Abstract
Chemical risk assessment relies on knowledge of hazard, the dose–response relationship, and exposure to characterize potential risks to public health and the environment. A chemical with minimal toxicity might pose a risk if exposures are extensive, repeated, and/or occurring during critical windows across the human life span. Exposure assessment involves understanding human activity, and this activity is confounded by interindividual variability that is both biological and behavioral. Exposures further vary between the general population and susceptible or occupationally exposed populations. Recent computational exposure efforts have tackled these problems through the creation of new tools and predictive models. These tools include machine learning to draw inferences from existing data and computer-enhanced screening analyses to generate new data. Mathematical models provide frameworks describing chemical exposure processes. These models can be statistically evaluated to establish rigorous confidence in their predictions. The computational exposure tools reviewed here are oriented toward ‘high-throughput’ application, that is, they are suitable for dealing with the thousands of chemicals in commerce with limited sources of chemical exposure information. These new tools and models are moving chemical exposure and risk assessment forward in the 21st century.
1). Introduction
Sources of chemical emissions surround us, including emissions from industry, building materials, furnishings, and consumer products. Given the multiplicity of domestic and occupational human activities, there is a wide diversity of chemical exposures across individuals and the human life span. In ‘Risk Assessment in the Federal Government,’ the U.S. National Research Council delineated three aspects that must be considered when assessing chemical risk: toxicological hazard, biological dose–response, and exposure [102]. Toxicological hazard is the potential of a chemical to cause a specific adverse effect at some dose; dose-response information characterizes the intensity and duration of doses needed to cause an adverse effect in an organism. Both toxicity and dose-response are functions of how chemicals move through and perturb the body [1]. Exposure is a measure of the amount of a chemical that reaches an individual and dose is the mass of a chemical that enters an organism over time by one or more routes of exposure [177]. Although traditional exposure assessment methods have been successful at addressing individual chemicals and specific scenarios [49], there remains a significant backlog of chemical exposures which have not yet been addressed [13, 38].
New approach methodologies (NAMs) are being developed to address and prioritize data needs for preliminary determination of the risk posed by chemicals to the public health [*75, 149]. We consider NAMs to broadly include new experimental, in silico, and informatic approaches that can rapidly inform chemical risk assessments [44, *75]. Exposure NAMs have been envisioned variously and under many names [6, 8, 31, 39, 104, 108, 142]. Here, we review examples of exposure NAMs largely surrounding research currently being conducted at or in collaboration with the U.S. Environmental Protection Agency (EPA)’s ExpoCast (‘Exposure Forecasting’) project [25, 132] and several collaborators, although additional important examples from other agencies are provided, including Health Canada, the European Centre for Ecotoxicology and Toxicology of Chemicals (ECETOC), and the European Network of Reference Laboratories, Research Centres and Related Organisations (NORMAN).
As illustrated in Figure 1, exposure to humans or ecological receptors result from a complex web of pathways. Here, we review advances in high throughput exposure NAMs. We start with an overview of the broad types of relevant exposure pathways. We then detail specific NAMs that have been developed to better characterize these pathways, noting where improvements are needed. We then describe how these NAMs can be combined with hazard information to set chemical priorities, including the identification of susceptible or highly exposed populations. Together these new approaches allow greater certainty about our environment and its impact on public health [149].
Figure 1:
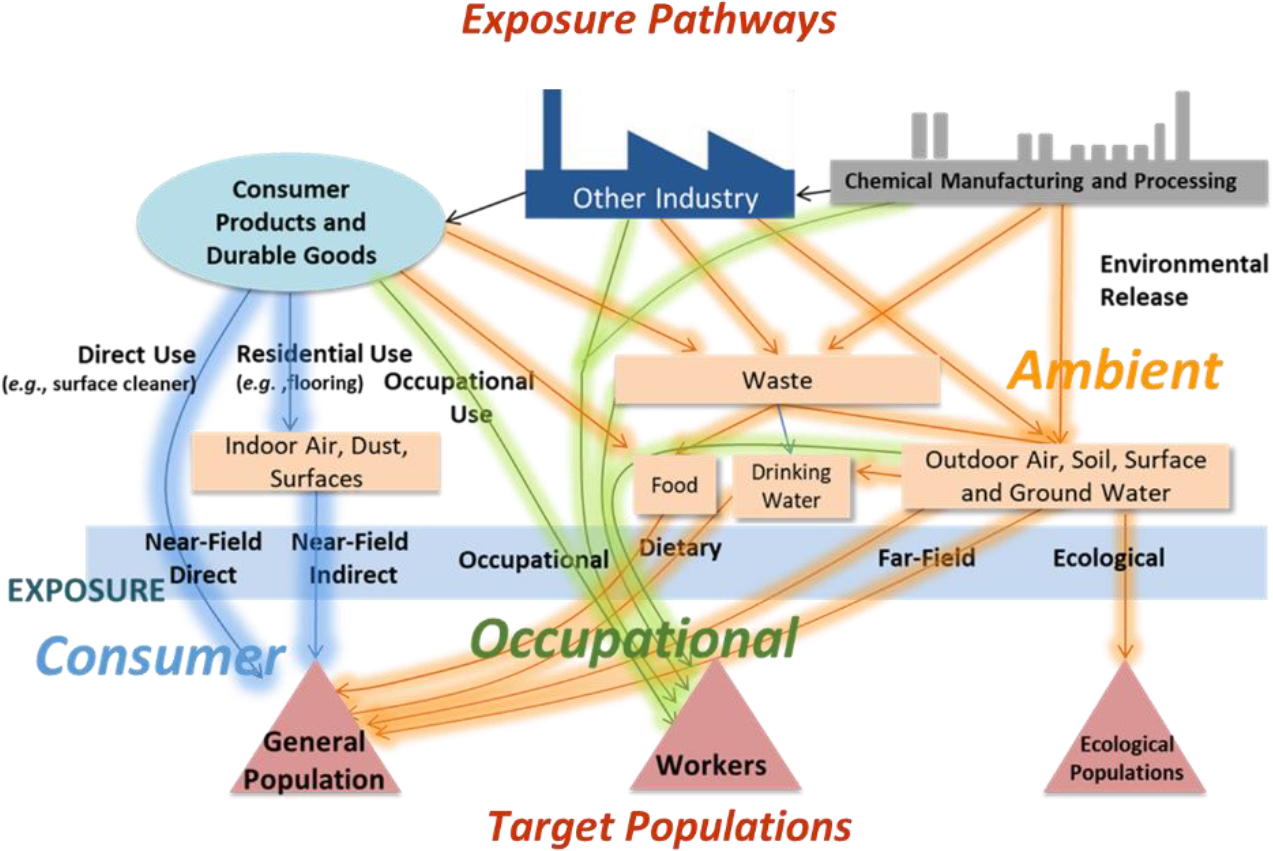
Chemical exposure arises from a diversity of pathways that involve human interactions and physical processes.
2). Exposure Pathways
As depicted in Figure 1, chemical exposure impacts both the public health and ecological endpoints. Human chemical exposures can be coarsely grouped into “near-field” sources in the home or at work (i.e., occupational sources) and in ‘far-field’ sources wherein individuals are exposed to chemicals that were released or used some distance away from the individual [3, 67]. Evaluation of near-field domestic chemical sources has emerged as a prominent topic in exposure research [63, 159]. Humans are exposed to these sources via consumer and occupational exposure pathways (Figure 1). However, describing far-field chemical releases and the resulting “ambient” exposures to humans who are not consumers or workers (i.e., the general population) remains critical to chemical risk assessment [47]. The methods described here are focused on public health, but many of the same tools can and have been adapted to address ecological impact of chemicals as well. We divide human exposure into following three broad categories.
Far-Field Sources of Chemical Exposure
Understanding chemical risk to public health requires detailed knowledge of the background concentration data in ambient environments and resulting exposure levels. Far-field sources include discharges to air, water, and soil by industrial activities and releases from end-of-life disposal of consumer products. Ambient exposure to chemicals from these sources occurs from inhalation of dust and vapors; dermal contact with water, soils, and dusts; and ingestion of food, water, and dust [111]. The parent compound released into the environment may itself be a toxicant of concern, or it may be a precursor to a toxicant that forms from the parent after release. Substances may be transformed by abiotic processes (for example, photolysis and hydrolysis) and biotic process (for example, microbial metabolism). The transformation of substances occurs almost everywhere so that the ‘transport’ (movement of a chemical in the environment) and ‘fate’ (transformation of the chemical) are linked, with fate being understood to include kinetics and equilibria. Thermodynamics underlie the chemical transformation of pollutants and their precursors. For example, raising temperature increases the rate of kinetics of a system.
The parent compound’s physical-chemical properties are the first determinant of how rapidly it will be transformed. These transformation processes parallel absorption, distribution, metabolism and excretion that take place inside an organism, albeit at different rates [90, 113, 144]. Environmental transport and fate processes involve the interrelationships between and within food webs, including humans [53]. The transfer of mass and energy up and down levels of biological organization can be measured and predicted. However, these data are not generally available for most compounds [38]. Currently, the international Human Toxicity Task Force recommends additional research to collect these data and reduce model and data uncertainties [165].
Near-Field Sources of Consumer Exposure
In contrast to “far-field” sources, “near-field” consumer chemical sources include consumer products, household furnishings, and building materials. Exposure to near-field sources may occur directly, as when consumer products are applied to the body, or indirectly via contact with contaminated residential air, dust, and/or surfaces. Volatile organic compounds (VOCs) and semi-volatile organic compounds (SVOCs) such as phthalates, flame retardant (FRs), and polychlorinated biphenyl (PCBs) are all classes of chemicals that have been found in indoor air, dust, and/or surfaces. As with far-field sources, however, the necessary exposure data are lacking [38]. Physical-chemical properties, information on identities and concentrations of chemicals present in consumer products or durable articles, and knowledge of how humans interact with chemicals in their environments are all examples of data required for exposure estimation that are often unknown.
Because adults spend roughly 80% of the day indoors [154] and have direct contact with many consumer products, exposures to near-field sources can contribute substantially to total exposure [157]. Using statistical analyses of biomonitoring data from the National Health and Nutrition Examination Survey (NHANES), Wambaugh et al. [159, 160] have demonstrated that use of a chemical in a near-field context is a predictor of higher levels of chemical intake. Efforts within EPA’s ExpoCast project and elsewhere have focused on compiling qualitative [33] and quantitative [*32, 52, 64, 101] data on the use of chemicals in consumer products. Chemicals may be used in multiple product types in different amounts. Therefore, characterizing the breadth of uses and the associated concentrations is critical to accurately assess potential aggregate exposure. While useful concentration information has been gleaned from Safety Data Sheets (SDS) [*32, 52, 64, 101] and reported ingredient lists [65] for consumer product formulations, these types of data sources do not include all chemicals in products. Undisclosed compounds may legally include chemicals present in small amounts (for example, <0.1 percent), proprietary ingredients, low-level contaminants of ingredients, components of ingredients that are themselves mixtures (for example, fixatives in fragrances) [*115], or degradation products. The composition of durable consumer goods (i.e., articles of commerce) and building materials prove to be particularly challenging because ingredient lists or SDS are not required. Therefore, analytical measurement of composition of relevant items and materials is still required to augment reported chemical data [7, 72, 77, *115, 174].
The presence of a chemical in, for example, a consumer product is a prerequisite for chemical exposure. However, chemicals must also be emitted from the matrix for exposure to occur [69, 83, 86, 175]. Emission properties can be understood using mass transfer models that describe the exchange of VOCs and SVOCs between sources, air, house dust, and interior surfaces in residential environments. To use these models, many empirical quantities must be known including adsorption/desorption rate constants, material/air partition coefficients, solid-phase diffusion coefficients, mass-transfer coefficients, and initial material phase concentration. The development of methods to measure emissions and determine these model parameters are essential to estimate indoor exposure. Relevant methodologies rely on a variety of emission chambers [151]. General principles and guidelines for expanding the overall experimental approaches and standardizing indoor exposure product testing protocols have been identified [151] to assess chemical exposures from indoor sources. The collected data and application of measurement-based modeling tools can then be used to refine and evaluate computational exposure models for rapidly predicting human exposures to chemicals [12].
