Abstract
Optically pure 1,2-diols and 1,3-diols are the most privileged structural motifs, widely present in natural products, pharmaceuticals and chiral auxiliaries or ligands. However, their synthesis relies on the use of toxic or expensive metal catalysts or suffer from low regioselectivity. Catalytic asymmetric synthesis of optically pure 1,n-diols from bulk chemicals in a highly stereoselective and atom-economical manner remains a formidable challenge. Here, we disclose a versatile and modular method for the synthesis of enantioenriched 1,2-diols and 1,3-diols from the high-production-volume chemicals ethane-1,2-diol (MEG) and 1,3-propanediol (PDO), respectively. The key to success is to temporarily mask the diol group as an acetonide, which imparts selectivity to the key step of C(sp3)-H functionalization. Additionally, 1,n-diols containing two stereogenic centers are also prepared through diastereoselective C(sp3)-H functionalization. The late-stage functionalization of biological active compounds and the expedient synthesis of chiral ligands and pharmaceutically relevant molecules further highlight the synthetic potential of this protocol.
Subject terms: Asymmetric synthesis, Synthetic chemistry methodology
Catalytic asymmetric synthesis of optically pure 1,n-diols from bulk chemicals in a stereoselective and atom-economical manner remains a challenge. Here, the authors report a modular method for the synthesis of enantioenriched 1,2-diols and 1,3-diols from the high-production-volume chemicals ethylene glycol and 1,3-propanediol, respectively.
Introduction
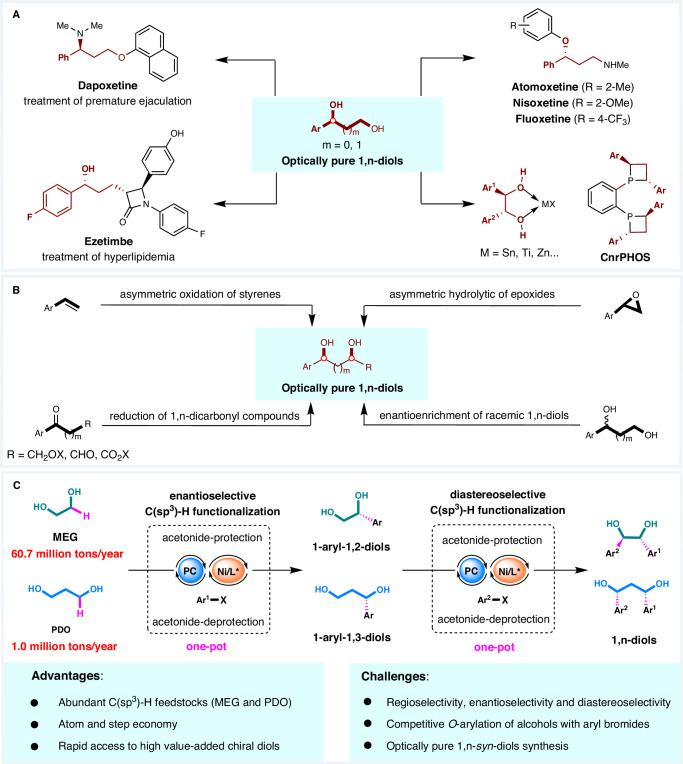
Optically pure 1,2-diols and 1,3-diols are exceptionally valuable as important and versatile building blocks in the pharmaceutical industry1. They are key intermediates in the synthesis of various pharmaceuticals, such as dapoxetine, ezetimibe, atomoxetine, etc.2–4. Moreover, optically pure diols are often serving as chiral auxiliaries and ligands for many asymmetric transformations5,6, further highlighting their potential and making them among the most sought-after motifs in asymmetric synthesis (Fig. 1A). To date, the production of optically pure 1,n-diols is mainly through oxidation of alkenes7–12, hydrolysis of epoxides13–18, pinacol coupling19,20, Aldol reaction21,22, reduction of carbonyl compounds23–25, Aldol-Tishchenko reaction26, hydrogen autotransfer27–29, and so on30–33 (Fig. 1B). Despite numerous milestones, these approaches often require the use of toxic or expensive metal catalysts or stereochemically pure olefins to achieve high selectivity or suffer from low regioselectivity. In stark contrast, methods capable of obtaining optically pure 1,n-diols from cheap and easily available bulk industrial feedstocks in a highly selective and atom-economical manner are rarely known and still pose significant challenges.
Fig. 1. The significance of optically pure 1,n-diols and the state of the art in their synthesis.
A Relevant drugs and chiral ligands synthesized from optically pure 1,n-diols. B Representative methods for the preparation of optically pure 1,n-diols. These protocols generally require the use of toxic or expensive metal catalysts. Methods capable of obtaining optically pure 1,n-diols from bulk chemicals with high stereoselectivity and atom economy are rarely known and still face significant challenges. C Working hypothesis: one-pot modular construction of chiral 1,n-syn-diols from high-production-volume chemicals ethylene glycol and 1,3-propanediol via photo-HAT/nickel dual catalysis.
The direct and enantioselective functionalization of C(sp3)–H bonds represents a transformative synthetic strategy as it allows efficient assembly of high value-added chiral molecules from readily available, inexpensive hydrocarbon feedstocks while minimizing waste generation34–39. In recent years, the expedient combination of photocatalysis and transition-metal catalysis, particularly dual photo-hydrogen atom transfer (HAT)/nickel catalysis, has become a powerful means for C(sp3)–H functionalization40–50. The HAT photocatalyst utilizes the energy of photons to trigger homolytic cleavage of the C(sp3)–H bonds51–53 without the need for pre-installed directing groups like traditional C–H activation reactions54. Taking advantage of nickel’s unique ability in alkyl radical coupling, the generated C-centered radicals can participate in a variety of nickel-catalyzed C–C bond-forming reactions55,56. Since the pioneering work of Molander57, MacMillan58, Doyle58 et al.59, extensive efforts have been devoted to the realm of photoredox/nickel dual-catalyzed C(sp3)–H functionalization. However, enantioselective variants remain largely underexplored60–67.
Ethylene glycol (MEG, annual production of 60.7 million tons) and 1,3-propanediol (PDO, annual production of 1.0 million tons) are both high-production-volume chemicals68,69. The pharmaceutical interest1,2 in optically pure 1,2-diols and 1,3-diols prompted us to consider a different strategy for the preparation of 1,n-diols: one that makes use of photo-HAT/nickel dual-catalyzed enantioselective C(sp3)–H functionalization of MEG and PDO (Fig. 1C). To achieve this appealing transformation, a series of challenging issues need to be addressed. First, Ni-catalyzed O-arylation of alcohols with aryl halides has been extensively studied70,71, and this reaction is highly competitive with the desired C(sp3)–H arylation, leading to chemoselectivity issues. In addition, controlling the enantioselective of C(sp3)–H arylation as well as the diastereoselective of 1,n-diols containing two stereogenic centers are challenges.
We envisioned a strategy to achieve selective C(sp3)–H functionalization of MEG and PDO by temporarily masking diol groups with easily introduced and removed protecting groups, which not only avoids the undesired C–O cross-coupling of alcohol and aryl halides, but also confers selectivity for the critical step of C(sp3)–H functionalization. Herein, we successfully executed this ideal by combining a decatungstate HAT photocatalyst with a chiral nickel catalyst while masking the diol as an acetonide that can be easily introduced and removed. This protocol provides a practical platform for the one-pot construction of enantiomerically enriched 1,2-diols and 1,3-diols from high-production-volume chemicals ethylene glycol and 1,3-propanediol, respectively. In addition, an efficient method to obtain 1,n-diols containing two stereogenic centers through diastereoselective C(sp3)–H arylation was also established. The utility of this method is further demonstrated in the late-stage functionalization of natural products and the synthesis of chiral ligands and many drug-relevant molecules (Fig. 1C).
Results
Reaction development
Initially, we aimed at the direct and enantioselective α-arylation of 1,2-diols. It is textbook knowledge that alcohols generally act as oxygen-centered nucleophiles through their lone electron pairs, whereas there are very few reports using unactivated alkyl alcohols as carbon-centered nucleophiles. MacMillan’s group developed direct α-functionalization of alcohols using zinc-mediated deprotonation of alcohol or using tetra-n-butylammonium phosphate as a hydrogen-binding catalyst to weaken α-hydroxy C–H bonds72,73. Inspired by these works, we tested these strategies to achieve direct α-arylation of unprotected ethylene glycol (MEG), however, the expected products were not obtained in the presence of chiral ligands.
