Abstract
Gamma interferon (IFN-γ)-activated macrophages use an alternative processing mechanism to present Salmonella antigens to CD8+ T lymphocytes. This pathway involves processing of antigen in a vacuolar compartment followed by secretion and loading of antigenic peptides to major histocompatibility complex class I (MHC-I) molecules on macrophage cell surface and bystander cells. In this study, we have shown that B lymphocytes are not able to process Salmonella antigens using this alternative pathway. This is due to differences in Salmonella enterica serovar Typhimurium-containing vacuoles (SCV) when comparing late endosomal-lysosomal processing compartments in B lymphocytes to those in macrophages. The IFN-γ-activated IC21 macrophage cell line and A-20 B-cell line were infected with live or dead Salmonella enterica serovar Typhimurium. The SCV in B cells were in a late endosomal-lysosomal compartment, whereas SCV in macrophages were remodeled to a noncharacteristic late endosomal-lysosomal compartment over time. Despite the difference in SCV within macrophages and B lymphocytes, S. enterica serovar Typhimurium survives more efficiently within the IFN-γ-activated B cells than in activated macrophage cell lines. Similar results were found during in vivo acute infection. We determined that a lack of remodeling of late endosomal-lysosomal compartments by live Salmonella infection in B lymphocytes is associated with the inability to use the alternative MHC-I antigen-processing pathway, providing a survival advantage to the bacterium. Our data also suggest that the B lymphocyte late endosome-lysosome environment allows the expression of Salmonella virulence mechanisms favoring B lymphocytes in addition to macrophages and dendritic cells as a reservoir during in vivo infection.
Macrophages and dendritic cells are considered to be target cells of Salmonella infection. The ability of this bacterium to survive and replicate within these cells constitutes a relevant pathogenic factor in the development of disseminated disease (32). On the other hand, macrophages, dendritic cells, and some other phagocytic cells are considered to be one of the first lines of defense against pathogens because of their microbicidal mechanisms (31, 61). This ability is linked with their function as professional antigen-presenting cells (APCs) to prime T-cell immune response (63). APCs are responsible for processing antigens (Ags) into peptides. These peptides subsequently bind to the major histocompatibility complex molecules to be transported to the plasma membrane and be recognized by specific T cells. Exogenous Ags including Salmonella proteins are processed into peptides recognized by CD4+ T lymphocytes presented by major histocompatibility complex class II (MHC-II) molecules. The role of CD4+ T cells in the induction of protective immunity against Salmonella infection has been well established (35, 36). However, CD8+ T lymphocytes are also fundamental in controlling Salmonella infection by promoting the lysis of infected macrophages and dendritic cells (60). Consequently, this event would then release bacteria from the replicative habitat, rendering them accessible to activated macrophages and to the humoral immune response. To induce a CD8+ T-lymphocyte response, APCs have to process and present Ags by the MHC-I molecules. In this regard, APCs present Ags from intracellular microorganisms using several processing mechanisms (classical and alternative). In the classical antigen presentation (27), MHC-I molecules bind peptides generated by proteolysis of cytosolic endogenous proteins by the proteasome. This classical pathway has been described as the main system for processing and presenting Ags from virus or intracellular bacteria such as Listeria monocytogenes (62).
In the alternative pathway, APC processes soluble exogenous Ags or those derived from microorganisms residing within vacuoles by at least two mechanisms: cross-presentation (cross-priming) (21, 24) and vacuolar alternative pathways (10, 11, 22, 30, 43, 45). One of these vacuolar pathways processes Ags in a noncharacterized endocytic compartment followed by secretion and loading of antigenic peptides into MHC-I molecules on the macrophages' cell surface and bystander cells (30, 43). This mechanism is different from the pathway used by cross-priming, where exogenous Ags are retrotranslocated from the phagosome to the cytosol to be degraded by proteosomes associated with the phagosomal membrane (21, 24). The peptides generated by this mechanism are then translocated to the phagosome lumen by transport-associated antigenic peptides to load intravacuolar MHC-I molecules (21, 24). We previously reported (30) that gamma interferon (IFN-γ)-activated macrophages were able to present Salmonella Ags to MHC-I molecules using the vacuolar alternative MHC-I processing pathway, but Salmonella-infected B lymphocytes and fibroblasts were not able to use this pathway. In addition, it has been determined that these cells can cross-present exogenous Ags with a low degree of efficiency (1). However, Salmonella is able to infect and survive within B lymphocytes and fibroblasts (30, 58), and infected B lymphocytes are able to present Salmonella Ags by MHC-II molecules to CD4+ T cells (55). Thus, the endocytic-lysosomal antigen degradation mechanism is functional in infected B lymphocytes. In this paper, we illustrate that Salmonella-infected B lymphocytes are not able to use the vacuolar alternative processing mechanism associated with a lack of remodeling of late endosomal-lysosomal compartments, such as live Salmonella does in macrophages. Our data also illustrate that the environment of IFN-γ-activated B lymphocytes favors a Salmonella reservoir during in vitro and in vivo infection.
MATERIALS AND METHODS
Antibodies.
The following culture supernatants from hybridomas were used for immunostaining: rat anti-mouse LAMP-1 clone ID4B (40) (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City), rat anti-mouse CD11b (Mac-1) clone M1/70.15.11.5.HL (30) (ATCC TIB-128; American Type Culture Collection, Rockville, MD), mouse anti-H-2Kb clone Y3 (gift from Gunter J. Hammerling, German Cancer Research Center, Heidelberg, Germany), rat anti-mouse B220 clone RA3-6B2 (gift from Leopoldo Santos-Argumedo, Departamento de Biomedicina Molecular, CINVESTAV, México D.F., Mexico), and biotin-conjugated goat anti-mouse CD19 (Zymed). Polyclonal anti-Salmonella serum generated by intravenous immunization of rabbits (New Zealand) with heat-killed Salmonella enterica serovar Typhimurium 14028 and polyclonal mouse anti-Salmonella serum generated by oral immunization of BALB/c mice with S. enterica serovar Typhimurium 14028 were used as primary antibodies to detect bacteria. Secondary antibodies were fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit immunoglobulin G (IgG), FITC-conjugated goat anti-mouse IgG (Sigma-Aldrich), tetramethyl rhodamine isothiocyanate (TRITC)-conjugated goat anti-mouse IgG, TRITC-conjugated goat anti-rat IgG (Dako, Denmark), biotin-conjugated goat anti-mouse IgG (Amersham Life Science), R-phycoerythrin (R-PE) goat anti-mouse IgG, R-PE-streptavidin, and PerCP-streptavidin (Zymed).
