Abstract
CBF1 is a cellular highly conserved DNA binding factor that is ubiquitously expressed in all tissues and acts as a repressor of cellular genes. In Epstein-Barr virus growth-transformed B-cell lines, CBF1 serves as a central DNA adaptor molecule for several viral proteins, including the viral transactivator Epstein-Barr virus nuclear antigen 2 (EBNA-2). EBNA-2 binds to CBF1 and thereby gains access to regulatory regions of target genes and activates transcription. We have inactivated the CBF1 gene by homologous recombination in the human B-cell line DG75 and characterized changes in cellular gene expression patterns upon loss of CBF1 and activation of EBNA-2. CBF1-negative DG75 cells were viable and proliferated at wild-type rates. Loss of CBF1 was not sufficient to release repression of the previously described EBNA-2 target genes CD21 or CCR7, whereas induction of both target genes by EBNA-2 required CBF1. In contrast, repression of immunoglobulin M by EBNA-2 was mainly CBF1 independent. CBF1-negative DG75 B cells thus provide an excellent tool to dissect CBF1-dependent and -independent functions exerted by the EBNA-2 protein in future studies.
Epstein-Barr virus (EBV) nuclear antigen 2 (EBNA-2) plays a key role in B-cell growth transformation by initiating and maintaining the proliferation of B cells upon Epstein-Barr virus infection in vitro. EBNA-2 is one of the first viral genes expressed after virus infection. By activating viral as well as cellular target genes, EBNA-2 initiates the transcription of a cascade of primary and secondary target genes, which eventually govern the activation of the resting B cell, cell cycle entry, and proliferation of the growth-transformed cells.
Since EBNA-2 lacks an intrinsic DNA binding function, it needs to gain access to target genes by using cellular adaptor proteins. So far, the best-studied cellular DNA adapter protein of EBNA-2 is CBF1, which was first identified as a downstream effector molecule of EBNA-2 in the context of viral promoter activation. CBF1 is a ubiquitously expressed protein and belongs to the group of CSL proteins CBF1, Su(H), and Lag1. CBF1 is a sequence-specific DNA binding protein which, in the absence of EBNA-2, recruits a corepressor complex to the promoter of target genes. Constituents of this corepressor complex are SMRT/N-CoR, CIR, SKIP, Sin3A, SAP30, and HDAC1, which either directly or indirectly interfere with histone acetylation of target gene chromatin, thereby repressing transcription (22, 23, 27, 55). Binding of EBNA-2 abolishes this repression by competition with corepressors as well as the recruitment of coactivator complexes (22).
EBNA-2-responsive elements within the viral LMP1 and LMP2 promoters all share functional binding motifs for CBF1 as a common denominator (17, 19, 33, 50, 57). To date, CD23, CD21, CCR7 (BLR2/EBI1), Hes-1, the proto-oncogene c-myc, AML-2 (RUNX3, CBFα-3), and BATF have been defined as direct or primary EBNA-2 target genes (3-5, 25, 26, 42, 45, 46, 52). CD23 is the human low-affinity Fc epsilon receptor II. Transcription of CD23 can be initiated from two alternative promoters, designated a and b, the former being predominantly used in B cells (54). An EBNA-2-responsive fragment could be delineated in the CD23 type a promoter and subsequently was shown to carry a functional CBF1 binding site (35, 51, 53). CD21, the complement receptor 2, is part of the B-cell receptor complex and a ligand for CD23 (12). CD21 promoter reporter constructs are activated by EBNA-2, but the relevant EBNA-2 response elements within this promoter have not been identified to date (41). The first intron of the human CD21 gene carries an intronic silencer which binds CBF1 (37, 38). Whether EBNA-2 can bind to this CBF1 site has not been described yet. The contribution of CBF1 signaling to target gene activation has been described for any of the other EBNA-2 cellular target genes.
The functional equivalent of the viral EBNA-2 protein is the activated Notch receptor. Upon ligand binding, the transmembrane receptor Notch is proteolytically processed, and an intracellular fragment (Notch-IC) translocates into the nucleus, binds to CBF1, and activates target gene expression by means of its intrinsic transactivation domain as well as by replacing corepressor complexes bound to CBF1. Notch/CBF1 signaling plays a key role in development and differentiation of multiple tissues (43). CBF1-negative mice die as embryos (7, 40). B-cell-specific disruption of the CBF1 gene causes a loss of marginal zone B-cell lineage but does not interfere with B-cell activation or maintenance (48). As a nuclear transactivator EBNA-2 shortcuts CBF1 signaling and activates a set of viral and cellular target genes, which at least partially overlap the target genes of activated Notch (15, 20, 21, 25, 47).
We have recently described the inactivation of a cellular gene, TB7, by homologous recombination in the EBV-negative Burkitt's lymphoma cell line DG75 (2, 13). Since EBV infects human B cells, EBNA-2 signaling and the impact of CBF1 function on gene expression should be preferentially studied in human B cells. We have thus inactivated the CBF1 gene in DG75. Here we show that targeted disruption of the CBF1 gene in this cell line did not cause any significant changes in the viability or proliferation of the cells. Also, loss of CBF1 did not cause activation of CD21, a gene which has been described previously to be repressed by CBF1 (38). While activation of CD21 as well as CCR7 by EBNA-2 was strictly CBF1 dependent, EBNA-2 could repress immunoglobulin M (IgM) expression efficiently even in the absence of CBF1.
MATERIALS AND METHODS
Antibodies.
In order to produce rat monoclonal antibodies specific for the CBF1 protein, human CBF1 was expressed in Escherichia coli BL21(DE3)RIL as a His/glutathione S-transferase (GST) fusion protein (VK91). The protein was purified by Ni-chelate affinity chromatography and used to immunize rats as described (32). The resulting CBF1-specific rat monoclonal antibody was designated RBP-J7A11-161. For Western blot analysis the EBNA-2-specific rat monoclonal antibody R3-1-3 (32), the novel CBF1-specific antibody RBP-J7A11-161, and the glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-specific mouse monoclonal antibody (MAB374, Chemicon) were used as primary antibodies. Rat-specific IgG-horseradish peroxidase (sc-2006, Santa Cruz) and mouse-specific IgG-horseradish peroxidase (NA931V, Amersham Biosciences) were used as secondary antibodies.
Plasmids.
To construct the targeting vectors using pBluescriptII SK- (pBSK, Stratagene) as the vector backbone, a 9-kb EcoRI fragment (1) containing exon 4 of the CBF1 gene and 6.4 and 2.6 kb of the 5′ and 3′ flanking intron sequences, respectively, was used. For negative selection a diphtheria toxin α (DTA) cassette, excised with RsrII from the vector pKO SelectDT V840 (Lexicon Genetics), was inserted in the unique PpuMI site at the 5′ end of the EcoRI fragment. A positive selection cassette flanked by two loxP sites and containing either neomycin resistance/DsRed2 or hygromycin resistance/enhanced green fluorescent protein (EGFP) replaced exon 4. Expression cassettes for neomycin resistance and DsRed2 were excised from pDsRed2-N1 (Clontech) using Bsu36I/EcoO109I and AflII/NsiI, respectively. The EGFP cassette was cut out from pEGFP-N1 (Clontech) with AflII/NsiI; an optimized hygromycin resistance cassette was taken from pTG76 (14) using BamHI/HindIII. The structures of the targeting vectors are shown in Fig. 1.
FIG. 1.
