Abstract
We have developed a system to analyze the specific protein kinase activity of herpes simplex virus 1 Us3 in vitro and shown that Us3 directly phosphorylates viral proteins UL34, ICP22, and Us9 and the cellular protein Bad, previously reported to be putative substrates. Using this system, we determined the phosphorylation sites of UL34 and identified UL31 as a previously unreported, novel substrate of Us3. This system will be useful for further identification of Us3 substrates and their phosphorylation sites, clarification of the role of Us3 in viral replication, and identification of additional Us3 function(s).
The herpes simplex virus (HSV) Us3 gene encodes a serine/threonine protein kinase (6, 8, 29). While not essential for viral growth in cell culture (26, 29), Us3 protein kinases are positive regulators of viral replication, based on studies showing that recombinant Us3 mutant viruses have impaired growth properties in cell cultures and mouse models (26, 32, 33). Accumulating evidence suggests that HSV Us3 has roles in viral replication by regulating apoptosis and in primary envelopment during viral egress. It has been reported that HSV Us3 protein kinase can prevent apoptosis induced by proapoptotic cellular proteins and by replication-incompetent mutant virus (1, 3, 4, 12, 20, 22, 23, 25, 27) and facilitate the nuclear egress of progeny nucleocapsids (32, 33).
Us3 may function by phosphorylating specific viral and cellular protein substrates. Identification of these substrates could clarify the mechanisms by which Us3 regulates apoptosis and progeny virus egress and elucidate other possible Us3 function(s). Thus far, UL34, ICP22, Us9, UL12, cytokeratin 17, Bad, and Bid have been reported to be putative substrates for Us3, based on several observations. First, the phosphorylation of viral membrane protein UL34 and the posttranslational processing of viral regulatory protein ICP22 have been reported to be reduced or obviated in cells infected with Us3 mutant viruses (30, 31, 33). Second, profiles of in vitro phosphorylated proteins from lysates of cells infected with a Us3 deletion mutant virus lack two phosphoproteins, which are probably the viral proteins encoded by the Us9 and UL12 genes (5, 7). Third, the cellular proapoptotic proteins Bad and Bid and a cellular intermediate filament protein, cytokeratin 17, have been shown to be phosphorylated by Us3 immunoprecipitates by use of a polyclonal antibody to Us3 (3, 4, 24). It also has been reported that the posttranslational modification of Bad was reduced in cells infected with a Us3 deletion mutant (4).
As described above, most data on Us3 substrates are from studies with Us3 mutant viruses or Us3 immunoprecipitates with polyclonal antibody. However, it is possible that the protein kinase activity detected in in vitro kinase assays using Us3 immunoprecipitates is due to a contaminating kinase(s) either associated with Us3 or coprecipitated by the antibody. Furthermore, in the experiments with Us3 mutant viruses, Us3 may directly phosphorylate the putative substrates, or it may either activate or induce other protein kinase(s) that phosphorylate these substrates. Consistent with the latter possibility, it has recently been reported that Us3 activates the cyclic AMP-dependent protein kinase, protein kinase A, whose phosphorylation sequence resembles that of Us3, and that both Us3 and protein kinase A phosphorylate the same residues in protein substrates (2). Therefore, it is not known whether Us3 directly phosphorylates the reported putative substrates. In this study, we attempted to establish a system to analyze the specific protein kinase activity of HSV-1 Us3 in vitro and to identify protein substrates that are phosphorylated directly by Us3 protein kinase.
Generation and purification of recombinant GST-Us3 and its kinase-negative mutant GST-Us3K220M.
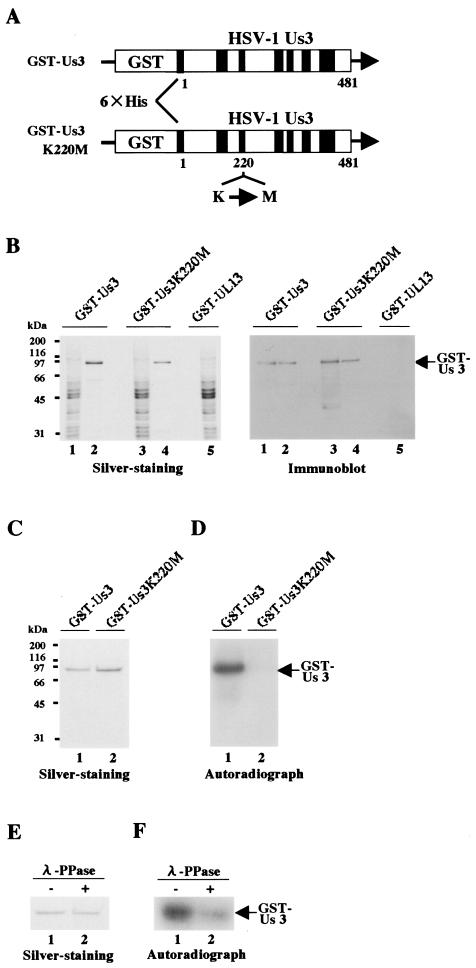
