Abstract
Claviceps purpurea, a biotrophic pathogen of cereals, has developed a unique pathogenic strategy including an extended period of unbranched directed growth in the host's style and ovarian tissue to tap the vascular system. Since the small GTPase Cdc42 has been shown to be involved in cytoskeleton organization and polarity in other fungi, we investigated the role of Cdc42 in the development and pathogenicity of C. purpurea. Expression of heterologous dominant-active (DA) and dominant-negative (DN) alleles of Colletotrichum trifolii in a wild strain of C. purpurea had significant impact on vegetative differentiation: whereas DA Ctcdc42 resulted in loss of conidiation and in aberrant cell shape, expression of DN Ctcdc42 stimulated branching and conidiation. Deletion of the endogenous Cpcdc42 gene was not lethal but led to a phenotype comparable to that of DN Ctcdc42 transformants. ΔCpcdc42 mutants were nonpathogenic; i.e., they induced no disease symptoms. Cytological analysis (light microscopy and electron microscopy) revealed that the mutants can penetrate and invade the stylar tissue. However, invasive growth was arrested in an early stage, presumably induced by plant defense reactions (necrosis or increased production of reactive oxygen species), which were never observed in wild-type infection. The data show a significant impact of Cpcdc42 on vegetative differentiation and pathogenicity in C. purpurea.
Small GTPases are molecular switches that are involved in numerous signal transduction pathways. They mediate signals by switching between active GTP- and inactive GDP-bound states. The entire process is regulated by guanine exchange factors, GTPase-activating proteins, and guanine nucleotide dissociation inhibitors. Guanine exchange factors activate GTPases by catalyzing the exchange of GDP to GTP. The activated GTP-bound form is stimulated by GTPase-activating proteins to hydrolyze GTP. This results in inactivation of the protein. Guanine nucleotide dissociation inhibitors inhibit the release of GDP from the GTPase (13, 8). The activated GTP-bound form mediates signals to a variety of downstream effectors. Since the CDC42 Rho GTPase was first identified in a Saccharomyces cerevisiae mutant strain and recognized to be necessary for polarized growth and bud emergence (1, 30), it has become increasingly apparent that homologs—which are found in all eukaryotes—are essential for cytoskeletal reorganization and gene expression in response to various signals (20, 24). In S. cerevisiae, Cdc42p not only regulates polarized growth and bud emergence but also transduces signals via the p21-activated serine/threonine kinase family members Ste20p (22) and Cla4p (11) to regulate gene expression and septin organization in filamentous growth, mating, and yeast cytokinesis. Existing in two different pools—cytosolic and membrane bound—Cdc42 can function in multiple pathways in the same cell (20).
In contrast to yeast in which cell polarity is mainly required for bud emergence, hyphal growth of filamentous fungi possesses unique features, such as maintaining new axes of polarity and an extremely rapid extension rate (14). These abilities enable them to react to external stimuli and, as a consequence, to colonize their habitats. In the case of phytopathogenic fungi, colonization often requires orientation and directed growth to locate natural entrances (e.g., Puccinia graminis [31]) or for orientation within their hosts. A well-described model system for the latter feature is the interaction Claviceps purpurea/rye (reviewed in reference 46).
C. purpurea is a ubiquitous biotrophic ascomycete which specifically colonizes only grass florets. For this purpose, hyphae invade the host at the stigma and follow the pollen tube path to reach vascular tissue at the ovarian base. After securing a stable nutrition supply by tapping the vascular bundles, the fungus colonizes the entire ovary. In this stage C. purpurea is able to maintain a continuous flow of phloem exudates for the production of conidia-containing honeydew. To complete pathogenic development, the ovary is replaced by a persisting sclerotium (43, 44). The growth pattern during the first stages of infection, penetration, and directed growth along the pollen tube path differs from that of later stages and from growth in axenic culture since hyphae are mostly unbranched and are clearly guided or attracted by external signals. To obtain a deeper insight into the molecular processes underlying directed growth, we have recently characterized different signal components, such as Cpcot1, a serine/threonine kinase (36) and the p21-activated serine/threonine kinase Cpcla4 (Y. Rolke and P. Tudzynski, unpublished). Deletion mutants of both genes were strongly affected in cell morphology/polarity and impaired in penetration and invasive growth.
As polarity is a prerequisite for orientated growth and Cdc42 is a primary switch mediating internal and external stimuli to favor polarized growth, we characterized the impact of Cpcdc42 on the pathogenicity of C. purpurea. Recently, a Cdc42 homolog (Ctcdc42) was identified and characterized in Colletotrichum trifolii, a filamentous fungal pathogen causing anthracnose disease in alfalfa. Results obtained by genetic approaches revealed that Ctcdc42 is involved in spore germination and proper hyphal growth and functions as a negative regulator of appressorium formation (M. Dickman et al., unpublished data).
In this paper we demonstrate the impact of Cpcdc42 on hyphal morphology and pathogenicity of C. purpurea. In a first step, we studied the effect of overexpression of constitutive active and negative forms of the highly homologous Ctcdc42 gene from C. trifolii. The activation by a glycine-to-valine exchange at position 12 (which corresponds to a G14V in Ctcdc42) was originally identified in oncogenic versions of human Ras (41). The mutation causes activation by arresting the protein in a form similar to the GTP-bound conformation (40). The Ctcdc42(T19N) mutation in C. trifolii is equivalent to the cdc42(T17N) mutation which causes an arrest in the GDP-bound status and hence a permanently negative state. Like the dominant negative form, this mutation was first analyzed in human Ras (39). Whereas the heterologous expression of dominant-active (DA) Ctcdc42 resulted in loss of conidiation and misshapen bulbous cells, the expression of dominant-negative (DN) Ctcdc42 stimulated branching and conidiation. Moreover, we found that deletion of Cpcdc42 was possible (i.e., not lethal) and led to a phenotype that was similar to the DN Ctcdc42 phenotype. DA Ctcdc42 expression in the deletion background resulted in the bulbous phenotype. In pathogenicity assays ΔCpcdc42 mutants were able to penetrate and invade the host, but invasive growth was arrested in the stylar tissue. Electron microscopic analyses detected elevated levels of reactive oxygen species (ROS) in the apoplast of the colonized plant tissue, and necrotic reactions were induced. The data presented here suggest a strong impact of Cpcdc42 not only on hyphal morphology but also on host pathogen interaction.
MATERIALS AND METHODS
Strains, media, and growth conditions.
The wild-type C. purpurea strain used in these experiments was 20.1, a putative haploid derivative of standard field isolate T5 (Fr.:Fr.) Tul., isolated from rye (Secale cereale L.; Hohenheim, Germany) and obtained by benomyl treatment (19). For conidia harvesting and DNA isolation, mycelia were cultivated on Mantle agar (16 g/liter of agar) with 100 g/liter of sucrose (23) at 28°C for 12 to 14 days. Escherichia coli strain TOP10F′ (Invitrogen) was used for all the subcloning experiments. E. coli strain LE392 (Stratagene) was employed for propagation of C. purpurea genomic lambda clones.
Nucleic acid extraction and analysis.
Standard recombinant DNA methods were performed according to Sambrook et al. (34) and Ausubel et al. (4). Genomic DNA from C. purpurea was prepared from lyophilized mycelium according to the method of Cenis (7). For Southern blot analysis, 5 to 10 μg of restriction-digested chromosomal DNA or PCR products was electrophoresed in 0.8 to 1.6% agarose gels with salt-free buffer (34), blotted onto positively charged nylon filters (Hybond N+; Amersham, Braunschweig, Germany), and hybridized to radioactivity-labeled DNA probes in Denhardt's hybridization solution (34). Filters were washed for 10 min in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.1% sodium dodecyl sulfate and for 10 min in 1× SSC-0.1% sodium dodecyl sulfate. The hybridization and washing temperatures used were 57 and 65°C for low- and high-stringency conditions, respectively. DNA sequencing was carried out as described by Moore et al. (25). Protein and DNA sequence alignment, editing, and organization were done with DNA Star (Madison, WI). Sequence analysis was done using BLAST at the National Center for Biotechnology Information, Bethesda, Md. (2). PCR was done as described by Sambrook and colleagues (34), using Red Taq Polymerase (Sigma, Milwaukee, WI). All primers were synthesized by MWG-Biotech (München, Germany). The amplification products were cloned with a PCR 2.1 TOPO-Cloning Kit from Invitrogen.