Occupational Exposure
Occupational exposures to chemicals are estimated to cause at least 290,000 deaths globally every year [84]. The primary occupational exposure route is inhalation, followed by dermal exposure [111]. There are both great variability across time in the occupational environment for any one individual, and significant differences in the exposures between different individuals performing different jobs within the same workplace [111]. Although public tools exist for performing occupational exposure assessment (for example, chemical screening tool for exposures and environmental releases ((ChemSTEER) [30]), these tools are not yet capable of rapidly running large numbers of chemicals with minimal information. Similarly, while data are available on occupational exposure (such as from the U.S. Occupational Safety and Health Administration, https://www.osha.gov/opengov/healthsamples.html), the interfaces and data are focused on specific chemicals and scenarios. Although attempts are being made to enhance the speed of occupational exposure assessment [76], the models and informatics needed to rapidly address thousands of chemicals in occupational exposure scenarios are largely missing [147].
3). NAMs for exposure
Here, we consider NAMs to broadly include advances in both computational and measurement approaches that can rapidly inform chemical risk assessments (European Chemicals Agency (ECHA), 2016); [*75]. Exposure measurements collected in different settings such as homes and workplaces can characterize exposure and allow model evaluation [2,35,63,159]. However, collecting exposure measurements in real-world settings are resource intensive because of the costs associated with chemical analysis, maintaining a cohort of participants, training field personnel, and designing and maintaining sampling equipment. “Computational exposure” science provides tools to supplement more traditional approaches [39]. Some of these approaches are purely computational such as machine learning, database development, and sophisticated algorithmics (for example, frequent itemset mining [55]). However, errors and other limitations of the available data and models can result in incorrect exposure model selection and consequently increase uncertainty in exposure assessment [134]. Other NAMs include more traditional scientific techniques enhanced by modern computational power (for example, suspect-screening mode mass spectrometry).
Exposure Measurement
Experimental measurements are the foundation of any science. Measurements of exposure can identify unique scenarios or sources that may not have been previously anticipated. For example, an important residential source of PCBs — namely, wood floor finish — was only identified through deeper investigation of uniquely high house dust and indoor air concentrations of PCBs in some residences enrolled in the Cape Cod Household Exposure Study [124]. Furthermore, biomonitoring revealed skin-lightning cream as an important and unrecognized source of inorganic mercury among the most highly exposed [93].
NAMs for exposure measurement include suspect screening and nontargeted mass spectrometry analysis [*143]. These advanced mass spectrometry techniques for chemical screening and identification commonly use high resolution mass spectrometry, fundamental chemistry concepts, and extensive chemical reference lists to simultaneously identify hundreds or thousands of substances within a sample [*153]. These methods hold promise for filling gaps in reported chemical data. Suspect screening mass spectrometry has allowed researchers to confirm the presence or absence of hundreds to thousands of known chemicals in dust [97, 119], water [98, *106, 130], consumer products [*115], and serum [162]. To facilitate the potential use of measurement NAMs in decision-making, EPA’s Non-Targeted Analysis Collaborative Trial (ENTACT) is working to characterize the performance of different approaches, establish benchmarks, and develop reporting standards [*139, *153]. In Europe, the NORMAN network has already made use of suspect screening analysis to detect emerging drinking water contaminants [130], while in the United States, researchers have also allowed the detection of previously unknown chemicals such as those recently introduced into commerce [88, *106].
Biomonitoring
Exposure biomonitoring involves measuring parent chemicals and/or their metabolites in accessible biological media (for example, blood, urine) and using these measurements as “exposure biomarkers” [103]. Biomonitoring surveys of human populations have provided an invaluable resource for inferring chemical exposures, although they are not without their limitations [6, 137]. Exposures must be inferred from biomarkers [140, 146] but research frameworks have been proffered to realize the many benefits of biomonitoring data for exposure and risk assessors [78, 142]. First, biomarkers aggregate exposure across all relevant routes (for example, ingestion, inhalation, dermal contact). Thus, a single biomarker measure can reflect the culmination of multiple exposure scenarios and provide a solid foundation for exposure model evaluation [*120, 159, 160]. Biomarker variability over time is often less than actual exposure variability [5, 85], and so a modest number of measurements may accurately serve as surrogate exposure measures in observational studies [96, 141]. Concurrent measurement of multiple exposure biomarkers from the same individual can measure the variability in time of exposures in the surveyed individuals [22] and can characterize correlations within and co-occurrence between exposures [*73]. Finally, exposure biomarkers can be measured concurrently with effect biomarkers using a single biological specimen [8].
Advances in laboratory techniques are also expanding the chemical space coverage of biomonitoring studies, leading to the discovery of contaminants-of-emerging-concern [57]. Sobus, et al. [*143] present a framework to integrate results from exposome [171] studies with high-throughput exposure (HTE) estimates, particularly how presence/absence and semi-quantitative information is useful in HTE prediction, where orders of magnitude uncertainty are unsurprisingly more useful than a complete lack of data. The framework describes the interrelationships [119] between screening-level mass spectrometry and efforts such as ExpoCast and ToxCast (the EPA’s Toxicity Forecaster Project [34]). For example, screening data can be used to calibrate models that predict exposure (see Figure 2), and these data are viewed as “one innovative approach for identifying and setting priorities among chemicals for additional exposure assessment, hazard testing, and risk assessment…” [*100]. As these new methods begin to inform public health risk assessment, ongoing efforts to characterize, standardize, and report the confidence of these data remain extremely important [129, *139, *153].
Figure 2.
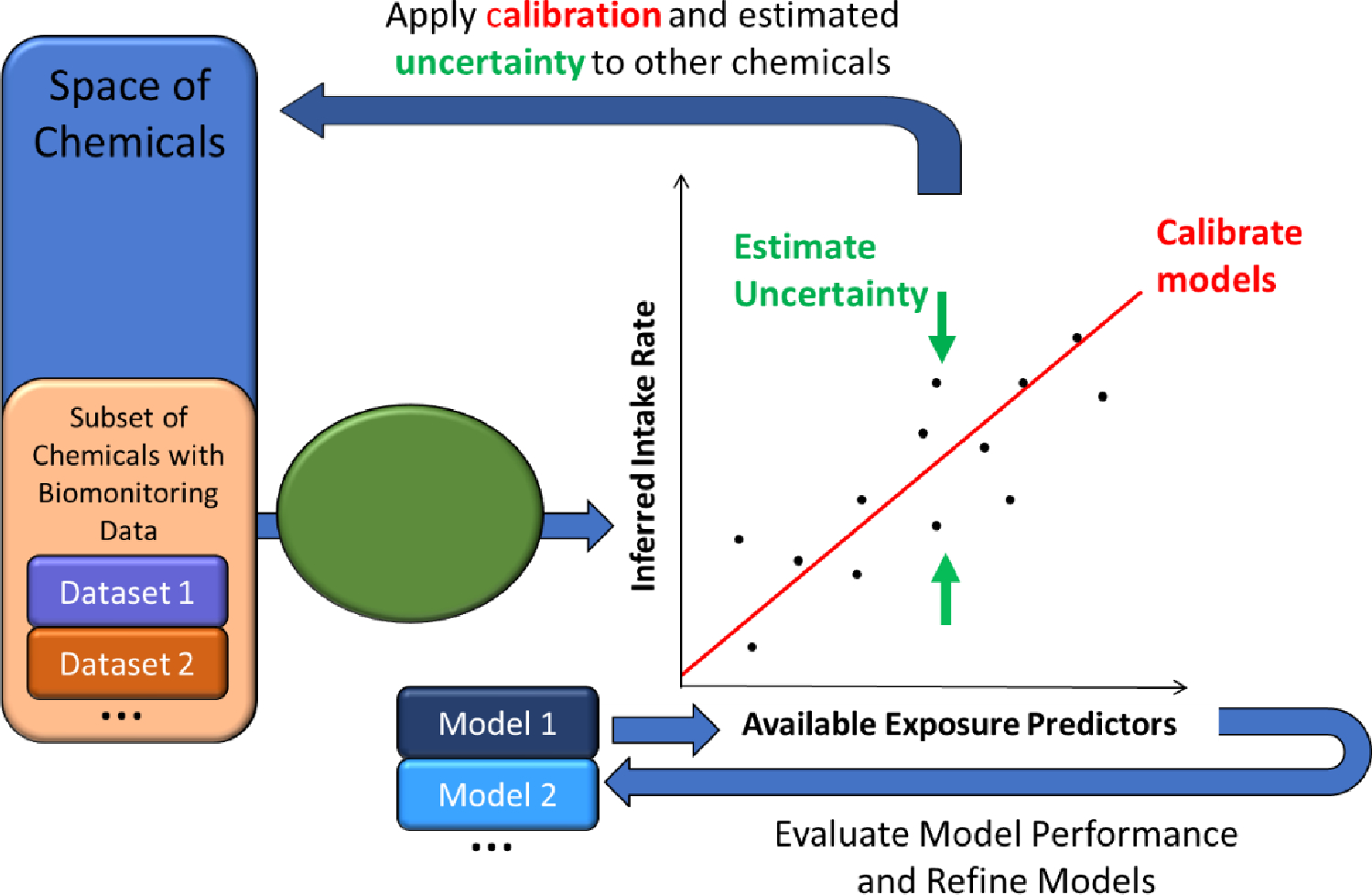
Systematic empirical evaluation of models (SEEM) uses Intake rates inferred from biomonitoring data to evaluate and calibrate exposure predictors across as many chemicals as possible. Exposure predictors include HTE models for predicting intake rates as well as presence on the chemicals on various lists (such as high production or banned). SEEM provides a quantitative estimate of uncertainty. HTE, high-throughput exposure.
The analysis of multiple biomarkers along an adverse outcome pathway enables molecular epidemiology investigations that can yield biomarker-based reference values for target chemicals [184]. Given the advantages of biomarkers, numerous national monitoring programs and large cohort studies have been deployed. Many of these programs routinely measure hundreds of biomarkers and aim to establish population reference ranges, track exposure trends, and examine links between exposure and disease [17, 79]. Guidance exists to enhance the utility of nationally representative study data for risk assessments [138]. However, newly discovered chemicals or chemicals with higher than expected exposures need to become priorities for toxicological screening [*143]. A recent workshop organized by the Helmholtz Centre for Environmental Research investigated ‘integrating the exposome approach with the adverse outcome pathway’ concept to better inform public health decision-making with respect to chemicals in the environment [43].
Toxicokinetics and Exposure Reconstruction
Toxicokinetics describes internal exposure, including the absorption, distribution, metabolism, and excretion of chemicals by the body and is important for understanding risk to public health [1. 26]. With toxicokinetic information, it is possible to predict internal tissue dosimetry via “forward” modeling, assuming a certain external exposure. Alternately, internal concentrations (such as from biomonitoring) can be used to predict an external exposure that would result in these internal concentrations (that is, “reverse dosimetry”) [50, 146]. Because most chemicals lack information on toxicokinetics [168, 170], high throughput toxicokinetics (HTTK) methods are needed. A series of studies have established HTTK methods for using in vitro assays to provide chemical-specific information allowing estimation of toxicokinetics, often within a factor of three [123, 158, 161, 164, 168, 169, 170, 178].
Toxicokinetics is heavily influenced by biological variability [66]. Quantifying human biological variability allows the development of HTTK models that can rapidly characterize the range and distribution of external and internal (tissue) exposures across individuals, allowing better identification of potentially sensitive subpopulations [*121, 167]. Data and tools to quantify human variability in exposure are increasingly available and accessible, for example the European Centre for Ecotoxicology and Toxicology of Chemicals (ECETOC) Human Exposure Assessment Tools Database (heatDB) (https://heatdb.cremeglobal.com/. In addition, the open-source R package httk [112] contains a module that uses NHANES data to simulate population physiological variability for use with HTTK modeling [121]. This tool simulates key aspects of biological variability in a correlated fashion, allowing the identification of the 95th percentile most “sensitive” individuals who experience higher plasma concentrations for the same exposure. These individuals require a lesser exposure to produce a given tissue concentration [166].
However, additional research is needed to reduce the uncertainty in the application of HTTK to exposure reconstruction [*135, 158, 161].
