Abstract
Purpose
We aimed to investigate the risk of developing depression in individuals with primary open-angle glaucoma with associated vision impairment.
Methods
We conducted a nationwide, population-based cohort study using data from the Korean National Health Information Database and National Disability Registry. We assessed baseline characteristics such as age, sex, income level, lifestyle factors, anthropometric data, lab results, and Charlson Comorbidity Index scores through diagnostic codes and health screening data. Depression risk in relation to glaucoma and vision impairment was analyzed using a multivariable-adjusted Cox proportional hazard model.
Results
Among 3,680,570 individuals screened through the Korean National Health Screening Program in 2009, 681,515 were newly diagnosed with depression during follow-up. Subjects with glaucoma showed a higher risk of depression than those without glaucoma, with an adjusted hazard ratio (HR) of 2.011 (95 % confidence interval [CI]: 1.946–2.078) pre-adjustment and 1.085 (95 % CI: 1.050–1.121) post-adjustment for covariates. For those with glaucoma and vision impairment, the adjusted HR increased to 1.164 (95 % CI: 1.045–1.297) and to 1.207 (95 % CI: 1.039–1.403) with severe vision impairment. The association between glaucoma and depression was more pronounced in men (P for interaction = 0.001) and those with a Charlson Comorbidity Index <3 (P for interaction = 0.008).
Conclusions
Primary open-angle glaucoma increased the risk of developing depression. The risk escalated gradually with the presence and severity of concurrent vision impairment. The impact of glaucoma and vision impairment on new-onset depression was greater in men and in those with less comorbidities.
Keywords: Depression, Primary open-angle glaucoma, Glaucoma, Vision impairment, Epidemiology
1. Introduction
Glaucoma is a progressive eye condition that can lead to permanent vision impairment (VI) [1,2]. In 2020, the disease accounted for 11 % of global blindness among those over 50 years, with 4.13 million people suffering moderate to severe VI due to glaucoma [3]. The chronic nature of glaucoma, lack of a definitive cure, and ongoing need for treatments can contribute to depressive symptoms [4,5]. Moreover, the VI caused by glaucoma can disrupt daily life, reduce mobility, and limit the ability to work or participate in routine activities, potentially impacting overall well-being and quality of life [6,7]. Additionally, glaucoma-related circadian rhythm disruptions may increase the risk of depression [8].
With an aging global population, glaucoma prevalence is expected to rise from 76 million cases in 2020 to 111.8 million by 2040, posing a public health challenge in terms of both healthcare costs and disease burden [9]. Given depression's significant impact on global health [10], understanding the link between glaucoma and depression and identifying contributing factors is crucial.
Prior studies have indicated a correlation between glaucoma and depression [1,[11], [12], [13], [14], [15], [16], [17]], though some findings are contradictory [[18], [19], [20], [21], [22]]. Furthermore, there is even greater disagreement on which factors contribute to the association [4]. Some studies have demonstrated that objective measures of vision, including the visual acuity or the visual field, are correlated with depression [13,23], but others have suggested that only self-reported measures of vision are associated with depression in glaucoma patients [1,24,25]. Some studies also have indicated that factors including age, sex, underlying comorbidities, education level, marital status, and others may be risks for depression in glaucoma patients, but previous studies did not stratify the disease according to accompanying vision impairment severity [15,26,27].
Therefore, the purpose of this study was to investigate whether primary open-angle glaucoma (POAG) and its related VI are associated with a higher risk of depression and to explore the influence of age, gender, and comorbidities on this relationship.
2. Method
2.1. Data source
In this nationwide, population-based, retrospective cohort study, we used authorized clinical data from the Korean National Health Insurance Service (KNHIS). The KNHIS requires all residents to be registered in this universal medical care system, which includes the following comprehensive medical information: i) demographic data such as age, sex, socioeconomic factors, and household income, ii) health claims data with diagnostic codes for admissions and outpatient care according to the International Classification of Diseases 10th revision (ICD-10), treatment procedures, and prescription records, and iii) data from the biennial Korean National Health Screening Program, covering anthropometric measurements, visual and hearing tests, blood pressure, basic laboratory results, and a standardized self-questionnaire on lifestyle factors related to health, including smoking, alcohol consumption, and physical activity.
We also used the National Disability Registry (NDR) to determine the presence and severity of VI. The Korean government established NDR to provide welfare benefits according to the type and severity of disabilities in 1989 [28]. The NDR categorizes VI based on ophthalmologic assessments reviewed by certified ophthalmologists, with a six-grade classification based on visual acuity and field of vision (Table 1). The registry requires reevaluation every 2, 3, or 4 years depending on the degree of impairment. In this study, grade 6 was regarded as mild VI, while grades 1–5 were regarded as severe VI [29].
Table 1.
Grading of vision impairment in the National Disability Registry.
| Grade 1 | Visual acuity of better eye is less than or equal to 20/1000 There should not be a title row. Grade 1 should be the same as the rest grades. |
|---|---|
| Grade 2 | Visual acuity of better eye is less than or equal to 20/500 |
| Grade 3 | Visual acuity of better eye is less than or equal to 20/320, or the visual field of each eye is less than 5° in any direction |
| Grade 4 | Visual acuity of better eye is less than or equal to 20/200, or the visual field of each eye is less than 10° in any direction |
| Grade 5 | Visual acuity of better eye is less than or equal to 20/100, or the sum of visual fields of both eyes is less than 50 % of normal |
| Grade 6 | Visual acuity of worse eye is less than or equal to 20/1000 |
2.2. Study population
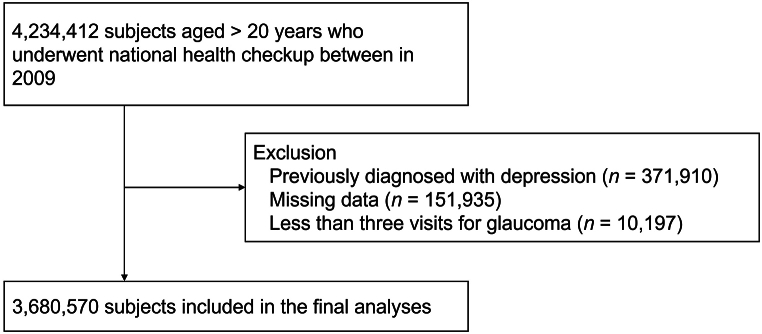
Using the KNHIS database, we identified 4,234,412 subjects aged 20 years or older who underwent national general health examination in 2009, which was defined as the index date. Exclusions included those previously diagnosed with depression (n = 371,910) and those missing essential data for covariate analysis (n = 151,935) (Fig. 1). Primary open-angle glaucoma was confirmed by at least three medical visits for POAG (ICD-10 code H01) to increase the validity of diagnosis as previously defined [30,31]. Those with less than three visits for glaucoma were excluded from the study (n = 10,197). Depression was defined using ICD-10 codes for depressive episode (F32) or recurrent depressive disorder (F33) as in previous epidemiologic studies [29,32,33]. Subjects were categorized according to the presence of glaucoma and the presence and severity of VI and were followed from the index date until the onset of depression, death, or December 31, 2021, whichever occurred first.
