ABSTRACT
Dispersal is a fundamental biological process, operating at multiple temporal and spatial scales. Despite an increasing understanding of fungal biodiversity, most research on fungal dispersal focuses on only a small fraction of species. Thus, any discussion of the dispersal dynamics of fungi as a whole is problematic. While abundant morphological and biogeographic data are available for hundreds of species, researchers have yet to integrate this information into a unifying paradigm of fungal dispersal, especially in the context of long-distance dispersal (LDD). Fungal LDD is mediated by multiple vectors, including meteorological phenomena (e.g., wind and precipitation), plants (e.g., seeds and senesced leaves), animals (e.g., fur, feathers, and gut microbiomes), and in many cases humans. In addition, fungal LDD is shaped by both physical constraints on travel and the ability of spores to survive harsh environments. Finally, fungal LDD is commonly measured in different ways, including by direct capture of spores, genetic comparisons of disconnected populations, and statistical modeling and simulations of dispersal data. To unify perspectives on fungal LDD, we propose a synthetic three-part definition that includes (i) an identification of the source population and a measure of the concentration of source inoculum and (ii) a measured and/or modeled dispersal kernel. With this information, LDD is defined as (iii) the distance found within the dispersal kernel beyond which only 1% of spores travel.
INTRODUCTION
The relative degree to which organisms move is a process operating at multiple temporal and physical scales (1). In recent years dispersal has received a great deal of attention in fields ranging from mathematics and physics to ecology and molecular biology, but only a patchy framework exists to explain dispersal over very large distances. Modeling patterns of long-distance dispersal (LDD) among macroorganisms, ranging from vertebrates and flying insects to seed plants, appears tractable, but documenting the geographic distributions and dispersal dynamics of microscopic propagules and microbes presents multiple theoretical and methodological challenges (2–4). The majority of empirical research directly measuring the dispersal of microbes or microscopic propagules is restricted to relatively short distances, and tracking dispersal at greater spatial scales involves mathematical or genetic models, e.g., in studies of moss (5–9), ferns (10–13), bacteria (14–19), and fungi (19–23). However, fitting dispersal data (e.g., from the tracking of spore movement) to mathematical functions often over- or underestimates LDD and imprecisely describes the trajectory of spore movement across large distances (24–28). Inferences based on population genetics data capture rare instances of successful LDD but incompletely describe underlying demographic processes and typically cannot speak to mechanisms of LDD (1). Besides the limitations of mathematical and genetic methods, important details about the natural history of species are often ignored or remain unknown, leaving many questions unanswered, including, e.g., how ephemeral propagules remain viable while exposed to harsh environments over extended periods of time.
Here we consider LDD as it relates to fungi. Although most research focuses on only a small number of fungi, the kingdom is extremely diverse, housing an estimated 1.5 to 10 million species (29). The ability of fungal dispersal structures (e.g., conidia, basidiospores, ascospores, sclerotia, etc.) to disperse over large distances may be highly context dependent (Fig. 1). Moreover, while LDD for a rust fungus, e.g., Puccinia graminis, may be over several kilometers, LDD for a bird’s nest fungus, e.g, Crucibulum laeve, may be only several dozen meters (30, 31). The delimitation of cryptic species by phylogenetic techniques has also in many cases revealed that a fungus once considered widespread in fact consists of several separate species, each with nearly indistinguishable morphological characteristics, and raises questions about the prevalence of LDD (32–34). Further complicating matters, direct evidence for LDD beyond several kilometers is lacking (35). Thus, any discussion of the dispersal dynamics of fungi as a whole is problematic, especially if comparisons are made or inferred between one fungal group and another (e.g., aquatic fungi compared to ectomycorrhizae) (19, 22, 23, 30, 36).
FIGURE 1.

A framework for understanding fungal LDD.
One common feature among sporulating fungi is the tremendous abundance of both sexual and asexual spores (e.g., a single gall of Ustilago maydis [corn smut] contains up to 25 billion spores, and a single sporangium of Rhizopus stolonifer [common bread mold], up to 50,000) (35). Fungal spores are orders of magnitude smaller than the smallest seeds—smaller than most moss and fern spores and comparable in size to some plant pollen (e.g., Triticum aestivum, or wheat, pollen) (37–40) (see Fig. 2 and 5). However, unlike pollen, many fungal spores are short-lived and highly susceptible to desiccation and UV radiation, and it is often unclear whether spores survive, e.g., transcontinental and oceanic transport (41–45).
FIGURE 2.

Sizes of fungal spores and other airborne particles. Some species are wind dispersed (e.g., P. graminis), while others have other means of dispersal (e.g., Gigaspora rosea). The smallest plant seed, Wolffia angusta, the pollen grains of Hibiscus syriacus and T. aestivum, and a glomerospore of the arbuscular mycorrhizal Gigaspora rosea are provided for comparison. Species labeled with an asterisk are not fungi.
FIGURE 5.

Images of various fungal spores. (A) Basidiospores of Agaricus bisporus (brown powder) next to seeds of Wolffia borealis (semicircles) and sugar crystals (white cubes). (B) Urediniospores of Puccinia menthae (Fig. 1 of reference 194). Conidia of (C) Alternaria solani and (D) A. alternata. A. alternata is a putative long-distance disperser, while A. solani (10× in size) is not (courtesy of Steve Jordan). (E) Glomerospore of Glomus irregulare (Fig. 5i of reference 195). (F) Conidium of C. herbarum (Fig. 5c of reference 196). (G) Teliospore of Tilletia controversa (Fig. 9 of reference 197). (H) Urediniospore of H. vastatrix (Fig. 1e of reference 198). (I) Urediniospore size, shape, and ornamentation of P. melanocephala (Fig. 1d of reference 199). (J) Zoospores of chytrid Rhizophydium elyensis (200). (K) Ascospores of Ascobolus denudatus (200). (L) Sporangiospores of Rhizopus microsporus var. chinensis (200). (M) Basidiospores of Boletellus taiwanensis still on soredia (200). (N) Conidia of the aquatic ascomycete Nawawi dendroides (Fig. 66 of reference 92).
Given these taxonomic, empirical, and methodological challenges, a sound conceptual framework to guide and synthesize research is urgently needed. Mycologists have yet to integrate the abundant physiological, morphological, and biogeographic data available for hundreds of species into a unifying paradigm of fungal LDD. If comparisons are to be effective or relevant, the highly relative nature of the spatial scales involved must be explicitly acknowledged in any discussion of LDD (46).
DEFINING FUNGAL LDD
In a general framework focused on dispersal, Nathan (28) highlights two general definitions of LDD that are often used in studies of animals and plants: movement exceeding (i) an absolute threshold equivalent to a chosen distance (e.g., 100 km) and (ii) a relative threshold based on a fraction of propagules found at the tail of a dispersal kernel (e.g., 99th percentile and above). However, a translation of these definitions to research on fungi is hindered by the incommensurate priority given to plants and animals in dispersal ecology (cf. 47–49) and by a lack of appropriate empirical data (e.g., spore sources are often inferred through reverse trajectory models that reveal little about source inoculum density, making inferences about fungal dispersal kernels [required by definition ii] difficult) (1, 28, 50). Moreover, definitions involving absolute threshold distances involve discretionary demarcations of LDD, resulting in a lack of consistency among studies. For example, definitions of LDD range from beyond 100 m (Fusarium graminearum), to beyond 1,000 m (Mycosphaerella fijiensis), to transoceanic transport (Aspergillus sydowii) (43, 51, 52). Using definitions based on a relative threshold facilitates comparisons of dispersal kernels of different species, but only if a common percentile is routinely used.
While it may be appropriate to have alternative definitions of fungal LDD for different species, at the moment there is no comprehensive approach to organizing the myriad methods used to think about fungal LDD. An accurate description of successful LDD must include, at a minimum, the magnitude of the source inoculum, the physical and biological probability of LDD (including, e.g., the vector[s] involved and the longevity of spores or tissues), the availability of suitable landing sites, and the probability of establishing a stable population and reproducing (Fig. 1). Any of these variables can prevent successful dispersal, perhaps explaining why fungal LDD appears extremely rare. Additionally, differences between stepwise vs. single-leap LDD must be distinguished. LDD involving sequential, shorter-distance dispersal is likely the more common phenomenon, while LDD involving a single successful spore moving a long distance is a very low-probability event that would coincide with optimal conditions for both fungus and vector.
To unify the disparate approaches used to describe and measure fungal LDD, we propose a synthetic three-part definition built on the framework presented by Nathan (1, 28). Any description of fungal LDD should include (i) identification of the source population and a measure of the concentration of source spores and (ii) a measured and/or modeled dispersal kernel. With this information LDD is defined as (iii) the distance found within the dispersal kernel beyond which only 1% of spores travel (Fig. 3). The 1% threshold provides a useful, common reference point; other choices are possible, but in any discussion the chosen threshold should be clearly identified. Using this standard definition, or discussing how any particular experiment relates to this definition, would facilitate an integrated approach to understanding fungal dispersal.
FIGURE 3.

To integrate the disparate approaches used to describe and measure fungal LDD, we propose a synthetic three-part definition building on the general framework presented by Nathan (1, 28). A description of fungal LDD should include (i) identification of a source population and measure of source inoculum concentration (e.g., the number of spores in a single rust pustule), (ii) a measured and/or modeled dispersal kernel, and (iii) a measure of the distance, based on the dispersal kernel, past which only 1% of spores travel. Adopting a standard approach would mitigate the confusion caused by differing definitions and measurements of LDD and facilitate comparisons among the dispersal kernels of different species. In the illustration, the blue and red dispersal kernels demonstrate idealized kernels for two hypothetical species. LDD is defined per species at distances A and B, respectively—the distance beyond which only 1% of spores travel. We next used our approach with real dispersal data of M. fijiensis (measured as the number of resistant lesions per square meter of banana leaf measured from a source to 1,000 m) (52), Fusarium graminearum (measured as the recovery rate of ascospores of a unique clone released from a source to 1,000 m) (58), and Lobaria pulmonaria (measured as the proportion of DNA from snow samples identical to an isolated source of soredia up to a distance of 40 m [193]) to estimate dispersal kernels and identify LDD for each species. We smoothed the published data to estimate an approximate dispersal kernel, and the distance beyond which 1% of spores traveled was found by integrating the area under each kernel from 0 m to the distance at which 99% of spores had been captured. Although both M. fijiensis and F. graminearum are capable of dispersing to approximately 1,000 m, the proportion of spores that fit our definition of LDD varies considerably, because LDD is defined past 714 m for F. graminearum and past 250 m for M. fijiensis. A holistic comparison of the two dispersal kernels suggests that different dynamics will shape the effective reach of each species. The dispersal kernel of L. pulmonaria illustrates how truncated experimental setups can impact measures of LDD. At the furthest collection point (40 m), a large proportion of samples tested positive, and the best dispersal kernel that can be modeled from the data (193) provides what is likely an underestimate of LDD, at approximately 39 m (15% of the positive samples collected at 0 m were detected). Ideally, the tail end of a modeled dispersal kernel should very closely approach a horizontal line at y = 0.
MEASURING LDD
Empirical measures of spore dispersal are difficult to make, but direct measures of movement remain critical to understanding the scale of a species’ dispersal as a whole (51–53). It cannot be assumed that spores traveling beyond the limits of an experimental setup are statistically and/or ecologically insignificant. Moreover, while spore viability is often ignored, successful LDD requires that, e.g., a spore that has crossed an ocean is also viable. Novel approaches to measuring both spore trajectories and the probabilities of survival are critically needed, and experiments involving creative thinking, and perhaps taking advantage of new technologies, will likely help to better address the many unanswered questions about fungal LDD.
Once a greater array of empirical dispersal data is available, new dispersal kernels can be developed to better quantify fungal LDD (for a review of kernel functions see reference 54). However, many kernel models are best suited to describe either the source or tail end of dispersal, but not both simultaneously, and when applied to entire trajectories, such models tend to either over- or underestimate LDD (24–27). Describing the mathematics behind these models is outside the scope of this review, but examples of their use can be found in many studies of fungi (31, 45, 55–58).
The majority of studies of fungal LDD employ molecular approaches to compare the genetics of populations across a geographic range. However, genetic inferences reveal little about the underlying biological, physiological, and ecological forces at play and may be less relevant to our proposed definition. Studies often compare allele frequencies among discrete populations to infer dispersal, e.g., among Southern Hemisphere populations of Ganoderma applanatum-australe, globally distributed populations of Tuber species, and pan-Arctic populations of many ectomycorrhizal fungi (20, 21, 59, 60). If two populations that are very far away from each other appear closely related in a phylogeny (e.g., Israeli populations appear more closely related to populations from Indiana than they are to Syrian populations), then LDD is inferred (Fig. 4) (61–64). Phylogenetic methods allow inferences of rare LDD to be made with less intensive sampling than the direct capture of spores but cannot provide critical information on spore longevity, nor information about the role of meteorological patterns, spore physiology, putative vectors, and human mediation. Genetic approaches cannot be used to model dispersal kernels and reveal little about dispersal mechanisms, regardless of geographic scale.
FIGURE 4.

