Abstract
Gas sensors present an alternative to traditional off-package food quality assessment, due to their high sensitivity and fast response without the need of sample pretreatment. The safe integration of gas sensors into packaging without compromising sensitivity, response rate, and stability, however, remains a challenge. Such packaging integration of spoilage sensors is crucial for preventing food waste and transitioning toward more sustainable supply chains. Here, we demonstrate a wide-ranging solution to enable the use of gas sensors for the continuous monitoring of food spoilage, building upon our previous work on paper-based electrical gas sensors (PEGS). By comparing various materials commonly used in the food industry, we analyze the optimal membrane to encapsulate PEGS for packaging integration. Focusing on spinach as a high-value crop, we assess the feasibility of PEGS to monitor the gases released during its spoilage at low and room temperatures. Finally, we integrated the sensors with wireless communication and batteryless electronics, creating a user-friendly system to evaluate the spoilage of spinach, operated by a smartphone via near-field communication (NFC). The work reported here provides an alternative approach that surpasses traditional on-site and in-line monitoring, ensuring comprehensive monitoring of food shelf life.
Keywords: smart packaging, encapsulation films, gas sensors, food spoilage, real-time monitoring
Food spoilage and waste pose formidable challenges to achieving a sustainable and efficient food supply chain. A volume of 1.3 billion tons of wasted food each year, with nearly half occurring during processing, distribution, and consumption, demands urgent attention.1,2
In response, there is growing interest in developing packaging systems that go beyond containment and actively monitor the quality, storage conditions, and shelf life of food products. Monitoring gas markers of food spoilage offers a clear advantage for packaging monitoring as it does not require sample pretreatment. For example, ammonia (NH3) and hydrogen disulfide (H2S) are marker gases for the spoilage of high-protein foods (e.g., eggs, dairy, and meat) and rotting vegetables (e.g., corn and spinach).3−8 The integration of colorimetric, electrical, or electrochemical sensors within food packaging holds promise for achieving continuous monitoring. Current approaches in packaging integration of sensors mainly involve indicator films or on-package sensors, which provide a colorimetric response to changes in gas concentration, pH, or accumulated time–temperature history.3,9−11 These provide qualitative detection and visualization of the freshness status. While such colorimetric sensors offer simplicity and cost-effectiveness, electrical and electrochemical sensors offer enhanced sensitivity and real-time response capabilities, enabling precise and dynamic assessment of food quality throughout the supply chain.6,12−15
Furthermore, integrating electrical sensors with wireless technology opens new avenues in continuous monitoring.15−17 With smart devices, wireless platforms comprising electrical sensors can transmit real-time data, enabling informed decisions regarding food quality control and waste reduction. Continuous monitoring systems integrating sensor technology and wireless communication facilitate traceability and enable rapid interventions to preserve food quality.
Such monitoring systems consist of the following units: (1) Sensing, where molecular interaction between the target and recognition element is converted to a sensor output, (2) Decision-making, which converts raw data from the sensing unit into a human-readable format, (3) Power, which provides the supply voltage to the system by using a battery or an energy harvesting mechanism.18−20
To enable the widespread adoption of sensor-based monitoring systems, attention must be directed toward optimizing their integration into packaging. Encapsulation membranes enable the incorporation of sensing systems into common packaging materials without compromising food safety.21−23 These membranes play an important role in preserving sensor integrity while allowing efficient gas and vapor permeability, crucial for accurate detection of spoilage markers, and must achieve a balance among selectivity, stability, and physical protection. There are, however, very few studies in the literature that extensively compare encapsulation membranes for food sensor applications, representing one of the bottlenecks in smart packaging.24,25
In this work, we address this challenge by providing a complete study of multiple protective membranes that can be used for the encapsulation of gas sensors into food packaging.26 We pivot on our previous research on low-cost and continuous food spoilage monitoring based on paper-based electric gas sensors (PEGS) to achieve the stage of packaging integration.27 PEGS are near-zero-cost electrical gas sensors using cellulose paper as a sensing material for the quantitative detection of water-soluble gases based on the hygroscopic nature of cellulose fibers and changes in conductivity due to dissolved aqueous species. The novelty of our research arises from the realization of long-term usage and stability of the sensors using protective membranes, paving the way for their widespread application in identifying spoilage in packed food with a particular focus on bagged spinach. Spinach is a high-value crop, but it is prone to spoilage, with over 35% of spinach production wasted during the household consumption phase.2 We evaluate the performance of the sensors to monitor spinach spoilage by correlating the outputs with the microbial counts of the samples. The operation of the sensors at cold (2–8 °C) and warm temperatures (24–26 °C) is also assessed to demonstrate the monitoring capacity of the system under real storage and transport conditions. The capability of sensors to distinguish between spoiled and fresh samples is demonstrated by measuring spinach samples before and after being stored in the refrigerator and at room temperature for several days. This system offers the potential to address challenges arising from disruptions in cold chain transport or exposure to adverse environmental conditions, thus ensuring the preservation of food freshness and reducing waste. Finally, the sensor system integrated with near-field communication-enabled technology operated by a smartphone is demonstrated as a proof-of-concept for the wireless, batteryless detection of spoiled samples.
Results and Discussion
Encapsulation of PEGS
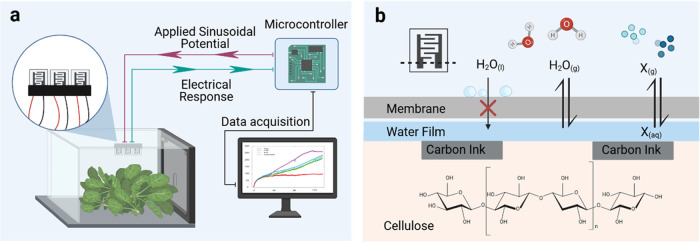
Our sensing system was based on PEGS, which has already demonstrated high sensitivity for monitoring water-soluble gases.27 The sensors consisted of two interdigitated carbon electrodes printed on filter paper with no other additives. A sinusoidal wave voltage was applied to the sensors at a low frequency operated by the microcontroller, and a transimpedance amplifier with a variable gain resistor was used to amplify and read the output signal (Figure 1a). Water-soluble gases partly dissolve in water according to Henry’s law, then dissociate into ions and change the ionic strength of the solution, thereby modifying the impedance of paper.28
Figure 1.
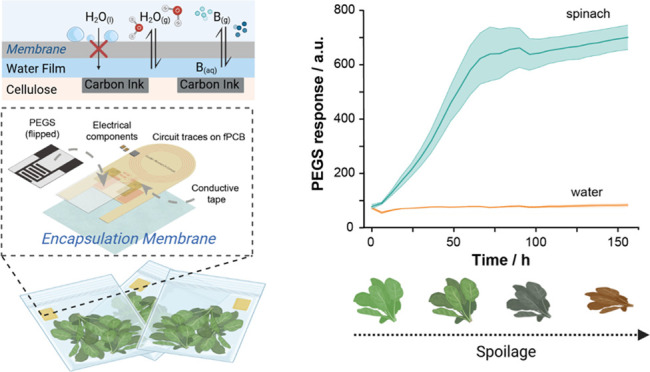
(a) Scheme of the overall system used to monitor the spoilage of spinach. Encapsulated PEGS are attached to the top of the food containers, and changes in total conductance proportional to the amount of water-soluble gas released during food spoilage are monitored. (b) Schematic representation of the surface of PEGS encapsulated with thin membranes that enable the permeation of molecules in a gas state but not in a liquid. The condensation of the water vapor on the PEGS surface creates a thin layer of liquid covering the cellulose fibers and enabling water-soluble gases (X(g)) to be dissolved (X(aq)). Created with BioRender.com.
