Abstract
Background
Aldehyde is a kind of important environmental pollutant, which has been demonstrated to be associated with increased risks of various chronic diseases with the economic and social development. However, the effects of aldehydes on serum uric acid (SUA) and hyperuricemia remained inexplicit, and the potential mediating pathways for this relationship needed to be addressed.
Methods
This study investigated the associations of individual and mixed aldehydes with SUA and hyperuricemia among 1588 U S. adults from the National Health and Nutrition Examination Survey 2013–2014. The generalized linear regression model was applied to assess the effects of individual aldehydes and the Bayesian kernel machine regression were incorporated to examine the impacts of mixed aldehydes. Mediation analyses were performed to explore the roles of inflammation and oxidative stress indices in aldehyde-induced SUA and hyperuricemia. Moreover, we conducted subgroup analyses for demographic and physical factors to detect disparity between groups.
Results
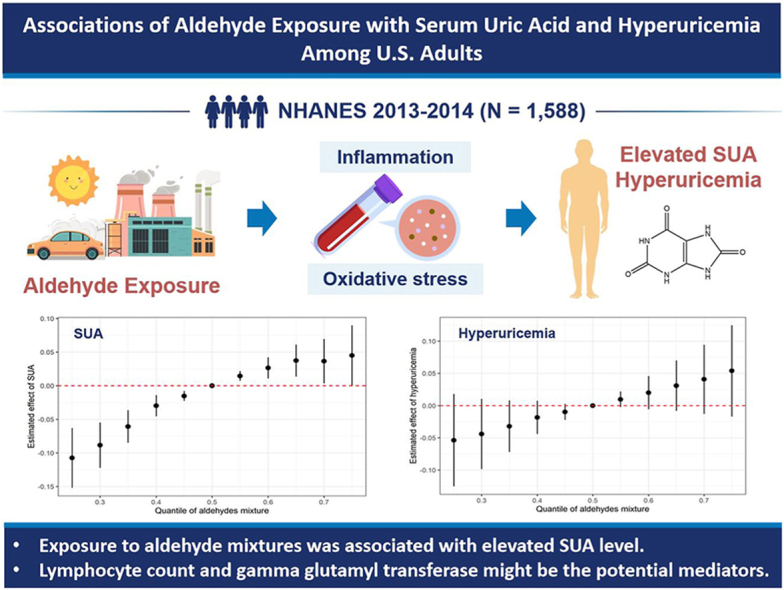
Propanaldehyde, butyraldehyde, and hexanaldehyde were associated with higher SUA level and butyraldehyde was correlated with increased hyperuricemia prevalence after multiple correction. Positive relationships between aldehyde mixtures and SUA level were also observed and hexanaldehyde contributed most. In addition, lymphocyte count and gamma glutamyl transferase partially mediated the associations between propanaldehyde, butyraldehyde, hexanaldehyde and SUA level, with mediation proportions ranging from 19.105 % to 27.316 %. Subgroup analyses showed that SUA level of participants with obesity, hypercholesterolemia, and hypertension tended to be more sensitive to aldehyde exposure.
Conclusions
Our results highlighted that multiple aldehydes mixtures exposure might increase SUA level, and revealed underlying mediating role of inflammation and oxidative stress. These findings provided crucial evidence for the impacts of environmental pollutants on human health and further prospective studies are still required to verify the findings.
Keywords: Aldehydes, Uric acid, Hyperuricemia, Bayesian kernel machine regression, Inflammation, Oxidative stress
Graphical abstract
Highlights
-
•
Individual aldehydes were associated with higher SUA level and hyperuricemia risk.
-
•
BKMR model showed aldehyde mixtures might increase SUA level.
-
•
Lymphocyte count and GGT mediated associations of aldehyde exposure with SUA.
-
•
Subjects with obesity, hypercholesterolemia, and hypertension were more vulnerable.
1. Introduction
Aldehydes are a class of ubiquitous organic compounds containing a terminal carbonyl group in surrounding environments. Environmental aldehydes originated from various sources, mainly formed as photochemical oxidation automobile exhaust products and as thermal degradation byproducts of gasoline combustion and e-cigarette vapors [[1], [2], [3]]. Other sources include organic matter from the pyrolysis of fuel, wood, and tobacco [4,5], as well as disinfection byproducts of ozonation [6]. Besides, aldehydes are endogenously generated in the human body through the reaction of free radicals with cell membrane lipids [7]. Researchers have showed that aldehydes can easily pass through cell membranes and create covalent bonds with cellular macromolecules, disrupting their function and potentially causing mutations [8,9]. Moreover, the toxic effects of aldehydes to the human body could contribute to multiple chronic and aging-related diseases, which should raise concern of investigators [9].
Uric acid (UA) is the end product of purine nucleotide metabolism, primarily produced in the liver and eliminated by the kidneys or intestines. Hyperuricemia would occur as a result of either overproduction or underexcretion of serum UA (SUA) [10], and has emerged as a common health problem with the prevalence of 20.1 % among U.S. people in 2015–2016 [11]. Despite stable prevalence rates over the latest decade, the disease burden remains substantial as ongoing population growth [11]. Increasing evidence indicated that environmental pollutants could also cause abnormal SUA level or hyperuricemia except for traditional risk factors [12,13]. Currently, aldehydes were demonstrated to be associated with the risk of cardiovascular diseases (CVD), hypertension, diabetes, and blood lipids [9,[14], [15], [16]]. Hyperuricemia is an important comorbidity of these diseases with insidious onset and deserves greater attention [17]. Researchers have found that aldehydes might result in kidney stone formation and kidney injury [18], while few studies addressed the relationship of aldehydes with SUA and hyperuricemia. Furthermore, investigators found that inflammation and oxidative stress might be the pathways linking aldehyde exposure to disease progression [9,19], and pathomechanisms of inflammation and oxidative stress also had potential impacts on UA metabolism [20,21]. Therefore, the potential role that inflammation and oxidative stress play in this association is warranted to be elucidated.
Considering single kind of aldehydes were typically assessed in previous studies [22], a more comprehensive evaluation of aldehydes should be estimated. In this case, based on the population data from the National Health and Nutrition Examination Survey (NHANES), we evaluated the associations of individual and combined exposure of aldehydes with SUA level and hyperuricemia, and whether these associations were potentially mediated by inflammation and oxidative stress indicators.
2. Methods
The study followed the recommendations of Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) (Supplementary Table S1).
2.1. Study design and population
Data were derived from the NHANES for years 2013–2014. The NHANES is a nationwide cross-sectional program conducted by the National Center for Health Statistics (NCHS) at the Centers for Disease Control and Prevention (CDC) to evaluate the physical condition and nutritional status of the U.S. population. The program gathers data through interviews, professional physical examinations, and laboratory tests, covering information including demographic factors, dietary conditions, examination and laboratory data, and individual medical status (Available online: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx). Approval for the NHANES has been obtained from the Institutional Review Board (IRB) of the NCHS, and informed consent forms have been signed by all participants or their representatives.
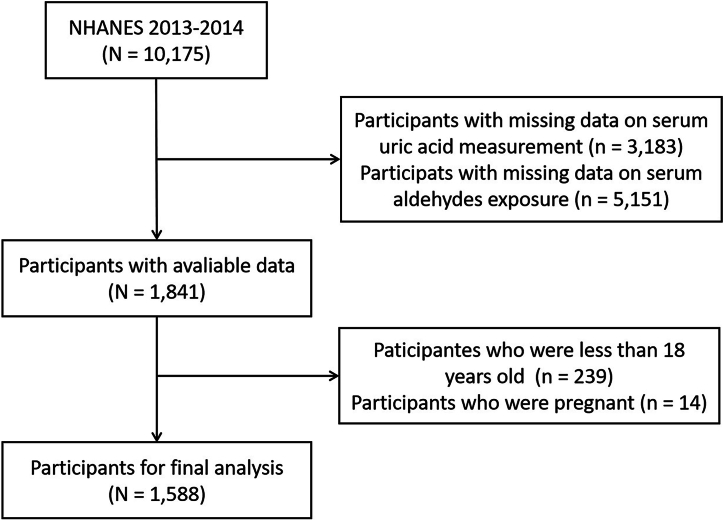
In NHANES 2013–2014, there were 10,175 participants attended the investigation. Initially, we excluded 3183 and 5151 participants due to a lack of data on SUA and serum aldehyde exposure, respectively. Subsequently, we further excluded 239 participants who were aged <18 years and 14 pregnant participants. Ultimately, a total of 1588 individuals were included in the data analysis. The entire process of inclusion and exclusion for study participants was shown in Fig. 1.