Bacterial strain and growth conditions.
Salmonella enterica serovar Typhimurium ATCC 14028 was grown in Luria-Bertani broth (LB; GIBCO-BRL) to logarithmic phase. The optical density at 0.6 nm was used to homogenize the multiplicity of infection (MOI). Heat-killed Salmonella was prepared by boiling. Bacterial death was confirmed by CFU detection.
Cell lines, bone marrow-derived macrophages, and culture conditions.
IC21 macrophages were obtained from the American Type Culture Collection (ATCC TIB-186) (33, 52, 59). RMA and RMA-S cells were donated by Gunter J. Hammerling (German Cancer Research Center, Heidelberg, Germany), A-20 B lymphocytes were donated by Jose Moreno (Unidad de Investigación Médica en Inmunobiología, CMN, IMSS). All cell lines were cultured in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum (GIBCO-BRL Life Technologies, Grand Island, NY) and 50 μM 2-β-mercaptoethanol (Sigma-Aldrich) (DMEM-10). Primary bone marrow macrophages were obtained from the femurs of adult BALB/c mice (Universidad Nacional Autónoma de México) as described before (51). After 6 days in culture, 7 × 104 differentiated macrophages were plated on 12-mm glass coverslips in DMEM-10 with no antibiotic for Salmonella invasion monitoring. Macrophages or B lymphocytes were activated using 20 U/ml of IFN-γ for 48 h (30).
Detection of MHC-I loaded with peptides.
Activated IC21 macrophages or A-20 B lymphocytes (5 × 105) infected for 30 min with live or heat-killed S. enterica serovar Typhimurium (MOI, 1:20) were cocultured with RMA-S cells at a ratio of 1:1 in DMEM-10 with 30 μg/ml gentamicin. The viability of infected cells was over 90% at the time of initiating the cocultures (30). After 18 h of coculture, RMA-S cells were harvested and immunolabeled with anti-Kb monoclonal antibody (MAb) and anti-Mac-1 supernatant. Secondary labeling was done with biotin-conjugated goat anti-mouse IgG, streptavidin-R-PE, and FITC-conjugated goat anti-rat IgG. Subsequently, flow analysis was performed with 104 cells per sample on a FACSort (Becton Dickinson, Mountain View, CA). Results were analyzed using WinMDI software (http://facs.scripps.edu/software.html). Cocultures of RMA-S cells in the presence of uninfected macrophages or A-20 cells were preformed in parallel. Preloaded RMA-S cells with 30 μg/ml of OVA254-267 synthetic peptide were used as positive controls of Kb binding peptides (14). Kinetics of bacterial survival within IFN-γ-activated IC21 and A-20 cells using a gentamicin protection assay were also determined in parallel experiments (4).
Infection of macrophages and B lymphocytes with metabolically labeled Salmonella enterica serovar Typhimurium.
A overnight culture of S. enterica serovar Typhimurium diluted 1:100 was subcultured in M9 minimal medium for 3 h (49). The bacterial pellet was washed and cultured again for an extra hour in M9 medium supplemented with 30 μCi of [3H]leucine, [3H]alanine, [3H]valine, and [3H]phenylalanine (NEN Life Science, Boston, MA). Radioactively labeled bacteria were washed extensively with phosphate-buffered saline (PBS) and used to infect IFN-γ-activated IC21 macrophage monolayers or IFN-γ-activated B lymphocytes in suspension. An MOI of 30 bacteria per cell was used. Invasion was allowed for 30 min; afterwards, extracellular bacteria were removed by extensive washing with PBS followed by reincubation of infected cells for 1, 3, and 18 h in the presence of DMEM-10 with 30 μg/ml gentamicin (30).
Isolation of SCV.
The Salmonella-containing vacuoles (SCV) were isolated from 108 infected macrophages and B cells with live radiolabeled Salmonella enterica serovar Typhimurium (MOI, 1:30). The procedure was a modification of the methods described by Tjelle et al. (53) and Claus et al. (12). Infected cells were harvested and resuspended in 2 ml of isosmotic buffer containing 10 mM Tris, 1 mM EDTA, 25 μg/ml leupeptin, 0.2 μg/ml aprotinin, 0.1 ng/ml pepstatin, 100 μM phenylmethylsulfonyl fluoride (Sigma-Aldrich), and 0.25 M sucrose (T. J. Baker, NJ). Cells were broken using a Dounce tissue grinder. To obtain an enriched-vacuole fraction, the homogenate was centrifuged at 800 × g for 5 min to get rid of unbroken cells and nuclei. The postnuclear supernatant was layered onto 8 ml of 30% Percoll (Amersham Pharmacia Biotech AB, Uppsala, Sweden), and a gradient was generated by ultracentrifugation at 170,000 × g for 1 h at 4°C in a Beckman SW40Ti rotor with slow acceleration and without a brake (Optima XL-100K; Beckman). The densities of the fractions were calculated using bean density markers (Amersham Pharmacia Biotech AB, Uppsala, Sweden) run in parallel with the postnuclear supernatant. The gradient was fractionated into 500-μl aliquots. Each one of these fractions was analyzed for the presence of radioactive material (Salmonella) in a beta-counter (LS 6500; Beckman). The Salmonella-derived radioactivity per fraction was expressed as a percentage of the total radioactivity detected in the Percoll gradient. In addition, the enzymatic activity of β-hexosaminidase was analyzed from each fraction using colorimetric detection of p-nitrophenyl N-acetyl-β-d-glucosaminide (Sigma-Aldrich) (15). The β-hexosaminidase activities of individual fractions are expressed as percentages of the total amounts retrieved from each Percoll gradient. To determine Salmonella CFU per fraction, 50 μl of each fraction was lysed in PBS-10% Triton X-100 (Sigma-Aldrich) and plated onto LB agar.
Indirect immunostaining and confocal microscopy.