We envisioned introducing appropriate protecting groups to achieve enantioselective α-arylation of 1,2-diols, but this is not trivial. Because the protecting groups, in addition to serving as a functional handle for enantioselectivity control, should also be easily removed to recover the chiral diols. Using a synergistic catalytic system consisting of a chiral PHOX-nickel catalyst and a triplet excited ketone PC photocatalyst67, we examined a series of diol-protecting groups (Table 1). Protection of MEG with benzoyl (1a) or silyl (1b) did not give the desired product. Considering the ease of introduction and removal of acetonides, we protected MEG into the corresponding cyclic acetal 1c. However, 3ca was obtained with a yield of 45%, while the ee value was only 60%. We further screened a variety of cyclic acetals (1d~1f) and borate ester (1g), but unfortunately no superior results were obtained. These results indicate that protective groups on the oxacycles have a significant impact on enantioselectivity control of this transformation.
Table 1.
Optimization of reaction conditions
 | |||||
|---|---|---|---|---|---|
| Entry | Photocatalyst | Ligand | Solvent | Yield of 3ca (%) | ee of 3ca (%) |
| 1b | PC (20 mol%) | L1 | EtOAc | 30 | 60 |
| 2 | PC (20 mol%) | L2 | EtOAc | 24 | 45 |
| 3 | PC (20 mol%) | L3 | EtOAc | 10 | 36 |
| 4 | PC (20 mol%) | L4 | EtOAc | 42 | 30 |
| 5 | PC (20 mol%) | L5 | EtOAc | 11 | 35 |
| 6 | PC (20 mol%) | L6 | EtOAc | 43 | 80 |
| 7 | TBADT (2 mol%) | L6 | Acetone | 47 | 85 |
| 8 | TBADT (2 mol%) | L6 | Acetone:PhCF3 = 1:1 | 47 | 90 |
| 9c | TBADT (5 mol%) | L6 | Acetone:PhCF3 = 1:1 | 71 | 90 |
All reactions were carried out on a 0.2 mmol scale with respect to aryl bromide (2a). Conditions: NiBr2.DME (10 mol%), chiral ligand L (15 mol%), 2a (0.2 mmol), 1 (1.0 mmol), K3PO4 (0.24 mmol), photocatalyst, solvent (2.0 mL, 0.1 M) under the irradiation of LEDs (10 W, 390 nm) at 25 °C for 60 h. Yields are for isolated and purified products. The ee values were determined by HPLC using a chiral stationary phase.
NP no product.
aThe reaction was performed in EtOAc at 25 °C using PC as photocatalyst and L1 as ligand.
bThe reaction was performed at −5 °C.
cAcetone/PhCF3 (1.0 mL, 0.2 M).
To further improve the efficiency and enantioselectivity of this reaction, a series of chiral ligands were then screened (entries 2–6, more details see supplementary Table S1). The bis(oxazoline) ligand L6 was found to be the most effective in terms of enantioselectivity (80% ee, entry 6). To our delight, higher efficiency and enantioselectivity were obtained using acetone as solvent and inexpensive TBADT (tetrabutylammonium decatungstate) as photocatalyst (85% ee, entry 7). The acetone/PhCF3 dual-solvent system was crucial for the success of this transformation, as the enantioselectivity of 3ca was increased to 90% (entry 8). Finally, we found that increasing the reaction concentration afforded the desired product 3ca in 71% yield with 90% ee (entry 9). As expected, control experiments confirmed that the nickel catalyst, light, and photocatalyst were indispensable for a successful outcome.
Substrate scope
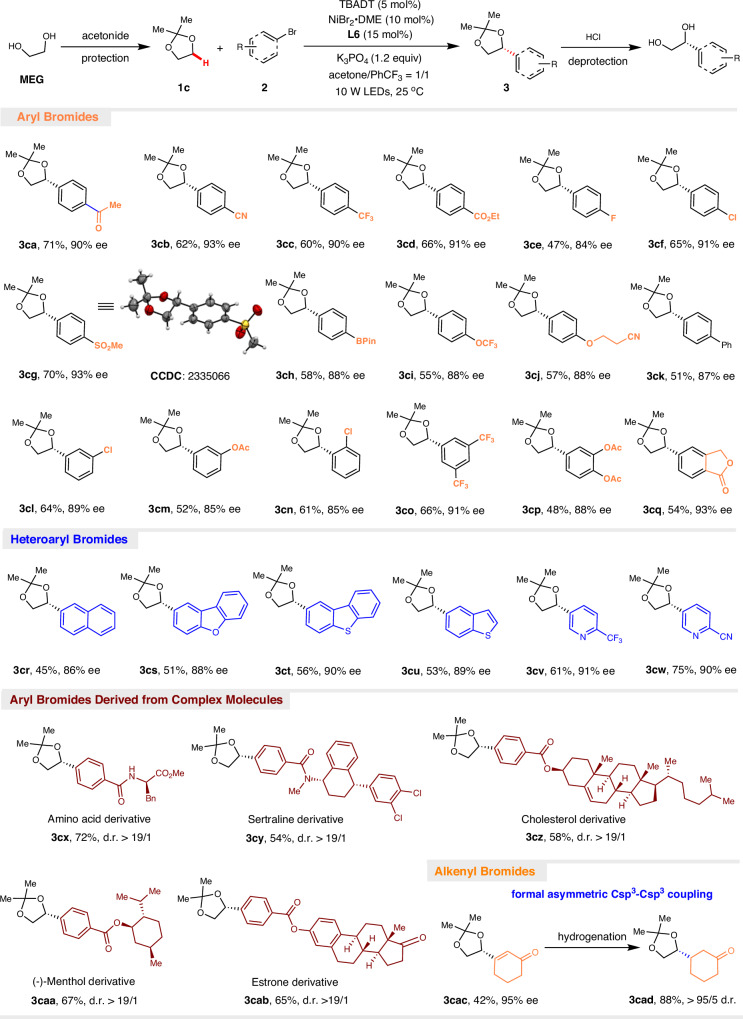
With the optimal conditions in hand, the generality of this transformation was investigated next (Fig. 2). Aryl bromides bearing a wide range of functional groups, such as ketone (3ca), cyano (3cb), trifluoromethyl (3cc), ester (3cd and 3cq), fluorine (3ce), sulfone (3cg), trifluoromethoxy (3ci), and ether (3cj), were perfectly tolerated, providing the corresponding arylated 1,2-diols in good yields with excellent enantioselectivities. Electron-deficient aryl bromides generally exhibited improved efficacy compared to electron-rich aryl bromides. The absolute configuration of 3cg was determined to be R by X-ray single crystal diffraction analysis, and the absolute configurations of all other products were assigned accordingly. Notably, aryl chloride (3cf and 3cl) and aryl boronic ester (3ch) were found to be accommodated, opening additional avenues for subsequent synthetic manipulations of the resulting products. The increase in steric hindrance did not significantly affect the reactivity and enantioselectivity, as meta- and ortho-substituted aryl bromides (3cl~3cn) as well as 3,5- and 3,4-disubstituted aryl bromides (3co–3cp) were compatible with established methods. 2-Naphthyl bromide is also a competent coupling partner (3cr). Heteroaryl bromides containing dibenzofuran (3cs), dibenzothiophene (3ct), benzothiophene (3cu), and pyridines (3cv and 3cw) could be successfully incorporated into the target 1,2-diols. Notably, aryl bromides derived from complex biologically important molecules such as sertraline (3cy), cholesterol (3cz), (-)-menthol (3caa), and estrone (3cab) were well tolerated, thus highlighting the potential of this approach for late-stage functionalization of pharmaceutical agents. Moreover, this asymmetric photochemical C(sp3)–H functionalization is not limited to aryl bromides. Alkenyl bromide such as 3-bromocyclohex-2-en-1-one was coupled with 1c to provide the corresponding optically active 1,2-diol 3cac with excellent enantioselectivity. Further reduction of the double bond by Pd–C afforded the product 3cad bearing two stereogenic centers in 88% yield with a diastereoselectivity greater than 95:5. This protocol provides an alternative strategy to address challenging enantioselective and diastereoselective C(sp3)–C(sp3) cross-couplings.
Fig. 2. Enantioselective synthesis of 1,2-diols.