Fig. 1.
Schematic flow diagram of inclusion and exclusion criteria for study participants.
2.2. Measurement of serum aldehyde exposure
The levels of 12 aldehydes from protein adducts in the serum were detected by an automated analytical method using solid phase microextraction (SPME) gas chromatography (GC) and high-resolution mass spectrometry (HRMS) with selected ion mass detection and isotope-dilution techniques. Each aldehyde has a specific limit of detection (LOD), and values below LOD were imputed by LOD divided by the square root of 2. To ensure the reliability and stability of the analysis results, similar to other studies, we excluded the aldehydes with missing data exceeding 20 % [23], and finally included 6 aldehydes in the analysis, including benzaldehyde, isopentanaldehyde, propanaldehyde, butyraldehyde, hexanaldehyde and heptanaldehyde. The LODs and characteristics of serum aldehyde exposure levels in the study population were summarized in Supplementary Table S2.
2.3. Evaluation of the outcome
Trained staffs collected serum samples from study participants and stored them at −30 °C until testing. The SUA level was tested using a timed endpoint method by DxC 800 (Beckman Coulter UniCel® DxC 800). Hyperuricemia was defined as SUA level ≥7.0 mg/dL for men and ≥6.0 mg/dL for women [24].
2.4. Measurement of inflammatory and oxidative stress biomarkers
The selected inflammatory biomarkers included alkaline phosphatase (ALP), white blood cell count (WBC), absolute neutrophil count (ANC), and lymphocyte count, and the selected oxidative stress biomarkers included total bilirubin and gamma glutamyl transferase (GGT) [25,26]. The levels of ALP, total bilirubin, and GGT were measured by DxC800 as mentioned above. The complete blood count on blood specimens were detected by instrument DxH 800 (Beckman Coulter UniCel® DxH 800.
2.5. Assessment of covariates
The covariates considered in this study encompassed a range of demographic and clinical factors including age, sex, race, education level, family poverty income ratio (PIR) index, body mass index (BMI), smoking status, alcohol consumption, physical activity, hypertension, diabetes, hypercholesterolemia and chronic kidney disease (CKD), which were collected through structured questionnaires, standard anthropometric measurement, and trained laboratory tests. The poverty-to-income ratio (PIR) was categorized into <1 (below poverty level) and ≥1 (above poverty level), which was calculated by dividing the annual household income by the poverty criterion for household size in the state of residence in a given year, to represent the level of household income. BMI was computed as the ratio of weight in kilograms and the height in meters squared. Smoking status was categorized as never smoking, current smoking, and former smoking (having a history of at least 100 cigarettes but currently not smoking). Alcohol consumption status was delineated as never drinking, current drinking and former drinking (having consumed a minimum of 12 alcoholic drinks in a lifetime but fewer than 12 in the last year). For physical activity (PA), we calculated the total amount of moderate to heavy work and leisure PA for each individual and divided them into ideal and non-ideal PA based on the criteria of achieving at 150 min/week. Hypertension was defined as a self-reported physician diagnosis of hypertension, and/or systolic blood pressure (SBP) ≥140 mmHg, and/or (DBP) ≥90 mmHg, and/or taking antihypertensive medications. Diabetes was determined by self-reported physician diagnosis of diabetes, and/or glycosylated hemoglobin (GHb) ≥6.5 %, and/or taking insulin or hypoglycemic agents. Hypercholesterolemia was characterized by a self-reported physician diagnosis of high cholesterol level and/or total cholesterol (TC) ≥240 mg/dL, and/or low-density lipoprotein cholesterol (LDLC) ≥160 mg/dL, and/or taking prescription for lowering cholesterol. CKD was defined as an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2, and/or albumin to creatinine ratio (ACR) > 30 mg/g [27,28]. When calculating the eGFR level, the serum-creatinine-based CKD-Epidemiology Collaboration (CKD-EPI) Equation was used [29]:
where represented serum creatinine, is 0.9 for male and 0.7 for female, is −0.411 for male and −0.329 for female.
2.6. Statistical analysis
The continuous variables were presented as means ± standard deviations (SD) and the categorical variables were displayed as frequencies (percentages). The chi-square test and t-test were used to compare the disparities between groups. All serum aldehydes levels and inflammatory biomarkers were transformed by the logarithmic method due to skewed distribution. Pearson correlation analysis was conducted to estimate the correlation among aldehyde exposure.
Following NHANES analytical guidelines, sample weights were calculated and applied in all analyses to deal with complex sampling design. The generalized linear regression model was used to assess the effect of individual aldehyde exposure on SUA (continuous) and hyperuricemia (dichotomous), and the results were displayed as estimated β (95 % confidence interval [CI]) or odds ratio (OR) (95%CI), respectively. The levels of aldehydes were included in the models as both continuous and categorical variables categorized by tertiles, with the first tertile as the reference. We constructed three models: model 1 was a crude model, model 2 was adjusted for age, sex and race, and model 3 as main model was further adjusted for educational level, family PIR, BMI, smoking status, alcohol consumption, physical activity, hypertension, diabetes, hypercholesterolemia, and CKD. A linear trend test was performed using each aldehyde categories as a continuous variable in the model. To account for multiple comparisons and reduce the risk of type I error, P values for models were corrected by false discovery rate (FDR) using Benjaminiand-Hochberg method [30]. Restricted cubic splines were used to characterize the exposure-response relationships of each aldehyde exposure with SUA level and hyperuricemia. To further estimate the effect of aldehyde mixtures, the Bayesian kernel machine regression (BKMR) with Markov chain Monte Carlo (MCMC) sampling was used, which could consider nonlinear relationships between exposures and outcomes as well as potential interactions among exposure components. The model was preformed 10,000 iterations in MCMC method and all covariates in the main model were adjusted. Fitting with variable selection, the BKMR model could estimate posterior inclusion probabilities (PIPs) for each aldehyde exposure to reflect their relative contribution of effect, and PIP >0.5 is usually considered important [31]. Additionally, the model also estimated the single effect of each aldehyde exposure on SUA and hyperuricemia when the other aldehydes were fixed at its 25th, 50th, and 75th percentile.
Furthermore, we explored whether biomarkers of inflammatory and oxidative stress mediated the associations between aldehydes and elevated SUA. We first used linear regression to investigate the associations of aldehydes with inflammatory and oxidative stress biomarkers, and inflammatory and oxidative stress biomarkers with SUA. Based on these results, potential mediating biomarkers were selected, and then mediation analysis was used to test and quantify the role of selected biomarkers. In the mediation analysis model, the total effect, average direct effect (ADE) and average causal mediation effect (ACME) were estimated as β (95%CI) and proportion mediated effect (95%CI) were calculated. The direct effect represents the effect of aldehyde exposure on SUA which is not mediated by mediator, and the indirect effect represents the effect of aldehyde exposure on SUA mediated by mediator [32]. The P value of ACME <0.05 was considered as indicative of significant mediation.
Additionally, several subgroup analyses were conducted stratified by age (≤50y vs > 50y), sex (men vs women), educational level (low: below college or AA degree vs high: college or AA degree or above), BMI (<25 kg/m2 vs ≥ 25 kg/m2), hypertension (no or yes) and hypercholesterolemia (no or yes). Product interaction terms were included in the model to test the significance of interaction effects. We also conducted sensitivity analyses by excluding participants with CKD (n = 247) and gout (n = 43) to reduce the effects of disease on SUA level. All analyses were performed using R (version 4.2.0), and the mediation analysis, BKMR model and FDR correction were conducted with “mediation”, “bkmr”, and “stats” package. A two-tailed P value < 0.05 was considered statistically significant.
