Abstract
The rapid development of delivery systems for cosmetics has revealed two critical challenges in the field: enhancing the solubility of active ingredients and ensuring the stability of natural materials used in cosmetics. Nanoemulsion technology has emerged as an indispensable solution for addressing these challenges, not only enhancing the stability of cosmetics but also improving the solubility of pharmaceuticals and active ingredients with poor solubility. Nanoemulsion formulations have reinforced stability and amended the bioavailability of hydrophobic drugs. Moreover, nanoemulsion exhibit excellent skin penetration and long-lasting effects, making them particularly appealing to consumers, especially in the cosmetic industry. This article aims to provide an overview of herbal nanoemulsion formulations as cosmetic products, covering formulation, production, and characterization. Herbal nanoemulsions is an effective, stable, and promising option for cosmetic delivery. The nanoemulsions were characterized by their key properties, such as particle size, polydisperse index (PDI), zeta potential, viscosity, stability and others. Techniques like zeta potential measurement, transmission electron microscopy (TEM) and scanning electronmicroscopy (SEM) were used to analyze the surface morphology, whereas stability tests were employed to evaluate nanoemulsion performance. This review also delves into the high-energy and the low-energy methods of manufacturing nanoemulsions. Additionally, we also explore the selection of appropriate surfactants, co-surfactants, and ingredients for creating herbal nanoemulsions with desirable attributes and qualities. Overall, this review consolidates the current knowledge on herbal nanoemulsion formulations for cosmetic preparations, designs, shedding light on their effectiveness, characteristics, and stability. These formulations hold promise in overcoming challenges related to meeting the increasing demand for effective herbal nanoemulsion and high-quality cosmetic products.
Keywords: Cosmetic, Formulation, Nanoemulsion, Natural product
1. Introduction
The Food and Drug Administration (FDA) defines cosmetics as substances intended to be applied to the human body or any part thereof for purposes such as cleansing, beautifying, promoting attractiveness, or altering appearance [1,2]. In a broader sense, cosmetics refer to products designed to improve skin appearance, enhance beauty, and facilitate thorough cleansing [3,4]. These definitions encompass a wide range of products falling under the cosmetics category, each serving specific functions related to personal care and aesthetics. Cosmetics consist of a range of commercial products, such as skin moisturizers, anti-aging products, facial makeup, shampoos, toothpaste, deodorants, hair dyes, and various other items utilized to smarten up one's appearance. This classification reflects the diverse nature of cosmetics, including items that cater to different aspects of personal grooming and aesthetic preferences [5,6]. In the cosmetic industry, cosmeceuticals are recognized as cosmetic products infused with biologically active ingredients that offer therapeutic benefits for enhancing the personal appearance [7,8]. These formulations cover a diverse range, spanning from skincare to body care to hair care, allowing for their application in various treatments [9,10]. Cosmeceuticals play a crucial role in addressing specific concerns such as skin aging, skin dryness, dark spots, pigmentation, hair damage, and many more [11,12]. This distinct category represents the fusion of cosmetic and pharmaceutical attributes, catering to individuals seeking beauty and personal care products with enhanced therapeutic properties [13–15].
The utilization of cosmetic products containing chemically active ingredients brings forth a spectrum of challenges and considerations. One primary concern lies in the potential skin sensitivity and allergic reactions, as certain individuals may experience adverse responses, including redness and irritation. Moreover, the disruption of the skin barrier function by certain chemical components raises concerns about susceptibility to environmental factors [16,17]. Prolonged and frequent use of cosmetics with specific chemical ingredients may cause unknown long-term effects due to the accumulation of potentially harmful substances in the body [18,19]. The environmental impact of these products during disposal, also contributes to water and land pollution, posing threats to our ecosystems. Additionally, certain chemical ingredients, such as parabens and phthalates, are endocrine disruptors, raising concerns about hormonal imbalances [20–22]. Ethical considerations, regulatory compliance, personalized reactions, and the lack of long-term safety data further compound the complex landscape of cosmetic use with chemical active ingredients. As public awareness grows, there is an increasing call for transparency, safety, and ethical practices in the cosmetic industry to address these multifaceted challenges and promote informed consumer decision-making [23,24].
The utilization of herbal remedies and medicinal plants to deal with diverse ailments traces back to ancient civilizations [25]. In modern times, spurred by the perceived health benefits of these natural remedies, this traditional practice has experienced a resurgence of interest among researchers and innovators globally [26]. The growing popularity of herbal medicines as alternative therapies in the western world emphasizes the necessity for further investigation, especially when combined with pharmaceutical nanotechnology, to ensure the safety and efficacy of these remedies [27,28]. In contemporary times, nano-drug delivery systems (NDDS) have emerged as a highly effective approach for delivering bioactive components or drugs within the human body [29,30]. These systems possess the ability to direct the drug or active molecule to specific tissue sites or cell types, ensuring the desired concentration is maintained throughout the effective treatment period within the therapeutic index [31,32]. The use of nanoparticles or nanodroplets enhances the drug's solubility and bioavailability. In this context, nanoencapsulation-based nanoemulsions, a process that utilizes nanocarriers to encapsulate active components within the lipidic or polymeric core of excipients, play a pioneering role in encapsulating herbal bioactives, resulting in improved solubility and bioavailability. The effectiveness of phytopharmaceuticals therefore depends on the development of efficient drug delivery systems [33–35].
A nanoemulsion is a type of emulsion, which is a mixture of two immiscible liquids (such as oil and water) stabilized by an emulsifying agent. It exists as a homogeneous, thermodynamically stable, and isotropic system composed of aqueous components, surfactants/co-surfactants (Smix), and an oil phase. Nanoemulsions are commonly used in various fields of industry such as pharmaceuticals, food, cosmetics, and agriculture [36,37]. In pharmaceuticals, for instance, nanoemulsions can be employed to improve the solubility and delivery of poorly soluble drugs. In the food industry, they are utilized to create products with a smoother texture and better flavor. The cosmetic industry uses them for skincare products to enhance the absorption of active ingredients [35,38].
A herbal nanoemulsion refers to a specialized form of nanoemulsion that incorporates herbal extracts or bioactives as its active components. This type of nanoemulsion is designed to address the challenges associated with the solubility, permeability, and bioavailability of herbal compounds [39,40]. By reducing the droplet size to the nanometer range, herbal nanoemulsions aim to enhance the stability and delivery of these bioactive components, thereby optimizing their therapeutic effects. All these advantages render the nanoemulsion technique promising for developing to be an effective and efficient delivery system for herbal medicines [38,41].
The use of natural herbal components, such as extracts derived from mangosteen rind, rhizome plants, essential oils, aloe vera, and phylanthus niruri, has experienced a notable increase in the field of cosmetic formulations [42,43]. This surge is motivated by the desire to create effective and high-quality products that prioritize both safety and nutritional benefits. The integration of herbal ingredients into cosmetic formulations reflects a growing trend, propelled by a discerning customer base that acknowledges the diverse benefits of natural components, particularly in the realm of skincare and cosmetics [44–46].
The nanoemulsion delivery system in cosmetics is a heterogeneous one, characterized by the presence of one or two immiscible liquids, where liquid molecules disperse as tiny droplets, typically measuring less than 500 nm in diameter. Nanoemulsions, unlike conventional formulations, along with other nanocarrier options, offer solutions to challenges such as creaming, flocculation, coalescence, sedimentation, and the troublesome phenomenon known as Ostwald ripening [47–49]. Ostwald ripening occurs when small, highly soluble droplets undergo diffusion, resulting in the formation of larger droplets a problem commonly encountered in formulations with large droplet particle size distributions [50,51].
Nanoemulsions in cosmetic science offer heightened aesthetic appeal, formulation customization of formulations, and reduced irritation as well, which make them popular in the cosmetic formulations and therapeutic products [52,53]. These characteristics contribute to the development of innovative and effective cosmetic products that align with consumer preferences and expectations [54,55]. The rapid expansion of the cosmetics industry in recent years highlights the practicality and effectiveness of integrating natural ingredients into cosmetic formulations [56–58]. Thus, given the significant demand for skincare and cosmetic products, this review focuses on the existing insights into the design, preparation, formulation, and characterization of potent herbal nanoemulsions in cosmetic applications. Additionally, particular attention will be paid to developments in nanoemulsion use in cosmetics over the past decade, particularly emphasizing products that utilize nanoemulsion technology in their formulations and incorporate active ingredients derived from herbal or natural sources. Nanoemulsion is an advanced technology that enhances the delivery and absorption of active ingredients into the skin, thereby increasing the efficacy of cosmetic products. Cosmetics that feature herbal or natural active ingredients are often perceived as gentler and more skin-friendly, potentially offering additional benefits depending on the specific herbs used. The combination of nanoemulsion technology with natural ingredients is generally expected to yield optimal results with minimal adverse effects.
To compile this information, we accessed the latest and pertinent references from reputable databases, including Science Direct, Google Scholar, MEDLINE (PubMed), Scopus, and SciFinder. We anticipate that this review will prove beneficial for researchers and serve as a valuable contribution to the field.
2. Cosmetic science and skin barrier
2.1. Cosmetic science
Cosmetic science is a multifaceted discipline that applies scientific principles to the intricate process of developing, formulating, and producing cosmetic and personal care products. Drawing from fields such as chemistry, biochemistry, biology, dermatology, and materials science, cosmetic scientists engage in a comprehensive exploration of raw materials, including active ingredients, preservatives, emollients, and surfactants [52]. The primary goal is to understand how these components interact to create stable and effective formulations that enhance the appearance and well-being of the skin, hair, and overall personal aesthetics [59,60].
A crucial aspect of cosmetic science involves the selection of ingredients based on their chemical properties, safety profiles, and intended effects. Cosmetic scientists conduct rigorous testing, including laboratory experiments and clinical studies, to evaluate product efficacy, safety, and stability over time. Ensuring regulatory compliance and ethical consideration is integral to the process, as is the constant pursuit of innovation to incorporate new technologies, ingredients, and trends into cosmetic formulations [61,62]. Collaborating with experts in dermatology, toxicology, and marketing, cosmetic scientists contribute to the continuous evolution of skincare, haircare, makeup, and fragrance products that align with both safety standards and consumer expectations [63,64].
2.2. Structure and function of skin
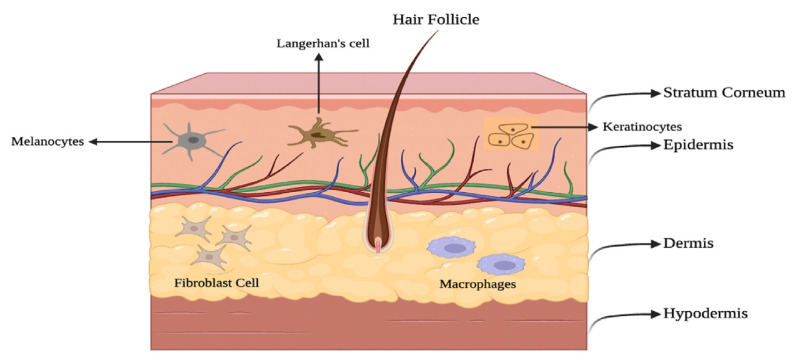
The skin, the body's largest organ, acts as a protective barrier between internal organs and the environment, shielding against potential damage. Consisting of three layers - epidermis, dermis, and hypodermis - each has unique cell compositions. The epidermis, the top layer, is mostly keratinocytes (95%), with the rest being melanocytes, Langerhans cells, and Merkel cells. These cells play crucial roles in skin structure and function, collectively contributing to its protective capabilities (Fig. 1) [65,66].
Fig. 1.
Schematic representation of human skin structure.
The epidermis can also be divided into four main layers: stratum basale, stratum spinosum, stratum granulosum, and stratum corneum (SC) from inner to upward layers respectively (Figs. 1 and 2). The stratum basale is a single layer of actively dividing keratinocytes, melanocytes, and Merkel cells. The stratum spinosum is a significant layer contributing to the skin structure and renewal [66]. Composed predominantly of keratinocytes, its distinct feature is the presence of desmosomes which provide strength and resilience to the skin, resisting mechanical stress. Actively dividing keratinocytes populate the stratum spinosum, as cells move upward from the stratum basale, ensuring a continual renewal process. The cells in this layer are metabolically engaged in RNA (ribonucleic acid) and protein synthesis, preparing for their transition into the next layer, the stratum granulosum. In essence, the stratum spinosum stands as a vital intermediary in the epidermal layers, balancing cell division, structural integrity, and the dynamic process of skin regeneration [67,68].
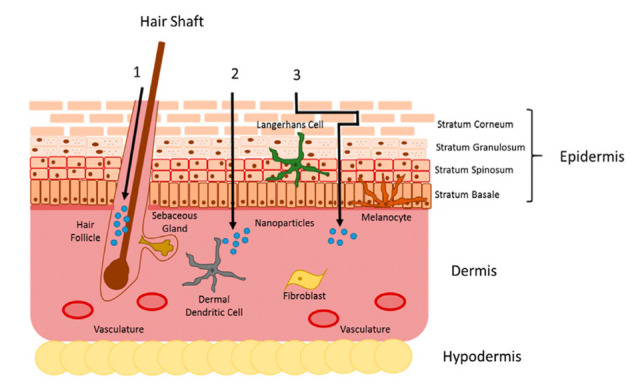
Fig. 2.
Illustration depicting skin penetration pathways of nanoparticles. Nanoparticles applied topically can penetrate the skin in one of three different routes: (1) the appendageal route, involving entry into hair follicles, sweat glands, or skin furrows for potential dermal penetration or enhanced drug release; (2) the intracellular route, which involves a direct passage through the cell membrane across multiple epidermal layers; or (3) the intercellular route, where nanoparticles traverse a more intricate route between epidermal cells. The selection of pathways is influenced by nanoparticle characteristics such as size, charge, morphology, and material. Figure and illustration credit [79].
The stratum granulosum is a pivotal layer that marks a transition in the process of keratinocyte differentiation. Comprising flattened and mature keratinocytes, the stratum granulosum is characterized by the presence of granules within the cells. These granules, known as keratohyalin granules, contain proteins that participate in the development of the skin's protective barrier. During this stage, the keratinocytes synthesize keratin filaments and the degrade of their cell nuclei [69]. This process is essential for forming a resilient, impermeable skin barrier against environmental stressors. As keratinocytes progress through the stratum granulosum, they further dehydrate, enhancing the compact structure. Ultimately, the stratum granulosum is vital for keratinocyte maturation, establishing skin barrier function and integrity [68,70–73].
The stratum corneum, situated at the outermost layer of the epidermis, is crucial for skin barrier function. Composed of flattened, keratin-filled cells called corneocytes, the stratum corneum acts as a protective shield, preventing the entry of pathogens and minimizing water loss from the body. These corneocytes are surrounded by a lipid-rich matrix, creating a water-resistant barrier that contributes to the impermeability of the skin [71]. The stratum corneum plays a pivotal role in maintaining skin hydration, preventing microbial invasion, and safeguarding the body against environmental stressors, thus ensuring the overall health and integrity of the skin [74,75]. The dermis, beneath the epidermis, comprises two layers enhancing its integrity and function. The upper layer, the papillary dermis, features finger-like projections called papillae extending into the epidermis, creating a unique interface. It contains blood vessels, nerve endings, and sensory receptors, crucial for regulating temperature, sensation, and skin nourishment [15,74].
2.3. Mechanism of nanocarrier skin transport
The skin also plays a role in regulating the transport of substances between the internal and external environments. This dynamic process involves various mechanisms that collectively contribute to the function and selective permeability of the skin barrier [76]. The skin allows certain molecules to pass through different routes. Lipophilic substances can penetrate the skin through the lipid-rich intercellular spaces, while hydrophilic molecules utilize aqueous channels formed by sweat ducts and hair follicles. Additionally, passive diffusion, facilitated by concentration gradients, plays a role in the transport of substances across the skin. The skin's pH, hydration level, and the presence of natural moisturizing factors influence these transport mechanisms. Understanding the intricate interplay of these factors is crucial in fields such as dermatology and drug delivery, as it impacts the absorption of topically applied substances and the overall health of the skin [73,77,78].
Nanoparticles penetrate the skin through one of three routes: Apendageal penetration, Intracellular penetration and Intercelluler penetration, by passing through corneocytes, by navigating around corneocytes, or by penetrating through dermal structures like hair follicles (Fig. 2). Appendageal penetration involves nanoparticles entering the skin via hair follicles and sweat glands, which act as natural openings that bypass the outermost layer of the skin, known as the stratum corneum. This route allows the nanoparticles to reach deeper skin layers more effectively. Intracellular penetration, on the other hand, involves nanoparticles entering skin cells through processes such as endocytosis, where the cell membrane engulfs the particles. Once inside the cells, the nanoparticles can be transported within vesicles or released into the cytoplasm, allowing them to interact with intracellular components. Lastly, intercellular penetration involves nanoparticles traveling between skin cells, navigating through the intercellular spaces in the stratum corneum and other skin layers. This movement is influenced by the extracellular matrix and tight junctions between cells, and factors such as particle size, charge, and hydrophobicity play a role in the effectiveness of this pathway. Each of these routes offers unique advantages for delivering drugs, cosmetics, or other therapeutic agents, depending on the properties of the nanoparticles and the intended application [73,77,78].
The process of nanoparticle skin penetration entails complex interactions and intricate mechanisms that depend on various factors, including nanoparticle characteristics and skin properties. Moreover, nanoparticles may exhibit skin penetration through multiple pathways, each influenced by distinct physical and chemical attributes [73,79].
♦ Apendageal penetration: Nanoparticles can also penetrate through skin appendages such as hair follicles, sweat glands, or skin furrows. This pathway provides an alternative route for nanoparticles to reach deeper layers of the skin, potentially facilitating enhanced drug delivery or retention.
♦ Intracellular penetration: Nanoparticles can directly penetrate through the outermost layer of the skin, known as the stratum corneum, by passing through corneocytes, which are the dead skin cells. This intracellular route involves the nanoparticles traversing multiple layers of the epidermis, eventually reaching the viable layers beneath.