Reaction conditions: 2 (0.2 mmol), 1c (1.0 mmol), NiBr2.DME (10 mol%), L6 (15 mol%), TBADT (5 mol%), K3PO4 (0.24 mmol) in acetone (0.5 mL) and PhCF3 (0.5 mL) under the irradiation of LEDs (10 W, 390 nm) at 25 °C for 60 h. Yields are for isolated and purified products. The ee values were determined by HPLC using a chiral stationary phase and d.r. ratios were determined by 1H NMR analysis.
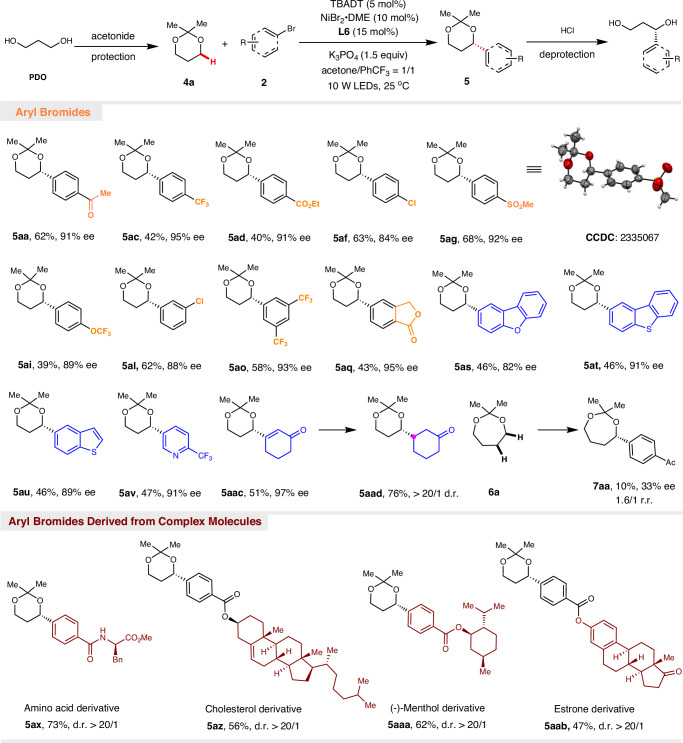
We are pleased to find that our protocol can be further extended for the enantioselective α-arylation of 1,3-diols (Fig. 3). Functional groups such as ketone (5aa), trifluoromethyl (5ac and 5ao), ester (5ad and 5aq), chloro (5af and 5al), sulfone (5ag), and trifluoromethoxy (5ai) were well tolerated, providing the corresponding 1,3-diols in moderate to good yields with excellent enantioselectivities. Heterocycles commonly found in drug molecules, such as furan (5as), thiophene (5at and 5au), and pyridine (5av), could be successfully embedded into the target 1,3-diols. Furthermore, many complex bioactive molecules such as cholesterol (5az), menthol (5aaa), and estrone (5aab) were successfully converted into the corresponding 1,3-diols, demonstrating the robustness of this asymmetric C(sp3–H) arylation reaction for late-state functionalization of drug molecules and natural products. Enantioselective α-alkenylation of 1,3-diol is also feasible (5aac), and further reduction can yield enantiomerically enriched α-alkylated 1,3-diol (5aad), which has extremely high synthetic value. Unfortunately, further attempts to extend the enantioselective α-arylation of 1,4-diol (6a) were unsuccessful, resulting in the formation of a mixture of regioisomers and reduced enantioselectivity and yields (7aa). This result suggests that ring conformation has a greater influence on the reaction outcome, probably because the seven-membered ring has greater strain than the five- and six-membered rings due to transannular crowding.
Fig. 3. Enantioselective synthesis of 1,3-diols.
Reaction conditions: 2 (0.2 mmol), 4a (2.0 mmol), NiBr2.DME (10 mol%), L6 (15 mol%), TBADT (5 mol%), K3PO4 (0.30 mmol) in acetone (0.5 mL) and PhCF3 (0.5 mL) under the irradiation of LEDs (10 W, 390 nm) at 25 °C for 60 h. Yields are for isolated and purified products. The ee values were determined by HPLC using a chiral stationary phase and d.r. ratios were determined by 1H NMR analysis.
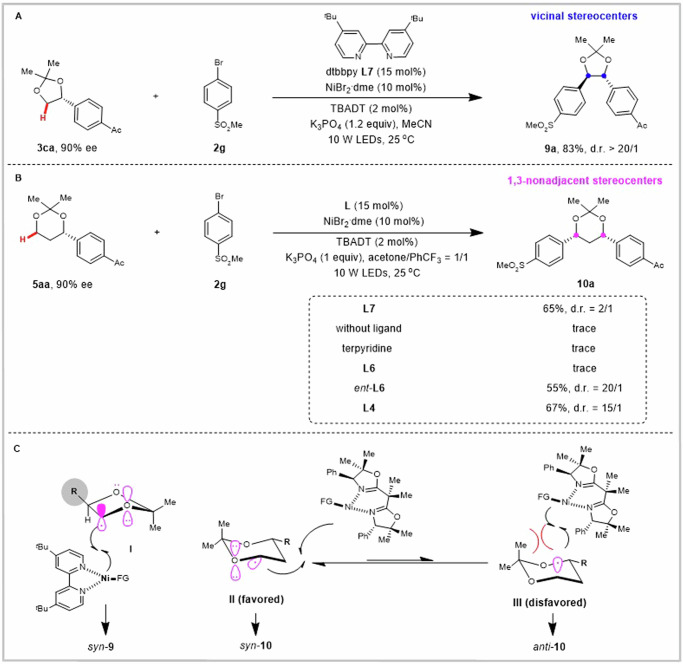
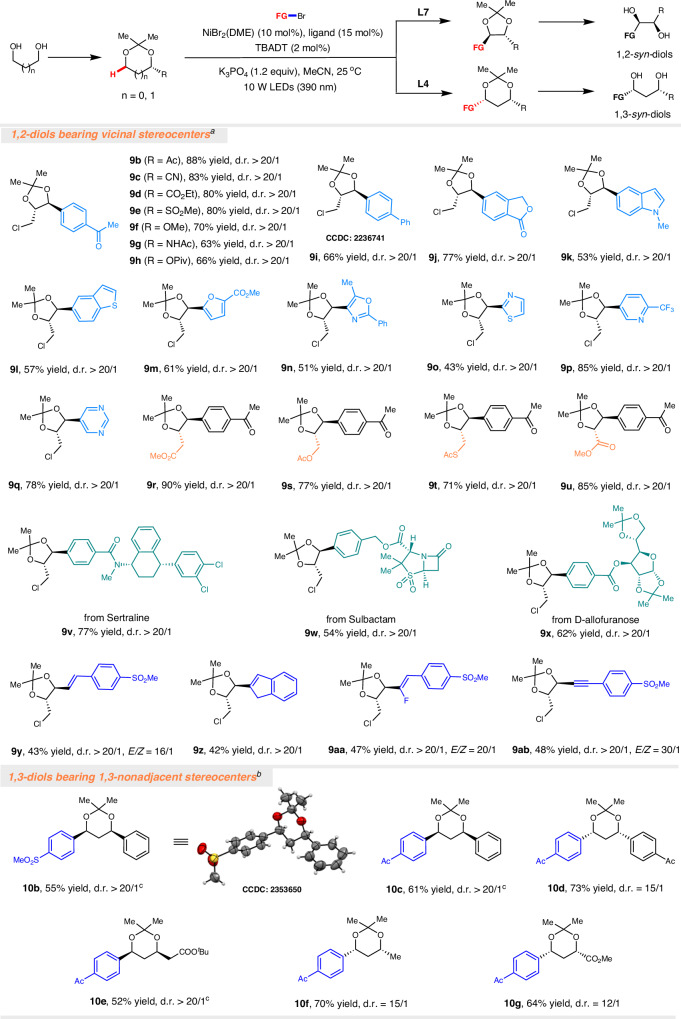
Given that many methods are available for the enantioselective synthesis of monosubstituted 1,n-diols7–32, it would be highly desirable to directly edit and convert them into 1,n-diols containing two stereogenic centers. We hypothesized that TBADT could further abstract the α-hydrogen atom of 1,n-diols to generate the corresponding α-oxy carbon-centered radical, which could be further engaged in Ni-catalyzed cross-couplings with aryl bromides. Inspired by earlier studies that the addition of β-substituted cyclopentyl radicals to alkenes produces predominantly anti-addition products74–77, we anticipated that arylation of 1,2-diols should also be highly diastereoselective, since the interaction of SOMO with adjacent oxygen lone pair orbital stabilizes the ring conformation of radical I, and the steric hindrance effect of the adjacent substituent (R) makes the catalyst complex to approach only from the opposite direction (Fig. 4C). As expected, C(sp3)–H arylation of 3ca with 2g in the presence of achiral ligand dtbbpy (L7) gave the 1,2-diaryl-1,2-syn-diol 9a with two adjacent stereocenters in 83% yield with greater than 20/1 diastereoselectivity (Fig. 4A).