The encapsulation of the sensors (Figure 1b) represents a dual step forward toward the application of our devices in food packaging: (i) provides physical separation between the samples and the sensor, avoiding possible contamination of the electrode surfaces by food items; (ii) prevents the sensors from being wet by water condensation inside food packages, which would affect their intrinsic impedance properties.27 Still, PEGS need a certain relative humidity (RH > 60%) to operate at its optimum, which conditions the type of material used for the encapsulation. We have tested 6 commercial membranes based on biocompatible materials such as poly(tetrafluoroethylene) (PTFE), polyurethane (PU), polyester (PET), and cellulose. These membranes permit the permeation of gases or vapors but not liquid samples.22,29,30 Material specifications for each membrane tested are listed in Table S1. PEGS were encapsulated with the membranes using double-sided tape to seal the edges and avoid water leakages inside the sensor reservoirs (see Encapsulation of Sensors section and Figure 2a). To evaluate the waterproofing properties of the encapsulation membranes while maintaining high RH in the sensors, encapsulated PEGS were dipped into water, and changes in conductance were recorded over time (Figures 2b and S1a). Optimal encapsulation membranes would enable changes in PEGS conductance similar to those observed by nonencapsulated sensors in a closed chamber saturated with water vapor. The response of nonencapsulated PEGS dipped into water is also shown in Figure 2b as a reference. In that case, the paper substrate is fully wet (instead of humid), increasing the baseline conductance and hindering the sensing properties of PEGS by masking the small conductance changes from gases dissolving into water.
Figure 2.
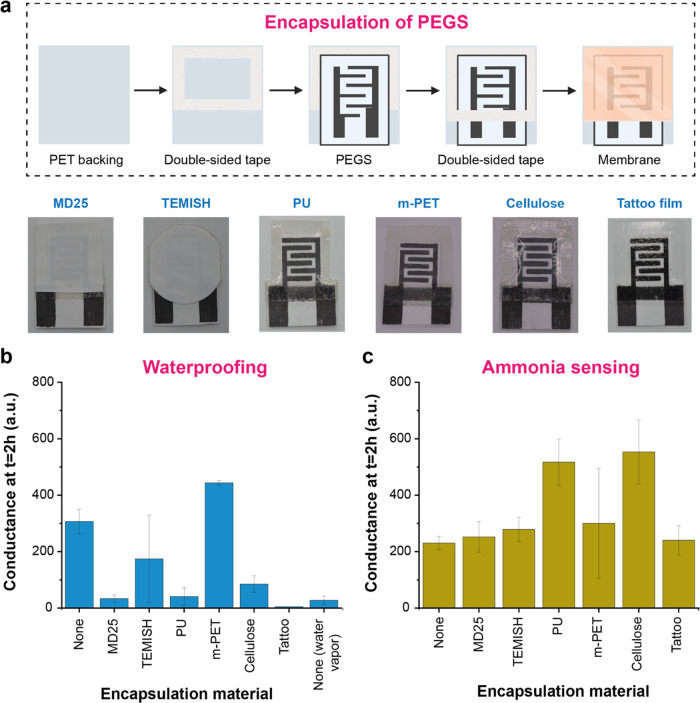
(a) Procedure followed to encapsulate PEGS with gas-permeable membranes. Bottom: pictures of encapsulated PEGS. (b) Changes in conductance of encapsulated PEGS after 2 h dipped into water and comparison with the response of nonencapsulated sensors to water and water vapor. (c) Changes in conductance of encapsulated PEGS when exposed to 1 mM NH4OH solution and comparison with the response of nonencapsulated sensors. PEGS are not dipped into the ammonia solution but placed above to study the determination of the gas formed in the headspace.
Only PEGS encapsulated with microperforated PET (mPET) exhibited changes in conductance higher than nonencapsulated PEGS. This could be explained by the low adhesivity of the membrane to the double-sided tape, enabling water to enter and stay in the reservoir in direct contact with the paper sensors. This phenomenon would affect the sensitivity of PEGS: wet sensors would translate into high baseline conductance, rendering PEGS unable to detect small changes in water-soluble gas concentrations. TEMISH membranes also showed high conductance and variability in the measurements, probably due to a decrease in the bonding properties of the adhesive layer in direct contact with water. Tattoo film membrane showed a very low response after 2 h, probably due to the low permeability of water vapor. This type of polyurethane-based membrane is designed to enable optimal moisture levels of the skin while preventing water buildup under the film to reduce infection. Increasing effective permeability (Peff), solubility (Seff), and diffusion (Deff) coefficients of polyurethane films have been, however, reported with increasing RH gradient due to water vapor and polymer interactions.31,32 The low initial water vapor permeability might represent an issue for short response time measurements, requiring rapid transduction of the response. For food monitoring, however, it is not critical as the release of spoilage gases is a relatively slow process, i.e., occurring within hours or days instead of minutes or seconds.33,34 The rest of the membranes showed good waterproofing properties and therefore potential as encapsulation materials.
We then compared the capacity of encapsulated PEGS to detect ammonia. The paper-based sensors have already shown an intrinsic selectivity toward ammonia gas in comparison to other gases tested, such as trimethylamine (TMA), hydrogen sulfide (H2S), or carbon dioxide (CO2).27 We chose ammonia sensing as a reference test to understand the effect of the encapsulation membranes on the sensing properties of PEGS before their application to food samples. Encapsulated and nonencapsulated PEGS were placed in the lids of vials containing 1 mM NH4OH (see Characterization of Encapsulated Sensors section and Figure S1b). Changes in the conductance of the sensors over time were due to the dissolution of ammonia gas from the headspace onto the paper-based sensors (Figure 2c). The response of nonencapsulated PEGS is shown as a reference. Encapsulated sensors should enable signals in the same range as nonencapsulated sensors to prevent significant drops in PEGS sensitivity. PEGS encapsulated with PTFE-based membranes (MD25 and TEMISH) and polyurethane-based tattoo film showed changes in conductance similar to those registered by the nonencapsulated sensors. This was expected since PTFE membranes are porous and hydrophobic and have been widely used in gas sensor applications. Tattoo films are designed to enable air permeation and the skin to breathe. The changes in conductance were, however, higher when the rest of the materials were used to encapsulate the sensors, showing higher variability too. Cellulose and mPET membranes showed high water condensation inside the paper-based sensor reservoir, which could explain the high changes in conductance. The presence of water inside the sensor reservoir would lead to a high response baseline, hindering the gas-sensing properties of the PEGS.
Overall, MD25 and tattoo film were optimal for the encapsulation of PEGS. They did not allow the permeation of liquid water, and their response when exposed to 1 mM NH4OH solution was similar to that from the nonencapsulated sensor. We then studied further applications of the sensor system in the monitoring of food spoilage.
Monitoring Spinach Spoilage Using Nonencapsulated PEGS
The spoilage of fresh spinach was first monitored using raw PEGS without any encapsulation layer. The scheme of the experimental setup is shown in Figure 1.
