Abstract
Background
Fibromyalgia (FM) is a generalized, widespread chronic pain disorder affecting 2.7% of the general population. In recent years, different studies have observed a strong association between FM and psychological trauma. Therefore, a trauma-focused psychotherapy, such as Eye Movement Desensitization and Reprocessing (EMDR), combined with a non-invasive brain stimulation technique, such as multifocal transcranial current stimulation (MtCS), could be an innovative adjunctive treatment option. This double-blind randomized controlled trial (RCT) analyzes if EMDR therapy is effective in the reduction of pain symptoms in FM patients, and if its potential is boosted with the addition of MtCS.
Methods
Ninety-six patients with FM and a history of traumatic events will be randomly allocated to the treatment as usual (TAU) condition, EMDR + active-MtCS condition, or EMDR + sham-MtCS condition. Therapists and patients will be kept blind to MtCS conditions, and raters will be kept blind to both EMDR and MtCS. All patients will be evaluated at baseline, post-treatment, and follow-up at 6 months after post-treatment. Evaluations will assess the following variables: sociodemographic data, pain, psychological trauma, sleep disturbance, anxiety and affective symptoms, wellbeing, self-care, emotional regulation, self-esteem, and cognitive functioning.
Discussion
This study will provide evidence of whether EMDR therapy is effective in reducing pain symptoms in FM patients, and whether the effect of EMDR can be enhanced by MtCS.
Trial registration number
This trial was registered at ClinicalTrials.gov on 2 August 2019, identifier: NCT04084795.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13063-024-08708-3.
Keywords: Fibromyalgia, Eye Movement Desensitization and Reprocessing, Multifocal transcranial current stimulation, Psychological trauma, Pain, Randomized controlled trial
Background
Fibromyalgia (FM) is a chronic pain (CP) disorder characterized by generalized and widespread pain, sleep disturbance, fatigue, cognitive dysfunction, and affective symptoms. The average prevalence of FM worldwide is 2.7%, affecting mainly female patients [1]. Although the etiology of FM remains unknown, it is currently conceptualized as a disorder involving the sensitization of the central nervous system (CNS) and impairments in endogenous pain inhibitory mechanisms, with genetic, hormonal, and immunological factors playing a role [2]. Different risk factors associated with FM onset have been highlighted, including the presence of traumatic experiences such as sexual and physical abuse, chronic stress, and adverse lifetime events [3–5]. In fact, posttraumatic stress disorder (PTSD) is considered an important and frequently present comorbidity in patients suffering from FM [6, 7], although it is often not given priority as a treatment objective. While the mechanism which links adverse events to FM development is not fully understood, evidence shows that allodynia and hyperalgesia can be stress-induced [8], and psychological trauma is thought to lead to dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis, linked to the aberrant cortisol secretion and epigenetic mechanisms [3, 4]. Therefore, psychological therapies focused on adaptive processing of traumatic experiences may improve both somatic and psychological symptoms and could be an innovative treatment option for FM.
Eye Movement Desensitization and Reprocessing (EMDR) is an integrative psychotherapeutic approach developed by Francine Shapiro at the end of the 1980s. While there is ongoing debate regarding the evidence for EMDR in treating PTSD [9], an individual data participant meta-analysis by the same group found no significant differences between EMDR and other trauma-focused interventions, such as trauma-focused cognitive behavioral psychotherapy [10]. Moreover, It has been recognized by the World Health Organization as a first-line therapeutic tool for PTSD [11] due to its efficacy in reducing clinical symptoms, evidenced in several meta-analyses in both adults [12–16] and children and adolescents [17, 18]. EMDR has shown its clinical applicability in the treatment of other pathologies, such as addictions [19], depression [20], anxiety [21], and CP [22]. In this last case, to date seven randomized controlled trials (RCT) have investigated the efficacy of EMDR in different conditions of CP: chronic pain [23], migraine headaches [24], chronic back pain [25], pain due to rheumatoid arthritis [26], acute pain after abdominal surgery [27], and pain due to FM or diffuse chronic pain [28]. These studies showed EMDR significantly reduced pain levels in comparison with TAU [23, 25–27] and in comparison with other treatment options, for example, standard pharmacology [24], guided imagery [26], or eclectic therapy [28]. Additionally, some systematic reviews also show positive outcomes for EMDR in non-PTSD conditions. Scelles and Bulnes [29] reported overall positive outcomes for EMDR in most cases (addictions, somatoform disorders, sexual dysfunction, eating disorders, personality disorders, mood disorders, pain, neurodegenerative disorders, sleep, etc.) and even being successful in usually difficult or unproductive cases (e.g., dementia or aphasia). Regarding pain specifically, Tesarz et al. [30]’s results suggest that EMDR may be a promising treatment line for chronic pain conditions with controlled trials demonstrating significant improvements in pain intensity, disability, depressive, and anxiety symptoms. However, the studies on this topic are few and contain methodological biases, such as a lack of active control subjects, variability in PTSD and depression diagnoses, and the high associated comorbidity.
Given the complex etiology of FM, combining psychotherapy with other treatment options might increase the effectiveness of the treatment and maximize therapeutic success. Due to the key role that CNS sensitivity plays in FM, pioneering non-invasive brain stimulation (NIBS) techniques, such as multifocal transcranial current stimulation (MtCS), are increasingly being investigated, as these can modify neural activities related to pain. This neurophysiological technique represents a promising intervention option, given its capacity to modulate cerebral excitability in a safe, not invasive, and painless manner [31]. The principal mechanism of action of MtCS is a subthreshold modulation of neuronal membrane potentials, modifying cortical excitability and activity dependent on the current direction of flow through the target neurons [32]. Based on this, MtCS modulates spontaneous neuronal activity without generating activity changes in resting neuronal networks [33], its effects depending on the previous state of physiological basal activity of the region of interest [34].
There is increasing evidence which shows that, in addition to psychotherapeutic and pharmacologic interventions, MtCS is useful in treating FM and CP in terms of inducing pain relief and improving quality of life [35–49]. Interestingly, MtCS can also increase the therapeutic potential of other therapies when used in conjunction with them [50]. In this regard, a recent review has shown that the combination of MtCS with Cognitive Therapies, such as Cognitive Control Therapy (CCT), increases its benefits when used for treating depressive disorders [50].
Taking into account the information presented above, we hypothesize that the combination of a trauma-focused therapy, such as EMDR, combined with MtCS targeted in the left dorsolateral prefrontal cortex (lDLPFC) could be a useful therapeutic option [51] for the treatment of patients with FM. The main reasons for selecting the lDLPFC are as follows: (1) this region is critical in the regulation of the limbic system, implicated in emotion, which is hyperactivated in traumatized patients [52]; (2) it has been seen to be relatively hypoactivated in patients with depression [52–54], anxiety disorders [53, 54] and PTSD [55]; (3) there is a proved efficacy of lDLPFC MtCS in the treatment of resistant and non-resistant depression [50, 55], which suggests that FM patients will also benefit due to their comorbidities; (4) the lDLPFC also plays a central role in executive functions [56], and MtCS has the potential to improve cognitive functions associated with cortical plasticity, such as therapy-related learning processing [57].
Although in two studies it was found that the application of MtCS on the lDLPFC was generally less effective in reducing pain in FM patients compared to the application of stimulation on the primary motor cortex [43, 49], other studies were able to demonstrate a short-term efficacy in terms of pain and life quality [38], pain and fatigue [41], and pain and improvement in executive attention and orientation [37]. In all the studies mentioned, the stimulation applied over lDLPFC was anodal.
Regarding the mechanisms underlying the effects of DLPFC/tDCS, while no studies have specifically explored them, it is plausible that the cognitive and affective effects of DLPFC stimulation could be induced through the connections with the limbic system (fronto-limbic network) [58]; while the effects on pain relief could be due to connectivity with the Diffuse Noxious Inhibitory Controls (DNIC) pathways that are involved in the inhibitory modulation of nociceptive input [59, 60].
Recently, multifocal transcranial current stimulation (MtCS) devices have been used to achieve more focal stimulation of specific cortical targets, because they use smaller electrodes compared to MtCS devices. Different methods have been designed to optimize the configuration of MtCS montage for stimulation of brain networks, represented by spatially extended cortical targets [61]. In this sense, MtCS could be more effective in modifying the functioning of the networks that are altered in patients with FM [58–60]. Previous studies with MtCS in FM have focused on the DLPFC; however, the effects of modulating the activity of this area with MtCS have not yet been explored.
Methods/design
Aims
The main objective of the study is to analyze whether EMDR therapy is effective in the reduction of pain symptoms in FM patients, and if its potential is boosted with the addition of MtCS. As secondary objectives, the study will analyze whether EMDR therapy is effective in reducing psychological trauma symptoms and comorbid symptoms of anxiety and depression, and in improving sleep quality and patient wellbeing, and if these effects are boosted by the addition of MtCS.
Study design
Within a double-blind randomized controlled design, patients will be randomized to (1) Waitlist Condition, 92) EMDR + active-MtCS (20 sessions), or (3) EMDR + sham-MtCS (20 sessions). All subjects will continue to receive their treatment as usual (TAU), regardless of the group to which they have been assigned during the study. If a participant does not attend 3 sessions consecutively, she will be withdrawn from the study. In this case, no follow-up will be carried out, all gathered data will be kept and used for pertinent analyses unless the patient rescinds their consent. If this is the case, all data will be erased. Psychotherapists and patients will be kept blind for MtCS treatment conditions until the end of the trial, and raters will be kept blind to both EMDR and MtCS conditions. It is not possible for patients to be blind to the EMDR condition due to its use of bilateral stimulation. The experimental condition assigned to the participants will only be revealed if the patient abandons the study. Otherwise, the blind condition will be maintained until the end of the study. All patients will be clinically evaluated at baseline, at post treatment and at 6 months from post treatment as follow-up.