IC21, primary bone marrow-derived macrophages, or A-20 B lymphocytes were infected with S. enterica serovar Typhimurium (MOI of 1:10) in DMEM-10 for 15 min. After the extracellular bacteria were washed, the cells were cultured in DMEM-10 with 30 μg/ml gentamicin. At 5 min, 30 min, 3 h, and 24 h post-gentamicin incubation, infected cells were treated with fixative buffer G-PLP (4% paraformaldehyde, 200 mM morpholineethanesulfonic acid, 700 mM NaCl, 50 mM KCl, 50 mM MgCl2, 20 mM EGTA, 20.1 mg of sodium periodate, and 700 mM lysine monohydrochloride) for 30 min at room temperature (2, 34). After fixation, cells were permeabilized with cold methanol for 1 min followed by 1 h of incubation in the presence of blocking buffer (PBS with 2% goat serum). Specific staining was performed by 1 h of incubation at 37°C with anti-LAMP1 or anti-Salmonella primary antibodies and secondary antibodies conjugated to FITC or TRITC fluorochromes (40). Coverslips with macrophage monolayers or cytospin-adhered B lymphocytes were mounted in VectaShield (Vector Labs) fluorescence microscopy mounting buffer. Images of infected cells were acquired at ×60 and ×100 on an epifluorescence microscope in line with a confocal system (Axiovert 100M). Ten to 20 sections per field were recovered (0.2 to 2 μm). Colocalization was detected by overlapping bacteria and marker-labeling fluorochromes. Z-sections were analyzed horizontally and vertically by direct visualization of overlapping and by graphical overlapping. The analysis was carried out with LSM 5 Image Browser software. Quantification of colocalization was determined in at least three experiments per variable. Macrophages loaded with ovalbumin Texas Red and heat-killed Salmonella were used as positive controls of intracellular kinetics of Lamp1 marker colocalization (40).
Quantification of live Salmonella from splenic macrophages and B lymphocytes.
Six- to 8-week-old male BALB/c mice were infected orogastrically with 103 CFU in 200 μl of PBS or intraperitoneally with 50 CFU in 150 μl of PBS (50). The inoculum dose was estimated by plating dilutions on LB agar plates. After days 3 and 5 postinfection, spleens from two mice per time point were taken and homogenized in DMEM-10. Splenocytes were stained with anti-Mac-1 MAb or with anti-B220 MAb. Secondary labeling was done with R-PE-conjugated goat anti-rat IgG. Macrophages and B lymphocytes were sorted in a FACSvantage (Becton Dickinson, Mountain View, CA). A sample of sorted B220 B lymphocytes was stained with biotin-conjugated anti-CD19 MAb, and secondary labeling was done with PerCP-streptavidin. The number of viable bacteria was determined by plating an aliquot of cells lysed in PBS-10% Triton X-100 (Sigma-Aldrich) on LB agar (4). CFU were counted, and the number of bacteria per 107 cells was calculated.
RESULTS
IFN-γ-activated B lymphocytes infected with Salmonella enterica serovar Typhimurium are not able to secrete peptides to load MHC-I molecules.
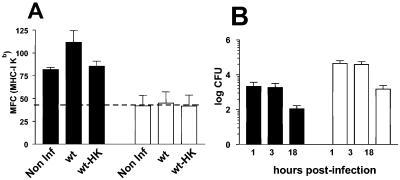
IFN-γ-activated macrophages are able to secrete Salmonella-derived peptides to load MHC-I molecules on their cell surface and bystander cells (30). To confirm whether activated B lymphocytes are able to process Salmonella Ags by this vacuolar Ag-processing mechanism, we cocultured RMA-S cells in the presence of infected A-20 B lymphocytes. Our data demonstrate that, in contrast to IFN-γ-activated macrophages infected with live Salmonella, B lymphocytes were not able to load Kb molecules on RMA-S cells (Fig. 1A). This difference was not caused by a limited amount of live intracellular Salmonella because, as shown in Fig. 1B, A-20 B lymphocytes internalized Salmonella as well as macrophages. Even more, after 18 h of infection, Salmonella survived more efficiently within activated B lymphocytes than within activated macrophages. The increased amount of Kb molecules loaded on RMA-S cells was not detected in the presence of uninfected or heat-killed Salmonella-infected macrophages or B lymphocytes.
FIG. 1.
Secreted peptides binding MHC-I through the alternative vacuolar pathway are present in IFN-γ-activated macrophages but not in activated B lymphocytes infected with Salmonella enterica serovar Typhimurium. IC21 macrophage monolayers or A-20 B lymphocytes in suspension were IFN-γ activated, infected with live (wt) or heat-killed (HK) Salmonella enterica serovar Typhimurium (MOI, 1:20), and cocultured with RMA-S cells. Kb molecules of RMA-S cells were detected by flow cytometry analysis using Y3 MAb. Macrophages and B lymphocytes were gated by Mac-1 and IgG surface antibodies, respectively. Cocultures of RMA-S cells in the presence of uninfected (Non Inf) macrophages or A-20 cells were run in parallel. Means and standard errors of the results from three different experiments are presented in panel A. The mean fluorescence channel is expressed on the y axis. The baseline of Kb expression fluorescence channel media in RMA-S is represented by the dotted horizontal line. Kinetics of bacterial survival within IFN-γ-activated IC21 and A-20 cells using a gentamicin protection assay were also determined in parallel experiments (B). Total numbers of CFU recovered per 5 × 105 cells infected are presented on the y axis. Black columns represent IC21 macrophages, and white columns are B lymphocytes.
Live S. enterica serovar Typhimurium is not able to remodel late endosomal-phagolysosomal vacuoles within B lymphocytes.