A phylogram of genetic distances among 15 geographic populations of Mycosphaerella graminicola. The fact that geographically distant populations of M. graminicola are grouped together, e.g., Uruguayan populations are grouped with Algerian and Syrian populations, likely suggests movement mediated by humans. M. graminicola infects one of the most traded agricultural products (wheat), and its ascospores cannot survive prolonged exposure to, e.g., dry air (183). Data adapted from Zhan et al. (64); similar clustering of geographically distant populations is found from data on Phaeosphaeria nodorum (129), Rhynchosporium secalis (131), and M. fijiensis (60).
However, the best examples of LDD are based on a variety of approaches. Considering the different limitations to direct sampling, statistical modeling, and genetic inference, it is not surprising that the best-known cases of fungal LDD are typically generated using these methods in combination. For example, several reports of fungal trans-Atlantic dispersal in Saharan dust manage to capture viable fungal material, describe a dispersal kernel, and use meteorological backtracking to identify air masses as having originated from Africa (42, 43, 65–67). For other taxa, researchers sample directly from within the planetary boundary layer using towers or aircraft, in addition to tracking the trajectories of air masses (68–71). Genetic methods are also combined with spore capture techniques, usually by first determining the genotype of a specific fungal strain and subsequently allowing it to release spores (58). The relative proportions of that genotype collected by spore traps are then used to construct a dispersal kernel.
DISPERSAL VECTORS
Wind
Wind is the most commonly considered vector of fungal dispersal. Aerosolized course particles (greater than 2.5 μm in diameter) have been photographed moving hundreds of kilometers from northern Africa across the Atlantic, depositing an estimated 500 million to over 1 billion tons of material per year in the Caribbean and Amazon basin (43, 66). Sand, soil, and, in smaller proportions, biological matter including bacteria and fungal spores have also been found in air samples retrieved from towers or aircraft (36, 68, 71). Further evidence that spores can move in the atmosphere is provided by tracking wind patterns. The biogeography of mosses and ferns, as well as lichens, in the Southern Hemisphere may be better described by wind patterns than by geographic distances between land masses (72), providing indirect evidence for LDD via “wind highways” (45). Wind patterns are also used to infer atmospheric LDD of fungal pathogens, e.g., the introduction of Hemileia vastatrix (coffee leaf rust) from Africa to Brazil, and of Puccinia melanocephala (sugarcane rust) from West Africa to the Caribbean and the United States (73, 74).
Samples of dust from surfaces are also used to infer fungal LDD. Metabarcoding data taken from North American dust reveal a low percentage of common species across regions, but some degree of overlap suggests that LDD is a real, albeit rare, phenomenon (19, 23). Alternative hypotheses posit that the ubiquity of some species is caused by short-distance dispersal over long time scales or that species appearing broadly distributed are complexes of cryptic species, each with restricted geographic ranges (75, 76).
Other studies track dispersal in wind by capturing spores. Peay et al. (30, 77) documented the dispersal of ectomycorrhizal assemblages at least 10 kilometers away from their source by placing uninfected “trap” seedlings at different distances from a source population. Data reveal that species richness and trap seedling colonization drop significantly beyond 1 kilometer. Additionally, viable ascospores of the wheat pathogen F. graminearum have been captured 50 meters to 1 kilometer above the Earth’s surface in all seasons in Virginia—even during winter, when its plant host is absent. The capture of spores during winter suggests that the source of spores is kilometers away, because there was no wheat in the vicinity of the experimental setup when the F. graminearum spores were collected (68, 70, 71).
The most complete picture of wind LDD emerges from research on A. sydowii, the causal agent of aspergillosis of the Caribbean sea fan, Gorgonia ventalina. An outbreak of the disease occurred during the 1980s and coincided with the highest recorded deposition of African dust in the Caribbean Sea (42). Air samples taken from African dust plumes revealed the presence of A. sydowii conidia, and by inoculating G. ventalina in laboratory assays, these same dust-borne conidia were shown to cause the same symptoms of aspergillosis as occurring in the Caribbean (42, 67). Furthermore, only samples derived from African air masses moving over the Caribbean contained viable A. sydowii material, while samples from air masses of different origins did not (78).
Atmospheric LDD may involve more than wind and may be facilitated by a combination of meteorological phenomena, including cloud, storms, and precipitation. In fact, spores may serve as rain- and cloud-forming nuclei, although the limited evidence for this phenomenon is debated, and it is still unclear how water can condense on the potentially hydrophobic outer surface of spores (see section below on morphological, biophysical, and physiological properties influencing LDD).
Fire may also play a role in initiating LDD because it can rapidly heat air and cause high-velocity updrafts (79). For example, back trajectory modeling of air masses suggests that viable spores captured from smoke over the Gulf Coast of Texas originated 1,500 km away in forest fires in the Yucatán Peninsula, Mexico (80). Sugarcane agriculture provides an excellent model for exploring how fire promotes LDD, because fields are frequently burned prior to harvest and are often plagued by one of the most widely referenced putative long-distance dispersers, P. melanocephala—among the first fungal species described as having undergone trans-Atlantic dispersal (79, 81). Fire-borne updrafts, perhaps often caused by humans, may facilitate the spread of P. melanocephala, challenging hypotheses of the unassisted dispersal of spores across oceans (cf. 73, 74).
Plants
Plants are another agent of fungal dispersal, and their ability to vector fungi is unsurprising given the close ecological association between the two kingdoms. Fungi inhabit both living and dead plant tissues, and there are many opportunities for fungi to codisperse, e.g., with seeds, senesced leaves, or branches.
Driftwood is an often overlooked substrate in which fungi disperse. Saprotrophic fungi are often found in decaying logs floating in bodies of water, and if hyphae or spores are able to withstand saline conditions, driftwood may be able to transport species across oceans. For example, Rämä et al. (82) sampled logs from across the North Sea and successfully cultured 147 fungal operational taxonomic units of Ascomycota, Basidiomycota, Mucormycotina, and Chytridiomycota, 50% of which were identified as terrestrial (nonmarine). Driftwood kept afloat by ice flows during the late Weichselian or early Holocene is suggested as a mediator of LDD for several trans-Arctic plant species and likely also their fungal symbionts (83). Data already support the long-distance movement of driftwood; e.g., Hellman et al. (84) show that logs collected in Greenland and Svalbard originated from western and central Siberia and North America. However, the majority of wood was logged, again suggesting that humans play a key role in many different kinds of LDD. The problem of driftwood-associated fungi remains a promising area for future research, and open questions concern patterns of driftwood movement and their possible relationship to fungal introductions and whether some logging practices increase the likelihood of LDD.
Living plant material transported by ocean currents is another putative mediator of fungal LDD. Symbiotic fungi are associated with plant roots as mycorrhizae and with leaves, stems, and seeds as endophytes. Thus, ocean-dispersed plant material, including floating seeds, asexual propagules, or entire root balls, may explain the geographic range of some fungi that are found on two sides of, e.g., an ocean. Little to no direct evidence for this phenomenon has been collected to date, and phylogenetic analyses testing whether plants and fungi disperse together are, surprisingly, lacking (85). Anecdotal evidence of arbuscular mycorrhizae occurring most frequently and with greater biomass on Hawaiian endemic beach grass species has been used to suggest fungus-plant long distance codispersal (86, 87). However, Koske and Gemma (87) provide alternative hypotheses such as concurrent, independent dispersal and sea bird-mediated dispersal of arbuscular mycorrhizae. A codispersal hypothesis is also suggested as an explanation for evidence of recent gene flow between island and mainland ectomycorrhizae, though vectors such as wind cannot be ruled out (85, 88).
Oceans, Rivers, and Lakes
Large bodies of water can act as vectors of fungal dispersal. Oceans and lakes provide large areas across which some fungi can freely travel by, e.g., moving with microcurrents and upwelling, while rivers and streams provide continuous movement in the direction of their flow. The number of fungi specifically adapted to an aquatic or amphibious lifestyle is estimated at more than 10,000 species, although only approximately 500 have been formally described (89). Aquatic species are informally divided into two major groups: Ingoldian fungi, found on decaying leaves in streams and lakes, and aquatic ascomycetes (traditionally referred to as hyphomycetes), found on submerged wood (90). The uncommon shapes of many aquatic fungal spores may facilitate dispersal, as well as adherence to various substrates, in aquatic environments. Conidia are typically sigmoid or tetraradiate, while ascospores are generally fusiform with bipolar mucilaginous pads (see Fig. 5N) (91).
Whether spores are passively or actively released remains unclear. Most marine ascomycetes appear to release their spores passively, while many tropical freshwater ascomycetes actively eject their spores away from the fungal thallus (92). A possible explanation for this pattern may involve wind dispersal of spores during seasonal drying of streams and rivers. Alternatively, storms can cause flooding and the accumulation of substrate on, e.g., riverbanks and subsequently expose ascocarps to airflows once waters subside (92). In both cases spores are hypothesized to be sometimes dispersed by air, although the interplay between aquatic and terrestrial habits of these kinds of fungi requires further study.
At least a few Ingoldian fungi appear to have cosmopolitan ranges, suggesting they may be capable of LDD (90). For example, recent phylogenetic methods have elucidated that there is no geographic structure to populations of the widely distributed marine fungus Lignicola laevis, hinting that the species may be a long-distance disperser. However, only two loci were used in the study, and including several more genetic regions may reveal more restricted population assemblages (75, 76, 89). Pang et al. (89) list several other species which seem to have similar cosmopolitan distributions—Aniptodera cheasapeakensis, Ceriosporopsis halima, Corollospora maritima, Savoryella lignicola, Torpedospora radiata, and Zalerion maritima—suggesting that aquatic fungal LDD may be an as yet undescribed phenomenon.
Aquatic environments may be an ideal place for LDD to occur considering that fungal dispersal is often limited by spore desiccation, UV damage, and harsh temperatures. Water temperature fluctuates more slowly than that of air, and water attenuates light penetration at relatively shallow depths (especially in highly trophic waters). Moreover, water provides a greater degree of buoyancy than air, increasing the time before spore sedimentation. Aquatic dispersal is perhaps the least commonly considered mechanism of LDD, and future studies might address the coupling of, e.g., spore hydrodynamics with river flow velocity, as well as the population structure of putatively cosmopolitan aquatic fungi.
Animals
Animals are also vectors of fungal dispersal. Many animals migrate across continents on an annual basis and may transport fungi either internally or externally as spores, hyphae, sclerotia, or symbionts (93–98). Fungal propagules sheltered deep within fur or feathers are potentially protected from some harsh environments as they move over large distances.
Flying animals clearly serve as fungal vectors, and there is a great deal of evidence for birds and insects as mediators of fungal dispersal, especially of pathogens. Examples in arthropods include the spread of Entomophaga maimaiga, an introduced pathogen of gypsy moths used for biocontrol in North America (99); Aspergillus flavus, which infects desert locusts in India (100); Sphaeropsis sapinea, a pathogen of conifers worldwide that is spread by the pine engraver beetle (101); Ophiostoma spp. and Knoxdaviesia proteae, commensal species of mites secondarily vectored by beetles in South Africa (102, 103); and many others. However, dispersal by insect vectors tends to be restricted within a localized range, e.g., a few hundred kilometers, while dispersal across, e.g., continents or oceans, is more commonly caused by the human-mediated movement of insects and fungi together (103, 104).
Migrating birds are another common agent of animal-mediated dispersal. Examples include Gibberella fujikuroi (Fusarium moniliforme), a pathogen of rice vectored by hummingbirds (105); Encephalitozoon and Enterocytozoon spp., microsporidian human pathogens collected from several bird species (106, 107); and 2,337 filamentous fungi isolated from 216 migrating Mediterranean birds, of which Cladosporium cladosporioides, Alternaria alternata, and Aspergillus niger were the most abundant (108).
The recent spread of white-nose syndrome in North America, caused by Pseudogymnoascus destructans (Geomyces destructans), is another example of flying animal-mediated fungal dispersal. The mycosis appears to be spread among congregating bats and by their subsequent movement to other caves (109). The recent emergence of the disease in North America has resulted in the death of millions of bats, but it is unclear if the epidemic has resulted from the introduction of a European species or from the recent emergence of a newly virulent North American strain (110). In either case, the disease is spreading on a continental scale, and in addition to bats, humans may play a role in its spread (109).
Finally, the spread of chytridiomycosis of amphibians, caused by Batrachochytrium dendrobatidis, is perhaps the most commonly cited example of putative animal-mediated LDD. In recent years chytridiomycosis has spread rapidly, perhaps facilitated by a changing climate, as shown in Central America (111). The disease is heterogeneously distributed across all continents except Antarctica, but the reasons for its disjointed distribution are unknown (112, 113). It is not entirely clear how the fungus moves over large spatial scales, but its spread may be caused by a combination of localized amphibian movement coupled with, again, human mediation via the international trade of Xenopus laevis (the African clawed frog) and other amphibians (114–116).
Humans
Ancient fungal dispersal mediated by human migrations is suggested by data on population structures (117–119). The range expansion of the fungal pathogen Coccidioides immitis into South America parallels human migration routes during the Pleistocene (120–122). Similarly, the diversification of Saccharomyces cerevisiae strains mirrors their use and movement with human populations (123).
Contemporary dispersal mediated by human vectors merits special consideration as the inadvertent transportation of biological materials continues at unprecedented spatial and temporal scales. Plant disease epidemics caused by introduced fungal pathogens are among the clearest examples of the impact of human-mediated LDD, e.g., Cryphonectria parasitica (chestnut blight), Ophiostoma ulmi and Ophiostoma novo-ulmi (Dutch elm disease), and Cronartium ribicola (white pine blister rust) (124–127). But human-mediated LDD is not restricted to pathogens. For example, Vellinga et al. (128) show that many genera of ectomycorrhizae have also been introduced to novel ranges and spread globally by the human movement of plants and soil.
