Abstract
The impact of arsenite [As(III)] on several levels of cellular metabolism and gene regulation was examined in Pseudomonas aeruginosa. P. aeruginosa isogenic mutants devoid of antioxidant enzymes or defective in various metabolic pathways, DNA repair systems, metal storage proteins, global regulators, or quorum sensing circuitry were examined for their sensitivity to As(III). Mutants lacking the As(III) translocator (ArsB), superoxide dismutase (SOD), catabolite repression control protein (Crc), or glutathione reductase (Gor) were more sensitive to As(III) than wild-type bacteria. The MICs of As(III) under aerobic conditions were 0.2, 0.3, 0.8, and 1.9 mM for arsB, sodA sodB, crc, and gor mutants, respectively, and were 1.5- to 13-fold less than the MIC for the wild-type strain. A two-dimensional gel/matrix-assisted laser desorption ionization-time of flight analysis of As(III)-treated wild-type bacteria showed significantly (>40-fold) increased levels of a heat shock protein (IbpA) and a putative allo-threonine aldolase (GlyI). Smaller increases (up to 3.1-fold) in expression were observed for acetyl-coenzyme A acetyltransferase (AtoB), a probable aldehyde dehydrogenase (KauB), ribosomal protein L25 (RplY), and the probable DNA-binding stress protein (PA0962). In contrast, decreased levels of a heme oxygenase (HemO/PigA) were found upon As(III) treatment. Isogenic mutants were successfully constructed for six of the eight genes encoding the aforementioned proteins. When treated with sublethal concentrations of As(III), each mutant revealed a marginal to significant lag period prior to resumption of apparent normal growth compared to that observed in the wild-type strain. Our results suggest that As(III) exposure results in an oxidative stress-like response in P. aeruginosa, although activities of classic oxidative stress enzymes are not increased. Instead, relief from As(III)-based oxidative stress is accomplished from the collective activities of ArsB, glutathione reductase, and the global regulator Crc. SOD appears to be involved, but its function may be in the protection of superoxide-sensitive sulfhydryl groups.
Arsenic is present in numerous disturbed and natural ecosystems and is a top-priority national pollutant. It can exist in multiple oxidation states, with the most common being arsenite [As(III)] and arsenate [As(V)]. As(V) is an analogue of phosphate, and its toxicity is due to the disruption of critical cellular functions or synthesis of essential building blocks. These include uncoupling of ATP phosphorylation that would directly impact energy flow, as well as nucleic acid and phospholipid synthesis. As(III) toxicity is thought to be due predominantly to its ability to covalently bind protein sulfhydryl groups. Of the two species, As(III) is considered the most toxic (16, 68).
Although some microorganisms can utilize As(V) for anaerobic respiration (81) or oxidize As(III) as a sole energy source (37, 75), arsenic generally is toxic to most microorganisms. Arsenic resistance in bacteria is due, in part, to plasmid- or chromosome-encoded ars genes. Typically, Ars-mediated resistance involves As(V) reduction to As(III) via a cytoplasmic As(V) reductase (ArsC), and the As(III) is extruded by a membrane-associated ArsB efflux pump that is efficient at removing As(III) and antimonite [Sb(II)] (56). In addition, since As(III) uptake is facilitated by the aquaglyceroporin GlpF (55), an absence or poor expression of this porin would perhaps also constitute an indirect form of resistance, as any mechanism contributing to reduced levels of As(III) in the cytoplasm would improve cell growth in the presence of As(III). This is very similar to an absence of porin activity in other gram-negative organisms that correlates with increased resistance to β-lactam antibiotics.
In Escherichia coli, the ars operon in plasmid R733 contains five genes referred to as arsR, arsD, arsA, arsB, and arsC (70), whereas the staphylococcal ars operon in pI258 is composed of but three genes: arsR, arsB, and arsC (39, 71). The arsR gene from both E. coli and Staphylococcus aureus encodes a regulatory protein that controls the expression of the ars operon, which can be induced by As(III), Sb(II), or bismuth (39, 71, 89). The arsD gene is constitutively expressed and encodes a regulatory protein that controls maximal expression of the ars operon (89). The arsA locus encodes the ATPase subunit (13) of a protein complex composed of an ArsA dimer bound to an ArsB polypeptide (17, 83), and arsC encodes an As(V) reductase (24, 38). ArsB alone is sufficient for As(III) resistance and proton motive force-dependent As(III) efflux, whereas ArsC is required for optimal resistance to As(V).
Other non-efflux-based mechanisms of arsenic detoxification have been examined. In a series of studies with Pseudomonas putida, Abdrashitova et al. (1-4) and Mynbaeva et al. (59) described As(III) resistance via a mechanism involving peroxidation of unsaturated fatty acids. It was suggested that this process leads to the generation of organic hydroperoxides and oxygen radicals, which, in turn, induce major components of the oxidative stress response, including superoxide dismutase (SOD) and catalase. More recently, arsenic toxicity in eukaryotic cells has also been shown to involve the generation of reactive oxygen intermediates (14, 32) or possibly even the formation of nitric oxide (47). Thus, it is likely that arsenic resistance in bacteria and eukaryotic cells involves multiple factors, at least two of which include As(III) efflux pumps and antioxidants.
In an attempt to extend beyond current ars gene-mediated resistance models and to improve our general understanding of how arsenic affects bacteria, we initiated a study to assess the relative contribution of multiple gene products that could be involved in As(III) sensitivity or resistance. Pseudomonas aeruginosa was selected for this work because this organism is (i) well studied in planktonic culture, (ii) a model organism for biofilm research (28, 29), and (iii) relatively simple to manipulate genetically. Further, it is an environmentally relevant organism, being found in soils as well as in mine tailings that are heavily contaminated with arsenic (52). Each of these features makes this organism an attractive choice for research involving arsenic tolerance and redox transformation activity. The results of this study suggest that the ArsB anion translocator, SOD, glutathione reductase, and the catabolite repressor control protein Crc are all important for optimal resistance of P. aeruginosa to As(III) under aerobic conditions.
MATERIALS AND METHODS
Chemicals.
Sodium arsenite [As(III)] was purchased from Matheson, Coleman, and Bell Manufacturing (Norwood, OH). Sodium arsenate [As(V)], nitroblue tetrazolium, ferric chloride, glutathione, riboflavin, and hydrogen peroxide (H2O2) were from Sigma Chemical Co. (St. Louis, MO).
Bacterial strains, plasmids and growth conditions.
All bacteria used in this study are listed in Table 1 and were grown in either Luria-Bertani (L) broth or M9 glucose minimal medium (74). Cultures were grown at 37°C with shaking at 300 rpm or on a roller wheel in 16- by 150-mm test tubes containing 5 ml of medium rotating at 70 rpm. Culture volumes were 1/10 of the total flask volume to ensure maximum aeration. Media were solidified with 1.5% Bacto agar. Frozen bacterial stocks were stored at −80°C in a 1:1 mixture of 25% glycerol and stationary-phase bacterial suspension.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype or characteristicsa | Source or reference |
|---|---|---|
| E. coli | ||
| DH5α | F− φ80dlacZΔM15 recA1 endA1 gyrA96 thi-1 relA1 supE44 hsdR17(rK− mK+) Δ(lacZYA-argF)U169 | Protein Express, Cincinnati, OH |
| SM10 | Mobilizer strain, Kmr, thi-1 thr leu tonA lacY supE recA::RP4-2-Tc::Mu pir | 80 |
| AB1157 | F−thr-1 ara-14 leuB6 DE(gpt-proA)62 lacY1 tsx-33 glnV44(AS) galK2(Oc) hisG4(Oc) rfbD1 mgl-51 rpoS396(Am) rpsL31(StrR) kdgK51 xylA5 mtl-1 argE3(Oc) thi-1 | J. Imlay |
| JI132 | As AB1157, but ΔsodA::Cm, ΔsodB::Km | J. Imlay |
| P. aeruginosa | ||
| PAO1 | Wild type, prototroph | 33 |
| FRD1 | Prototrophic, mucoid, cystic fibrosis isolate | 25 |
| ahpA | ahpA::Gm | This study |
| ahpA bfrB | ahpA bfrB::Gm | This study |
| ahpA bfrB katA bfrA | ahpA bfrB::Gm, katAbfrA::Tc | This study |
| ahpA katA | ahpA::Tc, katA::Gm | This study |
| ahpCF | ahpCF::Gm | 62 |
| ahpC katA | ahpCF::Gm, katA::Tc | This study |
| ahpC ohr | ahpCF::Gm, ohr::Tc | This study |
| ankB | ankB::Gm | 49 |
| anr (PAO6261) | anr::Tc | 90 |
| arsB | arsB::Gm | This study |
| atoB | atoB::Gm | This study |
| bfrA | bfrA::Gm | 49 |
| bfrB | bfrB::Tc | This study |
| bfrA bfrB | bfrA::Gm, bfrB::Tc | This study |
| crc (PAO8020) | crc::Tc | 50 |
| dps | dps::Gm | This study |
| dps bfrB | dps::Gm, bfrB::Tc | This study |
| fagA | fagA::Gm | This study |
| fpvA | fpvA::Tc | K. Poole |
| fumC | fumC::Gm | This study |
| furC6 | furC6, manganese resistant | 69 |