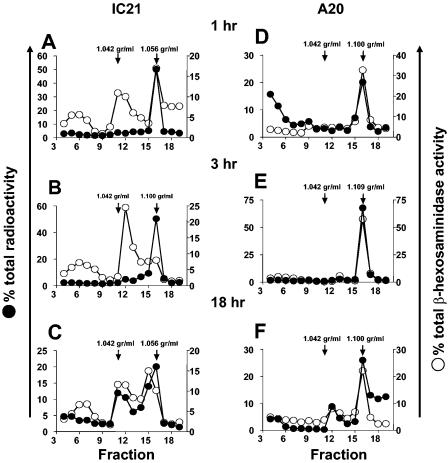
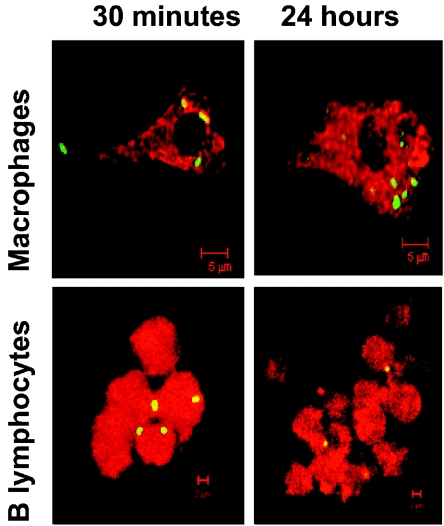
It has been reported that S. enterica serovar Typhimurium resides within a noncharacteristic late endosomal-lysosomal compartment (7, 25), but there is no evidence whether this event is preserved within IFN-γ-activated macrophages or B lymphocytes. To define whether remodeling of the late endosomal-lysosomal vacuoles by Salmonella was also present and maintained in activated macrophages and B lymphocytes, we analyzed the lysosomal activity kinetic of the SCV isolated by the Percoll density gradient. In Fig. 2A, we present live radioactive Salmonella or its products residing within a high-density vacuole with β-hexosaminidase enzymatic activity within macrophages at 1 h postinfection. However after 3 h postinfection (Fig. 2B), SCV exhibited higher density but with significantly lower β-hexosaminidase activity. After 18 h, we found different SCV densities with high or low β-hexosaminidase activity (Fig. 2C). These data confirm that Salmonella, even in IFN-γ-activated macrophages, is able to remodel late endosomal-lysosomal compartments, as has been described for inactivated cell lines or primary macrophages. On the other hand, SCV within IFN-γ-activated B lymphocytes, since early stages of infection up to 18 h postinfection, are characteristically high-density late-endosome-phagolysosome compartments. In addition, confocal fluorescence microscopy of activated B lymphocytes and macrophages infected with live and heat-killed Salmonella was performed to confirm intracellular bacterial localization within late endosomal-lysosomal compartments (Fig. 2D, E, and F). In Fig. 3 and Table 1, we present selected images and quantification of SCV colocalization kinetic with late endosomal-lysosomal marker Lamp1. As we described in the biochemical characterization mentioned above, most of the macrophage SCV excluded endocytic marker Lamp1 after 3 h postinfection. However, SCV from macrophages infected with heat-killed Salmonella, as in SCV from B lymphocytes infected with live Salmonella, always colocalized with the Lamp1 marker from the beginning to the last stage of infection (Table 1).
FIG. 2.
Late endosomal-lysosomal compartments of B lymphocytes lack Salmonella enterica serovar Typhimurium degradation and favor bacterial survival. After 1, 3, and 18 h postinfection with radiolabeled bacteria, intracellular SCV of activated macrophages and B lymphocytes were obtained by Percoll gradient separation. Salmonella-derived radioactivity within vacuoles was detected by beta scintillation analysis of the fractions. The distribution of late endosome-lysosome across the gradient was detected by β-hexosaminidase enzymatic activity. On the left y axis, we present the percentage of total radioactivity, the percentage of total β-hexosaminidase activity is displayed on the right y axis, and the x axis corresponds to the number of gradient density fractions. One representative experiment out of three is presented for β-hexosaminidase quantification and radioactive detection of SCV.
FIG. 3.
Confocal microscopy analysis of intracellular trafficking of Salmonella-containing vacuoles in macrophages and B lymphocytes. Monolayers of preactivated IC21 or bone marrow-derived macrophages from BALB/c mice or A-20 cells were infected with Salmonella enterica serovar Typhimurium (MOI, 1:10) for a period of 15 min. Selective images of colocalization with Lamp1 markers and bacteria within macrophages and B lymphocytes at 30 min and 24 h postinfection are shown.
TABLE 1.
Quantification of Salmonella colocalization with late endosomal-lysosomal marker Lamp1
| Cell line (type of Salmonella used) | No. of vacuoles analyzed at time:
|
% Colocalization with Lamp1 at time:
|
||||
|---|---|---|---|---|---|---|
| 30 min | 3 h | 24 h | 30 min | 3 h | 24 h | |
| IC21 (live) | 46 | 46 | 44 | 90 | 30 | 10 |
| IC21 (heat killed) | 10 | 30 | 22 | 90 | 100 | 91 |
| A-20 (live) | 100 | 54 | 74 | 100 | 100 | 100 |
S. enterica serovar Typhimurium survives within high-density late endosome-lysosome vacuoles of IFN-γ-activated B lymphocytes.
The ability of Salmonella to survive within macrophages has been considered to play a key role in pathogenesis. IFN-γ-activated macrophages are able to increase bacterial killing and favor antigen-processing mechanisms (6, 18, 38). To further determine whether Salmonella resides and survives within the late endosomal-lysosomal compartment of activated macrophages and B lymphocytes, we recovered bacterial CFU per vacuole fraction obtained in the Percoll gradient. After 3 and 18 h of infection, we found that live Salmonella resides mainly within high-density vacuoles with β-hexosaminidase activity in B cells (Fig. 4A and C). In contrast, Salmonella in macrophages persists within the remodeled compartments with decreased β-hexosaminidase activity (Fig. 4B). In addition, the total number of Salmonella CFU recovered from activated B lymphocytes after 18 h of infection was slightly increased within this late endosomal-lysosomal vesicle (Fig. 4C). In contrast, the Salmonella CFU recovered from macrophages decreased significantly (Fig. 4D). Salmonella at earlier time points, such as 1 hour postinfection, recovered from both cells came from high-density vacuoles with β-hexosaminidase activity (data not shown). These CFU data in combination with the total bacterium-derived radioactivity detected in activated macrophages show that Salmonella is more greatly degraded within activated macrophages than B lymphocytes. These Salmonella-derived protein products remain within high- and low-density intracellular vacuoles with lysosomal activity (Fig. 2, 4). Therefore, these data suggest that live bacteria reside within a compartment that is different from the compartment that contains processed protein products. This could be the reason why B lymphocytes are not able to use the alternative vacuolar processing mechanism observed in activated macrophages (Fig. 1).
FIG. 4.
Salmonella CFU recovered from intracellular vesicles from IFN-γ-activated macrophages and B lymphocytes. Aliquots of the Percoll gradient fractions were cultured in LB agar plates to detect bacterial survival. On the left y axis, we present the percentage of total β-hexosaminidase activity, CFU numbers are displayed on the right y axis, and the x axis corresponds to the number of density gradient fractions. Panels A and C show gradients obtained from B lymphocytes. Panels B and D show data obtained from macrophages. One representative experiment out of three is presented.
