Abstract
The emergence of single-atom catalysts offers exciting prospects for the green production of hydrogen peroxide; however, their optimal local structure and the underlying structure–activity relationships remain unclear. Here we show trace Fe, up to 278 mg/kg and derived from microbial protein, serve as precursors to synthesize a variety of Fe single-atom catalysts containing FeN5−xOx (1 ≤ x ≤ 4) moieties through controlled pyrolysis. These moieties resemble the structural features of nonheme Fe-dependent enzymes while being effectively confined on a microbe-derived, electrically conductive carbon support, enabling high-current density electrolysis. A comparative analysis involving catalysts derived from eleven representative microbes reveals that the presence of 0.05 wt% Fe single-atom sites leads to a significant 26% increase in hydrogen peroxide selectivity. Remarkably, the optimal catalyst featuring FeN3O2 sites demonstrates a selectivity of up to 93.7% and generates hydrogen peroxide in a flow cell at an impressive rate of 29.6 mol g−1 h−1 at 200 mA cm−2. This work achieves structural fine-tuning of metal single-atom sites at the trace level and provides topological insights into single-atom catalyst design to achieve cost-efficient hydrogen peroxide production.
Subject terms: Electrocatalysis, Materials for energy and catalysis, Electrocatalysis, Electrocatalysis
Single-atom catalysts hold great potential for the electrochemical oxygen reduction reaction in hydrogen peroxide production. Here, the authors report Fe single-atom catalysts derived from microbial protein precursors, which enhance both the selectivity and activity for hydrogen peroxide production.
Introduction
Hydrogen peroxide (H2O2) is highly valuable in various chemical, pharmaceutical, and environmental applications1,2. However, the current primary method for H2O2 production relies on an energy-intensive anthraquinone-based process3. A cleaner and more electrified alternative is clearly needed to achieve sustainability4. The electrochemical synthesis of H2O2 via the two-electron (2e−) oxygen reduction reaction (ORR) provides more possibilities for decentralized onsite production, which effectively mitigates production costs, transportation expenses, and safety risks5,6.
Single-atom catalysts (SACs) are cutting-edge electrocatalysts that enable cost-effective electrochemical production of H2O2, demonstrating superior catalytic activity and target product selectivity in the 2e− ORR7–10. Despite their ultralow active site loading, SACs exhibit notable performance11. These trace single-atom sites, often found in heteroatom-doped carbon materials, form spontaneously during pyrolysis and work synergistically with nonmetallic doping sites12,13. The use of microbes as precursors facilitates the scalable gram-level synthesis of heteroatom-doped carbon materials, bridging the gap between lab-scale advancements and industrial applications14. In microbial precursors, trace amounts of iron (Fe) act as metalloprotein centres confined by protein ligands with well-defined stereo-configurations, providing an ideal framework for the formation of Fe single-atom sites. While these trace Fe single-atom sites significantly contribute to ORR performance, the optimal local structure and the underlying structure–activity relationships remain unclear15,16. This lack of information is particularly evident for the active site H2O2 production, as Fe SACs are traditionally predisposed to reducing O2 to H2O via haem sites (FeN4 moiety). However, Fe SACs possess a moderate O2 binding affinity, allowing them to readily convert between various Fe–O intermediates17–20. By fine-tuning the coordination environment of Fe SACs, the selective conversion of different ORR products can be achieved21–23. When Fe SACs mimic the distinct metal environment of superoxide dismutase and superoxide reductase, they are endowed with the ability to undergo oxidative metabolism and protect against reactive oxygen species24–27. Their nonheme configurations, characterized by distorted square pyramidal geometries and electronic asymmetry, provide the structural basis for the selective reduction of Fe–O intermediates to H2O228,29. Mimicking these natural nonheme Fe-dependent enzymes or inheriting their characteristics at the atomic level30 is essential for unlocking their full potential for sustainable H2O2 production.
Designing multiple tailored active sites, especially those that transform metalloproteins into single-atom sites, is crucial for optimizing the performance of biomass-derived carbon materials. Here, we exploit thermally induced topological transformations of microbial Fe-dependent proteins to synthesize a series of Fe active sites, comprising FeN5−xOx (1 ≤ x ≤ 4) moieties, for efficient 2e− ORR. Upon heating, structural changes occur around the Fe species within the protein. The application of heat at different temperatures affects the degree of protein dehydration, deamination, and/or decarboxylation, resulting in various coordination environments of Fe SACs. This process allows continuous modulation of the N/O coordination ratio in FeN5−xOx moieties to optimize catalyst performance while providing insights into the structure−performance relationship by evaluating the catalyst series. Notably, the optimal catalyst, featuring FeN3O2 sites, demonstrated a selectivity of up to 93.7% and produced H2O2 in a flow cell at a high rate of 29.6 mol g−1 h−1 under a current density of 200 mA cm−2. Our computational analysis further revealed the significance of axial oxygen in FeN3O2 during the 2e−ORR. This work achieves structural fine-tuning of metal single-atom sites at the trace level and provides topological insights into SAC design to achieve cost-efficient H2O2 production.
Results
Prevalence of SACs transformed from microbial metalloproteins
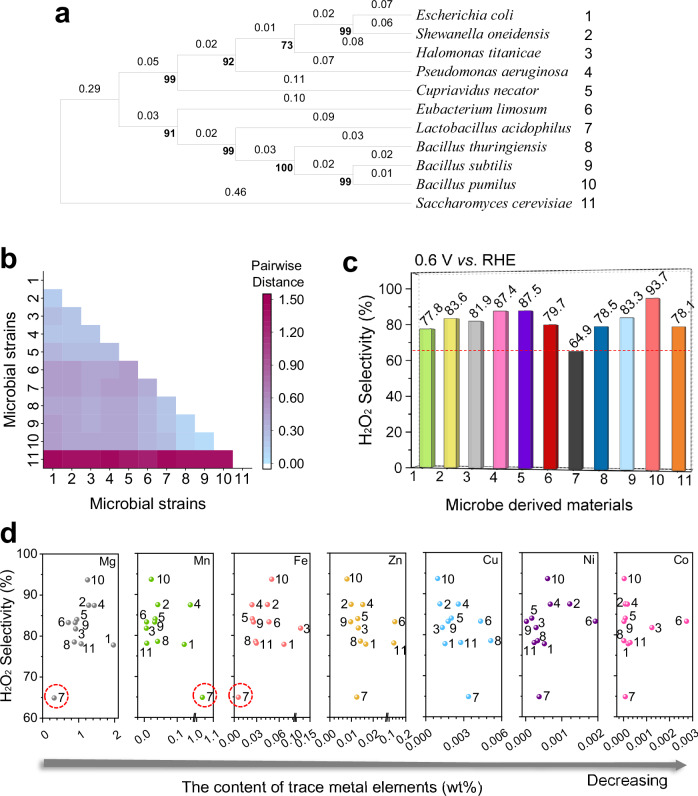
Microorganisms undergo pyrolysis to yield porous carbon-based materials that serve as effective ORR electrocatalysts. Eleven species of microorganisms commonly employed in both industrial bioproduction31 and scientific research32 were selected as pyrolysis precursors to obtain a comprehensive understanding of microbe-derived carbon materials. The microbial information and cultivation conditions are shown in Supplementary Table 1. The selected species included one eukaryotic strain and ten bacterial strains, representing diverse biological classifications, as depicted in Fig. 1a. The ten bacterial strains belong to the Proteobacteria and Firmicutes phyla, which are among the most common bacterial domains and include seven genera. Pairwise comparisons were performed to determine the genetic distances and assess the genetic diversity among these strains. The results of these comparisons are presented in Fig. 1b and Supplementary Table 2. The greater the genetic distance of pairwise comparisons is, the more significant the difference in genetic information. Among the selected microorganisms, Bacillus thuringiensis, Bacillus subtilis and Bacillus pumilus belong to the same genus, Bacillus. Escherichia coli, Shewanella oneidensis, Halomonas titanicae, Pseudomonas aeruginosa, Cupriavidus necator, Eubacterium limosum, Lactobacillus acidophilus and Saccharomyces cerevisiae are not closely related to the Bacillus genus in terms of biological affinity. Thus, these species can serve as representative examples of common microbial strains used for the preparation of microbe-derived carbon materials.
Fig. 1. Evaluation of microbe-derived electrocatalysts for H2O2 production.
a Molecular phylogenetic analysis of 16S (and 18S of Saccharomyces cerevisiae) rRNA gene sequences using the maximum likelihood method. b Genetic distances of amino acid sequences determined using the Poisson correction model. c H2O2 selectivity of various microbe-derived electrocatalysts. d, Relationships between the contents of metal elements in microbe-derived electrocatalysts and their H2O2 selectivity. The numerical ordinal numbers accompanying the strain names in (a) correspond to the microbial strains and their derived carbon materials in (b, d), respectively. During the electrochemical measurements, 85% iR compensation was applied.
