Abstract
1. Mutant strains of Clostridium pasteurianum were obtained, which are unable to synthesize granulose (an intracellularly accumulated amylopectin-like α-polyglucan). 2. These mutants lacked either (a) ADP-glucose pyrophosphorylase (EC 2.7.7.27), or (b) granulose synthase (i.e. ADP-glucose–α-1,4-glucan glucosyltransferase, EC 2.4.1.21). 3. Although both of these enzymes were constitutively synthesized by the wild-type organism, massive deposition of granulose in a sporulating culture coincided with a threefold increase in the specific activity of ADP-glucose pyrophosphorylase. 4. The soluble ADP-glucose pyrophosphorylase was partially purified (33-fold). Its ATP-saturation curve was not sigmoidal and its activity was not enhanced by phosphorylated intermediates of glycolysis, pyruvate, NAD(P)H or pyridoxal 5′-phosphate. ADP at relatively high concentrations acted as a competitive inhibitor (Ki=19mm). 5. The dependence of granulose synthase on a suitable polyglucan primer was demonstrated by using enzyme obtained from a granulose-free mutant strain (lacking ADP-glucose pyrophosphorylase). 6. Partial purification of granulose synthase from wild-type strains was facilitated by its being bound to the native particles of granulose. No activator was discovered, but ADP, AMP and pyridoxal 5′-phosphate were competitive inhibitors, ADP being most effective (Ki about 0.2mm). 7. It would appear that the synthesis of granulose in Cl. pasteurianum is not subject to the positive, fine control that is a feature of glycogen biosynthesis in most bacteria.
Full text
PDF



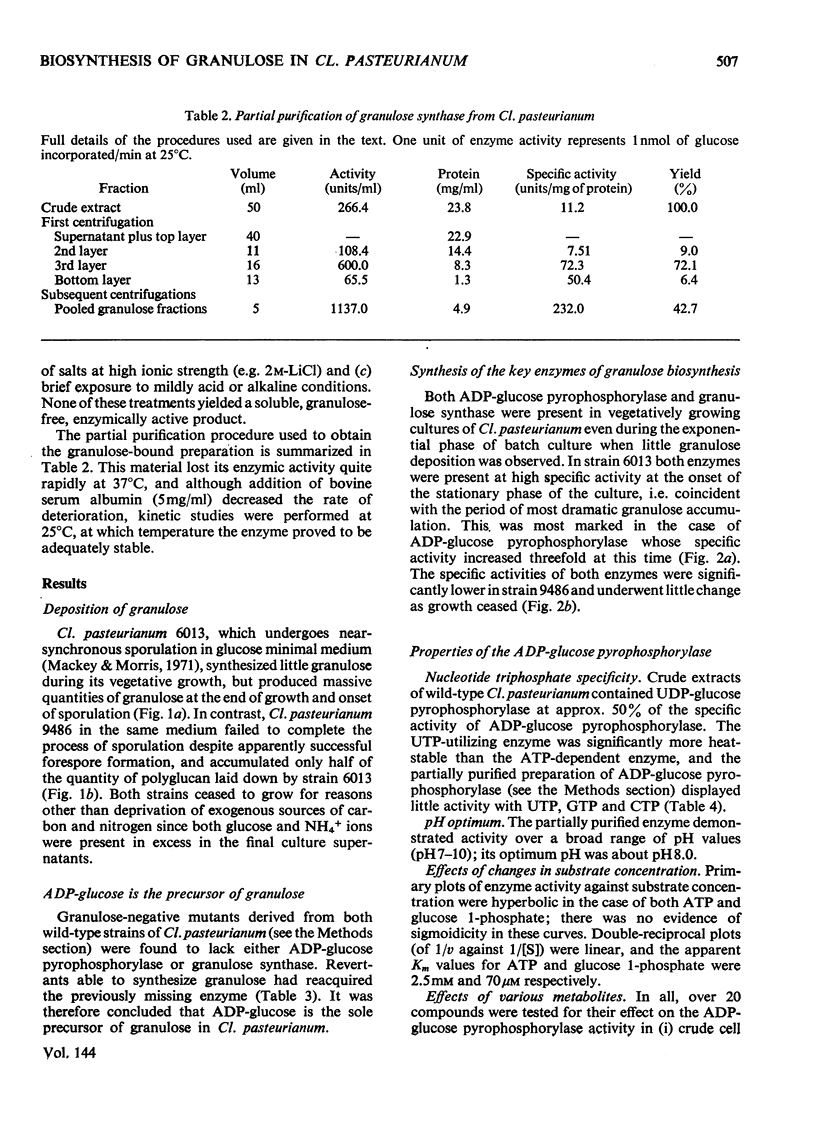
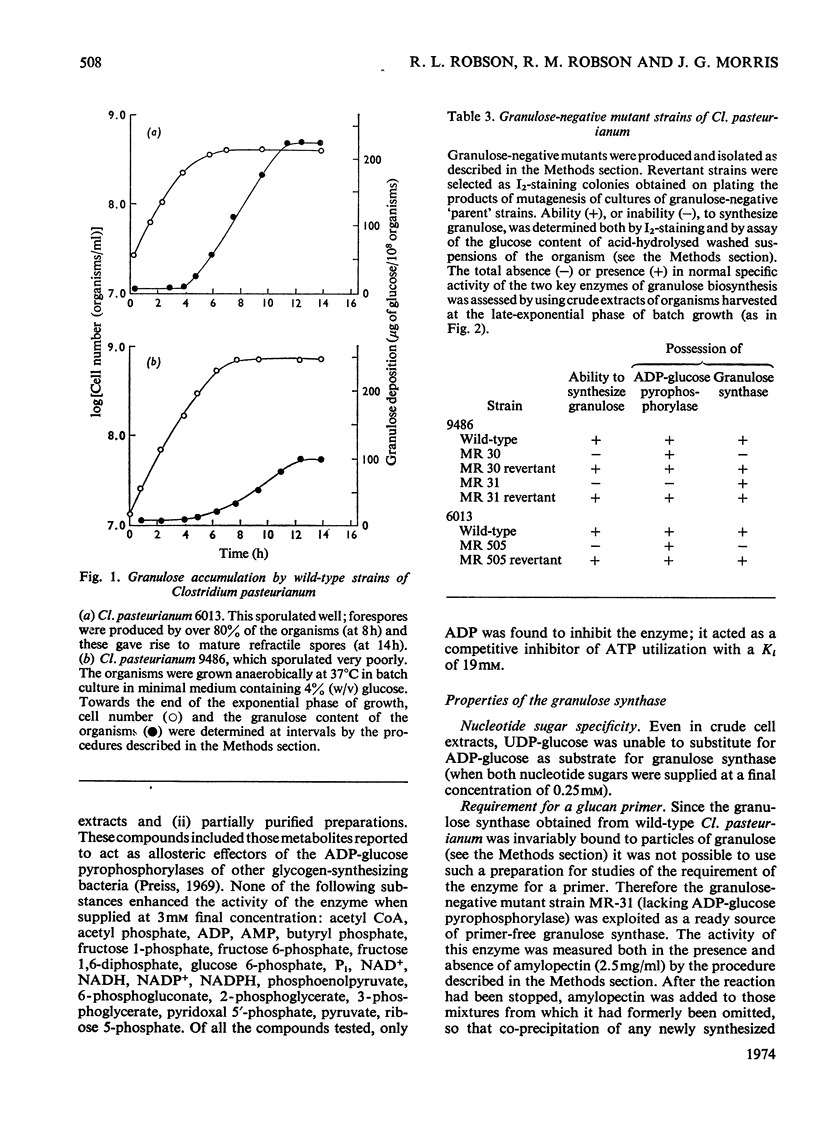