Humans also vary biologically with respect to their microbiome [37], which can significantly affect toxicokinetics [*183]. The microbiome is comprised of trillions of microbial cells [131] that collectively expands the metabolic capacity of the cooperative unit. A majority of bacteria reside in the intestinal tract [131] where they can interact with dietary compounds, pharmaceuticals, environmental chemicals, and xenobiotics present in consumer products [*19, 21, 29, 60, 114, 145]. While intestinal microbiota have increasingly been linked to the efficacy and toxicity of pharmacological compounds [173], the interaction between microbiota and environmental pollutants remains poorly understood. This is because it is difficult to separate the host from microbial metabolism. A recent report from the National Academies of Sciences, Engineering, and Medicine highlighted the need for risk assessment approaches to include an evaluation of the interaction between environmental chemicals and the microbiome [99]. If the impacts of host-associated microbes on tissue concentrations of chemicals can be further demonstrated, and the variation between human populations can be broadly characterized (for example, heavy antibiotic users, diets rich in fat and preservatives), then models may eventually be used to better characterize human exposure to chemicals [105].
High throughput Exposure (HTE) Models
Although biomonitoring and other exposure measurement studies may cover dozens or even hundreds of analytes, with tens of thousands of chemicals in commerce [155] a large number of known and unknown chemical–media combinations might best be addressed using models. These models can screen potential sources and routes of exposure to identify relevant chemicals beyond the usual suspects (for example, persistent organic pollutants, phthalates, and brominated flame retardants). While exposure modeling is a well-established field, newer ‘high-throughput’ models are a class of exposure NAMs whose development is ongoing. To be considered an HTE model, a model must:
be applicable to and capable of handling many chemicals with minimal descriptive information [3, 63, 122, 159];
cover one or more relevant exposure routes (for example, inhalation, food ingestion, mouthing, and dermal contact,) and sources (for example, industrial and residential use), accounting for the influential parameters relevant for the considered pathways [51, 179];
allow for integration with models for other pathways [47, 118, *120];
be scientifically plausible, respecting mass-balance principles and accounting for competing processes (for example volatilization versus dermal uptake) [90];
allow for the assessment of interindividual and intraindividual variation in exposure and impact of such variation on acute and chronic doses as the required input data becomes available [5, 117, *121];
be amenable to integration within statistical frameworks that quantify uncertainty for propagation into risk evaluations [2, *120, 159, 160, 168]; and
remain parsimonious, that is, no more complicated than necessary to reflect the data [20, 23, 46, 56].
This list was adapted from the study by Huang and Jolliet [59].
A series of HTE models have been developed for far-field environmental emissions such as primary and secondary fine particulates [9, 148], and for persistent organic pollutants [3, 94, 128, *165]. HTE models have also been constructed for the indoor environment [63, 81, 133], accounting for consumer product use and chemical release types and routes such as inhalation, dust ingestion, and dermal exposure. To further account for direct contact between household products and users, some product-specific models have been made available for cosmetics [28] or food contact materials [12, 42]. Many HTE models consider the fraction of the chemical inside a product that is taken in by users and overall population [59, 70].
HTE models can provide rough but quantitative estimates of exposure that can then be combined with quantitative estimates of toxicity [*121, *135, 168]. At the same time, substantial work is needed to consolidate model knowledge and accuracy of exposure pathways that are relatively poorly covered by high-throughput models such as mouthing, nondietary dust ingestion, air-to-skin dermal uptake, and occupational [76].
Exposed Populations in HTE Models
Human behavior is complex, and patterns such as consumer product use are difficult to quantify. However, it is important to continue to improve upon the description of these behaviors in HTE exposure models to provide the means to model population variability in exposure to chemicals. In general, these models use Monte Carlo (MC) simulation to draw from distributions for relevant model parameters based on substantial amounts of data regarding behavior. For example, the Stochastic Human Exposure and Dose Simulation High-Throughput (SHEDS-HT) model for residential and dietary exposures includes demographic data from the U.S. Census, dietary intake survey data from NHANES, and a large database of daily activity diaries covering more than 54,000 individual day entries [63]. Another example is the Creme Care model of consumer products exposures and Creme RIFM model of fragrance exposures which include a database of detailed consumer survey data collected from more than 36,000 consumers in the European Union and United States, characterizing variability in habits and practices regarding use of cosmetics, personal care products, and air freshener products [127]. Finally, the Probabilistic Aggregate Consumer Exposure Model (PACEM) for exposures to substances in personal care products includes use frequency and amount data from a survey of 512 Dutch adults [36].
An alternative approach to relying on survey data for human behavior is to model patterns of human behavior over time (longitudinal patterns). These models begin by defining the variation in the characteristics of the exposure population. An individual’s fixed characteristics are defined and used to simulate the individual’s behavior over time [117]. The simulation can be performed by combining records of daily activity patterns from multiple individuals [62], linking data from multiple surveys [180], or using agent based modeling [*15].
For any of these models, key parameters defining consumer product use patterns must be identified, for example, the percent of the population using the product (prevalence), frequency of use of the product, amount of product per use (mass), and duration of use. In addition, for some product categories, differentiation in use patterns by age, gender, ethnicity, or socioeconomic status may be appropriate and desired. In past studies, necessary input parameters to establish consumer product use patterns have been identified for individual product categories using a variety of sources, as no one established and authoritative source exists for data on consumer product use patterns. EPA’s Chemical and Products Database (CPDat) allows investigation of how and where chemicals are used [*32]. A handful of other sources also provide aggregated data [16, 27, 150]. However, there remain many product categories where availability of use pattern data are extremely limited or in some cases non-existent (for example, many arts and crafts, automotive care, and home maintenance related product categories), and in cases where data exist, they are often difficult to synthesize because findings are dependent on the survey design, population sampled, and method of data collection.
Chemical Descriptors
While the development of HTE models represents one class of NAMs, parallel advances in describing chemicals is a distinct class of NAMs. A key barrier to the use of HTE models is describing chemicals in a high-throughput manner, that is, how do we “parameterize” the models when we only know chemical structure [2]? Three types of input data describing each chemical are typically required for the HTE calculations: (1) physical—chemical properties, (2) how and how much chemical mass is applied, and (3), the characteristics of the exposed population [134].
Chemical Properties
Chemical structures and properties can be difficult to obtain; however, the EPA has compiled structures for more than 400,000 chemicals from lists with various degrees of curation (https://comptox.epa.gov/dashboard) [172]. While physical-chemical property measurements may not be available, properties can be predicted from structure [*92, 95, 125]. For chemicals that have the potential to dissociate (ionize) in ambient environments, most HTE models have to date made predictions based on only the neutral chemical properties of these chemicals in other high-throughput exposure model applications [2, 63, 159, 182]. Therefore, further measurements of chemical properties for these types of chemicals would improve exposure estimates [107, 144].
Chemical Release
Chemical mass released to the environment is important because exposure estimates from a single source are typically a direct linear function of the selected chemical mass applied, used, or released (Q), such that if the selected value for Q overestimates or underestimates actual chemical use/application quantity by a factor of n, then the exposure estimates will also include the factor of n error [2]. However, much of currently available data that can be used as surrogates for Q are either localized emissions or total (national) production volumes (TPVs). Several countries maintain some form of anthropogenic chemical release reporting [4, 41, 45, 152]. Production volume and industrial use data are often confidential or are only reported within large ranges that span several orders of magnitude for a given chemical. Furthermore, a portion of the produced volume of chemical might be used as an intermediate which is not released into the environment. Imports in and exports out of the country are also difficult to capture with TPVs. Finally, TPV estimates are not averaged values over multiple years, but from a single year. Thus, historical values accounting for import and export in a narrow range would significantly reduce uncertainty in exposure predictions.
A range of approaches have been developed to address chemical release data gaps, each with their own challenges and impacts on decision uncertainty. The approaches can be broadly categorized as “top-down” or “bottom-up”, although this terminology can vary in meaning. “Top-down” approaches include data mining [18], data mining with proxies [110], and inverse projection from environmental monitoring data [11]. Data mining can be a useful tool because it relies on existing data to model chemical releases by reconciling data in multiple reporting sources. This approach can be extended to groups of chemicals by using existing release data for a chemical as proxies for structurally similar chemicals. With inverse projection, releases are estimated by back-projecting chemical dispersion patterns from monitoring locations to known locations of emission point sources. “Bottom-up” approaches include process modeling and simulation [14, 30, 110, 136], production and consumption modeling [11, 82, 163, 181], and material (or substance) flow analysis [54, 80]. Process modeling and simulation can yield accurate release estimates provided sufficient detail is known about the activity being modeled. Production and consumption modeling uses chemical production or industrial use quantities and activity-specific emission factors to estimate releases. Substance flow analysis applies material balancing principles to the sequence of activities within a substance’s life cycle to determine the releases from each activity.
The chemical mass applied, used, or released is directly related to how the chemical is used. Possible exposure scenarios include direct oral intake of a product (for example, toothpaste), contact and contamination of food and beverages, direct dermal contact (for example, cosmetics applied to the skin), dermal contact to contaminants on indoor surfaces, and inhalation of contaminants in indoor and outdoor air [83, 86]. While the use scenarios, resulting exposure pathway, and relevant properties are available in some cases [*32], most often the necessary data are unknown. For example, the emission rate of a chemical from a product is critical but often unavailable information because it depends not only on the chemical but also on the properties of the matrix (product) itself [83, 86]. In these cases, the use of machine learning methods to predict chemical use from structure and properties can inform the potential exposure pathways in which a given chemical might be involved [*120].
Systematic Model Evaluation
To apply HTE models in a human health risk framework [*121, *135, 168], it is necessary to quantify the uncertainty in the HTE predictions [*120, 161]. One recent approach has been to treat chemicals for which monitoring data are available as representative of chemicals without such data. In this way, the uncertainty of HTE predictions for those chemicals may be estimated [109, 126, 159]. The predictions of models of human variability in exposure can be compared with population exposure biomonitoring data via toxicokinetic modeling, for purposes of evaluation and calibration [36, *120]. For example, the ExpoCast project has made use of the Systematic Empirical Evaluation of Models (SEEM) framework, as illustrated in Figure 2 [*120, 159, 160].
Machine Learning
One constant from exposure measurement, through modeling, to chemical properties is a lack of data. As existing data are compiled into databases and new data sets are generated, machine learning has become an increasingly common approach to fill remaining gaps and is therefore the final class of NAMs described here. At its most basic description, machine learning is the development of mathematical models to understand data (VanderPlas, 2016). The available information is used to build (“train”) and evaluate predictive models for extrapolation. A common application of machine learning models in exposure science (as well as drug-discovery and toxicology) is quantitative activity/structure-property relationships (QSAR/QSPRs) which use the measured or reported activity or property of known chemicals to predict that same activity or property for a chemical where it is unknown (Leach, 2001). Successful machine learning methods can identify complex, multivariate relationships among large numbers of chemical descriptors which might describe the presence or absence of thousands of structural features [176, 177]. Machine-learning models have been used to predict migration of chemicals from food packaging to food [12], chemical function within consumer products [64, *116], weight fractions of chemicals in consumer products [64], fraction of unbound xenobiotics in human plasma 61], physical-chemical properties used in exposure modeling [92], and chemical exposure pathways [*120]. As new exposure data are acquired, and subsequent data needs are identified, existing machine learning models can be re-trained with new and existing data to improve model accuracy and adjust the scope of the model (i.e., sensitive vs. general populations, consumer vs. industrial chemicals, or specific chemical class vs. broad chemical coverage).
4). Risk-based Prioritization with Exposure NAMs
All the classes of NAMs described in Table 1 can be used together when trying to identify chemicals for additional testing [40]. Given that there are thousands of chemicals in commerce that have undergone limited human health safety evaluation [156], it is hoped that high-throughput bioactivity screening (for example, ToxCast, Tox21), HTTK, and HTE models can, respectively, provide alternatives to traditional hazard, dose-response, and exposure data in priority setting. Early efforts to use ToxCast high-throughput toxicity testing data in chemical risk prioritization were limited by the inability to link bioactive in vitro concentrations to a meaningful exposure metric. Despite the inherent uncertainties in coverage of biological pathways, toxicokinetic processes, and exposure variability, if the uncertainty in these NAMs can be quantified, then it may be possible to prioritize chemicals with respect to potential risk, as in Figure 3. The value of these NAMs in prioritization efforts has been consistently recognized by experts and stakeholders across the sectors [*100].