3. Results
3.1. Population characteristics
The characteristics of 1588 participants were summarized in Table 1, categorized based on their hyperuricemia diagnosis. The prevalence of hyperuricemia was found to be 18.70 %. Average ages of individuals without and with hyperuricemia were 46.60 and 50.74 years, and male proportions were 48.64 % and 49.83 %, respectively. Non-Hispanic whites, college or associates (AA) degree, never married group, family income above poverty level made up the largest proportion of the included subjects. Patients with hyperuricemia were found to be older, exhibited higher levels of BMI, SBP, GHb, TC, LDLC, and were more likely to suffer from hypertension, diabetes, hypercholesterolemia, and CKD.
Table 1.
Characteristics of NAHNES participants in 2013–2014 in the study.
| Overall | No hyperuricemia | Hyperuricemia | P value | |
|---|---|---|---|---|
| Number of Participants | 1588 | 1291 | 297 | |
| Age, years | 47.38 ± 18.16 | 46.60 ± 18.09 | 50.74 ± 18.11 | <0.001 |
| Gender, n (%) | ||||
| Men | 776 (48.87) | 628 (48.64) | 148 (49.83) | 0.761 |
| Women | 812 (51.13) | 663 (51.36) | 149 (50.17) | |
| Race, n (%) | ||||
| Mexican American | 247 (15.55) | 221 (17.12) | 26 (8.75) | 0.001 |
| Other Hispanic | 133 (8.38) | 115 (8.91) | 18 (6.06) | |
| Non-Hispanic White | 735 (46.28) | 587 (45.47) | 148 (49.83) | |
| Non-Hispanic Black | 257 (16.18) | 198 (15.34) | 59 (19.87) | |
| Other Race | 216 (13.60) | 170 (13.17) | 46 (15.49) | |
| Educational level, n (%) | ||||
| Less than 9th grade | 139 (9.27) | 124 (10.22) | 15 (5.23) | 0.003 |
| 9–11th grade | 172 (11.47) | 144 (11.87) | 28 (9.76) | |
| High school graduate/GED or equivalent | 331 (22.07) | 255 (21.02) | 76 (26.48) | |
| Some college or AA degree | 466 (31.07) | 361 (29.76) | 105 (36.59) | |
| College graduate or above | 392 (26.13) | 329 (27.12) | 63 (21.95) | |
| Marital status, n (%) | 0.478 | |||
| Married | 771 (51.40) | 631 (52.02) | 140 (48.78) | |
| Widowed | 99 (6.60) | 73 (6.02) | 26 (9.06) | |
| Divorced | 175 (11.67) | 143 (11.79) | 32 (11.15) | |
| Separated | 46 (3.07) | 37 (3.05) | 9 (3.14) | |
| Never married | 291 (19.40) | 237 (19.54) | 54 (18.82) | |
| Living with partner | 118 (7.87) | 92 (7.58) | 26 (9.06) | |
| Family poverty income ratio, n (%) | ||||
| <1 | 344 (23.16) | 276 (22.87) | 68 (24.46) | 0.625 |
| ≥1 | 1141 (76.84) | 931 (77.13) | 210 (75.54) | |
| BMI, kg/m2 | 29.01 ± 7.17 | 28.21 ± 6.57 | 32.46 ± 8.50 | <0.001 |
| Smoking status, n (%) | ||||
| Never | 910 (57.30) | 740 (57.32) | 170 (57.24) | 0.622 |
| Former | 320 (20.15) | 265 (20.53) | 55 (18.52) | |
| Now | 358 (22.54) | 286 (22.15) | 72 (24.24) | |
| Drinking status, n (%) | ||||
| Never | 235 (57.74) | 189 (57.45) | 46 (58.97) | 0.945 |
| Former | 99 (24.32) | 80 (24.32) | 19 (24.36) | |
| Now | 73 (17.94) | 60 (18.24) | 13 (16.67) | |
| Moderate to high physical activity, n (%) | ||||
| <150 min/wk | 739 (46.68) | 585 (45.49) | 154 (51.85) | 0.055 |
| ≥150 min/wk | 844 (53.32) | 701 (54.51) | 143 (48.15) | |
| Hypertension, n (%) | ||||
| No | 920 (59.24) | 808 (63.92) | 112 (38.75) | <0.001 |
| Yes | 633 (40.76) | 456 (36.08) | 177 (61.25) | |
| Diabetes, n (%) | ||||
| No | 1375 (86.75) | 1134 (88.04) | 241 (81.14) | 0.002 |
| Yes | 210 (13.25) | 154 (11.96) | 56 (18.86) | |
| Hypercholesterolemia, n (%) | ||||
| No | 450 (40.98) | 368 (42.54) | 82 (35.19) | <0.001 |
| Yes | 648 (59.02) | 497 (57.46) | 151 (64.81) | |
| SBP, mmHg | 122.25 ± 17.95 | 121.44 ± 17.46 | 125.92 ± 19.59 | <0.001 |
| DBP, mmHg | 69.14 ± 12.55 | 69.13 ± 12.12 | 69.18 ± 14.37 | 0.953 |
| GHb, % | 5.67 ± 0.99 | 5.64 ± 1.00 | 5.80 ± 0.92 | 0.01 |
| TC, mg/dL | 186.78 ± 40.46 | 185.26 ± 39.55 | 193.35 ± 43.63 | 0.002 |
| LDLC, mg/dL | 109.31 ± 34.65 | 107.87 ± 33.73 | 114.96 ± 37.61 | 0.026 |
| Chronic kidney disease, n (%) | ||||
| No | 1327 (84.31) | 1117 (87.20) | 210 (71.67) | <0.001 |
| Yes | 247 (15.69) | 164 (12.80) | 83 (28.33) | |
| ALP, IU/L | 63.00 (26.00) | 63.00 (25.00) | 63.00 (26.00) | 0.208 |
| WBC, 1000 cells/uL | 7.10 (2.70) | 6.90 (2.60) | 7.60 (3.00) | 0.002 |
| ANC, 1000 cells/uL | 4.10 (2.10) | 4.00 (2.00) | 4.40 (2.20) | 0.022 |
| Lymphocyte count, 1000 cells/uL | 2.10 (0.90) | 2.10 (0.80) | 2.20 (1.10) | 0.016 |
| Total bilirubin, mg/dL | 0.60 (0.40) | 0.60 (0.40) | 0.60 (0.30) | 0.351 |
| GGT, U/L | 19.00 (16.00) | 18.00 (14.00) | 24.00 (23.00) | <0.001 |
BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; GHb: glycosylated hemoglobin; TC: total cholesterol; LDLC: low-density lipoprotein; ALP, alkaline phosphatase; WBC, white blood cell count; ANC, absolute neutrophil count; GGT, gamma-glutamyl transferase.
Except for ALP, WBC, ANC, lymphocyte count, total bilirubin and GGT, which were represented as the median (interquartile range), the other continuous variables were represented by the mean ± standard deviation. The categorical variables were presented as number (percentage).
Statistical characteristics of selected serum aldehyde exposure were shown in Supplementary Table S2. The median concentrations were 1.395, 0.426, 1.965, 0.512, 2.130, and 0.500 ng/mL for benzaldehyde, isopentanaldehyde, propanaldehyde, butyraldehyde, hexanaldehyde and heptanaldehyde. Serum aldehydes levels yielded mild to moderate correlation, as evidenced by Pearson correlation coefficients ranging from 0.01 to 0.55 (Supplementary Fig. S1). The strongest correlations were observed between isopentanaldehyde and propanaldehyde (r = 0.55), as well as between propanaldehyde and butyraldehyde (r = 0.51).
3.2. Associations of individual aldehydes with SUA and hyperuricemia
In the fully adjusted model (Table 2, Supplementary Fig. S2), positive associations were observed between log-transformed propanaldehyde (β: 0.263, 95%CI: 0.062, 0.464), butyraldehyde (β: 0.317, 95%CI: 0.173, 0.460), and hexanaldehyde (β: 0.360, 95%CI: 0.201, 0.518) with elevated SUA level after FDR correction. Compared with the lowest-tertile concentration groups, SUA increased 0.214 mg/dL (95%CI: 0.032, 0.397), 0.446 mg/dL (95%CI: 0.299, 0.593), 0.428 mg/dL (95%CI: 0.216, 0.640), and 0.213 mg/dL (95%CI: 0.051, 0.374) in the highest tertile groups of propanaldehyde, butyraldehyde, hexanaldehyde, and heptanaldehyde, respectively, with positive trends for latter three aldehydes (adjusted Ptrend<0.05). Similar relationships were also found between aldehydes and hyperuricemia (Table 3, Supplementary Fig. S3), and log-transformed butyraldehyde (β: 1.903, 95%CI: 1.373, 2.638) was associated with a higher prevalence of hyperuricemia. Compared with their counterparts, the ORs (95%CIs) of highest tertile groups for isopentanaldehyde, butyraldehyde, hexanaldehyde, and heptanaldehyde were 2.002 (1.055, 3.797), 2.438 (1.579, 3.765), 2.628 (1.676, 4.122), and 1.731 (1.127, 2.659), with positive trends for butyraldehyde and hexanaldehyde (adjusted Ptrend<0.05), respectively.
