Abstract
Crosstalk between autophagy, host cell death, and inflammatory host responses to bacterial pathogens enables effective innate immune responses that limit bacterial growth while minimizing coincidental host damage. Mycobacterium tuberculosis (Mtb) thwarts innate immune defense mechanisms in alveolar macrophages (AMs) during the initial stages of infection and in recruited bone marrow-derived cells during later stages of infection. However, how protective inflammatory responses are achieved during Mtb infection and the variation of the response in different macrophage subtypes remain obscure. Here, we show that the autophagy receptor Tax1bp1 plays a critical role in enhancing inflammatory cytokine production and increasing the susceptibility of mice to Mtb infection. Surprisingly, although Tax1bp1 restricts Mtb growth during infection of bone marrow-derived macrophages (BMDMs) (Budzik et al. 2020) and terminates cytokine production in response to cytokine stimulation or viral infection, Tax1bp1 instead promotes Mtb growth in AMs, neutrophils, and a subset of recruited monocyte-derived cells from the bone marrow. Tax1bp1 also leads to increases in bacterial growth and inflammatory responses during infection of mice with Listeria monocytogenes, an intracellular pathogen that is not effectively targeted to canonical autophagy. In Mtb-infected AMs but not BMDMs, Tax1bp1 enhances necrotic-like cell death early after infection, reprogramming the mode of host cell death to favor Mtb replication in AMs. Tax1bp1’s impact on host cell death is a mechanism that explains Tax1bp1’s cell type-specific role in the control of Mtb growth. Similar to Tax1bp1-deficiency in AMs, the expression of phosphosite-deficient Tax1bp1 restricts Mtb growth. Together, these results show that Tax1bp1 plays a crucial role in linking the regulation of autophagy, cell death, and pro-inflammatory host responses and enhancing susceptibility to bacterial infection.
Author Summary
Although macrophages are the first innate immune cells to encounter Mycobacterium tuberculosis during infection, M. tuberculosis has evolved the ability to persist in them. Recent studies highlight that some types of macrophages are more permissive to M. tuberculosis replication and survival than others, but the mechanisms for cell type-specific differences in M. tuberculosis growth are only beginning to be understood. We found that the host factor, Tax1bp1 (Tax-1 binding protein 1), supports M. tuberculosis growth during animal infection and in specific subsets of innate immune cells, including alveolar macrophages while restricting M. tuberculosis in bone marrow-derived macrophages. We also found that Tax1bp1 has a similar phenotype in enhancing the pathogenesis of another intracellular pathogen, Listeria monocytogenes. Compared to bone marrow-derived macrophages, in alveolar macrophages, Tax1bp1 enhances the release of inflammatory mediators and leads to necrotic-like host cell death, which is known to enhance M. tuberculosis growth. Phosphorylation of Tax1bp1 in alveolar macrophages promotes M. tuberculosis growth. Our research highlights that Tax1bp1 is a host target for host-directed therapy against M. tuberculosis and controls host responses to M. tuberculosis in a cell type-specific manner.
Introduction
Mycobacterium tuberculosis (Mtb), the causative agent of tuberculosis, has evolved the ability to circumvent host innate antimicrobial responses and survive within our immune cells (1,2). Understanding the host factors that limit antimicrobial immune responses to Mtb is critical for developing new host-directed antimicrobial therapies (3,4). The persistently high number of deaths from tuberculosis (1.13 million in 2023 (5)) highlights the need for new anti-TB treatments.
After inhalation of Mtb, alveolar macrophages (AMs) are the first immune cells to become infected with Mtb and provide a replicative niche for intracellular bacteria (6–8). To disseminate to other organs, Mtb must spread from AMs to different cell types, such as recruited monocyte-derived macrophages (6). In the mouse model of infection, this dissemination occurs at approximately 14–25 days post-infection upon recruitment of monocytes and their differentiation into macrophages in the lung parenchyma (9). Ultimately, live bacteria are transported from the lungs via lymphatics to the draining lymph nodes (9). In Mtb-infected lungs, monocytes have been shown to differentiate into two subsets that differ in their ability to control Mtb (10). The first subset is the CD11clo subset (MNC1, mononuclear cell subset 1), also known as recruited macrophages (11–13). The second subset is the CD11chi subset (MNC2), formerly known as dendritic cells but considered more closely related to macrophages (9,12–14). AMs, which are embryonically derived from the yolk sac, and monocyte-derived macrophages originating from circulating monocytes of bone marrow origin (15) exhibit divergent transcriptional responses against Mtb (8). AMs are impaired at mounting antibacterial responses against Mtb which lead to significant Mtb growth differences when compared to murine bone marrow-derived macrophages (BMDMs) (8). However, the host factors mediating different rates of Mtb growth in AMs compared to BMDMs are poorly understood.
In various macrophage subtypes, immune responses to Mtb are generated by the detection of Mtb lipoproteins and lipoglycans by macrophage surface receptors (e.g. TLR2 (16,17), Dectin 1 (18)), which mediate ERK (extracellular-signal regulated kinase) and NF-κB inflammatory signaling (19). Cytosolic sensors also recognize Mtb nucleic acids released during phagosomal perforation (20) and engage several downstream signaling pathways, each of which can have opposing impacts on Mtb growth. For example, cytosolic sensing of Mtb DNA by cGAS (cyclic GMP–AMP synthase) triggers type I interferon (IFN) production and autophagy (21). Autophagy is a protective response against Mtb that targets it for ubiquitylation and degradation in the lysosome (22–25). Conversely, type I IFN is a cytokine that protects against viruses but can be co-opted by Mtb to promote its growth (26–28). Another important cytosolic sensor for Mtb is the RIG (Retinoic acid-inducible gene I)-I-like pathway. Like the cGAS pathway, the RIG-I-like pathway’s cytosolic sensing of Mtb RNA induces host responses with opposing effects on Mtb growth. RIG-I induces apoptosis, a mode of cell death that leads to Mtb growth restriction, while also triggering pro-bacterial type I IFN and limiting NF-κB cytokine production such as TNF-α, IL-1β, and IL-6. The impacts of NF-κB-regulated cytokines can be pro- or anti-bacterial (29,30). For instance, TNF-α can restrict Mtb by activating phagocytes but, in excess, can enhance Mtb growth by mediating tissue damage (31–34). These Mtb-mediated signaling pathways are regulated by post-translational modifications through cascade signaling protein phosphorylation (20,22,25,35). Thus, Mtb infection triggers post-translationally regulated host responses that can have pro- or anti-bacterial effects. Nevertheless, we lack a complete understanding of host factors that drive these responses to thwart Mtb growth.
Tax1bp1 is an autophagy receptor at the nexus of multiple key immune responses critical for pathogen control. Tax1bp1 regulates inflammation and blocks apoptosis in response to cytokine stimulation, vesicular stomatitis virus (VSV), and Sendai virus infections by terminating NF-κB and RIG-I signaling (36–42). In infected Tax1bp1-deficient mice, respiratory syncytial virus (RSV) replication is decreased, whereas cytokine responses are enhanced (37). The anti-inflammatory function of Tax1bp1 has also been shown to impact non-infectious diseases through abrogating development of chemically-induced hepatocellular cancer (38), age-dependent dermatitis, and cardiac valvulitis (36). Tax1bp1 promotes selective autophagy that mediates lysosomal degradation of pathogens, such as Mtb in BMDMs (43) and Salmonella enterica Typhimurium (44). Additionally, Tax1bp1 mediates the clearance of aggregated neuronal proteins involved in neurodegenerative disease (45). Thus, Tax1bp1 has an anti-inflammatory function in several contexts, but the impact of Tax1bp1 in vivo during intracellular bacterial infection has hitherto not been described.