Targeted disruption of the CBF1 gene by deletion of exon 4. (a) Schematic view of the genomic structure of the CBF1 gene locus. Numbered bars represent the exons. A detailed view of the 9-kb EcoRI gene fragment encompassing exon 4 and flanking intron sequences (a, b, c, and d) used for construction of targeting constructs I, II, and III is shown as an insert. All three targeting constructs share a diphtheria toxin α expression cassette (DTA) and a dual positive selection cassette combining the neomycin (neo) or hygromycin (hyg) resistance gene with a fluorescent marker, EGFP or DsRed2, flanked by loxP sites. (b) Schematic overview of the NcoI fragments of the wild-type (F1) and the correctly targeted loci before (F2, F4, and F6) and after (F3 and F5) processing by Cre-recombinase detected by a 5′ external probe. Restriction enzyme sites: E, EcoRI; P, PpuMI; EN, EcoNI; A, ApaI; B, BsrGI; EV, EcoRV; K, KpnI; N, NcoI. (c) Southern blot of genomic DNA isolated from DG75 cellular clones, before and post targeting, digested with NcoI and hybridized to the 5′ external probe. The striped lines represent the sequences of the EcoRI fragment used for constructing the targeting vectors.
VK 60 and VK91 encode the open reading frame (ORF) of human CBF1 as His and His/GST fusion proteins, respectively, in pETM11 or pETM30 (Gunter Stier, EMBL, Germany). To obtain KG765, a plasmid encoding an EBNA-2-estrogen receptor fusion protein (ER-EBNA-2), the DsRed2 cassette from pDsRed2-N1 (Clontech) was excised using AseI/AflIII, blunted with Klenow, and ligated into the Eco47III digested p554-4 (30). The luciferase reporter plasmid pGa981-6 contains the hexamerized 50-bp EBNA-2 response element of the TP-1 promoter in front of the minimal β-globin promoter driving the luciferase gene (39). The CBF1 expression plasmid AJ247 was constructed by cloning the ORF of human CBF1 into pHACS1, a derivative of pcDNA3 with an N-terminal hemagglutinin (HA) tag. The CMVβgal plasmid was obtained from Clontech Laboratories. The EBNA-2 expression plasmid pSG5-EBNA-2 (36), carries the entire EBNA-2 open reading frame under the control of the simian virus 40 promoter element in the vector pSG5 (Stratagene).
Cell lines and culture and transfection conditions.
The EBV-negative Burkitt's lymphoma cell line DG75 (2) and the EBV-positive B-lymphocytic cell line 721 (28) were grown in RPMI 1640 medium supplemented with 10% fetal calf serum, 100 U penicillin, 100 μg streptomycin per ml, and 4 mM glutamine and maintained at 37°C in a 5% CO2 atmosphere. BJABK3 and BL41K3 cells have been described (29).
To target the CBF1 gene in DG75, 107 cells in 250 μl RPMI 1640 were transfected with 15 μg of linearized vector DNA by electroporation (250 V, 950 μF) using a Gene Pulser II (Bio-Rad). Transfectants were selected 48 h after transfection with either 0.4 mg/ml hygromycin B and/or 1.2 mg/ml G418. To delete the positive selection cassette flanked by loxP sites from correctly targeted clones, 15 μg of a Cre-recombinase expressing plasmid (pBS185, GIBCO/BRL) were transfected and the cells were seeded in single-cell dilution. Individual cell lines produced during the consecutive inactivation of the CBF1 gene are described in the Results section. The cell lines SM295 and SM296 were obtained after stable transfection of DG75 cells or the DG75 CBF1 knockout cell line SM224.9, respectively, with KG765 and selection with 1.2 mg/ml G418. To induce EBNA-2 in these cells, β-estradiol was added to the cell culture medium at a final concentration of 1 μM.
DNA isolation and Southern blots.
Genomic DNA was isolated by resuspending 107 cells in 3 ml lysis buffer (10 mM Tris/HCl, pH 8.0, 400 mM NaCl, 10 mM EDTA), adding 100 μl of 20% sodium dodecyl sulfate (SDS) and 0.2 mg/ml proteinase K, and incubation at 37°C for >2 h. One ml of 5 M NaCl was added and vortexed vigorously. After 30 min incubation on ice and centrifugation at room temperature at 2,500 × g for 30 min, the supernatant was transferred to a fresh tube and the DNA was precipitated by adding 0.6 volume of isopropanol. The DNA was washed twice with 70% ethanol, air dried briefly, and dissolved in TE (10 mM Tris, 1 mM EDTA, pH 8.0); 15 μg of genomic DNA was digested with NcoI, separated on 0.7% agarose gels in 1× Tris-acetate-EDTA, transferred to Hybond-N+ membrane (Amersham Biosciences) in 20× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) overnight, and cross-linked by baking at 80°C for 1 h. A 500-bp external probe specific for the 5′ flank was digoxigenin labeled by PCR of genomic DNA with the primers sense SM156-F9: 5′-GGTCCTTTTTTTTTTTCCCACGAAG-3′ and antisense SM156-B10:5′-TTCCTCCTCAATCCCTGCTC-3′ (AJ69VD). The filters were hybridized at 68°C overnight with 2.5 ng/ml of this probe and detected as described (9).
Western blot analysis.
For Western blot analysis total cell lysates were prepared by sonification in radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris/HCl, pH 7.5, 150 mM NaCl, 1% NP-40, 0.1% SDS, 0.5% Na-deoxycholate, 1× Complete protease inhibitor tablets). The protein concentration was determined, and 50 μg of protein were separated on Laemmli 10% polyacrylamide-SDS gels. Proteins were transferred onto a polyvinylidene difluoride membrane (Immobilon P, Millipore GmbH) and detected by using the enhanced chemiluminescence system (Amersham Biosciences) according to the manufacturer's instructions.
Reporter gene assay.
Transfections were done in triplicate and 107 cells were transfected with a mixture of 5 μg luciferase reporter plasmid pGa981-6, 1 μg CMVβgal, 5 μg pSG5-EBNA-2 or the empty vector pSG5, and 5 μg of the CBF1 expression plasmid AJ247. The DNA amounts were adjusted by adding pBluescriptII DNA. Cells were harvested 48 h after transfection, washed with phosphate-buffered saline, lysed in 100 μl LUC extraction buffer containing 10% (wt/vol) glycerine, 1% (wt/vol) Triton X-100, 2 mM EDTA, 25 mM Tris/HCl, pH 7.8, and 2 mM dithiothreitol. Cell debris was removed by centrifugation at 4°C and 16,000 × g for 15 min.
Luciferase activity was determined using 10 μl of the clarified lysates in duplicate followed by injection of 50 μl luciferase assay buffer containing 20 mM Tricine, 1.07 mM (MgCO3)4 · Mg(OH)2 · 5H2O), 2.67 mM MgSO4, 0.1 mM EDTA, 33.3 mM dithiothreitol, 270 μM coenzyme A, 470 μM luciferin, and 530 mM ATP. For the β-galactosidase assay 10 μl of the lysates in duplicate was incubated for 20 min at room temperature with 100 μl βGal assay buffer (100 mM Na-P, pH 8.0, 1% Galacton [Tropix, Bedford, MA], 1 mM MgCl2). The reaction was started by adding 50 μl of βGal acceleration buffer (0.2 M NaOH, 10% Emerald enhancer) and measured in a luminometer (LB9501, Berthold). Luciferase activity was normalized for variations in transfection efficiencies by cotransfection of a β-galactosidase reporter plasmid.
Northern blot analysis.
Total RNA was isolated using the peqGOLD TriFast kit (peqLAB, Erlangen, Germany) following the manufacturer's protocol; 5 μg of RNA was separated on 1.2% formaldehyde-agarose gels and transferred to Hybond-N+ membrane (Amersham) in 20× SSC overnight and cross-linked by baking at 80°C for 1 h. Fragments of the open reading frames of c-myc, CD21, CD23, CCR7, and CD83 were used as probes. The probe for IgM had been described (24). The probe for BATF was kindly provided by Gerhard Laux. All probes were digoxigenin labeled by Klenow polymerase or PCR according to the supplier's instructions (Roche). The filters were hybridized with 10 ng/ml of the specific probe and detected as described (9).