The global transport of agricultural products, as well as exotic plants, animals, and soil, all serve either indirectly or directly as platforms by which fungi can disperse over large distances at an accelerating rate. Modern transportation enables fungi—including fungal tissue that cannot independently disperse—to traverse continents in less than 24 hours. For instance, fruits and vegetables grown in North America typically spend a maximum of 5 days in intracontinental transit following harvest, and the transport time of produce grown in the Southern Hemisphere for U.S. consumption can take as little as a few days, depending on the mode of transportation (46).
Although many examples of human-mediated fungal dispersal are well documented, circumstantial evidence points to an even greater array of human-mediated dispersal events that are less well understood. Examples include M. fijiensis (black sigatoka) and Mycosphaerella graminicola (septoria leaf blotch), Puccinia striiformis f. sp. tritici (wheat yellow rust), P. melanocephala (sugarcane rust), H. vastatrix (coffee rust), and Rhynchosporium secalis (barley scald) (59, 63, 64, 73, 74, 129–131). Many of these species are intimately associated with agriculture, are planted over vast areas, and are regularly moved (either superficially on or within plant tissue) on a global scale. These same species are also frequently cited as prime examples of fungi capable of LDD (25). However, few to no data on fungal characteristics enabling or inhibiting LDD are available; to travel, e.g., across oceans, spores must presumably surmount considerable biophysical constraints. Many of these pathogens are also globally distributed—as are their crop hosts—and an alternative hypothesis explaining what appears as LDD would involve a global network of commerce that provides multiple opportunities for infectious material to be transported between locations.
Consider the global populations of M. graminicola studied by Zhan et al. (64), of which, e.g., populations in Syria and Uruguay are genetically less distant from each other than geographically close populations sampled from both eastern and western Australia (Fig. 4). The fact that geographically distant populations of M. graminicola are grouped together, e.g., Uruguayan populations are grouped with Algerian and Syrian populations, suggests movement by humans. M. graminicola infects a highly traded agricultural product, wheat, and its ascospores cannot survive prolonged exposure to, e.g., dry air (132). However, with enough time, if gene flow were to completely halt, geographic populations could diverge and no longer appear as nested populations, although relationships between genetic and geographic distances would remain difficult to interpret. Highlighting the connection between human-mediated LDD and its effects on population structure may prove itself as a key variable to consider when trying to determine vectors and mechanisms of fungal LDD.
MORPHOLOGICAL, BIOPHYSICAL, AND PHYSIOLOGICAL PROPERTIES INFLUENCING LDD
Spore Size and Shape
The most obvious and perhaps most important agent of dispersal is the spore, whose size and shape may critically affect movement over large distances (Fig. 5) (133). A spore’s ability to reach airflows, remain aloft, and then land in a suitable location is influenced by aerodynamic forces operating at a microscopic scale, and such forces may be harnessed by manipulating spore morphology (133, 134). Although Jenkins et al. (135) report no correlation between propagule size and dispersal distance in general cases, aspects of spore morphology are clearly optimized for movement. For example, Fritz et al. (136) have shown that among some Ascomycetes, spore dimensions precisely fit apical ring size to maximize launch distance with minimal energy. Others show that spore size can also be correlated to environmental parameters in ways that might maximize the probability of LDD. For example, Kauserud et al. (137, 138) show a relationship between spore size and the calendar date of sporulation and reason that spore morphology enables some fungi to take full advantage of seasonal wind velocities. However, when we compiled data on spore sizes and dispersal distances claimed as fungal LDD (Table 1) we found no relationship between spore morphology and dispersal distance (Fig. 6), but we hypothesize that the lack of any apparent correlation reflects the different measures and definitions of LDD used in the literature and not necessarily the lack of a biological relationship.
TABLE 1.
Spore parameters for putative long-distance dispersers
| Putative LDD fungal species | Spore type | Spore dimensionsa | Shape | Habit | Pigment | Clump | Reference |
|---|---|---|---|---|---|---|---|
| B. graminis f. sp. tritici | Ascospore | 20–30 μm (l) × 10–13 μm (w) | Ellipsoid | Plant pathogen | Hyaline | Yes | 201 |
| Gibberella zeae/Fusarium graminearum | Ascospore | 13–28 μm (l) × ∼4 μm (w) | Long ellipsoid | Plant pathogen | Light brown to hyaline | 202 | |
| M. fijiensis | Ascospore | 11.5–16.5 μm (l) × 2.5–5 μm (w) | Fusiform | Plant pathogen | Hyaline | 41 | |
| M. graminicola/Septoria tritici | Ascospore | 8–10 μm (l) × 2–2.5 μm (w) | Fusiform | Plant pathogen | Hyaline to light brown | 65 | |
| Mycosphaerella musicola | Ascospore | 14.9 μm (l) × 4.6 μm (w) | Fusiform | Plant pathogen | Dark brown | 203 | |
| Phaeosphaeria nodorum | Ascospore | 20–31 μm (l) × 4–5 μm (w) | Fusiform | Plant pathogen | Yellow-brown | 129 | |
| Sclerotinia sclerotiorum | Ascospore | 12 μm (l) × 6 μm (w) | Ellipsoid | Plant pathogen | Hyaline | Yes | 204 |
| Venturia inaequalis | Ascospore | 11–15 μm (l) × 5–7 μm (w) | Ellipsoid | Plant pathogen | Brown | 205 | |
| G. applanatum-australe | Basidiospore | Applanatum: 6.5–8.5 μm (l) × 4.5–6 μm (w); australe: 8–13 μm (l) × 5.5–9 μm (w) | Ellipsoid | Saprotroph | Brown | 88 | |
| Laccaria amethystina | Basidiospore | 6.16–8.47 μm (diam) | Globose, ornamented | Mycorrhiza | White | 206 | |
| A. alternata | Conidia | 20–63 μm (l) × 9–8 μm (w); often produced in chain of more than 5 conidia | Obclavate to obpyriform | Plant pathogen | Beige to brown | Yes | 19 |
| A.. sydowii | Conidia | 2.5–4.0 μm (diam) | Globose | Animal biotroph | Hyaline | 68 | |
| C. herbarum | Conidia | 1–12 μm (l) × 1–10 μm (w) with 50–100 nm × 50–400-nm bundle-like structures | Ellipsoid | Plant pathogen | Melanized | Yes | 19 |
| R. secalis | Conidia | 12–20 μm (l) × 2–4 μm (w) | Fusiform | Plant pathogen | Hyaline | 131 | |
| Peronospora hyoscyami f.sp. tabacina† | Oospore | 17–28 μm (l) × 13–17 μm (w) | Globose | Plant pathogen | Hyaline | 207 | |
| Sporisorium scitamineum | Teliospore | 5 μm (diam) | Ovoid | Plant pathogen | Brown | Yes | 208 |
| Ustilago nuda | Teliospore | 6.5 μm (l) × 5.8 μm (w) | Subglobose | Plant pathogen | Golden brown | 197 | |
| H. vastatrix | Urediniospore | 29.7–34.5 μm (l) × 18.9–37.3 μm (w) | Ellipsoid | Plant pathogen | Yellow | Yes | 198 |
| Phakospora pachyrhizi | Urediniospore | 18–34 μm (l) × 15–24 μm (w) | Globose | Plant pathogen | Pale yellow to hyaline | Yes | 209 |
| P. graminis f.sp. tritici | Urediniospore | 28.3 μm (l) × 17.5 μm (w) | Globose | Plant pathogen | Brown | Yes | 210 |
| P. melanocephala | Urediniospore | 28–33 μm (l) × 18–23 μm (w) | Obovoid | Plant pathogen | Cinnamon brown | 162 | |
| P. striiformis f.sp. tritici | Urediniospore | 14–36 μm (l) × 13–23 μm (w) | Ellipsoid | Plant pathogen | Yellow to brown | Yes | 211 |
| B. dendrobatidis | Zoospore | 3–5 μm (diam); posterior flagellum (19–20 μm long) | Ovoid | Animal pathogen | Hyaline | 212 |
Dimensions are given for major (l) and minor (w) axes or, when spherical, for diameter (diam). †, oomycete.
FIGURE 6.
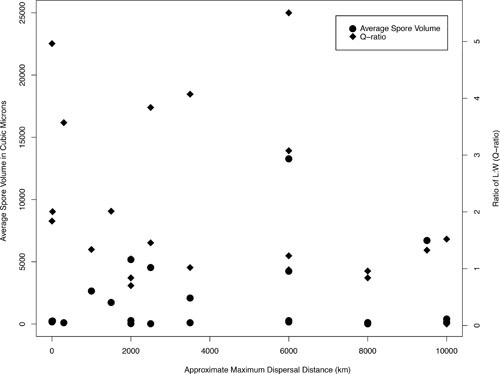
Comparing spore sizes to reported maximum dispersal distances. Spore volume in square micrometers is measured on the left-hand vertical axis, and spore Q-ratio (the ratio of spore length to width) is measured on the right. Data points were calculated from the parameters listed in Table 1. There is a poor correlation between approximate maximum dispersal distance and both average spore volumes (R2 = 0.0167, P = 0.5568) and Q-ratios (R2 = 0.1113, P = 0.1198). The lack of any correlation likely reflects inconsistent definitions and measurements of LDD, rather than any biological reality.
There is likely a compromise between small spore size, which can enable dispersal over longer distances, and large spore size, which can facilitate settling onto a favorable substrate (Fig. 5C, D). In principle, smaller spores should remain aloft for greater time intervals, but their reduced mass makes landing more difficult and increases their susceptibility to adverse environmental pressures, including UV exposure and desiccation (139). Greater and improved data on a range of spore parameters—emphasizing spore size, shape, longevity, and density—are required to further explore the tradeoffs involved in successful LDD. Often, the aerodynamic diameter (defined as the diameter of a spherical particle with equal density and terminal velocity to the particle of interest) of a spore is the sole parameter considered in estimates of spore dispersal (140). The focus on aerodynamic diameter may be problematic because many spores are not spheres, and also because density measurements specific to species of interest are not available but are necessary for accurate extrapolations of dispersal in heterogeneous airflows (140–142).
Successful fungal dispersal appears also to rely on a critical interplay between drag reduction (to maximize launch height) and drag maintenance (to maximize flight time). Roper et al. (143) have shown that explosively launched spores of many Ascomycetes have drag-minimizing shapes. Drag minimization enables spores to breach the boundary layer of still air surrounding sporocarps to reach more turbulent air layers. However, once aerosolized, successful LDD may require spores to remain aloft for extended periods (133). Wong et al. (144) have shown that remaining aloft is more a function of spore volume than of shape and have observed that the drag constants of spores are surprisingly proportional to their surface area (discounting shape and type of particle). Therefore, spore size appears to have, overall, a greater effect on settling velocity than do shape and density, suggesting that the latter characteristics may be less important in determining sedimentation rates (140, 142). An exciting direction for future research involves more thorough testing of whether or how fungi have adapted to take advantage of aerodynamic principles, especially among putative long-distance dispersers.
A Spore’s External Surface
Additional aspects of morphology that may influence spore dispersal include ornamentation and hydrophobicity, although these features appear to be more rarely studied than shape and size, despite limited data suggesting their key role in dispersal. For example, Halbwachs et al. (145) report that asymbiotic agaric species tend to be more ornamented than ectomycorhizal agarics, while the latter tend to have smoother, more pigmented spore walls; differences may reflect distinct dispersal dynamics; e.g., ectomycorrhizae may require more pigmentation for UV protection while dispersing greater distances to find a plant host (but see reference 146 for criticisms related to methodology). The many unanswered questions surrounding ornamentation and its potential impact on dispersal include, How does a spore’s outer morphology affect spore-to-spore aggregation, surface impaction, and dry or wet deposition (147) (Fig. 2)?
Slightly more is known about spore surface hydrophobicity. Aimanianda et al. (148) have shown that hydrophobic surface proteins on fungal spore walls allow many species to remain dormant inside animal lungs without causing an immune response. While spores rarely escape from lungs, spore hydrophobicity may protect spores in other animal cavities, e.g., the gut, and hydrophobins may enable survival over the relatively large distances covered by many animals. If spores remain undetected and viable in animal digestion tracts until excretion, spore hydrophobicity may well play a role in long-range movement of fungi within animals (149, 150).
Spore surface hydrophobicity also raises questions about whether spores can play a role in meteorological phenomena, either as cloud-condensing nuclei or ice nuclei (143, 151, 152). Spores can theoretically disperse within cloud formations, e.g., at the core of an ice particle, but how water would condense on hydrophobic spore walls remains an open question (153–155). Some kinds of plant pollen do act as cloud-condensing nuclei in high-humidity environments, despite a waxy outer layer. Pope (156) hypothesizes that the small pores found on pollen surfaces (approximately 1 μm in diameter) cause a localized reduction in vapor pressure and, as a result, capillary condensation. Similar morphologies are seen on ornamented fungal spores and, famously, on the adaxial surface of many basidiospores (143, 157–159). These structures may affect nucleation, although to date no study has tested whether spore wall ornamentation drives water condensation.
Do Spores “Clump?”
