Abstract
Background
Functional dependency may serve as a marker for positive SARC-F screen (Strength, Assistance with walking, Rise from a chair, Climb stairs and Falls) among older adults at the Emergency Department (ED). We compared functional dependency between SARC-F– (<4) and SARC-F+ (≥4) groups at the ED.
Methods
A secondary analysis of cohorts from two quasi-experimental studies among patients aged ≥65 years old presenting to the ED of a 1,700-bed tertiary hospital. We compared both groups for baseline characteristics using univariate analyses, and performed multiple linear regression to examine the association between Modified Barthel Index (MBI) and Lawton’s instrumental activities of daily living (IADL) against SARC-F, and binary logistic regression to examine the associations between individual ADL domains and SARC-F+. We compared the area under receiver operating characteristic curves (AUC) to detect SARC-F+ for MBI, IADL, frailty, age, cognition and comorbidity.
Results
SARC-F+ patients were older (86.4±7.6 years), predominantly female (71.5%) and frail (73.9%), more dependent on walking aids (77.2%), and had lower premorbid MBI (median 90.0 [interquartile range 71.0–98.0]) and IADL (4.0 [2.0–5.0]) (both p<0.001). MBI (β=–0.07, 95% confidence interval [CI] –0.086 to –0.055) and IADL (β=–0.533, 95% CI –0.684 to –0.381) were significantly associated with SARC-F. Dependency in finances (odds ratio [OR]=14.7, 95% CI 3.57–60.2, p<0.001), feeding (OR=12.4, 95% CI 1.45–106, p=0.022), and stair-climbing (OR=10.49, 95% CI 4.96–22.2, p<0.001) were the top three functional items associated with SARC-F. MBI (AUC=0.82, 95% CI 0.77–0.84) and IADL (AUC=0.78, 95% CI 0.72–0.84) showed superior discrimination for SARC-F+ compared to other measures (AUC=0.58–0.70).
Conclusion
Functional dependency is strongly associated with positive SARC-F screen among older adults at the ED. This highlights the need for increased vigilance, especially in the presence of dependency in relevant domains such as managing finances, feeding, and stair-climbing.
Keywords: Sarcopenia, Functional status, Geriatrics, Emergency medicine
INTRODUCTION
Sarcopenia is defined by the Asian Working Group for Sarcopenia (AWGS) as an age-related loss of muscle mass with diminished muscle strength and physical performance.1) Its prevalence by AWGS-recommended algorithm among community-dwelling persons ranged between 13.6% to 41%.2,3) Frailty, however, refers specifically to a broader syndrome characterized by multisystem impairment and increased vulnerability to stressors.4) Being well-recognized as ‘modern’ geriatric giants,4,5) sarcopenia and frailty appear to share similar clinical manifestations in physical and functional domains and are associated with a myriad of adverse outcomes including higher falls risk, functional decline and mortality.6-9) Additionally, sarcopenia is the antecedent and biological substrate of frailty.4) Hence, this underscores the importance of early identification of older adults at risk of sarcopenia during every healthcare encounter.9)
The SARC-F (Strength, Assistance with walking, Rise from a chair, Climb stairs and Falls) is recommended by the AWGS 2019, European Working Group on Sarcopenia in Older People 2 (EWGSOP2) and Singapore Clinical Practice Guidelines (CPG) for Sarcopenia as a case-finding measure to identify older persons at risk of sarcopenia.1,10-13) It comprises five components (0–2 points each): strength, assistance with walking, rise from a chair, climb stairs, and falls.14) At a cutoff of ≥4, the SARC-F is well-validated for use in various clinical settings including the community, outpatients, hemodialysis, cancer care, and the emergency department (ED).15-19) Whilst the SARC-F exhibits low sensitivity albeit high specificity for case detection of sarcopenia in the community setting, it is strongly predictive of adverse health outcomes including reduced physical performance, loss of functional independence, and low quality of life.20)
In 2020, the World Health Organization21) reported that an estimate of 14% of older persons globally are fully dependent in their basic needs. Additionally, they emphasized the importance of healthy aging, which is the process of developing and maintaining the functional ability that enables wellbeing in older age.21) Hence, functional measures should be routinely included as fundamental components of geriatric assessments to aid with the development of targeted and timely interventions that may mitigate further functional loss.22)
To the best of our knowledge, there remains a paucity of evidence examining the association between functional dependency and sarcopenia among older adults attending the ED. Our previous study highlighted the potential for sarcopenia case-finding using the SARC-F at the ED, which demonstrated excellent diagnostic ability good sensitivity-cum-specificity for frailty detection, and predictive validity for the outcomes of ED re-attendance and rehospitalization.19) Despite this, sarcopenia screening of older adults is often not done in the busy ED setting.
