Abstract
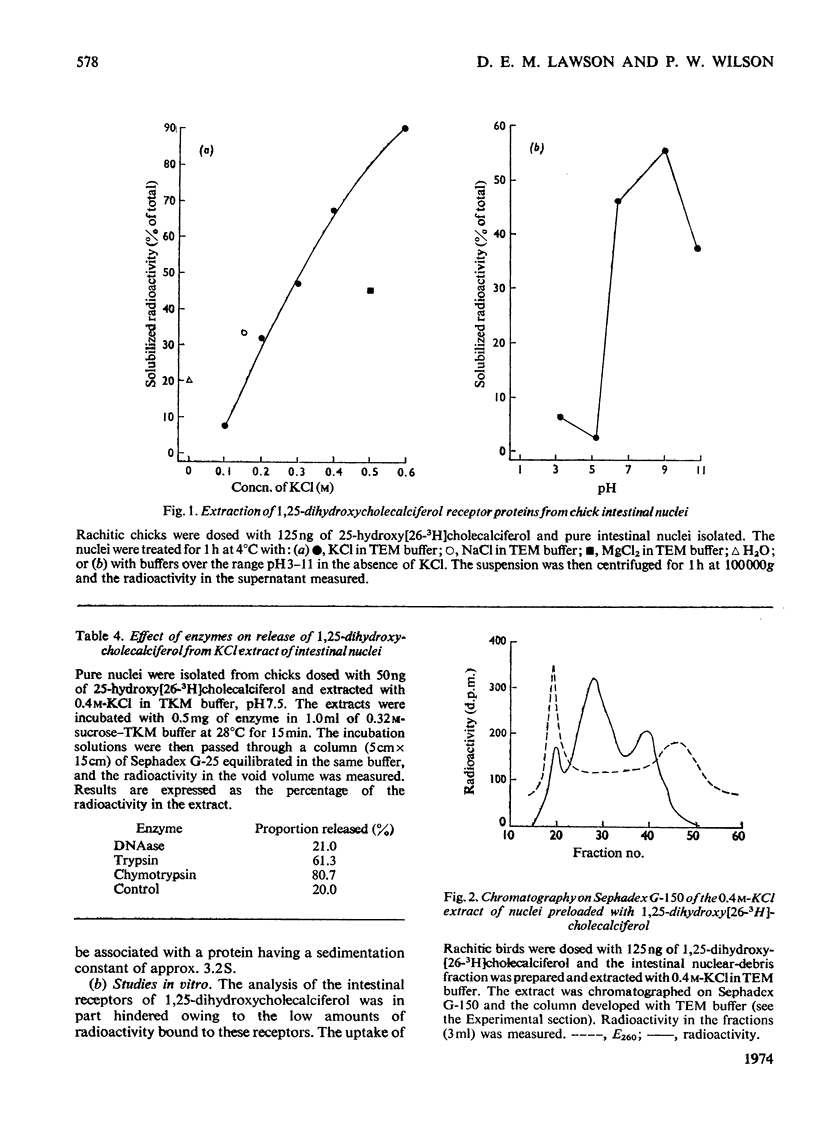
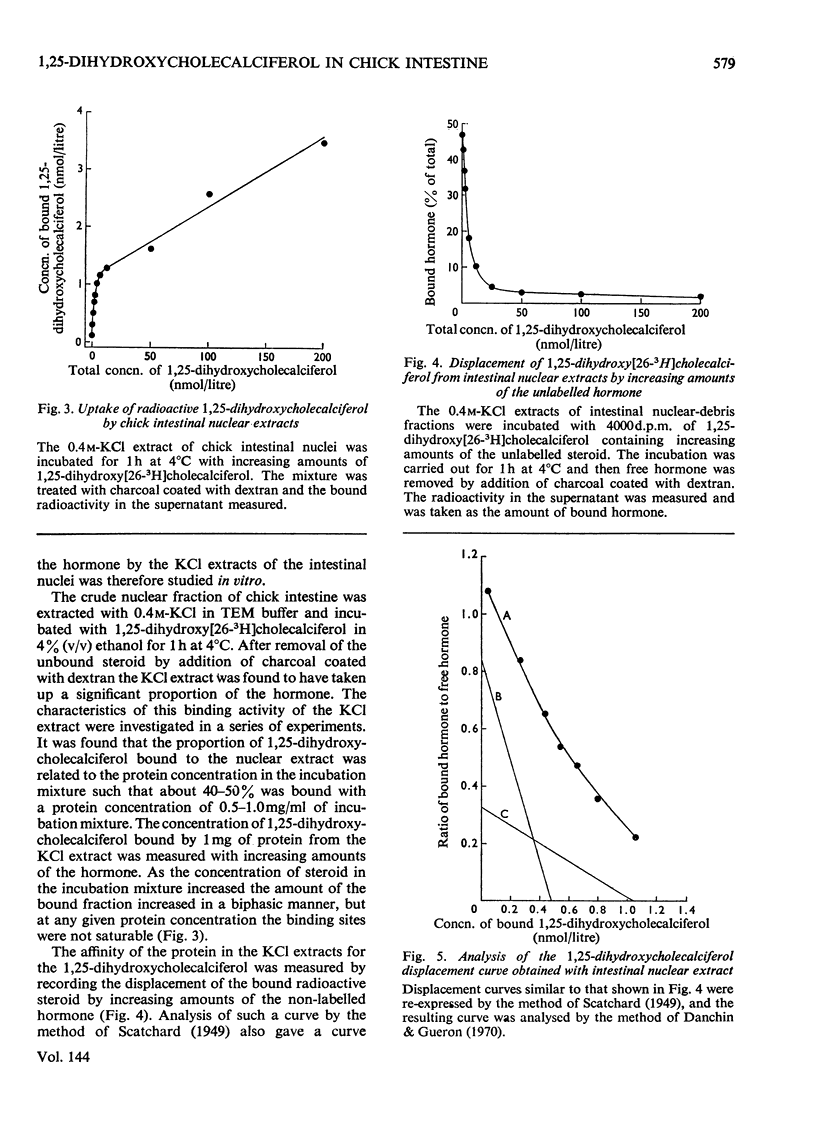
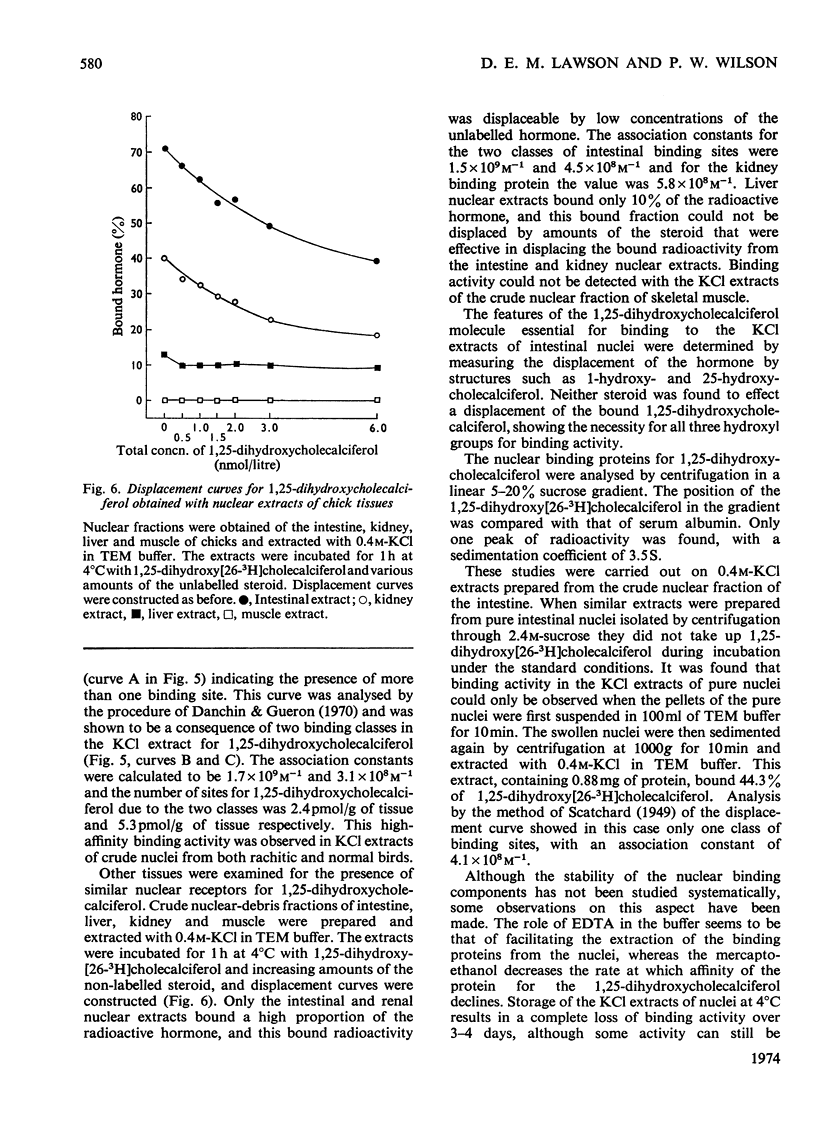
1. The intranuclear distribution of cholecalciferol and its metabolites was studied in the intestine of rachitic chicks. 2. At high doses of cholecalciferol the nuclei contain the vitamin and its 25-hydroxy metabolite, but over 80% of this is localized on the nuclear membranes. The hormone, 1,25-dihydroxycholecalciferol, is found within the cell nuclei irrespective of the intake of cholecalciferol, but significant amounts could not be found with chromatin isolated free of nuclear membranes. 3. 1,25-Dihydroxycholecalciferol is associated in the nucleus with an acidic protein. Since one of the actions of 1,25-dihydroxycholecalciferol is to control the synthesis of mRNA for calcium-binding protein it was to be expected that the hormone would be bound to chromatin, as with the other steroid hormones. It is suggested that the hormone–receptor complex exists as part of an equilibrium mixture of the complex bound to the DNA and in a free form. 4. A protein extract of nuclei was obtained, which when incubated at 4°C for 1h took up the 1,25-dihydroxycholecalciferol. The nature of this binding was studied. 5. There appear to be two nuclear proteins able to bind the hormone one of which is the intestinal nuclear receptor. The binding sites on this protein are saturable with the hormone, have an association constant of 2×109m−1 and show a high chemical specificity for the 1,25-dihydroxycholecalciferol. The number of nuclear binding sites for the hormone provided by this receptor is similar to the maximum intestinal hormone concentration so far observed. Its sedimentation coefficient is 3.5S, and is very close to that observed for the nuclear protein to which is attached the 1,25-dihydroxycholecalciferol formed in vivo from vitamin D. 6. The cytoplasmic protein has an association constant of 1×109m−1and a sedimentation coefficient of 3.0S, but its relation with the nuclear receptor is not yet clear.