Abstract
Leaf endospheres harbor diverse bacterial communities, comprising generalists and specialists, that profoundly affect ecosystem functions. However, the ecological dynamics of generalist and specialist leaf-endophytic bacteria and their responses to climate change remain poorly understood. We investigated the diversity and environmental responses of generalist and specialist bacteria within the leaf endosphere of mangroves across China. Our findings show a predominance of specialists in the mangrove leaf endosphere. Temperature is the key factor driving community dissimilarity in both groups, yet it negatively influences the alpha diversity. Soil nutritional factors, particularly phosphate for generalists and total organic carbon for specialists are critical in shaping the functional profiles. Interestingly, temperature has a limited impact on functional profiles. Stochastic processes govern community assembly in both bacterial groups, altering the β-nearest taxon indices as temperatures increase. Our findings indicate that the halophytic leaf endosphere favors microbial niche specialization, due to its unique microenvironment and discrete niches, showing thermal sensitivity in terms of the microbial community profile. This study provides insights into niche differentiation and environmental adaptation mechanisms of leaf endophytic microbes in woody halophytes in response to environmental perturbations.
Subject terms: Metagenomics
Thermal sensitivity of mangrove leaf endosphere microbiome reveals distinct niche adaptations in generalist and specialist bacteria. Specialists dominate, while temperature significantly drives diversity, impacting mangrove ecosystem resilience.
Introduction
Plant-microbial symbioses are essential for plant health and environmental adaptation. These partnerships can occur either on the plant surface (epiphytes) or within plant tissues (endophytes)1. Recent studies have highlighted that leaves, the primary interface between plants and the atmosphere, harbor a vast and diverse bacterial assemblage within their endosphere2. The leaf endosphere represents the internal tissues and intercellular spaces within leaves that are colonized by endophytic microorganisms3. These bacterial communities substantially impact ecosystem functions, including phytoremediation, pest management, stress tolerance, nitrogen cycling, and growth enhancement2. We hypothesized that (i) the leaf endosphere provides a stable niche for bacterial communities characterized by unique diversity patterns and functional profiles.
Mangrove ecosystems are indispensable for coastal protection and primary productivity and are highly valued for their unique ability to sustain harsh conditions, such as high salinity and anaerobic soils4. However, the mangrove ecosystems, are increasingly challenged by the impacts of global climate change5, which influences their growth, reproduction, and survival6, despite the relative resilience of mangroves compared to subtropical or temperate ecosystems. Additionally, it alters intra- and interspecies competition among mangroves and their associated microbiota, with profound implications for ecosystem functions and biological processes5. Bacterial diversity strongly influences the composition and function of mangrove ecosystems7. Mangrove leaves harbor substantial endophytic bacterial communities that promote plant development, stress tolerance, and nutrient cycling1. Moreover, research indicates that climatic and environmental factors may considerably alter bacterial communities in coastal woody halophytes8. For example, increasing temperatures can change the microbial species richness and biodiversity of diazotrophic communities in the mangrove rhizosphere9, thereby affecting photosynthesis and ecosystem productivity. Given the importance of bacterial communities in mangrove ecosystems and the potential impacts of climate change, we hypothesized that (ii) temperature would substantially affect the composition, diversity, assembly, and functional profile of leaf-endophytic bacterial communities in mangroves on a large-scale ecosystem.
Ecosystem functioning is profoundly influenced by the diverse dynamic interactions within microbial communities, comprising generalists and specialists10. Studies have shown that generalist and specialist bacteria exhibit distinct niche plasticity, which is the capacity to change and thrive in various environments11. Microbial communities can adapt to diverse environments using specialist and generalist dynamics2,12. Generalists are microbial taxa characterized by broad ecological niches, defined as having a niche breadth of ≥5, capable of thriving in diverse environmental conditions, and exhibiting functional flexibility, allowing them to adapt to varying ecological contexts13. Conversely, specialists possess narrow ecological niches, with a niche breadth of <1.2, and are sensitive to specific environmental factors, performing key ecological functions13. The balance between generalists and specialists and their respective ecological roles offers key insights into the adaptive capacity of mangrove ecosystems. Moreover, the dynamics of generalist and specialist microbial communities can inform more targeted and effective conservation and restoration strategies, crucial for maintaining the balance of microbial communities and preserve niche-specific bacteria14. Additionally, community assembly is a critical process in bacterial communities that enables them to disseminate and persist across various niches through environmental selection (deterministic processes) or dispersal (stochastic processes)11. Studies have demonstrated that the community assembly mechanisms of generalists, who exhibit broad habitat preferences, may differ from those of specialists, who have a limited range of habitats15. For instance, studies have reported that assemblages of generalists and specialists in alfalfa field soil16 and farmland soil2 are primarily driven by dispersal processes and environmental filtering, respectively. Studies have shown that the distinct ecological roles, tolerances, and niche plasticity of generalists and specialists allow microbial communities to adapt to diverse environments. However, global warming exacerbates environmental disturbances, disrupting microbial community assembly by altering the balance between deterministic and stochastic processes17. These environmental perturbations may differentially impact the community assembly of generalists and specialists, potentially leading to a shift in the stability and functional resilience of microbial communities18. Hence, we hypothesized that (iii) generalist versus specialist bacterial communities in the leaf endosphere may exhibit distinct responses to environmental perturbations, particularly in large biogeographic coastal habitats.
In response to environmental perturbation, nations worldwide have intensified efforts to conserve and restore mangrove ecosystems. For instance, in August 2020, China’s Ministry of Natural Resources, along with the Forestry and Grassland Administration, introduced the Special Action Plan for the Protection and Restoration of Mangrove Forests (2020–2025), aiming to establish mangrove forests in regions suitable for restoration. Specially, Kandelia obovata, notable for its exceptional cold tolerance, emerges as a key species in these restoration efforts, enabling the expansion of mangrove forests in subtropical regions and facilitating poleward migration in response to climate change19. Hence, to test these hypotheses, we employed high-throughput 16S rRNA gene sequencing to evaluate 250 leaf samples of K. obovata from ten coastal mangrove wetlands in China, spanning latitudes from 18.44 °N to 28.35 °N and longitudes from 108.24 °E to 121.18 °E. The present study aimed to elucidate the intricate dynamics and adaptive mechanisms of generalist and specialist bacterial communities in the mangrove leaf endosphere and to provide critical insights into their cascading ecological implications, providing valuable insights for mangrove conservation and restoration efforts in the context of climate change.
Results
Composition of generalists and specialists in leaf endosphere
From the 16,461,975 sequences identified, 1662 operational taxonomic units (OTUs) were identified. The leaf endosphere was dominated by Cyanobacteria, Proteobacteria, and Actinobacteria (Supplementary Fig. S1). Among the total OTUs, 1301 (78.28%) were classified as specialists, exhibiting high richness and relative abundance within narrow niches. In contrast, a total of 177 OTUs (10.65%) were classified as generalists, distributed across a wide range of niches (Fig. 1).
Fig. 1. Composition and distribution of generalist and specialist groups in mangrove leaf endosphere.
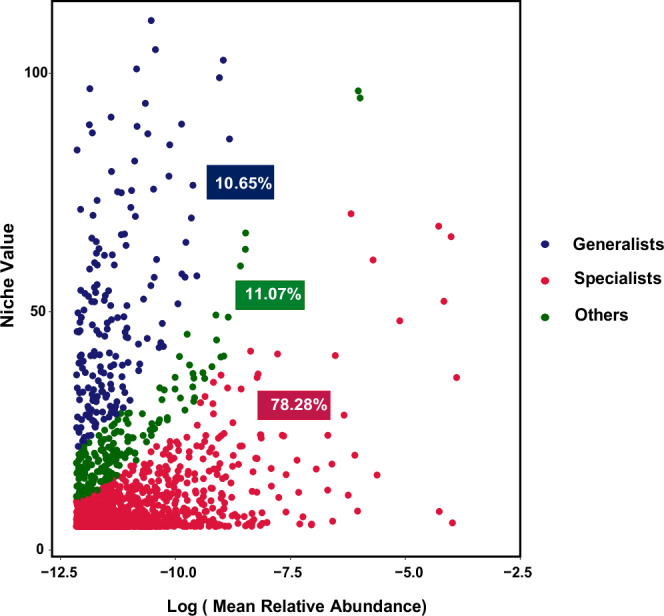
This figure illustrates the composition and distribution of generalist and specialist bacterial groups within the mangrove leaf endosphere. The niche value, indicative of species frequency, along with the abundance of specialist and generalist groups, is depicted. The x axis represents relative abundance on a logarithmic (log) scale. The percentage values denote the richness of operational taxonomic units (OTUs) within the generalist and specialist groups.