Table 2.
Associations between selected serum aldehydes exposure and level of SUA.
| Aldehydes | Log-transformed |
T1 |
T2 |
T3 |
Ptrend | Adjusted Ptrenda | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| β (95%CI) | P value | Adjusted P valuea | β (95%CI) | β (95%CI) | P value | β (95%CI) | P value | |||
| Benzaldehyde | ||||||||||
| Model 1 | −0.111 (−0.225, 0.003) | 0.075 | 1.000[Ref.] | −0.165 (−0.382, 0.053) | 0.159 | −0.268 (−0.485, −0.051) | 0.028 | 0.028 | ||
| Model 2 | −0.006 (−0.115, 0.102) | 0.911 | 1.000[Ref.] | −0.066 (−0.230, 0.098) | 0.442 | −0.096 (−0.290, 0.098) | 0.349 | 0.347 | ||
| Model 3 | 0.005 (−0.089, 0.099) | 0.924 | 0.944 | 1.000[Ref.] | 0.001 (−0.160, 0.161) | 0.994 | −0.031 (−0.211, 0.149) | 0.739 | 0.739 | 0.739 |
| Isopentanaldehyde | ||||||||||
| Model 1 | 0.025 (−0.112, 0.162) | 0.729 | 1.000[Ref.] | 0.237 (0.060, 0.414) | 0.019 | 0.154 (−0.046, 0.354) | 0.151 | 0.157 | ||
| Model 2 | −0.061 (−0.157, 0.035) | 0.233 | 1.000[Ref.] | 0.141 (−0.028, 0.310) | 0.123 | 0.021 (−0.105, 0.148) | 0.744 | 0.762 | ||
| Model 3 | 0.004 (−0.102, 0.109) | 0.944 | 0.944 | 1.000[Ref.] | 0.145 (−0.002, 0.293) | 0.065 | 0.086 (−0.098, 0.270) | 0.375 | 0.271 | 0.325 |
| Propanaldehyde | ||||||||||
| Model 1 | 0.313 (0.050, 0.577) | 0.034 | 1.000[Ref.] | 0.189 (0.006, 0.371) | 0.061 | 0.242 (0.017, 0.467) | 0.053 | 0.231 | ||
| Model 2 | 0.230 (0.028, 0.432) | 0.042 | 1.000[Ref.] | 0.097 (−0.062, 0.255) | 0.251 | 0.170 (−0.030, 0.370) | 0.117 | 0.702 | ||
| Model 3 | 0.263 (0.062, 0.464) | 0.022 | 0.044 | 1.000[Ref.] | 0.139 (−0.035, 0.312) | 0.138 | 0.214 (0.032, 0.397) | 0.036 | 0.214 | 0.321 |
| Butyraldehyde | ||||||||||
| Model 1 | 0.347 (0.194, 0.499) | <0.001 | 1.000[Ref.] | −0.003 (−0.226, 0.220) | 0.979 | 0.428 (0.270, 0.586) | <0.001 | <0.001 | ||
| Model 2 | 0.269 (0.134, 0.404) | 0.001 | 1.000[Ref.] | −0.005 (−0.219, 0.210) | 0.965 | 0.351 (0.205, 0.497) | <0.001 | <0.001 | ||
| Model 3 | 0.317 (0.173, 0.460) | 0.001 | 0.003 | 1.000[Ref.] | 0.046 (−0.102, 0.195) | 0.550 | 0.446 (0.299, 0.593) | <0.001 | <0.001 | <0.001 |
| Hexanaldehyde | ||||||||||
| Model 1 | 0.340 (0.100, 0.580) | 0.014 | 1.000[Ref.] | 0.072 (−0.056, 0.199) | 0.287 | 0.392 (0.124, 0.660) | 0.012 | 0.011 | ||
| Model 2 | 0.391 (0.206, 0.575) | 0.001 | 1.000[Ref.] | 0.182 (0.065, 0.298) | 0.008 | 0.458 (0.233, 0.684) | 0.001 | 0.001 | ||
| Model 3 | 0.360 (0.201, 0.518) | <0.001 | <0.001 | 1.000[Ref.] | 0.228 (0.044, 0.411) | 0.028 | 0.428 (0.216, 0.640) | 0.001 | 0.001 | 0.003 |
| Heptanaldehyde | ||||||||||
| Model 1 | 0.253 (0.067, 0.439) | 0.018 | 1.000[Ref.] | 0.057 (−0.211, 0.325) | 0.683 | 0.319 (0.140, 0.497) | 0.003 | 0.004 | ||
| Model 2 | 0.235 (−0.011, 0.482) | 0.081 | 1.000[Ref.] | 0.078 (−0.191, 0.347) | 0.578 | 0.242 (0.028, 0.456) | 0.043 | 0.043 | ||
| Model 3 | 0.195 (−0.008, 0.398) | 0.079 | 0.119 | 1.000[Ref.] | 0.079 (−0.140, 0.297) | 0.492 | 0.213 (0.051, 0.374) | 0.021 | 0.022 | 0.044 |
SUA: serum uric acid; OR: odds ratio; 95%CI: 95 % confidence interval; FRD: false discovery rate.
Model 1 was a crude model, model 2 was adjusted for age, sex and race, and model 3 was further adjusted for educational level, family poverty income ratio group, body mass index, smoking status, drinking status, hypertension, diabetes, hypercholesterolemia, and chronic kidney disease.
Serum aldehydes exposure was analyzed as both continuous and categorical variables. The categorical variables were grouped by tertiles, with T1, T2 and T3 representing the first, second and third tertiles, respectively.
P value was adjusted by FDR method.
Table 3.
Associations between selected serum aldehydes exposure and hyperuricemia.
