Abstract
Cancer remains a leading cause of mortality globally and a major health burden, with chemotherapy often serving as the primary therapeutic option for patients with advanced-stage disease, partially compensating for the limitations of non-curative treatments. However, the emergence of chemotherapy resistance significantly limits its efficacy, posing a major clinical challenge. Moreover, heterogeneity of resistance mechanisms across cancer types complicates the development of universally effective diagnostic and therapeutic approaches. Understanding the molecular mechanisms of chemoresistance and identifying strategies to overcome it are current research focal points. This review provides a comprehensive analysis of the key molecular mechanisms underlying chemotherapy resistance, including drug efflux, enhanced DNA damage repair (DDR), apoptosis evasion, epigenetic modifications, altered intracellular drug metabolism, and the role of cancer stem cells (CSCs). We also examine specific causes of resistance in major cancer types and highlight various molecular targets involved in resistance. Finally, we discuss current strategies aiming at overcoming chemotherapy resistance, such as combination therapies, targeted treatments, and novel drug delivery systems, while proposing future directions for research in this evolving field. By addressing these molecular barriers, this review lays a foundation for the development of more effective cancer therapies aimed at mitigating chemotherapy resistance.
Keywords: Chemotherapy resistance, Molecular mechanisms, Apoptosis evasion, Cancer stem cells, Epigenetic modifications, Targeted therapy
Introduction
In 2020, an estimated 19.3 million new cancer cases were reported globally, with approximately 10 million deaths attributed to cancer and related complications [1]. Cancer has become a leading cause of mortality worldwide, posing a significant challenge to life expectancy [2]. Radical resection remains the primary treatment option for many malignant tumors. However, due to the aggressive and often asymptomatic nature of some cancers, early diagnosis is difficult, leading to missed opportunities for timely intervention during the most effective treatment windows [3, 4]. This often reduces the potential for curative outcomes.
In this context, chemotherapy has emerged as a pivotal treatment strategy [5]. It is classified based on therapeutic goals into neoadjuvant, adjuvant, curative, and palliative chemotherapy [6–8]. The use of chemotherapy has proven effective in treating not only solid tumors but also hematologic malignancies, enhancing preoperative conditions and lowering postoperative recurrence rates, thus improving overall survival [9]. However, despite these advancements, chemotherapy resistance remains a critical obstacle to achieving effective treatment outcomes. This resistance can be divided into intrinsic resistance, present before treatment begins, and acquired resistance, which develops during the course of therapy [10]. Early identification of these resistance types is essential for optimizing treatment strategies and improving clinical outcomes [11]. Yet, the molecular basis of chemotherapy resistance remains incompletely understood, complicating efforts to overcome it.
This review aims to explore the complex molecular mechanisms driving chemotherapy resistance, including drug efflux, enhanced DNA damage repair, apoptosis evasion, epigenetic modifications, and the role of cancer stem cells. (Fig. 1) Unlike previous reviews, we not only summarize these mechanisms comprehensively but also highlight recent advances, such as the role of epigenetic modifications in chemoresistance [12, 13]. Additionally, the review outlines resistance mechanisms across major cancer types and identifies key therapeutic targets. Finally, we discuss strategies to overcome resistance, with a focus on emerging therapies. By addressing these molecular barriers, this review provides a foundation for developing more effective approaches to combat chemotherapy resistance in cancer treatment.
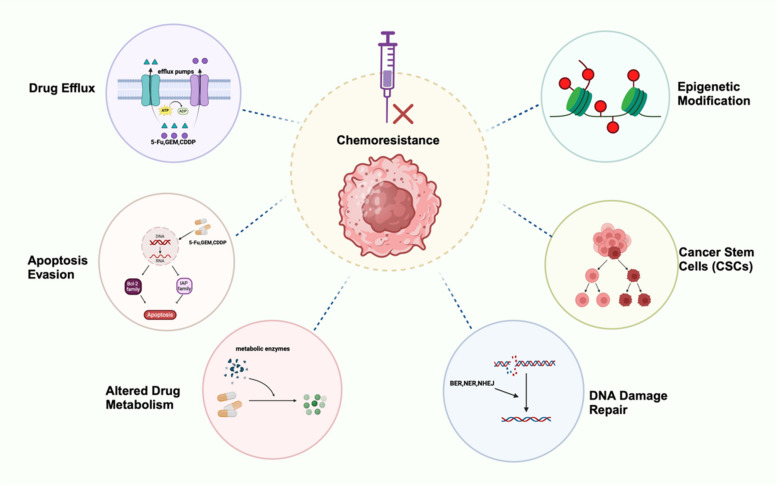
Fig. 1.
General mechanisms of cancer chemoresistance. Upon exposure to chemotherapeutic agents, tumors initiate multiple mechanisms that mediate chemoresistance. Here, we delineate the molecular pathways involved in cancer chemoresistance, including: drug efflux, DNA damage repair, apoptosis evasion, epigenetic modifications, Altered Drug Metabolism, Cancer Stem Cells (CSCs)
General mechanisms of chemotherapy resistance
Chemotherapy resistance in cancer is driven by a network of fundamental molecular mechanisms, including drug efflux, enhanced DNA damage repair, apoptosis evasion, epigenetic modifications, cancer stem cell dynamics, and alterations in intracellular drug metabolism. These mechanisms are highly interconnected and mutually influential, forming a complex landscape that remains only partially understood. This section systematically describes these molecular pathways, highlighting their roles in the development of chemotherapy resistance.
The excretion of chemotherapy drugs
The accelerated efflux of chemotherapy drugs from cancer cells significantly reduces their intracellular concentrations, contributing to chemotherapy resistance. This drug efflux is primarily facilitated by ATP-binding cassette (ABC) transporters, including ABCB1, ABCG2, and ABCC1, which are expressed in various organs such as the liver, intestines, kidneys, and brain [14–16]. These transporters use energy from ATP hydrolysis to expel a wide range of substrates, including chemotherapeutic agents, thereby decreasing the cellular exposure to these drugs.
One of the most well-studied efflux transporters is P-glycoprotein (P-gp), encoded by the ABCB1 (MDR1) gene, which is located on the apical membranes of various epithelial cells [17]. P-gp plays a crucial role in removing chemotherapeutic drugs from cancer cells, thus preventing their accumulation. Overexpression of MDR1 has been observed in gallbladder cancer (GBC) and is associated with increased resistance to drugs such as gemcitabine and 5-fluorouracil (5-FU) [18–21]. Elevated MDR1 expression has also been reported in other cancers, including colorectal cancer, breast cancer, osteosarcoma, and pancreatic cancer, where it contributes to multidrug resistance [22]. Another significant efflux pump is the Multidrug Resistance-associated Protein 1 (MRP1), part of the ABCC family. MRP1 plays a pivotal role in multidrug resistance by exporting chemotherapeutic agents out of cells [23, 24]. Targeting MRP1, for instance through the miR-145-MRP1 axis, shows potential in reversing chemoresistance [25]. In GBC, intracellular glutathione (GSH) can bind to cisplatin, forming GS-platinum complexes that are expelled by MRP1. Strategies that downregulate MRP1 or deplete GSH enhance the cytotoxicity of cisplatin, suggesting potential therapeutic approaches [26].
Breast Cancer Resistance Protein (BCRP), encoded by the ABCG2 gene, is another critical efflux transporter involved in drug resistance. BCRP protects cells by exporting toxic substances, including chemotherapy drugs, and is expressed in tissues like the intestines, bile ducts, placenta, blood-testis barrier, and blood–brain barrier [27]. Studies have linked increased BCRP expression to enhanced chemotherapy resistance [28, 29]. Notably, the overexpression of BCRP has been observed in metastatic cancer cells, leading to intrinsic resistance, even without prior exposure to chemotherapy [30].
In addition to ABC transporters, copper-transporting ATPases such as ATP7A and ATP7B help maintain intracellular copper balance but can also sequester platinum-based drugs like cisplatin. This sequestration reduces the drugs' efficacy and contributes to chemotherapy resistance [31]. Exploring the roles of ATP7A and ATP7B in GBC may provide insights into overcoming platinum-based drug resistance [32]. Although significant progress has been made in understanding drug excretion mechanisms, further research is required to fully elucidate the pathways involved in chemotherapy resistance, particularly in cancers like GBC. Developing therapeutic strategies targeting these efflux mechanisms may help overcome resistance and improve treatment outcomes.
DNA damage repair
When exposed to chemotherapeutic agents such as topoisomerase inhibitors, alkylating agents, or DNA cross-linking drugs like cisplatin and cyclophosphamide, tumor cells undergo various forms of DNA damage. In response, cells activate a network of DNA damage response (DDR) pathways to maintain genomic stability and integrity [33]. The most commonly observed DNA repair mechanisms include direct reversal of DNA lesions, base excision repair (BER), nucleotide excision repair (NER), and mismatch repair (MMR). For repairing DNA double-strand breaks (DSBs), two key pathways are involved: homologous recombination (HR) and non-homologous end joining (NHEJ) [34].
The efficiency of DNA damage repair plays a critical role in both tumor development and the sensitivity of cancer cells to chemotherapy. For example, cells with activated RAS and PI3K signaling pathways produce elevated levels of reactive oxygen species (ROS), which cause oxidative DNA damage. These cancer cells activate the BER pathway to repair oxidative lesions. However, this increased DNA repair capability can paradoxically enhance resistance to chemotherapy drugs like temozolomide and cisplatin [35]. Additionally, studies have shown that after DNA damage, the levels of Dicer, an enzyme involved in RNA processing, are significantly elevated. This promotes DNA repair through the NHEJ pathway, contributing to chemotherapy resistance in colorectal cancer [36]. This highlights the critical role of DDR pathways in developing chemotherapy resistance across various cancer types.
Overall, enhanced DNA repair mechanisms protect tumor cells from the cytotoxic effects of chemotherapy by rapidly repairing the DNA damage that these treatments induce. Understanding how these pathways function provides insight into potential targets for overcoming resistance. Inhibitors targeting specific components of DNA repair pathways, such as PARP inhibitors, have shown promise in sensitizing resistant cancer cells to chemotherapy by disrupting their ability to repair DNA damage effectively.
Apoptosis evasion
Apoptosis, or programmed cell death, is a tightly regulated process essential for eliminating damaged or harmful cells. Chemotherapeutic agents often induce apoptosis in cancer cells to exert their therapeutic effects [37, 38]. However, cancer cells frequently develop mechanisms to evade apoptosis, contributing significantly to chemotherapy resistance [39]. This evasion is driven by disruptions in apoptotic pathways, protective autophagy, and the overexpression of anti-apoptotic proteins. (Fig. 2).
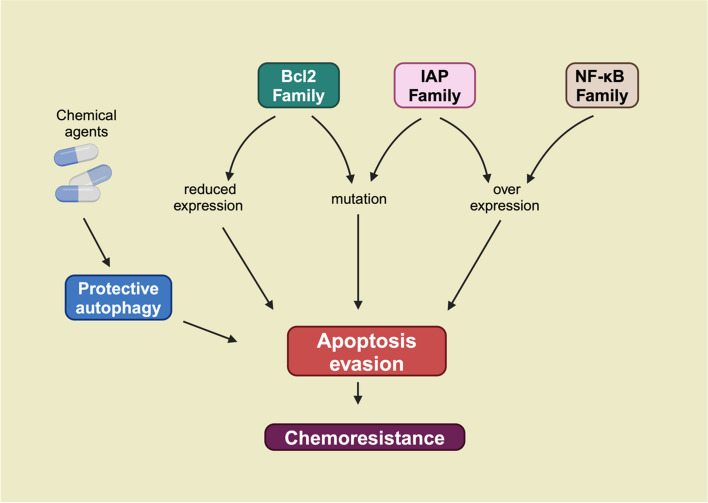
Fig. 2.
Apoptosis Evasion as a Mechanism of Chemoresistance. Chemotherapeutic intervention in tumor cells activates the intrinsic protective autophagy system. Concurrently, dysregulated expression and mutations in Bcl-2, IAP, and NF-κB family proteins impair chemotherapy-induced apoptosis in tumor cells. These factors collectively enable apoptosis evasion, leading to enhanced chemoresistance
One key mechanism by which cancer cells avoid apoptosis is through protective autophagy. Autophagy, a cellular self-degradation process, is activated in response to stress and serves as a survival mechanism in tumor cells [40]. Chemotherapy often triggers autophagy in cancer cells, which then inhibits apoptosis, reducing the effectiveness of treatment [41]. For instance, in cisplatin-resistant ovarian cancer cells, autophagy alleviates endoplasmic reticulum (ER) stress, thereby preventing mitochondrial-dependent apoptosis [42]. Similarly, in gallbladder cancer (GBC), nutrient deprivation, such as glucose starvation, triggers autophagy via the IL-6/STAT3 pathway, reducing apoptosis and increasing resistance to gemcitabine [43]. Similarly, in pancreatic cancer, Girdin activates protective autophagy by interacting with the autophagy-related protein p62/SQSTM1, which inhibits apoptotic pathways and promotes chemoresistance [44]. Evidence suggests that inhibiting autophagy may enhance the effectiveness of chemotherapy [45, 46]. Blocking autophagy with inhibitors like chloroquine has shown promise in restoring chemosensitivity.
In addition to autophagy, the dysregulation of apoptosis-related pathways plays a major role in chemotherapy resistance. The B-cell lymphoma 2 (Bcl-2) family of proteins regulates the mitochondrial outer membrane permeabilization, a key step in apoptosis. Overexpression of anti-apoptotic members of the Bcl-2 family, such as Myeloid Cell Leukemia-1 (Mcl-1) and Bcl-2, has been linked to chemotherapy resistance in several cancers, including leukemia, lung cancer, and breast cancer [47, 48]. In ovarian cancer, for example, cell lines with high Bcl-2 expression show increased resistance to chemotherapy [49]. Conversely, the knockdown of Mcl-1 restores chemosensitivity [50].
Mutations or reduced expression of pro-apoptotic proteins also contribute to resistance. For instance, mutations in the Bcl-2-associated X protein (BAX) gene, a pro-apoptotic protein, have been associated with colorectal cancer and therapeutic resistance [51]. Similarly, the downregulation of BAX and Phorbol-12-myristate-13-acetate-induced protein 1 (NOXA), other pro-apoptotic proteins, has been linked to resistance in acute myeloid leukemia (AML) [52–54]. Another important group of proteins involved in apoptosis regulation is the inhibitor of apoptosis proteins (IAPs). IAPs inhibit apoptosis by blocking caspase activity, thereby promoting cancer cell survival. Overexpression of IAP family members, such as X-linked inhibitor of apoptosis protein (XIAP), Survivin, and cIAP, has been associated with resistance to cisplatin and other chemotherapeutic agents [55]. Strategies targeting IAPs, such as second mitochondria-derived activator of caspases (SMAC) mimetics, are currently being explored to overcome apoptosis evasion [56]. Additionally, aberrant activation of the nuclear factor kappa-B (NF-κB) signaling pathway promotes apoptosis resistance in several cancers. NF-κB dysregulation has been shown to contribute to resistance to drugs like doxorubicin, cisplatin, and paclitaxel [57]. In GBC, co-administration of NF-κB inhibitors with chemotherapy has been found to enhance the sensitivity of cancer cells to gemcitabine [58].
In conclusion, evasion of apoptosis is a complex and multifaceted process in cancer cells that significantly contributes to chemotherapy resistance. Targeting key proteins involved in autophagy, anti-apoptotic signaling, and apoptosis regulation offers promising strategies to enhance the effectiveness of chemotherapy and overcome resistance.
Epigenetic modifications
Epigenetic modifications, which are heritable changes in gene expression without alterations to the DNA sequence, play a pivotal role in cancer development and progression. These changes are reversible and primarily occur through mechanisms like DNA methylation, histone modifications, and non-coding RNA regulation. (Fig. 3) Epigenetic alterations are closely linked to chemotherapy resistance by influencing the transcription and expression of key genes involved in drug response [59].