Proteobacteria and Cyanobacteria emerged as the dominant phyla in generalists (50%-Proteobacteria, 27%-Cyanobacteria) (Supplementary Fig. S2a) and specialists (34% Proteobacteria, 26% Cyanobacteria) (Supplementary Fig. S2b). Additionally, Proteobacteria in the mangrove leaf endosphere primarily consisted of Gammaproteobacteria (87%-generalists, 59%-specialists) and Alphaproteobacteria (13% generalists, 41%-specialists), with Gammaproteobacteria predominating in both generalist and specialist groups. At the species level, Vibrio xiamenensis and V. tritonius were more prevalent among generalists (Supplementary Fig. S2c), whereas Escherichia coli and Alphaproteobacterium CPCC100088 were more abundant in specialists (Supplementary Fig. S2d). Notably, at the species level, 82% of the leaf-endophytic bacterial taxa in K.obovata remained unclassified (Supplementary Fig. S2c, d). The classification success was substantially higher at the genus level (62% classified), family level (100% classified), and other broader taxonomic levels.
Bacterial diversity of generalists and specialists
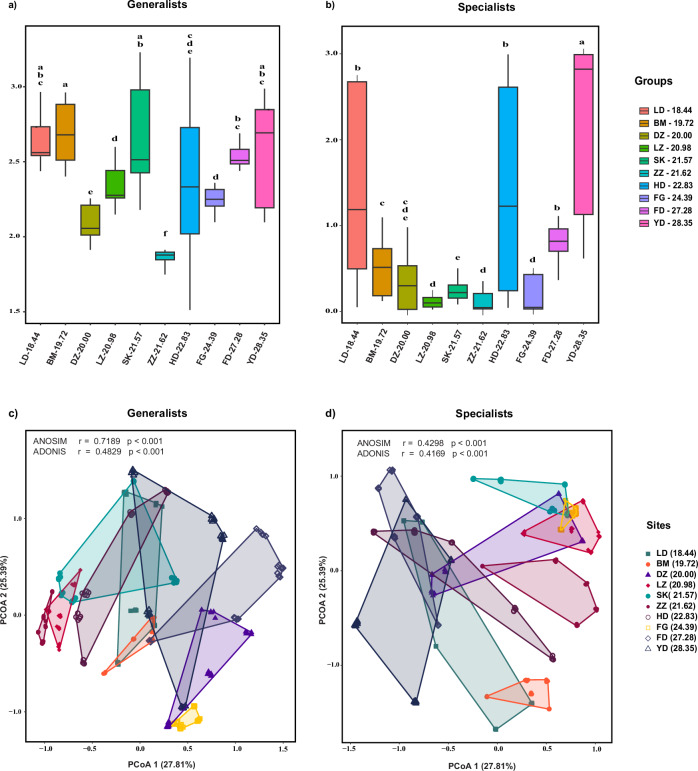
The alpha diversity of generalists and specialists exhibited marked differences across the sampling sites. According to the Shannon index, sites SK-21.57 and YD-28.35 harbored the most diverse groups of generalists (Fig. 2a) and specialists (Fig. 2b), respectively. In contrast, site ZZ-21.62 displayed the lowest alpha diversity for both groups (Fig. 2). Generalists exhibited higher alpha diversity indices and showed wider ranges of diversity values across samples compared to specialists. Interestingly, the alpha diversity indices of generalists and specialists exhibited distinct trends at different latitudes. Linear regression analysis indicated a positive significant correlation (p < 0.001) between specialists’ alpha diversity and spatial distance (Supplementary Fig. S3a). No significant changes were observed for the generalists (R2 = 0.0021, p > 0.05) (Supplementary Fig. S3b).
Fig. 2. Diversity analysis of leaf-endophytic bacterial communities across ten sites, comparing generalist and specialist taxa.
The alpha diversity is represented by the Shannon index for generalist (a) and specialist taxa (b). Boxplots show the distribution of diversity values for each site. Different letters above the boxes indicate statistically significant differences between sites (p < 0.05). Community dissimilarity analysis using principal coordinate analysis (PCoA) based on Bray–Curtis dissimilarity for generalist (c) and specialist taxa (d). Each point represents a sample colored according to the site. The polygons encompass all samples from the same site. The percentage variation explained by each principal coordinate is indicated on the axes.
The Bray–Curtis distance-based principal coordinate analysis (PCoA) revealed distinct structures of generalist communities among various sampling sites on the PCo1 and PCo2 axes, accounting for 27.81% and 25.39% of the variance, respectively (Fig. 2c, d). The analysis of similarities (ANOSIM) test results (p < 0.001) supported this pattern. The communities of generalists and specialists displayed geographical clustering within the mangrove ecosystems (ANOSIM, p < 0.001). Furthermore, specialists exhibited a significant increase in community dissimilarity with geographical distance (R2 < 0.01, p < 0.001) (Supplementary Fig. S3a), whereas generalists showed no significant changes (R2 < 0.01, p > 0.05) (Supplementary Fig. S3b).
Community assembly of bacterial generalists and specialists
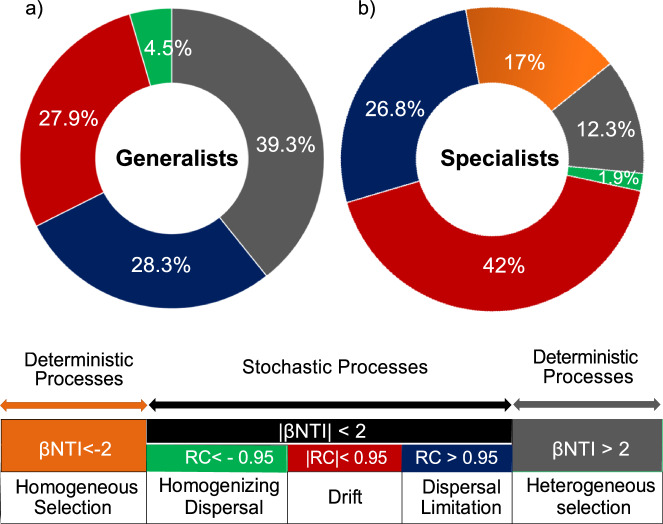
Stochastic processes had a more pronounced impact on the community assembly of generalists (60.7%) and specialists (70.7%), compared to deterministic processes. Specialists exhibited a higher prevalence of drift processes, accounting for 42% compared to 27.9% for generalists (Fig. 3). Among generalists, heterogeneous selection was the primary driver of the deterministic processes, explaining 39.3% of the variation in their assembly. Stochastic processes for generalists included homogeneous dispersal (4.5%), dispersal limitation (28.3%), and drift processes (27.9%) (Fig. 3a).
Fig. 3. Comparative analysis of community assembly processes for generalist and specialist species.
Group assembly analysis using a framework based on a null model. This figure illustrates the relative contributions of deterministic and stochastic processes in shaping the community assembly for generalist (a) and specialist (b) groups. Pie charts depict the percentage allocation of various ecological processes. The lower panel presents a conceptual framework for interpreting community assembly processes based on standardized effect sizes of the β-nearest taxon index (βNTI) and Raup–Crick metrics (RC).
Deterministic processes of specialists (29.3%) were attributed to heterogeneous (12.3%) and homogeneous selection (17%). Stochastic processes among specialists were influenced by homogenizing dispersal (1.9%), with minimal impact on dispersion and dispersal limitation (26.8%). Notably, drift (42%) played a significant role in shaping the community structure of specialists (Fig. 3b). Overall, homogenizing dispersal had negligible effects on the assembly processes of generalists (Fig. 3a) and specialists (Fig. 3b).
Influence of temperature on the alpha diversity and community dissimilarity
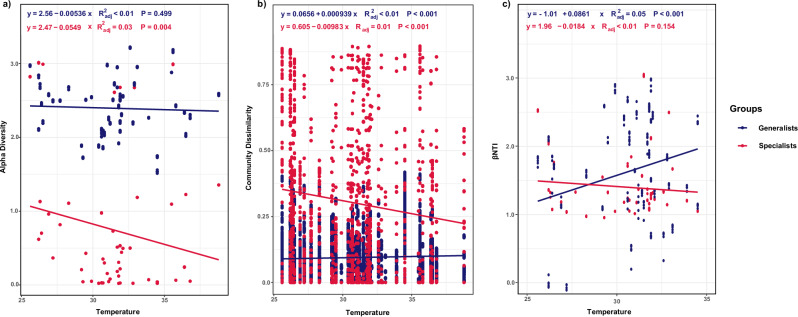
Mantel and partial mantel tests were conducted to assess the impact of environmental and biochemical factors on the dissimilarity of bacterial communities among generalists and specialists. Temperature showed a significant and positive correlation with community dissimilarity (Table 1), being the most significant factor (R = 0.1905, p < 0.001) in generalists. Among specialists, soil nutrient factor NO3−-N (R = 0.2276, p < 0.001) exerted the highest influence, followed by temperature (R = 0.1397, p < 0.001). The Bray–Curtis dissimilarity indices of generalists and specialists exhibited different trends across the temperature gradient. Community dissimilarity of generalists significantly increased along the temperature gradient (R2 < 0.01, p < 0.001) (Fig. 4b), whereas specialists demonstrated a significant decrease (R2 = 0.01, p < 0.001) (Fig. 4b).
Table 1.