Figure 1 shows a flow chart of the progress of the study.
Fig. 1.

SPIRIT checklist—Flow diagram: Schedule of enrollment, interventions and assessments. t1 = Baseline evaluation; 0 = randomization process; t2 = Post-treatment evaluation; t3 = Follow-up evaluation; t4 = Waitlist group participants receive treatment; VAS pain = Visual Analog Scale for pain; PCS = Pain Catastrophizing Scale; FIQ-R = Fibromyalgia Impact Questionnaire; BFI = Brief Fatigue Inventory; CTQ = Childhood Trauma Questionnaire; EGEP-5 = Evaluación General del Estrés Postraumático; TTE = Timeline of traumatic experiences; PCL-5 = Posttraumatic Stress Disorder Checklist, ITQ = The International Trauma Questionnaire; SUD = Subjective Units of Distress; CDS = Cambridge Depersonalization Scale; SDQ-20 = Somatoform Dissociation Questionnaire, MINI = MINI International Neuropsychiatric Scale; HADS = Hospital Anxiety and Depression Scale; AIS = Athens Insomnia Scale; SWLS = Satisfaction With Life Scale; ERSQ = Emotion Regulation Skills Questionnaire; RSE = Rosenberg Self-Esteem Scale; SCIP = Screen for cognitive impairment in psychiatry
Research setting
This multicenter collaborative project will involve the participation of the Centre Fòrum Research Unit of the Hospital del Mar as the entity responsible for coordinating the study and carrying out the evaluations, the Rheumatology Department of Parc de Salut Mar for patient diagnosis and referral to the study, the Institut d'investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) for randomization and data base management, and the Cognitive Neuro-Lab of Open University of Catalonia (UOC) for MtCS. External accredited EMDR psychotherapists, who have extensive experience and have received specific training for this study, will carry out the therapy, with supervision from the Centre Fòrum Research Unit (Barcelona, Spain). The study has been approved by the Ethics Committee of the IMIM, Parc de Salut Mar (2019/8772/I). All participants will sign the informed consent prior to enrollment in the baseline visit. Since this study involves a low-risk intervention, a Data Monitoring Committee will not be considered. However, AV (PI) and BA (co-PI) will be in charge of assessing the progress of the trial, data safety and, if needed, may access unblinded study information during the duration of the study in order to provide recommendations regarding study modification. Any deviation from the initial protocol will be communicated to the Ethics Committee through an official statement as well as to the Clinical Trials register.
Participants
The patient sample will consist of 96 females who have been diagnosed by the Rheumatology Department of the Hospital del Mar, Barcelona, Spain, through a clinical interview aligned with the 2016 American College of Rheumatology criteria for FM. According to the CDC [62], FM is twice as likely to happen to women than men. However, in a population-based cross-sectional study by Font Gayà et al. [63] evaluating the prevalence of fibromyalgia and associated factors in Spain, the distribution by sex prevalence was 4.49% in women (95% CI, 3.76–5.35); significantly higher than the 0.29% in men (95% CI, 0.14–0.60). Our own cross-sectional study about trauma prevalence in FM was offered to both sexes, but only female patients were finally referred and included [64]. As the distribution in the study’s country is so skewed towards the female population, we decided to include female patients only. Patients with this diagnosis will be referred from the Rheumatology Department, Anxiety Disorders Unit, Adult Mental Health Centers, and other departments of the Hospital del Mar. When a participant meets the study criteria, she will be informed about the study and asked whether she would like to participate. Once she accepts, the raters will contact her to schedule the baseline visit and then she will be randomized to one of the groups. Inclusion criteria will be as follows: (1) aged between 18 and 70 years, (2) mean pain score of at least 4 on the visual analog scale for pain (VAS pain ≥ 4) in the two weeks preceding the clinical trial, (3) presence of one or more traumatic events causing current trauma-related symptoms (detection of at least one traumatic event using the EGEP-5 initial list of traumatic events), (4) current clinical symptoms of depression and/or anxiety (Hospital Anxiety and Depression Scale ≥ 8), (5) stable medication regime over the previous 2 weeks, and (6) met internationally-established MtCS safety criteria [65]. Exclusion criteria will be as follows: (1) comorbid autoimmune or chronic inflammatory disease, (2) neurological or serious medical diseases, (3) bipolar disorder, schizoaffective disorder, or schizophrenia, (4) suicidal ideation, (5) previous EMDR therapy, (6) substance abuse/dependency within 1 month prior to participation (except for nicotine abuse/dependency), (7) pending FM-related litigation or disability, (8) metallic implants in the head, or (9) pregnancy.
Randomization
The main analysis will be the comparison between patients assigned to EMDR vs those not assigned to EMDR. The secondary analysis, only amongst patients assigned to EMDR, will be the comparison between patients assigned to active-MtCS vs patients assigned to sham-MtCS. Therefore, the individuals will not randomly be assigned to one of the three arms. Instead, the patients meeting the inclusion criteria will be randomized twice: first to EMDR vs non-EMDR, and then those in the EMDR group to active-MtCS or sham-MtCS. For the sake of brevity, only the randomization to EMDR vs non-EMDR is described here, because the randomization to active-MtCS vs. sham-MtCS is identical. The variables used for the randomization will be age, educational level, and pain intensity score. The first two patients will be randomly allocated to EMDR with p = 2/3. For each subsequent patient, the following biased coin algorithm will be applied: if a group includes at least two more patients than it would have to have to maintain the ratio 2 EMDR / 1 control, the patient will be randomly assigned to the other group with p = 0.6. Otherwise, there will be a simulation of the new patient as it was allocated to EMDR and calculate the between-group standardized difference in pain intensity variable, then simulate that the new patient is allocated to non-EMDR and recalculate the difference, and finally randomly allocate the patient to the group associated to the smallest difference with p = 0.6. This strategy decreases prognostic imbalances between groups because it decreases differences in potential co-founders, yet still includes randomization.
Once the randomization group has been obtained, the principal investigator (PI) of the study will inform the participants accordingly. If the participants have been assigned to the EMDR group, the coordinator will also contact the therapist responsible of the treatment.
Interventions
Eye Movement Desensitization and Reprocessing (EMDR)
EMDR therapy will consist of a maximum of 20 individual 60-min sessions of psychotherapy, principally using the EMDR protocol for FM [66]. This protocol begins by gathering information about all aspects of the patient related to FM (phase 1) and helps create a hierarchy of the targets that are going to be processed during the sessions (phase 2). The following phases (3 to 8) follow the same steps as the EMDR Standard protocol [67]. The protocol is briefly described below:
Patient history: The therapist collects information about the patient’s biography in relation to the following aspects: history of FM, psychological trauma history, pain as a trauma, and pain triggers. These memories will be therapeutic targets in the following sessions. Treatment aims will be agreed between the patient and therapist.
Preparation: The patient will receive an explanation of the EMDR approach and how the therapy functions. The therapist will get to know the patient’s personal resources and check the patient’s preferences with regard to bilateral stimulation. If eye movements are not well tolerated, tapping or auditory tones will be used. Positive resources for emotional regulation and self-care will be installed.
Assessment: The therapist will help the patient focus on a traumatic event selected from the first phase. The patient will select the image that represents the most traumatic part of the event and the positive and negative cognitions associated with the memory, as well as the validity given to these cognitions using the Validity of the Cognition Scale (VoC; ranging from 1 signifying “completely false” to 7 signifying “completely true”). Emotions, sensations in the body, and the level of distress generated by the memory will also be registered by using the Subjective Units of Disturbance scale (SUD; ranging from 0 indicating “neutral or no distress” to 10 indicating “maximum distress”).
Memory desensitization: The patient will focus on the traumatic image and will associate it with the negative cognition, emotions, and bodily sensations reported in the previous phase. At the same time, the therapist will apply bilateral stimulation and the patient will observe any changes. After every set the patient will inform the therapist about every change that has occurred. The role of the therapist will be to guide and accompany the patient during the processing until the SUD reaches 0.
Installing the positive cognition: The patient now focuses on the original memory and is asked to associate it with the positive cognition identified in the third phase. Bilateral stimulation will also be used to install the cognition.
Body scan: When the fifth phase is done, the patient will be asked to keep the memory and positive cognition in mind, and to scan their body for any sensations. If there is any negative sensation, bilateral stimulation will be applied until the sensation disappears. If there are only positive sensations, these will be installed through sets of bilateral stimulation.
Closure: When the session finishes, the therapist will explain that in the following days new material (such as new associations or memories) can arise, in which case the patient should register it for the following session.
Reevaluation: In the next session the therapist will assess the state of the distress caused by the memory processed during the last session. If the memory has been correctly processed and no longer causes distress, the therapist will then proceed to treat other memories following the same protocol.
When a patient appears to suffer intense pain during the regular EMDR session, and the pain is threatening the patient’s processing, the CP protocol will be used. Here, the target chosen is the current pain referred to by the patient. The main differences between the FM and the Pain protocols are the following: in phase 3, the patient must describe the pain felt and draw a representation of it, as well as assigning it personal characteristics; after phase 6, when improvements in levels of pain occur, the patient alongside the therapist will build a positive resource in order to reinforce the positive progress. It will then be followed by phases 7 and 8 of the standard protocol mentioned. Below is a brief description of the CP protocol:
Check that the patient’s pain is at a tolerable level by asking the patient to make a subjective assessment of their pain, evaluating their attitude to it, and ensuring that their pain is sufficiently controlled.
The medical diagnosis and the patient’s attitude towards it, including degree of acceptance, are reviewed.
The targets for EMDR reprocessing, and the treatment objectives (for example, pain relief or greater control over pain), are identified and put in order of priority and used to draw up a treatment plan. As pain is in many cases related, either directly or indirectly, to a traumatic or stressful event, these are treated first using the standard EMDR protocol explained above.
