ABSTRACT
For the majority of fungal species, the somatic body of an individual is a network of interconnected cells sharing a common cytoplasm and organelles. This syncytial organization contributes to an efficient distribution of resources, energy, and biochemical signals. Cell fusion is a fundamental process for fungal development, colony establishment, and habitat exploitation and can occur between hyphal cells of an individual colony or between colonies of genetically distinct individuals. One outcome of cell fusion is the establishment of a stable heterokaryon, culminating in benefits for each individual via shared resources or being of critical importance for the sexual or parasexual cycle of many fungal species. However, a second outcome of cell fusion between genetically distinct strains is formation of unstable heterokaryons and the induction of a programmed cell death reaction in the heterokaryotic cells. This reaction of nonself rejection, which is termed heterokaryon (or vegetative) incompatibility, is widespread in the fungal kingdom and acts as a defense mechanism against genome exploitation and mycoparasitism. Here, we review the currently identified molecular players involved in the process of somatic cell fusion and its regulation in filamentous fungi. Thereafter, we summarize the knowledge of the molecular determinants and mechanism of heterokaryon incompatibility and place this phenomenon in the broader context of biotropic interactions and immunity.
INTRODUCTION
Cell-cell fusion is an essential biological process that occurs in organisms throughout the tree of life. It is involved in both sexual and asexual developmental processes in most species and has been shown to occur in multicellular and in unicellular organisms. Somatic cell fusion events are widespread in eukaryotic organisms, including animals, where they are important for muscle differentiation, placental development, and formation of multinucleate giant cells in the immune system (1–4).
Somatic cell fusion is a highly regulated event that requires reciprocal recognition as well as coordinated molecular processes in fusion partners. However, indiscriminate cell fusion comes with risks, including fusion with infected, impaired, or genetically different fusion partners, which can result in a disadvantaged heterokaryon. Thus, cells have evolved mechanisms to discriminate genetically identical from genetically nonidentical (nonself) cells. There are diverse types of genetic recognition mechanisms that organisms use to distinguish self from nonself. In mammalian cells, self/nonself recognition mechanisms mediate pathogen defense, while in basal eukaryotic invertebrates, such as the colonial ascidian Botryllus schlosseri and the cnidarian Hydractinia symbiolongicarpus, self/nonself recognition functions when individuals fuse to form vascular and hematopoietic chimeras (5). In bacteria, such as Proteus mirabilis, swarming colonies can recognize each other as nonself and establish a visible boundary, whereas genetically identical swarms merge (6, 7). In protists such as Dictyostelium discoideum, self/nonself recognition limits “self” genotypes to sporulating fruiting bodies (8, 9). In plants, self/nonself recognition is important for both pathogen defense and the prevention of self-fertilization for out-crossing species (10, 11). In filamentous fungi, the syncytial lifestyle combined with cell fusion to form the interconnected hyphal networks that are the growth habit of these organisms makes them superb models for studies of self/nonself recognition mechanisms. In this chapter, we summarize the current knowledge about somatic cell fusion events in filamentous fungi that govern both self and nonself recognition processes and that act prior to and after cell fusion events.
SOMATIC FUSION IN FILAMENTOUS FUNGI AND ITS CONSEQUENCES: BENEFITS AND DETRIMENTS
In filamentous fungi, somatic cell fusion (anastomosis) is essential to develop the hallmark of filamentous fungal growth: an interconnected, multinucleated, syncytial network (Fig. 1). Fusion has been reported in more than 73 species of filamentous fungi covering over 20 genera (12). Unlike other organisms, such as plants or animals, fungi lack biological transport networks (vascular systems). Instead, these organisms build a mycelial network to distribute cytoplasm, organelles (including nuclei), nutrients, and other resources within the syncytium, facilitating growth and rapid spatial expansion of the fungal colony (13–16). In fact, filamentous fungi form the most extensive biological networks characterized so far (17). Anastomosis in hyphae is also critical for host colonization and virulence of pathogenic and symbiotic fungi (18, 19) and for mycoparasitism (20).
FIGURE 1.

Germling and hyphal fusion in Neurospora crassa. (A–C) Germinating spores undergo mutual attraction and fusion (A: 0 min; B: 40 min; C: 80 min). (D) Consecutive fusion events result in network formation. (E, F) Hyphal branches fuse and form cross connections. (E: DIC; F: cell walls stained with calcofluor white). Asterisks in all images indicate fusion points. Adapted from reference 38.
In addition to fusion within a colony, cell fusion can occur between genetically different colonies. Such fusion events of nonclone individuals can be viable and lead to the coexistence of genetically different nuclei in a common cytoplasm (heterokaryon). Heterokaryon formation in filamentous fungi has potential benefits; e.g., it results in functional diploidy and mitotic recombination during the parasexual cycle, which can be especially important for highly clonal species (21, 22). However, heterokaryon formation has risks, because infectious agents can be transmitted via fusion events (23–26). Within a heterokaryon, allorecognition processes determine the fate of the fused cells: compatible genotypes lead to viable heterokaryons indistinguishable from a homokaryotic colony, while heterokaryotic cells resulting from the fusion of incompatible genotypes are rapidly compartmentalized and undergo a programmed cell death (PCD) reaction, termed heterokaryon (or vegetative) incompatibility (HI or VI) (27, 28). It has been proposed that HI limits genome exploitation and represents a defense mechanism that has important adaptive value in the highly competitive biotopes inhabited by most filamentous fungi (29). HI has been shown to limit the propagation of deleterious agents such as mycoviruses, DNA transposons, and senescence plasmids (24, 30–32). However, a recent study by Bastiaans et al. showed that fusion among fungal colonies is mutually beneficial, relative to the absence of fusion upon nonself recognition, suggesting an interplay between beneficial aspects of cell fusion versus the risk factors associated with indiscriminate fusion with other partners (33).
Unlike somatic fusion, where genetic similarity between the fusion partners is favorable, in out-crossing species of filamentous fungi, fusion between genetically dissimilar partners is necessary for sexual reproduction. In heterothallic fungi, genetic dissimilarity is determined by the existence of at least two different mating types. Interestingly, in some fungal species, mating type also serves as an HI factor during somatic fusion, preventing the existence of different mating types within a heterokaryotic colony (34, 35). In species capable of outcrossing, individuals that are somatically incompatible undergo sexual reproduction and production of meiotic progeny, indicating that nonself recognition involved in HI processes must be inactive during the sexual cycle.
RECOGNITION, CELL FUSION, AND COLONY ESTABLISHMENT
In addition to hyphal fusion within a mature colony, somatic fusion events also contribute to the onset of colony formation. For example, cell fusion between germinated asexual spores (germlings) merges numerous individuals into one functional unit, providing the basis for the developing mycelial colony in a number of fungal species (Fig. 1) (36). The ability of germlings to form a network contributes to fitness, as indicated by more rapid colony establishment and subsequent asexual spore formation (36–39). However, fusion can be particularly risky for germlings. For example, a postfusion incompatibility reaction (see below) would likely result in the death of both germlings. Therefore, most HI reactions described so far are suppressed at the germling stage (40).
Germlings are especially tractable for analyzing the cellular mechanisms and components involved in the cell fusion process (2). In recent years, more than 60 proteins involved in germling fusion have been identified (41, 42). Most of the cellular components mediating germling fusion processes are also required for hyphal fusion in a mature colony, indicating that both processes share common molecular machinery. Mutual recognition and attraction of the fusion partners employs an intricate signaling network, which comprises conserved eukaryotic factors, such as mitogen-activated protein kinase (MAPK) modules, NADPH oxidases, the striatin-interacting phosphatase and kinase (STRIPAK) complex, and calcium-regulated factors, but also fungal-specific proteins (Fig. 2). A central hub within this network is a MAPK cascade homologous to the pheromone response pathway of Saccharomyces cerevisiae (43, 44). Analysis of the subcellular dynamics of the MAPK mitogen-acivated kinase-2 (MAK-2) during germling fusion in Neurospora crassa revealed an unusual mode of communication associated with localization of MAK-2 to cell tips during chemotropic interactions (45, 46). During the tropic interactions between germlings or hyphae, MAK-2 assembles with its two upstream kinases MEK-2 and NRC-1, the scaffold HAM-5, and the adaptor protein STE50 in an oscillating manner at the plasma membrane of cell tips (45, 47, 48). Within one cell, formation of the MAK-2 complexes at the cell tips alternates with the membrane recruitment of another protein, called SOFT (SO), a factor that is only conserved in filamentous ascomycete fungi and is essential for germling/hyphal fusion (45, 49). The membrane recruitment of the MAK-2 complex or SO alternates between the two fusion partners in a coordinated manner, with one phase lasting between 8 and 12 minutes. These observations indicate that the two fusion cells switch between two physiological states in a highly coordinated manner.
FIGURE 2.