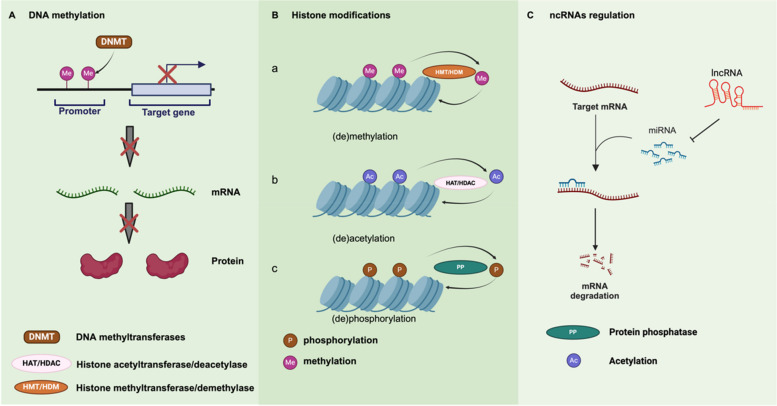
Fig. 3.
Overview of epigenetic modifications involved in cancer. (1) DNA methylation. Through the catalytic activity of DNMTs, cytosine residues are selectively methylated, producing 5-methylcytosine (5-mC). Methylation in promoter regions effectively downregulates the transcription of target genes, ultimately restricting the translation of mRNA into proteins. (2) Histone modifications. (a) (de)methylation. Histone methylation and demethylation, mediated by HMTs and HDMs, indirectly modulate chromatin accessibility and gene expression by altering the binding affinities of other epigenetic regulators. (b)(de)acetylation. Histone acetylation and deacetylation, catalyzed by HATs and HDACs, induce structural alterations in chromatin, thereby modulating gene expression. (c)(de)phosphorylation. Through the catalytic activities of PKs and PPs, histones undergo phosphorylation and dephosphorylation, facilitating rapid adjustments in chromatin structure and gene expression in response to cellular conditions and demands. (3) ncRNAs regulation. miRNA binds to the 3’ untranslated region (3’-UTR) of target mRNAs, typically leading to mRNA degradation or translational repression, which in turn reduces target protein expression. Conversely, lncRNA can act as a “miRNA sponge” by binding to miRNAs, preventing them from associating with their target mRNAs and thus promoting the expression of specific mRNAs
DNA methylation, the addition of methyl groups to CpG dinucleotides, is one of the earliest and most studied epigenetic mechanisms [60]. DNA methylation patterns in tumors often include global hypomethylation and site-specific hypermethylation of tumor suppressor genes [61]. For example, hypermethylation of the Breast Cancer 1 (BRCA1) gene in ovarian cancer cells is associated with increased sensitivity to platinum-based chemotherapy [62]. Conversely, the hypermethylation of genes like TGF-βRI in gastric cancer has been linked to resistance to transforming growth factor-β (TGF-β) signaling [63, 64]. Reversing these methylation patterns may help overcome chemotherapy resistance [65]. Histone modifications, including acetylation, methylation, ubiquitination, and phosphorylation, also regulate gene expression by altering chromatin structure. Histone acetylation, mediated by histone acetyltransferases (HATs) and reversed by histone deacetylases (HDACs), is particularly important in chemotherapy resistance [66]. Acetylation generally leads to chromatin relaxation, enhancing transcriptional activity, while deacetylation condenses chromatin, silencing genes [67, 68]. For example, the overexpression of p300, a HAT, has been associated with gemcitabine resistance in pancreatic cancer [69]. On the other hand, upregulation of HDACs is linked to resistance in various cancers, including melanoma and ovarian cancer [70, 71]. Inhibiting HDACs with histone deacetylase inhibitors (HDACi), such as belinostat, has shown promise in restoring chemotherapy sensitivity [72]. Histone methylation also plays a key role in drug resistance. The enzyme Enhancer of Zeste Homolog 2(EZH2), a methyltransferase, is overexpressed in cisplatin-resistant ovarian cancer cells. Reducing H3K27 trimethylation (mediated by EZH2) enhances sensitivity to cisplatin [73]. Similar findings have been reported in lung cancer, where histone methyltransferases contribute to chemotherapy resistance by regulating cancer stem cell genes, promoting epithelial-mesenchymal transition (EMT), and influencing cell migration [74].
Non-coding RNAs, particularly microRNAs (miRNAs) and long non-coding RNAs (lncRNAs), are also critical regulators of gene expression and have been increasingly implicated in chemoresistance [75]. MiRNAs, small non-coding RNAs that regulate gene expression post-transcriptionally, can act as either tumor suppressors or oncogenes [76]. In gallbladder cancer, miRNAs have been shown to regulate the expression of drug resistance genes [77, 78]. For instance, overexpression of certain miRNAs can suppress genes involved in drug metabolism or apoptosis, contributing to chemoresistance. Long non-coding RNAs (lncRNAs), which are longer RNA molecules that do not encode proteins, also play a role in drug resistance through interactions with various signaling pathways [79, 80]. For example, GBCDRlnc1 has been identified in doxorubicin-resistant gallbladder cancer cells, where it enhances autophagy and promotes drug resistance [81]. Similarly, in cholangiocarcinoma, the lncRNA HOTTIP modulates sensitivity to chemotherapy through the HOTTIP/miR-637/LASP1 axis [82]. (Table 1).
Table 1.
Chemoresistance mediated by noncoding RNA in GBC
| ncRNA type | Expression | Drug | Target | Reference |
|---|---|---|---|---|
| MicroRNA | ||||
| miR-145 | down | CDDP | MRP 1 | [25] |
| miR-125 b-5 p | down | CDDP | Bcl2 | [83] |
| miR-31 | down | DDP | Src | [84] |
| miR-205-5p | down | GEM | PRKCE | [85] |
| miR-218-5p | down | GEM | PRKCE/MDR1 | [19] |
| miR-193a-3p | down | trametinib | KRAS/ERK | [86] |
| miR-433 | down | GEM | miR-433/cyclin M | [87] |
| miR-223 | down | Docetaxel | STMN1 | [88] |
| LncRNA | ||||
| GBCDRlnc1 | up | DOX | PGK1 | [81] |
| MYLK-AS 1 | up | GEM | MYLK-AS 1/miR-217/EZH 2 | [89] |
| HOTTIP | up | GEM/CDDP | HOTTIP/miR 637/LASP 1 | [82] |
Epigenetic mechanisms offer potential therapeutic targets, as their reversible nature provides opportunities for intervention. By targeting specific epigenetic alterations, such as using DNA demethylating agents or histone deacetylase inhibitors, chemotherapy resistance may be mitigated.
Altered drug metabolism
Chemotherapeutic agents undergo extensive metabolism within tumor cells, often affecting their therapeutic efficacy. The enzymes responsible for drug metabolism are critical factors in the development of chemotherapy resistance. This process is generally divided into two phases: Phase I metabolism, which involves oxidation, reduction, or hydrolysis by enzymes such as cytochrome P450 (CYP), and Phase II metabolism, where drugs are conjugated by enzymes such as glutathione S-transferases (GSTs) [90]. Cytochrome P450 (CYP) enzymes play a key role in drug biotransformation. Alterations in CYP enzyme levels can significantly affect drug metabolism, leading to resistance. For example, overexpression of CYP1B1, CYP2E1, and CYP3A4 has been observed in non-rhabdomyosarcoma soft tissue sarcomas, contributing to reduced sensitivity to chemotherapy [91–93]. Conversely, decreased CYP expression has been linked to increased chemoresistance in human medulloblastoma cells [91].
The glutathione (GSH) metabolic system also plays a significant role in drug resistance. Elevated levels of intracellular GSH allow cancer cells to neutralize chemotherapy drugs by conjugating them with GSH, facilitating their removal from the cell [94]. This process is particularly relevant in cisplatin resistance, where GSH binds to the drug, inactivating it before it can exert its cytotoxic effects [95]. Overexpression of gamma-glutamyl transferase (GGT), which is involved in GSH metabolism, further enhances resistance by protecting cancer cells from oxidative stress and chemotherapeutic damage [96]. Other metabolic enzymes are also implicated in chemotherapy resistance. Deoxycytidine kinase (dCK), a critical enzyme in the activation of gemcitabine, is often downregulated in gemcitabine-resistant cancer cells. Mutations or reduced expression of dCK have been identified in resistant gallbladder cancer cell lines, where restoring dCK levels can re-sensitize cells to the drug [97–99].
The complexity of drug metabolism, including alterations in both Phase I and Phase II pathways, underscores its significant role in chemotherapy resistance. Targeting key metabolic enzymes such as CYPs, GSTs, or dCK offers potential strategies for overcoming drug resistance and improving the efficacy of chemotherapeutic agents.
Cancer stem cells (CSCs)
Cancer stem cells (CSCs) are a subpopulation of tumor cells characterized by their ability to self-renew, differentiate, and initiate tumor formation. These cells are also highly resistant to conventional chemotherapy, which primarily targets rapidly dividing cancer cells. As a result, CSCs often survive treatment, contributing to tumor recurrence and metastasis [100, 101]. (Fig. 4) CSCs express specific surface markers, such as CD133, CD44, and aldehyde dehydrogenase (ALDH), which help identify and isolate them [102].
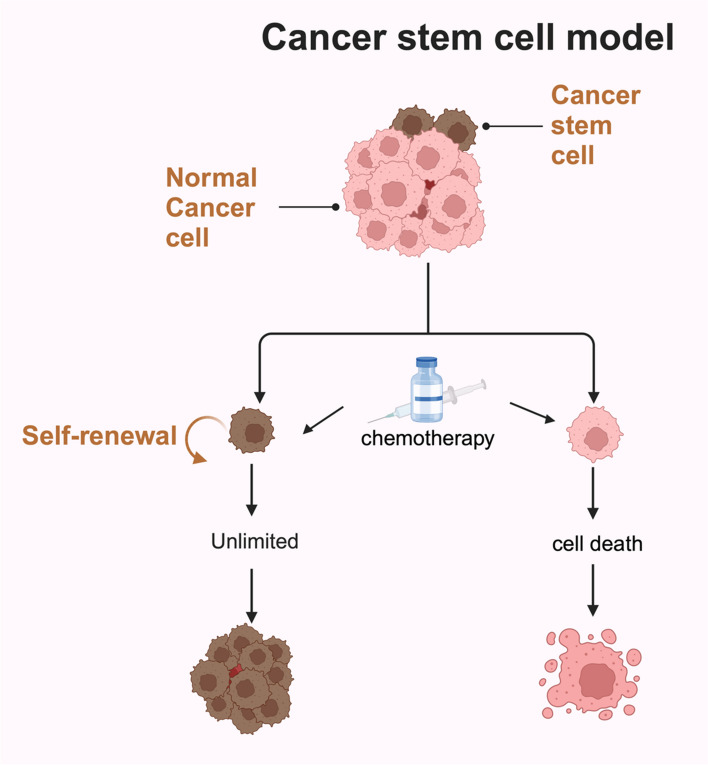
Fig. 4.
Cancer stem cell model. Tumor tissues contain CSCs, a subpopulation with self-renewing properties. When chemotherapy is applied, typical cancer cells undergo apoptosis, yet CSCs evade these effects, leading to their survival and the possibility of treatment resistance
One of the key mechanisms by which CSCs resist chemotherapy is through drug efflux, facilitated by ATP-binding cassette (ABC) transporters like ABCG2. These transporters actively pump chemotherapy drugs out of CSCs, reducing their intracellular concentration and thereby limiting their cytotoxicity [102, 103]. For instance, CD133 + CSCs in gallbladder cancer exhibit high levels of ABCG2 expression, making them resistant to drugs like gemcitabine and 5-fluorouracil (5-FU) [104]. Similarly, in lung cancer, CD133 + cells show resistance to DNA-damaging agents due to enhanced DNA repair capacity, mediated by increased expression of DNA repair proteins [105]. Enhanced DNA repair mechanisms also contribute to CSCs' ability to survive chemotherapy. CSCs often exhibit upregulated DNA repair pathways, allowing them to quickly resolve chemotherapy-induced DNA damage. For example, XRCC1, a DNA repair protein, is highly expressed in CD133 + GBC cells, contributing to 5-FU resistance [106]. In addition, CSCs are adept at maintaining low levels of reactive oxygen species (ROS), which allows them to evade the oxidative damage caused by certain chemotherapeutic agents. CSCs achieve this by upregulating antioxidant enzymes, which neutralize ROS and protect the cells from apoptosis [107]. This enhanced oxidative stress response helps CSCs survive therapies that induce ROS-mediated cell death [107–110].
Cellular quiescence, a state of dormancy in which cells exit the cell cycle (G0 phase), is another mechanism that protects CSCs from chemotherapy. Most chemotherapeutic agents target rapidly dividing cells, leaving quiescent CSCs unaffected. Once therapy ends, these quiescent CSCs can re-enter the cell cycle, leading to tumor regrowth [111, 112]. The ALDH enzyme, which detoxifies reactive aldehydes and reduces oxidative stress, is also highly expressed in quiescent CSCs, further contributing to their survival and resistance [113, 114]. The ability of CSCs to evade chemotherapy through these mechanisms makes them a critical focus of cancer research. Targeting CSC-specific pathways, such as drug efflux pumps, DNA repair systems, and ROS metabolism, could provide new therapeutic strategies to eliminate CSCs and reduce the likelihood of tumor relapse [115].
In this section, the molecular mechanisms underlying chemotherapy resistance in cancer are comprehensively described. These intricately connected pathways play a pivotal role in the failure of chemotherapeutic interventions, posing substantial challenges in clinical oncology. By gaining a deeper understanding of how these mechanisms contribute to resistance, researchers can more effectively identify novel therapeutic targets, paving the way for advanced treatment strategies and improved outcomes for cancer patients.
Chemotherapy resistance in major cancer types
While the previous section summarized the primary mechanisms of chemotherapy resistance, the molecular basis of resistance demonstrates considerable heterogeneity across different cancer types. Accordingly, this section systematically examines the resistance mechanisms in several clinically significant malignancies, including lung, breast, colorectal, and prostate cancers, as well as leukemia. These cancers collectively represent a substantial global health burden and are characterized by persistently high rates of therapeutic resistance. By elucidating both the convergent pathways and cancer-specific molecular targets implicated in resistance, this section underscores the complexity of these mechanisms. Critically, a comprehensive understanding of these processes is essential for refining therapeutic strategies and establishing a robust foundation for the development of innovative interventions to overcome resistance.
Lung cancer
Lung cancer is one of the most common malignancies worldwide, with over 2.2 million new cases and nearly 1.8 million deaths reported in 2020 [116]. It is classified into two main types: non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). While curative surgery is often the first-line treatment for early-stage tumors, many patients present with unresectable or advanced disease, requiring systemic therapies such as chemotherapy, targeted therapy, or immunotherapy [117]. In NSCLC, targeted therapies are preferred for patients with specific genetic mutations [118]. However, for those without such mutations, platinum-based chemotherapy remains the standard treatment [119].
Platinum drugs, such as cisplatin, work by forming DNA adducts that interfere with DNA synthesis and transcription, leading to cell death [120]. However, the development of resistance significantly reduces the efficacy of these therapies. Resistance mechanisms include reduced drug accumulation, enhanced DNA repair, and the activation of anti-apoptotic pathways. Reduced intracellular accumulation of platinum is often caused by increased drug efflux or impaired uptake via transport channels, such as copper transporters (CTR1/2) [121, 122]. Additionally, upregulation of efflux pumps like ATP7A/B and MRPs lowers intracellular platinum concentrations, contributing to resistance [123–125].
Enhanced DNA repair also plays a critical role in platinum resistance. Tumor cells with high DNA repair capacity can effectively remove platinum-induced DNA damage. For instance, NPAS2, which stabilizes H2AX (a key enzyme in homologous recombination repair), increases DNA repair activity, decreasing the sensitivity of lung adenocarcinoma cells to platinum drugs [126]. Likewise, the XRCC1/MACC1 complex activates the Akt signaling pathway, contributing to resistance through enhanced base excision repair [127]. In addition, the activation of anti-apoptotic pathways, such as NF-κB and Bcl-2 family proteins like MCL-1, also supports platinum resistance by inhibiting apoptosis [128–130].
Resistance to targeted therapies, particularly epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs), is another major challenge in NSCLC. While EGFR-TKIs improve outcomes in patients with EGFR mutations [131, 132], resistance, particularly due to EGFR-T790M mutations, limits their long-term efficacy [133]. Although third-generation EGFR inhibitors like osimertinib have been developed to address T790M-mediated resistance, new mutations, such as EGFR-C797S, have emerged [134–137]. These findings highlight the complexity of resistance mechanisms in lung cancer, underscoring the need for ongoing research to develop more effective treatments.
