Abstract
Bone marrow stimulation treatment by bone marrow stromal cells (BMSCs) released from the bone medullary cavity and differentiated into cartilage via microfracture surgery is a frequently employed technique for treating articular cartilage injuries, yet the treatment presents a main drawback of poor cartilage regeneration in the elderly. Prior research indicated that aging could decrease the stemness capacity of BMSCs, thus we made a hypothesis that increasing old BMSCs (OBMSCs) stemness might improve the results of microfracture in the elderly. First, we investigated the correlation between microfracture outcomes and BMSCs stemness using clinical data and animal experiments. The outcomes of microfracture surgery in the elderly were significantly decreased as compared with the young counterparts while the stemness capacity of OBMSCs was also significantly decreased, and they were positively correlated. To investigate the role of BMSCs stemness in microfracture, we developed microfracture-mimic cartilage regeneration organoid models. In vitro experiments identified SPI1 as a potential stemness target gene, which could enhance the stemness and chondrogenesis of OBMSCs. The implantation of cartilage regeneration organoids made by SPI1-overexpressed OBMSCs could notably enhance cartilage regeneration in the old rats as compared with the microfracture treatment alone. Furthermore, molecular docking suggested a possible interaction between SPI1 and 5-Aza-2′-deoxycytidine (5Aza). The application of 5Aza could significantly improve the result of microfracture by upregulating SPI1. In summary, we identified SPI1 as a novel stemness target of OBMSCs, which was beneficial for the improvement of microfracture-stimulated cartilage regeneration in the elderly.
Keywords: Microfracture, bone marrow stromal cells, stemness, aging, engineered organoids
Graphical abstract.
Introduction
Articular cartilage injuries are prevalent across various age groups, yet the self-repair capacity of cartilage is highly limited.1,2 Cartilage regeneration is challenging due to its lack of blood supply, complex extracellular matrix, and weak proliferative capacity of chondrocytes.1,2 Numerous efforts have been made by researchers to restore the functionality and quality of regenerated cartilage.3–5 Among the various techniques, microfracture is a frequently employed clinical method for cartilage regeneration, involving debridement of the damaged cartilage and the recruitment of stem cells from the medullary cavity.6,7 At a mean of 68 months follow-up after microfracture surgery, marked improvements in the clinical symptoms and functional scores were achieved. 8 Despite numerous studies indicating favorable clinical outcomes following microfracture, the long-term result of microfracture treatment is markedly decreased in the elderly.8–10 Scientists have undertaken extensive research to enhance microfracture outcomes, but little progress has been made for the improving the outcomes of microfracture in the elderly patients.11,12 Hence, the mechanisms by which aging results in diminished effectiveness of microfracture treatment and how to enhance aging-related poor cartilage regeneration need further investigation.
Given the notable influence of aging on microfracture outcomes, we concentrated on the aging-related phenotype changes of BMSCs, including cell proliferation, differentiation, senescence, stemness.13,14 Among them, stemness is a typical cellular phenotype. As aging progresses, stemness diminishes, thereby affecting cell destiny.14,15 Stemness has wide-ranging biological functions, and its disruption can result in dysfunction or disease. 16 Stemness-related genes can alleviate cellular senescence and promote the differentiation of mesenchymal stem cells.17,18 Studies have shown that BMSCs from elderly or young donors exhibit different gene expression profiles, including enrichment in stem cell differentiation regulation. 14 Short-term enforced expression of stemness genes OCT4, SOX2, KLF4, and c-MYC has been developed as a cell reprogramming method to rejuvenate aging cells, enhance tissue repair, and potentially extend lifespan. 19 This strategy underscores the critical message that identifying and manipulating certain stemness-associated genes could potentially reestablish youthful molecular programs, delay aging at the cellular and organismal levels, and enhance cellular function.20,21 However, there is currently little research on the relationship between the stemness capacity of BMSCs and the result of microfracture treatment in the people of different ages. It is an interesting question whether enhancing BMSCs stemness can improve the results of microfracture treatment in the elderly.
To investigate the effect of cellular stemness on microfracture-mediated cartilage regeneration, we thought of using the organoid model to identify the stemness-related target genes. Organoids, as an emerging technological model, have made significant progress in the field of oncology, particularly in the screening of novel therapeutic targets. Traditional tumor organoids combined with cDNA lentiviral libraries primarily assess effective targets based on cell survival rates. 22 Likewise, we aimed to develop a cartilage regeneration organoid model resembling microfracture and screen appropriate targets through the expression levels of chondrogenic genes. We intended to use organoid technology for the screening of stemness-related genes, aiming to identify genes that facilitate chondrogenesis, thereby improving cartilage regeneration in microfracture.
In the present study, we initially discovered that the outcomes of microfracture in the old animals were decreased and positively correlated with stemness impairment in the OBMSCs. Differential gene expression analysis identified certain stemness-related genes were downregulated in the OBMSCs, among which SPI1 was specifically screened out by following experiments using our established cartilage regeneration organoid model. Overexpressing SPI1 could increase the stemness and chondrogenesis of OBMSCs. Further, the in vivo cartilage defect animal models discovered that implantation of cartilage regeneration organoids made by SPI1-overexpressed OBMSCs could notably enhance cartilage regeneration in the old rats as compared with the microfracture treatment alone. Last, we identified a pharmacological DNA demethylating agent, 5Aza, 23 which could upregulate SPI1 expression and thus promote cartilage regeneration in the old animals. In summary, our findings demonstrated that the gene SPI1 could promote BMSCs chondrogenic differentiation by upregulating BMSCs stemness, indicating that SPI1 could be a potential therapeutic target for cartilage regeneration in the treatment of cartilage injury in the elderly.
Materials and methods
Animals and treatment procedure
All animal experiments were approved by the Ethics Committee of Nanjing First Hosptial (DWSY-22140266), and all procedures followed the Laboratory Animal Protection Law. For surgery, 2-month-old (young) and 12-month-old (old) Sprague-Dawley (SD) rats (male) were anesthetized with an intramuscular injection of ketamine hydrochloride (1.4 mL/kg of body weight) and xylazine (0.4 mL/kg of body weight). A longitudinal skin incision was made via the medial parapatellar approach to expose both knee joints, and a cartilage defect (2 mm diameter and 1 mm height) was meticulously created at the trochlea, ensuring no bleeding on the cartilage defect surface. Four microfracture holes were then made within the defect using a 0.4 mm K-wire, and the bleeding from each hole was immediately observed. The capsule and skin were closed in layers using 5-0 nylon sutures. Rats were sacrificed at 8 weeks after surgery. Additionally, three 12-month-old male rats were assigned to the sham surgery group. Twelve 12-month-old rats had different cartilage regeneration organoids implanted in the joint site following microfracture. 24 Six 12-month-old rats received intraperitoneal injections of 5Aza (0.4 mL/kg of body weight) for three consecutive days post-microfracture. 23 Four weeks after surgery, the treatment effects on the femoral condyle articular cartilage of SD rats were evaluated using the macroscopic International Cartilage Repair Society (ICRS) scoring system.
Histological staining
The femoral condyles were decalcified for 6 weeks and embedded in paraffin in the correct orientation. Following embedding, the samples were dehydrated using an ethanol gradient and sectioned into 5 μm thick slices. The sections were then stained with Safranin O/Fast Green and H&E, and the Wakitani scoring system was applied for histological grading. 25 All photographs were taken with an Olympus BX51 fluorescence microscope. The sections were stained with a Sirius Red Staining Kit (Biosharp), imaged using a polarized light microscope (NIKON Eclipse ci), and quantified.