Fig. 1.
Selection of study subjects.
Comorbidities were defined based on KNHIS diagnostic codes, prescription history within one year before the index date, and health examination results as in previous studies [29,34]. Hypertension was defined as having an ICD-10 code for hypertension (I10-I13 and I15) with at least one prescription for antihypertensive medication, or a systolic blood pressure of ≥140 mmHg or a diastolic blood pressure of ≥90 mmHg recorded during a national health examination. Diabetes was defined as at least one prescription of antidiabetic agent within one year prior to index date under ICD-10 code for diabetes (E11-E14) or fasting glucose ≥126 mg/dL on national health screening examination. Dyslipidemia was defined as ICD-10 code E78 for dyslipidemia and at least one prescription claim for lipid-lowering medications or serum total cholesterol level ≥240 mg/dL.
Data regarding health-related lifestyle factors were collected using self-questionnaires. Smoking status was categorized as non-smoker, ex-smoker, or current smoker. Alcohol consumption was classified as no alcohol (no alcohol intake within the past year), mild alcohol consumption (<30 g of alcohol per day), or heavy alcohol consumption (≥30 g of alcohol per day). Regular exercise was defined as moderate physical activity for more than 30 min a day for more than five times a week or strenuous physical activity for more than 20 min a day for more than three times a week. Subjects’ socioeconomic status was dichotomized into upper 75 % and lower 25 % based on household income. Body mass index (BMI) was calculated as weight (kg) divided by square of height (m2). Systolic and diastolic blood pressure were measured in a seated position after at least 5 min of rest. Serum fasting glucose and total cholesterol levels were measured with blood samples collected after overnight fasting. In addition, the Charlson comorbidity index (CCI), a weighted index widely used to indicate composite health status and to predict risk of death, was calculated using 19 comorbid diseases identified from KNHIS claims data using ICD-10 codes [29,35].
2.3. Statistical analysis
The baseline characteristics of the study participants were compared using a Student's t-test or ANOVA for continuous variables and X2 test for categorical variables. Normality of the data was assessed using various statistical methods, including quantile-quantile plots, due to the large sample size, as performed by our statistician (KH). The incidence rate of depression was determined by dividing the number of events by 1000 person-years. Hazard ratios (HR) and 95 % confidence intervals (CI) were calculated using Cox proportional hazards regression analysis. Fully adjusted model included age, sex, CCI, income, smoking status, drinking status, exercise status, and body mass index. Subgroup analyses considered the effect of age, sex, and comorbidities on depression risk. We used SAS (ver 9.4; SAS institute, Inc.) for all statistical analyses. Statistical significance was set at P < 0.05.
2.4. Ethics statement
The study adhered to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of the Yeouido St. Mary's Hospital (SC19ZESI0040), the Catholic University of Korea, which waived the requirement for informed consent because the data were anonymized public data.
3. Results
3.1. Baseline characteristics of the study population
This study included 3,680,570 adults aged 20 and above with no prior depression (Fig. 1). Table 2 presents baseline characteristics of the study population based on glaucoma and VI status. There were 10,163 subjects in the glaucoma group, of which 875 had accompanying VI. Compared to controls, individuals with glaucoma were less likely to be in the lowest income quartile, more likely to be former or non-smokers and non-drinkers, and more likely to have conditions like diabetes, hypertension, and dyslipidemia. Additionally, they were older and showed higher averages in BMI, glucose levels, blood pressure (both systolic and diastolic), and cholesterol. Those with glaucoma also had a significantly higher mean CCI (1.9 ± 1.93) compared to those without glaucoma (0.82 ± 1.3).
Table 2.
Baseline characteristics of study subjects according to glaucoma and vision impairment status.