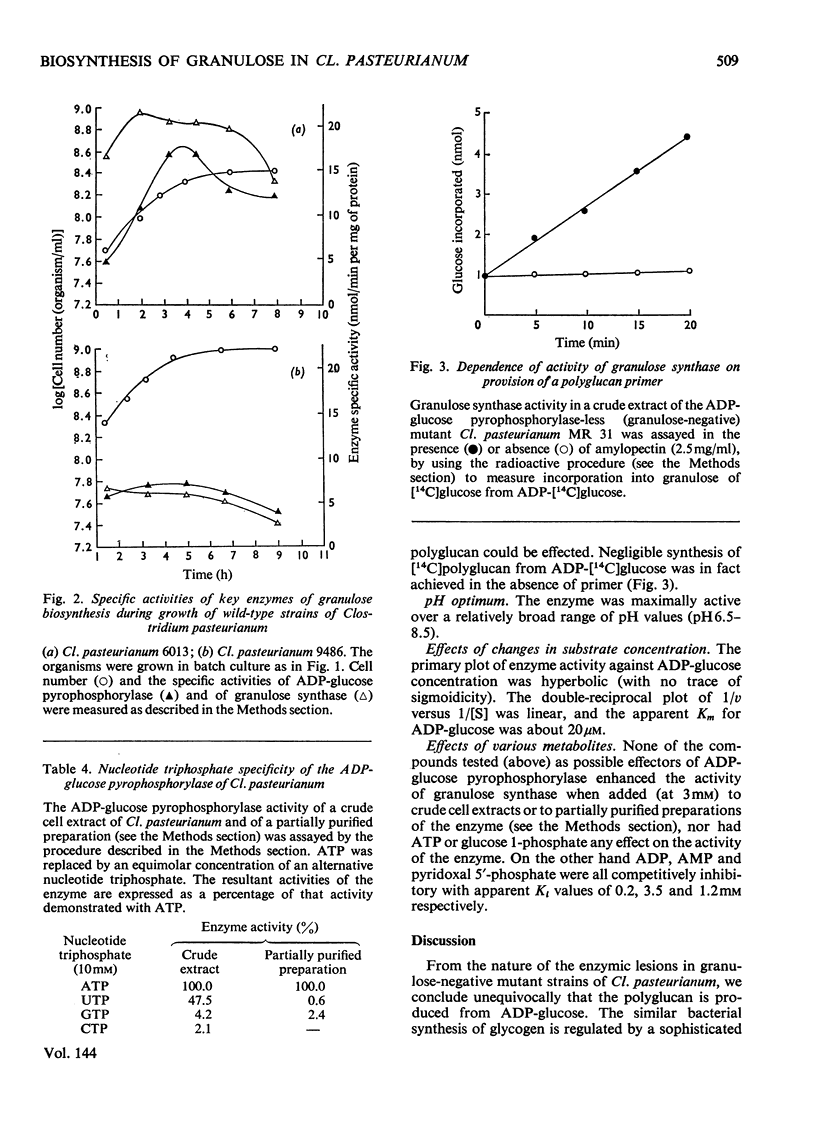


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson D. E., Walton G. M. Adenosine triphosphate conservation in metabolic regulation. Rat liver citrate cleavage enzyme. J Biol Chem. 1967 Jul 10;242(13):3239–3241. [PubMed] [Google Scholar]
- Dawes E. A., Senior P. J. The role and regulation of energy reserve polymers in micro-organisms. Adv Microb Physiol. 1973;10:135–266. doi: 10.1016/s0065-2911(08)60088-0. [DOI] [PubMed] [Google Scholar]
- Dietzler D. N., Strominger J. L. Purification and properties of the adenosine diphosphoglucose: glycogen transglucosylase of Pasteurella pseudotuberculosis. J Bacteriol. 1973 Feb;113(2):946–952. doi: 10.1128/jb.113.2.946-952.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbein A. D., Mitchell M. Levels of glycogen and trehalose in Mycobacterium smegmatis and the purification and properties of the glycogen synthetase. J Bacteriol. 1973 Feb;113(2):863–873. doi: 10.1128/jb.113.2.863-873.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENBERG E., PREISS J. BIOSYNTHESIS OF BACTERIAL GLYCOGEN. II. PURIFICATION AND PROPERTIES OF THE ADENOSINE DIPHOSPHOGLUCOSE:GLYCOGEN TRANSGLUCOSYLASE OF ARTHROBACTER SPECIES NRRL B1973. J Biol Chem. 1965 Jun;240:2341–2348. [PubMed] [Google Scholar]
- Krasil'nikov N. A., Duda V. I., Makar'eva E. D. Lokalizatsiia polisakharidov v kletkakh sporonosnykh anaérobnykh bakterii. Dokl Akad Nauk SSSR. 1972 Mar-Apr;203(1):216–218. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laishley E. J., Brown R. G., Otto M. C. Characteristics of a reserve alpha-glucan from Clostridium pasteurianum. Can J Microbiol. 1974 Apr;20(4):559–562. doi: 10.1139/m74-085. [DOI] [PubMed] [Google Scholar]
