Abstract
Triphala is a traditional Ayurvedic herbal formulation composed of three fruits: amla (Phyllanthus emblica), bibhitaki (Terminalia bellerica), and haritaki (Terminalia chebula). Triphala is a potent Ayurvedic remedy that promotes digestion, detoxification, and overall wellness, while also providing antioxidant benefits through its trio of nutrient-rich fruits. In order to elucidate the individual contributions of the three ingredients of Triphala from molecular perspective, the individual ingredients were used for the untargeted LCMS/MS analysis. Fresh fruits (PE, TC, and TB) were collected, processed into coarse powders, and sequentially extracted {hexane, chloroform, and ethyl acetate}. LCMS/MS data analysis was performed on the resultant metabolites, with bioinformatics tools employed for pathway enrichment, target prediction, and classification of identified compounds. Additionally, polyphenols were identified as key compounds with potential health benefits. LCMS analysis of the individual extracts identified a total of 10227 features, resulting in 2515 annotated metabolites, with PE contributing the highest number at 1286. Comparative analysis revealed 408 non-redundant metabolites, with 74.2% being unique to individual fruits, underscoring the complementary phytochemical profiles. Pathway enrichment analysis highlighted dominant phenylpropanoid biosynthesis pathways across all extracts, while a comprehensive polyphenol classification identified 71 polyphenols, with significant interactions predicted between polyphenols and gut microbiota. Additionally, five common polyphenols showed potential human targets related to antioxidant activity. These findings provide a deeper understanding of the phytochemical diversity and potential health benefits of Triphala, supporting its traditional use in promoting health.
Keywords: Triphala, Ayurveda, Antioxidants, Polyphenols
Subject terms: Metabolomics, Natural products, Biochemistry, Plant sciences, Diseases, Health care, Chemistry
Introduction
There is a growing understanding that phytochemical diversity in our food, beyond the macronutrients and micronutrients in the food we eat, is a cornerstone for good health1,2. Carlson (2010) estimates that there are more than 25000 bioactive food constituents3. These belong to three major phytochemical groups: the terpenoids, the alkaloids, and the phenylpropanoids and allied phenolic compounds. Terpenoids are derived from the five-carbon precursor isopentenyl diphosphate (IPP). Alkaloids are phytochemicals which contain one or more nitrogen atoms and are biosynthesized principally from amino acids. The phenolic compounds are formed by way of either the shikimic acid pathway or the mevalonate/acetate pathway. Within the body, the phytochemicals in food are pleiotropic and have multiple effects on areas such as cellular physiology, inflammation, insulin sensitization, stress response, cell signaling and epigenetic changes4–7. More recently, the mediation of gut in the effect of phytonutrients has been hypothesized. Well studied examples of such gut mediated transformation of phenolics into actives include the conversion of ellagic acid to Urolithin A8 and diadzein to equol9. Due to their ability to address multiple mechanisms simultaneously, it has been proposed that phytonutrients may be especially helpful in chronic diseases. For example, polyphenols have been suggested to be a potential nutraceutical intervention in type 2 diabetes10,11, where there are several dysfunctional processes related to glucose and lipid metabolism that impact several body systems. A phytochemically-rich diet could be an important pillar of preventive health strategies.
Polyherbal whole food supplements and nutraceuticals are rich in phytochemicals and can add phytonutrient diversity to our diet. Ayurveda has a number of polyherbal formulations that are recommended as food supplements and tonics. One of the popular formulations is ‘Triphala’ which is the Sanskrit word for ‘three fruits’. This food supplement combines dried and powdered fruits of the plants Terminalia chebula (TC), Terminalia bellerica (TB) and Phyllanthus emblica (PE) in equal proportions12. A typical daily consumption is 3 g a day of the dry powder, taken as is, or as a soup in water, or with other ingredients such as honey or ghee13. All the three fruits are highly astringent in their in-mouth experience accompanied with sourness and bitterness and hints of sweetness and pungency, indicating presence of phytochemicals with different functional groups.
There are many health benefits associated with daily consumption of Triphala in Ayurvedic literature. It is considered a longevity tonic—one that can make a person live to be a centenarian14. It is used for treatment of ulcers and wounds15, chronic fever, obesity, heart diseases and diabetes16. Triphala is said to help cleansing the mucus of the digestive villi, making for a more effective digestive process and support the elimination process. Individually, PE is a fruit that has been suggested to support intestinal repair, TB has been suggested to pull the old mucus off the wall and TC has been suggested to strengthen the intestinal muscles to contract more efficiently when the bowels need to move17,18.
The wide popularity of this polyherbal formulation amongst Indians and their diaspora, and its many benefits mentioned in Ayurveda, has led to scientific interest in it. Over the last two decades many research studies have focused on the bioactivity of the three fruits individually and of the mixed formulation. This includes evaluating their extracts in assays pertaining to cancer19, diabetes20–24, inflammation inhibition and immunomodulatory activity25–28, gastrointestinal disorders29–31, hepatoprotective activity32,33, antibacterial activity34, antiplasmodial activity and cardioprotective activity35. Extracts of PE have been studied for skin lightening, treatment of gonorrhea, prevention of greying of hair36, treatment of diabetes, hysteria, jaundice, eczema, piles, diarrhea, menorrhagia, scurvy, and increases red blood counts37–42. TB has been studied for treatment of cough, asthma, anorexia, vomiting, arthritis, fever, epilepsy, splenomegaly, piles, diarrhea, leprosy, brain tonic and laxative38,43–46.
The broad sensory and biological activity of the three ingredients suggests a large phytochemical diversity. Previous research has primarily focused on either the targeted identification of specific bioactive compounds or the overall profile of the Triphala formulation, without investigating how the individual ingredients contribute to its therapeutic effects. More recently, untargeted approaches using LCMS have been used to understand the overall phytochemical profile47 and identified more than 1500 compounds. However, a question that is left unanswered is the contribution of each of the three ingredients and the reason why they need to be combined as a single mix. Our study addresses this gap by performing a detailed untargeted metabolome analysis of the individual powders from the three fruits that comprise the Ayurvedic formulation Triphala—Terminalia chebula (TC), Terminalia bellerica (TB), and Phyllanthus emblica (PE)—using LCMS, and then comparing the metabolome profiles of the three fruits, allowing us to explore how their combined properties may work synergistically in the formulation. Additionally, we characterize the polyphenols in each fruit, predict their human targets, and assess their bioavailability, providing new insights into the molecular mechanisms underlying Triphala’s therapeutic potential.
This is the first study to profile each ingredient separately using a consistent analytical approach. This enables assessment of the distinct contributions of each fruit and an understanding of their complementary roles in the formulation. A key novelty of our study is the identification of complementary and supplementary phytochemical profiles, which helps explain the rationale behind the traditional combination of these three ingredients.
Materials and ethods
Sample preparation and metabolite extraction
Fresh PE fruits were collected from ICAR-Indian Institute of Horticultural Research (IIHR), Bengaluru, India. Fresh TC and TB fruits were collected from the medicinal plant garden of The University of Trans-Disciplinary Health Sciences and Technology (TDU), Bengaluru, India. The PE, TC & TB fruits were cut, deseeded and crushed and oven dried at 50 °C. The dried fruits were separately ground into a coarse powder. The coarse powders were stored in separate air tight containers for subsequent extraction.
Each of the three dried powders was extracted sequentially with three solvents, used in increasing order of polarity, in a Soxhlet apparatus following the methods of Singh et al. (2019)48. The three solvents used were hexane (boiling point- 68.7 °C and polarity ~ 0.1), chloroform (BP- 61.2 °C and polarity ~ 4.1) and ethyl acetate (BP- 77.1 °C and polarity ~ 4.4). Briefly, 10 g of dried powder was taken in the thimble of the Soxhlet apparatus and 100 ml of solvent was taken in the flask giving a dried powder to solvent ratio of 1:10 (w/v). The extraction was carried out till a colourless solvent was obtained and the number of cycles noted.
The solvent extracts obtained were centrifuged at 10000 rpm for 10 min and the supernatants collected. Each supernatant was evaporated separately to dryness at 30 to 40 °C and stored at -80 °C till further processing. Just before LCMS/MS, the dried samples were taken out of the freezer and brought to room temperature. The three dried solvent extracts of each fruit were then combined and suspended in 1 ml of 100% methanol and used for LCMS/MS analysis. All the solvents for the present study were from Merck and of HPLC grade.
LCMS/MS analysis
LCMS/MS was carried out on a QTRAP 6500 mass spectrometer (SCIEX, Framingham, MA, USA) interfaced with a 1290 Infinity HPLC system (Agilent Technologies). The samples were injected using a programmed auto sampler onto Poroshell 120 EC-C18 analytical column (2.1 mm × 100mm, 2.7µ) (Agilent Technologies, USA). Ultra performance liquid chromatography (UPLC; Waters ACQUITY UPLC system) Reverse phase, analytical column, HSS T3 C18 (100 × 2.1 mm i.d., 1.8-µm particle size; Waters) Q-Exactive Orbitrap-focus mass analyzer (Thermo Finnigan) PC machine with Windows operating system LCMS was used for the present analysis49. The Waters ACQUITY UPLC (Waters) coupled to a Q-Exactive Orbitrap-focus system (Thermo Finnigan) via an electrospray ionization (ESI) interface. Samples were run in both positive and negative ionization modes to provide the broadest coverage of the metabolome. Elution buffer A was prepared with a 0.1% solution (v/v) of ULC/MS grade formic acid (for liquid chromatography; Biosolve) and ultrapure water. Elution buffer B was prepared with a 0.1% solution (v/v) of ULC/MS grade formic acid (for liquid chromatography; Biosolve) and ultrapure acetonitrile (ULC/MS grade; Biosolve, cat. no. 01204102). The mobile phases used for the analysis included 0.1% formic acid in water (Solvent A) and 0.1% formic acid in 90% acetonitrile (Solvent B). The system was equilibrated by injecting 3–5 blank samples (methanol) and the samples were injected in a randomized manner. Each sample was analyzed in positive ionization mode, immediately followed by the same sample analyzed in negative ionization mode.