Salmonella survives within splenic B lymphocytes after in vivo infection.
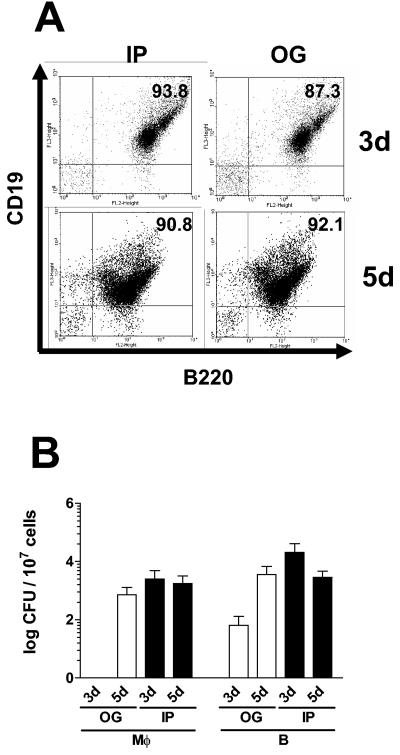
To investigate whether Salmonella can survive within B lymphocytes after in vivo infection, we sorted B220 splenic B cells which were CD19 positive (Fig. 5A). As shown in Fig. 5B, CFU of intracellular Salmonella were recovered from B cells after 3 or 5 days postinfection when bacteria were administrated either orogastrically or intraperitoneally. In accordance with in vitro-obtained data, Salmonella survives in B cells as well as in macrophages. These results suggested that not only macrophages but also B lymphocytes are involved in bacterial dissemination during the pathogenesis of this intracellular infection.
FIG. 5.
S. enterica serovar Typhimurium organisms survive in B lymphocytes during primary in vivo infection. BALB/c mice were infected orogastrically (OG) with 1,000 CFU or intraperitoneally (IP) with 50 CFU. On days (d) 3 and 5 postinfection, splenocytes were harvested and macrophages and B cells were sorted based on the expression of Mac-1 (macrophages) or B220 (B lymphocytes). In panel A, we exhibit a representative dot blot analysis of the sorted B lymphocytes using a CD19 marker. In panel B, we show Salmonela CFU obtained from splenic B cells and macrophages. The white bars correspond to CFU obtained from OG infected mice, and black bars correspond to IP infected mice. Data are presented as means and standard deviations of the results from three independent experiments.
DISCUSSION
It has been previously demonstrated that IFN-γ-activated macrophages are able to process Salmonella Ags using a vacuolar alternative MHC-I processing mechanism to induce immune response (30). In this pathway, we expect Salmonella-derived peptides produced within the vacuolar environment to load into this system. On the other hand, the ability of these bacteria to survive and replicate within professional phagocytic cells, such as macrophages and dendritic cells, is considered a key virulence determinant in developing disseminated disease (39, 41). At the same time, these cells are responsible for priming a specific immune response against Salmonella. It has been proposed that some Salmonella organisms can be degraded while others are able to induce in vivo expression of virulence mechanisms to survive within phagocytic cells (5, 42). In addition, it has been reported that IFN-γ-activated macrophages are able to kill Salmonella to a greater extent than nonactivated macrophages (16, 17). These data suggest that the balance between APC conditions of activation and bacterial virulence factors favors either survival, replication, or partial degradation to produce peptides able to induce immune response. In this work, we demonstrated that live Salmonella directs the trafficking of loading peptides to the vacuolar alternative MHC-I processing mechanism. These data suggest that, during infection of activated macrophages, some Salmonella factors induced in vivo are required to favor this processing mechanism.
Activated B lymphocytes are also professional APCs that mainly target CD4+ T cells (37, 44). Most information concerning B lymphocytes was generated using different B-cell lines such as A-20 because this cell line has many similarities with primary B lymphocytes (37, 44). Interestingly, Salmonella spp. are able to infect and survive within B lymphocytes (58). These B cells are also activated during the early immune response to Salmonella infection due to their ability to present antigens to T cells (55). Our data confirmed that activated B lymphocytes are efficiently infected with Salmonella, but in contrast to macrophages, they are not able to load peptides to the vacuolar alternative MHC-I processing mechanism. Therefore, in contrast to B lymphocytes, macrophages must have specific intracellular compartments favoring production and trafficking of Salmonella-antigenic peptides through the vacuolar alternative MHC-I processing pathway. Indeed, our data confirm differences found in the intracellular compartment where Salmonella and its products reside within macrophages and B lymphocytes. It has been demonstrated that, immediately after infection of macrophages, live Salmonella resides within spacious phagosomes (3, 4) with rapid maturation to late endosomal compartments. There is controversy in the literature addressing whether SCV within macrophages fuse with lysosomes (8, 40) or if they remain as late endosomes (7, 19, 23, 25, 46, 54). Some authors propose that, during the course of infection, the SCV is remodeled to a noncharacteristic late endosomal-lysosomal compartment (9, 47, 57). Our data mostly agree with the remodeling mechanism because we initially found rapid acquisition of late endosomal-lysosomal markers such as β-hexosaminidase activity and Lamp1. However, the SCV excluded these markers later on. Preliminary results (data not shown) suggest that Salmonella-derived peptides were mainly found in endocytic compartments with lysosomal enzymatic activity, suggesting that these peptides are sorted before loading MHC-I molecules by the alternative processing pathway. In contrast, activated B lymphocytes do not allow Salmonella to remodel the SCV remaining as late-endosome lysosomes. It is possible that lysosomal environments provide stronger signals to induce Salmonella virulence factors to survive and replicate within this intracellular niche. Further investigation using Salmonella-deficient mutants involved in endocytic traffic (54) would be necessary to define the specific role of the remodeled macrophage compartment in the sorting of Salmonella Ags to load MHC-I molecules through the vacuolar alternative pathway.