In microbe-derived carbon materials, both the content and the intrinsic activity of atomically dispersed metal sites are key factors determining differences in catalytic performance in the ORR. A rotating ring-disk electrode was used to test the ORR catalytic performance, and its collection efficiency was calibrated, as shown in Supplementary Fig. 1. The selected microorganisms were pyrolysed at 600 °C, resulting in ORR catalysts that predominantly catalysed the reaction via the 2e− pathway. A summary of their catalytic performance is presented in Supplementary Fig. 2 and Fig. 1c. With the exception of the notably inferior performance of the Lactobacillus acidophilus-derived carbon material, the remaining ten materials presented half-wave potentials ranging from 0.65 to 0.67 V vs. RHE and H2O2 selectivities ranging from 77.8% to 93.7%. Among them, the Bacillus pumilus-derived carbon material exhibited the optimal ORR activity and H2O2 selectivity. Compared with the Lactobacillus acidophilus-derived carbon material, the Bacillus pumilus-derived carbon material presented a positive shift of 0.1 V in the half-wave potential, reaching 0.65 V vs. RHE, and increased selectivity by 28.8% H2O2 at 0.6 V vs. RHE, reaching 93.7%. H2O2 selectivity was correlated with the metal content to explore whether these differences in ORR performance were induced by atomically dispersed metal sites. The H2O2 selectivities at 0.6 V vs. RHE are plotted against the contents of metal elements in microbe-derived carbon materials in Fig. 1d, including magnesium (Mg), manganese (Mn), Fe, zinc (Zn), copper (Cu), nickel (Ni) and cobalt (Co). The corresponding contents of each strain are presented in Supplementary Fig. 3. Specifically, in the case of the Lactobacillus acidophilus-derived carbon material, the contents of Mg and Fe were lower, whereas the content of Mn was significantly higher. Aside from the three metal elements that exhibited distinct variations, the contents of the remaining elements closely resembled those found in other microbe-derived carbon materials.
While Mg, Fe and Mn have been identified as possible influencing elements, determining whether they produce reactive atomic sites is still crucial. According to the elemental distribution of microbe-derived carbon materials (Supplementary Figs. 4–14), Mg tended to agglomerate and form nanoparticles, whereas Mn, Fe, Zn, Cu, Ni, and Co tended to disperse atomically. A sufficient number of highly active single-atom site must be formed using metal elements to improve the ORR catalytic performance. Mn, which had a content similar to that of Fe, is more likely to form 4-electron catalytic sites33,34. The Zn content was lower than that of Fe, whereas the contents of Cu, Ni, and Co were one to two orders of magnitude lower. Therefore, compared with other metal sites, Fe single-atom sites may have a greater impact on the catalytic performance of microbe-derived carbon materials. Fe is an abundant element on Earth and a biologically essential component of nearly all living organisms. Notably, Lactobacillus acidophilus is a lactic acid bacterium with unique metabolic characteristics that do not depend on Fe35, resulting in significantly lower iron contents in both Lactobacillus acidophilus and its derived carbon material. A horizontal comparison of the eleven microbe-derived carbon materials suggested that trace amounts of Fe may impact their ORR performance.
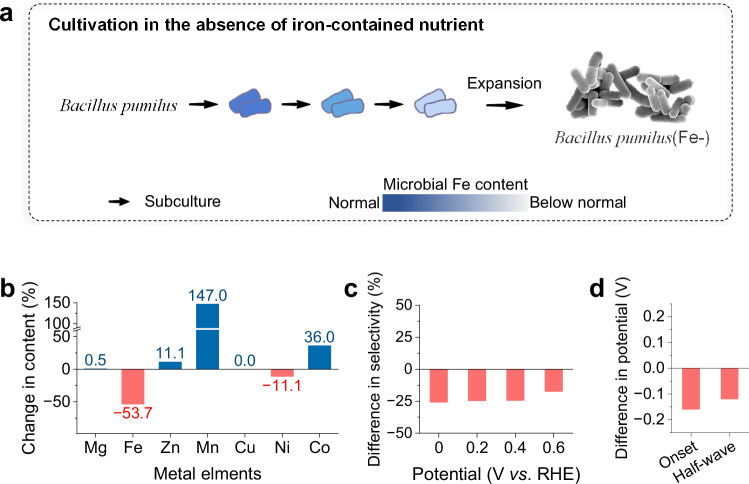
A vertical comparison of microbe-derived carbon materials was conducted to determine whether Fe-dependent proteins were transformed into active Fe sites for H2O2 production. Bacillus pumilus, which was selected for its superior catalytic performance among microbe-derived carbon materials, was investigated in detail. This robust probiotic strain, which is commonly sourced from chassis cells utilized in industrial biochemical production36, expresses a variety of Fe-dependent proteins37, including ferredoxin, cytochrome c oxidase, and Fe-dependent superoxide dismutase. The expression of these proteins is closely linked to the metabolic regulation of this microorganism, which enables the tailored cultivation of microbial strains with reduced iron‒protein contents for comparative studies. After four iterative cultures in customized iron-free media (see Supplementary Table 1), Bacillus pumilus was transformed into a mutant strain, designated Bacillus pumilus(Fe−), as shown schematically in Fig. 2a. The cellular structures of Bacillus pumilus and Bacillus pumilus(Fe−) are essentially indistinguishable under an electron microscope, as shown in Supplementary Fig. 15. However, Bacillus pumilus(Fe−) presented a markedly reduced iron content, with an approximately 72% decrease, as shown in Supplementary Fig. 16. During cultivation in the absence of iron sources, Bacillus pumilus(Fe−) compensated by assimilating other metal ions, which were used to express proteins that substitute for the deficient Fe-dependent proteins37,38. Consequently, the contents of Mg, Mn and Zn in Bacillus pumilus(Fe−) increased by 2.2, 6.6, and 0.1 times, respectively.
Fig. 2. Effects of trace Fe sites on the Bacillus pumilus-derived carbon material.
a Schematic of the preparation of microbial treatment samples of Bacillus pumilus(Fe−). b Changes in the metal content between the Bacillus pumilus- and Bacillus pumilus(Fe−)-derived carbon materials. c Differences in H2O2 selectivity between the Bacillus pumilus- and Bacillus pumilus(Fe−)-derived carbon materials. d Differences ORR potential between the Bacillus pumilus- and Bacillus pumilus(Fe−)-derived carbon materials. During the electrochemical measurements, 85% iR compensation was applied.
Bacillus pumilus(Fe−) cells were processed into the Bacillus pumilus(Fe−)-derived carbon material, which functioned as a comparative catalyst in contrast to the Bacillus pumilus-derived carbon material. Both catalysts underwent identical pyrolysis processes, leading to comparable levels of N and O doping and pore structures, as shown in Supplementary Figs. 17, 18. However, they presented distinct metal contents, consistent with the differences in the contents of microbial precursors, as depicted in Fig. 2b and Supplementary Fig. 16. Compared with the Bacillus pumilus-derived carbon material, Bacillus pumilus(Fe−)-derived carbon material resulted in a 53.7% decrease in the Fe content and a 147% increase in the Mn content. Additionally, the Mg content remained nearly unchanged, with only a marginal increase of 0.5%. This observation further corroborates that during pyrolysis, Mg tends to segregate and form MgO nanocrystallites, leading to its separation from the carbon matrix39. After assessing the corresponding difference in catalytic performance, the Bacillus pumilus(Fe−)-derived carbon material exhibited a maximum decrease in H2O2 selectivity to 59.0% at 0 V vs. RHE and a negative shift in the half-wave potential to 0.55 V vs. RHE, as presented in Fig. 2c, d and Supplementary Fig. 19. This drastic contrast illustrates that the increase of Mg does not contribute to the ORR catalytic performance. Despite the low Fe content of 0.05 wt%, the active iron sites significantly enhances H2O2 selectivity, resulting in a relate increase of at least 26%. This difference in catalytic behaviour underscores the crucial role of active Fe sites, which are pyrolytically transformed from Fe-dependent proteins, in catalysing the 2e− ORR.
Synthesis and characterization of catalysts featuring FeN5−xOx and FeN4 sites
During the pyrolysis of microbial cells to produce a porous carbon support, the atomic iron centres within the proteins are also modified to generate various atomic Fe sites. Further investigation is needed to understand the impact of their distinct coordination configurations on the performance of H2O2 electrosynthesis. Bacillus pumilus-derived carbon materials prepared through various thermal conversion processes were examined as examples, with a focus on their three-phase catalytic interfaces and the configurations of the trace Fe sites.
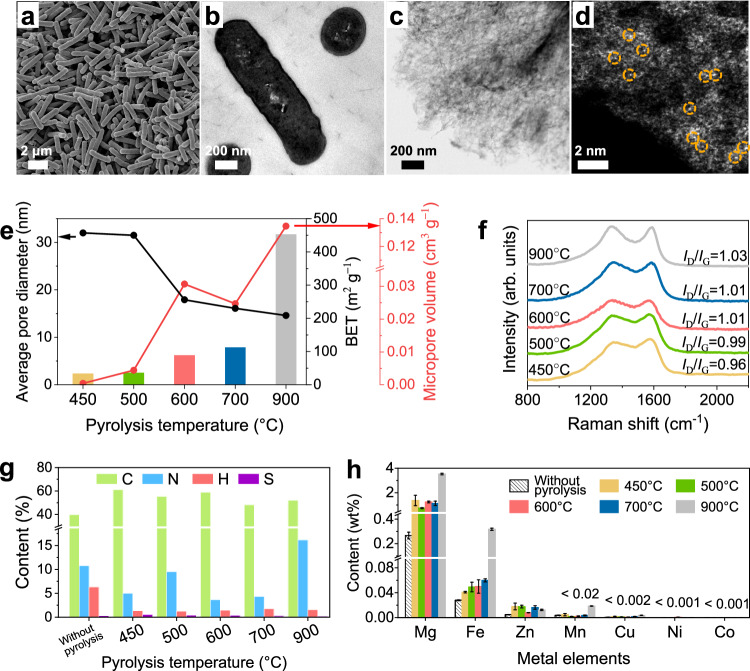
Prior to pyrolysis, the Bacillus pumilus cells, as shown in Fig. 3a, b and Supplementary Fig. 15, were approximately rods measuring 1–2 μm in length and 0.5 μm in diameter. After pyrolysis at temperatures ranging from 450 °C to 900 °C, the morphology of Bacillus pumilus cells changed significantly, condensing into porous carbon structures. The resulting Bacillus pumilus-derived carbon materials consisted of three-dimensional amorphous carbon with multilevel pore distributions. Scanning transmission electron microscopy images of the Bacillus pumilus-derived carbon material obtained at 600 °C are shown in Fig. 3c, d and Supplementary Fig. 13, whereas images obtained at 450 °C, 500 °C, 700 °C and 900 °C are provided in Supplementary Figs. 20–23. The pyrolysis temperature affected both the pore structure and the graphitic structure of the Bacillus pumilus-derived carbon materials. As the pyrolysis temperature increased, the specific surface area gradually increased from 31.7 m2 g −1 to 459.9 m2 g −1, accompanied by an increase in the distribution of micropores, as shown in Supplementary Fig. 24, Supplementary Table 1, and Fig. 3e. Moreover, the number of sp3-hybridized carbons in Bacillus pumilus-derived carbon materials increased as the intensity ratio of the D peak (ID, ~1400 cm −1) to the G peak (IG, ~1600 cm −1) in the Raman spectra increased40, as shown in Fig. 3f. The presence of multilevel pores and abundant carbon defects in Bacillus pumilus-derived carbon materials helped overcome the limitations associated with the mass diffusion of reactants and products during the ORR41,42.