Breast cancer
Breast cancer is the most common malignancy worldwide and remains a leading cause of death among women, posing a significant public health concern [138]. It is traditionally classified into molecular subtypes based on the expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). The main subtypes include hormone receptor-positive (HR +), HER2-positive (HER2 +), and triple-negative breast cancer (TNBC). Current therapeutic strategies are guided by these molecular subtypes [139, 140], although drug resistance mechanisms vary across these categories.
HR + tumors, defined by high expression of ER or PR, represent 70–80% of invasive breast cancer cases [141]. ER activation occurs via two pathways: the classical pathway, where estradiol binds to ER to stimulate proliferation, and the non-classical pathway, where ER is activated by mitogen-activated protein kinase (MAPK) or phosphatidylinositol-3-kinase (PI3K) signaling [142, 143]. This activation promotes gene transcription, driving breast cancer progression. As a result, endocrine therapies targeting the estrogen axis—such as the ER antagonist tamoxifen (TAM) and aromatase inhibitors (AIs) like letrozole and anastrozole—are standard treatments [144]. TAM is commonly used in premenopausal women, while AIs are prescribed for postmenopausal women. However, resistance to endocrine therapy remains a major challenge, with 50–60% of patients experiencing primary or acquired resistance [145]. This resistance is often linked to mutations in the ER ligand-binding domain (LBD) or alterations in signaling pathways. Notably, ESR1 gene mutations, which encode ERα, are rare in primary breast cancer but occur in about 20% of patients with metastatic breast cancer (MBC) who have undergone endocrine therapy [146–148]. The most frequent mutations, Y537 and D538, induce structural changes that reduce sensitivity to TAM and AIs, contributing to resistance [149]. These mutations also enhance cancer cell migration, invasion, and metastasis. Other genetic alterations in ESR1 and aberrations in the PI3K and MAPK pathways further complicate resistance mechanisms [150, 151]. For instance, mutations in PIK3CA or alterations in AKT1 or PTEN, which lead to abnormal activation of the PI3K pathway, are associated with endocrine resistance in ER + breast cancer [152]. Mutations in the MAPK pathway components, such as NF1, KRAS, and BRAF, also contribute to resistance, especially in metastatic cases [153, 154].
Receptor tyrosine kinases (RTKs) regulate pathways like PI3K/AKT and JAK/STAT, and upregulation of fibroblast growth factor receptors (FGFRs) can induce endocrine resistance. HER2 mutations acquired during endocrine therapy in HR + /HER2- patients may promote cross-talk with ER/PR-related pathways, furthering resistance [155]. The advent of cyclin-dependent kinase 4/6 (CDK4/6) inhibitors has improved treatment options, particularly for overcoming resistance to endocrine therapies. CDK4/6 inhibitors block cell cycle progression by preventing the phosphorylation of retinoblastoma protein (RB1), halting tumor cell growth [156, 157]. However, resistance to CDK4/6 inhibitors can occur due to RB1 loss, which allows cells to bypass the CDK4/6 blockade and continue proliferating through alternative pathways, such as E2F [158]. Thus, combining endocrine therapy with CDK4/6 inhibitors is a promising approach, but further clinical trials are necessary to optimize this strategy.
HER2 + breast cancer accounts for 25–30% of all cases and is associated with higher malignancy, recurrence, and poor prognosis. Trastuzumab (Herceptin), a monoclonal antibody targeting HER2, has dramatically improved outcomes in this subtype. However, resistance remains a major challenge [159, 160]. Up to one-third of patients exhibit intrinsic resistance to trastuzumab, and within the first year, 70% may develop acquired resistance [161]. Resistance mechanisms include HER2 gene mutations—such as the HER2-L755S mutation—which promote activation of the PI3K/AKT and MAPK pathways, negating trastuzumab’s efficacy [162, 163]. The presence of p95HER2, a splice variant of HER2, also limits anti-HER2 drug efficacy [164]. Additionally, Mucoprotein 4 (Muc4) can obscure trastuzumab’s binding site on HER2, exacerbating resistance [165]. Aberrant activation of the PI3K/AKT pathway, often through PIK3CA mutations, plays a central role in trastuzumab resistance [166].
Triple-Negative Breast Cancer (TNBC) represents 10–20% of breast cancer cases and lacks expression of ER, PR, and HER2, making it challenging to treat [167, 168]. Current therapies include chemotherapy, radiotherapy, immunotherapy, and targeted therapy, with chemotherapy being the cornerstone of treatment [169]. Despite chemotherapy, resistance remains common, prompting the need for novel therapeutic approaches. BRCA1 and BRCA2 mutations, which impair DNA repair, are significant in TNBC, as tumors with these mutations show heightened sensitivity to poly ADP-ribose polymerase (PARP) inhibitors [170, 171]. PARP inhibitors, such as olaparib, have proven effective in treating BRCA-mutated TNBC [172]. Moreover, mutations in the PI3K/AKT/mTOR pathway are the second most common in TNBC, occurring in 25–35% of cases [173]. Targeting this pathway, along with the androgen receptor (AR) pathway—which is expressed in 10–50% of TNBC cases—offers potential therapeutic avenues [174, 175]. The AR signaling pathway also exhibits significant cross-talk with the PI3K/Akt and MAPK pathways, creating a complex regulatory network that influences tumor biology. Additionally, AR interacts with the Wnt/β-catenin pathway, which plays a critical role in the initiation and progression of TNBC [176, 177]. Furthermore, the AR pathway promotes the transcription of genes involved in epithelial-mesenchymal transition (EMT), increasing the invasiveness and metastatic potential of cancer cells. Therefore, targeting the AR signaling pathway represents a promising therapeutic strategy for TNBC [178].
Multiple resistance mechanisms exist across breast cancer subtypes, driven by complex interactions between genetic mutations, signaling pathways, and tumor microenvironment factors. Despite advances in targeted therapies, the development of drug resistance continues to limit treatment efficacy. Ongoing research into these resistance mechanisms and large-scale clinical trials are essential for the development of precision medicine approaches, which will help to overcome therapeutic resistance and improve patient outcomes.
Colorectal cancer
Colorectal cancer (CRC) is the third most prevalent cancer globally and the second leading cause of cancer-related mortality, with 1,142,286 new cases reported in 2022 [138]. While surgery remains the primary treatment for CRC, the identification of key molecular targets involved in CRC pathogenesis and progression has led to the development of targeted therapies, offering promising prospects for improving patient outcomes [179]. Notable advancements include therapies targeting Kirsten rat sarcoma viral oncogene homolog (KRAS) mutations, such as KRAS inhibitors, and EGFR inhibitors like cetuximab [180, 181]. However, drug resistance poses a significant challenge to the effectiveness of these therapies, particularly resistance associated with KRAS and EGFR inhibitors.
KRAS mutations are among the most common oncogenic mutations in human cancers, present in approximately 35% to 49% of CRC cases, with the G12C mutation accounting for about 11% of all KRAS mutations. Recently, covalent inhibitors targeting the G12C subtype have shown promise in preclinical and clinical studies, becoming a focal point of KRAS inhibitor research [182]. However, as with other therapies, resistance remains a critical issue in clinical applications. Acquired resistance often arises from secondary mutations in the KRAS gene following treatment with KRAS G12C inhibitors. Genomic analyses of pre- and post-treatment samples from patients—including those with non-small cell lung cancer, CRC, and appendiceal cancer—revealed secondary KRAS mutations (such as G12D/R/V/W, G13D, Q61H, R68S, H95D/Q/R, or Y96C) and KRAS amplification as contributors to resistance. The activation of KRAS requires upstream signaling factors to engage downstream pathways, and reactivation or alteration of these factors can also drive resistance [183]. Notably, reactivation of receptor tyrosine kinase (RTK) signaling, particularly EGFR, plays a crucial role in resistance to KRAS G12C inhibitors in CRC. This mechanism may be due to the inherent RTK dependency in CRC and the reactivation of downstream effectors [184]. Combination therapies targeting both EGFR and KRAS G12C have shown promise in overcoming this resistance [185].
The MAPK pathway, a downstream component of the RAS signaling cascade, also plays a pivotal role in resistance to KRAS-targeted therapies. Feedback activation of this pathway can result in resistance to inhibitors targeting specific KRAS alleles [186, 187]. Additionally, epithelial-to-mesenchymal transition (EMT) may contribute to resistance against KRAS G12C inhibitors in CRC. EMT can alter signaling pathways upstream and downstream of RAS, reducing the effectiveness of KRAS inhibitors on the RAS signaling network [188].
In approximately 60% to 80% of CRC cases, EGFR is overexpressed, making it a critical therapeutic target [189]. Cetuximab, an EGFR-targeting monoclonal antibody, is approved for treating metastatic CRC [180]. The CRYSTAL trial demonstrated that combining cetuximab with first-line chemotherapy significantly improved progression-free survival (PFS) in patients with KRAS wild-type metastatic CRC [190]. However, resistance remains an unavoidable issue, often arising from mutations in the EGFR, KRAS, and BRAF genes. A mutation at codon 492 of EGFR, which changes serine to arginine (S492R), can alter the conformation of EGFR’s extracellular domain, leading to cetuximab resistance [191]. Additionally, cetuximab is effective only in patients with KRAS wild-type tumors, as KRAS mutations keep the MAPK signaling pathway activated, leading to resistance to EGFR-targeted therapies [192, 193]. BRAF mutations, particularly BRAFV600E, are also implicated in cetuximab resistance, resulting in poor treatment response and prognosis [191].
The Wnt/β-catenin pathway, a critical signaling cascade regulating various biological processes, including embryogenesis, stem cell maintenance, and tissue homeostasis, plays a key role in CRC initiation, progression, and resistance [194, 195]. Forkhead Box M1 (FOXM1) is involved in the malignant behavior of CRC by enhancing Wnt/β-catenin signaling. FOXM1 interacts with Dishevelled 2 (Dvl2), promoting its nuclear translocation and transcriptional activity, thereby driving metastasis and drug resistance [196]. Additionally, LGR5, a marker for cancer stem cells (CSCs) and a Wnt pathway target, contributes to chemoresistance by allowing CSCs to enter a quiescent state and evade drug treatments [197].
Resistance in CRC is closely linked to patient-specific variations and tumor heterogeneity. The mutation burden of resistance-associated genes significantly affects the efficacy of targeted therapies. By investigating the mechanisms behind CRC resistance, new therapeutic targets may be identified. Furthermore, a detailed evaluation of the molecular profiles of individual patients could enable the development of personalized adjuvant treatment strategies, ultimately improving clinical outcomes for advanced CRC patients.
Prostate cancer
Prostate cancer (PC) is the second most common malignancy among men worldwide, accounting for approximately 15% of all cancer diagnoses in men [198]. In recent decades, the treatment landscape has significantly evolved, with androgen deprivation therapy (ADT) becoming the standard approach for PC patients. ADT works by reducing androgen levels produced by the testes, depriving prostate cancer cells of their primary growth stimulus, leading to tumor regression or slower proliferation [199]. However, the effectiveness of ADT is often limited by the development of resistance. After 18 to 24 months of treatment, many patients progress to castration-resistant prostate cancer (CRPC), which no longer responds to ADT [200].
Resistance to ADT is primarily driven by adaptive changes in the androgen receptor (AR) signaling pathway. Mechanisms include AR amplification, point mutations in the AR gene, alterations in AR co-regulatory molecules, and changes beyond the AR pathway. Overexpression of AR allows tumor cells to survive and grow despite low androgen levels during therapy. In CRPC, increased AR mRNA and protein levels are commonly observed, likely due to epigenetic modifications [201, 202]. AR gene mutations, present in approximately 10–20% of CRPC cases, reduce the receptor’s specificity for testosterone, allowing it to bind other hormones like estrogens and progestins, diminishing the effectiveness of AR antagonists and contributing to chemotherapy resistance [203–205].
Changes in AR co-regulator expression also provide a survival advantage to cancer cells during ADT, promoting progression to CRPC. Co-regulators modulate AR transcriptional activity by acting as coactivators or corepressors [205]. Notably, steroid receptor coactivator-3 (SRC3) plays a critical role in AR activation and CRPC progression [206]. While AR reactivation is central to CRPC progression, alternative pathways also contribute to resistance. Heat shock protein Hsp27, for example, plays a key role in driving resistance. Inhibitors of Hsp27, such as antisense oligonucleotides (OGX-427) and small interfering RNA (siRNA), have shown potential to enhance chemotherapy efficacy. Hsp27 protects specific proteins involved in castration resistance, such as eIF4E, from degradation, promoting tumor survival [207, 208].
Neuroendocrine prostate cancer (NEPC) is a highly aggressive subtype that often develops following prolonged ADT. In NEPC, adenocarcinoma cells transform into neuroendocrine cells, a process driven by complex interactions among genomic, epigenetic, transcriptional, and post-translational changes [209–212]. NEPC is resistant to endocrine therapies, necessitating platinum-based chemotherapy as the first-line treatment. Since its introduction in 2004, docetaxel has become an essential therapy for metastatic castration-resistant prostate cancer (mCRPC) [213]. Clinical studies have shown that combining docetaxel with ADT and radiation significantly improves recurrence-free survival in patients with non-metastatic, locally advanced prostate cancer. However, resistance to docetaxel limits its long-term effectiveness [214]. Chemotherapy resistance in PC arises from various factors, including changes in drug targets, epigenetic modifications, DNA repair mechanisms, inhibition of apoptosis, and the epithelial-to-mesenchymal transition (EMT) process [215].
While endocrine therapy can prolong survival in advanced prostate cancer, the transition to CRPC marks a critical point in disease progression, increasing mortality risk. Resistance to established therapies, including immunotherapy and chemotherapy, remains a significant challenge. A deeper understanding of the dynamic changes in treatment targets and resistance mechanisms is essential for developing innovative treatment strategies. These insights can help identify new therapeutic approaches to improve outcomes for patients with advanced prostate cancer.
Leukemia
Leukemia is a group of cancers characterized by the uncontrolled growth of hematopoietic stem cells, and it is the most common cancer among children and adolescents [216]. Treatment strategies for leukemia include chemotherapy, radiotherapy, immunotherapy, and hematopoietic stem cell transplantation, with specific approaches tailored to different subtypes [217]. Acute myeloid leukemia (AML), a highly aggressive blood cancer, has a poor prognosis. Standard treatment for AML involves the "3 + 7" regimen, a combination of cytarabine (Ara-C) and anthracycline [218]. Recent advances have shed light on key molecular features that help guide targeted treatments, particularly mutations in the FMS-like tyrosine kinase 3 (FLT3) gene, which is altered in about one-third of AML patients [219, 220].
FLT3 inhibitors have improved treatment outcomes, but resistance remains a major challenge [221, 222]. Resistance can be caused by protective interactions in the bone marrow, mutations in the FLT3 gene, and mutations outside the FLT3 target region. The enzyme CYP3A4, which metabolizes FLT3 inhibitors, is highly concentrated in the bone marrow, reducing the drugs’ effectiveness by lowering their plasma levels [223, 224]. Studies show that using CYP3A4 inhibitors like clarithromycin can help overcome resistance to FLT3 inhibitors like sorafenib [225]. Bone marrow stromal cells also produce FLT3 ligand, which promotes AML cell survival and reduces their sensitivity to FLT3 inhibitors [226].
Mutations in the FLT3 gene, including internal tandem duplications (ITDs) and tyrosine kinase domain (TKD) mutations, further contribute to resistance, especially against type II FLT3 inhibitors [227]. Resistance is also driven by other pathways that compensate for FLT3 inhibition, such as the RAS/MEK/ERK and PI3K/AKT/mTOR pathways [228]. Insights from studies on chronic myeloid leukemia (CML) have helped in the development of next-generation FLT3 inhibitors to improve treatment outcomes in AML patients.
CML is caused by the fusion gene BCR-ABL1, which results from a translocation between chromosomes 9 and 22, forming the Philadelphia chromosome [229, 230]. This gene produces the BCR-ABL1 protein with uncontrolled tyrosine kinase activity, driving CML progression [231]. Tyrosine kinase inhibitors (TKIs), which block this activity, have significantly improved CML outcomes [232]. However, 30% of patients develop resistance, primarily due to BCR-ABL1 mutations or overexpression, which reduces TKI effectiveness [233]. Other resistance mechanisms include abnormal drug transport, activation of alternative pathways, and the persistence of leukemic stem cells [229]. These complexities highlight the need for new therapies to overcome resistance and improve survival.