|
N |
Glaucoma (−) |
Glaucoma (+) |
P-value | Glaucoma (−) |
Glaucoma (+) |
P-value | |
|---|---|---|---|---|---|---|---|
| Vision impairment (−) |
Vision impairment (+) |
||||||
| 3670407 | 10163 | 3670407 | 9288 | 875 | |||
| Age, years | 46.26 ± 13.9 | 60.95 ± 12.88 | <0.001 | 46.26 ± 13.9 | 60.72 ± 12.95 | 63.43 ± 11.81 | <0.001 |
| Sex, male | 2094969 (57.08) | 5890 (57.96) | 0.074 | 2094969 (57.08) | 5303 (57.1) | 587 (67.09) | <0.001 |
| Income, low 25%å | 786541 (21.43) | 1896 (18.66) | <0.001 | 786541 (21.43) | 1699 (18.29) | 197 (22.51) | <0.001 |
| Diabetes | 302125 (8.23) | 2498 (24.58) | <0.001 | 302125 (8.23) | 2256 (24.29) | 242 (27.66) | <0.001 |
| Hypertension | 892458 (24.31) | 4998 (49.18) | <0.001 | 892458 (24.31) | 4551 (49) | 447 (51.09) | <0.001 |
| Dyslipidemia | 609731 (16.61) | 3188 (31.37) | <0.001 | 609731 (16.61) | 2937 (31.62) | 251 (28.69) | <0.001 |
| Smoking | <0.001 | <0.001 | |||||
| Non | 2126205 (57.93) | 6435 (63.32) | 2126205 (57.93) | 5921 (63.75) | 514 (58.74) | ||
| Ex | 535970 (14.6) | 2150 (21.16) | 535970 (14.6) | 1938 (20.87) | 212 (24.23) | ||
| Current | 1008232 (27.47) | 1578 (15.53) | 1008232 (27.47) | 1429 (15.39) | 149 (17.03) | ||
| Drinking | <0.001 | <0.001 | |||||
| Non | 1821958 (49.64) | 6581 (64.75) | 1821958 (49.64) | 6005 (64.65) | 576 (65.83) | ||
| Mild | 1543289 (42.05) | 3020 (29.72) | 1543289 (42.05) | 2786 (30) | 234 (26.74) | ||
| Heavy | 305160 (8.31) | 562 (5.53) | 305160 (8.31) | 497 (5.35) | 65 (7.43) | ||
| Regular exercise | 662535 (18.05) | 2399 (23.61) | <0.001 | 662535 (18.05) | 2199 (23.68) | 200 (22.86) | <0.001 |
| Body mass index, kg/m2 | 23.69 ± 3.22 | 24.03 ± 3.03 | <0.001 | 23.69 ± 3.22 | 24.03 ± 3.03 | 24.03 ± 2.98 | <0.001 |
| Waist circumference, cm | 80.21 ± 9.09 | 83 ± 8.5 | <0.0001 | 80.21 ± 9.09 | 82.91 ± 8.52 | 83.96 ± 8.25 | <0.0001 |
| Fasting glucose, mg/dl | 97.01 ± 23.64 | 106.45 ± 31.51 | <0.001 | 97.01 ± 23.64 | 106.26 ± 31.24 | 108.43 ± 34.23 | <0.001 |
| Systolic blood pressure, mmHg | 122.36 ± 14.98 | 126.88 ± 15.86 | <0.001 | 122.36 ± 14.98 | 126.82 ± 15.88 | 127.5 ± 15.68 | <0.001 |
| Diastolic blood pressure, mmHg | 76.33 ± 10.05 | 77.51 ± 10.21 | <0.001 | 76.33 ± 10.05 | 77.47 ± 10.16 | 77.97 ± 10.69 | <0.001 |
| Total cholesterol, mg/dL | 194.75 ± 36.75 | 195.98 ± 38.87 | 0.005 | 194.75 ± 36.75 | 196.15 ± 38.88 | 194.07 ± 38.66 | 0.001 |
| Charlson Comorbidity Index | 0.82 ± 1.3 | 1.9 ± 1.93 | <0.001 | 0.82 ± 1.3 | 1.88 ± 1.93 | 2.05 ± 1.99 | <0.001 |
3.2. Incidence and risk of depression according to glaucoma and associated vision impairment
Table 3 provides data on depression incidence and risk based on glaucoma and VI. Across the follow-up period, 681,515 individuals were newly diagnosed with depression. The depression incidence rate was 40.620 per 1000 person-years in participants with glaucoma, compared to 20.264 in those without. Among those with glaucoma, participants with VI had a depression incidence of 45.499 per 1000 person-years, increasing to 48.558 for those with severe VI.
Table 3.
Incidence rate and hazard ratio of new onset depression according to previously diagnosed glaucoma and accompanying vision impairment (VI).
| N | Depression | Duration | Incidence rate (per 1000) | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|---|---|---|
| Glaucoma (−) | 3670407 | 743662 | 36698574.98 | 20.264 | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Glaucoma (+) | 10163 | 3585 | 88258.07 | 40.620 | 2.011 (1.946, 2.078) | 1.081 (1.046, 1.118) | 1.085 (1.050, 1.121) |
| Glaucoma (−) | 3670407 | 743662 | 36698574.98 | 20.264 | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Glaucoma (+), VI (−) | 9288 | 3255 | 81005.1 | 40.183 | 1.989 (1.922, 2.059) | 1.074 (1.037, 1.112) | 1.077 (1.041, 1.115) |
| Glaucoma (+), VI (+) | 875 | 330 | 7252.97 | 45.499 | 2.260 (2.029, 2.517) | 1.163 (1.044, 1.296) | 1.164 (1.045, 1.297) |
| Glaucoma (−) | 3670407 | 743662 | 36698574.98 | 20.264 | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Glaucoma (+), VI (−) | 9288 | 3255 | 81005.1 | 40.183 | 1.989 (1.922, 2.059) | 1.074 (1.037, 1.112) | 1.077 (1.041, 1.115) |
| Glaucoma (+), VI (+), milda | 446 | 160 | 3751.97 | 42.644 | 2.114 (1.811, 2.468) | 1.120 (0.959, 1.307) | 1.122 (0.961, 1.310) |
| Glaucoma (+), VI (+), severeb | 429 | 170 | 3501 | 48.558 | 2.420 (2.083, 2.811) | 1.207 (1.039, 1.403) | 1.207 (1.039, 1.403) |
Model 1: Unadjusted.
Model 2: Adjusted for age, gender, and Charlson Comorbidity Index.
Model 3: Adjusted for age, gender, Charlson Comorbidity Index, income, smoking, drinking, exercise, and body mass index.
Indicate grade 6 of vision impairment in the National Disability Registry.
Indicate grade 1–5 of vision impairment in the National Disability Registry.