Fig. 3. Characterization of catalysts featuring trace Fe sites.
a SEM image of Bacillus pumilus cells prior to pyrolysis. b TEM image of a biological slice from Bacillus pumilus cells before pyrolysis, showing various cross-sections of the cells. c Spherical aberration-corrected scanning transmission electron microscopy (STEM) image of a Bacillus pumilus-derived carbon material pyrolysed at 600 °C. d Spherical aberration-corrected high-angle annular dark field-scanning transmission electron microscopy (HAADF-STEM) image of a Bacillus pumilus-derived carbon material pyrolysed at 600 °C. e BET surface area and pore distribution of Bacillus pumilus-derived carbon materials pyrolysed at different temperatures. f Raman spectra of Bacillus pumilus-derived carbon materials pyrolysed at different temperatures. g Analysis of the nitrogen and oxygen contents of Bacillus pumilus-derived carbon materials pyrolysed at different temperatures. h Metal content of Bacillus pumilus-derived carbon materials pyrolysed at different temperatures, as measured by ICP‒MS. Error bars represent the standard deviation for three separate measurements.
In addition to their structural advantages as carriers for ORR catalysis, the catalytic performance of Bacillus pumilus-derived carbon materials fundamentally depends on the intrinsic catalytic activity of the various catalytic sites present. Trace FeN5−xOx sites and N/O doping sites synergistically contribute to the ORR catalytic performance, and their configurations are significantly affected by the pyrolysis temperature. The compositional changes in O and N in Bacillus pumilus-derived carbon materials were analysed and quantified via X-ray photoelectron spectrometer (XPS) and an elemental analyser, as depicted in Supplementary Figs. 25–26 and Fig. 3g. During the pyrolysis process, unstable organic structures are destroyed at different temperatures and undergo a series of reactions, including decomposition, reconstruction, dehydrogenation, dehydration, condensation, polymerization, oxidation, and gasification. The types of oxygen and nitrogen species progressively change with different pyrolysis temperatures43,44. Notably, the surfaces of these catalysts retained a significant amount of oxygen, resulting from the abundant oxygenated structures in microorganisms. The atomic ratio of O decreased from 19.02% to 11.35% as the temperature increased, primarily featuring C=O and O–C–O doping within the carbon layer. However, the stability of different nitrogen species varied significantly across temperature ranges, leading to more complex compositional changes45. The protein-bound nitrogen in microorganisms was initially destroyed, resulting in the formation of various nitrogen-containing products after dehydration and cleavage. The unstable nitrogen-containing products underwent secondary cracking at 500–700 °C, resulting in a significant decrease in the nitrogen content from 9.50% to 3.64%, followed by a slight increase to 4.31%. The stable nitrogen species in the catalysts were ultimately retained, primarily pyrrole nitrogen, pyridine nitrogen and graphite nitrogen. Among these species, the doping of oxygen and pyrrole nitrogen on carbon supports is crucial for the formation of heterogeneous catalytic sites and enhances their ORR performance for H2O2 production46–49.
In addition, metal elements—primarily Mg, Mn, Fe, Zn, Cu, Ni, and Co—were enriched in Bacillus pumilus-derived carbon materials. The content of Fe ranged from 0.04 wt% to 0.06 wt% in the temperature range of 450–700 °C and reached 0.32 wt% at 900 °C, as shown in Fig. 3h. This concentration was significantly lower than the typical content of active metals in SACs, which is approximately 1 wt%. Furthermore, no iron-containing crystalline structures were observed in Bacillus pumilus-derived carbon materials, as indicated by the XRD pattern in Supplementary Fig. 27. As shown in the spherical aberration-corrected high-angle annular dark field image in Fig. 3d, the trace metal atoms appeared as bright spots because their atomic mass was greater than that of N, O and C, as indicated by the yellow circles. The metal centres from the proteins remained atomically dispersed after pyrolysis, eliminating the need for any acid-leaching treatment. The combination of the low metal content, spatial confinement provided by protein ligands, and the amorphous porous carbon structure from microbe-derived carbon materials prevents the formation of agglomerations through sintering50,51.
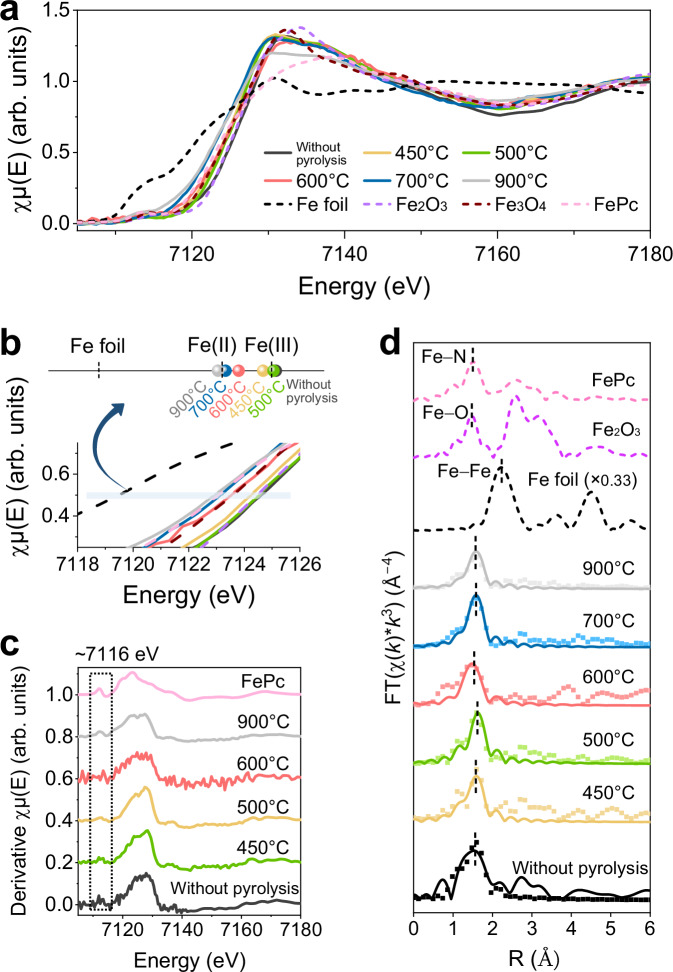
The coordination configurations of the trace Fe sites were elucidated using Fe K-edge X-ray absorption spectroscopy. The X-ray absorption near-edge structure (XANES) spectra of Fe are shown in Fig. 4a, b, with iron phthalocyanine (FePc), Fe3O4 and Fe2O3 serving as references. The valence state of the Fe species observed at 450 °C was approximately Fe(III). When the pyrolysis temperature increased to 500 °C, the Fe species were oxidized to Fe(III), and the XANES absorption edge overlapped with that of Fe2O3. The Fe species in the Bacillus pumilus-derived carbon material observed at 600 °C comprised a mixture of Fe(II) and Fe(III), similar to Fe3O4. As the pyrolysis temperature further increased, the Fe species were reduced to Fe(II), and the corresponding absorption edges shifted towards lower energies. The changes in the Fe valence state originate from various factors and are potentially influenced by the coordination structure of Fe single-atom sites52,53. The peak intensities of the Fe species pyrolysed at 450–700 °C were lower for the in-plane FeN4 structure than those of the Fe species pyrolysed at 900 °C and FePc at ~7116 eV, indicative of a diminution in the 1s–4pz shake-down transition characteristic of a square-planar configuration with high D4h symmetry54–56. This phenomenon can be attributed to the breaking of the symmetric in-plane Fe–N4 structure caused by axial coordination57,58. This property is more evident in the first derivative of the normalized XANES signals displayed in Fig. 4c, which are outlined with dashed boxes. The extended X-ray absorption fine structure (EXAFS) spectra of the Fe species, weighted by k3 and obtained after Fourier transformation from R-space data, are plotted as points in Fig. 4d. The spectra of the Fe species in Bacillus pumilus-derived carbon materials exhibited a prominent peak at ~1.6 Å (without phase correction in this study), which was assigned to Fe–N or Fe–O coordination59. The characteristic absorption peak of the Fe–Fe bond, typically located at 2.17 Å relative to the Fe foil, was not observed in the spectra of the Fe species in Bacillus pumilus-derived carbon materials. Therefore, we inferred that these Fe atoms were coordinated with N or O atoms, differing from adjacent N/O coordination conditions.
Fig. 4. Coordination analysis of Fe sites obtained at different pyrolysis temperatures.
a Normalized Fe K-edge XANES spectra of Bacillus pumilus-derived carbon materials. b The magnified corresponding near-edge region of Fe K-edge XANES spectra. c First derivative of the normalized Fe K-edge X-ray absorption spectra. d Fe K-edge EXAFS (points) and curve fit (lines) for Fe species in Bacillus pumilus-derived carbon materials shown in k3-weighted R-space after Fourier transformation; the data are not phase corrected.
