Abstract
Photocatalytic overall water splitting is a promising approach for a sustainable hydrogen provision using solar energy. For sufficient solar energy utilization, this reaction ought to be operated based on visible-light-active semiconductors, which is very challenging. In this work, an F-expedited nitridation strategy is applied to modify the wide-bandgap semiconductor Sr2TiO4 for visible-light-driven photocatalytic overall water splitting. Compared to the conventional nitridation approach, F-expedited nitridation introduces the desirable integration of a high concentration of N dopant for strong visible light absorption and a low concentration of defects (i.e. Ti3+ and oxygen vacancies) for effective separation of photocarriers. After being coated with Ti-oxyhydroxide protection layer and deposited with RhCrOy cocatalyst, the product from F-expedited nitridation can stably run photocatalytic overall water splitting with apparent quantum efficiency of 0.39% at 420 ± 20 nm and solar-to-hydrogen efficiency of 0.028%. These findings justify the effectiveness of F-expedited nitridation strategy and serve as a guidance to upgrade the photocatalytic activity of many other wide-bandgap semiconductors.
Subject terms: Nanoparticles, Photocatalysis, Artificial photosynthesis, Photocatalysis
Photocatalytic overall water splitting is a promising approach for sustainable hydrogen production, but it remains challenging under visible light. Here, the authors report an F-expedited nitridation strategy for Sr2TiO4 that enables visible-light-driven photocatalytic overall water splitting.
Introduction
Green hydrogen production technologies involving only solar irradiation, water, and photocatalysts are considered to enable a zero-carbon economic model and the development of clean energy facilities1–3. Such a promising scenario, nevertheless, awaits significant advancements in photocatalytic materials that can convert solar energy efficiently into hydrogen energy. Specifically, the photocatalytic materials should harvest appreciable amounts of solar photons to generate photocarriers (e− and h+) which in turn, move to the surface to catalyze water-splitting reactions in competing with the unproductive photocarrier recombination4. Layered compounds have been recognized as promising candidates for photocatalytic overall water splitting (POWS) due to the charge separation driving force derived from their structural laminations5–7. For instance, high POWS activities have been reported over A4Nb6O17 (A = K, Rb)8, A2-xLa2Ti3-xNbxO10·nH2O (A = K, Rb, Cs; x = 0, 0.5, 1)9, A′2ATa2O7 (A′ = H or K, A = La2/3 or Sr)10, Sr2M2O7 (M = Nb, Ta)11 etc., whose layered architectures play a pivotal role in expediting photocarrier dissociation and migration. However, most layered compounds are wide-bandgap semiconductors thereby having intrinsic limitations on POWS efficiency in terms of solar energy conversions12. Accordingly, it is highly desirable to exploit layered compounds with visible light sensitivity for POWS reactions.
As a typical Ruddlesden-Popper (RP) type layered perovskite, Sr2TiO4 has received extensive attention as it is a robust, easily producible compound comprising of cheap and low toxic elements. Aliovalent metal doping (e.g. Cr13,14, Fe15, Mn16, Ag17, La/Cr14, La/Fe18, La/Rh19, etc.), non-metal doping (e.g. F20, N21,22, etc.), and mixed doping (e.g. Nb/N21, La/N22, Cr/F23, etc.) tactics have been applied to extend the light absorption threshold of Sr2TiO4 into the visible light region. However, there is currently no viable approach to enable POWS activity over Sr2TiO4 under visible light illumination, which is the bottleneck for the development of this promising compound. Among various dopants studied, of note are N anions as they with high concentrations contribute to the hybridized 2p-orbital-based energy states of O and N, endorsing relatively higher mobility for the photo-generated holes. Doping N into metal oxides, i.e. nitridation, is normally conducted by high-temperature ammonolysis in which appropriate metal oxide precursors are calcined at elevated temperatures in the presence of flowing ammonia gas24,25. This is a typical non-equilibrium solid-gas reaction that involves multiple diffusion steps and sluggish kinetic processes as the replacement of oxygen by nitrogen is very slow26,27. A low N doping level (<1 at.%) at anion sites is usually observed28,29 and there is a strong likelihood that the product conceives of both low visible light absorbance and plentiful unwanted defects in order to balance the charge disturbance raised by O/N substitution26.
In this study, we adopted Sr2TiO3F2 as a precursor for F-expedited nitridation of Sr2TiO4. The choice of a fluorinated precursor is based on the following considerations: (1) F is of a higher electronegativity than O. The presence of F can weaken the Ti–O bonds according to the inductive effect30, facilitating O/N exchange. This is supported by the longer equatorial Ti–O bonds of Sr2TiO3F2 (~1.9737 Å) than that of Sr2TiO4 (~1.9433 Å)31,32. (2) F can serve as a co-dopant to N. Doping N and F together helps to minimize the charge variations at the anion sublattice, i.e. . Our results show that the presence of F is critical for increasing the N uptake, facilitating crystal growth, and inhibiting detrimental defects like Ti3+ and oxygen vacancies in the product. Notably, F-expedited nitridation of Sr2TiO4 delivers superior photocatalytic performance in overall water splitting, liberating H2 and O2 in a 2:1 ratio, under visible light.
Results
Different nitridation strategies
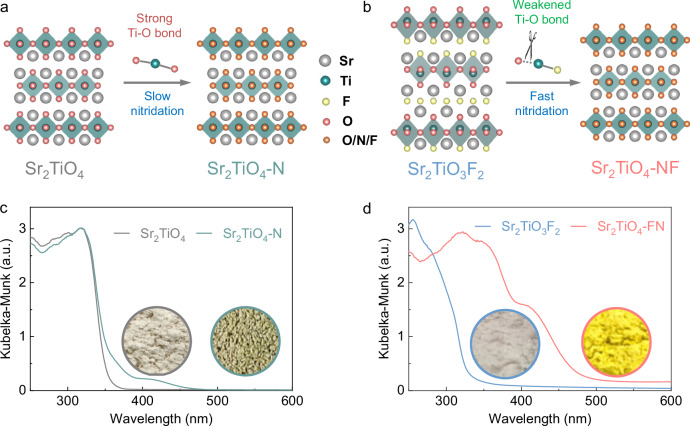
Considering the fact that direct nitridation of Sr2TiO4 by the conventional method (N-doped Sr2TiO4, denoted as Sr2TiO4-N, Fig. 1a) is subject to the slow kinetics due to the strong Ti–O bonds, Sr2TiO4 is firstly fluorinated into Sr2TiO3F2 to weaken the Ti–O bonds so as to facilitate O/N exchange during nitridation (N, F co-doped Sr2TiO4, denoted as Sr2TiO4-NF, Fig. 1b). The Sr2TiO3F2 has both interlayer and intralayer F anions in the structure which not only elongate the equatorial Ti–O bonds but also serve as the co-dopants to N. The morphology of product particles was inspected by field emission scanning electron microscope (FE-SEM, Supplementary Fig. 1). Although Sr2TiO4 and Sr2TiO3F2 both contain irregularly shaped particles, their nitridation products reveal dissimilar particle morphologies. Specifically, Sr2TiO4-N is composed of perforated particles with rough surfaces. This has been attributed to the slow O/N exchanging rate that results in poor crystal growth during high-temperature ammonolysis24. In contrast, Sr2TiO4-NF comprises plate-like particles with clear and smooth crystal facets, properly reflecting the crystal habit of a layered structure. Such distinct particle morphologies indicate that F helps to facilitate crystal growth during high-temperature ammonolysis.