Our previous work showed that the autophagy receptor, Tax1bp1, is phosphorylated during Mtb infection of BMDMs (43). Tax1bp1 restricts Mtb growth during ex vivo infection of BMDMs presumably because of the role of Tax1bp1 in antibacterial autophagy (43). Notably, Tax1bp1 does not significantly change the levels of NF-κB-regulated cytokines during Mtb infection of BMDMs (43). To expand our understanding of Tax1bp1’s function in other critical innate immune cell types and the presence of the complete immune system, here we employed the mouse infection models for Mtb and the intracellular pathogen Listeria monocytogenes. Surprisingly, this led to the discovery that Tax1bp1 promotes Mtb and Listeria growth during animal infection and enhances Mtb growth in several innate immune cell types including AMs, in contrast to the role of Tax1bp1 in the restriction of Mtb growth in BMDMs. Furthermore, Tax1bp1 had a pro-inflammatory function during Mtb and Listeria animal infection, compared to its anti-inflammatory role in viral infections. We found that Tax1bp1 enhances necrotic-like cell death and inflammatory mediator release during AM but not BMDM infection, which is a mechanism by which Tax1bp1 leads to cell type-specific changes in Mtb growth. To our knowledge, we are the first to study the function of Tax1bp1 in the context of mouse infections with these pathogens. Our findings from the animal infection model led us to uncover new relevant phenotypes compared to those revealed by the BMDM infection model and revealed Tax1bp1’s negative impact on immunity to intracellular pathogens.
Results
Tax1bp1 promotes Mtb virulence and inflammatory cytokine responses in vivo
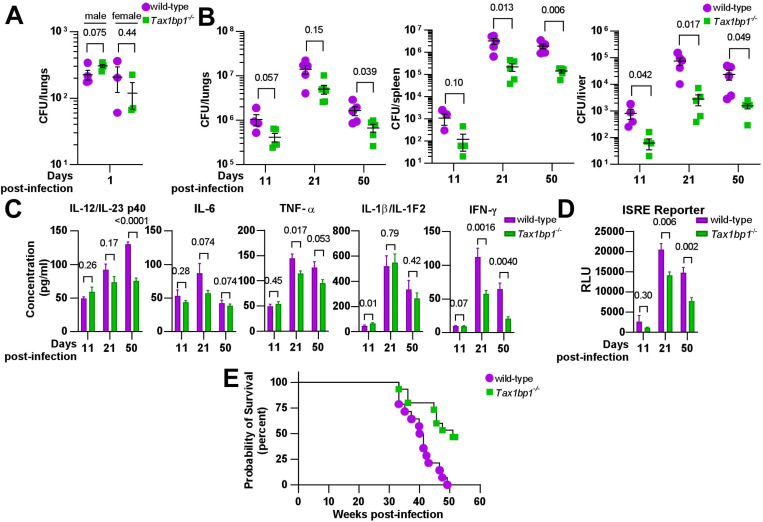
To assess the role of Tax1bp1’s contribution to controlling tuberculosis infection in vivo, we infected wild-type and Tax1bp1-deficient mice with virulent M. tuberculosis via the aerosol route and monitored the infection at different time points. First, we infected male and female wild-type and Tax1bp1−/− mice at a low dose and enumerated CFU at day one post-infection. This revealed no statistically significant difference in bacterial uptake (Figure 1A). Next, two independent mouse infections were performed on separate days to test the contribution of Tax1bp1 to Mtb growth (Figure 1B, Figure 1-figure supplement 1). On days 9 or 11, 21, and 50 post-infection, we harvested the lung, spleen, and liver for enumeration of bacterial CFU. In contrast to our previous results showing Tax1bp1 restricted Mtb growth in BMDMs infected ex vivo (43), in the mouse infection model, Tax1bp1 enhanced Mtb growth (Figure 1B, Figure 1-figure supplement 1). This unexpected difference manifests even after 11 days of infection, during the acute stage when Mtb replicates within AMs. Mtb’s improved growth was magnified in both peripheral organs, liver, and spleen, consistent with the differences in lung CFUs. These results contrast with the autophagy receptor function of Tax1bp1 since Tax1bp1 contributes to targeting Mtb to selective autophagy (43) and autophagy is required for controlling Mtb growth in vivo and ex vivo (22,46).
Figure 1. Tax1bp1 enhances M. tuberculosis virulence and inflammatory cytokine responses during mouse aerosol infection.
(A) Male and female mice were infected by the aerosol route with a mean Mtb CFU of 240 as determined by CFU enumeration from lung homogenates at 1-day post-infection. (B) Additional mice were euthanized at 11-, 21-, and 50 days post-infection for CFU enumeration. Results are the mean ± SEM from lung homogenates of 5 infected mice. (C) Cytokine levels from infected lung homogenates at 11-, 21-, and 50-days post-infection were measured by ELISA. Results are the mean ± SEM from five samples. (D) Levels of type I and II interferon-induced JAK/STAT signaling were measured by luminescence in relative light units (RLUs) from infected lung homogenates by the ISRE assay. Results are the mean ± SEM from five samples. Brackets indicate p-values from t-test comparisons. (E) Infected mice were monitored for death or 15% loss of maximum body weight, at which point they were euthanized. Log-rank (Mantel-Cox) test and Gehan-Breslow-Wilcoxon comparison test p-values for survival were 0.0008 and 0.0047, respectively.
Because Tax1bp1 has a known role in mediating inflammatory responses, we hypothesized that Tax1bp1 might be regulating inflammatory responses during Mtb infection. Indeed, analysis of a panel of pro-inflammatory cytokines from the lungs of infected mice revealed that, consistent with this idea, Tax1bp1 increased levels of IL-6, TNF-α, IL1-β, and IL-12/IL-23 p40 (Figure 1C, Figure 1-figure supplement 1). Type I and II IFN are particularly important for controlling Mtb infection (21,28,47). Therefore, we measured interferon levels using a type I and II IFN reporter cell line (ISRE) and a type II IFN (IFN-γ) by ELISA (Figure 1C, D). Consistent with other pro-inflammatory cytokines, we found that wild-type mice had significantly higher levels of type I and II IFN in the lungs compared to Tax1bp1−/− mice (Figure 1C, D). We also carried out survival studies and found that Tax1bp1 contributes to mortality during Mtb infection (Figure 1E). Although Tax1bp1 contributes to inflammatory cytokine synthesis during Mtb infection, microscopic examination of infected lung tissue did not reveal any significant differences in the cellular infiltrate of the lungs as reflected by lesion severity or tissue necrosis (Figure 1-figure supplement 2A, B) or by neutrophil recruitment reflected by myeloperoxidase staining (Figure 1-figure supplement 2C, D). During infection of BMDMs, we previously observed that Tax1bp1 reduces ubiquitin colocalization with Mtb (43). Thus, we sought to determine whether ubiquitin recruitment was regulated during infection in vivo. While Tax1bp1 led to a slight decrease in ubiquitin and Mtb colocalization in infected lung tissue samples at 50 days post-infection, this did not reach statistical significance (Figure 1-figure supplement 3). Our results suggest that Tax1bp1 amplifies host-detrimental inflammatory responses, which predominate over cell-intrinsic control of Mtb replication by autophagy in macrophages (48) and contribute to host susceptibility and mortality.
Tax1bp1 enhances Listeria monocytogenes growth, microabscess formation, and host inflammatory cytokine synthesis.