Overcoming chemotherapy resistance: therapeutic strategies and future directions
The persistence of chemotherapy resistance continues to challenge the efficacy of cancer treatment, underscoring the urgent need for innovative therapeutic strategies. In this section, we discuss four key approaches to addressing this issue: combination therapies, targeting the tumor microenvironment, advanced drug delivery systems, and personalized medicine. Specifically, combination therapies leverage multiple agents to counteract distinct resistance mechanisms, thereby enhancing therapeutic efficacy. Meanwhile, strategies targeting the tumor microenvironment aim to disrupt its supportive role in fostering resistance. In addition, emerging drug delivery technologies are designed to improve the precision, stability, and bioavailability of chemotherapeutic agents, thereby minimizing systemic toxicity and enhancing efficacy. Finally, personalized medicine focuses on tailoring treatment regimens to the unique molecular and genetic characteristics of individual patients, offering a precision-based approach to overcoming resistance. Collectively, these strategies provide a robust and comprehensive framework for combating chemotherapy resistance, representing critical directions for advancing cancer therapy.
Combination therapies
Since the first use of chemotherapy to treat cancer, the challenge of limited efficacy with monotherapy has persisted [234]. With the discovery of oncogenic targets in the 1980s, targeted therapies were developed. The emergence of immunotherapy, photodynamic therapy (PDT), and radiotherapy (RT) represents some of the most significant breakthroughs in the field of cancer treatment, have further advanced the field [235–237]. A key focus of current research is how to combine these therapies to overcome the limitations of monotherapy [238]. Cellular signaling pathways are complex, often involving feedback loops and crosstalk between pathways. This can lead to compensatory mechanisms, where inhibiting one pathway activates another. For example, combining BRAF and EGFR inhibitors has proven effective where single-agent therapy fails [239]. Inhibition of BRAF alone triggers a feedback loop that activates EGFR, but using both inhibitors together has shown significant efficacy [240]. The combination of cetuximab (an EGFR inhibitor) and encorafenib (a BRAF inhibitor) has been approved for treating BRAF-mutated colorectal cancer in the U.S. and Europe.
The integration of immunotherapy with other treatments has also shown great promise. Immune checkpoint inhibitors (PD-1, PD-L1, CTLA-4) enhance the immune system’s ability to fight cancer and have been approved for various cancers [235]. Combining checkpoint inhibitors with chemotherapy has demonstrated synergistic effects, improving outcomes. Chemotherapy-induced DNA damage can increase tumor antigenicity by enhancing antigen presentation via NF-κB activation and HLA expression [241]. For example, combining pembrolizumab with chemotherapy has improved survival rates in lung cancer compared to chemotherapy alone [242]. Additionally, combining targeted therapies with immunotherapy enhances the immune system’s ability to recognize and destroy cancer cells. BRAF and MEK inhibitors, for instance, have been shown to increase levels of interferon-γ (IFN-γ) and promote T cell activation, leading to enhanced tumor infiltration by CD8 + T cells [243–245]. The combination of immune checkpoint blockade (ICB) and MAPK inhibitors has been approved for treating melanoma [246].
Combining PDT and RT with chemotherapy has shown promising potential. PDT uses specific light wavelengths to activate intracellular compounds, generating reactive oxygen species (ROS) to destroy tumor cells. It also enhances chemotherapeutic drug permeability and modulates immune cell and cytokine infiltration, impacting distant disease sites. Clinical trials exploring PDT as a chemotherapy adjunct are ongoing [247]. PDT holds promise for enhancing treatment efficacy and overcoming chemotherapy resistance. RT, using high-energy X-rays or gamma rays, is essential for tumor control and improving quality of life [248]. Studies indicate that combining intensity-modulated radiotherapy (IMRT) with adjuvant immune checkpoint inhibitors significantly enhances efficacy, reduces postoperative adverse effects, and improves outcomes [249].
These combination strategies leverage the strengths of each modality, producing synergistic effects that can better address resistance and tumor heterogeneity, often achieving more than either treatment alone.
Targeting the tumor microenvironment
The tumor microenvironment (TME) is a complex network of cells and extracellular components surrounding tumor cells. It is characterized by a low pH, hypoxia, and metabolic byproducts, and includes endothelial cells, fibroblasts, immune cells, cytokines, growth factors, and the extracellular matrix [250]. The interactions between the TME and tumor cells not only promote tumor growth and invasion but also contribute to drug resistance by reducing drug permeability. Targeting the TME offers new opportunities to overcome resistance. For example, in hepatocellular carcinoma, the macrophage colony-stimulating factor (CSF-1) and its receptor regulate tumor-associated macrophages (TAMs). The CSF receptor inhibitor PLX3397 blocks CSF-1R tyrosine kinase activity and, while it doesn’t reduce TAM infiltration, it shifts TAMs toward the M1 phenotype, inhibiting tumor growth [251].
Improving drug delivery within the TME has also been explored as a way to enhance chemotherapy efficacy. One of the most studied methods is the nanoparticle delivery system, which uses cell-penetrating and tumor-targeting peptides designed to deliver drugs directly to the TME [252]. Understanding the TME’s role in tumor progression and resistance is crucial for developing new therapeutic strategies. Precisely targeting the TME could improve treatment outcomes and offer a promising approach to overcoming resistance in cancer therapy.
Drug delivery systems
Traditional chemotherapy faces several challenges in cancer treatment, such as reduced therapeutic efficacy due to poor drug targeting and increased toxicity from systemic exposure. To address these issues, drug delivery systems have emerged as a promising solution. Nanotechnology-based drug delivery systems encapsulate chemotherapeutic agents in nanoparticles, optimizing drug distribution and accumulation in the tumor microenvironment while minimizing exposure to healthy tissues [253]. Common nanoparticles include liposomes, micelles, and metal-based nanoparticles. Liposomes, composed of phospholipids, self-assemble into spherical vesicles that can carry therapeutic molecules. Their biocompatibility and safety make them ideal for drug delivery [254]. For example, folate-conjugated liposomes used to deliver 5-fluorouracil (5-FU) in colon cancer have shown increased cancer cell death and reduced tumor volume with lower doses of 5-FU [255]. Pegylated liposomal doxorubicin has also reduced cardiotoxicity compared to free doxorubicin, significantly lowering adverse effects [256]. Some nanoparticle-based systems can overcome resistance mechanisms by inhibiting drug efflux pumps and promoting apoptosis. Cationic nanoparticle complexes encapsulating paclitaxel and the Bcl-2 gene have been shown to inhibit drug-resistant liver cancer cells by disrupting P-glycoprotein (P-gp) drug efflux and activating apoptotic pathways [257]. These systems represent a major advance in overcoming drug resistance, improving both the efficacy and safety of cancer treatments by targeting tumor tissues more precisely and reducing off-target effects.
Personalized medicine
Personalized medicine (PM) is an innovative medical approach that customizes treatment plans based on an individual’s genetic makeup, environment, and lifestyle [258]. This approach aims to provide more effective care by accounting for individual variability. Advances in next-generation sequencing (NGS) have greatly improved genomic technologies, enabling the identification of actionable mutations in cancer patients. Personalized medicine has the potential to improve survival outcomes, particularly in patients with poor prognoses. A well-known example is the treatment of non-small cell lung cancer (NSCLC) in patients with EGFR mutations. NSCLC patients often develop resistance to first-generation EGFR inhibitors due to secondary mutations like EGFR T790M. The development of third-generation inhibitors, such as osimertinib, specifically targets these mutations [133, 136]. While many tumors share common resistance mechanisms, such as issues with drug transporters or DNA damage repair (DDR), each cancer type has unique molecular targets. Furthermore, individual patients vary significantly, making a one-size-fits-all approach ineffective. Therefore, treatments should be tailored to the molecular characteristics of each patient’s tumor, maximizing therapeutic benefit and minimizing toxicity. As genomic technologies continue to advance, personalized medicine will likely play a key role in overcoming resistance to cancer treatments.
Future directions and emerging therapies
Chemotherapy resistance arises from a complex interplay of multiple mechanisms, not just a single factor. Future research should focus on understanding and addressing these mechanisms, which include drug efflux, DNA damage repair, apoptosis evasion, epigenetic changes, intracellular drug metabolism, and tumor stem cells. Investigating these pathways will deepen our understanding of chemotherapy resistance and guide the development of more effective treatments. Advances in genomics, proteomics, and metabolomics have greatly facilitated the identification of resistance mechanisms across different cancers. Targeted therapies designed to address specific resistance pathways and immunotherapies that activate the immune system to destroy tumors are offering new strategies to combat resistance.
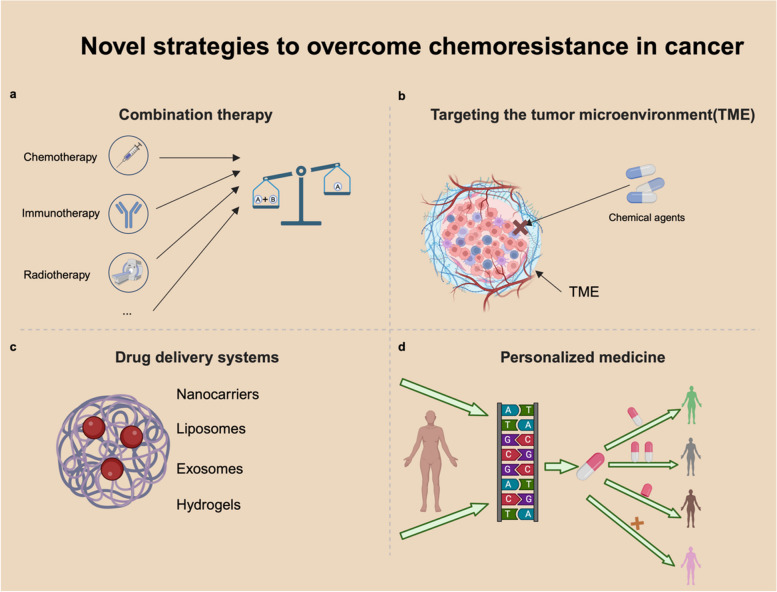
Combination therapies that integrate targeted treatments, immunotherapies, and traditional chemotherapy are showing great promise, as they exploit the synergistic effects between different modalities. Emerging therapies such as PDT, neural therapies, mRNA vaccines, and biologics are also rapidly progressing, expanding the arsenal against cancer. Going forward, cancer treatment will place greater emphasis on personalized medicine and optimizing drug delivery systems to enhance drug accumulation in tumors while reducing systemic toxicity. With a comprehensive understanding of chemotherapy resistance mechanisms, the field of oncology is poised to deliver more effective and tailored treatments, ultimately improving patient outcomes. (Fig. 5).
Fig. 5.
Novel strategies to overcome chemoresistance in cancer. a Combination therapy. Combining chemotherapy with other modalities improves therapeutic outcomes. b Targeting the tumor microenvironment (TME). The TME serves as a formidable barrier that chemotherapeutic agents must overcome to achieve therapeutic efficacy. c Drug delivery systems. Various carriers can enhance drug delivery, addressing issues of poor targeting and increased toxicity. d Personalized medicine. Analyzing genetic composition allows for the customization of therapies to achieve the greatest treatment benefit
Conclusion
Chemotherapy resistance remains a major challenge in cancer treatment. This review has explored the diverse molecular mechanisms underlying chemotherapy resistance, including DNA damage repair, apoptosis evasion, epigenetic modifications, intracellular drug metabolism, and the role of cancer stem cells. Each of these mechanisms contributes to the complexity of treatment resistance, creating significant obstacles for current therapeutic approaches. Additionally, we have examined resistance mechanisms in several major cancer types, highlighting key molecular targets.
Current efforts to overcome chemotherapy resistance focus on a range of strategies, including targeting multiple resistance pathways. Despite significant advancements, cancer continues to evolve, finding ways to evade the effects of chemotherapy. Future research should prioritize a deeper understanding of the molecular basis of resistance and leverage advanced technologies to identify novel therapeutic targets. Developing combination therapies that address multiple resistance mechanisms simultaneously will be essential. Moreover, improving drug delivery systems and adopting personalized medicine approaches will play a crucial role in reversing chemotherapy resistance. These strategies have the potential to offer patients more effective and tailored treatment options, ultimately improving outcomes in the battle against cancer.
Acknowledgements
The figures were created with BioRender.com.
Authors’ contributions
Conceptualization H.W. and L.Y.; methodology, Y.G. and R.Y.; data curation, Y.G. and Y.Z.; writing—original draft preparation Y.G.; writing—review and editing, H.W. and L.Y., K.T. and M.Z.; visualization, Y.G. and Y.Z and M.G.; supervision, H.W. and L.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Key R&D Program of China (2020YFA0210800) and National Natural Science Foundation of China (Grant No.82072668 and 2019XH004).
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yixiang Gu, Rui feng Yang and Yang Zhang contributed equally to this work.
Contributor Information
Linhua Yang, Email: yanglinhua1981@126.com.