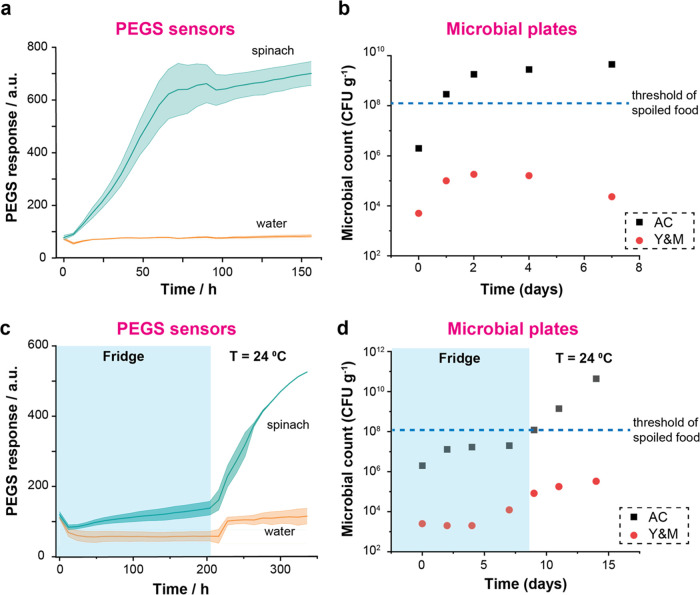
Changes in the conductance of the sensors associated with the spoilage of spinach were monitored over a week at room temperature (25 °C). Microbial plates were run in parallel to evaluate the correlation between the PEGS response and microbial content on the samples. PEGS response increased continuously during the first 3 days, which was attributed to an increase in the concentration of water-soluble gases released by the spinach samples during the spoilage mechanism (Figure 3a). The concentration of ammonia and volatile organic compounds (VOCs) such as ethanol, methanol, and organosulfur compounds (like dimethyl sulfide and methanethiol) has already been shown to increase significantly in the headspace of packaged baby spinach leaves when stored for 5 days at 21 °C.35 PEGS has already shown high sensitivity for the detection of ammonia and, to a less extent, to other gases such as CO2 and trimethylamine (TMA).27 The difference in sensitivity of PEGS toward several water-soluble gases was explained by the different levels of dissociation, solubility, and ion mobility of the gases in water. Here the overall response observed with PEGS during the spoilage of spinach corresponded to the additive effect of all of the water-soluble gases generated during the microbial breakdown of the food. Although the presence of multiple gases could alter the sensitivity of PEGS toward a specific gas, this is included in the total response of the sensors when applied to real samples. The overall conductance of the sensors in the food containers increased from 250 au (t = 24 h) to 650 au (t = 72 h), whereas the sensors in the water containers (blank) were stable at approximately 85 au for the entire analysis. After 72 h, the conductance of the paper-based electrodes in the presence of spinach reached a plateau, showing an overall conductance almost 8-fold higher than the blank sensors. This behavior was in line with the increase of microbial colonies in the food samples (Figure 3b). Aerobic colony count (ACC) is a food quality indicator that provides information about the remaining shelf life of a product or possible issues during handling and storage.36−39 Total ACC increased from 106 CFU g–1 (t = 0 h) to over 108 CFU g–1 (t = 48 h), the threshold for ACCs at which ready-to-eat food products such as salad vegetables are considered spoiled, reaching a plateau.40,41
Figure 3.
(a) Response of PEGS to spinach spoilage monitored over 6 days at room temperature (T = 25 °C, n = 3 for water, and n = 4 for spinach). (b) Microbial count (square filled, aerobic count (AC); red dot, yeast and mold (Y&M)) for the samples in (a). (c) Response of PEGS to spinach spoilage monitored for 9 days in the fridge (T = 2–8 °C) followed by 5 days at room temperature (T = 25 °C, n = 3 (water) and n = 4 (spinach)). (d) Microbial count (square filled, AC; red dot, Y&M) for the samples in (c).
Storage at low temperatures normally reduces the rate of bacterial growth and extends food shelf life.33,34,42 We monitored the spoilage of fresh spinach at low temperatures using raw PEGS (without encapsulation). The correlation between the increase in PEGS signals and the quantity of bacteria in the spinach samples was also observed at low temperatures. Figure 3c,d shows the change in conductance of PEGS and the bacterial activity, respectively, when spinach and blank samples were kept in the fridge (T = 2–8 °C). PEGS in spinach containers showed a reduced response at low temperatures compared to room temperature, with only a 2-fold higher response than blank sensors after 216 h compared to the 8-fold difference observed at T = 25 °C. This was in agreement with the data obtained from the microbial plates. The concentration of aerobic bacteria in spinach samples was constant for over 1 week when refrigerated, only increasing over the quality threshold after the food samples were placed at room T for a further 2 days. The reduced response of PEGS at low temperatures can be attributed to the combined effect of the temperature in (i) the growth rate of microorganisms in food; (ii) the behavior of the analyte (water-soluble gases); and (iii) the intrinsic properties of the sensor itself. Temperature affects the reaction rates and enzymatic activity. The growth rate of microorganisms and the rate of spoilage by microbial breakdown is, therefore, reduced at low temperatures.43 This expected phenomenon results in a decrease in the quantity of water-soluble gases released by the food; hence, the response of PEGS decreases (relative to room temperature). Regarding the behavior of the analyte, the solubility of gases in water decreases as temperature increases, leading to a reduction in the vapor pressure in the headspace and, therefore, a reduced dissolution of the gases on PEGS. Temperature also affects other phenomena, like the ammonia-ammonium equilibrium in water.44 Therefore, at low temperatures, it is expected that the total of gases generated by the microbial breakdown of food will be lower than at room temperature, and they will be retained longer in the food environment. Finally, the intrinsic properties of the sensor are also influenced by the temperature. Changes in the electrical properties of paper and, therefore, PEGS sensitivity are mainly due to the number and mobility of ions within the paper. The mobility of ions in a membrane, however, decreases as temperature decreases,45,46 contributing to the reduced signal observed in PEGS at low temperatures.
Overall, PEGS showed a distinct change in conductance during the spoilage of spinach in good correlation with the microbial plates, demonstrating their potential for monitoring food freshness by integration into packaging systems.
Monitoring Spinach Samples with Encapsulated PEGS
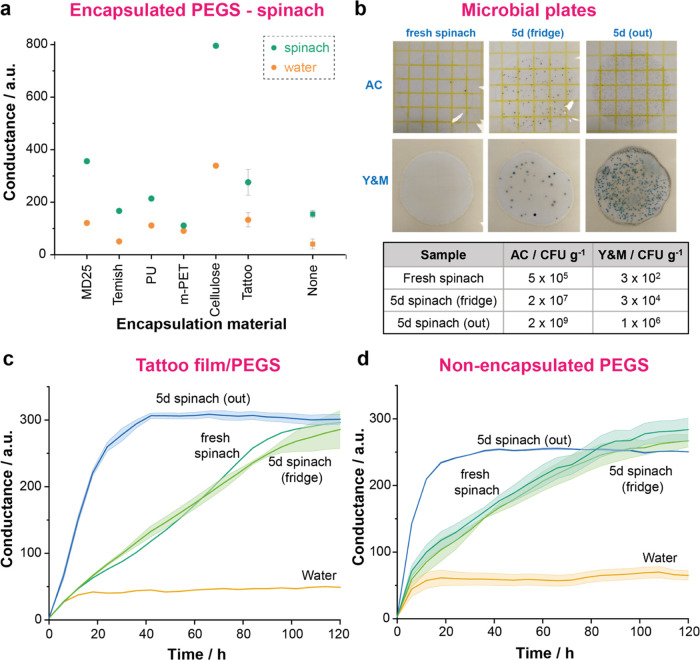
We then studied the effect of PEGS encapsulation on monitoring of the spoilage of spinach. Figure 4a compares the impact of encapsulation membranes on the sensitivity of PEGS to detect the spoilage of spinach stored in food boxes after 7 days at T = 25 °C. The changes in the conductance of encapsulated PEGS when exposed to only water are also shown as controls. Based on these results and their performance during the waterproofing and ammonia sensing tests (Encapsulation of PEGS section), we selected the most suitable membrane to encapsulate PEGS toward their integration into food packaging. The criteria we followed were: (i) high waterproofing, (ii) sensing properties toward ammonia and spinach (similar or better than nonencapsulated PEGS), and (iii) cost.