Electrophoretic mobility shift assay.
For preparation of nuclear extracts 4 × 107 to 5 × 107 cells were washed in ice-cold phosphate-buffered saline and centrifuged at 300 × g and 4°C for 10 min. The pellet was resuspended in 300 μl buffer A (10 mM HEPES, pH 7.9, 10 mM KCl, 1.5 mM MgCl2, 5 mM dithiothreitol, and protease inhibitors) and kept on ice for 60 min. The cell suspension was transferred to a 1-ml douncer, homogenized by douncing 20 times up and down with a tight pestle, and transferred to a microcentrifuge tube. After centrifugation at 4°C and maximal speed for 10 seconds 300 μl buffer A was added to the pellet and after brief vortexing centrifuged again as above. Nuclei were lysed by resuspending the pellet in 300 μl buffer B (20 mM HEPES, pH 7.9, 25% glycerol, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 5 mM dithiothreitol, and protease inhibitors), kept on ice for 30 min, vortexed, and centrifuged at 4°C and maximal speed for 20 min. The protein content of the supernatant was determined using Bio-Rad Protein Assay Kit II according to the manufacturer's protocol and aliquots were stored at −80°C.
The oligonucleotides Cp (SM316Cs: 5′-aagttggtgtaaacacgccgtgggaaaaaa-3′ and SM316Cas: 5′-gaaccataaattttttcccacgg-3′) were annealed by mixing equimolar ratios of sense and antisense oligonucleotides in annealing buffer (10 mM Tris/HCl, pH 7.4, 10 mM MgCl2, 50 mM NaCl), incubation at 90°C for 10 min, and cooling down to 37°C; 25 ng/μl of the annealed oligonucleotides were filled in with Klenow polymerase in the presence of [32P]dCTP (3,000 Ci/mmol) and unlabeled dATP, dGTP, and dTTP at 37°C for 1 h. The labeled probe was separated from unincorporated nucleotides using Nick Sephadex G50 columns (Amersham Biosciences). Two μg of nuclear extracts was incubated at room temperature for 30 min with 0.5 to 1 ng 32P-labeled oligonucleotide probe in a 20-μl reaction containing 10 mM HEPES, pH 7.9, 1 mM EDTA, 200 mM KCl, 4% Ficoll, 2 μg bovine serum albumin, 2 μg poly(dI-dC), 4 mM dithiothreitol, and proteinase inhibitors, with or without competitive unlabeled oligonucleotide or recombinant CBF1 protein.
His-tagged recombinant CBF1 protein was expressed in Escherichia coli BL21(DE3)RIL, purified by Ni-chelate affinity chromatography, and partially refolded in 50 mM Tris/HCl, pH 9, 1 M, arginine, 2 M NaCl, and 1% glycine. For competition assays the undiluted annealed oligonucleotides was incubated with deoxynucleoside triphosphates and Klenow fragment at 37°C for 1 h. Protein-DNA complexes were resolved on 4% polyacrylamide gels in 1× Tris-borate-EDTA buffer at room temperature for 3 h at 130 V. Gels were dried and exposed to X-ray films.
RESULTS
Inactivation of the CBF1 gene in the EBV-negative Burkitt's lymphoma cell line DG75.
The human CBF1 gene consists of 11 exons scattered over a region of almost 250 kb of the short arm of chromosome 4 (Fig. 1a). Four alternatively spliced transcripts can be generated, which differ in their first exon but share exons 2 to 11 (1). This exon/intron structure is conserved between mice and humans. Targeted disruption of exon 7 in mouse embryonic stem (ES) cells has been shown to cause a CBF1 null phenotype (7, 40). According to the published gene structure, deletion of exon 4 of the human CBF1 gene was predicted to cause a frameshift in the open reading frame of the resulting novel transcript, which encodes a truncated protein fragment of 75 amino acids. We decided to inactivate the human CBF1 gene by deletion of exon 4, thus shortening a potential residual CBF1 protein even further than in the published knockout mice.
Based on a genomic 9-kb EcoRI fragment spanning exon 4 of the CBF1 gene plus flanking homologous regions of 6.4-kb and 2.6-kb intronic sequences, three different targeting constructs were generated in order to meet the special requirements for gene targeting in somatic B cells (Fig. 1b). The targeting strategy established for inactivation of the CBF1 gene in mouse embryonic stem cells was modified in order to account for the need to target at least two alleles of the gene sequentially, the need to eliminate the marker genes postrecombination for subsequent molecular manipulations, as well as the potentially low frequency of homologous recombination events expected in a somatic B-cell line.
In all targeting constructs, exon 4 of CBF1 was replaced by a dual positive selection marker expression cassette consisting of a dominant selection marker (hygromycin resistance or neomycin resistance) as well as a fluorescent protein (DsRed2 or EGFP). These dual expression cassettes were flanked by loxP sites, which permit excision by Cre-recombinase and thus allowed us to reuse the same dominant marker gene for further rounds of homologous recombination. Use of the fluorescent protein in the dual expression cassette facilitated the identification of single-cell clones, which had successfully deleted the dominant selection marker post Cre-recombinase treatment. In order to avoid repeated targeting of the same allele, the targeting constructs were devised such that the first targeting event deleted an intronic gene fragment in the 5′ flanking region of exon 4 (Fig. 1b, denoted 5′ flanks b and c). This intronic gene fragment, deleted in the already targeted first allele, was then used for subsequent targeting steps as 5′ homologous flanking region of exon 4. In addition a negative selection marker, diphtheria toxin α (DTA), was introduced at the 5′ end of the EcoRI fragment, in order to decrease the frequency of random integrations.
DG75 cells were transfected with targeting construct I and double selected for G418 resistance and expression of DsRed2. Single-cell clones were tested for correct integration and recombination by Southern blot analysis (SM166C52 in Fig. 1c, lane 2) using a probe 5′ external to the 9-kb EcoRI fragment for detection. Correct integration of the targeting constructs was further confirmed by a 3′ external as well as an internal probe (data not shown). Next, the dual selection cassette was deleted by transient expression of Cre-recombinase in a single-cell clone, in which the first allele had been successfully disrupted. Single-cell clones which had lost expression of DsRed2 were screened by PCR for complete deletion of the positive selection cassette (data not shown) and confirmed by Southern blot analysis, as illustrated for clone SM224.9 (Fig. 1c, lane 3). Targeting construct II was then used to replace exon 4 of the second allele in SM224.9 by the dual selection cassette.
The restriction pattern of the genomic DNA proved that construct II was integrated correctly by homologous recombination. However, a fragment indicative of a residual wild-type exon 4 fragment, a potential third allele, could still be detected (Fig. 1c, lane 4, SM223E3). Again the dual selection cassette was excised by transient expression of Cre-recombinase. Single-cell clones were screened for loss of EGFP expression, tested by PCR for complete deletion of the positive selection cassette (data not shown), and confirmed by Southern blot analysis (SM267C18 in lane 5 in Fig. 1c). Finally, construct III was used to target the potential third allele in clone SM223E3 and eliminate the residual genomic wild-type fragment (SM261D11 in lane 6 in Fig. 1c). In summary, after three consecutive targeting steps, the genomic fragment encompassing exon 4 had been eliminated.