Next, each pain point is treated separately with the goal of helping the patient to relax and to notice changes in pain sensations. Bilateral stimulation is applied while the patient focuses on either current pain or a memory of pain. After each set, the patient explains their pain experience, and whether changes have occurred in the severity of the pain, its type, or where it is felt. The sets of bilateral stimulation are continued until the patient notices a positive change.
Finally, the patient is assisted in developing psychological pain-management resources, achieved by the cognitive integration of the positive changes in pain sensations. First the positive change is linked to an image, and this is reinforced through sets of bilateral stimulation. The patient then chooses a word to associate with the positive change, and this is reinforced through further sets of bilateral stimulation. The patient can then bring to mind the positive image and associated word and self-apply bilateral stimulation when they feel pain in the future, thus giving the patient pain management resources.
It is important to mention that this psychological intervention does not usually cause any risk to the health of the participants. However, due to remembering and reprocessing past traumatic experiences, it is possible that emotional discomfort will be felt during the evaluation and the therapeutic sessions. This discomfort usually disappears before the end of a session or, in exceptional cases, can also continue in the following day. If the discomfort continues, a new session with the patient should be immediately scheduled to assist her.
Would adverse effects other than those related to emotional discomfort from the therapeutic sessions occur, these shall be reported to the PI of the project. Additionally, if it is likely considered that EMDR was the cause, they will also be reported to the Ethics Committee department and relevant regulatory bodies, as required, indicating expectedness, seriousness, severity, and causality. However, as no problems that are detrimental to the participant are anticipated, no interim analyses or formal stopping rules have been planned.
Throughout the duration of the study, no participant may receive trauma-focused therapy sessions in parallel.
Multifocal transcranial current stimulation (MtCS)
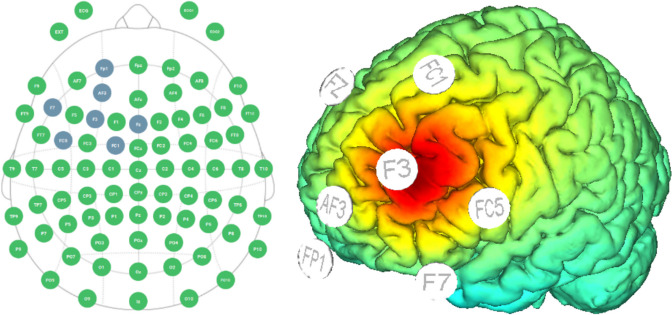
Multifocal transcranial current stimulation (MtCS) montage (F3 anodal; Fp1, F7, Fc5, AF3, Fc1, Fz return) will be used with the anode over the lDLPFC. This montage, guided by StarStim® computational modeling data (see Fig. 2), was planned with the intention of enhancing the activity of the lDLFPC. Active stimulation will consist of 2 mA MtCS for 20 min applied immediately before EMDR sessions. The same protocol and montage will be used for sham stimulation, but the protocol will be implemented by ramping down (slowly) the current immediately after the ramp up period, and by ramping up (slowly) the current right before the final ramp down portion of the session. This way, the subject will feel the ramp up and ramp down events, but will not receive a significant dose of stimulation. Thus, the patient will believe she is being stimulated normally, but there should not be any real effects, in order to control for placebo effects of the MtCS treatment. The device used will be the StarStim®, which is a wireless hybrid EEG/tCS 8-channel neurostimulator system. StarStim® is currently classified as an investigational device under US federal law.
Fig. 2.
Multifocal transcranial Current Stimulation (MtCS) montage
In the case of patients who have been prescribed medication with effects on the nervous system, such as antidepressants, anxiolytic drugs, anticonvulsants, or atypical antipsychotics, an individualized follow-up of the clinical outcomes will be carried out to ensure that there is no interaction with the brain stimulation [31]. Although the application of MtCS is painless, in the event that a participant, due to her medical condition, feels marked pain or very significant discomfort due to the application of the stimulation and asks to stop the stimulation, this decision will be respected and the participant will receive EMDR treatment only. Again, if other different adverse effects than those mentioned above occur, they will be reported to the PI, and, in the event they are considered to be related to MtCS, also to the Ethics Committee department and relevant regulatory bodies as required, indicating the expectedness, seriousness, severity, and causality of the adverse effect. However, as has been mentioned previously, no problems that are detrimental to the participant are anticipated, meaning no interim analyses or formal stopping rules have been planned.
During the time of the study, no participant may receive MtCS sessions in parallel.
Waitlist condition
The patients allocated to this condition will follow their usual treatment without receiving any other additional therapy. Treatment as usual consists of regular visits with the rheumatologist, psychiatrist, and general practitioners, who are responsible for prescribing and monitoring the pharmacological treatment, principally in form of analgesics such as non-steroidal anti-inflammatory drugs, opioids, paracetamol, and/or gabapentin, but also antidepressant drugs and anxiolytics/hypnotics. Health psychoeducation by the nursing service and therapeutic physical exercise are also included in the waitlist condition.
Outcomes
Demographic and clinical variables will be collected through a clinical interview using the medical history of the patients and a specific Case Report Form (CRF) designed for the study which will include age, educational level, personal and family history, drug use, current pharmacological treatment, and previous psychological treatment. We will also use the MINI International Neuropsychiatric Interview [68], Spanish validation [69] to explore the principal psychiatric disorders from Axis I of DSM-IV and CIE-10.
Pain intensity will be assessed using the following scales:
Visual analogue scale for pain (VAS pain) [70]: The VAS pain consists of a straight horizontal line, usually 10 cm long, anchored between 2 verbal descriptors: “No pain” on the left side and “Unbearable pain” on the right. Scores are interpreted as follows: no pain (0–2), mild pain [2–4], moderate pain [4–6], severe pain [6–8], and maximum pain [8–10]. This measure assesses the intensity of the perceived pain over the last 2 weeks.
The Revised Fibromyalgia Impact Questionnaire (FIQ-R) [71], Spanish validation [72]. The FIQ-R is a 9-item self-administered scale for measuring physical impairment due to FM over the last week. Higher scores indicate greater impact in functioning.
The Pain Catastrophizing Scale (PCS) [73], Spanish validation [74]. The PCS is a self-report questionnaire designed to assess catastrophic thinking related to pain experiences. It consists of 13 items that evaluate the extent to which individuals magnify, ruminate, and feel helpless about their pain. This scale helps clinicians and researchers understand how individuals interpret and respond to pain, which can inform treatment approaches for pain management.
The Brief Fatigue Inventory (BFI) [75], Spanish validation [76]. The BFI is a simple and quick self-report questionnaire used to assess the severity and impact of fatigue on individuals. It consists of nine items that measure the intensity of fatigue experienced in the past 24 h, as well as its interference with various aspects of daily life, such as mood, walking ability, work, relationships, and enjoyment of life.
Psychological trauma and trauma-related symptoms will be evaluated using the following scales:
Global Evaluation of Posttraumatic Stress (EGEP-5) [77]. The EGEP-5 is a 55-item clinician-applied scale to determine current PTSD diagnosis, based on DSM-V criteria. There are three different sections: presence of traumatic events, symptoms, and functioning. The scale can determine a diagnosis of PTSD, specifying the presence of dissociative symptoms (depersonalization and derealization) and delayed expression.
The International Trauma Questionnaire (ITQ) [78], Spanish validation [79]. This questionnaire is a comprehensive assessment tool used to evaluate and diagnose complex post-traumatic stress disorder (C-PTSD) and other trauma-related disorders, in terms of ICD-11 classification for mental disorders. It consists of 12 items that assess the presence and severity of PTSD symptoms, as well as disturbances in self-organization associated with C-PTSD. The ITQ is designed to provide a standardized measure for clinicians and researchers to assess trauma-related symptoms across different cultural and linguistic contexts.
Posttraumatic Stress Disorder Checklist (PCL-5) [80] Spanish validation [81]. This scale assesses DSM-5 PTSD symptoms using a 20-item questionnaire. Scores range from 0 to 80, with higher scores indicating greater severity. A cut-off score of 33 is used for diagnosis. The PCL-5 demonstrates high reliability (α = 0.94) and validity, making it a robust tool for PTSD screening, diagnosis, and symptom monitoring.
The Childhood Trauma Questionnaire (CTQ) [82], Spanish validation [83]. The CTQ is a self-applied scale which includes a 28-item test that measure 5 types of childhood maltreatment: emotional, physical and sexual abuse, and emotional or physical neglect. A 5-point Likert scale (from 1 to 5) is used for the responses which range from “never true” to “very often true”. The final scores provide a severity score for each subscale from “none to minimal,” “low to moderate,” “moderate to severe,” and “severe to extreme”.
The Cambridge Depersonalization Scale (CDS) [84] Spanish validation [85]. The CDS is a self-report questionnaire designed to assess the severity of depersonalization symptoms. It consists of 29 items that evaluate various aspects of depersonalization, including feelings of detachment from oneself, altered perceptions of time and space, and experiences of unreality. The CDS is used in clinical and research settings to measure the frequency and intensity of depersonalization symptoms in individuals with various psychiatric conditions, such as depersonalization disorder and dissociative disorders.
Somatoform Dissociation Questionnaire 20 (SDQ-20) [86], Spanish validation [87]. The SDQ-20 is a 20-item self-report questionnaire measuring somatoform dissociation. Items refer to somatic symptoms and then ask if there is a known cause for them. The items are answered on a 5-point Likert scale and the symptoms with no known cause are summed to achieve the total score.
Timeline of traumatic experiences: This tool was developed specifically for this study and consists of a table that qualitatively compiles different traumatic events that the person may have suffered both in childhood and in adulthood. The table is segmented into 5-year intervals ranging from 0–5 years old to 65–70 years old. Within each segment, participants are asked: “Do you recall experiencing any traumatic or stressful events during this age interval?”.