Mantel and Spearman test analysis of environmental factors
| Environmental factors | Mantel | Spearman | |||
|---|---|---|---|---|---|
| Generalists | Specialists | Generalists | Specialists | ||
| Soil properties | pH | 0.1662*** | 0.0612 | 0.17** | 0.46*** |
| Temperature | 0.1905*** | 0.1397*** | −0.05 | −0.18** | |
| Salinity | 0.1812*** | 0.0974*** | −0.12 | 0.11 | |
| Clay | 0.1361*** | 0.1324*** | 0.16* | 0.15* | |
| Silt | 0.1729*** | 0.168*** | 0.15* | 0.18** | |
| Sand | 0.1699*** | 0.1631*** | −0.14* | −0.18** | |
| Nutrients | TS | 0.1258*** | 0.0906* | 0.23*** | −0.07 |
| SO42− | 0.0103 | −0.0881 | −0.26*** | −0.4*** | |
| TP | 0.1032*** | 0.0659* | 0.2** | −0.07 | |
| PO43− | 0.1632*** | 0.0374 | 0.26*** | 0.18** | |
| TN | 0.0355* | −0.0239 | −0.19** | −0.37*** | |
| TC | 0.0084 | −0.0571 | −0.29*** | −0.43*** | |
| TOC | 0.0175 | −0.0424 | −0.27*** | −0.53*** | |
| TIC | 0.0526** | −0.0141 | −0.03 | 0.17** | |
| NO2−-N | 0.1044*** | −0.076 | 0.05 | 0.14* | |
| NO3−-N | 0.138*** | 0.2276*** | 0.02 | 0.33*** | |
| NH4+-N | 0.0265 | −0.0275 | −0.22*** | −0.32*** | |
| Climate | MAT | 0.0734*** | 0.0446 | −0.27*** | 0.12 |
| MAP | 0.1121*** | −0.0656 | 0.03 | 0.3*** | |
| MAE | 0.1628*** | 0.1028** | −0.13* | −0.13* | |
| MAR | 0.1059*** | 0.1421*** | −0.3*** | −0.06 | |
*p < 0.05; **p < 0.01; ***p < 0.001.
Mantel and Spearman test analysis of environmental factors associated with community diversity. The Mantel test was used for beta diversity, assessing the relationship between environmental factors and community dissimilarity across sites, while the Spearman correlation was applied to alpha diversity, examining rank-based associations between environmental factors and diversity within individual samples. Environmental factors include soil biochemical properties, such as temperature, salinity, pH, and soil texture (sand, silt, and clay fractions). Nutrient factors included total organic carbon (TOC), total inorganic carbon (TIC), total carbon (TC), total sulfur (TS), total phosphorus (TP), total nitrogen (TN), and concentrations of sulfate (SO42−), phosphate (PO43−). Additionally, nitrite (NO2−-N), nitrate (NO3−-N), ammonium (NH4+-N). Climate variables included the mean annual precipitation (MAP), mean annual temperature (MAT), mean annual relative humidity (MAR), mean annual sunshine duration (MAS), and mean annual evaporation (MAE). *p < 0.05; **p < 0.01; ***p < 0.001.
Fig. 4. Linear regression for temperature with alpha and beta diversity and community assembly.
Linear regression analysis of the alpha diversity versus temperature (a). Bray–Curtis similarity plotted against temperature (b). βNTI as a function of temperature represents the community assembly processes (c). Blue dots and lines represent generalist bacterial communities, whereas red dots and lines represent specialist communities. The R2 values indicate the proportion of variance explained by the linear models, and p values denote the statistical significance of the relationships.
The alpha diversity showed a negative relationship with temperature based on Spearman’s correlation analysis for generalists (R = −0.05, p > 0.05) and specialists (R = −0.18, p < 0.01) (Table 1). Specialists exhibited a significant negative relationship (p < 0.01), whereas generalists showed no significant shift (p > 0.05). As the temperature increased, the alpha diversity declined for both groups. However, specialists showed a significant relationship with the alpha diversity with increasing temperature (R2 = 0.03, p < 0.01) (Fig. 4a), contrasting with generalists (R2 < 0.01, p > 0.05) (Fig. 4a). The proportions of specialists and generalists exhibited contrasting patterns along the temperature gradient. With increasing temperature, specialists demonstrated a positive relationship with their proportional representation in the community (R2 < 0.01, p > 0.05) (Supplementary Fig. S4a), whereas generalists displayed a significant negative association between their proportional abundance and elevated temperatures (R2 < 0.01, p < 0.001) (Supplementary Fig. S4b).
Influence of temperature on the assembly and function profile
The correlation between pairwise comparisons of the β-nearest taxon index (βNTI) and temperature was employed to assess assembly changes along the temperature gradient. βNTI of generalists showed a significant positive correlation with temperature (R = 0.05, p < 0.001) (Fig. 4c), indicating that an increase in temperature promotes heterogeneous selection (βNTI > 2). Conversely, specialists exhibited a non-significant negative correlation with temperature (R < 0.01, p > 0.05), suggesting that the assembly processes shift from heterogeneous selection (βNTI > 2) to stochastic processes (−2 < βNTI < 2) with increasing temperature (Fig. 4c).
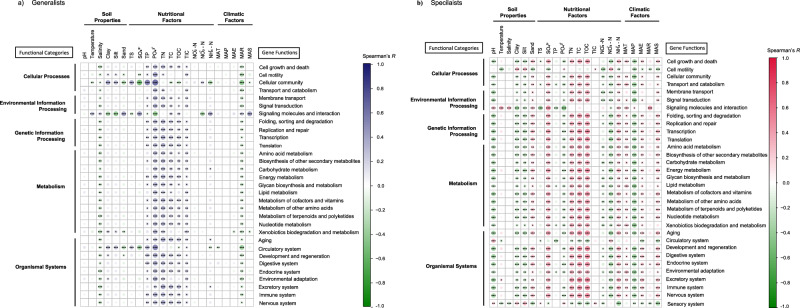
Correlations between environmental factors and the functions of generalists and specialists were analyzed (Fig. 5). Specialists exhibited greater sensitivity to environmental changes than generalists. Phosphate (PO43−) emerged as the most significant factor influencing generalists’ functions (Fig. 5a), while total organic carbon (TOC) and total carbon (TC) were pivotal for specialists (Fig. 5b). Interestingly, temperature had a limited direct impact on the functional profiles of generalists and specialists (Fig. 5). However, certain functions, such as cell motility, signaling molecules and interactions, sensory system (p < 0.001), circulatory system and excretory system (p < 0.05), showed significant correlations with temperature among specialists (Fig. 5b). In generalists, signaling molecules, degradation (p < 0.001) and cellular community (p < 0.01) were significantly correlated with temperature (Fig. 5a).
Fig. 5. Correlation of predictive functional profiles and environmental factors.
Spearman correlations between predictive functional profiles and environmental factors in generalists (a) and specialists (b). The functional profiles were categorized into five major functional categories. Cellular Processes: includes pathways involved in fundamental cellular activities, contributing to structural integrity, communication, and adaptability of microbial cells (e.g., cell growth, motility, and signaling). Environmental Information Processing: represents pathways related to the transport and signaling processes that mediate environmental sensing and interaction (e.g., transport and signaling pathways). Genetic Information Processing: encompasses pathways essential for the storage, transmission, and expression of genetic information, vital for maintaining genetic stability and microbial functionality under diverse conditions (e.g., DNA replication and protein translation). Metabolism: covers a broad range of metabolic pathways (e.g., energy, carbohydrate, and lipid metabolism) crucial for microbial survival and adaptation in nutrient-variable environments. Organismal Systems: Includes functional pathways related to environmental adaptation, immune responses, and other systemic-level functions (e.g., environmental adaptation and immune responses), reflecting advanced biological adaptations and interactions within the ecosystem. Environmental factors analyzed include temperature, salinity, pH, soil texture (sand, silt, and clay fractions), total organic carbon (TOC), total inorganic carbon (TIC), total carbon (TC), total sulfur (TS), total phosphorus (TP), and total nitrogen (TN), the concentrations of sulfate (SO42−), phosphate (PO43−). Additionally, nitrite (NO2−-N), nitrate (NO3−-N), ammonium (NH4+-N), mean annual precipitation (MAP), mean annual temperature (MAT), mean annual relative humidity (MAR), mean annual sunshine duration (MAS) and mean annual evaporation (MAE) were measured. *p < 0.05; **p < 0.01; ***p < 0.001.
Discussion
Global warming has the potential to substantially alter microbial community composition, including generalists with broad environmental tolerances and specialists adapted to specific niches20. However, our understanding of the niche plasticity of generalist and specialist leaf-endophytic bacteria in woody halophytes and their responses to climate change remains limited. In the present study, we investigated the composition, diversity, assembly, and functional processes of generalists and specialists inhabiting the leaf endosphere of K. obovata across various regions in China. Our findings revealed that specialists were the predominant members of the bacterial community in the K. obovata leaf endosphere. Our study showed that temperature significantly influenced community dissimilarity and the assembly of generalist and specialist bacteria. However, the functional profiles of generalists and specialists were influenced by soil nutrient factors, with temperature exerting a reduced impact. These findings underscore the profound effects of global warming on leaf endosphere microbial communities, potentially leading to cascading implications on the health and functioning of mangrove ecosystems.