Hui Wang, Email: wanghui801105@shsmu.edu.cn.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49. 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Laversanne M, Weiderpass E, Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127(16):3029–30. 10.1002/cncr.33587. [DOI] [PubMed] [Google Scholar]
- 3.Krell RW, Wei AC. Gallbladder cancer: surgical management. Chin Clin Oncol. 2019;8(4):36. 10.21037/cco.2019.06.06. [DOI] [PubMed] [Google Scholar]
- 4.Hickman L, Contreras C. Gallbladder Cancer: Diagnosis, Surgical Management, and Adjuvant Therapies. Surg Clin North Am. 2019;99(2):337–55. 10.1016/j.suc.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Vogel A, Bridgewater J, Edeline J, Kelley RK, Klümpen HJ, Malka D, et al. Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34(2):127–40. 10.1016/j.annonc.2022.10.506. [DOI] [PubMed] [Google Scholar]
- 6.Luo D, Yang Y, Zhang R, Li Q, Li X. Effect of interval between neoadjuvant chemoradiotherapy and surgery on oncological outcomes in poor responders with locally advanced rectal cancer: a retrospective cohort study. Int J Surg. 2023;109(7):1993–2000. 10.1097/js9.0000000000000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhuang Z, Yilihamu A, Li Z, Lei R, Li X, Han M et al. The Impact of Adjuvant Chemotherapy on the Long-Term Prognosis of Breast Malignant Phyllodes Tumors: A Propensity Score-Matched Study. J Natl Compr Canc Netw. 2024:1–7. 10.6004/jnccn.2024.7023. [DOI] [PubMed]
- 8.Noronha V, Patil VM, Menon N, Goud S, Singh A, Shah M, et al. Phase III randomized trial comparing palliative systemic therapy to best supportive care in advanced esophageal/GEJ cancer. Int J Cancer. 2024. 10.1002/ijc.35111. [DOI] [PubMed] [Google Scholar]
- 9.Yuan X, Guan D, Chen C, Guo S, Wu H, Bu H, et al. Development of an Imidazopyridazine-Based MNK1/2 Inhibitor for the Treatment of Lymphoma. J Med Chem. 2024;67(7):5437–57. 10.1021/acs.jmedchem.3c02008. [DOI] [PubMed] [Google Scholar]
- 10.Ramos A, Sadeghi S, Tabatabaeian H. Battling Chemoresistance in Cancer: Root Causes and Strategies to Uproot Them. Int J Mol Sci. 2021;22(17). 10.3390/ijms22179451. [DOI] [PMC free article] [PubMed]
- 11.Lippert TH, Ruoff HJ, Volm M. Intrinsic and acquired drug resistance in malignant tumors. The main reason for therapeutic failure. Arzneimittelforschung. 2008;58(6):261–4. 10.1055/s-0031-1296504. [DOI] [PubMed]
- 12.Zheng HC. The molecular mechanisms of chemoresistance in cancers. Oncotarget. 2017;8(35):59950–64. 10.18632/oncotarget.19048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan ST, Li ZL, He ZX, Qiu JX, Zhou SF. Molecular mechanisms for tumour resistance to chemotherapy. Clin Exp Pharmacol Physiol. 2016;43(8):723–37. 10.1111/1440-1681.12581. [DOI] [PubMed] [Google Scholar]
- 14.Liu X. ABC Family Transporters. Adv Exp Med Biol. 2019;1141:13–100. 10.1007/978-981-13-7647-4_2. [DOI] [PubMed] [Google Scholar]
- 15.Lusvarghi S, Robey RW, Gottesman MM, Ambudkar SV. Multidrug transporters: recent insights from cryo-electron microscopy-derived atomic structures and animal models. F1000Res. 2020;9. 10.12688/f1000research.21295.1. [DOI] [PMC free article] [PubMed]
- 16.Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, Gottesman MM. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer. 2018;18(7):452–64. 10.1038/s41568-018-0005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morau MV, Seguin CS, Visacri MB, Pincinato EC, Moriel P. Genetic Variants in the ABCB1 and ABCG2 Gene Drug Transporters Involved in Gefitinib-Associated Adverse Reaction: A Systematic Review and Meta-Analysis. Genes (Basel). 2024;15(5). 10.3390/genes15050591. [DOI] [PMC free article] [PubMed]
- 18.Li Q, Mou LJ, Tao L, Chen W, Sun XT, Xia XF, et al. Inhibition of mTOR suppresses human gallbladder carcinoma cell proliferation and enhances the cytotoxicity of 5-fluorouracil by downregulating MDR1 expression. Eur Rev Med Pharmacol Sci. 2016;20(9):1699–706. [PubMed] [Google Scholar]
- 19.Wang H, Zhan M, Xu SW, Chen W, Long MM, Shi YH, et al. miR-218-5p restores sensitivity to gemcitabine through PRKCE/MDR1 axis in gallbladder cancer. Cell Death Dis. 2017;8(5): e2770. 10.1038/cddis.2017.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisa A, Hanafy SM, Khalil H, Elshal MF. Sitagliptin synergizes 5-fluorouracil efficacy in colon cancer cells through MDR1-mediated flux impairment and down regulation of NFκB2 and p-AKT survival proteins. J Biochem Mol Toxicol. 2024;38(8): e23796. 10.1002/jbt.23796. [DOI] [PubMed] [Google Scholar]
- 21.Yang Z, Chen F, Wei D, Chen F, Jiang H, Qin S. EGR1 mediates MDR1 transcriptional activity regulating gemcitabine resistance in pancreatic cancer. BMC Cancer. 2024;24(1):268. 10.1186/s12885-024-12005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhan M, Wang H, Chen T, Chen W, Yang L, He M, et al. NOX1 mediates chemoresistance via HIF1α/MDR1 pathway in gallbladder cancer. Biochem Biophys Res Commun. 2015;468(1–2):79–85. 10.1016/j.bbrc.2015.10.161. [DOI] [PubMed] [Google Scholar]
- 23.Dong Q, Zhou C, Ren H, Zhang Z, Cheng F, Xiong Z, et al. Lactate-induced MRP1 expression contributes to metabolism-based etoposide resistance in non-small cell lung cancer cells. Cell Commun Signal. 2020;18(1):167. 10.1186/s12964-020-00653-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X, Gu G, Song L, Wang D, Xu Y, Yang S, et al. ID4 Promotes Breast Cancer Chemotherapy Resistance via CBF1-MRP1 Pathway. J Cancer. 2020;11(13):3846–57. 10.7150/jca.31988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhan M, Zhao X, Wang H, Chen W, Xu S, Wang W, et al. miR-145 sensitizes gallbladder cancer to cisplatin by regulating multidrug resistance associated protein 1. Tumour Biol. 2016;37(8):10553–62. 10.1007/s13277-016-4957-6. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Li X, Chen T, Wang W, Liu Q, Li H, et al. Mechanisms of verapamil-enhanced chemosensitivity of gallbladder cancer cells to platinum drugs: glutathione reduction and MRP1 downregulation. Oncol Rep. 2013;29(2):676–84. 10.3892/or.2012.2156. [DOI] [PubMed] [Google Scholar]
- 27.Pote MS, Gacche RN. ATP-binding cassette efflux transporters and MDR in cancer. Drug Discov Today. 2023;28(5): 103537. 10.1016/j.drudis.2023.103537. [DOI] [PubMed] [Google Scholar]
- 28.Feng M, Fan X, Shi J, Shan S, Li S, He S, et al. Terpenoids from quinoa reverse drug resistance of colon cancer by upregulating miR-495-3p. J Sci Food Agric. 2024. 10.1002/jsfa.13718. [DOI] [PubMed] [Google Scholar]
- 29.Zhao CC, Sun X, Chen J, Geng BD. NAT10-mediated mRNA N4-acetylcytidine modification of MDR1 and BCRP promotes breast cancer progression. Thorac Cancer. 2024;15(10):820–9. 10.1111/1759-7714.15262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uceda-Castro R, Margarido AS, Song JY, de Gooijer MC, Messal HA, Chambers CR, et al. BCRP drives intrinsic chemoresistance in chemotherapy-naïve breast cancer brain metastasis. Sci Adv. 2023;9(42):eabp9530. 10.1126/sciadv.abp9530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalayda GV, Wagner CH, Buss I, Reedijk J, Jaehde U. Altered localisation of the copper efflux transporters ATP7A and ATP7B associated with cisplatin resistance in human ovarian carcinoma cells. BMC Cancer. 2008;8:175. 10.1186/1471-2407-8-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao F, Li Y, Wan Y, Xue M. MircroRNA-139 sensitizes ovarian cancer cell to cisplatin-based chemotherapy through regulation of ATP7A/B. Cancer Chemother Pharmacol. 2018;81(5):935–47. 10.1007/s00280-018-3548-1. [DOI] [PubMed] [Google Scholar]
- 33.Federica G, Michela C, Giovanna D. Targeting the DNA damage response in cancer. MedComm. 2024;5(11):e788. 10.1002/mco2.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeppesen DK, Bohr VA, Stevnsner T. DNA repair deficiency in neurodegeneration. Prog Neurobiol. 2011;94(2):166–200. 10.1016/j.pneurobio.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vickridge E, Faraco CCF, Nepveu A. Base excision repair accessory factors in senescence avoidance and resistance to treatments. Cancer Drug Resist. 2022;5(3):703–20. 10.20517/cdr.2022.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen X, Li WF, Wu X, Zhang HC, Chen L, Zhang PY, et al. Dicer regulates non-homologous end joining and is associated with chemosensitivity in colon cancer patients. Carcinogenesis. 2017;38(9):873–82. 10.1093/carcin/bgx059. [DOI] [PubMed] [Google Scholar]
- 37.Wei S, Han C, Mo S, Huang H, Luo X. Advancements in programmed cell death research in antitumor therapy: a comprehensive overview. Apoptosis. 2024. 10.1007/s10495-024-02038-0. [DOI] [PubMed] [Google Scholar]
- 38.Fang J, Ye Z, Gu F, Yan M, Lin Q, Lin J, et al. DUSP1 enhances the chemoresistance of gallbladder cancer via the modulation of the p38 pathway and DNA damage/repair system. Oncol Lett. 2018;16(2):1869–75. 10.3892/ol.2018.8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto K, Iwadate D, Naito E, Tateishi K, Fujishiro M. Autophagy as a critical driver of metabolic adaptation, therapeutic resistance, and immune evasion of cancer. Curr Opin Biotechnol. 2023;84: 103012. 10.1016/j.copbio.2023.103012. [DOI] [PubMed] [Google Scholar]
- 41.Pimentel JM, Zhou JY, Wu GS. Autophagy and cancer therapy. Cancer Lett. 2024;605: 217285. 10.1016/j.canlet.2024.217285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu Y, Yu H, Qin H, Kang J, Yu C, Zhong J, et al. Inhibition of autophagy enhances cisplatin cytotoxicity through endoplasmic reticulum stress in human cervical cancer cells. Cancer Lett. 2012;314(2):232–43. 10.1016/j.canlet.2011.09.034. [DOI] [PubMed] [Google Scholar]
- 43.Yang B, Li Y, Zhang R, Liu L, Miao H, Li Y, et al. MOB1A regulates glucose deprivation-induced autophagy via IL6-STAT3 pathway in gallbladder carcinoma. Am J Cancer Res. 2020;10(11):3896–910. [PMC free article] [PubMed] [Google Scholar]
- 44.Wang S, Feng W, Wang W, Ye X, Chen H, Yu C. Girdin Knockdown Increases Gemcitabine Chemosensitivity to Pancreatic Cancer by Modulating Autophagy. Front Oncol. 2021;11: 618764. 10.3389/fonc.2021.618764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang FT, Wang H, Wang QW, Pan MS, Li XP, Sun W, et al. Inhibition of autophagy by chloroquine enhances the antitumor activity of gemcitabine for gallbladder cancer. Cancer Chemother Pharmacol. 2020;86(2):221–32. 10.1007/s00280-020-04100-5. [DOI] [PubMed] [Google Scholar]
- 46.Chen N, Qi Y, Ma X, Xiao X, Liu Q, Xia T, et al. Rediscovery of Traditional Plant Medicine: An Underestimated Anticancer Drug of Chelerythrine. Front Pharmacol. 2022;13: 906301. 10.3389/fphar.2022.906301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaur S, Mendonca P, Soliman KFA. The Anticancer Effects and Therapeutic Potential of Kaempferol in Triple-Negative Breast Cancer. Nutrients. 2024;16(15). 10.3390/nu16152392. [DOI] [PMC free article] [PubMed]
- 48.Urrutia S, Takahashi K. Precision medicine in AML: overcoming resistance. Int J Hematol. 2024. 10.1007/s12185-024-03827-8. [DOI] [PubMed] [Google Scholar]
- 49.Yuan J, Lan H, Jiang X, Zeng D, Xiao S. Bcl-2 family: Novel insight into individualized therapy for ovarian cancer (Review). Int J Mol Med. 2020;46(4):1255–65. 10.3892/ijmm.2020.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang J, Wang Y, Yin C, Gong P, Zhang Z, Zhao L, et al. Artesunate improves venetoclax plus cytarabine AML cell targeting by regulating the Noxa/Bim/Mcl-1/p-Chk1 axis. Cell Death Dis. 2022;13(4):379. 10.1038/s41419-022-04810-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miquel C, Borrini F, Grandjouan S, Aupérin A, Viguier J, Velasco V, et al. Role of bax mutations in apoptosis in colorectal cancers with microsatellite instability. Am J Clin Pathol. 2005;123(4):562–70. 10.1309/jq2x-3rv3-l8f9-tgyw. [DOI] [PubMed] [Google Scholar]
- 52.Carter JL, Su Y, Qiao X, Zhao J, Wang G, Howard M, et al. Acquired resistance to venetoclax plus azacitidine in acute myeloid leukemia: In vitro models and mechanisms. Biochem Pharmacol. 2023;216: 115759. 10.1016/j.bcp.2023.115759. [DOI] [PubMed] [Google Scholar]
- 53.Carter BZ, Mak PY, Tao W, Zhang Q, Ruvolo V, Kuruvilla VM, et al. Maximal Activation of Apoptosis Signaling by Cotargeting Antiapoptotic Proteins in BH3 Mimetic-Resistant AML and AML Stem Cells. Mol Cancer Ther. 2022;21(6):879–89. 10.1158/1535-7163.Mct-21-0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oakes SA, Scorrano L, Opferman JT, Bassik MC, Nishino M, Pozzan T, et al. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2005;102(1):105–10. 10.1073/pnas.0408352102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cetraro P, Plaza-Diaz J, MacKenzie A, Abadía-Molina F. A Review of the Current Impact of Inhibitors of Apoptosis Proteins and Their Repression in Cancer. Cancers (Basel). 2022;14(7). 10.3390/cancers14071671. [DOI] [PMC free article] [PubMed]
- 56.Rathore R, McCallum JE, Varghese E, Florea AM, Büsselberg D. Overcoming chemotherapy drug resistance by targeting inhibitors of apoptosis proteins (IAPs). Apoptosis. 2017;22(7):898–919. 10.1007/s10495-017-1375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guo Q, Jin Y, Lin M, Zeng C, Zhang J. NF-κB signaling in therapy resistance of breast cancer: Mechanisms, approaches, and challenges. Life Sci. 2024;348: 122684. 10.1016/j.lfs.2024.122684. [DOI] [PubMed] [Google Scholar]
- 58.Yang MH, Lee KT, Yang S, Lee JK, Lee KH, Moon IH, et al. Guggulsterone enhances antitumor activity of gemcitabine in gallbladder cancer cells through suppression of NF-κB. J Cancer Res Clin Oncol. 2012;138(10):1743–51. 10.1007/s00432-012-1254-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nwabo Kamdje AH, Dongmo Fogang HP, Mimche PN. Role of epigenetic in cancer biology, in hematologic malignancies and in anticancer therapy. Front Mol Med. 2024;4:1426454. 10.3389/fmmed.2024.1426454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Panda R, Mohan S, Vellapandian C. Harnessing Epigenetic Mechanisms to Overcome Immune Evasion in Cancer: The Current Strategies and Future Directions. Cureus. 2024;16(10): e70631. 10.7759/cureus.70631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Urban T, Pokorná P, Slabý O. Significance of aberrant DNA methylation for cancer diagnostics and therapy. Klin Onkol. 2024;38(2):88–94. 10.48095/ccko202488. [DOI] [PubMed] [Google Scholar]
- 62.Yang D, Khan S, Sun Y, Hess K, Shmulevich I, Sood AK, et al. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA. 2011;306(14):1557–65. 10.1001/jama.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kang SH, Bang YJ, Im YH, Yang HK, Lee DA, Lee HY, et al. Transcriptional repression of the transforming growth factor-beta type I receptor gene by DNA methylation results in the development of TGF-beta resistance in human gastric cancer. Oncogene. 1999;18(51):7280–6. 10.1038/sj.onc.1203146. [DOI] [PubMed] [Google Scholar]
- 64.Zhang X, Yashiro M, Ohira M, Ren J, Hirakawa K. Synergic antiproliferative effect of DNA methyltransferase inhibitor in combination with anticancer drugs in gastric carcinoma. Cancer Sci. 2006;97(9):938–44. 10.1111/j.1349-7006.2006.00253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stefansson OA, Esteller M. Epigenetic modifications in breast cancer and their role in personalized medicine. Am J Pathol. 2013;183(4):1052–63. 10.1016/j.ajpath.2013.04.033. [DOI] [PubMed] [Google Scholar]
- 66.Skouras P, Markouli M, Papadatou I, Piperi C. Targeting epigenetic mechanisms of resistance to chemotherapy in gliomas. Crit Rev Oncol Hematol. 2024;204: 104532. 10.1016/j.critrevonc.2024.104532. [DOI] [PubMed] [Google Scholar]