Dispersal may also be influenced by the ability of spores to aggregate, or form clumps. Clumping is reported for a variety of species, including P. graminis, P. striiformis, A. alternata (Fig. 5D), Cladosporium herbarum (Fig. 5F), Blumeria graminis, Phakopsora pachyrhizi (Fig. 5I), and H. vastatrix (Fig. 5H) (160–163). Clumping may improve individual fitness by stimulating germination (164) and may facilitate impaction on substrates by providing greater inertial mass (150, 165). Moreover, the outermost spores in airborne clumps may shield the innermost spores from harmful environmental conditions, e.g., solar radiation (163). However, whether clumping provides a net benefit to spores remains unknown. While the lower mass of a single spore may facilitate launch into turbulent air layers, the greater mass of clumped spores may shape horizontal displacement and deposition. Moreover, data on clumping are limited to a handful of fungal species (164, 165), and more research is needed to understand when, how, and how commonly spore clumps form.
The Physiological Hardiness of Spores
Fungal LDD may be constrained by the physiological tolerances of spores to solar radiation (especially UV), air moisture (relative humidity), and temperature. The resilience of a spore to any of these variables will vary according to species or taxonomic group. For example, urediniospores of P. striiformis var. tritici die quickly when exposed to high solar radiation, while ascospores of Gibberrella zeae are more susceptible to low relative humidities, and urediniospores of P. pachyrhizi cannot tolerate cold temperatures (166–168).
Because of the diversity of physiologies involved, the ability of species to withstand stresses must be tested individually. For example, if a species is hypothesized to have traveled from West Africa to Brazil by wind (cf. 73), verifying whether the spores of that particular species can withstand the UV, relative humidities, and temperatures likely encountered over the predicted path of flight is a critical and simple check on the plausibility of LDD. These kinds of data would complement evidence of inferred LDD, e.g., population genetics data, and enable a more comprehensive understanding of the likelihood of dispersal.
Sporocarp Properties Influencing LDD
Whether sporocarps influence LDD depends on the context in which dispersal occurs. Fungal species whose dispersal is animal-mediated, whether externally or internally, have evolved sporocarps with specific mechanisms to attract vectors. For example, Tuber spp. synthesize volatiles to encourage fungivory by mammals, while Phallus spp. produce foul aromas to attract insects (169–173).
Intriguingly, recent evidence suggests that sporocarps also play an active role in mediating wind dispersal. Within Ascomycetes, despite the diversity of spore and ascus apical ring shapes involved, the launch velocity of 90% of ascospores is within 2% of optimal energy conservation, so the morphology of the ascus facilitates ascospore penetration beyond the boundary layer of still air surrounding an ascocarp (Fig. 7F) (133, 136). Apothecia can also synchronize the release of spores, enabling groups of spores to move through still air to heights that could not be reached by the forcible discharge of a single spore (134). Basidiomycete mushrooms also appear to manipulate dispersal by using water evaporation from the pileus to generate convective airflows and move spores by at least several centimeters vertically (Fig. 7A) (174).
FIGURE 7.

Images of spore dispersal structures among fungi. (A) Basidiospores of Lentinula edodes carried vertically by evaporative airflows from mushroom cap (Fig. 1e of reference 174). (B) Hypogeous spore body of Tuber brumale (Wikimedia Commons Creative Commons Attribution-Share Alike 3.0 Unported [WC]). (C) Sporangium of Rhizopus oryzae releasing sporangiospores (courtesy of Andrii Gryganskyi). (D) Battarrea phalloides mushroom (Doug Collins, WC). (E) Synchronous spore release from Sclerotinia sclerotiorum apothecia (Fig. 1b of reference 135). (F) Asci of Amphisphaeria saccharicola (200). (G) Apothecia of Ascobolus scatigenus (200). (H) Typical gilled agaric mushroom with gills to increase surface area of spore-producing tissue (WC).
Less clear is whether sporocarps can control the timing of spore release to take advantage of local weather that might enhance the probability of LDD. Using a Lagrangian stochastic model, Savage et al. (175) show that fungal spores released during the hottest times of day are most likely to undergo LDD, presumably because updrafts formed by heated low-altitude air masses can lift spores into turbulent flows at higher altitudes. Extreme weather events, including thunderstorms and tornados, can also generate intense vertical updrafts and lift air into the upper troposphere. Anecdotal evidence suggests that fungi may release a greater concentration of spores just before thunderstorms (when updrafts are prevalent), and there are records of asthma outbreaks caused by fungal spores specifically prior to thunderstorms (40, 176–179). Efforts to track the timing and number of spores released during atmospheric updrafts, and in relation to other meteorological phenomena, remain an interesting direction for future research and may offer additional perspectives on the ability of fungi to manipulate LDD.
UNKNOWN AND CONFOUNDING VARIABLES: FREQUENCY OF LDD, VICARIANCE, AND CHANGING PATTERNS OF HUMAN-MEDIATED DISPERSAL
The frequency of LDD for any particular species often remains unknown. Frequent LDD requires movement to be unhindered by (i) physical barriers, (ii) lack of vectors, or (iii) unsuitable habitat. If LDD is frequent enough and occurs on a global scale, it results in what appears as a global population structure (32). Rare LDD might involve a single stochastic founding event and would be reflected in population structures where shared alleles become rare over time. Different rates of LDD are important because they result in very different dynamics when, e.g., a novel adaptive mutant (e.g., a genotype able to take advantage of a novel host) arises in one population.
When population structure is determined by geological events, rather than the movement of organisms themselves, the concept of vicariance is often invoked. Vicariance is typically defined as the fragmentation of a single population by changes in a landscape, causing limited to no gene flow between the resulting disjunct populations (180–183). Vicariance is relevant to a discussion of LDD because when data do not suggest vicariance, LDD often emerges as the default explanation for population structures. If populations appear related, despite a clear physical barrier (e.g., an ocean separating populations of the same species on two continents), LDD is often hypothesized to be the process causing the observed population structures (60, 184–187) (Fig. 4).
As discussed previously, when LDD is inferred from genetic data, the mechanism of LDD remains unknown. Natural vectors, especially wind, are typically invoked as an explanation, but the literature on vicariance and LDD may provide strong indirect evidence for the role humans play in mediating dispersal across extreme physical barriers, e.g., oceans and mountain ranges. This hypothesis is seldom discussed, but the inability of physical and/or genetic data to explain contemporary population structures for many species is highly suggestive of humans playing an expanding role in fungal LDD (20, 129, 131). As the entire field of invasion biology attests, there are different dynamics at play when humans become involved in mediating dispersal (188). Technology (e.g., commercial aircraft, ocean vessels, etc.) facilitates the movement of goods, and in recent times the spatial and temporal scales of potential fungal dispersal have clearly amplified. Understanding whether apparent gene flow is a function of changing human behaviors, and not part of an autonomous pattern more typical of the past thousands or millions of years, would usefully inform our understanding of, e.g., disease and changing patterns of biodiversity.
CONCLUSIONS: UNANSWERED QUESTIONS AND FUTURE DIRECTIONS
Many unanswered questions remain, including, What shapes the end stages of successful LDD (defined as the growth and reproduction of an individual following its dispersal) (Fig. 1)? Dispersal can mitigate intraspecific competition and parent-offspring conflict, but dispersal to new habitats also involves demographic risks, e.g., the lack of mates, inbreeding depression, and other problems of small populations. Difficulties may be especially acute for individuals establishing at the tail end of a dispersal kernel. Other unanswered questions include, Which characteristics have evolved in some fungi to optimize the likelihood of LDD, and do some fungi take advantage of stochastic meteorological events, e.g., storms, or other local environments, to facilitate LDD?
Our definition of fungal LDD simplifies comparisons among species because it provides a relative measure of LDD for any species under consideration. LDD may involve tens of meters (e.g., a soil yeast) to kilometers (e.g., a smut fungus), and exact scales depend on the measured dispersal kernel for any given fungus. While our definition does not explicitly account for differences in the number of propagules found in, e.g., a mushroom, an infected plant, a field, etc., theoretically, a well-defined dispersal kernel will scale proportionately with the number of spores released. But whether the concentration of spores at a source affects the shape of a fungal dispersal kernel remains an open question. Greater numbers of spores may change the shape of a kernel if more spores increase the likelihood of, e.g., clumping, spore-to-spore wind entrainment, and wet deposition.
As global change affects the current ecological ranges of species, and biological materials continue to be moved on a global scale, defining LDD and understanding the mechanisms by which it occurs emerge as key research priorities. Fungal LDD has the potential to impact international food security and public health, as witnessed by emerging threats to soybeans in the United States and increasing cases of mucormycosis across the globe (132, 189–192). Human-mediated dispersal may drive current fungal LDD (cf. 28), though mycologists seem reluctant to mention the likely role of humans as dispersal agents. A mushrooming realization of the breadth of fungal biodiversity suggests that much is still to be learned about fungal dispersal.
ACKNOWLEDGMENTS
J. G. is funded by a U.S. National Science Foundation Graduate Research Fellowship. A. P. gratefully acknowledges support from the Human Frontier Science Program.
The authors would also like to thank Daniel Levitis, Agnese Seminara, Martina Iapichino, and Emanuel Fiano for their editing and insights, and Andrii Gryganskyi for providing micrograph images.
REFERENCES
- 1.Nathan R. 2001. The challenges of studying dispersal. Trends Ecol Evol 16:481–483 10.1016/S0169-5347(01)02272-8. [DOI] [Google Scholar]
- 2.Finlay BJ. 2002. Global dispersal of free-living microbial eukaryote species. Science 296:1061–1063 10.1126/science.1070710. [DOI] [PubMed] [Google Scholar]
- 3.Hedlund B, Staley J. 2004. Microbial endemism and biogeography, p 225–231. In Bull AT (ed), Microbial Diversity and Bioprospecting. ASM Press, Washington, DC. [Google Scholar]
- 4.Martiny JBH, Bohannan BJM, Brown JH, Colwell RK, Fuhrman JA, Green JL, Horner-Devine MC, Kane M, Krumins JA, Kuske CR, Morin PJ, Naeem S, Ovreås L, Reysenbach AL, Smith VH, Staley JT. 2006. Microbial biogeography: putting microorganisms on the map. Nat Rev Microbiol 4:102–112 10.1038/nrmicro1341. [DOI] [PubMed] [Google Scholar]