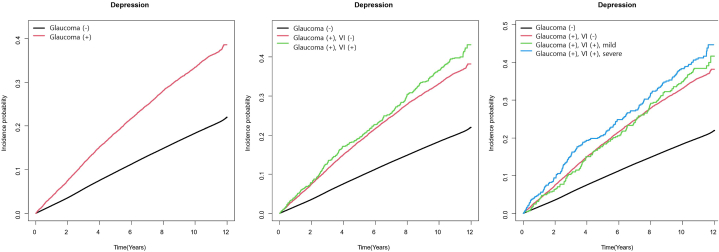
For those with glaucoma, the unadjusted HR for depression onset was 2.011 (95 % CI: 1.946–2.078), which decreased to an adjusted hazard ratio (aHR) of 1.085 (95 % CI: 1.050–1.121) after adjusting for variables such as age, sex, CCI, income, lifestyle factors, and BMI. In participants with glaucoma, those with accompanying VI showed a higher HR for depression onset (HR = 2.260, 95 % CI = 2.029–2.517; aHR = 1.164, 95 % CI = 1.045–1.297 after adjustment) than those without VI (HR = 1.989, 95 % CI = 1.922–2.059; aHR = 1.074, 95 % CI = 1.037–1.112 after adjustment). Furthermore, subjects with glaucoma and severe VI showed the highest risk of developing depression (HR = 2.420, 95 % CI = 2.083–2.811; aHR = 1.207, 95 % CI = 1.039–1.403 post-adjustment).). Depression risk was highest in the severe VI group (aHR = 1.207), followed by the mild VI (aHR = 1.122), and glaucoma-only (aHR = 1.077) groups. Fig. 2 shows the cumulative incidence of depression by glaucoma and VI status.
Fig. 2.
Cumulative incidence rate of depression according to glaucoma and associated vision impairment (VI) and its severity.
3.3. Subgroup analysis on depression risk
In analyses segmented by age, sex, and CCI (Table 4), the increased risk of depression associated with glaucoma and VI was evident across all groups. Glaucoma increased depression risk across all subgroups, with even higher risk in those with accompanying VI, especially severe VI. Age did not influence the association between glaucoma and depression (P for interaction = 0.984). The impact of glaucoma on depression was greater in men than in women (P for interaction = 0.001). Glaucoma's effect on depression was more pronounced in participants with a CCI <3 than those with CCI ≥3 (P for interaction = 0.008). However, for glaucoma patients with VI, those with a CCI ≥3 had a slightly higher risk of depression (HR = 1.181, 95 % CI = 1.003–1.392) compared to those with CCI <3 (HR = 1.155, 95 % CI = 1.000–1.333, P for interaction = 0.016).
Table 4.
Subgroup analyses of risk of new onset depression according to previously diagnosed glaucoma and accompanying vision impairment (VI) stratified by age, gender, and Charlson Comorbidity Index.
| A. Presence of previously diagnosed glaucoma | |||||||
|---|---|---|---|---|---|---|---|
| N | Depression | Duration | Incidence rate (per 1000) | Model 3 | P for interaction | ||
| Age <65 | Glaucoma (−) | 3244163 | 571173 | 33266525.29 | 17.170 | 1 (ref.) | 0.984 |
| Glaucoma (+) | 5524 | 1460 | 53009.7 | 27.542 | 1.085 (1.031, 1.142) | ||
| Age ≥65 | Glaucoma (−) | 426244 | 172489 | 3432049.69 | 50.258 | 1 (ref.) | |
| Glaucoma (+) | 4639 | 2125 | 35248.37 | 60.287 | 1.084 (1.039, 1.132) | ||
| Sex, male | Glaucoma (−) | 2094969 | 336164 | 21430106.62 | 15.687 | 1 (ref.) | 0.001 |
| Glaucoma (+) | 5890 | 1762 | 52707.8 | 33.430 | 1.149 (1.097, 1.204) | ||
| Sex, female | Glaucoma (−) | 1575438 | 407498 | 15268468.36 | 26.689 | 1 (ref.) | |
| Glaucoma (+) | 4273 | 1823 | 35550.27 | 51.280 | 1.029 (0.983, 1.078) | ||
| CCI <3 | Glaucoma (−) | 3307541 | 610109 | 33599825.27 | 18.158 | 1 (ref.) | 0.008 |
| Glaucoma (+) | 7040 | 2173 | 64251.44 | 33.820 | 1.127 (1.08, 1.175) | ||
| CCI ≥3 |
Glaucoma (−) | 362866 | 133553 | 3098749.7 | 43.099 | 1 (ref.) | |
| Glaucoma (+) |
3123 |
1412 |
24006.63 |
58.817 |
1.028 (0.975, 1.083) |
||
| B. Presence of previously diagnosed glaucoma and/or associated vision impairment | |||||||
| N | Depression | Duration | Incidence rate (per 1000) | Model 3 | P for interaction | ||
| Age <65 | Glaucoma (−) | 3244163 | 571173 | 33266525.29 | 17.170 | 1 (ref.) | 0.716 |
| Glaucoma, VI (−) | 5121 | 1348 | 49213.44 | 27.391 | 1.084 (1.028, 1.144) | ||
| Glaucoma, VI (+) | 403 | 112 | 3796.26 | 29.503 | 1.099 (0.913, 1.323) | ||
| Age ≥65 | Glaucoma (−) | 426244 | 172489 | 3432049.69 | 50.258 | 1 (ref.) | |
| Glaucoma (+), VI (−) | 4167 | 1907 | 31791.66 | 59.984 | 1.073 (1.025, 1.122) | ||
| Glaucoma (+), VI (+) | 472 | 218 | 3456.71 | 63.066 | 1.201 (1.051, 1.371) | ||
| Sex, male | Glaucoma (−) | 2094969 | 336164 | 21430106.62 | 15.687 | 1 (ref.) | 0.001 |
| Glaucoma (+), VI (−) | 5303 | 1558 | 47830.34 | 32.574 | 1.129 (1.074, 1.187) | ||
| Glaucoma (+), VI (+) | 587 | 204 | 4877.46 | 41.825 | 1.332 (1.161, 1.528) | ||
| Sex, female | Glaucoma (−) | 1575438 | 407498 | 15268468.36 | 26.689 | 1 (ref.) | |
| Glaucoma (+), VI (−) | 3985 | 1697 | 33174.76 | 51.153 | 1.034 (0.986, 1.085) | ||
| Glaucoma (+), VI (+) | 288 | 126 | 2375.51 | 53.041 | 0.967 (0.812, 1.152) | ||
