Abstract
Background
Intradialytic hypotension (IDH) is a common and serious complication in renal replacement therapy, especially in hospitalized patients. The absence of a standardized definition complicates data synthesis and the development of evidence-based guidelines. Current definitions vary, including different blood pressure thresholds, clinical symptoms, and the need for medical intervention during dialysis. IDH is linked to increased mortality and cardiovascular morbidity and may impede renal recovery in patients with acute kidney injury and chronic kidney disease.
Methods
A systematic review was conducted using MEDLINE via PubMed, Embase, and Web of Science to identify studies reporting IDH prevalence. A meta-analysis of proportions was performed to determine the global prevalence of IDH, with subgroup analyses to explore heterogeneity. The Joanna Briggs Institute's checklist was used to assess the risk of bias in prevalence studies. The PRISMA guidelines were followed to report the results of this study, PROSPERO registration number CRD42024500622.
Results
The meta-analysis found a global IDH prevalence of 31% (95% CI 0.18–0.44) across nine studies. Significant heterogeneity was observed (I²: 97.87%; p < 0.01), with prevalence rates ranging from 10.7% to 64% based on patient demographics and session characteristics. Sensitivity analysis suggested prevalence could range between 27% and 33% depending on study criteria.
Conclusions
IDH is a significant complication during hospital-based renal replacement therapy, with a global prevalence of 31%. These findings highlight the need for a standardized, evidence-based definition of IDH to improve diagnostic consistency and clinical outcomes through more accurate diagnosis, better treatment strategies, and tailored patient management.
Keywords: Dialysis, intradialytic hypotension, cardiovascular instability, systematic review, proportions meta-analysis
Introduction
Cardiovascular instability refers to the clinical state of abnormal vital signs, including blood pressure and heart rate, which can lead to inadequate tissue perfusion. 1 Intradialytic hypotension (IDH) is a common and distressing complication of renal replacement therapies; however, despite its clinical significance, there is no consensus medical definition for this condition. Over the years, numerous definitions have been implemented in clinical and research settings. The inconsistencies in definitions have difficulted data synthesis and the development of evidence-based guidelines for the prevention and treatment of IDH.2,3
The various definitions found in the literature incorporate three main components: a criterion for blood pressure, the occurrence of symptoms (nausea, dizziness, cramps, etc.), and the need for interventions by medical and nursing staff during the IDH session.2,3 The Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines propose a definition of IDH as a decrease of ≥20 mmHg in systolic blood pressure (SBP) or a decrease of ≥10 mmHg in mean arterial pressure (MAP), accompanied by symptoms of hypotension such as headache, fatigue, seizures, nausea, vomiting, and restlessness during dialysis. However, this definition is not applicable to critically ill patients who cannot report typical symptoms of hypotension or hypoperfusion and whose blood pressure is heavily influenced by concurrent illnesses (e.g. sepsis, cardiogenic shock) and treatments (e.g. mechanical ventilation, vasopressors). 4
It should be clear that IDH is a complication of all commonly used modalities of renal replacement therapy, including intermittent hemodialysis, sustained low-efficiency dialysis (SLED), and continuous renal replacement therapy (CRRT).5–8
The pathophysiology of IDH is diverse; it may be related to the underlying cause of acute kidney injury (AKI), decreased cardiac output from various origins (hypovolemia, hypocalcemia, diastolic dysfunction, etc.), or alterations in vasomotor tone related to membrane/circuit bio incompatibility, ultrafiltrate/dialysate, temperature, or ionic imbalance, among others.8–11
There is sufficient evidence suggesting that IDH negatively impacts the outcomes of patients with renal disease requiring renal replacement therapy. IDH is associated with higher mortality and cardiovascular morbidity and may limit renal recovery in patients with AKI.11–17
In this context, we conducted a systematic review to determine the prevalence of cardiovascular instability during hemodialysis in hospitalized patients to support the development of guidelines for the prevention or management of this complication during renal replacement therapy.
Methods
This study follows the PRISMA guidelines for reporting systematic reviews. 18 The systematic review was registered in PROSPERO with the following registration number (CRD42024500622).
Eligibility criteria
Primary studies related to renal replacement therapy as an exposure in a hospital or critical care setting adults (over 18 years old) were considered, regardless of publication status, language, and year of publication, including conference abstracts, case reports, original manuscripts, and those uploaded to preprint servers. Manuscripts that focused on ambulatory patients, as well as letters, comments, expert opinions, editorials, and other non-original studies, were excluded. Studies involving patients undergoing CRRT or SLED were also excluded.
Information sources
Search Strategies: Between January 2024 and February 20, 2024, a systematic search of information was conducted in the following databases: MEDLINE via PubMed, Embase, and Web of Science. Different combinations of terms, such as instability, hypotension, and dialysis, were used to identify eligible articles. The detailed search strategy is provided in Supplemental Appendix 1. Additionally, the search was supplemented by a manual exploration of the bibliographies of all included studies and a search of the grey literature through preprint repositories.
Study selection process
The selection process used the Rayyan–Intelligent Systematic Review software. 19 Two researchers (KA-V and MV-M) removed duplicate documents from all search results. Subsequently, an initial screening for relevance (whether the study addresses the research question) was independently performed by reading the title and abstract.
A second review was conducted on the selected manuscripts, in which the documents were assessed in full text and the selection criteria applied. A citation search was also conducted to obtain the final number of included documents. Two researchers (KA-V and MV-M) worked independently on including studies. A third investigator (CC-S) resolved disputes.
Data extraction and synthesis process
The data extraction form followed the Joanna Briggs Institute's Data extraction form for prevalence studies (JBI) 20 to record the following information: study details, study methodology, and results (authors, title, journal, year of publication, pages, date of data extraction completion, region and country, design, setting, number of participants and dialysis sessions, data collection period, inclusion and exclusion criteria, primary outcome, secondary outcomes, comorbidities, mean age, sex, severity scores, IDH definition, ethical approval, data analysis method, prevalence, confidence intervals (CI), ICU admission causes, hypotension by sex and age subgroups).
Data list
The form also included fields to capture relevant data for risk of bias (RoB) assessment. Prevalence figures and 95% CI were extracted or calculated from available data using the Wilson method. 21
RoB assessment of individual studies
The RoB in the selected studies was assessed through the Joanna Briggs Institute's critical appraisal checklist for studies reporting prevalence data.22–24 This tool evaluates nine domains 24 : (D1) adequacy of the sampling frame, (D2) recruitment method, (D3) sample size, (D4) study subjects and setting, (D5) coverage, (D6) diagnostic methods, (D7) reliability and standardization of measurements, (D8) statistical analysis, and (D9) response rate. Two evaluators (KA-V and MV-M) independently assessed the eligible studies and rated each question as “yes,” “no,” “unclear,” or “not applicable.” Discrepancies in the assessment were discussed until a consensus was reached. The final score for each study applied to the JBI questions was calculated based solely on the percentage of positive “yes” responses. The RoB for each study was categorized according to the final score as “high” (score of 49% or lower, leading to article exclusion), “moderate” (score ranging from 50% to 69%), or “low” (score higher than 70%).