The metabolites were eluted using the following gradient: 2% B for 1 min, 2–30% B for 9 min, 30–60% B for 1 min, 60–95% B for 2 min, 95% B for 4 min, 95–2% B for 12 s and 2% B for 3 min. The total run time was set to 20 min and the flow rate was set to 0.300 ml/min and the injection volume was set to 10 µl. Data were acquired in both positive and negative scan modes using the information dependent acquisition (IDA) method in the Enhanced MS (EMS) to Enhanced Product Ion (EPI) scan mode. The Analyst software (version 1.6.2, Sciex) was used for the acquisition of data. The ESI source parameters of QTRAP 6500 included Ion Source Gas 1 (GS1) as 40 psi, Ion Source Gas 2 (GS2) as 40 psi, Curtain gas (CUR) as 35 psi, source temperature 350 °C and Collisionally activated dissociation (CAD) gas at high. The ion spray voltage was set to 350 V and the declustering potential was set to 100 V in the negative as well as positive scan modes. The instrument was set to acquire m/z range 50–1000 Da. Data was searched against NIST spectral library 2017 using SCIEX OS 3.0 software.
Bioinformatics data analysis
The putatively identified metabolites were further filtered based on their library hit score. Metabolites with library hit score > 70 were considered for further analysis50–53. For the metabolites with library hit score > 70, the PubChem IDs were retrieved from PubChem using PubChem API. Corresponding SMILES were retrieved from PubChem Identifier Exchange Service (https://pubchem.ncbi.nlm.nih.gov/docs/identifier-exchange-service).
Pathway enrichment analysis
Pathway analysis of the identified metabolites was conducted using Metaboanalyst 5.054. The analysis utilized the Arabidopsis thaliana pathway library. For over-representation analysis, the hypergeometric test was employed, and pathway topology analysis was performed using relative-betweenness centrality. Venn diagrams were constructed using Calculate and draw custom Venn diagrams, an online tool (https://bioinformatics.psb.ugent.be/webtools/Venn/).
Prediction of antioxidant related targets from Triphala metabolites
The protein targets were predicted separately for exclusive metabolites of TC, PE and TB using the “Find my Compound’s Targets” tool (http://bindingdb.org/bind/chemsearch/marvin/FMCT.jsp) in BindingDB55. For the prediction of targets, a similarity criterion of 0.85 was employed. The metabolite-human target network was constructed using an open source software platform Cytoscape.
Classification of metabolites
The identified metabolites from all three plants were classified into super class, class and subclasses using ClassyFire56 a web based metabolite classification tool. Further metabolites from each plant have been classified into polyphenols using Phenol-Explorer 3.657. The ADME (Absorption, distribution, metabolism, and excretion) properties of polyphenols were predicted using SwissADME. The polyphenols between TC, PE and TB were compared and common polyphenols were used to predict potential human targets using SwissTargetPrediction58. The pathway enrichment of predicted human targets were performed using ShinyGO 0.8059. The overall workflow of the study is given in Supplement-1.
Results and discussion
LCMS/MS analysis of Triphala
This study investigated the individual contributions of the three components of Triphala—TC, TB and PE to elucidate the rationale behind their traditional formulation. Previous research by Subbannayya et al. (2018)47 did a global metabolite profiling of the extract of the mix of TC, PE and TB, using LCMS/MS, and identified 1897 putatively annotated metabolites. Since the profiling was done on an extract of the mix, the contribution of each fruit could not be done. As the goal of our study was to study the unique contribution of each fruit to the phytochemical profile of the Triphala mix, PE, TC and TB were extracted separately and profiling of the phytochemicals was done by LCMS/MS using electrospray ionization. The electrospray ionization technique produces positive and negative ions at high voltage by solvent evaporation, giving a broad coverage of the metabolome. By identifying complementary and supplementary sets of metabolites, we aimed to provide a molecular perspective on the synergistic benefits that justify the combined use of these three fruits in Triphala.
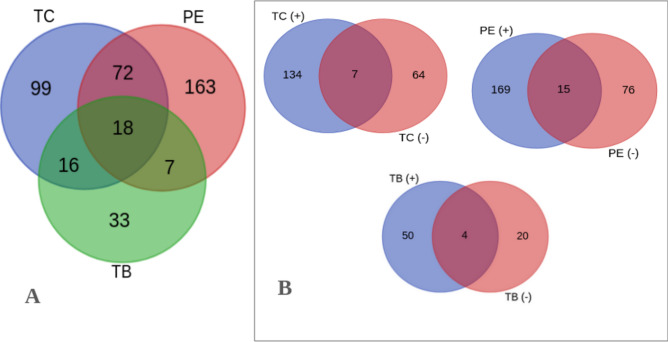
The LCMS/MS analysis identified a total of 10227 ions/features identified from PE, TC, and TB extracts. The features were searched against the National Institute of Standards and Technology (NIST) spectral library to identify the metabolites. This annotation resulted in a total of 2515 metabolites with TC having 975, PE having 1286 and TB having 254 metabolites. Further annotated metabolites were filtered using a library hit score of 70 as a cut-off. This yielded 787 metabolites, with TC having 295, PE having 396 and TB having 96 (Table 1). The positive ion mode released more ions in all the three extracts with 184 in PE, 141 in TC and 54 in TB. The negative ion mode identified 91 in PE of which 46 were new, 71 in TB, of which 64 were new, and 24 in TB of which 20 were new. 15 metabolites were identified by both + ve and -ve mode in PE, 7 in TC and 4 in TB (Fig. 1).
Table 1.
LC–MS data summary.
| Attribute | ESI | TC | PE | TB | Total |
|---|---|---|---|---|---|
| Total Ions/features | Positive | 2294 | 2787 | 398 | 10,227 |
| Negative | 2016 | 2374 | 358 | ||
| Total | 4310 | 5161 | 756 | ||
| Putatively annotated ions/features | Positive | 675 | 907 | 169 | 2515 |
| Negative | 300 | 379 | 85 | ||
| Total | 975 | 1286 | 254 | ||
| Ions/features with library hit score > 70 | Positive | 196 | 262 | 63 | 787 |
| Negative | 99 | 134 | 33 | ||
| Total | 295 | 396 | 96 |
Fig. 1.
Comparison of metabolites from Triphala sample. (A) Common and unique metabolites among PE, TC and TB; (B) Common and unique metabolites at different electrospray ionization (ESI + ve and –ve mode).
Further, the metabolites with library hit score > 70 were used for comparing the unique contribution of TC, PE and TB. A total of 408 non-redundant metabolites (Fig. 1) were identified. The highest number of metabolites, 260, was identified in PE extract. 205 metabolites were identified in TC extract and 74 in TB extract. The list of all metabolites is given in Supplement 2.
Eighteen metabolites were common to all the three fruit extracts. PE and TC shared an additional 72 metabolites, PE and TB 7 metabolites, while TC and TB shared an additional 16 metabolites beyond the 18 shared by all three. Thus, 74.2% of the metabolites are uniquely contributed by one fruit and only 4.4% are shared by all three fruits (Table 2). This underscores a high level of complementarity between the phytochemical profiles of these fruits. The unique and overlapping metabolites of TC, PE, and TB suggest a synergistic interaction where the combined phytochemical profiles enhance the overall therapeutic efficacy of Triphala. For instance, the complementary action of polyphenols from TC and flavonoids from PE can provide a broad spectrum of antioxidant and anti-inflammatory effects, which are more potent when combined than when used individually. This synergism is likely due to the multiple molecular targets and pathways modulated by the diverse set of metabolites.
Table 2.