Design of DA and DN CtCdc42.
Site-directed mutagenesis was used to generate mutant versions of CtCdc42 (DA CDC42 and DN CDC42) by Taq PCR-mediated DNA amplification. DA CDC42 was generated by substitution of the glycine 14 (G14) of CtCDC42 to valine. Similarly, DN CDC42 was generated by substitution of threonine 19 (T19) to asparagine. All mutagenized DNA fragments were amplified with Pfu polymerase (Stratagene, La Jolla, CA) and sequenced. Expression of DA CDC42, DN CDC42, and the wild-type CtCdc42 was driven by the constitutive glyceraldehyde 3-phosphate dehydrogenase (gpd) promoter from pNOM102 (32). For selection, the phleomycin resistance gene expression cassette from pAMPH520 (3) was subcloned in the two constructs as described above.
Cloning of Cpcdc42 and generation of a replacement vector.
For the amplification of an internal fragment of Cpcdc42, degenerate primers were designed using the CODEHOP program (http://blocks.fhcrc.org/blocks/codehop.html) (33) and the amino acid sequences of Cdc42 homologs of Aspergillus nidulans (AAF24513), S. cerevisiae (P19073), Schizosaccharomyces pombe (Q01112), Ashbya gossypii (AAG41247), Glomerella cingulata (AAD00177), and Suillus bovinus (AAF37871). With the deduced primers cdc42-3 (5′-GAC TAC GTC CCC ACC GTC TTY GAY AAY TA-3′) and cdc42-4 (5′-GGG CGG AGC ACT CGA CRT AYT TXA C-3′), a fragment of 495 bp was amplified showing high homology to Cdc42 in various fungi. With this fragment as a probe, we screened a genomic library of strain T5 (38) by plaque filter hybridization (34). From the 34,000 lambda clones screened, 10 hybridized to the PCR probe and 4 of them were further purified (34). They all contained an overlapping genomic region, as revealed by restriction and Southern blot analyses. From phage number 3 a 4.1-kb KpnI fragment was cloned into pUC19 resulting in pCDC42K1. This clone, containing the 1.0-kb open reading frame together with a 0.96-kb 5′ sequence and a 2.1-kb 3′ sequence, was subcloned and sequenced. Restriction analyses and sequencing revealed another KpnI site close to the 3′ end of pCDC42K1 due to partial restriction of phage number 3. For the construction of the Cpcdc42 replacement vector, the genomic regions upstream and downstream of Cpcdc42 (−816 to +294 bp relative to the start codon and −48 to +1,951 bp relative to the stop codon) were amplified by primers with integrated restriction sites. For the amplification of the upstream flank, NotI (within primer DCDCLF1) and XbaI (DCDCLF2) sites were generated, and for the downstream flank, EcoRV (DCDCRF1) and KpnI (CDCDRF2) were used. These PCR products were cloned into the PCR 2.1 TOPO vector, excised with NotI-XbaI and EcoRV-KpnI, respectively, and subcloned upstream and downstream of the hygromycin resistance cassette (hph) into the corresponding restriction sites of the pAN7-1UM (26) vector, producing the pΔcpcdc42 plasmid. The linear replacement construct was excised using NotI-KpnI and subsequently used to transform the C. purpurea wild-type strain 20.1 (see Fig. 3A). The vector for the complementation of ΔCpcdc42 mutants was obtained by cloning a 3.9-kb KpnI fragment of pCDC42K1 into the corresponding restriction site of the pAN8-1UM (26) vector (see Fig. 3A). This vector was termed pCcpcdc42 and was used to transform the ΔCpcdc42 mutant strain ΔCpcdc42-1.
FIG. 3.
Generation of ΔCpcdc42 mutants. (A) Gene replacement approach (Δ) and complementation construct (C) for Cpcdc42. For the replacement approach, the replacement vector pΔcpcdc42 was constructed by cloning 3′ and 5′ parts of Cpcdc42 on each side of a hygromycin resistance cassette (hph) in the pAN7-1UM plasmid (see Material and Methods for details). The resistance cassette, excised using a NotI-KpnI restriction, was used to transform the C. purpurea wild-type strain 20.1. Δcdc42 mutants were generated following the disruption of the wild-type gene (WT) by homologous recombination through a double crossover event. The Cpcdc42 coding sequence and introns are represented with black and white boxes, respectively. The ATG indicates the start of translation of Cpcdc42, and the black arrow indicates the orientation of the hygromycin resistance cassette within the replacement construct. Primers DCDCLF1/DCDCLF2 (a and b) and DCDCRF1/DCDCRF2 (c and d) used for the amplification of the upstream and downstream flanks, respectively, are indicated by black arrows. The positions of primers DCDC-hIL1/DCDC-hIL2 (e and f) and DCDC-hIR1/DCDC-hIR2 (g and h), used for the identification of homologous integrations, and primers DCDC-WT1/DCDC-WT2 (i and j), used for detection of the Cpcdc42 wild-type copy, are indicated with black triangles. The DNA fragment used as a probe in the Southern blot shown in panel B is indicated as a striped box. The full-length clone of Cpcdc42 for complementation is shown (see text for details). To check the reinsertion of Cpcdc42 for complementation, the primers DCDC-hIL1/CCDC42-1 (e and k) were used. Abbreviations for restriction enzymes: CI, ClaI; EV, EcoRV, KI, KpnI; NI, NotI; SI, SacI; XI, XbaI. (B) Southern analysis of deletion mutants ΔCpcdc42-1, ΔCpcdc42-2, complemented strain Ccpcdc42-1, and wild-type 20.1. Genomic DNA of selected strains was digested with ClaI, separated in an agarose gel, blotted, and probed with the right flank of the replacement vector pΔcpcdc42. A successful deletion by gene replacement is demonstrated by the shift of the wild-type band (2.6 kb) to 5.75 kb in the lanes of ΔCpcdc42-1 and ΔCpcdc42-2. The complementation leads to the reappearance of the 2.6-kb band and an additional band (0.64 kb). For restriction sites and location of the probe, see panel A.
Fungal transformation.
Protoplasts of C. purpurea generated with lysing enzymes from Trichoderma harzianum and Driselase (InterSpex) were transformed with 10 μg of the pΔcpcdc42 fragment (see Fig. 3) as described by Jungehülsing et al. (21). For hygromycin selection, protoplasts were incubated at 28°C for 24 h, after which they were overlaid with 10 ml of BII medium, pH 8, containing 1.5 mg/ml hygromycin to reach a final hygromycin concentration of 0.5 mg/ml in the petri dishes. Resistant colonies were transferred to fresh selective medium (BII, pH 8, containing 0.5 mg/ml hygromycin) and screened for homologous integration by PCR. To obtain homokaryotic strains, the transformants were subjected to at least one round of single spore isolation. Primer pairs DCDC-hIL1/DCDC-hIL2 (5′-GAA CGA AGC GAC GAG CAT CC-3′; 5′-TCC GGC GAA GAG AAG AAT AGC-3′) and DCDC-hIR1/DCDC-hIR2 (5′-GGC TGG CCC TGG CTG AGA AAG-3′; 5′-TCG GCC GAG CAA TGA CTA CTG ATA AAA-3′) were used to identify transformants with a homologous integration of the 5′ flank and 3′ flank, respectively (see Fig. 3A). The predicted 1,664- and 2,350-bp fragments could be amplified with two strains, termed ΔCpcdc42-1 and ΔCpcdc42-2. The lack of the wild-type gene copy in the ΔCpcdc42 mutants was checked using the primers DCDC-WT1 (5′-GGC GCC ACC TCC CCT ACT CC-3′) and DCDC-WT2 (5′-CGC CAT CTT ATC GCC TTC TTC CT-3′), which gave rise to a 1,279-bp fragment in the wild-type strain. Complementation and insertion of heterologous genes were done by transformation of circular plasmids carrying the phleomycin resistance gene (ble) as a selective marker. For phleomycin selection, phleomycin was directly applied to the protoplasts to a final concentration of 33 μg/ml of modified BII medium (pH 8, 20% sucrose and no FeSO4). Resistant colonies were transferred to fresh selective medium (BII, pH 8, containing 100 μg/ml phleomycin) and subjected to at least one round of single-spore isolation to obtain homokaryotic transformed strains. In the case of nonsporulating transformants, hyphal tips grown on selective medium were isolated and placed on fresh selective medium. Growing colonies were transferred to nonselective medium and finally to selective medium to confirm stable integration. Reintegration of the complete Cpcdc42 sequence including the promoter region of about 900 bp was determined by PCR with primers DCDC-hIL1 and CCDC42-1 (5′-AAG CCG GCA AAA GAC AAG AAA GAA-3′) and by Southern analyses (see Fig. 3B). To evaluate the integration of heterologous constructs [Ctcdc42(G14V), Ctcdc42(T19N), and Ctcdc42, all in the vector pNOM520] (32), PCR was done with the primers PANID1/PANID2 (5′-CCC CGA AGT GGA AAG GCT GGT GTG-3′; 5′-TTT CGG GCG TAT TGG GTG TTA-3′) annealing within the flanking gpdA promoter (A. nidulans gpd promoter) and trpC terminator (A. nidulans). PCR fragments were cloned into the PCR 2.1 TOPO vector and subsequently sequenced.