Fig. 4. Diastereoselective synthesis of 1,2-diaryl-1,2-syn-diols and 1,3-diaryl-1,3-syn-diols.
A Diastereoselective C(sp3)–H arylation of 3ca for the synthesis of 1,2-diaryl-1,2-syn-diol 9a bearing two adjacent stereogenic centers. B Diastereoselective C(sp3)–H arylation of 5aa for the synthesis of 1,3-diaryl-1,3-syn-diol 10a bearing two nonadjacent stereogenic centers. C Proposed stereochemical models for explaining the preferred selectivity.
In contrast, C(sp3)–H arylation of 5aa with 2g under identical conditions led to successful coupling and reversed the diastereoselectivity of the reaction to deliver 1,3-diaryl-1,3-syn-diol 10a as the major product (Fig. 4B). Nevertheless, the diastereoselectivity of the product 10a bearing 1,3-nonadjacent stereocenters was not ideal (d.r. = 2/1), which reinforces the notion that the 1,3-diastereoselective induction would be far from trivial78. We further screened a series of ligands in the hope of improving the diastereoselectivity of the product (Table S2). Using terpyridine as a ligand or in the absence of a ligand failed to obtain the target product 10a. To our delight, when ent-L6 was used as the ligand, 1,3-diaryl-1,3-syn-diol 10a bearing was obtained in 55% yield with greater than 20/1 diastereoselectivity. In addition, when L4 was used as the ligand, 10a was obtained in 67% yield with 15:1 diastereoselectivity. The use of ligand L6 resulted in low yield, suggesting that ligands are the key to regulating the stereoselectivity of the reaction (Fig. 4B). This diastereoselectivity could be explained by the subtle interplay between the steric effect of the nickel catalyst and the stereoelectronic effect of carbon radical. Due to the steric hindrance of nickel complex, we reason that equatorial substitution (radical II) is more favorable than axial substitution (radical III)79, thereby leading to the formation of predominantly 1,3-syn-diols (Fig. 4C).
The generality of the diastereoselective C(sp3)–H functionalization protocol for the synthesis of 1,2-diols and 1,3-diols containing two stereogenic centers was evaluated (Fig. 5). Electron-deficient, electron-neutral, and electron-rich aryl bromides were all viable substrates, and various functional groups such as ketone, nitrile, ester, sulfone, ether, and amide were perfectly tolerated (9a–9j). Heteroaryl bromides such as indole, benzothiophene, pyridine, and pyrimidine could be successfully incorporated into the target products (9k–9q). A variety of synthetically useful functional groups, such as ester, and thioester were found to accommodate, opening additional avenues for derivatization of the resulting products (9r–9u). Aryl bromides derived from complex biologically important molecules, such as sertraline (9v), sulbactam (9w), and D-allofuranose (9x), did not have any adverse effects on efficiency and diastereoselective. Remarkably, this method is not limited to aryl bromides, as alkenyl bromides, gem-difluoroalkenes, and alkynyl bromides were also effective coupling partners (9y–9z and 9aa–9ab). In addition, the C(sp3)–H arylation of aryl-substituted 1,3-diols afforded 1,3-diaryl-1,3-syn-diols 10b–10d bearing 1,3-nonadjacent stereocenters in good yields with excellent diastereoselectivity. It is worth noting that alkyl-substituted 1,3-diols could also undergo Csp3–H arylation with high diastereoselectivity to afford the corresponding 1,3-diols 10e–10g. To summarize, this C(sp3)–H functionalization protocol provides a convenient and practical solution for the stereoselective synthesis of various functionalized 1,2-diols and 1,3-diols.
Fig. 5. Stereoselective synthesis of 1,n-diols bearing two stereogenic centers.
aNiBr2.DME (10 mol%), dtbbpy L7 (15 mol%), TBADT (2 mol%), K3PO4 (0.24 mmol) in MeCN (1 mL) under the irradiation of LEDs (10 W, 390 nm) at 25 oC. bNiBr2.DME (10 mol%), L4 (15 mol%), TBADT (5 mol%), K3PO4 (0.30 mmol) in acetone (0.5 mL) and PhCF3 (0.5 mL) under the irradiation of LEDs (10 W, 390 nm) at 25 °C. cL6 was used as the ligand instead of L4. The diastereomeric ratios (d.r.) were determined by 1H NMR analysis.
Mechanistic studies
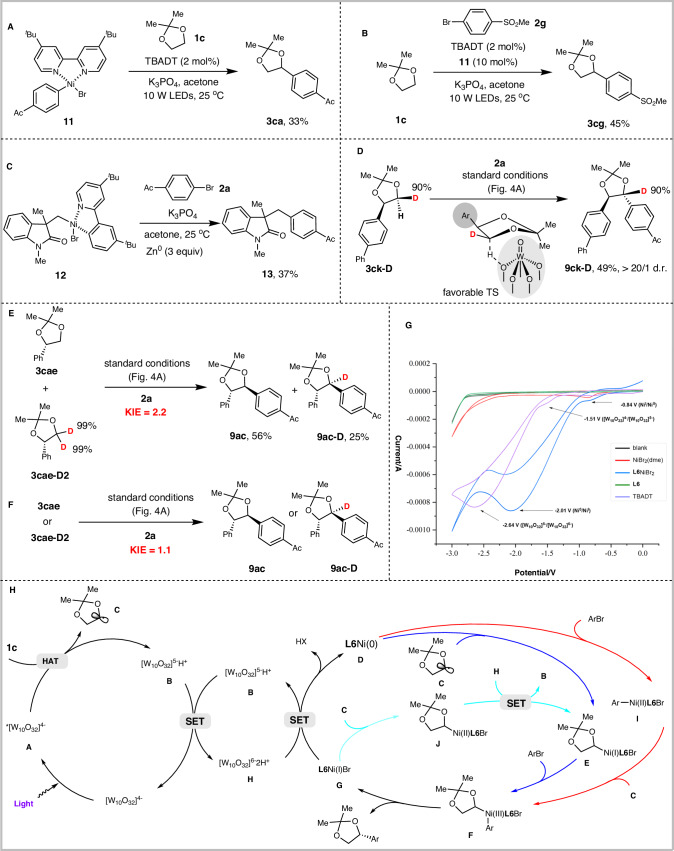
To gain insight into the reaction mechanism, a series of mechanistic experiments were conducted. The stoichiometric reaction of aryl-Ni(II) complex 11 with 1c under standard conditions afforded the desired product 3ca in 33% yield (Fig. 6A). Using 10 mol% of Ar-Ni(II) complex 11 as the catalyst, the reaction of 1c and 2g gave the desired product 3cg in 45% yield (Fig. 6B). These results suggest that aryl-Ni(II) complex may be reactive species in the catalytic cycle. In addition, we synthesized σ-alkyl-Ni(II) complex 12 according to the previously reported procedure80 and reacted with 2a in the presence of Zn0 power. The expected Csp3–Csp2 coupling product 13 was isolated in 37% yield (Fig. 6C), suggesting that alkyl-Ni(I) species generated by the reduction of alkyl-Ni(II) by Zn0 may participate in the catalytic cycle. Moreover, the reduction potentials of TBADT and L6NiBr2 were determined using cyclic voltammetry, which supports the feasibility of reducing Ni(I) to Ni(0) and Ni(II) to Ni(I) species by reduced TBADT, respectively. Because the reduction potential of [W10O32]5−/[W10O32]6− (E = −2.64 V vs Ag/Ag+ in MeCN) is more negative than NiI/Ni0 (E = −2.01 V vs Ag/Ag+ in MeCN) (Fig. 6G).
Fig. 6. Mechanistic studies and proposed mechanism.