Our study revealed that the bacterial composition, diversity, and distribution patterns within the leaf endosphere of K. obovata followed distinct biogeographical patterns. The bacterial population was primarily composed of Cyanobacteria, Proteobacteria, and Actinobacteria (Supplementary Fig. S1). Previous studies have reported that Proteobacteria, Cyanobacteria, and Actinobacteria are the primary endophytes in mangrove leaves such as those of K. candle and Bruguiera gymnorrhiza4. Mangrove leaf endosphere provides a stable niche for photosynthetic Cyanobacteria, allowing them to act as primary producers21. Cyanobacteria may dominate other microorganisms due to their ability to resilience against high salinity, oxidative stress, and desiccation and become dominant members of the mangrove ecosystem21. Previous studies have also found Proteobacteria to be the dominant phylum in the leaf endosphere of K. obovata22. These findings underscore the ecological significance of Proteobacteria due to their ability to tolerate high salinity, resist desiccation, and adapt to anaerobic conditions23. Generalists and specialists predominantly featured Proteobacteria and Cyanobacteria (Supplementary Fig. S2a, b), highlighting their adaptability to diverse niches within the leaf endosphere. Proteobacteria in the K. obovata leaf endosphere consist of Gammaproteobacteria (87%-generalists, 59%-specialists) and Alphaproteobacteria (13%-generalists, 41%-specialists), with Gammaproteobacteria predominating in both generalist and specialist groups. Gammaproteobacteria are known for their contributions to plant growth promotion via nitrogen fixation, phosphate solubilization, and their adaptations to salt tolerance, which are vital for survival in the fluctuating mangrove environment24. Alphaproteobacteria play key roles in nitrogen cycling and stress tolerance25. The predominance of Gammaproteobacteria suggests their greater adaptability to environmental stressors, while Alphaproteobacteria may thrive under more stable conditions. Recent metagenomic studies have shown that both classes possess genes involved in osmotic stress response, nutrient acquisition, and secondary metabolite production26, underscoring their adaptation to the unique physicochemical conditions of mangrove leaves. These class-level functional capacities are critical for understanding the complex microbial interactions in mangroves and informing conservation and restoration efforts. These phyla play crucial roles in supporting fundamental leaf endosphere functions for generalists and specialized functions and adaptations to distinct microenvironments for specialists.
Among the generalist OTUs, V. xiamenensis and V. tritonius were the most prevalent species (Supplementary Fig. S2a). Studies have shown that Vibrio species are highly adapted to marine and aquatic environments27 and V. xiamenensis and V. tritonius dominate mangrove soils28. Vibrio species exhibit a broad environmental tolerance and enhanced niche adaptation29, thriving under various environmental stresses, including temperature fluctuations, salinity changes, and nutrient variability. This adaptability further contributes to their success as generalists within K. obovata leaf endosphere. Despite representing different taxonomic levels and ecological strategies, more specialists were identified among E. coli and Alphaproteobacterium CPCC100088 (Supplementary Fig. S3b). Although E.coli is primarily associated with humans and animals30, it has also been identified as a plant endophyte31. Metabolic versatility and genetic adaptability allow E. coli to exploit unique nutritional resources within specific ecological niches, categorizing it as a specialist. The predominance of Alphaproteobacteria in the leaf endosphere is attributed to their ecological niche traits, including specific metabolic capacities, microaerobic adaptation, resistance to mangrove ecological stress, and symbiotic relationships with plant-associated bacteria32. Moreover, species-level classification is inherently challenging in 16S rRNA high-throughput sequencing due to the limited resolving power of marker genes for many bacterial groups33. This is evidenced by our much higher classification success at the genus level (62% classified), family level (100% classified), and broader taxonomic levels. The classification success is constrained by reference database coverage, which often underrepresents environmental and host-associated bacteria, particularly from marine and mangrove ecosystems34. The marked drop in classification success from genus (62%) to species level (18%), suggests that while niche specialization patterns are detectable at the genus level, finer taxonomic resolution remains challenging. Future studies incorporating culture-based methods or employing genome-resolved approaches might help better resolve the taxonomic identity of these unclassified mangrove endophytic bacteria.
Our findings revealed that in the leaf endosphere of K. obovata, bacterial communities were predominantly composed of specialists, with generalists exhibiting a low relative abundance but a more pronounced niche width (Fig. 1). Specialists, characterized by their narrower niche breadth, are highly adapted to specific environmental conditions, enabling them to thrive in resource-limited environments35. Several studies have provided evidence that leaf microbial communities are often dominated by specialists rather than generalists36,37. For instance, one study reported that the leaf endosphere provides a discrete and stable niche that facilitates specialists to adapt to specific circumstances and utilize resources efficiently38. The harsh environmental conditions of the mangrove ecosystem, including high salinity and regular tidal inundation, impose selective pressures on favor microorganisms with specialized adaptations4. The unique metabolic environment of mangrove leaves, characterized by specialized osmolytes such as glycine betaine, alongside limited nutrient availability in intertidal zones, intensifies the specialization observed in these microbial communities39. The dominance of specialists explained by theory of environmental filtering15, which suggests that strong selective pressures shape plant-associated microbiomes in challenging environments. Specialist-dominated bacterial communities have been observed in many environments, including subtropical marine bays13, plateau lakes11, coastal lakes40, and the soils of Mount Wutai41. Our findings suggest that the leaf endosphere acts as a selective niche, permitting colonization and survival only for bacteria with specific traits3. Furthermore, generalists are capable of utilizing a diverse range of resources, which can provide them with fitness advantages across varying environmental conditions. This metabolic versatility allows them to adapt to different niches and potentially outcompete specialists when resources fluctuate42. However, our results showed that the specialized and limited resources (plant-derived metabolites, abiotic stress) in the leaf endosphere of halophytic K. obovata43, may contribute to the dominance of specialists. These specialists may have co-evolved with the host plant, equipping them with the necessary adaptations to outcompete generalists41. These co-evolved symbiotic relationships between specialists and the mangrove host likely play a pivotal role in enhancing the resilience of mangrove ecosystems to environmental stressors.
Our study revealed distinct geographical patterns in the mangrove leaf endosphere for generalist and specialist bacterial species. The alpha diversity of specialists showed a strong positive correlation with geographical distance (Supplementary Fig. S3a), indicating that as the geographic distance between sampling sites increases, the alpha diversity of specialist species within each site correspondingly increases. Studies have shown that specialists are able to adapt to a variety of microhabitats promoting higher local diversity in more geographically isolated sites44. Conversely, the alpha diversity of generalists did not exhibit a significant correlation with spatial distance (Supplementary Fig. S3b), indicating that generalist bacteria are able to maintain similar levels of local diversity across a wider range of environmental conditions and geographic locations. Furthermore, we observed a strong positive correlation between community dissimilarity and geographical distance for specialist bacterial species within the mangrove leaf endosphere. This suggests that the composition and structure of specialist bacterial communities become increasingly distinct as the geographic separation between sites increases44,45. In contrast, generalist bacterial species did not exhibit a significant correlation between community dissimilarity and spatial distance. This indicates that the composition and structure of generalist bacterial communities remain more homogeneous across the range of geographic locations. Moreover, the PCoA and ANOSIM results revealed significant clustering of both generalists (r = 0.7189, p < 0.001) and specialists (r = 0.4298, p < 0.001) across different sites, indicating that spatial factors significantly influence the distribution of both groups. The distinct clustering of generalists showed their capacity to exploit a wide range of environmental conditions and resources, facilitating stable populations across diverse habitats. The higher ANOSIM r-value for generalists suggests their adaptability and resilience to geographical influences, such as climate variability and resource heterogeneity, enhancing their dispersal and colonization success in new environments12.
Conversely, the clustering of specialists is less pronounced (r = 0.4298) suggesting that their distribution is more constrained by specific environmental conditions and resource availability46. Studies found that specialists are more vulnerable to geographical barriers and environmental changes that limit their habitat range15,35. The geographical separation of specialist assemblages highlights the influence of localized ecological factors, such as soil composition, temperature, and specific host plants, on their survival. These findings imply that while generalists thrive across diverse environmental gradients, specialists exhibit more restricted distributions shaped by unique site-specific conditions. This distinction in dispersal patterns underscores the critical role of spatial dynamics in structuring microbial communities, with broader implications for understanding how environmental changes, including climate shifts, may differentially impact generalists and specialists.
The assembly patterns of specialists and generalists differ, due to their distinct ecological strategies and niche differentiation2. Our findings demonstrate that the assembly of generalists and specialists in the leaf endosphere of K. obovata was driven by stochastic mechanisms, mainly influenced by dispersion limitations and drift processes (Fig. 3). Generalists are less affected by environmental filtering due to their broad environmental tolerance, allowing stochastic dispersal16. Specialists in the leaf endosphere being assembled predominantly by stochastic processes suggests that even niche-specific organisms are subject to random variations in their environment. Despite their specialized niche requirements, high environmental variability and unpredictable biotic interactions in halophytic leaf endospheres contribute to the randomness of assembly, affecting the establishment and persistence of specialists47. However, some researchers have argued that deterministic processes exert an increased influence on specialist assembly processes, whereas stochastic processes dominate generalist community assemblies12,16. Studies found that the spatial heterogeneity of the leaf endosphere creates various microenvironments, allowing a wide range of microbes to persist without strong deterministic filtering and leading to stochastic processes18. Furthermore, the leaf endosphere, with its physical barriers, may limit bacterial transmission and enhance stochastic events48, suggesting that distinct tolerance to physical and chemical alterations at the plant leaf-air interface leads to less deterministic processes14.