Working model of the molecular events governing germling and hyphal fusion. The signal-emitting cell releases the ligand in a pulse-like manner, probably by exocytosis. Binding of the signal molecules to their cognate receptors results in assembly and activation of the MAK-2 module at the plasma membrane. MAK-2 phosphorylates MOB-3 of the STRIPAK complex, thereby promoting nuclear entry of MAK-1. In the nucleus MAK-2 activates the transcription factor PP-1, which controls cell fusion factor-encoding genes. Activation of MAK-2 involves reactive oxygen species production by the NADPH oxidase (NOX) complex either upstream or downstream of the MAP kinase cascade. Adapted from reference 38.
The current working model suggests that the cells take turns in signal sending and signal receiving. Mathematical modeling revealed that this unique mode of communication would allow signaling via a single receptor/ligand pair employed by both partner cells (50). The spatiotemporal coordination of signal sending versus signal receiving would enable genetically and developmentally identical cells to achieve mutual attraction and fusion, while avoiding self-stimulation. While the nature of the predicted signal and receptor activating the MAPK module remains unknown, the linkage of the MAK-2 module and the SO protein within the fusion signaling network is beginning to unfold. In Sordaria macrospora, a homolog to SO called PRO40 functions as a scaffold for a second MAPK module, the cell wall integrity pathway (51). Similar to the MAK-2 pathway, this MAPK module is also essential for somatic fusion in several filamentous fungi (43, 51–53). In N. crassa, nuclear entry of the MAPK of the cell wall integrity pathway, MAK-1, is controlled by the STRIPAK complex in a MAK-2-dependent manner (54). STRIPAK complexes are multiprotein assemblies that mediate a plethora of biological functions in eukaryotic organisms. Their presence in filamentous fungi was first shown in S. macrospora, and their essential role for somatic fusion has been established for several species (54–59). Besides the STRIPAK complex, an additional target of MAK-2 is the transcription factor PP-1, which in turn controls the expression of several fusion-mediating factors, including genes encoding the STRIPAK complex, so, mak-1, mek-1, and NADPH oxidase encoding genes (39). NADPH oxidase complexes are essential for somatic fusion in several filamentous fungi, and their potential interaction with the MAPK signaling modules as targets or activators is currently a major research topic in the cell fusion field (2, 60–62). In general, placing the numerous identified fusion factors into the described signaling network will be one of the main future challenges in this research field. Given, however, the experimental tractability of fungal model systems, hyphal and germling fusion may well advance as paradigms for studying eukaryotic signaling networks and their subcellular dynamics.
NONSELF RECOGNITION EVENTS ACTING PREFUSION
While the molecular basis of chemotropic interactions between genetically identical germlings has been studied extensively, there are only a few studies focusing on processes involved in chemotropic interactions between genetically nonidentical germlings. For example, prerecognition of incompatible strains has been reported in Tuber borchii and Glomus mosseae, in which incompatible hyphae avoid fusion with each other (63, 64). Although the genes and mechanisms behind these findings remain to be revealed, these observations suggest a link between fusion signaling and nonself recognition. This hypothesis was supported recently in a study by Heller et al. showing that the polymorphic “greenbeard” genes doc-1, doc-2, and doc-3 mediate kind discrimination in N. crassa populations and act at a distance during chemotropic interactions. Kind discrimination divides N. crassa populations into separate communication groups; only individuals with identical sets of doc genes (kind individuals) show chemotropic growth and cell fusion. Germlings from different communication groups grow past each other, even when in close proximity, to find a partner of their own communication group. If nonkind germlings are in close proximity, MAK-2 oscillation reinforcement is repressed, resulting in an inability of cells to establish chemotropic interactions (65). These findings show that cellular communication in fungi is even more complex than initially anticipated: due to nonself recognition mechanisms acting at a distance, cells must avoid not only self-stimulation during chemotropic interactions, but also stimulation by nonkind individuals.
NONSELF RECOGNITION MECHANISMS ACTING POSTFUSION
In spite of nonself recognition mechanisms that act prefusion, fusion between genetically different strains occurs frequently. Anastomosis between compatible genotypes results in formation of a viable heterokaryon, while anastomosis between incompatible genotypes triggers HI, resulting in a PCD reaction leading to death of the fusion cells (Fig. 3). This outcome is widespread in fungi and represents the most probable event after heterokaryon formation. Heterokaryon (or vegetative) incompatibility can be observed by the appearance of a demarcation line, which separates the genetically incompatible strains, termed “barrage.” One of the first accounts of this type of rejection of nonself in filamentous fungi was made in the early 20th century by the pioneering mycologist Dorothy M. Cayley on the plant pathogenic ascomycete Diaporthe perniciosa (66). After confronting mycelia of monosporic cultures that exhibited the barrage phenotype, Cayley noted that “like must meet like and unlike shows aversion to unlike.” HI reactions in nature, mostly on dead tree trunks, or on an agar plate under laboratory conditions, can be detected by assessing whether barrage lines form at interaction zones between genetically distinct individuals (Fig. 4) (67). Microscopically, incompatible heterokaryotic fusion cells are rapidly isolated from the rest of the mycelia by septal plugging (68, 69). Septal plugging in response to stress can be mediated by specialized peroxisome-derived organelles called Woronin bodies (70–73), although the role of the Woronin body in HI is unclear. However, a family of septal pore-associated proteins and the fusion protein SO have been associated with septal plugging during HI in N. crassa (74, 75). The HI reaction leads to vacuolization of the fusion cells accompanied by cell wall thickening, lipid droplet accumulation, reactive oxygen species production, and intracellular de novo formation of septa (Fig. 5) (70, 76). Furthermore, nuclear DNA degradation during cellular dismantling occurs, drawing parallels between fungal PCD and apoptosis in metazoans (77). The complete cellular lysis of an incompatible hyphal compartment can vary in duration, ranging from 20 minutes to more than 6 hours, depending on the genetic determinants triggering the reaction. Autophagy also plays a role in HI, although the autophagosomal process is not required for the execution of cell death (78, 79). It has been proposed that autophagy has a protective role during HI by limiting the spread of the PCD signals, similar to what has been reported in plant immune responses (80).
FIGURE 3.

Heterokaryosis and its possible outcomes. Genetically distinct individuals can undergo hyphal anastomosis. If there are no allelic specificity differences at het loci, a viable heterokaryon is established and nuclei (blue and brown dots) are exchanged. If allelic specificity is different between the two strains for any of the het loci, septal plugging isolates the heterokaryotic compartments and cell death occurs.
FIGURE 4.

Macroscopic visualization of vegetative incompatibility. The heterokaryon (vegetative) incompatibility reaction is visualized by the occurrence of a demarcation line called “barrage” that separates the incompatible strains. (A) Evidence of barrage on wood (spalted wood) occurring in the wild. (B) Barrage reaction (black arrows) between genetically incompatible Podospora anserina strains. Identical individuals fuse without inducing allorecognition PCD and do not form the barrage (white arrows).
FIGURE 5.
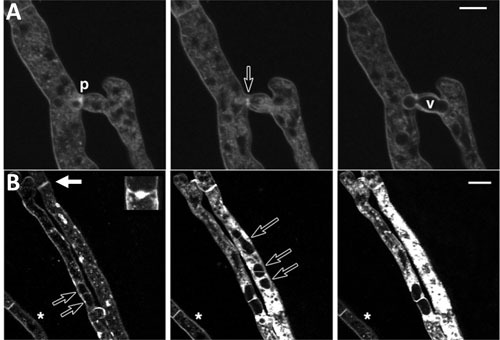
Microscopic visualization of programmed cell death during vegetative incompatibility. A time course of compatible and incompatible hyphal fusion in Neurospora crassa. The programmed cell death reaction is followed by the fluorescent vital dye (membrane staining) FM4-64. (A) Fusion between two N. crassa strains that have identical specificities at all het loci. Arrow shows the fusion pore (p). Nuclei or large vacuoles (v) are transported through the pore with the cytoplasmic flow. (B) Fusion between two N. crassa strains that differ in het specificity. Heterokaryotic cells are compartmentalized by septal plugs (solid arrow and insert). Permeabilization of the plasma membrane leads to increased cytoplasmic staining and vacuolization. Open arrows show large vacuoles within incompatible fusion cells, while the asterisk shows a nearby healthy cell. Bar = 10 μM. Adapted from reference 146.