Graphs considering each RoB domain across all studies were prepared using the ROBVIS R package v. 0.3.0.900. 25 Cohen's Kappa index was used to measure the agreement between the two evaluators; values between 0.61 and 0.8 were categorized as substantial inter-rater agreement, 0.81 and 0.88 as almost perfect agreement, and a Kappa index of 1 as perfect. 26
Synthesis methods
First, tables present a narrative synthesis of the general characteristics of all included studies. These characteristics include authors, title, journal, year of publication, region–country, design, setting, number of participants, number of renal replacement therapies, inclusion criteria, exclusion criteria, primary outcome, mean age, sex, IDH definition, and prevalence.
Given the relevance of the topic and the need to obtain an aggregate value for the prevalence of IDH, a quantitative synthesis is presented by a meta-analysis using a random-effects model, and a restricted maximum likelihood method was performed. Prior to the meta-analysis, a double arcsine transformation was conducted using the Freeman–Tukey formula to control the variance between studies. To improve comprehension, results were re-transformed to prevalence after the meta-analysis. Studies with a high RoB were excluded from the meta-analysis.
To assess heterogeneity, Cochran's Q test and I² were used. Cochran's Q test was considered statistically significant when it reached a value of <0.05. A univariable meta-regression analysis was conducted based on the covariates: RoB, sample size, and the number of sessions vs. the number of participants.
Finally, sensitivity analyses were conducted based on subgroups, correcting heterogeneity with an I² of 10%, a leave-one-out meta-analysis, and standard error corrections of the effect size (prevalence) using the Sidik–Jonkman and Knapp–Hartung truncated methods. All analyses were performed using Stata 18 statistical software. 27
Publication bias assessment
To objectively evaluate publication bias, Egger's test was measured, a funnel plot was graphed, and a trim-and-fill analysis was performed.
Certainty of evidence rating
This assessment was not performed because there is no specific instrument for evaluating the certainty of evidence in prevalence systematic reviews.
Results
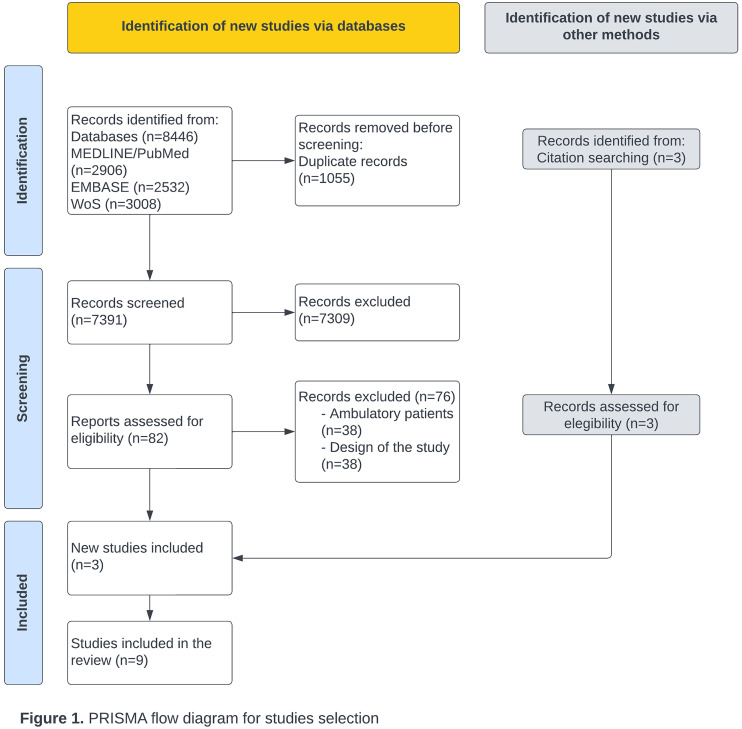
Figure 1 shows the flow diagram for article selection. The electronic search identified 8446 articles: 2906 from MEDLINE/PubMed, 2532 from Embase, and 3008 from Web of Science. After screening, 1055 duplicates were removed. A total of 7391 articles were examined by title and abstract, resulting in 82 full-text articles being assessed for eligibility. Of these, 76 articles were excluded for the following reasons: 38 were studies including ambulatory patients, and 38 had a different design. Additionally, three articles were identified from the reference lists of relevant studies. Ultimately, nine articles were included in this systematic review.
Figure 1.
PRISMA flow diagram for studies selection.
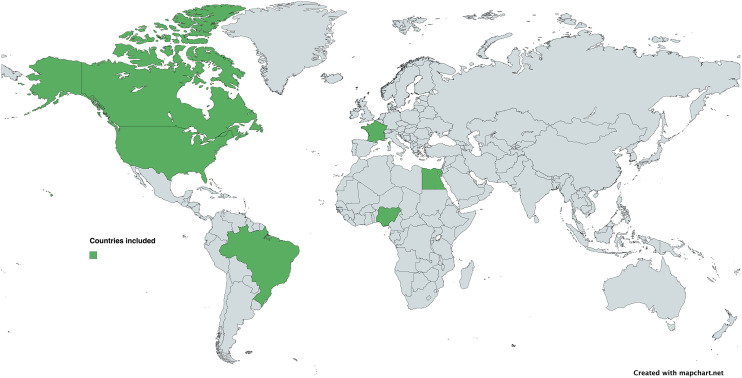
The nine included articles collected data from 2002 to 2021. Three articles (33.3%) were from Africa,28–30 three (33.3%) were from North America,31–33 one (11.1%) was from South America, 34 and two studies (22.2%) were from Europe.35,36 The countries where studies were conducted included Nigeria (n = 2; 22.2%), the United States (n = 2; 22.2%), France (n = 2; 22.2%), Canada (n = 1; 11.1%), Brazil (n = 1; 11.1%), and Egypt (n = 1; 11.1%) (see Figure 2).
Figure 2.
World map with the origin countries of the included studies.
Characteristics of the studies and individuals
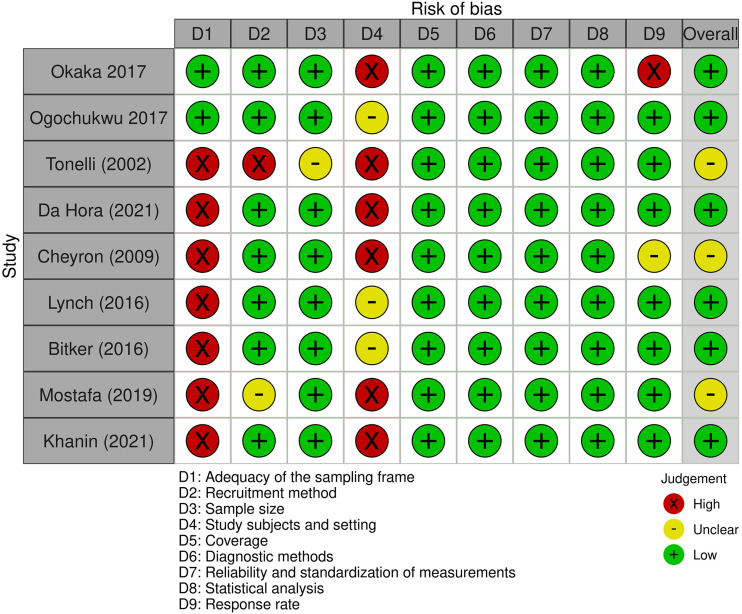
Six studies were found to have a low RoB,28,29,32–35 while three (n = 3; 33.3%) presented a moderate RoB.30,31,36 The selection process achieved a Cohen's kappa of 0.737, considered a substantial agreement (see Figures 3 and 4).