Deterministic processes also influenced the assembly of generalists (39.7%) and specialists (29.3%) (Fig. 3). The deterministic processes of specialists were driven by heterogeneous and homogeneous selection to adapt to niches and increase environmental tolerance. However, among the generalists, heterogeneous selection was the driving force, emphasizing that environmental instability was the primary factor affecting deterministic processes. Interestingly, homogenizing dispersal had minimal impact on generalists and specialists (Fig. 3). The development of adaptations by generalist and specialist bacteria to unique circumstances in their leaf endosphere microhabitats prevents homogeneous dispersal11,15.
We examined the effect of environmental conditions on the diversity of generalists and specialists within the K. obovata leaf endosphere. Our results showed that community dissimilarity among generalists was most influenced by temperature (R = 0.1905, p < 0.001). Among specialists, the soil nutrient factor NO3−-N (R = 0.2276, p < 0.001) had the strongest influence, followed by temperature (R = 0.1397, p < 0.001) (Table 1). Previous studies have confirmed that temperature significantly affects the diversity and composition of leaf-endophytic bacteria37,49. For instance, the flue-curing procedure, which involves changes in temperature, affects the diversity of endophytic bacteria in tobacco leaves15. Similarly, in a study in Antarctica, a simulation of global warming effects on Colobathus quitensis affected the composition of endophytic bacterial communities in the plant’s leaves50. Our results also revealed that bacterial communities in the leaf endosphere of halophytic mangrove plants were highly affected by temperature. Generalists and specialists exhibited different patterns of community dissimilarity as the temperature increased. Higher temperatures led to more diverse communities among generalists, as evidenced by the positive correlation between temperature and their community dissimilarity (Fig. 4b). Conversely, there was a significant decrease in the community dissimilarity of specialists across the temperature gradient (Fig. 4b), indicating high temperatures may have homogenized the environment, reducing niche differentiation. A previous study also found a strong negative correlation between temperature and relative abundance of rare taxa51. Thus, our results revealed that specialists are more sensitive to variations in temperature because they are adapted to certain environmental conditions, whereas generalists exhibit a diverse range of metabolic capabilities, enabling them to endure a broad range of temperatures.
We also observed that the proportions of specialists and generalists exhibited contrasting patterns along the temperature gradient. As temperatures increased, specialists showed a positive relationship with their proportional representation in the community (R2 < 0.01, p > 0.05) (Supplementary Fig. S4b). Previous research also suggested that specialist species are often better adapted to specific environmental conditions, allowing them to thrive in warmer climates where their niches are favored52. Conversely, the significant negative association observed for generalists (R2 < 0.01, p < 0.001) (Supplementary Fig. S4a) implies that their abundance may decline under rising temperatures. Although generalists are typically more adaptable to varying conditions, their ecological flexibility can become a disadvantage in highly specialized environments. For example, one study found that generalist species can experience reduced fitness under extreme temperature conditions, leading to a potential decline in their relative abundance due to competition with more specialized species that are better suited to these conditions53. In addition, we found that the alpha diversity of specialists had a significant negative relationship (R = −0.22, p < 0.001) with temperature. A negative relationship was also observed among the generalists, but the correlation was not statistically significant (R = −0.07, p > 0.05) (Table 1). This inverse relationship between increased temperature and decreased microbial diversity was further substantiated by linear regression analysis (Fig. 4a). Numerous studies have quantified the strength and direction of the association between temperature gradient and alpha diversity. For example, a study found that rising temperatures led to a decline in microbial diversity in terrestrial soil ecosystems across Scotland54. Our study revealed that the negative effects of increased temperature on microbial diversity extended to leaf endosphere-associated microbiomes, particularly in mangrove wetlands. Moreover, studies have indicated that thermal stress can reduce species richness and alpha diversity in specialist populations by decreasing their survival, metabolic activity, and reproduction at high temperatures12. In contrast, generalists, with their broad niche plasticity, can tolerate a wide range of temperatures, enabling them to survive shifting temperatures14. Our study underscores that specialists, due to their niche specialization and sensitivity to environmental conditions, may struggle to survive and compete when temperatures rise, resulting in reduced diversity compared to generalist communities. The observed differential responses of the alpha diversity and community dissimilarity to temperature can be interpreted through the lens of niche theory55 and the concept of environmental filtering56, which posit that the inherent characteristics and tolerances of taxa shape the diversity and composition of ecological communities. These theories also illustrate the distinct ecological strategies employed by generalists and specialists to adapt to changing environmental conditions. The diverse niche plasticity and adaptability of generalists, compared to specialists, explain their resilience to temperature gradients, allowing them to maintain stability amidst environmental shifts. Consequently, our results confirm that temperature exerts a significant influence on microbial diversity and composition, with unique mangrove temperature regimes imposing a more pronounced selective pressure on endophytic leaf communities. Furthermore, mangrove ecosystems are naturally resilient to moderate temperature fluctuations57. However, our results reveal that global warming could have profound effects on their associated microbial communities. The reduction in microbial diversity, particularly among specialist microbes, may impact crucial ecological processes and the overall health and resilience of mangrove plants. Specialist microbes often play key roles in plant-microbe interactions, facilitating nutrient exchange and protecting plants from pathogens15. The loss of these specialists due to rising temperatures could compromise the resilience of mangroves to environmental stressors. In contrast, while generalist microbes may provide some buffering capacity, their broader metabolic flexibility may not fully compensate for the specific functions lost with specialist taxa20.
Our findings demonstrated that the assembly mechanisms of generalists and specialists responded distinctly to increasing temperatures (Fig. 4c). The community assembly exhibited a significant positive correlation with temperature among generalists as shown by the βNTI indices, indicating that generalists experienced higher heterogeneous selection as temperatures increased than specialists (Fig. 4c). This suggests that rising temperatures foster diverse ecosystems, promoting adaptable inhabitants that can thrive under varying conditions and that environmental filtration increasingly shapes the community composition of generalists at higher temperatures15. The community assembly was negatively correlated with temperature among specialists (Fig. 4c), revealing that assembly processes shift from heterogeneous selection to stochastic processes as temperatures rise. This implies that specialists are less capable of responding to the growing environmental heterogeneity induced by global warming due to their narrow environmental tolerances. In contrast, generalists can exploit the increased niche availability created by heterogeneous conditions, leading to high community turnover (high βNTI) as temperatures rise, due to their ability to adapt to a wide range of conditions. Consequently, stochastic colonization, ecological drift, or dispersion limitation establishes specialist communities rather than environmental filtration at elevated temperatures58. These differences suggest that generalists and specialists employ different strategies and exhibit distinct temperature tolerances. Generalists can adapt to a wide range of habitats and temperatures, which is advantageous for resource utilization in high-temperature environments59. In contrast, the limited adaptability of specialists makes them more susceptible to stochastic processes41. Thus, our results reveal that specialists are more vulnerable to stochastic processes in warm environments, whereas generalists exhibit more resilient heterogeneous selection.
In our study, we explored the effects of environmental factors on the functional profiles of the generalists and specialists in K. obovata leaf endosphere (Fig. 5). We found that the functional profiles of specialists were more sensitive to environmental perturbations, whereas generalists exhibited a borderline functional resilience. The findings demonstrate that soil nutrient factors play a crucial role in influencing these functional profiles, with PO43− being crucial for the functions of generalists (Fig. 5a), TOC and TC being pivotal for specialists (Fig. 5b). Interestingly, despite temperature being a key driver of changes in diversity, our results revealed that it had less influence on the functional profiles of generalist and specialist communities (Fig. 5). This phenomenon may be explained by functional redundancy, where multiple taxa can perform similar roles or by functional plasticity, suggesting that generalists and specialists can adapt their activities to changes in nutrient availability60. Studies also found that generalists and specialists exhibit a range of physiological adaptations and regulatory systems that allow them to maintain their vital functions at various temperatures20. However, our findings revealed that several distinct processes significantly correlated with temperature in specialists, including cell motility, signaling molecules and interactions, sensation, circulation, and excretion (Fig. 5b). Previous studies have reported that temperature influences microbial metabolic rates, enzymatic activities, and overall community composition, which may explain the specific functional relationships with environmental interactions. Notably, generalists in our study exhibited less sensitivity to temperature in their functional response, as evidenced by significant correlations primarily observed in signaling molecules and degradation processes (Fig. 5a). Studies have shown that although species diversity is affected by temperature, generalists can exhibit metabolic flexibility based on nutritional availability, thus preserving functional stability61. Niche theory argues that resource availability and environmental circumstances affect species ranges and features55. This was supported by the differential responses of specialists and generalists to environmental influences. The functional resilience observed in mangrove microbial communities, despite reduced diversity, suggests that mangroves have inherent mechanisms to buffer environmental stress62. This resilience, likely driven by functional redundancy and plasticity, aligns with findings from other ecosystems. For example, in alpine regions, warming influences plant-microbe interactions without drastically altering ecosystem functions63. Similarly, grassland ecosystems show that microbial communities maintain functional stability despite compositional shifts due to drought64. These parallels indicate that microbial communities across ecosystems possess mechanisms to buffer environmental stress and sustain key functions.
