Abstract
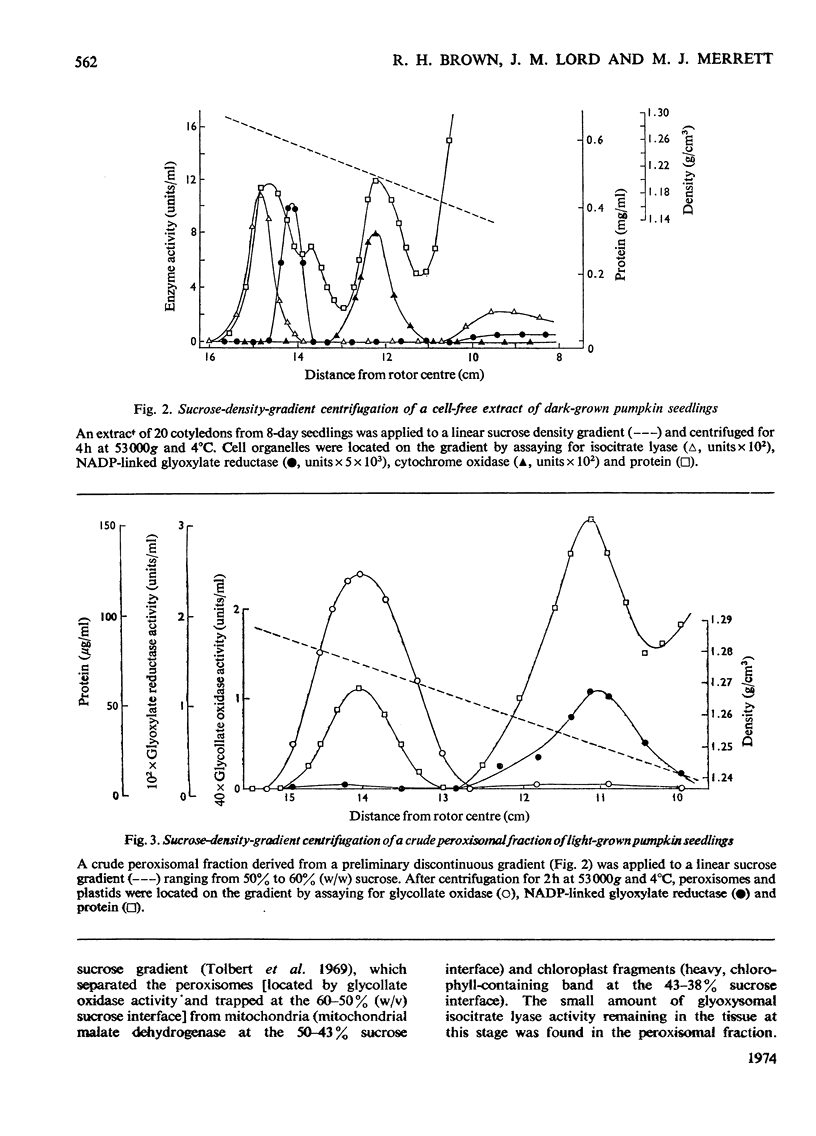
1. Glyoxysomes and peroxisomes have been isolated from dark- and light-grown seedlings of pumpkin (Cucurbita pepo) by sucrose-density-gradient centrifugation. 2. Pumpkin microbodies and castor-bean (Ricinus communis) glyoxysomes may be fractionated, by a combination of osmotic shock and treatment with KCl, into three distinct groups of proteins: readily soluble (matrix enzymes), solubilized in the presence of KCl (membrane-bound enzymes) and relatively insoluble (membrane `ghost' proteins). 3. Sodium dodecyl sulphate–polyacrylamide-gel electrophoresis of `ghost' fractions indicated that the membrane proteins were generally of low molecular weight; one gel band (mol.wt. 27000–28000) was common to all three microbodies. 4. Although there were major differences in the soluble protein components of pumpkin glyoxysomes and peroxisomes, electrophoresis of the pumpkin microbody `ghosts' indicated that the membrane proteins were similar, four main components being common to each class of microbody (monomer molecular weights 42000, 34000, 27000 and 17000).