Figure 3.
Risk of bias across studies through the Joanna Briggs Institute's critical appraisal checklist for studies reporting prevalence data.
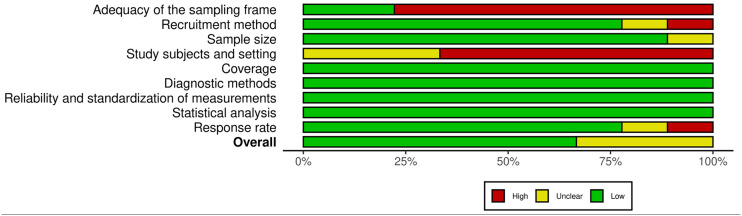
Figure 4.
Risk of bias across the domains of the Joanna Briggs Institute's critical appraisal checklist for studies reporting prevalence data.
Three types of study designs were used among the included studies: cross-sectional studies, prospective cohort studies, and retrospective studies. Most studies aimed to investigate the frequency of IDH and identify patient factors, treatment variables, or other variables associated with IDH.
Table 1 summarizes the patient characteristics of the nine articles. Five studies reported their findings by the number of patients included, which ranged from 20 to 404. On the other hand, four studies included the number of hemodialysis sessions.31,32,35,36 The mean age of patients ranged from 42.5 to 68 years. All studies specified the inclusion and exclusion criteria and reported the sex distribution. In seven studies, most patients were men28,29,32–34 and in two studies women.31,36
Table 1.
Characteristics of included studies.
| Authors | Title | Journal | Year of publication | Region–country | Design | Setting | Number of participants/renal replacement therapies | Inclusion criteria | Exclusion criteria | Primary outcome | Mean Age | Female | Male | IDH definition | Prevalence and 95% CI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Enajite I. Okaka, Chimezie G. Okwuonu 28 | Blood Pressure Variation and Its Correlates among Patients Undergoing Hemodialysis for Renal Failure in Benin City, Nigeria | Annals of African Medicine | 2017 | Africa- Nigeria | Single-center retrospective study | Patients admitted to an HD unit in a tertiary hospital in Benin City, Nigeria | 217 patients | All patients dialyzed in the unit during the study period | Patients who had <4 dialysis sessions | Determine the prevalence of blood pressure variation in patients undergoing hemodialysis | 42.5 ± 17.4 | 35.9% | 64.1% | Negative difference of >20 mmHg between the average SBP before HD and the average SBP after HD | 19.8% (15%–25.6%) |
| Ogochukwu Chinedum Okoye, Henry Enyinmisan Slater, Nilum Rajora 29 | Prevalence and risk factors of intradialytic hypotension: a 5-year retrospective report from a single Nigerian Centre | Pan African Medical Journal | 2017 | Africa-Nigeria | Single-center retrospective cross-sectional study | Patients admitted to a hemodialysis unit in a tertiary hospital | 404 patients | All adult patients on hemodialysis with acute or chronic kidney disease diagnosis | Patients with incomplete or incorrect data | Determine the crude prevalence of intradialytic hypotension | 48 ± 17 | 44.30% | 55.70% | If systolic blood pressure falls >20 mmHg at any time during a hemodialysis procedure, associated with clinical characteristics and need for intervention | 8.60% (6.2%–11.7%) |
| Marcello Tonelli, Paula Astephen, Pantelis Andreou, Stephen Beed, Peter Lundrigan, Kailash Jindal 31 | Blood volume monitoring in intermittent hemodialysis for acute renal failure | Kidney International | 2002 | North America- Canada | Prospective observational study | Medical ICU | Patients: 20, sessions: 57 | Consecutive adults receiving intermittent hemodialysis for ARF in the ICU | Patients who received packed red cells during hemodialysis treatment | Determine if relative blood volume measurement reduces intradialytic hypotension | 58.9 ± 16.5 | 55% | 45% | Mean arterial pressure of ≤70 mmHg for at least 5 min or SBP ≤100 mmHg as a secondary definition. Also considered as hypotension any interruption in ultrafiltration or need for intervention like intravenous fluid bolus or increased dose of vasoactive drugs | 30% (19.5%– 42.6%) |
| Rogerio da Hora Passos, Juliana Ribeiro Caldas, Joao Gabriel Rosa Ramos, Erica Batista dos Santos, Galvão de Melo, Marcelo Augusto Duarte Silveira, Paulo Benigno Pena Batista 34 | Prediction of hemodynamic tolerance of intermittent hemodialysis in critically ill patients: a cohort study | Nature Reviews Journals | 2021 | South America-Brazil | Single-center prospective observational study | 30-bed medical ICU in a tertiary hospital | 248 patients | Critically ill patients aged >18 years with ARF defined by KDIGO stage III requiring IHD, chronic kidney disease patients | Vascular disease or mediastinal disease | Evaluate the ability of clinical judgment and other variables to predict the occurrence of hypotension during intermittent hemodialysis | 68.0 (58.2–76) | 39.9% | 60.10% | First occurrence of a MAP below 65 mmHg during the session | 31.9% (26.4%–37.9%) |
| Damien du Cheyron, Olivier Lucidarme, Nicolas Terzi, Pierre Charbonneau 36 | Blood Volume- and Blood Temperature-Controlled Hemodialysis in Critically Ill Patients: A 6-Month, Case-Matched, Open-Label Study | Karger | 2009 | Europe-France | Single-center prospective open-label study | ICU | 62 patients, 572 sessions | All patients with oliguric ARF needing dialysis | Patients with end-stage renal disease and dialysis treatments involving red blood cell transfusions | Assess the feasibility and safety of online simultaneous blood volume and blood temperature monitoring during hemodialysis sessions in critically ill patients with acute kidney injury | 59 (58–70) | 52% | 48% | SBP of <90 mmHg or a drop of >40 mmHg from the baseline, requiring therapeutic interventions like fluid overload and vasopressor need to maintain adequate blood pressure | 26.40% (22.9%–30.1%) |
| Katherine E. Lynch, Fatimah Ghassemi, Jennifer E. Flythe, Mengling Feng, Marzyeh Ghassemi, Leo Anthony Celi, Steven M. Brunelli 32 | Sodium Modeling to Reduce Intradialytic Hypotension during Haemodialysis for Acute Kidney Injury in the Intensive Care Unit | Nephrology | 2016 | North America-USA | Prospective cohort study | ICU | 191 patients, 892 sessions | Adult patients undergoing at least one session of intermittent HD for ARF in the ICU | Patients with pre-existing end-stage renal disease requiring chronic renal replacement therapy or an estimated pre-admission glomerular filtration rate <15 mL/min/m² | Determine the association between sodium modeling and intradialytic hypotension | 62 | 39% | 61% | SBP of <80 mmHg or a drop of 50 mmHg from pre-HD BP | 10.7% (8.7%–12.8%) |