Genetic Control and Molecular Characterization of Heterokaryon Incompatibility
Early efforts to understand the HI reaction led to genetic mapping of loci involved in its control for a small number of ascomycete species, so-called het (heterokaryon) or vic (vegetative incompatibility) loci (81, 82). Genetic analyses revealed that the number of het/vic loci ranges from 7 to 12 for different species in sampled populations. Nonself recognition in HI systems may be mediated by alternate alleles at a single het locus (allelic HI systems) or by alternate alleles at more than one het locus (nonallelic HI systems). Within the three species (N. crassa, Podospora anserina, and Cryphonectria parasitica) which have served as model organisms for studying HI, nonallelic HI systems predominate (Fig. 6). Independent of the type of HI system, particular hallmarks of molecular evolution are shared between het loci. Foremost, alleles at a particular het locus are highly polymorphic in populations and are often found in hypervariable genomic regions (83). Second, alleles conferring alternate specificity are maintained in wild populations in nearly equal allelic frequencies, which is an indication of nonneutral balancing selection operating on these loci (83, 84). The observed signatures of balancing selection can be explained by the associated benefits of preserving incompatible alleles and are in accordance with the proposed adaptive value of HI. The underlying evolutionary process is an example of negative frequency-dependent selection in which the rare genotype is advantageous: strains carrying a less frequent het allele would potentially form a higher number of incompatible heterokaryons and therefore possess a fitness advantage by diminishing conspecific parasitism (85, 86). In some cases, the evolutionary pressure on HI determinants surpasses speciation events resulting in trans-species polymorphism in which closely related species share ancestral het alleles. Balancing selection and trans-species polymorphisms are described for other self/nonself defining loci such as the major histocompatibility complex in vertebrates and the S locus in flowering plants (87, 88).
FIGURE 6.

Incompatibility systems and genetically identified het (vic) loci in model filamentous ascomycete species. Round-headed arrows connecting the het/vic genes indicate nonallelic HI systems, and square-headed arrows indicate allelic HI systems. Blue arrows indicate that the incompatibility reaction influences the distribution of mycoviruses that result in hypovirulence in Cryphonectria parasitica. Genes in red encode for proteins with a HET domain, and boxed genes (loci) are still not identified molecularly.
In the next section we summarize the molecular details for the currently characterized het genes and HI systems of each of the three above-mentioned fungal models.
N. crassa
N. crassa is a heterothallic ascomycete with a haploid cell cycle and initially was used as a model organism to establish the bases of molecular biology and decipher metabolic pathways (89, 90). Classical genetics assays (complementation or dominance/recessiveness) with haploid monokaryotic strains carrying auxotrophic mutations revealed that heterokaryosis between strains of opposite mating types results in growth inhibition (91). Incompatibility based on differences at the mating type locus is not exclusive to N. crassa and is found in several other unrelated species (92, 93). The mating type locus in N. crassa is composed of genes that confer mating identity (mat A or mat a) and are evolutionarily unrelated (termed idiomorphs) (94). The mat-A1 and mat-a1 genes of the mat A or mat a idiomorphs, respectively, encode for transcriptional regulators which trigger HI when coexpressed in heterokaryotic cells (34, 95). The role of mating of the N. crassa mat locus is uncoupled with its HI function as demonstrated by strains carrying mutations in mat-A1 or mat-a1 that abolish HI but that do not impact mating competency (96, 97). Additional genetic analysis for suppressors of mating type incompatibility led to the identification of a recessive mutation called tol (tolerant) (98). Strains carrying the tol mutation mate normally but do not induce the HI phenotype. TOL actively regulates mating type-dependent HI, as shown by introgression experiments between N. crassa and the closely related pseudohomothallic species Neurospora tetrasperma (99, 100). The tol gene encodes for a putative 1,011-amino-acid polypeptide with a HET domain and coiled-coil and leucine-rich repeat regions. Transcriptional repression of tol during the sexual reproductive phase has been postulated to allow opposite mating type nuclei to coexist in the dikaryotic ascogenous hyphae (93).
Further investigations of heterokaryosis with nearly isogenic N. crassa strains or with the elegant use of strains containing partial chromosomal duplications revealed 10 other het loci in N. crassa (81, 101, 102). Three of these het genes have been cloned and their products examined at the molecular level. Two of the het genes, het-c and het-6, control HI in association with closely linked (adjacent) genes termed pin-c (partner in incompatibility of het-c) and un-24, respectively (81, 103, 104, 105). Nonallelic interactions between het-6 and un-24, the latter encoding the large subunit of ribonucleotide reductase, are considered crucial for the HI phenotype in the het-6/un-24 system (106). Haplotypes of het-c/pin-c and het-6/un-24 are under balancing selection, show severe linkage disequilibrium—haplotypes are inherited as blocks—and exhibit trans-species polymorphism (84, 107). The product of het-c is a glycine-rich cell wall protein, and the HI specificity of the allelic variants is determined by indels in a variable region (108, 109). The pin-c gene encodes a protein that shares a region of similarity with the products of het-6 and tol, termed the HET domain (∼150 amino acids). Genes encoding predicted HET domain proteins are ubiquitous in ascomycete genomes (83). A HET domain protein is also encoded by the recently characterized het-e locus (83), and coexpression of different het-e alleles triggers the HI reaction. Proteins encoding a HET domain are a common thread in all molecularly characterized HI systems in Neurospora, suggesting downstream similarities in PCD pathways. A combined population genomics and evolutionary approach was used to identify 15 additional candidate het loci, all encoding proteins with a HET domain and which displayed at least two long-diverged haplogroups (83). Additionally, deletion of a transcription factor, vib-1, suppresses the HI reaction mediated by genetic differences at all molecularly characterized het loci in N. crassa and was shown to be required for expression of some het genes encoding HET domain proteins (110–112).
P. anserina
P. anserina is an ascomycete in the same order (Sordariales) as Neurospora (68). Eight HI systems have been genetically defined in P. anserina and four have been molecularly investigated (70, 82). To date, none of the characterized het loci in P. anserina are orthologous to those described in N. crassa. As in N. crassa, three nonallelic HI systems (het-d/het-c, het-e/het-c, and het-r/het-v) depend on a gene encoding a HET domain protein (het-d, het-e, and het-r) (113, 114). The HET domain itself has been shown to play an essential role in the induction of PCD in P. anserina (115). The HET domain is situated at the N-terminus of these proteins next to a central nucleotide-binding NACHT domain and a C-terminal domain composed of WD40 repeats (116). The NACHT domain is associated with regulation of apoptosis and innate immunity in metazoans and mediates the oligomerization of proteins forming multimeric cell death platforms like the apoptosome (117, 118). The WD40 repeats form a doughnut-shaped beta-propeller structure where individual repeats of 40 to 42 amino acids, in beta-sheet conformation, revolve around a central axis and can function in molecular sensing as protein scaffolds (119). APAF-1, the human apoptotic factor assembling the apoptosome, also has a WD40 domain which binds to cytochrome c, thus regulating its apoptosis-inducing activity (120). The NACHT and WD40 domains have been shown to be functionally important in HET-E to trigger HI (121).
The coexpression of allelic variants of HET-D or HET-E with antagonistic variants of HET-C—a protein with a GLTP (glycolipid transfer protein) fold—induces a PCD reaction (122, 123). In this nonallelic incompatibility system, the het-c gene is not linked to het-e or to het-d (124). Eleven highly polymorphic het-c alleles are present in a population of 110 P. anserina strains, falling in seven distinct functional classes with polymorphic positions under positive diversifying selection (high ratio of nonsynonymous to synonymous substitutions per codon) overlapping with positions defining allelic specificity (125). The allelic specificity of HET-D, HET-E, and HET-R is defined by the variability of the WD40 domain (113). Exchange between the WD40 repeats between alleles from the same het locus and/or between different paralogous WD40 genes is hypothesized to aid in concerted evolution of alleles (126, 127). The current data support a molecular model for het-d/het-c and het-e/het-c HI systems in which direct binding of antagonistic HET-C allelic variants by the WD40 domain of HET-D/HET-E triggers the PCD reaction (128).
One HI system in P. anserina that has been extensively studied involves the het-s locus, which encodes two functional alleles, het-s and het-S (129). Importantly, the het-s allele encodes a prion protein (the HET-s protein) that can exist as a soluble monomer, in a state termed [Het-s*] or as infectious aggregates (a prion state) termed [Het-s] (130, 131). The appearance of the [Het-s] prion state occurs sporadically at a low rate and converts the [Het-s*] state to the [Het-s] prion, so that strains of the het-s genotype are exclusively prion-infected or prion-free. Only prion-infected [Het-s] strains trigger HI with [Het-S] strains (132). The HET-S protein is a pore-forming toxin that is activated upon interaction with the HET-s prion protein, resulting in death of the cell (133, 134). The two alleles het-s and het-S are under balancing selection and in a wild population of P. anserina, with 92% of the strains of het-s genotype being prion-infected, thus having the HI competent [Het-s] state (24). These observations underline the adaptive value of the allorecognition system in the wild—a conclusion supported in the same study by the correlated distribution of a deleterious plasmid with the frequency of the het-s and het-S genotypes in populations (24).