Comparison of metabolites between TC, PE and TB.
| Attribute | Number of molecules | % of total |
|---|---|---|
| Only TC | 99 | 24.3 |
| Only PE | 163 | 40.0 |
| Only TB | 33 | 8.1 |
| TC + PE but not TB | 72 | 17.6 |
| PE + TB but not TC | 7 | 1.7 |
| TB + TC but not PE | 16 | 3.9 |
| TB + PE + TC | 18 | 4.4 |
Metabolite Classification
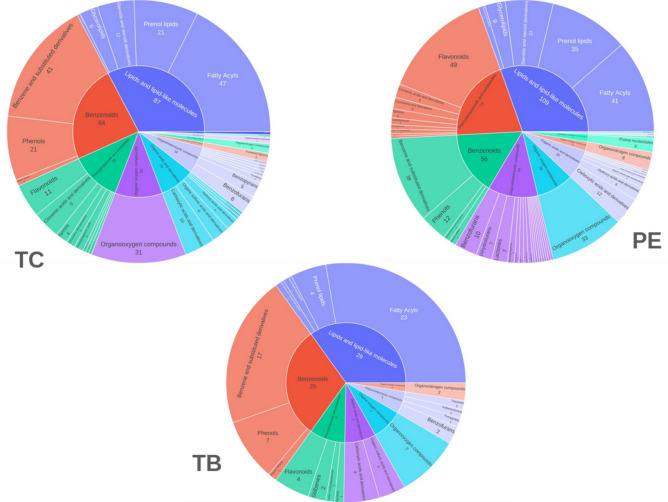
The extraction methods extracted both polar metabolites associated with metabolomics and some less polar compounds typically associated with lipidomics. To analyse further, the phytochemicals were classified into two big classes: the lipid metabolome and the non-lipid metabolome. They were further classified into super class, class and subclasses using ClassyFire56. Subsequently, the metabolites were further classified into primary (carbohydrates, lipids, amino acids, etc.) and secondary metabolites through manual inspection using class and subclasses identified through ClassyFire. 184 metabolites could be anotated using ClassyFire in the TC extract, 229 in the PE extract, and 65 in TB extract. Of these, the primary metabolites in TC and PE were 64 each, and TB had 25. TC had 120 secondary metabolites, PE 165, and TB 40. Furthermore, the functional group diversity within these secondary metabolites was highlighted, with TC exhibiting 11 superclasses and 27 classes, PE with 8 superclasses and 36 classes, and TB with 7 superclasses and 15 classes. Notably, TC and PE displayed a broader range of both superclass and class in secondary metabolites compared to TB. The diverse functional groups, including flavonoids, phenolic acids, and terpenoids, are associated with various health benefits. Flavonoids such as quercetin and kaempferol possess anti-inflammatory, anti-carcinogenic, and cardioprotective properties. Phenolic acids like gallic acid and ellagic acid exhibit strong antioxidant activities, reducing the risk of chronic diseases by neutralizing free radicals. Terpenoids, which were also present though to a smaller extent, may be contributing to the anti-microbial and anti-inflammatory effects of Triphala, supporting its traditional use in promoting gastrointestinal health and overall well-being.
In PE, the predominant non-lipidome metabolites belong to the phenylpropanoids and polyketides superclass, with flavonoids being the most abundant class. Additionally, benzenoids, including benzene and substituted derivatives were also identified in PE. TC had a significant presence of benzene derivatives. Phenols, prenol lipids, and steroids were also notable components. Flavonoids, carboxylic acids, and cinnamic acids were present in appreciable amounts as well. In TB, the chemical composition was less diverse, with a notable presence of benzene derivatives, and organooxygen compounds. Phenols and carboxylic acids were also present but to a lesser extent compared to the other extracts (Fig. 2). As mentioned above, the extraction method also extracted a number of lipids and lipid-like molecules. Fatty acyls and prenol lipids were noteworthy in PE. TC and TB also had notable fatty acyls. The presence of flavonoids, phenols, and fatty acyls, known for their health-promoting properties, aligns with the holistic therapeutic value attributed to Triphala in traditional medicine12.
Fig. 2.
Classification of metabolites into class and subclass level from TC, PE and TB.
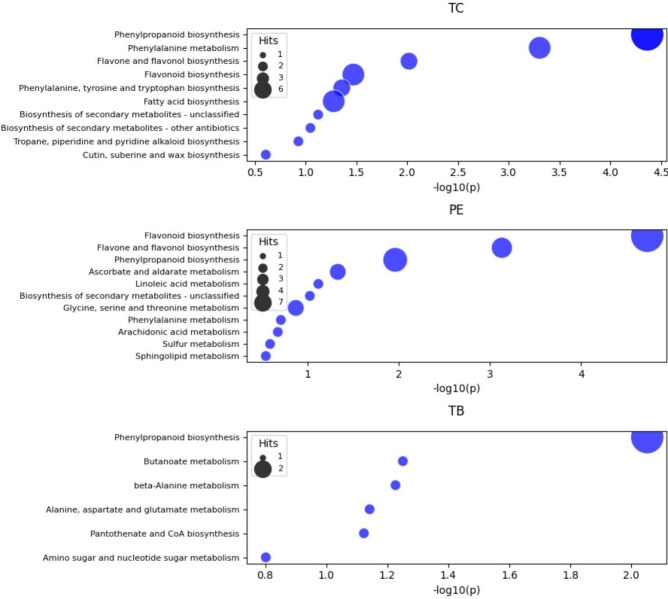
The secondary metabolites from three fruits were used for pathway enrichment analysis which revealed the dominance of phenylpropanoid and related biosynthetic pathways. Flavonoid, flavone & flavonol biosynthesis appearedin all three. These pathways lead to the secondary metabolites in classes of benzenoids, phenylpropanoid and polyketides. More specifically, in TC, the most significantly enriched pathways included phenylpropanoid biosynthesis, and flavone & flavonol biosynthesis, PE exhibited a pronounced emphasis on flavonoid biosynthesis, flavone and flavonol biosynthesis, and phenylpropanoid biosynthesis. TB had fewer pathways enriched than TC and PE but notably included the pathway for phenylpropanoid biosynthesis (Fig. 3). The pathways leading to the other major secondary metabolite groups such as alkaloids and terpenoids did not appear prominently in this analysis. The presence of the phenylpropanoid biosynthesis pathway in all three components of Triphala underscored its significance, as phenylpropanoids play an essential role in plant defense and exhibit notable antioxidant properties with potential health benefits. Additionally, the flavonoid biosynthesis pathway was specifically observed in PE and TC, indicating a rich flavonoid content within their metabolite profiles. Flavonoids are recognized for their anti-inflammatory, antioxidant, and anticancer activities, further enhancing the therapeutic potential of Triphala. The phenylpropanoid pathways are crucial for synthesizing various phenolic compounds known for their potent antioxidant and anti-inflammatory properties. These pathways generate metabolites that can scavenge free radicals, thereby protecting cells from oxidative damage.
Fig. 3.
Top 10 enriched pathways of metabolites identified from Triphala plants. Bubble size represents the number of metabolites identified from each pathway.
Key metabolites identified from TC, PE and TB using LCMS/MS
The untargeted metabolome study of Triphala churna (powder) by Subbannayya et al., 2018, had reported the metabolites chebulinic acid, methyl gallate, vanillic acid, ( +) catechin, epicatechin and intermediates of quercetin, cinnamic acid, kaempferol, luteolin, quinic acid, caffeic acid and coumaric acid. These phytochemicals have also been reported in previous HPLC and LCMS studies on PE60–62 and Terminalia species TC and TB60,63. All these metabolites were identified in our analysis as well. The full list of metabolites of Triphala curated from literature and its comparison with the metabolites identified in this study is given in Supplement-3.
To take the analysis further, we compared the top 20 metabolites, based on their signal strengths, in the three plant samples: TC, PE and TB (Table 3). Polyphenols figured in the top 20 list in all the three. TC had gallic acid and ethyl gallate in its top 20, PE had ellagic acid, quercetin, kaempferol, t-cinnamic acid, and quercitrin. TB had ethyl gallate. These molecules are known to be astringent with sour and bitter notes and may contribute significantly to the overall sensory profile of the three. Additionally, these molecules are also known for their antioxidant properties64 and may contribute to keeping the gut environment free of reactive oxygen species.
Table 3.
Top 20 high concentrated metabolites in TC, TB and PE.
