Abstract
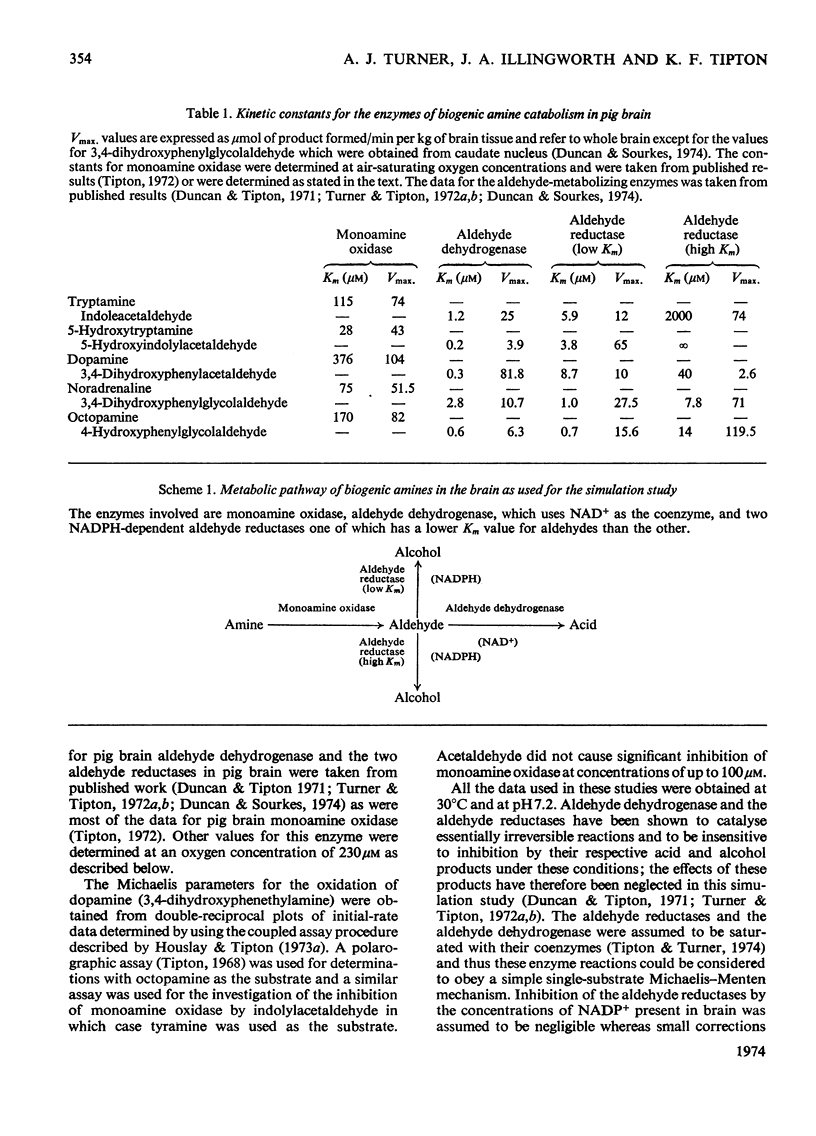
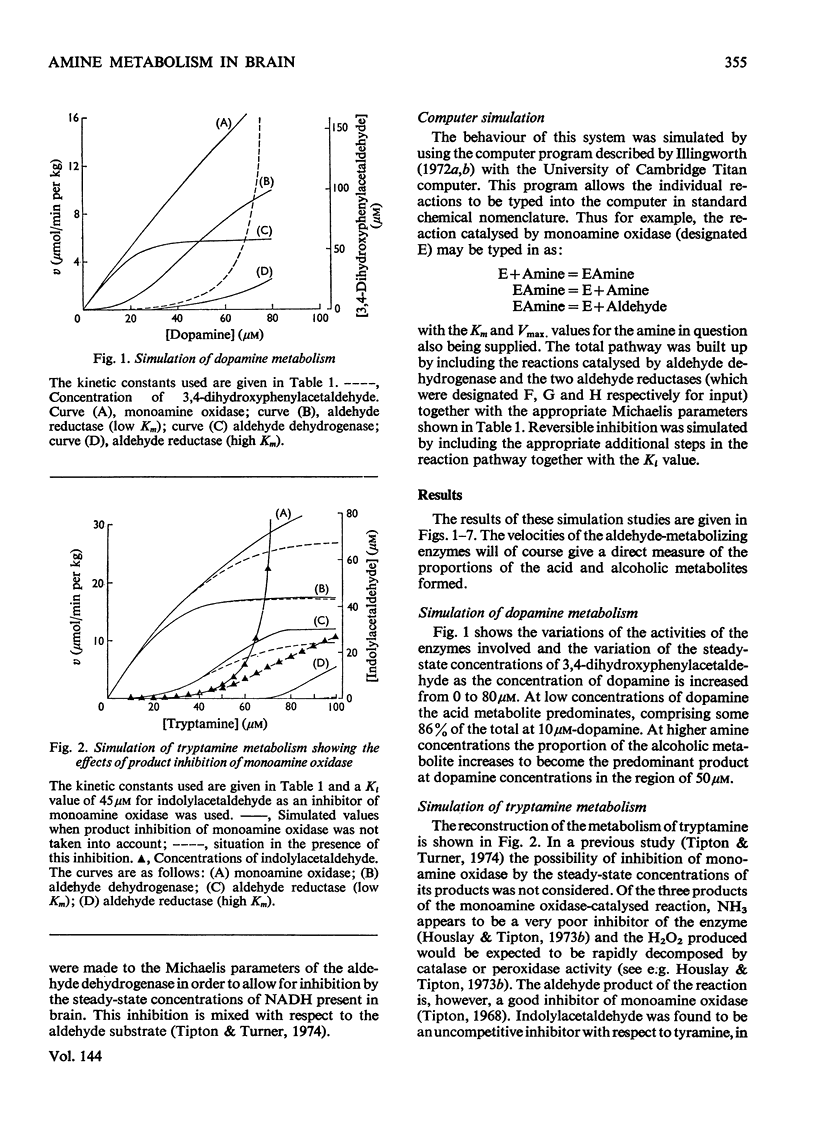
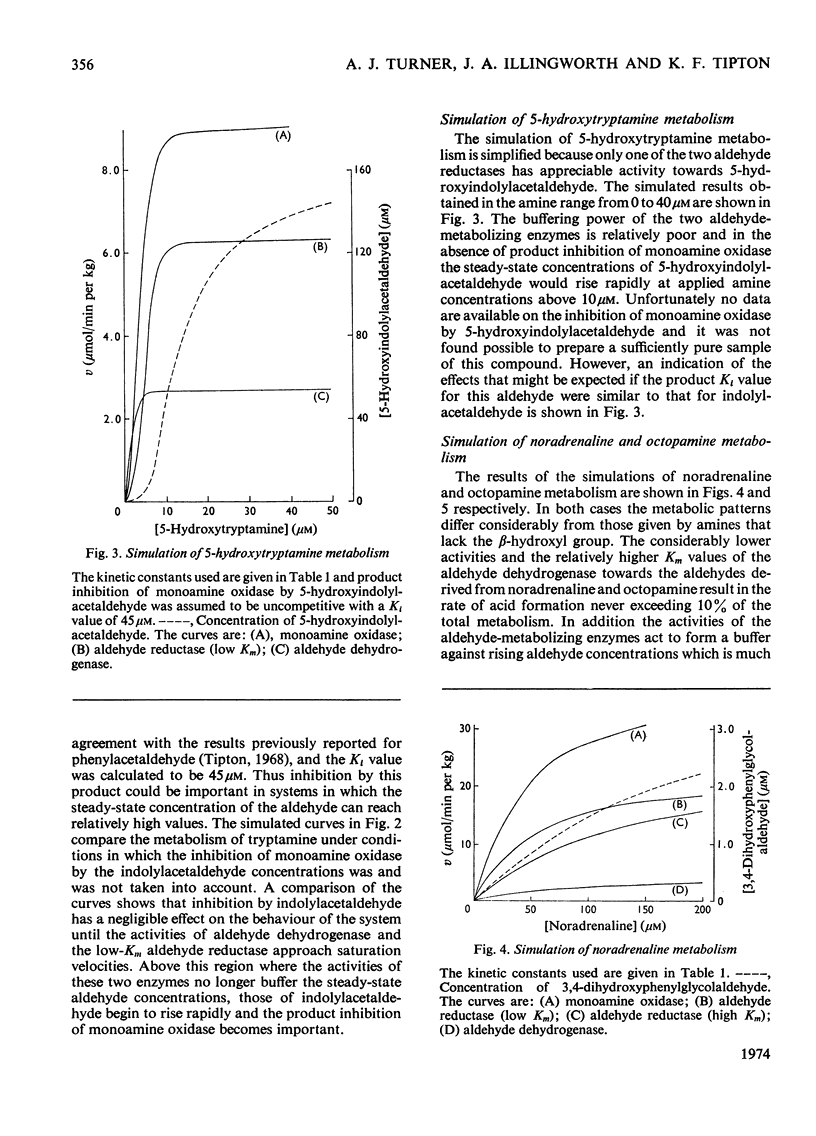
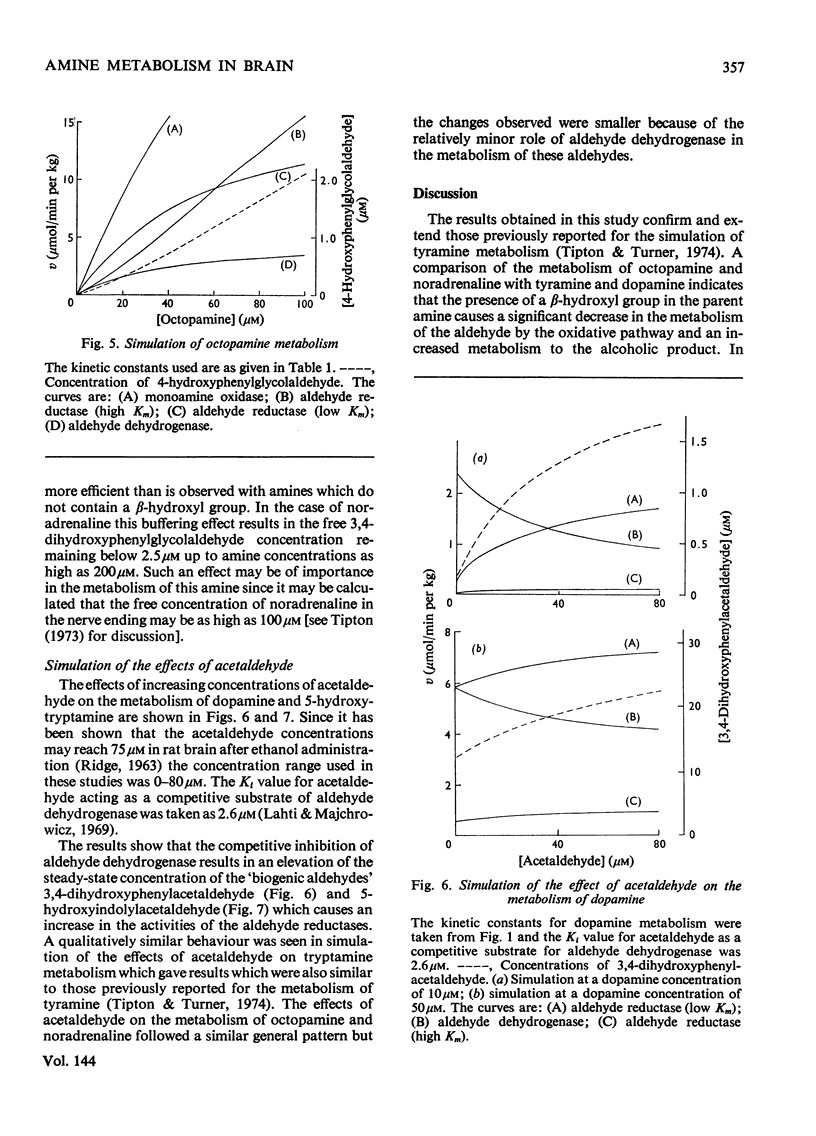
The metabolism of a number of biogenic amines has been simulated by using data obtained from studies of the individual enzymes from pig brain. It is shown that β-hydroxylated amines such as noradrenaline and octopamine are metabolized primarily to the alcoholic metabolite whereas amines lacking this group [e.g. dopamine (3,4-dihydroxyphenethylamine) and 5-hydroxytryptamine] are metabolized at low concentrations to give the corresponding acid. Increase in the amine concentration results in an increase in the proportion of the alcoholic metabolite formed and this may in part account for the effects of the drug reserpine on amine metabolism. The effects of disulfiram (Antabuse) and ethanol (acting through its metabolite acetaldehyde) on amine metabolism may be understood in terms of this simulated model. It is shown that drugs that affect this system also cause alterations in the steady-state concentrations of the intermediate aldehydes and the possible implications of this are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barofsky I., Feldstein A. Serotonin and its metabolites: their respective roles in the production of hypothermia in the mouse. Experientia. 1970 Sep 26;26(9):990–991. doi: 10.1007/BF02114149. [DOI] [PubMed] [Google Scholar]