The motifs of the Fe species were further analysed by matching the paths of Fe–N or Fe–O, and the corresponding fitting results are displayed as curve fits in Fig. 4d and Supplementary Figs. 28–30. The Fe atoms pyrolysed at 450–700 °C were coordinated with approximately five O or N atoms, labelled 'FeN5−xOx'. The adjacent coordination environments are proposed to be FeNO4, FeN2O3, and FeN3O2, as observed at 450 °C, 500 °C and 600 °C, respectively. When the temperature was 700 °C, the Fe sites were a mixture of FeN3O2 and FeN4O. For convenience, the Fe sites observed at 700 °C are referred to as FeN4O throughout the text. At 900 °C, the coordination number of the Fe atoms was approximately four, and the motifs were presumed to be FeN4 motifs. The corresponding fitting parameters of the FeN5−xOx and FeN4 sites are provided in Supplementary Table 3. Combining the analysis of the XANES results with DFT optimization, the predicted structural models are shown in Supplementary Fig. 31. The FeN5−xOx sites were composed of a FeN4−xOx (1 ≤ x ≤ 3) plane and an axial O ligand54,60, with the Fe atom slightly pulled out of the FeN4−xOx plane. During pyrolysis, the carbon layer surrounding the FeN5−xOx sites shrank and curled. The Fe–O bonds in the FeN5−xOx site broke down under heat. Then, the Fe atoms gradually bonded to the N atoms, and the FeN5−xOx configuration tended to be stable61. Upon reaching a pyrolysis temperature of 900 °C, the Fe atoms lost their axial O coordination and transformed into FeN4 planar structures55. As shown in Supplementary Fig. 31, at the FeN5−xOx and FeN4 sites, the degree of adjacent O coordination to Fe atoms was dynamically influenced by changes in the pyrolysis temperature.
Electrochemical ORR performance of catalysts featuring FeN5−xOx sites
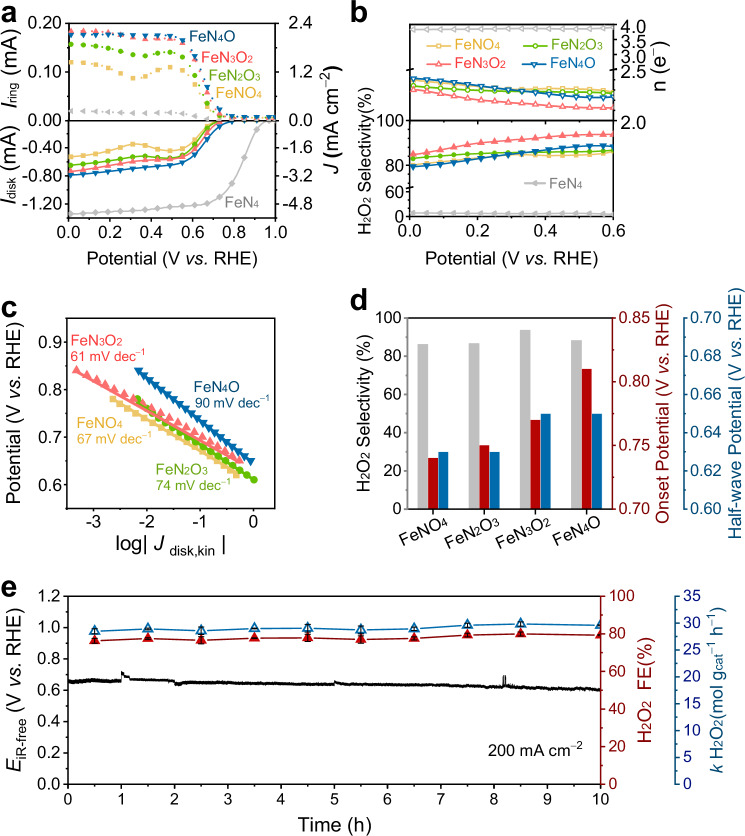
For convenience, the catalysts are labelled according to their featured Fe sites in Bacillus pumilus-derived carbon materials. The catalytic performance of these materials for the ORR was tested using a rotating ring-disk electrode, as shown in Supplementary Fig. 32, and the measured currents are summarized in the corresponding polarization curves in Fig. 5a. The FeN5−xOx catalysts tended to catalyse the ORR via the 2e− pathway, favouring H2O2 production, in sharp contrast to the FeN4 catalyst. The differences in catalytic performance were evident in the calculated results for the electron transfer number and H2O2 selectivity, as shown in Fig. 5b. In a basic electrolyte, the FeN3O2 catalyst exhibited an H2O2 selectivity of 93.7% at 0.6 V vs. RHE, with an onset potential of 0.77 V vs. RHE for H2O2 onset currents up to 0.1 mA cm−2. In a neutral electrolyte, the FeN3O2 catalyst still maintained an H2O2 selectivity of 88.8% at 0.6 V vs. RHE, as shown in Supplementary Fig. 33. Moreover, the FeN3O2 catalyst catalysed the ORR at currents up to the diffusion-limited value most rapidly, as reflected in the kinetic result of a lower Tafel slope in Fig. 5c. The catalytic performance of the FeN5−xOx catalysts was less affected by the hydrogen peroxide reduction reaction and mass loading, as shown in Supplementary Figs. 34–35. The differences in H2O2 selectivity, onset potential and half-wave potential for the FeN5−xOx catalysts are shown in Fig. 5d. Among them, the FeN3O2 catalyst exhibited efficient catalytic activity.
Fig. 5. ORR electrochemical performance of catalysts featuring FeN5−xOx sites.
a ORR polarization curve measured by a rotating ring-disk electrode at 1600 rpm in O2-saturated 0.1 M KOH. The disk electrode was used to measure oxygen reduction currents (solid lines), and the platinum ring electrode was used to measure H2O2 oxidation currents (dashed lines). The absolute mass loading of FeN5−xOx catalysts on the electrode was 0.1 mg cm−2, except for that of FeN4 catalyst, which was 0.4 mg cm−2. b Calculated ORR electron transfer number and H2O2 selectivity of FeN5−xOx catalysts at 0–0.6 V vs. RHE. c Tafel plots derived from linear sweep voltammetry curves. d Differences in H2O2 selectivity, the onset potential and half-wave potential in FeN5−xOx catalysts. e Chronopotentiometry test of FeN3O2 catalyst at 200 mA cm−2 in the flow cell. The interface impedance of the flow cell was measured to be 0.4 Ω. The absolute mass loading of FeN3O2 catalyst on the gas-diffusion electrode remained at 0.1 mg cm−2, while the electrolyte was 1 M KOH cycled at a rate of 0.01 L min−1 and was replaced every hour. An 85% iR compensation was applied for rotating ring-disk electrode tests, but not for flow cell tests.
Electrochemical H2O2 production was further evaluated in a flow cell using a gas-diffusion electrode to address the O2 mass transport issue and test the system at higher current densities. The FeN3O2 catalyst catalysed the ORR and steadily achieved an industrially relevant current density, as shown in Supplementary Fig. 37 and Fig. 5e. However, catalyst stability, arising from micropore flooding, demetallation and carbon oxidation62,63, remains an enormous challenge in Fe SACs. These problems were exacerbated during Fe SAC-catalysed H2O2 production, resulting in a steep decrease in flow cell performance at the beginning of the test. During the chronopotentiometry test, the cathode potential of the FeN3O2 catalyst changed at a rate of only 5 mV h−1. After 10 h, the FeN3O2 catalyst maintained a Faradaic efficiency for H2O2 production of 79.3% at 200 mA cm−2 without any loss of catalytic performance. Moreover, the FeN3O2 catalyst exhibited a high rate of H2O2 production, up to 29.6 mol g−1 h−1, which exceeded the previously reported values64. The microstructure of the FeN3O2 catalyst layer was favourable for oxygen transport, as it mitigated the unexpected local oxygen transport resistance65,66. In addition to the porous carbon support, the high yield of H2O2 can be attributed to the high activity of the FeN3O2 sites, which have a coordination structure similar to that of the Fe centre in superoxide dismutase, converting superoxide radicals into H2O2 at a nearly diffusion-controlled rate30. The multiple active sites and multilevel pores ensured consistent and reliable H2O2 production over an extended period, with the production of 1 L of H2O2 (3 wt%) requiring only 0.035 kWh of electricity. Considering its selectivity, activity, efficiency, and stability, the FeN3O2 catalyst can be employed as a cost-effective catalyst for the industrial electrosynthesis of H2O2.