Figure 4.
(a) Comparison of changes in conductance of PEGS encapsulated with different membranes and nonencapsulated PEGS (none) exposed to food boxes containing spinach (sample) or water (control) after 7 days (none: n = 3 water, n = 6 spinach; tattoo: n = 2 water, n = 4 spinach; other materials: n = 1). (b) Top: Microbial plates at 1:105 dilutions (AC) and 1:103 dilutions (Y&M) of fresh spinach, 5-day-old spinach stored in the fridge and 5-day-old spinach stored out of the fridge at T = 25 °C (all spinach bags were opened within 2 h of measuring the microbial content). Bottom: Table with microbial content in same samples, measured on the first day of the experiment (D0). Dynamic curve of PEGS conductance within food boxes containing water, fresh spinach, 5-day-old spinach stored in the fridge, or 5-day-old spinach stored when PEGS are (c) encapsulated with tattoo film (n = 1, water and fresh spinach; n = 2, 5d spinach fridge and out) and (d) nonencapsulated (n = 2).
m-PET did not show any difference between the water and spinach sensing. This membrane had already shown high variability in ammonia monitoring (Figure 2b) and lack of waterproofing with the current encapsulation approach (Figure 2a), making it unfeasible for this application. mPET behavior might be explained by the loss of adhesivity of the membrane to the double-sided tape after long exposure to a high-humidity environment. This accumulated water in the reservoir, in direct contact with the paper sensors, might potentially change the sensing mechanism. Additionally, PET has already been reported to be degraded by some of the gases generated during spoilage gases (e.g., the combination of ammonia and CO2), which might affect the reproducibility and sensing capabilities of PEGS.47
Cellulose showed high responses to both spinach spoilage and control water, translating into an overall higher sensitivity than nonencapsulated PEGS. This membrane also showed high water absorption, which was expected due to its cellulosic nature, leading to the accumulation of water in the PEGS reservoir. This might explain the high responses to the gases and the high variability observed in ammonia sensing. PEGS are paper-based, and their continuous contact with water might degrade the sensors over time. For this reason and despite its potential, the cellulose membrane was not selected for further long-term studies.
MD25 showed high potential as an encapsulation membrane with good waterproofing and sensing capabilities for ammonia and spinach spoilage. Its cost, however, makes it unsuitable for the encapsulation of low-cost PEGS as it would increase its total price by at least 20-fold.27,48
By comparing PEGS response to the spoilage of spinach with respect to water, PU, TEMISH, and tattoo film showed responses similar to those shown by the nonencapsulated sensors. PU, however, showed some disruptions in the encapsulation sealing after prolonged exposure to moist environments. This was not noticeable at short times, for example, after dipping for 2 h in water (Figure 2a), but it was observed in the sensors exposed to spoilage of spinach for 7 days. Like MD25, TEMISH is PTFE-based, and its high cost makes it unsuitable for this application. For these reasons, PU and TEMISH were discarded and only tattoo film was considered for further experiments toward integrating PEGS into food packaging.
The stability of PEGS encapsulated with a polyurethane-based tattoo film was also studied (Figure S2). Encapsulated PEGS did not show a decrease in the response to water (baseline) or ammonia during a week of continuous measurement. In contrast, the sensor response increased over time, probably due to the increase of water condensation on the outside surface of the encapsulation membrane. The increase in ammonia response over time was also observed with nonencapsulated sensors, which similarly showed an increase in sensor surface condensation over time. This would increase the humidity rate in the sensor and might contribute to accumulating ammonia on the sensor surface, leading to an increase in the conductance changes of PEGS and sensor response.
We then tested the capability of encapsulated PEGS (tattoo film) to differentiate between spoiled and fresh spinach. For this investigation, we used two bags of spinach from the same batch; one was stored for 5 days in the fridge (5d spinach (fridge)), and the other one was kept outside the fridge at 25 °C (5d spinach (out)). Fresh spinach purchased on the starting day of the test and water were both used as controls. Food boxes containing 20 g of each sample or 100 g of water were monitored over 5 days using encapsulated PEGS (tattoo film) and nonencapsulated. The microbial content (AC and Y&M) of the spinach samples at the start of the test was also recorded (Figure 4b). Spinach samples kept outside the fridge for 5 days showed a high level of AC and Y&M content, over the threshold considered for food safety. PEGS also showed a remarkable increase in the response when exposed to 5d spinach (out), which agreed with the results from the microbial plates (Figure 4c,d). PEGS response then reached a plateau after 30–40 h. The responses of PEGS for both 5d spinach (fridge) and fresh samples were similar. They both showed a mild but continuous increase of PEGS signal over time caused by the spoilage of the samples. The 5-day-old sample showed higher initial microbial content but still within the quality threshold, both samples being indistinguishable from each other by the naked eye. Samples stored at low temperatures had already shown a slow spoilage rate and, therefore, a PEGS response (Figure 3c,d). This agrees with PEGS responses for 5d spinach (fridge) and fresh samples. Both signals reached a plateau after 100–120h of monitoring, at the same level as 5d spinach (out). This is consistent with the standard pattern of bacterial growth in closed systems, which determines the VOCs released during food spoilage.49,50 The encapsulation of PEGS with a tattoo film did not seem to hinder their capacity to monitor the spoilage of spinach. Encapsulated PEGS showed a slight delay in the response in the first hours of the test since the presence of the membrane probably slows the process of reaching the RH value required for their optimum operation. This delay, however, did not interfere with the ability of the encapsulated sensors to provide an accurate pattern of spoilage.
NFC Integration
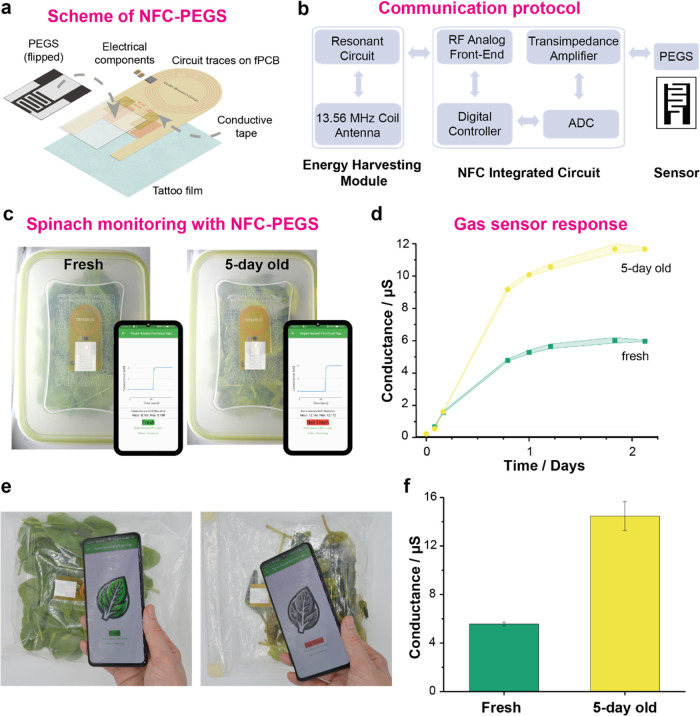
We then demonstrated the capacity of encapsulated PEGS to monitor food spoilage via its integration into food packaging. We designed a disposable NFC-powered device for the batteryless and wireless monitoring of food freshness on-site. Figure 5a,b shows the system architecture and design of the device, consisting of a planar single-coil copper antenna with a resonant frequency of 13.56 MHz. The SiliconCraft SIC4341 type 2 tag IC with a potentiostat sensor interface was used to obtain conductance readings and communicate with the smartphone simultaneously. Through a custom smartphone app, the IC was programmed to produce a 1.6 V peak-to-peak and 5 Hz square wave signal across the PEGS, and the conductance was measured over 20 s. The device was fabricated as a flexible PCB, with a “window” to expose PEGS, and then encapsulated using polyurethane-based tattoo film (the preexisting adhesive layer on the tattoo film also enabled mounting the device within spinach packages). By using low-cost, commercially available materials and electronic components, we proved the viability for widespread adoption.