- Breese G. R., Chase T. N., Kopin I. J. Metabolism of some phenylethylamines and their beta-hydroxylated analogs in brain. J Pharmacol Exp Ther. 1969 Jan;165(1):9–13. [PubMed] [Google Scholar]
- Breese G. R., Chase T. N., Kopin I. J. Metabolism of tyramine-3H and octopamine-3H by rat brain. Biochem Pharmacol. 1969 Apr;18(4):863–869. doi: 10.1016/0006-2952(69)90057-4. [DOI] [PubMed] [Google Scholar]
- Chase T. N., Breese G. R., Gordon E. K., Kopin I. J. Catecholamine metabolism in the dog: comparison of intravenously and intraventricularly administered [14C]dopamine and [3H]norepinephrine. J Neurochem. 1971 Jan;18(1):135–140. doi: 10.1111/j.1471-4159.1971.tb00175.x. [DOI] [PubMed] [Google Scholar]
- Davis V. E., Cashaw J. L., McLaughlin B. R., Hamlin T. A. Alteration of norepinephrine metabolism by barbiturates. Biochem Pharmacol. 1974 Jul 1;23(13):1877–1898. doi: 10.1016/0006-2952(74)90197-x. [DOI] [PubMed] [Google Scholar]
- Deitrich R. A., Erwin V. G. Mechanism of the inhibition of aldehyde dehydrogenase in vivo by disulfiram and diethyldithiocarbamate. Mol Pharmacol. 1971 May;7(3):301–307. [PubMed] [Google Scholar]
- Duncan R. J., Sourkes T. L. Some enzymic aspects of the production of oxidized or reduced metabolites of catecholamines and 5-hydroxytryptamine by brain tissues. J Neurochem. 1974 May;22(5):663–669. doi: 10.1111/j.1471-4159.1974.tb04278.x. [DOI] [PubMed] [Google Scholar]
- Duncan R. J., Tipton K. F. The kinetics of pig brain aldehyde dehydrogenase. Eur J Biochem. 1971 Oct 26;22(4):538–543. doi: 10.1111/j.1432-1033.1971.tb01574.x. [DOI] [PubMed] [Google Scholar]