A Stoichiometric reaction with Ar-Ni(II)Br complex. B Catalytic reaction with Ar-Ni(II)Br complex. C Stoichiometric reaction with alkyl-Ni(II)Br complex. D Intramolecular competition experiment of 3ck-D. E Intermolecular competition KIE experiment. F Side-by side KIE experiments. G Determining the reduction potentials of TBADT and L6NiBr2 by cyclic voltammetry. H Proposed mechanism.
We further prepared the monodeuterated substrate 3ck-D (99% D) and reacted it with aryl bromide 2a under standard conditions. The desired 1,2-diaryl-1,2-syn-diol 9ck-D was obtained in 49% yield. Interestingly, no loss of deuterium was observed during the reaction, indicating that the HAT process is stereoselective. This may be due to the large size of TBADT, which can only abstract hydrogen atoms that are in trans with the aryl group (Fig. 6D). Intermolecular competitive kinetic isotopic effect (KIE) experiments were performed using 3cae and 3cae-D2 in mixed mode (Fig. 6E) and parallel mode (Fig. 6F), providing KIE values of 2.2 and 1.1, respectively. These results indicate that the HAT process is not a rate-determining step.
On the basis of our mechanistic investigation and previous studies40–50, a plausible mechanism is proposed in Fig. 6H. Excited tetrabutylammonium decatungstate A abstracts an α-hydrogen atom from the acetonide-protected 1,n-diols, generating reduced decatungstate B and an α-oxygen carbon-centered radical C. Nucleophilic addition of radical C to Ni(0) species D generates alkyl-Ni(I) species E, which subsequently undergoes oxidative addition to aryl bromide to form alkyl-Ni(III)-aryl species F. Reductive elimination of intermediate F delivers the optically pure 1,n-diols and Ni(I) species G, which undergoes single-electron transfer with decatungstate H to regenerate the reduced decatungstate B and active Ni(0) catalyst D (Fig. 6H, blue). Alternatively, Ni(I) species E may be generated by addition of radical C to Ni(I) species G followed by single-electron reduction of the resulting alkyl-Ni(II) intermediate J by decatungstate H (Fig. 6H, green).
An alternative mechanism involves the oxidative addition of Ni(0) species D to aryl bromide to afford aryl-Ni(II) intermediate I, which intercepts radical C to form alkyl-Ni(III)-aryl species F (Fig. 6H, red). Previous studies via DFT calculations found that aryl-Ni(II) is thermodynamically easier to reduce than Ni(I) to Ni(0), suggesting that the reaction process may not involve aryl-Ni(II) species81. Nevertheless, given that the stoichiometric reaction supports the intermediacy of aryl-Ni(II), we cannot rule out this possibility.
Synthetic applications
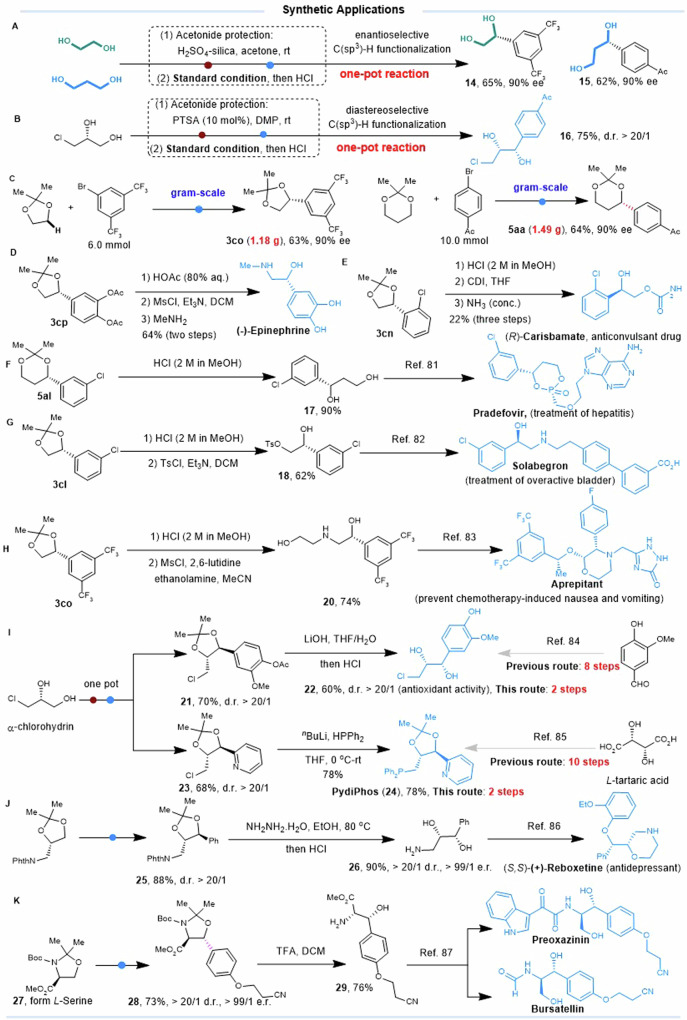
To demonstrate the utility of our developed catalytic regime, we developed a one-pot enantioselective approach to directly convert ethylene glycol and 1,3-propanediol to the corresponding optically pure 1,2-diols and 1,3-diols via acetonide-protection, C(sp3)–H arylation, and subsequent deprotection (Fig. 7A). Furthermore, a one-pot diastereoselective protocol was also established to transform diols into 1,2-diols with two adjacent stereocenters (Fig. 7B). Photo reactors cannot be scaled up by the conventional strategy of enlarging the dimensions due to the light attenuation effect. However, this C(sp3)–H arylation reaction can be scaled up and achieve the gram-scale preparation of optically pure 1,2-diol 3co and 1,3-diol 5aa without reducing yield and enantioselectivity (Fig. 7C).
Fig. 7. Applications in the synthesis of chiral ligands and natural products.
A One-pot enantioselective synthesis of optically pure 1,2-diol and 1,3-diol form ethylene glycol and 1,3-propanediol. B One-pot diastereoselective synthesis of 1,2-diol bearing two stereogenic centers. C Gram-scale preparation of optically pure 1,2-diol 3co and 1,3-diol 5aa. D Asymmetric synthesis of (-)-Epinephrine. E Asymmetric synthesis of (R)-Carisbamate. F Formal synthesis of Pradefovir. G Formal synthesis of Solabegron. H Formal synthesis of Aprepitant. I Concise and asymmetric synthesis of natural phenylpropanoid and chiral ligand PydiPhos. J Formal synthesis of (S,S)-(+)-Reboxetine. K Formal synthesis of (-)-Bursatellin and Preoxazinin. n-BuLi butyl lithium, PTSA p-toluene sulfonic acid, DMP 2,2-dimethoxypropane, Phth phthalimide, CDI 1,1′-carbonyldiimidazole, TsCl 4-toluenesulfonyl chloride, TFA trifluoroacetic acid.
The product, 3cp, was easily converted into Epinephrine in three steps with a yield of 64%, which can be used to treat a variety of conditions including anaphylaxis, cardiac arrest, and asthma (Fig. 7D). The product 3cn was readily transferred into the anticonvulsant drug (R)-Carisbamate in three steps via hydrolysis and carbamation (Fig. 7E). Additionally, the lead prodrug (Pradefovir) for the treatment of hepatitis B could be synthesized from 5al by a known procedure (Fig. 7F)82. Solabegron, a drug which acts as a selective agonist for the β3 adrenergic receptor83, could be synthesized from 3cl (Fig. 7G). Hydrolysis of product 3co followed by selective activation of the primary alcohol as a mesylate and displacement with ethanolamine afforded the amine 20. Following a reported procedure84, amine 20 can be used to synthesize aprepitant, a drug used to prevent chemotherapy-induced nausea and vomiting (Fig. 7H).
Threo-3-chloro-1-(4-hydroxyl-3-methoxyphenyl)propane-1,2-diol 22, a nature phenylpropanoid with antioxidant activity, was synthesized via one-pot C(sp3)–H arylation of (R)-α-chlorohydrin followed by hydrolysis to remove the protecting groups, which is much more concise than the known process85 (Fig. 7I). PydiPhos ligand 24 exhibits excellent regioselectivity in olefin hydroformylation, but its synthesis requires ten steps starting from L-tartaric acid86. Strikingly, using our protocol, PydiPhos 24 was synthesized from commercially available and inexpensive (R)-α-chlorohydrin in only two steps (Fig. 7I). The phthalimide-protecting group of 25 was removed with hydrazine to obtain aminodiol 26, which is an advanced intermediate for the synthesis of (S,S)-(+)-Reboxetine, a marketed drug for the treatment of major depressive disorder (Fig. 7J)87. Moreover, the product 28 obtained by the stereoselective arylation of amino acid derivative 27 was easily converted into 29, which is a common intermediate for the synthesis of natural products bursatellin and preoxazinin (Fig. 7K)88. To summarize, the C(sp3)–H functionalization protocol provides a convenient avenue to access chiral ligands and biologically active natural products.