C. parasitica
Six vic loci control the incompatibility reaction in C. parasitica, an ascomycete that causes the chestnut blight disease (135, 136). As in Podospora, HI in Cryphonectria is observed by the appearance of a barrage between incompatible strains. Moreover, a systematic exploration of the transmission of virulence-attenuating mycoviruses during heterokaryosis showed that genetic differences at five of the six vic loci reduced virus transmission between strains, suggesting fitness benefits associated with HI (31, 137). This approach makes Cryphonectria an exciting model to study the relationship between HI and its function as a barrier against mycovirus transmission. The six incompatibility systems in Cryphonectria are composed of closely linked polymorphic genes or idiomorphs, and their functional characterization indicates mostly nonallelic interactions (31). Three of the HI systems rely on proteins with HET domains, and one other involves a protein with NACHT-WD40 architecture. As above, the molecularly characterized vic genes are not orthologous to Podospora or Neurospora molecularly characterized het loci. Recently, as a consequence of the molecular characterization of the vic genes, a super mycovirus donor strain has been engineered that carries multiple deactivated vic genes, which could potentially serve as a more potent biocontrol agent against larger sets of wild isolates that are capable of causing the chestnut blight disease (138).
HI Determinants: at the Molecular Crossroad of Allo- and Xenorecognition
Experimental evidence supports the hypothesis that HI, an allorecognition process, has a beneficial role in nature, preventing conspecific parasitism and limiting harmful replicon propagation. However, similarities of some fungal HI-inducing determinants with plant and metazoan innate immunity factors, the latter mediating xenorecognition (nonself discrimination between different species), have prompted the idea that fungal allorecognition (HI) may originate from molecular networks with a primary role in heterospecific nonself recognition (139, 140). For example, HET-E (HET-D and HET-R), controlling HI in P. anserina, has a similar domain organization to APAF-1 and NLRC4 (a NOD-like receptor [NLR] protein), which are intracellular molecular sensors operating in mammalian apoptosis and innate immunity, respectively (Fig. 7) (141, 142). An extensive analysis of fungal NLR-like proteins—a protein superfamily that includes HET-E, HET-D, and HET-R from Podospora and VIC4-2 from Cryphonectria—suggests a similar role in xenorecognition for this class of proteins (143). Furthermore, fungal NLRs not directly involved in HI have been shown to regulate pore-forming toxins that function as bona fide HI-factors, like HET-S in Podospora (128, 144, 145). These data support the hypothesis regarding the relationship between allo- and xenorecognition systems in fungi and put the HI phenomenon in a broader context of fungal biotic interactions.
FIGURE 7.

Domain organization of fungal and metazoan NLRs (NOD-like receptors) and NLR-like proteins. The heterokaryon determinants HET-E (also HET-D and HET-R as paralogues of HET-E) and VIC4 present a typical NLR-like domain organization. NLRs have a tripartite domain organization with a central nucleotide-binding and oligomerization (NOD) domain, an N-terminal effector domain, and a C-terminal sensor domain. The sensor domain can be composed of various repeated motifs (LRR or WD40, in the examples presented here) that trigger the activation of the receptors upon recognition of defined molecular cues. The recognition of the signal activates the formation by the receptors of multimeric protein platforms. The oligomerization of the receptors is mediated by the NOD domain (NACHT or NB-ARC type) in characterized cases, such as APAF-1 (the human apoptosis-controlling factor) and NLRC4 (an innate immunity receptor). Abbreviations: CARD, caspase recruitment domain; LRR, leucine rich repeats.
CONCLUSIONS AND FUTURE OUTLOOK
Cell fusion is of capital importance for the fungal life style. It is not only related to mating, but it is also important during vegetative growth, facilitating colony establishment, network formation, and heterokaryon formation. Nonself recognition mechanisms that function pre- and postfusion restrict the development of heterokaryons to cells that are more genetically similar. Genetic analyses have identified a large number of genes required for cell fusion, but many of these have not been placed in a cellular pathway, nor is it understood how the different cellular pathways are integrated to accomplish cell fusion events. Another major outstanding question is the relationship between the molecular mechanisms regulating cell fusion events versus those that regulate nonself recognition, both pre- and postfusion. Unlike alleles at loci required for cell fusion, alleles at loci that function in nonself recognition often display features of balancing selection. The availability of complete fungal genome sequences on the population level will facilitate the identification and characterization of loci that display balancing selection and thus are presumptively involved in either xeno- or allorecognition.
As discussed above, the induction of PCD as a result of nonself recognition and HI seems to be conserved among species of filamentous fungi, even though the proteins used for nonself recognition can vary. However, with the exception of the het-s/het-S system of P. anserina, the link between the nonself recognition and the death reaction is still unclear for other HI systems. This raises the question of how death is induced on a molecular level. Identifying the signaling networks and exact mechanisms that control the PCD pathway will be at the center of interest for further research on fungal HI.
ACKNOWLEDGMENTS
This work was supported by a National Institute of General Medical Sciences grant (R01GM060468) and an Alexander von Humboldt Research Award to N.L.G. J.H. was supported by a research fellowship from the Deutsche Forschungsgemeinschaft (HE 7254/1-1). Work in the group of A.F. is supported by funding from the Deutsche Forschungsgemeinschaft (FL706-2) and the European Union (PITN-GA-2013-607963) to A.F.
REFERENCES
- 1.Huppertz B, Bartz C, Kokozidou M. 2006. Trophoblast fusion: fusogenic proteins, syncytins and ADAMs, and other prerequisites for syncytial fusion. Micron 37:509–517. 10.1016/j.micron.2005.12.011 [DOI] [PubMed] [Google Scholar]
- 2.Read ND, Goryachev AB, Lichius A. 2012. The mechanistic basis of self-fusion between conidial anastomosis tubes during fungal colony initiation. Fungal Biol Rev 26:1–11. 10.1016/j.fbr.2012.02.003 [DOI] [Google Scholar]
- 3.Richardson BE, Nowak SJ, Baylies MK. 2008. Myoblast fusion in fly and vertebrates: new genes, new processes and new perspectives. Traffic 9:1050–1059. 10.1111/j.1600-0854.2008.00756.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vignery A. 2008. Macrophage fusion: molecular mechanisms. Methods Mol Biol 475:149–161. 10.1007/978-1-59745-250-2_9 [PubMed] [DOI] [PubMed] [Google Scholar]
- 5.Cadavid LF, Powell AE, Nicotra ML, Moreno M, Buss LW. 2004. An invertebrate histocompatibility complex. Genetics 167:357–365. 10.1534/genetics.167.1.357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wenren LM, Sullivan NL, Cardarelli L, Septer AN, Gibbs KA. 2013. Two independent pathways for self-recognition in Proteus mirabilis are linked by type VI-dependent export. MBio 4:e00374-13. 10.1128/mBio.00374-13. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibbs KA, Urbanowski ML, Greenberg EP. 2008. Genetic determinants of self identity and social recognition in bacteria. Science 321:256–259. 10.1126/science.1160033 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strassmann JE, Zhu Y, Queller DC. 2000. Altruism and social cheating in the social amoeba Dictyostelium discoideum. Nature 408:965–967. 10.1038/35050087 [DOI] [PubMed] [Google Scholar]
- 9.Li SI, Purugganan MD. 2011. The cooperative amoeba: Dictyostelium as a model for social evolution. Trends Genet 27:48–54. 10.1016/j.tig.2010.11.003 [PubMed] [DOI] [PubMed] [Google Scholar]
- 10.Sanabria N, Goring D, Nürnberger T, Dubery I. 2008. Self/nonself perception and recognition mechanisms in plants: a comparison of self-incompatibility and innate immunity. New Phytol 178:503–514. 10.1111/j.1469-8137.2008.02403.x 10.1111/j.1469-8137.2008.02403.x [DOI] [PubMed] [Google Scholar]