Screening of stemness-associated genes from YBMSCs and OBMSCs
The RNA-Seq dataset GSE139073 including the transcriptomic data of BMSCs from young and elderly individuals was downloaded from the Gene Expression Omnibus (GEO) Database. The differentially expressed genes (DEGs) between the young and old groups were screened using the “limma” R package (R4.2.1), with |FoldChange| >1.5 and p < 0.05 as the criteria. The expression patterns of the classic stemness-related genes were visualized using a heatmap. 26 Venn diagram analysis of the up-regulated genes and the stemness-related gene set from GeneCards identified five stemness-related genes. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses were performed on the up-regulated genes using the “cluster Profiler” R software package. To construct a protein-protein interaction network (PPI) for the stemness-associated genes, we used the Search Tool for the Retrieval of Interacting Genes (STRING) database.
Lentiviral infection of rat BMSCs
Plasmid expressing shRNA targeting SPI1 was purchased from Miaoling Bio. Functional sequences in the shRNA vector were as follows: SPI1, GCGATCACTATTGGGATTTCTTTCAAGAGAAGAAATCCCAATAGTGATCGC. The expression plasmids for SPI1, CCR1, WNT1, SALL1, SNCA, and SOX9 (pLV3-CMV-SPI1(rat)-3Xflag-CopGFP-Puro, NM_001005892.2; pLV3-CMV-CCR1(rat)-3Xflag-CopGFP-Puro, NM_020542.2; pLV3-CMV-WNT1(rat)-3Xflag-CopGFP-Puro, NM_001105714.1; pLV3-CMV-SALL1(rat)-3Xflag-CopGFP-Puro, NM_001107415.2; pLV3-CMV-SNCA(rat)-3Xflag-CopGFP-Puro, NM_019169.3; pLV3-CMV-SOX9(rat)-3Xflag-CopGFP-Puro, NM_080403.2) were obtained from Miaoling Bio. A 293T cells were seeded in 6-well plates, and when they reached 60% confluence, they were transfected with ExFect Transfection Reagent (Vazyme) in OPTI-MEM medium (Life Technologies) at a DNA ratio of 1:2, along with 1 μg pMD2.G, 1 μg pRSV-Rev, and 2 μg pMDlg/pRRE packaging plasmids (SinoBiological). After transfection, the supernatant containing viral particles was collected and concentrated to 1/100 of its original volume using Universal Virus Concentration Kit (Beyotime). The culture of BMSCs is described in the Supplemental Material. For viral infection, BMSCs were seeded in 6-well plates, and when the cells reached 50% confluence, 10 μL of viral concentrate and 4 μg/mL polybrene were added to the complete medium. After 24 h of infection, the medium was replaced, and the cells were cultured for an additional 48 h before further processing. The efficiency of overexpression was evaluated by western blot (WB) and qRT-PCR.
Cartilage regeneration organoids culture
As previously described by Leijten et al., 27 we used photolithography to fabricate a mold of polydimethylsiloxane (PDMS, Dow Corning Sylgard 184 elastomer) cylinders with a diameter of 200 μm and a spacing of 200 μm (Wenhaochip). A 3% (w/v) agarose (Sigma-Aldrich) solution was poured onto the PDMS master mold. After the agarose solidified, it was demolded, and custom punchers were used to create micropores with an area of approximately 1.8 cm². These were placed in 24-well plates, filled with 1 mL PBS (Servicebio), and sterilized with UV light for 2 h. BMSCs were collected and seeded at a density of 5 × 105 cells per well, resulting in microspheres of approximately 500 cells through self-aggregation. For chondrogenic differentiation of microspheres, they were cultured in maintenance medium containing 1% Penicillin-Streptomycin (Beyotime), 1% Sodium Pyruvate (Pricella), 10−7 M Dexamethasone (Sigma-Aldrich), 1% ITS (Corning), 50 ng/mL Ascorbic Acid (Sigma-Aldrich), 40 ng/mL L-Proline (Sigma-Aldrich), 10 ng/mL TGF-β3 (Novoprotein), and 0.2 ng/mL FGF2 (Novoprotein) in high-glucose DMEM (KeyGEN) for 21 days. The medium was refreshed every 3 days.
Formation of engineered microtissue constructs
Cartilage regeneration organoids (approximately 3,000) from three wells of a 24-well plate at 21 days were collected and transferred to a low-adhesion 96-well plate (Engineering For Life), then centrifuged at 300× g for 5 min. The organoids were cultured in chondrogenic induction medium for 3 days with daily medium changes, resulting in the formation of a compact microtissue construct. The microtissue was extracted with a pipette, transferred into sterile PBS, and then implanted at the rat microfracture site for subsequent experiments.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total cellular RNA was extracted following the instructions provided by TRIzol Reagent (Invitrogen). cDNA synthesis was carried out in a 20 μL reaction volume using SweScript All-in-One RT SuperMix (Servicebio). qRT-PCR reactions were conducted with SYBR Green qPCR Master Mix (Servicebio) and detected using the Applied Biosystems QuantStudio 5 (ThermoFisher). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the reference gene, and the fold changes in mRNA expression were calculated using the 2−△△Ct method. The primer sequences are provided in Supplemental Table S1.
Statistical analysis
All data are displayed as mean ± SD. Comparisons between the two groups were performed using two-tailed Student’s t test, and comparisons among the three or more groups were performed using one-way ANOVA. Spearman correlation test was used to determine the correlation between stemness-related phenotype and histological grading. SPSS 13.0 or GraphPad 9 was used, and significance was confirmed at p < 0.05.
Results
The outcomes of microfracture treatment in the elderly were significantly decreased and positively correlated with the reduction of BMSCs stemness capacity
As reported, result discrepancy was found in the treatment of cartilage injuries by microfracture among the patients of different ages, particularly poor results found in the elderly ( >50 years). 8 Therefore, we first compared the outcomes of microfracture treatment in the animals of different ages. We performed microfracture surgery on the 2- and 12-month-old rats and observed their surgical outcomes. Histological staining indicated that the regeneration areas in the old rats had fewer regenerative cellularity and poorer chondrogenic tissue formation as compared with the young counterparts (Figure 1(a) and (b)). In addition, both macroscopic and histological scoring revealed poorer scores in the old rats as compared to the young counterparts (Figure 1(c)–(f)). Previous studies have shown that the BMSCs of human, rats, and mice undergo senescence and a decline in stemness with aging. 15 In light of this established phenomenon, we proposed that the level of cartilage regeneration was possibly related to the stemness capacity of the BMSCs released from the drilling sites during microfracture surgery. Initially, we extracted BMSCs from the marrow cavities of rats, and all these cells were analyzed using flow cytometry (Supplemental Figure S1(b)), along with trilineage differentiation to confirm multipotency (Supplemental Figure S1(c)). Subsequently, we investigated the stemness difference between the OBMSCs and YBMSCs. Cell Counting Kit-8 (CCK-8) and colony-forming unit (CFU) assays indicated that the OBMSCs exhibited lower proliferation and self-renewal capability than the YBMSCs (Figure 1(g) and (h)). Spheroid formation assays also verified that the quantity and diameter of the YBMSCs spheroids exceeded those of the OBMSCs spheroids (Figure 1(i)). As for chondrogenesis, immunofluorescence results also showed that the YBMSCs had a stronger chondrogenic capacity (Figure 1(j) and (k)). Further, all these stemness indices were significantly correlated with the histological scores of microfracture treatment by spearman analysis (Figure 1(l)).
Figure 1.