Methods
General procedure for the synthesis of 1-aryl-1,2-diols
An oven-dried 10-mL vial equipped with a PTFE-coated stir bar was charged with NiBr2(dme) (6.1 mg, 0.02 mmol, 10 mol%), L6 (11.7 mg, 0.03 mmol, 15 mol%) and anhydrous acetone (0.5 mL). This reaction mixture was stirred at room temperature for 1 h in an argon-filled glovebox. TBADT (33.5 mg, 0.02 mmol, 5 mol%), aryl or alkenyl bromide 2 (0.2 mmol, 1 equiv), 2,2-dimethyl-1,3-dioxolane 1c (102.1 mg, 1.0 mmol, 5 equiv), K3PO4 (50.9 mg, 0.24 mmol, 1.2 equiv) and PhCF3 (0.5 mL) was then added. The reaction mixture was stirred and irradiated with a 10 W 390 nm LED lamp at 25 °C for 60 h. The resulting mixture was removed from light, diluted with ethyl acetate, and passed through a pad of celite. The celite plug was further washed with ethyl acetate. The combined solvent was then evaporated under reduced pressure, and the residue was purified by column chromatography on silica gel to afford the corresponding products.
General procedure for the synthesis of 1-aryl-1,3-diols
An oven-dried 10-mL vial equipped with a PTFE-coated stir bar was charged with NiBr2(dme) (6.1 mg, 0.02 mmol, 10 mol%), L6 (11.7 mg, 0.03 mmol, 15 mol%) and anhydrous acetone (0.5 mL). This reaction mixture was stirred at room temperature for 1 h in an argon-filled glovebox. TBADT (33.5 mg, 0.02 mmol, 5 mol%), aryl or alkenyl bromide 2 (0.2 mmol, 1 equiv), 2,2-dimethyl-1,3-dioxane 4a (232.0 mg, 2.0 mmol, 10 equiv), K3PO4 (63.6 mg, 0.3 mmol, 1.5 equiv) and PhCF3 (0.5 mL) was then added. The reaction mixture was stirred and irradiated with a 10 W 390 nm LED lamp at 25 °C for 60 h. The resulting mixture was removed from light, diluted with ethyl acetate and passed through a pad of celite. The celite plug was further washed with ethyl acetate. The combined solvent was then evaporated under reduced pressure, and the residue was purified by column chromatography on silica gel to afford the corresponding products.
Supplementary information
Acknowledgements
This project was supported by the National Natural Science Foundation of China (22171215 to W.K. and 22301225 to Y.P.), Hubei Provincial Outstanding Youth Fund (2022CFA092 to K.W.), the Cultivation Program of Wuhan Institute of Photochemistry and Technology (GHY2023KF007 to K.W.), Hubei Provincial Natural Science Foundation (2023AFB034 to Y.P.), and Guangdong Basic and Applied Basic Research Foundation (2022A1515110113 to Y.P.). We thank the Core Facility of Wuhan University for X-ray single crystal diffraction analysis.
Author contributions
W.K. conceived and directed this project. S.X., Y.P., Y.S., H.G. and A.L. conducted the experimental investigations. S.X., Y.S. and Y.P. analyzed and interpreted the experimental data. W.K. wrote the manuscript with feedback from other authors. All authors contributed to discussions. S.X., Y.P. and Y.S. contributed equally.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
All data are available from the corresponding author upon request. The authors declare that all the data supporting the findings of this work are available within the article and its Supplementary Information files. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 2335066 (3cg), 2335067 (5ag), 2236741 (9g), and 2353650 (10b). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Sheng Xu, Yuanyuan Ping, Yinyan Su.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-55744-3.
References
- 1.Heravi, M. M., Zadsirjan, V., Esfandyari, M. & Lashaki, T. B. Applications of sharpless asymmetric dihydroxylation in the total synthesis of natural products. Tetrahedron Asymmetry28, 987–1043 (2017). [Google Scholar]
- 2.Ratovelomanana-Vidal, V. et al. Enantioselective hydrogenation of β-keto esters using chiral diphosphine-ruthenium complexes: optimization for academic and industrial purposes and synthetic applications. Adv. Synth. Catal.345, 261–274 (2003). [Google Scholar]
- 3.Khatik, G. L., Sharma, R., Kumar, V., Chouhan, M. & Nair, V. A. Stereoselective synthesis of (S)-dapoxetine: a chiral auxiliary mediated approach. Tetrahedron Lett.54, 5991–5993 (2013). [Google Scholar]
- 4.Goyal, S. et al. Stereoselective alkylation of imines and its application towards the synthesis of β-lactams. Asian J. Org. Chem.5, 1359–1367 (2016). [Google Scholar]
- 5.Genet, J. P., Marinetti, A. & Vidal, V. R. Recent advances in asymmetric catalysis. Synthetic applications to biologically active Compounds. Pure Appl. Chem.73, 299–303 (2001). [Google Scholar]
- 6.Ishihara, K., Nakashima, D., Hiraiwa, Y. & Yamamoto, H. The crystallographic structure of a Lewis acid-assisted chiral Brønsted acid as an enantioselective protonation reagent for silyl enol ethers. J. Am. Chem. Soc.125, 24–25 (2003). [DOI] [PubMed] [Google Scholar]
- 7.Kolb, H. C., Vannieuwenhze, M. S. & Sharpless, K. B. Catalytic asymmetric dihydroxylation. Chem. Rev.94, 2483–2547 (1994). [Google Scholar]
- 8.Bhunnoo, R. A., Hu, Y., Lainé, D. I. & Brown, R. C. D. An asymmetric phase-transfer dihydroxylation reaction. Angew. Chem. Int. Ed.41, 3479–3480 (2002). [DOI] [PubMed] [Google Scholar]
- 9.Morgan, J. B., Miller, S. P. & Morken, J. P. Rhodium-catalyzed enantioselective diboration of simple alkenes. J. Am. Chem. Soc.125, 8702–8703 (2003). [DOI] [PubMed] [Google Scholar]
- 10.Zhang, Y. & Sigman, M. S. Palladium(II)-catalyzed enantioselective aerobic dialkoxylation of 2-propenyl phenols: a pronounced effect of copper additives on enantioselectivity. J. Am. Chem. Soc.129, 3076–3077 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Suzuki, K., Oldenburg, P. D. & Que, L. Jr. Iron-catalyzed asymmetric olefin cis-dihydroxylation with 97% enantiomeric excess. Angew. Chem. Int. Ed.47, 1887–1889 (2008). [DOI] [PubMed] [Google Scholar]
- 12.Tian, B., Chen, P., Leng, X. & Liu, G. Palladium-catalysed enantioselective diacetoxylation of terminal alkenes. Nat. Catal.4, 172–179 (2021). [Google Scholar]
- 13.Yang, D. et al. A C2 symmetric chiral ketone for catalytic asymmetric epoxidation of unfunctionalized olefins. J. Am. Chem. Soc.118, 491–492 (1996). [Google Scholar]
- 14.Tokunaga, M., Larrow, J. F., Kakiuchi, F. & Jacobsen, E. N. Asymmetric catalysis with water: efficient kinetic resolution of terminal epoxides by means of catalytic hydrolysis. Science277, 936–938 (1997). [DOI] [PubMed] [Google Scholar]
- 15.Matsunaga, S. et al. Catalytic enantioselective meso-epoxide ring opening reaction with phenolic oxygen nucleophile promoted by gallium heterobimetallic multifunctional complexes. J. Am. Chem. Soc.122, 2252–2260 (2000). [Google Scholar]
- 16.Hickey, M., Goeddel, D., Crane, Z. & Shi, Y. Highly enantioselective epoxidation of styrenes: Implication of an electronic effect on the competition between spiro and planar transition states. Proc. Natl. Acad. Sci. USA101, 5794–5798 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barlan, A. U., Basak, A. & Yamamoto, H. Enantioselective oxidation of olefins catalyzed by a chiral bishydroxamic acid complex of molybdenum. Angew. Chem. Int. Ed.45, 5849–5852 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Monaco, M. R., Prévost, S. & List, B. Organocatalytic asymmetric hydrolysis of epoxides. Angew. Chem. Int. Ed.53, 8142–8145 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Bensari, A., Renaud, J.-L. & Riant, O. Enantioselective pinacol coupling of aldehydes mediated and catalyzed by chiral titanium complexes. Org. Lett.3, 3863–3865 (2001). [DOI] [PubMed] [Google Scholar]