- 67.Reyes ME, Pulgar V, Vivallo C, Ili CG, Mora-Lagos B, Brebi P. Epigenetic modulation of cytokine expression in gastric cancer: influence on angiogenesis, metastasis and chemoresistance. Front Immunol. 2024;15:1347530. 10.3389/fimmu.2024.1347530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seto E, Yoshida M. Erasers of histone acetylation: the histone deacetylase enzymes. Cold Spring Harb Perspect Biol. 2014;6(4): a018713. 10.1101/cshperspect.a018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ono H, Basson MD, Ito H. P300 inhibition enhances gemcitabine-induced apoptosis of pancreatic cancer. Oncotarget. 2016;7(32):51301–10. 10.18632/oncotarget.10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Emmons MF, Faião-Flores F, Sharma R, Thapa R, Messina JL, Becker JC, et al. HDAC8 Regulates a Stress Response Pathway in Melanoma to Mediate Escape from BRAF Inhibitor Therapy. Cancer Res. 2019;79(11):2947–61. 10.1158/0008-5472.Can-19-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim MG, Pak JH, Choi WH, Park JY, Nam JH, Kim JH. The relationship between cisplatin resistance and histone deacetylase isoform overexpression in epithelial ovarian cancer cell lines. J Gynecol Oncol. 2012;23(3):182–9. 10.3802/jgo.2012.23.3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vagapova E, Kozlov M, Lebedev T, Ivanenko K, Leonova O, Popenko V et al. Selective Inhibition of HDAC Class I Sensitizes Leukemia and Neuroblastoma Cells to Anticancer Drugs. Biomedicines. 2021;9(12). 10.3390/biomedicines9121846. [DOI] [PMC free article] [PubMed]
- 73.Hu S, Yu L, Li Z, Shen Y, Wang J, Cai J, et al. Overexpression of EZH2 contributes to acquired cisplatin resistance in ovarian cancer cells in vitro and in vivo. Cancer Biol Ther. 2010;10(8):788–95. 10.4161/cbt.10.8.12913. [DOI] [PubMed] [Google Scholar]
- 74.Zhang L, Zhang X, Shi Y, Ni Y, Fei J, Jin Z, et al. Role and potential therapeutic value of histone methyltransferases in drug resistance mechanisms in lung cancer. Front Oncol. 2024;14:1376916. 10.3389/fonc.2024.1376916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yan H, Bu P. Non-coding RNA in cancer. Essays Biochem. 2021;65(4):625–39. 10.1042/ebc20200032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rahdan F, Saberi A, Saraygord-Afshari N, Hadizadeh M, Fayeghi T, Ghanbari E, et al. Deciphering the multifaceted role of microRNAs in hepatocellular carcinoma: Integrating literature review and bioinformatics analysis for therapeutic insights. Heliyon. 2024;10(20): e39489. 10.1016/j.heliyon.2024.e39489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Long M, Zhan M, Xu S, Yang R, Chen W, Zhang S, et al. miR-92b-3p acts as a tumor suppressor by targeting Gabra3 in pancreatic cancer. Mol Cancer. 2017;16(1):167. 10.1186/s12943-017-0723-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shahin RK, Elkady MA, Abulsoud AI, Abdelmaksoud NM, Abdel Mageed SS, El-Dakroury WA, et al. miRNAs orchestration of gallbladder cancer - Particular emphasis on diagnosis, progression and drug resistance. Pathol Res Pract. 2023;248: 154684. 10.1016/j.prp.2023.154684. [DOI] [PubMed] [Google Scholar]
- 79.Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet. 2016;17(1):47–62. 10.1038/nrg.2015.10. [DOI] [PubMed] [Google Scholar]
- 80.Chen LL. Linking Long Noncoding RNA Localization and Function. Trends Biochem Sci. 2016;41(9):761–72. 10.1016/j.tibs.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 81.Cai Q, Wang S, Jin L, Weng M, Zhou D, Wang J, et al. Long non-coding RNA GBCDRlnc1 induces chemoresistance of gallbladder cancer cells by activating autophagy. Mol Cancer. 2019;18(1):82. 10.1186/s12943-019-1016-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gao K, Chen S, Yang X. HOTTIP Enhances Gemcitabine and Cisplatin Resistance Through Sponging miR-637 in Cholangiocarcinoma. Front Oncol. 2021;11: 664916. 10.3389/fonc.2021.664916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang D, Zhan M, Chen T, Chen W, Zhang Y, Xu S, et al. miR-125b-5p enhances chemotherapy sensitivity to cisplatin by down-regulating Bcl2 in gallbladder cancer. Sci Rep. 2017;7:43109. 10.1038/srep43109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li M, Chen W, Zhang H, Zhang Y, Ke F, Wu X, et al. MiR-31 regulates the cisplatin resistance by targeting Src in gallbladder cancer. Oncotarget. 2016;7(50):83060–70. 10.18632/oncotarget.13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang GF, Wu JC, Wang HY, Jiang WD, Qiu L. Overexpression of microRNA-205-5p exerts suppressive effects on stem cell drug resistance in gallbladder cancer by down-regulating PRKCE. Biosci Rep. 2020;40(9). 10.1042/bsr20194509. [DOI] [PMC free article] [PubMed]
- 86.Yang G, Xu Q, Wan Y, Zhang L, Wang Z, Meng F. miR-193a-3p Enhanced the Chemosensitivity to Trametinib in Gallbladder Carcinoma by Targeting KRAS and Downregulating ERK Signaling. Cancer Biother Radiopharm. 2023;38(6):371–9. 10.1089/cbr.2021.0016. [DOI] [PubMed] [Google Scholar]
- 87.Yu J, Zhang W, Lu B, Qian H, Tang H, Zhu Z, et al. miR-433 accelerates acquired chemoresistance of gallbladder cancer cells by targeting cyclin M. Oncol Lett. 2018;15(3):3305–12. 10.3892/ol.2017.7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lu W, Hu Y, Ma Q, Zhou L, Jiang L, Li Z, et al. miR-223 increases gallbladder cancer cell sensitivity to docetaxel by downregulating STMN1. Oncotarget. 2016;7(38):62364–76. 10.18632/oncotarget.11634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li Y, Tian M, Zhang D, Zhuang Y, Li Z, Xie S, et al. Long Non-Coding RNA Myosin Light Chain Kinase Antisense 1 Plays an Oncogenic Role in Gallbladder Carcinoma by Promoting Chemoresistance and Proliferation. Cancer Manag Res. 2021;13:6219–30. 10.2147/cmar.S323759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kaur G, Gupta SK, Singh P, Ali V, Kumar V, Verma M. Drug-metabolizing enzymes: role in drug resistance in cancer. Clin Transl Oncol. 2020;22(10):1667–80. 10.1007/s12094-020-02325-7. [DOI] [PubMed] [Google Scholar]
- 91.Tabur S, Oztuzcu S, Oguz E, Demiryürek S, Dagli H, Alasehirli B, et al. CYP gene expressions in obesity-associated metabolic syndrome. Obes Res Clin Pract. 2016;10(6):719–23. 10.1016/j.orcp.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 92.Valencia-Cervantes J, Huerta-Yepez S, Aquino-Jarquín G, Rodríguez-Enríquez S, Martínez-Fong D, Arias-Montaño JA, et al. Hypoxia increases chemoresistance in human medulloblastoma DAOY cells via hypoxia-inducible factor 1α-mediated downregulation of the CYP2B6, CYP3A4 and CYP3A5 enzymes and inhibition of cell proliferation. Oncol Rep. 2019;41(1):178–90. 10.3892/or.2018.6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Torres-Zárate C, Vences-Mejía A, Espinosa-Aguirre JJ, Díaz-Díaz E, Palacios-Acosta JM, Cárdenas-Cardós R, et al. Expression of Cytochrome P450 Enzymes in Pediatric Non-Rhabdomyosarcoma Soft Tissue Sarcomas: Possible Role in Carcinogenesis and Treatment Response. Int J Toxicol. 2022;41(3):234–42. 10.1177/10915818221085909. [DOI] [PubMed] [Google Scholar]
- 94.Marengo B, Pulliero A, Izzotti A, Domenicotti C. miRNA Regulation of Glutathione Homeostasis in Cancer Initiation. Progression and Therapy Resistance Microrna. 2020;9(3):187–97. 10.2174/2211536609666191218103220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Niu B, Liao K, Zhou Y, Wen T, Quan G, Pan X, et al. Application of glutathione depletion in cancer therapy: Enhanced ROS-based therapy, ferroptosis, and chemotherapy. Biomaterials. 2021;277: 121110. 10.1016/j.biomaterials.2021.121110. [DOI] [PubMed] [Google Scholar]
- 96.Zhang T, Yao C, Zhou X, Liu S, Qi L, Zhu S et al. Glutathione‑degrading enzymes in the complex landscape of tumors (Review). Int J Oncol. 2024;65(1). 10.3892/ijo.2024.5660. [DOI] [PMC free article] [PubMed]
- 97.Ueno H, Kiyosawa K, Kaniwa N. Pharmacogenomics of gemcitabine: can genetic studies lead to tailor-made therapy? Br J Cancer. 2007;97(2):145–51. 10.1038/sj.bjc.6603860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nakano T, Saiki Y, Kudo C, Hirayama A, Mizuguchi Y, Fujiwara S, et al. Acquisition of chemoresistance to gemcitabine is induced by a loss-of-function missense mutation of DCK. Biochem Biophys Res Commun. 2015;464(4):1084–9. 10.1016/j.bbrc.2015.07.080. [DOI] [PubMed] [Google Scholar]
- 99.Lu SC. Regulation of hepatic glutathione synthesis: current concepts and controversies. Faseb j. 1999;13(10):1169–83. [PubMed] [Google Scholar]
- 100.Aponte PM, Caicedo A. Stemness in Cancer: Stem Cells, Cancer Stem Cells, and Their Microenvironment. Stem Cells Int. 2017;2017:5619472. 10.1155/2017/5619472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Batlle E, Clevers H. Cancer stem cells revisited. Nat Med. 2017;23(10):1124–34. 10.1038/nm.4409. [DOI] [PubMed] [Google Scholar]
- 102.Gires O. Lessons from common markers of tumor-initiating cells in solid cancers. Cell Mol Life Sci. 2011;68(24):4009–22. 10.1007/s00018-011-0772-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Najafi M, Mortezaee K, Majidpoor J. Cancer stem cell (CSC) resistance drivers. Life Sci. 2019;234: 116781. 10.1016/j.lfs.2019.116781. [DOI] [PubMed] [Google Scholar]
- 104.Shi C, Tian R, Wang M, Wang X, Jiang J, Zhang Z, et al. CD44+ CD133+ population exhibits cancer stem cell-like characteristics in human gallbladder carcinoma. Cancer Biol Ther. 2010;10(11):1182–90. 10.4161/cbt.10.11.13664. [DOI] [PubMed] [Google Scholar]
- 105.Desai A, Webb B, Gerson SL. CD133+ cells contribute to radioresistance via altered regulation of DNA repair genes in human lung cancer cells. Radiother Oncol. 2014;110(3):538–45. 10.1016/j.radonc.2013.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu Z, Miao X, Zhang Y, Li D, Zou Q, Yuan Y, et al. XRCC1 Is a Promising Predictive Biomarker and Facilitates Chemo-Resistance in Gallbladder Cancer. Front Mol Biosci. 2020;7:70. 10.3389/fmolb.2020.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang Y, Qi H, Liu Y, Duan C, Liu X, Xia T, et al. The double-edged roles of ROS in cancer prevention and therapy. Theranostics. 2021;11(10):4839–57. 10.7150/thno.56747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Donadelli M, Dando I, Zaniboni T, Costanzo C, Dalla Pozza E, Scupoli MT, et al. Gemcitabine/cannabinoid combination triggers autophagy in pancreatic cancer cells through a ROS-mediated mechanism. Cell Death Dis. 2011;2(4): e152. 10.1038/cddis.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shibata T, Kokubu A, Gotoh M, Ojima H, Ohta T, Yamamoto M, et al. Genetic alteration of Keap1 confers constitutive Nrf2 activation and resistance to chemotherapy in gallbladder cancer. Gastroenterology. 2008;135(4):1358–68, 68.e1-4. 10.1053/j.gastro.2008.06.082. [DOI] [PubMed] [Google Scholar]
- 110.Sosa V, Moliné T, Somoza R, Paciucci R, Kondoh H, ME LL. Oxidative stress and cancer: an overview. Ageing Res Rev. 2013;12(1):376–90. 10.1016/j.arr.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 111.Sosa MS, Bragado P, Aguirre-Ghiso JA. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer. 2014;14(9):611–22. 10.1038/nrc3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.De Angelis ML, Francescangeli F, La Torre F, Zeuner A. Stem Cell Plasticity and Dormancy in the Development of Cancer Therapy Resistance. Front Oncol. 2019;9:626. 10.3389/fonc.2019.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vassalli G. Aldehyde Dehydrogenases: Not Just Markers, but Functional Regulators of Stem Cells. Stem Cells Int. 2019;2019:3904645. 10.1155/2019/3904645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pors K, Moreb JS. Aldehyde dehydrogenases in cancer: an opportunity for biomarker and drug development? Drug Discov Today. 2014;19(12):1953–63. 10.1016/j.drudis.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 115.Ma S, Lee TK, Zheng BJ, Chan KW, Guan XY. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene. 2008;27(12):1749–58. 10.1038/sj.onc.1210811. [DOI] [PubMed] [Google Scholar]
- 116.Huang J, Deng Y, Tin MS, Lok V, Ngai CH, Zhang L, et al. Distribution, Risk Factors, and Temporal Trends for Lung Cancer Incidence and Mortality: A Global Analysis. Chest. 2022;161(4):1101–11. 10.1016/j.chest.2021.12.655. [DOI] [PubMed] [Google Scholar]
- 117.Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS. Lung cancer. Lancet. 2021;398(10299):535–54. 10.1016/s0140-6736(21)00312-3. [DOI] [PubMed] [Google Scholar]
- 118.Yu L, Yang R, Long Z, Tao Q, Liu B. Targeted therapy of non-small cell lung cancer: mechanisms and clinical trials. Front Oncol. 2024;14:1451230. 10.3389/fonc.2024.1451230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hardavella G, Magouliotis DE, Chalela R, Januszewski A, Dennstaedt F, Putora PM, et al. Stage I and II nonsmall cell lung cancer treatment options. Breathe (Sheff). 2024;20(2): 230219. 10.1183/20734735.0219-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sahoo D, Deb P, Basu T, Bardhan S, Patra S, Sukul PK. Advancements in platinum-based anticancer drug development: A comprehensive review of strategies, discoveries, and future perspectives. Bioorg Med Chem. 2024;112: 117894. 10.1016/j.bmc.2024.117894. [DOI] [PubMed] [Google Scholar]
- 121.Jiang P, Wu X, Wang X, Huang W, Feng Q. NEAT1 upregulates EGCG-induced CTR1 to enhance cisplatin sensitivity in lung cancer cells. Oncotarget. 2016;7(28):43337–51. 10.18632/oncotarget.9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim ES, Tang X, Peterson DR, Kilari D, Chow CW, Fujimoto J, et al. Copper transporter CTR1 expression and tissue platinum concentration in non-small cell lung cancer. Lung Cancer. 2014;85(1):88–93. 10.1016/j.lungcan.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sone K, Oguri T, Uemura T, Takeuchi A, Fukuda S, Takakuwa O, et al. Genetic variation in the ATP binding cassette transporter ABCC10 is associated with neutropenia for docetaxel in Japanese lung cancer patients cohort. BMC Cancer. 2019;19(1):246. 10.1186/s12885-019-5438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Paramasivan P, Kumar JD, Baskaran R, Weng CF, Padma VV. Reversal of doxorubicin resistance in lung cancer cells by neferine is explained by nuclear factor erythroid-derived 2-like 2 mediated lung resistance protein down regulation. Cancer Drug Resist. 2020;3(3):647–65. 10.20517/cdr.2019.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nakagawa T, Inoue Y, Kodama H, Yamazaki H, Kawai K, Suemizu H, et al. Expression of copper-transporting P-type adenosine triphosphatase (ATP7B) correlates with cisplatin resistance in human non-small cell lung cancer xenografts. Oncol Rep. 2008;20(2):265–70. [PubMed] [Google Scholar]
- 126.Zhang Y, Chen Y, Huang W, Zhou Y, Wang Y, Fu K, et al. NPAS2 dampens chemo-sensitivity of lung adenocarcinoma cells by enhancing DNA damage repair. Cell Death Dis. 2024;15(1):101. 10.1038/s41419-023-06256-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Weaver DA, Crawford EL, Warner KA, Elkhairi F, Khuder SA, Willey JC. ABCC5, ERCC2, XPA and XRCC1 transcript abundance levels correlate with cisplatin chemoresistance in non-small cell lung cancer cell lines. Mol Cancer. 2005;4(1):18. 10.1186/1476-4598-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shen W, Qiu Y, Li J, Wu C, Liu Z, Zhang X, et al. IL-25 promotes cisplatin resistance of lung cancer cells by activating NF-κB signaling pathway to increase of major vault protein. Cancer Med. 2019;8(7):3491–501. 10.1002/cam4.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Qiu T, Zhou L, Wang T, Xu J, Wang J, Chen W, et al. miR-503 regulates the resistance of non-small cell lung cancer cells to cisplatin by targeting Bcl-2. Int J Mol Med. 2013;32(3):593–8. 10.3892/ijmm.2013.1439. [DOI] [PubMed] [Google Scholar]