Against this backdrop, functional dependency may serve as a marker for positive SARC-F screen amongst older adults presenting to the ED. Thus, using SARC-F to identify patients at risk of sarcopenia, we aimed to examine the association as well as the discriminatory ability of functional dependency and positive SARC-F screen (defined as a score of ≥4) among older adults attending the ED.
MATERIALS AND METHODS
Study Design and Recruitment Process
This is a secondary analysis of participants from two separate studies from the Emergency Department Interventions for Frailty (EDIFY) program.23,24) Both studies were conducted between July 2018 to August 2019 at the ED of a 1,700-bed tertiary hospital. Participants aged 65 years and above were recruited into either intervention or non-intervention groups, via alternating weekly blocks, within their respective studies. The first study evaluated the effectiveness of early geriatric specialist interventions in reducing potentially avoidable acute admissions (n=100; mean age 90.0±4.1 years),23) while the second study evaluated the effectiveness of a multicomponent frailty intervention in preserving or improving function among older ED attendees (n=140; mean age 79.7±7.6 years).24) Further details of the studies’ recruitment criteria can be found in Supplementary Fig. S1. Written informed consent was obtained from patients or their legally acceptable representative (if they lacked mental capacity). Ethics approval was granted by the Domain Specific Review Board of the National Healthcare Group, Singapore (Reference: 2017/01076). This study complied the ethical guidelines for authorship and publishing in the Annals of Geriatric Medicine and Research.25)
Functional & Sarcopenia Assessment
The Modified Barthel Index (MBI, range 0–100) was used to assess premorbid basic activities of daily living (ADL).26) The measure comprises various functional abilities including chair/bed transfers, ambulation/wheelchair, stair-climbing, toilet transfers, bowel control, bladder control, bathing, dressing, personal hygiene, and feeding. Each functional domain has different total scores with dependency for each item defined as any score less than the maximum achievable score. Premorbid instrumental ADL was evaluated using Lawton’s Instrumental Activities of Daily Living (IADL, range 0–8) comprising eight domains including telephone use, shopping, food preparation, housekeeping, laundry, transportation, medications, and handling finances.27) Each domain generates a score of 0 (dependent) or 1 (independent).
The SARC-F (range 0–10)14) was used for identifying patient at risk of sarcopenia. In a previous study of older patients at the ED, the SARC-F had good diagnostic performance for frailty identification and was able to predict acute hospitalization and ED reattendance at 3-month.19) The questionnaire was administered by a trained research assistant with participants being categorized into SARC-F– (score <4) and SARC-F+ (score ≥4) groups. The cut-off score of ≥4 has been reported to have moderate to high specificity (68.9%–88.9%) for case detection for sarcopenia.28)
Baseline Characteristics
We gathered information on demographics (age, sex, ethnicity, education, and smoking status), comorbidities (Charlson Comorbidity Index [CCI]),29) medications, cognitive status (Abbreviated Mental Test [AMT]),30) functional status (MBI and IADL),26,27) and frailty status (Clinical Frailty Scale [CFS]).31)
Statistical Analyses
Univariate analyses were performed using chi-square or Fisher exact test (when a cell has an expected value of ≤5) for categorical variables, and independent sample t-test (parametric) or Mann-Whitney U test (non-parametric) for continuous variables. Multiple linear regression was performed, adjusting for covariates—Model 1 (age and sex) and Model 2 (age, sex, education, smoking status, polypharmacy, AMT, CCI, and CFS)—to examine the association for MBI and IADL scores, against SARC-F. Additionally, multicollinearity test was performed examining tolerance and variance inflation factor (VIF). We then performed binary logistic regression, adjusting for the abovementioned covariates, to examine independent associations between dependency in individual functional domains for MBI and IADL, against SARC-F+. Lastly, we compared area under the operating characteristic curves (AUC) for age, CFS, CCI, MBI, IADL, and AMT against SARC-F and identified optimal cut-off scores using Youden’s index. Statistical analyses were performed using SPSS version 21.0 (IBM Corp., Armonk, NY, USA) and statistical significance was assessed using a threshold of 5%.
RESULTS
Recruitment and Baseline Characteristics
A total of 2,379 patients were screened: 1,520 patients were planned for acute admission while 859 patients were managed at the ED observation unit with an anticipated stay of <24 hours. Two-thousand-and-two patients did not meet study criteria and an additional 137 patients declined study participation. Hence, a sum of 240 participants were recruited into both the studies (Supplementary Fig. S1).
Overall, 123 participants (51.3%) were SARC-F+ with significantly higher SARC-F scores (mean, 5.7 vs. 1.7; p<0.001) (Table 1) compared to their SARC-F– counterparts. They were also significantly older (mean age, 86.4 vs. 81.4 years) and predominantly female (71.5% vs. 58.1%). Additionally, the SARC-F+ group had fever years of education (≥6 years, 26.0% vs. 44.4%), higher comorbidity burden (mean CCI, 2.6 vs. 2.0), higher prevalence of polypharmacy (80.5% vs. 64.1%), lower cognitive scores (mean AMT, 6.8 vs. 8.1), and greater frailty prevalence (73.9% vs. 41.9%) (all p<0.05).