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beevers H. Glyoxysomes of castor bean endosperm and their relation to gluconeogenesis. Ann N Y Acad Sci. 1969 Dec 19;168(2):313–324. doi: 10.1111/j.1749-6632.1969.tb43118.x. [DOI] [PubMed] [Google Scholar]
- Bieglmayer C., Graf J., Ruis H. Membranes of glyoxysomes from castor-bean endosperm. Enzymes bound to purified-membrane preparations. Eur J Biochem. 1973 Sep 3;37(3):553–562. doi: 10.1111/j.1432-1033.1973.tb03018.x. [DOI] [PubMed] [Google Scholar]
- Breidenbach R. W., Beevers H. Association of the glyoxylate cycle enzymes in a novel subcellular particle from castor bean endosperm. Biochem Biophys Res Commun. 1967 May 25;27(4):462–469. doi: 10.1016/s0006-291x(67)80007-x. [DOI] [PubMed] [Google Scholar]
- Breidenbach R. W. Characterization of some glyoxysomal proteins. Ann N Y Acad Sci. 1969 Dec 19;168(2):342–347. doi: 10.1111/j.1749-6632.1969.tb43120.x. [DOI] [PubMed] [Google Scholar]
- CANVIN D. T., BEEVERS H. Sucrose synthesis from acetate in the germinating castor bean: kinetics and pathway. J Biol Chem. 1961 Apr;236:988–995. [PubMed] [Google Scholar]
- Cook J. R. Properties of partially purified malate synthase for Euglena gracilis. J Protozool. 1970 May;17(2):232–235. doi: 10.1111/j.1550-7408.1970.tb02362.x. [DOI] [PubMed] [Google Scholar]
- Feierabend J., Beevers H. Developmental studies on microbodies in wheat leaves : I. Conditions influencing enzyme development. Plant Physiol. 1972 Jan;49(1):28–32. doi: 10.1104/pp.49.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. H., Beevers H. Localization of enzymes within microbodies. J Cell Biol. 1973 Aug;58(2):379–389. doi: 10.1083/jcb.58.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNBERG H. L., KREBS H. A. Synthesis of cell constituents from C2-units by a modified tricarboxylic acid cycle. Nature. 1957 May 18;179(4568):988–991. doi: 10.1038/179988a0. [DOI] [PubMed] [Google Scholar]
- Kagawa T., Lord J. M., Beevers H. The origin and turnover of organelle membranes in castor bean endosperm. Plant Physiol. 1973 Jan;51(1):61–65. doi: 10.1104/pp.51.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa T., McGregor D. I., Beevers H. Development of enzymes in the cotyledons of watermelon seedlings. Plant Physiol. 1973 Jan;51(1):66–71. doi: 10.1104/pp.51.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lord J. M., Kagawa T., Beevers H. Intracellular distribution of enzymes of the cytidine diphosphate choline pathway in castor bean endosperm. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2429–2432. doi: 10.1073/pnas.69.9.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J. M., Kagawa T., Moore T. S., Beevers H. Endoplasmic reticulum as the site of lecithin formation in castor bean endosperm. J Cell Biol. 1973 Jun;57(3):659–667. doi: 10.1083/jcb.57.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T. S., Lord J. M., Kagawa T., Beevers H. Enzymes of phospholipid metabolism in the endoplasmic reticulum of castor bean endosperm. Plant Physiol. 1973 Jul;52(1):50–53. doi: 10.1104/pp.52.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH L. Spectrophotometric assay of cytochrome c oxidase. Methods Biochem Anal. 1955;2:427–434. doi: 10.1002/9780470110188.ch13. [DOI] [PubMed] [Google Scholar]
- Tolbert N. E., Oeser A., Yamazaki R. K., Hageman R. H., Kisaki T. A survey of plants for leaf peroxisomes. Plant Physiol. 1969 Jan;44(1):135–147. doi: 10.1104/pp.44.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E., Yamazaki R. K. Leaf peroxisomes and their relation to photorespiration and photosynthesis. Ann N Y Acad Sci. 1969 Dec 19;168(2):325–341. doi: 10.1111/j.1749-6632.1969.tb43119.x. [DOI] [PubMed] [Google Scholar]
- Tolbert N. E., Yamazaki R. K., Oeser A. Localization and properties of hydroxypyruvate and glyoxylate reductases in spinach leaf particles. J Biol Chem. 1970 Oct 10;245(19):5129–5136. [PubMed] [Google Scholar]
- Trelease R. N., Becker W. M., Gruber P. J., Newcomb E. H. Microbodies (Glyoxysomes and Peroxisomes) in Cucumber Cotyledons: Correlative Biochemical and Ultrastructural Study in Light- and Dark-grown Seedlings. Plant Physiol. 1971 Oct;48(4):461–475. doi: 10.1104/pp.48.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigil E. L. Cytochemical and developmental changes in microbodies (glyoxysomes) and related organelles of castor bean endosperm. J Cell Biol. 1970 Sep;46(3):435–454. doi: 10.1083/jcb.46.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