| Laurent Bitker, Frédérique Bayle, Hodane Yonis, Florent Gober, Véronique Leray, Romain Taponnier, Sophie Debord, Alina Stoian-Cividjian, Claude Guérin, Jean-Christophe Richard 35 | Prevalence and risk factors of hypotension associated with preload-dependence during intermittent hemodialysis in critically ill patients | Critical Care | 2016 | Europe-France | Single-center prospective observational study | ICU | 47 patients, 107 sessions | Patients aged ≥18 years with an already installed PiCCO® device for acute circulatory insufficiency and acute kidney injury requiring renal replacement therapy | Pregnancy, lower limb amputation, excessive nurse workload, known inferior vena cava obstruction, and previous inclusion in the study during a prior ICU admission | Evaluate the prevalence and risk factors of hypotension associated with preload-dependence during intermittent hemodialysis in the ICU | 67 (58–78) | 32% | 68% | MAP of <65 mmHg | 57% (47.5%–65.9%) |
| Hanan Mostafa, Mohamed Shaban, Ahmed Hasanin, Hassan Mohamed, Shymaa Fathy, Hossam M. Abdelreheem, Ahmed Lotfy, Ayman Abougabal, Ahmed Mukhtar, Akram El-adawy 30 | Evaluation of peripheral perfusion index and heart rate variability as early predictors for intradialytic hypotension in critically ill patients | MC Anesthesiology | 2019 | Africa- Egypt | Single-center prospective observational study | ICU | 36 critically ill patients | Critically ill adult patients with KDIGO stage III AKI scheduled for the first session of intermittent hemodialysis | Patients with pre-existing end-stage renal disease, severe vascular disease compromising peripheral perfusion index measurements, and severe burns preventing electrical cardiometry electrode application | Evaluate the peripheral perfusion index and heart rate variability as early predictors for intradialytic hypotension in critically ill patients | 49 ± 13 | 22% | 78% | 20% reduction in MAP from the baseline, requiring initiation or increase in the norepinephrine infusion rate | 64% (47.6%– 77.6%) |
| Yuriy Khanin, Jamie S. Hirsch, Daniel Stalbow, Meng Zhang, Zubair Hasan, Daniel W. Ross 33 | Intradialytic Hypotension in Critically Ill Patients on Hemodialysis With A-Line versus B-Line Pattern on Lung Ultrasonography | Kidney International Reports | 2021 | North America-USA | Retrospective cohort study in two hospitals | ICU | 113 patients | All adults (≥18 years) with electronic health records and documented POCUS undergoing a hemodialysis procedure | Patients without lung ultrasound, presence of interstitial lung disease including ARDS or pneumonia diagnosed by POCUS or other imaging | Evaluate if patients with A-line (dry lungs) pattern on LUS have a higher incidence of IDH compared to patients with B-line pattern | 63.14 (16.45) | 41.59% | 58.41% | Decrease in SBP of ≥20 mmHg with any of the following: failure to achieve prescribed ultrafiltration goal, initiation or increase in vasopressor dose during HD, development of symptoms consistent with IDH (e.g. abdominal discomfort, nausea, vomiting, muscle cramps, dizziness, or fainting) | 46.02% (37.1%–55.1%) |
BP: systolic blood pressure; HD: hemodialysis; MAP: mean arterial pressure; PiCCO®: Pulsion Medical Systems, Feldkirchen, Germany; IDH: intradialytic hypotension; mmHg: millimeters of mercury; ICU: intensive care unit; mL/min/m²: milliliters per minute per square meter; AKI: acute kidney injury; KDIGO: Kidney Disease Improving Global Outcomes; ARDS: acute respiratory distress syndrome; LUS: lung ultrasound; POCUS: point-of-care ultrasound.
Seven studies were conducted in intensive care units (ICU),30–36 and two studies were conducted with patients admitted to tertiary hospital hemodialysis units.28,29 The seven ICU studies indicated the most common admission causes, including sepsis and cardiovascular causes. Six articles reported the percentage of individuals undergoing mechanical ventilation at the time of inclusion.30,31,33–36
Six articles reported prognostic and severity scores, such as Charlson, SAPS II, SOFA, and APACHE at the time of inclusion.30,31,33–36 Respectively, one study reported a statistically significant higher APACHE 30 and SOFA 35 score in patients with IDH; however, others found no differences regarding the IDH status.34,36
All articles described the cause of renal replacement therapy. In six articles, the patients had AKI,30–32,34–36 and in three, the patients had both AKI and chronic kidney disease.28,29,33 In five articles, patient comorbidities were detailed, with the most relevant being chronic glomerulonephritis, hypertension, diabetes mellitus, and heart failure.28,29,32,33,36
Definitions of IDH
Of the selected studies, one included a decrease in SBP of ≥20 mmHg 31 as a primary component of its definition; two other studies added clinical symptoms or the need for intervention to this element for diagnosing IDH.29,33 In two studies, hypotension was defined as the first occurrence of a MAP below 65 mmHg during the session.34,35
One defined IDH as a 20% reduction in MAP from the baseline, requiring the initiation or increase of the norepinephrine infusion rate. 30 Two studies identified IDH when SBP values were <80 and <90 mmHg or a drop of ≥50 and ≥40 mmHg, respectively, relative to pre-hemodialysis blood pressure.32,36
One study defined IDH when patients reached a MAP of <70 mmHg for at least 5 min or an SBP of <100 mmHg, or required any pause in ultrafiltration, or needed an intervention such as the administration of intravenous fluid bolus or an increase in vasoactive drug dosage. 31
Prevalence of IDH
From the studies that used the number of sessions, the prevalence of hemodialysis complicated with IDH varied from 10.7% to 57%, as shown in Table 2.
Table 2.
Prevalence by number of sessions.
| Author | Number of sessions | Prevalence |
|---|---|---|
| Tonelli (2002)31 | 57 | 30.0% |
| Cheyron (2009)36 | 572 | 26.4% |
| Lynch (2016)32 | 892 | 10.7% |
| Bitker (2016)35 | 107 | 57.0% |
Studies measuring the prevalence of IDH according to the number of patients showed values ranging from 8.6% in a study with 404 participants 28 to 64% in a study with 36 patients, 29 as shown in Table 3.
Table 3.