A significant correlation was observed between the outcomes of SD rats microfracture and the level of bone marrow stromal cells stemness. (a and b) Representative images of H&E, Safranin O/Fast green, immunohistochemical (IHC) staining of sections from rats microfracture models. n = 6. (c an d) Macroscopic scores and histological scores for assessment of the cartilage regeneration at 8 weeks after microfracture surgery. n = 6. (e and f) Quantification of COL2A1 and COL1A2 positive cells ratio in articular regenerated cartilage area. n = 6. (g) CCK-8 was performed to explore the proliferative capacity of rat YBMSCs and OBMSCs. n = 3. (h) The colony-forming abilities of YBMSCs and OBMSCs were explored by crystal violet after culture for 7 days. n = 3. (i) Spheroid formation capacity of YBMSCs and OBMSCs were explored after culture for 7 days. n = 3. (j and k) Representative immunofluorescence images and quantitative analysis of SOX9 or COL2A1 expression (green) in response to chondrogenesis treatment for 21 days. DAPI (blue) staining of nuclei. F-actin (red) staing of cytoskeleton. n = 3. (l) Strong correlation between in vitro biological properties of BMSCs, including CFU number, spheroid formation capacity and proliferative capacity, and cartilage repair measured by wakitani histology score. *p < 0.05. **p < 0.01. ***p < 0.001, ns: no significance. Values are means ± SD.
Meanwhile, to investigate the correlation between BMSCs stemness and microfracture outcomes clinically, preoperative and postoperative MRI from five patients who underwent microfracture surgery were collected (Supplemental Figure S1(a)). We simultaneously harvested bone marrow from these individuals during microfracture surgery, and performed gradient density centrifugation to extract BMSCs and further verified their identity by cell surface markers via flow cytometry, and trilineage differentiation (Supplemental Figure S1(d) and (e)). We then compared their stemness capacity and found that the BMSCs from the young individuals showed significantly higher levels of proliferation, self-renewal ability, and differentiation (Supplemental Figure S1(f) and (g)). These indices were correlated with the clinical outcomes of microfracture surgery. In summary, these findings revealed that both old rats and patients had poorer microfracture outcomes compared to their young counterparts, and the stemness of the OBMSCs was significantly inferior to the YBMSCs.
The expressions of stemness-related genes were downregulated in the OBMSCs and positively correlated with the decline of cartilage regeneration scores histologically in the microfracture treatment animal models
Based on the above proved correlation between BMSCs stemness and microfracture outcomes, we were curious about whether manipulation of some specific stemness genes could improve the outcomes of microfracture in the elderly. Firstly, the transcriptomic data (GSE139073) of human young BMSCs (<40 years) and old BMSCs (>60 years)was retrieved from the GEO database, and the DEGs were screened out using the ‘limma’ generalized linear model, with |FoldChange| >1.5 and p < 0.05 as the criteria. Compared to the OBMSCs, 117 genes were upregulated in the young groups (Figure 2(a)). Heatmap analysis was conducted for classic stemness-related genes between the two groups and we observed that the expression of stemness-related genes in the OBMSCs were generally decreased (Figure 2(b)).16,26 The up-regulated genes were subjected to GO and KEGG pathway analyses. A total of 429 significantly enriched GO terms with a p < 0.05 were identified, including cell differentiation and cellular developmental process (Figure 2(c)). Eleven significantly enriched KEGG pathways or related functions with a p < 0.05 were identified, including the signaling pathways regulating pluripotency of stem cells (Figure 2(d)). To further verify the correlation between BMSCs stemness and aging, we obtained the stemness-related gene set from the GeneCards database and created a venn diagram of the up-regulated gene set and the stemness-related gene set, identifying five stemness-associated molecules: SPI1, WNT1, CCR1, SALL1, and SNCA (Figure 2(e)). These stemness-related genes were notably downregulated due to the influence of aging (Figure 2(b)). In addition, we also briefly analyzed the gene regulatory network of these five specific molecules. Among them, SPI1 was significantly correlated with RUNX1, CSF1R, and CEBPA, which were involved in stem cell differentiation and normal skeletal development (Supplemental Figure S2(a)). This indicated that SPI1 was a stemness-related gene affected by aging and potentially facilitated BMSCs differentiation. To fully prove the expression changes of these stemness-related genes, BMSCs from the young and old rats were collected for qRT-PCR. In line with RNA-seq data, the expression of WNT1 and SPI1 was much lower in the OBMSCs as compared to the YBMSCs (Figure 2(f)). We then performed spearman analysis of histological scores for cartilage repair and the expression levels of these stemness-related genes in both the young and old microfracture treatment rats and discovered that the expression levels of SPI1, WNT1, and SALL1 were statistically correlated to the histological scores of cartilage repair (Figure 2(g)). Thus, our data revealed that the expression of certain stemness-related genes were aging-susceptible and were responsive for cartilage regeneration.
Figure 2.
In comparison to YBMSCs, the expression of some specific stemness-associated genes in OBMSCs was downregulated and associated with cartilage repair. (a) Volcano plot showing the significant DEGs between human young and old groups. (b) Heat map showing the classic stemness-associated genes in two BMSCs. (c) GO analysis showing biological process, cellular compartment and molecular function of the upregulated genes involved in YBMSCs. (d) KEGG Pathway analysis showing main signaling pathways of the up-regulated genes involved in YBMSCs. (e) Venn diagram showing the overlapping genes between upregulated genes and the stemness-associated gene set. (f) qRT-PCR was performed to detect the changes in five stemness-associated molecules mRNA levels in rat BMSCs. n = 3. (g) Strong correlation between the expression levels of SPI1, WNT1, and SALL1, and wakitani histology score. *p < 0.05. **p < 0.01. ***p < 0.001, ns: no significance. Values are means ± SD.
BMSCs stemness gene SPI1 was determined as the critical gene responsible for chondrogenesis using the in vitro microfracture treatment-mimic cartilage regeneration organoids
To identify which stemness-related genes could most effectively affect microfracture-mediated cartilage regeneration, we explored the chondrogenic potential of BMSCs by overexpressing the above found stemness-related genes under the environment of in vivo microfracture treatment. Since the result of the comparison test in the in vivo microfracture treatment animal models could be confused by the different genetics background of the animals, we decided to use the microfracture treatment-mimic cartilage regeneration organoids which were well-developed by us before and proved to be highly-mimic to the actual in vivo microfracture-induced cartilage regeneration. 8 In this model, different quantities of BMSCs self-aggregated and differentiated into cartilage, forming multiple organoids (Supplemental Figure S2(b)). Subsequently, we designed five overexpression plasmids for SPI1, WNT1, CCR1, SALL1, and SNCA, assembled them into lentiviruses, and infected the rat OBMSCs. The infection efficiency was visible to be high under fluorescence (Supplemental Figure S2(c) and (d)), and was further validated by qRT-PCR (Figure 3(a)). We cultured the organoids for 21 days and carried out histological observation. Alcian blue staining, specifically targeting glycosaminoglycans (GAGs), revealed the most intensive staining in the SPI1-overexpressed organoids together with mature chondrocyte morphology visible at the same time (Figure 3(b)). Also, immunostaining intensity for SOX9 was strong and scattered ACAN-positive areas were visible in the CCR1 and SPI1-overexpressed organoids. In addition, overexpression of CCR1, SPI1, and SALL1 intensified immunostaining for COL2A1, with SPI1 having the strongest effect (Figure 3(b) and (c)). At the level of mRNA expression, qRT-PCR identified a statistically significant increase in the ACAN expression at the groups of SPI1 and CCR1 overexpression. SPI1 also upregulated the mRNA expression of SOX9 and COL2A1, whereas CCR1 cannot. Likewise, western blot results revealed that SPI1 has the strongest effect on the upregulation of chondrogenic markers among the five genes groups (Figure 3(d) and (e)). Taken together, SPI1 was determined as the critical stemness-related gene responsible for the chondrogenesis of OBMSCs.
Figure 3.