Table 1.
Baseline characteristics between SARC-F– (<4) and SARC-F+ (≥4) groups
| Characteristic | All subjects (n=240) | SARC-F– (n=117) | SARC-F+ (n=123) | p-valuea) |
|---|---|---|---|---|
| SARC-F (total score) | 3.7±2.4 | 1.7±1.0 | 5.7±1.7 | <0.001 |
| Demographics | ||||
| Age (y) | 84±8.1 | 81.4±7.9 | 86.4±7.6 | <0.001 |
| Sex, female | 156 (65.0) | 68 (58.1) | 88 (71.5) | 0.040 |
| Ethnicity | 0.680 | |||
| Chinese | 213 (88.8) | 101 (86.3) | 112 (91.1) | |
| Indian | 16 (6.7) | 9 (7.7) | 7 (5.7) | |
| Malay | 8 (3.3) | 5 (4.3) | 3 (2.4) | |
| Eurasian | 3 (1.3) | 2 (1.7) | 1 (0.8) | |
| Years of education ≥6 | 84 (35.0) | 52 (44.4) | 32 (26.0) | 0.004 |
| Smoking status | 0.015 | |||
| Current smoker | 15 (6.3) | 11 (9.4) | 4 (3.3) | |
| Ex-smoker | 29 (12.1) | 19 (16.2) | 10 (8.1) | |
| Non-smoker | 196 (81.7) | 87 (74.4) | 109 (88.6) | |
| Co-morbidities | ||||
| Charlson Comorbidity Index | ||||
| Total score | 2.3±2.1 | 2.0±1.9 | 2.6±2.2 | 0.039 |
| Polypharmacy (≥5 medicines) | 174 (72.5) | 75 (64.1) | 99 (80.5) | 0.008 |
| Hyperpolypharmacy (≥10 medicines) | 54 (22.5) | 18 (7.6) | 36 (15.2) | 0.013 |
| Cognitive status | ||||
| Known dementia | 44 (18.3) | 12 (10.3) | 32 (26.0) | 0.003 |
| AMT total score | 7.5±2.7 | 8.1±2.1 | 6.8±3.1 | <0.001 |
| Functional status | ||||
| Locomotion | ||||
| Uses walking device | 134 (55.8) | 39 (33.3) | 95 (77.2) | <0.001 |
| Premorbid MBI | ||||
| Total score | 98.0 (89.0–100) | 100.0 (98.0–100) | 90.0 (71.0–98.0) | <0.001 |
| Premorbid Lawton’s IADLs | ||||
| Total score | 5.0 (3.0–7.0) | 6.0 (5.0–7.5) | 4.0 (2.0–5.0) | <0.001 |
| Frailty status | ||||
| Premorbid CFS | ||||
| Total score | 4.7±0.8 | 4.4±0.6 | 5.0±0.9 | <0.001 |
| Frail | 140 (58.3) | 49 (41.9) | 91 (73.9) | <0.001 |
| Categoryb) | ||||
| Robustc) (CFS 1–3) | 7 (2.9) | 3 (2.6) | 4 (3.3) | <0.001 |
| Pre-frail (CFS 4) | 93 (38.8) | 65 (55.6) | 28 (22.8) | |
| Mildly frail (CFS 5) | 98 (40.8) | 44 (37.6) | 54 (43.9) | |
| Moderately frail (CFS 6) | 39 (16.3) | 5 (4.3) | 34 (27.6) | |
| Severely frail (CFS 7) | 3 (1.3) | 0 (0.0) | 3 (2.4) |
Values are presented as mean±standard deviation or number (%) or median (interquartile range).
SARC-F, Strength, Assistance with walking, Rise from a chair, Climb stairs and Falls; AMT, Abbreviated Mental Test; CFS, Clinical Frailty Scale; IADLs, instrumental activities of daily living; MBI, Modified Barthel Index.
Chi-square test or Fisher exact test were performed (when expected value is less than 5).
There were no patients with CFS 8 or 9 in the cohort.
Includes patients with CFS category 1 (very fit), 2 (well), and 3 (managing well).
The SARC-F+ group had significantly lower premorbid MBI scores (median, 90 vs. 100; p<0.001) compared to their SARC-F– counterparts (Table 1). Furthermore, only 20 participants (16.3%) had full MBI scores compared to 71 participants (60.7%) in the SARC-F– group. SARC-F+ participants also had significantly lower premorbid IADL scores compared to their SARC-F– counterparts (median, 4.0 vs. 6.0; p<0.001).