| furA2 | furA2, manganese resistant | 69 |
| furA4 | furA4, manganese resistant | 69 |
| gly1 | gly1::Gm | This study |
| gor | gor::Gm | This study |
| grpE | grpE::Gm | This study |
| hemO/pigA | hemO::Gm | This study |
| hmp | hmp::Gm | This study |
| ibpA | ibpA::Gm | This study |
| katA | katA::Gm | 49 |
| katA katB | katA::Gm, katB::Tc | 28 |
| katA ohr | katA::Gm, ohr::Tc | This study |
| katB | katB::Gm | 10 |
| katB ankB | katB ankB::Gm | 35 |
| kauB | kauB::Gm | This study |
| lasI | lasI::Tn10 | 65 |
| lasI rhlI | lasI::Tc, rhlI::Tn501 | 66 |
| lasR | lasR::Tc | 20 |
| lasR rhlR | lasR::Tc, rhlR::Gm | This study |
| nadC (PAO4024) | nadC | P. Phibbs |
| ohr | ohr::Tc | 27 |
| orfX | orfX::Gm | This study |
| oxyR | oxyR::Gm | 61 |
| radA | radA::Gm | This study |
| recAb | recA::Tn501 | 34 |
| recC | recC::Gm | This study |
| rhlI | rhlI::Tn501-2 | 9 |
| rhlR | rhlR::Gm | This study |
| rplY | rplY::Gm | This study |
| rpoS | rpoS::Gm | 82 |
| snr-1 | snr-1::Gm | 41 |
| sodA | sodA::Gm | 30 |
| sodB | sodB::Cb | 30 |
| sodA sodBb | sodA::Gm, sodB::Cb | 30 |
| soxR | soxR::Gm | This study |
| vfr (PAO9001) | vfr::Gm | 86 |
| vfr (PAO9002) | vfr::Gm | 86 |
| Plasmids | ||
| pBluescript KS−/+ | Extended polylinker pUC derivative | Stratagene |
| pCRII | Apr, TA PCR cloning vector | Invitrogen |
| pCR2.1 | Apr, TA PCR cloning vector | Invitrogen |
| pUCGM | Apr, Gmr, pUC19 + 850-bp Gmr cassette | 76 |
| pUCP19 | Apr, broad-host-range expression vector | 87 |
| pUCP21T | Apr, broad-host-range expression vector | 87 |
| pEX100T | Apr, Cbr, mobilizable oriT sacB vector for mutant construction | 77 |
| pRK2013 | Kmr, Ori(ColE1) OriT (Mob+) Tra+ | 19 |
| pPZ-arsB | Apr, arsB::lacZ transcriptional fusion plasmid | This study |
| pPZ-arsR | Apr, arsR::lacZ transcriptional fusion plasmid | This study |
| pPZ-gor | Apr, gor::lacZ transcriptional fusion plasmid | This study |
| pPZ-sodB | Apr, sodB::lacZ transcriptional fusion plasmid | 29 |
Abbreviations used for genetic markers were as described by Holloway et al. (22). mob, mobilization site (ColE1); Tra+, conjugative phenotype; oriT, origin of transfer (RK2); Apr, ampicillin resistance; Cmr, chloramphenicol resistance; Gmr, gentamicin resistance. Isogenic mutants of mucoid cystic fibrosis isolate P. aeruginosa FRD1.
Manipulation of recombinant DNA and genetic techniques.
All plasmid and chromosomal nucleic acid manipulations were by standard techniques (74). Plasmid DNA was transformed into either E. coli strain DH5α-MCR (Gibco-BRL, Gaithersburg, MD) or strain SM10. To detect the presence of insert DNA, 5-bromo-4-chloro-3-indoyl-β-d-galactopyranoside (X-Gal, 40 μg/ml) was added to agar media. Restriction endonucleases, the Klenow fragment of DNA polymerase I, T4 DNA polymerase, and T4 DNA ligase were used as specified by the vendor (Invitrogen/Gibco-BRL Corporation, Gaithersburg, MD). Plasmid DNA was isolated using plasmid mini-prep isolation kits (QIAGEN), and restriction fragments were recovered from agarose gels using SeaPlaque low-melting-point agarose (FMC BioProducts, Rockland, ME). PCRs were performed using Taq DNA polymerase (BRL) and appropriate primers in an MJ Research thermal cycler, with 30 cycles of denaturation (2 min, 94°C), annealing (1 min, 54°C), and extension (1 min 30 sec, 72°C). Amplified DNA fragments were gel purified, cloned into either pCRII, pCR2.1 (both vectors from Invitrogen), or a pBluescript-based PCR vector (49), and then sequenced.
Construction of P. aeruginosa mutants.
The strategy for insertional inactivation of some of the genes listed in Table 1 was facilitated by gene disruption with an 850-bp Gmr cassette from pUCGM (76) and the gene replacement vector pEX100T (87), which allowed for selection of double-crossover events within putative recombinants cultured on agar containing 6% sucrose. Other mutants were constructed using the suicide vector pSUP203 (5). All mutants were confirmed by Southern blot analysis and/or PCR.
As(III) sensitivity assays.
For initial As(III) sensitivity screens, bacteria were grown aerobically for 17 h at 37°C in L broth. Cell suspensions were diluted 1:500 in fresh, prewarmed L broth with various concentrations of As(III) and incubated aerobically for an additional 17 h. Culture turbidity was recorded either with a Klett-Summerson colorimeter or by monitoring the absorbance of diluted suspensions at 600 nm using a Spectronics Genesys 5 spectrophotometer (Spectronic Instruments, Rochester, NY). For the mutant As(III) sensitivity experiments, the MIC of As(III) was determined. For these experiments, cultures were inoculated and incubated aerobically as described above, except that various amounts of As(III) were included in the medium. Culture growth was determined by measuring the optical density at 600 nm (OD600) as described above.
Sensitivity to H2O2 and PMS.
Wild-type, arsB, gor, crc, katA katB, and sodA sodB strains were grown overnight in L broth and diluted 1:100 in fresh L broth in triplicate. Cells were grown to an OD600 of 0.6 and then treated with 1 mM As(III). The arsB mutant was pretreated with 30 μM As(III). Triplicate sets of these above strains were also grown without As(III) treatment as controls. All cultures were grown overnight, and then 100 μl of As(III)-treated suspensions were plated on L agar plates containing 1 mM As(III). Control cells were plated on L agar plates without As(III). Filter paper disks (7.5 mm, Whatman) were impregnated with 10 μl of either 8.8 M H2O2 or 1 M phenazine methosulfate (PMS) and placed on the agar surface in triplicate. The zones of growth inhibition were determined after a 24-h incubation at 37°C.
β-Galactosidase reporter activity as influenced by As(III).
Bacteria containing plasmid-based lacZ reporter gene fusions were grown to mid-logarithmic phase in L broth plus 400 μg/ml carbenicillin (for plasmid maintenance), at which point they were exposed to 1 mM As(III) for 2 h at 37°C. Triplicate cell extracts were assayed for β-galactosidase reporter enzyme activity as previously described (48).
Two-dimensional gel electrophoresis and matrix-assisted laser desorption ionization-time of flight mass spectrometric protein analyses.
Wild-type bacteria were first grown aerobically overnight in L broth and then diluted 1:100 in fresh L broth. Cells were grown to mid-logarithmic phase, where they were treated with 1 mM As(III) and grown to an OD600 of 1.8. Control bacteria were similarly grown, but in the absence of As(III). Bacteria were harvested by centrifugation at 13,000 × g for 10 min at 4°C and subsequently washed twice in 10 mM Tris-HCl, pH 7.8. Cells were then lysed on ice for 5 min with lysis buffer (8 M urea, 4% CHAPS, 40 mM Tris-HCl, pH 7.8). To reduce viscosity, 10 mM Tris-Cl, pH 7.8, was added to the lysate, accounting for a 1.25-fold dilution, and the lysate was vortexed briefly. Immobiline Drystrips (Amersham) were used for isoelectric focusing of 80 μg of cell extract in the first dimension using the IPGphor isoelectric focusing system (Pharmacia Biotech). The strips were then equilibrated in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) buffer and separated by 12% SDS-PAGE in the second dimension using a Hoeffer SE 400 vertical gel electrophoresis unit. Mass spectrometric protein identification was performed as previously described (79, 91). Protein spots were excised from two-dimensional silver-stained polyacrylamide gels. Quantification of protein spots in two-dimensional gels was performed using Melanie 3.0 imaging software (Swiss Institute of Bioinformatics) and/or ImageQuaNT. Protein spots were digitized and quantified on a volumetric basis by mathematical integration of optical density over spot area. The final recorded changes in protein levels were based upon densitometric analyses of six different control and As(III)-treated samples.
Cell extract preparation, nondenaturing gel electrophoresis, and enzyme assays.
Cell extracts were prepared from cultures harvested by centrifugation at 13,000 × g for 10 min at 4°C. Cell extracts for native gel electrophoresis were prepared in 50 mM Tris-HCl, pH 7.8, as diluent. For catalase activity measurements, cell extracts were prepared in 50 mM potassium phosphate buffer, pH 7.0. Catalase activity was monitored by assessing the decomposition of 19.5 mM H2O2 in 50 mM potassium phosphate buffer, pH 7.0, at 240 nm (10, 49). One unit of activity is defined as that which decomposes 1 μmol of H2O2 min−1 mg protein−1. SOD activity was monitored by assessing the autoxidation of pyrogallol at 320 nm (72) using a modification of the original method described by Marklund and Marklund (53). Catalase and SOD activity staining in nondenaturing gels were performed as previously described (15, 84). Protein concentrations were estimated by the method of Bradford (8) using bovine serum albumin fraction V (Sigma) as standard. Where applicable, statistics were performed using Student's t test, with all assays being performed in triplicate.
RESULTS
Relative susceptibility of wild-type P. aeruginosa PAO1 to As(III).