- Eccleston D., Reading W. H., Ritchie I. M. 5-Hydroxytryptamine metabolism in brain and liver slices and the effect of ethanol. J Neurochem. 1969 Feb;16(2):274–276. doi: 10.1111/j.1471-4159.1969.tb05946.x. [DOI] [PubMed] [Google Scholar]
- Erwin V. G., Tabakoff B., Bronaugh R. L. Inhibition of a reduced nicotinamide adenine dinucleotide phosphate-linked aldehyde reductase from bovine brain by barbiturates. Mol Pharmacol. 1971 Mar;7(2):169–176. [PubMed] [Google Scholar]
- Feldstein A., Williamson O. 5-Hydroxytryptamine metabolism in rat brain and liver homogenates. Br J Pharmacol. 1968 Sep;34(1):38–42. doi: 10.1111/j.1476-5381.1968.tb07948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houslay M. D., Tipton K. F. The nature of the electrophoretically separable multiple forms of rat liver monoamine oxidase. Biochem J. 1973 Sep;135(1):173–186. doi: 10.1042/bj1350173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houslay M. D., Tipton K. F. The reaction pathway of membrane-bound rat liver mitochondrial monoamine oxidase. Biochem J. 1973 Dec;135(4):735–750. doi: 10.1042/bj1350735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonason J., Rutledge C. O. Metabolism of dopamine and noradrenaline in rabbit caudate nucleus in vitro. Acta Physiol Scand. 1968 Aug;73(4):411–417. doi: 10.1111/j.1365-201x.1968.tb10880.x. [DOI] [PubMed] [Google Scholar]
- Jouvet M. Biogenic amines and the states of sleep. Science. 1969 Jan 3;163(3862):32–41. doi: 10.1126/science.163.3862.32. [DOI] [PubMed] [Google Scholar]