Fig. 1. Different nitridation strategies.
a Direct nitridation of Sr2TiO4. b F-expedited nitridation of Sr2TiO4 using Sr2TiO3F2. c UV–visible absorption spectra of Sr2TiO4 and Sr2TiO4-N. d UV–visible absorption spectra of Sr2TiO3F2 and Sr2TiO4-NF, the insets show the digital photographs of sample powders. Source data for UV–visible absorption spectra are provided as a Source Data file.
Apart from the distinct particle morphologies, the products also reveal different light absorption capacities as illustrated in Fig. 1c, d. It is observed that the absorption edge of Sr2TiO4 occurs at 353 nm, indicative of a bandgap ~3.51 eV. The absorption edge position of Sr2TiO4-N closely resembles that of Sr2TiO4, with only a small absorption tail extending to 475 nm. In contrast, the light absorption range of Sr2TiO3F2, as shown in Fig. 1d, is blue-shifted compared to Sr2TiO4. Interestingly, the absorption edge of Sr2TiO4-NF experiences a large band-to-band redshift to 482 nm. This is also reflected by their distinct powder colors, i.e. greyish green for Sr2TiO4-N (Fig. 1c inset) and bright yellow for Sr2TiO4-NF (Fig. 1d inset). The much stronger visible light absorption can be ascribed to higher N content in Sr2TiO4-NF than in Sr2TiO4-N (see the following section) as N contributes to additional energy states above the top of the O-based valence band. The bandgap values of these compounds are then deduced according to the threshold of their light absorption (Supplementary Fig. 2). N doping notably reduces the bandgap of Sr2TiO4, being beneficial for an enhanced solar energy harvest. The band-edge positions of Sr2TiO4-N and Sr2TiO4-NF were explored based on the ultraviolet photoelectron spectra (UPS) technique using synchrotron radiation (Supplementary Fig. 3). The onset of UPS valence band spectra corresponds to the top of the valence band (VB) relative to the work function whose value can be deduced according to the UPS secondary electron cutoff spectra33. The band-edge positions can then be determined based on this information as well as the bandgap values. The sketched band-edge positions of Sr2TiO4-N and Sr2TiO4-NF are shown in Supplementary Fig. 4. Although both samples own a comparable work function (also indicated by Mott-Schottky analysis of Supplementary Fig. 5), Sr2TiO4-NF has higher levels of conduction band (CB) and VB positions than Sr2TiO4-N. This can be rationalized by the fact that a high N content in Sr2TiO4-NF uplifts the top of VB and F with a larger electronegativity contributing to the higher antibonding orbitals (i.e. CB). Nevertheless, both samples have straddled band-edge positions relative to water redox potentials, being thermodynamically feasible for overall water splitting.
Structure, composition, and coordination analysis
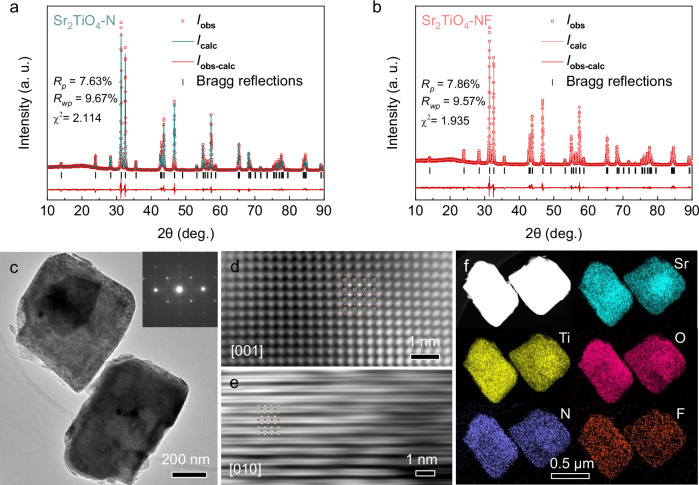
X-ray diffraction (XRD) analysis (Supplementary Fig. 6) suggests that Sr2TiO4-N and Sr2TiO4-NF exhibit similar patterns to the standard ones of Sr2TiO4 (JCPDS: 00-039-1471), indicating that the RP-type layered structure is maintained for both samples. This is also confirmed by Raman spectra that show identical vibration modes for Sr2TiO4, Sr2TiO4-N, and Sr2TiO4-NF (Supplementary Fig. 7). Rietveld refinements have been applied to investigate the crystal structure of Sr2TiO4-N and Sr2TiO4-NF. The refined XRD patterns are illustrated in Fig. 2a, b with the agreement factors included as the insets. The dopants, i.e. N and F, probably occupy randomly in the anion sublattice, as the agreement factors show no improvement in the case of ordered occupancies. The refined unit cell of Sr2TiO4-NF is slightly expanded when compared to Sr2TiO4 and Sr2TiO4-N, implying a higher N uptake in the presence of F (Supplementary Table 1). Transmission electron microscopy (TEM) analysis further indicates that each Sr2TiO4-NF particle is a single crystal with high crystallinity as revealed by the sharp selected area electron diffraction patterns as well as the well-defined lattice fringes in the high-resolution TEM images (Fig. 2c–e). All elements, including the N/F dopants, are uniformly distributed within the particles of Sr2TiO4-NF, as revealed by TEM-EDX element mapping analysis (Fig. 2f).
Fig. 2. Crystal structure analysis.
a Refined XRD patterns of Sr2TiO4-N, agreement factors (Rp, Rwp, and χ2) are inserted. b Refined XRD patterns of Sr2TiO4-NF, agreement factors (Rp, Rwp, and χ2) are inserted. c TEM image of Sr2TiO4-NF particles, inset shows the selected area electron diffraction patterns of a single particle in the upper part. d High-resolution TEM image of Sr2TiO4-NF particle along [001] direction, inset shows the projected crystal structure along [001] direction. e High-resolution TEM image of Sr2TiO4-NF particle along [010] direction, inset shows the projected crystal structure along [010] direction. f TEM-EDX element mapping images of Sr2TiO4-NF particles, all elements (Sr, Ti, O, F, N) are uniformly distributed in two typical particles. Source data for refined XRD are provided as a Source Data file.
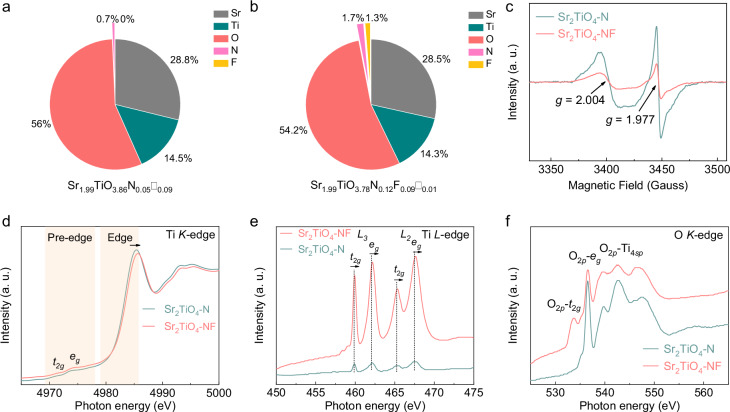
Element content analysis suggests that N and N/F are detected respectively in Sr2TiO4-N (Fig. 3a) and Sr2TiO4-NF (Fig. 3b), indicative of successful N doping and N/F co-doping. Accordant with previous expectations, the N content in the product is increased by a factor of 2.4 in the presence of F even though the nitridation conditions are kept the same for both samples. The oxygen vacancies, however, are decreased by a factor of 9 in the presence of F. These observations are analog to N/F co-doped TiO234 and N/F co-doped SrTiO335. The chemical formula of Sr2TiO4-N and Sr2TiO4-NF can be written as Sr1.99TiO3.86N0.05□0.09 and Sr1.99TiO3.78N0.12F0.09□0.01, respectively, where oxygen vacancies are represented as empty squares ‘□‘. Accordingly, Ti has an average valence of ~+3.9 in Sr2TiO4-N and ~+4.0 in Sr2TiO4-NF. The surface of sample powders is then studied by X-ray photoelectron spectroscopic (XPS, Supplementary Fig. 8) techniques. The XPS Ti 2p spectra involve a single spin-orbit doublet for all samples, being assignable to 2p3/2 and 2p1/2 states of Ti4+ species17,36. It is noteworthy that Ti3+ species, observed in N/F co-doped SrTiO335, are not detected here. This might correlate with the high Sr content in Sr2TiO4 that helps to stabilize Ti4+ species according to the inductive effect37,38. The successful N doping for Sr2TiO4-N and Sr2TiO4-NF is confirmed by a weak peak around 396 eV in XPS N 1s spectra which is assigned to N3− species in the lattice39. Likewise, the existence of F− species in Sr2TiO4-NF is also suggested by a strong peak around 685 eV in XPS F 1s spectra which is the same as its precursor Sr2TiO3F240. The XPS O 1s spectra of all samples comprise two overlapping peaks centered around 529 eV and 531 eV, assignable to lattice oxygen (O2−) and surface hydroxyl groups (OH−), respectively41. The strong signal for surface hydroxyl groups is consistent with the high hydrophilicity of Sr2TiO4-based compounds14,18,19.