| CCI <3 | Glaucoma (−) | 3307541 | 610109 | 33599825.27 | 18.158 | 1 (ref.) | 0.016 |
| Glaucoma (+), VI (−) | 6461 | 1986 | 59124.12 | 33.590 | 1.124 (1.076, 1.175) | ||
| Glaucoma (+), VI (+) | 579 | 187 | 5127.32 | 36.471 | 1.155 (1, 1.333) | ||
| CCI ≥3 |
Glaucoma (−) | 362866 | 133553 | 3098749.7 | 43.099 | 1 (ref.) | |
| Glaucoma (+), VI (−) | 2827 | 1269 | 21880.98 | 57.996 | 1.013 (0.959, 1.071) | ||
| Glaucoma (+), VI (+) |
296 |
143 |
2125.65 |
67.274 |
1.181 (1.003, 1.392) |
||
| C. Presence of previously diagnosed glaucoma and/or associated vision impairment and its severity | |||||||
| N | Depression | Duration | Incidence rate (per 1000) | Model 3 | P for interaction | ||
| Age <65 | Glaucoma (−) | 3244163 | 571173 | 33266525.29 | 17.170 | 1 (ref.) | 0.164 |
| Glaucoma (+), VI (−) | 5121 | 1348 | 49213.44 | 27.391 | 1.084 (1.028, 1.144) | ||
| Glaucoma (+), VI (+), milda | 208 | 46 | 2048.88 | 22.451 | 0.883 (0.662, 1.179) | ||
| Glaucoma (+), VI (+), severeb | 195 | 66 | 1747.38 | 37.771 | 1.325 (1.041, 1.686) | ||
| Age ≥65 | Glaucoma (−) | 426244 | 172489 | 3432049.69 | 50.258 | 1 (ref.) | |
| Glaucoma (+), VI (−) | 4167 | 1907 | 31791.66 | 59.984 | 1.073 (1.025, 1.122) | ||
| Glaucoma (+), VI (+), mild | 238 | 114 | 1703.09 | 66.937 | 1.26 (1.049, 1.514) | ||
| Glaucoma (+), VI (+), severe | 234 | 104 | 1753.62 | 59.306 | 1.143 (0.943, 1.385) | ||
| Sex, male | Glaucoma (−) | 2094969 | 336164 | 21430106.62 | 15.687 | 1 (ref.) | 0.002 |
| Glaucoma (+), VI (−) | 5303 | 1558 | 47830.34 | 32.574 | 1.129 (1.074, 1.187) | ||
| Glaucoma (+), VI (+), mild | 309 | 101 | 2591.56 | 38.973 | 1.263 (1.039, 1.535) | ||
| Glaucoma (+), VI (+), severe | 278 | 103 | 2285.91 | 45.059 | 1.408 (1.16, 1.708) | ||
| Sex, female | Glaucoma (−) | 1575438 | 407498 | 15268468.36 | 26.689 | 1 (ref.) | |
| Glaucoma (+), VI (−) | 3985 | 1697 | 33174.76 | 51.153 | 1.034 (0.986, 1.085) | ||
| Glaucoma (+), VI (+), mild | 137 | 59 | 1160.41 | 50.844 | 0.942 (0.73, 1.215) | ||
| Glaucoma (+), VI (+), severe | 151 | 67 | 1215.1 | 55.140 | 0.991 (0.78, 1.259) | ||
| CCI <3 | Glaucoma (−) | 3307541 | 610109 | 33599825.27 | 18.158 | 1 (ref.) | 0.020 |
| Glaucoma (+), VI (−) | 6461 | 1986 | 59124.12 | 33.590 | 1.124 (1.076, 1.175) | ||
| Glaucoma (+), VI (+), mild | 305 | 98 | 2704.17 | 36.240 | 1.179 (0.967, 1.437) | ||
| Glaucoma (+), VI (+), severe | 274 | 89 | 2423.15 | 36.729 | 1.129 (0.917, 1.39) | ||
| CCI ≥3 | Glaucoma (−) | 362866 | 133553 | 3098749.7 | 43.099 | 1 (ref.) | |
| Glaucoma (+), VI (−) | 2827 | 1269 | 21880.98 | 57.996 | 1.013 (0.959, 1.071) | ||
| Glaucoma (+), VI (+), mild | 141 | 62 | 1047.79 | 59.172 | 1.043 (0.813, 1.338) | ||
| Glaucoma (+), VI (+), severe | 155 | 81 | 1077.85 | 75.149 | 1.321 (1.063, 1.641) | ||
Model 3: Adjusted for age, gender, Charlson Comorbidity Index, income, smoking, drinking, exercise, and body mass index.
Indicate grade 6 of vision impairment in the National Disability Registry.
Indicate grade 1–5 of vision impairment in the National Disability Registry.
4. Discussion
In this nationwide population-based cohort study, we found that POAG and associated VI significantly increased the risk of new-onset depression. Individuals with POAG faced a heightened risk of depression, which increased further in those with VI, especially in cases of severe impairment. The risk patterns were consistent across age groups, although the association between POAG and depression was more pronounced in men and in those with CCI score less than 3. Glaucoma, a progressive and irreversible optic neuropathy [11], carries the threat of potential blindness, inducing anxiety and depression due to the disease's incurable nature and the lifetime commitment to medication [1,12]. Limitations on daily activities due to glaucoma-related VI can also negatively impact mental health, fostering a decrease in quality of life and well-being [11,13]. Glaucoma has also been associated with dysregulation of photo-dependent melatonin production, disturbing the circadian rhythm, which has been reported to play a role in the pathogenesis of depression [8]. These possible underlying mechanisms could explain our results that glaucoma increases the risk of depression development, and associated VI further escalates the risk. The association between glaucoma and depression has been reported in many previous studies. Although some studies found no significant association [13], most studies report that glaucoma patients are more depressed than those without [1], which we validated utilizing a large cohort data.