DFT calculations
DFT calculations were performed to gain atomic-level insights into the mechanism and further clarify the effect of adjacent O-coordinating dopants on the ORR catalytic performance of FeN5−xOx sites. In both the 4e−and 2e−catalytic pathways, the active site first activates O2, forming the key intermediate *OOH. The binding strength of the catalytic site to *OOH determines the activity and product selectivity of the ORR. In 0.1 M KOH, where H2O2 deprotonates to HO2− at pH > 11.6, the ORR catalytic process of FeN5−xOx sites via the 2e−pathway can be divided into two steps as follows:
| 1 |
| 2 |
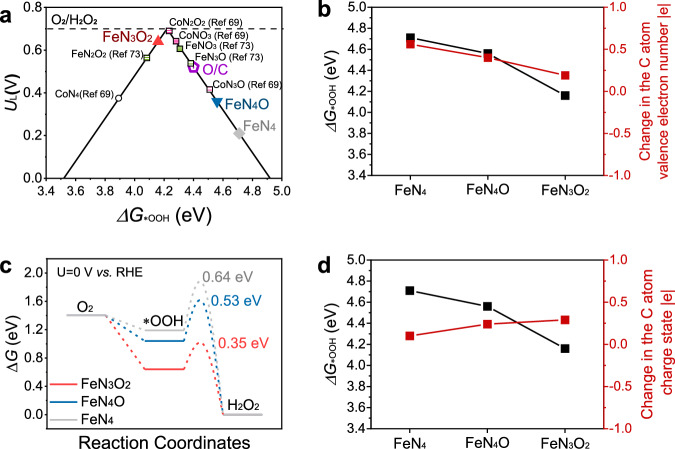
Before constructing the computational model, we determined the catalytic sites by performing potassium thiocyanate poisoning experiments67. After the Fe atoms were complexed with cyanate, the ORR catalytic currents of FeN5−xOx remained almost unaffected (Supplementary Fig. 39). The catalytic sites of FeN5−xOx motif were hypothesized to be the C atom adjacent to the axial O68,69, labelled the 'O-adjacent C atom', rather than the Fe atom. For comparison, the N-adjacent C atom was chosen as the catalytic site in the calculations for the FeN4 motif. Sketches of the structural models and catalytic sites of FeN3O2, FeN4O, FeN4, and metal-free oxygen-doped carbon (labelled 'O/C') are shown in Supplementary Figs. 40–44. Using *OOH as the intermediate in the reaction of O2 to H2O2, volcano plots for FeN3O2, FeN4O, FeN4, and the O/C site were constructed based on the Gibbs free energy of *OOH adsorption (ΔG*OOH) and the thermodynamic ultimate potential (UL), as shown in Fig. 6a. The volcano plot of the ideal 2e−pathway was located at the apex (ΔG*OOH = 4.2 eV), where the experimental equilibrium potential for H2O2 formation was 0.68 V vs. RHE, with no further potential correction for pH-dependent product changes in this work.
Fig. 6. Elucidation of the mechanism of efficient ORR catalysis by the FeN5−xOx sites for H2O2 production.
a Volcano plots of the computed activity of electrochemical H2O2 production via the 2e− ORR. The horizontal dash line indicates the equilibrium potential of O2/H2O2. Except for the FeN3O2, FeN4O, FeN4, and O/C sites, the sites labelled in black were adapted from the references. b Calculated *OOH adsorption energies and charges of the valence electron number of the C atom in the FeN3O2, FeN4O, and FeN4 sites. c Free energy diagram for the 2e− ORR of the FeN3O2, FeN4O, and FeN4 sites. The annotation values represent the computed kinetic barriers for *OOH to H2O2. d Calculated *OOH adsorption energies and changes in the C atom charge state at the FeN3O2, FeN4O, and FeN4 sites.
The FeN3O2 site exhibited a ΔG*OOH peak at 4.16 eV, close to the volcano plot apex, indicating impressive activity for the 2e− ORR pathway. In contrast, the FeN4 site exhibited significantly weak *OOH adsorption and had a UL of less than 0.3 V, indicating that its N-adjacent-C site was inactive in the ORR. The reactive site in FeN4 was found to be the iron atom, which tended to bind strongly to *OOH and cleave the O–O bond, leading to the production of H2O via the 4e− pathway. Doping of the O atom, an electron-withdrawing group, into the FeN5−xOx sites, induced more electron depletion from the Fe atoms64,70,71. In the FeN3O2 motif (Supplementary Figs. 45–46), the valence electron of the Fe atom decreased by 1.07|e|, whereas that of the O-adjacent C atom increased by 0.19 |e|. This modulation of the charge distribution was achieved through O bridging between the Fe and C atoms, primarily through the axial O. As a result, *OOH was induced to bind the O-adjacent C atom in the FeN5−xOx sites72, adjusting the ΔG*OOH to the optimal value for the 2e− pathway, as depicted in Fig. 6b. The migration of the catalytic site from the Fe atom to the O-adjacent C atom was essential for shifting the ORR catalytic pathway68,69,73. This migration phenomenon has also been observed in four-coordinate Fe SACs73 and Co SACs69, which are labelled in black in Fig. 6a. Proper doping of adjacent O coordination sites in planar FeN4−xOx (1 ≤ x ≤ 3) and CoN4−xO (1 ≤ x ≤ 3) sites improved the ORR performance for H2O2 production. Compared with the FeN2O2 site, the FeN3O2 site utilized axial O ligand coordination and exhibited better catalytic activity for the 2e− ORR pathway. The uneven distribution of the electronic structure at the FeN3O2 site substantially enhanced the electrocatalytic performance of the Fe SACs74,75.
The processes of *O2 protonation and *OOH desorption were analysed to further elucidate the high selectivity of the FeN5−xOx sites towards H2O2. Catalysed by the FeN3O2 site, the desorption process was the rate-limiting step (equation (2)) during the 2e− ORR pathway, consistent with the low Tafel slope observed in Fig. 5c and the discussion in Fig. 6a, b. The transition state energy for *OOH desorption was calculated, and the results are depicted in the reaction coordinates of Fig. 6c. FeN3O2 site exhibited a low catalytic energy barrier of 0.35 eV for *OOH desorption to HO2−, which was lower than the barriers of 0.53 eV and 0.64 eV observed for FeN4O and FeN4 site, respectively. The lower catalytic energy barrier of FeN5−xOx sites preserved the O–O bonds, thereby facilitating the desorption of *OOH76. Moreover, the strong binding affinity between the reactive sites and *OOH was reflected by the large number of electrons transferred during bonding. As shown in Fig. 6d, the charge state of the O-adjacent C atom in FeN3O2 site increased by 0.29 |e|. The charge states of the O-adjacent C atom in FeN4O site and the N-adjacent C atom in FeN4 site increased by 0.24 |e| and 0.10 |e|, respectively. Charge donation from the C atom to *OOH increased through O bridging, enhancing the binding strength between the C atom and *OOH. In summary, the adjacent O-coordinated dopant of FeN3O2 site enhanced its overall catalytic performance, promoting the efficient binding of the O-adjacent C atom to *OOH and catalysing its desorption with lower energy barriers.
Discussion
The FeN3O2 catalyst, as a representative of FeN5−xOx catalysts, demonstrates notable performance for H2O2 electrosynthesis; it achieves a high H2O2 selectivity of 93.7% and a remarkable yield of 29.6 mol g−1 h−1 at 200 mA cm−2 in a flow cell. These findings indicate that precisely regulating heteroatomic ligands within microbial Fe-dependent proteins is a sustainable, cost-effective approach for the production of Fe SACs. Adjacent O coordination modulates the charge distribution of FeN5−xOx sites, causing the reactive sites to migrate from Fe atoms to O-adjacent C atoms. When catalysed by FeN3O2 sites, the O-adjacent C atom effectively binds the intermediate *OOH, enabling notable H2O2 production. In addition to adjacent O coordination, the axial O ligands in the diverse coordinated configurations of FeN5−xOx sites provide steric hindrance, preventing excessive binding of *OOH to Fe atoms and thereby enhancing the ORR performance for H2O2 production. This research elucidates the influence of trace dopants, particularly metal elements, on biomass-derived carbon materials. By employing a rational design for the coordinated configurations of atomically active metal sites, highly active sites can enhance the catalytic performance of biomass-derived carbon materials under milder conditions. Moreover, this approach reduces the risks associated with accidents and eliminates the need for hazardous chemicals.
Methods
Chemicals and materials
Tryptone, yeast extract and beef extract (desiccant) were of FMB grade and were purchased from Sangon Biotech Co., Ltd. (Shanghai, China). Sodium chloride (NaCl), glucose, potassium dihydrogen phosphate, dipotassium hydrogen phosphate, potassium ferricyanide, diammonium citrate, sodium acetate, magnesium sulphate, manganese(II) chloride, polysorbate-80, ammonium sulphate, potassium chloride, tri(hydroxymethyl) amino methane hydrochloride (pH 7.5), calcium chloride, manganese sulphate, glutamate, potassium titanium oxide oxalate dehydrate (C4K2O9Ti·2H2O), urea, and isopropyl alcohol (99.9%), all of analytical grade, were purchased from Sinopharm Chemical Reagent Co., Ltd (Beijing, China). Potassium hydroxide (KOH, >95%), of excellent grade, was purchased from Sinopharm Chemical Reagent Co., Ltd (Beijing, China). Nafion solution (D520) at a concentration of 5 wt% was purchased from DuPont de Nemours, Inc (USA). All reagents were used without further purification. Ultrapure water (18.2 MΩ cm−1) generated with a Milli-Q system (Nihon Millipore Ltd., Japan) was used throughout the experiment. The culture conditions for Bacillus pumilus (No. 17816, China General Microbiological Culture Collection Center), Bacillus pumilus(Fe−) and other microorganisms are provided in Supplementary Table 1. Live cells were collected by centrifugation at 3500 × g for 10 min (Centrifuge 4530, Eppendorf, Germany). The collected cells were resuspended in a NaCl solution (0.09 wt%) three times to remove the residual medium on the cell surface. The cell pellets were freeze-dried to obtain the dried bacterial powder.
Synthesis of the ORR electrocatalysts
ORR electrocatalysts were synthesized by pyrolysing microbial cells at various temperatures. The pyrolysis precursor consisted of dried bacterial powder and urea that were mixed by milling in an agate mortar. The precursor dosages and pyrolysis temperatures used are shown in Table 1. The addition of urea improved the degree of nitrogen doping in the electrocatalysts. The tube furnace (SKGL-1200, Shanghai Jvjing Precision Instrument Manufacturing Co., Ltd., China) was programmed with a temperature ramp of 3 °C min−1 and maintained at the target temperature for 60 min in an argon atmosphere. After natural cooling, the carbon materials were collected and used as electrocatalysts.
Table 1.