Prevalence by number of participants.
| Author | Number of participants | Prevalence |
|---|---|---|
| Okaka (2017)28 | 217 | 19.8% |
| Ogochukwu (2017)29 | 404 | 8.6% |
| Da Hora (2021)34 | 248 | 31.9% |
| Mostafa (2019)30 | 36 | 64% |
| Ross (2021)33 | 113 | 46.02% |
Table 4 shows the prevalence of IDH by sex. In all studies, the prevalence was higher for men.
Table 4.
Prevalence of IDH by sex.
| Author | Prevalence |
|---|---|
| Da Hora (2021)34 | Male: 58.2% Female: 41.8% |
| Bitker (2016)35 | Male : 77% Female: 23% |
| Mostafa (2019)30 | Male: 87% Female: 13% |
Quantitative synthesis results
Heterogeneity
The heterogeneity among the nine included studies was assessed, and it was concluded that there is high heterogeneity across studies (I²: 97.87%; p < 0.01). It did not differ after subgroup analysis based on the RoB and the number of sessions or participants, where heterogeneity remained considerable. The Galbraith plot is shown in Supplemental Appendix 2.
A meta-regression analysis was performed with the variables sample size, RoB, and number of sessions versus number of participants.
Regarding the number of participants, an I² of 95.5% was identified, of which 46.6% corresponds to this variable. The meta-regression model was significant (p = 0.006). The corresponding bubble plot is shown in Supplemental Appendix 3. Other covariates, such as the RoB and the number of patients versus the number of sessions, showed no significance in the meta-regression model (p = 0.45 and p = 0.85, respectively).
Effect measure
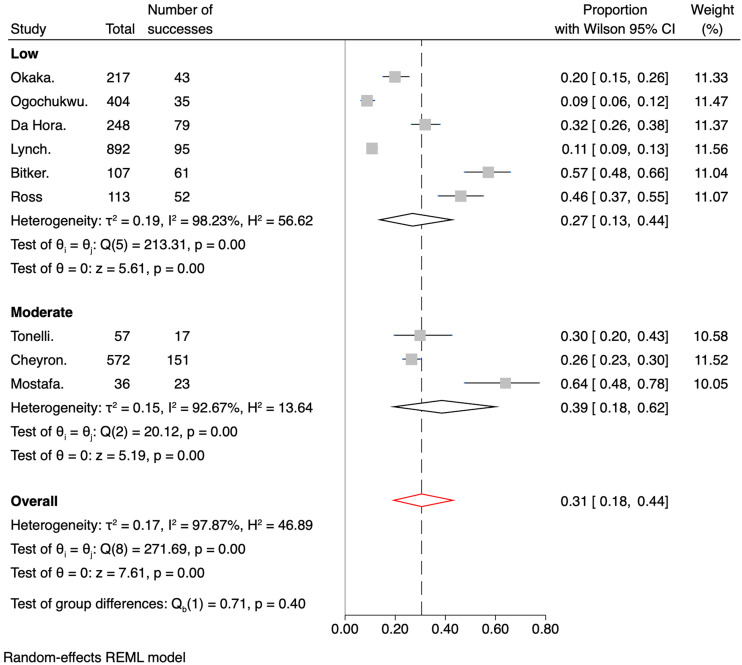
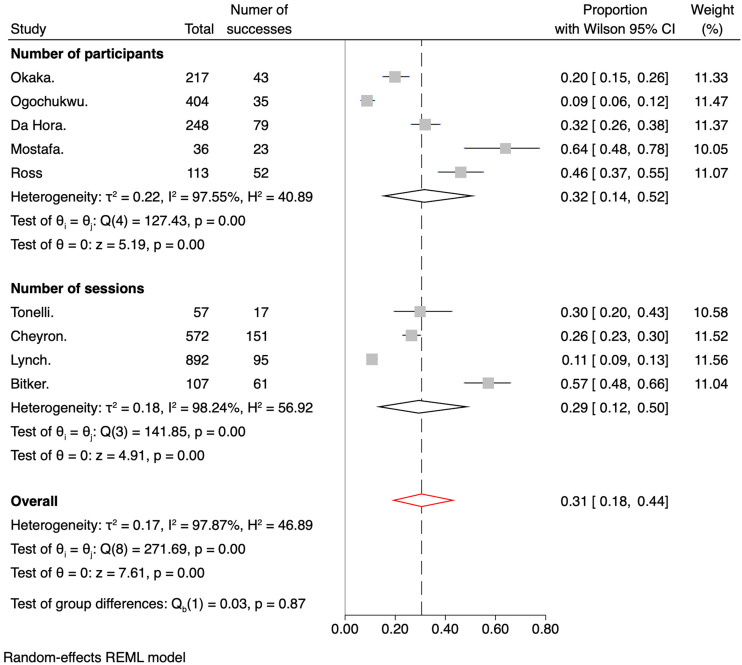
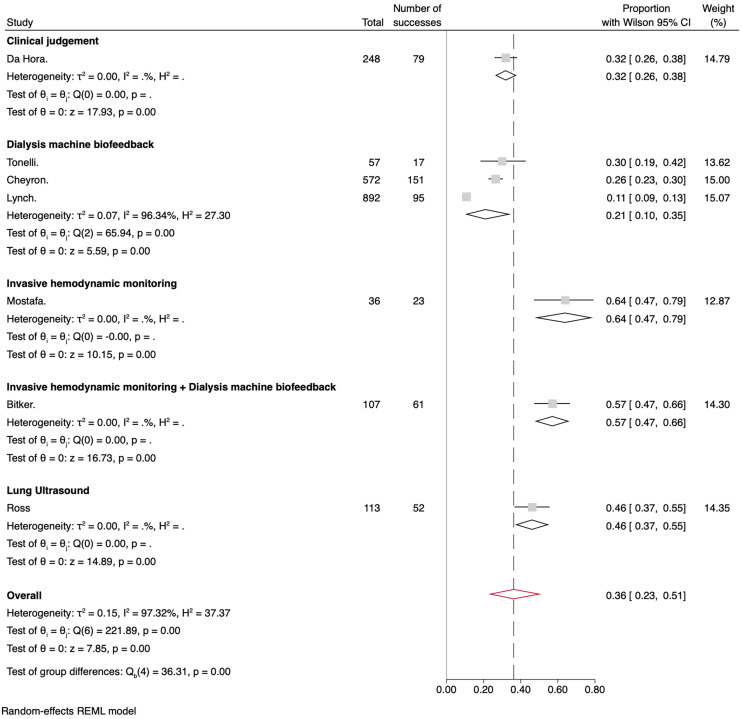
The meta-analysis's overall IDH prevalence across the nine included studies was 31% (95% CI 0.18–0.44). The cumulative prevalences by subgroups of RoB were 27% (95% CI 0.13–0.44) for the low bias subgroup and 39% (95% CI 0.18–0.62) for the medium bias subgroup, as shown in Figure 5. Regarding the number of sessions versus the number of participants, Figure 6 shows the pooled prevalence, which ranged from 32% (95% CI 0.14–0.52) for the number of patient subgroup to 29% (95% CI 0.12–0.50) for the number of dialysis session subgroup. Another analysis was performed with studies involving only patients admitted to the ICU (Figure 7). Subgroups were created based on the measures taken to prevent IDH. The overall prevalence was 36% (95% CI 0.23–0.51). The subgroup with the highest prevalence was the one who received any invasive hemodynamic monitoring (64%; 95% CI 0.47–0.79), and the one with the lowest prevalence was the group that received any dialysis machine biofeedback (21%; 95% CI 0.10–0.35).