Importantly, we found that glaucoma patients with VI, especially severe VI, showed higher risk of depression than those without VI. In line with our findings, a study performed by Gubin and associates reported that objective measures of visual function, especially retinal ganglion cell loss measured by Optical Coherence Tomography were strongly correlated with depression scores measured by Beck Depression Inventory II questionnaire [23]. In another study, Mabuchi and associates that older age and glaucoma severity, measured by mean deviation of Humphrey Visual Field Analyzer were associated with depression in patients with glaucoma [13]. However, some prior studies have not found a clear link between objective measures of vision in glaucoma and depression [1,24,25]. Jampel and associates found that visual acuity and visual field were not related with any items on the Center for Epidemiologic Studies Depression Scale [24]. Wang and associates also found that visual acuity and visual field were not associated with depression determined by the Patient Health Questionnaire-9 using the National Health and Nutrition Examination Survey [1]. The discrepancy among studies may be due to different ethnicities. There is higher incidence of normal tension glaucoma than high tension glaucoma in Asian POAG patients, including Korea [36]. It may also be due to different methods used to evaluate depression. Previous studies used various questionnaires to evaluate depressive symptoms [1,24,25]. Depression in our study was clinically diagnosed, which may indicate more severe and chronic symptoms. The difference may also be due to the different definitions to evaluate VI. More than 60 % of self-reported glaucoma patients had normal or early field defect in the worse eye and best corrected visual acuity in the worse eye was LogMAR 0.254 [1]. In addition, visual field loss in previous studies is usually expressed in decibels which does not indicate the location of visual field loss. We used NDR to evaluate the presence and severity of VI, in which the mildest VI grade indicates visual acuity of worse eye worse than or equal to 20/1000 (LogMAR 1.699). According to the NDR definition, visual field loss in the severe VI group indicates a loss of at least 50 % of the normal visual field in both eyes. Therefore, the VI group in our study indicates more severe VI, which may affect the impact on the patient's quality of life and mental status.
Furthermore, age differences between the glaucoma and control groups could have affected the results, as older age has been linked to higher depression risk [37]. However, in the multivariate models and subgroup analyses, age did not have a significant impact on the association between glaucoma and depression. Similar finding was reported by Zhang and associates [11], however, one study found that younger glaucoma patients had higher risk for depression than older patients [26]. In their study, they noted that depressive symptoms lessened during the first year after starting treatment, and other factors such as education level, marital status, income level, and substance abuse may also affect the relationship [15].
In addition, glaucoma patients had higher prevalence of comorbidities, including diabetes, hypertension, and dyslipidemia than the control group. Glaucoma has been previously associated with these comorbidities and several mechanisms have been postulated to contribute to the link [31,38,39]. Epidemiological studies have shown that hyperglycemia and hypertension have significant relationship with intraocular pressure, which is the most important risk factor for glaucoma. Other common risk factors and pathophysiologic mechanisms, such as vascular dysregulation and oxidative stress, have also been proposed as potential link. In subgroup analyses, those with fewer comorbidities (CCI <3) had a stronger glaucoma-depression association. However, glaucoma patients with VI, especially severe VI, the risk of depression was higher in patients with more comorbidities (CCI ≥3). In a prior study performed by Su and associates, high comorbidity burden and severe vascular diseases were associated with higher risk of depression in glaucoma patients, which may be associated with pathophysiology of underlying disease such as autonomic dysfunction or certain medications [40]. Our findings highlight the importance of monitoring mental health in POAG patients, particularly those with severe VI and multiple comorbid conditions. In stratified subgroup analyses, POAG had a greater impact on depression in men than in women. Although depression is generally more prevalent in women [41], a study by Zhou and associates indicated that female gender were not predictors of depression in glaucoma patients [27]. It has been suggested that men may be more prone to stressful life events due to potential gender differences in stress resilience and coping mechanisms [42].
Our study is strengthened by its large, population-based cohort and extended follow-up, establishing a robust temporal link between POAG and depression. In addition, the diagnosis of POAG and depression are claims-based, which indicate more validated diagnosis compared with self-reported questionnaires. Furthermore, the use of the NDR ensures rigorous criteria for defining and evaluating VI severity.
There are also limitations. As with other studies based on claims data, we did not have more detailed clinical data such as intraocular pressure, visual field, and extent of optic nerve damage. Second, as subjects with POAG only included those with clinically diagnosed POAG, those with undiagnosed POAG may have been included in the control group. However, the inclusion of undiagnosed POAG in the control group would have compelled the HR to move closer to the null.
In conclusion, this nationwide longitudinal cohort study revealed that POAG increased the risk of depression development. The risk escalated when accompanied by VI and even more when VI was severe. Age did not impact the association between glaucoma, VI, and depression incidence. However, the impact of glaucoma and VI on depression development was greater in men and in those with more comorbidities. These findings emphasize the need for healthcare providers to consider mental health assessments for POAG patients, particularly those with severe VI, facilitating timely psychiatric referrals where necessary.
CRediT authorship contribution statement
Sheng-Min Wang: Writing – original draft, Funding acquisition, Conceptualization. Younhea Jung: Writing – original draft, Supervision, Project administration, Investigation, Conceptualization. Kyungdo Han: Writing – review & editing, Visualization, Methodology, Formal analysis, Data curation, Conceptualization. Kyoung Ohn: Writing – review & editing, Visualization, Validation. Hae-young Lopilly Park: Writing – review & editing, Validation, Methodology. Chan Kee Park: Writing – review & editing. Jung Il Moon: Writing – review & editing, Validation, Methodology.
Data availability statement
Data are available from the Korea National Health Insurance Sharing Service Institutional Data Access Committee (https://nhiss.nhis.or.kr/bd/ay/bdaya001iv.do) for researchers who meet the access criteria.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Sheng-min Wang reports financial support was provided by National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) No. 2022R1A2C109321512. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
a. Funding/Support: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) No. 2022R1A2C109321512.
b. Financial disclosures: No financial disclosures.
c. Other Acknowledgments: none.