- Kellogg C., Vernadakis A., Rutledge C. O. Uptake and metabolism of [3H]norepinephrine in the cerebral hemispheres of chick embryos. J Neurochem. 1971 Oct;18(10):1931–1938. doi: 10.1111/j.1471-4159.1971.tb09599.x. [DOI] [PubMed] [Google Scholar]
- Lahti R. A., Majchrowicz E. Acetaldehyde--an inhibitor of the enzymatic oxidation of 5-hydroxyindoleacetaldehyde. Biochem Pharmacol. 1969 Feb;18(2):535–538. doi: 10.1016/0006-2952(69)90231-7. [DOI] [PubMed] [Google Scholar]
- Maas J. W., Landis D. H. In vivo studies of the metabolism of norepinephrine in the central nervous system. J Pharmacol Exp Ther. 1968 Sep;163(1):147–162. [PubMed] [Google Scholar]
- RIDGE J. W. The metabolism of acetaldehyde by the brain in vivo. Biochem J. 1963 Jul;88:95–100. doi: 10.1042/bj0880095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge C. O., Jonason J. Metabolic pathways of dopamine and norepinephrine in rabbit brain in vitro. J Pharmacol Exp Ther. 1967 Sep;157(3):493–502. [PubMed] [Google Scholar]
- SMITH A. A., WORTIS S. B. The effect of disulfiram on the metabolism of normetanephrine-1-14C in the guinea pig. Biochem Pharmacol. 1960 Jul;3:333–334. doi: 10.1016/0006-2952(60)90100-3. [DOI] [PubMed] [Google Scholar]
- Sabelli H. C., Giardina W. J., Alivisatos S. G., Seth P. K., Ungar F. Indoleacetaldehydes: serotonin-like effects on the central nervous system. Nature. 1969 Jul 5;223(5201):73–74. doi: 10.1038/223073a0. [DOI] [PubMed] [Google Scholar]