Figure 5.
(a) Schematic illustrating the exploded view of the NFC-powered batteryless device including fPCB, PEGS, and encapsulation. (b) Block diagram illustrating the key components comprising the NFC electronics of the device. (c) Pictures of the NFC-powered sensing device with encapsulated PEGS integrated into food boxes containing fresh (left) and 5-day-old (right) spinach. Inset: Pictures of the app were developed (research version) to run the measurements, plot the conductance, and communicate the fresh or not fresh result. (d) Conductance of PEGS over time within the food boxes containing fresh and 5-day-old spinach recorded by the NFC devices and smartphone app (n = 4). (e) Pictures of the NFC-powered sensing device with encapsulated PEGS integrated into polyethylene food bags containing fresh (left) and 5-day-old (right) spinach and a smartphone app (user-friendly version) showing the fresh or not fresh result of the measurement. (f) Comparison of PEGS conductance from (e) recorded after 24 h (n = 4).
Figure 5c shows a demonstration involving the NFC-powered devices placed within food boxes containing (i) fresh spinach (purchased on the day of the measurement) or (ii) 5-day-old spinach (unopened bagged spinach stored at 25 °C for 5 days). The real-time freshness of each package was determined based on the conductance reading of the PEGS, with thresholds determined from prior experiments, and communicated to the smartphone. We used a research version of the app, which showed the real-time conductance on-screen during the measurement (20 s) after tapping on the NFC-PEGS device. The data stored in the phone were then transferred to the cloud and used to plot the conductance readings (Figure 5d). As expected, data from both samples were indistinguishable for the first few hours, until the RH reached the optimum value for operation. After that, the system allowed the user to differentiate between fresh and spoiled spinach samples by a 2-fold conductance difference. The repeatability of the NFC-PEGS device and app operation was proven by recording subsequent readings (n = 4) of each sample at each time point, showing relative standard deviations (RSD) below 5% for all of the measurements.
We then demonstrated the potential of the NFC-PEGS tags to monitor food freshness by integrating the device into spinach packages of varying freshness (Figure 5e). 40 g of (i) fresh spinach or (ii) 5-day-old spinach were placed in polyethylene zip bags with the NFC-PEGS tag attached on the side. The samples were stored at 25 °C and measured over 3 days. A user-friendly version of the app was used here to communicate the level of spinach freshness by “Fresh” or “Not Fresh” messages. The research app was also used to collect the data and build the comparative graph (Figure 5f). The intra-assay repeatability, measured by subsequently recording (n = 4) each sample at each time value, was below 10% RSD.
Conclusions
We have presented here a system for monitoring spoilage in packed foods based on paper-based electric gas sensors integrated within packaging through gas-permeable membranes. This allows the permeation of analyte gases and vapors while preserving the stability of the sensors and protecting integrated electronics through waterproofing. Among the membranes we tested (MD25, TEMISH, PU, m-PET, cellulose, and tattoo film), the polyurethane-based tattoo film showed an optimal balance between response and waterproofing, with performance comparable to nonencapsulated sensors.
This work provides a standard method for using encapsulated PEGS that does not require highly specialized equipment and can be performed in most laboratories. As a proof-of-concept, we demonstrate NFC-enabled wireless, batteryless detection of spoilage in spinach. Given the efficacy and low cost of the full system (US $0.35), it could be implemented within food packages to enable dynamic assessment of spoilage beyond traditional expiry dates in a disposable manner.
The current packaging integration, however, has the following three challenges:
-
i.
In testing membranes, where a membrane did not have a preexisting adhesive layer, double-sided tape was used to attach the membranes onto PEGS. This made it difficult to judge long-term response in case of a decrease in the adhesive bonding over time; gases may penetrate the device via areas of reduced bonding rather than through the membrane.
-
ii.
In this work, microbial counting was used to evaluate the level of spinach spoilage detected by the encapsulated sensors as a consequence of the overall amount of gases released. For further studies exploring spoilage mechanisms in detail, instrumental analysis techniques like gas chromatography–mass spectrometry (GC-MS), selected-ion flow-tube mass spectrometry (SIFT-MS), and secondary electrospray ionization mass spectrometry (SESI-MS) may be required for gas identification and quantification. This would help researchers understand the differences in spoilage mechanisms and gas equilibrium occurring inside food boxes compared to bags, which would explain the high interassay variability observed when NFC-PEGS tags were tested in polyethylene zip bags.
-
iii.
Additional modifications of the paper-based sensors (e.g., with water, acids, and hydrogels) have been previously used to accelerate the detection of spoilage by PEGS.27,51 Here, only unmodified dry sensors have been used, which take longer to stabilize, thereby losing the initial response. Since our platform will be integrated into packed spinach at the packaging stations, the sensors will have time to equilibrate before the spoilage process starts. For future applications, modifications like hydrogels may enable the capture of the initial response by providing faster stabilization.
In this work, we focused on applications in spinach spoilage, but our system is flexible and can be easily applied to other packed foods or perishables. Further research on marker gases/VOCs released over spoilage of various foods and their permeation characteristics through encapsulation membranes can be used to optimize parameters such as membrane thickness and headspace within the encapsulated device. Despite this study’s focus on PEGS, the encapsulation scheme presented can easily be applied to other sensors. For example, encapsulation membranes can be used to create a thin layer of electrolyte near the sensors, where electrochemical reactions can be studied. With a broader scope, development of systems capable of simultaneous powering and communication with NFC tags in multiple packages (through anticollision protocols) can enable continuous monitoring of freshness throughout the supply chain.
Experimental Section
Reagents
Monobasic potassium phosphate, ammonium hydroxide (NH4OH), and sodium hydroxide were purchased from Sigma-Aldrich, U.K.
The paper-based electrical gas sensors (PEGS) were screen printed using 90% conductive carbon sensor paste (C2030519P4, SunChemical) and 10% thinner (CDSN4059, SunChemical) by Calder Screenprint Ltd., U.K. The electrodes were printed on Whatman grade 1 chromatography paper (20 cm × 20 cm, 0.18 mm thickness). We used the same size and configuration as previously optimized in our group.27 POREX Porous PTFE medical material (MD25) and 3 M Polyurethane medical film 9832F (PU) samples were kindly provided by Parafix, U.K. TEMISH porous PTFE S-NTF8031J samples were kindly provided by Nitto, Japan. Biaxially oriented polyester film (OCLF, mPET) and cellulose-based compostable sealing film (cellulose) samples were kindly provided by Bullseye Food Packaging, U.K. Polyurethane-based tattoo film (Tattoo) was purchased from Amito E-commerce Co., LTD, UK. 3 M 9086 Translucent double-sided tape (0.19 mm thick) was purchased from RS Components, U.K.