Subjective unit of distress (SUD) [88] this scale, ranging from 0 (no distress) to 10 (maximum distress), evaluates the level of subjective perturbation a person experiences when they bring to mind the traumatic event chosen in the EGEP-5 scale.
Anxiety and depression will be assessed using the following scale:
Hospital Anxiety and Depression Scale (HADS) [89], Spanish validation [90]. The HAD was created for detecting the presence of anxious and depressive disorders. It contains 14 items, 7 for each of the subscales (anxiety and depression), which can be rated from 0 to 3. A punctuation higher or equal to 11 indicates presence of affective disorder.
Quality of sleep will be assessed using the following scale:
Athens Insomnia Scale (AIS) [91], Spanish validation [92]. The AIS is a self-administered scale based on the ICD-10 criteria for insomnia. It measures sleep difficulties suffered over the previous three nights. It consists of 8 items evaluating sleep induction, awakenings during the night, final awakening, total sleep duration, sleep quality, well-being, functioning capacity, and sleepiness during the day. It is scored from 0 to 24 and higher scores mean greater difficulties.
Wellbeing will be assessed using the following scale:
Satisfaction With Life Scale (SWLS) [93], Spanish validation [94]. The SWLS is a 5-item self-administered scale measuring global cognitive judgment of satisfaction with one’s life. The items can be rated from 1 to 5, and lower scores indicate lower satisfaction.
Self-care will be assessed using the following scale:
González self-care scale [95]. The Self-Care Scale is a questionnaire that assesses how we treat ourselves. This Scale consists of 31 items grouped into six different components, addressing various aspects of self-care: (1) Self-Destruction: Refers to behaviors that harm us in some way; (2) Lack of Tolerance for Positive Affect: Refers to difficulty in receiving affectionate comments or behaviors from others; (3) Problems in Allowing Help: Refers to difficulty in allowing other people to help us; (4) Resentment for Non-Reciprocity: Refers to the anger felt because our needs or emotions have not been attended to or reciprocated; (5) Lack of Positive Activities: Refers to the absence of activities that make us feel good or are healthy for us; (6) Neglecting One’s Own Needs: Refers to the difficulty in identifying and satisfying our own needs. A total score above three on each factor is indicative of dysfunctionality.
Emotional regulation will be assessed using the following scale:
The Emotion-Regulation Skills Questionnaire (ERSQ) [96], Spanish validation [97]. This questionnaire is a self-report measure designed to assess an individual’s ability to regulate their emotions effectively. It consists of items that evaluate various emotion-regulation strategies, including cognitive reappraisal, acceptance, problem-solving, and suppression. The ERSQ provides insight into an individual’s skill level in managing their emotions, which can be useful in clinical settings for developing targeted interventions or in research settings for studying emotion regulation across different populations.
Self-esteem will be assessed using the following scale:
The Rosenberg Self-Esteem Scale (RSE) [98], Spanish validation [99]. The RSE consists of ten items that assess an individual’s overall feelings of self-worth and self-acceptance. The scale covers both positive and negative aspects of self-esteem, with higher scores indicating greater levels of self-esteem. The RSE is frequently utilized in research and clinical settings to evaluate self-esteem levels across various populations and to assess the impact of interventions aimed at improving self-esteem.
Cognitive functioning will be assessed using the following scale:
The Screen for Cognitive Impairment in Psychiatry (SCIP) [100], Spanish validation [101]. The SCIP is a screening tool used to assess cognitive function in psychiatric patients. It consists of a series of brief cognitive tests designed to evaluate various cognitive domains, including memory, attention, executive function, and language. The SCIP is used by mental health professionals to quickly identify cognitive deficits in patients with psychiatric disorders, which can help inform treatment planning and intervention strategies.
All patients will be clinically evaluated at baseline/enrollment (t1), post treatment (t2), and follow-up evaluation at 6 months from post-treatment (t3) (see Tables 1, 2, and 3). Personal participant data will be numerically coded and kept in a database in the Centre Forum Research Unit, who will be responsible for data maintenance and safety. Only the researchers involved in this trial will have access to this data. The database will contain the information of both the participants who finish the study as well as those who drop out, along with the corresponding causes for not completing the study. The Centre Forum Research Unit will be responsible for creating and maintaining the database.
Table 1.
Measurements to evaluate pain and FM impact
| Domain | Measure | Method of aggregation | Metric | Time point | ||
|---|---|---|---|---|---|---|
| t1 baseline | t2 post-treatment 6 months | t3 follow-up 12 months | ||||
| Pain intensity | VAS pain | Mean value of continuous variable | Change from baseline | x | x | x |
| Pain disability | PCS | Mean value of continuous variable | Change from baseline | x | x | x |
| Fatigue disability | BFI | Mean value of continuous variable | Change from baseline | x | x | x |
| FM impact | FIQ-R | Mean value of continuous variable | Change from baseline | x | x | x |
VAS pain Visual analogue scale for pain, PCS Pain Catastrophizing Scale, BFI The Brief Fatigue Inventory, FIQ-R The Revised Fibromyalgia Impact Questionnaire
Table 2.
Measurements to evaluate psychological trauma symptoms
| Domain | Measure | Method of aggregation | Metric | Time point | ||
|---|---|---|---|---|---|---|
| t1 baseline | t2 Post-treatment 6 months | t3 follow-up 12 months | ||||
| Childhood trauma | CTQ | Mean value of continuous variable | Baseline descriptive variable | x | ||
| TTE | Descriptive analysis (%) | Baseline descriptive variable | x | |||
| PTSD | EGEP-5 | Categorical variable, % of cases | Change from baseline | x | x | x |
| Complex PTSD | ITQ | Categorical variable, % of cases | Change from baseline | x | x | x |
| PTSD symptoms | PCL-5 | Mean value of continuous variable | Change from baseline | x | x | x |
| Distress associated to event | SUD | Mean value of continuous variable | Change from baseline | x | x | x |
| Dissociation | CDS | Mean value of continuous variable | Change from baseline | x | x | x |
| SDQ-20 | Mean value of continuous variable | Change from baseline | x | x | x | |
CTQ Childhood Trauma Questionnaire, TTE Timeline of Traumatic Experiences, PTSD Post-traumatic Stress Disorder, EGEP-5 Evaluación General del Estrés Postraumático, ITQ The International Trauma Questionnaire, PCL-5 Posttraumatic Stress Disorder Checklist, SUD Subjective Units of Distress, CDS The Cambridge Depersonalization Scale, SDQ-20 Somatoform Dissociation Questionnaire
Table 3.
Measurements to evaluate clinical symptoms, insomnia and quality of life, self-care, emotional regulation, self-esteem and cognitive functioning
| Domain | Measure | Method of aggregation | Metric | Time point | ||
|---|---|---|---|---|---|---|
| t1 baseline | t2 Post-treatment 6 months | t3 follow-up 12 months | ||||
| Comorbidity | MINI | Value at a time | x | |||
| Anxiety | HADS-A | Mean value of continuous variable | Change from baseline | x | x | x |
| Depression | HADS-D | Mean value of continuous variable | Change from baseline | x | x | x |
| Insomnia | AIS | Mean value of continuous variable | Change from baseline | x | x | x |
| Quality of life | SWLS | Mean value of continuous variable | Change from baseline | x | x | x |
| Self-care | González Self-care scale | Mean value of continuous variable | Change from baseline | x | x | x |
| Emotional Regulation | ERSQ | Mean value of continuous variable | Change from baseline | x | x | x |
| Self-esteem | RSE | Mean value of continuous variable | Change from baseline | x | x | x |
| Cognitive functioning | SCIP | Mean value of continuous variable | Change from baseline | x | x | x |
MINI MINI International Neuropsychiatric Scale, HADS-A Hospital Anxiety and Depression Scale-Anxiety, HADS-D Hospital Anxiety and Depression Scale-Depression, AIS Athens Insomnia Scale, SWLS Satisfaction With Life Scale, ERSQ The Emotion-Regulation Skills Questionnaire, RSE The Rosenberg Self-Esteem Scale, SCIP The Screen for Cognitive Impairment in Psychiatry
Sample size calculation
The main tests of the study will consist of assessing whether patients assigned to EMDR show different levels in the pain intensity variable using a standard formula for two-tailed t-tests. The total sample size required to detect large to very large effect size differences (Cohen’s d ≥ 1) between three groups with a significance level of 0.05 and statistical power of 80% is 28. Assuming 15% dropouts, we will aim to randomize 96 patients, meaning 32 per group.
Data analysis
The distribution of the sociodemographic and clinical variables between groups at baseline will be summarized by descriptive statistics. We will use t-tests to compare pain levels at post-treatment and follow up between groups (Waitlist vs EMDR; active-MtCS vs sham-MtCS). In order to avoid regression toward the mean and confusion effects, baseline levels of pain will be added as covariates, as well as age, depression and anxiety severity and number of years of education. Due to the sample size no further analysis is expected. The statistical software used for the analysis will be R. For the principal statistical analysis an intention to treat (ITT) analysis will be used, and multiple imputation for losses at follow-up. The dataset analyses during the current study will be available from the corresponding author on reasonable request.
Discussion
This scientific paper presents the first protocol of a double-blind RCT to investigate whether EMDR is effective in the treatment of FM and its comorbid symptoms, and if its potential is boosted with the addition of MtCS. Patients with FM can be a great clinical challenge for healthcare professionals, due to their high comorbidities and unexplained symptoms [102, 103]. The etiology of FM is poorly understood [104], consequently leading to an erroneous interpretation of the disorder and its manifestations, resulting in a pattern of excessive and non-beneficial therapy [105]. Patients usually feel frustrated about their treatment options [106] and dissatisfied with the support received: 38% of FM patients in Spain state that the public health system is the entity which gives them the least support, despite 72% of the FM patients only visiting doctors within this system. This lack of satisfaction with current diagnostic and treatment options could explain why 87% of FM patients prioritize scientific research as the means to a painless future [107]. It is the duty of researchers and clinicians to study precipitators and susceptible populations to build beneficial therapy protocols [4], and thus it is imperative to recognize the evidence showing the importance of the psychological trauma as a trigger in the development, maintenance, and chronification of FM, and provide suitable therapeutic options to address this.