The efficiency of the targeting frequency was partially quantified for the second targeting step. The fraction of viable and EGFP-positive cells was determined by fluorescence-activated cell sorting analysis either after transfection of an pEGFP-N1 control plasmid (Clontech) in order to judge the transfection efficiency or after transfection of targeting construct II in three independent transfection experiments (Table 1). Transfection of targeting construct II resulted in 3 to 4% EGFP-expressing cells 2 days after transfection, as opposed to 66% EGFP-expressing cells after transfection of pEGFP-N1; 2 to 3 weeks posttransfection, the clones which could be expanded under selective growth conditions were counted and EGFP-expressing clones were tested by Southern blot analysis, and 5 to 7% of EGFP-positive clones that were tested for targeted integration scored positive for correct integration of the selection cassette in the CBF1 gene locus.
TABLE 1.
Targeting efficiency for the second CBF1 allele in DG75
| Expt | % GFP+a | Total no. of clones grownb | No. of GFP+ clones tested | Correct integrationc |
|---|---|---|---|---|
| A | 3 | 186 | 83 | 5 (1) |
| B | 4 | 145 | 75 | 5 (1) |
| C | 4 | 239 | 142 | 10 (2) |
Percentage of EGFP-positive cells as determined by FACS analysis 2 days after transfection.
Clones growing under selection on 96-well plates, counted 2.5 weeks later.
Retargeting of the first allele is indicated by the number in parentheses.
CBF1 protein is expressed from a single allele in DG75 cells.
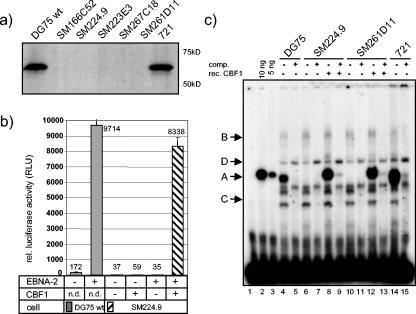
All cell lines derived by consecutive targeting of the CBF1 alleles in DG75 were perfectly viable and proliferated at wild-type rates (data not shown). We tested CBF1 expression by Western blot analysis and surprisingly found that CBF1 protein expression was already undetectable in DG75 cells after the first round of gene targeting (SM166C52 and SM224.9 in Fig. 2a). In order to confirm the absence of CBF1 protein in these cells we performed promoter reporter assays. DG75 and SM224.9 cells were cotransfected with EBNA-2 expression constructs and a luciferase reporter construct carrying six tandem CBF1 recognition motifs. Expression of EBNA-2 in DG75 cells increased the reporter gene activity up to 15-fold. In contrast, activation of the reporter construct by EBNA-2 in SM224.9 cells required coexpression of exogenous CBF1, confirming that CBF1 function was completely abolished in these cells (Fig. 2b). These findings indicated that the parental DG75 cells harbor a single functional CBF1 allele only.
FIG. 2.
SM224.9 cells are CBF1 negative and do not support EBNA-2 activation of CBF1 dependent reporter genes. (a) Whole-cell lysates prepared from the EBV positive B-cell line 721, DG75 wild-type and knockout (SM166C52, SM224.9, SM223E3, SM267C18, and SM261D11) cells were subjected to Western blot analysis by immunostaining using the CBF1-specific antibody RBP7A11-161. (b) DG75 wild-type (DG75) and knockout cells were cotransfected with a responsive CBF1 luciferase reporter plasmid (Ga981-6) and EBNA-2 (pSG5 EBNA-2) in the presence or absence of an expression vector for human CBF1 (AJ247). Results are presented as the means and standard deviations of triplicate experiments as relative light units (RLU) normalized for β-galactosidase activity. (c) Recombinant CBF1 and nuclear extracts of DG75 wild-type cells (lanes 2 and 3 and 4 and 5) were incubated with the 32P-labeled oligonucleotide from the C-promoter of EBV (Cp). Comparison with nuclear extracts from the CBF1 knockout cells SM224.9 (lanes 6 and 7) and SM261D11 (lanes 10 and 11) and after addition of recombinant CBF1 (lanes 8 and 9 and 12 and 13) reveals the position of the CBF1 complex (complex A). Nuclear extract of 721 cells was added as a control. Competition was performed with a 100-fold excess of the unlabeled Cp oligonucleotide (lanes 5, 7, 9, 11, 13, and 15). Lane 1 shows the 32P-labeled oligonucleotide alone.
For all further functional experiments the heterozygous clone SM224.9, which had lost CBF1 expression after the first round of targeting and which had deleted the selection cassette after Cre-recombinase treatment, was chosen, since we wanted to proceed to functional analyses with a cell line which had been subjected to a minimal number of manipulations.
CBF1 binding activity is abolished in SM224.9 cells.
The C promoter of EBV (Cp) carries a well-characterized CBF1 recognition site which binds CBF1 very efficiently (35). Since gel retardation experiments provide a very sensitive assay to analyze the activity of DNA binding factors, we compared nuclear extracts of DG75, SM224.9, and SM261D11 cells in gel retardation assays using the radiolabeled oligonucleotide Cp. Four protein complexes are formed by DG75 extracts (complexes A, B, C, and D; Fig. 2c, lane 4). Complexes A, B, and C could be competed by the unlabeled wild-type Cp oligonucleotide SM224.9 and SM261D11 cells lacked complex A, clearly indicating that this complex contains CBF1, while complexes B and C persisted and thus do not contain CBF1 (Fig. 2c, lanes 6 and 10). Formation of complex A by CBF1 was further confirmed by the addition of recombinant CBF1 protein to the extracts of the knockout cells (Fig. 2c, lanes 8 and 12).
Since complex C was competed by all, even completely unrelated, oligonucleotides tested (Fig. 2c and data not shown), this complex appeared to bind sequence unspecifically and thus was not analyzed further. In contrast, complex B was competed by Cp but also by other oligonucleotides that do not carry a CBF1 site (data not shown). This result suggested that the Cp oligonucleotide bound to an additional protein unrelated to CBF1 (to be published elsewhere). In summary, our results show that knockout cell lines SM224.9 and SM261D11 show the same pattern of complexes in gel retardation assays which can be reconstituted to wild-type patterns by the addition of recombinant purified CBF1 protein.
Induction of CD21 and CCR7 by EBNA-2 requires CBF1, while repression of IgM and c-Myc is not strictly dependent on CBF1.
CBF1 has been described to be a repressor which recruits histone deacetylases to the chromatin of target genes. In order to identify potential phenotypic alterations caused by the loss of repression by CBF1, a series of B-cell differentiation markers were tested by fluorescence-activated cell sorting analysis. We could not identify any significant alteration of CD10, CD19, CD20, CD21, CD23, CD30, CD32, CD37, CD38, CD39, CD44, CD48, CD70, CD77, CD79 a or b, CD80, CD83, CD86, CD95, or major histocompatibility complex (MHC) class I and II, cell surface expression in CBF1-negative cells. Importantly CD21, a B-cell activation marker, previously defined as a CBF1-repressed gene in K562 cells, was not induced upon loss of CBF1 in SM224.9 cells (data not shown).