- 130.Michels J, Obrist F, Vitale I, Lissa D, Garcia P, Behnam-Motlagh P, et al. MCL-1 dependency of cisplatin-resistant cancer cells. Biochem Pharmacol. 2014;92(1):55–61. 10.1016/j.bcp.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 131.Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378(2):113–25. 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 132.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–57. 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 133.Chhouri H, Alexandre D, Grumolato L. Mechanisms of Acquired Resistance and Tolerance to EGFR Targeted Therapy in Non-Small Cell Lung Cancer. Cancers (Basel). 2023;15(2). 10.3390/cancers15020504. [DOI] [PMC free article] [PubMed]
- 134.Vasconcelos P, Gergis C, Viray H, Varkaris A, Fujii M, Rangachari D et al. EGFR-A763_Y764insFQEA Is a Unique Exon 20 Insertion Mutation That Displays Sensitivity to Approved and In-Development Lung Cancer EGFR Tyrosine Kinase Inhibitors. JTO Clin Res Rep. 2020;1(3). 10.1016/j.jtocrr.2020.100051. [DOI] [PMC free article] [PubMed]
- 135.Oxnard GR, Hu Y, Mileham KF, Husain H, Costa DB, Tracy P, et al. Assessment of Resistance Mechanisms and Clinical Implications in Patients With EGFR T790M-Positive Lung Cancer and Acquired Resistance to Osimertinib. JAMA Oncol. 2018;4(11):1527–34. 10.1001/jamaoncol.2018.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Thress KS, Paweletz CP, Felip E, Cho BC, Stetson D, Dougherty B, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med. 2015;21(6):560–2. 10.1038/nm.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Katakami N, Atagi S, Goto K, Hida T, Horai T, Inoue A, et al. LUX-Lung 4: a phase II trial of afatinib in patients with advanced non-small-cell lung cancer who progressed during prior treatment with erlotinib, gefitinib, or both. J Clin Oncol. 2013;31(27):3335–41. 10.1200/jco.2012.45.0981. [DOI] [PubMed] [Google Scholar]
- 138.Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–63. 10.3322/caac.21834. [DOI] [PubMed] [Google Scholar]
- 139.Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–52. 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 140.Yin L, Duan JJ, Bian XW, Yu SC. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020;22(1):61. 10.1186/s13058-020-01296-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98(19):10869–74. 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.AlFakeeh A, Brezden-Masley C. Overcoming endocrine resistance in hormone receptor-positive breast cancer. Curr Oncol. 2018;25(Suppl 1):S18–s27. 10.3747/co.25.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Brufsky AM, Dickler MN. Estrogen Receptor-Positive Breast Cancer: Exploiting Signaling Pathways Implicated in Endocrine Resistance. Oncologist. 2018;23(5):528–39. 10.1634/theoncologist.2017-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Belachew EB, Sewasew DT. Corrigendum: Molecular Mechanisms of Endocrine Resistance in Estrogen-Receptor-Positive Breast Cancer. Front Endocrinol (Lausanne). 2021;12: 689705. 10.3389/fendo.2021.689705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Willman M, Willman J, Lucke-Wold B. Endocrine resistant breast cancer: brain metastasis. Explor Target Antitumor Ther. 2022;3(2):240–51. 10.37349/etat.2022.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Toy W, Shen Y, Won H, Green B, Sakr RA, Will M, et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat Genet. 2013;45(12):1439–45. 10.1038/ng.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jeselsohn R, Yelensky R, Buchwalter G, Frampton G, Meric-Bernstam F, Gonzalez-Angulo AM, et al. Emergence of constitutively active estrogen receptor-α mutations in pretreated advanced estrogen receptor-positive breast cancer. Clin Cancer Res. 2014;20(7):1757–67. 10.1158/1078-0432.Ccr-13-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Katzenellenbogen JA, Mayne CG, Katzenellenbogen BS, Greene GL, Chandarlapaty S. Structural underpinnings of oestrogen receptor mutations in endocrine therapy resistance. Nat Rev Cancer. 2018;18(6):377–88. 10.1038/s41568-018-0001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jeselsohn R, Bergholz JS, Pun M, Cornwell M, Liu W, Nardone A, et al. Allele-Specific Chromatin Recruitment and Therapeutic Vulnerabilities of ESR1 Activating Mutations. Cancer Cell. 2018;33(2):173–86.e5. 10.1016/j.ccell.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ferrando L, Vingiani A, Garuti A, Vernieri C, Belfiore A, Agnelli L, et al. ESR1 gene amplification and MAP3K mutations are selected during adjuvant endocrine therapies in relapsing Hormone Receptor-positive, HER2-negative breast cancer (HR+ HER2- BC). PLoS Genet. 2023;19(1): e1010563. 10.1371/journal.pgen.1010563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lei JT, Shao J, Zhang J, Iglesia M, Chan DW, Cao J, et al. Functional Annotation of ESR1 Gene Fusions in Estrogen Receptor-Positive Breast Cancer. Cell Rep. 2018;24(6):1434–44.e7. 10.1016/j.celrep.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li H, Prever L, Hirsch E, Gulluni F. Targeting PI3K/AKT/mTOR Signaling Pathway in Breast Cancer. Cancers (Basel). 2021;13(14). 10.3390/cancers13143517. [DOI] [PMC free article] [PubMed]
- 153.Pearson A, Proszek P, Pascual J, Fribbens C, Shamsher MK, Kingston B, et al. Inactivating NF1 Mutations Are Enriched in Advanced Breast Cancer and Contribute to Endocrine Therapy Resistance. Clin Cancer Res. 2020;26(3):608–22. 10.1158/1078-0432.Ccr-18-4044. [DOI] [PubMed] [Google Scholar]
- 154.Sokol ES, Feng YX, Jin DX, Basudan A, Lee AV, Atkinson JM, et al. Loss of function of NF1 is a mechanism of acquired resistance to endocrine therapy in lobular breast cancer. Ann Oncol. 2019;30(1):115–23. 10.1093/annonc/mdy497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Servetto A, Formisano L, Arteaga CL. FGFR signaling and endocrine resistance in breast cancer: Challenges for the clinical development of FGFR inhibitors. Biochim Biophys Acta Rev Cancer. 2021;1876(2): 188595. 10.1016/j.bbcan.2021.188595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Álvarez-Fernández M, Malumbres M. Mechanisms of Sensitivity and Resistance to CDK4/6 Inhibition. Cancer Cell. 2020;37(4):514–29. 10.1016/j.ccell.2020.03.010. [DOI] [PubMed] [Google Scholar]
- 157.Watt AC, Goel S. Cellular mechanisms underlying response and resistance to CDK4/6 inhibitors in the treatment of hormone receptor-positive breast cancer. Breast Cancer Res. 2022;24(1):17. 10.1186/s13058-022-01510-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kim S, Armand J, Safonov A, Zhang M, Soni RK, Schwartz G, et al. Sequential activation of E2F via Rb degradation and c-Myc drives resistance to CDK4/6 inhibitors in breast cancer. Cell Rep. 2023;42(11): 113198. 10.1016/j.celrep.2023.113198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–82. 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 160.Derakhshani A, Rezaei Z, Safarpour H, Sabri M, Mir A, Sanati MA, et al. Overcoming trastuzumab resistance in HER2-positive breast cancer using combination therapy. J Cell Physiol. 2020;235(4):3142–56. 10.1002/jcp.29216. [DOI] [PubMed] [Google Scholar]
- 161.Cao Y, Li Y, Liu R, Zhou J, Wang K. Preclinical and Basic Research Strategies for Overcoming Resistance to Targeted Therapies in HER2-Positive Breast Cancer. Cancers (Basel). 2023;15(9). 10.3390/cancers15092568. [DOI] [PMC free article] [PubMed]
- 162.Zuo WJ, Jiang YZ, Wang YJ, Xu XE, Hu X, Liu GY, et al. Dual Characteristics of Novel HER2 Kinase Domain Mutations in Response to HER2-Targeted Therapies in Human Breast Cancer. Clin Cancer Res. 2016;22(19):4859–69. 10.1158/1078-0432.Ccr-15-3036. [DOI] [PubMed] [Google Scholar]
- 163.Kancha RK, von Bubnoff N, Bartosch N, Peschel C, Engh RA, Duyster J. Differential sensitivity of ERBB2 kinase domain mutations towards lapatinib. PLoS ONE. 2011;6(10): e26760. 10.1371/journal.pone.0026760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Abraham J, Montero AJ, Jankowitz RC, Salkeni MA, Beumer JH, Kiesel BF, et al. Safety and Efficacy of T-DM1 Plus Neratinib in Patients With Metastatic HER2-Positive Breast Cancer: NSABP Foundation Trial FB-10. J Clin Oncol. 2019;37(29):2601–9. 10.1200/jco.19.00858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shokrollahi Barough M, Jalili N, Shafiee S, Salehi M, Naseri N, Javidi MA, et al. Anti-MUC1 nanobody can synergize the Tamoxifen and Herceptin effects on breast cancer cells by inducing ER, PR and HER2 overexpression. Int Immunopharmacol. 2023;124(Pt A): 110792. 10.1016/j.intimp.2023.110792. [DOI] [PubMed] [Google Scholar]
- 166.Sanz-Álvarez M, Martín-Aparicio E, Luque M, Zazo S, Martínez-Useros J, Eroles P et al. The Novel Oral mTORC1/2 Inhibitor TAK-228 Reverses Trastuzumab Resistance in HER2-Positive Breast Cancer Models. Cancers (Basel). 2021;13(11). 10.3390/cancers13112778. [DOI] [PMC free article] [PubMed]
- 167.Autier P, Boniol M, La Vecchia C, Vatten L, Gavin A, Héry C, et al. Disparities in breast cancer mortality trends between 30 European countries: retrospective trend analysis of WHO mortality database. BMJ. 2010;341: c3620. 10.1136/bmj.c3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yao Y, Chu Y, Xu B, Hu Q, Song Q. Risk factors for distant metastasis of patients with primary triple-negative breast cancer. Biosci Rep. 2019;39(6). 10.1042/bsr20190288. [DOI] [PMC free article] [PubMed]
- 169.Arvandi S, Razmjoo S, Zaheri AP. Risk factors and survival of triple-negative breast cancer among breast cancer patients: Ten-year cross-sectional study in the southwestern Iranian population. Health Sci Rep. 2023;6(12): e1767. 10.1002/hsr2.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Birkbak NJ, Wang ZC, Kim JY, Eklund AC, Li Q, Tian R, et al. Telomeric allelic imbalance indicates defective DNA repair and sensitivity to DNA-damaging agents. Cancer Discov. 2012;2(4):366–75. 10.1158/2159-8290.Cd-11-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Naipal KA, Verkaik NS, Ameziane N, van Deurzen CH, Ter Brugge P, Meijers M, et al. Functional ex vivo assay to select homologous recombination-deficient breast tumors for PARP inhibitor treatment. Clin Cancer Res. 2014;20(18):4816–26. 10.1158/1078-0432.Ccr-14-0571. [DOI] [PubMed] [Google Scholar]
- 172.Tung NM, Boughey JC, Pierce LJ, Robson ME, Bedrosian I, Dietz JR, et al. Management of Hereditary Breast Cancer: American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Guideline. J Clin Oncol. 2020;38(18):2080–106. 10.1200/jco.20.00299. [DOI] [PubMed] [Google Scholar]
- 173.Shah SP, Roth A, Goya R, Oloumi A, Ha G, Zhao Y, et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature. 2012;486(7403):395–9. 10.1038/nature10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lehmann BD, Bauer JA, Schafer JM, Pendleton CS, Tang L, Johnson KC, et al. PIK3CA mutations in androgen receptor-positive triple negative breast cancer confer sensitivity to the combination of PI3K and androgen receptor inhibitors. Breast Cancer Res. 2014;16(4):406. 10.1186/s13058-014-0406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gerratana L, Basile D, Buono G, De Placido S, Giuliano M, Minichillo S, et al. Androgen receptor in triple negative breast cancer: A potential target for the targetless subtype. Cancer Treat Rev. 2018;68:102–10. 10.1016/j.ctrv.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 176.Pohl SG, Brook N, Agostino M, Arfuso F, Kumar AP, Dharmarajan A. Wnt signaling in triple-negative breast cancer. Oncogenesis. 2017;6(4): e310. 10.1038/oncsis.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Raith F, O'Donovan DH, Lemos C, Politz O, Haendler B. Addressing the Reciprocal Crosstalk between the AR and the PI3K/AKT/mTOR Signaling Pathways for Prostate Cancer Treatment. Int J Mol Sci. 2023;24(3). 10.3390/ijms24032289. [DOI] [PMC free article] [PubMed]
- 178.Buyuk B, Jin S, Ye K. Epithelial-to-Mesenchymal Transition Signaling Pathways Responsible for Breast Cancer Metastasis. Cell Mol Bioeng. 2022;15(1):1–13. 10.1007/s12195-021-00694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, et al. NCCN guidelines insights: colon cancer, version 2.2018. J Natl Compr Canc Netw. 2018;16(4):359–69. 10.6004/jnccn.2018.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351(4):337–45. 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 181.Lito P, Solomon M, Li LS, Hansen R, Rosen N. Allele-specific inhibitors inactivate mutant KRAS G12C by a trapping mechanism. Science. 2016;351(6273):604–8. 10.1126/science.aad6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Papke B, Azam SH, Feng AY, Gutierrez-Ford C, Huggins H, Pallan PS, et al. Silencing of Oncogenic KRAS by Mutant-Selective Small Interfering RNA. ACS Pharmacol Transl Sci. 2021;4(2):703–12. 10.1021/acsptsci.0c00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Awad MM, Liu S, Rybkin II, Arbour KC, Dilly J, Zhu VW, et al. Acquired Resistance to KRAS(G12C) Inhibition in Cancer. N Engl J Med. 2021;384(25):2382–93. 10.1056/NEJMoa2105281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Misale S, Yaeger R, Hobor S, Scala E, Janakiraman M, Liska D, et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature. 2012;486(7404):532–6. 10.1038/nature11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ryan MB, Fece de la Cruz F, Phat S, Myers DT, Wong E, Shahzade HA, et al. Vertical pathway inhibition overcomes adaptive feedback resistance to KRAS(G12C) inhibition. Clin Cancer Res. 2020;26(7):1633–43. 10.1158/1078-0432.Ccr-19-3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Xue JY, Zhao Y, Aronowitz J, Mai TT, Vides A, Qeriqi B, et al. Rapid non-uniform adaptation to conformation-specific KRAS(G12C) inhibition. Nature. 2020;577(7790):421–5. 10.1038/s41586-019-1884-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Canon J, Rex K, Saiki AY, Mohr C, Cooke K, Bagal D, et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019;575(7781):217–23. 10.1038/s41586-019-1694-1. [DOI] [PubMed] [Google Scholar]
- 188.Park YL, Kim HP, Ock CY, Min DW, Kang JK, Lim YJ, et al. EMT-mediated regulation of CXCL1/5 for resistance to anti-EGFR therapy in colorectal cancer. Oncogene. 2022;41(14):2026–38. 10.1038/s41388-021-01920-4. [DOI] [PubMed] [Google Scholar]
- 189.Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358(11):1160–74. 10.1056/NEJMra0707704. [DOI] [PubMed] [Google Scholar]
- 190.Van Cutsem E, Köhne CH, Láng I, Folprecht G, Nowacki MP, Cascinu S, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29(15):2011–9. 10.1200/jco.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- 191.Van Emburgh BO, Sartore-Bianchi A, Di Nicolantonio F, Siena S, Bardelli A. Acquired resistance to EGFR-targeted therapies in colorectal cancer. Mol Oncol. 2014;8(6):1084–94. 10.1016/j.molonc.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, et al. Wild-Type KRAS Is Required for Panitumumab Efficacy in Patients With Metastatic Colorectal Cancer. J Clin Oncol. 2023;41(18):3278–86. 10.1200/jco.22.02758. [DOI] [PubMed] [Google Scholar]
- 193.Therkildsen C, Bergmann TK, Henrichsen-Schnack T, Ladelund S, Nilbert M. The predictive value of KRAS, NRAS, BRAF, PIK3CA and PTEN for anti-EGFR treatment in metastatic colorectal cancer: A systematic review and meta-analysis. Acta Oncol. 2014;53(7):852–64. 10.3109/0284186x.2014.895036. [DOI] [PubMed] [Google Scholar]