Associations between MBI and IADL scores with SARC-F
Using multiple linear regression, MBI scores were independently associated with SARC-F—Model 1 (unstandardized coefficient [β]=–0.078, standard error [SE]=0.006, T=–12.987, 95% confidence interval [CI] –0.09 to –0.066, p<0.001, tolerance=0.85, VIF=1.18) and Model 2 (β=–0.07, SE=0.008, T=–8.914, 95% CI –0.086 to –0.055, p<0.001, tolerance=0.55, VIF=1.82), such that higher MBI scores were associated with lower SARC-F scores.
IADL total scores were also independently associated with SARC-F—Model 1 (β=–0.606, SE=0.06, T=–10.06, 95% CI –0.725 to –0.488, p<0.001, tolerance=0.74, VIF=1.36) and Model 2 (β=–0.533, SE=0.077, T=–6.907, 95% CI –0.684 to –0.381, p<0.001, tolerance=0.45, VIF=2.23)—with higher IADL scores associated with lower SARC-F scores.
Associations between Individual ADL Domains and SARC-F+
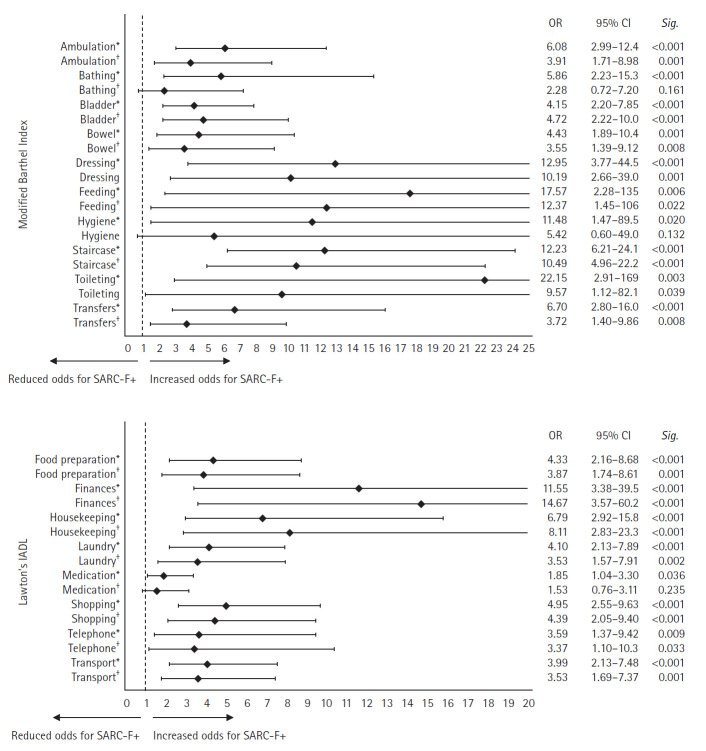
Most ADL domains were independently associated with SARC-F+ (Fig. 1). Using Model 2, we found that the top three domains that posed the greatest odds for SARC-F+ include dependency in feeding (odds ratio [OR]=12.37, 95% CI 1.45–106, p=0.022), stair-climbing (OR=10.49, 95% CI 4.96–22.2, p<0.001), and dressing (OR=10.19, 95% CI 2.66–39.0, p=0.001) for MBI, and dependency in finances (OR=14.67, 95% CI 3.57–39.5, p<0.001), housekeeping (OR=8.11, 95% CI 2.83–23.3, p<0.001), and shopping (OR=4.39, 95% CI 2.05–9.40, p<0.001) for IADL.
Fig. 1.
Logistic regression analysis examining the association between dependency in individual domains of basic (Modified Barthel Index) and instrumental ADL (Lawton’s IADL) and having sarcopenia risk (SARC-F+). ADL, activities of daily living; IADL, instrumental activities of daily living; SARC-F, Strength, Assistance with walking, Rise from a chair, Climb stairs and Falls; OR, odds ratio; CI, confidence interval. *Model 1: adjusted for age and sex. †Model 2: adjusted for age, sex, years of education, smoking status, polypharmacy, Abbreviated Mental Test, Charlson Comorbidity Index, and Clinical Frailty Scale.