- 20.Takenaka, N., Xia, G. & Yamamoto, H. Catalytic, highly enantio- and diastereoselective pinacol coupling reaction with a new tethered bis(8-quinolinolato) ligand. J. Am. Chem. Soc.126, 13198–13199 (2004). [DOI] [PubMed] [Google Scholar]
- 21.Notz, W. & List, B. Catalytic asymmetric synthesis of anti-1,2-diols. J. Am. Chem. Soc.122, 7386–7387 (2000). [Google Scholar]
- 22.Jiao, P., Kawasaki, M. & Yamamoto, H. A sequential O-nitrosoaldol and grignard addition process: an enantio- and diastereoselective entry to chiral 1,2-diols. Angew. Chem. Int. Ed.48, 3333–3336 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuwano, R., Sawamura, M., Shirai, J., Takahashi, M. & Ito, Y. Asymmetric hydrosilylation of symmetrical diketones catalyzed by a rhodium complex with trans-chelating chiral diphosphine EtTRAP. Tetrahedron Lett.36, 5239–5242 (1995). [Google Scholar]
- 24.Prasad, K. R. K. & Joshi, N. N. Stereoselective reduction of benzils: a new convenient route to enantiomerically pure 1,2-diarylethanediols. J. Org. Chem.61, 3888–3889 (1996). [DOI] [PubMed] [Google Scholar]
- 25.Koike, T., Murata, K. & Ikariya, T. Stereoselective synthesis of optically active α-hydroxy ketones and anti-1,2-diols via asymmetric transfer hydrogenation of unsymmetrically substituted 1,2-diketones. Org. Lett.2, 3833–3836 (2000). [DOI] [PubMed] [Google Scholar]
- 26.Gnanadesikan, V., Horiuchi, Y., Ohshima, T. & Shibasaki, M. Direct catalytic asymmetric aldol-Tishchenko reaction. J. Am. Chem. Soc.126, 7782–7783 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Ortiz, E., Chang, Y.-H., Shezaf, J. Z., Shen, W. & Krische, M. J. Stereo- and site-selective conversion of primary alcohols to allylic alcohols via ruthenium-catalyzed hydrogen auto-transfer mediated by 2-butyne. J. Am. Chem. Soc.144, 8861–8869 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ortiz, E., Spinello, B. J., Cho, Y., Wu, J. & Krische, M. J. Stereo- and site-selective crotylation of alcohol proelectrophiles via ruthenium-catalyzed hydrogen auto-transfer mediated by methylallene and butadiene. Angew. Chem. Int. Ed.61, e202212814 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saludares, C., Ortiz, E., Santana, C. G., Spinello, B. J. & Krische, M. J. Asymmetric ruthenium-catalyzed carbonyl allylations by gaseous allene via hydrogen auto-transfer: 1° versus 2° alcohol dehydrogenation for streamlined polyketide construction. ACS Catal.13, 1662–1668 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bodea, S. E., Wolbergb, M. & Müller, M. Stereoselective synthesis of 1,3-diols. Synthesis4, 557–588 (2006). [Google Scholar]
- 31.Gupta, P., Mahajan, N. & Taneja, S. C. Recent advances in the stereoselective synthesis of 1,3-diols using biocatalysts. Catal. Sci. Technol.3, 2462–2480 (2013). [Google Scholar]
- 32.Baer, K. et al. Sequential and modular synthesis of chiral 1,3-diols with two stereogenic centers: access to all four stereoisomers by combination of organo- and biocatalysis. Angew. Chem. Int. Ed.48, 9355–9358 (2009). [DOI] [PubMed] [Google Scholar]
- 33.Liu, T., Meng, W., Feng, X. & Du, H. Stereoselective hydrosilylation of 1,2-diketones catalyzed by chiral frustrated Lewis pairs. Angew. Chem. Int. Ed.63, e202313957 (2024). [DOI] [PubMed] [Google Scholar]
- 34.Labinger, J. A. & Bercaw, J. E. Understanding and exploiting C-H bond activation. Nature417, 507–514 (2002). [DOI] [PubMed] [Google Scholar]
- 35.Zhang, W. et al. Enantioselective cyanation of benzylic C-H bonds via copper-catalyzed radical relay. Science353, 1014–1018 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphy, J. J., Bastida, D., Paria, S., Fagnoni, M. & Melchiorre, P. Asymmetric catalytic formation of quaternary carbons by iminium ion trapping of radicals. Nature532, 218–222 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Li, Y., Lei, M. & Gong, L. Photocatalytic regio- and stereoselective C(sp3)–H functionalization of benzylic and allylic hydrocarbons as well as unactivated alkanes. Nat. Catal.2, 1016–1026 (2019). [Google Scholar]
- 38.Zhang, C., Li, Z., Gu, Q. & Liu, X. Catalytic enantioselective C(sp3)-H functionalization involving radical intermediates. Nat. Commun.12, 475–483 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olden, D. L., Suh, S. E. & Stahl, S. S. Radical C(sp3)-H functionalization and cross-coupling reactions. Nat. Rev. Chem.6, 405–427 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Twilton, J. et al. The merger of transition metal and photocatalysis. Nat. Rev. Chem.1, 0052 (2017). [Google Scholar]
- 41.Wang, C., Dixneuf, P. H. & Soulé, J. F. Photoredox catalysis for building C-C bonds from C(sp2)-H bonds. Chem. Rev.118, 7532–7585 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Milligan, J. A., Phelan, J. P., Badir, S. O. & Molander, G. A. Alkyl carbon-carbon bond formation by nickel/photoredox cross-coupling. Angew. Chem. Int. Ed.58, 6152–6163 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng, W. & Shang, R. Transition metal-catalyzed organic reactions under visible light: recent developments and future perspectives. ACS Catal.10, 9170–9196 (2020). [Google Scholar]
- 44.Zhu, C., Yue, H., Jia, J. & Rueping, M. Nickel-catalyzed C-heteroatom cross-coupling reactions under mild conditions via facilitated reductive elimination. Angew. Chem. Int. Ed.60, 17810–17831 (2021). [DOI] [PubMed] [Google Scholar]
- 45.Cheung, K. P. S., Sarkar, S. & Gevorgyan, V. Visible light-induced transition metal catalysis. Chem. Rev.122, 1543–1625 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lipp, A., Badir, S. O. & Molander, G. A. Stereoinduction in metallaphotoredox catalysis. Angew. Chem. Int. Ed.60, 1714–1726 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mondal, S. et al. Enantioselective radical reactions using chiral catalysts. Chem. Rev.122, 5842–5976 (2022). [DOI] [PubMed] [Google Scholar]
- 48.Kariofillis, S. K. & Doyle, A. G. Synthetic and mechanistic implications of chlorine photoelimination in nickel/photoredox C(sp3)-H cross-coupling. Acc. Chem. Res.54, 988–1000 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chan, A. Y. et al. Metallaphotoredox: the merger of photoredox and transition metal catalysis. Chem. Rev.122, 1485–1542 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holmberg-Douglas, N. & Nicewicz, D. A. Photoredox-catalyzed C-H functionalization reactions. Chem. Rev.122, 1925–2016 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cao, H., Tang, X., Tang, H., Yuan, Y. & Wu, J. Photoinduced intermolecular hydrogen atom transfer reactions in organic synthesis. Chem. Catal.1, 523–598 (2021). [Google Scholar]
- 52.Capaldo, L., Ravelli, D. & Fagnoni, M. Direct photocatalyzed hydrogen atom transfer (HAT) for aliphatic C-H bonds elaboration. Chem. Rev.122, 1875–1924 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang, Z., Chen, P. & Liu, G. Copper-catalyzed radical relay in C(sp3)-H functionalization. Chem. Soc. Rev.51, 1640–1658 (2022). [DOI] [PubMed] [Google Scholar]