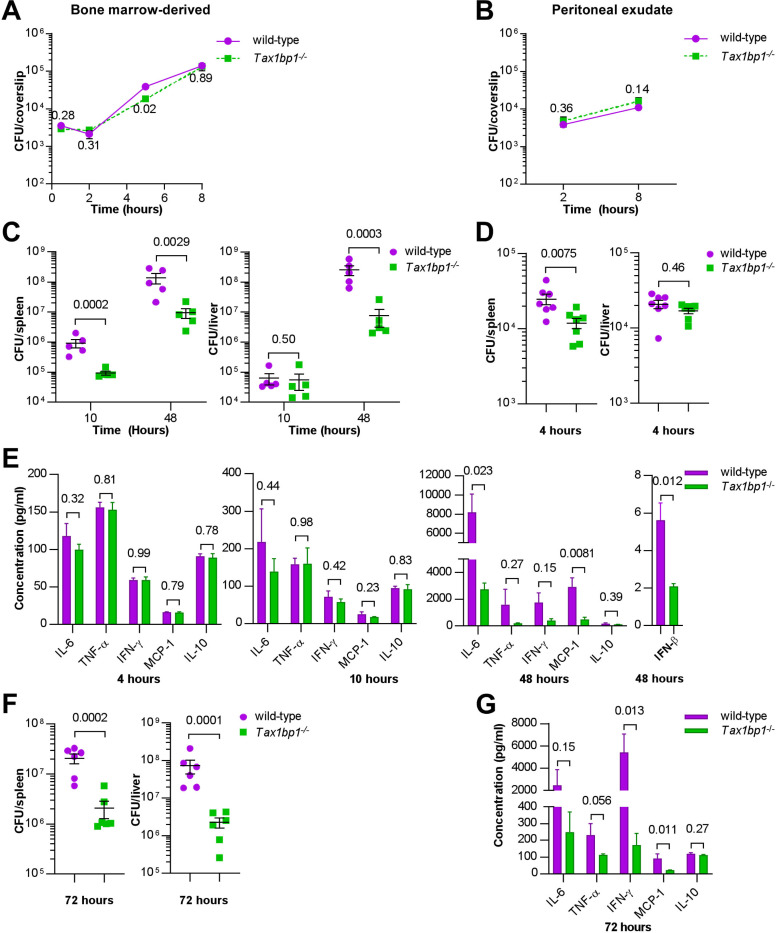
We then turned to a different intracellular bacterial pathogen, Listeria monocytogenes, which has potent autophagy-inhibiting mechanisms to avoid antibacterial autophagy (49–51). Using Listeria allowed us to assess the role of Tax1bp1 in inflammatory responses in isolation from its role in autophagy. As shown in Figures 2A and 2B, wild-type Listeria grew as well in both BMDMs and peritoneal macrophages harvested from wild-type and Tax1bp1−/− mice, indicating there is no defect in the ability of Listeria to replicate in Tax1bp1−/− macrophages ex vivo. In contrast, when we infected mice with Listeria via the intravenous (IV) route, we saw that over a 48-hour time course, Tax1bp1−/− mice were remarkably resistant to Listeria growth (Figure 2C). Consistent with Tax1bp1 playing a role early in the Listeria infection, we observed a statistically significant difference in Listeria CFU in the spleen, but not in the liver, at 4 hours post-infection (Figure 2D). This is consistent with the role of Tax1bp1 being manifested at the earliest stages of infection. To confirm the reproducibility of this growth phenotype, we repeated the Listeria mouse infections and observed similar results (Figure 2-figure supplement 1).
Figure 2. Tax1bp1 contributes to Listeria monocytogenes virulence and growth during murine but not ex vivo cellular infections.
(A and B) BMDMs or peritoneal exudate cells were infected with L. monocytogenes, and CFU were counted at 30 minutes, 2-, 5-, or 8 hours post-infection. Results are the mean ± SEM from three technical replicate samples. The p-values from t-test comparisons are shown. (C and D) CFU from spleen and liver homogenates from mice intravenously infected with L. monocytogenes were enumerated at 4-, 10-, or 48-hours post-infection. Results are the mean ± SEM from five mice. Brackets indicate p-values from t-test comparisons. (E & G) Cytokine levels were measured from the serum of mice infected with L. monocytogenes at 4-, 10-, and 48-hours post-infection by cytometric bead array (IL-6, TNF-α, IFN-γ, MCP-1, IL-10) or ELISA (IFN-β). Results are mean ± SEM from five samples. Brackets indicate p-values from t-test comparisons. (F) Spleen and liver homogenates from mice intraperitoneally infected with L. monocytogenes were enumerated for CFU 72 hours post-infection. Results are the mean ± SEM from five mice. Brackets indicate p-values from t-test comparisons. CFU data were logarithmically transformed prior to statistical analysis.
We measured inflammatory cytokine levels in the serum of the IV-infected mice and found that during the first 4- and 10 hours, inflammatory cytokines were low and were not significantly different between the wild-type and Tax1bp1−/− mice (Figure 2E). At 48 hours, Tax1bp1 significantly increased IL-6, TNF-α, IFN-γ, IFN-β and MCP-1 in the serum, indicating Tax1bp1 is required for augmenting pro-inflammatory cytokines and type I interferon (IFN-β) (Figure 2E). Infecting mice by the intraperitoneal route gave rise to very similar results, indicating that the route of infection is irrelevant to the infection outcome (Figure 2F). Indeed, Tax1bp1 increased CFU by approximately 1.5 logs in the liver and by 1 log in the spleen (Figure 2F). As with the IV infection, Tax1bp1 led to a considerable non-statistically significant increase in IL-6 production and a substantial increase in IFN-γ in the serum (Figure 2G). Likewise, Tax1bp1 augmented MCP-1 levels in the serum (Figure 2G). Consistent with the increased bacterial load mediated by Tax1bp1, histological examination of tissues from infected mice revealed that Tax1bp1 increased the number of microabscesses and led to lymphoid depletion (Figure 2-figure supplement 2). Tax1bp1 also reduced the occurrence and severity of hepatocyte coagulative necrosis (Figure 2-figure supplement 2). The lack of splenic microabscesses and lymphoid depletion of Tax1bp1−/− mice may correlate with the decrease in pro-inflammatory cytokine levels noted in the serum of Tax1bp1−/− mice compared to wild-type mice. These results suggest that Tax1bp1 enhances inflammatory cytokine signaling and bacterial growth during animal infection with Listeria monocytogenes, as observed during Mtb infection.
Tax1bp1 promotes Mtb growth in AMs, neutrophils, and recruited mononuclear cells in vivo
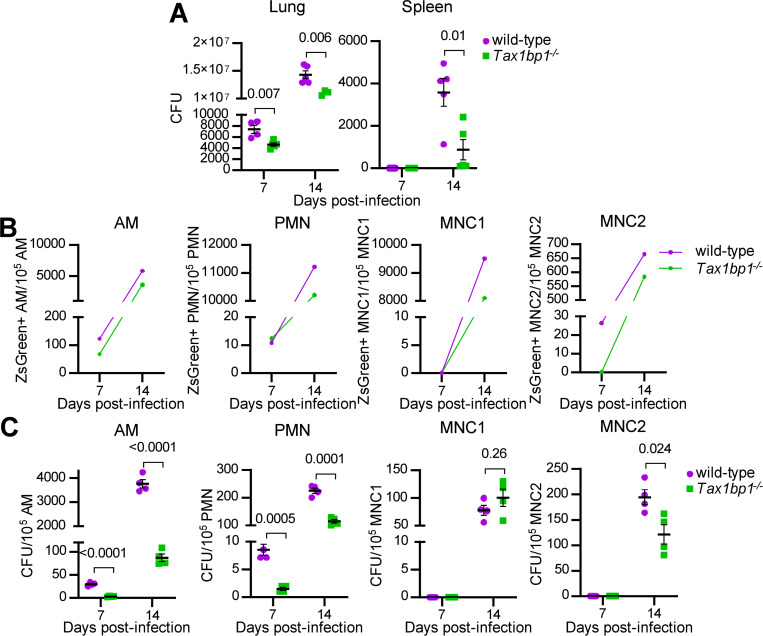
Since Tax1bp1 restricted Mtb growth in BMDMs infected ex vivo (43) but the opposite phenotype was observed during in vivo mouse infections, we hypothesized that Tax1bp1 promotes Mtb growth in other cell types. Following the transfer of Tax1bp1−/− mice from UC Berkeley to UC San Francisco and their rederivation, we infected wild-type and Tax1bp1−/− age- and sex-matched mice with a low dose of ZsGreen-expressing Mtb by the aerosol route. At 7- and 14 days post-infection, Mtb CFU were enumerated from aliquots of organ homogenates. Consistent with our previous results at UC Berkeley, Tax1bp1 promoted Mtb growth in rederived mice (Figure 3A). To test the hypothesis that Tax1bp1 enhances Mtb growth in distinct innate immune cells, lung cell suspensions were pooled from five mice of each genotype to obtain enough cells for downstream CFU analysis from sorted cell populations. After staining the pooled innate immune cells, we performed flow cytometry to sort CD11clo (MNC1) and CD11chi (MNC2) mononuclear cells, neutrophils (PMNs), and AMs using innate immune cell antibodies (Figure 3-figure supplement 1) (10). To account for differences in the number of cells sorted between genotypes, data were normalized by dividing the ZsGreen+ counts or CFU by the total number of each cell type sorted. Tax1bp1 increased the normalized ZsGreen+ counts in AMs, neutrophils, MNC1, and MNC2 (Figure 3B). We plated the sorted innate immune cells on agar plates in quadruplicate to determine the number of viable Mtb CFU in the sorted cells. Consistent with the ZsGreen+ counts, Tax1bp1 promoted Mtb growth by CFU counts in AMs by 11-fold and in PMNs by 6-fold at seven days post-infection (Figure 3C). At 14 days post-infection, the phenotype was more pronounced in AMs (43-fold increase) and less pronounced in PMNs (2-fold increase; Figure 3C). CFU were not detected from MNC1 and MNC2 at 7-days post-infection, consistent with a previous report (52). Although Tax1bp1 increases ZsGreen+ counts in MNC1 at 14-days post-infection, Tax1bp1 did not enhance Mtb CFU in MNC1 but did slightly in MNC2 (1.6-fold; Figure 3C). We performed a second independent aerosol infection to test the reproducibility of these findings and measure ZsGreen+ Mtb counts at an additional point 21 days post-infection. As observed in the first experiment, Tax1bp1 increased Mtb counts at the early time points in AMs, MNC2, and PMNs; however, at 21 days post-infection Tax1bp1 instead reduced Mtb ZsGreen+ counts in MNC1 (Figure 3-figure supplement 2). This suggests that Tax1bp1 can restrict Mtb growth in MNC1, consistent with our previous findings in BMDMs ex vivo. In summary, Tax1bp1 has a cell type-specific impact on Mtb growth with an overall effect of increasing Mtb growth in the major organs. In two independent aerosol infection experiments, Tax1bp1 enhances Mtb growth in AMs, PMNs, and MNC2, boosting Mtb growth in immune cells of various cell origins in vivo.