To study the specific protein kinase activity of HSV-1 Us3, we constructed a recombinant baculovirus (Bac-GST-Us3) expressing wild-type Us3 fused to glutathione S-transferase (GST) and purified GST-Us3 from Sf9 cells infected with Bac-GST-Us3 (Fig. 1A). To eliminate the possibility that the protein kinase activity detected in experiments using GST-Us3 is due to a kinase contaminant copurified by this procedure, we constructed a Us3 mutant with no intrinsic protein kinase activity but probably with retention of the overall Us3 structure. To generate this mutant, we constructed a recombinant baculovirus (Bac-GST-Us3K220M) expressing a Us3 mutant fused to GST (GST-Us3K220M), in which lysine at Us3 residue 220 (Lys-220) was replaced with methionine by site-directed mutagenesis (Fig. 1A). Lys-220 was chosen for mutagenesis because there is an invariant lysine at this position in the known protein kinases and the mutation of this invariant lysine in eukaryotic protein kinases has been shown to result in the loss of kinase activity (11). Bac-GST-Us3 and Bac-GST-Us3K220M were generated by cotransfection of pAcGHLT-Us3 and pAcGHLT-Us3K220M, respectively, with linearized baculovirus DNA baculoGold (Pharmingen) into Sf9 cells using Lipofectin (Invitrogen) (16). To construct pAcGHLT-Us3 and pAcGHLT-Us3K220M, the EcoRI-NotI fragments of pBS-Us3 and pBS-Us3K220M, respectively, were cloned into the EcoRI and NotI sites of pAcGHLT-B (Pharmingen) in frame with GST. pBS-Us3 was generated by amplifying the entire Us3 coding sequence (except the start codon) by PCR from pBC1013 (18) (a generous gift from B. Roizman, the University of Chicago) and cloning the DNA fragments into pBluescript II KS+ (Stratagene). pBS-Us3K220M, in which Us3 Lys-220 was replaced by methionine, was generated using the QuickChange site-directed mutagenesis kit according to the manufacturer's instructions (Stratagene). GST fusion proteins were purified from Sf9 cells infected with the recombinant baculoviruses (16), electrophoretically separated in a denaturing gel, and either silver stained or immunoblotted with rabbit anti-Us3 antiserum (6, 17). The anti-Us3 antiserum was generated by immunization of rabbits with a purified full length of Us3 fused to a polyhistidine tag (10). As shown in Fig. 1B, purified GST-Us3 and GST-Us3K220M contained one major band with an Mr of approximately 90,000 as detected by silver staining (left panel), and these proteins reacted with anti-Us3 antiserum (right panel). From Sf9 cells (106) infected with each baculovirus (either Bac-GST-Us3 or Bac-GST-Us3K220M), approximately 15 μg of GST fusion protein could be purified. To examine the enzymatic activity of purified GST-Us3 and GST-Us3K220M, the purified GST fusion proteins were used in in vitro kinase assays as described previously (14), except that the specific Us3 kinase buffer (50 mM Trs-HCl [pH 9.0], 20 mM MgCl2, 0.1% Nonidet P-40, and 1 mM dithiothreitol) was used (6). The results (Fig. 1D) showed that wild-type GST-Us3 was labeled with [γ-32P]ATP, while mutant GST-Us3K220M was not. To confirm that GST-Us3 labeling was due to phosphorylation, the labeled GST-Us3 was subjected to phosphatase treatment as described previously (13), except that lambda protein phosphatase (λ-PPase) (New England BioLabs) was used. As shown in Fig. 1F, GST-Us3 labeling was eliminated by phosphatase treatment, indicating that GST-Us3 was labeled with [γ-32P]ATP by autophosphorylation. The expression of each GST fusion protein and the identification of each radiolabeled band were verified by silver staining, as shown in Fig. 1C and E. These results show that enzymatically active GST-Us3 and its GST-Us3K220M kinase-negative mutant were purified and that Us3 residue Lys-220 is essential for protein kinase activity.
FIG. 1.
(A) Schematic diagram of the predicted amino acid sequence of GST-Us3 and its kinase-negative mutant GST-Us3K220M. The shaded areas represent subdomains I to VI, which are conserved in eukaryotic protein kinases (34). The K220M mutation is also indicated. (B) A silver-stained gel (left panel) and an immunoblot (right panel) of purified GST-Us3 and GST-Us3K220M from Sf9 cells infected with recombinant baculovirus Bac-GST-Us3 (lanes 1 and 2), Bac-GST-Us3K220M (lanes 3 and 4) and Bac-GST-UL13 (16) (lanes 5). Total cell extracts (lanes 1, 3, and 5) were subjected to affinity chromatography on glutathione-Sepharose and eluted (lanes 2 and 4). Proteins were separated on a denaturing gel and subjected to sliver staining (left panel) or transferred onto a nitrocellulose sheet and reacted with antibody to Us3 (right panel). Molecular masses (kDa) are shown on the left. (C) Purified GST-Us3 (lane 1) and GST-Us3K220M (lane 2) were incubated in kinase buffer containing [γ-32P]ATP, separated on a denaturing gel, and subjected to silver staining. Molecular masses (kDa) are shown on the left. (D) Autoradiograph of the gel in panel C. (E) Purified GST-Us3 was incubated in kinase buffer containing [γ-32P]ATP. The GST-Us3 sample was then either mock treated (lane 1) or treated with λ-PPase (lane 2), separated on a denaturing gel, and subjected to silver staining. (F) Autoradiograph of the gel in panel E.