- 11.Dodds PN, Rathjen JP. 2010. Plant immunity: towards an integrated view of plant-pathogen interactions. Nat Rev Genet 11:539–548. 10.1038/nrg2812 [DOI] [PubMed] [Google Scholar]
- 12.Gabriela Roca M, Read ND, Wheals AE. 2005. Conidial anastomosis tubes in filamentous fungi. FEMS Microbiol Lett 249:191–198. 10.1016/j.femsle.2005.06.048 [PubMed] [DOI] [PubMed] [Google Scholar]
- 13.Hickey PC, Jacobson D, Read ND, Glass NL. 2002. Live-cell imaging of vegetative hyphal fusion in Neurospora crassa. Fungal Genet Biol 37:109–119. 10.1016/S1087-1845(02)00035-X [PubMed] [DOI] [PubMed] [Google Scholar]
- 14.Pieuchot L, Lai J, Loh RA, Leong FY, Chiam KH, Stajich J, Jedd G. 2015. Cellular subcompartments through cytoplasmic streaming. Dev Cell 34:410–420. 10.1016/j.devcel.2015.07.017 [DOI] [PubMed] [Google Scholar]
- 15.Simonin A, Palma-Guerrero J, Fricker M, Glass NL. 2012. Physiological significance of network organization in fungi. Eukaryot Cell 11:1345–1352. 10.1128/EC.00213-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roper M, Simonin A, Hickey PC, Leeder A, Glass NL. 2013. Nuclear dynamics in a fungal chimera. Proc Natl Acad Sci USA 110:12875–12880. 10.1073/pnas.1220842110 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heaton L, Obara B, Grau V, Jones N, Nakagaki T, Boddy L, Fricker M. 2012. Analysis of fungal networks. Fungal Biol Rev 26:12–29. 10.1016/j.fbr.2012.02.001 [DOI] [Google Scholar]
- 18.Charlton ND, Shoji JY, Ghimire SR, Nakashima J, Craven KD. 2012. Deletion of the fungal gene soft disrupts mutualistic symbiosis between the grass endophyte Epichloë festucae and the host plant. Eukaryot Cell 11:1463–1471. 10.1128/EC.00191-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Craven KD, Vélëz H, Cho Y, Lawrence CB, Mitchell TK. 2008. Anastomosis is required for virulence of the fungal necrotroph Alternaria brassicicola. Eukaryot Cell 7:675–683. 10.1128/EC.00423-07 [PubMed][CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gruber S, Zeilinger S. 2014. The transcription factor Ste12 mediates the regulatory role of the Tmk1 MAP kinase in mycoparasitism and vegetative hyphal fusion in the filamentous fungus Trichoderma atroviride. PLoS One 9:e111636. 10.1371/journal.pone.0111636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pontecorvo G. 1956. The parasexual cycle in fungi. Annu Rev Microbiol 10:393–400. 10.1146/annurev.mi.10.100156.002141 [PubMed] [DOI] [PubMed] [Google Scholar]
- 22.Schoustra SE, Debets AJ, Slakhorst M, Hoekstra RF. 2007. Mitotic recombination accelerates adaptation in the fungus Aspergillus nidulans. PLoS Genet 3:e68. 10.1371/journal.pgen.0030068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Debets AJM, Griffiths AJF. 1998. Polymorphism of het-genes prevents resource plundering in Neurospora crassa. Mycol Res 102:1343–1349. 10.1017/S095375629800639X [DOI] [Google Scholar]
- 24.Debets AJ, Dalstra HJ, Slakhorst M, Koopmanschap B, Hoekstra RF, Saupe SJ. 2012. High natural prevalence of a fungal prion. Proc Natl Acad Sci USA 109:10432–10437. 10.1073/pnas.1205333109 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Son M, Yu J, Kim KH. 2015. Five questions about mycoviruses. PLoS Pathog 11:e1005172. 10.1371/journal.ppat.1005172 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearson MN, Beever RE, Boine B, Arthur K. 2009. Mycoviruses of filamentous fungi and their relevance to plant pathology. Mol Plant Pathol 10:115–128. 10.1111/j.1364-3703.2008.00503.x 10.1111/j.1364-3703.2008.00503.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saupe SJ. 2000. Molecular genetics of heterokaryon incompatibility in filamentous ascomycetes. Microbiol Mol Biol Rev 64:489–502. 10.1128/MMBR.64.3.489-502.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glass NL, Dementhon K. 2006. Non-self recognition and programmed cell death in filamentous fungi. Curr Opin Microbiol 9:553–558. 10.1016/j.mib.2006.09.001 [PubMed] [DOI] [PubMed] [Google Scholar]
- 29.Bastiaans E, Debets AJ, Aanen DK. 2016. Experimental evolution reveals that high relatedness protects multicellular cooperation from cheaters. Nat Commun 7:11435. 10.1038/ncomms11435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Diepeningen AD, Debets AJ, Hoekstra RF. 1997. Heterokaryon incompatibility blocks virus transfer among natural isolates of black Aspergilli. Curr Genet 32:209–217. 10.1007/s002940050268 [DOI] [PubMed] [Google Scholar]
- 31.Zhang DX, Spiering MJ, Dawe AL, Nuss DL. 2014. Vegetative incompatibility loci with dedicated roles in allorecognition restrict mycovirus transmission in chestnut blight fungus. Genetics 197:701–714. 10.1534/genetics.114.164574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Debets F, Yang X, Griffiths AJF. 1994. Vegetative incompatibility in Neurospora: its effect on horizontal transfer of mitochondrial plasmids and senescence in natural populations. Curr Genet 26:113–119. 10.1007/BF00313797 [PubMed] [DOI] [PubMed] [Google Scholar]
- 33.Bastiaans E, Debets AJ, Aanen DK. 2015. Experimental demonstration of the benefits of somatic fusion and the consequences for allorecognition. Evolution 69:1091–1099. 10.1111/evo.12626 [DOI] [PubMed] [Google Scholar]
- 34.Glass NL, Grotelueschen J, Metzenberg RL. 1990. Neurospora crassa A mating-type region. Proc Natl Acad Sci USA 87:4912–4916. 10.1073/pnas.87.13.4912 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glass NL, Kuldau GA. 1992. Mating type and vegetative incompatibility in filamentous ascomycetes. Annu Rev Phytopathol 30:201–224. 10.1146/annurev.py.30.090192.001221 [PubMed] [DOI] [PubMed] [Google Scholar]
- 36.Roca MG, Arlt J, Jeffree CE, Read ND. 2005. Cell biology of conidial anastomosis tubes in Neurospora crassa. Eukaryot Cell 4:911–919. 10.1128/EC.4.5.911-919.2005 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glass NL, Rasmussen C, Roca MG, Read ND. 2004. Hyphal homing, fusion and mycelial interconnectedness. Trends Microbiol 12:135–141. 10.1016/j.tim.2004.01.007 [PubMed] [DOI] [PubMed] [Google Scholar]
- 38.Herzog S, Schumann MR, Fleißner A. 2015. Cell fusion in Neurospora crassa. Curr Opin Microbiol 28:53–59. 10.1016/j.mib.2015.08.002 [PubMed][CrossRef] [DOI] [PubMed] [Google Scholar]
- 39.Leeder AC, Jonkers W, Li J, Glass NL. 2013. Early colony establishment in Neurospora crassa requires a MAP kinase regulatory network. Genetics 195:883–898. 10.1534/genetics.113.156984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishikawa FH, Souza EA, Shoji JY, Connolly L, Freitag M, Read ND, Roca MG. 2012. Heterokaryon incompatibility is suppressed following conidial anastomosis tube fusion in a fungal plant pathogen. PLoS One 7:e31175. 10.1371/journal.pone.0031175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lichius A, Lord KM. 2014. Chemoattractive mechanisms in filamentous fungi. Open Mycol J 8:28–57. [Google Scholar]
- 42.Weichert M, Fleissner A. 2015. Anastomosis and heterokaryon formation, p 3–21. In van den Berg M, Maruthachalam K (ed), Genetic Transformation Systems in Fungi, vol 2. Springer International Publishing, Cham, Switzerland. [Google Scholar]
- 43.Maerz S, Ziv C, Vogt N, Helmstaedt K, Cohen N, Gorovits R, Yarden O, Seiler S. 2008. The nuclear Dbf2-related kinase COT1 and the mitogen-activated protein kinases MAK1 and MAK2 genetically interact to regulate filamentous growth, hyphal fusion and sexual development in Neurospora crassa. Genetics 179:1313–1325. 10.1534/genetics.108.089425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pandey A, Roca MG, Read ND, Glass NL. 2004. Role of a mitogen-activated protein kinase pathway during conidial germination and hyphal fusion in Neurospora crassa. Eukaryot Cell 3:348–358. 10.1128/EC.3.2.348-358.2004 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fleissner A, Leeder AC, Roca MG, Read ND, Glass NL. 2009. Oscillatory recruitment of signaling proteins to cell tips promotes coordinated behavior during cell fusion. Proc Natl Acad Sci USA 106:19387–19392. 10.1073/pnas.0907039106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Read ND, Lichius A, Shoji JY, Goryachev AB. 2009. Self-signalling and self-fusion in filamentous fungi. Curr Opin Microbiol 12:608–615. 10.1016/j.mib.2009.09.008 [PubMed] [DOI] [PubMed] [Google Scholar]