- 194.Nusse R, Clevers H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell. 2017;169(6):985–99. 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 195.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149(6):1192–205. 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 196.Yang Y, Jiang H, Li W, Chen L, Zhu W, Xian Y, et al. FOXM1/DVL2/Snail axis drives metastasis and chemoresistance of colorectal cancer. Aging (Albany NY). 2020;12(23):24424–40. 10.18632/aging.202300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kobayashi S, Yamada-Okabe H, Suzuki M, Natori O, Kato A, Matsubara K, et al. LGR5-positive colon cancer stem cells interconvert with drug-resistant LGR5-negative cells and are capable of tumor reconstitution. Stem Cells. 2012;30(12):2631–44. 10.1002/stem.1257. [DOI] [PubMed] [Google Scholar]
- 198.Bergengren O, Pekala KR, Matsoukas K, Fainberg J, Mungovan SF, Bratt O, et al. 2022 Update on Prostate Cancer Epidemiology and Risk Factors-A Systematic Review. Eur Urol. 2023;84(2):191–206. 10.1016/j.eururo.2023.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wong YN, Ferraldeschi R, Attard G, de Bono J. Evolution of androgen receptor targeted therapy for advanced prostate cancer. Nat Rev Clin Oncol. 2014;11(6):365–76. 10.1038/nrclinonc.2014.72. [DOI] [PubMed] [Google Scholar]
- 200.Desai K, McManus JM, Sharifi N. Hormonal Therapy for Prostate Cancer. Endocr Rev. 2021;42(3):354–73. 10.1210/endrev/bnab002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Koivisto P, Kononen J, Palmberg C, Tammela T, Hyytinen E, Isola J, et al. Androgen receptor gene amplification: a possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res. 1997;57(2):314–9. [PubMed] [Google Scholar]
- 202.Powell SM, Christiaens V, Voulgaraki D, Waxman J, Claessens F, Bevan CL. Mechanisms of androgen receptor signalling via steroid receptor coactivator-1 in prostate. Endocr Relat Cancer. 2004;11(1):117–30. 10.1677/erc.0.0110117. [DOI] [PubMed] [Google Scholar]
- 203.Taplin ME, Bubley GJ, Shuster TD, Frantz ME, Spooner AE, Ogata GK, et al. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N Engl J Med. 1995;332(21):1393–8. 10.1056/nejm199505253322101. [DOI] [PubMed] [Google Scholar]
- 204.Tan J, Sharief Y, Hamil KG, Gregory CW, Zang DY, Sar M, et al. Dehydroepiandrosterone activates mutant androgen receptors expressed in the androgen-dependent human prostate cancer xenograft CWR22 and LNCaP cells. Mol Endocrinol. 1997;11(4):450–9. 10.1210/mend.11.4.9906. [DOI] [PubMed] [Google Scholar]
- 205.Watson PA, Arora VK, Sawyers CL. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat Rev Cancer. 2015;15(12):701–11. 10.1038/nrc4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Tien JC, Liu Z, Liao L, Wang F, Xu Y, Wu YL, et al. The steroid receptor coactivator-3 is required for the development of castration-resistant prostate cancer. Cancer Res. 2013;73(13):3997–4008. 10.1158/0008-5472.Can-12-3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Andrieu C, Taieb D, Baylot V, Ettinger S, Soubeyran P, De-Thonel A, et al. Heat shock protein 27 confers resistance to androgen ablation and chemotherapy in prostate cancer cells through eIF4E. Oncogene. 2010;29(13):1883–96. 10.1038/onc.2009.479. [DOI] [PubMed] [Google Scholar]
- 208.Le TK, Cherif C, Omabe K, Paris C, Lannes F, Audebert S, et al. DDX5 mRNA-targeting antisense oligonucleotide as a new promising therapeutic in combating castration-resistant prostate cancer. Mol Ther. 2023;31(2):471–86. 10.1016/j.ymthe.2022.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Aggarwal R, Huang J, Alumkal JJ, Zhang L, Feng FY, Thomas GV, et al. Clinical and Genomic Characterization of Treatment-Emergent Small-Cell Neuroendocrine Prostate Cancer: A Multi-institutional Prospective Study. J Clin Oncol. 2018;36(24):2492–503. 10.1200/jco.2017.77.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.The Molecular Taxonomy of Primary Prostate Cancer. Cell. 2015;163(4):1011–25. 10.1016/j.cell.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Beltran H, Prandi D, Mosquera JM, Benelli M, Puca L, Cyrta J, et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat Med. 2016;22(3):298–305. 10.1038/nm.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhang W, Liu B, Wu W, Li L, Broom BM, Basourakos SP, et al. Targeting the MYCN-PARP-DNA Damage Response Pathway in Neuroendocrine Prostate Cancer. Clin Cancer Res. 2018;24(3):696–707. 10.1158/1078-0432.Ccr-17-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zhao W, Lin ZX, Zhang ZQ. Cisplatin-induced premature senescence with concomitant reduction of gap junctions in human fibroblasts. Cell Res. 2004;14(1):60–6. 10.1038/sj.cr.7290203. [DOI] [PubMed] [Google Scholar]
- 214.Tucci M, Bertaglia V, Vignani F, Buttigliero C, Fiori C, Porpiglia F, et al. Addition of Docetaxel to Androgen Deprivation Therapy for Patients with Hormone-sensitive Metastatic Prostate Cancer: A Systematic Review and Meta-analysis. Eur Urol. 2016;69(4):563–73. 10.1016/j.eururo.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 215.Housman G, Byler S, Heerboth S, Lapinska K, Longacre M, Snyder N, et al. Drug resistance in cancer: an overview. Cancers (Basel). 2014;6(3):1769–92. 10.3390/cancers6031769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Whiteley AE, Price TT, Cantelli G, Sipkins DA. Leukaemia: a model metastatic disease. Nat Rev Cancer. 2021;21(7):461–75. 10.1038/s41568-021-00355-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kayser S, Levis MJ. Updates on targeted therapies for acute myeloid leukaemia. Br J Haematol. 2022;196(2):316–28. 10.1111/bjh.17746. [DOI] [PubMed] [Google Scholar]
- 218.Vago L, Gojo I. Immune escape and immunotherapy of acute myeloid leukemia. J Clin Invest. 2020;130(4):1552–64. 10.1172/jci129204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kennedy VE, Smith CC. FLT3 Mutations in Acute Myeloid Leukemia: Key Concepts and Emerging Controversies. Front Oncol. 2020;10: 612880. 10.3389/fonc.2020.612880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kiyoi H, Kawashima N, Ishikawa Y. FLT3 mutations in acute myeloid leukemia: Therapeutic paradigm beyond inhibitor development. Cancer Sci. 2020;111(2):312–22. 10.1111/cas.14274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wang Z, Cai J, Cheng J, Yang W, Zhu Y, Li H, et al. FLT3 Inhibitors in Acute Myeloid Leukemia: Challenges and Recent Developments in Overcoming Resistance. J Med Chem. 2021;64(6):2878–900. 10.1021/acs.jmedchem.0c01851. [DOI] [PubMed] [Google Scholar]
- 222.Wang J, Tomlinson B, Lazarus HM. Update on Small Molecule Targeted Therapies for Acute Myeloid Leukemia. Curr Treat Options Oncol. 2023;24(7):770–801. 10.1007/s11864-023-01090-3. [DOI] [PubMed] [Google Scholar]
- 223.Fujita K-i. Cytochrome P450 and Anticancer Drugs. Curr Drug Metab. 2006;7(1):23–37. 10.2174/138920006774832587. [DOI] [PubMed] [Google Scholar]
- 224.Dutreix C, Munarini F, Lorenzo S, Roesel J, Wang Y. Investigation into CYP3A4-mediated drug–drug interactions on midostaurin in healthy volunteers. Cancer Chemother Pharmacol. 2013;72(6):1223–34. 10.1007/s00280-013-2287-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Chang Y-T, Hernandez D, Alonso S, Gao M, Su M, Ghiaur G, et al. Role of CYP3A4 in bone marrow microenvironment–mediated protection of FLT3/ITD AML from tyrosine kinase inhibitors. Blood Adv. 2019;3(6):908–16. 10.1182/bloodadvances.2018022921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ghiaur G, Levis M. Mechanisms of Resistance to FLT3 Inhibitors and the Role of the Bone Marrow Microenvironment. Hematol Oncol Clin North Am. 2017;31(4):681–92. 10.1016/j.hoc.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Smith CC, Lin K, Stecula A, Sali A, Shah NP. FLT3 D835 mutations confer differential resistance to type II FLT3 inhibitors. Leukemia. 2015;29(12):2390–2. 10.1038/leu.2015.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.McMahon CM, Ferng T, Canaani J, Wang ES, Morrissette JJD, Eastburn DJ, et al. Clonal Selection with RAS Pathway Activation Mediates Secondary Clinical Resistance to Selective FLT3 Inhibition in Acute Myeloid Leukemia. Cancer Discov. 2019;9(8):1050–63. 10.1158/2159-8290.Cd-18-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Baykal-Köse S, Acikgoz E, Yavuz AS, Gönül Geyik Ö, Ateş H, Sezerman OU, et al. Adaptive phenotypic modulations lead to therapy resistance in chronic myeloid leukemia cells. PLoS ONE. 2020;15(2): e0229104. 10.1371/journal.pone.0229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2016 update on diagnosis, therapy, and monitoring. Am J Hematol. 2016;91(2):252–65. 10.1002/ajh.24275. [DOI] [PubMed] [Google Scholar]
- 231.Tyagi S, Singh A, Sharma N, Chaturvedi R, Kushwaha HR. Insights into existing and futuristic treatment approach for chronic myeloid leukaemia. Indian J Med Res. 2024;159(5):455–67. 10.25259/ijmr_1716_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ma J, Huang L, Han C. Expert consensus on the use of third-generation EGFR-TKIs in EGFR-mutated advanced non-small cell lung cancer with various T790M mutations post-resistance to first-/second-generation EGFR-TKIs. Ther Adv Med Oncol. 2024;16:17588359241289648. 10.1177/17588359241289648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Tang YJ, Chang JW, Chang CF, Huang CY, Yang CT, Kuo CS et al. Impact of T790M Mutation Status on Later-Line Osimertinib Treatment in Non-Small Cell Lung Cancer Patients. Cancers (Basel). 2022;14(20). 10.3390/cancers14205095. [DOI] [PMC free article] [PubMed]
- 234.Goodman LS, Wintrobe MM, et al. Nitrogen mustard therapy; use of methyl-bis (beta-chloroethyl) amine hydrochloride and tris (beta-chloroethyl) amine hydrochloride for Hodgkin’s disease, lymphosarcoma, leukemia and certain allied and miscellaneous disorders. J Am Med Assoc. 1946;132:126–32. 10.1001/jama.1946.02870380008004. [DOI] [PubMed] [Google Scholar]
- 235.Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, et al. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J Clin Oncol. 2015;33(17):1889–94. 10.1200/jco.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Viana Cabral F, Quilez Alburquerque J, Roberts HJ, Hasan T. Shedding Light on Chemoresistance: The Perspective of Photodynamic Therapy in Cancer Management. Int J Mol Sci. 2024;25(7). 10.3390/ijms25073811. [DOI] [PMC free article] [PubMed]
- 237.Baglan KL, Frazier RC, Yan D, Huang RR, Martinez AA, Robertson JM. The dose-volume relationship of acute small bowel toxicity from concurrent 5-FU-based chemotherapy and radiation therapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2002;52(1):176–83. 10.1016/s0360-3016(01)01820-x. [DOI] [PubMed] [Google Scholar]
- 238.Frei E 3rd, Holland JF, Schneiderman MA, Pinkel D, Selkirk G, Freireich EJ, et al. A comparative study of two regimens of combination chemotherapy in acute leukemia. Blood. 1958;13(12):1126–48. [PubMed] [Google Scholar]
- 239.Kopetz S, Desai J, Chan E, Hecht JR, O’Dwyer PJ, Maru D, et al. Phase II Pilot Study of Vemurafenib in Patients With Metastatic BRAF-Mutated Colorectal Cancer. J Clin Oncol. 2015;33(34):4032–8. 10.1200/jco.2015.63.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Corcoran RB, Ebi H, Turke AB, Coffee EM, Nishino M, Cogdill AP, et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2012;2(3):227–35. 10.1158/2159-8290.Cd-11-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wan S, Pestka S, Jubin RG, Lyu YL, Tsai YC, Liu LF. Chemotherapeutics and radiation stimulate MHC class I expression through elevated interferon-beta signaling in breast cancer cells. PLoS ONE. 2012;7(3): e32542. 10.1371/journal.pone.0032542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378(22):2078–92. 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 243.Offringa R, Kötzner L, Huck B, Urbahns K. The expanding role for small molecules in immuno-oncology. Nat Rev Drug Discov. 2022;21(11):821–40. 10.1038/s41573-022-00538-9. [DOI] [PubMed] [Google Scholar]
- 244.Ebert PJR, Cheung J, Yang Y, McNamara E, Hong R, Moskalenko M, et al. MAP Kinase Inhibition Promotes T Cell and Anti-tumor Activity in Combination with PD-L1 Checkpoint Blockade. Immunity. 2016;44(3):609–21. 10.1016/j.immuni.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 245.Hu-Lieskovan S, Mok S, Homet Moreno B, Tsoi J, Robert L, Goedert L, et al. Improved antitumor activity of immunotherapy with BRAF and MEK inhibitors in BRAF(V600E) melanoma. Sci Transl Med. 2015;7(279):279ra41. 10.1126/scitranslmed.aaa4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Gutzmer R, Stroyakovskiy D, Gogas H, Robert C, Lewis K, Protsenko S, et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAF(V600) mutation-positive melanoma (IMspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;395(10240):1835–44. 10.1016/s0140-6736(20)30934-x. [DOI] [PubMed] [Google Scholar]
- 247.A Multi-Center Randomised Open-Label Phase 2 Study to Assess the Safety, Tolerability and Efficacy of Fimaporfin-Induced Photochemical Internalisation of Gemcitabine Complemented by Gemcitabine/Cisplatin Chemotherapy Versus Gemcitabine/Cisplatin Alone in Patients With Inoperable Cholangiocarcinoma [database on the Internet]2019. Available from: https://clinicaltrials.gov/study/NCT04099888. Accessed:
- 248.Fadlallah H, El Masri J, Fakhereddine H, Youssef J, Chemaly C, Doughan S, et al. Colorectal cancer: Recent advances in management and treatment. World J Clin Oncol. 2024;15(9):1136–56. 10.5306/wjco.v15.i9.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Shirai K, Aoki S, Endo M, Takahashi Y, Fukuda Y, Akahane K, et al. Recent developments in the field of radiotherapy for the management of lung cancer. Jpn J Radiol. 2024. 10.1007/s11604-024-01663-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309–22. 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 251.Ao JY, Zhu XD, Chai ZT, Cai H, Zhang YY, Zhang KZ, et al. Colony-Stimulating Factor 1 Receptor Blockade Inhibits Tumor Growth by Altering the Polarization of Tumor-Associated Macrophages in Hepatocellular Carcinoma. Mol Cancer Ther. 2017;16(8):1544–54. 10.1158/1535-7163.Mct-16-0866. [DOI] [PubMed] [Google Scholar]
- 252.Qin H, Ding Y, Mujeeb A, Zhao Y, Nie G. Tumor Microenvironment Targeting and Responsive Peptide-Based Nanoformulations for Improved Tumor Therapy. Mol Pharmacol. 2017;92(3):219–31. 10.1124/mol.116.108084. [DOI] [PubMed] [Google Scholar]
- 253.Yao Y, Zhou Y, Liu L, Xu Y, Chen Q, Wang Y, et al. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front Mol Biosci. 2020;7:193. 10.3389/fmolb.2020.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65(1):36–48. 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 255.Sang R, Stratton B, Engel A, Deng W. Liposome technologies towards colorectal cancer therapeutics. Acta Biomater. 2021;127:24–40. 10.1016/j.actbio.2021.03.055. [DOI] [PubMed] [Google Scholar]
- 256.O’Brien ME, Wigler N, Inbar M, Rosso R, Grischke E, Santoro A, et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol. 2004;15(3):440–9. 10.1093/annonc/mdh097. [DOI] [PubMed] [Google Scholar]
- 257.Cheng H, Wu Z, Wu C, Wang X, Liow SS, Li Z, et al. Overcoming STC2 mediated drug resistance through drug and gene co-delivery by PHB-PDMAEMA cationic polyester in liver cancer cells. Mater Sci Eng C Mater Biol Appl. 2018;83:210–7. 10.1016/j.msec.2017.08.075. [DOI] [PubMed] [Google Scholar]
- 258.Denny JC, Collins FS. Precision medicine in 2030-seven ways to transform healthcare. Cell. 2021;184(6):1415–9. 10.1016/j.cell.2021.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.