Us3 directly phosphorylates substrates UL34, ICP22, Us9, and Bad, but not Bid, in vitro.
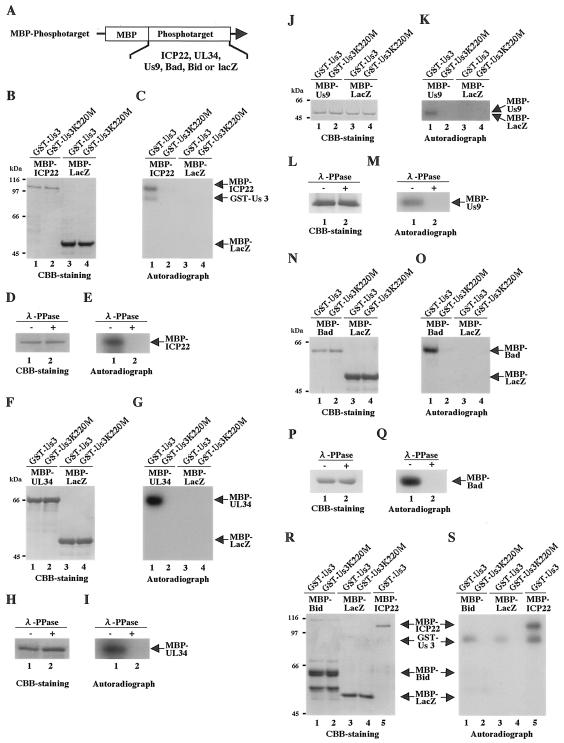
We examined whether the putative substrates reported earlier (3-5, 23, 30, 31) are in fact phosphorylated directly by Us3 in vitro. As substrates for these studies, we generated chimeric proteins, consisting of the maltose-binding protein (MBP) fused to the putative substrates (MBP-UL34, MBP-ICP22, MBP-Us9, MBP-Bad, and MBP-Bid) in Escherichia coli transformed with pMAL-UL34, pMAL-ICP22, pMAL-Us9, pMAL-Bad, and pMAL-Bid, respectively (15, 16). pMAL-ICP22 and pMAL-Us9 were constructed by amplifying the entire coding sequence of ICP22 and Us9, respectively, by PCR from pBC1013 (18) and cloning the DNA fragments into pMAL-c (New England BioLabs) in frame with MBP. pMAL-UL34 was generated by cloning the EcoRI-PstI fragment of pBS-UL34 into the EcoRI and PstI sites of pMAL-c in frame with MBP. pBS-UL34 was constructed by amplifying the entire coding sequence of UL34 by PCR from pBC1014 (18) (a kind gift from B. Roziman) and cloning the DNA fragments into pBluescript II KS+. To generate pMAL-Bid and pMAL-Bad, the entire coding sequence of Bid and Bad was amplified by PCR from a HeLa cDNA library (Clontech) and a human fetal-brain MATCHMAKER cDNA library (Clontech), respectively, and cloned into pMAL-c in frame with MBP. The MBP fusion proteins were captured on amylose beads and used as substrates in in vitro kinase assays with GST-Us3 and GST-Us3K220M. In these assays, MBP-UL34, MBP-ICP22, MBP-Us9, and MBP-Bad proteins were labeled with [γ-32P]ATP by purified GST-Us3, but neither MBP-lacZ nor MBP-Bid was labeled (Fig. 2C, G, K, O, and S). When the kinase-negative mutant GST-Us3K220M was used instead of GST-Us3 in the assays, none of the MBP fusion proteins were labeled (Fig. 2C, G, K, O, and S). Furthermore, labeling of the MBP fusion proteins by GST-Us3 was eliminated by phosphatase treatment (Fig. 2E, I, M, and Q). The expression of each MBP fusion protein and the identification of each radiolabeled MBP protein band were verified by Coomassie brilliant blue (CBB) staining as shown in Fig. 2B, D, F, H, J, L, N, P, and R. These results indicate that Us3 specifically and directly phosphorylates UL34, ICP22, Us9, and Bad proteins in vitro, while Bid does not appear to be a direct substrate for Us3 in this in vitro system.
FIG. 2.
CBB-stained and autoradiographic images of purified MBP fusion proteins used as substrates in in vitro kinase assays with purified GST-Us3 and GST-Us3K220M. (A) Schematic representation of target proteins fused to MBP. (B) Purified MBP-ICP22 (lanes 1 and 2) and MBP-lacZ (lanes 3 and 4) were incubated in kinase buffer containing [γ-32P]ATP and purified GST-Us3 (lanes 1 and 3) or GST-Us3K220M (lanes 2 and 4), separated on a denaturing gel, and stained with CBB. Molecular masses (kDa) are shown on the left. (C) Autoradiograph of the gel in panel B. (D) Purified MBP-ICP22 labeled as in panel B was then either mock treated (lane 1) or treated with λ-PPase (lane 2), separated on a denaturing gel, and stained with CBB. (E) Autoradiograph of the gel in panel D. (F to I) Experiments carried out as described in panels B to E, respectively, except that MBP-UL34 was used instead of MBP-ICP22. (J to M) Experiments carried out as described in panels B to E, respectively, except that MBP-Us9 was used instead of MBP-ICP22. (N to Q) Experiments were carried out as described in panels B to E, respectively, except that MBP-Bad was used instead of MBP-ICP22. (R and S) Experiments carried out as described in panels B and C, respectively, except that MBP-Bid was used in addition to MBP-ICP22.