To facilitate an efficient screen of the mutants used in this study, the relative sensitivity of wild-type P. aeruginosa PAO1 to As(III) was first determined to establish a maximum threshold for tolerance to As(III). Bacteria were incubated aerobically in L broth containing 0.5 to 100 mM As(III). The growth yield after a 24-h incubation was similar in control and 1 mM As(III)-treated bacteria but completely inhibited by 10 or 100 mM As(III) (data not shown). Based upon these results, a concentration of 1 mM As(III) was used for As(III) sensitivity screening of selected mutant strains.
Sensitivity of P. aeruginosa mutants to As(III).
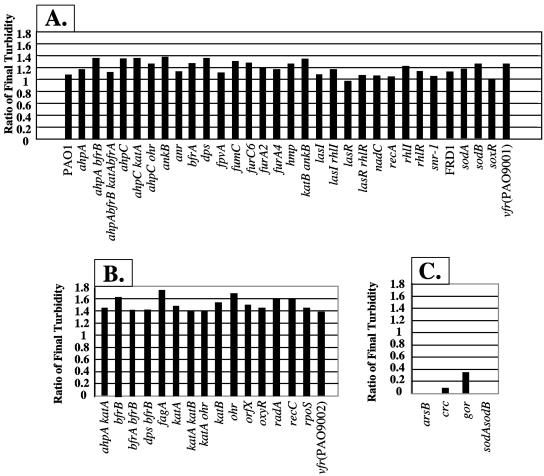
As(III) susceptibility under aerobic conditions was compared using mutants of P. aeruginosa PAO1 that lack specific antioxidants, DNA repair enzymes and binding proteins, bacterioferritins, membrane transporters, global regulators, metabolic enzymes, and quorum sensing circuitry. A description of the genes and gene products of the 57 mutants used in the following experiments is given in the supplemental information to this work (Table S1 at http://hassettdj.tripod.com). Screening these mutant strains for sensitivity to 1 mM As(III) revealed that most of the mutants displayed growth characteristics that were similar to wild-type bacteria (Fig. 1A). Interestingly, 16 mutants actually appeared to benefit from the presence of 1 mM As(III) in the medium (using an optical density ratio of 1.4 or greater as an arbitrary cutoff) (Fig. 1B). A smaller fraction of the mutants displayed severely impaired growth phenotypes (Fig. 1C). These included mutants devoid of the ArsB As(III) translocator, SOD (a sodA sodB double mutant of mucoid alginate-producing strain FRD1 [25]), glutathione reductase (gor), and the catabolite repressor protein Crc. All were significantly more sensitive to As(III) than the parental wild-type strain.
FIG. 1.
Sensitivity of P. aeruginosa mutants to 1 mM As(III). Results are segregated into three groups, (A) mutants which showed no apparent growth phenotype, (B) mutants whose growth was enhanced based upon an OD600 ratio of 1.4 as an arbitrary cutoff of As(III)-treated versus control bacteria, and (C) mutants which were severely inhibited by As(III). The turbidity of diluted suspensions was measured at 600 nm and expressed as the ratio of the culture density in the presence versus absence of As(III). The mutant designation is indicated below each bar.
Determination of As(III) MICs.
To quantify and compare the overall importance of ArsB, SOD, Gor, and Crc in cellular susceptibility to As(III), the MIC of As(III) was determined in arsB, sodA sodB, gor, and crc mutants. The As(III) MIC for wild-type strain PAO1 was 3.0 mM. In contrast, the MICs for the arsB and crc mutants were 0.2 and 0.8 mM, respectively. For wild-type strain FRD1, the MIC was 3.3 mM, while for the FRD1 sodA sodB mutant it was 0.3 mM. The sensitivity of the sodA sodB mutant suggested that exposure to As(III) results in an oxidative stress to the organism and, as such, is not unlike that concluded for the related organism P. putida (4).
As(III) exposure significantly reduces both catalase and SOD activity in arsB, gor, and crc mutants: a link to oxidative stress.
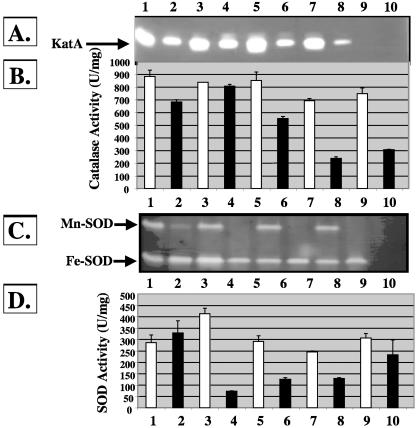
Because As(III) sensitivity was observed with mutants lacking various proteins involved in the oxidative stress response, we examined the effects of As(III) exposure on antioxidant expression. Wild-type bacteria and arsB, gor, and crc mutants were treated with 1 mM As(III) at the mid-logarithmic growth phase and allowed to recover to stationary phase to allow sufficient time for accumulation of enzymes important for the cellular response to As(III). Cell extracts were next examined for total catalase and SOD activity as well as isozyme profiles. As shown in Fig. 2A, catalase activity was reduced in the gor (Fig. 2, lane 6) and crc (Fig. 2, lane 8) mutant strains following exposure to As(III), while there was less of a reduction in the wild-type strain (Fig. 2, lane 2 versus lane 1) and no observed effect on total KatA activity in the arsB mutant (Fig. 2, lane 4 versus lane 3). Native gel activity staining revealed only expression of KatA activity; there was no apparent KatB activity, demonstrating that the reduction of total catalase activity was due to inhibition and/or destruction of the KatA isozyme (Fig. 2A and B). Similarly, total SOD activity was decreased in each mutant strain treated with As(III) but not in wild-type bacteria (Fig. 2C and D). The reduction in total SOD activity in the arsB, gor, crc, and katA katB mutants was largely due to the apparent complete loss of Mn-SOD activity, which also appeared targeted in the wild-type strain (Fig. 2C).
FIG. 2.
Effect of As(III) on catalase and SOD activity in P. aeruginosa. (A) Catalase isozyme activity stains based on 5 μg protein loaded per lane. Lane 1, wild-type PAO1; lane 2, PAO1 plus As(III); lane 3, arsB mutant; lane 4, arsB mutant plus As(III); lane 5, gor mutant; lane 6, gor mutant plus As(III); lane 7, crc mutant; lane 8, crc mutant plus As(III); lane 9, katA katB mutant; lane 10, katA katB mutant plus As(III). (B) Total catalase levels in cell extracts of wild-type and isogenic mutants that demonstrated sensitivity to As(III). Lane assignments are as in panel A except that: lane 9, sodA sodB mutant; lane 10, sodA sodB mutant plus As(III). (C) Nondenaturing gels stained for SOD activity gel based on 40 μg protein loaded per lane. Lane 1, wild-type PAO1; lane 2, PAO1 plus As(III); lane 3, arsB mutant; lane 4, arsB mutant plus As(III); lane 5, gor mutant; lane 6, gor mutant plus As(III); lane 7, crc mutant; lane 8, crc mutant plus As(III); lane 9, sodA sodB mutant; lane 10, sodA sodB mutant plus As(III). What appears to be a band in lane 10 is actually a notch cut in the gel to aid in the precise timing of electrophoresis. (D) Total SOD levels in cell extracts of wild-type and isogenic mutants that demonstrated sensitivity to As(III). Lane assignments are the same as in panel C except: lane 9, katA katB mutant; lane 10, katA katB mutant plus As(III). P values were obtained via Student's t test for PAO1 and the arsB, gor, crc, and sodA sodB mutants in the catalase activity assay, where activity of each strain is compared to that of the same strain that is As(III) treated, and are 0.35 × 10−5, 0.14 × 10−5, 0.023 × 10−5, 5.6 × 10−5, and 1.38 × 10−3, respectively. P values for PAO1 and the arsB, gor, crc, and katA katB mutants in the SOD assay are 0.61 × 10−4, 3.21 × 10−4, 6.01 × 10−3, 9.86 × 10−5, and 0.43, respectively.
Given that both total catalase and SOD levels were reduced in some of the As(III)-treated mutants, additional experiments were conducted to establish whether these mutants would also be more susceptible to oxidants such as H2O2 and O2− in the presence of As(III). SOD- and catalase-deficient P. aeruginosa have previously and predictably been shown to be exquisitely sensitive to O2− and H2O2, respectively (29, 30), and this was again observed in these experiments. Compared to the wild-type strain, the crc and gor mutants exhibited a statistically significant increase in sensitivity to H2O2 but not to the O2−-generating agent PMS (Table 2). A lack of Crc clearly caused the greatest problems for the bacteria in response to the dual stressors H2O2 and As(III), as shown by a doubling of the inhibition zone in the presence of either oxidative stress agent. Summarizing the experiments from Fig. 2 and Table 2, it appears that As(III) can inhibit the activity or paralyze the biosynthesis of both Mn-SOD and KatA but that only the latter inhibition resulted in a sensitized phenotype due to lack of additional catalase activity (i.e., no KatB induction). Apparently, Fe-SOD activity was adequate for protecting the cell against the O2−-generating agent PMS.
TABLE 2.