We also determined that Salmonella is able to survive and persist within activated B lymphocytes as well as in macrophages. We would expect to have greater microbicidal mechanisms developed in IFN-γ-activated cells, as happens in macrophages; however, B lymphocytes clearly do not control Salmonella infection. There is no information regarding microbicidal mechanisms within B lymphocytes to control Salmonella infection. It is also possible that other IFN-γ costimulation signals such as CD40 may be required to induce B-lymphocyte microbicidal activation. Furthermore, it has been proven that CD40 increases Ag-processing mechanisms in B lymphocytes and some other APCs (29). Our data clearly suggest that B lymphocytes are another Salmonella reservoir favoring bacterial survival, persistence, and dissemination. The murine model data supports that B lymphocytes are a Salmonella “niche” during in vivo infection because we detected Salmonella CFU from splenic B cells at 3 and 5 days postinfection regardless of whether inoculation was orogastric or intraperitoneal. Our data show a slightly greater amount of bacteria within splenic B lymphocytes with respect to splenic macrophages, in contrast to a recent publication (48), where the authors found only marginal splenic B-lymphocyte infection to a lesser extent than in macrophages. These authors used a significantly higher dose of a full virulent Salmonella strain than the regular 50% lethal dose previously reported (50), and they also used a different technique to identify the population of infected splenic cells. In our model, we purified the macrophages and B cells from spleens to obtain Salmonella CFU from the same number of live cells. One possible explanation for the difference in our data could be attributed to the apoptotic event induced by Salmonella in eukaryotic cells, as has been demonstrated with macrophages (28). Thus, if B lymphocytes are more susceptible to be infected by Salmonella, they could also be more susceptible to apoptosis. If bacteremia was significantly higher in their model, then the apoptotic events could increase as well, and the level of detectable infected B lymphocytes would be lower.
It would be interesting to define whether B lymphocytes mediate Salmonella transit from intestinal wall to spleen, liver, or bone marrow, the main targets for Salmonella persistence (13, 20, 26, 56). In fact, it has been demonstrated that activated B lymphocytes travel from lymphoid organs to bone marrow to become antibody-producing cells.
In summary, we demonstrated that, in contrast to macrophages, B cells are not able to use the vacuolar alternative antigen-processing mechanism to load Salmonella antigens to MHC-I molecules, associated with a lack of SCV remodeling by live Salmonella. Our data also suggest that the late endosome-lysosome environment of B lymphocytes allows bacterial survival and persistence, favoring other reservoirs and perhaps a mechanism of transportation during in vivo infection.
Acknowledgments
This work was supported by CONACYT grants 3595P-M9608 (to V.O.-N.) and 37899-M (to C.A.-A.). R.R.-R. was supported by CONACYT scholarship 95055 and CINVESTAV Scholarship.
We thank Victor Hugo Rosales García for assistance in flow cytometry.
Editor: F. C. Fang
REFERENCES
- 1.Ackerman, A. L., and P. Cresswell. 2004. Cellular mechanisms governing cross-presentation of exogenous antigens. Nat. Immunol. 5:678-684. [DOI] [PubMed] [Google Scholar]
- 2.Allen, L. H., and A. Aderem. 1995. A role for MARCKS, the alpha isozyme of protein kinase C and myosin I in zymosan phagocytosis by macrophages. J. Exp. Med. 182:829-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alpuche-Aranda, C. M., E. L. Racoosin, J. A. Swanson, and S. I. Miller. 1994. Salmonella stimulate macrophage macropinocytosis and persist within spacious phagosomes. J. Exp. Med. 179:601-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alpuche-Aranda, C. M., E. P. Berthiaume, B. Mock, J. A. Swanson, and S. I. Miller. 1995. Spacious phagosome formation within mouse macrophages correlates with Salmonella serotype pathogenicity and host susceptibility. Infect. Immun. 63:4456-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alpuche-Aranda, C. M., J. A. Swanson, W. P. Loomis, and S. I. Miller. 1992. Salmonella typhimurium activates virulence gene transcription within acidified macrophage phagosomes. Proc. Natl. Acad. Sci. USA 89:10079-10083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bao, S., K. W. Beagley, M. P. France, J. Shen, and A. J. Husband. 2000. Interferon-gamma plays a critical role in intestinal immunity against Salmonella typhimurium infection. Immunology 99:464-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchmeier, N. A., and F. Heffron. 1991. Inhibition of macrophage phagosome-lysosome fusion by Salmonella typhimurium. Infect. Immun. 59:2232-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carrol, M. E., P. S. Jackett, V. R. Aber, and D. B. Lowrie. 1979. Phagolysosome formation, cyclic adenosine 3′:5′-monophosphate and the fate of Salmonella typhimurium within mouse peritoneal macrophages. J. Gen. Microbiol. 110:421-429. [DOI] [PubMed] [Google Scholar]
- 9.Chakravortty, D., I. Hansen-Wester, and M. Hensel. 2002. Salmonella pathogenicity island 2 mediates protection of intracellular Salmonella from reactive nitrogen intermediates. J. Exp. Med. 195:1155-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chefalo, P. J., and C. V. Harding. 2001. Processing of exogenous antigens for presentation by class I MHC molecules involves post-golgi peptide exchange influenced by peptide-MHC complex stability and acidic pH. J. Immunol. 167:1274-1282. [DOI] [PubMed] [Google Scholar]
- 11.Chefalo, P. J., A. G. Grandea III, L. Van Kaer, and C. V. Harding. 2003. Tapasin−/− and TAP1−/− macrophages are deficient in vacuolar alternate class I MHC (MHC-I) processing due to decreased MHC-I stability at phagolysosomal pH. J. Immunol. 170:5825-5833. [DOI] [PubMed] [Google Scholar]