| Aldehydes | Log-transformed |
T1 |
T2 |
T3 |
Ptrend | Adjusted Ptrenda | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | P value | Adjusted P valuea | OR (95%CI) | OR (95%CI) | P value | OR (95%CI) | P value | |||
| Benzaldehyde | ||||||||||
| Model 1 | 0.975 (0.744, 1.276) | 0.855 | 1.000[Ref.] | 1.139 (0.826, 1.571) | 0.440 | 0.894 (0.586, 1.365) | 0.612 | 0.608 | ||
| Model 2 | 1.019 (0.778, 1.336) | 0.893 | 1.000[Ref.] | 1.221 (0.888, 1.677) | 0.238 | 0.965 (0.624, 1.494) | 0.877 | 0.891 | ||
| Model 3 | 1.061 (0.823, 1.370) | 0.653 | 0.653 | 1.000[Ref.] | 1.434 (0.949, 2.168) | 0.108 | 1.200 (0.783, 1.841) | 0.416 | 0.390 | 0.390 |
| Isopentanaldehyde | ||||||||||
| Model 1 | 1.221 (0.951, 1.567) | 0.139 | 1.000[Ref.] | 1.323 (0.763, 2.296) | 0.335 | 1.770 (1.163, 2.693) | 0.018 | 0.012 | ||
| Model 2 | 1.207 (0.937, 1.554) | 0.166 | 1.000[Ref.] | 1.293 (0.736, 2.271) | 0.386 | 1.734 (1.154, 2.607) | 0.018 | 0.012 | ||
| Model 3 | 1.314 (0.816, 2.117) | 0.279 | 0.335 | 1.000[Ref.] | 1.389 (0.720, 2.681) | 0.343 | 2.002 (1.055, 3.797) | 0.041 | 0.036 | 0.054 |
| Propanaldehyde | ||||||||||
| Model 1 | 1.485 (1.027, 2.145) | 0.045 | 1.000[Ref.] | 1.137 (0.769, 1.680) | 0.530 | 1.305 (0.955, 1.784) | 0.116 | 0.012 | ||
| Model 2 | 1.522 (1.049, 2.207) | 0.043 | 1.000[Ref.] | 1.149 (0.786, 1.680) | 0.484 | 1.333 (0.959, 1.854) | 0.108 | 0.012 | ||
| Model 3 | 1.474 (0.862, 2.519) | 0.176 | 0.264 | 1.000[Ref.] | 1.115 (0.678, 1.835) | 0.674 | 1.291 (0.809, 2.060) | 0.301 | 0.053 | 0.064 |
| Butyraldehyde | ||||||||||
| Model 1 | 1.676 (1.225, 2.293) | 0.006 | 1.000[Ref.] | 1.417 (0.892, 2.252) | 0.161 | 1.837 (1.247, 2.705) | 0.008 | 0.006 | ||
| Model 2 | 1.722 (1.270, 2.334) | 0.003 | 1.000[Ref.] | 1.434 (0.894, 2.297) | 0.155 | 1.884 (1.276, 2.782) | 0.006 | 0.005 | ||
| Model 3 | 1.903 (1.373, 2.638) | 0.002 | 0.012 | 1.000[Ref.] | 1.728 (1.133, 2.635) | 0.023 | 2.438 (1.579, 3.765) | 0.001 | 0.001 | 0.003 |
| Hexanaldehyde | ||||||||||
| Model 1 | 1.923 (1.141, 3.240) | 0.027 | 1.000[Ref.] | 1.218 (0.860, 1.725) | 0.284 | 2.307 (1.435, 3.708) | 0.004 | 0.006 | ||
| Model 2 | 2.017 (1.205, 3.377) | 0.018 | 1.000[Ref.] | 1.318 (0.886, 1.961) | 0.192 | 2.563 (1.618, 4.060) | 0.001 | 0.002 | ||
| Model 3 | 2.017 (1.201, 3.385) | 0.018 | 0.054 | 1.000[Ref.] | 1.432 (0.854, 2.401) | 0.194 | 2.628 (1.676, 4.122) | 0.001 | <0.001 | <0.001 |
| Heptanaldehyde | ||||||||||
| Model 1 | 1.614 (1.012, 2.574) | 0.043 | 1.000[Ref.] | 1.274 (0.819, 1.982) | 0.299 | 1.665 (1.159, 2.390) | 0.015 | 0.019 | ||
| Model 2 | 1.781 (1.133, 2.798) | 0.024 | 1.000[Ref.] | 1.335 (0.853, 2.090) | 0.225 | 1.736 (1.198, 2.516) | 0.011 | 0.013 | ||
| Model 3 | 1.598 (0.899, 2.841) | 0.131 | 0.262 | 1.000[Ref.] | 1.391 (0.903, 2.141) | 0.155 | 1.731 (1.127, 2.659) | 0.024 | 0.029 | 0.054 |
OR: odds ratio; 95%CI: 95 % confidence interval; FRD: false discovery rate.
Model 1 was a crude model, model 2 was adjusted for age, sex and race, and model 3 was further adjusted for educational level, family poverty income ratio group, body mass index, smoking status, drinking status, hypertension, diabetes, hypercholesterolemia, and chronic kidney disease.
Serum aldehydes exposure was analyzed as both continuous and categorical variables. The categorical variables were grouped by tertiles, with T1, T2 and T3 representing the first, second and third tertiles, respectively.
P value was adjusted by FDR method.
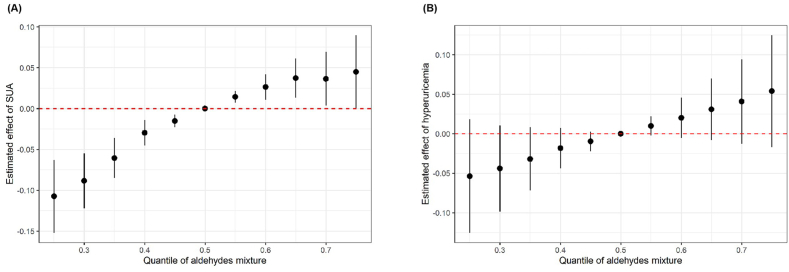
3.3. Associations of aldehyde mixture exposure with SUA and hyperuricemia
Since several single aldehydes were associated with SUA and hyperuricemia, we further assessed combined effects of aldehyde mixtures and the BKMR model showed that aldehyde mixtures exposure was associated with elevated SUA level (Fig. 2). Taking 50 % quantile as the reference, the SUA level showed a downward trend as quantiles decreased when all aldehyde mixture quantile <50 %. When all aldehyde mixture quantiles >50 %, the SUA value significantly increased with rising quantiles and estimated effect plateaued after 65 %. There was an apparent upward trend of hyperuricemia prevalence as the quantile of aldehyde mixture rose compared to the 50th percentiles, while no statistically significant associations were found. The BKMR model showed that hexanaldehyde had the highest PIP (0.995) for SUA, indicating it made the greatest contribution to the mixed exposure model (Supplementary Table S3).
Fig. 2.
Overall effect of the mixtures of selected serum aldehydes exposure on SUA and hyperuricemia using BKMR model
SUA: serum uric acid; BKMR: Bayesian kernel machine regression.
Models were adjusted for age, sex, race, educational level, family poverty income ratio group, body mass index, smoking status, drinking status, hypertension, diabetes, hypercholesterolemia, and chronic kidney disease. The estimated effects were assessed through comparing the aldehydes mixture at different percentiles with their median level.
By fixing other aldehydes at their 50th percentile exposure levels, we calculated univariate exposure-response functions of certain aldehydes in the mixture. The increasing trends were observed in SUA level with propanaldehyde, hexanaldehyde, and heptanaldehyde, as well as hyperuricemia risks with benzaldehyde, isopentanaldehyde, and hexanaldehyde (Supplementary Fig. S4). Meanwhile, we also assessed single effects of aldehydes to show changes in the effect of a certain aldehyde increasing from 25th to 75th percentiles when the other aldehydes were held at their 25th, 50th, and 75th percentiles, respectively (Supplementary Fig. S5). Propanaldehyde, hexanaldehyde, and heptanaldehyde were positively associated with SUA level and isopentanaldehyde showed a negative association. No significant associations were found between aldehydes and hyperuricemia. Moreover, as other aldehydes increasing from 25th to 75th percentiles, the effects of isopentanaldehyde, propanaldehyde, and heptanaldehyde on SUA level were increased, indicating correlations between aldehydes might influences effects of above three aldehydes.
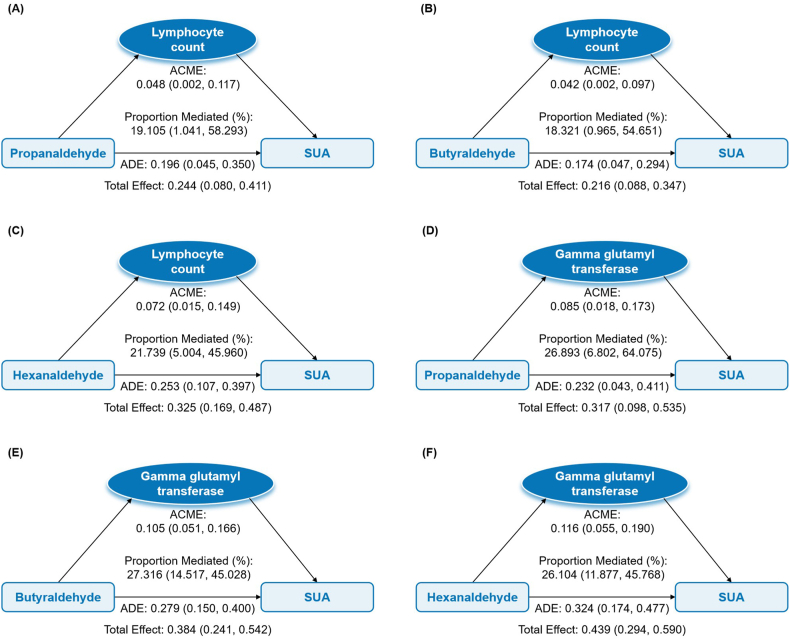
3.4. Mediating effects of inflammation and oxidative stress on associations between individual aldehydes and SUA and hyperuricemia
In the first step, there were several significantly positive associations between aldehydes and inflammation and oxidative stress indicators, which were mainly concentrated on lymphocyte count and GGT (Supplementary Table S4). In the second step, WBC, lymphocyte count, total bilirubin, and GGT were associated with elevated SUA, and GGT was also associated with hyperuricemia (Supplementary Table S5). Integrating associations in two steps, we found significant mediation effects of lymphocyte count and GGT for associations between three aldehydes and SUA. As shown in Fig. 3, lymphocyte count mediated 19.105 % (95%CI: 1.041, 58.293), 18.321 % (95%CI: 0.965, 54.651), and 21.739 % (95%CI: 5.004, 45.960) of the associations of propanaldehyde, butyraldehyde, and hexanaldehyde with SUA, respectively. Corresponding mediation proportions of GGT were 26.893 % (95%CI: 6.802, 64.075), 27.316 % (95%CI: 14.517, 45.028), and 26.104 % (95%CI: 11.877, 45.768), respectively.
