biol Chem. 1970 Oct 10;245(19):5166–5168. [PubMed] [Google Scholar]
- Forstner G. G. (1-14C)glucosamine incorporation by subcellular fractions of small intestinal mucosa. Identification by precursor labeling of three functionally distinct glycoprotein classes. J Biol Chem. 1970 Jul 25;245(14):3584–3592. [PubMed] [Google Scholar]
- Forstner G. G. Release of intestinal surface-membrane glycoproteins associated with enzyme activity by brief digestion with papain. Biochem J. 1971 Mar;121(5):781–789. doi: 10.1042/bj1210781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstner G. G., Sabesin S. M., Isselbacher K. J. Rat intestinal microvillus membranes. Purification and biochemical characterization. Biochem J. 1968 Jan;106(2):381–390. doi: 10.1042/bj1060381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDBARG J. A., RUTENBURG A. M. The colorimetric determination of leucine aminopeptidase in urine and serum of normal subjects and patients with cancer and other diseases. Cancer. 1958 Mar-Apr;11(2):283–291. doi: 10.1002/1097-0142(195803/04)11:2<283::aid-cncr2820110209>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Galand G., Forstner G. G. Soluble neutral and acid maltases in the suckling-rat intestine. The effect of cortisol and development. Biochem J. 1974 Nov;144(2):281–292. doi: 10.1042/bj1440281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galand G., Jacquot R. Effet de l'hydrocortisone et de la surreńalectomie sur l'activité sucrasique de l'intestin du rat au cours du sevrage. C R Acad Sci Hebd Seances Acad Sci D. 1970 Sep 28;271(13):1107–1110. [PubMed] [Google Scholar]
- Gorovsky M. A., Carlson K., Rosenbaum J. L. Simple method for quantitive densitometry of polyacrylamide gels using fast green. Anal Biochem. 1970 Jun;35(2):359–370. doi: 10.1016/0003-2697(70)90196-x. [DOI] [PubMed] [Google Scholar]
- Grey R. D., Moog F. Elevation of alkaline phosphatase activity in the intestine of the chick embryo by actinomycin D. Nature. 1966 Jul 23;211(5047):418–419. doi: 10.1038/211418b0. [DOI] [PubMed] [Google Scholar]
- Griffin M. J., Cox R. P. Studies on the mechanism of hormone induction of alkaline phosphatase in human cell cultures, II. Rate of enzyme synthesis and properties of base level and induced enzymes. Proc Natl Acad Sci U S A. 1966 Sep;56(3):946–953. doi: 10.1073/pnas.56.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALLIDAY R. The effect of steroid hormones on the absorption of antibody by the young rat. J Endocrinol. 1959 Jan;18(1):56–66. doi: 10.1677/joe.0.0180056. [DOI] [PubMed] [Google Scholar]
- Jirsová V., Heringová A. Effect of aldosterone and corticosterone on beta-galactosidase and invertase activity in the small intestine of rats. Nature. 1965 Apr 17;206(981):300–301. doi: 10.1038/206300a0. [DOI] [PubMed] [Google Scholar]
- Jones R. E. Intestinal absorption and gastro-intestinal digestion of protein in the young rat during the normal and cortisone-induced post-closure period. Biochim Biophys Acta. 1972 Aug 9;274(2):412–419. doi: 10.1016/0005-2736(72)90187-3. [DOI] [PubMed] [Google Scholar]
- Koldovský O., Sunshine P. Effect of cortisone on the developmental pattern of the neutral and the acid beta-galactosidase of the small intestine of the rat. Biochem J. 1970 Apr;117(3):467–471. doi: 10.1042/bj1170467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolínská J., Semenza G. Studies on intestinal sucrase and on intestinal sugar transport. V. Isolation and properties of sucrase-isomaltase from rabbit small intestine. Biochim Biophys Acta. 1967 Sep 12;146(1):181–195. doi: 10.1016/0005-2744(67)90085-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maestracci D., Schmitz J., Preiser H., Crane R. K. Proteins and glycoproteins of the human intestinal brush border membrane. Biochim Biophys Acta. 1973 Sep 27;323(1):113–124. doi: 10.1016/0005-2736(73)90435-5. [DOI] [PubMed] [Google Scholar]
- Moog F., Denes A. E., Powell P. M. Disaccharidases in the small intestine of the mouse: normal development and influence of cortisone, actinomycin D, and cycloheximide. Dev Biol. 1973 Nov;35(1):143–159. doi: 10.1016/0012-1606(73)90012-2. [DOI] [PubMed] [Google Scholar]
- Moog F. The regulation of alkaline phosphatase activity in the duodenum of the mouse from birth to maturity. J Exp Zool. 1966 Apr;161(3):353–367. doi: 10.1002/jez.1401610305. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- OVERTON J., SHOUP J. FINE STRUCTURE OF CELL SURFACE SPECIALIZATIONS IN THE MATURING DUODENAL MUCOSA OF THE CHICK. J Cell Biol. 1964 Apr;21:75–85. doi: 10.1083/jcb.21.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton J. Fine structure of the free cell surface in developing mouse intestinal mucosa. J Exp Zool. 1965 Jul;159(2):195–201. doi: 10.1002/jez.1401590205. [DOI] [PubMed] [Google Scholar]
- PENNINGTON R. J. Biochemistry of dystrophic muscle. Mitochondrial succinate-tetrazolium reductase and adenosine triphosphatase. Biochem J. 1961 Sep;80:649–654. doi: 10.1042/bj0800649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBINO A., ZIMBALATTI F., AURICCHIO S. INTESTINAL DISACCHARIDASE ACTIVITIES IN ADULT AND SUCKLING RATS. Biochim Biophys Acta. 1964 Nov 22;92:305–311. doi: 10.1016/0926-6569(64)90187-7. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Jackson R. L., Andrews E. P., Marchesi V. T. Human erythrocyte membrane glycoprotein: a re-evaluation of the molecular weight as determined by SDS polyacrylamide gel electrophoresis. Biochem Biophys Res Commun. 1971 Jul 16;44(2):390–395. doi: 10.1016/0006-291x(71)90612-7. [DOI] [PubMed] [Google Scholar]
- Welsh J. D., Preiser H., Woodley J. F., Crane R. K. An enriched microvillus membrane preparation from frozen specimens of human small intestine. Gastroenterology. 1972 Apr;62(4):572–582. [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]