- Sandler M., Carter S. B., Hunter K. R., Stern G. M. Tetrahydroisoquinoline alkaloids: in vivo metabolites of L-dopa in man. Nature. 1973 Feb 16;241(5390):439–443. doi: 10.1038/241439a0. [DOI] [PubMed] [Google Scholar]
- Sandler M., Youdim M. B. Promotion of 4-hydroxy-3-methoxyphenylglycol formation in man by reserpine. Nature. 1968 Feb 24;217(5130):771–772. doi: 10.1038/217771a0. [DOI] [PubMed] [Google Scholar]
- Squires R. F. Multiple forms of monoamine oxidase in intact mitochondria as characterized by selective inhibitors and thermal stability: a comparison of eight mammalian species. Adv Biochem Psychopharmacol. 1972;5:355–370. [PubMed] [Google Scholar]
- Tabakoff B., Anderson R., Alivisatos S. G. Enzymatic reduction of "biogenic" aldehydes in brain. Mol Pharmacol. 1973 Jul;9(4):428–437. [PubMed] [Google Scholar]
- Tacker M., Creaven P. J., McIsaac W. M. Alteration in tyramine metabolism by ethanol. Biochem Pharmacol. 1970 Feb;19(2):604–607. doi: 10.1016/0006-2952(70)90216-9. [DOI] [PubMed] [Google Scholar]
- Taylor K. M., Laverty R. The metabolism of tritiated dopamine in regions of the rat brain in vivo. II. The significance of the neutral metabolites of catecholamines. J Neurochem. 1969 Sep;16(9):1367–1376. doi: 10.1111/j.1471-4159.1969.tb05988.x. [DOI] [PubMed] [Google Scholar]
- Tipton K. F., Houslay M. D., Garrett N. J. Allotopic properties of human brain monoamine oxidase. Nat New Biol. 1973 Dec 19;246(155):213–214. doi: 10.1038/newbio246213a0. [DOI] [PubMed] [Google Scholar]