Us3 mediates the phosphorylation of UL34 at Thr-195 and Ser-198.
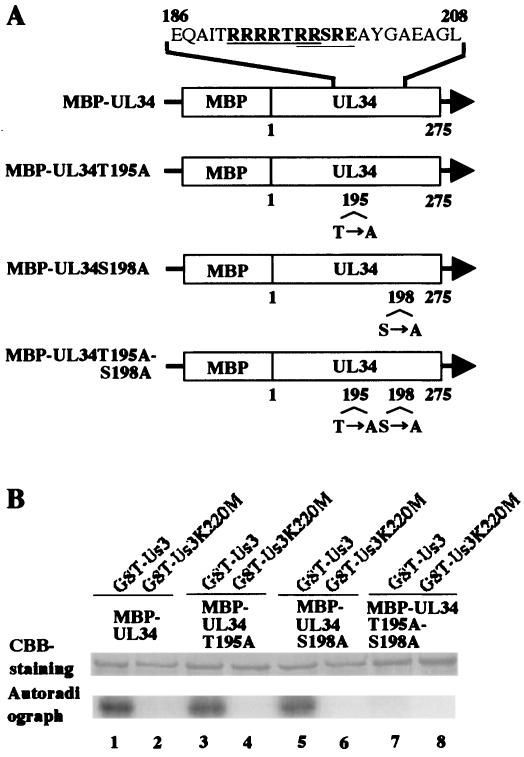
We then examined whether the system used to detect the specific kinase activity of Us3 could be applied to identify the phosphorylation sites of Us3 substrates. Purves et al. purified HSV-1 Us3 from infected cells using column chromatography and determined the sequence of the Us3 kinase phosphorylation site using the purified enzyme and synthetic polypeptides (19, 28). The consensus sequence is RnX(S/T)YY, where n is greater than or equal to 2, X can be Arg, Ala, Val, Pro, or Ser, and Y can be any residue except an acidic one (28). The predicted amino acid sequence of UL34 contains the consensus Us3 phosphorylation sites at UL34 codons 191 to 197 (RRRRTRR) and 196 to 200 (RRSRE) (21) (Fig. 3A). Therefore, we examined whether Us3 phosphorylates UL34 at Thr-195 and/or Ser-198. For this study, we generated mutant MBP-UL34 proteins (MBP-UL34-T195A, MBP-UL34-S198A, and MBP-UL34-T195A-S198A) in which Thr-195 and/or Ser-198 were substituted with alanine(s) by use of E. coli transformed with pMAL-UL34-T195A, pMAL-UL34-S198A, and pMAL-UL34-T195A-S198A, respectively (Fig. 3A). pMAL-UL34-T195A, pMAL-UL34-S198A, and pMAL-UL34-T195A-S198A were constructed by cloning the EcoRI-PstI fragments of pBS-UL34-T195A, pBS-UL34-S198A, and pBS-UL34-T195A-S198A, respectively, into the pMAL-c EcoRI and PstI sites in frame with MBP. pBS-UL34-T195A, pBS-UL34-S198A, and pBS-UL34-T195A-S198A were generated using the QuickChange site-directed mutagenesis kit with pBS-UL34 as the template. Wild-type and mutant UL34 proteins were used as substrates in the in vitro kinase assays. As shown in Fig. 3B, MBP-UL34, MBP-UL34T195A, and MBP-UL34S198A were labeled with [γ-32P]ATP, but MBP-UL34T195A-S198A was not labeled by GST-Us3. None of the MBP proteins were labeled by the kinase-negative mutant GST-Us3K220M. The expression of each MBP fusion protein and the identification of each MBP protein-radiolabeled band were verified by CBB staining. These results show that both UL34 Thr-195 and Ser-198 are phosphorylated by Us3.
FIG. 3.
(A) Schematic representation of MBP fusion proteins containing wild-type UL34 and mutant UL34 with alanine substitution(s) at Thr-195 and/or Ser-198. The amino acid sequence of UL34 residues 186 to 208 is shown. Sites with the consensus sequence for phosphorylation by Us3 are underlined. (B) CBB-stained and autoradiographic images of phosphorylated UL34. Purified MBP-UL34 (lanes 1 and 2), MBP-UL34T195A (lanes 3 and 4), MBP-UL34S198A (lanes 5 and 6), and MBP-UL34T195A-S198A (lanes 7 and 8) were incubated in kinase buffer containing [γ-32P]ATP and purified GST-Us3 (lanes 1, 3, 5, and 7) or GST-Us3K220M (lanes 2, 4, 6, and 8), separated on a denaturing gel, and stained with CBB (upper panel). The lower panel shows an autoradiograph of the gel in the upper panel.
Identification of UL31 as a novel substrate of Us3.