♦ Intercelluler penetration: Another pathway involves nanoparticles moving between the skin cells (keratinocytes) rather than penetrating through them. This intercellular route is characterized by a more tortuous path through the spaces between the skin cells. The nanoparticles navigate through these intercellular spaces to reach deeper layers of the skin.
Sungput and colleagues [80] in their study on mangosteen extracts loaded with coconut oil nanoemulsion, demonstrated that the size of particles is influenced by the inclusion of surfactant, cosurfactant, and the oil phase. Larger particle sizes result in faster skin penetration of the nanoemulsion. Furthermore, the study elucidated that nanoemulsion gel preparation exhibits superior penetration compared to emulsion gel preparation containing mangosteen extracts, with penetration rates of approximately 94.19% and 88.20%, respectively, for effectively delivering the active substances. Moreover, the oil phase and enhancer play a significant role in enhancing drug penetration into the lipophilic layer of the skin.
The success of nanoparticle skin penetration depends on several key factors such as nanoparticle size, charge, surface properties, morphology, and material composition. It is equally important to consider the potential safety implications associated with skin penetration. Current research endeavors aim to further elucidate these mechanisms and refine the design of nanoparticles to enhance the efficacy and safety across a spectrum of skin-related applications [81,82].
In the context of nanoemulsions and skin penetration, small and large particle sizes exhibit distinct characteristics and mechanisms that influence their penetration efficacy. Smaller particles benefit from a higher surface area to volume ratio, which enhances their interaction with the skin and facilitates diffusion through the skin's intercellular spaces. This increased surface contact allows them to navigate the tight junctions between skin cells more readily. However, smaller particles are more prone to aggregation, which can hinder their penetration efficiency. In contrast, larger particles can penetrate the skin more effectively through appendageal routes such as hair follicles and sweat glands, which can accommodate larger particles more readily and provide a pathway for deeper and faster penetration. Additionally, larger particles can create a higher osmotic gradient or pressure differential, driving them more forcefully into the skin layers and accelerating the penetration process. Larger particles may also disrupt the skin's lipid barriers more effectively, facilitating faster penetration. Despite the advantages of smaller particles in terms of surface area and diffusion, larger particles can sometimes achieve faster skin penetration due to their ability to utilize appendageal routes and create a higher driving force. Therefore, while both small and large particles have unique advantages, their effectiveness in penetrating the skin depends on their specific properties and the intended application of the nanoemulsion [15,74].
3. Design, formulation and techniques for making nanoemulsion
3.1. Nanoemulsion preparation
The production of nanoemulsions involves employing techniques that break down large droplets into significantly smaller ones, resulting in heightened stability and improved physical properties [80,83,84]. The nano-sized droplets within the emulsion create a larger surface area, providing advantages in diverse applications such as drug delivery, formulating food and beverages, creating cosmetics, and meeting the needs of industries seeking enhanced dispersion and absorption properties [85–87].
Nanoemulsions typically consist of several key components, all of which are pivotal in the formulation and stability of the emulsion. The main components of a nanoemulsion include [87,88]:
♦ Oil Phase: Nanoemulsions involve the dispersion of oil droplets in water or vice versa. The oil phase can be various oils, such as vegetable oils, mineral oils, or other lipids depending on the application.
♦ Water Phase: The continuous aqueous phase surrounds the dispersed oil droplets. It provides a medium for dispersion and is often responsible for carrying water-soluble components.
♦ Surfactants: They are molecules with hydrophilic (water-attracting) and hydrophobic (oil-attracting) regions. Surfactants reduce the interfacial tension between the oil and water phases, stabilizing the emulsion. They function in preventing the coalescence of droplets.
♦ Co-surfactants: In some formulations, co-surfactants are used along with the surfactants to enhance the stability and reduce the concentration of the primary surfactant.
♦ Co-solvents: These are optional components that can improve the solubility of certain ingredients and contribute to the overall stability of the nanoemulsion.
♦ Bioactive Compounds: In many cases, nanoemulsions are designed to carry specific active ingredients, such as drugs, herbs, nutrients, flavors, or fragrances. The small droplet size increases the surface area and enhances the bioavailability of these active compounds.
♦ Polymeric Stabilizers: Polymers can be added to further stabilize the nanoemulsion by providing a protective coating around the droplets.
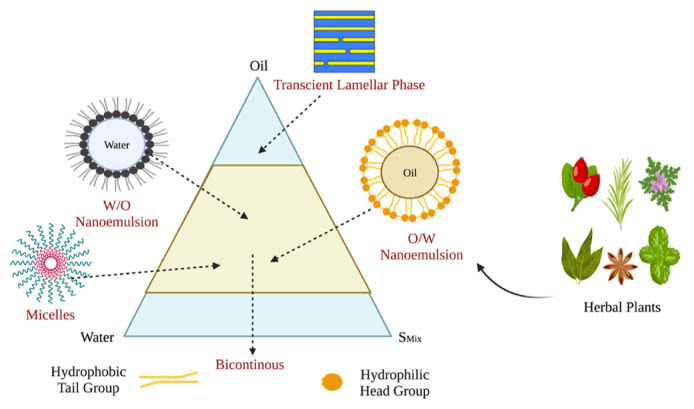
Screening excipients is important in the process of formulating nanoemulsions. The primary excipients involved in nanoemulsion formulation include oil, surfactant mixture (Smix), and water. Assessing drug solubility aids in the optimal selection of oils and surfactants for the development of nanoemulsion formulations. The use of a pseudoternary phase diagram is predominant in determining the proper ratio of water to oil to Smix in nanoemulsions. The actual ratio within the ternary system significantly influences the maximum drug solubility, encapsulation efficiency, and drug loading in the formulation [89,90]. Fig. 3 illustrates the distinct properties of the three components: oil, water, and Smix and their respective formulations.
Fig. 3.
An illustrative diagram depicting the interactions between oil, water, and Smix (surfactant mixture), highlighting the emulsification process and various formulations.
Understanding and optimizing the ratios and interplay between these elements are vital for creating a stable and efficient nanoemulsion customized for specific purposes, such as pharmaceuticals, food, cosmetics, or other industries [91,92].
3.2. Oil phase
The oil phase in a nanoemulsion refers to the component that comprises the dispersed oil droplets within the emulsion. This phase typically consists of various oils or lipids, depending on the desired properties and intended applications of the nanoemulsion. The choice of oil phase is the key point as it affects the emulsion's stability, texture, and compatibility with specific active ingredients [92–94]. Common types of oils used in the oil phase of nanoemulsions include vegetable oils such as soybean oil, sunflower oil, olive oil, or other plant-based oils. Mineral oils, derived from petroleum, can be used in certain applications. Lipids, including triglycerides or fatty acids, can be chosen based on their compatibility with the target formulation [95,96].
The selection of the oil phase depends on factors such as the intended use of the nanoemulsion, the desired characteristics of the final product, and the solubility of the active ingredients. Along with the water phase and emulsifying agents (surfactants and co-surfactants), the oil phase forms the fundamental structure of the nanoemulsion [57]. The goal is to create a stable dispersion of tiny oil droplets within the continuous water phase, and the choice of the oil phase significantly influences the overall performance and functionality of the nanoemulsion [97,98].
The nanoemulsion formulation conducted by Wuttikul and Sinakham [58] employed coconut oil as the oil phase, capitalizing on its inherent antioxidant properties. Additionally, the nanoemulsion preparation in this investigation featured Curcuma xanthorizza as a natural component, which also served as an active agent in cosmetic drug delivery [58].
Another study by Uchida and colleagues [99] focused on the development of nanoemulsions using Terminalia catappa Linn, employing crodamol as the oil phase, with concentrations of up to 3% of the oil carrier. Crodamol, an oil recently used for nanoemulsion formulations, has gained significant traction in cosmetics due to its versatile applications as an emollient, humectant, and more. This is attributed to crodamol's composition with medium-chain triglycerides, offering favorable properties such as colorlessness, odorlessness, stability, and antioxidant activity, rendering it suitable for both cosmetics and nanoemulsion preparations [100–102].
Another role for oil in nanoemulsions is to dissolve the lipophilic active substances. The choice of oil impacts the HLB due to variations in penetration abilities compared to the surfactant layer. Short to medium-chain oils exhibit superior penetration into the tail group area compared to long-chain alkanes, thereby reducing the HLB. Saturated fatty acids, such as lauric, myristic, and capric acids, along with unsaturated fatty acids like oleic and linoleic acids, have been demonstrated to enhance penetration [103]. In formulating, selecting lipophilic pharmaceuticals or those with high solubility in the oil phase is essential for size reduction in the formulation [104].
3.3. Surfactant
A surfactant, short for surface-active agent, is a chemical compound that reduces the surface tension between two immiscible substances, typically between a liquid and a solid or between two liquids. Surfactants have molecules with both hydrophilic (water-attracting) and hydrophobic (water-repelling) portions. This dual nature allows them to interact with and stabilize emulsions, which are colloidal dispersions of two immiscible liquids, such as oil and water [105,106].
Surfactants are widely used in various industries, including pharmaceuticals, cosmetics, food, and agriculture, due to their ability to improve the dispersal, solubility, and stability of substances that do not naturally mix. They are essential components in the formulation of products like emulsions, soaps, detergents, and foams [105,107].
Surfactants play a crucial role in reducing the interfacial tension between typically immiscible phases, facilitating their emulsification. These amphiphilic molecules contain both hydrophilic and lipophilic groups within a single molecule. Changes or adjustment to the interfacial layer can significantly affect factors influencing the emulsion system. As described by Lima and colleagues [108].
Emulsion breakdown primarily occurs via three mechanisms: creaming, flocculation, and coalescence. Creaming is a phase separation phenomenon resulting from the upward or downward movement of particles due to gravitational attraction in phases with varying density values. Flocculation is characterized by the agglomeration of droplets, and due to the lack of concentrated films between surfaces, it leads to quicker creaming formation. Coalescence, on the other hand, entails the merging of droplets into larger ones, accompanied by concentrated film formation between surfaces (see Fig. 3) [109,110].
Several surfactants, including Tween 80, Span 80, Span 85, Transcutol, and various natural surfactants, have been utilized in several studies discussed herein, typically at concentrations ranging from 4% to 15% in each formulation. Emulsion stability is influenced by various factors, including emulsion composition and processing techniques. Internal factors affecting emulsion stability include the type and concentration of emulsifying agents, the nature and concentration of components in the dispersed and continuous phases, the viscosity of the continuous phase, the ratio of dispersed phase to continuous phase, and particle size. External factors that impact emulsion stability include agitation, evaporation, and temperature variations [111,112].
In the development of nanoemulsions for herbal cosmetics, newer surfactants such as Transcutol® and Labrasol® have emerged as significant advancements beyond traditional agents like Tween and Span. Transcutol® (diethylene glycol monoethyl ether) is utilized for its exceptional skin penetration enhancement and solvent properties, which facilitate the effective delivery of active ingredients into the dermal layers. Labrasol® (caprylocaproic triglyceride) serves as both an emulsifier and solubilizer, stabilizing nanoemulsions and improving the dispersion of hydrophobic compounds, thereby enhancing their bioavailability. Additionally, caprylic/capric triglycerides, derived from coconut oil and glycerin, are employed for their emulsifying and moisturizing properties, contributing to a smooth texture and better delivery of actives. Polysorbate 80 and lecithin are also used for their stabilizing effects; polysorbate 80 improves dispersion and reduces surface tension, while lecithin, a natural emulsifier, enhances skin compatibility and formulation stability [97,98]. Polyglyceryl-10 oleate and sodium lauroyl glutamate are innovative possibilities for surfactants that are both natural and gentle. These choices are in line with the current movement towards using cosmetic chemicals that are more friendly to the skin and the environment. Together, these contemporary surfactants enhance the effectiveness and user satisfaction of herbal cosmetic products by improving performance in nanoemulsions [105,107].
3.4. Co-surfactant/co-solvent
In cosmetic nanoemulsions, various surfactants are utilized to stabilize the formulation and enhance its cosmetic properties. Commonly employed surfactants include Tween 20 (Polysorbate 20) and Tween 80 (Polysorbate 80), which are nonionic surfactants known for their emulsifying and solubilizing properties. Additionally, Span 20 (Sorbitan Monolaurate) and Span 80 (Sorbitan Monooleate) are often used as lipophilic surfactants to stabilize oil-in-water nanoemulsions. Cremophor EL (Polyoxyl 35 Castor Oil) is another nonionic surfactant frequently employed in cosmetic formulations due to its compatibility with a wide range of substances. Sodium lauryl sulfate (SLS) and cetyltrimethylammonium bromide (CTAB) are examples of anionic and cationic surfactants, respectively, which may be used in specific formulations to achieve desired properties. Additionally, Poloxamer 188, a nonionic surfactant with both hydrophilic and lipophilic properties, is often utilized for its emulsifying and stabilizing effects in cosmetic nanoemulsions. These surfactants play essential roles in stabilizing the emulsion, enhancing the solubility of active ingredients, and improving the overall cosmetic performance of the product [113,114].
Guzman and colleagues [48] investigated the production of nanoemulsions from Passiflora edulis var. edulis using Span 85 and Tween 80 as surfactants and cosurfactants. In addition to one surfactant, multiple studies [45,115,116] have demonstrated that the use of co-surfactants is necessary for the creation of nanoparticles. This is because a mixture of surfactants provides better stability during storage compared to nanoemulsions that use only a single surfactant.
In a study conducted by Harimurti and colleagues [117] (Table 2), the utilization of two surfactants such as Tween 80 and Span 20 in the formulation of nanoemulsions involving Curcuma xanthoriza and Hylocereus polyrhizus yielded improved properties and stability [117]. This was evidenced by favorable characteristics, starting with particle size and the polydisperse index value, which met the criteria for nanoemulsion preparations. Moreover, the combination of low HLB values for surfactants and cosurfactants with high HLB values for surfactants and co-surfactants may enhance the stability of nanoemulsion preparations [118]. HLB serves as an empirical measure for assessing the interaction between hydrophilic and hydrophobic groups in surfactants or co-surfactants. This technique is also used to gauge surfactant emulsification of the oil and water phases, with HLB values ranging from 1 to 40. Lower values indicate oil solubility, while higher values signify water solubility. According to Almeida and colleagues [118], HLB value differentials can be employed to calculate surfactant combinations and percentages [118,119].
Table 2.
Formulation and methodology of cosmetic nanoemulsions from natural/herbal sources: Insights from previous studies.
| Herbal plant | Properties | Formulation | Methods | Ref | |
|---|---|---|---|---|---|
|
| |||||
| Oil-phase | Surfactant & Co-surfactant | ||||
| Rhodiola rosea | Antioxidant | NMa | Tween 80 | Low-energy method | [46] |
| Lonicera japonica | Stiring (Spontaneous emulsification) | ||||
| Curcuma aromatica | Antioxidant | Coconut oil | Tween 80, Span 80 | Low-energy method | [58] |
| Anti-aging | Stirring (spontaneous emulsification) | ||||
| Garcinia mangostana L. | Antioxidant | Virgin coconut oil | Span 20 | High-energy method | [80] |
| Tween 80 | High speed homogenization | ||||
| Propylene glycol | |||||
| Terminalia catappa Linn | Antioxidant | Crodamol | Tween 80 | Low-energy method | [99] |
| Anti-aging | Stirring (spontaneous emulsification) | ||||
| Dipteryx alata vog | Anti-aging | Palmitic acid | Tween 80 | High-energy method | [103] |
| Linoleic acid | High speed homogenization | ||||
| Passiflora edulis var. edulis | Antioxidant | NMa | Tween 80, Span 85 | High-energy method | [116] |
| Anti-aging | Ultrasonication | ||||
| Curcuma xanthoriza | Antioxidant | Corn oil | Tween 80 | High-energy method | [117] |
| Hylocereus polyrhizus | Anti-wrinkle | High speed homogenization | |||
| Rhodiola rosea | Whitening | Soybean oil, | Transcutol, Labrasol, | Low-energy method | [128] |
| Anti-aging | Labrafac oil | Tween 80, Span 80 | Stiring (Spontaneous emulsification) | ||
| Sunflower oil | Sunscreen | Sunflower oil | Tween 80 | Low-energy method | [137] |
| Span 80 | Sorbitol | Stiring (Spontaneous emulsification) | |||
| Psoralea corylifolia | Anti-aging | NMa | Coco bectaine, | Low-energy method | [140] |
| Surfactin | Stirring (spontaneous emulsification) | ||||
| Curcumin | Antioxidant | Virgin coconut oil | Tween 80, Span | High-energy method | [141] |
| Anti-aging | High speed homogenization | ||||
| Agave sisalana | Moisturizer | Ethylhexyl palmitate | Crodamol | High-energy method | [142] |
| Anti-aging | Tween 80 | Ultrasonic probe sonication | |||
| Span 80 | |||||
| Mauritia flexuosa | Sunscreen | Buriti oil | Tween 80, Span 80 | High-energy method | [145] |
| Antioxidant | High pressure homogenization | ||||
| Curcumin | Antioxidant | Echium oil | Tween 80 | High-energy method | [146] |
| High pressure homogenization | |||||
| Brown mustard seed | Moisturizer | Rice bran oil | Sorbitan oleate, | Low-energy method | [167] |
| Anti-aging | Polyethylene glycol | Stirring (spontaneous emulsification) | |||
| Pea protein | Antioxidant | NMa | Tween 80 | Low-energy method | [197] |
| Stirring (spontaneous emulsification) | |||||
| Cordyceps militaris | Antioxidant | Sea buckthorn oil (fruit oil) | Tween 80 | High-energy method | [198] |
| Chitosan | High pressure homogenization | ||||
| Opuntia ficus-indica | Moisturizer | Capric/Caprilic | Tween 80 | High-energy method | [199] |
| Triglycerides, Ethylhexyl Palmitate | Sorbitan oleate | (Ultra turrax mod) | |||
| Vellozia squamata | Antioxidant | Babacu oil | Span 60 | Low-energy method | [182] |
| PEG-40 | Stirring (spontaneous emulsification) | ||||
| Hydrogenated castor oil | |||||
| Phyllanthus niruri | Antioxidant | Citrullus lannatus seed oil | Tween 80 | Low-energy method | [200] |
| Stirring (spontaneous emulsification) | |||||
| Punica grantum var. Rabab | Antioxidant | Flaxseed oil | Tween 80 | High-energy method | [188] |
| High-pressure homogenization and Ultrasonication | |||||
NM: Not mentioned.