- 12.Claus, V., A. Jahraus, T. Tjelle, T. Berg, H. Kirschke, H. Faulstich, and G. Griffiths. 1998. Lysosomal enzyme trafficking between phagosomes, endosomes, and lysosomes in J774 macrophages. J. Biol. Chem. 273:9842-9851. [DOI] [PubMed] [Google Scholar]
- 13.Dance, D., J. E. Richens, M. Ho, G. Acharya, B. Pokhrel, and N. R. Tuladhar. 1991. Blood and bone marrow cultures in enteric fever. J. Clin. Pathol. 44:1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diaz-Quinonez, A., N. Martin-Orozco, A. Isibasi, and V. Ortiz-Navarrete. 2004. Two Salmonella OmpC Kb-restricted epitopes for CD8+-T-cell recognition. Infect. Immun. 72:3059-3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Driessen, C., R. A. Bryant, A. M. Lennon-Dumenil, J. A. Villadangos, P. W. Bryant, G. P. Shi, H. A. Chapman, and H. L. Ploegh. 1999. Cathepsin S controls the trafficking and maturation of MHC class II molecules in dendritic cells. J. Cell Biol. 147:775-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards, C. K., III, S. M. Ghiasuddin, L. M. Yunger, R. M. Lorence, S. Arkins, R. Dantzer, and K. W. Kelley. 1992. In vivo administration of recombinant growth hormone or gamma interferon activates macrophages: enhanced resistance to experimental Salmonella typhimurium infection is correlated with generation of reactive oxygen intermediates. Infect. Immun. 60:2514-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foster, N., S. D. Hulme, and P. A. Barrow. 2003. Induction of antimicrobial pathways during early-phase immune response to Salmonella spp. in murine macrophages: gamma interferon (IFN-γ) and upregulation of IFN-γ receptor alpha expression are required for NADPH phagocytic oxidase gp91-stimulated oxidative burst and control of virulent Salmonella spp. Infect. Immun. 71:4733-4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fruh, K., and Y. Yang. 1999. Antigen presentation by MHC class I and its regulation by interferon gamma. Curr. Opin. Immun. 11:76-81. [DOI] [PubMed] [Google Scholar]
- 19.Garvis, S. G., C. R. Beuzon, and D. W. Holden. 2001. A role for the PhoP/Q regulon in inhibition of fusion between lysosomes and Salmonella-containing vacuoles in macrophages. Cell. Microbiol. 3:731-744. [DOI] [PubMed] [Google Scholar]
- 20.Gasem, M. H., M. Keuter, W. M. Dolmans, J. Van Der Ven-Jongekrijg, R. Djokomoeljanto, and J. W. Van Der Meer. 2003. Persistence of Salmonellae in blood and bone marrow: randomized controlled trial comparing ciprofloxacin and chloramphenicol treatments against enteric fever. Antimicrob. Agents Chemother. 47:1727-1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guermonprez, P., L. Saveanu, M. Kleijmeer, J. Davoust, P. Van Endert, and S. Amigorena. 2003. ER-phagosome fusion defines an MHC class I crosspresentation compartment in dendritic cells. Nature 245:397-402. [DOI] [PubMed] [Google Scholar]
- 22.Harding, C. V., and R. Song. 1994. Phagocytic processing of exogenous particulate antigens by macrophages for presentation by class I MHC molecules. J. Immunol. 153:4925-4933. [PubMed] [Google Scholar]
- 23.Hashim, S., K. Mukherjee, M. Raje, S. K. Basu, and A. Mukhopadhyay. 2000. Live Salmonella modulate expression of Rab proteins to persist in a specialized compartment and escape transport to lysosomes. J. Biol. Chem. 275:16281-16288. [DOI] [PubMed] [Google Scholar]
- 24.Houde, M., S. Bertholet, E. Gagnon, S. Brunet, G. Goyette, A. Laplante, M. F. Princiotta, P. Thibault, D. Sacks, and M. Desjardins. 2003. Phagosomes are competent organelles for antigen cross-presentation. Nature 245:402-406. [DOI] [PubMed] [Google Scholar]
- 25.Ishibashi, Y., and T. Arai. 1990. Specific inhibition of phagosome-lysosome fusion in murine macrophages mediated by Salmonella typhimurium infection. FEMS Microbiol. Immunol. 2:35-43. [DOI] [PubMed] [Google Scholar]
- 26.Kirby, A. C., U. Yrlid, M. Svensson, and M. J. Wick. 2001. Differential involvement of dendritic cell subsets during acute Salmonella infection. J. Immunol. 166:6802-6811. [DOI] [PubMed] [Google Scholar]
- 27.Kloetzel, P. M. 2004. Generation of major histocompatibility complex class I antigens: functional interplay between proteasomes and TPPII. Nat. Immunol. 5:661-669. [DOI] [PubMed] [Google Scholar]
- 28.Knodler, L. A., and B. B. Finlay. 2001. Salmonella and apoptosis: to live or let die? Microbes Infect. 3:1321-1326. [DOI] [PubMed] [Google Scholar]
- 29.Marriott, I., E. K. Thomas, and K. L. Bost. 1999. CD40-CD40 ligand interactions augment survival of normal mice, but not CD40 ligand knockout mice, challenged orally with Salmonella dublin. Infect. Immun. 67:5253-5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin-Orozco, N., A. Isibasi, and V. Ortiz-Navarrete. 2001. Macrophages present exogenous antigens by class I major histocompatibility complex molecules via a secretory pathway as a consequence of interferon-gamma activation. Immunology 103:41-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mastroeni, P. 2002. Immunity to systemic Salmonella infections. Curr. Mol. Med. 2:393-406. [DOI] [PubMed] [Google Scholar]
- 32.Mastroeni, P., and M. Sheppard. 2004. Salmonella infections in the mouse model: host resistance factors and in vivo dynamics of bacterial spread and distribution in the tissues. Microbes Infect. 6:398-405. [DOI] [PubMed] [Google Scholar]
- 33.Mauel, J., and V. Defendi. 1971. Infection and transformation of mouse peritoneal macrophages by simian virus. J. Exp. Med. 134:335-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McLean, I. W., and P. K. Nakane. 1974. Periodate-lysine-paraformaldehyde fixative. J. Histochem. Cytochem. 22:1077-1083. [DOI] [PubMed] [Google Scholar]
- 35.McSorley, S. J., B. T. Cookson, and M. K. Jenkins. 2000. Characterization of CD4+ T cell responses during natural infection with Salmonella typhimurium. J. Immunol. 164:986-993. [DOI] [PubMed] [Google Scholar]
- 36.Mittrucker, H. W., and S. H. Kaufmann. 2000. Immune response to infection with Salmonella typhimurium in mice. J. Leukoc. Biol. 67:457-463. [DOI] [PubMed] [Google Scholar]
- 37.Myers, C. D. 1991. Role of B cell antigen processing and presentation in the humoral immune response. FASEB J. 5:2547-2553. [DOI] [PubMed] [Google Scholar]