Fig. 3. Coordination states of elements.
Element content of a Sr2TiO4-NF and b Sr2TiO4-N by ICP, ONH, and ion chromatograph analysis, deduced chemical formula are shown at the bottom (oxygen vacancies are represented by empty squares ‘□‘); c EPR spectra of Sr2TiO4-N and Sr2TiO4-NF measured at 100 K. d XAS spectra at Ti K-edges of Sr2TiO4-N and Sr2TiO4-NF, the shading areas correspond to the pre-edge and edge region; e XAS spectra at Ti L-edges of Sr2TiO4-N and Sr2TiO4-NF, L3 and L2 denote signals due to spin-orbit interactions whilst t2g and eg denote signals due to octahedral crystal field splitting; f XAS spectra at O K-edges of Sr2TiO4-N and Sr2TiO4-NF, O2p-t2g, O2p-eg, and O2p-Ti4sp denote the hybridized states between O 2p and Ti 3d and 4sp orbitals. Source data for element content, ESR spectra, and XAS spectra are provided as a Source Data file.
Although XPS spectra suggest no significant difference in terms of the surface state, Sr2TiO4-N and Sr2TiO4-NF are essentially different in defect concentrations, as revealed by EPR and X-ray absorption spectra (XAS). Both Sr2TiO4-N and Sr2TiO4-NF show detectable EPR signals at g values of ~2.004 and ~1.977 (Fig. 3c), corresponding to oxygen vacancies and Ti3+ species42,43, respectively. Notably, Sr2TiO4-NF has much weaker EPR signals than Sr2TiO4-N, corresponding to a much lower concentration of both defects. This is further supported by XAS. XAS spectra at Ti K-edges show that the transition energy of Sr2TiO4-NF is positively shifted compared to Sr2TiO4-N at both pre-edge and edge region (Fig. 3d), indicating a higher valence state of Ti in Sr2TiO4-NF than in Sr2TiO4-N. Similar observations are also noticed in XAS spectra at Ti L-edges which reveal transitions from occupied Ti 2p orbitals to unoccupied Ti 3d orbitals (Fig. 3e). The two doublets, arising from spin-orbit interactions (2p3/2(L3), 2p1/2(L2)) and octahedral crystal field splitting (t2g, eg), shift slightly to higher energy when comparing Sr2TiO4-NF with Sr2TiO4-N, affirming again the higher Ti valence in the former. Moreover, the eg states at Ti L-edges are known to be more sensitive to octahedral distortion than t2g states due to the fact that dz2 and dx2-y2 orbitals are pointing towards ligand anions44. It can be seen from Fig. 3e that eg peaks of Sr2TiO4-N have a higher level of asymmetry than those of Sr2TiO4-NF. In other words, the dz2 and dx2-y2 orbitals of Ti 3d states are less degenerated in Sr2TiO4-N, being consistent with its higher concentration of defects (e.g. oxygen vacancies) that break the octahedral symmetry. In addition, the XAS spectra at O K-edges also provide useful information for the Ti states due to the strong hybridization between O 2p and Ti 3d and 4sp orbitals. Figure 3f shows the XAS spectra at O K-edges for Sr2TiO4-NF and Sr2TiO4-N. The pre-edge absorption at the low-energy side (<540 eV) is assignable to the transitions from O 1s orbitals to the hybridized O 2p and Ti 3d orbitals which split into O2p-t2g and O2p-eg hybridized states due to octahedral crystal field splitting45. Compared to Sr2TiO4-NF, Sr2TiO4-N has a poorly resolved O2p-t2g state, probably because of its high Ti3+ concentration that quenches the transition by filling electrons into the empty t2g orbitals. These results jointly confirm that Sr2TiO4-NF has a much lower defect concentration than Sr2TiO4-N, being consistent with the composition analysis. The decrement of defect concentration is fully consistent with earlier expectations: the presence of F not only facilitates O/N exchange by weakening Ti–O bonds but also balances the charge variations by serving as a co-dopant to N, both of which reduce the risks of defect formation.
Photocarrier separation
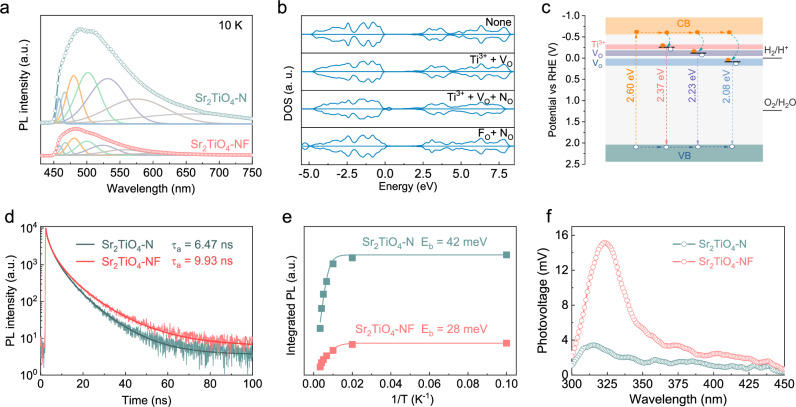
The photocarrier behavior in Sr2TiO4-N and Sr2TiO4-NF were initially examined through photoluminescence (PL) spectroscopy by cooling the sample to 10 K to suppress the phonon-assisted nonradiative recombination46. For both samples, PL spectra showed a broad emission band in the visible light region, corresponding to a variety of radiative photocarrier recombination events. The broad PL emission band can be deconvolved into a series of emission peaks that have different origins, as shown in Fig. 4a. The peaks centered below 500 nm are probably due to interband emissions as their photon energy exceeds the light absorption threshold of Sr2TiO4-N and Sr2TiO4-NF. The strong peak around 500 nm can be assigned to the band-edge emissions as it is close to the light absorption edges. The rest peaks above 500 nm are attributable to the defect-mediated emissions within the bandgap because of their low photon energy. Similar defect-mediated emissions have also been noticed in N/F doped and N/F co-doped TiO2 and SrTiO3, and originated from Ti3+ species and oxygen vacancies that are singly and doubly ionized47,48. Notably, Sr2TiO4-NF is characterized by much weaker emission intensity at this region than Sr2TiO4-N, being accordant with its lower concentration of Ti3+ and oxygen vacancies. Density Functional Theory (DFT) calculation further suggests that Ti3+ and oxygen vacancies tend to form additional energy states below the conduction band and N dopants help to uplift the top of the valence band (Fig. 4b and Supplementary Figs. 9–12). The energy levels of these defect states can then be deduced based on PL data. A schematic illustration for Sr2TiO4-N is depicted in Fig. 4c. Although Sr2TiO4-N is feasible for overall water splitting, the separation of photocarriers is severely impaired by defects, i.e. Ti3+ and oxygen vacancies. These defects align relatively deep below the conduction band and thereby are efficient trapping/recombination centers for photocarriers, being detrimental to photocatalytic activity. It is generally considered that defects with shallow energy states (i.e. shallow defects) may play a positive role in photocarrier separation, particularly when they are located at the surface49. However, defects with deep energy states (i.e. deep defects) generally undermine photocarrier separation as photocarriers can be tightly trapped and are unlikely to escape from these defects for surface reactions49. This is particularly true for oxygen vacancies here as they form deep defects whose energy states lie close to or lower than the water reduction potential. Presumably, the trapped electrons by oxygen vacancies can hardly escape and would have an inadequate driving force for water reduction reactions. From these considerations, co-doping N/F into Sr2TiO4 is more preferable than doping N alone due to a lower concentration of both Ti3+ and oxygen vacancies that is beneficial for photocarrier separation.