- Tipton K. F. Some properties of monoamine oxidase. Adv Biochem Psychopharmacol. 1972;5:11–24. [PubMed] [Google Scholar]
- Tipton K. F., Spires I. P. The homogeneity of pig brain mitochondrial monoamine oxidase. Biochem Pharmacol. 1968 Oct;17(10):2137–2141. doi: 10.1016/0006-2952(68)90188-3. [DOI] [PubMed] [Google Scholar]
- Tipton K. F. The reaction pathway of pig brain mitochondrial monoamine oxidase. Eur J Biochem. 1968 Aug;5(3):316–320. doi: 10.1111/j.1432-1033.1968.tb00372.x. [DOI] [PubMed] [Google Scholar]
- Tipton K. F., Turner A. J. Computer reconstruction of tyramine breakdown in brain. Biochem Pharmacol. 1974 Jul 1;23(13):1906–1910. doi: 10.1016/0006-2952(74)90200-7. [DOI] [PubMed] [Google Scholar]
- Turner A. J., Tipton K. F. The characterization of two reduced nicotinamide-adenine dinucleotide phosphate-linked aldehyde reductases from pig brain. Biochem J. 1972 Dec;130(3):765–772. doi: 10.1042/bj1300765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A. J., Tipton K. F. The purification and properties of an NADPH-linked aldehyde reductase from pig brain. Eur J Biochem. 1972 Oct;30(2):361–368. doi: 10.1111/j.1432-1033.1972.tb02106.x. [DOI] [PubMed] [Google Scholar]
- Watson M. J., Tyler J. M., Buchanan J. G., Baddiley J. The type-specific substance from Pneumococcus type 13. Biochem J. 1972 Nov;130(1):45–54. doi: 10.1042/bj1300045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youdim M. B. Multiple forms of monoamine oxidase and their properties. Adv Biochem Psychopharmacol. 1972;5:67–77. [PubMed] [Google Scholar]