- 38.Nauciel, C., and F. Espinasse-Maes. 1992. Role of gamma interferon and tumor necrosis factor alpha in resistance to Salmonella typhimurium infection. Infect. Immun. 60:450-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niedergang, F., J. C. Sirard, C. T. Blanc, and J. P. Kraehenbuhl. 2000. Entry and survival of Salmonella typhimurium in dendritic cells and presentation of recombinant antigens do not require macrophage-specific virulence factors. Proc. Natl. Acad. Sci. USA 97:14650-14655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oh, Y. K., C. Alpuche-Aranda, E. Berthiaume, T. Jinks, S. I. Miller, and J. A. Swanson. 1996. Rapid and complete fusion of macrophage lysosomes with phagosomes containing Salmonella typhimurium. Infect. Immun. 64:3877-3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohl, M. E., and S. I. Miller. 2001. Salmonella: a model for bacterial pathogenesis. Annu. Rev. Med. 52:259-274. [DOI] [PubMed] [Google Scholar]
- 42.Pfeifer, C. G., S. L. Marcus, O. Steele-Mortimer, L. A. Knodler, and B. B. Finlay. 1999. Salmonella typhimurium virulence genes are induced upon bacterial invasion into phagocytic and nonphagocytic cells. Infect. Immun. 67:5690-5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfeifer, J. D., M. J. Wick, R. L. Roberts, K. Findlay, S. J. Normark, and C. V. Harding. 1993. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature 361:359-362. [DOI] [PubMed] [Google Scholar]
- 44.Pierce, S. K., J. F. Morris, M. J. Grusby, P. Kaumaya, A. van Buskirk, M. Srinivasan, B. Crump, and L. A. Smolenski. 1988. Antigen-presenting function of B lymphocytes. Immunol. Rev. 106:149-180. [DOI] [PubMed] [Google Scholar]
- 45.Potter, N. S., and C. V. Harding. 2001. Neutrophils process exogenous bacteria via an alternate class I MHC processing pathway for presentation of peptides to T lymphocytes. J. Immunol. 167:2538-2546. [DOI] [PubMed] [Google Scholar]
- 46.Rathman, M., L. P. Barker, and S. Falkow. 1997. The unique trafficking pattern of Salmonella typhimurium-containing phagosomes in murine macrophages is independent of the mechanism of bacterial entry. Infect. Immun. 65:1475-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roy, D., D. R. Liston, V. J. Idone, A. Di, D. J. Nelson, C. Pujol, J. B. Bliska, S. Chakrabarti, and N. W. Andrews. 2004. A process for controlling intracellular bacterial infections induced by membrane injury. Science 304:1515-1518. [DOI] [PubMed] [Google Scholar]
- 48.Salcedo, S. P., M. Noursadeghi, J. Cohen, and D. W. Holden. 2001. Intracellular replication of Salmonella typhimurium strains in specific subsets of splenic macrophages in vivo. Cell. Microbiol. 3:587-597. [DOI] [PubMed] [Google Scholar]
- 49.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed., p. A.1-1.13. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 50.Shea, J. E., C. R. Beuzon, C. Gleeson, R. Mundy, and D. W. Holden. 1999. Influence of the Salmonella typhimurium pathogenicity island 2 type III secretion system on bacterial growth in the mouse. Infect. Immun. 67:213-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swanson, J. A. 1989. Phorbol esters stimulate macropinocytosis and solute flow through macrophages. J. Cell Sci. 94:135-142. [DOI] [PubMed] [Google Scholar]
- 52.Takao, S., E. H. Smith, D. Wang, C. K. Chan, G. B. Bulkey, and A. S. Klein. 1996. Role of reactive oxygen metabolites in murine peritoneal macrophage phagocytosis and phagocytic killing. Am. J. Physiol. 271:C1278-C1284. [DOI] [PubMed] [Google Scholar]
- 53.Tjelle, T. E., A. Brech, L. K. Juvet, G. Griffiths, and T. Berg. 1996. Isolation and characterization of early endosomes, late endosomes and terminal lysosomes: their role in protein degradation. J. Cell Sci. 109:2905-2914. [DOI] [PubMed] [Google Scholar]
- 54.Uchiya, K., M. A. Barbieri, K. Funato, A. H. Shah, P. D. Stahl, and E. A. Groisman. 1999. A Salmonella virulence protein that inhibits cellular trafficking. EMBO J. 18:3924-3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ugrinovic, S., N. Menager, N. Goh, and P. Mastroeni. 2003. Characterization and development of T-cell immune responses in B-cell-deficient (Igh-6−/−) mice with Salmonella enterica serovar Typhimurium infection. Infect. Immun. 71:6808-6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vazquez-Torres, A., J. Jones-Carson, A. J. Bäumler, S. Falkow, R. Valdivia, W. Brown, M. Le, R. Berggren, W. T. Parks, and F. C. Fang. 1999. Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature 401:804-808. [DOI] [PubMed] [Google Scholar]
- 57.Vazquez-Torres, A., Y. Xu, J. Jones-Carson., D. W. Holden, S. M. Lucia, M. C. Dinauer, P. Mastroeni, and F. C. Fang. 2000. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science 287:1655-1658. [DOI] [PubMed] [Google Scholar]
- 58.Verjans, G. M., J. H. Ringrose, L. van Alphen, T. E. Feltkamp, and J. G. Kusters. 1994. Entrance and survival of Salmonella typhimurium and Yersinia enterocolitica within human B-and T-cell lines. Infect. Immun. 62:2229-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walker, W. S., and A. Demus. 1975. Antibody-dependent cytolysis of chicken erythrocytes by an in vitro established line of mouse peritoneal macrophages. J. Immunol. 114:765-769. [PubMed] [Google Scholar]
- 60.Wick, M. J., and H. G. Ljunggren. 1999. Processing of bacterial antigens for peptide presentation on MHC class I molecules. Immunol. Rev. 172:153-162. [DOI] [PubMed] [Google Scholar]
- 61.Wick, M. J. 2004. Living in the danger zone: innate immunity to Salmonella. Curr. Opin. Microbiol. 7:51-57. [DOI] [PubMed] [Google Scholar]
- 62.Wong, P., and E. G. Pamer. 2003. CD8 T cell responses to infectious pathogens. Annu. Rev. Immunol. 21:29-70. [DOI] [PubMed] [Google Scholar]
- 63.Yrlid, U., M. Svensson, C. Johansson, and M. J. Wick. 2000. Salmonella infection of bone marrow-derived macrophages and dendritic cells: influence on antigen presentation and initiating an immune response. FEMS Immunol. Med. Microbiol. 27:313-320. [DOI] [PubMed] [Google Scholar]