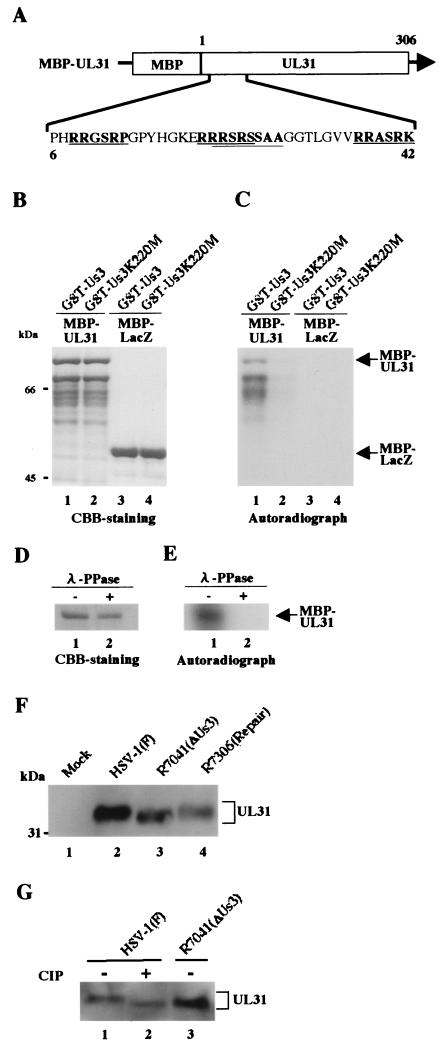
Based on the consensus Us3 phosphorylation site sequence, we identified putative Us3 phosphorylation sites in UL31 (Fig. 4A), at UL31 codons 8 to 13 (RRGSRP), 21 to 26 (RRRSRS), 22 to 29 (RRSRSSAA), and 37 to 42 (RRASRK) (21). To investigate whether UL31 can be a substrate for Us3, we generated a chimeric protein consisting of MBP fused to full-length UL31 (MBP-UL31) by use of E. coli transformed with pMAL-UL31. pMAL31 was generated by amplifying the entire UL31 coding sequence by PCR from pBR1014 and cloning the DNA fragments into pMAL-c in frame with MBP. As shown in Fig. 4C, in the reaction with purified GST-Us3, MBP-UL31 was labeled with [γ-32P]ATP, but MBP-lacZ was not. When the GST-Us3K220M kinase-negative mutant was used instead of GST-Us3, neither of the MBP fusion proteins was labeled. MBP-UL31 labeling by GST-Us3 was eliminated by phosphatase treatment (Fig. 4E). The expression of each MBP fusion protein and the identification of each MBP protein-radiolabeled band were verified by CBB staining as shown in Fig. 4B and D. These results show that Us3 directly phosphorylates UL31 in vitro. Furthermore, UL31 proteins produced by cells infected with a Us3 deletion mutant virus R7041 (29) migrated in a denaturing gel faster than those produced by cells infected with wild-type HSV-1 strain F [HSV-1(F)] (35) or a recombinant virus R7306 in which the Us3 gene was repaired (Fig. 4F) (29). R7041 and R7306 were kindly provided by B. Roizman. After phosphatase treatment (36) of the lysate from cells infected with HSV-1(F), UL31 proteins migrated as fast as those from cells infected with R7041 (Fig. 4G). These results indicate that Us3 mediates phosphorylation of UL31 in infected cells.
FIG. 4.
(A) Schematic diagram of the amino acid sequence of UL31 fused to MBP. The amino acid sequence of UL31 residues 6 to 42 is shown. Sites with the consensus sequence for phosphorylation by Us3 are underlined. (B) CBB-stained and autoradiographic images of purified MBP-UL31 (lanes 1 and 2) and MBP-lacZ (lanes 3 and 4) incubated in kinase buffer containing [γ-32P]ATP and purified GST-Us3 (lanes 1 and 3) or GST-Us3K220M (lanes 2 and 4), separated on a denaturing gel, and stained with CBB. Molecular masses (kDa) are shown on the left. (C) Autoradiograph of the gel in panel B. (D) Purified MBP-UL31 incubated in kinase buffer containing [γ-32P]ATP and purified GST-Us3 was then either mock treated (lane 1) or treated with λ-PPase (lane 2), separated on a denaturing gel, and stained with CBB. (E) Autoradiograph of the gel in panel D. (F) An immunoblot of electrophoretically separated lysates from Vero cells mock infected (lane 1) or infected with HSV-1(F) (lane 2), R7041 (lane 3), or R7306 (lane 4) at multiplicities of infection of 3 PFU per cell. The infected cells were harvested at 12 h after infection and subjected to immunoblotting with a polyclonal antibody to UL31 (37). Molecular masses (kDa) are shown on the left. (G) The lysates used for panel F were mock treated (lanes 1 and 3) or treated with alkaline phosphatase (lane 2), separated on a denaturing gel, and subjected to immunoblotting with the antibody to UL31.