Table 1.
New approach methodologies for exposure science.
| Exposure NAM class | Description | Traditional Approach | Makes use of |
|||||
|---|---|---|---|---|---|---|---|---|
| Measurement | Toxicokinetics | Models | Descriptors | Evaluation | Machine learning | |||
|
| ||||||||
| Measurements | New techniques including screening analyses capable of detecting hundreds of chemicals present in a sample | Targeted (chemical-specific) analyses | – | • | • | • | • | |
| Toxicokinetics | High-throughput methods using in vitro data to generate chemical-specific models | Analyses based on in vivo animal studies | • | – | • | • | ||
| HTE models | Models capable of making predictions for thousands of chemicals | Models requiring detailed, chemical- and scenario- specific information | • | • | – | • | ||
| Chemical descriptors | Informatic approaches for organizing chemical information in a machine- readable format | Tools targeted at single- chemical analyses by humans | – | • | ||||
| Evaluation | Statistical approaches that use the data from many chemicals to estimate the uncertainty in a prediction for a new chemical | Comparison of model predictions to data on a per- chemical basis | • | • | • | • | – | • |
| Machine learning | Computer algorithms to identify patterns | Manual inspection of the data | • | • | • | – | ||
| Prioritization | Integration of exposure and other NAMs to identify chemicals for follow-up study | Expert decision-making | • | • | • | • | • | • |
HTE, high-throughput exposure; NAMs, new approach methodologies.
We describe six broad categories of NAMs that are being used or applied to inform exposure along with other chemical risk prioritization NAMs (Table 1).
• indicates that the NAM also makes use of the NAM in the indicated column, while - indicates that these are the same NAM.
Figure 3:
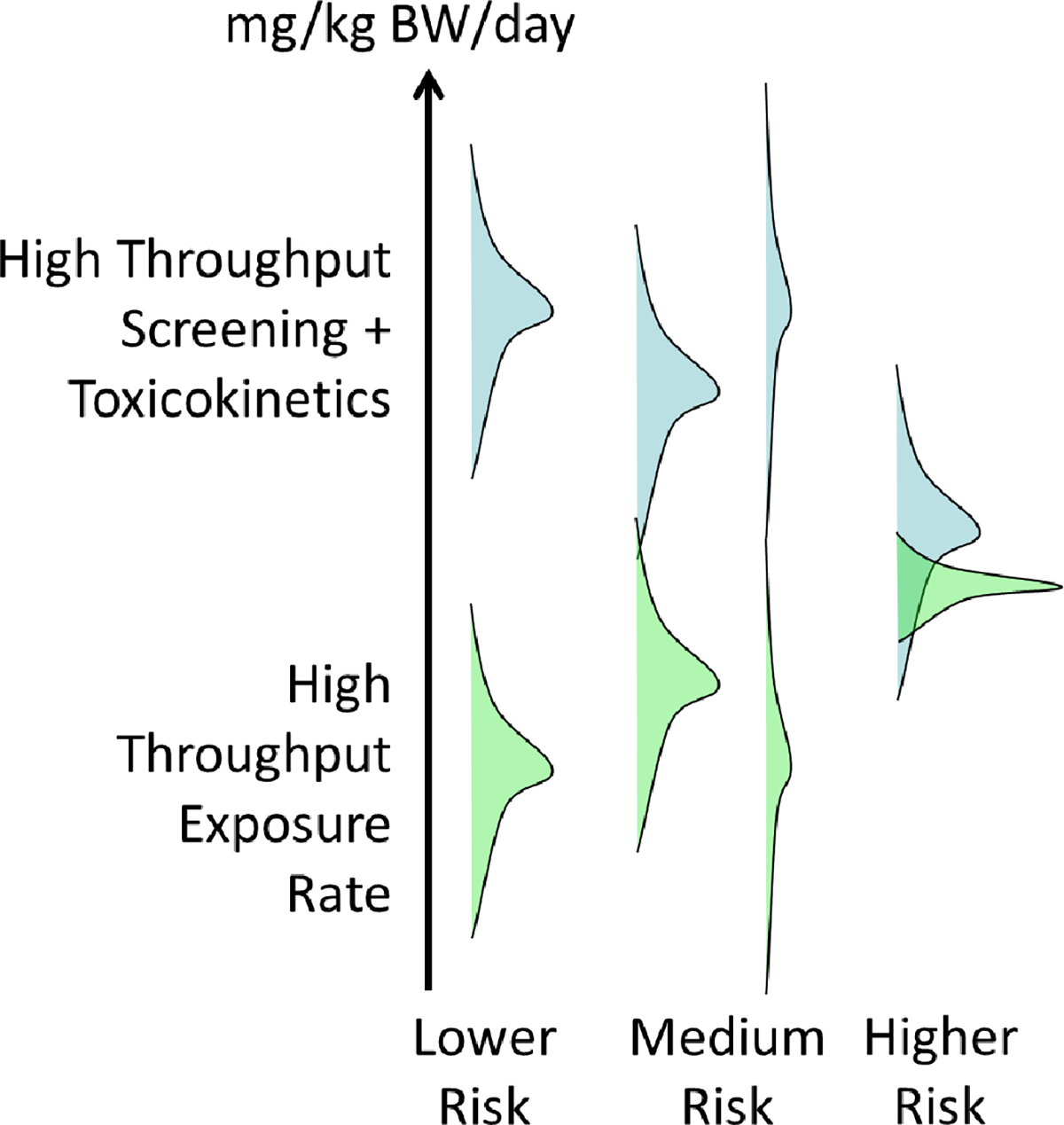
High-throughput methods may trade off precisions for speed, but if the uncertainty in the methods can be quantified, then the methods may still be useful for separating chemicals based on likelihood of risk.
For example, Health Canada has moved from focusing on a single chemical at a time to higher throughput methods applicable to most chemicals in commerce [13]. Bonnell et al. [13] write that regulatory prioritizations “…can therefore greatly benefit from inclusion of exposure descriptors and, by extension, exposure modeling to improve targeting of chemicals of highest concern.” HTE models for fate and human exposure [3] were used by Health Canada when the relevant environmental concentration and biomonitoring data were not available to identify the chemicals of ‘highest concern’ [13]. Recently, the EPA has proposed a working approach for selecting chemical candidates for chemical prioritization that includes risk-based metrics developed from both toxicity and exposure NAMs (U.S. Environmental Protection Agency, 2018).
Considerations for Susceptible Populations
In the United States, the recently updated Frank R. Lautenberg Chemical Safety for the 21st Century Act specifically calls for consideration of “potentially exposed or susceptible subpopulations,” defined as a groups “who, due to either greater susceptibility or greater exposure, may be at greater risk than the general population of adverse health effects from exposure to a chemical substance or mixture, such as infants, children, pregnant women, workers, or the elderly’’. Susceptibility to harmful effects to environmental chemicals is greater at certain periods in the life span and is influenced by genetic factors as well as exposure to other stressors [48, 91]. Periods of the life span known to be more susceptible to perturbation than others include during fetal, infant, and child development, spermatogenesis and oocyte maturation before conception, puberty, pregnancy, postpartum, and menopause [10, 48, 58, 68]. In addition to greater susceptibility, greater exposures may be experienced across the life span. For example, children and infants have unique exposure pathways that result in higher exposure than the general population for some chemicals (U.S. EPA, 2006). These pathways include soil and dust ingestion [185], breast milk and formula ingestion [186], and enhanced hand-to-mouth and object-to-mouth chemical transfer (U.S. EPA, 2006). Children also consume more food and water and have higher inhalation rates per unit body weight than adults (U.S. EPA, 2002), which also results in higher exposure rates. Therefore, comprehensive understanding of exposure across the life span, including in the testicular, ovarian, and fetal compartments, is necessary to fully characterize risk for susceptible populations. Various toxicokinetic (TK) models have been built to link exposure during gestation [89] and breast-feeding [24] to tissue concentrations, but these models continue need revision [74] and are typically tailored to a single chemical (that is, not high throughput). Generic TK models do exist for some routes important to occupational exposure, such as inhalation [71], but chemical-specific parameters for these models cannot yet be rapidly generated. Significant work remains in characterizing susceptible and highly exposed individuals with high-throughput methods [∗121, 167].
The only HTE models with quantified uncertainty have large (orders of magnitude) uncertainties when only chemical structure can be provided as input [*120]. Furthermore, while different median intake rates can be estimated for various population demographics [160], these approaches do not yet assess individuals who experience “greater exposure” because of limitations in sample sizes of the biomonitoring data used for evaluation. Finally, exposure to chemicals is often correlated, with various combinations of chemical exposures tending to be significantly more or less likely [*73]. Uncertainty in the HTE model, results for various age groups can be reduced by the development of improved pathway-specific HTE models that examine exposure variability across the life cycle (for example, refined consumer models) or for pathways that are specific to a susceptible population (for example, occupational pathways). Improved understanding of highly exposed populations and chemical co-exposures must be addressed by via the development of new biomonitoring data (for example, from next-generation mass spectrometry) and its integration into evaluation frameworks. Although such approaches may be more uncertain than traditional monitoring, the collection of information covering a larger sample size or an expanded chemical space can aid in identifying and quantifying exposures for highly exposed groups.
5). Conclusion
The exposure data available for chemicals in commerce are limited, which contributes to uncertainties in risk assessment [13, 38]. It is impractical, if not impossible, to acquire all the needed exposure data using traditional methods, and thus, there is a focus on developing higher throughput NAMs. For example, while HTE models can currently characterize exposure for exposure from ambient (far-field) sources and consumer product sources, additional work is needed for occupational and susceptible population scenarios and dietary pathways. Better characterization (including measurements) of the breadth of pathways, scenarios, and human biological variability will allow continued advances in HTE modeling and reduction in uncertainty.
Here, we have reviewed six broad classes of NAMs for exposure, starting with measurement methods. Additional measurement data are needed to improve HTE models. Screening analyses of environmental media are still in their infancy and not without limitations, yet they have already contributed greatly to reducing uncertainty in exposure data in two ways: 1) confirming the presence/absence of known chemicals; and 2) identifying previously unknown chemicals. We then reviewed exposure inference, which requires HTTK to address the large number of chemicals in commerce. We reviewed HTE models, and how they can fill gaps that cannot be addressed even with NAMs for measurement. Just as importantly, we reviewed NAMs for chemical descriptors, including the quantitative chemical concentration and emission data required for using HTE models. Because the measured presence of a chemical in environmental media is only a prerequisite to exposure [69, 83, 87, 175], it remains of critical importance to characterizing the emissivity of chemicals, which depends both on the chemical and the media in which it is found. We then reviewed how systematic statistical evaluation of HTE models using inferred exposures allows for the uncertainty quantification needed for prioritization. Finally, we reviewed how all other exposure NAMs can be informed by machine learning. We ended up with examples drawing together the six classes of NAMs to perform exposure- and risk-based prioritization of chemicals.
While great strides have been made to advance high-throughput methods for hazard identification, continued advances in exposure assessment will help to establish the real-world, human health consequences for chemicals in our environment.
Acknowledgements:
The authors thank Drs. Peter Egeghy and Maureen Gwinn for their helpful reviews of the manuscript.
Footnotes
Disclaimer: The United States Environmental Protection Agency through its Office of Research and Development primarily funded the research described here. The views expressed in this publication are those of the authors and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency. Reference to commercial products or services does not constitute endorsement.
References
- 1.Andersen ME: Toxicokinetic modeling and its applications in chemical risk assessment. Toxicol Lett 2003, 138:9–27. [DOI] [PubMed] [Google Scholar]
- 2.Arnot JA, Brown TN, Wania F, Breivik K, McLachlan MS: Prioritizing chemicals and data requirements for screening-level exposure and risk assessment. Environ Health Perspect 2012, 120:1565–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnot JA, MacKay D, Webster E, Southwood JM: Screening level risk assessment model for chemical fate and effects in the environment. Environ Sci Technol 2006, 40:2316–2323. [DOI] [PubMed] [Google Scholar]
- 4.Australian Government Department of the Environment and Energy: National Pollutant Inventory. 2019. Available at: http://www.npi.gov.au/ [Accessed].