Sensitivity of P. aeruginosa gor, crc, katA katB, and sodA sodB mutants to H2O2 and PMSa
| Strain | Clearance zone (mm) or P value
|
|||||
|---|---|---|---|---|---|---|
| H2O2
|
PMS
|
|||||
| −As(III) +H2O2 | +As(III) +H2O2 | P value | −As(III) +PMS | +As(III) +PMS | P value | |
| PAO1 | 10.17 ± 0.24 | 16.17 ± 0.24 | 1.41 × 10−5 | 33.83 ± 1.03 | 34 ± 0.41 | 0.84 |
| gor | 12.67 ± 0.94 | 20 ± 0 | 3.88 × 10−4 | 34.83 ± 0.24 | 37.83 ± 1.18 | 0.02 |
| crc | 10.33 ± 1.55 | 31.17 ± 0.85 | 7.53 × 10−5 | 34.83 ± 0.24 | 35.33 ± 2.49 | 0.79 |
| katA katB | 44.5 ± 0.41 | 43 ± 1.08 | 0.14 | ND | ND | ND |
| sodA sodB | ND | ND | ND | 53.5 ± 0.5 | 44.17 ± 0.24 | 2.15 × 10−4 |
| PAO1b | 17.33 ± 1.25 | 19.5 ± 0.71 | 0.099 | 35.33 ± 0.47 | 35 ± 0 | 0.37 |
| arsBb | 15.67 ± 1.70 | 37.33 ± 1.70 | 2.18 × 10−4 | 34.33 ± 0.24 | 37 ± 0 | 8.92 × 10−5 |
ND, not determined. Filter paper disks (7.5 mm) containing 10 μl of 8.8 M H2O2 or 1 M PMS were placed in triplicate on L agar surfaces, and the zones of growth inhibition were measured after 24 h at 37°C.
Because of the exquisite sensitivity of the arsB mutant to As(III), the As(III) pretreatment was reduced to only 30 μM As(III), a sublethal dose for the arsB mutant.
Influence of Gor, SOD, and Crc on the As(III)-mediated activation of arsR and arsB.
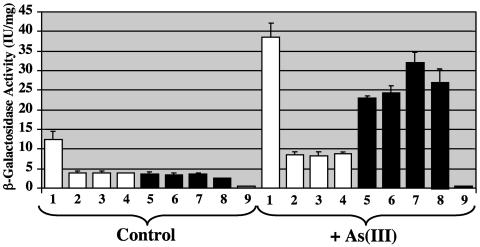
In E. coli and S. aureus, transcription of arsR and arsB is increased when the bacteria are exposed to As(III) (38, 64). To assess the potential effect of Gor, SOD, and Crc on arsR and arsB transcription, plasmids harboring arsR-lacZ and arsB-lacZ fusions were transformed into wild-type bacteria and into the gor, crc, and sodA sodB mutants. As shown in Fig. 3, in culture conditions lacking As(III), arsR-lacZ reporter activity was reduced ∼75% in the gor, crc, and sodA sodB mutants (Fig. 3, white bars) relative to the wild-type strain. However, apparent arsR transcription in these mutants was still sensitive to As(III), increasing approximately twofold in bacteria exposed to As(III), although the As(III)-sensitive arsR induction in these mutants was proportionally much smaller in comparison to the wild-type strain. As expected, arsB-lacZ reporter enzyme activity was greater in the wild-type strain exposed to As(III) (Fig. 3). In addition, although somewhat more variable, apparent As(III)-induced arsB expression levels were consistently increased in all of these mutants relative to the wild-type strain (Fig. 3). As(III) exposure increased arsB transcription roughly sixfold in the wild type and the crc mutant, whereas As(III)-induced transcription in the gor and sodAB mutants increased 8- and 11-fold, respectively, relative to untreated controls. β-Galactosidase activity in control samples was consistently low, averaging ∼1 IU/mg (Fig. 3).
FIG. 3.
Effect of As(III) on transcription of arsR and arsB in wild-type bacteria and crc, gor, and sodA sodB mutants. Bacteria were grown to mid-logarithmic phase and exposed to 1 mM As(III) for 2 h at 37°C, and cell extracts were prepared and assayed for β-galactosidase activity in triplicate as described in Materials and Methods. White bars, arsR-lacZ; black bars, arsB-lacZ. Lanes 1 and 5, wild type; lanes 2 and 6, crc mutant; lanes 3 and 7, gor mutant; lanes 4 and 8, sodA sodB mutant. Lane 9 represents the lacZ plasmid control.
Proteomic analysis of As(III)-treated P. aeruginosa.
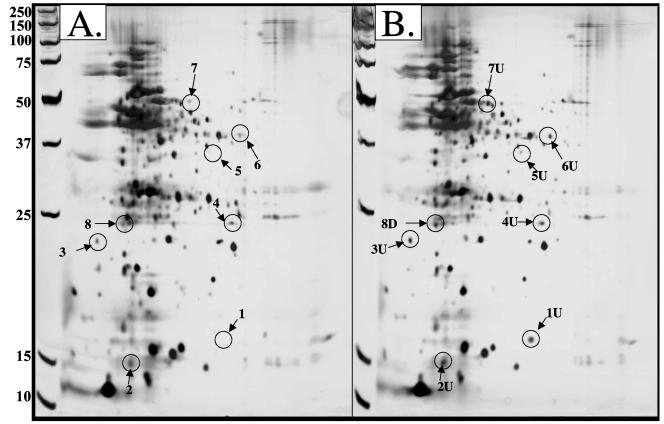
Additional experiments were conducted to initiate a more global assessment of the cellular physiology of As(III)-treated P. aeruginosa. Whole-cell lysates from control bacteria and those treated with 1 mM As(III) were separated by two-dimensional gel electrophoresis (Fig. 4). Based on internal control protein standards, and using the default parameter setting in the Melanie 3.0 and ImageQuaNT software, an average of 240 protein spots were detected in the control samples, while protein extracts from As(III)-treated cells averaged 213 protein spots. Of the 26 proteins excised from these gels, 8 were confidently identified by matrix-assisted laser desorption ionization-time of flight analyses (Table 3). These included the following: (i) the heat shock protein IbpA; (ii) a probable DNA-binding stress protein; (iii) ribosomal protein L25, RplY; (iv) heat shock protein GrpE; (v) a putative allo-threonine aldolase; (vi) acetyl-coenzyme A acetyltransferase; (vii) probable aldehyde dehydrogenase KauB; and (viii) a hypothetical protein.
FIG. 4.
Two-dimensional SDS polyacrylamide gel of control (A) and As(III)-treated (B) P. aeruginosa PAO1. Bacteria were grown to mid-logarithmic phase and exposed to 1 mM As(III) until subrecovery at OD600 of 1.8. Cells were harvested at 4°C and proteins prepared immediately for separation via two-dimensional gel electrophoresis and staining with silver nitrate. Proteins up-regulated are labeled “U” and numbered; proteins down-regulated are labeled “D” and numbered. Proteins targeted for mutational analysis are circled and are listed in Table 3. Molecular weights are shown to the left of panel A.
TABLE 3.
Identification of P. aeruginosa proteins modulated by As(III)a
| Spot no. or PAO1 | Gene name | Protein name | PA no. | Modulation level | MOWSE score | MW/pI | MIC (mM) | Lag phase (h) (P value) |
|---|---|---|---|---|---|---|---|---|
| 1 | ibpA | Heat shock protein IbpA | PA3126 | ▴51 | 856 | 16.6/5.8 | 2.3 | 14.3 (0.038) |
| 2 | PA0962 | Probable DNA-binding stress protein | PA0962 | ▴1.9 | 69,790 | 17.5/5.0 | 2.3 | 13.9 (0.081) |
| 3 | grpE | Heat shock protein GrpE | PA4762 | ▴2.6 | 6,943 | 20.7/4.5 | ND | |
| 4 | rplY | Ribosomal protein L25, RplY | PA4671 | ▴2.5 | 265 | 21.9/5.8 | 2.0 | 20.6 (0.006) |
| 5 | glyI | Putative allo-threonine aldolase | PA0902 | ▴48 | 610 | 35.4/6.0 | ND | |
| 6 | atoB | Acetyl-CoA acetyltransferase | PA2001 | ▴3.1 | 1,276 | 40.4/6.0 | 2.7 | 14.4 (0.050) |
| 7 | kauB | Probable aldehyde dehydrogenase | PA5312 | ▴1.7 | 200 | 53.1/5.4 | 2.5 | 16.2 (0.012) |
| 8 | hemO/pigA | Heme oxygenase | PA0672 | ▾3.3 | 733 | 21.9/5.0 | 14.3 (0.109) | |
| PAO1 | 3.0 | 12.9 |
Twenty-six reproducibly represented proteins containing at least 1 pmol of protein were selected for mass spectrometric analysis. Eight of these proteins were identified with significant certainty (MOWSE score of 200 or greater). The modulation level is the average densitometric level compared with untreated controls of six different two-dimensional gels. The symbols ▴ and ▾ are meant to indicate up- and down-regulated proteins, respectively. ND, not determined because an isogenic mutant could not be constructed.
To examine the relative importance of these eight gene products in protection against As(III) toxicity, the genes encoding these proteins were cloned, insertionally inactivated with an 850-bp Gmr cassette, and then recombined into the genome of the wild-type strain in order to create isogenic mutants for each gene. Mutants for six of these genes were successfully constructed, and their As(III) tolerance was examined using both a modified screening procedure that assessed the time required for these mutants to recover from a treatment with 1 mM As(III) as well as an MIC assessment. All mutants displayed an extended lag period relative to the parental wild-type strain (12.9 h), with the longest lag periods being observed with the rplY (20.6 h) and kauB (16.2 h) mutants (Table 3). However, once growth began, growth rates for all mutants appeared similar to the wild-type strain (data not shown). In general, the MIC for each of these mutants was less than that for wild-type bacteria, ranging from 0.3 to 2.7 mM (Table 3).