- 5.Crum H. 1972. The geographic origins of the mosses of North America & eastern deciduous forest. J Hattori Bot Lab 35:269–298. [Google Scholar]
- 6.Frahm JP. 2008. Diversity, dispersal and biogeography of bryophytes mosses. Biodivers Conserv 17:277–284 10.1007/s10531-007-9251-x. [DOI] [Google Scholar]
- 7.McDaniel SF, Shaw AJ. 2003. Phylogeographic structure and cryptic speciation in the trans-Antarctic moss Pyrrhobryum mnioides. Evolution 57:205–215 10.1111/j.0014-3820.2003.tb00256.x. [DOI] [PubMed] [Google Scholar]
- 8.Piñeiro R, Popp M, Hassel K, Listl D, Westergaard KB, Flatberg KI, Stenøien HK, Brochmann C. 2012. Circumarctic dispersal and long-distance colonization of South America: the moss genus Cinclidium. J Biogeogr 39:2041–2051 10.1111/j.1365-2699.2012.02765.x. [DOI] [Google Scholar]
- 9.Szövényi P, Sundberg S, Shaw AJ. 2012. Long-distance dispersal and genetic structure of natural populations: an assessment of the inverse isolation hypothesis in peat mosses. Mol Ecol 21:5461–5472 10.1111/mec.12055. [DOI] [PubMed] [Google Scholar]
- 10.Wolf PG, Schneider H, Ranker TA. 2001. Geographic distributions of homosporous ferns: does dispersal obscure evidence of vicariance? J Biogeogr 28:263–270 10.1046/j.1365-2699.2001.00531.x. [DOI] [Google Scholar]
- 11.Pryer KM, Schuettpelz E, Wolf PG, Schneider H, Smith AR, Cranfill R. 2004. Phylogeny and evolution of ferns (monilophytes) with a focus on the early leptosporangiate divergences. Am J Bot 91:1582–1598 10.3732/ajb.91.10.1582. [DOI] [PubMed] [Google Scholar]
- 12.Perrie L, Brownsey P. 2007. Molecular evidence for long-distance dispersal in the New Zealand pteridophyte flora. J Biogeogr 34:2028–2038 10.1111/j.1365-2699.2007.01748.x. [DOI] [Google Scholar]
- 13.Schuettpelz E, Pryer KM. 2009. Evidence for a Cenozoic radiation of ferns in an angiosperm-dominated canopy. Proc Natl Acad Sci USA 106:11200–11205 10.1073/pnas.0811136106. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gage SH, Isard SA, Colunga-G M. 1999. Ecological scaling of aerobiological dispersal processes. Agric Meteorol 97:249–261 10.1016/S0168-1923(99)00070-2. [DOI] [Google Scholar]
- 15.Staley JT, Gosink JJ. 1999. Poles apart: biodiversity and biogeography of sea ice bacteria. Annu Rev Microbiol 53:189–215 10.1146/annurev.micro.53.1.189. [DOI] [PubMed] [Google Scholar]
- 16.Jones AM, Harrison RM. 2004. The effects of meteorological factors on atmospheric bioaerosol concentrations: a review. Sci Total Environ 326:151–180 10.1016/j.scitotenv.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 17.Vos M, Velicer GJ. 2008. Isolation by distance in the spore-forming soil bacterium Myxococcus xanthus. Curr Biol 18:386–391. [PubMed] [DOI] [PubMed] [Google Scholar]
- 18.Smith DJ, Griffin DW, McPeters RD, Ward PD, Schuerger AC. 2011. Microbial survival in the stratosphere and implications for global dispersal. Aerobiologia 27:319–332 10.1007/s10453-011-9203-5. [DOI] [Google Scholar]
- 19.Barberán A, Ladau J, Leff JW, Pollard KS, Menninger HL, Dunn RR, Fierer N. 2015. Continental-scale distributions of dust-associated bacteria and fungi. Proc Natl Acad Sci USA 112:5756–5761 10.1073/pnas.1420815112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown JKM, Hovmøller MS. 2002. Aerial dispersal of pathogens on the global and continental scales and its impact on plant disease. Science 297:537–541 10.1126/science.1072678. [DOI] [PubMed] [Google Scholar]
- 21.Bonito GM, Gryganskyi AP, Trappe JM, Vilgalys R. 2010. A global meta-analysis of Tuber ITS rDNA sequences: species diversity, host associations and long-distance dispersal. Mol Ecol 19:4994–5008 10.1111/j.1365-294X.2010.04855.x. [DOI] [PubMed] [Google Scholar]
- 22.Talbot JM, Bruns TD, Taylor JW, Smith DP, Branco S, Glassman SI, Erlandson S, Vilgalys R, Liao H-L, Smith ME, Peay KG. 2014. Endemism and functional convergence across the North American soil mycobiome. Proc Natl Acad Sci USA 111:6341–6346 10.1073/pnas.1402584111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grantham NS, Reich BJ, Pacifici K, Laber EB, Menninger HL, Henley JB, Barberán A, Leff JW, Fierer N, Dunn RR. 2015. Fungi identify the geographic origin of dust samples. PLoS One 10:e0122605 10.1371/journal.pone.0122605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shigesada N, Kawasaki K. 1997. Biological Invasions: Theory and Practice. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- 25.Cain ML, Milligan BG, Strand AE. 2000. Long-distance seed dispersal in plant populations. Am J Bot 87:1217–1227 10.2307/2656714. [DOI] [PubMed] [Google Scholar]
- 26.Clark JS. 1998. Why trees migrate so fast: confronting theory with dispersal biology and the paleorecord. Am Nat 152:204–224 10.1086/286162. [DOI] [PubMed] [Google Scholar]
- 27.Nathan R, Muller-Landau HC. 2000. Spatial patterns of seed dispersal, their determinants and consequences for recruitment. Trends Ecol Evol 15:278–285 10.1016/S0169-5347(00)01874-7. [DOI] [PubMed] [Google Scholar]
- 28.Nathan R. 2006. Long-distance dispersal of plants. Science 313:786–788 10.1126/science.1124975. [DOI] [PubMed] [Google Scholar]
- 29.Hawksworth D. 2001. The magnitude of fungal diversity: the 1.5 million species estimate revisited. Mycol Res 105:1422–1432 10.1017/S0953756201004725. [DOI] [Google Scholar]
- 30.Peay KG, Garbelotto M, Bruns TD. 2010. Evidence of dispersal limitation in soil microorganisms: isolation reduces species richness on mycorrhizal tree islands. Ecology 91:3631–3640 10.1890/09-2237.1. [DOI] [PubMed] [Google Scholar]
- 31.Hassett MO, Fischer MWF, Sugawara ZT, Stolze-Rybczynski J, Money NP. 2013. Splash and grab: biomechanics of peridiole ejection and function of the funicular cord in bird’s nest fungi. Fungal Biol 117:708–714 10.1016/j.funbio.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 32.Pringle A, Baker DM, Platt JL, Wares JP, Latgé JP, Taylor JW. 2005. Cryptic speciation in the cosmopolitan and clonal human pathogenic fungus Aspergillus fumigatus. Evolution 59:1886–1899 10.1111/j.0014-3820.2005.tb01059.x. [DOI] [PubMed] [Google Scholar]
- 33.Taylor JW, Turner E, Townsend JP, Dettman JR, Jacobson D. 2006. Eukaryotic microbes, species recognition and the geographic limits of species: examples from the kingdom Fungi. Philos Trans R Soc Lond B Biol Sci 361:1947–1963 10.1098/rstb.2006.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geml J, Tulloss RE, Laursen GA, Sazanova NA, Taylor DL. 2008. Evidence for strong inter- and intracontinental phylogeographic structure in Amanita muscaria, a wind-dispersed ectomycorrhizal basidiomycete. Mol Phylogenet Evol 48:694–701 10.1016/j.ympev.2008.04.029. [DOI] [PubMed] [Google Scholar]
- 35.Ingold CT. 1965. Spore Liberation. Clarendon Press, Oxford, United Kingdom. [PubMed] [Google Scholar]
- 36.Li D-W. 2005. Release and dispersal of basidiospores from Amanita muscaria var. alba and their infiltration into a residence. Mycol Res 109:1235–1242 10.1017/S0953756205003953. [DOI] [PubMed] [Google Scholar]
- 37.Whittier P, Wagner WH. 1971. The variation in spore size and germination in Dryopteris taxa. Am Fern J 61:123–127 10.2307/1546642. [DOI] [Google Scholar]
- 38.Brown HM, Irving KR. 1973. The size and weight of common allergenic pollens. An investigation of their number per microgram and size distribution. Acta Allergol 28:132–137 10.1111/j.1398-9995.1973.tb01319.x. [DOI] [PubMed] [Google Scholar]
- 39.Sundberg S. 2010. Size matters for violent discharge height and settling speed of Sphagnum spores: important attributes for dispersal potential. Ann Bot 105:291–300 10.1093/aob/mcp288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pringle A. 2013. Asthma and the diversity of fungal spores in air. PLoS Pathog 9:e1003371 10.1371/journal.ppat.1003371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parnell M, Burt PJA, Wilson K. 1998. The influence of exposure to ultraviolet radiation in simulated sunlight on ascospores causing black sigatoka disease of banana and plantain. Int J Biometeorol 42:22–27 10.1007/s004840050079. [DOI] [Google Scholar]
- 42.Shinn EA, Smith GW, Prospero JM, Betzer P, Hayes ML, Garrison V, Barber RT. 2000. African dust and the demise of Caribbean coral reefs. Geophys Res Lett 27:3029–3032 10.1029/2000GL011599. [DOI] [Google Scholar]
- 43.Griffin DW, Kellogg CA, Shinn EA. 2001. Dust in the wind: long range transport of dust in the atmosphere and its implications for global public and ecosystem health. Glob Change Hum Health 2:20–33 10.1023/A:1011910224374. [DOI] [Google Scholar]
- 44.Kellogg CA, Griffin DW. 2006. Aerobiology and the global transport of desert dust. Trends Ecol Evol 21:638–644 10.1016/j.tree.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 45.Schmale DG III, Ross SD. 2015. Highways in the sky: scales of atmospheric transport of plant pathogens. Annu Rev Phytopathol 53:591–611 10.1146/annurev-phyto-080614-115942. [PubMed] [DOI] [PubMed] [Google Scholar]
- 46.Barrett D. 2007. Maximizing the nutritional value of fruits & vegetables. Food Technol 61:40–44. [Google Scholar]
- 47.Ingold CT. 1953. Dispersal in Fungi. Clarendon Press, Oxford, United Kingdom. [Google Scholar]
- 48.Bullock J, Kenward R, Hails R. 2002. Dispersal Ecology. 42nd Symposium of the British Ecological Society, University of Reading, 2001. Blackwell Science, Malden, MA. [Google Scholar]
- 49.Clobert J, Baguette M, Benton TG, Bullock JM (ed). 2012. Dispersal Ecology and Evolution. Oxford University Press, Oxford, United Kingdom. 10.1093/acprof:oso/9780199608898.001.0001 [DOI] [Google Scholar]
- 50.Holmer L, Stenlid J. 1993. The importance of inoculum size for the competitive ability of wood decomposing fungi. FEMS Microbiol Ecol 12:169–176 10.1111/j.1574-6941.1993.tb00029.x. [DOI] [Google Scholar]
- 51.Prussin AJ, Marr LC, Schmale DG III, Stoll R, Ross SD. 2015. Experimental validation of a long-distance transport model for plant pathogens: application to Fusarium graminearum. Agric For Meteorol 460:1117–1121. [Google Scholar]
- 52.Rieux A, Soubeyrand S, Bonnot F, Klein EK, Ngando JE, Mehl A, Ravigne V, Carlier J, de Lapeyre de Bellaire L. 2014. Long-distance wind-dispersal of spores in a fungal plant pathogen: estimation of anisotropic dispersal kernels from an extensive field experiment. PLoS One 9:e103225 10.1371/journal.pone.0103225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aylor DE. 1986. A framework for examining inter-regional aerial transport of fungal spores. Agric Meteorol 38:263–288 10.1016/0168-1923(86)90017-1. [DOI] [Google Scholar]
- 54.Nathan R, Klein E, Robledo-Arnuncio J, Revilla E. 2012. Dispersal kernels: review, p 187–210. In Clobert J, Baguette M, Benton TG, Bullock JM (ed), Dispersal Ecology and Evolution. Oxford University Press, Oxford, United Kingdom. 10.1093/acprof:oso/9780199608898.003.0015 [DOI] [Google Scholar]
- 55.Singh RP, Hodson DP, Huerta-Espino J, Jin Y, Bhavani S, Njau P, Herrera-Foessel S, Singh PK, Singh S, Govindan V. 2011. The emergence of Ug99 races of the stem rust fungus is a threat to world wheat production. Annu Rev Phytopathol 49:465–481 10.1146/annurev-phyto-072910-095423. [DOI] [PubMed] [Google Scholar]
- 56.Dam N. 2013. Spores do travel. Mycologia 105:1618–1622 10.3852/13-035. [DOI] [PubMed] [Google Scholar]
- 57.Aylor DE, Taylor GS, Raynor GS. 1982. Long-range transport of tobacco blue mold spores. Agric Meteorol 27:217–232 10.1016/0002-1571(82)90007-3. [DOI] [Google Scholar]
- 58.Aylor DE. 2003. Spread of plant disease on a continental scale: role of aerial dispersal of pathogens. Ecology 84:1989–1997 10.1890/01-0619. [DOI] [Google Scholar]
- 59.Prussin AJ II, Li Q, Malla R, Ross SD, Schmale DG III. 2013. Monitoring the long-distance transport of Fusarium graminearum from field-scale sources of inoculum. Plant Dis 98:504–511 10.1094/PDIS-06-13-0664-RE. [DOI] [PubMed] [Google Scholar]