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baulieu E. E., Alberga A., Jung I., Lebeau M. C., Mercier-Bodard C., Milgrom E., Raynaud J. P., Raynaud-Jammet C., Rochefort H., Truong H. Metabolism and protein binding of sex steroids in target organs: an approach to the mechanism of hormone action. Recent Prog Horm Res. 1971;27:351–419. doi: 10.1016/b978-0-12-571127-2.50033-x. [DOI] [PubMed] [Google Scholar]
- Berezney R., Funk L. K., Crane F. L. The isolation of nuclear membrane from a large-scale preparation of bovine liver nuclei. Biochim Biophys Acta. 1970 Jun 2;203(3):531–546. doi: 10.1016/0005-2736(70)90190-2. [DOI] [PubMed] [Google Scholar]
- Chen T. C., DeLuca H. F. Receptors of 1,25-dikydroxycholecalciferol in rat intestine. J Biol Chem. 1973 Jul 25;248(14):4890–4895. [PubMed] [Google Scholar]
- Chen T. C., Weber J. C., DeLuca H. F. On the subcellular location of vitamin D metabolites in intestine. J Biol Chem. 1970 Aug 10;245(15):3776–3780. [PubMed] [Google Scholar]
- DINGMAN C. W., SPORN M. B. STUDIES ON CHROMATIN. I. ISOLATION AND CHARACTERIZATION OF NUCLEAR COMPLEXES OF DEOXYRIBONUCLEIC ACID, RIBONUCLEIC ACID, AND PROTEIN FROM EMBRYONIC AND ADULT TISSUES OF THE CHICKEN. J Biol Chem. 1964 Oct;239:3483–3492. [PubMed] [Google Scholar]
- Danchin A., Guéron M. Cooperative binding of manganese (II) to transfer RNA. Eur J Biochem. 1970 Nov;16(3):532–536. doi: 10.1111/j.1432-1033.1970.tb01113.x. [DOI] [PubMed] [Google Scholar]
- Fraser D. R., Kodicek E. Regulation of 25-hydroxycholecalciferol-1-hydroxylase activity in kidney by parathyroid hormone. Nat New Biol. 1973 Feb 7;241(110):163–166. doi: 10.1038/newbio241163a0. [DOI] [PubMed] [Google Scholar]