DISCUSSION
P. aeruginosa, the model organism for this study, harbors a chromosomal arsR-arsB-arsC-arsH operon. These genes encode an ArsR regulator, the ArsB As(III) translocator protein, an ArsC As(V) reductase, and a gene homologous to Thiobacillus ferrooxidans arsH encoding a protein of unknown function that was shown not to confer arsenic resistance in E. coli (11) (for a schematic diagram, see Fig. 5 [12]). A second putative arsC gene is located on the P. aeruginosa chromosome (accession no. NP 249641), but there is no information available regarding expression of this alternative As(V) reductase or its potential role in arsenic resistance (58). The goals of the present study were to (i) extend beyond this basic resistance paradigm, (ii) establish a greater understanding of how arsenic affects bacteria, and ultimately (iii) contribute to a better conceptual model of a global cellular response involved in bacteria-arsenic interactions. Two approaches were used: (i) screening of a large panel of defined mutants and (ii) a limited proteomics-based assessment to identify other proteins that may be differentially modulated as a result of As(III) exposure. The latter was accompanied by a second round of mutant construction and analysis that directly assessed the relative importance of the identified proteins in As(III) resistance in P. aeruginosa. From a total of 57 mutants studied, several were identified as having increased As(III) sensitivity (Fig. 1C), and somewhat surprisingly, other mutants seemed to grow better in the presence of As(III) (Fig. 1B).
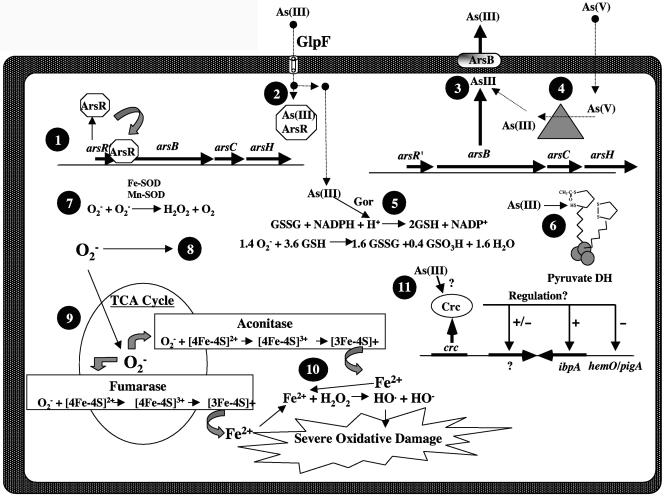
FIG. 5.
Model of P. aeruginosa cellular events that could occur upon exposure to As(III). 1. The main defense against As(III) exposure is induction of the arsR-arsB-arsC operon, encoding the ArsR repressor, the ArsB anion translocator, and the ArsC As(V) reductase (12). Prior to As(III) exposure, the ArsR repressor prevents transcription of the arsB and arsC genes. 2. Upon entry of As(III) via potentially a homolog of the mammalian GlpF aquaporin that is also present in E. coli (55), the ArsR repressor binds As(III) and no longer acts as a repressor, allowing for synthesis of ArsB and ArsC. 3. ArsB then extrudes As(III) from the cell. 4. If the cell is exposed to arsenate [As(V)], ArsC reduces As(V) to As(III), which is then extruded by ArsB. 5. In an arsB mutant, As(III) accumulates and then depletes the reduced glutathione (GSH) pools that are essential to keep sulfhydryl groups on proteins reduced. As(III) would also oxidize the same sulfhydryl groups being reduced by GSH. 6. Example: the sulfhydryl groups on the lipoamide arms of the pyruvate dehydrogenase complex (PDH) which is sensitive to As(III) at micromolar levels (36). 7. In wild-type cells, the role of SOD is to detoxify O2−. 8. However, in a sodA sodB mutant, elevated concentrations of O2− can react with GSH to form GSSG and sulfonate (88), reducing the cells' capacity to protect against As(III) exposure. 9. O2− can also react with many [4Fe-4S]-containing enzymes, such as aconitase and fumarase, and thus inhibit the TCA cycle. 10. This causes oxidation of the iron in the cluster, rendering the cluster unstable. This results in the release of reactive iron (Fe2+) in the cytoplasm. The Fe2+ is then free to react with H2O2 in a Fenton reaction to form the very destructive hydroxyl radical (HO·). 11. Putative regulatory function of Crc in response to As(III) exposure. Loci potentially regulated by Crc are compressed together to conserve space in this figure but are not transcriptionally linked, as might be inferred by their adjacent location as depicted in the figure.
Because P. aeruginosa does not possess the ArsA ATPase, which provides enhanced As(III) resistance (MIC ≈ 10 mM) to E. coli (18), it is probably not surprising that the As(III) MIC for wild-type P. aeruginosa was found to be only ∼3.0 mM. However, the 13-fold-greater sensitivity of an isogenic arsB mutant relative to the wild-type strain confirmed the importance of ArsB for As(III) resistance in P. aeruginosa and in bacteria in general. Other mutants, such as the sodA sodB double mutant and the crc and gor mutants, also exhibited substantially increased sensitivity to As(III) (Fig. 1C). A closer analysis of the function(s) of these proteins in As(III) resistance is clearly warranted in future experiments, but the results obtained in this study are consistent with those conducted previously with both eukaryotic and prokaryotic organisms that suggest that elements of an oxidative stress response are involved (4, 16, 38, 60).
Function of glutathione reductase (Gor) and SOD.
In eukaryotic cells, As(III) exposure results in increased levels of reduced glutathione (GSH) (60), and an As(III)-triglutathione complex has been found in human liver excreta (40), leading to the conclusion that GSH is important in reducing As(III) toxicity in humans (26). These cellular activities are thought to be part of a general set of reactions involved in responding to As(III)-mediated production of reactive oxygen species (14, 54) and are consistent with our observations and those made in previous studies with prokaryotes. Experiments with P. putida showed that Gor levels increase upon exposure to As(III) (4). Further, E. coli mutants lacking Gor or enzymes essential for glutathione synthesis (gshA and gshB) were also found to be more sensitive to As(V) than the wild-type strain (62). One function of Gor in E. coli is to recycle oxidized glutathione (GSSG) back to GSH, which is the reductant for As(V) reductase (62) that converts As(V) to As(III). The latter is then actively removed from the cell by ArsB and, as such, establishes the primary mechanism for As(III) resistance.
Results from the present study suggest that Gor has another as-yet-undocumented function(s) in arsenic resistance. In all experiments, arsenic was supplied as As(III), so the bacteria would not require GSH per se as a reductant for reducing As(V) prior to extrusion via ArsB. Indeed, presumably ArsB should be no less functional in the gor mutant than the wild type, as arsB induction in the gor mutant was increased by about one-third relative to the parental wild-type strain (Fig. 3). Gor could also be important for maintaining near-optimal GSH-to-GSSG ratios, with GSH itself providing a “sacrificial” sulfhydryl (—SH) group that helps titrate cellular As(III) and thus indirectly protect the sulfhydryl groups of critical proteins, such as the As(III)-sensitive lipoamide-containing pyruvate dehydrogenase (36). Other GSH maintenance-related Gor functions may be linked to cellular responses to As(III)-mediated production of O2−. O2− production in As(III)-treated P. aeruginosa was clearly implied by the acute As(III) sensitivity of the sodA sodB double mutant. Elevated intracellular O2− levels would poison important [4Fe-4S]2+ cluster-containing proteins, including the aconitases (23), fumarases (46), and various dehydratases (22, 45) (Fig. 5). The O2− poisoning of the aforementioned proteins has been shown to be reduced by the addition of GSH into the growth medium (7, 21) and likely results from two important functions: GSH helps scavenge O2− (88), and it could potentially provide a source of sulfur for reconstitution of the Fe-S centers via the activity of cysteine desulfurases (78). In the experiments reported herein, lower cellular GSH-to-GSSG ratios would be expected in both the gor and sodA sodB mutants, but for different reasons. Recycling of GSSG back to GSH would predictably be impaired in a gor mutant. In a SOD-limited bacterium, however, GSH could be rapidly depleted by O2− (O2− reacts with GSH at a rate estimated at 105 M−1 s−1 [85]). We suggest that our data are not inconsistent with the hypothesis that the As(III)-sensitive phenotypes of the gor and sodA sodB mutants have a common basis in that both are involved in maintaining or protecting cellular GSH. In E. coli (6), Salmonella enterica serovar Typhimurium (44), and Saccharomyces cerevisiae (67), both Gor and SOD have also been shown to be important for resistance to selenite toxicity, which has also been suggested to result from oxidative damage (44). P. aeruginosa possesses two SODs, one of which incorporates iron as cofactor (Fe-SOD, encoded by sodB [31]) and the other manganese (Mn-SOD, encoded by sodA [31]). Collectively, their function is to disproportionate O2− to H2O2 and O2. To remove H2O2, P. aeruginosa possesses at least three catalases, KatA (49), KatB (10), and KatC (D. J. Hassett and U. A. Ochsner, unpublished data). KatA activity is the major catalase activity that is detectable in all phases of growth but is at a maximum in stationary phase (49). In contrast, KatB activity is detectable in P. aeruginosa only after exposure to significant levels of H2O2 (10).
Catabolite repressor control protein (Crc).