Fig. 3.
Mediating effects of inflammation on associations between selected serum aldehydes exposure and SUA
SUA: serum uric acid; ACME, average causal mediation effect; ADE, average direct effect.
Models were adjusted for age, sex, race, educational level, family poverty income ratio group, body mass index, smoking status, drinking status, hypertension, diabetes, hypercholesterolemia, and chronic kidney disease.
3.5. Subgroup and sensitivity analyses
In subgroup analyses (Supplementary Figs. S6–S7), stronger associations with significant interactions (Pinteraction<0.05) were observed in overweight participants (BMI≥25) and hypercholesterolemia patients between hexanaldehyde and SUA, and overweight subjects between isopentanaldehyde and hyperuricemia. Marginally greater effects (Pinteraction<0.1) of several aldehydes on SUA or hyperuricemia were also discovered among overweight participants, hypertensive population, and hypercholesterolemia patients. Sensitivity analyses showed no substantial changes in associations of the individual aldehyde and mixed aldehydes with SUA and hyperuricemia after excluding participants with CKD and gout, which proved the result robustness (Supplementary Tables S6–S7, Fig. S8).
4. Discussion
The study demonstrated that several individual aldehydes were associated with higher SUA level (propanaldehyde, butyraldehyde, and hexanaldehyde) and hyperuricemia risks (butyraldehyde). The BKMR models ulteriorly illustrated that aldehyde mixtures were significantly associated with elevated SUA concentrations, in which hexanaldehyde contributed most. Furthermore, mediation effects of lymphocyte count and GGT were found in relationships between propanaldehyde, butyraldehyde, hexanaldehyde and SUA. Associations of aldehydes and SUA were more pronounced among individuals with obesity, hypercholesterolemia, and hypertension. Overall, our study provided the latest population-based evidence on the effects of aldehyde exposure on SUA level and hyperuricemia among U.S. adults.
As part of environmental pollution, aldehydes are reactive electrophiles that can induce toxicity by generating oxidative stress, lipid peroxidation, DNA adducts, protein adduct toxicity, and advanced glycation end-products [9]. The protein adducts resulting from aldehydes are typically reversible, but they have the potential to degrade into reactive advanced lipoxidation or glycoxidation end-products. These compounds can covalently cross-link proteins, leading to their accumulation with age and contributing to the disruption of protein and cellular function [9]. Increased endogenous carbonylated proteins have been found with aging and in various pathological conditions, including premature diseases, rheumatoid arthritis, and atherosclerosis [33]. Since aldehydes in the body are volatile and highly reactive, it is challenging to detect their internal exposure levels and few studies have assessed their impacts on human health before [14]. Benefited from the detection technique promotion, epidemiological studies on aldehydes have risen in recent years [34]. Currently, aldehydes have been observed to be associated with increased risks of CVDs, hypertension, diabetes, metabolic syndrome, and hypertriglyceridemia, which demonstrated their adverse impacts on human cardiometabolic health [[14], [15], [16],35,36]. Considering the close associations of SUA level and hyperuricemia with progression of CVDs, metabolic diseases, and renal diseases [37,38], we thus performed the population-based analysis to illustrate the positive associations of aldehydes with SUA level and hyperuricemia, and provided research evidence for this unaddressed scientific issue.
Based on the significant effect of individual aldehyde exposure, we further evaluated associations of aldehyde mixtures with SUA level and hyperuricemia using BKMR model. BKMR model is a novel hierarchical variable selection approach which could identify crucial mixture components and address the correlated nature of the mixture in complex, high-dimensional settings. This approach is adept at capturing the uncertainty in the exposure-response relationship and offer greater power for detecting important mixture components compared with previous methods [39]. Our results showed aldehyde mixtures were positively associated with SUA level and hexanaldehyde contributed most. As the combined exposure was more approximate to the realistic situation, our results indicated harmful effects of aldehydes on SUA and identified the most important component. Although no significant association was found between aldehyde mixtures and hyperuricemia, nearly linear and positive trends were found in Fig. 2. The possible reason might be that insufficient sample size resulted in lower statistical power, so that future studies could expand aldehyde detection items and enlarge study sample size to further examine the effect of mixed aldehydes.
When exploring mediating pathways, we found lymphocyte count and GGT could significantly mediate associations between aldehydes and SUA. The positive relationships of aldehydes with lymphocyte count and GGT in our study were consistent with previous studies [36,40]. Lymphocytes serve as indicators of chronic inflammation, coordinate immune system response and hold pivotal roles in cell-mediated immunity [41]. GGT is a widely distributed enzyme critical for maintaining glutathione homeostasis and providing protection against oxidative stress [42]. The underlying mechanism proposed that aldehydes might trigger inflammation and oxidative stress through diverse pathological mechanisms and lead to the increase of corresponding indicator levels [40]. Besides, aldehyde could cause immune system activation and immunotoxicity by affecting production and expression of cytokines, and then resulted in lymphocyte count change since cytokines play an important part in inducing cell-mediated immunity and humoral immunity [43]. Moreover, aldehyde exposure could lead to liver injury through disrupting mitochondrial energy metabolism or generating adducts, thus increasing the serum level of GGT which was linked with liver dysfunction [36].
As for the relationships between lymphocyte count, GGT and SUA, the plausible inference was that chronic inflammatory responses might influence UA metabolism. Endogenous sources are responsible for about two-thirds of the body's daily UA production. Chronic inflammation and inflammation-related states induce cell death and produce purine, which consequently contribute to increased UA concentration [44,45]. In addition, about 70 % of the daily UA produced in humans is excreted by the kidneys [46]. Since inflammation and oxidative stress were associated with kidney disease progression, the subsequent kidney injury might result in UA excretion dysfunction [47,48]. In turn, UA could induce inflammation response and oxidative stress, creating a vicious cycle [49,50].
In subgroup analyses, we found that highly exposed aldehydes were more strongly associated with higher level of SUA in participants with obesity, hypercholesterolemia, and hypertension. Similarly, other previous studies have reported that the association of aldehyde exposure with CVD and metabolic syndrome was more pronounced in those who were overweight or obese, and had hypertension or dyslipidemia [14,35]. These people might be more susceptible to the harmful effects of aldehyde exposure. In addition, aldehydes and obesity, hypertension and hypercholesterolemia might share common pathologic pathways, such as inflammation response, oxidative stress, myocardial dysfunction and liver injury, and thus produce synergistic effects [9,51]. Our findings suggested that future strategies to deal with aldehyde hazards should pay great attention to higher-risk groups with metabolic diseases and effectively protect them by controlling weight, blood pressure and lipid levels.
Many factors such as high-purine diet, alcohol consumption, obesity, and genetic risk could lead to elevated SUA level [52]. Besides, in this study, we identified aldehyde exposure as a novel risk factor for elevated SUA level and hyperuricemia, which could provide references for some unexplained SUA elevation. Meanwhile, exploring the etiology of elevated UA might help identify new intervention targets. At present, the disease burden of hyperuricemia is increasing, and the prevalence has not been effectively controlled, especially in developed countries such as the U.S [11,53]. Increasing UA is a risk factor for hypertension, CVDs, non-alcoholic fatty liver disease, and CKD, and the analysis of hyperuricemia etiology has important public health significance for reducing the burden of disease [54,55].