This protocol is innovative in combining the EMDR approach for treating psychological trauma and reducing pain levels with MtCS. This approach should also help treat comorbidity, which, according to the results of recently published works, may also improve FM symptoms [105, 108–110]. The stress-related antecedents of FM include early-life traumas, PTSD, depression, anxiety, and major life stress [111]. Therefore, since traumatic events can predispose individuals to FM, mood, and anxiety disorders and influence mental and physical health [107, 110], the interventions appearing in this protocol seem beneficial.
Although there are several studies showing high comorbidity between FM and PTSD [4], very few studies have been carried out testing interventions targeting trauma-related comorbidity, and those which exist are of low quality [4], due to factors such as a lack of control subjects, variability in PTSD and depression diagnoses, and the high associated comorbidity [6]. Thus, this study will be a useful addition to the extant literature, and it will shed light on the possibility of developing a larger RCT using a new psychotherapeutic approach for the treatment of FM.
Limitations
Some limitations of our trial have been taken into account. Firstly, the lack of control regarding drug treatment is a potential source of bias. To partly overcome this limitation, the current medication regimen should not be changed, as far as possible once patients have been included in the study. Secondly, this study only includes females. Further studies would be needed to see if the findings of this study can be replicated in the much lower proportion of male FM patients. Thirdly, this is an exploratory and pragmatic trial with a relatively moderate size, meaning findings will later need to be replicated in a larger sample. Finally, another possible limitation could be the scalability of the MtCS treatment.
Trial status
In September 25th, 2019, we initiated a pilot study with the objective of enlisting 45 participants to be randomly assigned to one of three groups: (1) EMDR + tDCS active (n = 15), (2) EMDR + tDCS sham (n = 15), or (3) TAU (n = 15). Unfortunately, the onset of the COVID-19 pandemic led to the suspension of the trial, as the intervention could not be administered remotely. Subsequently, AVG and BA secured funding for a randomized controlled trial (RCT) which allowed the previous pilot study to expanded to a full study, increasing the cohort to 96 patients. Additionally, author MFM was added as her involvement in this manuscript has been significant due to her current and future dedication to the project, which will be the main paper of her PhD. As for the change in outcomes (self-care, emotional regulation, self-esteem, and cognitive functioning), while reflecting on the population this study is targeting and other potential troubles they encounter, MFM and AMA hypothesized that the intervention should help in alleviating patient’s troubles in these areas. Therefore, evaluating these new outcomes is relevant in order to identify if EMDR therapy + MtDCS is an effective treatment for said aspects of general mental health.
Supplementary Information
Acknowledgements
The investigators want to thank to the EMDR therapists (TC, AA, MC, and KC) for their future implications in the present project and to the Spanish EMDR Association for the training support. AMA wants to thank to “Secretaria d′Universitats i Recerca del Departament d′Economia i Coneixement (2017 SGR 46 to “Unitat de Recerca del Centre Fòrum”), Generalitat de Catalunya (Government of Catalonia)” for the recognition as an emerging research group. VP wants to thank unrestricted research funding from “Secretaria d′Universitats i Recerca del Departament d′Economia i Coneixement (2017 SGR 134 to “Mental Health Research Group”), Generalitat de Catalunya (Government of Catalonia)”. He also acknowledges the continuous support by the CIBERSAM (Centro de Investigación Biomédica en Red de Salud Mental), Madrid, Spain. AVG wants to thank to Instituto Carlos III for supporting her research thanks to the Juan Rodés Research Contract (JR19/00001) in the period between 2020 and 2024. None of the acknowledged funders have played a part in the study design; collection, management, analysis, and interpretation of data; writing of the report; or in the decision of submitting the report for publication.
Abbreviations
- FM
Fibromyalgia
- EMDR
Eye Movement Desensitization and Reprocessing
- MtCS
Multifocal transcranial Current Stimulation
- TAU
Therapy As Usual
- RCT
Randomized controlled trial
- CP
Chronic pain
- PTSD
Postraumatic stress disorders
- HPA
Hypothalamic-pituitary adrenal
- NIBS
Non-invasive brain stimulation
- MtCS
Transcranial direct current stimulation
- CCT
Cognitive Control Therapy
- lDPFC
Left dorsolateral prefrontal cortex
- DNIC
Diffuse noxious inhibitory controls
- CRF
Case Report Form
- VAS
Visual analogue scale
- PDI
Pain Disability Index
- FIQ
Fibromyalgia Impact Questionnaire
- HADS
Hospital Anxiety and Depression Scale
- AIS
Athens Insomnia Scale
- EGEP-5
Evaluación Global de Estrés Postraumático-5
- IES-R
Impact of Event Scale-Revised
- CTQ
Childhood Trauma Questionnaire
- DES
Dissociative Experiences Scale
- SDQ
Somatoform Dissociation Questionnaire-20
- SWLS
Satisfaction With Life Scale
- ITT
Intention to treat
- LOCF
Last Observation Carried Forward
Authors’ contributions
AMA and BLA had the idea of the project. IGS, VP, WL, BLA, and AMA contributed to the design of the study. AVG will be the coordinator of the study. OMT and DRR will apply MtCS stimulation to the patients. JR will carry out the randomization and all statistical analysis. IGS, BH, LMS, and MFM will blindly evaluate the participants. IGS wrote the first draft of the manuscript with the supervision of AMA and BLA. IGS, OMT, BH, DRR, AVG, LMS, MFM, JMB, WL, VP, JR, BLA, and AMA contributed to the revisions and modifications of the manuscript and all have approved the final version. No professional writers have been involved in the manuscript.
Funding
This work has been supported by a grant for AVG and BLA from the Plan Nacional de I + D + i and co-funded by the Instituto de Salud CarlosIII-Subdirección General de Evaluación y Fomento de la Investigación, Plan Nacional 2024–2026 with the following Research Project (PI23/00072).
Data availability
Personal data of the participant will be numerically coded and kept in a database at the Centre Forum Research Unit. Only the researchers involved in the trial will have access to this data, however, the dataset analysis of the current study will be available from the corresponding author upon reasonable request. When the study finishes and results are obtained, we will send a copy of the article in Spanish to all, together with a plain language summary, as well as providing individual feedback about progress during the study if any participant requests it. The results of the study will also be published in peer review journals, and national and international conferences. The datasets analyzed during the current study will be available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study has been approved by the Ethics Committee of the Parc de Salut Mar (2019/8772/I) and re-approved with all changes for the new study by the same Ethics Committe in April 10th 2024. All participants will sign the informed consent prior to enrollment in the baseline visit. The informed consent will be available from the corresponding author upon request. Details of the design of the trial can be also gathered from Supplementary Material (Standard Protocol Items: Recommendations for Interventional Trials [SPIRIT] Checklist and Schedule of enrolment, interventions and assessments [SPIRIT] Flow diagram). The trial is registered at ClinicalTrials.gov, identifier: NCT04084795 (2nd of August of 2019).
Consent for publication
Not applicable.