Figure 3. Tax1bp1 promotes Mtb growth in AMs, PMNs, and MNC2 following low-dose aerosol infection.
Mice were infected with aerosolized Mtb expressing ZsGreen (calculated dose of 100 CFU per mouse), and five wild-type and 5 Tax1bp1−/− mice were euthanized at 7- and 14-days post-infection. (A) Lung and spleen homogenates from 5 wild-type and 5 Tax1bp1−/− mice at each time point were plated for CFU. (B) Lung cells were pooled and stained for AMs, neutrophils (PMNs), and recruited monocyte 1 and 2 subsets (MNC1, 2). ZsGreen-positive innate immune cell subsets were quantified by analytical flow cytometry. (C) Innate immune cells were sorted. The sorted cells were plated for Mtb CFU in quadruplicate. Data were normalized to the number of cells sorted. SEM and p-values from the t-test are displayed.
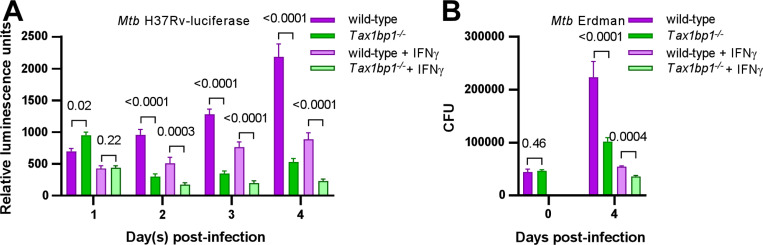
To test whether Tax1bp1 also enhances Mtb growth in AMs infected ex vivo, we obtained murine AMs by bronchoalveolar lavage (BAL), discarded the suspension cells, and cultured the remaining adherent AMs. We infected the AMs with either luminescent or wild-type Mtb in the presence or absence of IFN-γ, a cytokine that activates macrophage antibacterial host responses (53). As reflected by changes in luminescence (Figure 4A), Tax1bp1 began to promote Mtb growth at two days post-infection in the presence or absence of IFN-γ. Similarly, Tax1bp1 augmented Mtb growth as measured by CFU (Figure 4B). This data implicates Tax1bp1 in enhancing Mtb growth during AM infection and is consistent with our in vivo studies, although it is in sharp contrast to our results in Mtb-infected BMDMs (43) and MNC1. Therefore, we focused on understanding Tax1bp1’s function in AMs as this model better represents the overall impact of Tax1bp1 on real-world infections and in most innate immune cell types. Even though Tax1bp1’s phenotype in BMDMs does not model the phenotype in several other cell types (i.e., AMs, MNC2, and PMNs), we took advantage of Tax1bp1’s phenotype in BMDMs by using BMDMs as a control.
Figure 4. Tax1bp1 enhances Mtb growth in AMs infected ex vivo.
AMs were infected ex vivo with luciferase-expressing Mtb H37Rv (A) or wild-type Mtb Erdman (B) at a M.O.I. of 1 in the presence or absence of IFN-γ added at the time of infection. (A) Monolayer luminescence was measured daily. (B) CFU were measured immediately after infection (day 0) or 4 days post-infection. Displayed are the mean, SEM, and FDR-adjusted p-values from the t-test.
Tax1bp1 promotes Mtb autophagosome maturation in AMs ex vivo
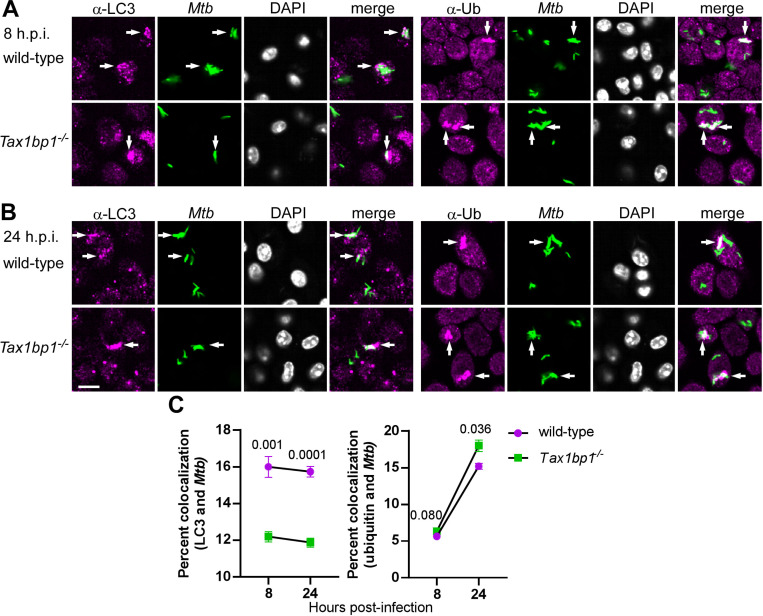
Since we previously showed that Tax1bp1 promoted Mtb autophagosome maturation in BMDMs, we questioned if Tax1bp1 played a similar role in mediating selective autophagy in AMs. To test this, we performed confocal fluorescence microscopy of wild-type and Tax1bp1−/− AMs infected with fluorescent Mtb and assessed the colocalization of Mtb and autophagy markers. At 24 hours post-infection, Tax1bp1 slightly decreased Mtb colocalization with ubiquitin by 16% (Figure 5A-C), whereas Tax1bp1 increased Mtb colocalization with LC3 by 26% (Figure 5A-C). The decrease of ubiquitylated Mtb autophagosomes and the increase in LC3-Mtb colocalization indicate that Tax1bp1 promotes Mtb autophagosome formation and maturation in AMs to a small degree. This is the same pattern of autophagy marker staining as we previously observed in wild-type and Tax1bp1−/− BMDMs (43). Since Tax1bp1’s impact on Mtb growth was cell type-specific but its effect on autophagy targeting of Mtb was not, we reasoned that Tax1bp1 enhanced Mtb growth in AMs by a different mechanism.
Figure 5. Tax1bp1 targets Mtb to autophagy in AMs.
AMs were infected ex vivo with ZsGreen-expressing Mtb Erdman at a M.O.I of 2. At 8- and 24-hours post-infection, monolayers were fixed and stained with primary antibodies for autophagy markers, secondary Alexa-Fluor 647 antibodies, and DAPI. Immunofluorescence microscopy was performed at 63X magnification in 69 x/y positions and 4 z planes each in quadruplicate wells. Immunofluorescence microscopy images at 8- (A) and 24- (B) hours post-infection are displayed. Arrows denote Mtb that colocalized with LC3 or ubiquitin. The white bar denotes 10 μm. (C) Quantification of Mtb and autophagy marker colocalization is displayed. Mean percent colocalization in each well, SEM, and p-values from the t-test are depicted.