Identification of the physiological substrate of a viral protein kinase requires demonstration that the substrate is specifically and directly phosphorylated by the enzyme in vitro and that the phosphorylation of the substrate in cells infected with a mutant virus lacking protein kinase activity is altered. In studies of HSV Us3, several viral and cellular proteins have been identified as putative substrates for Us3 (3-5, 7, 23, 24, 30, 31, 33), although none could be definitively shown to be a substrate for Us3 in vitro because of the difficulty in obtaining specific in vitro protein kinase activity. However, as described above, we have developed a system for the expression of large amounts of recombinant Us3 in insect cells by use of a recombinant baculovirus and have obtained highly purified Us3 with kinase activity. We also have generated a kinase-negative Us3 mutant to use as a control to eliminate the possibility that the kinase activity detected using purified recombinant Us3 is due to a copurified contaminating kinase(s). The availability of purified recombinant Us3 and its mutant enabled us to investigate the specific activity of Us3 and show that the previously reported putative substrates for Us3 (UL34, ICP22, Us9, and Bad) (4, 5, 23, 30, 31) are phosphorylated directly by Us3. Furthermore, these studies identified UL31, a previously unreported Us3 substrate, as a novel substrate of Us3 in vitro. Of these substrates, UL34, ICP22, and Bad have been shown to have reduced phosphorylation in cells infected with Us3 kinase-negative mutant viruses (4, 30, 31). In the studies described here, we demonstrated that the phosphorylation of UL31 was obviated in cells infected with Us3 deletion mutant virus. It is therefore likely that UL34, ICP22, UL31, and Bad are physiological substrates of Us3 in infected cells. In contrast, it is not clear at present whether Us9 is a natural substrate of Us3, because phosphorylation of the viral protein has not been analyzed thus far in Us3 kinase-negative mutant virus-infected cells.
It has been reported that Us3 can directly phosphorylate the cellular protein Bid in assays of Us3 immunoprecipitates with polyclonal antibody to the protein (3). However, that study did not show clearly that Us3 was the only kinase present or that phosphorylation of Bid was not carried out by a kinase(s) contaminant. Using the in vitro system developed in the studies reported here, we demonstrated that Us3 was not able to directly phosphorylate Bid. Since we used Bid fused to MBP as the substrate in the in vitro kinase assays instead of the nonfusion Bid protein as in the previously reported study (3), we cannot completely exclude the possibility that the lack of Bid phosphorylation by Us3 in our studies resulted from the steric hindrance of Bid due to its fusion to MBP. Further studies are needed to clarify whether Bid is a direct substrate for Us3.
The in vitro system described here enables studies to determine the phosphorylation site(s) of Us3 substrates. The identification of phosphorylation site(s) is a crucial step in the elucidation of the biological significance of the phosphorylation. Once the phosphorylation site(s) of the substrate has been determined, the biological significance of the phosphorylation can be investigated using substrate mutants with amino acid substitution(s) in the phosphorylation site(s) (33, 36). We have demonstrated that both UL34 Thr-195 and Ser-198 are sites for Us3 phosphorylation. In agreement with this conclusion, it has been reported that the phosphorylation of UL34 in cells infected with recombinant viruses carrying amino acid substitutions in UL34 Thr-195 and/or Ser-198 of UL34 is completely abolished (31, 33). Taken together, these observations indicate that Us3 phosphorylates UL34 Thr-195 and Ser-198 both in vitro and in vivo.
In conclusion, we have established a system for analyzing the specific protein kinase activity of Us3 in vitro. This system is useful for the identification of direct Us3 substrates and their phosphorylation site(s). It is also noteworthy that the system is easily applied to expression screening methods for identifying protein kinase substrates, such as phosphorylation screening (9). However, proteins identified by this system are only in vitro substrates and must be shown by in vivo experiments to be physiological substrates. The utilization of these methods can help to identify physiological Us3 substrates and, by extension, contribute to clarifying the mechanisms by which Us3 expresses its functions in viral replication, including possible novel function(s) of Us3.
Acknowledgments
We thank B. Roizman for pBC1013, pBC1014, R7041, and R7306. We thank H. Noma, T. Tsuruguchi, and E. Iwata for technical assistance.
This study was supported in part by Grants-in-Aid for Scientific Research and Grants-in-Aid for Scientific Research in Priority Areas from the Ministry of Education, Culture, Science, Sports, and Technology (MEXT) of Japan and Japan Society for the Promotion of Science (JSPS).