- 5.Aylward LL, Kirman CR, Adgate JL, McKenzie LM, Hays SM: Interpreting variability in population biomonitoring data: role of elimination kinetics. J Expo Sci Environ Epidemiol 2012a, 22:398. [DOI] [PubMed] [Google Scholar]
- 6.Aylward LL, Kirman CR, Schoeny R, Portier CJ, Hays SM: Evaluation of biomonitoring data from the CDC National Exposure Report in a risk assessment context: perspectives across chemicals. Environ Health Perspect 2012b, 121: 287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartsch J, Uhde E, Salthammer T: Analysis of odour compounds from scented consumer products using gas chromatography-mass spectrometry and gas chromatography-olfactometry. Anal Chim Acta 2016, 904:98–106. [DOI] [PubMed] [Google Scholar]
- 8.Bell SM, Edwards SW: Identification and prioritization of relationships between environmental stressors and adverse human health impacts. Environ Health Perspect 2015, 123: 1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bey I, Jacob DJ, Yantosca RM, Logan JA, Field BD, Fiore AM, Li Q, Liu HY, Mickley LJ, Schultz MG: Global modeling of tropospheric chemistry with assimilated meteorology: model description and evaluation. J Geophys Res Atmos 2001, 106:23073–23095. [Google Scholar]
- 10.Bhardwaj J, Saraf P: Influence of toxic chemicals on female reproduction: a review. Cell Biol Res Ther 2014, 3:2. [Google Scholar]
- 11.Bie P, Fang X, Li Z, Wang Z, Hu J: Emissions estimates of carbon tetrachloride for 1992–2014 in China. Environ Pollut 2017, 224:670–678. [DOI] [PubMed] [Google Scholar]
- 12.Biryol D, Nicolas CI, Wambaugh J, Phillips K, Isaacs K: High-throughput dietary exposure predictions for chemical migrants from food contact substances for use in chemical prioritization. Environ Int 2017, 108:185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonnell MA, Zidek A, Griffiths A, Gutzman D: Fate and expo-sure modeling in regulatory chemical evaluation: new directions from retrospection. Environ Sci: Proc Impact 2018, 20:20–31. [DOI] [PubMed] [Google Scholar]
- 14.Bouarar I, Brasseur G, Petersen K, Granier C, Fan Q, Wang X, Wang L, Ji D, Liu Z, Xie Y: Influence of anthropogenic emission inventories on simulations of air quality in China during winter and summer 2010. Atmos Environ 2019, 198: 236–256. [Google Scholar]
- 15. Brandon N, Dionisio KL, Isaacs K, Tornero-Velez R, Kapraun D, Setzer RW, Price PS: Simulating exposure-related behaviors using agent-based models embedded with needs-based artificial intelligence. J Expo Sci Environ Epidemiol 2018, 1. * The authors created an agent-based model (ABM) for simulating longitudinal human exposure to chemicals and other stressors. The ABM simulates human decision-making in how to meet needs in order allowing detailed analysis of the duration, location of exposures as well as co-occurring exposures.
- 16.Bremmer H, Prud’homme de Lodder L, van Engelen J: Cosmetics Fact Sheet: to assess the risks for the consumer; updated version for ConsExpo 4. 2011:1–77. Report No. RIVM 320104001/2006; 2006. [PubMed] [Google Scholar]
- 17.Calafat AM: The U.S. National Health and Nutrition Examination Survey and human exposure to environmental chemicals. Int J Hyg Environ Health 2012, 215:99–101. [DOI] [PubMed] [Google Scholar]
- 18.Cashman SA, Meyer DE, Edelen AN, Ingwersen WW, Abraham JP, Barrett WM, Gonzalez MA, Randall PM, Ruiz-Mercado G, Smith RL: Mining available data from the United States Environmental Protection Agency to support rapid life cycle inventory modeling of chemical manufacturing. Environ Sci Technol 2016, 50:9013–9025. [DOI] [PubMed] [Google Scholar]
- 19. Catron TR, Swank A, Wehmas LC, Phelps D, Keely SP, Brinkman NE, et al. : Microbiota alter metabolism and mediate neurodevelopmental toxicity of 17b-estradiol. Sci Rep 2019, 9:7064. * The authors used two groups of zebra fish that were either colonized (present and functioning microbiome) or axenic (microbe-free). The authors demonstrated that microbial colonization status may influence chemical toxicity.
- 20.Chiu WA, Barton HA, DeWoskin RS, Schlosser P, Thompson CM, Sonawane B, Lipscomb JC, Krishnan K: Evaluation of physiologically based pharmacokinetic models for use in risk assessment. J Appl Toxicol 2007, 27:218–237. [DOI] [PubMed] [Google Scholar]
- 21.Cho I, Yamanishi S, Cox L, Methe BA, Zavadil J, Li K, Gao Z, Mahana D, Raju K, Teitler I, et al. : Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012, 488:621–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christensen KL, Makris SL, Lorber M: Generation of hazard indices for cumulative exposure to phthalates for use in cumulative risk assessment. Regul Toxicol Pharmacol 2014, 69: 380–389. [DOI] [PubMed] [Google Scholar]
- 23.Clewell RA, Clewell III HJ: Development and specification of physiologically based pharmacokinetic models for use in risk assessment. Regul Toxicol Pharmacol 2008, 50:129–143. [DOI] [PubMed] [Google Scholar]
- 24.Clewell RA, Gearhart JM: Pharmacokinetics of toxic chemicals in breast milk: use of PBPK models to predict infant exposure. Environ Health Perspect 2002, 110:A333–A337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen Hubal EA: Biologically relevant exposure science for 21st century toxicity testing. Toxicol Sci 2009, 111:226–232. [DOI] [PubMed] [Google Scholar]
- 26.Cohen Hubal EAC, Wetmore BA, Wambaugh JF, El-Masri H, Sobus JR, Bahadori T: Advancing internal exposure and physiologically-based toxicokinetic modeling for 21st-century risk assessments. J Expo Sci Environ Epidemiol 2019, 29: 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Consumer Product Ingredient Safety: Exposure and risk screening methods for consumer product ingredients. American Cleaning Institute; 2010. [Google Scholar]
- 28.Csiszar SA, Ernstoff AS, Fantke P, Meyer DE, Jolliet O: High-throughput exposure modeling to support prioritization of chemicals in personal care products. Chemosphere 2016, 163:490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daisley BA, Trinder M, McDowell TW, Collins SL, Sumarah MW, Reid G: Microbiota-mediated modulation of organophosphate insecticide toxicity by species-dependent interactions with lactobacilli in a Drosophila melanogaster insect model. Appl Environ Microbiol 2018, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daniels W, Lee S, Miller A: EPA’s exposure assessment tools and models. Appl Occup Environ Hyg 2003, 18:82–86. [DOI] [PubMed] [Google Scholar]
- 31.Dennis KK, Auerbach SS, Balshaw DM, Cui Y, Fallin MD, Smith MT, Spira A, Sumner S, Miller GW: The importance of the biological impact of exposure to the concept of the expo-some. Environ Health Perspect 2016, 124:1504–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dionisio K, Phillips K, Price P, Grulke C, Williams A, Biryol D, Hong T, Isaacs K: The Chemical and Products Database, a resource for exposure-relevant data on chemicals in consumer products. Scientific Data; 2018. * The authors created a new, publicly searchable database identifying 75,000 chemicals in more than 15,000 consumer products. The database is accessible at: https://comptox.epa.gov/dashboard
- 33.Dionisio KL, Frame AM, Goldsmith M-R, Wambaugh JF, Liddell A, Cathey T, Smith D, Vail J, Ernstoff AS, Fantke P: Exploring consumer exposure pathways and patterns of use for chemicals in the environment. Toxicology Reports 2015, 2: 228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dix DJ, Houck KA, Martin MT, Richard AM, Setzer RW, Kavlock RJ: The ToxCast program for prioritizing toxicity testing of environmental chemicals. Toxicol Sci 2007, 95: 5–12. [DOI] [PubMed] [Google Scholar]
- 35.Dodson RE, Camann DE, Morello-Frosch R, Brody JG, Rudel RA: Semivolatile organic compounds in homes: strategies for efficient and systematic exposure measurement based on empirical and theoretical factors. Environ Sci Technol 2014, 49:113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dudzina T, Delmaar CJ, Biesterbos JW, Bakker MI, Bokkers BG, Scheepers PT, van Engelen JG, Hungerbuehler K, Von Goetz N: The probabilistic aggregate consumer exposure model (PACEM): validation and comparison to a lower-tier assessment for the cyclic siloxane D5. Environ Int 2015, 79:8–16. [DOI] [PubMed] [Google Scholar]
- 37.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA: Diversity of the human intestinal microbial flora. Science 2005, 308: 1635–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Egeghy PP, Judson R, Gangwal S, Mosher S, Smith D, Vail J, Cohen Hubal EA: The exposure data landscape for manufactured chemicals. Sci Total Environ 2012, 414:159–166. [DOI] [PubMed] [Google Scholar]
- 39.Egeghy PP, Sheldon LS, Isaacs KK, Özkaynak H, Goldsmith M-R, Wambaugh JF, Judson RS, Buckley TJ: Computational expo-sure science: an emerging discipline to support 21st-century risk assessment. Environ Health Perspect 2016, 124:697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Egeghy PP, Vallero DA, Hubal EAC: Exposure-based prioritization of chemicals for risk assessment. Environ Sci Pol 2011, 14:950–964. [Google Scholar]
- 41.Environment and Climate Change Canada: Air pollutant emis-sions inventory. Available at: https://www.canada.ca/en/environment-climate-change/services/pollutants/air-emissions-inventory-overview.html; 2019. [Accessed].
- 42.Ernstoff A, Niero M, Muncke J, Trier X, Rosenbaum RK, Hauschild M, Fantke P: Challenges of including human expo-sure to chemicals in food packaging as a new exposure pathway in life cycle impact assessment. Int J Life Cycle Assess 2018:1–10. [Google Scholar]
- 43.Escher BI, Hackermüller J, Polte T, Scholz S, Aigner A, Altenburger R, Böhme A, Bopp SK, Brack W, Busch W: From the exposome to mechanistic understanding of chemical-induced adverse effects. Environ Int 2017, 99:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.European Chemicals Agency (ECHA): New approach method-ologies in regulatory science. In Proceedings of a scientific workshop; 2016. [Helsinki]. [Google Scholar]
- 45.European Environment Agency: National inventories for green-house gases and other air pollutants. 2019. Available at: https://www.eea.europa.eu/data-and-maps/data/national-emissions-reported-to-the-convention-on-long-range-transboundary-air-pollution-lrtap-convention-12 [Accessed].