| Library Hit | Experimental m/z | RT | Area | Adduct/charge | Library Score |
|---|---|---|---|---|---|
| Terminalia chebula (TC) | |||||
| Phytanic acid | 313.277 | 34.25 | 1.83E+08 | [M+H]+ | 88.2 |
| 6-Ketoprostaglandin F1.alpha | 353.269 | 34.25 | 8.24E+07 | [M+H]+ | 100 |
| 1-Palmitoylglycerol | 331.287 | 34.25 | 5.11E+07 | [M+H]+ | 91.9 |
| 1-Stearoyl-rac-glycerol | 359.319 | 37.88 | 2.33E+07 | [M]+ | 94.7 |
| Muscone | 239.239 | 34.25 | 1.93E+07 | [M+H]+ | 84.5 |
| cis,cis-9,12-Octadecadien-1-ol | 267.271 | 37.97 | 1.46E+07 | [M+H]+ | 96.2 |
| Methyl hexadecanoate | 271.266 | 34.13 | 1.06E+07 | [M+CH3OH+H]+ | 86 |
| 1,2,3-Benzenetriol | 125.024 | 3.71 | 8.85E+06 | [M-H]- | 99.7 |
| Shikimic acid | 173.045 | 2.69 | 8.72E+06 | [M-H]- | 74.4 |
| 1,4-D-Xylobiose | 281.248 | 38.19 | 8.49E+06 | [M-H]- | 100 |
| Hexadecanoic acid | 257.250 | 34.25 | 8.29E+06 | [M+H]+ | 97.8 |
| cis-7-Hexadecenoic acid | 253.216 | 33.69 | 6.76E+06 | [M-H]- | 100 |
| 2,6-Di-tert-butylphenol | 205.160 | 35.38 | 6.43E+06 | [M-H]- | 93.9 |
| N2-Trifluoroacetyl-L-glutamine | 241.217 | 34.92 | 4.92E+06 | [M-H]- | 87.4 |
| DL-Malic acid | 133.014 | 2.93 | 4.67E+06 | [M-H]- | 89.1 |
| Gallic acid | 171.031 | 20.77 | 3.94E+06 | [M+H]+ | 98.3 |
| Ethyl gallate | 199.062 | 20.77 | 3.55E+06 | [M+H]+ | 98.2 |
| L-(-)-3-Phenyllactic acid | 165.055 | 21.38 | 3.20E+06 | [M-H]- | 90.6 |
| 5-Methylisoxazol-3-amine | 98.976 | 1.81 | 3.05E+06 | [M+H]+ | 71.9 |
| Phyllanthus emblica | |||||
| 1-Palmitoylglycerol | 331.287 | 33.75 | 4.29E+07 | [M+H]+ | 96 |
| D-Saccharic acid | 209.036 | 2.64 | 4.19E+07 | [M-H]- | 96.3 |
| Ellagic acid | 301.005 | 24.01 | 2.58E+07 | [M-H]- | 97 |
| D-Saccharic acid 1,4-lactone | 191.025 | 2.9 | 2.34E+07 | [M-H2O-H]- | 99.3 |
| Muscone | 239.239 | 33.69 | 2.22E+07 | [M+H]+ | 82.9 |
| 2-Bromo-4,6-di(tert-butyl)phenol | 283.270 | 35.92 | 2.00E+07 | [M-H]- | 71.2 |
| DL-Malic acid | 133.018 | 5.44 | 1.57E+07 | [M-H]- | 99.6 |
| Loganin | 413.072 | 5.3 | 1.41E+07 | [M+H]+ | 100 |
| Xanthosine | 285.063 | 3.64 | 1.30E+07 | [M+H]+ | 85 |
| cis,cis-9,12-Octadecadien-1-ol | 267.271 | 37.69 | 9.48E+06 | [M+H]+ | 93.6 |
| Vitamin C | 175.030 | 2.69 | 8.91E+06 | [M-H]- | 89.5 |
| Quercetin | 303.053 | 28.22 | 8.81E+06 | [M+H]+ | 93.3 |
| Kaempferol | 285.046 | 29.8 | 6.52E+06 | [M-H]- | 86.8 |
| 1,2,3-Benzenetriol | 125.028 | 3.66 | 6.45E+06 | [M-H]- | 99.3 |
| Phytosphingosine | 318.304 | 32.48 | 6.06E+06 | [M+H]+ | 82.7 |
| trans-Cinnamic acid | 147.050 | 28.9 | 5.88E+06 | [M-H]- | 86.4 |
| Glibornuride | 367.030 | 3.53 | 5.69E+06 | [M+H]+ | 90.7 |
| 2-Pyrrolidinone, 1-methyl- | 100.077 | 5.81 | 5.13E+06 | [M+H]+ | 96.6 |
| Decylbenzenesulfonic acid | 297.159 | 35.29 | 4.93E+06 | [M-H]- | 98.1 |
| Quercitrin | 447.063 | 24.05 | 4.89E+06 | [M-H]- | 92.9 |
| Terminalia bellerica | |||||
| Erucamide | 338.342 | 37.52 | 1.78E+08 | [M+H]+ | 89.6 |
| Pristanic acid | 299.294 | 36.25 | 6.21E+07 | [M+CH3OH+H]+ | 79.6 |
| Methyl hexadecanoate | 271.263 | 37.82 | 1.98E+07 | [M+H]+ | 73.3 |
| 17-Phenyltrinorprostaglandin E2 | 369.299 | 35.62 | 1.37E+07 | [M+H]+ | 72.6 |
| Benzyl hexadecyl dimethyl ammonium cation | 360.323 | 37.52 | 1.03E+07 | [M+Na]+ | 100 |
| Ac-Pro-Gly-Pro (PGP ) | 312.325 | 36.99 | 7.85E+06 | [M+H]+ | 82.6 |
| Methyl tetradecanoate | 243.231 | 36.92 | 7.83E+06 | [M+H]+ | 98.9 |
| 1,2-Dilinoleoyl-sn-glycero-3-phosphocholine | 782.568 | 37.57 | 7.18E+06 | [M+H]+ | 88.7 |
| Docosanamide | 340.345 | 37.52 | 7.07E+06 | [M+H]+ | 87.1 |
| N-(sec-Butyl)-N-(4-(sec-butyl(trifluoroacetyl)amino)phenyl-2,2,2-trifluoroacetamide | 413.265 | 35.55 | 5.71E+06 | [M+H]+ | 72.2 |
| 8(9)-Epoxy-5Z,11Z,14Z-eicosatrienoic acid | 321.314 | 37.52 | 4.64E+06 | [M+H]+ | 91.2 |
| Dioctyl phthalate | 391.283 | 35.62 | 4.58E+06 | [M+Na]+ | 92.5 |
| N-Acetylneuraminic acid, 2,3-dehydro-2-deoxy- | 274.274 | 30.65 | 3.74E+06 | [M+H]+ | 70.8 |
| cis,cis-9,12-Octadecadien-1-ol | 267.267 | 36.23 | 2.61E+06 | [M+H]+ | 99.5 |
| Phthalic anhydride | 149.022 | 35.55 | 2.36E+06 | [M+H]+ | 99.8 |
| Peonidin cation | 301.141 | 34.7 | 2.36E+06 | [M+H]+ | 93.9 |
| Dodecyl sulfate | 265.135 | 30.57 | 2.00E+06 | [M-H]- | 78.4 |
| Di(propylene glycol) propyl ether | 174.958 | 37.59 | 1.85E+06 | [M-H]- | 77.8 |
| (.+-.)7-epi-Jasmonic acid | 228.196 | 31.57 | 1.63E+06 | [M+NH4]+ | 80 |
| Ethyl gallate | 197.048 | 16.23 | 1.56E+06 | [M-H]- | 100 |
Besides polyphenols there were other functional groups dominant in the LCMS/MS scans of the three extracts. TC had a notable abundance of fatty acids and glycerol derivatives, including prominent metabolites such as phytanic acid and 1-stearoyl-rac-glycerol. This suggests a potential emphasis on lipid-related pathways or biosynthesis. The metabolome profile of PE showcased a rich assortment of compounds, ranging from essential acids like DL-malic acid to flavonoids such as quercetin and kaempferol, and rejuvenating agents like loganin. This indicated a broad spectrum of biochemical pathways active within PE. . PE has been regarded as a good source of ascorbic acid65 and its identification in the top 20 metabolite list supported this. The metabolic profile of TB revealed presence of fatty acid derivatives such as pristanic acid and methyl hexadecanoate to specialized molecules such as the benzyl hexadecyl dimethyl ammonium cation and 17-phenyltrinorprostaglandin E2.
Overall, these distinct metabolic fingerprints contributed to the nuanced understanding of the individual plant’s roles within the Triphala formulation, paving the way for deeper investigations into their health-promoting properties and synergistic effects.
Polyphenol classification
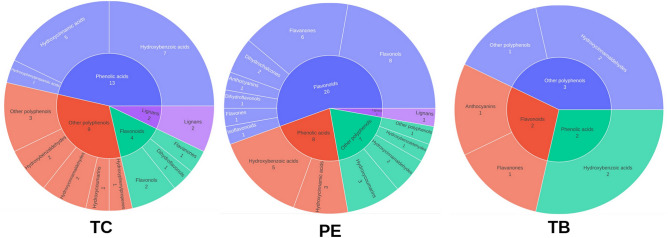
The dominance of polyphenols was apparent from the list of phytochemicals identified in the three fruits and the pathway enrichment exercise. Polyphenols are recognized as a class of molecules that are generally astringent in taste66 and have antioxidant property67. This is in line with the Ayurveda pharmacology wherein the three fruits PE, TC, and TB are classified as ‘Kashaya’ (Sanskrit word for astringent) group of medicinal and food ingredients in Ayurveda literature68–70. Equally, modern literature finds the antioxidant activity of these three fruits interesting and there are a number of published articles documenting this for the individual fruit extracts71–74 as well as for the mix75. We therefore studied the phenolic components of PE, TC and TB in greater detail. The polyphenols were classified using the Phenol-Explorer database57. Total 71 metabolites were classified as polyphenols from all three plants (28 from TC, 36 from PE and 7 from TB). The list of all polyphenols along with their classification is given in Supplement-4.
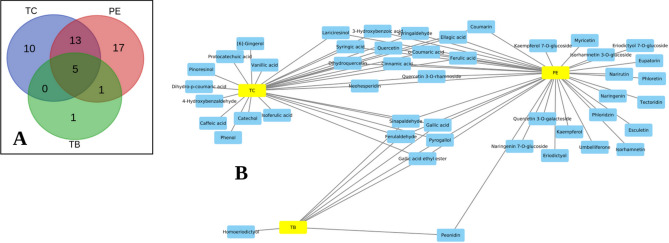
PE had the most phytochemicals in the flavonoid class, specifically flavanones and flavonols, TC was rich in phenolic acid class, with notable metabolites including hydroxybenzoic acids and hydroxybenzaldehydes. TB’s polyphenols included hydroxybenzoic acids and hydroxycinnamaldehyde, amongst others. Notably, lignans and stilbenes maintained minimal levels across all three fruit extracts (Fig. 4). TC exhibited ten unique polyphenols, while TB had one unique polyphenol, and PE had 17 unique polyphenols (Fig. 5). Pyrogallol, gallic acid, ferulaldehyde, gallic acid ethyl ester (ethyl gallate) and sinapaldehyde were found in all three.
Fig. 4.