Host and pathogen expression analysis of Mtb-infected Tax1bp1−/− AMs ex vivo
To broadly query host effector responses regulated by Tax1bp1 and simultaneously determine if Tax1bp1 triggers upregulation of Mtb genes required for intracellular Mtb replication, we performed host and pathogen dual transcriptional profiling of Mtb-infected wild-type and Tax1bp1−/− AMs. Differential gene expression analysis of Mtb transcripts showed no significant changes in gene expression using an adjusted p-value cutoff of 0.05. However, using a less stringent p-value threshold for statistical significance of an unadjusted p-value of 0.05, similar to other reports of Mtb transcriptional profiling (54), Tax1bp1 upregulated two Mtb genes, mmpL4 and mbtE (Figure 6-figure supplement 1A). The upregulated Mtb genes included several required for intracellular Mtb replication, including mmpL4 (55) and genes with a p-value >0.05, including the extracellular repeat protein TB18.5 (56) and transketolase tkt (57) (Figure 6-figure supplement 1A). These results are consistent with our observation that Tax1bp1 enhances increased intracellular Mtb growth during AM infection (Figure 4).
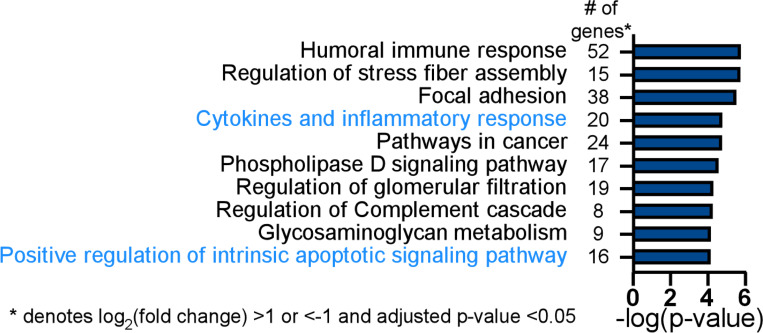
From the 140 differentially expressed host genes with a log2(fold change) of greater than 1 or less than −1 and an adjusted p-value <0.05, gene ontogeny enrichment analysis identified cytokines and inflammatory response (20 genes, -log (p-value) = 4.8) as a significant functional pathway controlled by Tax1bp1 in Mtb-infected AMs, including the genes Cd4, Cxcl1, Pf4, Pdgfa, Kitl, and Cxcl3 (Figure 6; Figure 6-figure supplement 1B). Gene ontogeny enrichment analysis also identified positive regulation of the intrinsic apoptotic signaling pathway (16 genes, -log (p-value) = 4.1), including the genes encoding for Bok, an apoptotic regulator, and prostaglandin-endoperoxide synthase 2 (Ptgs2, also known as COX-2; Figure 6-figure supplement 1B). The differentially expressed gene of the greatest magnitude in Mtb-infected Tax1bp1−/− AMs compared to wild-type AMs was Sox7 (log2(fold change) = 5.4, adjusted p-value = 2.94 × 10−12). Sox7 is a transcription factor that induces apoptosis through the MAP kinase ERK-BIM (BCL2-interacting mediator of cell death) pathway (58). These results from gene expression analysis indicate that Tax1bp1 regulates the expression of inflammatory signaling and host cell death genes, both of which can contribute to the control of Mtb growth. Therefore, we further tested the hypothesis that Tax1bp1 impacts these two host responses during Mtb infection of AMs.
Figure 6. Tax1bp1 contributes to differential expression of inflammatory response and apoptotic signaling pathway genes during Mtb infection of AMs.
Wild-type and Tax1bp1−/− AMs were infected in biological triplicate with Mtb at a M.O.I. of 2. The RNA was harvested at 36-hours post-infection for differential pathogen and host gene expression analysis by RNAseq. Gene ontogeny enrichment analysis of statistically significant differentially expressed host genes (log2(fold change) >1 or <−1, adj. p-values <0.05) during Mtb infection of wild-type and Tax1bp1−/− AMs was performed with Metascape (93). The top ten enriched pathways and the number of genes in each functional pathway are displayed.
Tax1bp1 promotes inflammatory cytokines signaling during AM infection ex vivo
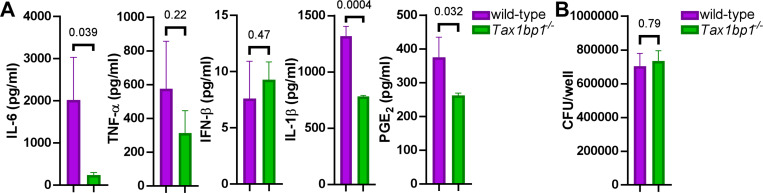
We next aimed to investigate whether the effects of Tax1bp1 on cytokines and inflammatory responses during AM infection would align with our previous observation that Tax1bp1 promoted inflammatory cytokine production during infection in vivo (Figure 1 C, D, and Figure 1-figure supplement 1B). Tax1bp1 promoted gene expression of IL-1β (Fig. 6-figure supplement 1B) and synthesis of several inflammatory cytokines, including IL-1β, but not IFN-β as measured by ELISA (Figure 7A). As previously noted, Tax1bp1 also led to an increase in the expression of Ptgs2 (prostaglandin-endoperoxide synthase 2; Fig. 6-figure supplement 1B), which is involved in the production of inflammatory prostaglandins. Notably, prostaglandin E2 (PGE2) is an inflammatory eicosanoid that promotes Mtb growth by blocking efferocytosis (59,60). In addition to enhancing cytokines regulated by NF-κB, Tax1bp1 also promoted the production of PGE2 during Mtb infection of AMs (Figure 7A). To our knowledge, PGE2 was not previously known to be regulated by Tax1bp1. Importantly, these cytokine and eicosanoid levels were measured early after infection (24 hours) when the Mtb CFU were the same in wild-type and Tax1bp1−/− AMs (Figure 7B), before any differences in Mtb growth were observed at later time points. Therefore, these findings suggest that the increased cytokine and PGE2 production mediated by Tax1bp1 happens independently of bacterial burden. In summary, Tax1bp1 promotes the production of proinflammatory cytokines and eicosanoids during Mtb infection of AMs. These findings are consistent with our cytokine analysis during in vivo infection (Figures 1 and 2) and in contrast to our previous report in BMDMs in which Tax1bp1 did not impact inflammatory cytokine production during Mtb infection (43).
Figure 7. Tax1bp1 enhances IL-6, IL-1β, and PGE2 secretion during AM infection.
AMs were seeded at 100,000 cells/well and infected in triplicate wells with Mtb at a M.O.I. of 5. At 24 hours post-infection, (A) the supernatants were collected for cytokine measurement by ELISA, and (B) AM monolayers were lysed and plated for Mtb CFU. Mean, SEM, and p-values from the t-test are displayed.