Competing interests
BLA was the Research Committee Chair of EMDR Europe between 2017 and 2021 and has been invited as speaker to various national and international congresses of EMDR. AVG is member of the Research Committee of EMDR Europe since May 2023 until now. AMA is member of the Research Committee of the Spanish EMDR Organization since September 2023 until now. The other authors declare they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A. Valiente-Gómez and A. Moreno-Alcázar have shared senior authorship
References
- 1.Queiroz LP. Worldwide epidemiology of fibromyalgia topical collection on fibromyalgia. Curr Pain Headache Rep. 2013;17. Available from: 10.1007/s11916-013-0356-5 [DOI] [PubMed]
- 2.Galvez-Sánchez CM, Duschek S, Paso GAR. Psychological impact of fibromyalgia: current perspectives. Psychol Res Behav Manag. 2019;12. Available from: 10.2147/PRBM.S178240 [DOI] [PMC free article] [PubMed]
- 3.Burke NN, Finn DP, McGuire BE, Roche M. Psychological stress in early life as a predisposing factor for the development of chronic pain: Clinical and preclinical evidence and neurobiological mechanisms. J Neurosci Res. 2017Jun;95(6):1257–70. [DOI] [PubMed] [Google Scholar]
- 4.Yavne Y, Amital D, Watad A, Tiosano S, Amital H. A systematic review of precipitating physical and psychological traumatic events in the development of fibromyalgia. Semin Arthritis Rheum. 2018;48. Available from: 10.1016/j.semarthrit.2017.12.011 [DOI] [PubMed]
- 5.Gündüz N, Polat A, Erzincan E, Turan H, Sade I, Tural Ü. Psychiatric comorbidity and childhood trauma in fibromyalgia syndrome. Turk J Phys Med Rehabil. 2018;64. Available from: 10.5606/tftrd.2018.1470 [DOI] [PMC free article] [PubMed]
- 6.Cohen H, Neumann L, Haiman Y, Matar MA, Press J, Buskila D. Prevalence of post-traumatic stress disorder in fibromyalgia patients: overlapping syndromes or post-traumatic fibromyalgia syndrome? Semin Arthritis Rheum. 2002;32. Available from: 10.1053/sarh.2002.33719 [DOI] [PubMed]
- 7.Peres JFP, Gonçalves AL, Peres MFP. Psychological trauma in chronic pain: implications of PTSD for fibromyalgia and headache disorders. Curr Pain Headache Rep. 2009;13. Available from: 10.1007/s11916-009-0057-2 [DOI] [PubMed]
- 8.Crettaz B, Marziniak M, Willeke P, Young P, Hellhammer D, Stumpf A. Stress-induced allodynia - evidence of increased pain sensitivity in healthy humans and patients with chronic pain after experimentally induced psychosocial stress. PLoS One. 2013;8. Available from: 10.1371/journal.pone.0069460 [DOI] [PMC free article] [PubMed]
- 9.Cuijpers P, van Veen SC, Sijbrandij M, Yoder W, Cristea IA. Eye movement desensitization and reprocessing for mental health problems: a systematic review and meta-analysis. Cogn Behav Ther. 2020May;49(3):165–80. [DOI] [PubMed] [Google Scholar]
- 10.Wright SL, Karyotaki E, Cuijpers P, Bisson J, Papola D, Witteveen A, et al. EMDR v. other psychological therapies for PTSD: a systematic review and individual participant data meta-analysis. Psychol Med. 2024;54(8):1580–8. [DOI] [PubMed] [Google Scholar]
- 11.WHO. Guidelines for the Management of Conditions Specifically Related to Stress. Assess Manag Cond Specifically Relat to Stress mhGAP Interv Guid Modul (version 10). 2013;1–273. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24649518
- 12.Davidson PR, Parker KCH. Eye movement desensitization and reprocessing (EMDR): a meta-analysis. J Consult Clin Psychol. 2001;69. Available from: 10.1037/0022-006X.69.2.305 [DOI] [PubMed]
- 13.Seidler GH, Wagner FE. Comparing the efficacy of EMDR and trauma-focused cognitive-behavioral therapy in the treatment of PTSD: a meta-analytic study. Psychol Med. 2006;36. Available from: 10.1017/S0033291706007963 [DOI] [PubMed]
- 14.Cusack K, Jonas DE, Forneris CA, Wines C, Sonis J, Middleton JC. Psychological treatments for adults with posttraumatic stress disorder: a systematic review and meta-analysis. Clin Psychol Rev. 2016;43. Available from: 10.1016/j.cpr.2015.10.003 [DOI] [PubMed]
- 15.Chen L, Zhang G, Hu M, Liang X. Eye movement desensitization and reprocessing versus cognitive-behavioral therapy for adult posttraumatic stress disorder: systematic review and meta-analysis. J Nerv Ment Dis. 2015;203. Available from: 10.1097/NMD.0000000000000306 [DOI] [PubMed]
- 16.Chen YR, Hung KW, Tsai JC, Chu H, Chung MH, Chen SR. Efficacy of eye-movement desensitization and reprocessing for patients with posttraumatic-stress disorder: a meta-analysis of randomized controlled trials. PLoS One. 2014;9. Available from: 10.1371/journal.pone.0103676 [DOI] [PMC free article] [PubMed]
- 17.Moreno-Alcázar A, Treen D, Valiente-Gómez A, Sio-Eroles A, Pérez V, Amann BL, et al. Efficacy of Eye Movement Desensitization and Reprocessing in Children and Adolescent with Post-traumatic Stress Disorder: A Meta-Analysis of Randomized Controlled Trials. Front Psychol. 2017;8:1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodenburg R, Benjamin A, Roos C, Meijer AM, Stams GJ. Efficacy of EMDR in children: a meta-analysis. Clin Psychol Rev. 2009;29. Available from: 10.1016/j.cpr.2009.06.008 [DOI] [PubMed]
- 19.Pilz R, Hartleb R, Konrad G, Reininghaus E, Unterrainer HF. The role of eye movement desensitization and reprocessing (EMDR) in substance use disorders: a systematic review. Fortschritte der Neurol Psychiatr. 2017;85. Available from: 10.1055/s-0043-118338 [DOI] [PubMed]
- 20.Wood E, Ricketts T, Parry G. EMDR as a treatment for long-term depression: a feasibility study. Psychol Psychother Theory Res Pr. 2018;91. Available from: 10.1111/papt.12145 [DOI] [PMC free article] [PubMed]
- 21.Bandelow B, Reitt M, Röver C, Michaelis S, Görlich Y, Wedekind D. Efficacy of treatments for anxiety disorders: a meta-analysis. Int Clin Psychopharmacol. 2015;30. Available from: 10.1097/YIC.0000000000000078 [DOI] [PubMed]
- 22.Tesarz J, Wicking M, Bernardy, Seidler GH. EMDR therapy’s efficacy in the treatment of pain. J EMDR Pr Res. 2019;13. Available from: 10.1891/1933-3196.13.4.337
- 23.Arias-Suárez N, Moreno-Pérez J, Redolar-Ripoll D, Hogg BM, Gardoki-Souto I, García-Guerrero F, et al. EMDR versus treatment-as-usual in patients with chronic non-malignant pain: a randomized controlled pilot study. J EMDR Pract Res. 2020;14(4):190–205.
- 24.Marcus S V. Phase 1 of integrated EMDR: an abortive treatment for migraine headaches. J EMDR Pr Res. 2008;2. Available from: 10.1891/1933-3196.2.1.15
- 25.Gerhardt A, Leisner S, Hartmann M, Janke S, Seidler GH, Eich W, et al. Eye movement desensitization and reprocessing vs treatment-as-usual for non-specific chronic back pain patients with psychological trauma: a randomized controlled pilot study. 2016;7(201):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nia NG, Afrasiabifar A, Behnammoghadam M. Comparing the effect of eye movement desensitization and reprocessing (EMDR) with guided imagery on pain severity in patients with rheumatoid arthritis. J Pain Res. 2018;11. Available from: 10.2147/JPR.S158981 [DOI] [PMC free article] [PubMed]
- 27.Maroufi M, Zamani S, Izadikhah Z, Marofi M, O’Connor P. Investigating the effect of eye movement desensitization and reprocessing (EMDR) on postoperative pain intensity in adolescents undergoing surgery: a randomized controlled trial. J Adv Nurs. 2016;72. Available from: 10.1111/jan.12985 [DOI] [PubMed]
- 28.Brennstuhl MJ, Tarquinio C, Bassan F. Utilisation de la thérapie EMDR-Eye Movement Desensitization and Reprocessing-dans le cadre de ladouleur chronique: étude pilote. Prat Psychol. 2016;22.
- 29.Scelles C, Bulnes LC. EMDR as Treatment Option for Conditions Other Than PTSD: A Systematic Review. Front Psychol. 2021;12: 644369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tesarz J, Leisner S, Gerhardt A, Janke S, Seidler GH, Eich W, et al. Effects of eye movement desensitization and reprocessing (EMDR) treatment in chronic pain patients: a systematic review. Pain Med. 2014Feb;15(2):247–63. [DOI] [PubMed] [Google Scholar]
- 31.Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T. Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stimul. 2016;9. Available from: 10.1016/j.brs.2016.06.004 [DOI] [PMC free article] [PubMed]
- 32.Das S, Holland P, Frens MA, Donchin O. Impact of transcranial direct current stimulation (tDCS) on neuronal functions. Front Neurosci. 2016;10. Available from: 10.3389/fnins.2016.00550 [DOI] [PMC free article] [PubMed]
- 33.Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron. 2010;66. Available from: 10.1016/j.neuron.2010.03.035 [DOI] [PMC free article] [PubMed]
- 34.Antal A, Ambrus GG, Chaieb L. The impact of electrical stimulation techniques on behavior. Wiley Interdiscip Rev Cogn Sci. 2014;5. Available from: 10.1002/wcs.1319 [DOI] [PubMed]
- 35.Brighina F, Curatolo M, Cosentino G, Tommaso M, Battaglia G, Sarzi-Puttini PC. Brain modulation by electric currents in fibromyalgia: a structured review on non-invasive approach with transcranial electrical stimulation. Front Hum Neurosci. 2019;13. Available from: 10.3389/fnhum.2019.00040 [DOI] [PMC free article] [PubMed]