REFERENCES
- 1.Asano, S., T. Honda, F. Goshima, D. Watanabe, Y. Miyake, Y. Sugiura, and Y. Nishiyama. 1999. US3 protein kinase of herpes simplex virus type 2 plays a role in protecting corneal epithelial cells from apoptosis in infected mice. J. Gen. Virol. 80:51-56. [DOI] [PubMed] [Google Scholar]
- 2.Benetti, L., and B. Roizman. 2004. Herpes simplex virus protein kinase US3 activates and functionally overlaps protein kinase A to block apoptosis. Proc. Natl. Acad. Sci. USA 101:9411-9416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cartier, A., E. Broberg, T. Komai, M. Henriksson, and M. G. Masucci. 2003. The herpes simplex virus-1 Us3 protein kinase blocks CD8T cell lysis by preventing the cleavage of Bid by granzyme B. Cell Death Differ. 10:1320-1328. [DOI] [PubMed] [Google Scholar]
- 4.Cartier, A., T. Komai, and M. G. Masucci. 2003. The Us3 protein kinase of herpes simplex virus 1 blocks apoptosis and induces phosphorylation of the Bcl-2 family member Bad. Exp. Cell Res. 291:242-250. [DOI] [PubMed] [Google Scholar]
- 5.Daikoku, T., R. Kurachi, T. Tsurumi, and Y. Nishiyama. 1994. Identification of a target protein of US3 protein kinase of herpes simplex virus type 2. J. Gen. Virol. 75:2065-2068. [DOI] [PubMed] [Google Scholar]
- 6.Daikoku, T., Y. Yamashita, T. Tsurumi, K. Maeno, and Y. Nishiyama. 1993. Purification and biochemical characterization of the protein kinase encoded by the US3 gene of herpes simplex virus type 2. Virology 197:685-694. [DOI] [PubMed] [Google Scholar]
- 7.Daikoku, T., Y. Yamashita, T. Tsurumi, and Y. Nishiyama. 1995. The US3 protein kinase of herpes simplex virus type 2 is associated with phosphorylation of the UL12 alkaline nuclease in vitro. Arch. Virol. 140:1637-1644. [DOI] [PubMed] [Google Scholar]
- 8.Frame, M. C., F. C. Purves, D. J. McGeoch, H. S. Marsden, and D. P. Leader. 1987. Identification of the herpes simplex virus protein kinase as the product of viral gene US3. J. Gen. Virol. 68:2699-2704. [DOI] [PubMed] [Google Scholar]
- 9.Fukunaga, R., and T. Hunter. 1997. MNK1, a new MAP kinase-activated protein kinase, isolated by a novel expression screening method for identifying protein kinase substrates. EMBO J. 16:1921-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goshima, F., T. Daikoku, H. Yamada, S. Oshima, T. Tsurumi, and Y. Nishiyama. 1998. Subcellular localization of the US3 protein kinase of herpes simplex virus type 2. Arch. Virol. 143:613-622. [DOI] [PubMed] [Google Scholar]
- 11.Hanks, S. K., A. M. Quinn, and T. Hunter. 1988. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science 241:42-52. [DOI] [PubMed] [Google Scholar]
- 12.Jerome, K. R., R. Fox, Z. Chen, A. E. Sears, H. Lee, and L. Corey. 1999. Herpes simplex virus inhibits apoptosis through the action of two genes, Us5 and Us3. J. Virol. 73:8950-8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kato, K., Y. Kawaguchi, M. Tanaka, M. Igarashi, A. Yokoyama, G. Matsuda, M. Kanamori, K. Nakajima, Y. Nishimura, M. Shimojima, H. T. Phung, E. Takahashi, and K. Hirai. 2001. Epstein-Barr virus-encoded protein kinase BGLF4 mediates hyperphosphorylation of cellular elongation factor 1delta (EF-1delta): EF-1delta is universally modified by conserved protein kinases of herpesviruses in mammalian cells. J. Gen. Virol. 82:1457-1463. [DOI] [PubMed] [Google Scholar]
- 14.Kato, K., A. Yokoyama, Y. Tohya, H. Akashi, Y. Nishiyama, and Y. Kawaguchi. 2003. Identification of protein kinases responsible for phosphorylation of Epstein-Barr virus nuclear antigen leader protein at serine-35, which regulates its coactivator function. J. Gen. Virol. 84:3381-3392. [DOI] [PubMed] [Google Scholar]
- 15.Kawaguchi, Y., R. Bruni, and B. Roizman. 1997. Interaction of herpes simplex virus 1 α regulatory protein ICP0 with elongation factor 1δ: ICP0 affects translational machinery. J. Virol. 71:1019-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawaguchi, Y., K. Kato, M. Tanaka, M. Kanamori, Y. Nishiyama, and Y. Yamanashi. 2003. Conserved protein kinases encoded by herpesviruses and cellular protein kinase cdc2 target the same phosphorylation site in eukaryotic elongation factor 1δ. J. Virol. 77:2359-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawaguchi, Y., K. Nakajima, M. Igarashi, T. Morita, M. Tanaka, M. Suzuki, A. Yokoyama, G. Matsuda, K. Kato, M. Kanamori, and K. Hirai. 2000. Interaction of Epstein-Barr virus nuclear antigen leader protein (EBNA-LP) with HS1-associated protein X-1: implication of cytoplasmic function of EBNA-LP. J. Virol. 74:10104-10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawaguchi, Y., C. Van Sant, and B. Roizman. 1997. Herpes simplex virus 1 α regulatory protein ICP0 interacts with and stabilizes the cell cycle regulator cyclin D3. J. Virol. 71:7328-7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leader, D. P., A. D. Deana, F. Marchiori, F. C. Purves, and L. A. Pinna. 1991. Further definition of the substrate specificity of the alpha-herpesvirus protein kinase and comparison with protein kinases A and C. Biochim. Biophys. Acta 1091:426-431. [DOI] [PubMed] [Google Scholar]