Polyphenol classification of Triphala metabolites (TC, PE and TB).
Fig. 5.
Comparison of polyphenols from Triphala ingredients (TC, PE and TB). (A) Venn diagram showing the common and unique metabolites between TC, PE and TB; (B) Network depicting the Triphala plants and and corresponding containing polyphenols.
Prediction of absorption, distribution, metabolism and excretion (ADME) properties and in silico bioactivity of polyphenols
In general, polyphenols have poor absorption in the small intestine76,77. This suggests that a significant proportion of dietary polyphenols are expected to reach the colon where they may interact with the gut microbiome78–81.
The association between polyphenols identified in TC, PE & TB and gut microbes was investigated using GMMAD2 (Gut Microbial Metabolite Association with Disease; http://guolab.whu.edu.cn/GMMAD2). This platform provides comprehensive information on metabolite-related interactions between gut microbes and human diseases. We downloaded the interactions between microbes and metabolites and extracted the polyphenol-microbe interactions based on the PubChem IDs of Triphala polyphenols.
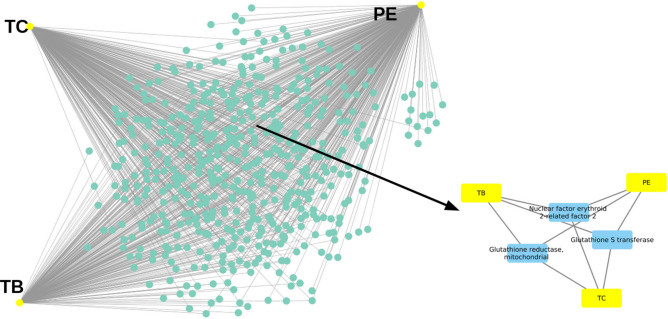
In total, 439 gut bacterial species (corresponding to 174 bacterial genera) demonstrated interactions with 19 polyphenols. Among the bacterial genera, Escherichia showed the highest number of interactions with polyphenols (8), followed by Eubacterium (6), Enterococcus (6), Bifidobacterium (6), Clostridium (5), Bacteroides (5), and others (Supplement 5; Fig. 1). These findings were in accordance with the Ma and Chen (2020)82. The interactions observed in our study between gut bacterial species and Triphala polyphenols further elucidated the significant impact of dietary polyphenols on microbial dynamics. These interactions suggest a significant role of polyphenols in modulating the gut microbiota composition. Polyphenols such as catechol and 3,4-dihydroxybenzoic acid, which interact with multiple bacterial genera, can enhance gut health by promoting the growth of beneficial bacteria and inhibiting pathogenic species. This modulation can lead to improved gut barrier function and reduced inflammation, thereby contributing to overall metabolic regulation and health benefits8,82.
Regarding polyphenols, phenol exhibited the highest number of interactions, with 133 bacterial genera, followed by catechol (61), 3,4-dihydroxybenzoic acid (39), 4-hydroxybenzaldehyde (21), and others (Supplement-5; Fig. 2). The complete list of bacterial genera and their associated polyphenols is provided in the supplementary material (Supplement-6).
Further, the ADME characteristics of the 47 classified polyphenols were examined. These characteristics encompassed physicochemical properties, lipophilicity, water solubility, pharmacokinetics, adherence to drug-like criteria, and relevance to medicinal chemistry. Oral bioavailability for potential active compounds was assessed using both Lipinski’s Rule of Five83 and Veber’s Rule84. The molecular weight of the polyphenols ranged from 94.11 (phenol with the least number of heavy atoms—7) to 610.56 g/mol (neohesperidin with maximum number of heavy atoms—43). Phenol was identified only in TC whereas neohesperidin was identified in both TC and PE (Supplement-7).
Out of 47 polyphenols, 33 polyphenols showed high GI (Gastrointestinal) absorption, 17 showed BBB (Blood Brain Barrier) permeant, 14 polyphenols identified as PGP (P-glycoprotein) substrate. The BBB potential was previously reported from amla extract for ischemia injury in rats85. The Log Kp (skin permeation) values for 47 metabolites ranged from − 10.54 to − 5.69 cm/s, with more negative values indicating lower skin permeation potential and less negative values suggesting higher permeation potential. With respect to water solubility, one polyphenol was predicted as moderately soluble, and 36 polyphenols predicted as water soluble. Ten polyphenols were predicted as highly soluble ( syringaldehyde, gallic acid, syringic acid, pyrogallol, caffeic acid, dihydro-p-coumaric acid, protocatechuic acid, catechol, 4-hydroxybenzaldehyde, and phenol).
In terms of drug-likeliness properties, 36 polyphenols exhibited adherence to Lipinski’s rule of five with no violations, while three polyphenols had a single violation, six polyphenols had two violations, and two polyphenols showed three violations. Additionally, with respect to Veber’s rule, 35 polyphenols demonstrated full compliance, while 12 polyphenols exhibited a single violation (Supplement-7).
A deeper assessment on oral bioavailability was done for five polyphenols that were found to be common to all the three fruit extracts (TC, PE, and TB), using the bioavailability radar device. The bioavailability radar device considered key parameters, including lipophilicity, size, polarity, solubility, flexibility, and saturation, to gauge the affinity of the drug and potential absorption. Among the five polyphenols, pyrogallol, gallic acid, ferulaldehyde, ethyl gallate, and sinapaldehyde, the saturation values for all except ethyl gallate suggested that they fell outside the desired range for optimal bioavailability. Saturation is a crucial factor influencing the absorption and effectiveness of orally administered drugs. On the positive side, all five polyphenols demonstrated favourable values for other bioavailability parameters such as lipophilicity, size, polarity, solubility, and flexibility. Despite the saturation challenge, these polyphenols possessed characteristics that could contribute to some absorption and utilization within the body (Supplement-5; Fig. 3).
The above results revealed insights into the potential mechanisms of absorption and distribution within the body, and the sites of action for the polyphenols. Firstly, the observation that the majority of these polyphenols exhibited minimal skin transport suggested limited permeability through the skin barrier. Furthermore, the high-water solubility predicted for most of the polyphenols suggested that they were likely to dissolve readily in aqueous environments. Surprisingly, a significant proportion of these polyphenols showed high gastrointestinal (GI) absorption potential, suggesting there may be some absorption through the small intestine80. Moreover, the identification of polyphenols capable of crossing the blood–brain barrier (BBB) further underscored their potential systemic effects86. This is supported by previous rat model studies demonstrating the presence of polyphenols in the brain following administration85.
Triphala has been reported to exhibit the capability to effectively neutralize various reactive oxygen species (ROS), including superoxide anion, hydrogen peroxide, and hydroxyl radicals through scavenging activity. Takauji et al., (2016) showed the eliminated reactive oxygen species (ROS) in HeLa cells using Triphala formulation87. Umapathi et al., (2019) assessed the effects of Triphala churna (powder) on the free radical scavenging activities such as hydrogen peroxide, nitric oxide (NO) radical, and superoxide anion radical scavenging activities in vitro88,89 and studied the free radical scavenging property of Triphala using phenyl) -(2,4,6-trinitrophenyl) iminoazanium (DPPH) scavenging of ferric ion, O2-, NO, H2O2 models88. Triphala has also been reported to have a regulatory impact on signaling pathways associated with oxidative stress. Specifically, it was shown to influence transcription factors like Nrf2, thereby promoting the expression of genes responsible for antioxidant production90. Since a few of the polyphenol molecules may have some absorption into the serum, they could potentially play a role in these activities. To understand the molecular basis of above reported results in literature, a network pharmacology approach was undertaken and metabolites were used to predict the human targets related to antioxidant activity. From this study, the Nrf2 transcription factor was one of the targets identified from metabolites of TC, PE and TB. Observations also revealed the identification of targets associated with antioxidant activity, including enzymes like glutathione reductase and glutathione transferase (Fig. 6). The antioxidants present in TC, PE and TB can stimulate the endogenous production of crucial enzymes like superoxide dismutase (SOD), catalase, and glutathione peroxidase. These enzymes, in turn, contribute to reinforcing the body’s ability to counteract oxidative stress and maintain cellular health12. The multifaceted antioxidant properties of molecules found in TC, PE and TB could potentially be responsible for protecting cells and promoting the overall well-being promise of Triphala.
Fig. 6.
Human target predicted from metabolites of TC, PE and TB.
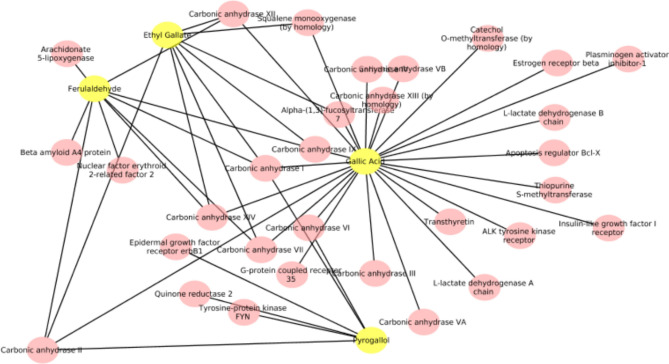
The five polyphenols which were common between all three plants were used to predict the potential human targets using SwissTarget prediction. Total 48 targets (probability > 0.1) were identified for 5 polyphenols common in TC, PE and TB (Fig. 7). Carbonic anhydrase I (CA I) and carbonic anhydrase II (CA II) enzymes were identified as potential targets for all four polyphenols analysed. Chandran et al. (2015) conducted a network pharmacology analysis of Triphala, utilizing reported metabolites and identifying human targets associated with these compounds. The study highlighted common metabolites such as ellagic acid, quercetin, chebulanin, methyl gallate, among others, across the three fruits, and reported their associated human targets and diseases. Interestingly, the authors also identified carbonic anhydrase as one of the targets for Triphala metabolites, which aligns with the findings of our study91.