Pathogenicity tests.
Rye plants were cultivated in growth chambers as described by Smit and Tudzynski (38). Florets of blooming ears (30 to 40 florets/ear) were inoculated with 5 μl of a suspension containing 2 × 106/ml conidia collected from Mantle agar, as described by Tenberge et al. (42). In the case of nonsporulating strains, suspensions of mycelium were used for infection. To avoid cross contamination, the ears were covered with paper bags equipped with cellophane windows directly after inoculation. In order to infect the plant (“wounding test”), rye florets were bisected and inoculated with the conidial suspension. Pictures were taken 4 weeks postinoculation.
Microscopic analyses.
For microscopic analyses an in vitro infection system was used (modified from reference 15; J. Scheffer, unpublished): rye pistils were isolated from blooming rye ears and put onto Hoagland solution (16, 17) modified for barley shoot culture [for 1 liter: 5 ml MES buffer (19.5 g/liter 2-morpholino ethansulfonic acid, 2.0 g/liter NaOH, pH 6.5), 94 mg of Ca(NO3)2 · 4 H2O, 66 mg of KNO3, 52 mg of MgSO4 · 7 H2O, 38 mg of KH2PO4, 2.86 mg of H3BO3, 0.22 mg of ZnSO4 · 7H2O, 0.1 mg of CuSO4 · 5H2O, 0.05 mg of Na2MoO4 · 2 H2O, 12 mg of FeEDTA] and solidified with 16 g/liter agar. Stigmas were infected with suspensions containing 2 × 106 conidia/ml with an inoculation loop and were incubated at room temperature for 5 days. The KOH-aniline blue staining of the fungus was realized as described by Hood and Shew (18), except that the pistils were incubated in 1 M KOH overnight at room temperature. The infected ovaries were observed by epifluorescence microscopy using a Leica DMRBE microscope with a PixelFly digital camera (PCO Computer Optics GmbH) and filter set A (BP 340 to 380, RKP 400, LT 425, UV light 340 to 380 nm). Embedding, sectioning, and conventional light microscopic analyses were performed as described in Oeser et al. (28); detection of H2O2 (using the cerium chloride technique [5]) was done according to Nathues et al. (27).
RESULTS
Cpcdc42 encodes a highly conserved Cdc42 homolog.
Using the CODEHOP program, we designed two degenerated primers (Cdc42-1 and Cdc42-2) based on a theory of primer design in which the degree of degeneration is minimized using an invariant core region at the 5′ end and a variable clamp region at the 3′ end (33). The derived amino acid sequence of the PCR fragment showed high homology to the Cdc42-like proteins CflA of Penicillium marneffei (AAK56917) and MgCdc42 of Magnaporthe grisea (AAF73431), and this fragment was used to screen an EMBL3 genomic library of C. purpurea strain T5. By Southern analyses of putative lambda phages, subcloning of a positive lambda clone, and sequence analyses an open reading frame of 588 bp, interrupted by three introns of 256 bp, 105 bp, and 88 bp (confirmed by reverse transcription-PCR; data not shown) was identified, encoding a polypeptide of about 21.6 kDa (Fig. 1). The derived amino acid sequence is 92% identical to Ctcdc42 and shows high homology to several Cdc42 homologs (Fig. 1). The sequence contains all domains previously shown to be necessary in Cdc42 function of S. cerevisiae, S. pombe, and others (for review, see reference 20). These domains include four GTP binding and hydrolysis domains, an effector domain, a Rho insert domain, and a membrane localization domain (see Fig. 1). Therefore, the gene was termed Cpcdc42. Southern blot analyses under low-stringency conditions, using digested genomic DNA from the wild-type strain 20.1 and the PCR product used for the screening of the genomic library as a probe, confirmed that Cpcdc42 is a single copy gene (data not shown).
FIG. 1.
Protein sequence alignment of the CDC42 homologs (with accession numbers) from C. purpurea (CpCDC42; AJ879079), C. trifolii (CtCdc42; AAK31624), Gibberella zeae (CDC42; EAA75264), M. grisea (MgCdc42; AAF73431); S. cerevisiae (Cdc42p; NP_013330), and H. sapiens (CDC42, AAH18266). Amino acids mutated in the Ctcdc42 alleles (see text) are indicated by arrows. The known functional domains are indicated as follows: dashed box, GTP binding/hydrolysis domains; bold black box, effector domain; dotted box, Rho insert domain; and thin black box, membrane localization domain.
Heterologous expression of dominant active Ctcdc42(G14V) and dominant negative Ctcdc42(T19N) in C. purpurea.
Since deletion of Cdc42 was found to be lethal in many eukaryotes (20), we used an alternative approach to characterize CpCDC42 by expressing dominant active and dominant negative forms of this protein. Based on the significantly high similarity between Cpcdc42 and Ctcdc42, we transformed C. purpurea wild-type strain 20.1 with several Ctcdc42 constructs, including the DA Ctcdc42(G14V), the DN Ctcdc42(T19N) and—as a control—the wild-type Ctcdc42, all under the control of the constitutive gpdA promoter (32). This promoter was previously shown to be functional in C. purpurea (e.g., reference 48). For simplicity, the obtained mutants were named DN, DA, and DCtWT, respectively (Table 1). Phleomycin-resistant transformants were checked by PCR for the integration of the complete promoter-gene constructs. For each DNA construct, four independent fungal strains were further analyzed. To separate transformed from nontransformed nuclei of possible heterokaryotic strains, one round of single spore isolation or—with strains impaired in conidiation—isolation of hyphal tips grown on selective medium was performed. One passage of growth on nonselective medium followed by one on selective medium was done to confirm stable integration of the transgenes. All strains bearing the same DNA construct were phenotypically similar. This observation together with the severity of the phenotypes represents sufficient evidence that the transgenes were expressed in the transformants.
TABLE 1.
Overview of CDC42-transformation experiments
| Abbreviation in texta | Strain | Constructb | Morphological phenotype | Pathogenicityc |
|---|---|---|---|---|
| DN | Wild type | Oe; DN CtCdc42 | Enhanced branching, increased conidiation | + |
| DA | Wild type | Oe; DA CtCdc42 | Bulbous hyphae, no conidia | (+) |
| DCtWT | Wild type | Oe; WT CtCdc42 | Comparable to wild type | + |
| Δ | ΔCpcdc42 | Like DN, but more pronounced | − | |
| ΔDA | ΔCpcdc42 | Oe; DA CtCdc42 | Like DA, but more pronounced | − |
| ΔDCtWT | ΔCpcdc42 | Oe; WT CtCdc42 | Restoration of wild type | + |
| ΔCpWT | ΔCpcdc42 | CpCDC42 | Restoration of wild type | + |
For simplicity, these abbreviations are used to describe the mutants in the text.