Pyrolysis precursors and temperatures for electrocatalyst synthesis
| Sample | Pyrolysis temperature (°C) | Bacteria dry powder to urea ratio (g:g) |
|---|---|---|
| FeNO4 | 450 | 0.1: 0.4 |
| FeN2O3 | 500 | 0.1: 0.4 |
| FeN3O2 | 600 | 0.1: 0.5 |
| FeN4O | 700 | 0.1: 0.5 |
| FeN4 | 900 | 0.1: 0.5 |
| FeN5−xOx(Fe−) | 600 | 0.1: 0.5 |
Characterization of electrocatalysts
The morphology of the electrocatalysts was examined using a field-emission scanning electron microscope (s4800, Hitachi, Japan) and a transmission electron microscope (Talos F200i S/TEM, Thermo Fisher, USA). Metal atoms were captured using an aberration-corrected TEM (Titan G2 Cube 60–300 kV, Thermo Fisher, USA) operating at an accelerating voltage of 200 kV. Energy-dispersive X-ray (EDX) spectroscopy was performed using an Oxford IE250 system (Oxford Instruments, UK). An X-ray photoelectron spectrometer (Axis Supra, Shimadzu, Japan) was used to determine the surface composition of the electrocatalysts. The X-ray diffraction (XRD) analysis was performed using an X’Pert Pro diffractometer (PANalytical, the Netherlands) to characterize the electrocatalysts. Microporous physical and chemical adsorption measurements were conducted using an ASAP 2020 M + C instrument (Micromeritics, USA). Raman spectra were recorded with a laser confocal Raman spectrometer (LabRAM, Horiba Jobin Yvon S.A.S.) using a 532 nm laser source. The elemental composition of C, N, and O in each sample was determined using an elemental analyser (Vario EL cube, Elementar, Germany). Concurrently, the concentrations of major metal content were quantified via inductively coupled plasma–mass spectrometer (ICP‒MS 7700, Agilent, USA).
X-ray absorption spectroscopy (XAS) experiments were conducted at the iron K-edge (7112 eV) using the 1W1B-XAFS beamline at the Beijing Synchrotron Radiation Facility with a double-crystal monochromator. FeN5−xOx and FeN4 data were acquired in fluorescence mode by a Lytle detector at room temperature, while reference data were collected in transmission mode. Each sample was mixed with a binder of polyvinylidene fluoride and crushed into a disk (13 mm diameter, ~1 mm thick). The E0 value of XANES was corrected via energy calibration of the Fe foil.
X-ray absorption spectra were analysed using the Demeter software package77. The 3-power weighted χ(k) data were Fourier transformed after applying a K window function (Δk = 3.5–12.1). During the fitting procedure, only the adjacent coordination shell was fitted. The coordination numbers (CNs), interatomic distances (Reff), and edge-energy shifts (ΔE0) were obtained by nonlinear fitting, with least-squares refinement, of the EXAFS equation to the Fourier-transformed data in R-space. The amplitude reduction factor (S02) was set to 0.916, which was based on the value fitted from the spectra of the Fe foil. The Debye–Waller factor (σ2) was fixed, and a single ΔE0 value was applied uniformly for each sample.
Electrochemical measurements
The assessment of the FeN5−xOx catalysts’ catalytic performance for the ORR was evaluated in a custom three-electrode cell setup, as illustrated in Supplementary Fig. 36, utilizing a commercial bi-potentiostat device (CHI 760E, Shanghai) under ambient conditions (25 °C and atmospheric pressure). A rotating ring-disk electrode (RRDE) from PINE Research Instrumentation was served as the working electrode. The disk electrode consisted of a glassy carbon disk with a geometric area of 0.2475 cm2 (diameter = 5.61 mm), and the associated platinum ring electrode featured inner and external diameters of 6.25 mm and 7.92 mm, respectively. The collection number (N) of the RRDE was calibrated to 34.1% using a potassium ferricyanide solution, as detailed in Supplementary Fig. 1. A graphite rod functioned as the counter electrode, while le the reference electrode was selected based on the electrolyte, either a Hg|HgO electrode in 0.1 M KOH solution (pH = 13 ± 0.1) or a saturated calomel electrode in 0.1 M PBS solution (pH = 7.2 ± 0.1). The electrolytes were prepared in advance of the electrochemical tests, with a continuous flow of N2 or O2 flow to achieve N2- or O2-saturated solutions.
For the RRDE, the catalyst ink was formulated by dispersing 5 mg of catalyst powder into a solution comprising 800 μL of isopropanol, 170 μL of water, and 30 μL of Nafion. The mixture underwent ultrasonication for over an hour to ensure uniform dispersion and was then applied to prepare the working electrode. Either 5 or 20 μL of the catalyst ink was drop-coated onto the disk electrode to achieve mass loading of 0.1 or 0.4 mg cm−2, respectively. The electrode was subsequently dehydrated under an infra-red heat source, ready for subsequent electrochemical analysis.
The H2O2 productivity and durability of the FeN3O2 catalyst were assessed in a flow-through cell, with the schematic representation provided in Supplementary Fig. 36. The tests were also monitored using a CHI 760e electrochemical workstation with a C211035 current amplifier under room temperature (20 °C) and pressure. A gas diffusion electrode (2 cm2 in area, YSL-30T, Sinerosz Co., Ltd.), nickel foam (3 cm2 in area and 0.1 mm thick, 99.95% purity), and an Ag/AgCl electrode functioned as the cathode, anode, and reference electrode, respectively. The anolyte and catholyte were both 1 M KOH (50 mL each, with a pH of 14 ± 0.1), segregated by an anion exchange membrane (4.2 cm2 in area, Fumasep FAB-PK-130, Sinerosz Co., Ltd.). The electrolyte flow rate through the chambers was set at 10 mL min−1. Oxygen was passed through the cathodic microporous layer in the gas channel at a flow rate of 50 mL min−1. The anion exchange membrane was pre-treated by soaking in a 1 M KOH solution at 60 °C for 3 hours, followed by storage at room temperature for 24 h. To ensure a leak-proof setup, silicone gaskets were employed to seal the cell. The working area of the cathode and anode was 1 cm2, and the interface impedance of the flow cell was measured to be 0.4 Ω.
For the gas diffusion electrode, the catalyst ink was prepared by mixing 5 mg of catalyst powder with the solution of 4 mL isopropanol and 1 mL water, sonicated for 1 h to ensure homogeneity. Nafion (100 μL) was then incorporated into the mixture, maintaining a 1:1 weight ratio between the ionomer and the catalyst. After stirring for 12 hours, the catalyst ink was drop-coated onto the cathodic microporous layer, with a catalyst loading of 0.1 mg cm−2. The coated gas diffusion electrode was air-dried before being immersed in a 1 M KOH solution for 24 hours. Throughout this soaking process, the H+-form Nafion was exchanged to the K+-form Nafion, preventing acid‒base reactions between H+-form Nafion and OH− during the ORR process.
The tested electrode potentials refer to the reversible hydrogen electrode (RHE) according to Equation (3):
| 3 |
ΔE represents the difference in potential between the reference electrode and the RHE. The reference electrode was calibrated to a RHE in the same electrolyte before each test. For the Hg|HgO, saturated calomel and Ag|AgCl reference electrodes, the values of ΔE were 0.098 V, 0.241 V and 0.197 V, respectively.
ORR polarization curves of RRDE were recorded using linear sweep voltammetry, ranging from 0 to 1.0 V vs. RHE at 1600 r.p.m. and a scan rate of 10 mV s−1. The measured data were adjusted to account for solution ohmic losses, with the impedance spectrum resistance measured by the PINE system and automatically compensated by 85%. The current response in the O2-saturated electrolyte was background-corrected by subtracting the current from the N2-saturated electrolyte, which accounts for the capacitive current. Identical test parameters were maintained for both measurements. The H2O2 selectivity and electron transfer number were calculated using the current from disk electrode (Idisk) and ring electrode (Iring) based on Equations (4) and (5):
| 4 |
| 5 |
The Tafel slope was calculated from the Tafel equation (6) based on the kinetic current densities calculated from the Koutecky‒Levich equation (7):
| 6 |
| 7 |
Jdisk,kin is the kinetic current density (A cm−2), whereas Jdisk is the measured disk current density, and Jdisk,kin is the diffusion-limited current density, which was obtained by assuming a disk current with no diffusion limitation at 0.2 V vs. RHE as the limit value.
The H2O2 production efficiency and stability of the FeN3O2 catalyst were assessed using polarization and current-time curves in a flow-through cell. Chronopotentiometry was conducted at a constant current density of 200 mA cm−2, with the catalyst’s stability monitored by observing potential changes over time. A holding time of 3600 s was implemented during the measurements to ensure a steady-state current density. To prevent product accumulation that could hinder the reaction, the electrolyte was refreshed periodically at consistent intervals. The concentration of H2O2 produced in the cathodic electrolyte was determined using a spectrophotometric titration method. Titanium(IV) sulphate displayed a maximum absorbance at 398 nm, measured with a visible spectrophotometer (GENESYS 30, Thermo Fisher). The corresponding calibration curve, which relates its absorbance to known H2O2 concentrations, is presented in Supplementary Fig. 38. The TiOSO4 titrant was prepared by fully dissolving 0.1 M C4K2O9Ti·2H2O in 50% v/v H2SO4, then stored in a sealed container at room temperature. Both the standard H2O2 solutions and test samples underwent the same titration procedures. To ensure test accuracy, aliquots of catholyte were collected and diluted to the concentration between 3 to 12 mM. A 0.2 mL portion of diluted catholyte was mixed with 0.8 mL of titrant by vortexing. During the reaction, colourless tetravalent titanium ions were reduced by H2O2 to trivalent titanium ions, producing the characteristic yellow solution.