Fig. 4. Separation of photocarriers.
a PL spectra of Sr2TiO4-N and Sr2TiO4-NF by the 420 nm excitation at 10K. b DFT-calculated DOS of Sr2TiO4 with different defects, i.e. Ti3+, oxygen vacancies (VO), nitrogen at the oxygen site (NO), and fluorine at the oxygen site (FO), the fermi level is adjusted properly for better comparisons. c Schematic illustration of photocarrier behaviors in Sr2TiO4-N, i.e. generation, transportation, being captured by defects, defect-mediated radiative recombination, Ti3+ species, and oxygen vacancies that are singly and doubly ionized are labeled as Ti3+, VO•, and VO, respectively. d Time-resolved PL spectra of Sr2TiO4-N and Sr2TiO4-NF, sample powders are excited by 420 nm photons at 10K, and amplitude-weighted average PL decay lifetime (τa) is shown as inset. e Integrated PL emission intensity as a function of temperature and the fitted exciton binding energy (Eb) curves for Sr2TiO4-N and Sr2TiO4-NF. f SPV spectra of Sr2TiO4-N and Sr2TiO4-NF. Source data for PL, DFT-calculated DOS, TRPL, integrated PL, and SPV spectra are provided as a Source Data file.
As a support to the above statements, time-resolved photoluminescence (TRPL) decay curves and PL emission intensity at different temperatures are analyzed and fitted based on a double first-order exponential decay model (equation (1))50 and Arrhenius relation (equation (2))51, respectively:
| 1 |
| 2 |
where A(t) is PL intensity at time t, I(T) is integrated PL intensity at temperature T, Eb is the exciton binding energy, kB is the Boltzmann constant. The amplitude-weighted average PL decay lifetime (τa) is then determined by equation (3):
| 3 |
It can be seen from Fig. 4d and e that Sr2TiO4-NF reveals a longer TPRL decay lifetime (τa = 9.93 ns) and a smaller exciton binding energy (Eb = 28 meV) than Sr2TiO4-N (τa = 6.47 ns, Eb = 42 meV). The Eb of Sr2TiO4-NF is comparable to the thermal energy (25 meV at 293 K), indicating that excitons can be readily dissociated into free photocarriers within Sr2TiO4-NF at ambient conditions. Correspondingly, Sr2TiO4-NF exhibits a notably higher surface photovoltage (SPV) signal compared to Sr2TiO4-N, as illustrated in the SPV spectra (Fig. 4f). The larger photocurrent (Supplementary Fig. 13) and slower open-circuit voltage decay process (Supplementary Fig. 14) also revealed that Sr2TiO4-NF has more efficient photocarrier separation efficiency than Sr2TiO4-N, resulting in more effective quenching of the triplet EPR signals of TEMPO under light illumination (Supplementary Fig. 15).
Photocatalytic overall water splitting
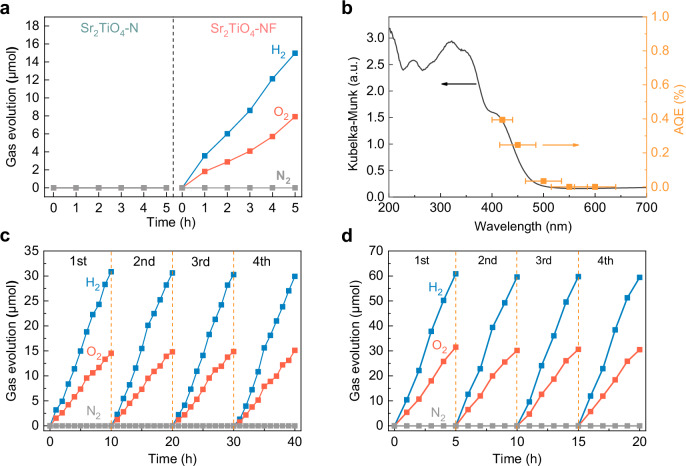
After loading Rh-Cr bimetallic oxide (i.e. RhCrOy) as a cocatalyst, Sr2TiO4-NF, characterized by wide spectral response and low defect concentration, demonstrates the capability to achieve photocatalytic overall water splitting with a stoichiometric ratio of hydrogen to oxygen of 2:1 under visible light illumination (λ ≥ 420 nm, Supplementary Fig. 16). However, apart from H2 and O2, there was a simultaneous production of N2 during the experiment, suggesting that oxidative self-corrosion reactions occurred over Sr2TiO4-NF. To address this issue, Sr2TiO4-NF was coated with a thin protection layer of amorphous Ti-oxyhydroxide (denoted as TiOXH) against self-corrosion52. This treatment not only inhibited N2 evolution but also facilitated H2/O2 evolution by a factor of 2.4 as illustrated in Supplementary Fig. 16. The TiOXH is reported to have dual functions: (1) it serves as a permeation barrier for O2, therefore suppressing the occurrence of water-splitting reverse reactions; (2) it facilitates hole injection from photocatalyst to the TiOXH layer, therefore avoiding excessive buildup of holes at the photocatalyst’s surface and reducing the likelihood of photocorrosion52,53. Therefore, TiOXH was coated onto sample powders after loading RhCrOy unless otherwise specified. In contrast to Sr2TiO4-NF, Sr2TiO4-N was completely inactive for POWS reactions under the same conditions (Fig. 5a). Applying additional annealing treatment has no improvement on the photocatalytic activity of Sr2TiO4-N (Supplementary Fig. 17). This is in good accordance with many other N-doped metal oxides that remain inert to POWS or are only moderately active for water-splitting half-reactions39,54. In addition, swapping the fluorination and nitridation orders for sample synthesis, i.e. fluorination after nitridation, substantially annihilates the POWS activity both under visible light and simulated sunlight (Supplementary Fig. 18). Structure and composition analysis reveals that this modification generates an F-rich and N-poor sample with a high similarity to Sr2TiO4-N (Supplementary Fig. 18). From these control experiments, one can realize that F serves to enhance the N uptake during nitridation, and N rather than F plays the vital role for POWS activity. The successful POWS reactions over Sr2TiO4-NF justify the effectiveness of the F-expedited nitridation strategy in opening up the photocatalytic potential of otherwise inactive semiconductors. The POWS activity of Sr2TiO4-NF was further investigated by tuning some important parameters including RhCrOy content, TiOXH content, and catalyst dosage (Supplementary Fig. 19). Under optimized conditions, Sr2TiO4-NF delivers apparent quantum efficiency (AQE) as high as 0.39% at 420 ± 20 nm and solar-to-hydrogen (STH) efficiency as high as 0.028%. These values are quite competitive when compared to the reported photocatalysts that are active to the visible-light-driven POWS (Supplementary Table 2 and Supplementary Fig. 20). Moreover, the activity and AQE at specific wavelengths show clear wavelength-dependence, closely matching the UV-vis DRS spectra (Fig. 5b and Supplementary Fig. 21). The stability of photocatalytic overall water splitting was evaluated by extended usage under both visible light and simulated AM1.5 G illumination. As shown in Fig. 5c, d, Sr2TiO4-NF does not show activity attenuation after four consecutive cycles, indicating superior stability of the material, which is further verified by the XRD, XPS, and SEM before and after the reaction (Supplementary Figs. 22–24).