3 M Petrifilm Aerobic count (AC) plates and 3 M Petrifilm Yeast and Mold (Y&M) count plates were purchased from Scientific Laboratory Supplies, U.K.
Encapsulation of Sensors
The procedure followed to encapsulate PEGS with the membranes under study is indicated in Figure 2a. Briefly, double-sided tape (3 M 9086, 0.19 mm thick) was used to fix PEGS to a PET substrate and seal the edges, delimiting the sensing area and avoiding water leakages inside the sensor reservoirs. After the paper-based sensors were placed, an extra layer of tape was added to the front part of the sensor to ensure the sensor reservoir was fully sealed once the encapsulation membrane was brought into contact. After encapsulation, the sensor reservoir was 1.2 cm × 1.8 cm. The encapsulation membranes covered only one side of the sensors to facilitate the study.
Characterization of Encapsulated Sensors
Waterproofing
Encapsulated PEGS were placed in 28 mL vials containing 25 mL of deionized (DI) water, leaving the sensors dipped into water (Figure S1a). Unless otherwise stated, changes in PEGS conductance were then recorded by applying a sinusoidal voltage signal with an amplitude of 4 V and 10 Hz to the sensors and using a transimpedance amplifier with a gain resistor of 50 kΩ to amplify and read the output signal (current). We measured the amplitude of this signal, which corresponded to the magnitude |Z| of the impedance of the sensor. Measurements were taken every 2.5 s.
Ammonia Sensing
Encapsulated PEGS were placed in 28 mL vials containing 10 mL of 1 mM NH4OH, where the sensors were not in contact with the solution but in the headspace (Figure S1b). Changes in PEGS conductance were recorded by using a sinusoidal voltage signal of 4 V and 10 Hz for at least 24 h.
Monitoring Spinach Spoilage
For sample monitoring, 20 g of fresh bagged spinach was placed in 1 L food containers with PEGS attached to the lids. Additional containers with 100 g of DI water to create 100% RH were used as controls. Each container accommodated two sensors. Each experiment consisted of six plastic containers (12 sensors evaluated in total), four with spinach, and two with water as controls. For experiments involving nonencapsulated PEGS, 20 μL of DI water was deposited onto PEGS immediately before placing the sensors into the food containers to enhance signal recording.27 For experiments with encapsulated PEGS, they were used dry from the start to mimic food packaging procedures. Unless otherwise stated, a sinusoidal voltage signal with an amplitude of 2 V and 10 Hz was applied to the sensors, and a transimpedance amplifier with a gain resistor of 50 kΩ was used to amplify and read the output signal.
Microbial Plate Counting
Potassium dihydrogen phosphate stock solution was prepared by adding 34 g of monobasic potassium phosphate and 175 mL of 1 N sodium hydroxide to 825 mL of deionized water. Butterfield’s buffer (pH 7.2) was prepared by adding 1.25 mL of potassium dihydrogen phosphate stock solution to 1 L of deionized water, followed by sterilization.52 To measure the microbial content of spinach samples, 10 g of spinach was first blended with 90 g of Butterfield’s buffer. 1:10 dilutions were subsequently made up to 1:108 dilutions. For each dilution, we added 1 mL of the sample to each of the 3 M Petrifilm (AC or Y&M) plates and distributed them evenly using the plate spreaders provided. AC plates were incubated at 35 °C for 48 h. Y&M plates were incubated at 25 °C for 5 days. Unless otherwise stated, the estimation of microbial contamination was done by the naked eye. Sample dilutions and microbial plates were prepared in the safety cabinet to minimize cross-contamination.
NFC-Tag Design and Fabrication
The disposable NFC-tag comprises a flexible Printed Circuit Board (fPCB) and Android app for data visualization and communication with a cloud server. The fPCB (5.7 cm × 1.8 cm) was designed in KiCAD and manufactured by JiaLiChuang Co. Ltd. to enable data acquisition and wireless transmission to a mobile device through NFC communication. An SIC4341 chip with a potentiostat sensor interface and NFC capacity was provided by Silicon Craft Technology PLC. The NFC capacity was used to harvest energy from and communicate with the user’s smartphone. The passive components (2 × 0.1 μF capacitors) were obtained from Digi-Key Electronics. 3 M Electrically Conductive Adhesive Transfer Tape 9703 was used to mount the PEGS on the fPCB. The fPCB with the sensor was attached to the 1 L food containers or PE zip bags (24 × 25 cm2) with polyurethane-based tattoo film (8 × 5 cm2). A sensor window (1.7 cm × 1.3 cm) was designed in the fPCB to enable gas and water vapor to reach the surface of the sensors through the tattoo film.
Acknowledgments
The authors thank the Department of Bioengineering at Imperial College London. F.G. and L.G.-M. thank the US Army (grant number W911QY-20-R-0022) and the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement no. 101025390. F.G. and J.H. thank the EPSRC (EP/L016702/1). F.G. and J.H. acknowledge Imperial College Centre for Processable Electronics and the Centre for Doctoral Training in Plastic Electronics. H.S.L. thanks London Interdisciplinary Social Science Doctoral Training Partnership. F.G. and L.G.-M. thank the Bill and Melinda Gates Foundation (Grand Challenges Explorations scheme under grant numbers OPP1212574 and INV-038695). F.G. thanks Bezos Earth Fund through the Bezos Centre for Sustainable Protein (BCSP/IC/001).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acssensors.4c02510.
The authors declare the following competing financial interest(s): F.G. is a shareholder of Blakbear Ltd. G.B. is an operating member of BlakBear Ltd.
Supplementary Material
References
- FAO . Food Wastage Footprint Impacts on Natural Resources 2013. https://www.fao.org/4/i3347e/i3347e.pdf.