- 36. Deus-Yela J, Soler MD, Pelayo-Vergara R, Vidal-Samsó J. Estimulación transcraneal por corriente directa en la fibromialgia: Revisión sistemática. Rev Neurol. 2017;65. [PubMed]
- 37.Silva AF, Zortea M, Carvalho S, Leite J, Torres IL, Fregni F, Caumo W. Anodal transcranial direct current stimulation over the left dorsolateral prefrontal cortex modulates attention and pain in fibromyalgia: randomized clinical trial. Sci Rep. 2017;7(1):135. 10.1038/s41598-017-00185-w. [DOI] [PMC free article] [PubMed]
- 38.Valle A, Roizenblatt S, Botte S, Zaghi S, Riberto M, Tufik S. Efficacy of anodal transcranial direct current stimulation (tDCS) for the treatment of fibromyalgia: results of a randomized, sham-controlled longitudinal clinical trial. J Pain Manag. 2009;2(3):353–61. [PMC free article] [PubMed]
- 39.Yoo HB, Ost J, Joos W, Havenbergh T, Ridder D, Vanneste S. Adding prefrontal transcranial direct current stimulation before occipital nerve stimulation in fibromyalgia. Clin J Pain. 2018; 34(5):421–7. [DOI] [PubMed]
- 40.Zhu CE, Yu B, Zhang W, Chen WH, Qi Q, Miao Y. Effectiveness and safety of transcranial direct current stimulation in fibromyalgia: a systematic review and meta-analysis. J Rehabil Med. 2017;49. Available from: 10.2340/16501977-2179 [DOI] [PubMed]
- 41.To WT, James E, Ost J, Hart J, Ridder D, Vanneste S. Differential effects of bifrontal and occipital nerve stimulation on pain and fatigue using transcranial direct current stimulation in fibromyalgia patients. J Neural Transm. 2017;124. Available from: 10.1007/s00702-017-1714-y [DOI] [PubMed]
- 42.Fagerlund AJ, Hansen OA, Aslaksen PM. Transcranial direct current stimulation as a treatment for patients with fibromyalgia: a randomized controlled trial. Pain. 2015;156. Available from: 10.1016/j.pain.0000000000000006 [DOI] [PubMed]
- 43.Fregni F, Gimenes R, Valle AC, Ferreira MJL, Rocha RR, Natalle L. A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheum. 2006;54. Available from: 10.1002/art.22195 [DOI] [PubMed]
- 44.Jales Junior LH, Costa M d. DL, Jales Neto LH, Ribeiro JPM, Freitas WJS d. N, Teixeira MJ. Transcranial direct current stimulation in fibromyalgia: effects on pain and quality of life evaluated clinically and by brain perfusion scintigraphy. Rev Dor. 2015;16. Available from: 10.5935/1806-0013.20150008
- 45.Khedr EM, Omran EAH, Ismail NM, El-Hammady DH, Goma SH, Kotb H. Effects of transcranial direct current stimulation on pain, mood and serum endorphin level in the treatment of fibromyalgia: a double blinded, randomized clinical trial. Brain Stimul. 2017;10. Available from: 10.1016/j.brs.2017.06.006 [DOI] [PubMed]
- 46.Mendonca ME, Simis M, Grecco LC, Battistella LR, Baptista AF, Fregni F. Transcranial direct current stimulation combined with aerobic exercise to optimize analgesic responses in fibromyalgia: a randomized placebo-controlled clinical trial. Front Hum Neurosci. 2016;10. Available from: 10.3389/fnhum.2016.00068 [DOI] [PMC free article] [PubMed]
- 47.O’Connell NE, Marston L, Spencer S, Desouza LH, Wand BM. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst Rev. 2018;4. [DOI] [PMC free article] [PubMed]
- 48.Riberto M. Efficacy of transcranial direct current stimulation coupled with a multidisciplinary rehabilitation program for the treatment of fibromyalgia. Open Rheumatol J. 2011;5:45–50. [DOI] [PMC free article] [PubMed]
- 49.Roizenblatt S, Fregni F, Gimenez R, Wetzel T, Rigonatti SP, Tufik S. Site-specific effects of transcranial direct current stimulation on sleep and pain in fibromyalgia: a randomized, sham-controlled study. Pain Pr. 2007;7. Available from: 10.1111/j.1533-2500.2007.00152.x [DOI] [PubMed]
- 50.Sathappan A V, Luber BM, Lisanby SH. The dynamic duo: combining noninvasive brain stimulation with cognitive interventions. Prog Neuropsychopharmacol Biol Psychiatry. 2019;89. Available from: 10.1016/j.pnpbp.2018.10.006 [DOI] [PubMed]
- 51.Chalah MA, Ayache SS. Noninvasive brain stimulation and psychotherapy in anxiety and depressive disorders: a viewpoint. Brain Sci. 2019;9. Available from: 10.3390/brainsci9040082 [DOI] [PMC free article] [PubMed]
- 52.Moffa AH, Brunoni AR, Nikolin S, Loo CK. Transcranial direct current stimulation in psychiatric disorders: a comprehensive review. Psychiatr Clin North Am. 2018;41. Available from: 10.1016/j.psc.2018.05.002 [DOI] [PubMed]
- 53.Hampstead BM, Briceño EM, Mascaro N, Mourdoukoutas A, Bikson M. Current status of transcranial direct current stimulation in posttraumatic stress and other anxiety disorders. Curr Behav Neurosci Rep. 2016;3. Available from: 10.1007/s40473-016-0070-9 [DOI] [PMC free article] [PubMed]
- 54.Kuo MF, Chen PS, Nitsche MA. The application of tDCS for the treatment of psychiatric diseases. Int Rev Psychiatry. 2017;29. Available from: 10.1080/09540261.2017.1286299 [DOI] [PubMed]
- 55.Mutz J, Edgcumbe DR, Brunoni AR, Fu CHY. Efficacy and acceptability of non-invasive brain stimulation for the treatment of adult unipolar and bipolar depression: a systematic review and meta-analysis of randomised sham-controlled trials. Neurosci Biobehav Rev. 2018;92. Available from: 10.1016/j.neubiorev.2018.05.015 [DOI] [PubMed]
- 56.Imburgio MJ, Orr JM. Effects of prefrontal tDCS on executive function: methodological considerations revealed by meta-analysis. Neuropsychologia. 2018;117. Available from: 10.1016/j.neuropsychologia.2018.04.022 [DOI] [PubMed]
- 57.D’Urso G, Mantovani A, Micillo M, Priori A, Muscettola G. Transcranial direct current stimulation and cognitive-behavioral therapy: evidence of a synergistic effect in treatment-resistant depression. Brain Stimul. 2013;6. Available from: 10.1016/j.brs.2012.09.003 [DOI] [PubMed]
- 58.Kregel J, Coppieters I, Depauw R, Malfliet A, Danneels L, Nijs J. Does conservative treatment change the brain in patients with chronic musculoskeletal pain? A systematic review. Pain Physician. 2017;20. [PubMed]
- 59.Ceko M, Bushnell MC, Gracely RH. Neurobiology underlying fibromyalgia symptoms. Pain Res Treat. 2012;585419. [DOI] [PMC free article] [PubMed]
- 60.Lee YC, Nassikas NJ, Clauw DJ. The role of the central nervous system in the generation and maintenance of chronic pain in rheumatoid arthritis, osteoarthritis and fibromyalgia. Arthritis Res Ther. 2011;13(2):211. [DOI] [PMC free article] [PubMed]
- 61.Ruffini G, Fox MD, Ripolles O, Miranda PC, Pascual-Leone A. Optimization of multifocal transcranial current stimulation for weighted cortical pattern targeting from realistic modeling of electric fields. Neuroimage. 2014;89. Available from: 10.1016/j.neuroimage.2013.12.002 [DOI] [PMC free article] [PubMed]
- 62.CDC. Fibromyalgia. 2022. p. 2–5. Available from: https://archive.cdc.gov/www_cdc_gov/arthritis/types/fibromyalgia.htm
- 63.Font Gayà T, Bordoy Ferrer C, Juan Mas A, Seoane-Mato D, Álvarez Reyes F, Delgado Sánchez M, et al. Prevalence of fibromyalgia and associated factors in Spain. Clin Exp Rheumatol. 2020;38 Suppl 1(1):47–52. [PubMed] [Google Scholar]
- 64.Gardoki-Souto I, Redolar-Ripoll D, Fontana M, Hogg B, Castro MJ, Blanch JM, et al. Prevalence and Characterization of Psychological Trauma in Patients with Fibromyalgia: A Cross-Sectional Study. Pain Res Manag. 2022;2022:2114451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Iorio R. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. committee. Clin Neurophysiol. 2015;126. Available from: 10.1016/j.clinph.2015.02.001 [DOI] [PMC free article] [PubMed]
- 66.Konuk E, Zat Z, Kavakçı Ö. Fibromyalgia syndrome treatment with EMDR therapy. In: Luber M, editor. Eye movement desensitization and reprocessing EMDR therapy scripted protocols and summary sheets treating eating disorders, chronic pain and maladaptive self-care behaviors. New York: Springer Publishing Company; 2018.
- 67.Shapiro F. Eye movement desensitization and reprocessing: basic principles, protocols, and procedures. New York: Guilford Press; 2001. [Google Scholar]
- 68.Lecrubier Y, Sheehan D V, Weiller E, Amorim P, Bonora I, Sheehan KH. The MINI international neuropsychiatric interview (MINI). A short diagnostic structured interview: reliability and validity according to the CIDI. Eur Psychiatry. 1997;12. Available from: 10.1016/S0924-9338(97)83296-8
- 69.Ferrando L, Bobes J, Gibert J. MINI. Mini International Neuropsychiatric Interview. Versión en Español 5.0.0 DSM-IV. Instrumentos detección y orientación diagnóstica. 2000;0.
- 70.Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17(1):45–56. [DOI] [PubMed]
- 71.Burckhardt CS, Clark SR, Bennett RM. The fibromyalgia impact questionnaire: development and validation. J Rheumatol. 1991;18. [PubMed]
- 72.Monterde S. Validación de la versión española del Fibromyalgia Impact Questionnaire. Rev española Reum Órgano la Soc Española Reum. 2004;31(9):507–13.