Using a nationally representative sample, we explored the effect of individual and mixed aldehyde exposure on UA and hyperuricemia for the first time, as well as identified the relative importance of each aldehyde in the mixture. In addition, we performed sufficient subgroup analyses to provide evidence across different populations. Further, we also explored the role of inflammation and oxidative stress biomarkers in the increase of SUA caused by aldehydes with significant public health significance for future intervention and prevention. However, several limitations also need to be considered in this study. First, it was difficult to determine the causality between aldehydes and SUA or hyperuricemia in a cross-sectional study. More longitudinal researches are required to validate our findings in the future. Second, a single measurement of aldehyde exposure might not adequately reflect individual long-term exposure level. Aldehydes have a long half-life within the body, so their influence on the outcomes might be underestimated. Third, despite implementing multiple adjustment, bias still persisted due to unmeasured confounders, such as high purine diet and medication that affects metabolism of SUA.
5. Conclusions
In brief, we found the positive associations of individual aldehydes with SUA level and hyperuricemia prevalence, and BKMR model indicated combined effects arising from aldehyde mixtures on higher SUA level among U.S. adults. Significant mediating effects of lymphocyte count and GGT were observed in associations between aldehydes with SUA. These findings provided evidence on potential deleterious effects of both single and mixed aldehydes on UA metabolism and indicated underlying pathophysiologic pathways. Given the complexity of assessing health risks of aldehyde mixtures, further longitudinal studies with larger sample sizes are still demanded to verify our results.
CRediT authorship contribution statement
Jinyue Li: Writing – review & editing, Writing – original draft, Visualization, Software, Resources, Methodology, Formal analysis, Data curation, Conceptualization. Hanping Ma: Writing – review & editing, Validation. Jingyang Wang: Writing – review & editing, Validation. Han Ma: Writing – review & editing, Writing – original draft, Supervision, Resources, Project administration, Methodology, Conceptualization.
Data availability statement
Publicly available datasets were analyzed in this study. These data can be uploaded at https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
Ethics statement
NHANES data collection was approved by the Institutional Review Board of the National Center for Health Statistics, and informed consent forms have been signed by all participants or their representatives before the study began.
Funding
None.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors acknowledge the staff and participants from the NHANES program for their important participation and contribution.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e39707.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Destaillats H., Spaulding R.S., Charles M.J. Ambient air measurement of acrolein and other carbonyls at the Oakland-San Francisco Bay Bridge toll plaza. Environ. Sci. Technol. 2002;36:2227–2235. doi: 10.1021/es011394c. [DOI] [PubMed] [Google Scholar]
- 2.Khlystov A., Samburova V. Flavoring compounds dominate toxic aldehyde production during E-cigarette vaping. Environ. Sci. Technol. 2016;50:13080–13085. doi: 10.1021/acs.est.6b05145. [DOI] [PubMed] [Google Scholar]
- 3.Cecinato A., Yassaa N., Di Palo V., Possanzin M. Observation of volatile and semi-volatile carbonyls in an Algerian urban environment using dinitrophenylhydrazine/silica-HPLC and pentafluorophenylhydrazine/silica-GC-MS. J. Environ. Monit. 2002;4:223–228. doi: 10.1039/b110616n. [DOI] [PubMed] [Google Scholar]
- 4.Elghawi U.M., Mayouf A.M. Carbonyl emissions generated by a (SI/HCCI) engine from winter grade commercial gasoline. Fuel. 2014;116:109–115. doi: 10.1016/j.fuel.2013.07.124. [DOI] [Google Scholar]
- 5.Lipari F., Dasch J.M., Scruggs W.F. Aldehyde emissions from wood-burning fireplaces. Environ. Sci. Technol. 1984;18:326–330. doi: 10.1021/es00123a007. [DOI] [PubMed] [Google Scholar]
- 6.Richardson S.D., Thruston A.D., Caughran T.V., et al. Identification of new ozone disinfection byproducts in drinking water. Environ. Sci. Technol. 1999;33:3368–3377. doi: 10.1021/es981218c. [DOI] [Google Scholar]
- 7.Bruce W.R., Lee O., Liu Z., Marcon N., Minkin S., O'Brien P.J. Biomarkers of exposure to endogenous oxidative and aldehyde stress. Biomarkers. 2011;16:453–456. doi: 10.3109/1354750x.2011.580369. [DOI] [PubMed] [Google Scholar]
- 8.Furuhata A., Nakamura M., Osawa T., Uchida K. Thiolation of protein-bound carcinogenic aldehyde. An electrophilic acrolein-lysine adduct that covalently binds to thiols. J. Biol. Chem. 2002;277:27919–27926. doi: 10.1074/jbc.M202794200. [DOI] [PubMed] [Google Scholar]
- 9.O'Brien P.J., Siraki A.G., Shangari N. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit. Rev. Toxicol. 2005;35:609–662. doi: 10.1080/10408440591002183. [DOI] [PubMed] [Google Scholar]
- 10.Yu W., Cheng J.D. Uric acid and cardiovascular disease: an update from molecular mechanism to clinical perspective. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.582680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen-Xu M., Yokose C., Rai S.K., Pillinger M.H., Choi H.K. Contemporary prevalence of gout and hyperuricemia in the United States and decadal trends: the national health and nutrition examination Survey, 2007-2016. Arthritis Rheumatol. 2019;71:991–999. doi: 10.1002/art.40807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma Y., Hu Q., Yang D., et al. Combined exposure to multiple metals on serum uric acid in NHANES under three statistical models. Chemosphere. 2022;301 doi: 10.1016/j.chemosphere.2022.134416. [DOI] [PubMed] [Google Scholar]
- 13.Zhang F., Wang H., Cui Y., et al. Association between mixed dioxin exposure and hyperuricemia in U.S. adults: a comparison of three statistical models. Chemosphere. 2022;303 doi: 10.1016/j.chemosphere.2022.135134. [DOI] [PubMed] [Google Scholar]
- 14.Xu C., Liang J., Xu S., Liu Q., Xu J., Gu A. Increased serum levels of aldehydes are associated with cardiovascular disease and cardiovascular risk factors in adults. J. Hazard Mater. 2020;400 doi: 10.1016/j.jhazmat.2020.123134. [DOI] [PubMed] [Google Scholar]
- 15.Weng X., Chen J., Fei Q., et al. The association of aldehydes exposure with diabetes mellitus in US population: NHANES 2013-2014. Chemosphere. 2022;291 doi: 10.1016/j.chemosphere.2021.133019. [DOI] [PubMed] [Google Scholar]
- 16.Zhu Y., Liu M., Fu W., Bo Y. Association between serum aldehydes and hypertension in adults: a cross-sectional analysis of the national health and nutrition examination Survey. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.813244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi Y., Williamson G. Quercetin lowers plasma uric acid in pre-hyperuricaemic males: a randomised, double-blinded, placebo-controlled, cross-over trial. Br. J. Nutr. 2016;115:800–806. doi: 10.1017/s0007114515005310. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y., Shen X., Li G., Yue S., Liang C., Hao Z. Association between aldehyde exposure and kidney stones in adults. Front. Public Health. 2022;10 doi: 10.3389/fpubh.2022.978338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ong F.H., Henry P.J., Burcham P.C. Prior exposure to acrolein accelerates pulmonary inflammation in influenza A-infected mice. Toxicol. Lett. 2012;212:241–251. doi: 10.1016/j.toxlet.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Xie F., Wu Z., Feng J., Li K., Li M., Wu Y. Association between systemic immune-inflammation index and serum uric acid in U.S. adolescents: a population-based study. Nutr Metab Cardiovasc Dis. 2023 doi: 10.1016/j.numecd.2023.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Packer M. Uric acid is a biomarker of oxidative stress in the failing heart: lessons learned from trials with allopurinol and SGLT2 inhibitors. J. Card. Fail. 2020;26:977–984. doi: 10.1016/j.cardfail.2020.08.015. [DOI] [PubMed] [Google Scholar]