Abbreviations: Oe, overexpression; DA, dominant active; DN, dominant negative; WT, wild-type; CpCDC42, CDC42 homologue of C. purpurea; CtCdc42, CDC42 homologue of C. trifolii.
+, fully pathogenic on rye, including honeydew production and sclerotia formation; (+), limited infection rate; −, apathogenic.
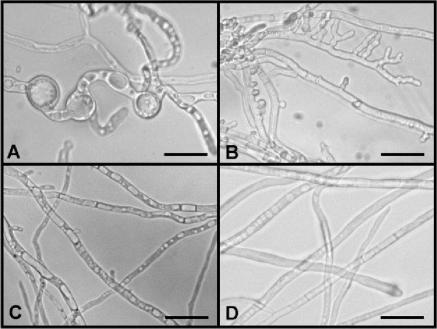
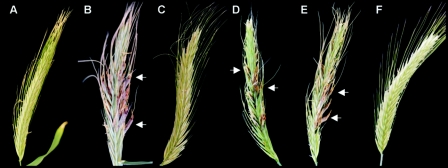
C. purpurea is able to grow in axenic culture on solid and liquid media. On rich solid medium such as Mantle medium (23), a growth rate of about 2 to 3 mm/day is reached. Under these conditions conidiation starts after 7 days. Grown in axenic culture on solid Mantle medium, the transformants carrying the wild-type Ctcdc42 (DCtWT) showed no differences in germination, hyphal development, and growth rate. Conidiation was unaffected and comparable to the wild-type 20.1 (Fig. 2C). However, both the DA Ctcdc42 strains and the DN Ctcdc42 strains were drastically altered in growth behavior. DA strains revealed extremely distorted growth: formation of hyphae was unequal due to the appearance of extremely enlarged vacuoles (Fig. 2A). Conidiation was never observed in these strains. This growth behavior resembles the bulbous storage cells in the sclerotial stage of in planta infection (43). In addition, after 7 days in axenic culture, autolysis started from the center of the colony (data not shown). The DN transformants had a completely different phenotype: they showed enhanced branching along with massive production of conidia. Conidiation already appeared after 2 days; germination of conidia was not affected. In contrast to the DA phenotype, proportion of the hyphae did not differ from wild type (Fig. 2B). To further analyze these transformants, we performed pathogenicity assays. As a first indication of successful infection, the period of time until appearance of honeydew, a sugar-rich fluid serving as distributor of conidia, is measured. Inoculation with C. purpurea wild-type 20.1 leads to honeydew formation after 6 to 7 days postinoculation (dpi). About 14 dpi the development of sclerotia is initiated. For pathogenicity tests, rye florets were inoculated with conidial suspensions of DN and DCtWT strains and, as a control, wild-type strain 20.1. Inoculation with the nonsporulating DA strain was carried out by using suspensions of mycelium, together with equally treated wild-type strain 20.1. For each strain, 150 rye ears were infected. As a control for viability, the conidial or mycelial suspensions that were used for infection were plated on solid medium. All strains germinated and grew in axenic culture; only the DA strains showed reduced viability, probably due to altered cell architecture (see above). Surprisingly, neither the DCtWT transformants nor the DN transformants showed a distinct alteration in virulence. Time course of honeydew and sclerotia formation was equal to that of the wild type. In contrast, most of the tested DA trains did not infect at all; only the infection with one strain (DA3) led to a (retarded) production of honeydew (10 to 12 dpi) in two out of five ears. Mycelium regenerated from honeydew of these ears still showed phleomycin resistance but had a rather variable phenotype, indicating a heterokaryotic status of this transformant (data not shown).
FIG. 2.
Morphology of C. purpurea transformants overexpressing Ctcdc42(G14V) (A), Ctcdc42(T19N) (B), and unmodified Ctcdc42 (C). C. purpurea wild-type strain 20.1 (D). Strains were grown for 5 days on Mantle medium. For details see text. Scale bars, 10 μm.
Generation of ΔCpcdc42 mutants.
Although the DN strains showed drastic morphological alterations in axenic growth, no reduction of pathogenicity could be observed, possibly due to the presence of the Cpcdc42 wild-type gene. Therefore, we implemented a gene replacement approach. As outlined in Fig. 3A, 5′ and 3′ fragments of the gene were cloned upstream and downstream, respectively, of the hygromycin resistance cassette of pAN7-1UM (for details, see Material and Methods). A total of 210 hygromycin-resistant colonies were screened for a homologous integration of the pΔcpcdc42 fragment by PCR. A homologous integration and thus a knockout event could be demonstrated in two strains. After purification by one round of single spore isolation, the wild-type gene was no longer detectable by PCR. Finally, Southern blot analyses confirmed the deletion of Cpcdc42 in these strains (Fig. 3B): the two mutants are characterized by a shift of the wt band (2.6 kb) to the homologous integration band (5.75 kb). The knockout strains were termed ΔCpcdc42-1 and ΔCpcdc42-2.
Cpcdc42 has impact on conidiation and branching.
The ΔCpcdc42 mutants showed drastic phenotypes in axenic culture. Already 2 days after inoculation on solid medium, massive conidiation was initiated. This phenomenon was compared to the wild type in detail. In axenic culture the wild type first colonizes the medium by forming long hyphae with rare branches (Fig. 2D), and later phialidic conidia arranged in heads called conidiophores start to form (characterized in detail by reference 29). In contrast, the ΔCpcdc42 mutants differ in terms of conidiation in two aspects: (i) branching was very early and mainly to form conidiophores and (ii) the occurrence of conidiophores was considerably more frequent (Fig. 4A to C). The germination rates of conidia and the size and proportions of hyphae did not differ from the wild type. This phenotype in axenic culture corresponds to that of the DN form but is much more pronounced.
FIG. 4.
Axenic growth of the ΔCpcdc42-1 mutant (A to C), complemented mutant Ccpcdc42-1 (D and E), ΔCpcdc42 mutant overexpressing DA Ctcdc42(G14V) (ΔCpcdc42-DA Ctcdc42-1) (F to I), and ΔCpcdc42 mutant overexpressing wild-type Ctcdc42 (ΔCpcdc42-WT Ctcdc42-1) (J to L). For ΔCpcdc42-1 young mycelium with phialidic branches are shown in panels A and B in detail. Massive conidiation occurs after 2 days (C). In the complemented strain Ccpcdc42-1, complementation restored hyphal morphology, as shown in panel D (E, detailed view). For ΔCpcdc42-DA Ctcdc42-1, swollen hyphae (F to H) and bulbous hyphal tips are shown (I). In ΔCpcdc42-WT Ctcdc42-1, hyphal morphology is restored (J and K). Occasionally, an unusual branching pattern was observed (L), which, however, did not form conidia as did ΔCpcdc42-1. Compare wild type with Fig. 2D. Strains were grown on Mantle medium. Pictures were taken 5 dpi. Scale bars, 10 μm.
Cpcdc42 is essential for pathogenicity.