Cartilage regeneration organoid models revealed that SPI1 was the most critical stemness-related gene for chondrogenesis in OBMSCs. (a) RT-qPCR was performed to verify overexpression efficiency in rat OBMSCs. n = 3. (b) Representative sections of alcian blue, SOX9, COL2A1, ACAN immunostaining. n = 3. (c) Quantification of SOX9, COL2A1, ACAN positive BMSCs in organoids. n = 3. (d and e) Representative qRT-PCR and WB of SOX9, COL2A1, and ACAN on cartilage regeneration organoids. n = 3. *p < 0.05. **p < 0.01. ***p < 0.001, ns: no significance. Values are means ± SD.
Manipulation of SPI1 expression could influence cartilage formation through regulating the stemness of BMSCs
Since SPI1 overexpression could enhance chondrogenesis of the OBMSCs in our in vitro microfracture treatment-mimic cartilage regeneration organoids, we questioned whether this enhancement was from the upregulation of stemness of OBMSCs. Therefore, we manipulated SPI1 expression in the BMSCs using lentiviral vectors to observe the effect of SPI1 gain and loss on BMSCs stemness capacities. WB analysis after transfection revealed that SPI1 protein expression was downregulated in the YBMSCs but upregulated in OBMSCs, with the SPI1 expression in overexpressed OBMSCs exceeding those in YBMSCs (Figure 4(a)). Next, we compared the in vitro stemness capacities of four groups of BMSCs: YBMSCs, SPI1-knockdown YBMSCs, OBMSCs, and SPI1-overexpressed OBMSCs. Excitingly, the gain of SPI1 markedly delayed the age-induced loss of stemness, as shown by increased proliferation and colony-forming capability, although still less robust than those of the YBMSCs (Figure 4(b) and (c)). Knockdown of SPI1 also weakened the proliferation and colony-forming ability of the YBMSCs. In accordance with the proliferation and CFU assay, osteogenesis differentiation assay revealed that the numbers of alizarin red s (ARS) and alkaline phosphatase (ALP) positive staining cells were also significantly decreased after SPI1 knockdown compared to those in the YBMSCs and obviously elevated after SPI1 overexpression compared to the OBMSCs (Figure 4(d) and (e)).
Figure 4.
SPI1 regulated the stemness and chondrogenic potential of BMSCs. (a) Western blot assessed the protein expression of SPI1 on rat BMSCs after lentivirus infection. n = 3. (b) CCK-8 was performed to explore the proliferative capacity of YBMSCs, YBMSCs after knockdown of SPI1, OBMSCs, and OBMSCs after overexpression of SPI1. n = 3. (c) The colony-forming abilities of YBMSCs, YBMSCs after knockdown of SPI1, OBMSCs, and OBMSCs after overexpression of SPI1 were explored by crystal violet after culture for 7 days. n = 3. (d-e) After osteogenic induction for 21 days, ARS and ALP staining was performed in YBMSCs, YBMSCs after knockdown of SPI1, OBMSCs, and OBMSCs after overexpression of SPI1. n = 3. (f) After chondrogenic induction for 21 days, alcian blue staining was performed in YBMSCs, YBMSCs after knockdown of SPI1, OBMSCs, and OBMSCs after overexpression of SPI1. n = 3. (g and h) Representative immunofluorescence images and quantitative analysis of SOX9 (green) and COL2A1 (red) in cartilage regeneration organoids. DAPI (blue) staining of nuclei. n = 3. (i and j) Representative qRT-PCR and WB of SOX9, COL2A1, ACAN,SPI1 on four distinct types of cartilage regeneration organoids. n = 3. *p < 0.05. **p < 0.01. ***p < 0.001, ns: no significance. Values are means ± SD.
To further validate the role of SPI1 in the chondrogenesis of BMSCs, we evaluated chondrogenic markers (SOX9, COL2A1, and ACAN) in 2D monolayer and 3D organoid culture separately. Alcian blue staining of GAGs showed that SPI1 knockdown in the YBMSCs decreased staining intensity, whereas overexpression of SPI1 in the OBMSCs significantly enhanced staining intensity, indicating the positive impact of SPI1 on cartilage matrix regeneration (Figure 4(f)). Additionally, we performed immunofluorescence staining for SOX9 and COL2A1 in the BMSCs and the results revealed that increased expression of SOX9 and COL2A1 were exhibited in the OBMSCs after SPI1 overexpression (Supplemental Figure S2(e) and (f)). Interestingly, immunofluorescence staining in 3D culture demonstrated a substantial increase in COL2A1 expression in the SPI1-overexpressed OBMSCs, approaching the level observed in YBMSCs (Figure 4(g) and (h)). Likewise, we performed qRT-PCR and WB analysis on chondrogenic markers of four different groups of organoids. The results showed that overexpression of SPI1 augmented, while SPI1 knockdown suppressed the expression of SOX9, COL2A1, and ACAN at both the mRNA and protein levels (Figure 4(i) and (j)).
At the same time, we confirmed the beneficial effect of SPI1 in BMSCs from three patients. We assembled SPI1 gene ORF cDNA clone into lentiviruses and infected the human OBMSCs. Positive Human Nuclear Antigen (HNA) staining confirmed its origin from human cells (Supplemental Figure S3(a)). The infection efficiency was visible to be high under fluorescence (Supplemental Figure S3(a)), and western blot results validated the successful overexpression of SPI1 in the human OBMSCs (Supplemental Figure S3(b)). To verify whether SPI1 overexpression enhanced the stemness and chondrogenic capacity of old human BMSCs (HBMSCs), we conducted a CFU assay and organoid culture. The CFU assay showed that BMSCs from all three elderly patients exhibited an increased number of CFU, suggesting improved stemness in HBMSCs (Supplemental Figure S3(c)). Histological staining was conducted on 14-day human cartilage regeneration organoids. Slight morphological variations were noted in organoids from different patient BMSCs. Alcian blue staining demonstrated differences in staining intensity across organoids from different patients, with all intensities increasing after SPI1 overexpression. Immunohistochemical staining for ACAN also validated the positive effect of SPI1 in promoting chondrogenic potential (Supplemental Figure S3(c)).
Furthermore, to confirm that high stemness was essential for chondrogenic differentiation, we performed SOX9 overexpression and chondrogenic differentiation in YBMSCs and OBMSCs. Western blot analysis at day 0 confirmed the high expression of SOX9 in BMSCs (Supplemental Figure S4(a)). Western blot analysis at day 14 showed that upregulation of SOX9 in both YBMSCs and OBMSCs enhanced the expression of COL2A and ACAN. However, the expression of COL2A1 and ACAN in YBMSCs showed a significant increase, outperforming the limited upregulation seen in OBMSCs (Supplemental Figure S4(b)). This suggested that increased cellular stemness promotes the action of SOX9, with SPI1 being the most effective stemness-related gene. Taken together, the in vitro results suggested that manipulation of SPI1 expression could influence cartilage formation by regulating the stemness of BMSCs.