AUC for MBI and IADL against SARC-F
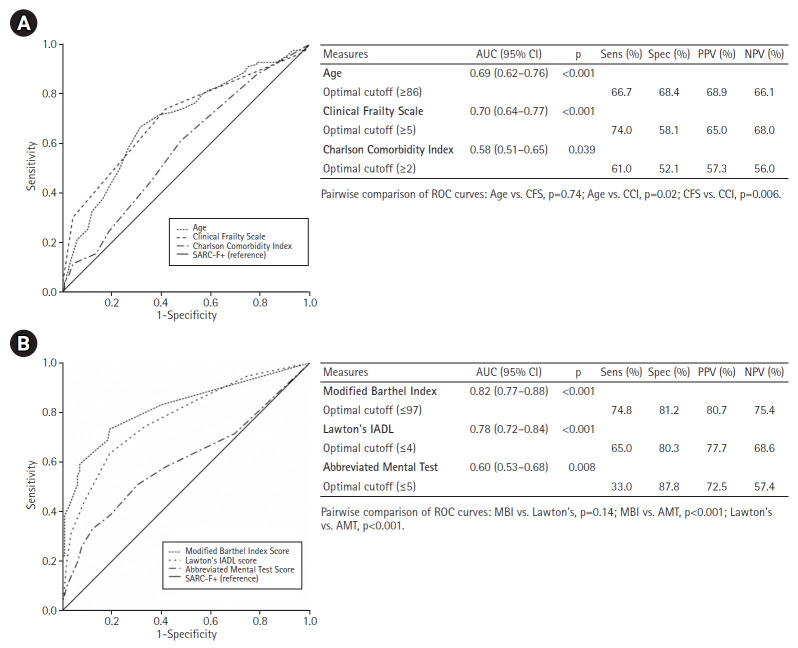
MBI (AUC=0.82, 95% CI 0.77–0.88, p<0.001) and IADL (AUC=0.78, 95% CI 0.72–0.84, p<0.001) performed best for SARC-F+ screen (SARC-F ≥4), with their optimal cut-off scores being ≤97 for MBI (sensitivity=74.8%, specificity=81.2%, positive predictive value [PPV]=80.7%), and ≤4 for IADL (sensitivity=65.0%, specificity=80.3%, PPV=77.7%). Other measures including CFS (AUC=0.70), age (AUC=0.69), AMT (AUC=0.60), and CCI (AUC=0.58) had lower discriminatory performance (Fig. 2).
Fig. 2.
Areas under receiver-operating characteristic curve (AUC) for various measures against SARC-F+. (A) Measures with higher scores indicating a more positive test. (B) Measures with lower scores indicating a more positive test. SARC-F, Strength, Assistance with walking, Rise from a chair, Climb stairs and Falls; AMT, Abbreviated Mental Test; CCI, Charlson Comorbidity Index; CFS, Clinical Frailty Scale; IADL, instrumental activities of daily living; MBI, Modified Barthel Index; ROC, receiver operating characteristic; Sens, sensitivity; Spec, specificity; NPV, negative predictive value; PPV, positive predictive value; CI, confidence interval.
DISCUSSION
Our findings suggest that functional dependency, especially in feeding, stair-climbing, dressing, finances, housekeeping, and shopping is strongly associated with positive SARC-F screen among older adults attending the ED. While prevalence of sarcopenia and its association with ADLs have been previously established in community-dwelling older adults, nursing home residents, and rehabilitation ward patients,32-34) our study is the first to examine the association between individual ADL domains and SARC-F+ in older ED attendees.
We observed that dependency in managing finances conferred the greatest odds for being SARC-F+. This observation may partly be contributed by the significantly greater proportion of cognitively-impaired patients among the SARC-F+ group, although this was adjusted for in Model 2. Similar results were noted even when AMT was substituted for dementia diagnosis (OR=12.9, 95% CI 3.30–50.61, p<0.001). A recent cross-sectional study of 201 participants found that older adults with cognitive impairment had a significantly higher prevalence of sarcopenia when compared to those with normal cognitive functions (15.4% vs. 3.7%; p=0.006).35) Additionally, a systematic review and meta-analysis study reported that the pooled adjusted OR for cognitive impairment for patients with sarcopenia was 2.25, when compared to those without sarcopenia.36) Therefore, we postulate that the synergistic effect of physical disability and cognitive impairment in persons with sarcopenia may result in significant challenges in managing finances independently.
Sarcopenia & oral health is fast becoming a growing interest in the scientific field, with conditions such as “sarcopenic dysphagia,” “malnutrition,” and “oral frailty” sharing many phenotypically overlapping features.37) A study reported significantly poorer swallowing functions among sarcopenia patients when five swallowing assessment tools including the dysphagia severity scale, repetitive saliva swallowing test, genio-thyroid distance, thyroid-sternum distance, and genio-sternum grade were used.38) Another study investigating 18,782 participants of the Korean National Health and Nutrition Examination Survey from 2008 to 2011 found that there was a significant association between loss of natural teeth and sarcopenia, reporting an adjusted OR for sarcopenia among older participants with <20 natural teeth of 1.92 in males and 2.63 in females.39) On a related note, a study of community-dwelling older adults reported that SARC-F+ patients had the most prevalent difficulty in stair-climbing (96.8%) and strength (81.1%), and an inclination for at least one IADL disability.40) Hence, our findings build on growing evidence that sarcopenia is closely related to the loss of physical abilities such as feeding and stair-climbing.