- 60.Rivas G-G, Zapater M-F, Abadie C, Carlier J. 2004. Founder effects and stochastic dispersal at the continental scale of the fungal pathogen of bananas Mycosphaerella fijiensis. Mol Ecol 13:471–482 10.1046/j.1365-294X.2003.02043.x. [DOI] [PubMed] [Google Scholar]
- 61.Geml J, Timling I, Robinson CH, Lennon N, Nusbaum HC, Brochmann C, Noordeloos ME, Taylor DL. 2012. An arctic community of symbiotic fungi assembled by long-distance dispersers: phylogenetic diversity of ectomycorrhizal basidiomycetes in Svalbard based on soil and sporocarp DNA. J Biogeogr 39:74–88 10.1111/j.1365-2699.2011.02588.x. [DOI] [Google Scholar]
- 62.Matheny PB, Aime MC, Bougher NL, Buyck B, Desjardin DE, Horak E, Kropp BR, Lodge DJ, Soytong K, Trappe JM, Hibbett DS. 2009. Out of the palaeotropics? Historical biogeography and diversification of the cosmopolitan ectomycorrhizal mushroom family Inocybaceae. J Biogeogr 36:577–592 10.1111/j.1365-2699.2008.02055.x. [DOI] [Google Scholar]
- 63.Peterson KR, Pfister DH, Bell CD. 2010. Cophylogeny and biogeography of the fungal parasite Cyttaria and its host Nothofagus, southern beech. Mycologia 102:1417–1425 10.3852/10-048. [DOI] [PubMed] [Google Scholar]
- 64.Zhan J, Pettway RE, McDonald BA. 2003. The global genetic structure of the wheat pathogen Mycosphaerella graminicola is characterized by high nuclear diversity, low mitochondrial diversity, regular recombination, and gene flow. Fungal Genet Biol 38:286–297 10.1016/S1087-1845(02)00538-8. [DOI] [PubMed] [Google Scholar]
- 65.Linde CC, Zhan J, McDonald BA. 2002. Population structure of Mycosphaerella graminicola: from lesions to continents. Phytopathology 92:946–955 10.1094/PHYTO.2002.92.9.946. [DOI] [PubMed] [Google Scholar]
- 66.Prospero JM. 1999. Long-term measurements of the transport of African mineral dust to the southeastern United States: implications for regional air quality. J Geophys Res 104(D13):15917–15927 10.1029/1999JD900072. [DOI] [Google Scholar]
- 67.Moulin C, Lambert CE, Dulac F, Dayan U. 1997. Control of atmospheric export of dust from North Africa by the North Atlantic oscillation. Nature 387:691–694 10.1038/42679. [DOI] [Google Scholar]
- 68.Weir-Brush JR, Garrison VH, Smith GW, Shinn EA. 2004. The relationship between gorgonian coral Cnidaria: Gorgonacea diseases and African dust storms. Aerobiologia 20:119–126 10.1023/B:AERO.0000032949.14023.3a. [DOI] [Google Scholar]
- 69.Hirst JM, Stedman OJ, Hurst GW. 1967. Long-distance spore transport: vertical sections of spore clouds over the sea. J Gen Microbiol 48:357–377 10.1099/00221287-48-3-357. [DOI] [PubMed] [Google Scholar]
- 70.Maldonado-Ramirez SL, Schmale DG III, Shields EJ, Bergstrom GC. 2005. The relative abundance of viable spores of Gibberella zeae in the planetary boundary layer suggests the role of long-distance transport in regional epidemic. Agric Meteorol 132:20–27 10.1016/j.agrformet.2005.06.007. [DOI] [Google Scholar]
- 71.Schmale DG, Ross SD, Fetters TL, Tallapragada P, Wood-Jones AK, Dingus B. 2012. Isolates of Fusarium graminearum collected 40–320 meters above ground level cause Fusarium head blight in wheat and produce trichothecene mycotoxins. Aerobiologia 28:1–11 10.1007/s10453-011-9206-2. [DOI] [Google Scholar]
- 72.Muñoz J, Felicísimo ÁM, Cabezas F, Burgaz AR, Martínez I. 2004. Wind as a long-distance dispersal vehicle in the Southern Hemisphere. Science 304:1144–1147 10.1126/science.1095210. [DOI] [PubMed] [Google Scholar]
- 73.Bowden J, Gregory PH, Johnson CG. 1971. Possible wind transport of coffee leaf rust across the Atlantic Ocean. Nature 229:500–501 10.1038/229500b0. [DOI] [PubMed] [Google Scholar]
- 74.Purdy LH. 1985. Introduction of sugarcane rust into the Americas and its spread to Florida. Plant Dis 69:689 10.1094/PD-69-689. [DOI] [Google Scholar]
- 75.Unterseher M, Jumpponen A, Opik M, Tedersoo L, Moora M, Dormann CF, Schnittler M. 2011. Species abundance distributions and richness estimations in fungal metagenomics: lessons learned from community ecology. Mol Ecol 20:275–285 10.1111/j.1365-294X.2010.04948.x. [DOI] [PubMed] [Google Scholar]
- 76.Tedersoo L, Anslan S, Bahram M, Põlme S, Riit T, Liiv I, Kõljalg U, Kisand V, Nilsson H, Hildebrand F, Bork P, Abarenkov K. 2015. Shotgun metagenomes and multiple primer pair-barcode combinations of amplicons reveal biases in metabarcoding analyses of fungi. MycoKeys 10:1–43 10.3897/mycokeys.10.4852. [DOI] [Google Scholar]
- 77.Peay KG, Schubert MG, Nguyen NH, Bruns TD. 2012. Measuring ectomycorrhizal fungal dispersal: macroecological patterns driven by microscopic propagules. Mol Ecol 21:4122–4136 10.1111/j.1365-294X.2012.05666.x. [DOI] [PubMed] [Google Scholar]
- 78.Prospero JM, Blades E, Mathison G, Naidu R. 2015. Interhemispheric transport of viable fungi and bacteria from Africa to the Caribbean with soil dust. Aerobiologia 21:1–19 10.1007/s10453-004-5872-7. [DOI] [Google Scholar]
- 79.Paugam R, Wooster M, Freitas S, Val Martin M. 2016. A review of approaches to estimate wildfire plume injection height within large-scale atmospheric chemical transport models. Atmos Chem Phys 16:907–925 10.5194/acp-16-907-2016. [DOI] [Google Scholar]
- 80.Mims SA, Mims FM III. 2004. Fungal spores are transported long distances in smoke from biomass fires. Atmos Environ 38:651–655 10.1016/j.atmosenv.2003.10.043. [DOI] [Google Scholar]
- 81.de Resende AS, Xavier RP, de Oliveira OC, Urquiaga S, Alves BJR, Boddey RM. 2006. Long-term effects of pre-harvest burning and nitrogen and vinasse applications on yield of sugar cane and soil carbon. Plant Soil 281:339–351 10.1007/s11104-005-4640-y. [DOI] [Google Scholar]
- 82.Rämä T, Nordén J, Davey ML, Mathiassen GH, Spatafora JW, Kauserud H. 2014. Fungi ahoy! Diversity on marine wooden substrata in the high North. Fungal Ecol 8:46–58 10.1016/j.funeco.2013.12.002. [DOI] [Google Scholar]
- 83.Johansen S, Hytteborn H. 2001. A contribution to the discussion of biota dispersal with drift ice and driftwood in the North Atlantic. J Biogeogr 28:105–115 10.1046/j.1365-2699.2001.00532.x. [DOI] [Google Scholar]
- 84.Hellmann L, Tegel W, Eggertsson Ó, Schweingruber FH, Blanchette R, Kirdyanov A, Gärtner H, Büntgen U. 2013. Tracing the origin of Arctic driftwood. J Geophys Res D Atmospheres 118:68–76 10.1002/jgrg.20022. [DOI] [Google Scholar]
- 85.Hayward J, Hynson NA. 2014. New evidence of ectomycorrhizal fungi in the Hawaiian Islands associated with the endemic host Pisonia sandwicensis Nyctaginaceae. Fungal Ecol 12:62–69 10.1016/j.funeco.2014.09.001. [DOI] [Google Scholar]
- 86.Nicolson TH, Johnston C. 1979. Mycorrhiza in the Gramineae. III. Glomusfasiculatus as the endophyte of pioneer grasses in maritime dunes. Trans Br Mycol Soc 72:261–268 10.1016/S0007-1536(79)80041-8. [DOI] [Google Scholar]
- 87.Koske RE, Gemma JN. 1990. VA mycorrhizae in strand vegetation of Hawaii: evidence for long-distance codispersal of plants and fungi. Am J Bot 77:466–474 10.2307/2444380. [DOI] [PubMed] [Google Scholar]
- 88.Moncalvo J-M, Buchanan PK. 2008. Molecular evidence for long distance dispersal across the Southern Hemisphere in the Ganoderma applanatum-australe species complex (Basidiomycota). Mycol Res 112:425–436 10.1016/j.mycres.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 89.Pang KL, Vrijmoed LLP, Jones EBG. 2013. Genetic variation within the cosmopolitan aquatic fungus Lignincola laevis (Microascales, Ascomycota). Org Divers Evol 13:301–309 10.1007/s13127-013-0132-8. [DOI] [Google Scholar]
- 90.Wong MKM, Goh TK, Hodgkiss IJ, Hyde KD, Ranghoo VM, Tsui CKM, Ho WH, Wong WSW, Yuen TK. 1998. Role of fungi in freshwater ecosystems. Biodivers Conserv 7:1187–1206 10.1023/A:1008883716975. [DOI] [Google Scholar]
- 91.Chauvet E, Cornut J, Sridhar KR, Selosse MA, Bärlocher F. 2016. Beyond the water column: aquatic hyphomycetes outside their preferred habitat. Fungal Ecol 19:112–127 10.1016/j.funeco.2015.05.014. [DOI] [Google Scholar]
- 92.Goh TK, Hyde KD. 1996. Biodiversity of freshwater fungi. J Ind Microbiol Biotechnol 17:328–345 10.1007/BF01574764. [DOI] [Google Scholar]
- 93.Colgan W III, Claridge AW. 2002. Mycorrhizal effectiveness of Rhizopogon spores recovered from faecal pellets of small forest-dwelling mammals. Mycol Res 106:314–320 10.1017/S0953756202005634. [DOI] [Google Scholar]
- 94.D’Alva T, Lara C, Estrada-Torres A, Castillo-Guevara C. 2007. Digestive responses of two omnivorous rodents (Peromyscus maniculatus and P. alstoni) feeding on epigeous fungus (Russula occidentalis). J Comp Physiol B 177:707–712 10.1007/s00360-007-0188-x. [DOI] [PubMed] [Google Scholar]
- 95.Greif MD, Currah RS. 2007. Patterns in the occurrence of saprophytic fungi carried by arthropods caught in traps baited with rotted wood and dung. Mycologia 99:7–19 10.1080/15572536.2007.11832595. [DOI] [PubMed] [Google Scholar]
- 96.Rudolphi J. 2009. Ant-mediated dispersal of asexual moss propagules. Bryologist 112:73–79 10.1639/0007-2745-112.1.73. [DOI] [Google Scholar]
- 97.de Vega C, Arista M, Ortiz PL, Herrera CM, Talavera S. 2011. Endozoochory by beetles: a novel seed dispersal mechanism. Ann Bot 107:629–637 10.1093/aob/mcr013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Piattoni F, Amicucci A, Iotti M, Ori F, Stocchi V, Zambonelli A. 2014. Viability and morphology of Tuber aestivum spores after passage through the gut of Sus scrofa. Fungal Ecol 9:52–60 10.1016/j.funeco.2014.03.002. [DOI] [Google Scholar]
- 99.Weseloh RM. 2003. Short and long range dispersal in the gypsy moth Lepidoptera: Lymantriidae fungal pathogen, Entomophaga maimaiga Zygomycetes: Entomophthorales. Environ Entomol 32:111–122 10.1603/0046-225X-32.1.111. [DOI] [Google Scholar]
- 100.Venkatesh MV, Joshi KR, Harjai SC, Ramdeo IN. 1975. Aspergillosis in desert locust (Schistocerka gregaria Forsk). Mycopathologia 57:135–138 10.1007/BF00551419. [DOI] [PubMed] [Google Scholar]
- 101.Whitehill JG, Lehman JS, Bonello P. 2007. Ips pini (Curculionidae: Scolytinae) is a vector of the fungal pathogen, Sphaeropsis sapinea (Coelomycetes), to Austrian pines, Pinus nigra (Pinaceae). Environ Entomol 36:114–120 10.1603/0046-225X(2007)36[114:IPCSIA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 102.Roets F, Wingfield MJ, Crous PW, Dreyer LL. 2009. Fungal radiation in the Cape Floristic region: an analysis based on Gondwanamyces and Ophiostoma. Mol Phylogenet Evol 51:111–119 10.1016/j.ympev.2008.05.041. [DOI] [PubMed] [Google Scholar]
- 103.Aylward J, Dreyer LL, Steenkamp ET, Wingfield MJ, Roets F. 2014. Panmixia defines the genetic diversity of a unique arthropod-dispersed fungus specific to Protea flowers. Ecol Evol 4:3444–3455 10.1002/ece3.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Koch FH, Smith WD. 2008. Spatio-temporal analysis of Xyleborus glabratus (Coleoptera: Curculionidae [corrected] Scolytinae) invasion in eastern U.S. forests. Environ Entomol 37:442–452 10.1093/ee/37.2.442. [DOI] [PubMed] [Google Scholar]
- 105.Lara C, Ornelas JF. 2003. Hummingbirds as vectors of fungal spores in Moussonia deppeana (Gesneriaceae): taking advantage of a mutualism? Am J Bot 90:262–269 10.3732/ajb.90.2.262. [DOI] [PubMed] [Google Scholar]
- 106.Barton CE, Phalen DN, Snowden KF. 2003. Prevalence of microsporidian spores shed by asymptomatic lovebirds: evidence for a potential emerging zoonosis. J Avian Med Surg 17:197–202 10.1647/2002-011. [DOI] [Google Scholar]
- 107.Lallo MA, Calábria P, Milanelo L. 2012. Encephalitozoon and Enterocytozoon (microsporidia) spores in stool from pigeons and exotic birds: microsporidia spores in birds. Vet Parasitol 190:418–422 10.1016/j.vetpar.2012.06.030. [DOI] [PubMed] [Google Scholar]
- 108.Alfonzo A, Francesca N, Sannino C, Settanni L, Moschetti G. 2013. Filamentous fungi transported by birds during migration across the Mediterranean sea. Curr Microbiol 66:236–242 10.1007/s00284-012-0262-9. [DOI] [PubMed] [Google Scholar]