The differential responses of specialists and generalists to environmental factors reflect broader ecological principles. In marine ecosystems, generalists exhibit greater resilience to ocean acidification65, mirroring our findings where generalists showed more functional stability across environmental gradients. In contrast, the sensitivity of specialists to factors such as TOC and TC is consistent with findings from tropical rainforests66, where specialist species are more vulnerable to habitat changes. These comparisons highlight the role of functional redundancy and plasticity in maintaining ecosystem stability under environmental stress, underscoring the importance of studying both generalist and specialist microbial communities to better understand ecosystem responses. Our findings in mangrove ecosystems contribute to this broader understanding, emphasizing microbial communities’ roles in sustaining ecosystem resilience across diverse biomes.
Given China’s ongoing mangrove restoration efforts, our findings are particularly timely. Understanding about key species such as K. obovata, which is pivotal in the poleward expansion of mangroves due to its cold tolerance respond to climate change is critical for guiding conservation and restoration strategies. The thermal sensitivity of microbial communities associated with K. obovata offers valuable insights into the potential responses of mangrove ecosystems to future environmental shifts, helping to sustain overall mangrove health. As remote sensing and advanced technologies are increasingly used to monitor and manage mangrove ecosystems, our study contributes to the scientific foundation needed to guide adaptive management strategies in the face of climate change.
Conclusion
In this study, we investigated the ecological dynamics of specialist and generalist bacteria in the leaf endosphere of K. obovata plants in China. The leaf endosphere offers a unique and stable niche for specialized bacteria to adapt to specific conditions. Stochastic processes determined the community assembly of both groups compared to deterministic processes. Temperature exerted as a significant factor, influencing community dissimilarity and promoting the expansion of generalists over specialists. Notably, high temperatures led to decreased alpha diversity in both taxa. The functional capabilities of generalists and specialists were strongly influenced by soil nutritional factors, maintaining stability despite temperature changes. These findings improve our understanding of microbial ecology within the K. obovata leaf endosphere, highlighting the importance of niche differentiation employed by generalist and specialist bacteria for environmental adaptation mechanisms, maintaining resilience and plant health against global warming in coastal woody halophytes. Given the high risk of homogenization due to rising temperatures, future studies should focus on simulating IPCC-predicted temperature scenarios to assess long-term impacts on microbial assemblages, particularly in mangrove ecosystems.
Material and methods
Sampling sites
The present study was conducted in coastal mangrove wetlands spanning the geographical coordinates of 18.44 to 28.35 °N and 108.24 to 121.18 °E across China (Supplementary Fig. S5). The latitudinal transect spans nearly 10 degrees, enabling the study of thermal sensitivity across a diverse range of climatic conditions, from tropical to subtropical zones. The southernmost point (18.44 °N) represents the tropical mangrove ecosystems of Hainan Island, while the northernmost boundary (28.35 °N) in Fujian Province marks the species’ upper latitudinal limit, approaching its thermal tolerance threshold. A total of 250 individual leaf samples of K. obovata were collected. The sampling process involved creating five 5 × 5 m2 plots in each region. Subsequently, K. obovata leaf specimens were individually packed in sterile polyethylene bags and stored in ice-packed coolers. To eliminate surface microbes, all leaves were subjected to surface sterilization within 48 h of sampling. The surface sterilization procedure included immersing each leaf for 30 s in sterile water, followed by 1 min immersion in a solution of 75% ethanol (v/v), then 3 min in a solution of 3.25% sodium hypochlorite, and finally another 30 s immersion in a solution of 75% ethanol (v/v). The sterilization process was completed after three consecutive 2 min rinses in sterile water. The effectiveness of surface sterilization was assessed by placing leaf samples on 90 mm Petri dishes containing malt extract agar (2%) and incubating at 25 °C for 48 h to detect any microbial colonies. Leaf samples were subsequently pulverized into a fine powder using liquid nitrogen67.
DNA extraction and polymerase chain reaction (PCR) amplification
Leaf samples weighing ~0.25 g were subjected to DNA extraction using the GeneJET Genomic DNA Purification Kit (Thermo Scientific, Waltham, USA)68. The extracted DNA was pooled and stored at −80 °C for subsequent analyses. The bacterial 16S rRNA gene from the leaf endosphere was amplified using 169F and 680R primer pairs. PCR was performed in a 20 μL reaction mixture comprising 10 μL of 2× Taq PCR Mastermix (TIANGEN, China), 1 μL of each primer (10 mM concentration), 1 μL of template DNA (~50 ng), and 7 μL of nuclease-free water. The amplification protocol included an initial denaturation at 94 °C for 3 min, followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 45 s, with a final extension step at 72 °C for 5 min. After confirming the PCR product integrity through 1% agarose gel electrophoresis and quantifying them with a Nanodrop 2000 Spectrophotometer (Thermo Scientific), DNA samples were prepared for sequencing. The preparation was performed according to the guidelines specified by the TruSeq DNA Kit manufacturer (Illumina, USA).
Bioinformatics analysis
High-throughput sequencing was performed using a NovaSeq 6000 PE150 platform (Shanghai Majorbio Bio-Pharm Technology Co., Ltd., Shanghai, China). Raw sequence processing initially involved filtering out sequences with primer mismatches (lengths <275 bp), low-quality reads (quality scores < 30), and barcode sequences. Subsequently, chimera filtering and quality control were performed using the Trimmomatic tool and the DADA2 denoising method within the QIIME2 framework69.
Processed reads underwent further analyses, including de-replication, removal of chimeric sequences, and clustering of OTUs using de novo methods in QIIME2 at a 97% similarity threshold70. OTUs were chosen for their ability to capture ecological patterns without over-segmenting microbial populations, which is essential for understanding broad niche differentiation and for broad ecological pattern detection71. OTUs also allow consistency across datasets, supporting meaningful comparisons in studies focused on functional traits and habitat specialization across environmental gradients. Representative sequences were annotated, and species-level classifications were assigned only to sequences that satisfied stringent criteria, including a sequence similarity threshold of ≥97% and a minimum alignment length of 275 base pairs and verification through local Blastn searches against the RDP database (Release 14). Taxonomic assignments for sequences that did not achieve these confidence thresholds were made at the highest hierarchical level that satisfied our classification criteria. Organelle-derived sequences (chloroplast and mitochondria) were identified and removed from the dataset. Sequence data generated in this study have been deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) database under Bio-Project Accession number PRJNA1130664.
Environmental factor analyses
Environmental factors in coastal mangrove wetlands were quantitatively assessed. Climate variables were obtained from the China Meteorological Data Sharing Service System (https://data.cma.cn), including the mean annual precipitation (MAP), mean annual temperature (MAT), mean annual relative humidity (MAR), mean annual sunshine duration (MAS), and mean annual evaporation (MAE). Soil biochemical properties, including temperature, salinity, pH, and soil texture (sand, silt, and clay fractions), were determined using a Malvern Mastersizer 2000 (Malvern, United Kingdom), as described by ref. 72. Nutrient factors assessed in this study included total organic carbon (TOC), total inorganic carbon (TIC), total carbon (TC), total sulfur (TS), total phosphorus (TP), total nitrogen (TN), and concentrations of sulfate (SO42−) and, phosphate (PO43−). Additionally, concentrations of nitrite (NO2−-N), nitrate (NO3−-N), and ammonium (NH4+-N) were determined as inorganic nitrogen in the laboratory.
Identification of specialists and generalists
Generalist and specialist species were classified based on findings outlined in recent studies11,13 at the OTU level. Classification thresholds were determined using observed distributions with random distribution patterns. To assess the adaptability of OTUs to their respective environmental groups while maintaining the observed OTU richness within each sample, 10,000 random shuffles of the OTU dataset were performed. The OTUs that dominated in various environmental groups, likely evolved to adapt to their specific environments, compared to the random distribution considered as the widely distributed generalists. Conversely, OTUs found in a narrower range of habitats than expected based on random distribution were classified as specialists. The ‘niche width’ function from the ‘spaa’ package in R was employed to analyze the niche breadth of species.
Data analysis
Statistical analyses were conducted using R. The composition of the leaf endosphere bacterial community, species richness, and Shannon index (indicating alpha diversity at genus level) were calculated using the ‘vegan’ package. PCoA, ANOSIM, and permutation multivariate analysis of variance (PERMANOVA) were used to evaluate differences in community structure based on Bray–Curtis dissimilarity. Mantel and partial Mantel tests, implemented in the ‘vegan’ package, were used to identify metabolic parameters distinguishing generalists and specialists. Data correlations were evaluated using Spearman and Pearson rank algorithms with the ‘psych’ software. Linear regression analysis, visualized with the ‘ggplot2’ R package, examined the relationship between different factors. Statistical significance was determined set at p < 0.05.