- 46.Fantke P, Aylward L, Bare J, Chiu WA, Dodson R, Dwyer R, Ernstoff A, Howard B, Jantunen M, Jolliet O: Advancements in life cycle human exposure and toxicity characterization. Environ Health Perspect 2018, 126:125001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fantke P, Ernstoff AS, Huang L, Csiszar SA, Jolliet O: Coupled near-field and far-field exposure assessment framework for chemicals in consumer products. Environ Int 2016, 94: 508–518. [DOI] [PubMed] [Google Scholar]
- 48.Faustman EM, Silbernagel SM, Fenske RA, Burbacher TM, Ponce RA: Mechanisms underlying Children’s susceptibility to environmental toxicants. Environ Health Perspect 2000, 108(suppl 1):13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Furtaw EJ: An overview of human exposure modeling activities at the USEPA’s National Exposure Research Laboratory. Toxicol Ind Health 2001, 17:302–314. [DOI] [PubMed] [Google Scholar]
- 50.Georgopoulos PG, Sasso AF, Isukapalli SS, Lioy PJ, Vallero DA, Okino M, Reiter L: Reconstructing population exposures to environmental chemicals from biomarkers: challenges and opportunities. J Expo Sci Environ Epidemiol 2009, 19:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Georgopoulos PG, Wang S-W, Yang Y-C, Xue J, Zartarian VG, Mccurdy T, Özkaynak H: Biologically based modeling of multimedia, multipathway, multiroute population exposures to arsenic. J Expo Sci Environ Epidemiol 2008, 18:462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goldsmith MR, Grulke CM, Brooks RD, Transue TR, Tan YM, Frame A, Egeghy PP, Edwards R, Chang DT, Tornero-Velez R, et al. : Development of a consumer product ingredient database for chemical exposure screening and prioritization. Food Chem Toxicol 2014, 65:269–279. [DOI] [PubMed] [Google Scholar]
- 53.Graedel TE: On the concept of industrial ecology. Annu Rev Energy Environ 1996, 21:69–98. [Google Scholar]
- 54.Guzman AC: Environmental exposure of nanomaterials: methodological advances and new predictions. ETH Zurich; 2017. [Google Scholar]
- 55.Han J, Pei J, Yin Y. Mining frequent patterns without candidate generation, vol. 29. ACM; 2000:1–12. [Google Scholar]
- 56.Hauschild MZ, Huijbregts M, Jolliet O, MacLeod M, Margni M, van de Meent D, Rosenbaum RK, McKone TE: Building a model based on scientific consensus for life cycle impact assessment of chemicals: the search for harmony and parsimony. ACS Publications; 2008. [DOI] [PubMed] [Google Scholar]
- 57.Hollender J, Schymanski EL, Singer HP, Ferguson PL: Nontarget screening with high resolution mass spectrometry in the environment: ready to go? Environ Sci Technol 2017, 51: 11505–11512. [DOI] [PubMed] [Google Scholar]
- 58.Holmes P, Rumsby P, Harrison PT: Endocrine disrupters and menopausal health. British Menopause Society Journal 2004, 10:54–59. [DOI] [PubMed] [Google Scholar]
- 59.Huang L, Jolliet O: A parsimonious model for the release of volatile organic compounds (VOCs) encapsulated in products. Atmos Environ 2016, 127:223–235. [Google Scholar]
- 60.Hubbard TD, Murray IA, Nichols RG, Cassel K, Podolsky M, Kuzu G, Tian Y, Smith P, Kennett MJ, Patterson AD, et al. : Dietary broccoli impacts microbial community structure and attenuates chemically induced colitis in mice in an ah receptor dependent manner. JFunct Foods 2017, 37: 685–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ingle BL, Veber BC, Nichols JW, Tornero-Velez R: Informing the human plasma protein binding of environmental chemicals by machine learning in the pharmaceutical space: applicability domain and limits of predictability. J Chem Inf Model 2016, 56:2243–2252. [DOI] [PubMed] [Google Scholar]
- 62.Isaacs K, McCurdy T, Glen G, Nysewander M, Errickson A, Forbes S, Graham S, McCurdy L, Smith L, Tulve N: Statistical properties of longitudinal time-activity data for use in human exposure modeling. J Expo Sci Environ Epidemiol 2013, 23: 328. [DOI] [PubMed] [Google Scholar]
- 63.Isaacs KK, Glen WG, Egeghy P, Goldsmith M-R, Smith L, Vallero D, Brooks R, Grulke CM, Özkaynak Hk: SHEDS-HT: an integrated probabilistic exposure model for prioritizing exposures to chemicals with near-field and dietary sources. Environ Sci Technol 2014, 48:12750–12759. [DOI] [PubMed] [Google Scholar]
- 64.Isaacs KK, Goldsmith M-R, Egeghy P, Phillips K, Brooks R, Hong T, Wambaugh JF: Characterization and prediction of chemical functions and weight fractions in consumer products. Toxicol Rep 2016, 3:723–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Isaacs KK, Phillips KA, Biryol D, Dionisio KL, Price PS: Con-sumer product chemical weight fractions from ingredient lists. J Expo Sci Environ Epidemiol 2018, 28:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jamei M, Dickinson GL, Rostami-Hodjegan A: A framework for assessing inter-individual variability in pharmacokinetics using virtual human populations and integrating general knowledge of physical chemistry, biology, anatomy, physiology and genetics: a tale of ‘bottom-up’ vs ‘top-down’-recognition of covariates. Drug Metab Pharmacokinet 2009, 24: 53–75. [DOI] [PubMed] [Google Scholar]
- 67.Jayjock MA, Chaisson CF, Franklin CA, Arnold S, Price PS: Using publicly available information to create exposure and risk-based ranking of chemicals used in the workplace and consumer products. J Expo Sci Environ Epidemiol 2009, 19: 515. [DOI] [PubMed] [Google Scholar]
- 68.Jenardhanan P, Panneerselvam M, Mathur PP. Effect of environmental contaminants on spermatogenesis, vol. 59. Elsevier; 2016:126–140. [DOI] [PubMed] [Google Scholar]
- 69.Jo S-H, Lee M-H, Kim K-H, Kumar P: Characterization and flux assessment of airborne phthalates released from polyvinyl chloride consumer goods. Environ Res 2018, 165:81–90. [DOI] [PubMed] [Google Scholar]
- 70.Jolliet O, Ernstoff AS, Csiszar SA, Fantke P: Defining product intake fraction to quantify and compare exposure to consumer products. Environ Sci Technol 2015, 49:8924–8931. [DOI] [PubMed] [Google Scholar]
- 71.Jongeneelen FJ, Berge WFT: A generic, cross-chemical predictive PBTK model with multiple entry routes running as application in MS Excel; design of the model and comparison of predictions with experimental results. Ann Occup Hyg 2011, 55:841–864. [DOI] [PubMed] [Google Scholar]
- 72.Jonker W, Ballesteros-Gómez A, Hamers T, Somsen GW, Lamoree MH, Kool J: Highly selective screening of estrogenic compounds in consumer-electronics plastics by liquid chromatography in parallel combined with nanofractionation-bioactivity detection and mass spectrometry. Environ Sci Technol 2016, 50:12385–12393. [DOI] [PubMed] [Google Scholar]
- 73. Kapraun DF, Wambaugh JF, Ring CL, Tornero-Velez R, Setzer RW: A method for identifying prevalent chemical combinations in the US population. Environ Health Perspect 2017, 125, 087017. * Using frequent itemset mining (FIM), the authors determined that a handful of the billions of possible combinations of chemical exposures are much more common than the rest, allowing more targeted chemical mixtures research.
- 74.Kapraun DF, Wambaugh JF, Setzer RW, Judson RS: Empirical models for anatomical and physiological changes in a human mother and fetus during pregnancy and gestation. PLoS One 2019, 14, e0215906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kavlock RJ, Bahadori T, Barton-Maclaren TS, Gwinn MR, Rasenberg M, Thomas RS: Accelerating the pace of chemical risk assessment. Chem Res Toxicol 2018, 31:287–290. * The authors report on an international collaboration of public health regulators to adopt “new approach methodologies” or NAMs, which include high-throughput tools for toxicity screening and exposure.
- 76.Gl Kijko, Margni M, Partovi-Nia V, Doudrich G, Jolliet O: Impact of occupational exposure to chemicals in life cycle assessment: a novel characterization model based on measured concentrations and labor hours. Environ Sci Technol 2015, 49:8741–8750. [DOI] [PubMed] [Google Scholar]
- 77.Kotthoff M, Müller J, Jürling H, Schlummer M, Fiedler D: Perfluoroalkyl and polyfluoroalkyl substances in consumer products. Environ Sci Pollut Control Ser 2015, 22:14546–14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.LaKind JS, Sobus JR, Goodman M, Barr DB, Furst P, Albertini RJ, Arbuckle TE, Schoeters G, Tan YM, Teeguarden J, et al. : A proposal for assessing study quality: biomonitoring, Environmental Epidemiology, and Short-lived Chemicals (BEES-C) instrument. Environ Int 2014, 73:195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee S, Tan YM, Phillips MB, Sobus JR, Kim S: Estimating methylmercury intake for the general population of South Korea using physiologically based pharmacokinetic modeling. Toxicol Sci 2017, 159:6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li L, Wania F: Tracking chemicals in products around the world: introduction of a dynamic substance flow analysis model and application to PCBs. Environ Int 2016, 94:674–686. [DOI] [PubMed] [Google Scholar]
- 81.Li L, Westgate JN, Hughes L, Zhang X, Givehchi B, Toose L, Armitage JM, Wania F, Egeghy P, Arnot JA: A model for risk-based screening and prioritization of human exposure to chemicals from near-field sources. Environ Sci Technol 2018, 52:14235–14244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li L, Zhai Z, Liu J, Hu J: Estimating industrial and domestic environmental releases of perfluorooctanoic acid and its salts in China from 2004 to 2012. Chemosphere 2015, 129: 100–109. [DOI] [PubMed] [Google Scholar]
- 83.Liang Y, Liu X, Allen MR: Measurements of parameters controlling the emissions of organophosphate flame retardants in indoor environments. Environ Sci Technol 2018, 52: 5821–5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, et al. : A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet (London, England) 2012, 380:2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lin YS, Kupper LL, Rappaport SM: Air samples versus biomarkers for epidemiology. Occup Environ Med 2005, 62: 750–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu X: Characterise sources for exposure assessment of chemicals in indoor environment. London, England: SAGE Publications Sage UK; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu X, Guo Z, Krebs KA, Stinson RA, Nardin JA, Pope RH, Roache NF: Chamber study of PCB emissions from caulking materials and light ballasts. Chemosphere 2015a, 137: 115–121. [DOI] [PubMed] [Google Scholar]
- 88.Liu Y, Pereira ADS, Martin JW: Discovery of C5–C17 poly-and perfluoroalkyl substances in water by in-line SPE-HPLC-Orbitrap with in-source fragmentation flagging. Anal Chem 2015b, 87:4260–4268. [DOI] [PubMed] [Google Scholar]
- 89.Luecke RH, Wosilait WD, Pearce BA, Young JF: A physiologically based pharmacokinetic computer model for human pregnancy. Teratology 1994, 49:90–103. [DOI] [PubMed] [Google Scholar]
- 90.MacLeod M, Scheringer M, McKone TE, Hungerbuhler K: The state of multimedia mass-balance modeling in environmental science and decision-making. ACS Publications; 2010. [DOI] [PubMed] [Google Scholar]
- 91.Makri A, Goveia M, Balbus J, Parkin R: Children’s susceptibility to chemicals: a review by developmental stage. J Toxicol Environ Health Part B 2004, 7(6):417–435. [DOI] [PubMed] [Google Scholar]
- 92. Mansouri K, Grulke CM, Judson RS, Williams AJ: OPERA models for predicting physicochemical properties and environmental fate endpoints. J Cheminf 2018, 10:10. * The authors develop public, open-source tools for predicting chemical properties from structure. A key enhancement that is introduced is the inclusion of “domain of applicability” assessment that identifies structures for which the tools are appropriate.
- 93.McKelvey W, Jeffery N, Clark N, Kass D, Parsons PJ: Population-based inorganic mercury biomonitoring and the identification of skin care products as a source of exposure in New York City. Environ Health Perspect 2010, 119: 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McKone T: CalTOX, a multimedia total exposure model for hazardous-waste sites. 1993. [Google Scholar]
- 95.McKone T: The precision of QSAR methods for estimating intermedia transfer factors in exposure assessments. SAR QSAR Environ Res 1993b, 1:41–51. [Google Scholar]
- 96.Morgan MK, Nash M, Barr DB, Starr JM, Scott Clifton M, Sobus JR: Distribution, variability, and predictors of urinary bisphenol A levels in 50 North Carolina adults over a six-week monitoring period. Environ Int 2018, 112:85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Moschet C, Anumol T, Lew BM, Bennett DH, Young TM: Household dust as a repository of chemical accumulation: new insights from a comprehensive high-resolution mass spectrometric study. Environ Sci Technol 2018, 52: 2878–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moschet C, Lew BM, Hasenbein S, Anumol T, Young TM: LC-and GC-QTOF-MS as complementary tools for a comprehensive micropollutant analysis in aquatic systems. Environ Sci Technol 2017, 51:1553–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.National Academies of Sciences, E., and Medicine: Environmental chemicals, the human microbiome, and health risk: a research strategy. Washington (DC): The National Academies Press; 2018. 10.17226/24960. [DOI] [PubMed] [Google Scholar]
- 100. National Academies of Sciences, E., and Medicine: Using 21st century science to improve risk-related evaluations. Washington, DC: The National Academies Press; 2017. 10.17226/24635. * In this document the U.S. National Academies of Sciences, Engineering, and Medicine outline how new tools for toxicity testing, exposure estimation, and toxicokinetics might one day transform chemical risk decision-making.