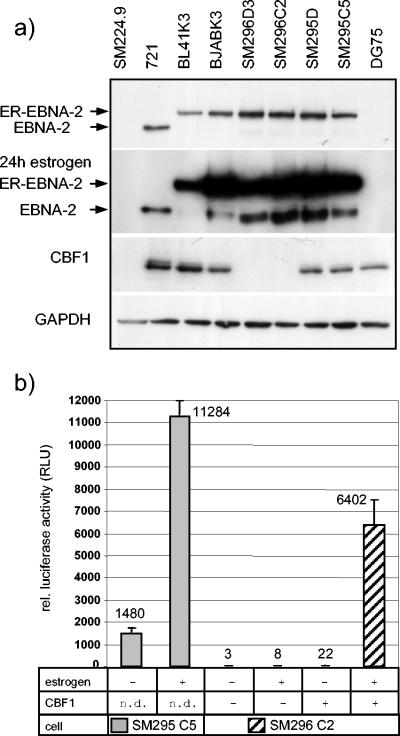
Northern blot analysis of SM244.9 cells and the complete knockout cell line SM261D11 also showed no induction of CD21 or CCR7 expression (see Fig. 4a, left). Since CD21 has been described to be an EBNA-2 target gene, we tested whether CD21 can be induced by EBNA-2 in our DG75 cellular background. We generated stable transfectants of DG75 and SM224.9 expressing an inducible ER-EBNA-2 fusion protein. This ER-EBNA-2 fusion protein is only active in the presence of estrogen in the cell culture medium (29, 30). DG75 ER-EBNA-2 transfectants (single-cell clones SM295 D6 and C5), SM224.9 ER-EBNA-2 transfectants (single-cell clones SM296 D3 and C2), and the previously published ER-EBNA-2 transfectants BL41K3 and BJABK3 were analyzed for EBNA-2 and CBF1 expression (24, 31). All transfectants express an estrogen receptor (ER)-EBNA-2 protein of the expected size which is seen at elevated levels in the presence of estrogen. Estrogen also causes the appearance of an EBNA-2 fragment similar in size to the EBNA-2 wild-type protein detected in 721. This fragment is frequently seen in ER-EBNA-2 expressing cells upon estrogen induction. ER-EBNA-2 expression is most prominent in the DG75 transfectants. The same lysates were also tested for GAPDH protein expression in order to control for equal loading of all lanes (Fig. 3a).
FIG. 4.
Induction of CD21 and CCR7 expression by EBNA-2 requires CBF1, while EBNA-2 downregulation of IgM and c-Myc is only partially CBF1 dependent. Different B-cell lines and ER-EBNA-2 transfectants which had been cultivated in the presence of estrogen for various time periods were tested for CD21 and CCR7 (a), CD23 and BATF (b), and IgM and c-Myc (c) expression. The amount of RNA loaded onto each lane is visualized by the ethidium bromide staining of the 28S rRNA below each blot.
FIG. 3.
Conditional activation of EBNA-2 in DG75 and SM224.9 (a) Total cell lysates of ER-EBNA-2 expressing DG75 (SM295D6 and C5), SM224.9 (SM296D3 and C2), BL41 (BL41K3) and BJAB (BJABK3) cells, and from 721, DG75 and 224.9 cells were tested for EBNA-2, CBF1 and GAPDH expression by Western blot analysis. (b) SM295 C5 and SM296 C2 cells were tested for activation of the responsive CBF1 luciferase reporter construct (Ga981-6) upon activation of ER-EBNA-2 by addition of estrogen to the cell culture medium and expression of human CBF1 (AJ247) where indicated. Results of a representative experiment, presented as the means and standard deviations of triplicate experiments are given as relative light units (RLU) normalized for β-galactosidase activity.
Two ER-EBNA-2 transfectants either positive or negative for CBF1 were tested for ER-EBNA-2 activation by estrogen in transient reporter gene assays using a CBF1-responsive reporter plasmid. One representative experiment is shown in Fig. 3b. Addition of β-estradiol to SM295 cells increased the reporter gene activity up to 7.6-fold (Fig. 4). As expected, activation of the reporter construct in SM296 cells required not only β-estradiol but also coexpression of exogenous CBF1 (Fig. 3b). The results obtained for cell lines SM295 and SM296 in the absence of β-estradiol but presence of endogenous or exogenous CBF1 are not identical. The elevated activities in cell line SM295 could indicate a residual background activity of the conditional system or reflect differential transfection efficiencies of the two cell lines SM295 and SM296. A more stringent test for conditional ER/EBNA-2 activity can be performed by assaying the activation of EBNA-2 endogenous target genes in response to estrogen.
Induction of endogenous target genes by EBNA-2 was then compared in SM295, SM296, BJABK3, and BL41K3 upon estrogen induction. In parallel, DG75 and SM224.9 and RNA isolated from the EBV-positive B-cell line 721 were analyzed in Northern blots. CD21 and CCR7 were induced by ER-EBNA-2 in all cell lines, but induction was strictly dependent on CBF1 expression (Fig. 4a). While CD21 shows a strong induction in BJABK3 and BL41K3 at 6 h post-estrogen treatment, the induction in the DG75 ER-EBNA-2 transfectant SM295 C5 was weaker and seen only 48 h postinduction. Induction levels varied significantly for CCR7, ranging from strong induction in BL41K3 and weak induction levels in SM295 DG75 transfectants to hardly detectable levels in BJABK3. Neither CD21 nor CCR7 were induced in untransfected DG75 cells by estrogen (data not shown). CD23 and BATF were induced in BL41K3 but not in BJABK3, SM295, and SM296. CD83, which has recently been shown to be an LMP1 target gene, scored as an EBNA-2 target gene in our experiments as well (8). Induction was transient and most prominently seen in BL41K3 while less pronounced in BJABK3 and undetectable in DG75 transfectants. Thus, unfortunately, the requirement for CBF1 in CD23, CD83, or BATF induction could not be tested in our system, since the cellular background of DG75 is not permissive for induction of these genes (Fig. 4b).
We have previously described that EBNA-2 can downregulate IgM expression. In Burkitt's lymphoma cell lines which carry the t(8;14) chromosomal translocation juxtaposing the c-myc and the IgM heavy-chain locus, c-myc transcription is coregulated and also repressed. In BJAB, a non-Burkitt's lymphoma cell line, IgM repression and c-myc expression are uncoupled (24). In order to analyze whether downregulation of IgM by EBNA-2 is CBF1 dependent, we analyzed DG75 wild-type and knockout cell lines in parallel with BL41K3 and BJABK3. IgM expression was completely downregulated in SM295 and to a significant extent in SM296 cells, indicating that this function of EBNA-2 is at least partially CBF1 independent. Expression of c-myc was coregulated in all Burkitt's lymphoma cell lines, while IgM and c-myc expression was uncoupled in BJABK3 cells, and indeed c-myc could be induced by EBNA-2 (Fig. 4c).
DISCUSSION
Here we show that CBF1 can be inactivated in the Burkitt's lymphoma cell line DG75 and prove the loss of CBF1 in these cells by three independent experimental approaches, Western blot, electrophoretic mobility shift assay, and reporter gene assays. Since a limited number of clones were obtained, only a semiquantitative assessment of targeting efficiencies could be performed. Within the limits of these experiments, we report here targeting efficiencies in DG75 cells were about 5% for those clones that grew under selection conditions and expressed the fluorescent marker genes. These targeting frequencies are gene specific, since in a recent parallel study up to 69% of the clones were shown to carry correctly integrated targeting constructs (13). Previous studies describing the inactivation of target genes in other B-cell lines, Nalm-6 and BL2, have reported frequencies of approximately 1 to 2% (10, 16). Unexpectedly, inactivation of the first allele abolished protein expression of CBF1, but two consecutive further rounds of gene targeting were required to delete the wild-type genomic fragment. We assume, but have not analyzed in detail, that the second and third allele of CBF1 were nonfunctional due to genetic alterations in DG75.
In our hands only a minority of the integration events retargeted the first allele. This fact might indicate that our targeting strategy, which aimed at preventing targeting of the identical allele by using alternate homologous flanks of the targeted region, was successful.
Next, targeting constructs which had been efficiently used to target CBF1 in DG75 cells were also tested for inactivation of CBF1 in BJAB, 721, or BL41. However, we were unable to identify correctly targeted clones after transfection of these cell lines. Transfection efficiencies were not significantly reduced in those other cell lines when tested by transfection of the cytomegaloviru-driven EGFP expression construct. In contrast, EGFP-expressing cells could only be detected at significant levels 2 days after transfection, when the targeting constructs were introduced into DG75 cells. Formally, we cannot exclude that the failure to inactivate CBF1 in cell lines other than DG75 was due to polymorphisms of the genomic loci of these cells. However, nonisogenic targeting constructs have been successfully used before and thus do not seem to be a general problem in gene targeting approaches (44).