- 47.Dettmann A, Heilig Y, Valerius O, Ludwig S, Seiler S. 2014. Fungal communication requires the MAK-2 pathway elements STE-20 and RAS-2, the NRC-1 adapter STE-50 and the MAP kinase scaffold HAM-5. PLoS Genet 10:e1004762. 10.1371/journal.pgen.1004762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jonkers W, Leeder AC, Ansong C, Wang Y, Yang F, Starr TL, Camp DG, Smith RD, Glass NL. 2014. HAM-5 functions as a MAP kinase scaffold during cell fusion in Neurospora crassa. PLoS Genet 10:e1004783. 10.1371/journal.pgen.1004783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fleissner A, Sarkar S, Jacobson DJ, Roca MG, Read ND, Glass NL. 2005. The so locus is required for vegetative cell fusion and postfertilization events in Neurospora crassa. Eukaryot Cell 4:920–930. 10.1128/EC.4.5.920-930.2005 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goryachev AB, Lichius A, Wright GD, Read ND. 2012. Excitable behavior can explain the “ping-pong” mode of communication between cells using the same chemoattractant. BioEssays 34:259–266. 10.1002/bies.201100135 [PubMed] [DOI] [PubMed] [Google Scholar]
- 51.Teichert I, Steffens EK, Schnaß N, Fränzel B, Krisp C, Wolters DA, Kück U. 2014. PRO40 is a scaffold protein of the cell wall integrity pathway, linking the MAP kinase module to the upstream activator protein kinase C. PLoS Genet 10:e1004582. 10.1371/journal.pgen.1004582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Becker Y, Eaton CJ, Brasell E, May KJ, Becker M, Hassing B, Cartwright GM, Reinhold L, Scott B. 2015. The fungal cell-wall integrity MAPK cascade is crucial for hyphal network formation and maintenance of restrictive growth of Epichloë festucae in symbiosis with Lolium perenne. Mol Plant Microbe Interact 28:69–85. 10.1094/MPMI-06-14-0183-R [DOI] [PubMed] [Google Scholar]
- 53.Hou Z, Xue C, Peng Y, Katan T, Kistler HC, Xu JR. 2002. A mitogen-activated protein kinase gene (MGV1) in Fusarium graminearum is required for female fertility, heterokaryon formation, and plant infection. Mol Plant Microbe Interact 15:1119–1127. 10.1094/MPMI.2002.15.11.1119 [DOI] [PubMed] [Google Scholar]
- 54.Dettmann A, Heilig Y, Ludwig S, Schmitt K, Illgen J, Fleißner A, Valerius O, Seiler S. 2013. HAM-2 and HAM-3 are central for the assembly of the Neurospora STRIPAK complex at the nuclear envelope and regulate nuclear accumulation of the MAP kinase MAK-1 in a MAK-2-dependent manner. Mol Microbiol 90:796–812. 10.1111/mmi.12399 [DOI] [PubMed] [Google Scholar]
- 55.Bloemendal S, Bernhards Y, Bartho K, Dettmann A, Voigt O, Teichert I, Seiler S, Wolters DA, Pöggeler S, Kück U. 2012. A homologue of the human STRIPAK complex controls sexual development in fungi. Mol Microbiol 84:310–323. 10.1111/j.1365-2958.2012.08024.x [DOI] [PubMed] [Google Scholar]
- 56.Nordzieke S, Zobel T, Fränzel B, Wolters DA, Kück U, Teichert I. 2015. A fungal sarcolemmal membrane-associated protein (SLMAP) homolog plays a fundamental role in development and localizes to the nuclear envelope, endoplasmic reticulum, and mitochondria. Eukaryot Cell 14:345–358. 10.1128/EC.00241-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simonin AR, Rasmussen CG, Yang M, Glass NL. 2010. Genes encoding a striatin-like protein (ham-3) and a forkhead associated protein (ham-4) are required for hyphal fusion in Neurospora crassa. Fungal Genet Biol 47:855–868. 10.1016/j.fgb.2010.06.010 [DOI] [PubMed] [Google Scholar]
- 58.Wang CL, Shim WB, Shaw BD. 2015. The Colletotrichum graminicola striatin ortholog Str1 is necessary for anastomosis and is a virulence factor. Mol Plant Pathol 10.111/mpp.12339. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pöggeler S, Kück U. 2004. A WD40 repeat protein regulates fungal cell differentiation and can be replaced functionally by the mammalian homologue striatin. Eukaryot Cell 3:232–240. 10.1128/EC.3.1.232-240.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dirschnabel DE, Nowrousian M, Cano-Domínguez N, Aguirre J, Teichert I, Kück U. 2014. New insights into the roles of NADPH oxidases in sexual development and ascospore germination in Sordaria macrospora. Genetics 196:729–744. 10.1534/genetics.113.159368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takemoto D, Kamakura S, Saikia S, Becker Y, Wrenn R, Tanaka A, Sumimoto H, Scott B. 2011. Polarity proteins Bem1 and Cdc24 are components of the filamentous fungal NADPH oxidase complex. Proc Natl Acad Sci USA 108:2861–2866. 10.1073/pnas.1017309108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roca MG, Weichert M, Siegmund U, Tudzynski P, Fleissner A. 2012. Germling fusion via conidial anastomosis tubes in the grey mould Botrytis cinerea requires NADPH oxidase activity. Fungal Biol 116:379–387. 10.1016/j.funbio.2011.12.007 [PubMed] [DOI] [PubMed] [Google Scholar]
- 63.Sbrana C, Nuti MP, Giovannetti M. 2007. Self-anastomosing ability and vegetative incompatibility of Tuber borchii isolates. Mycorrhiza 17:667–675. 10.1007/s00572-007-0144-3 [PubMed] [DOI] [PubMed] [Google Scholar]
- 64.Giovannetti M, Sbrana C, Strani P, Agnolucci M, Rinaudo V, Avio L. 2003. Genetic diversity of isolates of Glomus mosseae from different geographic areas detected by vegetative compatibility testing and biochemical and molecular analysis. Appl Environ Microbiol 69:616–624. 10.1128/AEM.69.1.616-624.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heller J, Zhao J, Rosenfield G, Kowbel DJ, Gladieux P, Glass NL. 2016. Characterization of greenbeard genes involved in long-distance kind discrimination in a microbial eukaryote. PLoS Biol 14:e1002431. 10.1371/journal.pbio.1002431 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cayley DM. 1923. The phenomenon of mutual aversion between mono-spore mycelia of the same fungus (Diaporthe perniciosa, Marchal). With a discussion of sex-heterothallism in fungi. J Genet 13:353–370. 10.1007/BF02983069 [DOI] [Google Scholar]
- 67.Todd NK, Rayner ADM. Fungal individualism. Sci Progr (1933-). 66:331–354. http://www.jstor.org/stable/43420507. [Google Scholar]
- 68.Espagne E, Lespinet O, Malagnac F, Da Silva C, Jaillon O, Porcel BM, Couloux A, Aury JM, Ségurens B, Poulain J, Anthouard V, Grossetete S, Khalili H, Coppin E, Déquard-Chablat M, Picard M, Contamine V, Arnaise S, Bourdais A, Berteaux-Lecellier V, Gautheret D, de Vries RP, Battaglia E, Coutinho PM, Danchin EG, Henrissat B, Khoury RE, Sainsard-Chanet A, Boivin A, Pinan-Lucarré B, Sellem CH, Debuchy R, Wincker P, Weissenbach J, Silar P. 2008. The genome sequence of the model ascomycete fungus Podospora anserina. Genome Biol 9:R77. 10.1186/gb-2008-9-5-r77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jacobson DJ, Beurkens K, Klomparens KL. 1998. Microscopic and ultrastructural examination of vegetative incompatibility in partial diploids heterozygous at het loci in Neurospora crassa. Fungal Genet Biol 23:45–56. 10.1006/fgbi.1997.1020 [DOI] [PubMed] [Google Scholar]
- 70.Pinan-Lucarré B, Paoletti M, Clavé C. 2007. Cell death by incompatibility in the fungus Podospora. Semin Cancer Biol 17:101–111. 10.1016/j.semcancer.2006.11.009 [PubMed] [DOI] [PubMed] [Google Scholar]
- 71.Jedd G, Chua NH. 2000. A new self-assembled peroxisomal vesicle required for efficient resealing of the plasma membrane. Nat Cell Biol 2:226–231. 10.1038/35008652 [PubMed] [DOI] [PubMed] [Google Scholar]
- 72.Jedd G, Pieuchot L. 2012. Multiple modes for gatekeeping at fungal cell-to-cell channels. Mol Microbiol 86:1291–1294. 10.1111/mmi.12074 [PubMed] [DOI] [PubMed] [Google Scholar]
- 73.Maruyama J, Juvvadi PR, Ishi K, Kitamoto K. 2005. Three-dimensional image analysis of plugging at the septal pore by Woronin body during hypotonic shock inducing hyphal tip bursting in the filamentous fungus Aspergillus oryzae. Biochem Biophys Res Commun 331:1081–1088. 10.1016/j.bbrc.2005.03.233 [DOI] [PubMed] [Google Scholar]
- 74.Lai J, Koh CH, Tjota M, Pieuchot L, Raman V, Chandrababu KB, Yang D, Wong L, Jedd G. 2012. Intrinsically disordered proteins aggregate at fungal cell-to-cell channels and regulate intercellular connectivity. Proc Natl Acad Sci USA 109:15781–15786. 10.1073/pnas.1207467109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fleissner A, Glass NL. 2007. SO, a protein involved in hyphal fusion in Neurospora crassa, localizes to septal plugs. Eukaryot Cell 6:84–94. 10.1128/EC.00268-06 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hutchison E, Brown S, Tian C, Glass NL. 2009. Transcriptional profiling and functional analysis of heterokaryon incompatibility in Neurospora crassa reveals that reactive oxygen species, but not metacaspases, are associated with programmed cell death. Microbiology 155:3957–3970. 10.1099/mic.0.032284-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marek SM, Wu J, Glass NL, Gilchrist DG, Bostock RM. 2003. Nuclear DNA degradation during heterokaryon incompatibility in Neurospora crassa. Fungal Genet Biol 40:126–137. 10.1016/S1087-1845(03)00086-0 [DOI] [PubMed] [Google Scholar]