The community assembly of the generalist and specialist communities was assessed following the methodology outlined by a recent study73. Predicted functional profiles of bacterial communities were generated using the PICRUSt (Phylogenetic Investigation of Communities by Reconstruction of Unobserved States based on 16S rDNA high-throughput sequencing data). Through metagenomic function predictions, predictive functional gene clusters were identified and categorized into Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways. Further comprehensive analyses were performed on specific KEGG pathways representing candidate predictive functional categories in the level 2 KEGG classification.
Statistics and reproducibility
All statistical analyses were conducted using R and relevant packages such as ‘vegan,’ ‘psych,’ and ‘ggplot2.’ The 250 leaf samples represented independent biological samples collected from different individual trees across diverse geographical locations, ensuring genetic and environmental heterogeneity. Unless otherwise stated, the data presented were derived from five independent biological replicates, with each replicate representing a unique sample collected from different sites across the study region. In addition, five technical replicates were performed for each biological sample during DNA extraction and PCR amplification to ensure reproducibility. The replicate samples were treated as independent biological entities, and statistical analyses were performed on the aggregated data from each biological replicate. Alpha diversity (Shannon index and species richness) was calculated using the ‘vegan’ package. For multivariate analyses, PCoA, ANOSIM, and PERMANOVA were applied to assess community structure differences based on Bray–Curtis dissimilarity. The Mantel and partial Mantel tests, implemented in the ‘vegan’ package, were used to identify environmental factors associated with generalist and specialist OTUs. Correlations between different environmental variables and bacterial community composition were analyzed using Spearman or Pearson rank correlation, depending on data distribution. Statistical significance was defined as p < 0.05, with additional levels of significance denoted as *p < 0.01, **p < 0.01, ***p < 0.0001.
The data were reproducible across multiple biological and technical replicates, ensuring reliability in our findings. The details of statistical tests employed for each analysis are provided in the respective figure legends and methods descriptions. Reproducibility of the experimental protocols was ensured by conducting all sample collection, DNA extraction, PCR amplification, and bioinformatics analysis according to standardized, previously validated procedures.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
This research was jointly funded by the Guangdong Ocean University Innovative Team (Early-warning of marine disasters) (Grant number 2023KCXTD015), Guangxi Key Research and Development Program (Grant number AB24010109), the Scientific Research Start Funds of Guangdong Ocean University. The Natural Science Foundation of Guangxi Province (Grant number 2022GXNSFBA035591) and the National Research Foundation of Korea under project number NRF-2022R1F1A1066643. China-Sri Lanka Joint Center for Education & Research, Chinese Academy of Sciences.
Author contributions
R.T.N.: conceptualization, methodology, writing—review & editing, supervision. H.Z.: conceptualization and methodology. L.P.: investigation, visualization, supervision. X.Q.: investigation, formal analysis, supervision. J.H.: investigation, supervision. Q.He: writing—review & editing. X.S.: writing—review & editing. G.J.: formal analysis, visualization. Q.Hou: writing—review & editing. Q.C.: writing—review & editing, supervision. X.L., K.D., L.X.: writing—review & editing. N.L.: conceptualization, methodology, formal analysis, investigation, visualization, writing—review & editing.
Peer review
Peer review information
Communications Biology thanks Tallita Tavares and Alexandre Rosado for their contribution to the peer review of this work. Primary Handling Editors: Anna Heintz-Buschart and David Favero.
Data availability
The sequence data were deposited in SRA at BioProject Accession: PRJNA1130664. The source data for plotting figures and tables (Supplementary Data 1.xlsx) and the processed data from the 16S rRNA sequencing can be archived in the supplementary material (Supplementary Data 2.xlsx). Climate variables can be obtained from the China Meteorological Data Sharing Service System (https://data.cma.cn).
Code availability
The R (R-4.1.2) codes used in this study for data analysis and graphs drawing are available at the repository Zenodo (10.5281/zenodo.14259420).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-024-07446-1.
References
- 1.Afzal, I., Shinwari, Z. K., Sikandar, S. & Shahzad, S. Plant beneficial endophytic bacteria: mechanisms, diversity, host range and genetic determinants. Microbiol. Res.221, 36–49 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Xu, Q. et al. Microbial generalists and specialists differently contribute to the community diversity in farmland soils. J. Adv. Res.40, 17–27 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Compant, S. et al. The plant endosphere world – bacterial life within plants. Environ. Microbiol.23, 1812–1829 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Yuan, Z., Zeng, Z. & Liu, F. Community structures of mangrove endophytic and rhizosphere bacteria in Zhangjiangkou National Mangrove Nature Reserve. Sci. Rep.13, 1–14 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fanous, M., Eden, J. M., Remesan, R. & Daneshkhah, A. Challenges and prospects of climate change impact assessment on mangrove environments through mathematical models. Environ. Model. Softw.162, 105658 (2023). [Google Scholar]
- 6.Otundo, R. M. Blue carbon and the role of mangroves in carbon sequestration: its mechanisms, estimation, human impacts and conservation strategies for economic incentives among African Countries Along the Indian Ocean Belt. SSRN Electronic Journal10.2139/ssrn.4912339 (2024).
- 7.Miura, T., Sánchez, R., Castañeda, L. E., Godoy, K. & Barbosa, O. Shared and unique features of bacterial communities in native forest and vineyard phyllosphere. Ecol. Evol.9, 3295–3305 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rahman, M. M. et al. Co-benefits of protecting mangroves for biodiversity conservation and carbon storage. Nat. Commun.12, 1–9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alfaro-Espinoza, G. & Ullrich, M. S. Bacterial N2-fixation in mangrove ecosystems: Insights from a diazotroph-mangrove interaction. Front. Microbiol.6, 445 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng, W. et al. SOC mediates the contribution of generalists and specialists to changes in soil nirK bacterial diversity: evidence from apple orchards in main production areas of China. Appl. Soil Ecol.182, 104713 (2023).
- 11.Liao, J. et al. The importance of neutral and niche processes for bacterial community assembly differs between habitat generalists and specialists. FEMS Microbiol. Ecol.92, fiw174 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Zuo, J. et al. Patterns of bacterial generalists and specialists in lakes and reservoirs along a latitudinal gradient. Glob. Ecol. Biogeogr.32, 2017–2032 (2023). [Google Scholar]
- 13.Mo, Y. et al. Biogeography and co-occurrence patterns of bacterial generalists and specialists in three subtropical marine bays. Limnol. Oceanogr.66, 793–806 (2021). [Google Scholar]
- 14.Yan, Q. et al. Distinct strategies of the habitat generalists and specialists in sediment of Tibetan lakes. Environ. Microbiol.24, 4153–4166 (2022). [DOI] [PubMed] [Google Scholar]
- 15.Hu, A. et al. Environmental filtering drives the assembly of habitat generalists and specialists in the coastal sand microbial communities of Southern China. Microorganisms7, 598 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu, Q. et al. Microbial generalist or specialist: Intraspecific variation and dormancy potential matter. Mol. Ecol.31, 161–173 (2022). [DOI] [PubMed] [Google Scholar]
- 17.de Gabriel Hernando, M. et al. Trends in weather conditions favor generalist over specialist species in rear-edge alpine bird communities. Ecosphere13, e3953 (2022). [Google Scholar]
- 18.Dini-Andreote, F., Stegen, J. C., Van Elsas, J. D. & Salles, J. F. Disentangling mechanisms that mediate the balance between stochastic and deterministic processes in microbial succession. Proc. Natl Acad. Sci. USA112, E1326–E1332 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zou, C., Wang, Y., Zhou, R. & Tang, T. Genetic basis of local adaptation in the cold-tolerant mangrove Kandelia obovata. Front. Plant Sci.15, 1385210 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen, Y. J. et al. Metabolic flexibility allows bacterial habitat generalists to become dominant in a frequently disturbed ecosystem. ISME J.15, 2986–3004 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alvarenga, D. O., Rigonato, J., Branco, L. H. Z. & Fiore, M. F. Cyanobacteria in mangrove ecosystems. Biodivers. Conserv.24, 799–817 (2015). [Google Scholar]
- 22.Hong, Y. et al. Diversity of endophytic and rhizoplane bacterial communities associated with exotic Spartina alterniflora and native mangrove using Illumina amplicon sequencing. Can. J. Microbiol.61, 723–733 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Stevens, V., Thijs, S. & Vangronsveld, J. Diversity and plant growth-promoting potential of (un)culturable bacteria in the Hedera helix phylloplane. BMC Microbiol.21, 1–11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang, C.-J. et al. Prokaryotic diversity in mangrove sediments across southeastern China fundamentally differs from that in other biomes. MSystems4, e00442-19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fiebig, A., Herrou, J., Willett, J. & Crosson, S. General stress signaling in the Alphaproteobacteria. Annu. Rev. Genet.49, 603–625 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li, Y., Zheng, L., Zhang, Y., Liu, H. & Jing, H. Comparative metagenomics study reveals pollution induced changes of microbial genes in mangrove sediments. Sci. Rep.9, 1–10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mandal, S. & Mandal, M. Vibrio: vibrio cholerae. In: Encyclopedia of Food Microbiology: 2nd Edn. 708–716 (2014).