Tax1bp1 enhances necrotic-like cell death and delays apoptosis of Mtb-infected AMs
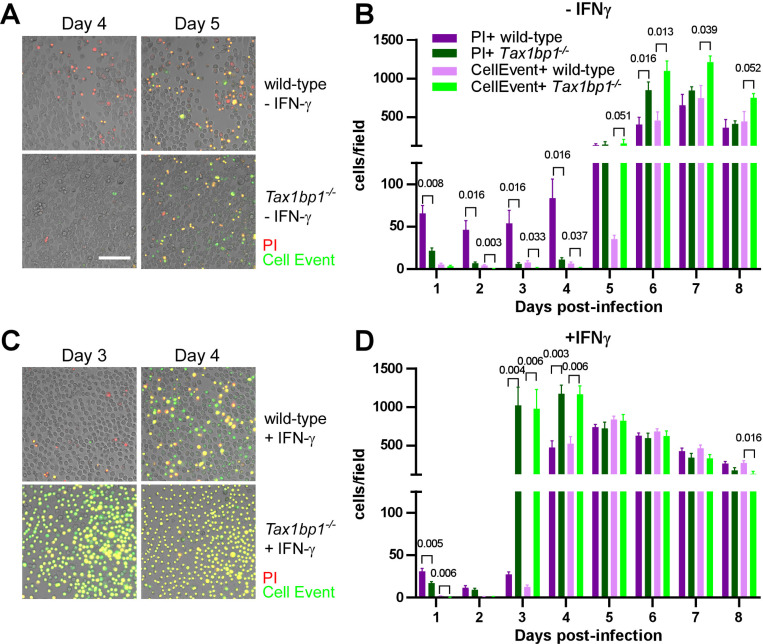
In addition to regulating cytokine and inflammatory responses, our gene expression analysis suggested that Tax1bp1 regulates apoptotic gene expression during AM infection. Tax1bp1 was previously shown to regulate apoptosis following cytokine stimulation (61) and viral infection (42). Furthermore, apoptosis is a mode of cell death that leads to restriction of Mtb growth through efferocytosis, in which Mtb-infected apoptotic cells are phagocytosed by neighboring macrophages (59,62). This is in contrast to necrosis, which is a form of uncontrolled cell death that is highly immunostimulatory and enhances Mtb growth (46,63–66). To test if Tax1bp1 impacts cell death during Mtb infection of AMs and BMDMs, we analyzed cells during Mtb infection by live cell fluorescence microscopy using CellEvent caspase 3/7 to detect apoptotic cells and propidium iodide (PI) for necrotic/late apoptotic cells. In the absence of IFN-γ stimulation, Tax1bp1 leads to necrotic-like cell death and a delay in apoptosis (Figure 8A, B; Figure 8-figure supplement 1). In IFN-γ stimulated cells, Tax1bp1 also delays apoptosis (Figure 8C, D; Figure 8-figure supplement 2). In contrast to AMs, Tax1bp1 did not impact the amount of apoptotic or necrotic-like cell death in infected BMDMs in the absence of IFN-γ (Figure 8-figure supplement 2A, C). Only when stimulated with IFN-γ, Tax1bp1 delayed apoptotic cell death during Mtb infection of BMDMs on day four post-infection (Figure 7-figure supplement 2B, D). Together, these results show that Tax1bp1 leads to necrotic-like cell death of AMs, but not BMDMs, and causes a delay in apoptosis during Mtb infection. These results also highlight an important difference in cell death modality between Mtb-infected wild-type AMs and BMDMs. AMs begin to undergo necrotic-like cell death early after infection, followed by apoptosis later, whereas Mtb-infected BMDMs also die from apoptosis later but do not undergo early necrosis.
Figure 8. Tax1bp1 promotes necrotic-like cell death and delays apoptosis in Mtb-infected AMs.
AMs were infected with Mtb at a M.O.I. of 1 in the presence of PI (propidium iodide) and CellEvent without (A, B) or with (C, D) IFN-γ added to the media. Fluorescence images were obtained at 20X magnification in two positions per well in three replicate wells. (A, C) Representative fluorescence and brightfield microscopy images were merged, cropped, and scaled. (B, D) The number of fluorescent cells in each field was quantified in the green (CellEvent) and red fluorescence (PI) channels. Mean, SEM, and statistically significant FDR-adjusted p-values from t-test comparisons are displayed. For clarity, only statistically significant p-values (p ≤ 0.05) are shown. The white bar is 100 μm.
Expression of phosphosite-deficient Tax1bp1 restricts Mtb growth in AMs
We next investigated the function of Tax1bp1 phosphorylation because Tax1bp1 phosphorylation controls inflammatory responses (40). In data obtained from murine embryonic fibroblasts and in vitro kinase assays, Tax1bp1 is phosphorylated at 13 amino acids by the non-canonical IκB kinase IKKi (or IKK epsilon) (41), eight amino acids by the non-canonical IκB kinase TBK1 (67), and two amino acids by the canonical IKK kinase IKKα (40,41). Phosphorylation of Tax1bp1 by IKKα terminates inflammatory signaling triggered by cytokine or LPS stimulation (40). We initially discovered that Tax1bp1 is significantly phosphorylated during Mtb infection compared to mock infection at the IKKα substrate serine-693 in a global phosphoproteomic analysis of BMDMs (43). Having found that Tax1bp1 enhances inflammatory signaling during Mtb infection of AMs (Figure 7A), we hypothesized that phosphorylation of Tax1bp1 has a critical functional role during Mtb infection.
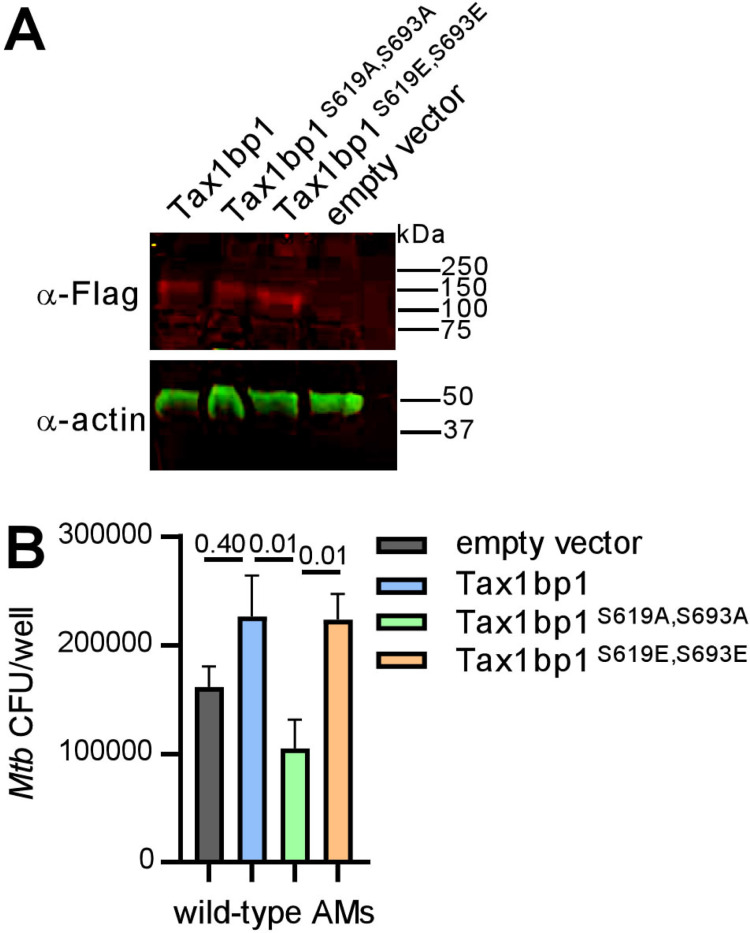
To test if Tax1bp1 phosphorylation impacts Mtb growth, we took advantage of the expanded AM (exAM) model in which primary murine AMs replicate in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) (68,69). We initially transduced primary murine Tax1bp1−/− exAMs with lentivirus for overexpression of Flag-tagged wild-type, a double phosphomutant Tax1bp1 alleles incapable of phosphorylation by IKKα (alanine substitution; Flag-Tax1bp1S619A, S693A), or another that mimics phosphorylation (glutamic acid substitution; Tax1bp1S619E, S693E). However, we could not recover enough transduced Tax1bp1−/− exAMs for subsequent assays (data not shown). Therefore, we overexpressed the Flag-Tax1bp1 alleles in wild-type exAMs, as confirmed by immunoblot of cellular lysates with anti-Flag antibodies (Figure 9A). We then infected the transduced exAMs with Mtb and measured intracellular growth of Mtb by CFU analysis four days post-infection. Compared to wild-type exAMs transduced with empty vector, overexpression of Flag-Tax1bp1 in wild-type exAMs led to a non-significant increase in Mtb growth (Figure 9B). However, compared to Flag-Tax1bp1, overexpression of Flag-Tax1bp1S619A, S693A but not Flag-Tax1bp1S619E, S693E restricted Mtb growth in a statistically significant manner (Figure 9B). We acknowledge several limitations of this approach. First, Tax1bp1 phosphomutants may interact with wild-type Tax1bp1 still present in these cells. Second, the exAM model does not include all the metabolic cues found in vivo (e.g., TGF-β) (70). In summary, these results reveal that expression of phosphosite-deficient Tax1bp1 inhibits Mtb growth in exAMs, similar to our finding that Tax1bp1−/− AMs also restrict Mtb growth compared to wild-type AMs.
Figure 9. Overexpression of phosphosite-deficient Tax1bp1 restricts Mtb growth in AMs.
(A) Cell lysates from wild-type AMs transduced with lentivirus for overexpression of Flag-tagged wild-type or phosphomutant Tax1bp1 were separated by SDS-PAGE. Immunoblot was performed with primary antibodies for the Flag epitope or actin and secondary antibodies conjugated to IRdye 680RD or IRdye 800CW, respectively. Fluorescence images in the 680 (red) and 800 (green) channels are displayed. The mobility of the molecular weight marker is displayed. (B) Transduced AMs were infected with Mtb (M.O.I. 0.5) in 5 technical replicate wells, and CFU enumerated at 4 days post-infection. Mean, SEM and adjusted p-values from Tukey’s multiple comparison test (ordinary one-way ANOVA) are displayed.