The H2O2 faradaic efficiency (FEH2O2) and electricity consumption (kWh kg−1 for 3 wt% H2O2) were calculated using Equations (8, 9):
| 9 |
| 10 |
where is the concentration of produced H2O2 (mol L−1), V is the volume of electrolyte (L), F is the Faraday constant (96485 C mol−1), Q is the amount of passed charge (C), and I and E represent the applied current (A) and cell potential (V), respectively. MH2O2 is the molecular weight of H2O2 (34 g mol−1).
Computational method
Density functional theory (DFT) calculations were carried out using the Vienna Ab initio Simulation Package (VASP)78. The projector augmented wave (PAW) method was adopted79. Transition states (TS) were identified through a constrained optimization approach80. The exchange-correlation energy was calculated with the generalized gradient approximation (GGA), based on the Perdew–Burke–Emzerhof (PBE) functional81. The plane-wave basis set had an energy cut-off fixed at 400 eV. Partial occupancies of Kohn–Sham orbitals were managed using the Gaussian smearing scheme with a width of 0.2 eV. A Monkhorst–Pack k-point grid of 2 × 2 × 1 was employed to sample the Brillouin zone. This grid was used for FeN3O2, FeN4O, FeN4 and O/C moieties. Self-consistent calculations used a convergence threshold of 10−4 eV for energy. The force convergence limit was set to 0.05 eV Å−1.
The following free energy corrections were considered at 298 K:
| 11 |
where ΔE, ΔGZPE, ΔGU, and ΔS refer to the DFT-calculated energy change, the correction from the zero-point energy, the correction from the inner energy, and the correction from the entropy, respectively.
The solvent effect was evaluated to account for stabilization of adsorbates through the hydrogen bonding network in water, which was computed using VASPsol. An intermediate (*OOH) stabilization energy of −0.20 eV was incorporated.
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant numbers 22025603, F.Z. 22236007, F.Z. and 42021005, F.Z.). We acknowledge the support from the SSRF (beamline 11B) and BSRF (beamline 1W1B) for the allocation of synchrotron beamtime. We thank Prof. Maozi Lin of Fujian Polytechnic Normal University for providing the Bacillus pumilus strains.
Author contributions
X.-F.X. performed the experiments, collected and analysed the data, and composed the manuscript. Z.-C.Z. conceived the idea, designed the experiments, performed the XAFS measurements, analysed the data, and revised the manuscript. S.-H.Y. helped test the flow cell and analysed the catalytic performance. Z.-J.X. and T.G. performed the XAFS data analysis. R.-H.Y. and J.-S.W helped test and analyse the AC-STEM results. X.-C.T. helped revise the manuscript. Y.-X.J. provided the experimental instruments for the electrochemical tests. D.-S.W. and F.Z. designed the research projects and experiments, performed the integrated analyses, and revised the manuscript. All the authors commented on the manuscript and approved the final version of the manuscript.
Peer review
Peer review information
Nature Communications thanks Shichao Ding and Xie Quan for their contribution to the peer review of this work. A peer review file is available.
Data availability
The data that substantiate the study’s findings and contribute to the assessment of the paper’s conclusions can be accessed within the paper and its Supplementary Information. All other relevant data supporting the findings of this study are available from the corresponding authors upon request. Source data file has been deposited in Figshare, 10.6084/m9.figshare.27653034. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xiaofeng Xiao, Zechao Zhuang.
Contributor Information
Dingsheng Wang, Email: wangdingsheng@mail.tsinghua.edu.cn.
Feng Zhao, Email: fzhao@iue.ac.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-55041-z.
References
- 1.Ciriminna, R., Albanese, L., Meneguzzo, F. & Pagliaro, M. Hydrogen peroxide: a key chemical for today’s sustainable development. ChemSusChem9, 3374–3381 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Tang, J. et al. Selective hydrogen peroxide conversion tailored by surface, interface, and device engineering. Joule5, 1432–1461 (2021). [Google Scholar]
- 3.Campos‐Martin, J. M., Blanco‐Brieva, G. & Fierro, J. L. Hydrogen peroxide synthesis: an outlook beyond the anthraquinone process. Angew. Chem. Int. Ed.45, 6962–6984 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Wang, Y., Zheng, X. & Wang, D. Design concept for electrocatalysts. Nano Res.15, 1730–1752 (2022). [Google Scholar]
- 5.Siahrostami, S. et al. Enabling direct H2O2 production through rational electrocatalyst design. Nat. Mater.12, 1137–1143 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Jiang, Y. et al. Selective electrochemical H2O2 production through two-electron oxygen electrochemistry. Adv. Energy Mater.8, 1801909 (2018). [Google Scholar]
- 7.Gan, T. & Wang, D. Atomically dispersed materials: Ideal catalysts in atomic era. Nano Res. 17, 18–38 (2023).
- 8.Jung, E. et al. Atomic-level tuning of Co–N–C catalyst for high-performance electrochemical H2O2 production. Nat. Mater.19, 436–442 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Chen, J. et al. Kinetically restrained oxygen reduction to hydrogen peroxide with nearly 100% selectivity. Nat. Commun.13, 2808 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu, S. et al. High activity and selectivity of single palladium atom for oxygen hydrogenation to H2O2. Nat. Commun.13, 4737 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.An, Z. et al. Highly active, ultra-low loading single-atom iron catalysts for catalytic transfer hydrogenation. Nat. Commun.14, 6666 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dessalle, A., Quílez-Bermejo, J., Fierro, V., Xu, F. & Celzard, A. Recent progress in the development of efficient biomass-based ORR electrocatalysts. Carbon203, 237–260 (2023). [Google Scholar]
- 13.Borghei, M., Lehtonen, J., Liu, L. & Rojas, O. J. Advanced biomass-derived electrocatalysts for the oxygen reduction reaction. Adv. Mater.30, 1703691 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Tiwari, J. N. et al. Multi-heteroatom-doped carbon from waste-yeast biomass for sustained water splitting. Nat. Sustain.3, 556–563 (2020). [Google Scholar]
- 15.Wang, L., Ambrosi, A. & Pumera, M. “Metal-free” catalytic oxygen reduction reaction on heteroatom-doped graphene is caused by trace metal impurities. Angew. Chem.125, 14063–14066 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Liu, J. et al. High-performance oxygen reduction electrocatalysts based on cheap carbon black, nitrogen, and trace iron. Adv. Mater.25, 6879–6883 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Pan, Y. et al. Regulating the coordination structure of single-atom Fe–NxCy catalytic sites for benzene oxidation. Nat. Commun.10, 4290 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun, K., Shan, H., Neumann, H., Lu, G.-P. & Beller, M. Efficient iron single-atom catalysts for selective ammoxidation of alcohols to nitriles. Nat. Commun.13, 1848 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu, H. et al. Adjacent single-atom irons boosting molecular oxygen activation on MnO2. Nat. Commun.12, 5422 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang, L., Chen, J., Gan, L., Wang, J. & Dong, S. Single-atom nanozymes. Sci. Adv.5, eaav5490 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long, X. et al. Graphitic phosphorus coordinated single Fe atoms for hydrogenative transformations. Nat. Commun.11, 4074 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qin, J. et al. An enzyme-mimic single Fe–N3 atom catalyst for the oxidative synthesis of nitriles via C–C bond cleavage strategy. Sci. Adv. 8, eadd1267 (2022). [DOI] [PMC free article] [PubMed]
- 23.Li, R. & Wang, D. Understanding the structure-performance relationship of active sites at atomic scale. Nano Res.15, 6888–6923 (2022). [Google Scholar]
- 24.Wodrich, M. D. & Hu, X. Natural inspirations for metal–ligand cooperative catalysis. Nat. Rev. Chem.2, 0099 (2017). [Google Scholar]
- 25.Wang, A., Li, J. & Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem.2, 65–81 (2018). [Google Scholar]
- 26.Huang, X. & Groves, J. T. Oxygen activation and radical transformations in heme proteins and metalloporphyrins. Chem. Rev.118, 2491–2553 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bullock, R. M. et al. Using nature’s blueprint to expand catalysis with Earth-abundant metals. Science369, eabc3183 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whittaker, J. W. & Solomon, E. I. Spectroscopic studies on ferrous nonheme iron active sites: magnetic circular dichroism of mononuclear iron sites in superoxide dismutase and lipoxygenase. J. Am. Chem. Soc.110, 5329–5339 (1988). [Google Scholar]
- 29.Kitagawa, T. et al. A functional model for the cysteinate-ligated non-heme iron enzyme superoxide reductase (SOR). J. Am. Chem. Soc.128, 14448–14449 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheng, Y. et al. Superoxide dismutases and superoxide reductases. Chem. Rev.114, 3854–3918 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu, J., Wu, X., Yao, M., Xiao, W. & Zha, J. Chassis engineering for microbial production of chemicals: From natural microbes to synthetic organisms. Curr. Opin. Biotechnol.66, 105–112 (2020). [DOI] [PubMed] [Google Scholar]
- 32.Yang, C. et al. Carbon dots-fed Shewanella oneidensis MR-1 for bioelectricity enhancement. Nat. Commun.11, 1379 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han, X. et al. Mn−N4 oxygen reduction electrocatalyst: operando investigation of active sites and high performance in zinc–air battery. Adv. Energy Mater.11, 2002753 (2021). [Google Scholar]
- 34.Li, J. et al. Atomically dispersed manganese catalysts for oxygen reduction in proton-exchange membrane fuel cells. Nat. Catal.1, 935–945 (2018). [Google Scholar]
- 35.Andrews, S. C., Robinson, A. K. & Rodríguez-Quiñones, F. Bacterial iron homeostasis. FEMS Microbiol. Rev.27, 215–237 (2003). [DOI] [PubMed] [Google Scholar]
- 36.Xu, X., Liu, Y., Du, G., Ledesma-Amaro, R. & Liu, L. Microbial chassis development for natural product biosynthesis. Trends Biotechnol.38, 779–796 (2020). [DOI] [PubMed] [Google Scholar]
- 37.Neilands, J. B. Microbial Iron Metabolism: a Comprehensive Treatise. (Academic Press, New York, 1974).