- Fraser D. R., Kodicek E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature. 1970 Nov 21;228(5273):764–766. doi: 10.1038/228764a0. [DOI] [PubMed] [Google Scholar]
- Garabedian M., Holick M. F., Deluca H. F., Boyle I. T. Control of 25-hydroxycholecalciferol metabolism by parathyroid glands. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1673–1676. doi: 10.1073/pnas.69.7.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussler M. R., Myrtle J. F., Norman A. W. The association of a metabolite of vitamin D3 with intestinal mucosa chromatin in vivo. J Biol Chem. 1968 Aug 10;243(15):4055–4064. [PubMed] [Google Scholar]
- Jensen E. V., Suzuki T., Numata M., Smith S., DeSombre E. R. Estrogen-binding substances of target tissues. Steroids. 1969 Apr;13(4):417–427. doi: 10.1016/0039-128x(69)90053-1. [DOI] [PubMed] [Google Scholar]
- KODICEK E. VITAMIN D AND CALCIUM HOMEOSTASIS. Adv Fluorine Res. 1965;21:39–52. [PubMed] [Google Scholar]
- Lawson D. E., Bell P. A. Metabolism of dihydrotachysterol and 5,6-trans-cholecalciferol in the chick and the rat. Biochem J. 1974 Jul;142(1):37–46. doi: 10.1042/bj1420037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson D. E., Pelc B., Bell P. A., Wilson P. W., Kodicek E. Synthesis of (1,2- 3 H 2 )cholecalciferol and metabolism of (4- 14 C,1,2- 3 H 2 )- and (4- 14 C,1- 3 H)-cholecalciferol in rachitic rats and chicks. Biochem J. 1971 Feb;121(4):673–682. doi: 10.1042/bj1210673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson D. E., Wilson P. W., Barker D. C., Kodicek E. Isolation of chick intestinal nuclei. Effect of vitamin D3 on nuclear metabolism. Biochem J. 1969 Nov;115(2):263–268. doi: 10.1042/bj1150263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson D. E., Wilson P. W., Kodicek E. Metabolism of vitamin D. A new cholecalciferol metabolite, involving loss of hydrogen at C-1, in chick intestinal nuclei. Biochem J. 1969 Nov;115(2):269–277. doi: 10.1042/bj1150269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marushige K., Bonner J. Template properties of liver chromatin. J Mol Biol. 1966 Jan;15(1):160–174. doi: 10.1016/s0022-2836(66)80218-8. [DOI] [PubMed] [Google Scholar]