The discovery that the Crc protein is somehow involved in As(III) resistance (Fig. 1) and the oxidative stress response (Fig. 2) represents another development in the general understanding of bacterial arsenic resistance. Although the crc gene was discovered in 1991 (51), relatively little is known about the regulatory breadth of its gene product. Previous studies have indicated that when a crc mutant is grown on succinate, it does not repress synthesis of proteins involved in mannitol and glucose transport or the enzymes glucose-6-phosphate dehydrogenase, glucokinase, or Entner-Doudoroff-controlled dehydratase, aldolase, and amidase enzymes (50). Other studies have shown that Crc is involved in regulating production of the hemolytic phospholipase PlcH (73), repression of aromatic compound catabolism (57), and an as-yet-uncharacterized feature(s) of biofilm formation (63). We also found the crc mutant to be more sensitive to H2O2 in the presence of As(III) (Table 2), a phenotype very likely linked to the reduced levels of catalase under these growth conditions (Fig. 2A and B). SOD activity was also reduced in crc mutant bacteria (Fig. 2C and D), but this did not translate into increased PMS sensitivity, likely because there was still enough residual Fe-SOD activity (even without Mn-SOD) to protect the bacterium. Given the regulatory nature of Crc, the heightened As(III) sensitivity of the crc mutant is assumed to be due to abnormal regulation (either underexpression or overexpression) of genes under Crc control. The results of the present study clearly suggest that this Crc-regulated gene(s) appears nearly as important as arsB in conferring maximal As(III) resistance (Fig. 1C).
Previous studies with P. putida demonstrated that catalase activity increased in response to the presence and oxidation of As(III) (3, 4). In addition, selenite exposure studies with S. enterica serovar Typhimurium (44) and E. coli (6) also demonstrated that catalase may be an important cellular response to this similar-acting metalloid. However, the results of the present study demonstrated that this may not be a universal response among bacteria. The activity of the major housekeeping catalase, KatA, was reduced in most of the As(III)-treated strains examined in the present study. In some cases, such as the crc mutant, the inhibition of activity or biosynthesis was significant. The reduced catalase activity in all As(III)-treated organisms correlated with increased sensitivity to H2O2 (Table 2).
Other As(III)-affected proteins.
Proteomic experiments identified other proteins whose expression is affected by As(III) exposure. Mutants were isolated for the encoding genes and were found to exhibit growth phenotypes that suggested some involvement in the initial cellular response to As(III) that aids in adaptation to a more resistant state. None of the encoded proteins have obvious direct regulatory functions that could help the bacteria in the transition to a full As(III) resistance response. However, in at least one instance, the specific role of one of these genes might be inferred to involve some form of antioxidant activity. IbpA was up-regulated 51-fold in As(III)-treated cells and absent in control cells (Table 3). In E. coli, IbpA is important for protecting proteins from inactivation by both heat shock and oxidative stress (42, 43).
Finally, a novel yet puzzling observation from the latter experiments identified the apparent requirement for down-regulation of certain genes for optimum As(III) resistance. The long lag phase of the heme oxygenase HemO/PigA mutant (Table 3) implies that this As(III)-down-regulated protein is somehow important in the wild-type cell. At present, it is unclear why the absence of proteins that are actually down-regulated would have negative consequences for As(III) resistance, although a negative regulatory function is plausible.
Summary.
The various experiments in this study contributed to several novel observations regarding arsenic-microbe interactions. Novel functions for Gor can be inferred, and a novel regulatory element (Crc) was discovered. The presence or absence of several proteins correlated with optimum As(III) resistance in P. aeruginosa (depicted in Fig. 5). Some mutants exhibited enhanced growth in the presence of As(III) and, similarly, a requirement for down-regulation of some loci for optimum As(III) resistance (Fig. 1). Improved selenite response phenotypes have been reported for various E. coli mutant combinations, particularly a gshA sodA sodB triple mutant (6), and our work serves to demonstrate a similar and, as such, confirmatory response to As(III). Improved resistance to As(III) (or selenite) in mutants lacking antioxidant enzymes was unexpected and is clearly inconsistent with the concept that oxidative damage is a primary toxic effect of As(III) poisoning in bacteria. Still, elements of an oxidative stress-like response were evident and included SOD and Gor, although catalase genes were not induced, and indeed, catalase activity levels were reduced in most strains exposed to As(III). Induction of additional SOD activity (i.e., sodA) was likewise not apparent in our experiments (absence of the Mn-SOD isozyme in Fig. 2C), and it is likely that the “housekeeping” enzyme Fe-SOD is essential for normal arsenic resistance in P. aeruginosa but that in its absence another SOD-like activity is required. Inducible SODs could, however, be important for bacteria that do not constitutively maintain robust SOD levels, as in P. aeruginosa, or under some environmental conditions where As(III) levels might overwhelm the capacity of ArsB to remove As(III) from the cell. The As(III) levels used to examine mutant susceptibility in this study (i.e., 1 mM) would be representative of some of the more extreme cases of arsenic contamination, such as in soils surrounding mine smelters (T. R. McDermott and D. J. Hassett, unpublished data), or in natural ecosystems, such as in some geothermal environments [2 to 3 mM As(III) in some thermal areas in Yellowstone National Park (H. Langner and W. P. Inskeep, personal communication)].
Acknowledgments
This work was supported in part by grant AI-40541 from the National Institutes of Health (D.J.H.), funds from the Department of Molecular Genetics, Biochemistry and Microbiology at the University of Cincinnati College of Medicine (D.J.H.), and grants from the Environmental Protection Agency (R827457-01-0) and the USDA-NRI Soils and Soil Biology Grant Program (2002-35107-12268) to T.R.M.
We also acknowledge the Mass Spectrometry Facility at the Veteran's Administration Hospital (Cincinnati, OH).
REFERENCES
- 1.Abdrashitova, S. A., G. G. Abdulline, and A. N. Ilyaletdinov. 1986. Role of arsenites in lipid peroxidation in Pseudomonas putida cells oxidizing arsenite. Mikrobiologiya 55:212-216. [Google Scholar]
- 2.Abdrashitova, S. A., G. G. Abdulline, and A. N. Ilyaletdinov. 1986. Role of lipids in the oxidation of arsenites by a culture of Pseudomonas putida. Mikrobiologiya 55:582-585. [Google Scholar]
- 3.Abdrashitova, S. A., A. N. Ilialetdinov, B. N. Mynbaeva, and G. G. Abdullina. 1982. Catalase activity of a Pseudomonas putida strain oxidizing arsenic. Mikrobiologiya 51:34-37. [PubMed] [Google Scholar]
- 4.Abdrashitova, S. A., B. N. Mynbaeva, B. B. Aidarkhanov, and A. N. Ilyaletdinov. 1990. Effect of arsenite on lipid peroxidation and on activity of antioxidant enzymes in arsenite-oxidizing microorganisms. Mikrobiologiya 59:234-240. [Google Scholar]
- 5.Bagdasarian, M., R. Lurz, B. Rueckert, F. C. H. Franklin, M. M. Bagdasarian, J. Frey, and K. N. Timmis. 1981. Specific purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene 16:237-247. [DOI] [PubMed] [Google Scholar]
- 6.Bebien, M., G. Lagniel, J. Garin, D. Touati, A. Vermeglio, and J. Labarre. 2002. Involvement of superoxide dismutases in the response of Escherichia coli to selenium oxides. J. Bacteriol. 184:1556-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benov, L., and I. Fridovich. 1998. Growth in iron-enriched medium partially compensates Escherichia coli for the lack of manganese and iron superoxide dismutase. J. Biol. Chem. 273:10313-10316. [DOI] [PubMed] [Google Scholar]
- 8.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 9.Brint, J. M., and D. E. Ohman. 1995. Synthesis of multiple exoproducts in Pseudomonas aeruginosa is under the control of RhlR-RhlI, another set of regulators in strain PAO1 with homology to the autoinducer-responsive LuxR-LuxI family. J. Bacteriol. 177:7155-7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown, S. M., M. L. Howell, M. L. Vasil, A. J. Anderson, and D. J. Hassett. 1995. Cloning and characterization of the katB gene of Pseudomonas aeruginosa encoding a hydrogen peroxide-inducible catalase: purification of KatB, cellular localization, and demonstration that it is essential for optimal resistance to hydrogen peroxide. J. Bacteriol. 177:6536-6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butcher, B. G., S. M. Deane, and D. E. Rawlings. 2000. The chromosomal arsenic resistance genes of Thiobacillus ferrooxidans have an unusual arrangement and confer increased arsenic and antimony resistance to Escherichia coli. Appl. Environ. Microbiol. 66:1826-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai, J., K. Salmon, and M. S. DuBow. 1998. A chromosomal ars operon homologue of Pseudomonas aeruginosa confers increased resistance to arsenic and antimony in Escherichia coli. Microbiology 144:2705-2713. [DOI] [PubMed] [Google Scholar]