References
- 1.Wang S.Y., Singh K., Lin S.C. Prevalence and predictors of depression among participants with glaucoma in a nationally representative population sample. Am. J. Ophthalmol. 2012;154(3):436–444.e432. doi: 10.1016/j.ajo.2012.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Gestel A., Webers C.A., Beckers H.J., van Dongen M.C., Severens J.L., Hendrikse F., et al. The relationship between visual field loss in glaucoma and health-related quality-of-life. Eye (Lond) 2010;24(12):1759–1769. doi: 10.1038/eye.2010.133. [DOI] [PubMed] [Google Scholar]
- 3.Blindness G.B.D., Vision Impairment C. S. Vision loss expert group of the global burden of disease, causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the right to sight: an analysis for the global burden of disease study. Lancet Global Health. 2021;9(2):e144–e160. doi: 10.1016/S2214-109X(20)30489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stamatiou M.E., Kazantzis D., Theodossiadis P., Chatziralli I. Depression in glaucoma patients: a review of the literature. Semin. Ophthalmol. 2022;37(1):29–35. doi: 10.1080/08820538.2021.1903945. [DOI] [PubMed] [Google Scholar]
- 5.Goldberg I., Moloney G., McCluskey P. Topical ophthalmic medications: what potential for systemic side effects and interactions with other medications? Med. J. Aust. 2008;189(7):356–357. doi: 10.5694/j.1326-5377.2008.tb02077.x. [DOI] [PubMed] [Google Scholar]
- 6.Sabel B.A., Wang J., Cardenas-Morales L., Faiq M., Heim C. Mental stress as consequence and cause of vision loss: the dawn of psychosomatic ophthalmology for preventive and personalized medicine. EPMA J. 2018;9(2):133–160. doi: 10.1007/s13167-018-0136-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burton M.J., Ramke J., Marques A.P., Bourne R.R.A., Congdon N., Jones I., et al. The lancet global health commission on global eye health: vision beyond 2020. Lancet Global Health. 2021;9(4):e489–e551. doi: 10.1016/S2214-109X(20)30488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agorastos A., Huber C.G. The role of melatonin in glaucoma: implications concerning pathophysiological relevance and therapeutic potential. J. Pineal Res. 2011;50(1):1–7. doi: 10.1111/j.1600-079X.2010.00816.x. [DOI] [PubMed] [Google Scholar]
- 9.Tham Y.C., Li X., Wong T.Y., Quigley H.A., Aung T., Cheng C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Collaborators G.B.D.M.D. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatr. 2022;9(2):137–150. doi: 10.1016/S2215-0366(21)00395-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X., Olson D.J., Le P., Lin F.C., Fleischman D., Davis R.M. The association between glaucoma, anxiety, and depression in a large population. Am. J. Ophthalmol. 2017;183:37–41. doi: 10.1016/j.ajo.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 12.Zheng Y., Wu X., Lin X., Lin H. The prevalence of depression and depressive symptoms among eye disease patients: a systematic review and meta-analysis. Sci. Rep. 2017;7 doi: 10.1038/srep46453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mabuchi F., Yoshimura K., Kashiwagi K., Yamagata Z., Kanba S., Iijima H., et al. Risk factors for anxiety and depression in patients with glaucoma. Br. J. Ophthalmol. 2012;96(6):821–825. doi: 10.1136/bjophthalmol-2011-300910. [DOI] [PubMed] [Google Scholar]
- 14.Chen V.C., Ng M.H., Chiu W.C., McIntyre R.S., Lee Y., Lin T.Y., et al. Effects of selective serotonin reuptake inhibitors on glaucoma: a nationwide population-based study. PLoS One. 2017;12(3) doi: 10.1371/journal.pone.0173005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y.Y., Lai Y.J., Wang J.P., Shen Y.C., Wang C.Y., Chen H.H., et al. The association between glaucoma and risk of depression: a nationwide population-based cohort study. BMC Ophthalmol. 2018;18(1):146. doi: 10.1186/s12886-018-0811-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mabuchi F., Yoshimura K., Kashiwagi K., Shioe K., Yamagata Z., Kanba S., et al. High prevalence of anxiety and depression in patients with primary open-angle glaucoma. J. Glaucoma. 2008;17(7):552–557. doi: 10.1097/IJG.0b013e31816299d4. [DOI] [PubMed] [Google Scholar]
- 17.Wang S.Y., Singh K., Lin S.C. Prevalence and predictors of depression among participants with glaucoma in a nationally representative population sample. Am. J. Ophthalmol. 2012;154(3) doi: 10.1016/j.ajo.2012.03.039. 436-444 e432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eramudugolla R., Wood J., Anstey K.J. Co-morbidity of depression and anxiety in common age-related eye diseases: a population-based study of 662 adults. Front. Aging Neurosci. 2013;5:56. doi: 10.3389/fnagi.2013.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jonas J.B., Wei W.B., Xu L., Rietschel M., Streit F., Wang Y.X. Self-rated depression and eye diseases: the Beijing Eye Study. PLoS One. 2018;13(8) doi: 10.1371/journal.pone.0202132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rezapour J., Nickels S., Schuster A.K., Michal M., Munzel T., Wild P.S., et al. Prevalence of depression and anxiety among participants with glaucoma in a population-based cohort study: the Gutenberg Health Study. BMC Ophthalmol. 2018;18(1):157. doi: 10.1186/s12886-018-0831-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thau A.J., Rohn M.C.H., Biron M.E., Rahmatnejad K., Mayro E.L., Gentile P.M., et al. Depression and quality of life in a community-based glaucoma-screening project. Can. J. Ophthalmol. 2018;53(4):354–360. doi: 10.1016/j.jcjo.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Wilson M.R., Coleman A.L., Yu F., Fong Sasaki I., Bing E.G., Kim M.H. Depression in patients with glaucoma as measured by self-report surveys. Ophthalmology. 2002;109(5):1018–1022. doi: 10.1016/s0161-6420(02)00993-4. [DOI] [PubMed] [Google Scholar]