- Lin H.; Black M. J.; Walsh L.; Giordano F. S.; Borrion A. Life Cycle Assessment of Baby Leaf Spinach: Reduction of Waste through Interventions in Growing Treatments and Packaging. J. Cleaner Prod. 2024, 449, 141723 10.1016/j.jclepro.2024.141723. [DOI] [Google Scholar]
- Zhai X.; Li Z.; Shi J.; Huang X.; Sun Z.; Zhang D.; Zou X.; Sun Y.; Zhang J.; Holmes M.; Gong Y.; Povey M.; Wang S. A Colorimetric Hydrogen Sulfide Sensor Based on Gellan Gum-Silver Nanoparticles Bionanocomposite for Monitoring of Meat Spoilage in Intelligent Packaging. Food Chem. 2019, 290, 135–143. 10.1016/j.foodchem.2019.03.138. [DOI] [PubMed] [Google Scholar]
- Schaude C.; Meindl C.; Fröhlich E.; Attard J.; Mohr G. J. Developing a Sensor Layer for the Optical Detection of Amines during Food Spoilage. Talanta 2017, 170, 481–487. 10.1016/j.talanta.2017.04.029. [DOI] [PubMed] [Google Scholar]
- Siribunbandal P.; Osotchan T.; Kim Y.-H.; Jaisutti R. Highly Sensitive Colorimetric Ammonia Sensors Based on Polydiacetylene/Zinc Oxide Nanopellet-Embedded PDMS Films for Meat Spoilage Detection. ACS Appl. Polym. Mater. 2023, 5 (10), 7786–7794. 10.1021/acsapm.3c00993. [DOI] [Google Scholar]
- Yao Z.; Coatsworth P.; Shi X.; Zhi J.; Hu L.; Yan R.; Güder F.; Yu H.-D. Paper-Based Sensors for Diagnostics, Human Activity Monitoring, Food Safety and Environmental Detection. Sens. Diagn. 2022, 1 (3), 312–342. 10.1039/D2SD00017B. [DOI] [Google Scholar]
- Yuan Z.; Bariya M.; Fahad H. M.; Wu J.; Han R.; Gupta N.; Javey A. Trace-Level, Multi-Gas Detection for Food Quality Assessment Based on Decorated Silicon Transistor Arrays. Adv. Mater. 2020, 32 (21), 1908385 10.1002/adma.201908385. [DOI] [PubMed] [Google Scholar]
- Park S. J.; Lee S. M.; Oh M.-H.; Huh Y. S.; Jang H. W. Food Quality Assessment Using Chemoresistive Gas Sensors: Achievements and Future Perspectives. Sustainable Food Technol. 2024, 2 (2), 266–280. 10.1039/D3FB00196B. [DOI] [Google Scholar]
- Liu B.; Zhuang J.; Wei G. Recent Advances in the Design of Colorimetric Sensors for Environmental Monitoring. Environ. Sci. Nano 2020, 7 (8), 2195–2213. 10.1039/D0EN00449A. [DOI] [Google Scholar]
- Kuswandi B.; Moradi M.; Ezati P. Food Sensors: Off-Package and on-Package Approaches. Packag. Technol. Sci. 2022, 35 (12), 847–862. 10.1002/pts.2683. [DOI] [Google Scholar]
- Jafarzadeh S.; Yildiz Z.; Yildiz P.; Strachowski P.; Forough M.; Esmaeili Y.; Naebe M.; Abdollahi M. Advanced Technologies in Biodegradable Packaging Using Intelligent Sensing to Fight Food Waste. Int. J. Biol. Macromol. 2024, 261, 129647 10.1016/j.ijbiomac.2024.129647. [DOI] [PubMed] [Google Scholar]
- Shi Z.; Xia L.; Li G. Recent Progress of Electrochemical Sensors in Food Analysis. Chemosensors 2023, 11, 478 10.3390/chemosensors11090478. [DOI] [Google Scholar]
- Ayerdurai V.; Cieplak M.; Kutner W. Molecularly Imprinted Polymer-Based Electrochemical Sensors for Food Contaminants Determination. TrAC, Trends Anal. Chem. 2023, 158, 116830 10.1016/j.trac.2022.116830. [DOI] [Google Scholar]
- Dincer C.; Bruch R.; Costa-Rama E.; Fernández-Abedul M. T.; Merkoçi A.; Manz A.; Urban G. A.; Güder F. Disposable Sensors in Diagnostics, Food, and Environmental Monitoring. Adv. Mater. 2019, 31 (30), 1806739 10.1002/adma.201806739. [DOI] [PubMed] [Google Scholar]
- Barandun G.; Gonzalez-Macia L.; Lee H. S.; Dincer C.; Güder F. Challenges and Opportunities for Printed Electrical Gas Sensors. ACS Sens 2022, 7 (10), 2804–2822. 10.1021/acssensors.2c01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Macia L.; Li Y.; Zhang K.; Nunez-Bajo E.; Barandun G.; Cotur Y.; Asfour T.; Olenik S.; Coatsworth P.; Herrington J.; Güder F. NFC-Enabled Potentiostat and Nitrocellulose-Based Metal Electrodes for Electrochemical Lateral Flow Assay. Biosens. Bioelectron. 2024, 251, 116124 10.1016/j.bios.2024.116124. [DOI] [PubMed] [Google Scholar]
- Olenik S.; Lee H. S.; Güder F. The Future of Near-Field Communication-Based Wireless Sensing. Nat. Rev. Mater. 2021, 6 (4), 286–288. 10.1038/s41578-021-00299-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo J.; Feng J.; Gameiro M. G.; Tian Y.; Liang J.; Wang Y.; Ding J.; He Q. RFID-Based Sensing in Smart Packaging for Food Applications: A Review. Future Foods 2022, 6, 100198 10.1016/j.fufo.2022.100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuertes G.; Soto I.; Carrasco R.; Vargas M.; Sabattin J.; Lagos C. Intelligent Packaging Systems: Sensors and Nanosensors to Monitor Food Quality and Safety. J. Sens. 2016, 2016, 4046061 10.1155/2016/4046061. [DOI] [Google Scholar]
- Ates H. C.; Nguyen P. Q.; Gonzalez-Macia L.; Morales-Narváez E.; Güder F.; Collins J. J.; Dincer C. End-to-End Design of Wearable Sensors. Nat. Rev. Mater. 2022, 7 (11), 887–907. 10.1038/s41578-022-00460-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahab Y. A.; Al-Ani L. A.; Khalil I.; Schmidt S.; Tran N. N.; Escribà-Gelonch M.; Woo M. W.; Davey K.; Gras S.; Hessel V.; Muhd Julkapli N. Nanomaterials: A Critical Review of Impact on Food Quality Control and Packaging. Food Control 2024, 163, 110466 10.1016/j.foodcont.2024.110466. [DOI] [Google Scholar]
- Siracusa V. Food Packaging Permeability Behaviour: A Report. Int. J. Polym. Sci. 2012, 2012, 302029 10.1155/2012/302029. [DOI] [Google Scholar]
- Kiryukhin M. V.; Lau H. H.; Goh S. H.; Teh C.; Korzh V.; Sadovoy A. A Membrane Film Sensor with Encapsulated Fluorescent Dyes towards Express Freshness Monitoring of Packaged Food. Talanta 2018, 182, 187–192. 10.1016/j.talanta.2018.01.085. [DOI] [PubMed] [Google Scholar]
- Yousefi H.; Su H.-M.; Imani S. M.; Alkhaldi K.; Filipe C. D. M.; Didar T. F. Intelligent Food Packaging: A Review of Smart Sensing Technologies for Monitoring Food Quality. ACS Sens. 2019, 4 (4), 808–821. 10.1021/acssensors.9b00440. [DOI] [PubMed] [Google Scholar]
- Andre R. S.; Mercante L. A.; Facure M. H. M.; Sanfelice R. C.; Fugikawa-Santos L.; Swager T. M.; Correa D. S. Recent Progress in Amine Gas Sensors for Food Quality Monitoring: Novel Architectures for Sensing Materials and Systems. ACS Sens. 2022, 7 (8), 2104–2131. 10.1021/acssensors.2c00639. [DOI] [PubMed] [Google Scholar]
- Naik A.; Lee H. S.; Herrington J.; Barandun G.; Flock G.; Güder F.; Gonzalez-Macia L.. Enabling Packaging Integration of Paper-Based Electrical Gas Sensors for the Monitoring of Spoilage in Fresh Spinach bioRxiv 2024 10.1101/2024.07.22.604629. (accessed July 25, 2024). [DOI]