In vivo implantation of SPI1-overexpressed cartilage regeneration organoids could enhance cartilage regeneration in old rats
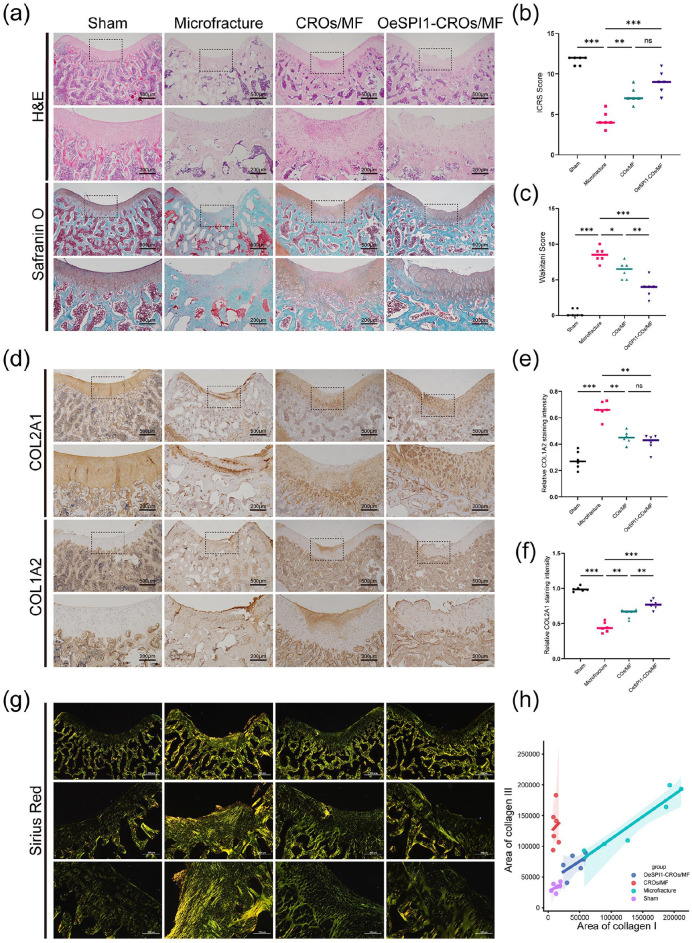
Since the role of SPI1 on enhancement of cartilage regeneration was demonstrated in our in vitro microfracture treatment-mimic cartilage regeneration organoids, we sought to determine whether SPI1 overexpression could improve the outcomes of microfracture treatment in vivo. To investigate this enhancement effect, we developed an in vivo cartilage regeneration organoids implantation rat model. Firstly, we harvested about 3000 cartilage regeneration organoids and cultured them for three days to develop the microtissues (Supplemental Figure S5(a)). We implanted microtissues onto the cartilage lesions of the 12-month-old rats that had received microfracture treatment. The rats were randomly divided into 4 groups: sham operation; microfracture treatment(MF); MF plus OBMSCs cartilage regeneration organoids implantation (CROs/MF) and MF plus SPI1-overexpressed OBMSCs cartilage regeneration organoids implantation (OeSPI1-CROs/MF). Macroscopic and histological observations were conducted at 4 weeks post-surgery. According to the items of the ICRS and Wakitani scoring systems, we continued to apply these two systems for macroscopic and histological evaluations.24,25 Macroscopically, the two organoid implantation groups exhibited higher gross scores, characterized by 75% or more repair of the defect depth and smoother surface as compared to the other two groups. Histologically, the OeSPI1-CROs/MF group exhibited most intensive cartilage matrix staining and the appearance of chondrocyte-like cells were abundantly visible in the regeneration area (Figure 5(a)–(c)). As the good outcomes of microfracture therapy were determined by the good quality of the regenerated cartilage which could be evaluated by the increased ratio of type II to type I collagen, we chose to evaluate the quality of regeneration cartilage in all groups by immunohistochemical staining of COL2A1/COL1A2. 28 Our results revealed that COL2A1 expression was significantly higher in the two organoid implantation groups compared to the MF group and especially the OeSPI1-CROs/MF group achieved the highest quality of regenerated cartilage tissue as demonstrated by the highest ratio of COL2A1 to COL1A2 expression (Figure 5(d)–(f)). To further assess the composition and alignment of collagen fibers in the regenerated cartilage, we conducted sirius red staining on the four groups of animals. These results indicated that the collagens in the superficial region of the regenerated cartilage in the two organoids implantation groups tended to be horizontally oriented, akin to normal cartilage, whereas the collagen entanglement orientation in the MF group was dispersed between 0° and 45° (Figure 5(g)). Furthermore, we assessed the collagen composition by its color, where yellow indicated type I collagen and green indicated type III collagen. According to the quantitative results, the type I and type III collagen content in the OeSPI1-CROs/MF group was similar to that in normal cartilage, whereas the CROs/MF group exhibited higher type III collagen, and the MF group showed elevated levels of both type I and type III collagen (Figure 5(h)). Our results indicated that microfracture often lead to extensive fibrocartilage regeneration, whereas organoids implantation, particularly modified organoids implantation, increased hyaline cartilage content. Taken together, in vivo implantation of cartilage regeneration organoids could enhance cartilage regeneration in rats and the general cartilage repair effect in the OeSPI1-CROs/MF was better than in the other three groups. These data strongly suggested that SPI1 is an important enhancer for chondrogenesis in vivo.
Figure 5.
In vivo implantation of SPI1-overexpressed cartilage regeneration organoids could enhance cartilage repair in the old rats. (a) Representative images of H&E, Safranin O/Fast green staining of sections at 4 weeks after organoids implantation. n = 6. (b and c) Macroscopic scores and histological scores for assessment of the cartilage regeneration at 4 weeks after organoids implantation. n = 6. (d) Representative images of IHC staining of sections at 4 weeks after organoids implantation. n = 6. (e and f) Quantification of COL2A1 and COL1A2 positive cells ratio in articular regenerated cartilage area. n = 6. (g) Representative images of sirius red staining of sections at 4 weeks after organoids implantation. n = 6. (h) Quantification of type I and III collagen ratio in articular regenerated cartilage area. n = 6. *p < 0.05. **p < 0.01. ***p < 0.001, ns: no significance. Values are means ± SD.
5Aza enhances the stemness of OBMSCs and cartilage regeneration by upregulating SPI1 expression
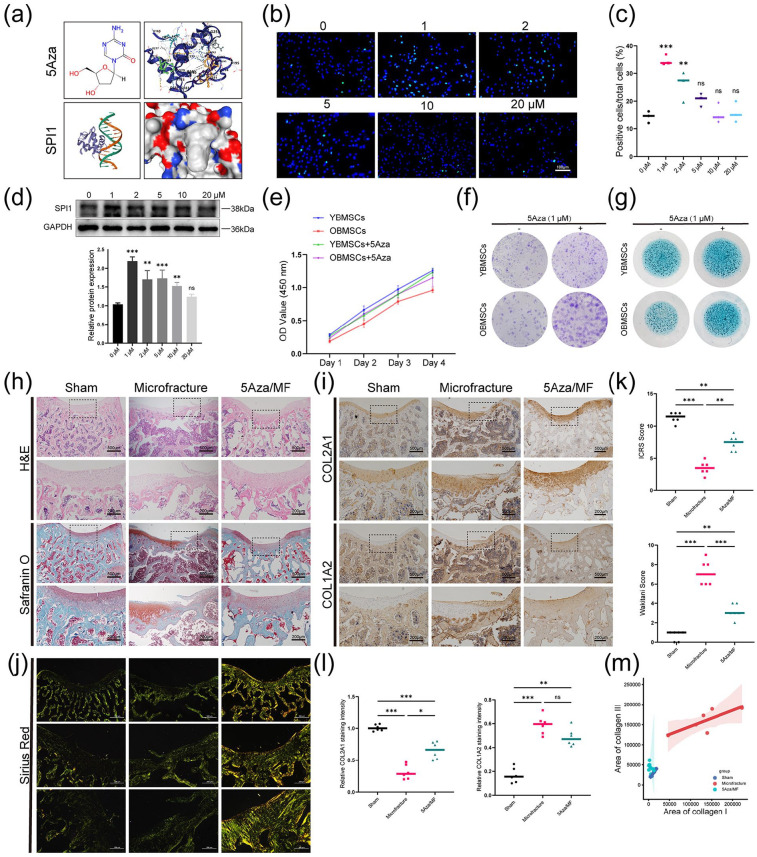
Given the beneficial effects of SPI1 on the result improvement for microfracture treatment were clarified both in vitro and in vivo, we aimed to discover certain small molecule compound that could upregulate SPI1 expression and thus be used clinically. Under the help of docking prediction tools, we discovered that the small molecule DNA methyltransferase inhibitor 5-Aza-2′-deoxycytidine (5Aza) could interact with the SPI1 protein (Figure 6(a)). 29 Previous research has indicated that the promoter region of SPI1 is methylated in classical Hodgkin lymphoma cell line, and SPI1 expression is upregulated in cells treated with 5Aza. 30 To prove that 5Aza could also upregulate SPI1 expression in the BMSCs, we performed immunocytochemistry staining and WB assay for the cells stimulated by various concentrations of 5Aza. The results indicated that 1, 2, and 5 μM of 5Aza increased the numbers of SPI1-positive cells in the OBMSCs (Figure 6(b) and (c)). WB analysis showed that SPI1 reached the highest expression at a concentration of 1 μM (Figure 6(d)). In addition, we tested the influence of different concentrations of 5Aza on the viability of OBMSCs and the live/dead staining showed that the concentrations above 5 μM could induce cell death (Supplemental Figure S6(a and b)). Similarly, the CCK-8 assay revealed that 1 and 2 μM of 5Aza had no significant effect on cell viability in both short-term and long-term cultures (Supplemental Figure S6(c)). Thus, we chose 1 μM 5Aza for the following test. To demonstrate that 5Aza could enhance BMSCs stemness by upregulating SPI1 expression, we treated the YBMSCs and OBMSCs with 5Aza and then evaluated their stemness abilities. CCK-8 and CFU assays indicated that 5Aza markedly increased the proliferation and colony-forming ability of OBMSCs, while its effect on YBMSCs was not statistically significant (Figure 6(e) and (f)). Similar result was reached as for sphere formation assay that 5Aza could significantly increase the size and numbers of the OBMSCs spheres but had little impact on the YBMSCs spheres (Supplemental Figure S6(d)). The expression levels of the stemness-related genes were evaluated after 5Aza treatment and their levels were significantly upregulated in the OBMSCs as compared to untreated group (Supplemental Figure S6(e)). Additionally, the impact of 5Aza treatment on the chondrogenic capacity of BMSCs was observed in the in vitro monolayer cell culture and much more intensive alcian blue staining was found in the OBMSCs while little change was found in the YBMSCs (Figure 6(g)).