Fig. 5. Photocatalytic activity and stability.
a Temporal gas evolution (H2, O2 and N2) over Sr2TiO4-NF and Sr2TiO4-N under visible light illumination (λ ≥ 420 nm). The samples are coated with TiOXH (1 wt%) after loading RhCrOy (0.5 wt%). b Action spectra (AQE vs. λ) of Sr2TiO4-NF for POWS reactions, the error bars correspond to the bandwidth of the band-pass filter used, UV-vis DRS spectra are included for comparisons. c Extended cycles of POWS reactions over Sr2TiO4-NF under visible light illumination (λ ≥ 420 nm). d Extended cycles of POWS reactions over Sr2TiO4-NF under AM1.5 G (100 mW·cm−2). Reaction conditions: 0.4 g catalysts, 100 mL deionized water. Source data for gas evolution and action spectra are provided as a Source Data file.
Discussion
In summary, the RP-type layered perovskite with a wide spectral response and high-efficiency POWS activity have been obtained through F-expedited nitridation of Sr2TiO4. The presence of F not only increases the N uptake but also facilitates crystal growth during high-temperature ammonolysis. Notably, the involvement of F for nitridation reduces the concentration of deep-level defects in the product, such as Ti3+ and oxygen vacancies, which can seriously undermine the activity. Correspondingly, Sr2TiO4-NF demonstrates a much stronger visible light absorption and much-improved photocarrier separation capabilities than Sr2TiO4-N. As a result, Sr2TiO4-NF displays a stable POWS activity under both visible light and simulated sunlight illumination, delivering an AQE as high as 0.39% at 420 ± 20 nm and STH efficiency as high as 0.028%. These findings provide a useful guideline for the modification of the wide-bandgap semiconductors that are potentially active for POWS. For a more general application of the F-expedited nitridation strategy, an F-involving amorphous precursor can be easily prepared by the Chimie douce method, which shall work in a similar way as the crystallized precursor Sr2TiO3F2 used in this work and will be our future study.
Methods
Preparation of Sr2TiO4 powders
Strontium nitrate (1.9142 g, SCR, 99.5%), citric acid (8.6888 g, Aladdin, 99.5%), and tetrabutyl titanate (1.5496 g, Aladdin, 99%) were dispersed in a mixture of ethylene glycol (17 mL, Aladdin, GC grade) and ultrapure water, and stirred continuously until fully dissolved. The transparent solution was evaporated (573 K, 5 h) to a brown resin, followed by pyrolysis (823 K, 15 h) to remove organic components. The resulting white powders were thoroughly ground and pressed into pellets at 5 MPa, and further calcined at 1373 K for 10 h. The final product was ground again for characterization and further processing.
Conventional nitridation of Sr2TiO4
The Sr2TiO4 powders (0.2 g) were loaded into a tube furnace using an alumina boat and subjected to heating 1273 K for 48 h under flowing ammonia gas (200 mL·min−1, Jiaya Chemicals, 99.999%). Subsequently, the product powders were allowed to cool to room temperature naturally, followed by thorough rinsing with deionized water and drying in an oven overnight, and denoted as Sr2TiO4-N. A double annealing sample has been prepared, i.e. annealing in air at 573 K for 14 h and 623 K for 14 h with intermediate grinding before nitridation. This sample was denoted as Sr2TiO4-N2 in order to study whether or not the annealing history improves the photocatalytic activity.
F-expedited nitridation of Sr2TiO4
Sr2TiO4 powders (1.0 g) and PVDF (0.2 g, Arkema, M.W. ~1,100,000) were mixed and pressed into pellets, followed by stepwise calcination at 573 K for 14 h and 623 K for 14 h with intermediate grinding. The product powders were identified to be Sr2TiO3F2. The subsequent high-temperature ammonolysis process was consistent with the conventional nitridation method, except for the use of Sr2TiO3F2 powders as a precursor. The final product is denoted as Sr2TiO4-NF. In addition, the Sr2TiO4-N sample was also fluorinated in a similar way using PVDF, i.e. fluorination after nitridation, to produce a control sample denoted as Sr2TiO4-N@F.
Sample characterizations
X-ray powder diffraction (XRD) patterns of sample powders were recorded using a Bruker D8 Focus diffractometer (Bruker, Germany). The General Structure Analysis System (GSAS) software package was employed for Rietveld refinement. A UV-Vis spectrophotometer (JASCO V750, Japan) was adopted to collect the UV–visible diffuse reflection spectra. The microstructures of sample powders were monitored using a field emission scanning electron microscope (FE-SEM, JSM-7900F, Japan) and a transmission electron microscope (TEM, JEOL JEM-2100, Japan). Inductively coupled plasma optical emission spectrometry (ICP-OES, PE 8300, USA) was used to determine the content of Sr and Ti within the samples. The O and N content of samples was determined using an oxygen-nitrogen-hydrogen analyzer (ONH2000, USA). The F content within the sample powders was determined by dissolving the sample powders in aqua regia which was then analyzed using an ion chromatograph (ICS-1100, USA). X-ray photoelectron spectra (XPS) were collected using an X-ray photoelectron spectrometer (Thermo Escalab 250, USA). Brunauer-Emmett-Teller (BET) surface area of sample powders was evaluated by a surface analyzer (TriStar II 3020, Micromeritics, USA). A Raman spectrometer (Renishaw InVia, UK) was employed to record the Raman spectra of different samples. A laser with a wavelength of 514.5 nm was used for sample excitation during spectra collection. For the photoluminescence (PL) spectroscopic and time-resolved PL (TRPL) decay spectroscopic analysis, the samples were cooled down to the targeted temperature by a cryostat system (ARS DE-202, USA). A picosecond pulsed laser at a wavelength of 420 nm was then introduced to excite the sample powders and the PL and TRPL signals generated were collected by a fluorescence spectrometer (PicoQuant FluoTime 300, Germany). For the electron paramagnetic resonance (EPR) spectroscopic analysis, the samples were frozen down to 100 K using a liquid nitrogen variable temperature unit. The EPR spectra were then collected by an X-band benchtop EPR spectrometer (CIQTEK EPR200M, China). The hard X-ray absorption spectra of sample powders were recorded in the Shanghai Synchrotron Radiation Facility (SSRF) at Beamline BL11B. Ultraviolet photoelectron spectra (UPS) and the soft X-ray absorption spectra were collected at the Catalysis and Surface Science Endstation (Beamline BL11U) of the National Synchrotron Radiation Laboratory (NSRL, Hefei, China). The photon energy for UPS is 40.0 eV. The samples were electrically biased by –5 V for the collection of the secondary electron cutoff (SEC) spectra. The work function was deduced based on the energy difference between the photon energy and SEC binding energy. For the surface photovoltage (SPV) analysis, the samples were enclosed into a photovoltaic