- 78.Pinan-Lucarré B, Balguerie A, Clavé C. 2005. Accelerated cell death in Podospora autophagy mutants. Eukaryot Cell 4:1765–1774. 10.1128/EC.4.11.1765-1774.2005 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pinan-Lucarré B, Paoletti M, Dementhon K, Coulary-Salin B, Clavé C. 2003. Autophagy is induced during cell death by incompatibility and is essential for differentiation in the filamentous fungus Podospora anserina. Mol Microbiol 47:321–333. 10.1046/j.1365-2958.2003.03208.x [PubMed] [DOI] [PubMed] [Google Scholar]
- 80.Liu Y, Schiff M, Czymmek K, Tallóczy Z, Levine B, Dinesh-Kumar SP. 2005. Autophagy regulates programmed cell death during the plant innate immune response. Cell 121:567–577. 10.1016/j.cell.2005.03.007 [DOI] [PubMed] [Google Scholar]
- 81.Mylyk OM. 1975. Heterokaryon incompatibility genes in Neurospora crassa detected using duplication-producing chromosome rearrangements. Genetics 80:107–124. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Labarére J, Bègueret J, Bernet J. 1974. Incompatibility in Podospora anserina: comparative properties of the antagonistic cytoplasmic factors of a nonallelic system. J Bacteriol 120:854–860. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao J, Gladieux P, Hutchison E, Bueche J, Hall C, Perraudeau F, Glass NL. 2015. Identification of allorecognition loci in Neurospora crassa by genomics and evolutionary approaches. Mol Biol Evol 32:2417–2432. 10.1093/molbev/msv125 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hall C, Welch J, Kowbel DJ, Glass NL. 2010. Evolution and diversity of a fungal self/nonself recognition locus. PLoS One 5:e14055. 10.1371/journal.pone.0014055 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Richman A. 2000. Evolution of balanced genetic polymorphism. Mol Ecol 9:1953–1963. 10.1046/j.1365-294X.2000.01125.x [DOI] [PubMed] [Google Scholar]
- 86.Charlesworth D. 2006. Balancing selection and its effects on sequences in nearby genome regions. PLoS Genet 2:e64. 10.1371/journal.pgen.0020064 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roux C, Pauwels M, Ruggiero MV, Charlesworth D, Castric V, Vekemans X. 2013. Recent and ancient signature of balancing selection around the S-locus in Arabidopsis halleri and A. lyrata. Mol Biol Evol 30:435–447. 10.1093/molbev/mss246 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Apanius V, Penn D, Slev PR, Ruff LR, Potts WK. 1997. The nature of selection on the major histocompatibility complex. Crit Rev Immunol 17:179–224. 10.1615/CritRevImmunol.v17.i2.40 [PubMed] [DOI] [PubMed] [Google Scholar]
- 89.Perkins DD. 1992. Neurospora: the organism behind the molecular revolution. Genetics 130:687–701. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Beadle GW, Tatum EL. 1941. Genetic control of biochemical reactions in Neurospora. Proc Natl Acad Sci USA 27:499–506. 10.1073/pnas.27.11.499 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Beadle GW, Coonradt VL. 1944. Heterocaryosis in Neurospora crassa. Genetics 29:291–308. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kown KJ, Raper KB. 1967. Heterokaryon formation and genetic analyses of color mutants in Aspergillus heterothallicus. Am J Bot 54:49–60. 10.2307/2440886 [DOI] [PubMed] [Google Scholar]
- 93.Shiu PK, Glass NL. 1999. Molecular characterization of tol, a mediator of mating-type-associated vegetative incompatibility in Neurospora crassa. Genetics 151:545–555. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Metzenberg RL, Glass NL. 1990. Mating type and mating strategies in Neurospora. BioEssays 12:53–59. 10.1002/bies.950120202 [PubMed] [DOI] [PubMed] [Google Scholar]
- 95.Glass NL, Vollmer SJ, Staben C, Grotelueschen J, Metzenberg RL, Yanofsky C. 1988. DNAs of the two mating-type alleles of Neurospora crassa are highly dissimilar. Science 241:570–573. 10.1126/science.2840740 [PubMed] [DOI] [PubMed] [Google Scholar]
- 96.Griffiths AJ, Delange AM. 1978. Mutations of the a mating-type gene in Neurospora crassa. Genetics 88:239–254. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Philley ML, Staben C. 1994. Functional analyses of the Neurospora crassa mt a-1 mating type polypeptide. Genetics 137:715–722. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Newmeyer D. 1970. A suppressor of the heterokaryon-incompatibility associated with mating type in Neurospora crassa. Can J Genet Cytol 12:914–926. 10.1139/g70-115 [PubMed] [DOI] [PubMed] [Google Scholar]
- 99.Jacobson DJ. 1992. Control of mating type heterokaryon incompatibility by the tol gene in Neurospora crassa and N. tetrasperma. Genome 35:347–353. 10.1139/g92-053 [PubMed] [DOI] [PubMed] [Google Scholar]
- 100.Metzenberg RL, Ahlgren SK. 1973. Behaviour of Neurospora tetrasperma mating-type genes introgressed into N. crassa. Can J Genet Cytol 15:571–576. 10.1139/g73-068 [PubMed] [DOI] [PubMed] [Google Scholar]
- 101.Garnjobst L, Wilson JF. 1956. Heterocaryosis and protoplasmic incompatibility in Neurospora crassa. Proc Natl Acad Sci USA 42:613–618. 10.1073/pnas.42.9.613 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Perkins DD. 1975. The use of duplication-generating rearrangements for studying heterokaryon incompatibility genes in Neurospora. Genetics 80:87–105. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kaneko I, Dementhon K, Xiang Q, Glass NL. 2006. Nonallelic interactions between het-c and a polymorphic locus, pin-c, are essential for nonself recognition and programmed cell death in Neurospora crassa. Genetics 172:1545–1555. 10.1534/genetics.105.051490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Smith ML, Micali OC, Hubbard SP, Mir-Rashed N, Jacobson DJ, Glass NL. 2000. Vegetative incompatibility in the het-6 region of Neurospora crassa is mediated by two linked genes. Genetics 155:1095–1104. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sarkar S, Iyer G, Wu J, Glass NL. 2002. Nonself recognition is mediated by HET-C heterocomplex formation during vegetative incompatibility. EMBO J 21:4841–4850. 10.1093/emboj/cdf479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lafontaine DL, Smith ML. 2012. Diverse interactions mediate asymmetric incompatibility by the het-6 supergene complex in Neurospora crassa. Fungal Genet Biol 49:65–73. 10.1016/j.fgb.2011.11.001 [DOI] [PubMed] [Google Scholar]
- 107.Mir-Rashed N, Jacobson DJ, Dehghany MR, Micali OC, Smith ML. 2000. Molecular and functional analyses of incompatibility genes at het-6 in a population of Neurospora crassa. Fungal Genet Biol 30:197–205. 10.1006/fgbi.2000.1218 [DOI] [PubMed] [Google Scholar]
- 108.Saupe SJ, Kuldau GA, Smith ML, Glass NL. 1996. The product of the het-C heterokaryon incompatibility gene of Neurospora crassa has characteristics of a glycine-rich cell wall protein. Genetics 143:1589–1600. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Saupe SJ, Glass NL. 1997. Allelic specificity at the het-c heterokaryon incompatibility locus of Neurospora crassa is determined by a highly variable domain. Genetics 146:1299–1309. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xiang Q, Glass NL. 2002. Identification of vib-1, a locus involved in vegetative incompatibility mediated by het-c in Neurospora crassa. Genetics 162:89–101. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xiang Q, Glass NL. 2004. The control of mating type heterokaryon incompatibility by vib-1, a locus involved in het-c heterokaryon incompatibility in Neurospora crassa. Fungal Genet Biol 41:1063–1076. 10.1016/j.fgb.2004.07.006 [DOI] [PubMed] [Google Scholar]