Information:
HLBr: the ratio of the combined value of 2 surfactants
HLBA: the ratio HLB of lipophilic surfactant
HLBB: the ratio HLB of hydrophilic surfactant
A%: percentage of lipophilic surfactant
B%: percentage of hydrophilic surfactant
Co-surfactants, also known as co-emulsifiers, cosolubilizers, or co-solvents, typically have a similar molecular structure to surfactants. The addition of a co-surfactant can improve the formulation by providing additional stability, reducing the required concentration of the primary surfactant, or modifying the characteristics of the emulsion, such as its droplet size or viscosity [120]. In summary, co-surfactants work in conjunction with primary surfactants to fine-tune the emulsification process, thereby improving the overall stability and performance of emulsions across various applications [121,122].
3.5. Herbal active ingredients
Herbal active ingredients refer to bioactive compounds derived from plants that have therapeutic or beneficial properties. These active ingredients are often extracted from different parts of medicinal plants, including leaves, roots, stems, flowers, and seeds. Herbs have been used for centuries in traditional medicine for their natural healing properties [41,85].
Examples of herbal active ingredients include:
Alkaloids: Potent compounds found in plants like opium poppy (morphine), cinchona (quinine), and caffeine-containing plants (coffee, tea).
Flavonoids: Antioxidant compounds present in fruits, vegetables, and herbs such as quercetin in onions, catechins in green tea, and rutin in buckwheat.
Terpenes: A diverse group of compounds found in plant-based essential oils, like menthol in mint, limonene in citrus fruits, and cannabinoids in cannabis.
Glycosides: Plant compounds with sugar molecules attached, such as cardiac glycosides in foxglove (used for heart conditions) or saponins in various plants.
Polyphenols: Broadly occurring compounds with antioxidant properties, including resveratrol in grapes, curcumin in turmeric, and epigallocatechin gallate (EGCG) in green tea.
Essential Oils: Volatile aromatic compounds found in plants, like eucalyptus, lavender, or peppermint, which are often used in aromatherapy or as topical agents.
These herbal active ingredients have various pharmacological effects including anti-inflammatory, antioxidant, antimicrobial, analgesic, and other therapeutic properties [123]. The active compounds are also frequently used as herbal medicine and natural remedies to improve the health and well-being of individuals. Nevertheless, it's essential to acknowledge that the effectiveness and safety of herbal products may vary, highlighting the importance of validating the therapeutic claims with extensive scientific research [85,124].
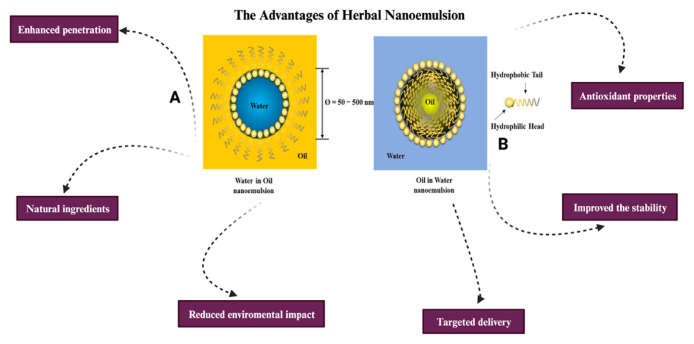
A herbal nanoemulsion, derived from plants, incorporates active compounds such as bioactive compounds, essential oils, or other therapeutic substances extracted from medicinal plants. In essence, herbal nanoemulsions offer a technologically advanced approach to delivering herbal active ingredients in a more efficient and bioavailable manner, promising potential advantages across diverse health and wellness applications [124–126].
Herbal nanoemulsions offer several unique advantages over conventional nanoemulsions in cosmetic sciences, primarily due to the incorporation of natural, plant-derived active ingredients. One of the main benefits is enhanced safety, as herbal nanoemulsions generally pose a reduced risk of adverse reactions due to the use of natural ingredients, which are less likely to cause skin irritation or sensitization compared to synthetic chemicals found in conventional nanoemulsions. Additionally, herbal nanoemulsions demonstrate improved efficacy, with the natural active compounds exhibiting better skin compatibility and enhanced penetration, ensuring higher bioavailability and effectiveness in delivering therapeutic benefits [100–102].
Moreover, herbal nanoemulsions are rich in natural antioxidants, providing superior protection against oxidative stress and environmental damage. They also possess natural anti-inflammatory properties, which help to reduce inflammation and provide soothing and calming effects on the skin. This anti-inflammatory benefit is often absent in conventional nanoemulsions that rely on synthetic ingredients. Furthermore, certain herbal extracts have inherent antimicrobial properties, making herbal nanoemulsions effective against various skin pathogens without the need for additional antimicrobial agents [121,122].
Consumer preference is another significant advantage of herbal nanoemulsions, as there is a growing trend towards natural and organic products. This preference makes herbal nanoemulsions more appealing in the cosmetic market. By highlighting these benefits—enhanced safety, improved efficacy, antioxidant properties, anti-inflammatory effects, antimicrobial activity, and consumer preference—the superiority of herbal nanoemulsions in cosmetic applications can be clearly demonstrated, showcasing their potential to deliver safer, more effective, and multifunctional skincare solutions [41,85].
4. Preparation method of herbal nanoemulsion
The preparation of herbal nanoemulsions involves several critical steps aimed at achieving a stable and effective formulation. Below is a general outline of the preparation method [126,127]:
-
Selection of Ingredients:
- Choose a suitable oil phase containing herbal extracts or essential oils.
- Select an aqueous phase, usually composed of water or an herbal aqueous extract.
- Pick appropriate surfactants and co-surfactants based on compatibility and stability considerations.
-
Determination of Ratios:
- Evaluate the optimal proportions among the oil phase, aqueous phase, surfactants, and cosurfactants. This assessment often involves utilizing a pseudoternary phase diagram to identify the appropriate ratios conducive to stable nanoemulsion formulation.
-
Preparation of Oil Phase:
- Combine the herbal extracts or essential oils with the chosen oil phase, possibly requiring heating and stirring to achieve homogeneity.
-
Preparation of Aqueous Phase:
- Combine the components of the aqueous phase, including water or herbal extracts.
-
Emulsification:
- Merge the oil phase and aqueous phase.
- Employ a low-energy method for spontaneous emulsification or a high-energy method such as high-pressure homogenization, ultrasonication, or microfluidization to break down droplets into nanoscale sizes, a crucial step for nanoemulsion formation.
-
Addition of Surfactants and Co-surfactants:
- Introduce the surfactants and co-surfactants to stabilize the nanoemulsion. This step helps prevent droplet coalescence and ensures long-term stability.
-
Post-processing:
- Allow the nanoemulsion to cool if heating was involved during the preparation.
- Check for any signs of phase separation or instability.
-
Characterization:
- Evaluate the characteristics of the nanoemulsion, including droplet size, polydispersity, zeta potential, pH and stability storage time.
-
Storage:
- Store the herbal nanoemulsion in appropriate conditions to maintain its stability until use.
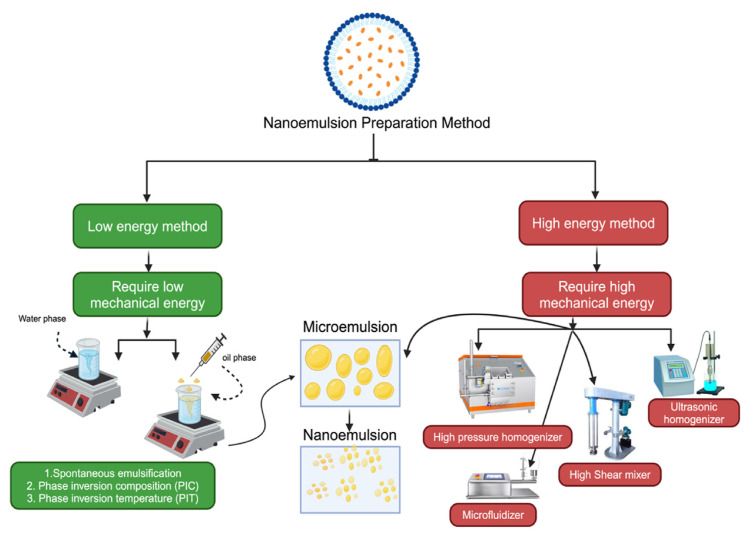
It's important to note that the specific details of the preparation method may vary depending on selected ingredients, intended application, and available equipment. Furthermore, adherence to Good Manufacturing Practices (GMP) and proper documentation are crucial for consistent and reproducible preparation of herbal nanoemulsions. Additionally, the creation of herbal nanoemulsions typically employs two primary methods: low-energy and high-energy methods, each offering distinct advantages and serving different purposes (Fig. 4). Below is an overview of several frequently utilized methods for preparing nanoemulsions, as outlined by Wuttikul and colleagues [58,128,129].
Fig. 4.
Study of nanoemulsion preparation method.
4.1. Low energy method preparation
In the context of formulations, a “low energy method” typically refers to processes or techniques that do not involve high levels of mechanical energy or intense processing conditions. This approach is often preferred for formulating pharmaceuticals, cosmetics, or emulsions, where the goal is to achieve a stable product without subjecting it to excessive energy that could compromise its stability or integrity [130,131].
A formulation utilizing the low-energy method focuses on minimizing the use of high-energy procedures (Fig. 4). This approach emphasizes gentle and controlled techniques to ensure the stability and quality of the end product. It begins with the careful selection of suitable components, taking into account their physicochemical characteristics. Moderate temperatures are employed to facilitate liquid phase mixing, eliminating the necessity for high-speed mixers or homogenizers.
The gradual addition of solid components is achieved through gentle agitation, minimizing degradation or undesired alterations in formulation qualities. If needed, minor adjustments in temperature and viscosity are made using low-energy techniques such as gentle heating or cooling. Emulsification methods, such as the surfactant utilization, enable the creation of stable emulsions without the need for high-intensity homogenization [132].
To achieve a more refined texture, filtration or straining techniques can be employed, while stringent quality control measures ensure stability, uniformity, and desired attributes. The packaging process is meticulously carried out to preserve product quality, and efforts are made to ensure that energy-efficient techniques can be effectively scaled up for expanded production.
This strategy is particularly beneficial in industries where minimize stress on the formulation to maintain the desired qualities of the final product [133,134]. For instance, in emulsion production, a low-energy approach could entail employing mild agitation, mixing, or homogenization techniques to attain the desired emulsion without subjecting the ingredients to excessive heat or strain. This is especially important when handling delicate substances or when preservation of formulation integrity is crucial [135,136].
The formulation of a low-energy method can vary depending on product characteristics and constituents involved, often requiring a delicate balance of components and a gradual approach to prevent undesired alterations during production [114]. For instance, Arianto and colleagues investigated a low-energy method employing spontaneous emulsification (3000–4000 rpm for 30 min) in their study of sunflower oil emulsification [137], They successfully produced three distinct nanoemulsions of sunflower oil with particle sizes less than 500 nm, resulting in a transparent solution. This study demonstrated the efficacy of the low-energy technique in reducing particle size and fulfilling the requirements for nanoemulsion preparation [106,138].
Similarly, Uchida and co-workers [99] conducted research on T. catappa Linn nanoemulsion utilizing the low-energy method of spontaneous emulsification. This method produced nanoemulsions with particle sizes less than 70 nm, along with clear and stable nanoemulsions. Another advantage of this method is its simplicity, quickness, and lack of requirement for special or expensive equipment. Spontaneous emulsification is a modification of the solvent evaporation method, where a water-soluble solvent (methanol or acetone) is added to a water-insoluble organic solvent (dichloromethane or chloroform). Interfacial turbulence formed between the two phases facilitates the formation of smaller particles through spontaneous emulsification of the water-soluble solvent. Additionally, diffusion of water-soluble solvents contributes to particle size reduction [98,139].
In essence, the low-energy approach involves employing mild processing techniques that minimize mechanical strain and energy consumption, resulting in the production of stable and superior products.
In a study by Lewinska et al. [140] investigating the formulation of nanoemulsion Psoralea corylifolia for anti-ageing cosmetic applications, the formulation employed spontaneous emulsification with stirring at 1000 rpm for 25 min. The modification and expansion of the liquid and air phases were achieved through dripping at a temperature of 25 °C [140,141]. The spontaneous emulsification method uses the chemical energy released during the dilution process to create a continuous phase at a constant temperature with no phase transitions during emulsification (Table 1). During dilution, water-soluble components (solvent, surfactant, or cosurfactant) diffuse from the organic phase into the aqueous phase, creating an oil-in-water nanoemulsion, as a result significantly increasing interfacial area and establishing a metastable emulsion state. Achieving nanoscale droplets through spontaneous diffusion emulsification requires careful consideration of the solvent or oil ratio. Turbulence facilitates solvent diffusion, promoting the formation of nanoscale droplets [98,138].
Table 1.
| Provision | Low energy methods | High energy methods |
|---|---|---|
| Equipment/instrument | Generally not required |
|
| Energy input Pressure | 103–105 W/kg Not applied | >108 W/kg for diameter below than 200 nm
|
| Droplet size distribution | Up to 50 nm |
|
| Total production cost | Low production cost |
|
4.2. High energy method preparation
Numerous scientific studies have demonstrated the formation of micellar nanoparticles within nanoemulsion systems, particularly within the cosmetic field. Characterization methods and pre-clinical investigations have been employed to validate the effectiveness of micellar nanoparticles as a delivery system for cosmeceuticals [114,132].
One notable study by Gomes et al. [142] focused on formulating a skin moisturizer based on nanoemulsion, utilizing ultrasonication techniques to incorporate micelles of Agave sisalana droplets as the active ingredient. The nanoemulsion system was initially developed by combining A. sisalana with various non-ionic surfactant types. The researchers successfully achieved a stable nanoemulsion formulation by using surfactants such as crodamol, Tween 80 and Span 80. This formulation contributed to the creation of smaller micellar particles, ranging between 83 and 155 nm in size. This innovative approach highlights the potential of micellar nanoparticles within nanoemulsions for enhancing the delivery and efficacy of active ingredients in cosmeceutical applications [143,144].
In another study by Paulo et al. [103], almond nanoemulsion formulations were examined using high-energy methods. Utilizing a high-pressure homogenizer (Omni Macro ES Digital Programmable Homogenizer, Kennesaw, USA) operating at 7500 rpm for 3 min resulted in the smallest particle size of 219 nm. This formulation exhibited favorable nanoemulsion characteristics and passed the stability testing. High-energy methodology is commonly employed for the preparation of nanoemulsions, utilizing high-temperature pressure devices to induce elevated temperatures and velocities for nanoemulsion formulation (Table 1) [139,145].
Furthermore, Inal et al. [146] used a high-energy method with the Ultra-Turrax T18 (IKA, Germany) instrument operating at a speed of 12500 rpm for 10 min at room temperature in the production of curcumin nanoemulsion and echium seed oil. The use of Ultra-Turrax in this formulation process is highly advantageous and proficient in generating nanoemulsions with sizes below 200 nm, aligning with the specific attributes of nanoemulsions. The efficacy of high-energy techniques in the manufacturing process, coupled with the optimal combination of surfactants, water phase, and oil phase, are key factors in achieving desirable features and stability in this nanoemulsion [146,147].
A study conducted by Gauthier and colleagues [148] demonstrated that the high-energy production process can be delineated into numerous alternatives, encompassing ultrasonication, high-pressure homogenizer, microfluidization, and rotorstator methods. The selection of nanoemulsion creation method hinges on factors such as the dosage formulation objective, material selection, instrument availability, and the economic considerations. This choice is pivotal for subsequent generation and characterization of nanoemulsion preparations that meet specified requirements [43,148,149].
5. Characterization of nanoemulsion
Previous studies have illustrated that cosmetic nanoemulsion formulations derived from herbal sources exhibit notable attributes. These formulations undergo nanoemulsion production and testing, as detailed in Tables 2 and 3.
Table 3.