Understanding associations between individual domains of ADL and SARC-F+ may potentially aid in contextualizing clinical care through a deeper appreciation of its impact on one’s ability to meet basic needs due to having risk of sarcopenia. This allows for the design and delivery of person-centered care and interventions to optimize functional ability of older persons with risk of sarcopenia. The AWGS 2019 recommends an algorithmic approach for sarcopenia diagnosis comprising skeletal muscle mass measurement and assessments of handgrip strength or physical performance in the hospital setting.1) While the SARC-F has promising potential for use as a case-finding tool for sarcopenia,19) the fast-paced and often chaotic environment at the ED may not support the incorporation of additional tools to their routine assessment battery.
A potential approach would be to place emphasis on functional assessments as means for triggering further assessment in sarcopenia. For example, dependency in the "high-risk" ADL or IADL categories in our study should alert the ED physician to look out for, and address potential issues, that are associated with sarcopenia. This can be achieved using the 4Ds approach of drugs (medications such as statin or steroids that can result in myalgia and proximal muscle weakness), diabetes mellitus, other diseases (chronic diseases of the lungs, kidneys, liver or heart, osteoporosis, progressive neurological diseases, and others) and deficiency (poor dentition or oral health, swallowing impairment, vitamin D deficiency, and others).11) Additionally, EDs who aspire to be frailty-ready may consider adopting the Quadruple Aim framework, which comprises four key objectives: improving patient health outcomes, reducing cost, improving patient experience, and improving healthcare team experience.41) This approach will aid health-care systems in addressing any mismatch between existing care delivery and evidence-based best practices for older persons.
Our study had several limitations and results should be interpreted with care. First, this is a secondary analysis combining data from two separate studies, which had different aims and population demographics. Thus, the sample size may not be adequately powered for the intended purposes of this study. Nevertheless, combining both cohorts enhance generalizability due to the inclusion of a wider range of participants’ age and presentation to the ED. Second, despite combining cohorts, many participants had a presentation of fall or recurrent falls (n=112; 46.7%). The majority of sarcopenic patients required assistance with walking (52.0%) and had a history of fall (68.2%). This may potentially promote higher SARC-F scores, especially for items 2 (assistance with walking) and 5 (falls), and limit applicability of our findings to patients with other illness presentations. Last, there are overlapping elements between the SARC-F and MBI—items 3 (rise from a chair) and 4 (climb stairs), and "chair/bed transfers" and "stair-climbing," respectively. Nevertheless, when we excluded stair-climbing from total MBI score, MBI remained independently associated with SARC-F—Model 1 (β=–0.082, SE=0.007, T=–11.222, 95% CI –0.096 to –0.068, p<0.001, tolerance=0.87, VIF=1.15) and Model 2 (β=–0.068, SE=0.009, T=–7.398, 95% CI –0.086 to –0.050, p<0.001, tolerance=0.59, VIF=1.70). Hence, this is not necessary a limitation as it explains why the SARC-F and MBI were reported to be most predictive for frailty (both AUC >0.90) in a recent study.19) Additionally, it was anticipated that strong associations between the abovementioned tools, which share common functional components, were observed in our study.
In conclusion, functional dependency in older adults at the ED is strongly associated with positive SARC-F screen, indicating patients are at risk of sarcopenia. This highlights the need for increased vigilance, especially in the presence of dependency in relevant domains such as managing finances, feeding, and stair-climbing. Further studies with more robust methodologies are required to build on evidence to support our novel finding of functional dependency and positive SARC-F screen among older adults at the front-door of acute hospitals.
Footnotes
The authors wish to express their gratitude to the staff of the Emergency Department of Tan Tock Seng Hospital for their unwavering support in the EDIFY programme. We also thank the staff of the Institute of Geriatrics and Active Ageing and the Health Services and Outcomes Research for ensuring the quality and integrity of the study were always upheld. In addition, we thank Dr J Baldevarona-Llego, Ms B.Y. Ooi, Ms S Cheong, Ms Zhu B, Ms A. Ho, and Ms Y.C. Yeoh for playing a vital role in the success of the EDIFY programme.
CONFLICT OF INTEREST
The researchers claim no conflicts of interest.
FUNDING
This work was supported by the Ng Teng Fong Healthcare Innovation Programme (Project Code: NTF_JUL2017_I_C2_CQR_02), which had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
AUTHOR CONTRIBUTIONS
Conceptualization, EC, WSL; Data curation, EC, EFG; Funding acquisition, EC; Investigation, EC, EFG; Methodology, EC, WSL; Project administration, EFG; Supervision, WSL; Writing–original draft, EC, EFG; Writing–review & editing, WSL.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.4235/agmr.24.0091.
Emergency Department Interventions for Frailty (EDIFY) study criteria and recruitment diagram.