Discussion
The autophagy receptor Tax1bp1 plays a role in multiple stages of intracellular pathogen infections, including autophagy and regulation of cytokine responses. More specifically, Tax1bp1 was implicated in the termination of inflammatory NF-κB signaling during Sendai virus and VSV infection (39) as well as the restriction of Mtb growth in BMDMs (43). Here we show that Tax1bp1, unexpectedly, also plays a role in promoting inflammation in vivo and in Mtb growth in AMs, neutrophils, and MNC2 (Figure 3, Figure 3-figure supplement 2). While Tax1bp1 did not impact Listeria growth in macrophage ex vivo, Tax1bp1 supports Listeria and Mtb infection in mice (Figure 2C, D, F, and Figure 2-figure supplement 1; Figure 1B, E, and Figure 1-figure supplement 1A). In addition to Mtb and Listeria, differing results between ex vivo and in vivo pathogen replication were also reported during RSV infection (37). Viral replication is restricted during Tax1bp1−/− murine infection but only slightly changed in cultured A549 Tax1bp1 knockdown cells (37). Collectively, the differences in pathogen replication in mice and cultured cells suggest that the cell type and the tissue environment in vivo can play a critical role in the function of Tax1bp1. Indeed, transplanted bone marrow precursors and terminally differentiated macrophages can change their chromatin landscape in various tissue environments (71). This has significant consequences in the lungs, where the microenvironment impacts macrophage activation and function (72). The discovery that Tax1bp1 promotes Mtb infection in AMs, MNC2, and neutrophils, implies that Tax1bp1’s function supports Mtb replication in several innate immune cell types, including those from different embryonic origins. In contrast, Tax1bp1 restricts Mtb replication in BMDMs and, likely, MNC1. One major difference between BMDMs and AMs is that M-CSF (macrophage colony stimulating factor (73,74)) is used as a stimulating factor for the differentiation of the former, whereas GM-CSF (75–77) is thought to be more crucial for the differentiation and maintenance of the latter (78,79). Since these stimulating factors induce phenotypic changes in macrophages (80) and GM-CSF can be a bactericidal effector against Mtb (81), testing whether the stimulating factor present during immune cell differentiation enables Tax1bp1 to promote or restrict Mtb growth may shed light on an underlying mechanism that drives Tax1bp1’s cell type-specific function.
In addition to inducing inflammatory signaling, we discovered that Tax1bp1 controls the mode of host cell death by initially promoting necrotic-like cell death in the first four days of Mtb infection in AMs but not BMDMs, while delaying apoptosis in the later stages of infection. Since Mtb growth is enhanced in necrotic macrophages (46,82) and apoptosis leads to restriction of Mtb growth via efferocytosis (59), these results indicate that Tax1bp1’s impact on the mode of host cell death is a mechanism by which Tax1bp1 enhances Mtb growth in AMs. Indeed, Tax1bp1 was previously shown to mediate ferroptosis, a programmed type of necrosis, in response to copper stress-induced reactive oxygen species (83). Interestingly, ferroptosis enhances Mtb dissemination (84). In addition to promoting ferroptosis, Tax1bp1 is known to impact apoptotic signaling by restraining apoptosis during VSV and Sendai virus infection (42). During viral infection, termination of RIG-I mediated mitochondrial antiviral signaling proteins (MAVS) signaling blocked apoptosis and type I IFN signaling (42). Because we did not observe altered levels of type I IFN during AM infection with Mtb, we hypothesize Tax1bp1 signals through a different pathway during Mtb infection in these cells. Tax1bp1 also blocks apoptosis by acting as an adaptor for TNFAIP3 (also known as A20) to bind and inactivate its substrates RIPK1 (receptor (TNFRSF)-interacting serine-threonine kinase 1) in the TNFR signaling pathway (85). Further experiments are needed to determine the downstream signaling pathway by which Tax1bp1 blocks apoptosis and whether it promotes a programmed form of necrosis during Mtb infection.
Tax1bp1 is an autophagy adaptor, and autophagy plays a role in cell-autonomous immunity to microbial pathogens. Autophagy also affects immune cell development and inflammatory responses (86–90). The Tax1bp1-deficient knockout mice used in this study are deficient in Tax1bp1 in all cells. While our results indicate that the effect of Tax1bp1 is mediated during the innate immune responses, other cells may likely require Tax1bp1 for their function and that might impact Mtb infection. For example, Tax1bp1 is important for the metabolic transition of activated T cells (91). Tax1bp1 also terminates ERK signaling in B cells to mediate B cell differentiation and antigen-specific antibody production (92). Therefore, Tax1bp1 may be needed for the normal function of other immune cells that undergo proliferation and activation in addition to AMs.
In conclusion, Tax1bp1 plays a unique role in controlling host cell death and promoting inflammatory responses during Mtb and Listeria infection in contrast to its function in terminating NF-κB signaling during viral infection. We discovered that Tax1bp1 is a host factor contributing to differences in Mtb growth in AMs compared to BMDMs (8). While multiple autophagy receptors, including Tax1bp1 and p62, target Mtb for selective autophagy (22,43), this work reveals that different autophagy receptors may play fundamentally distinct roles in Mtb pathogenesis even depending on the host cell type. In contrast to Tax1bp1, the primary autophagy receptor, p62, is not involved in survival from Mtb infection (48). These findings would suggest that one could alter the inflammatory responses and augment protective host responses to pathogens by blocking Tax1bp1 function or inhibiting Tax1bp1 phosphorylation. A better understanding of the mechanism by which Tax1bp1 regulates host cell death, autophagy, and inflammation during infection may enable the development of Tax1bp1 as a target for anti-bacterial therapies.
Methods
Ethics statement
Animal infections were performed in accordance with the animal use protocol (AUP-2015–11-8096, AN192778–01) approved by the Animal Care and Use Committee at the University of California, Berkeley, and the Institutional Animal Care and Use Program at the University of California, San Francisco, in adherence with the federal regulations provided by the National Research Council and National Institutes of Health.
M. tuberculosis mouse infections at UC Berkeley
Tax1bp1−/− mice were provided by Dr. Hidekatsu Iha, Oita University, Japan. Low-dose aerosol infection (100 CFU) of age- and sex-matched wild-type or Tax1bp1-deficient mice (male and female, age 8–12 weeks) was performed with M. tuberculosis Erdman strain using the Glass-Col Inhalation Exposure System. One day after infection, infected mice were euthanized, the lungs were homogenized, and CFU were enumerated on 7H10 agar plates supplemented with 10% OADC and 0.5% glycerol to determine the inoculum. On days 9, 11, 21, and 50 days after infection, the lung was divided into portions. The superior lobe of the right lung was fixed in 10% buffered formalin for histologic analysis. The remainder of the right lung and left lung were combined and homogenized in 1 ml of PBS containing 0.05% Tween80 in a Bullet Blender Tissue Homogenizer (Next Advance). The spleen and liver were homogenized in 400 μl or 2 ml of PBS containing 0.05% Tween80, respectively. For measurement of CFU, organ homogenates were serially diluted and plated on 7H10 agar plates supplemented with 10% OADC and 0.5% glycerol. For measurement of cytokines from the lung homogenate, protein extraction was performed by combining 700 μl of homogenate with 700 μl of Tissue Protein Extraction Reagent (T-PER; Thermo Scientific) containing cOmplete, mini, EDTA-free protease inhibitor cocktail (Roche 11836170001) at 2X concentration. Samples were incubated for 20 min at 4°C, vortexed, and centrifuged at 12,000 x g for 10 min. The supernatants were filter sterilized with a 0.22 μm filter and stored at −80°C until further analysis. For survival experiments, mice were sacrificed after 15% loss of maximum body weight.
Cytokine measurements
Cytokines from lung lysates were measured using DuoSet ELISA kits (R&D systems) following the manufacturer’s protocol. Interferon levels were measured from the Mtb-infected lung lysates using L929 ISRE-luciferase reporter cells as previously described (94). Luciferase reporter cells were seeded in a 96-well plate for 24 hours. Lung lysates were incubated with the reporter cells for 8 hours, and luciferase activity was measured with the Luciferase Assay Report Assay (Promega) using the manufacturer’s protocol.