- Murphy B. E. Some studies of the protein-binding of steroids and their application to the routine micro and ultramicro measurement of various steroids in body fluids by competitive protein-binding radioassay. J Clin Endocrinol Metab. 1967 Jul;27(7):973–990. doi: 10.1210/jcem-27-7-973. [DOI] [PubMed] [Google Scholar]
- NORMAN A. W., DELUCA H. F. THE SUBCELLULAR LOCATION OF H3 VITAMIN D3 IN KIDNEY AND INTESTINE. Arch Biochem Biophys. 1964 Jul;107:69–77. doi: 10.1016/0003-9861(64)90270-x. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Wong M., Bikle D., Goodman D. B. Hormonal control of the renal conversion of 25-hydroxycholecalciferol to 1,25-dihydroxycholecalciferol. J Clin Invest. 1972 Sep;51(9):2502–2504. doi: 10.1172/JCI107065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein G. S., Spelsberg T. C., Kleinsmith L. J. Nonhistone chromosomal proteins and gene regulation. Science. 1974 Mar 1;183(4127):817–824. doi: 10.1126/science.183.4127.817. [DOI] [PubMed] [Google Scholar]
- Stohs S. J., DeLuca H. F. Subcellular location of vitamin D and its metabolites in intestinal mucosa after a 10-IU dose. Biochemistry. 1967 Nov;6(11):3338–3349. doi: 10.1021/bi00863a002. [DOI] [PubMed] [Google Scholar]
- Tsai H. C., Norman A. W. Studies on calciferol metabolism. 8. Evidence for a cytoplasmic receptor for 1,25-dihydroxy-vitamin D3 in the intestinal mucosa. J Biol Chem. 1973 Sep 10;248(17):5967–5975. [PubMed] [Google Scholar]
- Zbarsky I. B., Perevoshchikova K. A., Delektorskaya L. N., Delektorsky V. V. Isolation and biochemical characteristics of the nuclear envelope. Nature. 1969 Jan 18;221(5177):257–259. doi: 10.1038/221257a0. [DOI] [PubMed] [Google Scholar]