- Barandun G.; Soprani M.; Naficy S.; Grell M.; Kasimatis M.; Chiu K. L.; Ponzoni A.; Güder F. Cellulose Fibers Enable Near-Zero-Cost Electrical Sensing of Water-Soluble Gases. ACS Sens. 2019, 4 (6), 1662–1669. 10.1021/acssensors.9b00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander R. Compilation of Henry’s Law Constants (Version 4.0) for Water as Solvent. Atmos. Chem. Phys. 2015, 15 (8), 4399–4981. 10.5194/acp-15-4399-2015. [DOI] [Google Scholar]
- Khan S.; Monteiro J. K.; Prasad A.; Filipe C. D. M.; Li Y.; Didar T. F. Material Breakthroughs in Smart Food Monitoring: Intelligent Packaging and On-Site Testing Technologies for Spoilage and Contamination Detection. Adv. Mater. 2024, 36 (1), 2300875 10.1002/adma.202300875. [DOI] [PubMed] [Google Scholar]
- Baschetti M. G.; Minelli M. Test Methods for the Characterization of Gas and Vapor Permeability in Polymers for Food Packaging Application: A Review. Polym. Test. 2020, 89, 106606 10.1016/j.polymertesting.2020.106606. [DOI] [Google Scholar]
- Xu R.; Xia H.; He W.; Li Z.; Zhao J.; Liu B.; Wang Y.; Lei Q.; Kong Y.; Bai Y.; Yao Z.; Yan R.; Li H.; Zhan R.; Yang S.; Luo G.; Wu J. Controlled Water Vapor Transmission Rate Promotes Wound-Healing via Wound Re-Epithelialization and Contraction Enhancement. Sci. Rep. 2016, 6 (1), 24596 10.1038/srep24596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turan D. Water Vapor Transport Properties of Polyurethane Films for Packaging of Respiring Foods. Food Eng. Rev. 2021, 13 (1), 54–65. 10.1007/s12393-019-09205-z. [DOI] [Google Scholar]
- Giannakourou M. C.; Tsironi T. N. Application of Processing and Packaging Hurdles for Fresh-Cut Fruits and Vegetables Preservation. Foods 2021, 10, 830 10.3390/foods10040830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond S. T.; Brown J. H.; Burger J. R.; Flanagan T. P.; Fristoe T. S.; Mercado-Silva N.; Nekola J. C.; Okie J. G. Food Spoilage, Storage, and Transport: Implications for a Sustainable Future. Bioscience 2015, 65 (8), 758–768. 10.1093/biosci/biv081. [DOI] [Google Scholar]
- Dryahina K.; Som S.; Smith D.; Španěl P. Characterization of Spoilage-Related Volatile Organic Compounds in Packaged Leaf Salads. Flavour Fragr J. 2020, 35 (1), 24–33. 10.1002/ffj.3535. [DOI] [Google Scholar]
- Food Safety Authority of Ireland . Guidelines for the Interpretation of Results of Microbiological Testing of Ready-to-Eat Foods Placed on the Market (Revision 4); Dublin, 2020.
- European Commission . COMMISSION REGULATION (EC) No 2073/20005 on Microbial Criteria for Foodstuffs 2005. https://www.legislation.gov.uk/eur/2005/2073/data.pdf.
- Valentin-Bon I.; Jacobson A.; Monday S. R.; Feng P. C. H. Microbiological Quality of Bagged Cut Spinach and Lettuce Mixes. Appl. Environ. Microbiol. 2008, 74 (4), 1240–1242. 10.1128/AEM.02258-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Łepecka A.; Zielińska D.; Szymański P.; Buras I.; Kołożyn-Krajewska D. Assessment of the Microbiological Quality of Ready-to-Eat Salads—Are There Any Reasons for Concern about Public Health?. Int. J. Environ. Res. Public Health 2022, 19, 1582 10.3390/ijerph19031582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food Safety Authority of Ireland . Guidelines for the Interpretation of Results of Microbiological Testing of Ready-to-Eat Foods Placed on the Market Revision 4; Dublin, 2020. https://www.fsai.ie/getmedia/74524294-d92c-4471-9d90-9633d1915c35/guidance-note-3-guidelines-for-the-interpretation-of-results-of-microbiological-testing-of-ready-to-eat-foods-placed-on-the-market-4.pdf?ext=.pdf.
- Health Protection Agency . Guidelines for Assessing the Microbiological Safety of Ready-to-Eat Foods; London, 2009.
- Jay J. M.Low-Temperature Food Preservation and Characteristics of Psychrotrophic Microorganisms BT - Modern Food Microbiology; Jay J. M., Ed.; Springer US: Boston, MA, 1995; pp 328–346. [Google Scholar]
- Yurdakos O. B.; Cihanbegendi O. System Design Based on Biological Olfaction for Meat Analysis Using E-Nose Sensors. ACS Omega 2024, 9 (30), 33183–33192. 10.1021/acsomega.4c04791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z.-G.; Fan J.-T.; Zheng X.; Wang S.-P.; Guo X.-S.; Zhang T.-X.; Yang S.-W.; Zhang Y.-Z. Neglect of Temperature and PH Impact Leads to Underestimation of Seasonal Ecological Risk of Ammonia in Chinese Surface Freshwaters. J. Chem. 2019, 2019 (1), 3051398 10.1155/2019/3051398. [DOI] [Google Scholar]
- Roy Y.; Warsinger D. M.; Lienhard J. H. Effect of Temperature on Ion Transport in Nanofiltration Membranes: Diffusion, Convection and Electromigration. Desalination 2017, 420, 241–257. 10.1016/j.desal.2017.07.020. [DOI] [Google Scholar]
- Al-Salih H.; Baranova E. A.; Abu-Lebdeh Y. Unraveling the Phase Diagram-Ion Transport Relationship in Aqueous Electrolyte Solutions and Correlating Conductivity with Concentration and Temperature by Semi-Empirical Modeling. Commun. Chem. 2023, 6 (1), 195 10.1038/s42004-023-00993-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.; Sharma S.; Di Bernardo C.; Rossi E.; Lima R.; Kamounah F. S.; Poderyte M.; Enemark-Rasmussen K.; Ciancaleoni G.; Lee J.-W. Catalytic Fabric Recycling: Glycolysis of Blended PET with Carbon Dioxide and Ammonia. ACS Sustainable Chem. Eng. 2023, 11 (30), 11294–11304. 10.1021/acssuschemeng.3c03114. [DOI] [Google Scholar]
- Fisherbran PTFE Filter Membranes—Fisher Scientific. https://www.fishersci.co.uk/shop/products/ptfe-filter-membranes/p-8003193. (access on May 31, 2024).
- Maier R. M.Bacterial Growth. In Environmental Microbiology, 2nd ed.; Maier R. M.; Pepper I. L.; Gerba C. P., Eds.; Academic Press: San Diego, 2009. Chapter 3, pp 37–54. [Google Scholar]
- Franke C.; Hilgarth M.; Vogel R. F.; Petermeier H.; Langowski H.-C. Characterization of the Dynamics of Volatile Organic Compounds Released by Lactic Acid Bacteria on Modified Atmosphere Packed Beef by PTR-MS. Food Packag. Shelf Life 2019, 22, 100400 10.1016/j.fpsl.2019.100400. [DOI] [Google Scholar]
- Grell M.; Barandun G.; Asfour T.; Kasimatis M.; Collins A. S. P.; Wang J.; Güder F. Point-of-Use Sensors and Machine Learning Enable Low-Cost Determination of Soil Nitrogen. Nat. Food 2021, 2 (12), 981–989. 10.1038/s43016-021-00416-4. [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration . Food and Drug Administration. Bacteriological AnalyticalManual. BAM R11: Butterfield’s Phosphate-Buffered Dilution Water. https://www.fda.gov/food/laboratory-methods-food/bam-r11-butterfields-phosphate-buffered-dilution-water. (accessed on June 2021).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.