Evaluation of the characteristics and functions of herbal nanoemulsions.
| Herbal plant | Characteristics Evaluation | Experimental Results | Functional Testing | Ref |
|---|---|---|---|---|
| Curcuma aromatica | Organoleptic PZ PDI Viscosity pH |
|
Antioxidant activity assessment, lipid peroxidation inhibition assay, protein denaturation inhibition test, heating-cooling cycle evaluation | [58] |
| Terminalia catappa Linn | Organoleptic PZ PDI ZP pH |
|
Carotenoid assay, <-amylase inhibition assay, antioxidant activity assay | [99] |
| Dipteryx alata vog | PZ PDI Stability |
|
None | [103] |
| Mauritia flexuosa | PZ PDI pH SM |
|
In vitro phototoxicity test, SPF test, radiation exposure assessment | [145] |
| Curcumin | Organoleptic PZ PDI ZP pH |
|
Solubility assessment, antioxidant capacity evaluation, in vitro simulated digestion analysis, fatty acid composition determination | [146] |
| Passiflora edulis var. edulis | PZ PDI Viscosity SM |
|
determination of >-anisidine value in nanoemulsion, conductivity test, skin viscoelasticity and firmness evaluation, transpidermal water loss measurement | [116] |
| Curcuma xanthoriza Hylocereus polyrhizus | PZ PDI ZP Stability Surface morphology |
|
Antoxodant activity, Fourier-Transform Infrared Spectroscopy (FTIR) examination | [117] |
| Rhodiola rosea | Organoleptic PZ PDI ZP pH Stability Surface morphology |
|
Skin irritation assessment, antioxidant activity evaluation, viscosity analysis, FTIR spectroscopy examination, centrifugation test, encapsulation efficiency determination | [128] |
| Curcumin | Organoleptic PZ PDI ZP Surface morphology |
|
Antioxidant activity assessment, skin penetration test, uorescent imaging analysis, scratch assay for wound healing assessment, cell cultivation and proliferation assay | [141] |
| Agave sisalana | PZ PDI ZP pH Stability Surface morphology |
|
Allergy testing, centrifugation analysis, skin hydration test | [142] |
| Rice bran oil | Organoleptic PZ Stability Surface morphology |
|
In vitro simulated lipid digestion and bioavailability assessment, measurement of free fatty acid release, ex vivo bioactivity analysis, assessment of prophylactic mitigation against mitogen (LPS) induced oxidative stress, uptake studies | [167] |
| Cordyceps militaris | PZ PDI ZP pH Stability Surface morphology |
|
FTIR, antioxidant activity, in vitro cytotoxicity, antibacterial activity, | [198] |
| Opuntia ficus-indica | PZ PDI pH Stability Surface morphology |
|
Electrical conductivity, evaluation of in vivo moisturizing properties, transepidermal water loss | [199] |
| Vellozia squamata | PZ PDI pH Stability |
|
Antioxidant activity, rheology test, total phenolic content | [182] |
| Phyllanthus niruri | PZ PDI ZP Stability Surface morphology |
|
Antioxidant activity, penetration test by cumulative percentage of release, refractive index and viscosity, release kinetic study | [200] |
| Punica grantum var. Rabab | PZ PDI ZP Stability |
|
Antioxidant activity, creaming stability, Ostwald ripening test, chemical stability | [188] |
| Garcinia mangostana L. | PZ PDI ZP pH Stability Surface morphology |
|
Antioxidant activity, clarity, glitter, viscosity | [201] |
PZ = Particle size.
PDI = Polydispersity index.
ZP = Zeta potential.
pH = Acidity.
SM = Surface morphology.
TEM = Transmission electron microscopy.
FTIR = Fourier-transform infrared spectroscopy.
Nanoemulsions, characterized in Table 3, typically exhibit stability and transparent appearance, with small droplet sizes typically less than 500 nm. Furthermore, they demonstrate enhanced stability, often with particle sizes falling within the range of 10–250 nm [150]. Nanoemulsions are formulated by combining oil and water phases using surfactants and cosurfactants, effectively reducing the interfacial surface tension [116,140,142,145].
6. Characteristics of nanoemulsion
Various methods were employed in the preparation of nanoemulsions using different herbal plants and ingredients (Table 2). Each method is associated with characteristic assessments to evaluate the quality and stability of the resulting nanoemulsions. Nanoemulsions were prepared using two primary methods: high energy and low energy methods [151].
To verify the fundamental characteristics of herbal nanoemulsions including particle size, PDI, organoleptic properties, visual inspection, viscosity, acidity, zeta potential, surface morphology and stability [152,153]. Several tests such as stirring (spontaneous emulsification), phase inversion composition (PIC), phase inversion temperature for low energy methods, high-pressure homogenization, ultrasonication and high-speed homogenization for high energy method were conducted (Fig. 4). The methods and corresponding results are integrated into Table 3.
The results varied among the different herbal plants and methods used, with some formulations exhibiting stability during storage, while others experienced separation or changes in characteristic properties. These findings provide valuable insights into the formulation and characterization of nanoemulsions, which have applications in the pharmaceutical and food industries for encapsulating bioactive compounds. In conclusion, Table 3 provides a comprehensive overview of the preparation methods, experimental models and characteristic testing results of nanoemulsions involving various herbal plants and ingredients. The selection of the preparation method and the distinct characteristics of each nanoemulsion differed, influencing their stability and quality over the course of storage [154,155].
6.1. Visual inspection and organoleptic test
Visual inspection and organoleptic tests are crucial techniques for evaluating the quality and sensory characteristics of nanoemulsions, especially in industries such as cosmetics, medicines, and food, where nanoscale formulations are widely used. Nanoemulsions, consisting of droplets finely scattered in the nanoscale range, undergo thorough visual examination to assess hue, clarity, and overall uniformity [156,157]. Anomalies such as phase separation, sedimentation, or visual irregularities are carefully observed as they can provide valuable information about formulation difficulties or instability. Visual examination ensures that the nanoemulsion satisfies aesthetic criteria andcomplies with rigorous quality requirements, delivering a visually appealing product [82,118].
In a study conducted by Paulo and colleagues [103] on Dypteryx alata vog. nanoemulsion for macroscopic stability, the creaming effect, a frequently observed destabilizing mechanism in O/W nanoemulsions, was examined visually at various salt concentrations and pH levels over a 28-day period, with evaluations conducted weekly. A creaming index approaching 0% indicates that the emulsified system maintains stability during the 28-day storage period, a distinctive characteristic of nanoemulsions being their small particle size, evident by their clear and stable visual appearance [158,159].
Organoleptic tests play a vital role in assessing the sensory characteristics of nanoemulsions, engaging human senses such as taste, smell, and touch. In skincare or cosmetics applications, parameters like texture, smoothness, and absence of grittiness are evaluated; whereas for pharmaceutical or food products, taste, odor, and mouthfeel are crucial factors. Deviations from expected sensory attributes may indicate formulation issues or the presence of undesirable elements. Organoleptic testing ensures that the nanoemulsion delivers a satisfactory sensory experience, aligning with consumer expectations [77,134,160].
The subjective nature of human sensory perception underscores the importance of these assessments in quality assurance. Visual inspection and organoleptic tests collectively contribute to ensuring that nanoemulsions not only meet technical benchmarks, such as droplet size and stability, but also deliver a satisfying sensory experience to consumers. These rigorous evaluations play a crucial role in maintaining the overall quality and market acceptance of nanoemulsions in industries characterized by constant innovation and dynamic consumer preferences [62,107]. Both visual inspection and organoleptic tests rely on subjective human perceptions, making them valuable tools for quality assurance. The combined use of these methods ensures that products not only meet technical specifications but also align with consumer expectations by delivering a satisfactory sensory experience [82,161].
6.2. Particle size and PDI (polydispersity index)
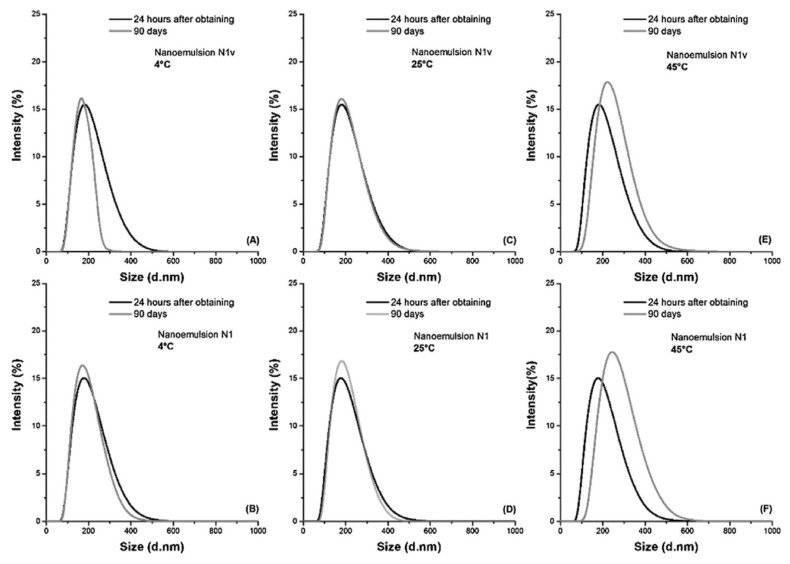
Barreto and colleagues [142] discussed the evaluation of droplet size distribution by intensity, revealing the presence of a monomodal peak with high intensity (Fig. 5). Moreover, the formulations exhibited lower PDI values, indicating the stability of the systems and the effectiveness of the ultrasound method in achieving stable nanoemulsions. Over a 90-day storage period at 4 °C, 25 °C, and 45 °C, no significant alteration in zeta potential values was observed, indicating the stability of the nanoemulsions. The lower values of zeta potential may be attributed to the steric stabilization mechanism.
Fig. 5.
Dynamic light scattering analysis revealing the distribution of droplet sizes (intensity plotted against droplet diameter) in Agave sisalana nanoemulsion [142].
Nanoparticle characterization involves determining the particle size, particle size distribution, and PDI values. Particle size analysis using laser diffraction operates on the principle that particles passing through diffracted light exhibit varying angles of diffraction corresponding to their size. As the diffraction angle increases, the particle size decreases. A narrow beam of monochromatic light, emitted from a He–Ne laser (λ = 633 nm), is transmitted through the suspension, and the diffracted light is concentrated on the detector. This procedure is grounded in the principles of scattered light energy [92,111].
In a study conducted by Saari and colleagues [141], specifically focusing on curcumin nanoemulsion formulation, the particle size was measured at 19.92 nm, with a corresponding PDI value of 0.17. This measurement is of paramount importance, ensuring that the size of the formulated preparation falls within the nanoscale range [141]. Another investigation, carried out by Inal and colleagues [146], involved curcumin nanoemulsions. Particle size analysis comparing curcumin-containing and curcumin-free nanoemulsions showed variations between the two, yet both remained within the particle size distribution range typical of curcumin nanoemulsions, namely <50 nm [48,146].
The PDI serves as a vital metric for assessing the size dispersion of oil droplets within nanoemulsions. Monodisperses is indicated when the PDI falls within the range of 0.1–0.7, denoting a narrow particle size distribution. The PDI value functions as a parameter for evaluating the uniformity or homogeneity of nanoparticles. Smaller PDI values correspond to greater particle homogeneity [142,162]. A PDI value approaching 0 signifies a high degree of particle size homogeneity, with values below 0.3 indicating monodispersity. PDI values ranging from 0.3 to 0.7 reflect polydispersity
In a study conducted by Barreto and colleagues [142], eight nanoemulsion formulations were developed, resulting in particle size distribution test measurements ranging from 83.57 nm to 155.80 nm, with PDI values ranging from 0.09 to 0.23. These findings confirm that the nanoemulsion formulations adhere to the specified criteria and align with typical nanoemulsion characteristics. It's important to highlight that this examination not only verifies particle sizes are within the nano range but also evaluates the appropriate distribution of particle sizes. This assessment is critical to prevent issues such as aggregation, sedimentation, and other factors that may jeopardize the stability of the formulations [98,142,163].
6.3. Zeta potential
Zeta potential values are indicative of the surface charge of nanoparticles in liquid formulations and are vital for evaluating their stability. This measurement relies on assessing the surface tension of nanoparticles in liquid preparations, offering valuable insights into the stability of the liquid formulation [164,165].
In a study conducted by Tran and colleagues [98] investigating nanoemulsions and the impact of zeta potential values on stability, it was observed that all nanoemulsion preparations exhibited negative (−) zeta potential values. Notably, the most stable nanoparticles displayed zeta potential values ranging from 25 mV to −25 mV. The assessment of zeta potential for a sample was performed using the Zetasizer-Nano Malvern Instrument, which monitored the direction and velocity of droplet movement in response to an applied electrical field after a diluted sample was introduced into the instrument chamber [58,98,166].
6.4. Surface morphology
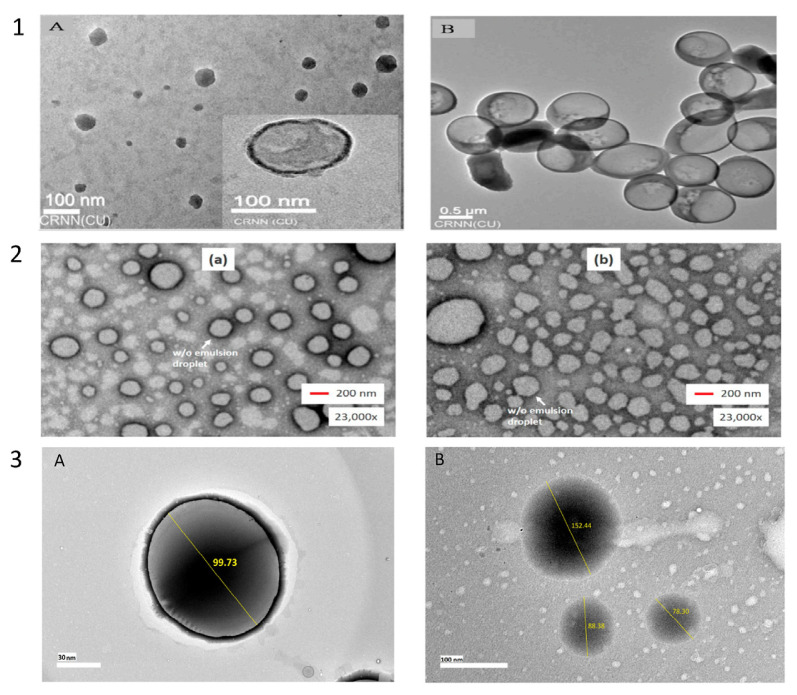
Harimurti and colleagues [117] conducted an investigation employing Transmission Electron Microscopy (TEM) for morphology testing, revealing that nanoscale dimensions could be determined by observing the shape and morphology of particles at a magnification of 200 nm. The TEM results allowed for a more detailed examination of the shape and size of the nanoemulsion preparations. The impact of oil addition to the nanoemulsion preparation was evident as a distinct dark outline around the globules. Additionally, TEM data provided valuable insights into particle size distribution [55,117,140].
Das and colleagues [167] (Fig. 6. (1A & 1B)) observed spherical droplets of the selected rice bran oil nanoemulsion, evenly distributed across the entire field, as revealed by TEM pictures. The average droplet diameter obtained from TEM analysis can be compared to the hydrodynamic droplet diameters obtained from dynamic light scattering (DLS) analysis. Typically, the hydrodynamic diameters of nanoemulsions are slightly larger than the sizes of dry droplets. Additionally, TEM allowed for the observation of aggregation of larger droplets in the conventional emulsion. In another study by Harimurti and colleagues [117] (Fig. 6. (2A & 2B)), TEM analysis complemented particle size and distribution assessments performed using the Zetasizer, providing a comprehensive characterization of the nanoparticles [105, 138]. Fig. 6 (3A & 3B) displays TEM images of the optimized nanoemulsion incorporating KMO. The TEM findings revealed that the optimized nanoemulsion containing KMO exhibited a polydisperse system, with droplet sizes ranging from 70 to 160 nm. The droplets appeared spherical, with no observable aggregation within the system [168].
Fig. 6.
(1A & 1B) Morphological examination of Rice bran oil-in-water nanoemulsions NE2 (MPI: Tween 20 – 1:1) and the corresponding conventional emulsion. Nanoemulsion analysis conducted using A-TEM, while traditional emulsion analysis performed using B-TEM [167]. (2A & 2B) TEM images depicting Nanoemulsions Containing Temulawak (Curcuma xanthorriza Roxb) and Red Dragon Fruit (Hylocereus polyrhizus) [117]. (3A & 3B) TEM images of kojic monooleate (KMO) nanoemulsion [168].
6.5. Stability study
The stability assessment performed by Paulo et al. [103] underscores the significance of evaluating nanoemulsion formulations, particularly due to the presence of oil content, which can render nanoemulsions vulnerable to instability. Many prior studies have used stability testing protocols to evaluate various particle properties, including size, PDI, zeta potential, pH, and viscosity, under different conditions and storage periods. This comprehensive assessment ensures that nanoemulsions demonstrate extended durability and consistent stability over time. Paulo et al. note that stability testing is essential for assessing the efficacy of cosmetics throughout their shelf-life.
The study noted an increase in particle size from 230 nm initially to 253 nm after 28 days, along with a rise in PDI value from 0.29 to 0.34 on the first day, although statistically insignificant. The observed change indicates potential particle aggregation and repulsion, impacting both particle size and PDI values [55,57,150].
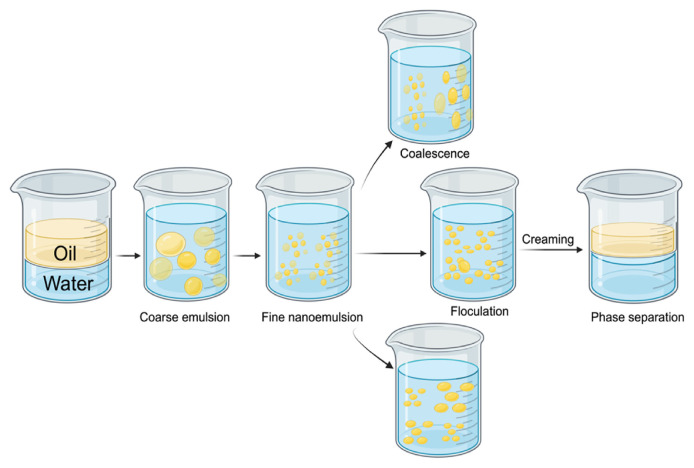
Nanoemulsions, despite their promising properties and applications, can have issues related to instability. Various factors, including formulation components and external conditions, influence nanoemulsion stability. External conditions refer to environmental factors outside of the nanoemulsion formulation that can affect its stability. These conditions may include temperature fluctuations, exposure to light or oxygen, pH changes, mechanical agitation, and interactions with other substances present in the surrounding environment [169]. Temperature variations can lead to phase separation or changes in viscosity, while exposure to light or oxygen can cause degradation of sensitive ingredients or oxidation of lipid components [170,171]. pH changes can impact the stability of the emulsion by affecting the charge of the droplets and the interactions between surfactant molecules. Mechanical agitation, such as mixing or shaking, can disrupt the emulsion structure and lead to coalescence or droplet size changes. Interactions with other substances, such as salts or additives, can also influence the stability of the nanoemulsion. Overall, external conditions play a critical role in determining the stability and performance of nanoemulsions in various applications [157,172]. One common issue that compromises the stability of nanoemulsion is coalescence, where smaller droplets combine into larger ones [173]. Additional stability concerns include flocculation, the aggregation of droplets into loose clusters, and creaming or sedimentation, where droplets migrate to the top or bottom of the formulation. Ostwald ripening, a process where larger droplets grow at the expense of smaller ones, can also reduce stability. Chemical instability, such as oxidation or hydrolysis, temperature fluctuations, and pH variations, exacerbate instability risks (Fig. 7) [82,103,110].