- 23.Gubin D., Neroev V., Malishevskaya T., Kolomeichuk S., Cornelissen G., Yuzhakova N., et al. Depression scores are associated with retinal ganglion cells loss. J. Affect. Disord. 2023;333:290–296. doi: 10.1016/j.jad.2023.04.039. [DOI] [PubMed] [Google Scholar]
- 24.Jampel H.D., Frick K.D., Janz N.K., Wren P.A., Musch D.C., Rimal R., et al. Depression and mood indicators in newly diagnosed glaucoma patients. Am. J. Ophthalmol. 2007;144(2):238–244. doi: 10.1016/j.ajo.2007.04.048. [DOI] [PubMed] [Google Scholar]
- 25.Skalicky S., Goldberg I. Depression and quality of life in patients with glaucoma: a cross-sectional analysis using the Geriatric Depression Scale-15, assessment of function related to vision, and the Glaucoma Quality of Life-15. J. Glaucoma. 2008;17(7):546–551. doi: 10.1097/IJG.0b013e318163bdd1. [DOI] [PubMed] [Google Scholar]
- 26.Musch D.C., Niziol L.M., Janz N.K., Gillespie B.W. Trends in and predictors of depression among participants in the collaborative initial glaucoma treatment study (CIGTS) Am. J. Ophthalmol. 2019;197:128–135. doi: 10.1016/j.ajo.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou C., Qian S., Wu P., Qiu C. Anxiety and depression in Chinese patients with glaucoma: sociodemographic, clinical, and self-reported correlates. J. Psychosom. Res. 2013;75(1):75–82. doi: 10.1016/j.jpsychores.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Shin D.W., Lee J.W., Jung J.H., Han K., Kim S.Y., Choi K.S., et al. Disparities in cervical cancer screening among women with disabilities: a national database study in South Korea. J. Clin. Oncol. 2018;36(27):2778–2786. doi: 10.1200/JCO.2018.77.7912. [DOI] [PubMed] [Google Scholar]
- 29.Hwang S., Kang S.W., Kim S.J., Han K., Kim B.S., Jung W., et al. Impact of age-related macular degeneration and related visual disability on the risk of depression: a nationwide cohort study. Ophthalmology. 2023;130(6):615–623. doi: 10.1016/j.ophtha.2023.01.014. [DOI] [PubMed] [Google Scholar]
- 30.Jung Y., Han K., Ohn K., Kim D.R., Moon J.I. Association between diabetes status and subsequent onset of glaucoma in postmenopausal women. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-97740-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jung Y., Han K., Park H.Y.L., Lee S.H., Park C.K. Metabolic health, obesity, and the risk of developing open-angle glaucoma: metabolically healthy obese patients versus metabolically unhealthy but normal weight patients. Diabetes Metab. J. 2020;44(3):414–425. doi: 10.4093/dmj.2019.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park M.J., Yoo J., Han K., Shin D.W., Fava M., Mischoulon D., et al. High body weight variability is associated with increased risk of depression: a nationwide cohort study in South Korea. Psychol. Med. 2023;53(8):3719–3727. doi: 10.1017/S003329172200040X. [DOI] [PubMed] [Google Scholar]
- 33.Baek J.H., Shin D.W., Fava M., Mischoulon D., Kim H., Park M.J., et al. Increased metabolic variability is associated with newly diagnosed depression: a nationwide cohort study. J. Affect. Disord. 2021;294:786–793. doi: 10.1016/j.jad.2021.07.006. [DOI] [PubMed] [Google Scholar]
- 34.Jung Y., Han K., Lee J.M., Park H.Y., Moon J.I. Impact of vision and hearing impairments on risk of cardiovascular outcomes and mortality in patients with type 2 diabetes: a nationwide cohort study. J Diabetes Investig. 2022;13(3):515–524. doi: 10.1111/jdi.13689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quan H., Li B., Couris C.M., Fushimi K., Graham P., Hider P., et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011;173(6):676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 36.Cho H.K., Kee C. Population-based glaucoma prevalence studies in Asians. Surv. Ophthalmol. 2014;59(4):434–447. doi: 10.1016/j.survophthal.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Valvanne J., Juva K., Erkinjuntti T., Tilvis R. Major depression in the elderly: a population study in Helsinki. Int. Psychogeriatr. 1996;8(3):437–443. doi: 10.1017/s1041610296002797. [DOI] [PubMed] [Google Scholar]
- 38.Nislawati R., Taufik Fadillah Zainal A., Ismail A., Waspodo N., Kasim F., Gunawan A. Role of hypertension as a risk factor for open-angle glaucoma: a systematic review and meta-analysis. BMJ Open Ophthalmol. 2021;6(1) doi: 10.1136/bmjophth-2021-000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song B.J., Aiello L.P., Pasquale L.R. Presence and risk factors for glaucoma in patients with diabetes. Curr. Diabetes Rep. 2016;16(12):124. doi: 10.1007/s11892-016-0815-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Su C.C., Chen J.Y., Wang T.H., Huang J.Y., Yang C.M., Wang I.J. Risk factors for depressive symptoms in glaucoma patients: a nationwide case-control study. Graefes Arch. Clin. Exp. Ophthalmol. 2015;253(8):1319–1325. doi: 10.1007/s00417-015-3032-0. [DOI] [PubMed] [Google Scholar]
- 41.Djernes J.K. Prevalence and predictors of depression in populations of elderly: a review. Acta Psychiatr. Scand. 2006;113(5):372–387. doi: 10.1111/j.1600-0447.2006.00770.x. [DOI] [PubMed] [Google Scholar]
- 42.Assari S., Lankarani M.M. Stressful life events and risk of depression 25 Years later: race and gender differences. Front. Public Health. 2016;4:49. doi: 10.3389/fpubh.2016.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the Korea National Health Insurance Sharing Service Institutional Data Access Committee (https://nhiss.nhis.or.kr/bd/ay/bdaya001iv.do) for researchers who meet the access criteria.