Fig. 7.
Polyphenols (TC, PE and TB)—human target network.
Triphala may contribute to the overall antioxidant defense within the body. In the context of the target prediction analysis for the five polyphenols, it was observed that ferulaldehyde specifically targets Nrf2. This interaction implied that ferulaldehyde, one of the polyphenols, may play a role in activating Nrf2, consequently enhancing the expression of antioxidant response elements (ARE) and contributing to the overall cellular antioxidant defense90,92.
Carbonic anhydrase I (CA I) and carbonic anhydrase II (CA II) are two crucial isoforms within the carbonic anhydrase enzyme family. These enzymes, which are zinc metalloenzymes, play vital roles in various physiological processes, including the regulation of acid–base balance, carbon dioxide transport, and maintenance of cellular pH93,94. Both CA I and CA II are integral members of the broader carbonic anhydrase family, responsible for catalysing the interconversion of carbon dioxide and water to bicarbonate and protons94. Their activities are essential for preserving physiological pH, regulating ion transport, and facilitating diverse metabolic processes throughout the body. The intricate roles of CA I and CA II underscore their significance in maintaining homeostasis and supporting fundamental cellular functions.
These human targets were classified into different classes. Among these, lyase was the most prevalent, with 27 potential targets, suggesting a frequent association between polyphenols and targets classified under this category, while kinase and transferase were associated with four and three potential targets, respectively. Unspecified enzymes were identified as potential targets in six cases. Other target types such as secreted protein, family A G protein-coupled receptor, membrane receptor, nuclear receptor, other ion channel, oxidoreductase, and unclassified protein each represented one potential target in the dataset (Supplement-5; Fig. 4). This distribution provided insights into the diversity of target classifications linked to the polyphenols, highlighting the potential multifaceted roles of these compounds across various biological processes and molecular pathways.
Pathway analysis of human targets associated with five common polyphenols in TC, PE, and TB revealed potential effects on various cellular functions. These polyphenols demonstrated enrichment in pathways related to cellular structural integrity, such as Adherens junction and proximal tubule bicarbonate reclamation, suggesting an impact on cellular transport processes.
Furthermore, these polyphenols showed enrichment in pathways linked to metabolic regulation, exemplified by central carbon metabolism in cancer. This mirrors traditional use of Triphala as a digestive aid and metabolism regulator12, indicating a potential role in influencing cellular metabolic processes for overall health promotion. Additionally, there was significant enrichment in pathways related to nitrogen metabolism, EGFR tyrosine kinase inhibitor resistance, HIF-1 signaling pathway, and Endocrine resistance, suggesting a broader impact on cellular signaling and nitrogen-related processes (Supplement-5; Fig. 5).
The findings of this study lay a robust foundation for future research and development of Triphala-based herbal supplements by providing a comprehensive framework for the profiling of its constituent ingredients. This enhances the understanding of their synergistic interactions and facilitates targeted therapeutic applications, while also aiding in the standardization and quality control of Triphala formulations. Additionally, it supports the integration of traditional herbal medicine with modern pharmacotherapy, offering insights from ADME analysis and network pharmacology. These insights elucidate the pharmacokinetics and potential molecular targets of the bioactive compounds present in Triphala, thereby informing the rational design of supplements with optimized bioavailability and therapeutic efficacy. Furthermore, the study clarifies the rationale for combining the distinct botanicals of Triphala, highlighting their collective therapeutic potential and underscoring the need for continued investigation into the pharmacokinetic properties and molecular interactions of its constituents.
Conclusion
In this study we have done the metabolomeanalysis of the individual powders of the three fruits that comprise the popular Ayurveda formulation calledTriphala by untargeted profiling using LCMS/MS. The study elucidates the individual contributions of the three ingredients of Triphala and provides an understanding of the reason for combining them into a single formulation. By identifying the complementary and the supplementary molecule sets we are able to state why the three fruits have been combined in a formulation from a molecular perspective. Our results show that of the three main groups of secondary metabolites (alkaloids, phenolics & isoprenoids), the phytoprofile of the three fruits is dominated by phenolic acids. Therefore, we have characterized the polyphenols from all the three fruits and also identified potential human targets from the polyphenols as well as predicted the bioavailability of the identified polyphenols. This is the first holistic study of metabolome of Triphala (three species separately) with special reference to the contribution of polyphenols for therapeutic purposes. The study provides strong evidence for the complementary and synergistic roles of TC, TB, and PE in Triphala. Each ingredient contributes unique and valuable phytochemicals that, when combined, enhance the overall medicinal potential of Triphala. This comprehensive profiling supports the traditional use of Triphala in Ayurveda for promoting health.
The ADME analysis on the polyphenols in these botanicals reveals that most are water soluble and have at least some potential to be absorbed into the bloodstream in the small intestine. This suggests three points of action & absorption—bioactivity in the gut (such as antioxidant activity to keep the colon environment reactive oxygen species free), transformation by gut bacteria to forms that have better absorbability in the colon (such as urolithin A from ellagic acid), and direct absorption into bloodstream through small intestine. Together, the wide-spectrum polyphenols resulting from the blend of the three fruits and the three points of action & absorption, explain the multiple benefits associated with this polyherbal whole food supplement. The insights will also be useful in standardizing processing and products.
Supplementary Information
Acknowledgements
Authors acknowledge Rural India Supporting Trust (RIST) for the faculty support.
Author contributions
GS involved in designing study, initiating and heading the project. GS and LDK involved in data generation. SNH involved in data analysis, interpretation and drafting manuscript. MC and NM involved in sample collection and solvent extraction protocols. GS and LDK were involved in editing the manuscript and providing inputs.
Data availability
The raw data used during the current study available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Santhosh N. Hegde and Lavanya Devi K.
Contributor Information
Lavanya Devi K, Email: lavanya@tdu.edu.in.
Gurmeet Singh, Email: gurmeet.singh@tdu.edu.in.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-80544-6.
References
- 1.Grusak, M. A. Genomics-assisted plant improvement to benefit human nutrition and health. Trends Plant Sci.4, 164–166 (1999). [DOI] [PubMed] [Google Scholar]
- 2.Minich, D. M. A review of the science of colorful, plant-based food and practical strategies for “eating the rainbow”. J. Nutr. Metab.2019, 1–19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carlsen, M. H. et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr. J.9 (2010). [DOI] [PMC free article] [PubMed]
- 4.Banudevi, S., Swaminathan, S. & Maheswari, K. U. Pleiotropic role of dietary phytochemicals in cancer: Emerging perspectives for combinational therapy. Nutr. Cancer67, 1021–1048 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Minich, D. M. & Bland, J. S. Dietary management of the metabolic syndrome beyond macronutrients. Nutr. Rev.66(8), 429–444 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Remely, M. et al. Therapeutic perspectives of epigenetically active nutrients. Br. J. Pharmacol.172, 2756–2768 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sundin, T. & Hentosh, P. InTERTesting association between telomerase, mTOR and phytochemicals. Expert Rev. Mol. Med.14, e8 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Kikuchi, H., Harata, K., Madhyastha, H. & Kuribayashi, F. Ellagic acid and its fermentative derivative urolithin A show reverse effects on the gp91-phox gene expression, resulting in opposite alterations in all-trans retinoic acid-induced superoxide generating activity of U937 cells. Biochem. Biophys. Rep.25, 100891 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayo, B., Vázquez, L. & Flórez, A. B. Equol: A bacterial metabolite from the daidzein isoflavone and its presumed beneficial health effects. Nutrients11, 2231 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bahadoran, Z., Mirmiran, P. & Azizi, F. Dietary polyphenols as potential nutraceuticals in management of diabetes: A review. J. Diabetes Metab. Disord.12, 43 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun, C. et al. Dietary polyphenols as antidiabetic agents: Advances and opportunities. Food Front.1, 18–44 (2020). [Google Scholar]
- 12.Peterson, C. T., Denniston, K. & Chopra, D. Therapeutic uses oftriphalain ayurvedic medicine. J. Altern. Complement. Med.23, 607–614 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Momin, M., Amin, A. F. & Pundarikakshudu, K. Development and evaluation of triphala formulations. Indian J. Pharm. Sci66, 427–432 (2004). [Google Scholar]
- 14.Agniveśa, C., Śarm, R. M. & Dash, B. Agniveaś’s Caraka sahita: Text with English Translation & Critical Exposition Based on Cakrapi Datta’s yurveda dpik. Chowkhamba Sanskrit Series Office (1976).
- 15.Bhishagratna, K. An English Translation of the Sushruta Samhita, Based on Original Sanskrit Text, with a Full and Comprehensive Introd. in Additional Texts, Different Readings, Notes, Comparative Views, Index, Glossary And Plates (Varanasi, India, 1963).
- 16.Vagbhata. Ashtanga Hridayam with Commentaries of Arunadatta and of Hemadri Edited by Bhisagacharya Harisastri Paradkara Vaidya, Chaukhambha Orientalia. (2005).