- 54.Saint-Denis, T. G., Zhu, R. Y., Chen, G., Wu, Q. F. & Yu, J. Q. Enantioselective C(sp)3-H bond activation by chiral transition metal catalysts. Science359, 4798–4809 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choi, J. & Fu, G. C. Transition metal-catalyzed alkyl-alkyl bond formation: another dimension in cross-coupling chemistry. Science356, eaaf7230 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fu, G. C. Transition-metal catalysis of nucleophilic substitution reactions: a radical alternative to SN1 and SN2 processes. ACS Cent. Sci.3, 692–700 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tellis, J. C., Primer, D. N. & Molander, G. A. Single-electron transmetalation in organoboron cross-coupling by photoredox/nickel dual catalysis. Science345, 433–436 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zuo, Z. et al. Merging photoredox with nickel catalysis: coupling of α-carboxyl sp3-carbons with aryl halides. Science345, 437–440 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shen, Y., Gu, Y. & Martin, R. sp3 C-H arylation and alkylation enabled by the synergy of triplet excited ketones and nickel catalysts. J. Am. Chem. Soc.140, 12200–12209 (2018). [DOI] [PubMed] [Google Scholar]
- 60.Cheng, X., Lu, H. & Lu, Z. Enantioselective benzylic C-H arylation via photoredox and nickel dual catalysis. Nat. Commun.10, 3549 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rand, A. X. et al. Dual catalytic platform for enabling sp3 α C-H arylation and alkylation of benzamides. ACS Catal.10, 4671–4676 (2020). [Google Scholar]
- 62.Shu, X., Huan, L., Huang, Q. & Huo, H. Direct enantioselective C(sp3)-H acylation for the synthesis of α-amino ketones. J. Am. Chem. Soc.142, 19058–19064 (2020). [DOI] [PubMed] [Google Scholar]
- 63.Huan, L., Shu, X., Zu, W., Zhong, D. & Huo, H. Asymmetric benzylic C(sp3)–H acylation via dual nickel and photoredox catalysis. Nat. Commun.12, 3536 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheng, X., Li, T., Liu, Y. & Lu, Z. Stereo- and Enantioselective Benzylic C-H Alkenylation via Photoredox/Nickel Dual Catalysis. ACS Catal.11, 11059–11065 (2021). [Google Scholar]
- 65.Xu, J., Li, Z., Xu, Y., Shu, X. & Huo, H. Stereodivergent synthesis of both Z- and E-alkenes by photoinduced, Ni-catalyzed enantioselective C(sp3)-H alkenylation. ACS Catal.11, 13567–13574 (2021). [Google Scholar]
- 66.Shu, X., Zhong, D., Lin, Y., Qin, X. & Huo, H. Modular access to chiral α-(hetero)aryl amines via Ni/photoredox catalyzed enantioselective cross-coupling. J. Am. Chem. Soc.144, 8797–8806 (2022). [DOI] [PubMed] [Google Scholar]
- 67.Xu, S. et al. Enantioselective C(sp3)-H functionalization of oxacycles via photo-HAT/nickel dual catalysis. J. Am. Chem. Soc.145, 5231–5241 (2023). [DOI] [PubMed] [Google Scholar]
- 68.Mordor IntelligenceTM. Glycol Market Size & Share Analysis—Growth Trends & Forecasts (2024–2029), https://www.mordorintelligence.com/industry-reports/glycol-market (2024).
- 69.Mordor IntelligenceTM. Propylene Glycol Market Size & Share Analysis—Growth Trends & Forecasts (2024–2029), https://www.mordorintelligence.com/industry-reports/propylene-glycol-market (2024).
- 70.Terrett, J. A., Cuthbertson, J. D., Shurtleff, V. W. & MacMillan, D. W. C. Switching on elusive organometallic mechanisms with photoredox catalysis. Nature524, 330–334 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morrison, K. M. & Stradiotto, M. Advances in nickel-catalyzed O-arylation of aliphatic alcohols and phenols with (hetero)aryl electrophiles. Synthesis56, 229–238 (2024). [Google Scholar]
- 72.Jeffrey, J. L., Terrett, J. A. & MacMillan, D. W. C. O-H hydrogen bonding promotes H-atom transfer from α C-H bonds for C-alkylation of alcohols. Science349, 1532–1536 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Twilton, J. et al. Selective hydrogen atom abstraction through induced bond polarization: direct α-arylation of alcohols through photoredox, HAT, and nickel catalysis. Angew. Chem. Int. Ed.57, 5369–5373 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barton, D. H. R. et al. Stereospecificity in radical carbon-carbon bond formation reactions based on tartaric acid. J. Chem. Soc. Chem. Commun.1987, 1790–1792 (1987). [Google Scholar]
- 75.Masuda, K., Nagatomo, M. & Inoue, M. Direct assembly of multiply oxygenated carbon chains by decarbonylative radical–radical coupling reactions. Nat. Chem.9, 207–212 (2017). [DOI] [PubMed] [Google Scholar]
- 76.Raguž, L. et al. Total synthesis and functional evaluation of IORs, sulfonolipid-based inhibitors of cell differentiation in Salpingoeca rosetta. Angew. Chem. Int. Ed.61, e202209105 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu, S. et al. Stereoselective and site-divergent synthesis of C-glycosides. Nat. Chem.16, 2054–2065 (2024). [DOI] [PubMed] [Google Scholar]
- 78.Pan, Q. et al. Ligand-controlled, nickel-catalyzed stereodivergent construction of 1,3-nonadjacent stereocenters. J. Am. Chem. Soc.146, 15453–15463 (2024). [DOI] [PubMed] [Google Scholar]
- 79.Zhang, B. et al. Complex molecule synthesis by electrocatalytic decarboxylative cross-coupling. Nature623, 745–751 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang, K., Ding, Z., Zhou, Z. & Kong, W. Ni-catalyzed enantioselective reductive diarylation of activated alkenes by domino cyclization/cross-coupling. J. Am. Chem. Soc.140, 12364–12368 (2018). [DOI] [PubMed] [Google Scholar]
- 81.Maity, B. et al. Mechanistic insight into the photoredox-nickel-HAT triple catalyzed arylation and alkylation of α‑amino Csp3-H bonds. J. Am. Chem. Soc.142, 16942–16952 (2020). [DOI] [PubMed] [Google Scholar]
- 82.Reddy, K. R. et al. Pradefovir: a prodrug that targets adefovir to the liver for the treatment of hepatitis B. J. Med. Chem.51, 666–676 (2008). [DOI] [PubMed] [Google Scholar]
- 83.Hertzberg, R., Santiago, G. M. & Moberg, C. Synthesis of the β3-adrenergic receptor agonist solabegron and analogous N-(2-ethylamino)-β-amino alcohols from O-acylated cyanohydrins-expanding the scope of minor enantiomer recycling. J. Org. Chem.80, 2937–2941 (2015). [DOI] [PubMed] [Google Scholar]
- 84.Pye, P. J. et al. Crystallization-induced diastereoselection: asymmetric synthesis of substance P inhibitors. Chem. Eur. J.8, 1372–1376 (2002). [DOI] [PubMed] [Google Scholar]
- 85.Wang, Q. et al. Enantioselective synthesis and absolute configuration of the natural threo-3-chloro-1-(4-hydroxy-3-methoxyphenyl)propane-1,2-diol. J. Chem. Res.2004, 504–505 (2004). [Google Scholar]
- 86.Chelucci, G., Cabras, M. A., Botteghi, C. & Marchetti, M. (-)-(4S,5R)-4-(2-pyridyl)-5-(diphenylphosphino) methyl-2,2-dimethyl-1,3-dioxolane a new chiral ligand for enantioselective catalysis. Tetrahedron Asymmetry5, 299–302 (1994). [Google Scholar]
- 87.Métro, T., Pardo, D. G. & Cossy, J. Syntheses of (S,S)-reboxetine via a catalytic stereospecific rearrangement of β-amino alcohols. J. Org. Chem.73, 707–710 (2008). [DOI] [PubMed] [Google Scholar]
- 88.Dattatraya, H. D., Alok, R. & Vijendra, H. P. Asymmetric first total syntheses and assignment of absolute configuration of oxazinin-5, oxazinin-6 and preoxazinin-7. Org. Biomol. Chem.9, 7990–7992 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available from the corresponding author upon request. The authors declare that all the data supporting the findings of this work are available within the article and its Supplementary Information files. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 2335066 (3cg), 2335067 (5ag), 2236741 (9g), and 2353650 (10b). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.