REFERENCES
- 1.Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21:300–7. doi: 10.1016/j.jamda.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 2.Chang HK, Lee JY, Gil CR, Kim MK. Prevalence of sarcopenia in community-dwelling older adults according to simplified algorithms for sarcopenia consensus based on Asian Working Group for Sarcopenia. Clin Interv Aging. 2020;15:2291–9. doi: 10.2147/CIA.S281131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pang BW, Wee SL, Lau LK, Jabbar KA, Seah WT, Ng DH, et al. Prevalence and associated factors of sarcopenia in Singaporean adults: the Yishun study. J Am Med Dir Assoc. 2021;22:885. doi: 10.1016/j.jamda.2020.05.029. [DOI] [PubMed] [Google Scholar]
- 4.Morley JE. Frailty and sarcopenia: the new geriatric giants. Rev Invest Clin. 2016;68:59–67. [PubMed] [Google Scholar]
- 5.Lee SY. Sarcopenia: a geriatric giant facing a huge transition. Ann Geriatr Med Res. 2021;25:1–3. doi: 10.4235/agmr.21.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cesari M, Landi F, Vellas B, Bernabei R, Marzetti E. Sarcopenia and physical frailty: two sides of the same coin. Front Aging Neurosci. 2014;6:192. doi: 10.3389/fnagi.2014.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dent E, Lien C, Lim WS, Wong WC, Wong CH, Ng TP, et al. The Asia-Pacific clinical practice guidelines for the management of frailty. J Am Med Dir Assoc. 2017;18:564–75. doi: 10.1016/j.jamda.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 8.Malmstrom TK, Miller DK, Simonsick EM, Ferrucci L, Morley JE. SARC-F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle. 2016;7:28–36. doi: 10.1002/jcsm.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang M, Hu X, Wang H, Zhang L, Hao Q, Dong B. Sarcopenia predicts readmission and mortality in elderly patients in acute care wards: a prospective study. J Cachexia Sarcopenia Muscle. 2017;8:251–8. doi: 10.1002/jcsm.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cesari M, Kuchel GA. Role of sarcopenia definition and diagnosis in clinical care: moving from risk assessment to mechanism-guided interventions. J Am Geriatr Soc. 2020;68:1406–9. doi: 10.1111/jgs.16575. [DOI] [PubMed] [Google Scholar]
- 11.Lim WS, Cheong CY, Lim JP, Tan MM, Chia JQ, Malik NA, et al. Singapore clinical practice guidelines for sarcopenia: screening, diagnosis, management and prevention. J Frailty Aging. 2022;11:348–69. doi: 10.14283/jfa.2022.59. [DOI] [PubMed] [Google Scholar]
- 12.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baek JY, Jung HW, Kim KM, Kim M, Park CY, Lee KP, et al. Korean Working Group on Sarcopenia guideline: expert consensus on sarcopenia screening and diagnosis by the Korean Society of Sarcopenia, the Korean Society for Bone and Mineral Research, and the Korean Geriatrics Society. Ann Geriatr Med Res. 2023;27:9–21. doi: 10.4235/agmr.23.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malmstrom TK, Morley JE. SARC-F: a simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc. 2013;14:531–2. doi: 10.1016/j.jamda.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 15.Woo J, Leung J, Morley JE. Validating the SARC-F: a suitable community screening tool for sarcopenia? J Am Med Dir Assoc. 2014;15:630–4. doi: 10.1016/j.jamda.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 16.Tan LF, Lim ZY, Choe R, Seetharaman S, Merchant R. Screening for frailty and sarcopenia among older persons in medical outpatient clinics and its associations with healthcare burden. J Am Med Dir Assoc. 2017;18:583–7. doi: 10.1016/j.jamda.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Lin YL, Hou JS, Lai YH, Wang CH, Kuo CH, Liou HH, et al. Association of SARC-F questionnaire and mortality in prevalent hemodialysis patients. Diagnostics (Basel) 2020;10:890. doi: 10.3390/diagnostics10110890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams GR, Al-Obaidi M, Dai C, Bhatia S, Giri S. SARC-F for screening of sarcopenia among older adults with cancer. Cancer. 2021;127:1469–75. doi: 10.1002/cncr.33395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chong E, Bao M, Goh EF, Lim WS. SARC-F at the emergency department: diagnostic performance for frailty and predictive performance for reattendances and acute hospitalizations. J Nutr Health Aging. 2021;25:1084–9. doi: 10.1007/s12603-021-1676-5. [DOI] [PubMed] [Google Scholar]
- 20.Manrique-Espinoza B, Salinas-Rodriguez A, Rosas-Carrasco O, Gutierrez-Robledo LM, Avila-Funes JA. Sarcopenia is associated with physical and mental components of health-related quality of life in older adults. J Am Med Dir Assoc. 2017;18:636. doi: 10.1016/j.jamda.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization . Decade of healthy ageing: baseline report. Geneva, Switzerland: World Health Organization; 2020. [Google Scholar]