To test if the deletion of Cpcdc42 alters the virulence of C. purpurea, conidial suspensions of the two mutant strains and the wild-type strain were used for the infection of rye ears. Interestingly, with the ΔCpcdc42 mutants no macroscopic symptoms of infection were detectable, neither the occurrence of honeydew nor the formation of sclerotia. The appearance of these florets was comparable to noninfected florets as they withered after about 1 week (Fig. 5C). To pinpoint the block of infection, detailed microscopic analyses were necessary. For this purpose we infected in vitro cultivated rye ovaries and incubated them for different periods of time. After cutting and staining (for details see Material and Methods), we traced the fungal infection route by microscopic observation. Normally C. purpurea wild-type germinates and penetrates the plant's stigmatic hairs during the first 2 dpi. Three to five days postinfection, hyphae enter the ovarian transmitting tissue, followed by the growth toward the basal part of the ovary where the fungus taps the vascular bundles and starts to colonize the whole ovarian tissue (>5 dpi). Comparable to the wild type, the ΔCpcdc42 mutants were able to germinate and penetrate the stigmatic hairs (Fig. 6B). Following the pollen tube path, the invading hyphae headed for the ovary (Fig. 6C and E). However, no hyphae could be detected in the basal part of the stylar tissue and the ovarian cap, the region where the style inserts the ovary. Even after an extended period of time (up to 7 dpi), no further growth of the mutants was visible. For additional confirmation of these results, we microscopically analyzed infected ovaries on an ultrastructural level (for procedure, see Material and Methods). Rye ovaries infected with conidia of wild type and ΔCpcdc42 mutant were incubated for 5 days. In Fig. 7 tissues infected by wild-type strain 20.1 and the mutant ΔCpcdc42-1 are compared. Toluidine blue-stained semithin sections (Fig. 7A to C, G, and H) showed that the ΔCpcdc42 mutant penetrates and grows intercellulary in the stigmatic hairs and in the subepidermal tissue in the upper part of the style, though with much lower density than the wild type. However, in the basal part of the style, no hyphae of the ΔCpcdc42 mutant were detected. Instead, the stylar cells were collapsed, as demonstrated in Fig. 7C.
FIG. 5.
Pathogenicity assays of C. purpurea transformants on rye. Rye florets were infected with water (A) and conidial suspensions from wild type (B), ΔCpcdc42-1 mutant (C), the complemented strain Ccpcdc42-1 (D), a ΔCpcdc42 mutant overexpressing wild-type Ctcdc42 (E), and a ΔCpcdc42 mutant overexpressing Ctcdc42(G14V) (F). Pictures were taken 4 weeks postinoculation. Arrows indicate sclerotia.
FIG. 6.
Effect of the inactivation of Cpcdc42 on pathogenicity of C. purpurea. Ovaries were infected in vitro with conidial suspensions from wild type (A, D, and F) and ΔCpcdc42-1 mutant (B, C, E, and H). A schematic overview of a rye ovary is given in panel G. The solid-lined box represents details shown in pictures panels A to E, while the dotted box represents pictures in panels F and H. Hyphal growth within the stigmas and stigmatic hairs is visible in both the wild-type (A and D) and the ΔCpcdc42-1 mutant (B, C, and E). In contrast to the massive colonization of the transmitting tissue by the wild type (F), no hyphae could be detected in this area after infection with the ΔCpcdc42-1 mutant. Ovaries were collected at 5 days postinoculation, stained with aniline blue, and observed using epifluorescence microscopy. Arrows indicate hyphae.
FIG. 7.
Light and electron microscopic analyses of rye stylar tissues infected by wild type and ΔCpcdc42. (A and B) longitudinal section of a style colonized by wild-type C. purpurea showing mostly intercellular growth between the prosenchymatic host tissue. (C) Rye stylar tissue inoculated with ΔCpcdc42. The transition from normal-looking, highly vacuolated host cells to an area of obviously collapsed host cells showing dense staining of the whole cell compartment is visible. (D to F and I to K) In situ detection of H2O2 using the CeCl3 technique. Electron-dense precipitate of ceriumperhydroxide represents the areas where H2O2 was formed. (D) Subcuticular wild-type hypha which shows cell wall-bound and secreted H2O2. Note that the host cell completely lacks any signs of H2O2 generation. (E) Wild-type hyphae, growing both epicuticular (asterisk) and subcuticular, showing intense formation of H2O2. Note also that the host epidermal cells produce H2O2 at this interaction site. (F) Wild-type hyphae among the prosenchymatic transmitting tissue. In this tissue virtually no generation of H2O2 takes place. (G) A ΔCpcdc42 hypha inside the stigmatic trichomes. (H) Sparse colonization of stylar tissue by ΔCpcdc42 hyphae (arrow). (I) H2O2 production in noncollapsed prosenchymatic host tissue (as depicted in the upper part of panel C adjacent to ΔCpcdc42 hyphae). Strong signals can be found in the area of the plasmalemma, vacuoles, and host cell wall (arrow). The host cells show clear signs of breakdown of cell compartments like disintegration of the vacuolar system. (J) H2O2 detection in collapsed host tissue (as depicted in the lower part of panel C). A plasma membrane-bound signal is visible. (K) Collapsed host tissue (CeCl3 treatment was omitted). Granular electron-dense particles are visible in large parts of the cell compartments pointing to the occurrence of phenolic/tannic substances in this area. (L) Noncollapsed host tissue (CeCl3 treatment was omitted). No electron-dense precipitation structures are detectable. Toluidine blue (pH 6.8) staining was used in panels A to C, G, and H. Scale bars: 150 μm (A and C), 10 μm (B, G, and H), 5 μm (D, E, and J to L), and 2 μm (F and I). f, fungus; hc, host cell.
Absence of Cpcdc42 triggers H2O2 production in planta.
Using ultrathin sections treated with cerium chloride, H2O2 production was detectable in early stages of subcuticular growth of the wild type (visible as an electron-dense precipitate of cerium perhydroxide); the peroxide seems to stem from both interaction partners (Fig. 7D and E). This is the first time that ROS production has been demonstrated in rye tissue infected by a wild strain of C. purpurea; analyses of later infection stages had indicated that the fungus is not recognized by the plant (see reference 27). Accordingly, in the subepidermal stylar tissue, no signal could be detected, indicating that the wild type represses (or does not induce) H2O2 generation in this stage of infection; both the plant tissue and the hyphae look healthy (Fig. 7F). In contrast, during infection with the ΔCpcdc42 mutant H2O2 occurs in high concentration also in subepidermal tissue, probably stemming from both partners (Fig. 7I). Additionally, a breakdown of plant cell compartments can be observed (Fig. 7J). This degeneration of plant cells is accompanied by the occurrence of granular electron-dense particles (phenolic/tannic substances?), as is visible in non-CeCl2 treated samples (Fig. 7K).
Complementation of ΔCpcdc42 mutants.
To prove that the described phenotype of the ΔCpcdc42 mutants was due to the deletion of Cpcdc42, we complemented a ΔCpcdc42 mutant by ectopic integration of the complete gene, including the promoter region of about 1 kb (see Material and Methods). Phleomycin-resistant strains were checked for complete integration of the gene by PCR and Southern analyses (Fig. 3B). It was demonstrated that in these strains the phenotype in axenic culture and the pathogenicity on rye were restored (Fig. 4D and E and 5D, respectively).
Heterologous expression of Ctcdc42 in a ΔCpcdc42 background restores wild-type hyphal morphology and pathogenicity.
Since in axenic culture the phenotype of the ΔCpcdc42 mutants is very similar to that of transformants carrying the DN form of Ctcdc42, we wanted to know if a heterologous complementation with Ctcdc42 is possible. We introduced the wild-type Ctcdc42 under the control of the gpdA promoter into a ΔCpcdc42 mutant using the same construct as described above. Transformants were purified by single spore isolation and evaluated by PCR for complete integration of the promoter and Ctcdc42. Five out of eight strains analyzed were similar in phenotype to C. purpurea wild type, and conidiation was reduced to the normal extent. However, hyphae tended to branch more often than in the wild type (Fig. 4L). To test if this heterologous complementation could also restore pathogenicity, rye ears were infected with conidia of strains showing restored growth characteristics (see above). In two of the tested strains, pathogenicity was fully restored (Fig. 5E): production of honeydew was comparable to the wild type. To ensure that this restoration of pathogenicity was not due to cross-contamination with wild type, we analyzed mycelia derived from honeydew; they were still hygromycin and phleomycin resistant. In addition, the presence of Ctcpc42 could be demonstrated by PCR (data not shown). The successful complementation of the ΔCpcdc42 mutant by the heterologous gene proves that Ctcdc42 is functional in C. purpurea.
Heterologous expression of the dominant active Ctcdc42(G14V) allele in a ΔCpcdc42 background.