- 28.Rim Kang, S., Srinivasan, S. & Lee, S. S. Vibrio oceanisediminis sp. nov., a nitrogen-fixing bacterium isolated from an artificial oil-spill marine sediment. Int. J. Syst. Evolut. Microbiol.65, 3552–3557 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Ceccarelli, D. & Colwell, R. R. Vibrio ecology, pathogenesis, and evolution. Front. Microbiol.5, 1–2 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Umpiérrez, A. et al. Non-O157 Shiga toxin-producing Escherichia coli with potential harmful profiles to humans are isolated from the faeces of calves in Uruguay. Austral J. Vet. Sci.54, 45–53 (2022). [Google Scholar]
- 31.Rosenblueth, M. & Martínez-Romero, E. Bacterial endophytes and their interactions with hosts. Mol. Plant Microbe Interact.19, 827–837 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Poole, P., Ramachandran, V. & Terpolilli, J. Rhizobia: from saprophytes to endosymbionts. Nat. Rev. Microbiol.16, 291–303 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Pascoal, F., Duarte, P., Assmy, P., Costa, R. & Magalhães, C. Full-length 16S rRNA gene sequencing combined with adequate database selection improves the description of Arctic marine prokaryotic communities. Ann. Microbiol.74, 1–12 (2024). [Google Scholar]
- 34.Parks, D. H. et al. GTDB: An ongoing census of bacterial and archaeal diversity through a phylogenetically consistent, rank normalized and complete genome-based taxonomy. Nucleic Acids Res.50, D785–D794 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Meijenfeldt, F. A. B., Hogeweg, P. & Dutilh, B. E. A social niche breadth score reveals niche range strategies of generalists and specialists. Nat. Ecol. Evol.7, 768–781 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rastogi, G., Coaker, G. L. & Leveau, J. H. J. New insights into the structure and function of phyllosphere microbiota through high-throughput molecular approaches. FEMS Microbiol. Lett.348, 1–10 (2013). [DOI] [PubMed] [Google Scholar]
- 37.Bodenhausen, N., Horton, M. W. & Bergelson, J. Bacterial communities associated with the leaves and the roots of Arabidopsis thaliana. PLoS One8, e56329 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hardoim, P. R., van Overbeek, L. S. & van Elsas, J. D. Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol.16, 463–471 (2008). [DOI] [PubMed] [Google Scholar]
- 39.Pramono, H. et al. Bacterial endophytes from mangrove leaves with antibacterial and enzymatic activities. Malays. J. Microbiol.15, 543–553 (2019). [Google Scholar]
- 40.Logares, R. et al. Biogeography of bacterial communities exposed to progressive long-term environmental change. ISME J.7, 937–948 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo, Z. et al. Biogeographic patterns and assembly mechanisms of bacterial communities differ between habitat generalists and specialists across elevational gradients. Front. Microbiol.10, 1–12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muscarella, M. E., Boot, C. M., Broeckling, C. D. & Lennon, J. T. Resource heterogeneity structures aquatic bacterial communities. ISME J.13, 2183–2195 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dragojević, M., Stanković, N., Djokić, L., Raičević, V. & Jovičić-Petrović, J. Endorhizosphere of indigenous succulent halophytes: a valuable resource of plant growth promoting bacteria. Environ. Microbiome18, 1–15 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Devictor, V., Julliard, R. & Jiguet, F. Distribution of specialist and generalist species along spatial gradients of habitat disturbance and fragmentation. Oikos117, 507–514 (2008). [Google Scholar]
- 45.Gianuca, A. T., Declerck, S. A. J., Lemmens, P. & De Meester, L. Effects of dispersal and environmental heterogeneity on the replacement and nestedness components of β-diversity. Ecology98, 525–533 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Mo, Y. et al. Biogeography and co occurrence patterns of bacterial generalists and specialists in three subtropical marine bays. Limnol. Oceanogr.66, 793–806 (2021). [Google Scholar]
- 47.Zhang, K. et al. Salinity is a key determinant for soil microbial communities in a desert ecosystem. MSystems4, e00225-18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Müller, D. B., Vogel, C., Bai, Y. & Vorholt, J. A. The plant microbiota: systems-level insights and perspectives. Annu. Rev. Genet.50, 211–234 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Vacher, C. et al. The phyllosphere: microbial jungle at the plant-climate interface. Annu. Rev. Ecol. Evol. Syst.47, 1–24 (2016). [Google Scholar]
- 50.Perazzolli, M. et al. Simulated global warming affects endophytic bacterial and fungal communities of Antarctic pearlwort leaves and some bacterial isolates support plant growth at low temperatures. Sci. Rep.12, 18839 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shade, A. et al. Conditionally rare taxa disproportionately contribute to temporal changes in microbial diversity. MBio5, e01371-14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jacob, S. et al. Habitat choice meets thermal specialization: competition with specialists may drive suboptimal habitat preferences in generalists. Proc. Natl Acad. Sci. USA115, 11988–11993 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fontúrbel, F. E., Nespolo, R. F., Amico, G. C. & Watson, D. M. Climate change can disrupt ecological interactions in mysterious ways: Using ecological generalists to forecast community-wide effects. Clim. Change Ecol.2, 100044 (2021). [Google Scholar]
- 54.Delgado-Baquerizo, M. et al. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun.7, 1–8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hutchinson, G. E. Concluding remarks. Cold Spring Harb. Symp. Quant. Biol.22, 415–427 (1957). [Google Scholar]
- 56.Keddy, P. A. & Keddy, P. Assembly and response rules: two goals for predictive community ecology. J. Veg. Sci.3, 157–164 (1992). [Google Scholar]
- 57.Buffington, K. J. et al. Projecting mangrove forest resilience to sea-level rise on a Pacific Island: species dynamics and ecological thresholds. Estuaries Coasts47, 1–12 (2024). [Google Scholar]
- 58.Ning, D. et al. A quantitative framework reveals ecological drivers of grassland microbial community assembly in response to warming. Nat. Commun.11, 1–11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bell, T. H. Many roads to bacterial generalism. FEMS Microbiol. Ecol.97, fiaa240 (2021). [DOI] [PubMed] [Google Scholar]
- 60.Louca, S. et al. Function and functional redundancy in microbial systems. Nat. Ecol. Evol.2, 936–943 (2018). [DOI] [PubMed] [Google Scholar]
- 61.Roller, B. R. K., Stoddard, S. F. & Schmidt, T. M. Exploiting rRNA operon copy number to investigate bacterial reproductive strategies. Nat. Microbiol.1, 1–7 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Avila-Jimenez, M. L. et al. Functional associations and resilience in microbial communities. Microorganisms8, 1–14 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ruan, Y. et al. Warming and altered precipitation independently and interactively suppress alpine soil microbial growth in a decadal-long experiment. ELife12, e89392 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leizeaga, A., Hicks, L. C., Manoharan, L., Hawkes, C. V. & Rousk, J. Drought legacy affects microbial community trait distributions related to moisture along a savannah grassland precipitation gradient. J. Ecol.109, 3195–3210 (2021). [Google Scholar]
- 65.Colossi Brustolin, M. et al. Future ocean climate homogenizes communities across habitats through diversity loss and rise of generalist species. Glob. Change Biol.25, 3539–3548 (2019). [DOI] [PubMed] [Google Scholar]
- 66.Amora-Nogueira, L. et al. Tropical forests as drivers of lake carbon burial. Nat. Commun.13, 1–12 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qian, X. et al. Leaf and root endospheres harbor lower fungal diversity and less complex fungal co-occurrence patterns than rhizosphere. Front. Microbiol.10, 1–12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bibi, F. et al. Isolation, diversity, and biotechnological potential of rhizo- and endophytic bacteria associated with mangrove plants from Saudi Arabia. Genet. Mol. Res.16, 1–8 (2017). [DOI] [PubMed] [Google Scholar]
- 69.Bolyen, E. et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol.37, 852–857 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao, H. et al. Abundant and rare taxa of planktonic fungal community exhibit distinct assembly patterns along coastal eutrophication gradient. Microb. Ecol.85, 495–507 (2023). [DOI] [PubMed] [Google Scholar]
- 71.Glassman, S. I. & Martiny, J. B. H. Broadscale ecological patterns are robust to use of exact sequence variants versus operational taxonomic units. MSphere3, e00148-18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sen, B. T. C. & Olesen, J. E. Nitrogen mineralization potential of organomineral size separates from soils with annual straw incorporation. Eur. J. Soil Sci.49, 25–36 (1998). [Google Scholar]
- 73.Jia, X., Dini-Andreote, F. & Falcão Salles, J. Community assembly processes of the microbial rare biosphere. Trends Microbiol.26, 738–747 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The sequence data were deposited in SRA at BioProject Accession: PRJNA1130664. The source data for plotting figures and tables (Supplementary Data 1.xlsx) and the processed data from the 16S rRNA sequencing can be archived in the supplementary material (Supplementary Data 2.xlsx). Climate variables can be obtained from the China Meteorological Data Sharing Service System (https://data.cma.cn).
The R (R-4.1.2) codes used in this study for data analysis and graphs drawing are available at the repository Zenodo (10.5281/zenodo.14259420).