Figure 6.
5Aza could upregulate SPI1 expression and enhance cartilage regeneration in vivo and in vitro. (a) Using the RCSB model server to predict SPI1 protein structure and using CB-DOCK2 to simulate 5Aza docking with SPI1. (b and c) Representative immunofluorescence images and quantitative analysis of SPI1 in OBMSCs treated with varying concentrations of 5Aza. n = 3. (d) Representative WB images of OBMSCs treated with varying concentrations of 5Aza. n = 3. (e) CCK-8 was performed to explore the proliferative capacities of four different types of BMSCs. n = 3. (f) The colony-forming abilities of four different types of BMSCs were explored by crystal violet. n = 3. (g) Representative alcian blue staining images of four different types of BMSCs. n = 3. (h) Representative images of H&E, Safranin O/Fast green staining of sections at 4 weeks after intramuscular injection. n = 6. (i) Representative images of IHC staining of sections at 4 weeks after intramuscular injection. n = 6. (j) Representative images of sirius red staining of sections at 4 weeks after intraperitoneal injection of 5Aza. n = 6. (k) Macroscopic scores and histological scores for assessment of the cartilage regeneration at 4 weeks after intramuscular injection. n = 6. (l) Quantification of COL2A1 and COL1A2 positive cells ratio in articular regenerated cartilage area. n = 6. (m) Quantification of type I and III collagen ratio in articular regenerated cartilage area. n = 6. *p < 0.05. **p < 0.01. ***p < 0.001, ns: no significance. Values are means ± SD.
We then investigated the effect of 5Aza treatment on the outcomes of microfracture in vivo. The 12-month-old rats were randomly assigned to three groups: sham operation; microfracture treatment alone and combined microfracture and 5Aza treatment (5Aza/MF). Four weeks after surgery, we conducted pathological staining and scoring of the knee joint. Both H&E and Safranin O staining confirmed that the regenerated cartilage layer showed a fiber-like structure and rough joint surface in the MF group. In contrast, in the 5Aza/MF group, the cartilage structure showed normal hyaline-like morphology and the regenerated cartilage surface was smooth (Figure 6(h)). Similarly, the macroscopic and histological scores in the 5Aza/MF group were superior to those in the MF group, providing initial evidence of the advantages of 5Aza for microfracture (Figure 6(k)). Immunohistochemical staining showed that 5Aza also increased COL2A1/COL1A2 ratio in articular cartilage (Figure 6(i) and (l)). Using sirius red staining, we found that the type I and type III collagen areas in the 5Aza/MF group were reduced compared to the MF group, nearing the levels observed in the sham group. Therefore, 5Aza could reduce the fibrocartilage phenotype (type I and type III collagen) in microfracture and increase the hyaline cartilage phenotype (type II collagen), which was beneficial for the outcomes of microfracture (Figure 6(j) and (m)). This indicated that 5Aza might promote hyaline cartilage regeneration in vivo. Collectively, both in vivo and in virto data strongly suggested that 5Aza had a significant role in modulating cellular stemness and chondrogenic differentiation.
Discussion
As reported, the outcome of microfracture in the elderly is limited. 31 Aging is a complicated process and often accompanied by a loss of cellular function. Notably, cellular stemness, as a cell property significantly affected by aging, has been widely studied in regulating osteogenic differentiation, but there are few studies concerning its regulation of chondrogenesis.13,14 In our study, we concentrated on the relationship between cellular stemness and the outcome of microfracture in the elderly. 32 We discovered that SPI1 expression decreased with aging and was closely linked to the diminished stemness and chondrogenic potential of BMSCs. We further demonstrated that the stemness of BMSCs plays a significant role in the outcomes of microfracture, and upregulating SPI1 expression, including via 5Aza treatment, could enhance the stemness of effector cells and improve microfracture-mediated cartilage regeneration.
SPI1, known as a transcriptional regulator, dictates cell fate by decompacting stem cell heterochromatin and allowing other transcription factors to enter otherwise inaccessible genomic sites.33,34 At the cellular level, the activities of SPI1 are involved in regulating various biological processes, such as cell proliferation, differentiation, and tumorigenesis, and is particularly essential for myeloid and lymphoid development.35,36 In this study, we discovered that SPI1 expression in BMSCs declined with aging and further confirmed that the stemness capacity of BMSCs and related genes, such as WNT1 and SALL1, were also age-affected. Despite previous reports on numerous stemness genes, such as OCT4 and SOX2, that influence cell differentiation, our gain and loss of function experiments confirmed that SPI1 can upregulate chondrogenic marker expression in OBMSCs. 21 Moreover, protein interaction network analysis also validated that SPI1-related genes were significantly associated with stem cell differentiation and cartilage regeneration. Collectively, SPI1 was a stemness-related gene that truly affected cellular stemness and chondrogenic differentiation.
To further validate that chondrogenic ability of SPI1 surpassed other stemness-related genes, we proposed a strategy to compare various stemness-related genes based on organoid models. Prior organoid models were mainly used in tumor heterogeneity research and drug screening, but we had integrated cellular stemness with organoid technology to investigate the roles of various targets. 37 Notably, we created a cartilage regeneration organoid model using a microporous plate method to mimic the microfracture-mediated cartilage regeneration process, using cells overexpressing various stemness-associated genes for organoid culture and comparing their chondrogenesis.27,38 We found that SPI1 had the most significant effect on the expression of chondrogenic genes, including the transcription factor SOX9, and cartilage matrix components COL2A1 and ACAN. This is an innovative approach to using engineered organoids in the field of cartilage repair.