The effect of expression of the DA Ctcdc42 in C. purpurea wild type (Table 1, DA) on pathogenicity was not uniform in all transformants tested (see above). Therefore, expression of the DA form of Ctcdc42 in the ΔCpcdc42 background was analyzed. For introduction of DA Ctcdc42 in a ΔCpcdc42 mutant, we used the same DA construct as for the transformation of the wild type. The correct integration of the construct was determined by PCR. After one round of hyphal tip isolation followed by growth on selective medium, four independent transformants were characterized. They showed a similar phenotype as the DA transformants of the wild type: swollen vacuoles and an aberrant cell shape and complete suppression of conidiation (Fig. 4F to I). In comparison to the DA form in wild-type background, however, this phenotype was much more severe, and segments of normally shaped hyphae were not detected. In pathogenicity assays (with mycelial suspensions, 150 florets per strain) no symptoms of infection could be detected (Fig. 5F). However, analysis of the mycelial suspension used for infection on agar plates showed reduced viability. Therefore, the observed (in this case uniform) nonpathogenicity of the transformants could be mainly due to reduced fitness.
DISCUSSION
In this study we describe characterization of the Cpcdc42 gene encoding a highly conserved Cdc42p-like protein using two strategies: (i) the overexpression of constitutive active and negative forms of Ctcdc42, a Cdc42 ortholog from C. trifolii and (ii) the deletion of Cpcdc42.
As a prerequisite for using dominant active and negative alleles of the heterologous Ctcdc42 gene, the heterologous constructs were proved to be functional in C. purpurea: (i) the overexpression of wild-type Ctcdc42 in C. purpurea wild-type background did not lead to an impaired phenotype with respect to vegetative growth and pathogenicity, (ii) the dominant-negative construct induced the same phenotype—albeit less severe—as the deletion of Cpcdc42, and (iii) a ΔCpcdc42 mutant could be complemented by the heterologous expression of wild-type Ctcdc42. As shown here and in several previous studies (e.g., references 6 and 47), the use of a constitutive promoter instead of the original promoter did not affect the function of the GTPase. Thus, increased concentration of the protein does not alter the ability to transduce signals as long as the proper function is secured. However, if the original protein competes with a gene product of an additionally introduced gene, a competitive situation is created and the proportion of the two proteins is decisive. As described above, the phenotype of transformants of the wild-type strain expressing the dominant negative Ctcdc42(T19N) was less severe than the effect of deleting Cpcdc42. In addition, the effect of the DA form in a ΔCpcdc42 background was more pronounced than in the wild-type background.
Nevertheless, heterologous expression of the DA and DN constructs resulted in significant phenotypic alterations. The DA transformants showed misshapen hyphae: cells were swollen and had a spherical rather than elongated shape, autolysis started prematurely, and conidiation was not observed. These findings partially parallel results in other fungi. The activation of homologs of Cdc42 in Wangiella dermatitidis and P. marneffei resulted in swollen hyphae very similar to the DA transformants of C. purpurea (6, 50). In contrast, a lethal phenotype was observed by introducing the dominant activated form into Candida albicans (47) and Saccharomyces cerevisiae (51). Considering the similarity in cell deformation in DA transformants of C. purpurea to the phenotypes of W. dermatitidis and P. marneffei DA strains, it can be assumed that the dominant activation in C. purpurea also leads to a similar deregulation of cell polarity via actin cytoskeleton organization. Further investigations of this phenotype are necessary to verify this hypothesis.
Regarding the impact on pathogenicity, a partial reduction of virulence in the DA strains with wild-type background and a complete loss in the DA strains with ΔCpcdc42 background could be due to the defect in cell architecture caused by the influence of activated Cdc42. This instability could prevent proper growth and penetration. Since mycelial suspensions used for infection hardly regenerated after plating, it is not surprising that hyphae of these strains do not possess the power to invade the host. Therefore, concerning pathogenicity, attention should rather be focused on transformants with dominant-negative and null Cdc42, respectively.
As mentioned above, the phenotypes of the DN strains with wild-type background and the ΔCpcdc42 mutants were similar, but in the ΔCpcdc42 mutants the effect was much more pronounced. Both strains were viable. Hyphal morphology was not affected in terms of hyphal shape, colony growth, and germ tube emergence. However, a higher frequency of branching combined with an enormous increase of conidiation occurred. To our knowledge this morphological phenotype was never described before. In contrast, the heterologous expression in A. nidulans of both the DA (G14V) and the DN (D120A) version of CflA, which is a homolog to Cdc42 in P. marneffei, eliminated conidiation completely. It was concluded that proper Cdc42 function is required for conidiation in A. nidulans, which is not the case in P. marneffei (6). Given the full restoration of wild-type phenotype in a ΔCpcdc42 mutant by the heterologous Ctcdc42 gene, it is interesting that the effects of the DA and DN alleles in the two fungi are not identical. In C. trifolii, the effect of DA Ctcdc42 expression is observed only in minimal medium (and can be reversed by addition of proline); transformants show heavily distorted hyphal growth and lack of appressoria formation, similar to the dominant-active Ras mutant (M. Dickman, et al., unpublished). In contrast to C. purpurea, DN Ctcdc42 transformants show dramatically reduced conidial germination. In addition, they display increased appresoria formation under noninducible conditions such as soft agar surface, suggesting that Ctcdc42 is a negative regulator of appressoria formation (M. Dickman et al., unpublished).
Infection assays on rye with the Δcdc42 mutants revealed a loss of pathogenicity: symptoms of infection (honeydew and sclerotia) were completely absent. This observation was remarkable since hyphal growth of the mutants obviously was not impaired, and the mutants were still able to invade the host. Microscopic analyses demonstrated that after germination, hyphae directly penetrate the stigmatic hairs. Additionally, we could observe hyphae within the stigmas following the wild-type infection path. As hyphal growth of the Δcdc42 strains never occurred within the ovarian tissue, even after a prolonged period of time, fungal growth stops in the basal part of the style. Until this infection stage the fungus has to overcome different barriers within its host, such as penetrating the cuticle which covers the epidermis of the stigmatic cells, growing between epidermal cell walls, orientating within the host's apoplast (reviewed in reference 45). Since the Δcdc42 mutants can cope with all these barriers in a period of time comparable to that of wild-type infection, the mutants seem to face new aspects of resistance in the transmitting tissue. Light- and electron microscopic analyses showed that, in contrast to the wild type, during infection with the ΔCpcdc42 mutant the stylar cells became necrotic; a comparable degeneration of stylar tissue has been observed after successful pollination, obviously in order to prevent competition of multiple pollen tubes (15). Thus, the ΔCpcdc42 mutant evokes a necrotic reaction that obviously prevents further progress of infection. This plant defense reaction and detection of H2O2 was unexpected, since the primary function of cdc42 generally involves the cytoskeleton.
The role of ROS in the C. purpurea/rye interaction has been studied by our group in detail (reviewed in reference 46). Cytological analyses of wild-type-infected ovarian tissue had never detected significant levels of ROS formation, indicating that the biotroph C. purpurea effectively hides itself and is not recognized by the plant; i.e., it provokes no defense reactions. In this study we can show for the first time a plant reaction. The epidermal cells produce H2O2, but after penetration and during growth in the center of the style, no further reactions are detectable. Recently we showed that deletion of the gene cptf1, encoding a Bzip-transcription factor in C. purpurea, led to a drastic reduction of activity of all known catalase isoforms (27). In addition, the mutants showed significantly reduced virulence. In contrast to the wild type, infection by the Δcptf1 mutants triggered an oxidative burst during colonization. Interestingly, the fungus itself also secreted H2O2 in significant amounts. It was postulated that the down-regulation of catalase activity in the Δcptf1 mutants reduces the decomposition of H2O2 secreted by the fungus. This could cause a host defense reaction, which is similar to that caused by the ΔCpcdc42 mutants, but occurs in later stages (in ovarian tissue). It will be interesting to reveal the molecular background for the effect of CpCDC42 on the ROS status in planta. Preliminary northern analyses showed that the expression of a CPTF1-target gene, the catalase gene Cpcat1, is not affected by the Cpcdc42 deletion; i.e., the similar effect of the ΔCpcdc42 and Δcptf1 mutants is not due to a direct effect of CpCDC42 on the transcription factor. If the observed oxidative-burst-like phenotype is caused by increased H2O2 secretion by the fungus (as postulated for Δcptf1 [27]), this could also be due to an increased ROS production, e.g., by enhanced levels of O2− produced by an NADPH oxidase (Nox). Nox activity and ROS production have been shown to be controlled by a second small GTPase, Rac, in mammalian systems and also in a filamentous fungus (9). Recently it could be shown that Cdc42 interacts antagonistically with Rac in control of O2− production: Cdc42 can act as an inhibitor of the Rac-mediated activation of Nox in mammalian cells (12); our finding of increased ROS levels in a cdc42 knockout is consistent with this idea.