- 101.National Library of Medicine: The household products database. 2018. Available at: https://hpd.nlm.nih.gov/ [Accessed]. [Google Scholar]
- 102.National Research Council: Risk assessment in the federal government: managing the process. Washington (DC): National Academies Press; 1983. 10.17226/317. [DOI] [PubMed] [Google Scholar]
- 103.National Research Council: Human biomonitoring for environmental chemicals. National Academies Press; 2006. [Google Scholar]
- 104.National Research Council: Exposure science in the 21st century: a vision and a strategy. 2012. [PubMed] [Google Scholar]
- 105.Nazaroff WW: Embracing microbes in exposure science. J Expo Sci Environ Epidemiol 2019, 29:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Newton SR, McMahen RL, Sobus JR, Mansouri K, Williams AJ, McEachran AD, Strynar MJ: Suspect screening and non-targeted analysis of drinking water using point-of-use filters. Environ Pollut 2018, 234:297–306. * The authors performed screening analysis of household drinking water filters. Out of roughly 15,000 molecular features that could be observed, high-throughput toxicity screening, exposure forecasts, and consumer product data helped identify 91 compounds for follow-up study.
- 107.Nicolas CI, Mansouri K, Phillips KA, Grulke CM, Richard AM, Williams AJ, Rabinowitz J, Isaacs KK, Yau A, Wambaugh JF: Rapid experimental measurements of physicochemical properties to inform models and testing. Sci Total Environ 2018, 636:901–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Niedzwiecki MM, Walker DI, Vermeulen R, Chadeau-Hyam M, Jones DP, Miller GW: The exposome: molecules to populations. Annu Rev Pharmacol Toxicol 2019, 59:107–127. [DOI] [PubMed] [Google Scholar]
- 109.Oreskes N: Evaluation (not validation) of quantitative models. Environ Health Perspect 1998, 106(Suppl 6):1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Parvatker A, Tunceroglu H, Sherman JD, Coish P, Anastas PT, Zimmerman JB, Eckelman MJ: Cradle-to-gate greenhouse gas emissions for twenty anesthetic active pharmaceutical ingredients based on process scale-up and process design calculations. ACS Sustainable Chem Eng 2019, 7:6580–6591. [Google Scholar]
- 111.Paustenbach DJ: The practice of exposure assessment: a state-of-the-art review. J Toxicol Environ Health B Crit Rev 2000, 3(3):179–291. [DOI] [PubMed] [Google Scholar]
- 112.Pearce RG, Setzer RW, Strope CL, Sipes NS, Wambaugh JF: Httk: R package for high-throughput toxicokinetics. J Stat Softw 2017, 79:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Peyret T, Poulin P, Krishnan K: A unified algorithm for predicting partition coefficients for PBPK modeling of drugs and environmental chemicals. Toxicol Appl Pharmacol 2010, 249:197–207. [DOI] [PubMed] [Google Scholar]
- 114.Phelps D, Brinkman NE, Keely SP, Anneken EM, Catron TR, Betancourt D, Wood CE, Espenschied ST, Rawls JF, Tal T: Microbial colonization is required for normal neurobehavioral development in zebrafish. Sci Rep 2017, 7:11244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Phillips K, Yau AY, Favela KA, Isaacs KK, McEachran A, Grulke CM, Richard AM, Williams A, Sobus JR, Thomas RS, et al. : Suspect screening analysis of chemicals in consumer products. Environ Sci Technol 2018, 52:3125–3135. * The authors used screening mass spectrometry analysis to examine one hundred consumer products. Over 1600 chemicals were confirmed or tentatively identified, out of which only roughly 200 were previously known to be present in consumer products.
- 116. Phillips KA, Wambaugh JF, Grulke CM, Dionisio KL, Isaacs KK: High-throughput screening of chemicals as functional substitutes using structure-based classification models. Green Chem 2017, 19:1063–1074. * The authors used machine learning to relate chemical structure to chemical use, allowing prediction of functions such as hair dyes. High-throughput toxicity screening data was then used to identify alternative chemicals with lesser bioactivity than those currently used.
- 117.Price PS, Chaisson CF: A conceptual framework for modeling aggregate and cumulative exposures to chemicals. J Expo Sci Environ Epidemiol 2005, 15:473. [DOI] [PubMed] [Google Scholar]
- 118.Price PS, Young JS, Chaisson CF: Assessing aggregate and cumulative pesticide risks using a probabilistic model. Ann Occup Hyg 2001, 45(suppl_1):S131–S142. [DOI] [PubMed] [Google Scholar]
- 119.Rager JE, Strynar MJ, Liang S, McMahen RL, Richard AM, Grulke CM, Wambaugh JF, Isaacs KK, Judson R, Williams AJ, et al. : Linking high resolution mass spectrometry data with exposure and toxicity forecasts to advance high-throughput environmental monitoring. Environ Int 2016, 88:269–280. [DOI] [PubMed] [Google Scholar]
- 120. Ring CL, Arnot JA, Bennett DH, Egeghy PP, Fantke P, Huang L, Isaacs KK, Jolliet O, Phillips KA, Price PS: Consensus modeling of median chemical intake for the US population based on predictions of exposure pathways. Environ Sci Technol 2018, 53:719–732. * The authors use machine learning and chemical structure to predict the types of exposure scenarios relevant to a chemical. This allows twelve different predictors of exposure to be organized in a way that prediction of daily intake rate can be made for ~480,000 chemicals.
- 121. Ring CL, Pearce RG, Setzer RW, Wetmore BA, Wambaugh JF: Identifying populations sensitive to environmental chemicals by simulating toxicokinetic variability. Environ Int 2017, 106: 105–118. * The authors coupled high-throughput toxicity screening data, high-throughput toxicokinetics, and high-throughput exposure predictions to identify specific U.S. demographic groups that were more vulnerable to either specific chemicals or chemical exposure in general.
- 122.Rosenbaum RK, Bachmann TM, Gold LS, Huijbregts MA, Jolliet O, Juraske R, Koehler A, Larsen HF, MacLeod M, Margni M: USEtox—the UNEP-SETAC toxicity model: recommended characterisation factors for human toxicity and freshwater ecotoxicity in life cycle impact assessment. Int J Life Cycle Assess 2008, 13:532–546. [Google Scholar]
- 123.Rotroff DM, Wetmore BA, Dix DJ, Ferguson SS, Clewell HJ, Houck KA, LeCluyse EL, Andersen ME, Judson RS, Smith CM, et al. : Incorporating human dosimetry and exposure into high-throughput in vitro toxicity screening. Toxicol Sci 2010, 117:348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rudel RA, Seryak LM, Brody JG: PCB-containing wood floor finish is a likely source of elevated PCBs in residents’ blood, household air and dust: a case study of exposure. Environ Health 2008, 7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sabljic A: QSAR models for estimating properties of persistent organic pollutants required in evaluation of their environmental fate and risk. Chemosphere 2001, 43:363–375. [DOI] [PubMed] [Google Scholar]
- 126.Sadd J, Hall E, Pastor M, Morello-Frosch R, Lowe-Liang D, Hayes J, Swanson C: Ground-truthing validation to assess the effect of facility locational error on cumulative impacts screening tools. Geography Journal 2015, 2015. [Google Scholar]
- 127.Safford B, Api A, Barratt C, Comiskey D, Ellis G, McNamara C, O’Mahony C, Robison S, Rose J, Smith B: Application of the expanded Creme RIFM consumer exposure model to fragrance ingredients in cosmetic, personal care and air care products. Regul Toxicol Pharmacol 2017, 86:148–156. [DOI] [PubMed] [Google Scholar]
- 128.Scheringer M, Wegmann F, Fenner K, Hungerbühler K: Investigation of the cold condensation of persistent organic pollutants with a global multimedia fate model. Environ Sci Technol 2000, 34:1842–1850. [Google Scholar]
- 129.Schymanski EL, Jeon J, Gulde R, Fenner K, Ruff M, Singer HP, Hollender J: Identifying small molecules via high resolution mass spectrometry: communicating confidence. Environ Sci Technol 2014, 48:2097–2098. [DOI] [PubMed] [Google Scholar]
- 130.Schymanski EL, Singer HP, Slobodnik J, Ipolyi IM, Oswald P, Krauss M, Schulze T, Haglund P, Letzel T, Grosse S: Non-target screening with high-resolution mass spectrometry: critical review using a collaborative trial on water analysis. Anal Bioanal Chem 2015, 407:6237–6255. [DOI] [PubMed] [Google Scholar]
- 131.Sender R, Fuchs S, Milo R: Revised estimates for the number of human and bacteria cells in the body. PLoS Biol 2016, 14, e1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sheldon LS, Cohen Hubal EA: Exposure as part of a systems approach for assessing risk. Environ Health Perspect 2009, 117:1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shin H-M, McKone TE, Bennett DH: Intake fraction for the indoor environment: a tool for prioritizing indoor chemical sources. Environ Sci Technol 2012, 46:10063–10072. [DOI] [PubMed] [Google Scholar]
- 134.Shin HM, Ernstoff A, Arnot JA, Wetmore BA, Csiszar SA, Fantke P, Zhang XM, McKone TE, Jolliet O, Bennett DH: Risk-based high-throughput chemical screening and prioritization using exposure models and in vitro bioactivity assays. Environ Sci Technol 2015, 49:6760–6771. [DOI] [PubMed] [Google Scholar]
- 135. Sipes NS, Wambaugh JF, Pearce R, Auerbach SS, Wetmore BA, Hsieh J-H, Shapiro AJ, Svoboda D, DeVito MJ, Ferguson SS: An intuitive approach for predicting potential human health risk with the Tox21 10k library. Environ Sci Technol 2017, 51: 10786–10796. * The authors used high-throughput exposure and toxicokinetic predictions to place high-throughput toxicity screening data in a more realistic context. They demonstrated that of 3925 with in vitro bioactivity, only 36 might plausibly have exposures warranting follow-up study.
- 136.Smith RL, Ruiz-Mercado GJ, Meyer DE, Gonzalez MA, Abraham JP, Barrett WM, Randall PM: Coupling computer-aided process simulation and estimations of emissions and land use for rapid life cycle inventory modeling. ACS Sus-tainable Chem Eng 2017, 5:3786–3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sobus JR, DeWoskin RS, Tan Y-M, Pleil JD, Phillips MB, George BJ, Christensen K, Schreinemachers DM, Williams MA, Hubal EAC: Uses of NHANES biomarker data for chemical risk assessment: trends, challenges, and opportunities. Environ Health Perspect 2015a, 123:919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sobus JR, DeWoskin RS, Tan YM, Pleil JD, Phillips MB, George BJ, Christensen K, Schreinemachers DM, Williams MA, Hubal EA, et al. : Uses of NHANES biomarker data for chemical risk assessment: trends, challenges, and opportunities. Environ Health Perspect 2015b, 123:919–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Sobus JR, Grossman JN, Chao A, Singh R, Williams AJ, Grulke CM, Richard AM, Newton SR, McEachran AD, Ulrich EM: Using prepared mixtures of ToxCast chemicals to evaluate non-targeted analysis (NTA) method performance. Anal Bioanal Chem 2019, 411:835–851. * EPA’s Non-Targeted Analysis Collaborative Trial (ENTACT) was launched in 2016 to evaluate the ability to use mass spectrometry to discover contaminants of emerging concern. The authors demonstrate that any one method can likely characterize only a subset of substances.
- 140.Sobus JR, Morgan MK, Pleil JD, Barr DB: Chapter 45 - biomonitoring: uses and considerations for assessing nonoccupational human exposure to pesticides. Krieger R (Ed.), Hayes’ handbook of pesticide toxicology (3rd ed.), Academic Press, New York: (2010), pp. 1021–1036. 10.1016/B978-0-12-374367-1.00045-8. [DOI] [Google Scholar]
- 141.Sobus JR, Pleil JD, McClean MD, Herrick RF, Rappaport SM: Biomarker variance component estimation for exposure surrogate selection and toxicokinetic inference. Toxicol Lett 2010b, 199:247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sobus JR, Tan Y-M, Pleil JD, Sheldon LS: A biomonitoring framework to support exposure and risk assessments. Sci Total Environ 2011, 409:4875–4884. [DOI] [PubMed] [Google Scholar]
- 143. Sobus JR, Wambaugh JF, Isaacs KK, Williams AJ, McEachran AD, Richard AM, Grulke CM, Ulrich EM, Rager JE, Strynar MJ: Integrating tools for non-targeted analysis research and chemical safety evaluations at the US EPA. J Expo Sci Environ Epidemiol 2018, 28:411. * The authors characterize how new advances in mass spectrometry allow the rapid characterization of thousands of never-before-studied compounds in a wide variety of environmental, residential, and biological media.