Fig. 7.
Process of nanoemulsion preparation and various forms of stability changes [195].
To address and mitigate instability issues, formulation adjustments, incorporation of stabilizing agents, and optimization of manufacturing procedures are common practices. Stabilizers, including surfactants and co-surfactants, play a crucial role in preserving nanoemulsion stability by preventing droplet coalescence and forming a protective barrier [174]. Furthermore, the stability of nanoemulsions over time depends on factors such as storage conditions, packaging, and the careful selection of ingredients in the formulation [175].
In the formulation of nanoemulsions, stabilizers such as surfactants and co-surfactants are pivotal in maintaining droplet stability and preventing coalescence. Surfactants like Polysorbate 80, an example of a nonionic surfactant, function by reducing interfacial tension between the oil and water phases, thereby facilitating the formation and stabilization of smaller droplets. This is achieved through the formation of a protective monolayer around the droplets, which prevents coalescence via steric hindrance and reduced surface tension. Anionic surfactants, such as Sodium Lauryl Sulfate (SLS), impart a negative charge to the droplet surfaces, creating electrostatic repulsion that inhibits droplet aggregation [168]. Conversely, cationic surfactants like Cetyltrimethylammonium Bromide (CTAB) provide a positive charge, similarly contributing to stability through electrostatic repulsion. Co-surfactants, including amphoteric surfactants like Cocamidopropyl Betaine and nonionic cosurfactants like Polyglyceryl-10 Oleate, enhance the emulsification process by further reducing interfacial tension and providing additional stabilization [157,172]. The combined effect of these surfactants and co-surfactants ensures the formation of a stable nanoemulsion through mechanisms of reduced interfacial tension, protective barrier formation, electrostatic repulsion, and steric stabilization. This multifaceted approach is crucial for maintaining the uniformity, efficacy, and shelf-life of nanoemulsion formulations in various applications [82,103,110].
6.6. In vitro release study
In their investigation on phylanthus niruri nanoemulsion, Pathania and colleagues [176] conducted an in vitro drug release study comparing the blank nanoemulsion (control) without extract with the phylanthus niruri-based nanoemulsion in phosphate buffer. Their findings indicate that the blank nanoemulsion, owing to its lipophilic nature and low water solubility, exhibited the highest in vitro drug release of 38.22 ± 1.74% within 12 h (Fig. 8A). This phenomenon may be attributed to the small droplet size, providing a larger surface area for drug release. Consequently, the nanoemulsion demonstrated a significantly higher release profile (P < 0.05) compared to the control nanoemulsion release, highlighting the rapid release of drugs from unmodified oil-in-water (o/w) emulsions [73,74,176].
Fig. 8.
In vitro drug release study of control and phylanthus niruri-based nanoemulsion (A). Skin penetration investigation of curcumin-loaded test formulations (S75-jojo-curc, S75-mtg-curc, and S75-sun-curc) using porcine ear skin (B). Values represent means of n = 4 ± standard deviation (SD). Statistically significant differences are denoted by asterisks (*p < 0.05, ***p < 0.001), determined by one-way ANOVA followed by post-hoc Tukey test, with significance set at p < 0.05 [176,177,196].
Vater and team [177] investigated the in vitro release study, comparing three optimized formulations of curcumin nanoemulsion. The curcumin nanoemulsion formulation, specifically s75-mtgcurc, exhibited the highest penetration depth (%) compared to the other two optimized formulas. It was observed that as the particle size of the curcumin nanoemulsion decreased, the resulting percentage of penetration increased (Fig. 8B). This underscores the relationship between particle size reduction in curcumin nanoemulsion and the enhanced percentage of penetration, as highlighted in the study.
The nanoemulsion is subjected to a carefully selected release medium, mirroring physiological conditions or a specific target environment. This medium serves as a representative milieu to illuminate how the nanoemulsion behaves in contexts relevant to its intended application. Sampling at defined intervals allows for the measurement of the concentration of the released substance. Analytical techniques such as spectrophotometry or chromatography are deployed for precise quantification [119,178]. After the completion of data collection, an in-depth analysis is initiated to construct release profiles, unveiling the rate and extent of substance release over time. Parameters such as initial burst release, sustained release, and total release undergo meticulous examination. Statistical analyses are employed to determine the significance of observed differences in release profiles or behaviors, especially when comparing various formulations or conditions [92,179–181].
This in vitro release study serves as a pivotal step in understanding the performance, stability, and potential applications of nanoemulsions. In drug delivery systems, where controlled release is of paramount importance, these investigations offer crucial insights for optimizing formulations and predicting the behavior of nanoemulsions in vivo. By shedding light on the nuanced dynamics of substance release, this study aids in fine-tuning nanoemulsion formulations to meet specific requirements and enhance their efficacy in targeted applications [23,92].
7. Nanoemulsion for herbal cosmetic application
7.1. Nanoemulsion preparation as a delivery system for cosmetics
Barreto et al. [142] conducted a comprehensive assessment of A. sisalana nanoemulsion employed as a moisturizing and anti-aging cosmetic formulation. The evaluation included anti-wrinkle, spot reduction, antioxidant, and skin moisture tests, showing potentially faster effects compared to marker formulations [23,139]. Iskandar and colleagues [182] discussed the potential antioxidant activity of Rhodiola rosea nanoemulsion, indicating a positive correlation between extract concentration and antioxidant activity, highlighting its efficacy as an antioxidant by quenching free redicals [99,182].
Herbal nanoemulsions and normal nanoemulsions, while similar in their basic formulation and structure, differ primarily in the type of active ingredients they incorporate. Herbal nanoemulsions are specifically designed to include extracts from herbal plants as their active components. These extracts are derived from natural sources and contain a variety of beneficial compounds, such as antioxidants, anti-inflammatory agents, and antimicrobial substances. The use of herbal extracts not only enhances the therapeutic efficacy of the nanoemulsions but also aligns with the growing consumer preference for natural and organic skincare products [82,103,110].
On the other hand, normal nanoemulsions typically contain synthetic or non-herbal active ingredients. These could be various chemical compounds designed to deliver specific cosmetic benefits, such as moisturization, anti-aging effects, or UV protection. While these ingredients can be highly effective, they may not offer the additional benefits associated with natural plant extracts, such as a reduced risk of irritation and enhanced biocompatibility [142].
The primary distinction between herbal and normal nanoemulsions lies in the source and type of their active ingredients. Herbal nanoemulsions utilize plant-derived extracts, which often provide multifaceted benefits, including antioxidant, anti-inflammatory, and antimicrobial properties. In contrast, normal nanoemulsions rely on synthetic or non-herbal active ingredients, which can be effective for targeted benefits but may not offer the same range of multifaceted properties [92,179–181].
In terms of safety, herbal nanoemulsions are generally considered safer with a reduced risk of irritation, owing to the natural origin of their active ingredients. Normal nanoemulsions, however, may have a potential for irritation depending on the specific synthetic ingredients used. The efficacy of herbal nanoemulsions is often enhanced by the presence of natural compounds that offer multiple benefits, whereas normal nanoemulsions are typically effective for specific, targeted cosmetic benefits but may lack the broader range of advantages found in herbal formulations.
Additionally, herbal nanoemulsions tend to have high antioxidant properties due to the natural antioxidants present in plant extracts, while the antioxidant properties of normal nanoemulsions can vary depending on the synthetic ingredients included. Herbal nanoemulsions also naturally incorporate anti-inflammatory agents, whereas normal nanoemulsions typically require the addition of specific anti-inflammatory additives [117].
From a consumer preference perspective, herbal nanoemulsions are often favored for their alignment with natural and organic product trends, while normal nanoemulsions are preferred for their ability to deliver specific, targeted cosmetic benefits. By clearly defining and differentiating these two types of nanoemulsions, we can highlight the unique advantages that herbal nanoemulsions bring to cosmetic formulations, particularly in terms of safety, efficacy, and consumer appeal [176,182].
Insights from existing literature [55–57,103, 116,117,139,183], emphasize the high antioxidant activity of plant-derived compounds in nanoemulsion cosmetics, particularly in anti-aging formulations. Nanoemulsion cosmetics enriched with potent antioxidants show promise for enhancing anti-aging efficacy compared to conventional preparations [94,108].
7.2. Antioxidants in herbal nanoemulsion cosmetic
In herbal cosmetics, plant extracts with antioxidant properties are important for promoting skin health and combating oxidative stress. Green tea extract, derived from Camellia sinensis, is notable for its rich polyphenol content, providing potent antioxidant protection to neutralize free radicals and alleviate inflammation [184]. Phyllanthus niruri, containing flavonoids and terpenoids, contributes antioxidant benefits that aid in skin protection and anti-aging effects. Vellozia squamata extract, abundant in proanthocyanidins, exhibits strong antioxidant activity, promoting collagen formation and improving skin elasticity [176,182].
Mauritia flexuosa extract, derived from M. flexuosa plant, offers antioxidant and anti-inflammatory advantages for herbal skincare formulations [145]. Turmeric extract, containing curcumin as its active component, demonstrates potent antioxidant and anti-inflammatory properties, enhancing skin health. Rosemary extract, with antioxidants such as rosmarinic acid and carnosic acid, provides free radical-scavenging capabilities and protective effects to the skin [141,185]. Pomegranate extract, rich in polyphenols and anthocyanins, act as a potent source of antioxidants, promoting skin regeneration and protection. Incorporating these plant extracts into herbal cosmetic formulations embraces a natural and holistic approach to skincare, delivering antioxidants that shield the skin from environmental damage and promote a healthy, radiant complexion [186–188].
7.3. Advantages and challenges of herbal nanocosmetics
Herbal nanocosmetics represent a compelling fusion of natural plant-based ingredients with advanced nanotechnology, offering a range of potential advantages. By incorporating herbal extracts, these formulations leverage the inherent benefits of botanical compounds, including vitamins, antioxidants, and other bioactive molecules [189]. Notably, they hold promise for enhanced penetration and targeted delivery of these herbal constituents, facilitated by the nano-sized particles. This improved delivery mechanism may contribute to heightened efficacy in addressing various skincare concerns. Additionally, the appeal of natural ingredients aligns with consumer preferences for cleaner and more sustainable beauty products, potentially reducing the environmental impact associated with certain synthetic alternatives (Fig. 9) [95,190].
Fig. 9.
Schematic representations of: The Advantages of Herbal Nanoemulsion Formulation. (A) Water-in-Oil and Oil-in-Water Nanoemulsion structures and (B).
Herbal nanoemulsion for cosmetics offer notable advantages, including enhanced delivery of active ingredients, improved formulation stability, and increased skin penetration. Nanoemulsion technology facilitates the encapsulation of natural extracts into nano-sized droplets, which can significantly improve the efficacy and absorption of cosmetic products. However, the integration of nanotechnology into cosmetic formulations also presents distinct challenges, particularly concerning the safety assessment of these products [55–57,103,116,117].
The safety evaluation of herbal nanoemulsion for cosmetics is crucial due to the unique physicochemical properties of nanoparticles, which can alter their biological interactions compared to their bulk forms. Nano-sized particles may penetrate deeper into the skin, raising concerns about potential systemic exposure and accumulation in tissues. Comprehensive safety assessments are necessary to evaluate the potential for toxicity, irritation, or allergic reactions, and to ensure that nanoparticles do not induce adverse long-term effects. Additionally, regulatory compliance demands rigorous safety testing to meet the standards set by authorities such as the FDA (Food and Drug Administration) or EMA (European Medicines Agency). Ensuring consumer safety requires thorough investigation into both immediate and chronic effects of these novel formulations. By addressing these safety concerns, researchers and manufacturers can optimize the benefits of herbal nanocosmetics while safeguarding public health [95,190].
However, the journey of herbal cosmetics nanoemulsion is not without its challenges. Ensuring consistent quality and standardization of herbal extracts poses a significant hurdle due to variations in plant sources, growing conditions, and extraction methods. Stability issues, such as the potential for particle aggregation or degradation of active ingredients, need to be carefully addressed to maintain the efficacy of these formulations over time. Furthermore, manufacturers must navigate the changing regulatory landscape for nanocosmetics and address consumer concerns through education as critical aspects [191–193]. The potential for higher production costs associated with advanced technologies and the need for affordability further add to the complex landscape of herbal cosmetics nanoemulsion. Striking a balance between harnessing the natural benefits of herbal extracts and overcoming these challenges is essential for the successful development and widespread acceptance of these innovative cosmetic products [190,194].
8. Conclusion
The review concludes that nanoemulsion preparations show great potential as a drug delivery method, particularly for direct-to-skin applications, especially in the field of cosmetics. This is attributed to the distinct properties of nanoemulsions, including their excellent stability, distinctive characteristics, and ability to easily penetrate and remain in the skin for extended periods. Consequently, nanoemulsions enhance the efficacy of the applied preparations. Furthermore, the utilization of natural ingredients is noteworthy due to their multifunctional properties in cosmetics. These ingredients possess various activities, such as antioxidant effects to neutralize free radicals, anti-aging and anti-wrinkle properties, moisturizing capabilities, sun protection, and more. Additionally, progress has been made in developing diverse manufacturing methods and formulations. The primary allure for researchers in developing nanoemulsion formulations from natural substances, particularly for herbal cosmetic treatments, lies in the elevated level of safety.
Supplementary Information
Footnotes
Conflict of interest: The authors have declared no conflict of interest.