cell which was then irradiated using a Xenon lamp (Perfect Light, PLS-LAX500, China). The output of the lamp was controlled by a monochromator (Zolix, SBP500, China) and a shutter (Stanford Research Systems, SR540, USA) whose chopping frequency was set at 23 Hz. The signal generated by the photovoltaic cell was strengthened by a lock-in amplifier (Stanford Research Systems, SR830, USA) before being collected for analysis. To probe the photo-generated electrons in the sample powders under light illumination, 2,2,6,6-tetramethylpiperidinyloxy (TEMPO) was used as an electron scavenger and was monitored by EPR. The photoelectrochemical (PEC) properties of sample powders were investigated by a standard three-electrode setup in a commercial single-compartment PEC cell comprising the photoelectrode, Pt foil (1 × 1 cm), and Ag/AgCl electrode as the working, counter, and reference electrode, respectively55,56. The Ag/AgCl reference electrode was calibrated by measuring the reversible hydrogen electrode (RHE) potential of Pt foil in an H2-saturated electrolyte. The photoelectrode used for PEC measurements was fabricated by the electrophoretic method57: two pieces of clean fluorine-doped tin oxide (FTO) glass (1 × 3 cm) were immersed in parallel, with a separation distance of 1 cm and conductive sides facing inward, into 40 mL acetone solution containing 40 mg samples and 10 mg iodine. A potentiostatic control (Keithley 2450 Source Meter, USA) was applied to create a constant bias of 10 V between the two pieces of FTO glass for 3 min. This treatment deposited ~ 5 mg sample powders onto FTO glass with an area of 1 cm2. The as-deposited FTO glass was heated at 473 K for 2 h in N2 to eliminate adsorbed iodine. The as-deposited FTO glass was then dipped into TiCl4 methanol solution (0.01 M) and was calcined at 623 K for 15 min. This measure helps to strengthen the interconnection between sample particles deposited on the FTO glass. The PEC measurements were conducted using a Zahner electrochemical workstation. KH2PO4/K2HPO4 buffer solution (pH = 7.3 ± 0.2) was used as the electrolyte. The buffer solution was prepared by dissolving 0.1361 g KH2PO4 (Aladdin, 99.5%) and 0.1742 g K2HPO4 (Aladdin, 99%) into 10 mL deionized water. The buffer solution was deaerated and stored in a sealed volumetric flask before use. A 300 W Xenon lamp (Perfect Light, PLX-SXE300, China) was employed as the light source whose output was filtered through an ultraviolet cut-off filter (λ ≥ 420 nm). An electronic timer and shutter (DAHNG, GCI-73) were introduced to generate chopped light illumination. The linear scan voltammetry (LSV) of the photoelectrode was recorded under chopped light illumination from negative potential to positive potential at a scan speed of ~20 mV/s. It is worth mentioning that the potential reported in this work was not iR corrected. For better comparisons, the potentials were adjusted based on the Nernst relation (equation 4) for the reference to the reversible hydrogen electrode (RHE) potential:
| 4 |
The flat band potential of sample powders was determined by the Mott-Schottky (MS) analysis and the capacitance was deduced from electrochemical impedance spectra using an a. c. signal of 1000 Hz and an amplitude of 10 mV. The electrochemical impedance spectra were recorded using the same setup for PEC measurement.
Cocatalyst deposition
The cocatalyst was deposited onto the photocatalyst according to the following procedures53: specifically, 0.4 g of the photocatalyst was dispersed in an aqueous solution containing rhodium(III) chloride trihydrate (Aladdin, 98%) and chromium(III) nitrate nonahydrate (Aladdin, 99%) and evaporated at 343 K. The resulting powder was subjected to a calcination process at 623 K for 1 h under flowing N2 (~100 mL·min−1, Jiaya Chemicals, 99.999%). To prevent the photocorrosion, an amorphous layer of Ti-oxyhydroxide (TiOXH) was deposited after samples were loaded with RhCrOy49: briefly, 18 µL titanium tetraisopropoxide (Aladdin, 95%) and 50 µL aqueous H2O2 solution (SCR, 30 wt% in H2O) were dissolved in 2 mL distilled water. The solution was then added to the sample aqueous suspension (0.1 L). TiOXH was deposited onto the sample powders by illuminating the above suspensions using a 300 W Xenon lamp (Perfect Light, PLX-SXE300, λ ≥ 300 nm) for 10-15 h.
Photocatalyst evaluation
The performance of POWS reactions was evaluated in a commercial photocatalytic testing system (Perfect Light Labsolar-6A, China)58. The system employed a top-illumination-type quartz reactor for measurements whose temperature was maintained by a water jacket at 281 K. Typically, 0.4 g sample powders, loaded with RhCrOy and coated with TiOXH, were suspended into 0.1 L deionized water. After the air in the system was thoroughly evacuated, a 300 W Xenon lamp (Perfect Light, PLX-SXE300, China) was employed for light illumination. An ultraviolet cut-off filter (λ ≥ 420 nm) or an AM1.5G filter was applied to generate visible light or simulated sunlight. The gas content in the reactor was examined at targeted time points by an online gas chromatograph (SHIMADZU, GC2014C, Japan). Ultrapure Argon gas (Jiaya Chemicals, 99.999%) was used as the carrier gas.
Apparent quantum Efficiency (AQE) and solar-to-hydrogen conversion efficiency (STH) measurements
The monochromatic light (420 nm, 450 nm, 500 nm, 550 nm, and 600 nm) was generated by filtering the output of a 300 W Xenon lamp using a band-pass filter. The photon flux of each monochromatic light and the bandwidth of individual band-pass filters was measured by a quantum meter (Apogee MP-300, USA).
The AQE was determined by the following equation (5):
| 5 |
where n = 2 and 4 for H2 and O2, respectively.
The STH was calculated using the following equation (6):
| 6 |
where ∆Gr is the reaction Gibbs energy for the photocatalytic reaction (~237 kJ·mol−1), P is the power of simulated sunlight illumination (100 mW·cm−2), and S is the illumination area (~28.3 cm2).
Theoretical calculations
The electronic structures of Sr2TiO4 containing different dopants and defects were calculated based on density functional theory (DFT). The Vienna ab initio simulation package (VASP) was adopted for the calculation59,60. The generalized gradient approximation (GGA) and Perdew–Burke–Ernzerhof (PBE) functional were chosen for the description of the exchange-correlation between electrons61,62. The projector augmented wave (PAW) pseudopotentials were introduced for calculation63. The plane-wave cutoff energy was 450 eV. A 2 × 2 × 1 tetragonal unit cell (a = b = 7.77 Å, c = 12.59 Å) was built as the structure model for calculation. The structures were relaxed to reduce the force on each atom at a value lower than 0.02 eV/Å. The atomic coordinates of the optimized crystal structures can be found in Supplementary Data 1. A Monkhorst-Pack k-points mesh of 8 × 8 × 3 was sampled.