Figure 5.
Forest plot for the prevalence of IDH divided by risk of bias.
Figure 6.
Forest plot for the prevalence of IDH divided by the number of participants or sessions.
Figure 7.
Forest plot for the prevalence of IDH in critical patients divided by the measures adopted to prevent IDH.
In the sensitivity analysis, assuming an I2 of 10%, the prevalence of IDH decreased to 20%. Analyzing the effect size (prevalence) by removing one study at a time, the prevalence ranged from 27% to 33% (Table 5).
Table 5.
Leave-one-out meta-analysis.
| Omitted study | Proportion | [95% confidence interval] | p-value |
|---|---|---|---|
| Okaka | 0.321 | 0.185–0.473 | <0.001 |
| Ogochukwu | 0.339 | 0.217–0.474 | <0.001 |
| Tonelli | 0.307 | 0.172–0.460 | <0.001 |
| Da Hora | 0.304 | 0.169–0.459 | <0.001 |
| Cheyron | 0.312 | 0.175–0.467 | <0.001 |
| Lynch | 0.336 | 0.209–0.475 | <0.001 |
| Bitker | 0.274 | 0.160–0.405 | <0.001 |
| Mostafa | 0.271 | 0.162–0.396 | <0.001 |
| Ross | 0.287 | 0.161–0.433 | <0.001 |
| Proportion | 0.305 | 0.185–0.441 | <0.001 |
Through sensitivity analysis using the Sidik–Jonkman and the Knapp–Hartung truncated method for adjusting the standard error of the effect size, the prevalence of IDH was 30.5%.
Publication bias
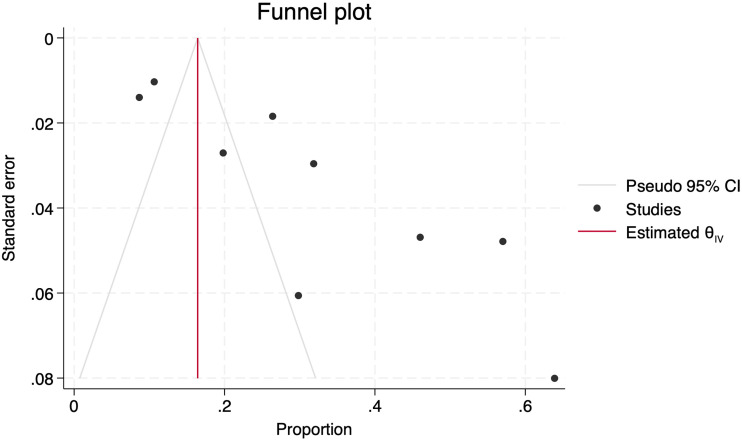
The effect of small studies was assessed using Egger's test, which reached a significance level of p = 0.0015, subjectively confirmed by the asymmetric distribution observed in the Funnel plot (Figure 8). Finally, a Trim-and-fill analysis was conducted, and no new articles were imputed.
Figure 8.
Funnel plot with asymmetric distribution of studies.
Discussion
In our systematic review, the overall incidence of IDH in hospitalized patients was 31%, with minimal variability when considering the study RoB and prevalence measured according to the different approaches for renal replacement therapy. However, the prevalence is higher than that reported in a large 2019 study that included non-hospitalized patients 36 and similar to the reported in other publications.14,37–41 Our prevalence significantly decreased to 20% when we performed the sensitivity analysis, assuming an I2 of 10%.
The wide range of prevalence rates also suggests the existence of multifactorial factors contributing to the occurrence of hypotension during dialysis sessions and the variety of definitions.2,3 Currently, there is no general consensus on the best evidence-based indicators of IDH; however, our report found definitions that include a reduction in SBP of ≥20 mmHg with or without additional symptoms or as a drop in MAP.
Cardiovascular instability during dialysis is linked to severe complications such as intestinal ischemia, myocardial infarction, and access thrombosis. 42 This condition may also hasten the decline of residual renal function and adversely affect renal recovery in patients with severe AKI.43,44 Given the high prevalence observed in our study, the critical challenge for nephrologists, intensivists, and nurses is to implement early interventions to prevent these complications.
We identified the demographic and clinical characteristics of patients, as well as causes and pre-existing conditions associated with IDH. Chronic glomerulonephritis, primary hypertension, diabetes mellitus, and heart failure were the most prevalent. From our results, we could also infer that sepsis was the most common cause of IDH in ICU-admitted patients. In addition, men were most affected by IDH, which differs from results previously published where the female sex is considered a risk factor for IDH.45–50
Some of the included studies also suggest measures to prevent IDH.29–36 However, we believe an individualized assessment based on the severity and the risk of IDH should be performed before and during renal replacement therapy in a hospitalization or ICU setting.
The data presented in this systematic review should be interpreted in terms of its limitations and strengths. The available evidence has limitations, such as heterogeneity between studies and the lack of standardization in the diagnostic criteria for IDH. We recommend an initial assessment of the patient before the onset of the renal replacement therapy and consecutive hemodynamic evaluations to determine the origin of the shock and to individualize the treatment based on current guidelines. 51
Among the strengths of our systematic review are that the search strategy was exhaustive and conducted according to a previously developed protocol and that studies with a broad demographic distribution were included, which ensures a global approximation of IDH prevalence. Finally, all patients included were hospitalized or admitted to the ICU, providing a clearer picture of IDH in these settings.
Conclusions
In our systematic review and meta-analysis, the prevalence of hospital-based renal replacement therapies with IDH in hospitalized patients was 31%.
Although it is challenging to establish a uniform definition of IDH that encompasses decreases in blood pressure, symptoms, and interventions taken in response to hemodynamic instability, standardizing a single definition would facilitate understanding and homogenization of diagnoses and improve decision-making by healthcare personnel, making it more appropriate and timelier.