- 17.Alwadhi, A. Triphala: A wonder drug of Ayurveda. Int. J. Health Sci. Res.11, 314–316 (2021). [Google Scholar]
- 18.Singh Shridevi Gothe Nadana, G. Smriti Chawala Pharmacological potential and phytochemical evaluation of Emblica officinalis—A wonder herb in Ayurveda. in Chemistry, Biological Activities and Therapeutic Applications of Medicinal Plants in Ayurvedaby (eds. Amalraj, A. & Kuttappan, S.) vol. 10 (Karthik Varma Royal Society of Chemistry, Cambridge, CB40WF, UK).
- 19.Vadde, R., Radhakrishnan, S., Reddivari, L. & Vanamala, J. K. P. Triphala extract suppresses proliferation and induces apoptosis in human colon cancer stem cells via suppressing c-Myc/cyclin D1 and elevation of Bax/Bcl-2 ratio. Biomed Res. Int.2015, 1–12 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Latha, R. C. R. & Daisy, P. Therapeutic potential of octyl gallate isolated from fruits of Terminalia bellerica in streptozotocin-induced diabetic rats. Pharm. Biol.51, 798–805 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Eltimamy, M., Elshamarka, M., Aboelsaad, M., Sayed, M. & Moawad, H. Effects of alcoholic extract of Terminalia chebula dried fruit on blood biochemical profile in diabetic rats. J. Diabetes Metab. Disord.21, 159–170 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar, G. P. S., Arulselvan, P., Kumar, D. S. & Subramanian, S. P. Anti-diabetic activity of fruits of Terminalia chebula on streptozotocin induced diabetic rats. J. Health Sci.52, 283–291 (2006). [Google Scholar]
- 23.Huang, Y.-N. et al. Anti-hyperglycemic effect of chebulagic acid from the fruits of Terminalia chebula Retz. Int. J. Mol. Sci.13, 6320–6333 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shyni, G. L. et al. Chebulagic acid from Terminalia chebula enhances insulin mediated glucose uptake in 3T3-L1 adipocytes via PPARγ signaling pathway. Biofactors40, 646–657 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Shanmuganathan, S. & Angayarkanni, N. Chebulagic acid Chebulinic acid and Gallic acid, the active principles of Triphala, inhibit TNFα induced pro-angiogenic and pro-inflammatory activities in retinal capillary endothelial cells by inhibiting p38, ERK and NFkB phosphorylation. Vascul. Pharmacol.108, 23–35 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Aher, V. & Wahi, A. Immunomodulatory activity of alcohol extract of Terminalia chebula Retz combretaceae. Trop. J. Pharm. Res.10 (2011)
- 27.Yang, M. H., Ali, Z., Khan, I. A. & Khan, S. I. Anti-inflammatory activity of constituents isolated from Terminalia chebula. Nat. Prod. Commun.9, 965–968 (2014). [PubMed] [Google Scholar]
- 28.Ou, L. et al. Terminalia chebula Retz. aqueous extract inhibits the Helicobacter pylori-induced inflammatory response by regulating the inflammasome signaling and ER-stress pathway. J. Ethnopharmacol.320, 117428 (2024). [DOI] [PubMed] [Google Scholar]
- 29.Dusi, S. & Department of Pharmaceutical Chemistry, Delhi Pharmaceutical Sciences and Research University, New Delhi - 110017. Crude extract of Terminalia chebula fruit effect on Gastro intestinal disorders using different animal models. ijpbms 01, (2021).
- 30.Chatterjee, A., Chatterjee, S. & Bandyopadhyay, S. K. H. pylori-induced gastric ulcer: Pathophysiology and herbal remedy. Int. J. Biol. Med. Res.3, 1461–1465 (2012). [Google Scholar]
- 31.Mehra, D. & Vyas, N. Role of Terminalia chebula on gastrointestinal mucosa. Res. J. Pharm. Technol.5, 1183–1186 (2012). [Google Scholar]
- 32.Feng, X.-H. et al. In vivo hepatoprotective activity and the underlying mechanism of chebulinic acid from Terminalia chebula fruit. Phytomedicine83, 153479 (2021). [DOI] [PubMed] [Google Scholar]
- 33.Gupta, R. & Singh, R. Hepatoprotective activities of triphala and its constituents. Int. J. Pharma Res. Rev.4, 34–55 (2015). [Google Scholar]
- 34.Kannan, S. R. & Hopper, W. Antibacterial activity of Terminalia chebula Retz fruit extract. Afr. J. Microbiol. Res.3, 180–184 (2009). [Google Scholar]
- 35.Dodke, P. C. & Pansare, T. A. Ayurvedic and modern aspect of Terminalia chebula Retz. Haritaki an overview. Int. J. Ayurvedic Herbal Med.7, 2508–2517 (2017). [Google Scholar]
- 36.Mirunalini, S. & Krishnaveni, M. Therapeutic potential of Phyllanthus emblica (amla): the ayurvedic wonder. J. Basic Clin. Physiol. Pharmacol.21, 93–105 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Fatima, N., Usharani, P. & Muralidhar. Effects of Phyllanthus emblica extract on endothelial dysfunction and biomarkers of oxidative stress in patients with type 2 diabetes mellitus: a randomized, double-blind, controlled study. Diabetes Metab. Syndr. Obes. 275 (2013) 10.2147/dmso.s46341. [DOI] [PMC free article] [PubMed]
- 38.Barbara, G. et al. Mucosal permeability and immune activation as potential therapeutic targets of probiotics in irritable bowel syndrome. J. Clin. Gastroenterol.46, S52–S55 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Wasilewski, A., Zielińska, M., Storr, M. & Fichna, J. Beneficial effects of probiotics, prebiotics, synbiotics, and psychobiotics in inflammatory bowel disease. Inflamm. Bowel Dis.21, 1674–1682 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Basturk, A. et al. Efficacy of synbiotic, probiotic, and prebiotic treatments for irritable bowel syndrome in children: A randomized controlled trial. Turk. J. Gastroenterol.27, 439–443 (2020). [DOI] [PubMed] [Google Scholar]
- 41.Kumar, N. S., Nair, A. S., Nair, A. M. & Murali, M. Pharmacological and therapeutic effects of triphala-a literature review. J. Pharmacogn. Phytochem. JPP23, 23–27 (2016). [Google Scholar]
- 42.Gupta, H., Kumar, S., Roy, S. & Gaud, R. S. Patent protection strategies. J. Pharm. Bioallied Sci.2, 2 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kilpatrick, L. A. et al. Mo2030 neurobiology of psychological resilience in irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD) patients. Gastroenterology148, S-774 (2015). [Google Scholar]
- 44.Grundmann, O. Complementary and alternative medicines in irritable bowel syndrome: An integrative view. World J. Gastroenterol.20, 346 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lauche, R. et al. Efficacy and safety of Ayurvedic herbs in diarrhoea-predominant irritable bowel syndrome: A randomised controlled crossover trial. Complement. Ther. Med.26, 171–177 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Joos, S. et al. Use of complementary and alternative medicine in Germany—A survey of patients with inflammatory bowel disease. BMC Complement. Altern. Med.6, (2006). [DOI] [PMC free article] [PubMed]
- 47.Subbannayya, Y. et al. Global metabolite profiling and network pharmacology of triphala identifies neuromodulatory receptor proteins as potential targets. J. Proteins Proteomics9, 101–114 (2018). [Google Scholar]
- 48.Singh, S., Bala, E. K. L., Singh, D. S. S. & Kumari, E. A. Effect of processing conditions on solvent extraction of Amla (Emblica officinalis) seed oil. Pharma Innov.8, 128–131 (2019). [Google Scholar]
- 49.Glauser, G., Veyrat, N., Rochat, B., Wolfender, J.-L. & Turlings, T. C. J. Ultra-high pressure liquid chromatography–mass spectrometry for plant metabolomics: A systematic comparison of high-resolution quadrupole-time-of-flight and single stage Orbitrap mass spectrometers. J. Chromatogr. A1292, 151–159 (2013). [DOI] [PubMed] [Google Scholar]
- 50.Silva, E. et al. Untargeted metabolomics analysis by UHPLC-MS/MS of soybean plant in a compatible response to Phakopsora pachyrhizi infection. Metabolites11, 179 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Siddaiah, C. et al. Metabolite profiling of Alangium salviifolium bark using advanced LC/MS and GC/Q-TOFTechnology. Cells10, 1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shah, S. M. Z. et al. Untargeted screening of plant metabolites based on data-independent and data-dependent acquisition modes using LC-ESI-QTOF-MS: Tribulusterrestris L. as a case study. Arab. J. Chem.16, 104978 (2023). [Google Scholar]
- 53.Chen, Y., Guo, J., Xing, S., Yu, H. & Huan, T. Global-scale metabolomic profiling of human hair for simultaneous monitoring of endogenous metabolome, short- and long-term exposome. Front. Chem.9 (2021). [DOI] [PMC free article] [PubMed]
- 54.Pang, Z. et al. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res.49, W388–W396 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nicola, G., Berthold, M. R., Hedrick, M. P. & Gilson, M. K. Connecting proteins with drug-like compounds: Open source drug discovery workflows with BindingDB and KNIME. Database (Oxford)2015, bav087 (2015). [DOI] [PMC free article] [PubMed]