In summary, DG75 cells appear to be exceptionally well suited as a somatic human B-cell line for targeted gene inactivation. Transfection efficiencies and survival rates posttransfection might contribute to targeting efficiencies but cannot solely account for this phenotype, suggesting that an unknown genetic lesion which promotes homologous recombination might have occurred in DG75. A drawback of this cellular system is the fact that targeting efficiencies in DG75 can vary depending on the gene locus which is targeted and minor genetic lesions, like the third allele of CBF1 in the genome of DG75, can hamper the interpretation of the results.
Inactivation of CBF1 in DG75 cells did not interfere with viability or proliferation rate of the cells. Our results are in concurrence with previously published findings showing that only a limited subpopulation of B cells, the marginal zone B cells, require CBF1 for development (49). In contrast, proliferation of EBV-infected B cells can be abolished by peptides which block EBNA-2/CBF1 signaling, proving that this signal transduction pathway is essential in EBV growth-transformed B cells (11). However, the proliferation of EBV-infected B cells is controlled by the concerted action of EBV proteins, including EBNA-2, which target CBF1. In contrast, proliferation of the Burkitt's lymphoma cell line DG75 is driven by a growth program initiated by activated Myc, which is independent of CBF1 function.
Notably, neither CD21 nor CCR7 was induced in the SM224.9 cell line or the complete knockout cell line SM261D11, indicating that abolishing repression is not sufficient to induce expression of these genes in the cellular background of the DG75 cell line. Obviously, other cellular pathways dominate the expression of target genes like CD21 and CCR7 in DG75. Since repression of CD21 has been previously described in K562 cells, our results suggest that the cellular background might affect CD21 expression (38). In general BL41 supports the induction of cellular target genes by EBNA-2 more efficiently than DG75. Induction of all cellular target genes by EBNA-2 was weaker in DG75 cells compared to BL41. CD23 or BATF induction was seen in BL41K3 cells, only, while CD83 was induced in BJABK3 and BL41K3. CD83 has been previously described to be activated by the viral LMP-1 protein via activation of the NF-κB pathway (8). Activation of cellular target genes by EBNA-2 and by LMP-1 has also been described for CD23, and we show here again that activation of cellular target genes by both proteins is not mutually exclusive for CD83 induction.
It has been shown before that the cellular context can significantly influence target gene activation in response to EBNA-2 (6, 51). We report here that DG75 expresses CBF1 from a single allele. In fact, CBF1 protein levels reach about half the levels seen in 721 and BL41 but equal the levels seen in BJAB. Thus, limiting amounts of CBF1 might partially contribute to the weak EBNA-2 responses seen in DG75 but other epigenetic factors might influence target gene activation as well. Further analysis of cis-regulatory EBNA-2-responsive elements within these genes will be required to fully elucidate the requirements for gene transactivation.
Repression of IgM by EBNA-2 was readily seen in all cell lines tested. As described before, in Burkitt's lymphoma cell lines, IgM repression correlated with c-myc repression (24). Unlike our previous studies, which indicated that c-myc expression is not affected in the non-Burkitt's lymphoma cell line BJAB, we can show now that c-myc is even upregulated in response to EBNA-2, providing strong evidence that IgM and c-myc are coregulated in Burkitt's lymphoma cells while regulation is uncoupled in BJAB cells. Importantly, in this study we have shown that this function of EBNA-2 is mainly CBF1 independent. This result was unexpected, since it has been shown recently that constitutively activated Notch can downregulate IgM (47). However, CBF1 is not the only common denominator of Notch or EBNA-2 signaling. Thus, further common interaction partners of both Notch and EBNA-2, such as SKIP (55, 56) or Nur77 (34), could potentially contribute to this function. A genomewide screen for EBNA-2 target genes dissecting CBF1-dependent and -independent functions can now be performed in the near future.
The inactivation of cellular genes by homologous recombination in somatic cell lines of specific origin is a potent strategy to facilitate the biochemical analysis of signal transduction pathways, in particular when ubiquitously expressed proteins are essential transducers. CBF1 is a signal-transducing protein which is targeted by several viral factors. Apart from EBNA-2, the EBV proteins EBNA-3A, -B, and -C and RPMS1, the Kaposi’s sarcoma-associated herpesvirus protein Rta, and the adenoviral 13SE1A can bind to CBF1 (18). The contribution of CBF1 signaling to the functions of each of these viral factors as well as to the function of cellular proteins such as Notch or SKIP can now be analyzed in the context of a human B-cell line.
Acknowledgments
We thank Tasuko Honjo for the human genomic clone 5CB, Roland Schmidt for the human CBF1 cDNA clone, Gerhard Laux for the BATF molecular probe, and Karen Henning for recombinant His-tagged CBF1 protein.
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 455), Die Deutsche Krebshilfe (grant 10-1963-Ke-I), and Wilhelm-Sanderstiftung (grant 2003. 143.1) to B.K.
REFERENCES
- 1.Amakawa, R., W. Jing, K. Ozawa, N. Matsunami, Y. Hamaguchi, F. Matsuda, M. Kawaichi, and T. Honjo. 1993. Human Jk recombination signal binding protein gene (IGKJRB): comparison with its mouse homologue. Genomics 17:306-315. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Bassat, H., N. Goldblum, S. Mitrani, T. Goldblum, J. M. Yoffey, M. M. Cohen, Z. Bentwich, B. Ramot, E. Klein, and G. Klein. 1977. Establishment in continuous culture of a new type of lymphocyte from a “Burkitt like” malignant lymphoma (line D.G.-75). Int. J. Cancer 19:27-33. [DOI] [PubMed] [Google Scholar]
- 3.Burgstahler, R., B. Kempkes, K. Steube, and M. Lipp. 1995. Expression of the chemokine receptor BLR2/EBI1 is specifically transactivated by Epstein-Barr virus nuclear antigen 2. Biochem. Biophys. Res. Commun. 215:737-743. [DOI] [PubMed] [Google Scholar]
- 4.Calender, A., M. Cordier, M. Billaud, and G. M. Lenoir. 1990. Modulation of cellular gene expression in B lymphoma cells following in vitro infection by Epstein-Barr virus (EBV). Int. J. Cancer 46:658-663. [DOI] [PubMed] [Google Scholar]
- 5.Cordier, M., A. Calender, M. Billaud, U. Zimber, G. Rousselet, O. Pavlish, J. Banchereau, T. Tursz, G. Bornkamm, and G. M. Lenoir. 1990. Stable transfection of Epstein-Barr virus (EBV) nuclear antigen 2 in lymphoma cells containing the EBV P3HR1 genome induces expression of B-cell activation molecules CD21 and CD23. J. Virol. 64:1002-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cordier-Bussat, M., M. Billaud, A. Calender, and G. M. Lenoir. 1993. Epstein-Barr virus (EBV) nuclear-antigen-2-induced up-regulation of CD21 and CD23 molecules is dependent on a permissive cellular context. Int J. Cancer 53:153-160. [DOI] [PubMed] [Google Scholar]