- 13.Chen, C. M., T. K. Misra, S. Silver, and B. P. Rosen. 1986. Nucleotide sequence of the structural genes for an anion pump. The plasmid-encoded arsenical resistance operon. J. Biol. Chem. 261:15030-15038. [PubMed] [Google Scholar]
- 14.Chen, Y. C., S. Y. Lin-Shiau, and J. K. Lin. 1998. Involvement of reactive oxygen species and caspase 3 activation in arsenite-induced apoptosis. J. Cell. Physiol. 177:324-333. [DOI] [PubMed] [Google Scholar]
- 15.Clare, D. A., M. N. Duong, D. Darr, F. Archibald, and I. Fridovich. 1984. Effects of molecular oxygen on the detection of superoxide radical with nitroblue tetrazolium and an activity stain for catalase. Anal. Biochem. 140:532-537. [DOI] [PubMed] [Google Scholar]
- 16.Cullen, W. R., and K. J. Reimer. 1989. Arsenic speciation in the environment. Chem. Rev. 89:713-764. [Google Scholar]
- 17.Dey, S., D. Dou, L. S. Tisa, and B. P. Rosen. 1994. Interaction of the catalytic and membrane subunits of an oxyanion-translocating ATPase. Arch. Biochem. Biophys. 311:418-424. [DOI] [PubMed] [Google Scholar]
- 18.Dey, S., and B. P. Rosen. 1995. Dual mode of energy coupling by the oxyanion-translocating ArsB protein. J. Bacteriol. 177:385-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Figurski, D., and D. R. Helinski. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. USA 76:1648-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gambello, M. J., and B. H. Iglewski. 1991. Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transciptional activator of elastase expression. J. Bacteriol. 173:3000-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gardner, P. R., and I. Fridovich. 1993. Effect of glutathione on aconitase in Escherichia coli. Arch. Biochem. Biophys. 301:98-102. [DOI] [PubMed] [Google Scholar]
- 22.Gardner, P. R., and I. Fridovich. 1991. Superoxide sensitivity of the Escherichia coli 6-phosphogluconate dehydratase. J. Biol. Chem. 266:1478-1483. [PubMed] [Google Scholar]
- 23.Gardner, P. R., and I. Fridovich. 1991. Superoxide sensitivity of the Escherichia coli aconitase. J. Biol. Chem. 266:19328-19333. [PubMed] [Google Scholar]
- 24.Gladysheva, T. B., K. L. Oden, and B. P. Rosen. 1994. The ArsC arsenate reductase of plasmid R773. Biochemistry 33:7287-7293. [DOI] [PubMed] [Google Scholar]
- 25.Goldberg, J. B., and D. E. Ohman. 1984. Cloning and expression in Pseudomonas aeruginosa of a gene involved in the production of alginate. J. Bacteriol. 158:1115-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gyurasics, A., F. Varga, and Z. Gregus. 1991. Effect of arsenicals on biliary excretion of endogenous glutathione and xenobiotics with glutathione-dependent hepatobiliary transport. Biochem. Pharmacol. 41:937-944. [DOI] [PubMed] [Google Scholar]
- 27.Hassett, D. J., E. Alsabbagh, K. Parvatiyar, M. L. Howell, R. W. Wilmott, and U. A. Ochsner. 2000. A protease-resistant catalase, KatA, that is released upon cell lysis during stationary phase, is essential for aerobic survival of a Pseudomonas aeruginosa oxyR mutant at low cell densities. J. Bacteriol. 182:4557-4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hassett, D. J., J. G. Elkins, J.-F. Ma, and T. R. McDermott. 1999. Pseudomonas aeruginosa biofilm sensitivity to biocides: use of hydrogen peroxide as model antimicrobial agent for examining resistance mechanisms. Methods Enzymol. 310:599-608. [DOI] [PubMed] [Google Scholar]
- 29.Hassett, D. J., J.-F. Ma, J. G. Elkins, T. R. McDermott, U. A. Ochsner, S. E. H. West, C.-T. Huang, J. Fredericks, S. Burnett, P. S. Stewart, G. McPheters, L. Passador, and B. H. Iglewski. 1999. Quorum sensing in Pseudomonas aeruginosa controls expression of catalase and superoxide dismutase genes and mediates biofilm susceptibility to hydrogen peroxide. Mol. Microbiol. 34:1082-1093. [DOI] [PubMed] [Google Scholar]
- 30.Hassett, D. J., H. P. Schweizer, and D. E. Ohman. 1995. Pseudomonas aeruginosa sodA and sodB mutants defective in manganese- and iron-cofactored superoxide dismutase activity demonstrate the importance of the iron-cofactored form in aerobic metabolism. J. Bacteriol. 177:6330-6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hassett, D. J., W. A. Woodruff, D. J. Wozniak, M. L. Vasil, M. S. Cohen, and D. E. Ohman. 1993. Cloning of the sodA and sodB genes encoding manganese and iron superoxide dismutase in Pseudomonas aeruginosa: demonstration of increased manganese superoxide dismutase activity in alginate-producing bacteria. J. Bacteriol. 175:7658-7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hei, T. K., S. X. Liu, and C. Waldren. 1998. Mutagenicity of arsenic in mammalian cells: role of reactive oxygen species. Proc. Natl. Acad. Sci. USA 95:8103-8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holloway, B. W. 1969. Genetics of Pseudomonas. Bacteriol. Rev. 33:419-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horn, J. M., and D. E. Ohman. 1988. Autogenous regulation and kinetics of induction of Pseudomonas aeruginosa recA transcription as analyzed with operon fusions. J. Bacteriol. 170:4699-4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howell, M. L., E. Alsabbagh, J. F. Ma, U. A. Ochsner, M. G. Klotz, T. J. Beveridge, K. M. Blumenthal, E. C. Niederhoffer, R. E. Morris, D. Needham, G. E. Dean, M. A. Wani, and D. J. Hassett. 2000. AnkB, a periplasmic ankyrin-like protein in Pseudomonas aeruginosa, is required for optimal catalase B (KatB) activity and resistance to hydrogen peroxide. J. Bacteriol. 182:4545-4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu, Y., L. Su, and E. T. Snow. 1998. Arsenic toxicity is enzyme specific and its affects on ligation are not caused by the direct inhibition of DNA repair enzymes. Mutat. Res. 408:203-218. [DOI] [PubMed] [Google Scholar]
- 37.Ilialetdinov, A. N., and S. A. Abdrashitova. 1981. Autotrophic arsenic oxidation by a Pseudomonas arsenitoxidans culture. Mikrobiologiya 50:197-204. [PubMed] [Google Scholar]
- 38.Ji, G., and S. Silver. 1992. Reduction of arsenate to arsenite by the ArsC protein of the arsenic resistance operon of Staphylococcus aureus plasmid pI258. Proc. Natl. Acad. Sci. USA 89:7974-7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ji, G., and S. Silver. 1992. Regulation and expression of the arsenic resistance operon from Staphylococcus aureus plasmid pI258. J. Bacteriol. 174:3684-3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kala, S. V., M. W. Neely, G. Kala, C. I. Prater, D. W. Atwood, J. S. Rice, and M. W. Lieberman. 2000. The MRP2/cMOAT transporter and arsenic-glutathione complex formation are required for biliary excretion of arsenic. J. Biol. Chem. 275:33404-33408. [DOI] [PubMed] [Google Scholar]
- 41.Kerschen, J., V. R. Irani, D. J. Hassett, and J. J. Rowe. 2001. The snr-1 gene is required for nitrate reduction in Pseudomonas aeruginosa. J. Bacteriol. 183:2125-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kitagawa, M., Y. Matsumura, and T. Tsuchido. 2000. Small heat shock proteins, IbpA and IbpB, are involved in resistances to heat and superoxide stresses in Escherichia coli. FEMS Microbiol. Lett. 184:165-171. [DOI] [PubMed] [Google Scholar]
- 43.Kitagawa, M., M. Miyakawa, Y. Matsumura, and T. Tsuchido. 2002. Escherichia coli small heat shock proteins, IbpA and IbpB, protect enzymes from inactivation by heat and oxidants. Eur. J. Biochem. 269:2907-2917. [DOI] [PubMed] [Google Scholar]
- 44.Kramer, G. F., and B. N. Ames. 1988. Isolation and characterization of a selenium metabolism mutant of Salmonella typhimurium. J. Bacteriol. 170:736-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuo, C.-F., T. Mashino, and I. Fridovich. 1987. α,β-Dihydroxyisovalerate dehydratase. A superoxide-sensitive enzyme. J. Biol. Chem. 262:4724-4727. [PubMed] [Google Scholar]
- 46.Liochev, S. I., and I. Fridovich. 1992. Fumarase C, the stable fumarase of Escherichia coli, is controlled by the soxRS regulon. Proc. Natl. Acad. Sci. USA 89:5892-5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lynn, S., J. N. Shiung, J. R. Gurr, and K. Y. Jan. 1998. Arsenite stimulates poly(ADP-ribosylation) by generation of nitric oxide. Free Radic. Biol. Med. 24:442-449. [DOI] [PubMed] [Google Scholar]
- 48.Ma, J.-F., P. W. Hager, M. L. Howell, P. V. Phibbs, and D. J. Hassett. 1998. Cloning and characterization of the Pseudomonas aeruginosa zwf gene encoding glucose-6-phosphate dehydrogenase, an enzyme important in resistance to methyl viologen (paraquat). J. Bacteriol. 180:1741-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma, J.-F., U. A. Ochsner, M. G. Klotz, V. K. Nanayakkara, M. L. Howell, Z. Johnson, J. Posey, M. L. Vasil, J. J. Monaco, and D. J. Hassett. 1999. Bacterioferritin A modulates catalase A (KatA) activity and resistance to hydrogen peroxide in Pseudomonas aeruginosa. J. Bacteriol. 181:3730-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.MacGregor, C. H., J. A. Wolff, S. K. Arora, P. B. Hylemon, and P. V. Phibb, Jr. 1992. Catabolite repression control in Pseudomonas aeruginosa, p. 198-206. In E. Galli, S. Silver, and B. Witholt (ed.), Pseudomonas molecular biology and biotechnology. American Society for Microbiology, Washington, D.C.