- Laishley E. J., MacAlister T. J., Clements I., Young C. Isolation and morphology of native intracellular polyglucose granules from Clostridium pasteurianum. Can J Microbiol. 1973 Aug;19(8):991–994. doi: 10.1139/m73-157. [DOI] [PubMed] [Google Scholar]
- O'Brien R. W., Morris J. G. Oxygen and the growth and metabolism of Clostridium acetobutylicum. J Gen Microbiol. 1971 Nov;68(3):307–318. doi: 10.1099/00221287-68-3-307. [DOI] [PubMed] [Google Scholar]
- Preiss J., Ozbun J. L., Hawker J. S., Greenberg E., Lammel C. ADPG synthetase and ADPG- -glucan 4-glucosyl transferase: enzymes involved in bacterial glycogen and plant starch synthesis. Ann N Y Acad Sci. 1973 Feb 9;210:265–278. doi: 10.1111/j.1749-6632.1973.tb47578.x. [DOI] [PubMed] [Google Scholar]
- Rousseau M., Hermier J., Bergere J. L. Structure de certains Clostridium du groupe butyrique. I. Sporulation de Clostridium butyricum et Clostridium saccharobutyricum. Ann Inst Pasteur (Paris) 1971 Jan;120(1):23–32. [PubMed] [Google Scholar]
- Ryman B. E., Whelan W. J. New aspects of glycogen metabolism. Adv Enzymol Relat Areas Mol Biol. 1971;34:285–443. doi: 10.1002/9780470122792.ch6. [DOI] [PubMed] [Google Scholar]
- Whyte J. N., Strasdine G. A. An intracellular alpha-D-glucan from Clostridium botulinum, type E. Carbohydr Res. 1972 Dec;25(2):435–441. doi: 10.1016/s0008-6215(00)81655-9. [DOI] [PubMed] [Google Scholar]
- Wilson G. S. The Proportion of Viable Bacteria in Young Cultures with Especial Reference to the Technique Employed in Counting. J Bacteriol. 1922 Jul;7(4):405–446. doi: 10.1128/jb.7.4.405-446.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]