- 7.de la Pompa, J. L., A. Wakeham, K. M. Correia, E. Samper, S. Brown, R. J. Aguilera, T. Nakano, T. Honjo, T. W. Mak, J. Rossant, and R. A. Conlon. 1997. Conservation of the Notch signalling pathway in mammalian neurogenesis. Development 124:1139-1148. [DOI] [PubMed] [Google Scholar]
- 8.Dudziak, D., A. Kieser, U. Dirmeier, F. Nimmerjahn, S. Berchtold, A. Steinkasserer, G. Marschall, W. Hammerschmidt, G. Laux, and G. W. Bornkamm. 2003. Latent membrane protein 1 of Epstein-Barr virus induces CD83 by the NF-κB signaling pathway. J. Virol. 77:8290-8298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engler-Blum, G., M. Meier, J. Frank, and G. A. Muller. 1993. Reduction of background problems in nonradioactive northern and Southern blot analyses enables higher sensitivity than 32P-based hybridizations. Anal. Biochem. 210:235-244. [DOI] [PubMed] [Google Scholar]
- 10.Faili, A., S. Aoufouchi, Q. Gueranger, C. Zober, A. Leon, B. Bertocci, J. C. Weill, and C. A. Reynaud. 2002. AID-dependent somatic hypermutation occurs as a DNA single-strand event in the BL2 cell line. Nat. Immunol. 3:815-821. [DOI] [PubMed] [Google Scholar]
- 11.Farrell, C. J., J. M. Lee, E. C. Shin, M. Cebrat, P. A. Cole, and S. D. Hayward. 2004. Inhibition of Epstein-Barr virus-induced growth proliferation by a nuclear antigen EBNA2-TAT peptide. Proc. Natl. Acad. Sci. USA 101:4625-4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fearon, D. T., and M. C. Carroll. 2000. Regulation of B lymphocyte responses to foreign and self-antigens by the CD19/CD21 complex. Annu. Rev. Immunol. 18:393-422. [DOI] [PubMed] [Google Scholar]
- 13.Feederle, R., H. J. Delecluse, J. P. Rouault, A. Schepers, and W. Hammerschmidt. 2004. Efficient somatic gene targeting in the lymphoid human cell line DG75. Gene 343:91-97. [DOI] [PubMed] [Google Scholar]
- 14.Giordano, T. J., and W. T. McAllister. 1990. Optimization of the hygromycin B resistance-conferring gene as a dominant selectable marker in mammalian cells. Gene 88:285-288. [DOI] [PubMed] [Google Scholar]
- 15.Gordadze, A. V., R. Peng, J. Tan, G. Liu, R. Sutton, B. Kempkes, G. W. Bornkamm, and P. D. Ling. 2001. Notch1IC partially replaces EBNA2 function in B cells immortalized by Epstein-Barr virus. J. Virol. 75:5899-5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grawunder, U., D. Zimmer, S. Fugmann, K. Schwarz, and M. R. Lieber. 1998. DNA ligase IV is essential for V(D)J. recombination and DNA double-strand break repair in human precursor lymphocytes. Mol. Cell 2:477-484. [DOI] [PubMed] [Google Scholar]
- 17.Grossman, S. R., E. Johannsen, X. Tong, R. Yalamanchili, and E. Kieff. 1994. The Epstein-Barr virus nuclear antigen 2 transactivator is directed to response elements by the J. kappa recombination signal binding protein. Proc. Natl. Acad. Sci. USA 91:7568-7572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayward, S. D. 2004. Viral interactions with the Notch pathway. Semin. Cancer Biol. 14:387-396. [DOI] [PubMed] [Google Scholar]
- 19.Henkel, T., P. D. Ling, S. D. Hayward, and M. G. Peterson. 1994. Mediation of Epstein-Barr virus EBNA2 transactivation by recombination signal-binding protein J kappa. Science 265:92-95. [DOI] [PubMed] [Google Scholar]
- 20.Hofelmayr, H., L. J. Strobl, G. Marschall, G. W. Bornkamm, and U. Zimber-Strobl. 2001. Activated Notch1 can transiently substitute for EBNA2 in the maintenance of proliferation of LMP1-expressing immortalized B cells. J. Virol. 75:2033-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofelmayr, H., L. J. Strobl, C. Stein, G. Laux, G. Marschall, G. W. Bornkamm, and U. Zimber-Strobl. 1999. Activated mouse Notch1 transactivates Epstein-Barr virus nuclear antigen 2-regulated viral promoters. J. Virol. 73:2770-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsieh, J. J., and S. D. Hayward. 1995. Masking of the CBF1/RBPJ kappa transcriptional repression domain by Epstein-Barr virus EBNA2. Science 268:560-563. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh, J. J., S. Zhou, L. Chen, D. B. Young, and S. D. Hayward. 1999. CIR, a corepressor linking the DNA binding factor CBF1 to the histone deacetylase complex. Proc. Natl. Acad. Sci. USA 96:23-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jochner, N., D. Eick, U. Zimber-Strobl, M. Pawlita, G. W. Bornkamm, and B. Kempkes. 1996. Epstein-Barr virus nuclear antigen 2 is a transcriptional suppressor of the immunoglobulin mu gene: implications for the expression of the translocated c-myc gene in Burkitt's lymphoma cells. EMBO J. 15:375-382. [PMC free article] [PubMed] [Google Scholar]
- 25.Johansen, L. M., C. D. Deppmann, K. D. Erickson, W. F. Coffin, 3rd, T. M. Thornton, S. E. Humphrey, J. M. Martin, and E. J. Taparowsky. 2003. EBNA2 and activated Notch induce expression of BATF. J. Virol. 77:6029-6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaiser, C., G. Laux, D. Eick, N. Jochner, G. W. Bornkamm, and B. Kempkes. 1999. The proto-oncogene c-myc is a direct target gene of Epstein-Barr virus nuclear antigen 2. J. Virol. 73:4481-4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kao, H. Y., P. Ordentlich, N. Koyano-Nakagawa, Z. Tang, M. Downes, C. R. Kintner, R. M. Evans, and T. Kadesch. 1998. A histone deacetylase corepressor complex regulates the Notch signal transduction pathway. Genes Dev. 12:2269-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kavathas, P., F. H. Bach, and R. DeMars. 1980. Gamma ray-induced loss of expression of HLA and glyoxalase I alleles in lymphoblastoid cells. Proc. Natl. Acad. Sci. USA 77:4251-4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kempkes, B., M. Pawlita, U. Zimber-Strobl, G. Eissner, G. Laux, and G. W. Bornkamm. 1995. Epstein-Barr virus nuclear antigen 2-estrogen receptor fusion proteins transactivate viral and cellular genes and interact with RBP-J kappa in a conditional fashion. Virology 214:675-679. [DOI] [PubMed] [Google Scholar]
- 30.Kempkes, B., D. Spitkovsky, P. Jansen-Durr, J. W. Ellwart, E. Kremmer, H. J. Delecluse, C. Rottenberger, G. W. Bornkamm, and W. Hammerschmidt. 1995. B-cell proliferation and induction of early G1-regulating proteins by Epstein-Barr virus mutants conditional for EBNA2. EMBO J. 14:88-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kempkes, B., U. Zimber-Strobl, G. Eissner, M. Pawlita, M. Falk, W. Hammerschmidt, and G. W. Bornkamm. 1996. Epstein-Barr virus nuclear antigen 2 (EBNA2)-oestrogen receptor fusion proteins complement the EBNA2-deficient Epstein-Barr virus strain P3HR1 in transformation of primary B cells but suppress growth of human B-cell lymphoma lines. J. Gen. Virol. 77:227-237. [DOI] [PubMed] [Google Scholar]