- 109.Puechmaille SJ, Wibbelt G, Korn V, Fuller H, Forget F, Mühldorfer K, Kurth A, Bogdanowicz W, Borel C, Bosch T, Cherezy T, Drebet M, Görföl T, Haarsma AJ, Herhaus F, Hallart G, Hammer M, Jungmann C, Le Bris Y, Lutsar L, Masing M, Mulkens B, Passior K, Starrach M, Wojtaszewski A, Zöphel U, Teeling EC. 2011. Pan-European distribution of white-nose syndrome fungus (Geomyces destructans) not associated with mass mortality. PLoS One 6:e19167 10.1371/journal.pone.0019167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hayes MA. 2012. The Geomyces fungi: ecology and distribution. Bioscience 62:819–823 10.1525/bio.2012.62.9.7. [DOI] [Google Scholar]
- 111.Pounds JA, Bustamante MR, Coloma LA, Consuegra JA, Fogden MPL, Foster PN, La Marca E, Masters KL, Merino-Viteri A, Puschendorf R, Ron SR, Sánchez-Azofeifa GA, Still CJ, Young BE. 2006. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 439:161–167 10.1038/nature04246. [DOI] [PubMed] [Google Scholar]
- 112.Whittaker K, Vredenburg V. 2011. An Overview of Chytridiomycosis. Amphibiaweb, Berkeley, CA. [Google Scholar]
- 113.Olson DH, Aanensen DM, Ronnenberg KL, Powell CI, Walker SF, Bielby J, Garner TWJ, Weaver G, Group TBM, Fisher MC. 2013. Mapping the global emergence of Batrachochytrium dendrobatidis, the amphibian chytrid fungus complex. Mol Ecol 21:281–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Weldon C, du Preez LH, Hyatt AD, Muller R, Speare R. 2004. Origin of the amphibian chytrid fungus. Emerg Infect Dis 10:2100–2105 10.3201/eid1012.030804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ron SR. 2005. Predicting the distribution of the amphibian pathogen Batrachochytrium dendrobatidis in the New World. Biotropica 37:209–221 10.1111/j.1744-7429.2005.00028.x. [DOI] [Google Scholar]
- 116.Swei A, Rowley JJL, Rödder D, Diesmos MLL, Diesmos AC, Briggs CJ, Brown R, Cao TT, Cheng TL, Chong RA, Han B, Hero JM, Hoang HD, Kusrini MD, Le DTT, McGuire JA, Meegaskumbura M, Min MS, Mulcahy DG, Neang T, Phimmachak S, Rao DQ, Reeder NM, Schoville SD, Sivongxay N, Srei N, Stöck M, Stuart BL, Torres LS, Tran DTA, Tunstall TS, Vieites D, Vredenburg VT. 2011. Is chytridiomycosis an emerging infectious disease in Asia? PLoS One 6:e23179 10.1371/journal.pone.0023179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Falush D, Wirth T, Linz B, Pritchard JK, Stephens M, Kidd M, Blaser MJ, Graham DY, Vacher S, Perez-Perez GI, Yamaoka Y, Mégraud F, Otto K, Reichard U, Katzowitsch E, Wang X, Achtman M, Suerbaum S. 2003. Traces of human migrations in Helicobacter pylori populations. Science 299:1582–1585 10.1126/science.1080857. [DOI] [PubMed] [Google Scholar]
- 118.Araujo A, Reinhard KJ, Ferreira LF, Gardner SL. 2008. Parasites as probes for prehistoric human migrations? Trends Parasitol 24:112–115 10.1016/j.pt.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 119.Breurec S, Guillard B, Hem S, Brisse S, Dieye FB, Huerre M, Oung C, Raymond J, Tan TS, Thiberge JM, Vong S, Monchy D, Linz B. 2011. Evolutionary history of Helicobacter pylori sequences reflect past human migrations in Southeast Asia. PLoS One 6:e22058 10.1371/journal.pone.0022058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Slatkin M. 1987. Gene flow and the geographic structure of natural populations. Science 236:787–792 10.1126/science.3576198. [DOI] [PubMed] [Google Scholar]
- 121.Fisher MC, Koenig GL, White TJ, San-Blas G, Negroni R, Alvarez IG, Wanke B, Taylor JW. 2001. Biogeographic range expansion into South America by Coccidioides immitis mirrors New World patterns of human migration. Proc Natl Acad Sci USA 98:4558–4562 10.1073/pnas.071406098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Goebel T, Waters MR, O’Rourke DH. 2008. The late Pleistocene dispersal of modern humans in the Americas. Science 319:1497–1502 10.1126/science.1153569. [DOI] [PubMed] [Google Scholar]
- 123.Legras J-L, Merdinoglu D, Cornuet J-M, Karst F. 2007. Bread, beer and wine: Saccharomyces cerevisiae diversity reflects human history. Mol Ecol 16:2091–2102 10.1111/j.1365-294X.2007.03266.x. [DOI] [PubMed] [Google Scholar]
- 124.Milgroom MG, Lipari SE. 1995. Population differentiation in the chestnut blight fungus, Cryphonectria parasitica, in eastern North America. Phytopathology 85:155–160 10.1094/Phyto-85-155. [DOI] [Google Scholar]
- 125.Milgroom MG, Wang K, Zhou Y, Lipari SE, Kaneko S. 1996. Intercontinental population structure of the chestnut blight fungus, Cryphonectria parasitica. Mycologia 88:179–190 10.2307/3760921. [DOI] [Google Scholar]
- 126.Brasier CM, Buck KW. 2001. Rapid evolutionary changes in a globally invading fungal pathogen Dutch elm disease. Biol Invasions 3:223–233 10.1023/A:1015248819864. [DOI] [Google Scholar]
- 127.Et-touil K, Bernier L, Beaulieu J, Bérubé JA, Hopkin A, Hamelin RC. 1999. Genetic structure of Cronartium ribicola populations in eastern Canada. Phytopathology 89:915–919 10.1094/PHYTO.1999.89.10.915. [DOI] [PubMed] [Google Scholar]
- 128.Vellinga EC, Wolfe BE, Pringle A. 2009. Global patterns of ectomycorrhizal introductions. New Phytol 181:960–973 10.1111/j.1469-8137.2008.02728.x. [DOI] [PubMed] [Google Scholar]
- 129.Stukenbrock EH, Banke S, McDonald BA. 2006. Global migration patterns in the fungal wheat pathogen Phaeosphaeria nodorum. Mol Ecol 15:2895–2904 10.1111/j.1365-294X.2006.02986.x. [DOI] [PubMed] [Google Scholar]
- 130.Hovmøller MS, Yahyaoui AH, Milus EA, Justesen AF. 2008. Rapid global spread of two aggressive strains of a wheat rust fungus. Mol Ecol 17:3818–3826 10.1111/j.1365-294X.2008.03886.x. [DOI] [PubMed] [Google Scholar]
- 131.Linde CC, Zala M, McDonald BA. 2009. Molecular evidence for recent founder populations and human-mediated migration in the barley scald pathogen Rhynchosporium secalis. Mol Phylogenet Evol 51:454–464 10.1016/j.ympev.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 132.Gough FJ, Lee TS. 1985. Moisture effects on the discharge and survival of conidia of Septoria tritici. Phytopathology 75:180–182 10.1094/Phyto-75-180. [DOI] [Google Scholar]
- 133.Pringle A, Brenner MP, Fritz JA, Roper M, Seminara A. 2017. Reaching the wind: boundary layer escape as a constraint on ascomycete spore shooting, p •••–•••. In Dighton J, White JF (ed), The Fungal Community: Its Organization and Role in the Ecosystem, 4th ed. CRC Press, Boca Raton, FL. [Google Scholar]
- 134.Roper M, Seminara A, Bandi MM, Cobb A, Dillard HR, Pringle A. 2010. Dispersal of fungal spores on a cooperatively generated wind. Proc Natl Acad Sci USA 107:17474–17479 10.1073/pnas.1003577107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jenkins DG, Brescacin CR, Duxbury CV, Elliott JA, Evans JA, Grablow KR, Hillegass M, Lyon BN, Metzger GA, Olandese ML, Pepe D, Silvers G, Suresch HN, Thompson TN, Trexler CM, Williams GE, Williams NC, Williams SE. 2007. Does size matter for dispersal distance? Glob Ecol Biogeogr 16:415–425 10.1111/j.1466-8238.2007.00312.x. [DOI] [Google Scholar]
- 136.Fritz JA, Seminara A, Roper M, Pringle A, Brenner MP. 2013. A natural O-ring optimizes the dispersal of fungal spores. J R Soc Interface 10:20130187 10.1098/rsif.2013.0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kauserud H, Colman JE, Ryvarden L. 2008. Relationship between basidiospore size, shape and life history characteristics: a comparison of polypores. Fungal Ecol 1:19–23 10.1016/j.funeco.2007.12.001. [DOI] [Google Scholar]
- 138.Kauserud H, Heegaard E, Halvorsen R, Boddy L, Høiland K, Stenseth NC. 2011. Mushroom’s spore size and time of fruiting are strongly related: is moisture important? Biol Lett 7:273–276 10.1098/rsbl.2010.0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Norros V, Rannik U, Hussein T, Petäjä T, Vesala T, Ovaskainen O. 2014. Do small spores disperse further than large spores? Ecology 95:1612–1621 10.1890/13-0877.1. [DOI] [PubMed] [Google Scholar]
- 140.Hussein T, Norros V, Hakala J, Petäjä T, Aalto PP, Rannik Ü, Vesala T, Ovaskainen O. 2013. Species traits and inertial deposition of fungal spores. J Aerosol Sci 61:81–98 10.1016/j.jaerosci.2013.03.004. [DOI] [Google Scholar]
- 141.Reponen T, Willeke K, Ulevicius V, Reponen A, Grinshpun SA. 1996. Effect of relative humidity on the aerodynamic diameter and respiratory deposition of fungal spores. Atmos Environ 30:3967–3974 10.1016/1352-2310(96)00128-8. [DOI] [Google Scholar]
- 142.Tesmer J, Schnittler M. 2007. Sedimentation velocity of myxomycete spores. Mycol Prog 6:229–234 10.1007/s11557-007-0539-8. [DOI] [Google Scholar]
- 143.Roper M, Pepper RE, Brenner MP, Pringle A. 2008. Explosively launched spores of ascomycete fungi have drag-minimizing shapes. Proc Natl Acad Sci USA 105:20583–20588 10.1073/pnas.0805017105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wong LT, Yu HC, Mui KW, Chan WY. 2015. Drag constants for common indoor bioaerosols. Indoor Built Environ 24:401–413 10.1177/1420326X13515897. [DOI] [Google Scholar]
- 145.Halbwachs H, Brandl R, Bässler C. 2015. Spore wall traits of ectomycorrhizal and saprotrophic agarics may mirror their distinct lifestyles. Fungal Ecol 17:197–204 10.1016/j.funeco.2014.10.003. [DOI] [Google Scholar]
- 146.Pringle A, Vellinga E, Peay K. 2015. The shape of fungal ecology: does spore morphology give clues to a species’ niche? Fungal Ecol 17:213–216 10.1016/j.funeco.2015.04.005. [DOI] [Google Scholar]
- 147.Trunov M, Trakumas S, Willeke K, Grinshpun SA, Reponen T. 2001. Collection of bioaerosol particles by impaction: effect of fungal spore agglomeration and bounce. Aerosol Sci Technol 34:490–498 10.1080/02786820121411. [DOI] [Google Scholar]
- 148.Aimanianda V, Bayry J, Bozza S, Kniemeyer O, Perruccio K, Elluru SR, Clavaud C, Paris S, Brakhage AA, Kaveri SV, Romani L, Latgé JP. 2009. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature 460:1117–1121 10.1038/nature08264. [DOI] [PubMed] [Google Scholar]
- 149.Fisher MC, Gow NAR, Gurr SJ. 2016. Tackling emerging fungal threats to animal health, food security and ecosystem resilience. Philos Trans R Soc Lond B Biol Sci 371:20160332 10.1098/rstb.2016.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Whiteford JR, Spanu PD. 2002. Hydrophobins and the interactions between fungi and plants. Mol Plant Pathol 3:391–400 10.1046/j.1364-3703.2002.00129.x. [DOI] [PubMed] [Google Scholar]
- 151.Huffman JA, Prenni AJ, DeMott PJ, Pöhlker C, Mason RH, Robinson NH, Fröhlich-Nowoisky J, Tobo Y, Després VR, Garcia E, Gochis DJ, Harris E, Müller-Germann I, Ruzene C, Schmer B, Sinha B, Day DA, Andreae MO, Jimenez JL, Gallagher M, Kreidenweis SM, Bertram AK, Pöschl U. 2013. High concentrations of biological aerosol particles and ice nuclei during and after rain. Atmos Chem Phys 13:6151–6164 10.5194/acp-13-6151-2013. [DOI] [Google Scholar]
- 152.Iannone R, Chernoff DI, Pringle A, Martin ST, Bertram AK. 2011. The ice nucleation ability of one of the most abundant types of fungal spores found in the atmosphere. Atmos Chem Phys 11:1191–1201 10.5194/acp-11-1191-2011. [DOI] [Google Scholar]
- 153.Hassett MO, Fischer MWF, Money NP. 2015. Mushrooms as rainmakers: how spores act as nuclei for raindrops. PLoS One 10:e0140407 10.1371/journal.pone.0140407. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Morris CE, Sands DC, Glaux C, Samsatly J, Asaad S, Moukahel AR, Gonçalves FLT, Bigg EK. 2013. Urediospores of rust fungi are ice nucleation active at > −10 °C and harbor ice nucleation active bacteria. Atmos Chem Phys 13:4223–4233 10.5194/acp-13-4223-2013. [DOI] [Google Scholar]
- 155.Fröhlich-Nowoisky J, Hill TCJ, Pummer BG, Yordanova P, Franc GD, Pöschl U. 2015. Ice nucleation activity in the widespread soil fungus Mortierella alpina. Biogeosciences 12:1057–1071 10.5194/bg-12-1057-2015. [DOI] [Google Scholar]