Supplemental Material
Supplemental material, sj-docx-1-sci-10.1177_00368504241308982 for Prevalence of cardiovascular instability during hemodialysis therapy in hospitalized patients: A systematic review and meta-analysis by Karla Arcentales-Vera, María Fernanda Vera-Mendoza, Cristian Cevallos-Salas, María Fernanda García-Aguilera and Luis Fuenmayor-González in Science Progress
Supplemental material, sj-jpg-2-sci-10.1177_00368504241308982 for Prevalence of cardiovascular instability during hemodialysis therapy in hospitalized patients: A systematic review and meta-analysis by Karla Arcentales-Vera, María Fernanda Vera-Mendoza, Cristian Cevallos-Salas, María Fernanda García-Aguilera and Luis Fuenmayor-González in Science Progress
Supplemental material, sj-jpg-3-sci-10.1177_00368504241308982 for Prevalence of cardiovascular instability during hemodialysis therapy in hospitalized patients: A systematic review and meta-analysis by Karla Arcentales-Vera, María Fernanda Vera-Mendoza, Cristian Cevallos-Salas, María Fernanda García-Aguilera and Luis Fuenmayor-González in Science Progress
Acknowledgements
We extend our sincere gratitude to Dr. Fabricio Picoita for his guidance throughout the course of this research. We also wish to acknowledge the support of the Universidad Central del Ecuador and the Consejo de Postgrado Rodrigo Yépez for providing the necessary resources and facilities that made this study possible.
Footnotes
Author contributions: A-V. K. contributed to data curation, formal analysis, resources, investigation, writing the original draft, and writing, reviewing, and editing the final draft. V-M. M. contributed to data curation, formal analysis, resources, investigation, writing the original draft, and writing, reviewing, and editing the final draft. C-S. C. contributed to project administration, investigation, validation, and writing, reviewing, and editing of the final draft. G-A. M. contributed to the formal analysis, methodology, validation, visualization, and reviewing and editing of the final draft. F-G. L. contributed to the conceptualization, formal analysis, methodology, project administration, validation, visualization, and writing, reviewing and editing of the final draft.
Data availability: Data will be made available upon reasonable request following communication with the corresponding author.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Luis Fuenmayor-González https://orcid.org/0000-0001-6141-7692
Supplemental material: Supplemental material for this article is available online.
References
- 1.Yildiz AB, Vehbi S, Covic A, et al. An update review on hemodynamic instability in renal replacement therapy patients. Int Urol Nephrol 2023; 55: 929–942. [DOI] [PubMed] [Google Scholar]
- 2.Assimon MM, Flythe JE. Definitions of intradialytic hypotension. Semin Dial 2017; 30: 464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laurence Saint LSQ, Larizza C, Nocera A, et al. A comparative study of the definitions of intradialytic hypotension correlated with increased mortality to identify universal predictors. Int J Med Inform 2023; 173: 104975. [DOI] [PubMed] [Google Scholar]
- 4.K/DOQI Workgroup. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis 2005; 45: 16–153. [PubMed] [Google Scholar]
- 5.Shawwa K, Kompotiatis P, Jentzer JC, et al. Hypotension within one-hour from starting CRRT is associated with in-hospital mortality. J Crit Care 2019; 54: 7–13. [DOI] [PubMed] [Google Scholar]
- 6.Beaubien-Souligny W, Yang Y, Burns KEA, et al. Intra-dialytic hypotension following the transition from continuous to intermittent renal replacement therapy. Ann Intensive Care 2021; 11. DOI: 10.1186/S13613-021-00885-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chazot G, Bitker L, Mezidi M, et al. Prevalence and risk factors of hemodynamic instability associated with preload-dependence during continuous renal replacement therapy in a prospective observational cohort of critically ill patients. Ann Intensive Care 2021; 11. DOI: 10.1186/S13613-021-00883-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douvris A, Zeid K, Hiremath S, et al. Mechanisms for hemodynamic instability related to renal replacement therapy: a narrative review. Intensive Care Med 2019; 45: 1333–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Douvris A, Malhi G, Hiremath S, et al. Interventions to prevent hemodynamic instability during renal replacement therapy in critically ill patients: a systematic review. Crit Care 2018; 22. DOI: 10.1186/S13054-018-1965-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villa G, Husain-Syed F, Saitta T, et al. Hemodynamic instability during acute kidney injury and acute renal replacement therapy: pathophysiology and clinical implications. Blood Purif 2021; 50: 729–739. [DOI] [PubMed] [Google Scholar]
- 11.Tsujimoto Y, Tsujimoto H, Nakata Y, et al. Dialysate temperature reduction for intradialytic hypotension for people with chronic kidney disease requiring haemodialysis. Cochrane Database Syst Rev 2019; 2019. DOI: 10.1002/14651858.CD012598.PUB2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silversides JA, Pinto R, Kuint R, et al. Fluid balance, intradialytic hypotension, and outcomes in critically ill patients undergoing renal replacement therapy: a cohort study. Crit Care 2014; 18. DOI: 10.1186/S13054-014-0624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burton JO, Jefferies HJ, Selby NMet al. et al. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol 2009; 4: 914–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sands JJ, Usvyat LA, Sullivan T, et al. Intradialytic hypotension: frequency, sources of variation and correlation with clinical outcome. Hemodial Int 2014; 18: 415–422. [DOI] [PubMed] [Google Scholar]
- 15.Shoji T, Tsubakihara Y, Fujii Met al. et al. Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int 2004; 66: 1212–1220. [DOI] [PubMed] [Google Scholar]
- 16.Stefánsson BV, Brunelli SM, Cabrera C, et al. Intradialytic hypotension and risk of cardiovascular disease. Clin J Am Soc Nephrol 2014; 9: 2124–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tislér A, Akócsi K, Borbás B, et al. The effect of frequent or occasional dialysis-associated hypotension on survival of patients on maintenance haemodialysis. Nephrol Dial Transplant 2003; 18: 2601–2605. [DOI] [PubMed] [Google Scholar]
- 18.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J 2021; 372: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ouzzani M, Hammady H, Fedorowicz Zet al. et al. Rayyan-a web and mobile app for systematic reviews. Syst Rev 2016; 5: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.JBI. Anexo 5.2: Formulario de extracción de datos para estudios de prevalencia - Manual del JBI para la Síntesis de la Evidencia - JBI Global Wiki, https://jbi-global-wiki.refined.site/space/MDJPLSDLE/237503135/Anexo+5.2%3A+Formulario+de+extracci%C3%B3n+de+datos+para+estudios+de+prevalencia (2024, accessed 6 August 2024).
- 21.Statistics Kingdom. Proportion confidence interval calculator - normal approximation (Wilson interval), Clopper–Pearson, Wilson score interval, https://www.statskingdom.com/proportion-confidence-interval-calculator.html (2024, accessed 6 May 2024).