- 32.Kremmer, E., B. R. Kranz, A. Hille, K. Klein, M. Eulitz, G. Hoffmann-Fezer, W. Feiden, K. Herrmann, H. J. Delecluse, G. Delsol, G. W. Bornkamm, N. Mueller-Lantzsch, and F. A. Grassert. 1995. Rat monoclonal antibodies differentiating between the Epstein-Barr virus nuclear antigens 2A (EBNA2A) and 2B (EBNA2B). Virology 208:336-342. [DOI] [PubMed] [Google Scholar]
- 33.Laux, G., F. Dugrillon, C. Eckert, B. Adam, U. Zimber-Strobl, and G. W. Bornkamm. 1994. Identification and characterization of an Epstein-Barr virus nuclear antigen 2-responsive cis element in the bidirectional promoter region of latent membrane protein and terminal protein 2 genes. J. Virol. 68:6947-6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee, J. M., K. H. Lee, M. Weidner, B. A. Osborne, and S. D. Hayward. 2002. Epstein-Barr virus EBNA2 blocks Nur77-mediated apoptosis. Proc. Natl. Acad. Sci. USA 99:11878-11883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ling, P. D., J. J. Hsieh, I. K. Ruf, D. R. Rawlins, and S. D. Hayward. 1994. EBNA-2 upregulation of Epstein-Barr virus latency promoters and the cellular CD23 promoter utilizes a common targeting intermediate, CBF1. J. Virol. 68:5375-5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ling, P. D., D. R. Rawlins, and S. D. Hayward. 1993. The Epstein-Barr virus immortalizing protein EBNA-2 is targeted to DNA by a cellular enhancer-binding protein. Proc. Natl. Acad. Sci. USA 90:9237-9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Makar, K. W., C. T. Pham, M. H. Dehoff, S. M. O'Connor, S. M. Jacobi, and V. M. Holers. 1998. An intronic silencer regulates B lymphocyte cell- and stage-specific expression of the human complement receptor type 2 (CR2, CD21) gene. J. Immunol. 160:1268-1278. [PubMed] [Google Scholar]
- 38.Makar, K. W., D. Ulgiati, J. Hagman, and V. M. Holers. 2001. A site in the complement receptor 2 (CR2/CD21) silencer is necessary for lineage specific transcriptional regulation. Int. Immunol. 13:657-664. [DOI] [PubMed] [Google Scholar]
- 39.Minoguchi, S., Y. Taniguchi, H. Kato, T. Okazaki, L. J. Strobl, U. Zimber-Strobl, G. W. Bornkamm, and T. Honjo. 1997. RBP-L, a transcription factor related to RBP-Jκ. Mol. Cell. Biol. 17:2679-2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oka, C., T. Nakano, A. Wakeham, J. L. de la Pompa, C. Mori, T. Sakai, S. Okazaki, M. Kawaichi, K. Shiota, T. W. Mak, and T. Honjo. 1995. Disruption of the mouse RBP-J. kappa gene results in early embryonic death. Development 121:3291-3301. [DOI] [PubMed] [Google Scholar]
- 41.Radkov, S. A., M. Bain, P. J. Farrell, M. West, M. Rowe, and M. J. Allday. 1997. Epstein-Barr virus EBNA3C represses Cp, the major promoter for EBNA expression, but has no effect on the promoter of the cell gene CD21. J. Virol. 71:8552-8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakai, T., Y. Taniguchi, K. Tamura, S. Minoguchi, T. Fukuhara, L. J. Strobl, U. Zimber-Strobl, G. W. Bornkamm, and T. Honjo. 1998. Functional replacement of the intracellular region of the Notch1 receptor by Epstein-Barr virus nuclear antigen 2. J. Virol. 72:6034-6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schweisguth, F. 2004. Notch signaling activity. Curr. Biol. 14:R129-138. [PubMed] [Google Scholar]
- 44.Sedivy, J. M., and A. Dutriaux. 1999. Gene targeting and somatic cell genetics-a rebirth or a coming of age? Trends Genet. 15:88-90.10203800 [Google Scholar]
- 45.Spender, L. C., G. H. Cornish, B. Rowland, B. Kempkes, and P. J. Farrell. 2001. Direct and indirect regulation of cytokine and cell cycle proteins by ebna-2 during epstein-barr virus infection. J. Virol. 75:3537-3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spender, L. C., G. H. Cornish, A. Sullivan, and P. J. Farrell. 2002. Expression of transcription factor AML-2 (RUNX3, CBF(alpha)-3) is induced by Epstein-Barr virus EBNA-2 and correlates with the B-cell activation phenotype. J. Virol. 76:4919-4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strobl, L. J., H. Hofelmayr, G. Marschall, M. Brielmeier, G. W. Bornkamm, and U. Zimber-Strobl. 2000. Activated Notch1 modulates gene expression in B cells similarly to Epstein-Barr viral nuclear antigen 2. J. Virol. 74:1727-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanigaki, K., H. Han, N. Yamamoto, K. Tashiro, M. Ikegawa, K. Kuroda, A. Suzuki, T. Nakano, and T. Honjo. 2002. Notch-RBP-J. signaling is involved in cell fate determination of marginal zone B cells. Nat. Immunol. 3:443-450. [DOI] [PubMed] [Google Scholar]
- 49.Tanigaki, K., K. Kuroda, H. Han, and T. Honjo. 2003. Regulation of B-cell development by Notch/RBP-J signaling. Semin. Immunol. 15:113-119. [DOI] [PubMed] [Google Scholar]
- 50.Waltzer, L., F. Logeat, C. Brou, A. Israel, A. Sergeant, and E. Manet. 1994. The human J kappa recombination signal sequence binding protein (RBP-J kappa) targets the Epstein-Barr virus EBNA2 protein to its DNA responsive elements. EMBO J. 13:5633-5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang, F., C. Gregory, C. Sample, M. Rowe, D. Liebowitz, R. Murray, A. Rickinson, and E. Kieff. 1990. Epstein-Barr virus latent membrane protein (LMP1) and nuclear proteins 2 and 3C are effectors of phenotypic changes in B lymphocytes: EBNA-2 and LMP1 cooperatively induce CD23. J. Virol. 64:2309-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang, F., C. D. Gregory, M. Rowe, A. B. Rickinson, D. Wang, M. Birkenbach, H. Kikutani, T. Kishimoto, and E. Kieff. 1987. Epstein-Barr virus nuclear antigen 2 specifically induces expression of the B-cell activation antigen CD23. Proc. Natl. Acad. Sci. USA 84:3452-3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang, F., H. Kikutani, S. F. Tsang, T. Kishimoto, and E. Kieff. 1991. Epstein-Barr virus nuclear protein 2 transactivates a cis-acting CD23 DNA element. J. Virol. 65:4101-4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yokota, A., H. Kikutani, T. Tanaka, R. Sato, E. L. Barsumian, M. Suemura, and T. Kishimoto. 1988. Two species of human Fc epsilon receptor II (Fc epsilon RII/CD23): tissue-specific and IL-4-specific regulation of gene expression. Cell 55:611-618. [DOI] [PubMed] [Google Scholar]
- 55.Zhou, S., M. Fujimuro, J. J. Hsieh, L. Chen, and S. D. Hayward. 2000. A role for SKIP in EBNA2 activation of CBF1-repressed promoters. J. Virol. 74:1939-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou, S., M. Fujimuro, J. J. Hsieh, L. Chen, A. Miyamoto, G. Weinmaster, and S. D. Hayward. 2000. SKIP, a CBF1-associated protein, interacts with the ankyrin repeat domain of NotchIC To facilitate NotchIC function. Mol. Cell. Biol. 20:2400-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zimber-Strobl, U., L. J. Strobl, C. Meitinger, R. Hinrichs, T. Sakai, T. Furukawa, T. Honjo, and G. W. Bornkamm. 1994. Epstein-Barr virus nuclear antigen 2 exerts its transactivating function through interaction with recombination signal binding protein RBP-J. kappa, the homologue of Drosophila Suppressor of Hairless. EMBO J. 13:4973-4982. [DOI] [PMC free article] [PubMed] [Google Scholar]