- 156.Pope FD. 2010. Pollen grains are efficient cloud condensation nuclei. Environ Res Lett 5:44015 10.1088/1748-9326/5/4/044015. [DOI] [Google Scholar]
- 157.Buller AHR. 1909. Researches on Fungi. Longmans, Green and Co, London, United Kingdom. 10.5962/bhl.title.5397 [DOI] [Google Scholar]
- 158.Ingold CT. 1971. Fungal Spores: Their Liberation and Dispersal. Clarendon Press, Oxford, United Kingdom. [Google Scholar]
- 159.Pringle A, Patek SN, Fischer M, Stolze J, Money NP. 2005. The captured launch of a ballistospore. Mycologia 97:866–871 10.1080/15572536.2006.11832777. [DOI] [PubMed] [Google Scholar]
- 160.Sache I. 2000. Short-distance dispersal of wheat rust spores. Agronomie 20:757–767 10.1051/agro:2000102. [DOI] [Google Scholar]
- 161.McCartney HA, Bainbridge A. 1987. Deposition of Erysiphe graminis conidia on a barley crop. J Phytopathol 118:243–257 10.1111/j.1439-0434.1987.tb00453.x. [DOI] [Google Scholar]
- 162.Lacey M, West J. 2006. The Air Spora: a Manual for Catching and Identifying Airborne Biological Particles. Springer, Dordrecht, The Netherlands. 10.1007/978-0-387-30253-9 [DOI] [Google Scholar]
- 163.Leite B, Navaez D, Marois J, Wright D. 2007. Clumping of Phakopsora pachyrhizi urediniospores and its significance in spore biology. Phytopathology 97:S63. [Google Scholar]
- 164.Richard F, Glass NL, Pringle A. 2012. Cooperation among germinating spores facilitates the growth of the fungus, Neurospora crassa. Biol Lett 8:419–422 10.1098/rsbl.2011.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Nix-Stohr S, Moshe R, Dighton J. 2008. Effects of propagule density and survival strategies on establishment and growth: further investigations in the phylloplane fungal model system. Microb Ecol 55:38–44 10.1007/s00248-007-9248-8. [DOI] [PubMed] [Google Scholar]
- 166.Maddison AC, Manners JG. 1972. Sunlight and viability of cereal rust uredospores. Trans Br Mycol Soc 59:429–443 10.1016/S0007-1536(72)80124-4. [DOI] [Google Scholar]
- 167.Fernando WG, Miller JD, Seaman WL, Seifert K, Paulitz TC. 2000. Daily and seasonal dynamics of airborne spores of Fusarium graminearum and other Fusarium species sampled over wheat plots. Can J Bot 78:497–505 10.1139/b00-027. [DOI] [Google Scholar]
- 168.Park S, Chen Z-Y, Chanda AK, Schneider RW, Hollier CA. 2008. Viability of Phakopsora pachyrhizi urediniospores under simulated southern Louisiana winter temperature conditions. Plant Dis 92:1456–1462 10.1094/PDIS-92-10-1456. [DOI] [PubMed] [Google Scholar]
- 169.Borg-Karlson A-K, Englund FO, Unelius CR. 1994. Dimethyl oligosulphides, major volatiles released from Sauromatum guttatum and Phallus impudicus. Phytochemistry 35:321–323 10.1016/S0031-9422(00)94756-3. [DOI] [Google Scholar]
- 170.Pelusio F, Nilsson T, Montanarella L, Tilio R, Larsen B, Facchetti S, Madsen J. 1995. Headspace solid-phase microextraction analysis of volatile organic sulfur compounds in black and white truffle aroma. J Agric Food Chem 43:2138–2143. [Google Scholar]
- 171.Sleeman DP, Jones P, Cronin JN. 1997. Investigations of an association between the stinkhorn fungus and badger setts. J Nat Hist 31:983–992 10.1080/00222939700770481. [DOI] [Google Scholar]
- 172.Johnson SD, Jürgens A. 2010. Convergent evolution of carrion and faecal scent mimicry in fly-pollinated angiosperm flowers and a stinkhorn fungus. S Afr J Bot 76:796–807 10.1016/j.sajb.2010.07.012. [DOI] [Google Scholar]
- 173.Schigel DS. 2012. Fungivory and host associations of Coleoptera: a bibliography and review of research approaches. Mycology 3:258–272. [Google Scholar]
- 174.Dressaire E, Yamada L, Song B, Roper M. 2016. Mushrooms use convectively created airflows to disperse their spores. Proc Natl Acad Sci USA 113:2833–2838 10.1073/pnas.1509612113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Savage D, Barbetti MJ, MacLeod WJ, Salam MU, Renton M. 2012. Seasonal and diurnal patterns of spore release can significantly affect the proportion of spores expected to undergo long-distance dispersal. Microb Ecol 63:578–585 10.1007/s00248-011-9949-x. [DOI] [PubMed] [Google Scholar]
- 176.Troutt C, Levetin E. 2001. Correlation of spring spore concentrations and meteorological conditions in Tulsa, Oklahoma. Int J Biometeorol 45:64–74 10.1007/s004840100087. [DOI] [PubMed] [Google Scholar]
- 177.Burch M, Levetin E. 2002. Effects of meteorological conditions on spore plumes. Int J Biometeorol 46:107–117 10.1007/s00484-002-0127-1. [DOI] [PubMed] [Google Scholar]
- 178.Grinn-Gofroń A, Strzelczak A. 2013. Changes in concentration of Alternaria and Cladosporium spores during summer storms. Int J Biometeorol 57:759–768 10.1007/s00484-012-0604-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dales RE, Cakmak S, Judek S, Dann T, Coates F, Brook JR, Burnett RT. 2003. The role of fungal spores in thunderstorm asthma. Chest 123:745–750 10.1378/chest.123.3.745. [DOI] [PubMed] [Google Scholar]
- 180.Lieberman BS. 2005. Geobiology and paleobiogeography: tracking the coevolution of the Earth and its biota. Palaeogeogr Palaeoclimatol Palaeoecol 219:23–33 10.1016/j.palaeo.2004.10.012. [DOI] [Google Scholar]
- 181.Mao K, Milne RI, Zhang L, Peng Y, Liu J, Thomas P, Mill RR, Renner SS. 2012. Distribution of living Cupressaceae reflects the breakup of Pangea. Proc Natl Acad Sci USA 109:7793–7798 10.1073/pnas.1114319109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.De Queiroz A. 2014. The Monkey’s Voyage: How Improbable Journeys Shaped the History of Life. Basic Books, Philadelphia, PA. [Google Scholar]
- 183.Gutiérrez EE, Boria RA, Anderson RP. 2014. Can biotic interactions cause allopatry? Niche models, competition, and distributions of South American mouse opossums. Ecography 37:741–753 10.1111/ecog.00620. [DOI] [Google Scholar]
- 184.Lichtwardt RW. 1995. Biogeography and fungal systematics. Can J Bot 73(S1):731–737 10.1139/b95-316. [DOI] [Google Scholar]
- 185.Moyersoen B, Beever RE, Martin F. 2003. Genetic diversity of Pisolithus in New Zealand indicates multiple long-distance dispersal from Australia. New Phytol 160:569–579 10.1046/j.1469-8137.2003.00908.x. [DOI] [PubMed] [Google Scholar]
- 186.Coetzee MPA, Bloomer P, Wingfield MJ, Wingfield BD. 2011. Paleogene radiation of a plant pathogenic mushroom. PLoS One 6:e28545 10.1371/journal.pone.0028545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Theodoro RC, Teixeira MM, Felipe MSS, Paduan KS, Ribolla PM, San-Blas G, Bagagli E. 2012. Genus paracoccidioides: species recognition and biogeographic aspects. PLoS One 7:e37694 10.1371/journal.pone.0037694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Davis MA. 2009. Invasion Biology. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- 189.Nucci M, Marr KA. 2005. Emerging fungal diseases. Clin Infect Dis 41:521–526 10.1086/432060. [DOI] [PubMed] [Google Scholar]
- 190.Schneider RW, Hollier CA, Whitam HK, Palm ME, McKemy JM, Hernández JR, Levy L, DeVries-Paterson R. 2005. First report of soybean rust caused by Phakopsora pachyrhizi in the continental United States. Plant Dis 89:774.1. 10.1094/PD-89-0774A. [DOI] [PubMed] [Google Scholar]
- 191.Yorinori JT, Paiva WM, Frederick RD, Costamilan LM, Bertagnolli PF, Hartman GE, Godoy CV, Nunes J Jr. 2005. Epidemics of soybean rust Phakopsora pachyrhizi in Brazil and Paraguay from 2001 to 2003. Plant Dis 89:675–677 10.1094/PD-89-0675. [DOI] [PubMed] [Google Scholar]
- 192.Goellner K, Loehrer M, Langenbach C, Conrath U, Koch E, Schaffrath U. 2010. Phakopsora pachyrhizi, the causal agent of Asian soybean rust. Mol Plant Pathol 11:169–177 10.1111/j.1364-3703.2009.00589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Werth S, Wagner HH, Gugerli F, Holderegger R, Csencsics D, Kalwij JM, Scheidegger C. 2006. Quantifying dispersal and establishment limitation in a population of an epiphytic lichen. Ecology 87:2037–2046 10.1890/0012-9658(2006)87[2037:QDAELI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 194.Johnson DA, Ball TA, Hess WM. 1999. Image analysis of urediniospores that infect Mentha. Mycologia 91:1016–1020 10.2307/3761633. [DOI] [Google Scholar]
- 195.Marleau J, Dalpé Y, St-Arnaud M, Hijri M. 2011. Spore development and nuclear inheritance in arbuscular mycorrhizal fungi. BMC Evol Biol 11:51 10.1186/1471-2148-11-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wittmaack K, Wehnes H, Heinzmann U, Agerer R. 2005. An overview on bioaerosols viewed by scanning electron microscopy. Sci Total Environ 346:244–255 10.1016/j.scitotenv.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 197.Piepenbring M, Bauer R, Oberwinkler F. 1998. Teliospores of smut fungi teliospore walls and the development of ornamentation studied by electron microscopy. Protoplasma 204:170–201 10.1007/BF01280323. [DOI] [Google Scholar]
- 198.Carvalho CR, Fernandes RC, Carvalho GMA, Barreto RW, Evans HC. 2011. Cryptosexuality and the genetic diversity paradox in coffee rust, Hemileia vastatrix. PLoS One 6:e26387 10.1371/journal.pone.0026387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Dixon LJ, Castlebury LA, Aime MC, Glynn NC, Comstock JC. 2010. Phylogenetic relationships of sugarcane rust fungi. Mycol Prog 9:459–468 10.1007/s11557-009-0649-6. [DOI] [Google Scholar]
- 200.Tzean SS, Hsieh WH, Chang TT, Wu SH, Ho HM. 2015. Mycobiota Taiwanica, 3rd ed. National Taiwan University, TaiPei, Taiwan. [Google Scholar]
- 201.Liu N, Gong G, Zhang M, Zhou Y, Chen Z, Yang J, Chen H, Wang X, Lei Y, Liu K. 2012. Over-summering of wheat powdery mildew in Sichuan Province, China. Crop Prot 34:112–118 10.1016/j.cropro.2011.12.011. [DOI] [Google Scholar]
- 202.Leslie JF, Summerell BA (ed). 2006. The Fusarium Laboratory Manual. Blackwell, Ames, IA. 10.1002/9780470278376 [DOI] [Google Scholar]
- 203.Stover RH. 1963. Leaf spot of bananas caused by Mycosphaerella musicola: associated ascomycetous fungi. Can J Bot 41:1481–1485 10.1139/b63-128. [DOI] [Google Scholar]
- 204.Qandah IS, del Río Mendoza LE. 2011. Temporal dispersal patterns of Sclerotinia sclerotiorum ascospores during canola flowering. Can J Plant Pathol 33:159–167 10.1080/07060661.2011.554878. [DOI] [Google Scholar]
- 205.Aylor DE. 1992. Release of Venturia inaequalis ascospores during unsteady rain: relationship to spore transport and deposition. Phytopathology 82:532–540 10.1094/Phyto-82-532. [DOI] [Google Scholar]
- 206.Vincenot L, Nara K, Sthultz C, Labbé J, Dubois M-P, Tedersoo L, Martin F, Selosse M-A. 2012. Extensive gene flow over Europe and possible speciation over Eurasia in the ectomycorrhizal basidiomycete Laccaria amethystina complex. Mol Ecol 21:281–299 10.1111/j.1365-294X.2011.05392.x. [DOI] [PubMed] [Google Scholar]
- 207.Aylor DE, Taylor G. 1983. Escape of Peronospora tabacina spores from a field of diseased tobacco plants. Phytopathology 73:525–529 10.1094/Phyto-73-525. [DOI] [Google Scholar]
- 208.Simmonds NW. 1994. Some speculative calculations of the dispersal of sugarcane smut disease. Sugar Cane 1:2–5. [Google Scholar]
- 209.Isard SA, Gage SH, Comtois P, Russo JM. 2005. Principles of the atmospheric pathway for invasive species applied to soybean rust. Bioscience 55:851–861 10.1641/0006-3568(2005)055[0851:POTAPF]2.0.CO;2. [DOI] [Google Scholar]
- 210.Anikster Y, Eilam T, Bushnell WR, Kosman E. 2005. Spore dimensions of Puccinia species of cereal hosts as determined by image analysis. Mycologia 97:474–484 10.1080/15572536.2006.11832823. [DOI] [PubMed] [Google Scholar]
- 211.Ali S, Gladieux P, Leconte M, Gautier A, Justesen AF, Hovmøller MS, Enjalbert J, de Vallavieille-Pope C. 2014. Origin, migration routes and worldwide population genetic structure of the wheat yellow rust pathogen Puccinia striiformis f.sp. tritici. PLoS Pathog 10:e1003903 10.1371/journal.ppat.1003903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lawrence D. 2008. Batrachochytrium dendrobatidis: Chytrid Disease. Oregon State University, Corvallis, OR. [Google Scholar]