- 22.Munn Z, Moola S, Lisy K, et al. Systematic reviews of prevalence and incidence. In: Aromataris E, Munn Z. (eds) JBI Manual for Evidence Synthesis. JBI, 2020, pp.177–217. [Google Scholar]
- 23.Borges Migliavaca C, Stein C, Onica Colpani V, et al. Quality assessment of prevalence studies: a systematic review. J Clin Epidemiol 2020; 127: 59–68. [DOI] [PubMed] [Google Scholar]
- 24.Munn Z, MClinSc SM, Lisy K, et al. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc 2015; 13: 147–153. [DOI] [PubMed] [Google Scholar]
- 25.McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods 2021; 12: 55–61. [DOI] [PubMed] [Google Scholar]
- 26.McHugh ML. Interrater reliability: the kappa statistic. Biochem Med 2012; 22: 276–282. https://pubmed.ncbi.nlm.nih.gov/23092060/ (accessed 7 August 2024). [PMC free article] [PubMed] [Google Scholar]
- 27.StataCorp. Stata statistical software: release 18. College Station, TX: StataCorp LLC, 2023. [Google Scholar]
- 28.Okaka E, Okwuonu C. Blood pressure variation and its correlates among patients undergoing hemodialysis for renal failure in Benin City, Nigeria. Ann Afr Med 2017; 16: 65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okoye OC, Slater HE, Rajora N. Prevalence and risk factors of intra-dialytic hypotension: a 5 year retrospective report from a single Nigerian Centre. Pan Afr Med J 2017; 28. DOI: 10.11604/PAMJ.2017.28.62.13743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mostafa H, Shaban M, Hasanin A, et al. Evaluation of peripheral perfusion index and heart rate variability as early predictors for intradialytic hypotension in critically ill patients. BMC Anesthesiol 2019; 19. DOI: 10.1186/S12871-019-0917-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tonelli M, Astephen P, Andreou P, et al. Blood volume monitoring in intermittent hemodialysis for acute renal failure. Kidney Int 2002; 62: 1075–1080. [DOI] [PubMed] [Google Scholar]
- 32.Lynch KE, Ghassemi F, Flythe JE, et al. Sodium modelling to reduce intradialytic hypotension during haemodialysis for acute kidney injury in the intensive care unit. Nephrology (Carlton) 2016; 21: 870–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khanin Y, Hirsch JS, Stalbow D, et al. Intradialytic hypotension in critically ill patients on hemodialysis with A-line versus B-line pattern on lung ultrasonography. Kidney Int Rep 2021; 6: 1969–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Da R, Passos H, Caldas JR, et al. Prediction of hemodynamic tolerance of intermittent hemodialysis in critically ill patients: a cohort study. Sci Rep 2021; 11: 23610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bitker L, Bayle F, Yonis H, et al. Prevalence and risk factors of hypotension associated with preload-dependence during intermittent hemodialysis in critically ill patients. Crit Care 2016; 20. DOI: 10.1186/S13054-016-1227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DuCheyron D, Lucidarme O, Terzi Net al. et al. Blood volume- and blood temperature-controlled hemodialysis in critically ill patients: a 6-month, case-matched, open-label study. Blood Purif 2010; 29: 245–251. [DOI] [PubMed] [Google Scholar]
- 37.Davenport A. Can advances in hemodialysis machine technology prevent intradialytic hypotension? Semin Dial 2009; 22: 231–236. [DOI] [PubMed] [Google Scholar]
- 38.Perazella MA. Pharmacologic options available to treat symptomatic intradialytic hypotension. Am J Kidney Dis 2001; 38: S26–S36. [DOI] [PubMed] [Google Scholar]
- 39.Dubin R, Owens C, Gasper W, et al. Associations of endothelial dysfunction and arterial stiffness with intradialytic hypotension and hypertension. Hemodial Int 2011; 15: 350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Passauer J, Bussemaker E, Gross P. Dialysis hypotension: do we see light at the end of the tunnel? Nephrol Dial Transplant 1998; 13: 3024–3029. [DOI] [PubMed] [Google Scholar]
- 41.Zucchelli P, Santoro A. Dialysis-induced hypotension: a fresh look at pathophysiology. Blood Purif 1993; 11: 85–98. [DOI] [PubMed] [Google Scholar]
- 42.Reilly RF. Attending rounds: a patient with intradialytic hypotension. Clin J Am Soc Nephrol 2014; 9: 798–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Misra M, Vonesh E, Van Stone JC, et al. Effect of cause and time of dropout on the residual GFR: a comparative analysis of the decline of GFR on dialysis. Kidney Int 2001; 59: 754–763. [DOI] [PubMed] [Google Scholar]
- 44.Daugirdas JT. Pathophysiology of dialysis hypotension: an update. Am J Kidney Dis 2001; 38: S11–S17. [DOI] [PubMed] [Google Scholar]
- 45.Degoulet P, Réach I, Di Giulio S, et al. Epidemiology of dialysis induced hypotension. Proc Eur Dial Transplant Assoc 1981; 18: 133–138. https://pubmed.ncbi.nlm.nih.gov/7329960/ (accessed 7 August 2024). [PubMed] [Google Scholar]
- 46.Rehman Awan AU, Shafi T, Mand Ahmed A, et al. Frequency of intradialytic hypotension among patients on maintenance heamodialysis. Pak J Med Health Sci 2011; 5: 461–465. [Google Scholar]
- 47.Kuipers J, Oosterhuis JK, Krijnen WP, et al. Prevalence of intradialytic hypotension, clinical symptoms and nursing interventions - a three-months, prospective study of 3818 haemodialysis sessions. BMC Nephrol 2016; 17. DOI: 10.1186/S12882-016-0231-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu J, Liu Z, Shen B, et al. Intradialytic hypotension as an independent risk factor for long-term mortality in maintaining hemodialysis patients: a 5-year follow-up cohort study. Blood Purif 2018; 45: 320–326. [DOI] [PubMed] [Google Scholar]
- 49.Orofino L, Marcen R, Quereda C, et al. Epidemiology of symptomatic hypotension in hemodialysis: is cool dialysate beneficial for all patients? Am J Nephrol 1990; 10: 177–180. [DOI] [PubMed] [Google Scholar]
- 50.Steinwandel U, Gibson N, Towell-Barnard M, et al. Measuring the prevalence of intradialytic hypotension in a satellite dialysis clinic: are we too complacent? J Clin Nurs 2018; 27: e1561–e1570. [DOI] [PubMed] [Google Scholar]
- 51.Hasanin A, Sanfilippo F, Dünser MW, et al. The MINUTES bundle for the initial 30 min management of undifferentiated circulatory shock: an expert opinion. Int J Emerg Med 2024; 17: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-sci-10.1177_00368504241308982 for Prevalence of cardiovascular instability during hemodialysis therapy in hospitalized patients: A systematic review and meta-analysis by Karla Arcentales-Vera, María Fernanda Vera-Mendoza, Cristian Cevallos-Salas, María Fernanda García-Aguilera and Luis Fuenmayor-González in Science Progress
Supplemental material, sj-jpg-2-sci-10.1177_00368504241308982 for Prevalence of cardiovascular instability during hemodialysis therapy in hospitalized patients: A systematic review and meta-analysis by Karla Arcentales-Vera, María Fernanda Vera-Mendoza, Cristian Cevallos-Salas, María Fernanda García-Aguilera and Luis Fuenmayor-González in Science Progress
Supplemental material, sj-jpg-3-sci-10.1177_00368504241308982 for Prevalence of cardiovascular instability during hemodialysis therapy in hospitalized patients: A systematic review and meta-analysis by Karla Arcentales-Vera, María Fernanda Vera-Mendoza, Cristian Cevallos-Salas, María Fernanda García-Aguilera and Luis Fuenmayor-González in Science Progress