References
- 1. Thompson BM, Scott BI, Boiani JA. Understanding the food and drug administration's jurisdiction over laboratory-developed tests and divisions between food, drug, and cosmetic act–regulated and clinical laboratory improvement amendments of 1988–regulated activities. Clin Lab Med. 2016;36:575–85. doi: 10.1016/j.cll.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Kapp RW. Food, drug, and cosmetic act, US. In: Wexler P, editor. Encyclopedia of toxicology. 4th ed. Oxford: Academic Press; 2024. pp. 801–12. [Google Scholar]
- 3.Van Tran V, Lee Y-C. 11 - nanoemulsion: Application in body-care products. In: Mohd Setapar SH, Ahmad A, Jawaid M, editors. Nanotechnology for the preparation of cosmetics using plant-based extracts. Elsevier; 2022. pp. 283–300. [Google Scholar]
- 4. Implications for pediatric dermatologists: FDA-reported cosmetic adverse events and baby products. J Am Acad Dermatol. 2018;79:AB112. [Google Scholar]
- 5. FDA granted more powers to tighten up safety of cosmetics throughout the United States via MoCRA. Focus Pigments. 2023;2023:6. [Google Scholar]
- 6. Raszewska-Famielec M, Flieger J. Nanoparticles for topical application in the treatment of skin dysfunctions-an overview of dermo-cosmetic and dermatological products. Int J Mol Sci. 2022;23:15980. doi: 10.3390/ijms232415980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rente D, Cvjetko Bubalo M, Panić M, Paiva A, Caprin B, Radojčić Redovniković I, et al. Review of deep eutectic systems from laboratory to industry, taking the application in the cosmetics industry as an example. J Clean Prod. 2022;380:135147. [Google Scholar]
- 8. Prajaputra V, Isnaini N, Maryam S, Ernawati E, Deliana F, Haridhi HA, et al. Exploring marine collagen: sustainable sourcing, extraction methods, and cosmetic applications. S Afr J Chem Eng. 2024;47:197–211. [Google Scholar]
- 9. Tang Z, Wang Y, Xie X, Pei Y, Zhao L, Zhao P. Evaluation of biosafety and skin-care efficacy for “Four white” medicinal materials used in cosmetics. J Holis Integr Pharm. 2022;3:153–62. [Google Scholar]
- 10. Alshehrei FM. Microbiological quality assessment of skin and body care cosmetics by using challenge test. Saudi J Biol Sci. 2024;31:103965. doi: 10.1016/j.sjbs.2024.103965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gupta V, Mohapatra S, Mishra H, Farooq U, Kumar K, Ansari MJ, et al. Nanotechnology in cosmetics and cosmeceuticals-A review of latest advancements. Gels. 2022:8. doi: 10.3390/gels8030173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Santos AC, Morais F, Simões A, Pereira I, Sequeira JAD, Pereira-Silva M, et al. Nanotechnology for the development of new cosmetic formulations. Expet Opin Drug Deliv. 2019;16:313–30. doi: 10.1080/17425247.2019.1585426. [DOI] [PubMed] [Google Scholar]
- 13. Suphasomboon T, Vassanadumrongdee S. Multi-stakeholder perspectives on sustainability transitions in the cosmetic industry. Sustain Prod Consum. 2023;38:225–40. [Google Scholar]
- 14. Rocca R, Acerbi F, Fumagalli L, Taisch M. Sustainability paradigm in the cosmetics industry: state of the art. Clean Waste Syst. 2022;3:100057. [Google Scholar]
- 15. Craddock N, Spotswood F, Rumsey N, Diedrichs PC. “We should educate the public that cosmetic procedures are as safe as normal medicine”: understanding corporate social responsibility from the perspective of the cosmetic procedures industry”. Body Image. 2022;43:75–86. doi: 10.1016/j.bodyim.2022.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Adeel S, Abrar S, Ozomay M, Fazalur R, Hussaan M, Batool F. Chapter 15 - evolving role of plant pigments in the cosmetic industry. In: Ul Islam S, editor. Renewable dyes and pigments. Elsevier; 2024. pp. 307–19. [Google Scholar]
- 17.Kian LK, Jawaid M. 1 - introduction of nanotechnology in cosmetic formulation. In: Mohd Setapar SH, Ahmad A, Jawaid M, editors. Nanotechnology for the preparation of cosmetics using plant-based extracts. Elsevier; 2022. pp. 1–12. [Google Scholar]
- 18. Zhang L, Zhang F, Fan Z, Liu B, Liu C, Meng X. DHA and EPA nanoemulsions prepared by the low-energy emulsification method: process factors influencing droplet size and physicochemical stability. Food Res Int. 2019;121:359–66. doi: 10.1016/j.foodres.2019.03.059. [DOI] [PubMed] [Google Scholar]
- 19. Basudkar V, Gharat SA, Momin MM, Shringarpure M. A review of anti-aging nanoformulations: recent developments in excipients for nanocosmeceuticals and regulatory guidelines. Crit Rev Ther Drug Carrier Syst. 2022;39:45–97. doi: 10.1615/CritRevTherDrugCarrierSyst.2021039544. [DOI] [PubMed] [Google Scholar]
- 20. Akbay HEG, Akarsu C, Isik Z, Belibagli P, Dizge N. Investigation of degradation potential of polyethylene micro-plastics in anaerobic digestion process using cosmetics industry wastewater. Biochem Eng J. 2022;187:108619. [Google Scholar]
- 21. Liu M, Yang C, Liu E, Zhang F, Meng X, Liu B. Effect of environmental stresses on physicochemical properties of ALA oil-in-water nanoemulsion system prepared by emulsion phase inversion. Food Chem. 2021;343:128475. doi: 10.1016/j.foodchem.2020.128475. [DOI] [PubMed] [Google Scholar]
- 22. Begum F, Chutia H, Bora M, Deb P, Mahanta CL. Characterization of coconut milk waste nanocellulose based curcumin-enriched Pickering nanoemulsion and its application in a blended beverage of defatted coconut milk and pineapple juice. Int J Biol Macromol. 2024:129305. doi: 10.1016/j.ijbiomac.2024.129305. [DOI] [PubMed] [Google Scholar]
- 23.Chuo SC, Mohd Setapar SH. 15 - application of nanoemulsion in cosmetics. In: Mohd Setapar SH, Ahmad A, Jawaid M, editors. Nanotechnology for the preparation of cosmetics using plant-based extracts. Elsevier; 2022. pp. 355–71. [Google Scholar]
- 24. Pandey VK, Srivastava S, Ashish, Dash KK, Singh R, Dar AH, et al. Bioactive properties of clove (Syzygium aromaticum) essential oil nanoemulsion: a comprehensive review. Heliyon. 2024;10:e22437. doi: 10.1016/j.heliyon.2023.e22437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saikia AP, Ryakala VK, Sharma P, Goswami P, Bora U. Ethnobotany of medicinal plants used by Assamese people for various skin ailments and cosmetics. J Ethnopharmacol. 2006;106:149–57. doi: 10.1016/j.jep.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 26. Arora H, Naaz F, Sharma A, Dubey S, Sharma S, Rajauria G. Thyme-licorice nanoemulsion for anthracnose management in Capsicum annuum L. and life cycle assessment of its production. Biocatal Agric Biotechnol. 2024;56:103029. [Google Scholar]
- 27. Xie M, Jiang Z, Lin X, Wei X. Application of plant extracts cosmetics in the field of anti-aging. J Dermatol Sci Cosmet Technol. 2024:100014. [Google Scholar]
- 28. Mugale MN, Dev K, More BS, Mishra VS, Washimkar KR, Singh K, et al. A comprehensive review on preclinical safety and toxicity of medicinal plants. Clin Complement Med Pharmacol. 2024;4:100129. [Google Scholar]
- 29.Sohail M, Rabbi F, Younas A, Hussain A, Yu B, Li Y, et al. Chapter 6 - herbal bioactive–based nano drug delivery systems. In: Bakshi IS, Bala R, Madaan R, Sindhu RK, editors. Herbal bioactive-based drug delivery systems. Academic Press; 2022. pp. 169–93. [Google Scholar]
- 30. Arundina I, Diyatri I, Juliastuti WS, Budhy TI, Surboyo MDC, Iskandar B, et al. Osteoblast viability of liquid smoke rice hull and nanoparticles form as periodontitis treatment. Eur J Dermatol. 2022;17:450–5. doi: 10.1055/s-0042-1745772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kolling C, Ribeiro JLD, de Medeiros JF. Performance of the cosmetics industry from the perspective of corporate social responsibility and design for sustainability. Sustain Prod Consum. 2022;30:171–85. [Google Scholar]
- 32. Ren G, Sun Z, Wang Z, Zheng X, Xu Z, Sun D. Nanoemulsion formation by the phase inversion temperature method using polyoxypropylene surfactants. J Colloid Interface Sci. 2019;540:177–84. doi: 10.1016/j.jcis.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 33.Ganjali M, Ganjali M, Aljabali AAA, Barhoum A. Chapter 15 - drug delivery systems based on nano-herbal medicine. In: Barhoum A, Jeevanandam J, Danquah MK, editors. Bionanotechnology : emerging applications of bio-nanomaterials. Elsevier; 2022. pp. 491–530. [Google Scholar]
- 34. Reolon JB, Sari MHM, Marchiori C, Dallabrida KG, Santos JARd, Almeida IdFRd, et al. Herbal drugs-loaded soft nanoparticles for treating skin disorders: where do we stand? Ind Crop Prod. 2023;206:117602. [Google Scholar]
- 35.Attama AA, Agbo CP, Onokala OB, Kenechukwu FC, Ugwueze ME, Mbah CC, et al. Chapter 6 - applications of nanoemulsions as drug delivery vehicle for phytoconstituents. In: Thomas S, Oyedeji AO, Oluwafemi OS, Jaquilin PjR, editors. Nanotechnology in herbal medicine. Woodhead Publishing; 2023. pp. 119–94. [Google Scholar]
- 36. Wang D, Bao A, Yuan Y, Wang Y, Li L, Song G, et al. Whey protein isolate-stabilized gardenia fruit oil nanoemulsions: ultrasonic preparation, characterization and applications in nutraceuticals delivery. Ind Crop Prod. 2024;212:118345. [Google Scholar]
- 37. Hassanabadi N, Mahdavi Meymand Z, Ashrafzadeh A, Sharififar F. Antioxidant and cytotoxicity activity of a nanoemulsion from Satureja kermanica (Lamiaceae) Annales Pharmaceutiques Françaises. 2024 doi: 10.1016/j.pharma.2024.01.005. [DOI] [PubMed] [Google Scholar]
- 38.Okole B, Pillai SK, Ndzotoyi P. Phasha 8- use of herbal extract-based nanoemulsions for hair care application. In: Mohd Setapar SH, Ahmad A, Jawaid M, editors. Nanotechnology for the preparation of cosmetics using plant-based extracts. Elsevier; 2022. pp. 203–33. [Google Scholar]
- 39. Wang X, Lu J, Cao Y, Liang Y, Dai X, Liu K, et al. Does binary blend emulsifier enhance emulsifier performance? Preparation of baicalin nanoemulsions using tea saponins and glycyrrhizic acid as binary blend emulsifier. J Drug Deliv Sci Technol. 2023;84:104398. [Google Scholar]
- 40. Panwar A, Kumar V, Dhiman A, Thakur P, Sharma V, Sharma A, et al. Nanoemulsion based edible coatings for quality retention of fruits and vegetables-decoding the basics and advancements in last decade. Environ Res. 2024;240:117450. doi: 10.1016/j.envres.2023.117450. [DOI] [PubMed] [Google Scholar]
- 41. Gumus ZP, Guler E, Demir B, Barlas FB, Yavuz M, Colpankan D, et al. Herbal infusions of black seed and wheat germ oil: their chemical profiles, in vitro bio-investigations and effective formulations as Phyto-Nanoemulsions. Colloids Surf B Biointerfaces. 2015;133:73–80. doi: 10.1016/j.colsurfb.2015.05.044. [DOI] [PubMed] [Google Scholar]
- 42. Tong K, Zhao C, Sun D. Formation of nanoemulsion with long chain oil by W/O microemulsion dilution method. Colloids Surf A Physicochem Eng Asp. 2016;497:101–8. [Google Scholar]
- 43. Safaya M, Rotliwala YC. Nanoemulsions: a review on low energy formulation methods, characterization, applications and optimization technique. Mater Today Proc. 2020;27:454–9. [Google Scholar]
- 44. Ganesan NG, Singh RD, Dwivedi D, Rangarajan V. Synergy evaluation between diverse biosurfactants toward the formulation of green oil-in-water nanoemulsions by ultrasonication method. J Clean Prod. 2023;400:136735. [Google Scholar]
- 45. Cizauskaite U, Bernatoniene J. Innovative natural ingredients-based multiple emulsions: the effect on human skin moisture, sebum content, pore size and pigmentation. Molecules. 2018;23 doi: 10.3390/molecules23061428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen YS, Liou HC, Chan CF. Tyrosinase inhibitory effect and antioxidative activities of fermented and ethanol extracts of Rhodiola rosea and Lonicera japonica. Sci World J. 2013;2013:612739. doi: 10.1155/2013/612739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jarzębski M, Fathordoobady F, Guo Y, Xu M, Singh A, Kitts DD, et al. Pea protein for hempseed oil nanoemulsion stabilization. Molecules. 2019;24:4288. doi: 10.3390/molecules24234288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Guzmán E, Fernández-Peña L, Rossi L, Bouvier M, Ortega F, Rubio RG. Nanoemulsions for the encapsulation of hydrophobic actives. Cosmetics. 2021;8:45. [Google Scholar]
- 49. Prakash V, Parida L. Characterization and rheological behavior of vitamin E nanoemulsions prepared by phase inversion composition technique. Result Eng. 2023;18:101175. [Google Scholar]
- 50. Jarzębski M, Siejak P, Smułek W, Fathordoobady F, Guo Y, Pawlicz J, et al. Plant extracts containing saponins affects the stability and biological activity of hempseed oil emulsion system. Molecules. 2020:25. doi: 10.3390/molecules25112696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Xue F, Li X, Qin L, Liu X, Li C, Adhikari B. Anti-aging properties of phytoconstituents and phyto-nanoemulsions and their application in managing aging-related diseases. Adv Drug Deliv Rev. 2021;176:113886. doi: 10.1016/j.addr.2021.113886. [DOI] [PubMed] [Google Scholar]
- 52. Kongkaneramit L, Sitthithaworn W, Phattanaphakdee W, Sarisuta N. Physicochemical properties and stability of nanoemulsions containing Clinacanthus nutans extract for postherpetic neuralgia. J Drug Deliv Sci Technol. 2022;68:103116. [Google Scholar]
- 53. Iskandar B, Karsono SJ. Preparation of spray nanoemulsion and cream containing vitamin e as anti-aging product tested in vitro and in vivo method. Int J PharmaTech Res. 2016;9:307–8. [Google Scholar]
- 54. Sedaghat Doost A, Van Camp J, Dewettinck K, Van der Meeren P. Production of thymol nanoemulsions stabilized using Quillaja Saponin as a biosurfactant: antioxidant activity enhancement. Food Chem. 2019;293:134–43. doi: 10.1016/j.foodchem.2019.04.090. [DOI] [PubMed] [Google Scholar]
- 55. Li M, Bi D, Yao L, Yi J, Fang W, Wu Y, et al. Optimization of preparation conditions and in vitro sustained-release evaluation of a novel nanoemulsion encapsulating unsaturated guluronate oligosaccharide. Carbohydr Polym. 2021;264:118047. doi: 10.1016/j.carbpol.2021.118047. [DOI] [PubMed] [Google Scholar]
- 56. Mahdi WA, Hussain A, Bukhari SI, Alshehri S, Singh B, Ali N. Removal of clarithromycin from aqueous solution using water/triton X-100/ethanol/olive oil green nanoemulsion method. J Water Proc Eng. 2021;40:101973. [Google Scholar]
- 57. Ren G, Li B, Lu D, Di W, Ren L, Tian L, et al. Preparation of polyoxypropylene surfactant-based nanoemulsions using phase inversion composition method and their application in oil recovery. J Mol Liq. 2021;342:117469. [Google Scholar]
- 58. Wuttikul K, Sainakham M. In vitro bioactivities and preparation of nanoemulsion from coconut oil loaded Curcuma aromatica extracts for cosmeceutical delivery systems. Saudi J Biol Sci. 2022;29:103435. doi: 10.1016/j.sjbs.2022.103435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Luengo GS, Fameau AL, Léonforte F, Greaves AJ. Surface science of cosmetic substrates, cleansing actives and formulations. Adv Colloid Interface Sci. 2021;290:102383. doi: 10.1016/j.cis.2021.102383. [DOI] [PubMed] [Google Scholar]
- 60. Goyal A, Sharma A, Kaur J, Kumari S, Garg M, Sindhu RK, et al. Bioactive-based cosmeceuticals: an update on emerging trends. Molecules. 2022:27. doi: 10.3390/molecules27030828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Moghassemi S, Dadashzadeh A, Azevedo RB, Amorim CA. Nanoemulsion applications in photodynamic therapy. J Contr Release. 2022;351:164–73. doi: 10.1016/j.jconrel.2022.09.035. [DOI] [PubMed] [Google Scholar]
- 62. Rohilla S, Rohilla A, Narwal S, Dureja H, Bhagwat DP. Global trends of cosmeceutical in nanotechnology: a review. Pharm Nanotechnol. 2023;11:410–24. doi: 10.2174/2211738511666230508161611. [DOI] [PubMed] [Google Scholar]
- 63. Musazzi UM, Franzè S, Minghetti P, Casiraghi A. Emulsion versus nanoemulsion: how much is the formulative shift critical for a cosmetic product? Drug Deliv Transl Res. 2018;8:414–21. doi: 10.1007/s13346-017-0390-7. [DOI] [PubMed] [Google Scholar]
- 64. Mushtaq A, Mohd Wani S, Malik AR, Gull A, Ramniwas S, Ahmad Nayik G, et al. Recent insights into Nanoemulsions: their preparation, properties and applications. Food Chem X. 2023;18:100684. doi: 10.1016/j.fochx.2023.100684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wong R, Geyer S, Weninger W, Guimberteau JC, Wong JK. The dynamic anatomy and patterning of skin. Exp Dermatol. 2016;25:92–8. doi: 10.1111/exd.12832. [DOI] [PubMed] [Google Scholar]
- 66. Rittié L, Fisher GJ. Natural and sun-induced aging of human skin. Cold Spring Harb Perspect Med. 2015;5:a015370. doi: 10.1101/cshperspect.a015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hirao T. Chapter 40 - structure and function of skin from a cosmetic aspect. In: Sakamoto K, Lochhead RY, Maibach HI, Yamashita Y, editors. Cosmetic science and technology. Amsterdam: Elsevier; 2017. pp. 673–83. [Google Scholar]
- 68. Dutta S, Kumar SPJ, Banerjee R. A comprehensive review on astaxanthin sources, structure, biochemistry and applications in the cosmetic industry. Algal Res. 2023;74:103168. [Google Scholar]
- 69. Masjedi M, Montahaei T. An illustrated review on nonionic surfactant vesicles (niosomes) as an approach in modern drug delivery: fabrication, characterization, pharmaceutical, and cosmetic applications. J Drug Deliv Sci Technol. 2021;61:102234. [Google Scholar]