We initiated these studies to characterize the role of the Cpcdc42 gene encoding a highly conserved Cdc42p-like protein. A high degree of structural conservation is common to Cdc42p-like proteins in eukaryotic organisms of distant relationship such as yeasts, flies, and mammals (Fig. 1). This was demonstrated in recent studies by heterologous complementation of S. cerevisiae cdc42 mutants with orthologs of W. dermatitidis (50) or A. gossypii (49) and also Caenorhabditis elegans (10), Drosophila melanogaster (35), and Homo sapiens (37). The structural similarities coincide with common functions within signal transduction pathways in all organisms investigated so far (20). However, these similarities (homology of interacting partners and influence on the cytoskeleton) do not reflect the differences in biological functions. Unlike in S. cerevisiae, S. pombe, and the closely related fungi C. albicans and A. gossypii, a deletion of Cdc42 is not lethal in more distantly related fungi such as W. dermatitidis, M. grisea, and P. marneffei. The data presented in our studies support the idea that in contrast to yeast, Cdc42 is not essential in filamentous fungi. The multitude of processes for which Cdc42 of yeasts acts as a signal transducer seems to be divided in filamentous fungi to Cdc42 and a second Rho GTPase, Rac (14). So far, to our knowledge nothing is known about the signal pathways in which CDC42 is integrated and its downstream targets in filamentous fungi. Since the presence of CDC42 in C. purpurea has impact on, but is not essential for, vegetative growth and is indispensable for pathogenicity, we possess a valuable tool for the identification of downstream components of Cpcdc42 and especially of those involved in pathogenesis of C. purpurea.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 629 “Molecular cell dynamics” to P.T.).
REFERENCES
- 1.Adams, A. E., D. I. Johnson, R. M. Longnecker, B. F. Sloat, and J. R. Pringle. 1990. CDC42 and CDC43, two additional genes involved in budding and the establishment of cell polarity in the yeast Saccharomyces cerevisiae. J. Cell Biol. 111:131-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 3.Austin, B., R. M. Hall, and B. M. Tyler. 1990. Optimized vectors and selection for transformation of Neurospora crassa and Aspergillus nidulans to bleomycin and phleomycin resistance. Gene 93:157-162. [DOI] [PubMed] [Google Scholar]
- 4.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidmann, J. A. Smith, and K. Struhl. 1987. Current protocols in molecular biology. John Wiley and Sons, New York, N.Y.
- 5.Bestwick, C. S., I. R. Brown, M. H. R. Bennett, and J. W. Mansfield. 1997. Localization of hydrogen peroxide accumulation during the hypersensitive reaction of lettuce cells to Pseudomonas syringae pv phaseolicola. Plant Cell 9:209-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyce, K. J., M. J. Hynes, and A. Andrianopoulos. 2001. The CDC42 homolog of the dimorphic fungus Penicillium marneffei is required for correct cell polarization during growth but not development. J. Bacteriol. 183:3447-3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cenis, J. L. 1992. Rapid extraction of fungal DNA for PCR amplification. Nucleic Acid Res. 20:2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chant, J., and L. Stowers. 1995. GTPase cascades choreographing cellular behavior: movement, morphogenesis, and more. Cell 81:1-4. [DOI] [PubMed] [Google Scholar]
- 9.Chen, C. B., and M. B. Dickman. 2004. Dominant active Rac and dominant negative Rac revert the dominant active Ras phenotype in Colletotrichum trifolii by distinct signalling pathways. Mol. Microbiol. 51:1493-1507. [DOI] [PubMed] [Google Scholar]
- 10.Chen, W., H. H. Lim, and L. Lim. 1993. The CDC42 homologue from Caenorhabditis elegans. Complementation of yeast mutation. J. Biol. Chem. 268:13280-13285. [PubMed] [Google Scholar]
- 11.Cvrckova, F., C. De Virgilio, E. Manser, J. R. Pringle, and K. Nasmyth. 1995. Ste20-like protein kinases are required for normal localization of cell growth and for cytokinesis in budding yeast. Genes Dev. 9:1817-1830. [DOI] [PubMed] [Google Scholar]
- 12.Diebold, B. A., B. Fowler, J. Lu, M. C. Dinauer, and G. M. Bokoch. 2004. Antagonistic cross-talk between Rac and Cdc42 GTPases regulates generation of reactive oxygen species. J. Biol. Chem. 279:28136-28281. [DOI] [PubMed] [Google Scholar]
- 13.Hakoshima, T., T. Shimizu, and R. Maesaki. 2003. Structural basis of the Rho GTPase signaling. J. Biochem. (Tokyo) 134:327-331. [DOI] [PubMed] [Google Scholar]
- 14.Harris, S. D., and M. Momany. 2004. Polarity in filamentous fungi: moving beyond the yeast paradigm. Fungal Genet. Biol. 41:391-400. [DOI] [PubMed] [Google Scholar]
- 15.Heslop-Harrison, J., and Y. Heslop-Harrison. 1981. The pollen-stigma interaction in the grasses. 2. Pollen-tube penetration and the stigma response in Secale. Acta Bot. Neerl. 30:289-307. [Google Scholar]
- 16.Hewitt, E. J. 1966. Sand and water culture methods used in the study of plant nutrition. Technical communication no. 22, rev. 2nd ed. Commonwealth Bureau of Horticulture and Plantation Crops. The Eastern Press, Ltd., London, United Kingdom.
- 17.Hoagland, D. R., and D. I. Arnon. 1938. The water-culture method for growing plants without soil. University of California Agricultural Experiment Station, circular 347. University of California, Berkeley, Calif.