- 73.Osman A, Barrios FX, Gutierrez PM, Kopper BA, Merrifield T, Grittmann L. The Pain Catastrophizing Scale: further psychometric evaluation with adult samples. J Behav Med. 2000;23(4):351–65. [DOI] [PubMed] [Google Scholar]
- 74.García Campayo J, Rodero B, Alda M, Sobradiel N, Montero J, Moreno S. Validación de la versión española de la escala de la catastrofización ante el dolor (Pain Catastrophizing Scale) en la fibromialgia. Med Clin (Barc). 2008;131(13):487–93. Available from: https://www.elsevier.es/es-revista-medicina-clinica-2-articulo-validacion-version-espanola-escala-catastrofizacion-13127277 [DOI] [PubMed]
- 75.Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999Mar;85(5):1186–96. [DOI] [PubMed] [Google Scholar]
- 76.Lorca LA, Sacomori C, Puga B. Assessment of a brief fatigue inventory in patients with hematologic malignancies. Rev Med Chil. 2016Jul;144(7):894–9. [DOI] [PubMed] [Google Scholar]
- 77.Crespo M, Gomez MM. La Evaluación del Estrés Postraumático: Presentación de la Escala de Evaluación Global de Estrés Postraumático (EGEP). Clínica y Salud. 2012;23. Available from: 10.5093/cl2012a4
- 78.Hyland P, Shevlin M, Brewin CR, Cloitre M, Downes AJ, Jumbe S, et al. Validation of post-traumatic stress disorder (PTSD) and complex PTSD using the international trauma questionnaire. Acta Psychiatr Scand. 2017;136(3):313–22. 10.1111/acps.12771. Cited 2024 Mar 19. [DOI] [PubMed] [Google Scholar]
- 79.Fresno A, Ramos Alvarado N, Núñez D, Ulloa JL, Arriagada J, Cloitre M, et al. Initial validation of the International Trauma Questionnaire (ITQ) in a sample of Chilean adults. Eur J Psychotraumatol. 2023 [cited 2024 Mar 19];14(2). Available from: https://pubmed.ncbi.nlm.nih.gov/37815059/ [DOI] [PMC free article] [PubMed]
- 80.Blevins CA, Weathers FW, Davis MT, Witte TK, Domino JL. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): Development and Initial Psychometric Evaluation. J Trauma Stress. 2015Dec;28(6):489–98. [DOI] [PubMed] [Google Scholar]
- 81.Jiménez-Fernández R, Herraiz Soria ME, Peña Granger M, Losa-Iglesias ME, Becerro de Bengoa-Vallejo R, Corral-Liria I. Reliability and validity of the posttraumatic stress disorder checklist for DSM-5 (PCL-5) test for post-traumatic stress disorder in mental health nurses in Spain. Arch Psychiatr Nurs. 2024;50:122–8. [DOI] [PubMed] [Google Scholar]
- 82.Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. 1994;151. Available from: 10.1176/ajp.151.8.1132 [DOI] [PubMed]
- 83.Hernandez A, Gallardo-Pujol D, Pereda N, Arntz A, Bernstein DP, Gaviria AM. Initial validation of the Spanish Childhood Trauma Questionnaire-Short Form: factor structure, reliability and association with parenting. J Interpers Violence. 2013;28. Available from: 10.1177/0886260512468240%255 [DOI] [PubMed]
- 84.Sierra M, Berrios GE. The Cambridge Depersonalisation Scale: a new instrument for the measurement of depersonalisation. Psychiatry Res. 2000Mar 6;93(2):153–64. [DOI] [PubMed] [Google Scholar]
- 85.Molina Castillo JJ, Martínez de la Iglesia J, Albert Colomer C, Berrios G, Sierra M, Luque Luque R. Adaptación y validación al castellano de la Escala de Despersonalización de Cambridge. Actas esp Psiquiatr. 2006;185–92. [PubMed]
- 86.Nijenhuis ERS, Spinhoven P, Dyck R, Hart O, Vanderlinden J. The development and psychometric characteristics of the Somatoform Dissociation Questionnaire (SDQ-20). J Nerv Ment Dis. 1996;184(11):688–94. [DOI] [PubMed]
- 87.González-Vázquez AI, Del Río-Casanova L, Seijo-Ameneiros N, Cabaleiro-Fernández P, Seoane-Pillado T, Justo-Alonso A, et al. Validity and reliability of the Spanish version of the Somatoform Dissociation Questionnaire (SDQ-20). Psicothema. 2017May;29(2):275–80. [DOI] [PubMed] [Google Scholar]
- 88.Wolpe J. The practice of behavior therapy. 1969 [cited 2024 Mar 19];314. Available from: https://books.google.com/books/about/The_Practice_of_Behavior_Therapy.html?hl=es&id=2XtHAAAAMAAJ
- 89.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Occup Med (Chic Ill). 2014;64. Available from: 10.1093/occmed/kqu024
- 90.De las Cuevas Castresana C, García-Estrada Perez A, Gonzalez de Rivera JL. “Hospital Anxiety and Depression Scale” Y Psicopatologia Afectiva. An Psiquiatr. 1995;11(4):126–30. [Google Scholar]
- 91.Soldatos CR, Dikeos DG, Paparrigopoulos TJ. Athens Insomnia Scale: validation of an instrument based on ICD-10 criteria. J Psychosom Res. 2000;48. Available from: 10.1016/S0022-3999(00)00095-7 [DOI] [PubMed]
- 92.Gómez-Benito J, Ruiz C, Guilera G. A Spanish version of the Athens Insomnia Scale. Qual Life Res. 2011;20. Available from: 10.1007/s11136-010-9827-x [DOI] [PubMed]
- 93.Diener E, Emmons RA, Larsen RJ, Griffin S. The satisfaction with life scale. 1985. [DOI] [PubMed]
- 94.Atienza FL, Pons D, Balaguer I, García-Merita M. Propiedades Psicométricas de la Escala de Satisfacción con la Vida en Adolescentes. Psicothema. 2000;13(2):314–9.
- 95.González-Vazquez AI., Mosquera-Barral D, Knipe J, Leeds AM. Self-Care Scale. 2018 [cited 2024 Mar 19]. Available from: https://psycnet.apa.org/Landing?doi=10.1037%2Ft71094-000
- 96.Grant M, Salsman NL, Berking M. The assessment of successful emotion regulation skills use: Development and validation of an English version of the Emotion Regulation Skills Questionnaire. PLoS One. 2018 Oct 1 [cited 2024 Mar 19];13(10):e0205095. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0205095 [DOI] [PMC free article] [PubMed]
- 97.Orozco-Vargas AE, García-López GI, Aguilera-Reyes U, Venebra-Muñoz A. Versión en Español del Emotion Regulation Skills Questionnaire: Análisis de su Fiabilidad y Validez The Spanish Version of the Emotion Regulation Skills Questionnaire: Analysis of Reliability and Validity. Rev Iberoam Diagnóstico y Evaluación. 2021 [cited 2024 Mar 19];4:189–203. Available from: 10.21865/RIDEP61.4.13
- 98.Rosenberg M. Conceiving the self. Publ 1979 New York by Basic Books. 1979 [cited 2024 Mar 19];xvi, 319 s., fig. Available from: https://lib.ugent.be/catalog/rug01:000357281
- 99.Gómez-Lugo M, Espada JP, Morales A, Marchal-Bertrand L, Soler F, Vallejo-Medina P. Adaptation, Validation, Reliability and Factorial Equivalence of the Rosenberg Self-Esteem Scale in Colombian and Spanish Population. Span J Psychol. 2016 Oct 14 [cited 2024 Mar 19];19:E66. Available from: https://www.cambridge.org/core/journals/spanish-journal-of-psychology/article/abs/adaptation-validation-reliability-and-factorial-equivalence-of-the-rosenberg-selfesteem-scale-in-colombian-and-spanish-population/18FA95CBC0511034D0526239501D7481 [DOI] [PubMed]
- 100.Purdon SE. SCIP Manual. 2005 [cited 2024 Mar 19]. Available from: https://www.researchgate.net/publication/309310482_Purdon_2005_SCIP_Manual
- 101.Pino O, Guilera G, Rojo JE, Gómez-Benito J, Bernardo M, Crespo-Facorro B, et al. Spanish version of the Screen for Cognitive Impairment in Psychiatry (SCIP-S): Psychometric properties of a brief scale for cognitive evaluation in schizophrenia. Schizophr Res. 2008Feb;99(1–3):139–48. [DOI] [PubMed] [Google Scholar]
- 102.Higgs JB. Fibromyalgia in primary care. Prim Care Clin Off Pr. 2018;45. Available from: 10.1016/j.pop.2018.02.008 [DOI] [PubMed]
- 103.Uclés-Juárez R, Fernández-Carreño D, Fernández-Miranda López S, Cangas DA. Conceptualización de la Fibromialgia: ¿Consenso o discrepancia entre clínicos en España? Rev Esp Salud Pública. 2020;94. Available from: 10.4321/S1135-57272020000100018 [PubMed]
- 104.Burke NN, Finn DP, McGuire BE, Roche M. Psychological stress in early life as a predisposing factor for the development of chronic pain: clinical and preclinical evidence and neurobiological mechanisms. J Neurosci Res. 2017;95. Available from: 10.1002/jnr.23802 [DOI] [PubMed]
- 105.Lichtenstein A, Tiosano S, Amital H. The complexities of fibromyalgia and its comorbidities. Curr Opin Rheumatol. 2018;30. Available from: 10.1097/BOR.0000000000000464 [DOI] [PubMed]
- 106.Briones-Vozmediano E. The social construction of fibromyalgia as a health problem from the perspective of policies, professionals, and patients. Glob Heal Action. 2017;10. Available from: 10.1080/16549716.2017.1275191 [DOI] [PMC free article] [PubMed]
- 107.Collado A, Gomez E, Coscolla R, Sunyol R, Solé E, Rivera J. Work, family and social environment in patients with fibromyalgia in Spain: an epidemiological study: EPIFFAC study. BMC Heal Serv Res. 2014;14. Available from: 10.1186/s12913-014-0513-5 [DOI] [PMC free article] [PubMed]
- 108.Morgan B, Wooden S. Diagnosis and treatment of common pain syndromes and disorders. Nurs Clin North Am. 2018;53. Available from: 10.1016/j.cnur.2018.04.004 [DOI] [PubMed]
- 109.Gota CE. What you can do for your fibromyalgia patient. Cleve Clin J Med. 2018;85. Available from: 10.3949/ccjm.85gr.18002 [DOI] [PubMed]
- 110.Dell’Osso L, Carmassi C, Consoli G, Conversano C, Ramacciotti CE, Musetti L. Lifetime post-traumatic stress symptoms are related to the health-related quality of life and severity of pain/fatigue in patients with fibromyalgia. Clin Exp Rheumatol. 2011;29. [PubMed]
- 111.Wolfe F, Ablin J, Guymer EK, Littlejohn GO, Rasker JJ. The relation of physical comorbidity and multimorbidity to fibromyalgia, widespread pain and fibromyalgia-related variables. J Rheumatol. 2020;47(4):624–31. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Personal data of the participant will be numerically coded and kept in a database at the Centre Forum Research Unit. Only the researchers involved in the trial will have access to this data, however, the dataset analysis of the current study will be available from the corresponding author upon reasonable request. When the study finishes and results are obtained, we will send a copy of the article in Spanish to all, together with a plain language summary, as well as providing individual feedback about progress during the study if any participant requests it. The results of the study will also be published in peer review journals, and national and international conferences. The datasets analyzed during the current study will be available from the corresponding author on reasonable request.