Furthermore, we were curious whether enhancing BMSCs stemness, particularly overexpression of SPI1, could also promote their chondrogenic differentiation. Previous studies had confirmed that enhancing the stemness of BMSCs could promote their osteogenic differentiation. Liu et al. 38 enhanced OCT4 and NANOG expression in BMSCs using a lentiviral system and found that their colony-forming and osteogenic abilities were significantly increased. In our study, we overexpressed SPI1 in OBMSCs via lentivirus and compared them with YBMSCs, discovering that SPI1 significantly impacts the stemness capacity of BMSCs, especially their colony-forming ability. Likewise, overexpression of SPI1 resulted in a marked enhancement of the chondrogenic differentiation in both BMSCs and their derived organoids. We observed that BMSCs with increased stemness show significantly enhanced chondrogenic differentiation capacity, because overexpressing stemness-related genes in BMSCs was a form of reprogramming that rejuvenates the stem cells. 38 Under the same TGFβ induction conditions, the new BMSCs exhibited better chondrogenic effects. Fan et al. 39 also confirmed that OCT4 and SOX2 overexpression enhances the differentiation of BMSCs. Whether SPI1 overexpression directly influences through regulation of cartilage-related transcription factors like SOX9 and RUNX1 remains to be further explored. 40
To facilitate clinical application and translation, we aimed to design SPI1-overexpressed organoid explants and seek SPI1-targeting drugs to achieve comparable chondrogenic effects in the elderly. We had improved microfracture outcomes using organoid technology and the known stemness gene SPI1. Shen et al. 24 previously reported a case where cartilage organoids were implanted in rat cartilage defects, resulting in successful repair. Thus, We implanted both regular organoids and SPI1-overexpressed organoids, finding that the organoid implants integrated well with MF exudate, especially with both showing significantly higher repair cartilage quality compared to the MF group. The type II to type I collagen ratio in the OeSPI1-CROs/MF group was higher, closer to that of normal hyaline cartilage. Our findings suggested that organoids implantation, particularly of engineered organoids, during the MF process might be an effective strategy to improve the outcomes of microfracture in elderly individuals. The SPI1-overexpressed organoid explants served as an excellent alternative to cartilage transplantation, and this was significant for tissue regeneration.
5-Aza-2′-deoxycytidine (5Aza) is an orally active deoxycytidine analog antimetabolite and DNA methyltransferase inhibitor. Zhu et al. 23 have shown that 5Aza can inhibit osteoarthritis-related excessive inflammatory cytokines and deficiencies in antioxidant enzymes, both in vivo in mice and in vitro in chondrocytes; this effect can be blocked by PPARγ-specific inhibitors and PPARγ knockout in mice. Ushmorov et al. 30 discovered that 5Aza can significantly enhance SPI1 expression in B cells. Small molecule drugs are frequently used in articular cartilage regeneration, and we are curious about the effects of 5Aza on articular cartilage regeneration. 41 Interestingly, our study found that 5Aza could bind to SPI1, enhance its expression in BMSCs, and further improve stemness and promote chondrogenic differentiation. We also observed that 5Aza treatment can improve microfracture outcomes in old rats and partially promote the expression of hyaline cartilage in regenerated cartilage of them. Notably, 5Aza enhanced SPI1 expression in BMSCs, emphasizing the importance of subchondral bone drilling to recruit BMSCs for its effectiveness. In cases of pure cartilage injury without microfracture treatment, additional in vivo studies are required to validate the effectiveness of 5Aza. As stem and progenitor cells may be present in the synovium and cartilage of the joint, it is still unclear if 5Aza facilitates cartilage repair by boosting their stemness. In this study, we did not explore other molecular mechanisms, so we cannot rule out effects of 5Aza on articular cartilage beyond SPI1. As 5Aza typically inhibits DNA methyltransferases, further epigenetic experiments are required to fully validate the effects of 5Aza, which is a limitation of this study. 42 Furthermore, the efficacy and safety of 5Aza need to be further evaluated in larger animals. We believed that 5Aza could be a potential drug to enhance cellular stemness and microfracture-mediated cartilage regeneration in the elderly, which has significant clinical implications.
Conclusions
Our study indicates that SPI1 functions as a stemness-enhancing factor regulating the chondrogenic capacity of BMSCs, and via organoid implantation and 5Aza treatment, can significantly improve microfracture outcomes in aged rats. Thus, SPI1 may emerge as a potential therapeutic target for enhancing microfracture outcomes in the elderly. Engineered organoid implantation and 5Aza may provide novel insights into the therapeutic strategies for articular cartilage regeneration.
Supplemental Material
Supplemental material, sj-docx-1-tej-10.1177_20417314241311073 for SPI1 facilitates microfracture-mediated cartilage regeneration in the elderly by enhancing bone marrow stromal cells ctemness by Changjiang Wang, Yishu Wang, Yueqiang Gu, Yi Zhu, Rui Yin, Yang Li and Jianchao Gui in Journal of Tissue Engineering
Footnotes
Author contributions: Changjiang Wang and Yishu Wang: Conceptualization, Methodology, Formal Analysis, Investigation, and Writing—Original Draft Preparation. Yueqiang Gu: Methodology and Formal Analysis. Yi Zhu: Methodology and Investigation. Rui Yin: Conceptualization and Methodology. Yang Li: Conceptualization, Methodology, and Writing—Review & Editing. Jianchao Gui: Conceptualization, Formal Analysis, and Writing—Review & Editing. Changjiang Wang and Yishu Wang contributed equally to this study.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Project supported by Nanjing Medical Science and Technique Development Foundation (YKK21136) and the National Natural Science Foundation of China (81672210).
Ethical approval: Experimental methods and tissue procurement were conducted with approval from the Ethics Committee of Nanjing First Hosptial (DWSY-22140266, KY20210328-KS-02).
ORCID iDs: Changjiang Wang  https://orcid.org/0009-0003-2726-4049
https://orcid.org/0009-0003-2726-4049
Jianchao Gui  https://orcid.org/0000-0002-0876-5265
https://orcid.org/0000-0002-0876-5265
Supplemental material: Supplemental material for this article is available online.