- 18.Hood, M. E., and H. D. Shew. 1996. Applications of KOH-aniline blue fluorescence in the study of plant-fungal interactions. Phytopathoglogy 86:704-708. [Google Scholar]
- 19.Hüsgen, U., P. Büttner, U. Müller, and P. Tudzynski. 1999. Variation in karyotype and ploidy level among field isolates of Claviceps purpurea. J. Phytopathol. 147:591-597. [Google Scholar]
- 20.Johnson, D. I. 1999. Cdc42: an essential Rho-type GTPase controlling eukaryotic cell polarity. Microbiol. Mol. Biol. Rev. 63:54-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jungehülsing, U., C. Arntz, R. Smit, and P. Tudzynski. 1994. The Claviceps purpurea glyceraldehyde-3-phosphate dehydrogenase gene: cloning, characterization and use for improvement of a dominant selection system. Curr. Genet. 25:101-106. [DOI] [PubMed] [Google Scholar]
- 22.Manser, E., T. Leung, H. Salihuddin, Z. S. Zhao, and L. Lim. 1994. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature 367:40-46. [DOI] [PubMed] [Google Scholar]
- 23.Mantle, P. G., and L. J. Nisbet. 1976. Differentiation of Claviceps purpurea in axenic culture. J. Gen. Microbiol. 93:321-334. [DOI] [PubMed] [Google Scholar]
- 24.Matozaki, T., H. Nakanishi, and Y. Takai. 2000. Small G-protein networks: their crosstalk and signal cascades. Cell Signal 12:515-524. [DOI] [PubMed] [Google Scholar]
- 25.Moore, S., O. de Vries, and P. Tudzynski. 2002. The major Cu Zn SOD of the phytopathogen Claviceps purpurea is not essential for pathogenicity. Mol. Plant Pathol. 3:9-22. [DOI] [PubMed] [Google Scholar]
- 26.Müller, U., K. B. Tenberge, B. Oeser, and P. Tudzynski. 1997. Cel1, probably encoding a cellobiohydrolase lacking the substrate binding domain, is expressed in the initial infection phase of Claviceps purpurea on Secale cereale. Mol. Plant-Microbe Interact. 10:268-279. [DOI] [PubMed] [Google Scholar]
- 27.Nathues, E., S. Joshi, K. B. Tenberge, M. von den Driesch, B. Oeser, N. Bäumer, M. Mihlan, and P. Tudzynski. 2004. CPTF1 a CREB-like transcription factor is involved in the oxidative stress response in the phytopathogen Claviceps purpurea and modulates ROS level in its host Secale cereale. Mol. Plant-Microbe Interact. 17:383-393. [DOI] [PubMed] [Google Scholar]
- 28.Oeser, B., P. Heidrich, U. Müller, K. B. Tenberge, and P. Tudzynski. 2002. Polygalacturonase is a pathogenicity factor in the Claviceps purpurea/rye interaction. Fungal Genet. Biol. 36:176-186. [DOI] [PubMed] [Google Scholar]
- 29.Pažoutová, S., M. Kolarik, and R. Kolinska. 2004. Pleomorphic conidiation in Claviceps. Mycol. Res. 108:126-135. [PubMed] [Google Scholar]
- 30.Pruyne, D., and A. Bretscher. 2000. Polarization of cell growth in yeast. J. Cell Sci. 113:571-585. [DOI] [PubMed] [Google Scholar]
- 31.Read, N. D., L. J. Kellock, H. Knight, and A. J. Trewavas. 1992. Contact sensing during infection by fungal pathogens, p. 137-192. In J. A. Callow and J. R. Green (ed.), Perspectives in plant cell recognition. Cambridge University Press, Cambridge, United Kingdom
- 32.Roberts, I. N., R. P. Oliver, P. J. Punt, and C. A. van den Hondel. 1989. Expression of the Escherichia coli beta-glucuronidase gene in industrial and phytopathogenic filamentous fungi. Curr. Genet. 15:177-180. [DOI] [PubMed] [Google Scholar]
- 33.Rose, T. M., E. R. Schultz, J. G. Henikoff, S. Pietrokovski, C. M. McCallum, and S. Henikoff. 1998. Consensus-degenerate hybrid oligonucleotide primers for amplification of distantly related sequences. Nucleic Acids Res. 26:1628-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molacular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 35.Sasamura, T., T. Kobayashi, S. Kojima, H. Qadota, Y. Ohya, I. Masai, and Y. Hotta. 1997. Molecular cloning and characterization of Drosophila genes encoding small GTPases of the Rab and Rho families. Mol. Gen. Genet. 254:486-494. [DOI] [PubMed] [Google Scholar]
- 36.Scheffer, J., C. Ziv, O. Yarden, and P. Tudzynski. 2005. The COT homologue CPCOT1 regulates polar growth and branching and is essential for pathogenicity in Claviceps purpurea. Fungal Genetics Biol. 42:107-118. [DOI] [PubMed] [Google Scholar]
- 37.Shinjo, K., J. G. Koland, M. J. Hart, V. Narasimhan, D. I. Johnson, T. Evans, and R. A. Cerione. 1990. Molecular cloning of the gene for the human placental GTP-binding protein Gp (G25K): identification of this GTP-binding protein as the human homolog of the yeast cell-division-cycle protein CDC42. Proc. Natl. Acad. Sci. USA 87:9853-9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smit, R., and P. Tudzynski. 1992. Efficient transformation of Claviceps purpurea using pyrimidine auxotrophic mutants: cloning of the OMP decarboxylase gene. Mol. Gen. Genet. 234:297-305. [DOI] [PubMed] [Google Scholar]
- 39.Stacey, D. W., M. Roudebush, R. Day, S. D. Mosser, J. B. Gibbs, and L. A. Feig. 1991. Dominant inhibitory Ras mutants demonstrate the requirement for Ras activity in the action of tyrosine kinase oncogenes. Oncogene 6:2297-2304. [PubMed] [Google Scholar]
- 40.Sweet, R. W., S. Yokoyama, T. Kamata, J. R. Feramisco, M. Rosenberg, and M. Gross. 1984. The product of ras is a GTPase and the T24 oncogenic mutant is deficient in this activity. Nature 311:273-275. [DOI] [PubMed] [Google Scholar]
- 41.Tabin, C. J., S. M. Bradley, C. I. Bargmann, R. A. Weinberg, A. G. Papageorge, E. M. Scolnick, R. Dhar, D. R. Lowy, and E. H. Chang. 1982. Mechanism of activation of a human oncogene. Nature 300:143-149. [DOI] [PubMed] [Google Scholar]
- 42.Tenberge, K. B., V. Homann, B. Oeser, and P. Tudzynski. 1996. Structure and expression of two polygalacturonase genes of Claviceps purpurea oriented in tandem and cytological evidence for pectinolytic enzyme activity during infection of rye. Phytopathology 86:1084-1097. [Google Scholar]
- 43.Tenberge, K. B. 1999. Biology and life strategy of the ergot fungi, p. 25-56. In V. Køen and L. Cvak (ed.), Ergot: the genus Claviceps. Medicinal and aromatic plants, industrial profiles, vol. 6. CRC Press, Inc., Boca Raton, Fla.
- 44.Tudzynski, P., K. B. Tenberge, and B. Oeser. 1995. Claviceps purpurea, p. 161-187. In K. Kohmoto, U. S. Singh, and R. P. Singh (ed.), Pathogenesis and host specificity in plant diseases: histopathological, biochemical, genetic and molecular bases. Eukaryotes, vol. II. Elsevier Science Ltd., New York, N.Y
- 45.Tudzynski, P., and K. B. Tenberge. 2003. Molecular aspects of host pathogen interaction and of ergot alkaloid biosynthesis in Claviceps, pp. 445-473. In J. White, C. Bacon, N. L. Hywel-Jones, and I. W. Spatafora (ed.), The clavicipitalean fungi: evolutionary biology, chemistry, biocontrol and cultural impacts. Marcel Dekker, New York, N.Y.
- 46.Tudzynski, P., and J. Scheffer. 2004. Claviceps purpurea: molecular aspects of a unique pathogenic lifestyle. Mol. Plant Pathol. 5:377-388. [DOI] [PubMed] [Google Scholar]
- 47.Ushinsky, S. C., D. Harcus, J. Ash, D. Dignard, A. Marcil, J. Morchhauser, D. Y. Thomas, M. Whiteway, and E. Leberer. 2002. CDC42 is required for polarized growth in human pathogen Candida albicans. Eukaryot. Cell 1:95-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Engelenburg, F., R. Smit, T. Goosen, H. van den Broek, and P. Tudzynski. 1989. Transformation of Claviceps purpurea using a bleomycin resistance gene. Appl. Microbiol. Biotechnol. 30:364-370. [Google Scholar]
- 49.Wendland, J., and P. Philippsen. 2001. Cell polarity and hyphal morphogenesis are controlled by multiple rho-protein modules in the filamentous ascomycete Ashbya gossypii. Genetics 157:601-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ye, X., and P. J. Szaniszlo. 2000. Expression of a constitutively active Cdc42 homologue promotes development of sclerotic bodies but represses hyphal growth in the zoopathogenic fungus Wangiella (Exophiala) dermatitidis. J. Bacteriol. 182:4941-4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ziman, M., J. M. O'Brien, L. A. Ouellette, W. R. Church, and D. I. Johnson. 1991. Mutational analysis of CDC42Sc, a Saccharomyces cerevisiae gene that encodes a putative GTP-binding protein involved in the control of cell polarity. Mol. Cell. Biol. 11:3537-3544. [DOI] [PMC free article] [PubMed] [Google Scholar]