- 144.Strope CL, Mansouri K, Clewell HJ, Rabinowitz JR, Stevens C, Wambaugh JF: High-throughput in-silico prediction of ionization equilibria for pharmacokinetic modeling. Sci Total Environ 2018, 615:150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tal T, Phelps D, Weitekamp C, Swank A, Mccord J, Sobus J, Catron T, Keely S, Brinkman N, Wheaton E. Triclosan-resistant host-associated microbiota perform xenobiotic biotransformations in larval Zebrafish, vol. 111. 111 RIVER ST, HOBOKEN 07030–5774, NJ USA: WILEY; 2019. 476–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tan Y-M, Sobus J, Chang D, Tornero-Velez R, Goldsmith M, Pleil J, Dary C: Reconstructing human exposures using biomarkers and other “clues”. J Toxicol Environ Health Part B 2012, 15(1):22–38. [DOI] [PubMed] [Google Scholar]
- 147.Teschke K, Olshan A, Daniels J, De Roos A, Parks C, Schulz M, Vaughan T: Occupational exposure assessment in case–control studies: opportunities for improvement. Occup Environ Med 2002, 59:575–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tessum CW, Hill JD, Marshall JD: InMAP: a model for air pollution interventions. PLoS One 2017, 12, e0176131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Thomas RS, Bahadori T, Buckley TJ, Cowden J, Deisenroth C, Dionisio KL, Frithsen JB, Grulke CM, Gwinn MR, Harrill JA: The next generation blueprint of computational toxicology at the US Environmental Protection Agency. Toxicol Sci 2019, 169: 317–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.U.S. Environmental Protection Agency: Exposure factors handbook: 2011 edition. Office of Research and Development National Center for Environmental; 2011. [Google Scholar]
- 151.U.S. Environmental Protection Agency: Indoor exposure product testing protocols. 2017. [Google Scholar]
- 152.U.S. Environmental Protection Agency: Toxics release inventory. 2019. Available at: https://www.epa.gov/toxics-release-inventory-tri-program [Accessed].
- 153. Ulrich EM, Sobus JR, Grulke CM, Richard AM, Newton SR, Strynar MJ, Mansouri K, Williams AJ: EPA’s non-targeted analysis collaborative trial (ENTACT): genesis, design, and initial findings. Anal Bioanal Chem 2019, 411:853–866. * The authors describe the design of EPA’s Non-Targeted Analysis Collaborative Trial (ENTACT). Participants include nearly 30 laboratories using a variety of chromatography, mass spectrometry, and data processing approaches to characterize ten synthetic chemical mixtures.
- 154.USEPA: Exposure factors handbook 2011 edition (final). 2011. [Google Scholar]
- 155.USGAO. In Chemical regulation: options for enhancing the effectiveness of the toxic substances control Act. Edited by G. A. Office; 2009. [Google Scholar]
- 156.USGAO. In Toxic substances: EPA has increased efforts to assess and control chemicals but could strengthen its approach. Edited by U. S. G. A. Office; 2013. [Google Scholar]
- 157.Wallace LA, Pellizzari ED, Hartwell TD, Sparacino C, Whitmore R, Sheldon L, Zelon H, Perritt R: The TEAM study: personal exposures to toxic substances in air, drinking water, and breath of 400 residents of New Jersey, North Carolina, and North Dakota. Environ Res 1987, 43:290–307. [DOI] [PubMed] [Google Scholar]
- 158.Wambaugh JF, Hughes MF, Ring CL, MacMillan DK, Ford J, Fennell TR, Black SR, Snyder RW, Sipes NS, Wetmore BA, et al. : Evaluating in vitro-in vivo extrapolation of toxicokinetics. Toxicol Sci 2018, 163:152–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wambaugh JF, Setzer RW, Reif DM, Gangwal S, Mitchell-Blackwood J, Arnot JA, Joliet O, Frame A, Rabinowitz J, Knudsen TB, et al. : High-throughput models for exposure-based chemical prioritization in the ExpoCast project. Environ Sci Technol 2013, 47:8479–8488. [DOI] [PubMed] [Google Scholar]
- 160.Wambaugh JF, Wang A, Dionisio KL, Frame A, Egeghy P, Judson R, Setzer RW: High throughput heuristics for prioritizing human exposure to environmental chemicals. Environ Sci Technol 2014, 48:12760–12767. [DOI] [PubMed] [Google Scholar]
- 161.Wambaugh JF, Wetmore BA, Pearce R, Strope C, Goldsmith R, Sluka JP, Sedykh A, Tropsha A, Bosgra S, Shah I, et al. : Toxicokinetic triage for environmental chemicals. Toxicol Sci 2015, 147:55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang A, Gerona RR, Schwartz JM, Lin T, Sirota M, Morello-Frosch R, Woodruff TJ: A suspect screening method for characterizing multiple chemical exposures among a demographically diverse population of pregnant women in San Francisco. Environ Health Perspect 2018, 126, 077009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang T, Wang P, Meng J, Liu S, Lu Y, Khim JS, Giesy JP: A review of sources, multimedia distribution and health risks of perfluoroalkyl acids (PFAAs) in China. Chemosphere 2015, 129:87–99. [DOI] [PubMed] [Google Scholar]
- 164.Wang Y-H: Confidence assessment of the simcyp time-based approach and a static mathematical model in predicting clinical drug-drug interactions for mechanism-based CYP3A inhibitors. Drug Metab Dispos 2010, 38:1094–1104. [DOI] [PubMed] [Google Scholar]
- 165. Wannaz C, Fantke P, Jolliet O: Multiscale spatial modeling of human exposure from local sources to global intake. Environ Sci Technol 2018, 52:701–711. * The authors describe Pangea, a new multiscale, spatial multimedia fate and exposure assessment model. The model suggests that, by neglecting distant low-level exposure, local assessments might only account for small fractions of global cumulative chemical intakes.
- 166.Wetmore BA: Quantitative in vitro-to-in vivo extrapolation in a high-throughput environment. Toxicology 2015, 332:94–101. [DOI] [PubMed] [Google Scholar]
- 167.Wetmore BA, Allen B, Clewell HJ 3rd, Parker T, Wambaugh JF, Almond LM, Sochaski MA, Thomas RS: Incorporating population variability and susceptible subpopulations into dosimetry for high-throughput toxicity testing. Toxicol Sci 2014, 142: 210–224. [DOI] [PubMed] [Google Scholar]
- 168.Wetmore BA, Wambaugh JF, Allen B, Ferguson SS, Sochaski MA, Setzer RW, Houck KA, Strope CL, Cantwell K, Judson RS, et al. : Incorporating high-throughput exposure predictions with dosimetry-adjusted in vitro bioactivity to inform chemical toxicity testing. Toxicol Sci 2015, 148:121–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wetmore BA, Wambaugh JF, Ferguson SS, Li L, Clewell HJ 3rd, Judson RS, Freeman K, Bao W, Sochaski MA, Chu TM, et al. : Relative impact of incorporating pharmacokinetics on predicting in vivo hazard and mode of action from high-throughput in vitro toxicity assays. Toxicol Sci 2013, 132:327–346. [DOI] [PubMed] [Google Scholar]
- 170.Wetmore BA, Wambaugh JF, Ferguson SS, Sochaski MA, Rotroff DM, Freeman K, Clewell HJ 3rd, Dix DJ, Andersen ME, Houck KA, et al. : Integration of dosimetry, exposure, and high-throughput screening data in chemical toxicity assessment. Toxicol Sci 2012, 125:157–174. [DOI] [PubMed] [Google Scholar]
- 171.Wild CP: Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomark Prev 2005, 14:1847–1850. [DOI] [PubMed] [Google Scholar]
- 172.Williams AJ, Grulke CM, Edwards J, McEachran AD, Mansouri K, Baker NC, Patlewicz G, Shah I, Wambaugh JF, Judson RS: The CompTox Chemistry Dashboard: a community data resource for environmental chemistry. J Cheminf 2017, 9:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wilson ID, Nicholson JK: Gut microbiome interactions with drug metabolism, efficacy, and toxicity. Transl Res 2017, 179: 204–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wu J, Hu C, Li C, Cai Z, Hu D: Determination of 2, 8-dichlorodibenzo-p-dioxin in toothpaste and mouthwash consumer products using GC-MS. Environ Sci Pollut Control Ser 2015, 22:18927–18932. [DOI] [PubMed] [Google Scholar]
- 175.Wu Y, Xie M, Cox SS, Marr LC, Little JC: A simple method to measure the gas-phase SVOC concentration adjacent to a material surface. Indoor Air 2016, 26:903–912. [DOI] [PubMed] [Google Scholar]
- 176.Yang C, Tarkhov A, Marusczyk Jr, Bienfait B, Gasteiger J, Kleinoeder T, Magdziarz T, Sacher O, Schwab CH, Schwoebel J: New publicly available chemical query language, CSRML, to support chemotype representations for application to data mining and modeling. J Chem Inf Model 2015, 55:510–528. [DOI] [PubMed] [Google Scholar]
- 177.Yap CW: PaDEL-descriptor: an open source software to calculate molecular descriptors and fingerprints. J Comput Chem 2011, 32:1466–1474. [DOI] [PubMed] [Google Scholar]
- 178.Yoon M, Efremenko A, Blaauboer BJ, Clewell HJ: Evaluation of simple in vitro to in vivo extrapolation approaches for environmental compounds. Toxicol In Vitro 2014, 28:164–170. [DOI] [PubMed] [Google Scholar]
- 179.Zartarian V, Bahadori T, McKone T: Adoption of an official ISEA glossary. J Expo Anal Environ Epidemiol 2005, 15. [DOI] [PubMed] [Google Scholar]
- 180.Zartarian V, Xue J, Glen G, Smith L, Tulve N, Tornero-Velez R: Quantifying children’s aggregate (dietary and residential) exposure and dose to permethrin: application and evaluation of EPA’s probabilistic SHEDS-Multimedia model. J Expo Sci Environ Epidemiol 2012, 22:267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhang L, Liu J, Hu J, Liu C, Guo W, Wang Q, Wang H: The inventory of sources, environmental releases and risk assessment for perfluorooctane sulfonate in China. Environ Pollut 2012, 165:193–198. [DOI] [PubMed] [Google Scholar]
- 182.Zhang XM, Arnot JA, Wania F: Model for screening-level assessment of near-field human exposure to neutral organic chemicals released indoors. Environ Sci Technol 2014, 48: 12312–12319. [DOI] [PubMed] [Google Scholar]
- 183. Zimmermann M, Zimmermann-Kogadeeva M, Wegmann R, Goodman AL: Separating host and microbiome contributions to drug pharmacokinetics and toxicity. Science 2019, 363. * Using genetic analysis of metabolism in various tissue of mice, the authors demonstrated that the responses of individuals to the same drug depended on the genetics of each individual’s microbiome.
- 184.Hays Sean M, Aylward Lesa L: Interpreting human bio-monitoring data in a public health risk context using Bio-monitoring Equivalents. Int J Hygiene Environ Health 2012, 215(2):145–148. [DOI] [PubMed] [Google Scholar]
- 185.Jacqueline Moya, Phillips Linda: A review of soil and dust ingestion studies for children. J Expos Sci Environ Epidemiol 2014, 24(6):545. [DOI] [PubMed] [Google Scholar]
- 186.Lehmann Geniece M, et al. : Improving the risk assessment of lipophilic persistent environmental chemicals in breast milk. Crit Rev Toxicol 2014, 44(7):600–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.VanderPlas Jake; Python Data Science Handbook, 2016, O’Reilly Media, Inc., ISBN: 978–1-4949–1205-8 [Google Scholar]
- 188.Leach Andrew R: Molecular Modelling: Principles and Applications, 2nd Edition, 2001, Pearson, ISBN: 978–0582382107 [Google Scholar]