References
- 1. Murphy MP, Koepke LS, Lopez MT, et al. Articular cartilage regeneration by activated skeletal stem cells. Nat Med 2020; 26: 1583–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gao Y, Liu S, Huang J, et al. The ECM-Cell Interaction of Cartilage Extracellular Matrix on Chondrocytes. BioMed Res Int 2014; 2014: 648459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Muthu S, Korpershoek JV, Novais EJ, et al. Failure of cartilage regeneration: emerging hypotheses and related therapeutic strategies. Nat Rev Rheumatol 2023; 19: 403–416. [DOI] [PubMed] [Google Scholar]
- 4. Valderrabano V, Miska M, Leumann A, et al. Reconstruction of osteochondral lesions of the talus with autologous spongiosa grafts and autologous matrix-induced chondrogenesis. Am J Sports Med 2013; 41: 519–527. [DOI] [PubMed] [Google Scholar]
- 5. McIlwraith CW, Frisbie DD, Rodkey WG, et al. Evaluation of intra-articular mesenchymal stem cells to augment healing of microfractured chondral defects. Arthroscopy 2011; 27: 1552–1561. [DOI] [PubMed] [Google Scholar]
- 6. Hashimoto Y, Nishida Y, Takahashi S, et al. Transplantation of autologous bone marrow-derived mesenchymal stem cells under arthroscopic surgery with microfracture versus microfracture alone for articular cartilage lesions in the knee: a multicenter prospective randomized control clinical trial. Regen Ther 2019; 11: 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Min B-H, Choi WH, Lee YS, et al. Effect of different bone marrow stimulation techniques (BSTs) on MSCs mobilization. J Orthop Res 2013; 31: 1814–1819. [DOI] [PubMed] [Google Scholar]
- 8. Asik M, Ciftci F, Sen C, et al. The microfracture technique for the treatment of full-thickness articular cartilage lesions of the knee: midterm results. Arthroscopy 2008; 24: 1214–1220. [DOI] [PubMed] [Google Scholar]
- 9. Weber AE, Locker PH, Mayer EN, et al. Clinical outcomes after microfracture of the knee: midterm follow-up. Orthop J Sports Med 2018; 6: 232596711775357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Philippon MJ, Schenker ML, Briggs KK, et al. Can microfracture produce repair tissue in acetabular chondral defects? Arthroscopy 2008; 24: 46–50. [DOI] [PubMed] [Google Scholar]
- 11. Theruvath AJ, Mahmoud EE, Wu W, et al. Ascorbic acid and iron supplement treatment improves stem cell-mediated cartilage regeneration in a minipig model. Am J Sports Med 2021; 49: 1861–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Park S, Na JY, Gwon Y, et al. Transplantable stem cell nanobridge scaffolds for accelerating articular cartilage regeneration. Biomaterials 2023; 301: 122287. [DOI] [PubMed] [Google Scholar]
- 13. Xiao Y, Cai G-P, Feng X, et al. Splicing factor YBX1 regulates bone marrow stromal cell fate during aging. EMBO J 2023; 42: e111762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hu M, Xing L, Zhang L, et al. NAP1L2 drives mesenchymal stem cell senescence and suppresses osteogenic differentiation. Aging Cell 2022; 21: e13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yi L, Ju Y, He Y, et al. Intraperitoneal injection of Desferal® alleviated the age-related bone loss and senescence of bone marrow stromal cells in rats. Stem Cell Res Ther 2021; 12: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Katajisto P, Döhla J, Chaffer CL, et al. Asymmetric apportioning of aged mitochondria between daughter cells is required for stemness. Science 2015; 348: 340–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huyghe A, Trajkova A, Lavial F. Cellular plasticity in reprogramming, rejuvenation and tumorigenesis: a pioneer TF perspective. Trends Cell Biol 2024; 34: 255–267. [DOI] [PubMed] [Google Scholar]
- 18. Lin Y, Xiao Y, Lin C, et al. SALL1 regulates commitment of odontoblast lineages by interacting with RUNX2 to remodel open chromatin regions. Stem Cells 2021; 39: 196–209. [DOI] [PubMed] [Google Scholar]
- 19. Ocampo A, Reddy P, Martinez-Redondo P, et al. In vivo amelioration of age-associated hallmarks by partial reprogramming. Cell 2016; 167: 1719–1733.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dong Y, Long T, Wang C, et al. NOTCH-mediated maintenance and expansion of human bone marrow stromal/stem cells: a technology designed for orthopedic regenerative medicine. Stem Cells Transl Med 2014; 3: 1456–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jing Y, Jiang X, Ji Q, et al. Genome-wide CRISPR activation screening in senescent cells reveals SOX5 as a driver and therapeutic target of rejuvenation. Cell Stem Cell 2023; 30: 1452–1471.e10. [DOI] [PubMed] [Google Scholar]
- 22. Salahudeen AA, Seoane JA, Yuki K, et al. Functional screening of amplification outlier oncogenes in organoid models of early tumorigenesis. Cell Reports 2023; 42: 113355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhu X, Chen F, Lu K, et al. PPARγ preservation via promoter demethylation alleviates osteoarthritis in mice. Ann Rheum Dis 2019; 78: 1420–1429. [DOI] [PubMed] [Google Scholar]
- 24. Shen C, Wang J, Li G, et al. Boosting cartilage repair with silk fibroin-DNA hydrogel-based cartilage organoid precursor. Bioact Mater 2024; 35: 429–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wakitani S, Goto T, Pineda SJ, et al. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am 1994; 76: 579–592. [DOI] [PubMed] [Google Scholar]
- 26. Wu D, Zhao L, Sui B, et al. An appearance data-driven model visualizes cell state and predicts mesenchymal stem cell regenerative capacity. Small Methods 2022; 6: e2200087. [DOI] [PubMed] [Google Scholar]
- 27. Leijten J, Teixeira LSM, Bolander J, et al. Bioinspired seeding of biomaterials using three dimensional microtissues induces chondrogenic stem cell differentiation and cartilage formation under growth factor free conditions. Sci Rep 2016; 6: 36011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Armiento AR, Alini M, Stoddart MJ. Articular fibrocartilage – why does hyaline cartilage fail to repair? Adv Drug Deliv Rev 2019; 146: 289–305. [DOI] [PubMed] [Google Scholar]
- 29. Liu Y, Yang X, Gan J, et al. CB-Dock2: improved protein-ligand blind docking by integrating cavity detection, docking and homologous template fitting. Nucleic Acids Res 2022; 50: W159–W164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ushmorov A, Leithäuser F, Sakk O, et al. Epigenetic processes play a major role in B-cell-specific gene silencing in classical Hodgkin lymphoma. Blood 2006; 107: 2493–2500. [DOI] [PubMed] [Google Scholar]
- 31. Lee K-B, Bai L-B, Chung J-Y, et al. Arthroscopic microfracture for osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc 2010; 18: 98. [DOI] [PubMed] [Google Scholar]
- 32. Tao W, Yu Z, Han J-DJ. Single-cell senescence identification reveals senescence heterogeneity, trajectory, and modulators. Cell Metab 2024; 36: 1126–1143.e5. [DOI] [PubMed] [Google Scholar]
- 33. Pham T-H, Minderjahn J, Schmidl C, et al. Mechanisms of in vivo binding site selection of the hematopoietic master transcription factor PU.1. Nucleic Acids Res 2013; 41: 6391–6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weng H, Huang H, Wu H, et al. Mettl14 inhibits hematopoietic stem/progenitor differentiation and promotes leukemogenesis via mRNA m6A modification. Cell Stem Cell 2018; 22: 191–205.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Le Coz C, Nguyen DN, Su C, et al. Constrained chromatin accessibility in PU.1-mutated agammaglobulinemia patients. J Exp Med 2021; 218: e20201750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lai Y, Guo Y, Liao C, et al. Osteoclast differentiation and dynamic mRNA expression during mice embryonic palatal bone development. Sci Rep 2023; 13: 15170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hansen SL, Larsen HL, Pikkupeura LM, et al. An organoid-based CRISPR-Cas9 screen for regulators of intestinal epithelial maturation and cell fate. Sci Adv 2023; 9: eadg4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu TM, Wu YN, Guo XM, et al. Effects of ectopic Nanog and Oct4 overexpression on mesenchymal stem cells. Stem Cells Dev 2009; 18: 1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fan YX, Gu CH, Zhang YL, et al. Oct4 and Sox2 overexpression improves the proliferation and differentiation of bone mesenchymal stem cells in Xiaomeishan porcine. Genet Mol Res 2013; 12: 6067–6079. [DOI] [PubMed] [Google Scholar]
- 40. Mao S, Frank RC, Zhang J, et al. Functional and physical interactions between AML1 proteins and an ETS protein, MEF: implications for the pathogenesis of t(8;21)-positive leukemias. Mol Cell Biol 1999; 19: 3635–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jin Y, Liu Q, Chen P, et al. A novel prostaglandin E receptor 4 (EP4) small molecule antagonist induces articular cartilage regeneration. Cell Discov 2022; 8: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Long S, Huang G, Ouyang M, et al. Epigenetically modified AP-2α by DNA methyltransferase facilitates glioma immune evasion by upregulating PD-L1 expression. Cell Death Dis 2023; 14: 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tej-10.1177_20417314241311073 for SPI1 facilitates microfracture-mediated cartilage regeneration in the elderly by enhancing bone marrow stromal cells ctemness by Changjiang Wang, Yishu Wang, Yueqiang Gu, Yi Zhu, Rui Yin, Yang Li and Jianchao Gui in Journal of Tissue Engineering