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
We thank the National Natural Science Foundation of China (Grant No. 52332009 (G.L.), 52172225 (X.X.), 52425201 (G.L.)), the National Key R&D Program of China (no. 2021YFA1500800 (G.L.)), and the Fundamental Research Funds for the Central Universities for funding. G.L. thanks the financial support from the New Cornerstone Science Foundation through the XPLORER PRIZE. We also thank the BL11B beamline of Shanghai Synchrotron Radiation Facility (SSRF, Shanghai, China) and the BL11U beamline of the National Synchrotron Radiation Laboratory (NSRL, Hefei, China) for the XAS and UPS measurements.
Author contributions
G.L. and X.X. led the project. J.Y. designed and performed experiments. R.L. and Y.L. collected the PL and TRPL data. X.X. analyzed the data and wrote the manuscript. J.H. and G.L. revised the manuscript. All authors were involved in the data discussion.
Peer review
Peer review information
Nature Communications thanks Jinlin Long, Zaicheng Sun and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
The data supporting the findings of this study are reported in the main text or the Supplementary Information. Raw data are provided as a Source Data file. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jinxing Yu, Jie Huang.
Contributor Information
Gang Liu, Email: gangliu@imr.ac.cn.
Xiaoxiang Xu, Email: xxxu@tongji.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-55748-z.
References
- 1.Wang, Q. & Domen, K. Particulate photocatalysts for light-driven water splitting: mechanisms, challenges, and design strategies. Chem. Rev.120, 919–985 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Nishiyama, H. et al. Photocatalytic solar hydrogen production from water on a 100-m2 scale. Nature598, 304–307 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Tao, X., Zhao, Y., Wang, S., Li, C. & Li, R. Recent advances and perspectives for solar-driven water splitting using particulate photocatalysts. Chem. Soc. Rev.51, 3561–3608 (2022). [DOI] [PubMed] [Google Scholar]
- 4.Chen, R. et al. Spatiotemporal imaging of charge transfer in photocatalyst particles. Nature610, 296–301 (2022). [DOI] [PubMed] [Google Scholar]
- 5.Miseki, Y., Kato, H. & Kudo, A. Water splitting into H2 and O2 over niobate and titanate photocatalysts with (111) plane-type layered perovskite structure. Energy Environ. Sci.2, 306–314 (2009). [Google Scholar]
- 6.Allen, M. R. et al. Evolution of physical and photocatalytic properties in the layered titanates A2Ti4O9 (A = K, H) and in nanosheets derived by chemical exfoliation. Chem. Mater.22, 1220–1228 (2010). [Google Scholar]
- 7.Rodionov, I. A. & Zvereva, I. A. Photocatalytic activity of layered perovskite-like oxides in practically valuable chemical reactions†. Russ. Chem. Rev.85, 248 (2016). [Google Scholar]
- 8.Kudo, A. et al. Nickel-loaded K4Nb6O17 photocatalyst in the decomposition of H2O into H2 and O2: structure and reaction mechanism. J. Catal.120, 337–352 (1989). [Google Scholar]
- 9.Takata, T. et al. Photocatalytic decomposition of water on spontaneously hydrated layered perovskites. Chem. Mat.9, 1063–1064 (1997). [Google Scholar]
- 10.Shimizu, K.-I. et al. Photocatalytic water splitting on Ni-intercalated Ruddlesden-Popper tantalate H2La2/3Ta2O7. Chem. Mater.17, 5161–5166 (2005). [Google Scholar]
- 11.Kudo, A., Kato, H. & Nakagawa, S. Water splitting into H2 and O2 on new Sr2M2O7 (M = Nb and Ta) photocatalysts with layered perovskite structures: factors affecting the photocatalytic activity. J. Phys. Chem. B104, 571–575 (2000). [Google Scholar]
- 12.Chen, Z. et al. Accelerating materials development for photoelectrochemical hydrogen production: standards for methods, definitions, and reporting protocols. J. Mater. Res.25, 3–16 (2010). [Google Scholar]
- 13.Lehuta, K. A., Haldar, A. & Kittilstved, K. R. Spectroscopic study of the reversible chemical reduction and reoxidation of substitutional Cr ions in Sr2TiO4. Inorg. Chem.56, 9177–9184 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Sun, X. et al. Photocatalytic hydrogen production over chromium doped layered perovskite Sr2TiO4. Inorg. Chem.54, 7445–7453 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Han, S. et al. Modification of the band gap of Ruddlesden‒Popper perovskites Srn+1TinO3n+1 (n=1, 2, 3, and ∞) by Fe ion irradiation doping. Ceram. Int.49, 7396–7403 (2023). [Google Scholar]
- 16.Kato, H., Takeda, Y., Kobayashi, M., Kobayashi, H. & Kakihana, M. Photoluminescence properties of layered perovskite-type strontium scandium oxyfluoride activated with Mn4+. Front. Chem.6, 467 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao, H. et al. Enhancing the photocatalytic activity of Ruddlesden-Popper Sr2TiO4 for hydrogen evolution through synergistic silver doping and moderate reducing pretreatment. Mater. Today Energy23, 100899 (2022). [Google Scholar]
- 18.Zhang, H., Ni, S., Mi, Y. & Xu, X. Ruddlesden-Popper compound Sr2TiO4 co-doped with La and Fe for efficient photocatalytic hydrogen production. J. Catal.359, 112–121 (2018). [Google Scholar]
- 19.Sun, X. & Xu, X. Efficient photocatalytic hydrogen production over La/Rh co-doped Ruddlesden-Popper compound Sr2TiO4. Appl. Catal. B Environ. Energy210, 149–159 (2017). [Google Scholar]
- 20.Han, X. et al. Non-metal fluorine doping in Ruddlesden–Popper perovskite oxide enables high-efficiency photocatalytic water splitting for hydrogen production. Mater. Today Energy23, 100896 (2022). [Google Scholar]
- 21.Wang, J., Li, P., Zhao, Y. & Zeng, X. Nb/N co-doped layered perovskite Sr2TiO4: preparation and enhanced photocatalytic degradation tetracycline under visible light. Int. J. Mol. Sci.23, 10927 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun, X., Mi, Y., Jiao, F. & Xu, X. Activating layered perovskite compound Sr2TiO4 via La/N codoping for visible light photocatalytic water splitting. ACS Catal.8, 3209–3221 (2018). [Google Scholar]
- 23.Yu, J. & Xu, X. Fluorination over Cr doped layered perovskite Sr2TiO4 for efficient photocatalytic hydrogen production under visible light illumination. J. Energy Chem.51, 30–38 (2020). [Google Scholar]
- 24.Ebbinghaus, S. G. et al. Perovskite-related oxynitrides – Recent developments in synthesis, characterisation and investigations of physical properties. Prog. Solid. State Chem.37, 173–205 (2009). [Google Scholar]
- 25.Fuertes, A. Metal oxynitrides as emerging materials with photocatalytic and electronic properties. Mater. Horiz.2, 453–461 (2015). [Google Scholar]
- 26.Bao, Y. et al. Metallic powder promotes nitridation kinetics for facile synthesis of (oxy)nitride photocatalysts. Adv. Mater.35, 2302276 (2023). [DOI] [PubMed] [Google Scholar]
- 27.Jacob, K. T., Verma, R. & Mallya, R. M. Nitride synthesis using ammonia and hydrazine—a thermodynamic panorama. J. Mater. Sci.37, 4465–4472 (2002). [Google Scholar]
- 28.Irie, H., Watanabe, Y. & Hashimoto, K. Nitrogen-concentration dependence on photocatalytic activity of TiO2-xNx powders. J. Phys. Chem. B107, 5483–5486 (2003). [Google Scholar]