- 38.Puig, S., Askeland, E. & Thiele, D. J. Coordinated remodeling of cellular metabolism during iron deficiency through targeted mRNA degradation. Cell120, 99–110 (2005). [DOI] [PubMed] [Google Scholar]
- 39.Li, X., Guan, B. Y., Gao, S. & Lou, X. W. D. A general dual-templating approach to biomass-derived hierarchically porous heteroatom-doped carbon materials for enhanced electrocatalytic oxygen reduction. Energy Environ. Sci.12, 648–655 (2019). [Google Scholar]
- 40.Kim, H. W. et al. Efficient hydrogen peroxide generation using reduced graphene oxide-based oxygen reduction electrocatalysts. Nat. Catal.1, 282–290 (2018). [Google Scholar]
- 41.Asset, T. & Atanassov, P. Iron–nitrogen–carbon catalysts for proton exchange membrane fuel cells. Joule4, 33–44 (2020). [Google Scholar]
- 42.Zhao, Z. et al. Defect engineering in carbon materials for electrochemical energy storage and catalytic conversion. Mater. Adv.4, 835–867 (2023). [Google Scholar]
- 43.Bagri, A. et al. Structural evolution during the reduction of chemically derived graphene oxide. Nat. Chem.2, 581–587 (2010). [DOI] [PubMed] [Google Scholar]
- 44.Li, X. et al. Simultaneous nitrogen doping and reduction of graphene oxide. J. Am. Chem. Soc.131, 15939–15944 (2009). [DOI] [PubMed] [Google Scholar]
- 45.Chen, W. et al. Transformation of nitrogen and evolution of N-containing species during algae pyrolysis. Environ. Sci. Technol.51, 6570–6579 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Lu, Z. et al. High-efficiency oxygen reduction to hydrogen peroxide catalysed by oxidized carbon materials. Nat. Catal.1, 156–162 (2018). [Google Scholar]
- 47.Chen, S. et al. Chemical identification of catalytically active sites on oxygen-doped carbon nanosheet to decipher the high activity for electro-synthesis hydrogen peroxide. Angew. Chem. Int. Ed.60, 16607–16614 (2021). [DOI] [PubMed] [Google Scholar]
- 48.Lim, J. S. et al. Designing highly active nanoporous carbon H2O2 production electrocatalysts through active site identification. Chem7, 3114–3130 (2021). [Google Scholar]
- 49.Zhang, J., Yang, H. & Liu, B. Coordination engineering of single-atom catalysts for the oxygen reduction reaction: a review. Adv. Energy Mater.11, 2002473 (2021). [Google Scholar]
- 50.Hwang, J. et al. Controlling the morphology of metal–organic frameworks and porous carbon materials: metal oxides as primary architecture-directing agents. Chem. Soc. Rev.49, 3348–3422 (2020). [DOI] [PubMed] [Google Scholar]
- 51.Datye, A. K., Xu, Q., Kharas, K. C. & McCarty, J. M. Particle size distributions in heterogeneous catalysts: what do they tell us about the sintering mechanism? Catal. Today111, 59–67 (2006). [Google Scholar]
- 52.Zhang, H. et al. Single atomic iron catalysts for oxygen reduction in acidic media: particle size control and thermal activation. J. Am. Chem. Soc.139, 14143–14149 (2017). [DOI] [PubMed] [Google Scholar]
- 53.Yin, S. et al. Seizing gaseous Fe2+ to densify O2-accessible Fe–N4 sites for high-performance proton exchange membrane fuel cells. Energy Environ. Sci.15, 3033–3040 (2022). [Google Scholar]
- 54.Li, J. et al. Structural and mechanistic basis for the high activity of Fe–N–C catalysts toward oxygen reduction. Energy Environ. Sci.9, 2418–2432 (2016). [Google Scholar]
- 55.Liu, W. et al. Discriminating catalytically active FeNx species of atomically dispersed Fe–N–C catalyst for selective oxidation of the C–H bond. J. Am. Chem. Soc.139, 10790–10798 (2017). [DOI] [PubMed] [Google Scholar]
- 56.Fei, H. et al. General synthesis and definitive structural identification of MN4C4 single-atom catalysts with tunable electrocatalytic activities. Nat. Catal.1, 63–72 (2018). [Google Scholar]
- 57.Cao, R. et al. Promotion of oxygen reduction by a bio-inspired tethered iron phthalocyanine carbon nanotube-based catalyst. Nat. Commun.4, 2076 (2013). [DOI] [PubMed] [Google Scholar]
- 58.Chen, K. et al. Iron phthalocyanine with coordination induced electronic localization to boost oxygen reduction reaction. Nat. Commun.11, 4173 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marshall-Roth, T. et al. A pyridinic Fe–N4 macrocycle models the active sites in Fe/N-doped carbon electrocatalysts. Nat. Commun.11, 5283 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang, H. et al. A graphene-supported single-atom FeN5 catalytic site for efficient electrochemical CO2 reduction. Angew. Chem.131, 15013–15018 (2019). [DOI] [PubMed] [Google Scholar]
- 61.Li, J. et al. Thermally driven structure and performance evolution of atomically dispersed FeN4 sites for oxygen reduction. Angew. Chem. Int. Ed.58, 18971–18980 (2019). [DOI] [PubMed] [Google Scholar]
- 62.Shao, Y., Dodelet, J.-P., Wu, G. & Zelenay, P. PGM-free cathode catalysts for PEM fuel cells: a mini-review on stability challenges. Adv. Mater.31, 1807615 (2019). [DOI] [PubMed] [Google Scholar]
- 63.Zhao, L. et al. Materials engineering toward durable electrocatalysts for proton exchange membrane fuel cells. Adv. Energy Mater.12, 2102665 (2022). [Google Scholar]
- 64.Cao, P. et al. Metal single-site catalyst design for electrocatalytic production of hydrogen peroxide at industrial-relevant currents. Nat. Commun.14, 172 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Islam, M. N. et al. Designing fuel cell catalyst support for superior catalytic activity and low mass-transport resistance. Nat. Commun.13, 6157 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu, Z. Y. et al. A general synthesis of single atom catalysts with controllable atomic and mesoporous structures. Nat. Synth.1, 658–667 (2022). [Google Scholar]
- 67.Jiang, W. J. et al. Understanding the high activity of Fe–N–C electrocatalysts in oxygen reduction: Fe/Fe3C nanoparticles boost the activity of Fe–Nx. J. Am. Chem. Soc.138, 3570–3578 (2016). [DOI] [PubMed] [Google Scholar]
- 68.Zhu, Y. et al. Switching theoxygen reduction reaction pathway via tailoring the electronic structure of FeN4/C catalysts. ACS Catal.11, 13020–13027 (2021). [Google Scholar]
- 69.Tang, C. et al. Tailoring acidic oxygen reduction selectivity on single-atom catalysts via modification of first and second coordination spheres. J. Am. Chem. Soc.143, 7819–7827 (2021). [DOI] [PubMed] [Google Scholar]
- 70.Fan, W. et al. Rational design of heterogenized molecular phthalocyanine hybrid single-atom electrocatalyst towards two-electron oxygen reduction. Nat. Commun.14, 1426 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee, B.-H. et al. Supramolecular tuning of supported metal phthalocyanine catalysts for hydrogen peroxide electrosynthesis. Nat. Catal.6, 234–243 (2023). [Google Scholar]
- 72.Ramaswamy, N., Tylus, U., Jia, Q. & Mukerjee, S. Activity descriptor identification for oxygen reduction on nonprecious electrocatalysts: linking surface science to coordination chemistry. J. Am. Chem. Soc.135, 15443–15449 (2013). [DOI] [PubMed] [Google Scholar]
- 73.Jiang, K. et al. Highly selective oxygen reduction to hydrogen peroxide on transition metal single atom coordination. Nat. Commun.10, 3997 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xin, C. et al. Integration of morphology and electronic structure modulation on atomic iron–nitrogen–carbon catalysts for highly efficient oxygen reduction. Adv. Funct. Mater.32, 2108345 (2022). [Google Scholar]
- 75.Dai, Y. et al. Tailoring the d-orbital splitting manner of single atomic sites for enhanced oxygen reduction. Adv. Mater.35, 2210757 (2023). [DOI] [PubMed] [Google Scholar]
- 76.Li, X. et al. Molecule confined isolated metal sites enable the electrocatalytic synthesis of hydrogen peroxide. Adv. Mater.34, 2104891 (2022). [DOI] [PubMed] [Google Scholar]
- 77.Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat.12, 537–541 (2005). [DOI] [PubMed] [Google Scholar]
- 78.Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci.6, 15–50 (1996). [DOI] [PubMed] [Google Scholar]
- 79.Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B50, 17953–17979 (1994). [DOI] [PubMed] [Google Scholar]
- 80.Liu, Z. P. & Hu, P. General rules for predicting where a catalytic reaction should occur on metal surfaces: a density functional theory study of C–H and C–O bond breaking/making on flat, stepped, and kinked metal surfaces. J. Am. Chem. Soc.125, 1958–1967 (2003). [DOI] [PubMed] [Google Scholar]
- 81.Perdew, J. P. et al. Atoms, molecules, solids, and surfaces: applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B46, 6671–6687 (1992). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The data that substantiate the study’s findings and contribute to the assessment of the paper’s conclusions can be accessed within the paper and its Supplementary Information. All other relevant data supporting the findings of this study are available from the corresponding authors upon request. Source data file has been deposited in Figshare, 10.6084/m9.figshare.27653034. Source data are provided with this paper.