- 20.Leopardi, R., C. Van Sant, and B. Roizman. 1997. The herpes simplex virus 1 protein kinase US3 is required for protection from apoptosis induced by the virus. Proc. Natl. Acad. Sci. USA 94:7891-7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGeoch, D. J., M. A. Dalrymple, A. J. Davison, A. Dolan, M. C. Frame, D. McNab, L. J. Perry, J. E. Scott, and P. Taylor. 1988. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J. Gen. Virol. 69:1531-1574. [DOI] [PubMed] [Google Scholar]
- 22.Munger, J., A. V. Chee, and B. Roizman. 2001. The US3 protein kinase blocks apoptosis induced by the d120 mutant of herpes simplex virus 1 at a premitochondrial stage. J. Virol. 75:5491-5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munger, J., and B. Roizman. 2001. The US3 protein kinase of herpes simplex virus 1 mediates the posttranslational modification of BAD and prevents BAD-induced programmed cell death in the absence of other viral proteins. Proc. Natl. Acad. Sci. USA 98:10410-10415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murata, T., F. Goshima, Y. Nishizawa, T. Daikoku, H. Takakuwa, K. Ohtsuka, T. Yoshikawa, and Y. Nishiyama. 2002. Phosphorylation of cytokeratin 17 by herpes simplex virus type 2 US3 protein kinase. Microbiol. Immunol. 46:707-719. [DOI] [PubMed] [Google Scholar]
- 25.Murata, T., F. Goshima, Y. Yamauchi, T. Koshizuka, H. Takakuwa, and Y. Nishiyama. 2002. Herpes simplex virus type 2 US3 blocks apoptosis induced by sorbitol treatment. Microbes Infect. 4:707-712. [DOI] [PubMed] [Google Scholar]
- 26.Nishiyama, Y., Y. Yamada, R. Kurachi, and T. Daikoku. 1992. Construction of a US3 lacZ insertion mutant of herpes simplex virus type 2 and characterization of its phenotype in vitro and in vivo. Virology 190:256-268. [DOI] [PubMed] [Google Scholar]
- 27.Ogg, P. D., P. J. McDonell, B. J. Ryckman, C. M. Knudson, and R. J. Roller. 2004. The HSV-1 Us3 protein kinase is sufficient to block apoptosis induced by overexpression of a variety of Bcl-2 family members. Virology 319:212-224. [DOI] [PubMed] [Google Scholar]
- 28.Purves, F. C., A. D. Deana, F. Marchiori, D. P. Leader, and L. A. Pinna. 1986. The substrate specificity of the protein kinase induced in cells infected with herpesviruses: studies with synthetic substrates [corrected] indicate structural requirements distinct from other protein kinases. Biochim. Biophys. Acta 889:208-215. [DOI] [PubMed] [Google Scholar]
- 29.Purves, F. C., R. M. Longnecker, D. P. Leader, and B. Roizman. 1987. Herpes simplex virus 1 protein kinase is encoded by open reading frame US3 which is not essential for virus growth in cell culture. J. Virol. 61:2896-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Purves, F. C., W. O. Ogle, and B. Roizman. 1993. Processing of the herpes simplex virus regulatory protein alpha 22 mediated by the UL13 protein kinase determines the accumulation of a subset of alpha and gamma mRNAs and proteins in infected cells. Proc. Natl. Acad. Sci. USA 90:6701-6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Purves, F. C., D. Spector, and B. Roizman. 1991. The herpes simplex virus 1 protein kinase encoded by the US3 gene mediates posttranslational modification of the phosphoprotein encoded by the UL34 gene. J. Virol. 65:5757-5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reynolds, A. E., E. G. Wills, R. J. Roller, B. J. Ryckman, and J. D. Baines. 2002. Ultrastructural localization of the herpes simplex virus type 1 UL31, UL34, and US3 proteins suggests specific roles in primary envelopment and egress of nucleocapsids. J. Virol. 76:8939-8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryckman, B. J., and R. J. Roller. 2004. Herpes simplex virus type 1 primary envelopment: UL34 protein modification and the US3-UL34 catalytic relationship. J. Virol. 78:399-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith, R. F., and T. F. Smith. 1989. Identification of new protein kinase-related genes in three herpesviruses, herpes simplex virus, varicella-zoster virus, and Epstein-Barr virus. J. Virol. 63:450-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanaka, M., H. Kagawa, Y. Yamanashi, T. Sata, and Y. Kawaguchi. 2003. Construction of an excisable bacterial artificial chromosome containing a full-length infectious clone of herpes simplex virus type 1: viruses reconstituted from the clone exhibit wild-type properties in vitro and in vivo. J. Virol. 77:1382-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yokoyama, A., M. Tanaka, G. Matsuda, K. Kato, M. Kanamori, H. Kawasaki, H. Hirano, I. Kitabayashi, M. Ohki, K. Hirai, and Y. Kawaguchi. 2001. Identification of major phosphorylation sites of Epstein-Barr virus nuclear antigen leader protein (EBNA-LP): ability of EBNA-LP to induce latent membrane protein 1 cooperatively with EBNA-2 is regulated by phosphorylation. J. Virol. 75:5119-5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu, H. Y., H. Yamada, Y. M. Jiang, M. Yamada, and Y. Nishiyama. 1999. Intracellular localization of the UL31 protein of herpes simplex virus type 2. Arch. Virol. 144:1923-1935. [DOI] [PubMed] [Google Scholar]