- 22.Morley JE, Arai H, Cao L, Dong B, Merchant RA, Vellas B, et al. Integrated care: enhancing the role of the primary health care professional in preventing functional decline: a systematic review. J Am Med Dir Assoc. 2017;18:489–94. doi: 10.1016/j.jamda.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 23.Chong E, Zhu B, Tan H, Molina JC, Goh EF, Baldevarona-Llego J, et al. Emergency Department Interventions for Frailty (EDIFY): front-door geriatric care can reduce acute admissions. J Am Med Dir Assoc. 2021;22:923–8. doi: 10.1016/j.jamda.2021.01.083. [DOI] [PubMed] [Google Scholar]
- 24.Chong E, Zhu B, Ng SH, Tan H, Goh EF, Molina JC, et al. Emergency department interventions for frailty (EDIFY): improving functional outcomes in older persons at the emergency department through a multicomponent frailty intervention. Age Ageing. 2022;51:afab251. doi: 10.1093/ageing/afab251. [DOI] [PubMed] [Google Scholar]
- 25.Noh JH, Jung HW, Ga H, Lim JY. Ethical guidelines for publishing in the Annals of Geriatric Medicine and Research. Ann Geriatr Med Res. 2022;26:1–3. doi: 10.4235/agmr.22.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah S, Vanclay F, Cooper B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidemiol. 1989;42:703–9. doi: 10.1016/0895-4356(89)90065-6. [DOI] [PubMed] [Google Scholar]
- 27.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–86. [PubMed] [Google Scholar]
- 28.Voelker SN, Michalopoulos N, Maier AB, Reijnierse EM. Reliability and concurrent validity of the SARC-F and its modified versions: a systematic review and meta-analysis. J Am Med Dir Assoc. 2021;22:1864–76. doi: 10.1016/j.jamda.2021.05.011. [DOI] [PubMed] [Google Scholar]
- 29.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 30.Sahadevan S, Lim PP, Tan NJ, Chan SP. Diagnostic performance of two mental status tests in the older chinese: influence of education and age on cut-off values. Int J Geriatr Psychiatry. 2000;15:234–41. doi: 10.1002/(sici)1099-1166(200003)15:3<234::aid-gps99>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 31.Chong E, Chia JQ, Law F, Chew J, Chan M, Lim WS. Validating a standardised approach in administration of the clinical frailty scale in hospitalised older adults. Ann Acad Med Singap. 2019;48:115–24. [PubMed] [Google Scholar]
- 32.Tanimoto Y, Watanabe M, Sun W, Sugiura Y, Tsuda Y, Kimura M, et al. Association between sarcopenia and higher-level functional capacity in daily living in community-dwelling elderly subjects in Japan. Arch Gerontol Geriatr. 2012;55:e9–13. doi: 10.1016/j.archger.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 33.Kamo T, Ishii H, Suzuki K, Nishida Y. Prevalence of sarcopenia and its association with activities of daily living among Japanese nursing home residents. Geriatr Nurs. 2018;39:528–33. doi: 10.1016/j.gerinurse.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 34.Yoshimura Y, Wakabayashi H, Bise T, Tanoue M. Prevalence of sarcopenia and its association with activities of daily living and dysphagia in convalescent rehabilitation ward inpatients. Clin Nutr. 2018;37:2022–8. doi: 10.1016/j.clnu.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 35.Yigit B, Oner C, Cetin H, Simsek EE. Association between sarcopenia and cognitive functions in older individuals: a cross-sectional study. Ann Geriatr Med Res. 2022;26:134–9. doi: 10.4235/agmr.22.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng TC, Chen WL, Wu LW, Chang YW, Kao TW. Sarcopenia and cognitive impairment: a systematic review and meta-analysis. Clin Nutr. 2020;39:2695–701. doi: 10.1016/j.clnu.2019.12.014. [DOI] [PubMed] [Google Scholar]
- 37.de Sire A, Ferrillo M, Lippi L, Agostini F, de Sire R, Ferrara PE, et al. Sarcopenic dysphagia, malnutrition, and oral frailty in elderly: a comprehensive review. Nutrients. 2022;14:982. doi: 10.3390/nu14050982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shiozu H, Higashijima M, Koga T. Association of sarcopenia with swallowing problems, related to nutrition and activities of daily living of elderly individuals. J Phys Ther Sci. 2015;27:393–6. doi: 10.1589/jpts.27.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han CH, Chung JH. Association between sarcopenia and tooth loss. Ann Geriatr Med Res. 2018;22:145–50. doi: 10.4235/agmr.2018.22.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marincolo JC, Aprahamian I, Corona LP, Neri AL, Yassuda MS, Borim FS. Three definitions of probable sarcopenia and associations with falls and functional disability among community-dwelling older adults. Osteoporos Sarcopenia. 2021;7:69–74. doi: 10.1016/j.afos.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chong E, Ong T, Lim WS. Editorial: front-door geriatrics: frailty-ready emergency department to achieve the quadruple aim. J Frailty Aging. 2023;12:254–7. doi: 10.14283/jfa.2023.42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Emergency Department Interventions for Frailty (EDIFY) study criteria and recruitment diagram.