- 29.Asahi, R., Morikawa, T., Ohwaki, T., Aoki, K. & Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science293, 269–271 (2001). [DOI] [PubMed] [Google Scholar]
- 30.Etourneau, J., Portier, J. & Ménil, F. The role of the inductive effect in solid state chemistry: how the chemist can use it to modify both the structural and the physical properties of the materials. J. Alloy. Compd.188, 1–7 (1992). [Google Scholar]
- 31.Slater, P. R. & Gover, R. K. B. Synthesis and structure of the new oxide fluoride Sr2TiO3F2 from the low temperature fluorination of Sr2TiO4: an example of a staged fluorine substitution/insertion reaction. J. Mater. Chem.12, 291–294 (2002). [Google Scholar]
- 32.Kawamura, K. et al. Structural origin of the anisotropic and isotropic thermal expansion of K2NiF4-type LaSrAlO4 and Sr2TiO4. Inorg. Chem.54, 3896–3904 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Tian, B. et al. Correlation between interfacial structures and device performance: the double-edged sword effect of lead iodide in perovskite solar cells. ChemPhysChem24, e202300400 (2023). [DOI] [PubMed] [Google Scholar]
- 34.Maeda, K. et al. Studies on TiNxOyFz as a visible-light-responsive photocatalyst. J. Phys. Chem. C.111, 18264–18270 (2007). [Google Scholar]
- 35.Yoon, S. et al. Bandgap tuning in SrTi(N,O,F)3 by anionic-lattice variation. J. Solid. State Chem.206, 226–232 (2013). [Google Scholar]
- 36.Xu, X., Wang, R., Sun, X., Lv, M. & Ni, S. Layered perovskite compound NaLaTiO4 modified by nitrogen doping as a visible light active photocatalyst for water splitting. ACS Catal.10, 9889–9898 (2020). [Google Scholar]
- 37.Wei, S., Zhang, G. & Xu, X. Activating BaTaO2N by Ca modifications and cobalt oxide for visible light photocatalytic water oxidation reactions. Appl. Catal. B Environ. Energy237, 373–381 (2018). [Google Scholar]
- 38.Wang, Y. et al. Switching on efficient photocatalytic water oxidation reactions over CaNbO2N by Mg modifications under visible light illumination. Appl. Catal. B Environ. Energy245, 10–19 (2019). [Google Scholar]
- 39.Lv, M. et al. Ultrathin lanthanum tantalate perovskite nanosheets modified by nitrogen doping for efficient photocatalytic water splitting. ACS Nano11, 11441–11448 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Nefedov, V. I., Salyn, Y. V., Leonhardt, G. & Scheibe, R. A comparison of different spectrometers and charge corrections used in X-ray photoelectron spectroscopy. J. Electron. Spectrosc. Relat. Phenom.10, 121–124 (1977). [Google Scholar]
- 41.Wei, S. & Xu, X. Boosting photocatalytic water oxidation reactions over strontium tantalum oxynitride by structural laminations. Appl. Catal. B Environ. Energy228, 10–18 (2018). [Google Scholar]
- 42.Zou, F. et al. Template-free synthesis of mesoporous N-doped SrTiO3 perovskite with high visible-light-driven photocatalytic activity. Chem. Commun.48, 8514–8516 (2012). [DOI] [PubMed] [Google Scholar]
- 43.Li, D., Ohashi, N., Hishita, S., Kolodiazhnyi, T. & Haneda, H. Origin of visible-light-driven photocatalysis: a comparative study on N/F-doped and N–F-codoped TiO2 powders by means of experimental characterizations and theoretical calculations. J. Solid. State Chem.178, 3293–3302 (2005). [Google Scholar]
- 44.Braun, A. et al. Nitrogen doping of TiO2 photocatalyst forms a second eg state in the oxygen 1s NEXAFS pre-edge. J. Phys. Chem. C.114, 516–519 (2010). [Google Scholar]
- 45.Kumar, V. et al. Origin of intense blue-green emission in SrTiO3 thin films with implanted nitrogen ions: An investigation by synchrotron-based experimental techniques. Phys. Rev. B103, 024104 (2021). [Google Scholar]
- 46.Fu, J. et al. Identifying performance-limiting deep traps in Ta3N5 for solar water splitting. ACS Catal.10, 10316–10324 (2020). [Google Scholar]
- 47.Miyauchi, M., Takashio, M. & Tobimatsu, H. Photocatalytic activity of SrTiO3 codoped with nitrogen and lanthanum under visible light illumination. Langmuir20, 232–236 (2004). [DOI] [PubMed] [Google Scholar]
- 48.Lian, J. H. et al. A band-to-band transition visible-light-responsive anatase titania photocatalyst by N,F-codoping for water splitting and CO2 reduction. J. Mater. Chem. A11, 141–148 (2022). [Google Scholar]
- 49.Yu, J. et al. Single-crystalline LaTiO2N nanosheets with regulated defects for photocatalytic overall water splitting under visible light up to 600 nm. ACS Catal.14, 608–618 (2024). [Google Scholar]
- 50.Phivilay, S. P. et al. Anatomy of a visible light activated photocatalyst for water splitting. ACS Catal.8, 6650–6658 (2018). [Google Scholar]
- 51.Li, X. et al. CsPbX3 quantum dots for lighting and displays: room-temperature synthesis, photoluminescence superiorities, underlying origins and white light-emitting diodes. Adv. Funct. Mater.26, 2435–2445 (2016). [Google Scholar]
- 52.Xu, J., Pan, C., Takata, T. & Domen, K. Photocatalytic overall water splitting on the perovskite-type transition metal oxynitride CaTaO2N under visible light irradiation. Chem. Commun.51, 7191–7194 (2015). [DOI] [PubMed] [Google Scholar]
- 53.Pan, C. et al. A complex perovskite-type oxynitride: the first photocatalyst for water splitting operable at up to 600|nm. Angew. Chem. Int. Ed.54, 2955–2959 (2015). [DOI] [PubMed] [Google Scholar]
- 54.Liu, G. et al. A red anatase TiO2 photocatalyst for solar energy conversion. Energy Environ. Sci.5, 9603–9610 (2012). [Google Scholar]
- 55.Govindaraju, G. V., Wheeler, G. P., Lee, D. & Choi, K.-S. Methods for electrochemical synthesis and photoelectrochemical characterization for photoelectrodes. Chem. Mater.29, 355–370 (2017). [Google Scholar]
- 56.Wei, S. et al. Stable and efficient solar-driven photoelectrochemical water splitting into H2 and O2 based on a BaTaO2N photoanode decorated with CoO microflowers. Chem. Commun.57, 4412–4415 (2021). [DOI] [PubMed] [Google Scholar]
- 57.Yu, J., Chang, S., Shi, L. & Xu, X. Single-crystalline Bi2YO4Cl with facet-aided photocarrier separation for robust solar water splitting. ACS Catal.13, 3854–3863 (2023). [Google Scholar]
- 58.Huang, J. et al. Gradient tungsten-doped Bi3TiNbO9 ferroelectric photocatalysts with additional built-in electric field for efficient overall water splitting. Nat. Commun.14, 7948 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B54, 11169–11186 (1996). [DOI] [PubMed] [Google Scholar]
- 60.Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B59, 1758–1775 (1999). [Google Scholar]
- 61.Perdew, J. P. et al. Atoms, molecules, solids, and surfaces: applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B46, 6671–6687 (1992). [DOI] [PubMed] [Google Scholar]
- 62.Perdew, J. P. & Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B45, 13244–13249 (1992). [DOI] [PubMed] [Google Scholar]
- 63.Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B50, 17953–17979 (1994). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The data supporting the findings of this study are reported in the main text or the Supplementary Information. Raw data are provided as a Source Data file. Source data are provided with this paper.