- 56.Djoumbou Feunang, Y. et al. ClassyFire: automated chemical classification with a comprehensive, computable taxonomy. J. Cheminform.8 (2016). [DOI] [PMC free article] [PubMed]
- 57.Neveu, V. et al. Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database (Oxford)2010, bap024–bap024 (2010). [DOI] [PMC free article] [PubMed]
- 58.Daina, A., Michielin, O. & Zoete, V. SwissTargetPrediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res.47, W357–W364 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ge, S. X., Jung, D. & Yao, R. ShinyGO: A graphical gene-set enrichment tool for animals and plants. Bioinformatics36, 2628–2629 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Avula, B., Wang, Y.-H., Wang, M., Shen, Y.-H. & Khan, I. Simultaneous determination and characterization of tannins and triterpene saponins from the fruits of various species of Terminalia and phyllantus emblica using a UHPLC-UV-MS method: Application to triphala. Planta Med.79, 181–188 (2013). [DOI] [PubMed] [Google Scholar]
- 61.Kumar, S., Singh, A. & Kumar, B. Identification and characterization of phenolics and terpenoids from ethanolic extracts of Phyllanthus species by HPLC-ESI-QTOF-MS/MS. J. Pharm. Anal.7, 214–222 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang, B., Kortesniemi, M., Liu, P., Karonen, M. & Salminen, J.-P. Analysis of hydrolyzable tannins and other phenolic compounds in emblic leafflower (Phyllanthusemblica L.) fruits by high performance liquid chromatography–electrospray ionization mass spectrometry. J. Agric. Food Chem.60, 8672–8683 (2012). [DOI] [PubMed] [Google Scholar]
- 63.Dhanani, T., Shah, S. & Kumar, S. A validated high-performance liquid chromatography method for determination of tannin-related marker constituents Gallic acid, corilagin, chebulagic acid, ellagic acid and chebulinic acid in four Terminalia species from India. J. Chromatogr. Sci.53, 625–632 (2015). [DOI] [PubMed] [Google Scholar]
- 64.Youssef, A. M. M., Maaty, D. A. M. & Al-Saraireh, Y. M. Phytochemical Analysis and Profiling of Antioxidants and Anticancer Compounds from Tephrosiapurpurea (L.) subsp. apollinea Family Fabaceae. Molecules28, 3939 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Majeed, M., Bhat, B., Jadhav, A. N., Srivastava, J. S. & Nagabhushanam, K. Ascorbic acid and tannins from emblica officinalis Gaertn. Fruits—A revisit. J. Agric. Food Chem.57, 220–225 (2009). [DOI] [PubMed] [Google Scholar]
- 66.Choi, K.-O., Lee, D. H., Park, S. J., Im, D. & Hur, Y. Y. Correlations between phenolic composition and perceived astringency of wines. Appl. Sci. (Basel)10, 8020 (2020). [Google Scholar]
- 67.Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem.44 (2020). [DOI] [PubMed]
- 68.Meher, S. K., Panda, P., Das, B., Bhuyan, G. C. & Rath, K. K. Pharmacological Profile of Terminalia chebula Retz. and Willd. (Haritaki) in Ayurveda with evidences. Res. J. Pharmacol. Pharmacodyn.10, 115 (2018). [Google Scholar]
- 69.Garg, N. & Jain, A. Therapeutic and medicinal uses of vibhitaka: A review. Asian J. Sci. Technol. 4776–4781 (2017).
- 70.Timudom, T., Chaiyasut, C., Sivamaruthi, B. S., Tiampasook, P. & Nacapunchai, D. Anti-sebum efficacy of Phyllanthus emblica L. (emblica) toner on facial skin. Appl. Sci. (Basel)10, 8193 (2020). [Google Scholar]
- 71.Na Takuathung, M., Wongnoppavich, A., Jaijoy, K., Soonthornchareonnon, N. & Sireeratawong, S. Antioxidant and antitumorigenic activities of the standardized water extract from fruit of Terminalia chebula Retz. var. chebula. Nat. Prod. Commun.18 (2023).
- 72.Suksaeree, J., Wunnakup, T. & Monton, C. Synergistic antioxidant activity of plant compositions contained in Chatuphalathika herbal recipe: Terminalia chebula Retz. var. chebula, Terminalia arjuna Wight and Arn., Terminalia bellirica (Gaertn.) Roxb., and Phyllanthus emblica L. Adv. Tradit. Med.22, 547–556 (2022).
- 73.Hazra, B., Sarkar, R., Biswas, S. & Mandal, N. Comparative study of the antioxidant and reactive oxygen species scavenging properties in the extracts of the fruits of Terminalia chebula, Terminalia belerica and Emblica officinalis. BMC Complement. Altern. Med.10 (2010). [DOI] [PMC free article] [PubMed]
- 74.Khan, A. et al. Antioxidant activity and inhibitory effect of some commonly used medicinal plants against lipid per-oxidation in mice brain. Afr. J. Tradit. Complement. Altern. Med.11, 83 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Naik, G. H. et al. In vitro antioxidant studies and free radical reactions of triphala, an ayurvedic formulation and its constituents. Phytother. Res.19, 582–586 (2005). [DOI] [PubMed] [Google Scholar]
- 76.Chen, L., Cao, H. & Xiao, J. Polyphenols. In Polyphenols: Properties, Recovery, and Applications 45–67 (Elsevier, 2018).
- 77.Swallah, M. S., Fu, H., Sun, H., Affoh, R. & Yu, H. The impact of polyphenol on general nutrient metabolism in the monogastric gastrointestinal tract. J. Food Qual.2020, 1–12 (2020). [Google Scholar]
- 78.Scalbert, A., Morand, C., Manach, C. & Rémésy, C. Absorption and metabolism of polyphenols in the gut and impact on health. Biomed. Pharmacother.56, 276–282 (2002). [DOI] [PubMed] [Google Scholar]
- 79.Ray, S. K. & Mukherjee, S. Evolving interplay between dietary polyphenols and gut Microbiota—An emerging importance in healthcare. Front. Nutr.8 (2021). [DOI] [PMC free article] [PubMed]
- 80.Manach, C., Williamson, G., Morand, C., Scalbert, A. & Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr.81, 230S-242S (2005). [DOI] [PubMed] [Google Scholar]
- 81.Aura, A.-M. Microbial metabolism of dietary phenolic compounds in the colon. Phytochem. Rev.7, 407–429 (2008). [Google Scholar]
- 82.Ma, G. & Chen, Y. Polyphenol supplementation benefits human health via gut microbiota: A systematic review via meta-analysis. J. Funct. Foods66, 103829 (2020). [Google Scholar]
- 83.Lipinski, C. A. Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov. Today Technol.1, 337–341 (2004). [DOI] [PubMed] [Google Scholar]
- 84.Veber, D. F. et al. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem.45, 2615–2623 (2002). [DOI] [PubMed] [Google Scholar]
- 85.Tripathi, D., Sunita & Bhattacharya, S. Effect of Emblica Officinalis (Amla) on blood brain barrier disruption and serum cytokines level following ischemia reperfusion injury in rats. Cerebrovascu. Dis. 44 (2017).
- 86.Youdim, K. A., Shukitt-Hale, B. & Joseph, J. A. Flavonoids and the brain: interactions at the blood–brain barrier and their physiological effects on the central nervous system. Free Radic. Biol. Med.37, 1683–1693 (2004). [DOI] [PubMed] [Google Scholar]
- 87.Takauji, Y. et al. Triphala, a formulation of traditional Ayurvedic medicine, shows protective effect against X-radiation in HeLa cells. J. Biosci.41, 569–575 (2016). [DOI] [PubMed] [Google Scholar]
- 88.Umapathy, D., Bhuvaneswarri, J., Jayamathi, G., Sadhana & Preethe Jayaraman, S. Free radical scavenging potentials of Triphala: A medicinal herb used in Indian Ayurvedic system of medicine. Drug Invention Today12, 407–411 (2019).
- 89.Babu, D., Gurumurthy, P. & Sai Cherian, K. M. Antioxidant and free radical scavenging activity of triphala determined by using different in vitro models. J. Med. Plants Res. 2898–2905 (2013).
- 90.Prasad, S. & Srivastava, S. K. Oxidative stress and cancer: Chemopreventive and therapeutic role of Triphala. Antioxidants (Basel)9, 72 (2020). [DOI] [PMC free article] [PubMed]
- 91.Chandran, U., Mehendale, N., Tillu, G. & Patwardhan, B. Network pharmacology of Ayurveda formulation triphala with special reference to anti-cancer property. Comb. Chem. High Throughput Screen.18, 846–854 (2015). [DOI] [PubMed] [Google Scholar]
- 92.Giudice, A., Arra, C. & Turco, M. C. Review of molecular mechanisms involved in the activation of the Nrf2-ARE signaling pathway by chemopreventive agents. In Methods in Molecular Biology 37–74 (Humana Press, Totowa, NJ, 2010). [DOI] [PubMed]
- 93.Occhipinti, R. & Boron, W. F. Role of carbonic anhydrases and inhibitors in acid–base physiology: Insights from mathematical modeling. Int. J. Mol. Sci.20, 3841 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sharker, M. R. et al. Molecular characterization of carbonic anhydrase II (CA II) and its potential involvement in regulating shell formation in the Pacific abalone, Haliotis discus hannai. Front. Mol. Biosci.8 (2021). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data used during the current study available from the corresponding author on reasonable request.