- 22.Cho Y., Song M.K., Kim T.S., Ryu J.C. Identification of novel cytokine biomarkers of hexanal exposure associated with pulmonary toxicity. Environ Pollut. 2017;229:810–817. doi: 10.1016/j.envpol.2017.06.041. [DOI] [PubMed] [Google Scholar]
- 23.Liao S., Wu N., Gong D., et al. Association of aldehydes exposure with obesity in adults. Ecotoxicol. Environ. Saf. 2020;201 doi: 10.1016/j.ecoenv.2020.110785. [DOI] [PubMed] [Google Scholar]
- 24.Feig D.I., Kang D.H., Johnson R.J. Uric acid and cardiovascular risk. N. Engl. J. Med. 2008;359:1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu X., Kong X., Chen M., et al. Blood ethylene oxide, systemic inflammation, and serum lipid profiles: results from NHANES 2013-2016. Chemosphere. 2022;299 doi: 10.1016/j.chemosphere.2022.134336. [DOI] [PubMed] [Google Scholar]
- 26.Huang Q., Wan J., Nan W., Li S., He B., Peng Z. Association between manganese exposure in heavy metals mixtures and the prevalence of sarcopenia in US adults from NHANES 2011-2018. J. Hazard Mater. 2024;464 doi: 10.1016/j.jhazmat.2023.133005. [DOI] [PubMed] [Google Scholar]
- 27.Webster A.C., Nagler E.V., Morton R.L., Masson P. Chronic kidney disease. Lancet. 2017;389:1238–1252. doi: 10.1016/s0140-6736(16)32064-5. [DOI] [PubMed] [Google Scholar]
- 28.Levey A.S., Eckardt K.U., Tsukamoto Y., et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 29.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Roy. Stat. Soc. B: Methodological. 1995;57:289–300. [Google Scholar]
- 31.Coker E., Chevrier J., Rauch S., et al. Association between prenatal exposure to multiple insecticides and child body weight and body composition in the VHEMBE South African birth cohort. Environ. Int. 2018;113:122–132. doi: 10.1016/j.envint.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imai K., Keele L., Tingley D. A general approach to causal mediation analysis. Psychol. Methods. 2010;15:309–334. doi: 10.1037/a0020761. [DOI] [PubMed] [Google Scholar]
- 33.Chevion M., Berenshtein E., Stadtman E.R. Human studies related to protein oxidation: protein carbonyl content as a marker of damage. Free Radic. Res. 2000;33(Suppl):S99–S108. Published 2001/02/24. [PubMed] [Google Scholar]
- 34.Silva L.K., Hile G.A., Capella K.M., et al. Quantification of 19 aldehydes in human serum by headspace SPME/GC/High-Resolution mass spectrometry. Environ. Sci. Technol. 2018;52:10571–10579. doi: 10.1021/acs.est.8b02745. [DOI] [PubMed] [Google Scholar]
- 35.Ba Y., Guo Q., Du A., et al. Association between serum aldehyde concentrations and metabolic syndrome in adults. Environ. Sci. Pollut. Res. Int. 2023;30:74290–74300. doi: 10.1007/s11356-023-27459-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li D., Chen Z., Shan Y., et al. Liver enzymes mediate the association between aldehydes co-exposure and hypertriglyceridemia. Ecotoxicol. Environ. Saf. 2023;263 doi: 10.1016/j.ecoenv.2023.115346. [DOI] [PubMed] [Google Scholar]
- 37.Nishizawa H., Maeda N., Shimomura I. Impact of hyperuricemia on chronic kidney disease and atherosclerotic cardiovascular disease. Hypertens. Res. 2022;45:635–640. doi: 10.1038/s41440-021-00840-w. [DOI] [PubMed] [Google Scholar]
- 38.Yanai H., Adachi H., Hakoshima M., Katsuyama H. Molecular biological and clinical understanding of the pathophysiology and treatments of hyperuricemia and its association with metabolic syndrome, cardiovascular diseases and chronic kidney disease. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22179221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bobb J.F., Valeri L., Claus Henn B., et al. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics. 2015;16:493–508. doi: 10.1093/biostatistics/kxu058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zang X., Qin W., Xiong Y., et al. Using three statistical methods to analyze the association between aldehyde exposure and markers of inflammation and oxidative stress. Environ. Sci. Pollut. Res. Int. 2023;30:79437–79450. doi: 10.1007/s11356-023-27717-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bosire E.M., Nyamache A.K., Gicheru M.M., Khamadi S.A., Lihana R.W., Okoth V. Population specific reference ranges of CD3, CD4 and CD8 lymphocyte subsets among healthy Kenyans. AIDS Res. Ther. 2013;10:24. doi: 10.1186/1742-6405-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ndrepepa G., Colleran R., Kastrati A. Gamma-glutamyl transferase and the risk of atherosclerosis and coronary heart disease. Clin. Chim. Acta. 2018;476:130–138. doi: 10.1016/j.cca.2017.11.026. [DOI] [PubMed] [Google Scholar]
- 43.Wei H., Tan K., Sun R., Yin L., Zhang J., Pu Y. Aberrant production of Th1/Th2/Th17-related cytokines in serum of C57BL/6 mice after short-term formaldehyde exposure. Int J Environ Res Public Health. 2014;11:10036–10050. doi: 10.3390/ijerph111010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.George C., Leslie S.W., Minter D.A. StatPearls Publishing LLC.; 2023. Hyperuricemia. Treasure Island (FL) Ineligible Companies. [Google Scholar]
- 45.van Loo G., Bertrand M.J.M. Death by TNF: a road to inflammation. Nat. Rev. Immunol. 2023;23:289–303. doi: 10.1038/s41577-022-00792-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li D., Yuan S., Deng Y., et al. The dysregulation of immune cells induced by uric acid: mechanisms of inflammation associated with hyperuricemia and its complications. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1282890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anders H.J., Schaefer L. Beyond tissue injury-damage-associated molecular patterns, toll-like receptors, and inflammasomes also drive regeneration and fibrosis. J. Am. Soc. Nephrol. 2014;25:1387–1400. doi: 10.1681/asn.2014010117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ho H.J., Shirakawa H. Oxidative stress and mitochondrial dysfunction in chronic kidney disease. Cells. 2022;12 doi: 10.3390/cells12010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jung S.W., Kim S.M., Kim Y.G., Lee S.H., Moon J.Y. Uric acid and inflammation in kidney disease. Am J Physiol Renal Physiol. 2020;318:F1327–f1340. doi: 10.1152/ajprenal.00272.2019. [DOI] [PubMed] [Google Scholar]
- 50.Gherghina M.E., Peride I., Tiglis M., Neagu T.P., Niculae A., Checherita I.A. Uric acid and oxidative stress-relationship with cardiovascular, metabolic, and renal impairment. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms23063188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luo J., Hill B.G., Gu Y., et al. Mechanisms of acrolein-induced myocardial dysfunction: implications for environmental and endogenous aldehyde exposure. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H3673–H3684. doi: 10.1152/ajpheart.00284.2007. [DOI] [PubMed] [Google Scholar]
- 52.Li L., Zhang Y., Zeng C. Update on the epidemiology, genetics, and therapeutic options of hyperuricemia. Am J Transl Res. 2020;12:3167–3181. Published 2020/08/11. [PMC free article] [PubMed] [Google Scholar]
- 53.Singh G., Lingala B., Mithal A. Gout and hyperuricaemia in the USA: prevalence and trends. Rheumatology. 2019;58:2177–2180. doi: 10.1093/rheumatology/kez196. [DOI] [PubMed] [Google Scholar]
- 54.Johnson R.J., Bakris G.L., Borghi C., et al. Hyperuricemia, acute and chronic kidney disease, hypertension, and cardiovascular disease: report of a scientific workshop organized by the national kidney foundation. Am. J. Kidney Dis. 2018;71:851–865. doi: 10.1053/j.ajkd.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Darmawan G., Hamijoyo L., Hasan I. Association between serum uric acid and non-alcoholic fatty liver disease: a meta-analysis. Acta Med. Indones. 2017;49:136–147. Published 2017/08/10. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available datasets were analyzed in this study. These data can be uploaded at https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.