- 70.Knottenbelt DC, Patterson-Kane JC, Snalune KL. 14 - other epithelial neoplasms: cutaneous, mucocutaneous and ocular adnexal. In: Knottenbelt DC, Patterson-Kane JC, Snalune KL, editors. Clinical equine oncology. Elsevier; 2015. pp. 247–80. [Google Scholar]
- 71.Scott DW, Miller WH. Chapter 1 - structure and function of the skin. In: Scott DW, Miller WH, editors. Equine dermatology. 2nd ed. W.B. Saunders; Saint Louis: 2011. pp. 1–34. [Google Scholar]
- 72. Landau M, Landau SB. Hacking the international nomenclature of cosmetic ingredients list- how to read ingredients in cosmetic products and what is important for a dermatologist to know? Dermatol Clin. 2024;42:7–11. doi: 10.1016/j.det.2023.06.006. [DOI] [PubMed] [Google Scholar]
- 73. Burgeson RE, Christiano AM. The dermal-epidermal junction. Curr Opin Cell Biol. 1997;9:651–8. doi: 10.1016/s0955-0674(97)80118-4. [DOI] [PubMed] [Google Scholar]
- 74.Barbieri JS, Wanat K, Seykora J. Skin: basic structure and function. In: McManus LM, Mitchell RN, editors. Pathobiology of human disease. San Diego: Academic Press; 2014. pp. 1134–44. [Google Scholar]
- 75. Mohd Nordin UU, Ahmad N, Salim N, Tajuddin HA, Abu Bakar NF, Narasimhan AK. Performance of alkyl β-D-maltosides in molecular self-assembly and formation of oil-in-water nanoemulsions as drug delivery systems. Colloids Surf A Physicochem Eng Asp. 2023;674:131886. [Google Scholar]
- 76. Firmansyah F, Muhtadi WK, Indriani S, Ulhaq MD, Auliya SR, Iskandar B, et al. Iai special edition: development of novel curcumin nanoemulgel: Optimisation, characterisation, and ex vivo permeation. Pharm Educ. 2022;22:98–103. [Google Scholar]
- 77. Wang C, Cao M, Zhao J, Hu A, Liu X, Chen Z, et al. Epidermal and dermal cells from adult rat eccrine sweat gland-containing skin can reconstruct the three-dimensional structure of eccrine sweat glands. Acta Histochem. 2024;126:152120. doi: 10.1016/j.acthis.2023.152120. [DOI] [PubMed] [Google Scholar]
- 78. Nakazawa H, Imai T, Hatta I, Kato S. Low-flux electron diffraction study on body site dependence of stratum corneum structures in human skin. Biochim Biophys Acta Biomembr. 2022;1864:183933. doi: 10.1016/j.bbamem.2022.183933. [DOI] [PubMed] [Google Scholar]
- 79. Badhe Y, Sharma P, Gupta R, Rai B. Elucidating collective translocation of nanoparticles across the skin lipid matrix: a molecular dynamics study††Electronic supplementary information (ESI) available. See. Nanoscale Adv. 2023;5:1978–89. doi: 10.1039/d2na00241h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sungpud C, Panpipat W, Chaijan M, Sae Yoon A. Techno-biofunctionality of mangostin extract-loaded virgin coconut oil nanoemulsion and nanoemulgel. PLoS One. 2020;15:e0227979. doi: 10.1371/journal.pone.0227979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Abd E, Namjoshi S, Mohammed YH, Roberts MS, Grice JE. Synergistic skin penetration enhancer and nanoemulsion formulations promote the human epidermal permeation of caffeine and naproxen. J Pharmaceut Sci. 2016;105:212–20. doi: 10.1002/jps.24699. [DOI] [PubMed] [Google Scholar]
- 82. A N, Kovooru L, Behera AK, Kumar KPP, Srivastava P. A critical review of synthesis procedures, applications and future potential of nanoemulsions. Adv Colloid Interface Sci. 2021;287:102318. doi: 10.1016/j.cis.2020.102318. [DOI] [PubMed] [Google Scholar]
- 83. Rai VK, Mishra N, Yadav KS, Yadav NP. Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: formulation development, stability issues, basic considerations and applications. J Contr Release. 2018;270:203–25. doi: 10.1016/j.jconrel.2017.11.049. [DOI] [PubMed] [Google Scholar]
- 84. Roda Zitha Vilanculos J, Silva de Farias B, Inês Engelmann J, Silveira Ribeiro E, Diaz de Oliveira P, Roberto Sant'Anna Cadaval T, et al. Physicochemical evaluation of chitosan–xanthan gum nanoemulsions as polyunsaturated enriched lipid–carrier. J Mol Liq. 2023;386:122533. [Google Scholar]
- 85. Harwansh RK, Deshmukh R, Rahman MA. Nanoemulsion: promising nanocarrier system for delivery of herbal bioactives. J Drug Deliv Sci Technol. 2019;51:224–33. [Google Scholar]
- 86. Yan Z, Wang X, Zhao P, He Y, Meng X, Liu B. The effect of octenyl succinic anhydride-modified chitosan coating on DHA-loaded nanoemulsions: physichemical stability and in vitro digestibility. Food Chem. 2024;441:138289. doi: 10.1016/j.foodchem.2023.138289. [DOI] [PubMed] [Google Scholar]
- 87. Singh Y, Meher JG, Raval K, Khan FA, Chaurasia M, Jain NK, et al. Nanoemulsion: concepts, development and applications in drug delivery. J Contr Release. 2017;252:28–49. doi: 10.1016/j.jconrel.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 88. Yuan L, Pan M, Shi K, Hu D, Li Y, Chen Y, et al. Nanocarriers for promoting skin delivery of therapeutic agents. Appl Mater Today. 2022;27:101438. [Google Scholar]
- 89. Inmuangkham N, Sukjarernchaikul P, Thepwatee S, Iemsam-Arng J. Fatty acid profiles of olive oil and moringa oil and their influence on nanoemulsion formation via D-phase emulsification using bakuchiol as a model active compound. Ind Crop Prod. 2024;209:118052. [Google Scholar]
- 90. Pandey VK, Srivastava S, Singh R, Dar AH, Dash KK. Effects of clove essential oil (Caryophyllus aromaticus L.) nanoemulsion incorporated edible coating on shelf-life of fresh cut apple pieces. J Agric Food Res. 2023;14:100791. [Google Scholar]
- 91. Schreiner TB, Santamaria-Echart A, Colucci G, Plasencia P, Santos Costa P, Dias MM, et al. Saponin-based natural nanoemulsions as alpha-tocopherol delivery systems for dermal applications. J Mol Liq. 2023;391:123371. [Google Scholar]
- 92. Mushtaq A, Mohd Wani S, Malik AR, Gull A, Ramniwas S, Ahmad Nayik G, et al. Recent insights into Nanoemulsions: their preparation, properties and applications. Food Chem X. 2023;18:100684. doi: 10.1016/j.fochx.2023.100684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Masiero JF, Knirsch MC, Barreto T, Arantes GJ, Stephano MA, Ishida K, et al. Unveiling the optimal path: high-pressure homogenization and D-phase emulsification methods for borage oil nanoemulsion design. Ind Crop Prod. 2024;208:117853. [Google Scholar]
- 94. Le X-T, Tuan Le M, Manh Do V, Minh Bui Q, Tam Nguyen A, Cuong Luu X, et al. Fabrication of cajeput essential oil nanoemulsions by phase inversion temperature process. Mater Today Proc. 2022;59:1178–82. [Google Scholar]
- 95. Ahuja A, Bajpai M. Novel Arena of nanocosmetics: applications and their remarkable contribution in the management of dermal disorders, topical delivery, future trends and challenges. Curr Pharm Des. 2024;30:115–39. doi: 10.2174/0113816128288516231228101024. [DOI] [PubMed] [Google Scholar]
- 96. Silva RCSd, de Souza Arruda IR, Malafaia CB, de Moraes MM, Beck TS, Gomes da Camara CA, et al. Synthesis, characterization and antibiofilm/antimicrobial activity of nanoemulsions containing Tetragastris catuaba (Burseraceae) essential oil against disease-causing pathogens. J Drug Deliv Sci Technol. 2022;67:102795. [Google Scholar]
- 97. Kumar N, Verma A, Mandal A. Formation, characteristics and oil industry applications of nanoemulsions: a review. J Petrol Sci Eng. 2021;206:109042. [Google Scholar]
- 98. Van Tran V, Loi Nguyen T, Moon J-Y, Lee Y-C. Core-shell materials, lipid particles and nanoemulsions, for delivery of active anti-oxidants in cosmetics applications: challenges and development strategies. Chem Eng J. 2019;368:88–114. [Google Scholar]
- 99. Uchida VH, de Araújo Padilha CE, Rios NS, dos Santos ES. Enzymatic inhibition of α-amylase and encapsulation of bioactive compounds by nanoemulsion from pulp extract Terminalia catappa Linn fruit. Result Chem. 2023;5:100736. [Google Scholar]
- 100. Abboosh TS, Kassaba AC, Al-Dogmi AM, Safhi FA, Alshehri E, Alotaibi AM, et al. Identification, forensic evidences and effect of the most used lip cosmetics on the human STR profiling at kingdom of Saudi arabi. Forensic Sci Int. 2023:111684. doi: 10.1016/j.forsciint.2023.111684. [DOI] [PubMed] [Google Scholar]
- 101. de Meneses AC, Marques EBP, Leimann FV, Gonçalves OH, Ineu RP, de Araújo PHH, et al. Encapsulation of clove oil in nanostructured lipid carriers from natural waxes: preparation, characterization and in vitro evaluation of the cholinesterase enzymes. Colloids Surf A Physicochem Eng Asp. 2019;583:123879. [Google Scholar]
- 102. Cipolatti EP, Valério A, Nicoletti G, Theilacker E, Araújo PHH, Sayer C, et al. Immobilization of Candida Antarctica lipase B on PEGylated poly(urea-urethane) nanoparticles by step miniemulsion polymerization. J Mol Catal B Enzym. 2014;109:116–21. [Google Scholar]
- 103. Paulo LAdO, Fernandes RN, Simiqueli AA, Rocha F, Dias MMdS, Minim VPR, et al. Baru oil (Dipteryx alata vog.) applied in the formation of O/W nanoemulsions: a study of physical-chemical, rheological and interfacial properties. Food Res Int. 2023;170:112961. doi: 10.1016/j.foodres.2023.112961. [DOI] [PubMed] [Google Scholar]
- 104. Yang Y, Yan S, Yu B, Gao C, Wang J. Hydrophobically modified inulin based nanoemulsions for enhanced stability and transdermal delivery of retinyl propionate. Colloids Surf A Physicochem Eng Asp. 2022;653:129883. [Google Scholar]
- 105.Pandey V, Shukla R, Garg A, Kori ML, Rai G. Chapter 17 - nanoemulsion in cosmetic: from laboratory to market. In: Nanda A, Nanda S, Nguyen TA, Rajendran S, Slimani Y, editors. Nanocosmetics. Elsevier; 2020. pp. 327–47. [Google Scholar]
- 106.Sonneville-Aubrun O, Yukuyama MN, Pizzino A. Chapter 14 - application of nanoemulsions in cosmetics. In: Jafari SM, McClements DJ, editors. Nanoemulsions. Academic Press; 2018. pp. 435–75. [Google Scholar]
- 107. Okamoto T, Tomomasa S, Nakajima H. Preparation and thermal properties of fatty alcohol/surfactant/oil/water nanoemulsions and their cosmetic applications. J Oleo Sci. 2016;65:27–36. doi: 10.5650/jos.ess15183. [DOI] [PubMed] [Google Scholar]
- 108. Lima TS, Silva MFS, Nunes XP, Colombo AV, Oliveira HP, Goto PL, et al. Cineole-containing nanoemulsion: development, stability, and antibacterial activity. Chem Phys Lipids. 2021;239:105113. doi: 10.1016/j.chemphyslip.2021.105113. [DOI] [PubMed] [Google Scholar]
- 109. Ribeiro CM, Souza M, Pelegrini BL, Palacios RS, Lima SM, Sato F, et al. Ex vivo UV–vis and FTIR photoacoustic spectroscopy of natural nanoemulsions from cellulose nanocrystals and saponins topically applied into the skin: diffusion rates and physicochemical evaluation. J Photochem Photobiol B Biol. 2022;236:112587. doi: 10.1016/j.jphotobiol.2022.112587. [DOI] [PubMed] [Google Scholar]
- 110. Kour P, Ahmad Dar A. Effect of interfacially-engineered nanoemulsions of linoleic acid stabilized by mixed surfactant systems and curcumin on their physicochemical properties, induction times, and peroxidation stability. J Mol Liq. 2023;384:122243. [Google Scholar]
- 111. Kim GW, Yun S, Jang J, Lee JB, Kim SY. Enhanced stability, formulations, and rheological properties of nanoemulsions produced with microfludization for eco-friendly process. J Colloid Interface Sci. 2023;646:311–9. doi: 10.1016/j.jcis.2023.05.005. [DOI] [PubMed] [Google Scholar]
- 112. Ganesan NG, Singh RD, Rangarajan V. Optimization of processing parameters for the improved production of surfactin from B. Subtilis using a statistical approach and demonstration of its imminent potential in cosmetic applications. Mater Today Proc. 2023;76:70–80. [Google Scholar]
- 113. Ceresa C, Fracchia L, Sansotera AC, De Rienzo MAD, Banat IM. Harnessing the potential of biosurfactants for biomedical and pharmaceutical applications. Pharmaceutics. 2023:15. doi: 10.3390/pharmaceutics15082156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ashaolu TJ. Nanoemulsions for health, food, and cosmetics: a review. Environ Chem Lett. 2021;19:3381–95. doi: 10.1007/s10311-021-01216-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Argudo PG, Spitzer L, Ibarboure E, Jerome F, Cramail H, Lecommandoux S. Mannose-based surfactant as biofunctional nanoemulsion stabilizer. Colloids Surf B Biointerfaces. 2022;220:112877. doi: 10.1016/j.colsurfb.2022.112877. [DOI] [PubMed] [Google Scholar]
- 116. Guzmán C, Rojas MA, Aragón M. Optimization of ultrasound-assisted emulsification of emollient nanoemulsions of seed oil of Passiflora edulis var. edulis. Cosmetics. 2021;8:1. [Google Scholar]
- 117. Harimurti N, Nasikin M, Mulia K. Water-in-Oil-in-Water nanoemulsions containing Temulawak (Curcuma xanthorriza Roxb) and red Dragon fruit (Hylocereus polyrhizus) extracts. Molecules. 2021;26:196. doi: 10.3390/molecules26010196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Almeida F, Corrêa M, Zaera AM, Garrigues T, Isaac V. Influence of different surfactants on development of nanoemulsion containing fixed oil from an Amazon palm species. Colloids Surf A Physicochem Eng Asp. 2022;643:128721. [Google Scholar]
- 119. Sharma M, Bains A, Sridhar K, Nayak PK, Sarangi PK, Ali N, et al. Sustainable design and characterization of Aegle marmelos fruit nanomucilage-flaxseed oil nanoemulsion: shelf-life of coated fresh-cut papaya. Sustain Chem Pharm. 2024;37:101409. [Google Scholar]
- 120. Luciano WA, Pimentel TC, Bezerril FF, Barão CE, Marcolino VA, de Siqueira Ferraz Carvalho R, et al. Effect of citral nanoemulsion on the inactivation of Listeria monocytogenes and sensory properties of fresh-cut melon and papaya during storage. Int J Food Microbiol. 2023;384:109959. doi: 10.1016/j.ijfoodmicro.2022.109959. [DOI] [PubMed] [Google Scholar]
- 121. Dini I. Contribution of Nanoscience research in antioxidants delivery used in nutricosmetic sector. Antioxidants. 2022:11. doi: 10.3390/antiox11030563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Yukuyama MN, Ghisleni DD, Pinto TJ, Bou-Chacra NA. Nanoemulsion: process selection and application in cosmetics–a review. Int J Cosmet Sci. 2016;38:13–24. doi: 10.1111/ics.12260. [DOI] [PubMed] [Google Scholar]
- 123. Ma Q, Davidson PM, Zhong Q. Nanoemulsions of thymol and eugenol co-emulsified by lauric arginate and lecithin. Food Chem. 2016;206:167–73. doi: 10.1016/j.foodchem.2016.03.065. [DOI] [PubMed] [Google Scholar]
- 124. Kiattisin K, Srithongchai P, Jaiyong W, Boonpisuttinant K, Ruksiriwanich W, Jantrawut P, et al. Preparation and characterization of ultrasound-assisted nanoemulsions containing natural oil for anti-aging effect. J Agric Food Res. 2024:101004. [Google Scholar]
- 125.Silva AM, Luís AS, Macedo C, Ferreira AS, Costa PC, Delerue-Matos C, et al. Chapter 17 - cosmetic applications of herbal products and encapsulated herbal active extracts. In: Thomas S, Oyedeji AO, Oluwafemi OS, Jaquilin PjR, editors. Nanotechnology in herbal medicine. Woodhead Publishing; 2023. pp. 447–90. [Google Scholar]
- 126. Yien RMK, Matos APdS, Gomes ACC, Garófalo DdA, Santos-Oliveira R, Simas NK, et al. Nanotechnology promoting the development of products from the biodiversity of the asteraceae family. Nutrients. 2023;15:1610. doi: 10.3390/nu15071610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kumari S, Goyal A, Sönmez Gürer E, Algõn Yapar E, Garg M, Sood M, et al. Bioactive loaded novel nano-formulations for targeted drug delivery and their therapeutic potential. Pharmaceutics. 2022;14:1091. doi: 10.3390/pharmaceutics14051091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Iskandar B, Mei H-C, Liu T-W, Lin H-M, Lee C-K. Evaluating the effects of surfactant types on the properties and stability of oil-in-water Rhodiola rosea nanoemulsion. Colloids Surf B Biointerfaces. 2024;234:113692. doi: 10.1016/j.colsurfb.2023.113692. [DOI] [PubMed] [Google Scholar]
- 129. Zhou Q, Li X, Wang X, Shi D, Zhang S, Yin Y, et al. Vanillic acid as a promising xanthine oxidase inhibitor: extraction from amomum villosum lour and biocompatibility improvement via extract nanoemulsion. Foods. 2022;11:968. doi: 10.3390/foods11070968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Hashemnejad SM, Badruddoza AZM, Zarket B, Ricardo Castaneda C, Doyle PS. Thermoresponsive nanoemulsion-based gel synthesized through a low-energy process. Nat Commun. 2019;10:2749. doi: 10.1038/s41467-019-10749-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Venkatraman PD, Sayed U, Parte S, Korgaonkar S. Development of advanced textile finishes using nano-emulsions from herbal extracts for organic cotton fabrics. Coatings. 2021;11:939. [Google Scholar]
- 132. Petralito S, Garzoli S, Ovidi E, Laghezza Masci V, Trilli J, Bigi B, et al. Long-term stability of lavandula x intermedia essential oil nanoemulsions: can the addition of the ripening inhibitor impact the biocidal activity of the nanoformulations? Pharmaceutics. 2024;16:108. doi: 10.3390/pharmaceutics16010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Ahmad U, Ahmad Z, Khan AA, Akhtar J, Singh SP, Ahmad FJ. Strategies in development and delivery of nanotechnology based cosmetic products. Drug Res. 2018;68:545–52. doi: 10.1055/a-0582-9372. [DOI] [PubMed] [Google Scholar]
- 134. Gupta A, Eral HB, Hatton TA, Doyle PS. Nanoemulsions: formation, properties and applications. Soft Matter. 2016;12:2826–41. doi: 10.1039/c5sm02958a. [DOI] [PubMed] [Google Scholar]
- 135. Gharibzahedi SMT, Mohammadnabi S. Characterizing the novel surfactant-stabilized nanoemulsions of stinging nettle essential oil: thermal behaviour, storage stability, antimicrobial activity and bioaccessibility. J Mol Liq. 2016;224:1332–40. [Google Scholar]