- 112.Dementhon K, Iyer G, Glass NL. 2006. VIB-1 is required for expression of genes necessary for programmed cell death in Neurospora crassa. Eukaryot Cell 5:2161–2173. 10.1128/EC.00253-06 [PubMed][CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Espagne E, Balhadère P, Penin ML, Barreau C, Turcq B. 2002. HET-E and HET-D belong to a new subfamily of WD40 proteins involved in vegetative incompatibility specificity in the fungus Podospora anserina. Genetics 161:71–81. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chevanne D, Bastiaans E, Debets A, Saupe SJ, Clavé C, Paoletti M. 2009. Identification of the het-r vegetative incompatibility gene of Podospora anserina as a member of the fast evolving HNWD gene family. Curr Genet 55:93–102. 10.1007/s00294-008-0227-5 [DOI] [PubMed] [Google Scholar]
- 115.Paoletti M, Clavé C. 2007. The fungus-specific HET domain mediates programmed cell death in Podospora anserina. Eukaryot Cell 6:2001–2008. 10.1128/EC.00129-07 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Saupe S, Turcq B, Bégueret J. 1995. A gene responsible for vegetative incompatibility in the fungus Podospora anserina encodes a protein with a GTP-binding motif and G beta homologous domain. Gene 162:135–139. 10.1016/0378-1119(95)00272-8 [DOI] [PubMed] [Google Scholar]
- 117.Koonin EV, Aravind L. 2000. The NACHT family: - a new group of predicted NTPases implicated in apoptosis and MHC transcription activation. Trends Biochem Sci 25:223–224. 10.1016/S0968-0004(00)01577-2 [DOI] [PubMed] [Google Scholar]
- 118.Yuan S, Akey CW. 2013. Apoptosome structure, assembly, and procaspase activation. Structure 21:501–515. 10.1016/j.str.2013.02.024 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stirnimann CU, Petsalaki E, Russell RB, Müller CW. 2010. WD40 proteins propel cellular networks. Trends Biochem Sci 35:565–574. 10.1016/j.tibs.2010.04.003 [PubMed] [DOI] [PubMed] [Google Scholar]
- 120.Zhou M, Li Y, Hu Q, Bai XC, Huang W, Yan C, Scheres SH, Shi Y. 2015. Atomic structure of the apoptosome: mechanism of cytochrome c- and dATP-mediated activation of Apaf-1. Genes Dev 29:2349–2361. 10.1101/gad.272278.115 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Espagne E, Balhadère P, Bégueret J, Turcq B. 1997. Reactivity in vegetative incompatibility of the HET-E protein of the fungus Podospora anserina is dependent on GTP-binding activity and a WD40 repeated domain. Mol Gen Genet 256:620–627. 10.1007/s004380050610 [DOI] [PubMed] [Google Scholar]
- 122.Saupe S, Descamps C, Turcq B, Bégueret J. 1994. Inactivation of the Podospora anserina vegetative incompatibility locus het-c, whose product resembles a glycolipid transfer protein, drastically impairs ascospore production. Proc Natl Acad Sci USA 91:5927–5931. 10.1073/pnas.91.13.5927 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kenoth R, Kamlekar RK, Simanshu DK, Gao Y, Malinina L, Prendergast FG, Molotkovsky JG, Patel DJ, Venyaminov SY, Brown RE. 2011. Conformational folding and stability of the HET-C2 glycolipid transfer protein fold: does a molten globule-like state regulate activity? Biochemistry 50:5163–5171. 10.1021/bi200382c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Saupe S, Turcq B, Bégueret J. 1995. Sequence diversity and unusual variability at the het-c locus involved in vegetative incompatibility in the fungus Podospora anserina. Curr Genet 27:466–471. 10.1007/BF00311217 [PubMed] [DOI] [PubMed] [Google Scholar]
- 125.Bastiaans E, Debets AJ, Aanen DK, van Diepeningen AD, Saupe SJ, Paoletti M. 2014. Natural variation of heterokaryon incompatibility gene het-c in Podospora anserina reveals diversifying selection. Mol Biol Evol 31:962–974. 10.1093/molbev/msu047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chevanne D, Saupe SJ, Clavé C, Paoletti M. 2010. WD-repeat instability and diversification of the Podospora anserina hnwd non-self recognition gene family. BMC Evol Biol 10:134. 10.1186/1471-2148-10-134 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Paoletti M, Saupe SJ, Clavé C. 2007. Genesis of a fungal non-self recognition repertoire. PLoS One 2:e283. 10.1371/journal.pone.0000283 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Daskalov A, Habenstein B, Martinez D, Debets AJ, Sabaté R, Loquet A, Saupe SJ. 2015. Signal transduction by a fungal NOD-like receptor based on propagation of a prion amyloid fold. PLoS Biol 13:e1002059. 10.1371/journal.pbio.1002059 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Saupe SJ. 2011. The [Het-s] prion of Podospora anserina and its role in heterokaryon incompatibility. Semin Cell Dev Biol 22:460–468. 10.1016/j.semcdb.2011.02.019 [PubMed] [DOI] [PubMed] [Google Scholar]
- 130.Maddelein ML, Dos Reis S, Duvezin-Caubet S, Coulary-Salin B, Saupe SJ. 2002. Amyloid aggregates of the HET-s prion protein are infectious. Proc Natl Acad Sci USA 99:7402–7407. 10.1073/pnas.072199199 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Beisson-Schecroun J. 1962. Cellular incompatibility and nucleo-cytoplasmic interactions in the “barrage” phenomena in Podospora anserina. Ann Genet 4:4–50. [PubMed] [PubMed] [Google Scholar]
- 132.Wickner RB. 1997. A new prion controls fungal cell fusion incompatibility. Proc Natl Acad Sci USA 94:10012–10014. 10.1073/pnas.94.19.10012 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Seuring C, Greenwald J, Wasmer C, Wepf R, Saupe SJ, Meier BH, Riek R. 2012. The mechanism of toxicity in HET-S/HET-s prion incompatibility. PLoS Biol 10:e1001451. 10.1371/journal.pbio.1001451 [PubMed][CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mathur V, Seuring C, Riek R, Saupe SJ, Liebman SW. 2012. Localization of HET-S to the cell periphery, not to [Het-s] aggregates, is associated with [Het-s]-HET-S toxicity. Mol Cell Biol 32:139–153. 10.1128/MCB.06125-11 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cortesi P, McCulloch CE, Song H, Lin H, Milgroom MG. 2001. Genetic control of horizontal virus transmission in the chestnut blight fungus, Cryphonectria parasitica. Genetics 159:107–118. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cortesi P, Milgroom MG. 1998. Genetics of vegetative incompatibility in Cryphonectria parasitica. Appl Environ Microbiol 64:2988–2994. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Choi GH, Dawe AL, Churbanov A, Smith ML, Milgroom MG, Nuss DL. 2012. Molecular characterization of vegetative incompatibility genes that restrict hypovirus transmission in the chestnut blight fungus Cryphonectria parasitica. Genetics 190:113–127. 10.1534/genetics.111.133983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang DX, Nuss DL. 2016. Engineering super mycovirus donor strains of chestnut blight fungus by systematic disruption of multilocus vic genes. Proc Natl Acad Sci USA 113:2062–2067. 10.1073/pnas.1522219113 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Paoletti M, Saupe SJ. 2009. Fungal incompatibility: evolutionary origin in pathogen defense? BioEssays 31:1201–1210. 10.1002/bies.200900085 [DOI] [PubMed] [Google Scholar]
- 140.Rinkevich B. 2004. Allorecognition and xenorecognition in reef corals: a decade of interactions. Hydrobiologia 530-531:443–450. 10.1007/s10750-004-2686-0 [DOI] [Google Scholar]
- 141.Proell M, Riedl SJ, Fritz JH, Rojas AM, Schwarzenbacher R. 2008. The Nod-like receptor (NLR) family: a tale of similarities and differences. PLoS One 3:e2119. 10.1371/journal.pone.0002119 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Daskalov A, Paoletti M, Ness F, Saupe SJ. 2012. Genomic clustering and homology between HET-S and the NWD2 STAND protein in various fungal genomes. PLoS One 7:e34854. 10.1371/journal.pone.0034854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dyrka W, Lamacchia M, Durrens P, Kobe B, Daskalov A, Paoletti M, Sherman DJ, Saupe SJ. 2014. Diversity and variability of NOD-like receptors in fungi. Genome Biol Evol 6:3137–3158. 10.1093/gbe/evu251 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Daskalov A, Habenstein B, Sabaté R, Berbon M, Martinez D, Chaignepain S, Coulary-Salin B, Hofmann K, Loquet A, Saupe SJ. 2016. Identification of a novel cell death-inducing domain reveals that fungal amyloid-controlled programmed cell death is related to necroptosis. Proc Natl Acad Sci USA 113:2720–2725. 10.1073/pnas.1522361113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen D, Yu J, Zhang L. 2016. Necroptosis: an alternative cell death program defending against cancer. Biochim Biophys Acta 1865:228–236. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Glass NL, Kaneko I. 2003. Fatal attraction: nonself recognition and heterokaryon incompatibility in filamentous fungi. Eukaryot Cell 2:1–8. 10.1128/EC.2.1.1-8.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]