- 51.MacGregor, C. H., J. A. Wolff, S. K. Arora, and P. V. Phibbs, Jr. 1991. Cloning of a catabolite repression control (crc) gene from Pseudomonas aeruginosa, expression of the gene in Escherichia coli, and identification of the gene product in Pseudomonas aeruginosa. J. Bacteriol. 173:7204-7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Macur, R. E., J. T. Wheeler, T. R. McDermott, and W. P. Inskeep. 2001. Microbial populations associated with the reduction and enhanced mobilization of arsenic in mine tailings. Environ. Sci. Technol. 35:3676-3682. [DOI] [PubMed] [Google Scholar]
- 53.Marklund, S., and G. Marklund. 1974. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 47:469-474. [DOI] [PubMed] [Google Scholar]
- 54.Matsui, M., C. Nishigori, S. Toyokuni, J. Takada, M. Akaboshi, M. Ishikawa, S. Imamura, and Y. Miyachi. 1999. The role of oxidative DNA damage in human arsenic carcinogenesis: detection of 8-hydroxy-2′-deoxyguanosine in arsenic-related Bowen's disease. J. Investig. Dermatol. 113:26-31. [DOI] [PubMed] [Google Scholar]
- 55.Meng, Y. L., Z. Liu, and B. P. Rosen. 2004. As(III) and Sb(III) uptake by GlpF and efflux by ArsB in Escherichia coli. J. Biol. Chem. 279:18334-18341. [DOI] [PubMed] [Google Scholar]
- 56.Mobley, H. L., and B. P. Rosen. 1982. Energetics of plasmid-mediated arsenate resistance in Escherichia coli. Proc. Natl. Acad. Sci. USA 79:6119-6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morales, G., J. F. Linares, A. Beloso, J. P. Albar, J. L. Martinez, and F. Rojo. 2004. The Pseudomonas putida Crc global regulator controls the expression of genes from several chromosomal catabolic pathways for aromatic compounds. J. Bacteriol. 186:1337-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mukhopadhyay, R., B. P. Rosen, T. Phung le, and S. Silver. 2002. Microbial arsenic: from geocycles to genes and enzymes. FEMS Microbiol. Rev. 26:311-325. [DOI] [PubMed] [Google Scholar]
- 59.Mynbaeva, B. N., S. A. Abdrashitova, B. B. Aidarkhanov, and A. N. Ilyaletdinov. 1990. Effect of linetol and antioxidants on oxidation of arsenite by Pseudomonas putida. Mikrobiologiya 59:570-574. [Google Scholar]
- 60.Ochi, T. 1997. Arsenic compound-induced increases in glutathione levels in cultured Chinese hamster V79 cells and mechanisms associated with changes in gamma-glutamylcysteine synthetase activity, cystine uptake and utilization of cysteine. Arch. Toxicol. 71:730-740. [DOI] [PubMed] [Google Scholar]
- 61.Ochsner, U. A., M. L. Vasil, E. Alsabbagh, K. Parvatiyar, and D. J. Hassett. 2000. Role of the Pseudomonas aeruginosa oxyR-recG operon in oxidative stress defense and DNA repair: OxyR-dependent regulation of katB, ahpB, and ahpCF. J. Bacteriol. 182:4533-4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oden, K. L., T. B. Gladysheva, and B. P. Rosen. 1994. Arsenate reduction mediated by the plasmid-encoded ArsC protein is coupled to glutathione. Mol. Microbiol. 12:301-306. [DOI] [PubMed] [Google Scholar]
- 63.O'Toole, G. A., K. A. Gibbs, P. W. Hager, P. V. Phibbs, Jr., and R. Kolter. 2000. The global carbon metabolism regulator Crc is a component of a signal transduction pathway required for biofilm development by Pseudomonas aeruginosa. J. Bacteriol. 182:425-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Owolabi, J. B., and B. P. Rosen. 1990. Differential mRNA stability controls relative gene expression within the plasmid-encoded arsenical resistance operon. J. Bacteriol. 172:2367-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pearson, J. P., E. C. Pesci, and B. H. Igkewski. 1997. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J. Bacteriol. 179:5756-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pesci, E. C., J. P. Pearson, P. C. Seed, and B. H. Iglewski. 1997. Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 179:3127-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pinson, B., I. Sagot, and B. Daignan-Fornier. 2000. Identification of genes affecting selenite toxicity and resistance in Saccharomyces cerevisiae. Mol. Microbiol. 36:679-687. [DOI] [PubMed] [Google Scholar]
- 68.Pontius, F. W., K. G. Brown, and C.-J. Chen. 1994. Health implications of arsenic in drinking water. J. Am. Water Works Assoc. 86:52-63. [Google Scholar]
- 69.Prince, R. W., C. D. Cox, and M. L. Vasil. 1992. Molecular cloning and sequencing of the Pseudomonas aeruginosa fur gene: coordinate regulation of siderophore and exotoxin A production. J. Bacteriol. 175:2589-2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosen, B. P., S. Dey, D. Dou, G. Ji, P. Kaur, M. Y. Ksenzenko, S. Silver, and J. Wu. 1992. Evolution of an ion-translocating ATPase. Ann. N. Y. Acad. Sci. 671:257-272. [DOI] [PubMed] [Google Scholar]
- 71.Rosenstein, R., A. Peschel, B. Wieland, and F. Gotz. 1992. Expression and regulation of the antimonite, arsenite, and arsenate resistance operon of Staphylococcus xylosus plasmid pSX267. J. Bacteriol. 174:3676-3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sadosky, A. B., J. W. Wilson, H. M. Steinman, and H. A. Shuman. 1994. The iron superoxide dismutase of Legionella pneumophila is essential for viability. J. Bacteriol. 176:3790-3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sage, A. E., and M. L. Vasil. 1997. Osmoprotectant-dependent expression of plcH, encoding the hemolytic phospholipase C, is subject to novel catabolite repression control in Pseudomonas aeruginosa PAO1. J. Bacteriol. 179:4874-4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 75.Santini, J. M., L. I. Sly, R. D. Schnagl, and J. M. Macy. 2000. A new chemolithoautotrophic arsenite-oxidizing bacterium isolated from a gold mine: phylogenetic, physiological, and preliminary biochemical studies. Appl. Environ. Microbiol. 66:92-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schweizer, H. P. 1993. Small broad-host-range gentamicin resistance gene cassettes for site-specific insertion and deletion mutagenesis. BioTechniques 15:831-833. [PubMed] [Google Scholar]
- 77.Schweizer, H. P., and T. T. Hoang. 1995. An improved system for gene replacement and xylE fusion analysis in Pseudomonas aeruginosa. Gene 158:15-22. [DOI] [PubMed] [Google Scholar]
- 78.Schwyn, B., and J. B. Neilands. 1987. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 160:47-56. [DOI] [PubMed] [Google Scholar]
- 79.Shevchenko, A., O. N. Jensen, A. V. Podtelejnikov, F. Sagliocco, M. Wilm, O. Vorm, P. Mortensen, H. Boucherie, and M. Mann. 1996. Linking genome and proteome by mass spectrometry: large-scale identification of yeast proteins from two dimensional gels. Proc. Natl. Acad. Sci. USA 93:14440-14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Simon, R., U. Priefer, and A. Puehler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 81.Stolz, J. F., and R. S. Oremland. 1999. Bacterial respiration of arsenic and selenium. FEMS Microbiol. Rev. 23:615-627. [DOI] [PubMed] [Google Scholar]
- 82.Suh, S. J., L. Silo-Suh, D. E. Woods, D. J. Hassett, S. E. West, and D. E. Ohman. 1999. Effect of rpoS mutation on the stress response and expression of virulence factors in Pseudomonas aeruginosa. J. Bacteriol. 181:3890-3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tisa, L. S., and B. P. Rosen. 1989. Molecular characterization of an anion pump: the ArsB protein is the membrane anchor for the ArsA protein. J. Biol. Chem. 265:190-194. [PubMed] [Google Scholar]
- 84.Wayne, L. G., and G. A. Diaz. 1986. A double staining method for differentiating between two classes of mycobacterial catalase in polyacrylamide gels. Anal. Biochem. 157:89-92. [DOI] [PubMed] [Google Scholar]
- 85.Wefers, H., and H. Sies. 1983. Oxidation of glutathione by the superoxide radical to the disulfide and the sulfonate yielding singlet oxygen. Eur. J. Biochem. 137:29-36. [DOI] [PubMed] [Google Scholar]
- 86.West, S. E., A. K. Sample, and L. J. Runyen-Janecky. 1994. The vfr gene product, required for Pseudomonas aeruginosa exotoxin A and protease production, belongs to the cyclic AMP receptor protein family. J. Bacteriol. 176:7532-7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.West, S. E. H., H. P. Schweizer, C. Dall, A. K. Sample, and L. J. Runyen-Janecky. 1994. Construction of improved Escherichia-Pseudomonas shuttle vectors derived from pUC18/19 and sequence of the region required for their replication in Pseudomonas aeruginosa. Gene 148:81-86. [DOI] [PubMed] [Google Scholar]
- 88.Winterbourn, C. C., and D. Metodiewa. 1994. The reaction of superoxide with reduced glutathione. Arch. Biochem. Biophys. 314:284-290. [DOI] [PubMed] [Google Scholar]
- 89.Wu, J., and B. P. Rosen. 1993. The arsD gene encodes a second trans-acting regulatory protein of the plasmid-encoded arsenical resistance operon. Mol. Microbiol. 8:615-623. [DOI] [PubMed] [Google Scholar]
- 90.Ye, R. W., D. Haas, J.-O. Ka, V. Krishnalillai, A. Zimerman, C. Baird, and J. M. Tiedje. 1995. Anaerobic activation of the entire denitrification pathway in Pseudomonas aeruginosa requires Anr, an analog of Fnr. J. Bacteriol. 177:3606-3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yoon, S. S., R. F. Hennigan, G. M. Hilliard, U. A. Ochsner, K. Parvatiyar, M. C. Kamani, H. L. Allen, T. R. DeKievit, P. R. Gardner, U. Schwab, J. J. Rowe, B. H. Iglewski, T. R. McDermott, R. P. Mason, D. J. Wozniak, R. E. Hancock, M. R. Parsek, T. L. Noah, R. C. Boucher, and D. J. Hassett. 2002. Pseudomonas aeruginosa anaerobic respiration in biofilms: relationships to cystic fibrosis pathogenesis. Dev. Cell 3:593-603. [DOI] [PubMed] [Google Scholar]