- 136. Silva HD, Cerqueira MA, Vicente AA. Influence of surfactant and processing conditions in the stability of oil-in-water nanoemulsions. J Food Eng. 2015;167:89–98. [Google Scholar]
- 137. Arianto A, Cindy C. Preparation and evaluation of sunflower oil nanoemulsion as a sunscreen. Open Access Maced J Med Sci. 2019;7:3757–61. doi: 10.3889/oamjms.2019.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Ozogul Y, Karsli GT, Durmuş M, Yazgan H, Oztop HM, McClements DJ, et al. Recent developments in industrial applications of nanoemulsions. Adv Colloid Interface Sci. 2022;304:102685. doi: 10.1016/j.cis.2022.102685. [DOI] [PubMed] [Google Scholar]
- 139. Iskandar B, Iskandar B, Karsono Silalahi J, editors. Preparation of spray nanoemulsion and cream containing vitamin E as anti-aging product tested in vitro and in vivo method. 2016 [Google Scholar]
- 140. Lewińska A, Domżał-Kędzia M, Maciejczyk E, Łukaszewicz M, Bazylińska U. Design and engineering of “green” nanoemulsions for enhanced topical delivery of bakuchiol achieved in a sustainable manner: a novel eco-friendly approach to bioretinol. Int J Mol Sci. 2021;22:10091. doi: 10.3390/ijms221810091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Md Saari NH, Chua LS, Hasham R, Yuliati L. Curcumin-loaded nanoemulsion for better cellular permeation. Sci Pharm. 2020;88:44. [Google Scholar]
- 142. Barreto SMAG, Maia MS, Benicá AM, de Assis HRBS, Leite-Silva VR, da Rocha-Filho PA, et al. Evaluation of in vitro and in vivo safety of the by-product of Agave sisalana as a new cosmetic raw material: development and clinical evaluation of a nanoemulsion to improve skin moisturizing. Ind Crop Prod. 2017;108:470–9. [Google Scholar]
- 143. Mascarenhas-Melo F, Mathur A, Murugappan S, Sharma A, Tanwar K, Dua K, et al. Inorganic nanoparticles in dermo-pharmaceutical and cosmetic products: properties, formulation development, toxicity, and regulatory issues. Eur J Pharm Biopharm. 2023;192:25–40. doi: 10.1016/j.ejpb.2023.09.011. [DOI] [PubMed] [Google Scholar]
- 144. Chaiyana W, Inthorn J, Somwongin S, Anantaworasakul P, Sopharadee S, Yanpanya P, et al. The fatty acid compositions, irritation properties, and potential applications of teleogryllus mitratus oil in nanoemulsion development. Nanomaterials. 2024;14:184. doi: 10.3390/nano14020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Mansur MCPPR, Campos C, Vermelho AB, Nobrega J, da Cunha Boldrini L, Balottin L, et al. Photoprotective nanoemulsions containing microbial carotenoids and buriti oil: efficacy and safety study. Arab J Chem. 2020;13:6741–52. [Google Scholar]
- 146. Inal A, Yenipazar H, Şahin-Yeşlçubuk N. Preparation and characterization of nanoemulsions of curcumin and echium oil. Heliyon. 2022;8:e08974. doi: 10.1016/j.heliyon.2022.e08974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Santamaría E, Maestro A, González C. Use of double gelled microspheres to improve release control of cinnamon-loaded nanoemulsions. Molecules. 2024;29:158. doi: 10.3390/molecules29010158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Gauthier G, Capron I. Pickering nanoemulsions: an overview of manufacturing processes, formulations, and applications. JCIS Open. 2021;4:100036. [Google Scholar]
- 149. Kumar M, Bishnoi RS, Shukla AK, Jain CP. Techniques for formulation of nanoemulsion drug delivery system: a review. Prev Nutr Food Sci. 2019;24:225–34. doi: 10.3746/pnf.2019.24.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Montenegro L, Lai F, Offerta A, Sarpietro MG, Micicchè L, Maccioni AM, et al. From nanoemulsions to nanostructured lipid carriers: a relevant development in dermal delivery of drugs and cosmetics. J Drug Deliv Sci Technol. 2016;32:100–12. [Google Scholar]
- 151. Santamaría E, Maestro A, Vilchez S, González C. Study of nanoemulsions using carvacrol/MCT-(Oleic acid-potassium oleate)/Tween 80 ®- water system by low energy method. Heliyon. 2023;9:e16967. doi: 10.1016/j.heliyon.2023.e16967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. He J-L, Liu B-H, Zhang H-L, Xu D, Shi B-M, Zhang Y-H. Improvement of hydrolysis efficiency and interfacial properties of zein using nanoemulsions prepared by a low energy emulsification method. Food Biosci. 2023;54:102922. [Google Scholar]
- 153.Verma R, Vaidya S. 1 - basics of nanoemulsion: synthesis and characterization. In: Jha M, Hussain CM, Kailasam K, editors. Industrial applications of nanoemulsion. Elsevier; 2024. pp. 1–16. [Google Scholar]
- 154. Sampaio CI, Bourbon AI, Gonçalves C, Pastrana LM, Dias AM, Cerqueira MA. Low energy nanoemulsions as carriers of thyme and lemon balm essential oils. LWT (Lebensm-Wiss & Technol) 2022;154:112748. [Google Scholar]
- 155. Wathoni N, Rusdin A, Motoyama K, Joni IM, Lesmana R, Muchtaridi M. Nanoparticle drug delivery systems for α-mangostin. Nanotechnol Sci Appl. 2020;13:23–36. doi: 10.2147/NSA.S243017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Luangapai F, Iwamoto S. Influence of blending and layer-by-layer assembly methods on chitosan–gelatin composite films enriched with curcumin nanoemulsion. Int J Biol Macromol. 2023;249:126061. doi: 10.1016/j.ijbiomac.2023.126061. [DOI] [PubMed] [Google Scholar]
- 157. Fu X, Gao Y, Yan W, Zhang Z, Sarker S, Yin Y, et al. Preparation of eugenol nanoemulsions for antibacterial activities. RSC Adv. 2022;12:3180–90. doi: 10.1039/d1ra08184e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Li H, Lu H, Zhang Y, Liu D, Chen J. Oil-in-water nanoemulsion with reversible charge prepared by the phase inversion composition method. J Mol Liq. 2021;336:116174. [Google Scholar]
- 159. Sonneville-Aubrun O, Simonnet JT, L'Alloret F. Nanoemulsions: a new vehicle for skincare products. Adv Colloid Interface Sci. 2004;108–109:145–9. doi: 10.1016/j.cis.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 160. Li G, Zhang Z, Liu H, Hu L. Nanoemulsion-based delivery approaches for nutraceuticals: fabrication, application, characterization, biological fate, potential toxicity and future trends. Food Funct. 2021;12:1933–53. doi: 10.1039/d0fo02686g. [DOI] [PubMed] [Google Scholar]
- 161. Howe AM, Pitt AR. Rheology and stability of oil-in-water nanoemulsions stabilised by anionic surfactant and gelatin 1) addition of nonionic, cationic and ethoxylated-cationic co-surfactants. Adv Colloid Interface Sci. 2008;144:24–9. doi: 10.1016/j.cis.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 162.Borthakur P, Boruah PK, Sharma B, Das MR. 5 - nanoemulsion: preparation and its application in food industry. In: Grumezescu AM, editor. Emulsions. Academic Press; 2016. pp. 153–91. [Google Scholar]
- 163. Delforce L, Ontiveros JF, Nardello-Rataj V, Aubry J-M. Rational design of O/W nanoemulsions based on the surfactant dodecyldiglyceryl ether using the normalized HLD concept and the formulation-composition map. Colloids Surf A Physicochem Eng Asp. 2023;671:131679. [Google Scholar]
- 164. Tran E, Mapile AN, Richmond GL. Peeling back the layers: investigating the effects of polyelectrolyte layering on surface structure and stability of oil-in-water nanoemulsions. J Colloid Interface Sci. 2021;599:706–16. doi: 10.1016/j.jcis.2021.04.115. [DOI] [PubMed] [Google Scholar]
- 165. Souto EB, Cano A, Martins-Gomes C, Coutinho TE, Zielińska A, Silva AM. Microemulsions and nanoemulsions in skin drug delivery. Bioengineering. 2022:9. doi: 10.3390/bioengineering9040158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Fürst A, Shahzadi I, Akkuş-Dağdeviren ZB, Schöpf AM, Gust R, Bernkop-Schnürch A. Zeta potential shifting nanoemulsions comprising single and gemini tyrosine-based surfactants. Eur J Pharmaceut Sci. 2023;189:106538. doi: 10.1016/j.ejps.2023.106538. [DOI] [PubMed] [Google Scholar]
- 167. Das T, Chatterjee N, Chakraborty A, Banerjee A, Haiti SB, Datta S, et al. Fabrication of rice bran oil nanoemulsion and conventional emulsion with Mustard Protein Isolate as a novel excipient: focus on shelf-life stability, lipid digestibility and cellular bioavailability. Food Hydrocolloid Health. 2023;4:100143. [Google Scholar]
- 168. Roselan MA, Ashari SE, Faujan NH, Mohd Faudzi SM, Mohamad R. An improved nanoemulsion formulation containing kojic monooleate: optimization, characterization and in vitro studies. Molecules. 2020;25:2616. doi: 10.3390/molecules25112616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Rai VK, Mishra N, Yadav KS, Yadav NP. Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: formulation development, stability issues, basic considerations and applications. J Contr Release. 2018;270:203–25. doi: 10.1016/j.jconrel.2017.11.049. [DOI] [PubMed] [Google Scholar]
- 170. Chen BH, Stephen Inbaraj B. Nanoemulsion and nano-liposome based strategies for improving anthocyanin stability and bioavailability. Nutrients. 2019;11 doi: 10.3390/nu11051052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Singh Y, Meher JG, Raval K, Khan FA, Chaurasia M, Jain NK, et al. Nanoemulsion: concepts, development and applications in drug delivery. J Contr Release. 2017;252:28–49. doi: 10.1016/j.jconrel.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 172. Khan NU, Ali A, Khan H, Khan ZU, Ahmed Z. Stability studies and characterization of glutathione-loaded nanoemulsion. J Cosmet Sci. 2018;69:257–67. [PubMed] [Google Scholar]
- 173. Onaizi SA. Effect of oil/water ratio on rheological behavior, droplet size, zeta potential, long-term stability, and acid-induced demulsification of crude oil/water nanoemulsions. J Petrol Sci Eng. 2022;209:109857. [Google Scholar]
- 174. Vitória Minzoni de Souza Iacia M, Eduarda Ferraz Mendes M, Cristiny de Oliveira Vieira K, Cristine Marques Ruiz G, José Leopoldo Constantino C, da Silva Martin C, et al. Evaluation of curcumin nanoemulsion effect to prevent intestinal damage. Int J Pharm. 2024;650:123683. doi: 10.1016/j.ijpharm.2023.123683. [DOI] [PubMed] [Google Scholar]
- 175. Hou X, Sheng JJ. Properties, preparation, stability of nanoemulsions, their improving oil recovery mechanisms, and challenges for oil field applications—a critical review. Geoenergy Sci Eng. 2023;221:211360. [Google Scholar]
- 176. Pathania R, Najda A, Chawla P, Kaushik R, Khan MA. Lowenergy assisted sodium alginate stabilized Phyllanthus niruri extract nanoemulsion: characterization, in vitro antioxidant and antimicrobial application. Biotechnol Rep (Amst) 2022;33:e00711. doi: 10.1016/j.btre.2022.e00711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Vater C, Hlawaty V, Werdenits P, Cichoń MA, Klang V, Elbe-Bürger A, et al. Effects of lecithin-based nanoemulsions on skin: short-time cytotoxicity MTT and BrdU studies, skin penetration of surfactants and additives and the delivery of curcumin. Int J Pharm. 2020;580:119209. doi: 10.1016/j.ijpharm.2020.119209. [DOI] [PubMed] [Google Scholar]
- 178. Wong CK, Wong JQX, Rosli NSB, Jamaluddin J, Hasham R, Bohari SPM, et al. Sponge-like characteristic of cellulose hydrogel for transport of rosmarinic acid nanoemulsion. Materials Today: Proceedings. 2023;96:35–9. [Google Scholar]
- 179. Wang Z, Tang W, Sun Z, Liu F, Wang D. An innovative Pickering W/O/W nanoemulsion co-encapsulating hydrophilic lysozyme and hydrophobic Perilla leaf oil for extending shelf life of fish products. Food Chem. 2024;439:138074. doi: 10.1016/j.foodchem.2023.138074. [DOI] [PubMed] [Google Scholar]
- 180. Zhang M, Cao Q, Yuan Y, Guo X, Pan D, Xie R, et al. Functional nanoemulsions: controllable low-energy nano-emulsification and advanced biomedical application. Chin Chem Lett. 2024;35:108710. [Google Scholar]
- 181. Adil M, Onaizi SA. Pickering nanoemulsions and their mechanisms in enhancing oil recovery: a comprehensive review. Fuel. 2022;319:123667. [Google Scholar]
- 182. Quintão FJO, Tavares RSN, Vieira-Filho SA, Souza GHB, Santos ODH. Hydroalcoholic extracts of Vellozia squamata: study of its nanoemulsions for pharmaceutical or cosmetic applications. Revista Brasileira de Farmacognosia. 2013;23:101–7. [Google Scholar]
- 183. Kumari S, Sharma V, Soni S, Sharma A, Thakur A, Kumar S, et al. Layered double hydroxides and their tailored hybrids/composites: progressive trends for delivery of natural/synthetic-drug/cosmetic biomolecules. Environ Res. 2023;238:117171. doi: 10.1016/j.envres.2023.117171. [DOI] [PubMed] [Google Scholar]
- 184. Lante A, Friso D. Oxidative stability and rheological properties of nanoemulsions with ultrasonic extracted green tea infusion. Food Res Int. 2013;54:269–76. [Google Scholar]
- 185. Bashir O, Amin T, Hussain SZ, Naik HR, Goksen G, Wani AW, et al. Development, characterization and use of rosemary essential oil loaded water-chestnut starch based nanoemulsion coatings for enhancing post-harvest quality of apples var. Golden delicious. Curr Res Food Sci. 2023;7:100570. doi: 10.1016/j.crfs.2023.100570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Gull A, Bhat N, Wani SM, Masoodi FA, Amin T, Ganai SA. Shelf life extension of apricot fruit by application of nano-chitosan emulsion coatings containing pomegranate peel extract. Food Chem. 2021;349:129149. doi: 10.1016/j.foodchem.2021.129149. [DOI] [PubMed] [Google Scholar]
- 187. Soltanzadeh M, Peighambardoust SH, Ghanbarzadeh B, Mohammadi M, Lorenzo JM. Chitosan nanoparticles as a promising nanomaterial for encapsulation of pomegranate (Punica granatum L.) peel extract as a natural source of antioxidants. Nanomaterials. 2021:11. doi: 10.3390/nano11061439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Sadeghian SF, Majdinasab M, Nejadmansouri M, Hosseini SMH. Effects of natural antioxidants and high-energy fabrication methods on physical properties and oxidative stability of flaxseed oil-in-water nanoemulsions. Ultrason Sonochem. 2023;92:106277. doi: 10.1016/j.ultsonch.2022.106277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Nanda S, Nanda A, Lohan S, Kaur R, Singh B. Chapter 3 - nanocosmetics: performance enhancement and safety assurance. In: Grumezescu AM, editor. Nanobiomaterials in Galenic formulations and cosmetics. William Andrew Publishing; 2016. pp. 47–67. [Google Scholar]
- 190.Banik B, Borkotoky S, Das MK. Chapter 14 - biosynthesized colloidal metallic nanoparticles-based nanocosmetic formulations. In: Das MK, editor. Nanocosmeceuticals. Academic Press; 2022. pp. 369–88. [Google Scholar]
- 191. Liew SN, Utra U, Alias AK, Tan TB, Tan CP, Yussof NS. Physical, morphological and antibacterial properties of lime essential oil nanoemulsions prepared via spontaneous emulsification method. LWT (Lebensm-Wiss & Technol) 2020;128:109388. [Google Scholar]
- 192. Pandey AS, Bawiskar D, Wagh V. Nanocosmetics and skin health: a comprehensive review of nanomaterials in cosmetic formulations. Cureus. 2024;16:e52754. doi: 10.7759/cureus.52754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Mavridi-Printezi A, Guernelli M, Menichetti A, Montalti M. Bio-applications of multifunctional melanin nanoparticles: from nanomedicine to nanocosmetics. Nanomaterials. 2020:10. doi: 10.3390/nano10112276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Dubey SK, Dey A, Singhvi G, Pandey MM, Singh V, Kesharwani P. Emerging trends of nanotechnology in advanced cosmetics. Colloids Surf B Biointerfaces. 2022;214:112440. doi: 10.1016/j.colsurfb.2022.112440. [DOI] [PubMed] [Google Scholar]
- 195. Aswathanarayan JB, Vittal RR. Nanoemulsions and their potential applications in food industry. Front Sustain Food Syst. 2019;3 [Google Scholar]
- 196. Kobra K, Wong SY, Mazumder MAJ, Li X, Arafat MT. Xanthan and gum acacia modified olive oil based nanoemulsion as a controlled delivery vehicle for topical formulations. Int J Biol Macromol. 2023;253:126868. doi: 10.1016/j.ijbiomac.2023.126868. [DOI] [PubMed] [Google Scholar]
- 197. Akkam Y, Rababah T, Costa R, Almajwal A, Feng H, Laborde JEA, et al. Pea protein nanoemulsion effectively stabilizes vitamin D in food products: a potential supplementation during the COVID-19 pandemic. Nanomaterials. 2021;11:887. doi: 10.3390/nano11040887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Rupa EJ, Li JF, Arif MH, Yaxi H, Puja AM, Chan AJ, et al. Cordyceps militaris fungus extracts-mediated nanoemulsion for improvement antioxidant, antimicrobial, and anti-inflammatory activities. Molecules. 2020;25:5733. doi: 10.3390/molecules25235733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Ribeiro RCdA, Barreto SMAG, Ostrosky EA, Rocha-Filho PAd, Veríssimo LM, Ferrari M. Production and characterization of cosmetic nanoemulsions containing opuntia ficus-indica (L.) mill extract as moisturizing agent. Molecules. 2015;20:2492–509. doi: 10.3390/molecules20022492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Pathania R, Najda A, Chawla P, Kaushik R, Khan MA. Lowenergy assisted sodium alginate stabilized Phyllanthus niruri extract nanoemulsion: characterization, in vitro antioxidant and antimicrobial application. Biotechnol Rep. 2022;33:e00711. doi: 10.1016/j.btre.2022.e00711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Krisanti EA, Kirana DP, Mulia K. Nanoemulsions containing Garcinia mangostana L. pericarp extract for topical applications: development, characterization, and in vitro percutaneous penetration assay. PLoS One. 2021;16:e0261792. doi: 10.1371/journal.pone.0261792. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.