Histology sample processing and quantitative analysis
Formalin-fixed specimens were washed three times in PBS and stored in 70% ethanol. Histologic processing was performed by Histowiz. Serial ultrathin sections were stained for hematoxylin & eosin (H&E), ubiquitin (anti-ubiquitylated antibody, AB1690, EMD-Millipore, 1:100 dilution), tuberculosis (Abcam ab214721, 1:1000 dilution), or myeloperoxidase (Abcam ab9535, 1:50 dilution). Primary antibodies were detected with 3,3’-diaminobenzidine (DAB) staining. The ubiquitin and tuberculosis IHC images were aligned and combined using image registration scripts in QuPath. The MPO staining analysis was analyzed using Indica Labs Halo image analysis software. Cells were segmented using the Multiplex IHC algorithm v3.1.4 in Halo and MPO-positive cells were determined by thresholding the DAB channel. Positive cells were further sub-divided into Low intensity, Medium Intensity and High intensity bins to allow for subsequent calculation of a H-score based on percentage of cells positive for Low, Medium and High using the following formula: H-Score = (1 x % positive cells low) + (2 x % positive cells medium) + (3 x % positive cells high). Cells in all the intensity bins were considered positive for myeloperoxidase staining.
Images of tuberculosis lesions were exported from QuPath into ImageJ. A minimum threshold of two standard deviations above the mean signal was applied to filter positive pixels for ubiquitin and tuberculosis immunohistochemistry images. Colocalization was calculated from pixel overlap in images of the tuberculosis and ubiquitin immunohistochemistry. A veterinary pathologist analyzed the H&E histopathology images.
M. tuberculosis mouse infections at UC San Francisco
Tax1bp1−/− mice were imported from UC Berkeley and rederived by the UC San Francisco Rederivation Core to eliminate the potential for any interinstitutional murine pathogen transmission. Low-dose aerosol infections (100–200 CFU) of age- and sex-matched wild-type and Tax1bp1−/− mice were performed with a Glass-col inhalation chamber. At the indicated time points, mice were euthanized with CO2, and their lungs were minced with scissors and digested in 3 ml of RPMI-1640 with 5% heat-inactivated FBS containing 1 mg/ml collagenase D (Sigma) and 30 μg/ml DNAseI (Sigma) for 30 min at 37°C. Cells were processed with a gentleMACS dissociator (Miltenyi Biotec, lung program 2) and filtered through a 70 μm strainer. The samples were rinsed with 1 ml of FACS buffer (PBS with 3% heat-inactivated FBS, 2 mM EDTA). Residual tissue on the cell strainer was further processed using a syringe plunger and rinsed with 1 ml of FACS buffer. The cell suspension was then centrifuged at 650 ˣ g for 3 minutes at 4 °C, and the supernatant was discarded. The cell pellet was resuspended in 3 ml of ACK lysis buffer (Gibco) to lyse the RBCs, and lysis was quenched with 3 ml of FACS buffer solution. After centrifuging the cell suspension at 650 ˣ g for 3 minutes at 4 °C, the supernatant was removed, and the cells were resuspended in 1 ml of FACS buffer. Each cell suspension was pooled (from 5 mice) and passed through a 50-μm strainer.
Single-cell lung suspensions were stained with the Zombie Aqua Fixable Viability kit (1:200 dilution, BioLegend, #423101) and treated with CD16/CD32 Fc block (1:100 dilution, BD 553142) in PBS (1 ml) for 15 min. The samples were centrifuged at 650 ˣ g for 3 minutes at 4 °C, and the supernatants were removed. Cells were stained with 2 ml of antibody mixture diluted in Brilliant Stain Buffer (Table 1; Invitrogen #00–4409-42) for 30 min at 4°C. Antibodies diluted in Brilliant Stain Buffer (BD, #566349) were added to the cells. Antibody staining was performed for 30 minutes at 4°C. Subsequently, the cell suspensions were centrifuged at 650 g for 3 minutes at 4°C, washed with 1 ml of FACS buffer solution, resuspended in 3 ml of FACS buffer, and passed through a 50-μm strainer. Cell subsets were sorted using a BD Aria Fusion Sorter through a 100 μm nozzle using the 4-way purity mode.
Table 1.
Key Resources.
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Biological sample (M. musculus) | Primary bone marrow-derived macrophages, AMs, and peritoneal exudate cells, wild-type C57BL/6J | Jackson Laboratory | Stock # 000664 | |
| Biological sample (M. musculus) | Primary bone marrow-derived macrophages, AMs, and peritoneal exudate cells, Tax1bp1−/− | (36) | Tax1bp1 −/− | |
| Antibody | Anti-ubiquitylated antibody | EMD-Millipore | AB1690 | 1:100 dilution |
| Antibody | Anti-Mycobacterium tuberculosis antibody | Abcam | ab214721 | 1:1000 dilution |
| Antibody | Anti-Myeloperoxidase antibody | Abcam | ab9535 | 1:50 dilution |
| Antibody | Anti-LC3 (2G6) | NanoTools | 0260-100/LC3-2G6 | 1:200 |
| Antibody | Anti-ubiquitinylated proteins antibody (FK2) | Sigma-Aldrich | 04–263 | 1:400 |
| Antibody | PE Rat Anti-Mouse Siglec-F | BD Biosciences | BDB552126 | 1:200 |
| Antibody | Anti-mouse CD16/CD32 Fc block | BD Biosciences | BD553142 | 1:100 |
| Antibody | PE/Cyanine5 anti-mouse CD90.2 (Thy1.2) | BioLegend | 105314 | 1:300 |
| Antibody | PE/Cyanine5 anti-mouse CD19 | BioLegend | 115510 | 1:300 |
| Antibody | PE/Cyanine5 anti-mouse NK-1.1 | BioLegend | 108716 | 1:200 |
| Antibody | PE/Cyanine7 anti-mouse Ly-6C | BioLegend | 128018 | 1:300 |
| Antibody | Brilliant Violet 421 anti-mouse Ly-6G | BioLegend | 127628 | 1:200 |
| Antibody | Brilliant Violet 605 anti-mouse CD11c | BioLegend | 117334 | 1:200 |
| Antibody | Brilliant Violet 711 anti-mouse/human CD11b | BioLegend | 101242 | 1:200 |
| Antibody | Alexa Fluor 647 anti-mouse MHCII | BioLegend | 107618 | 1:300 |
| Commercial assay | Luciferase assay system | Promega | E1500 | |
| Commercial kit | Mouse IL-6 DuoSet ELISA | R and D | DY406 | |
| Commercial kit | Mouse TNF-α DuoSet ELISA | R and D | DY410 | |
| Commercial kit | Mouse IL-12/IL-23 p40 allele-specific DuoSet ELISA | R and D | DY499 | |
| Commercial kit | Cytometric Bead Array Mouse Inflammation Kit | BD Biosciences | 552364 | |
| Commercial kit | Mouse IFN-β ELISA Kit, high sensitivity | PBL Assay Science | 42410–1 | |
| Commercial kit | Prostaglandin E2 Express ELISA Kit | Cayman Chemicals | 500141 | |
| Commercial kit | Q5 Site Directed Mutagenesis kit | New England Biolabs | E0554S | |
| Commercial kit | NEBuilder HiFi DNA Assembly Cloning Kit | New England Biolabs | E5520S | |
| Commercial kit | LR Clonase II Enzyme Mix | Invitrogen | 11791020 | |
| Strain | Lenti-X 293T | TakaraBio | 632180 | |
| Strain | Mtb: Erdman | ATCC | 35801 | |
| Strain | Listeria monocytogenes 10403S | (99) | ||
| Strain | Mtb: H37Rv (pMV306hsp-LuxG13) | Plasmid from Addgene | 26161 | |
| Strain | Mtb; Erdman (pMV261::ZsGreen) | (10) |
Bacteria were quantified from sorted cells by serial dilution in PBS containing 0.05% Tween80 and plated on 7H10 agar plates supplemented with 10% Middlebrook OADC, 0.5% glycerol, and PANTA antibiotic mixture at a 1:500 dilution to reduce contamination risk from non-mycobacteria during organ dissection. BD PANTA antibiotic mixture (BD, B4345114) containing polymyxin B, amphotericin B, nalidixic acid, trimethoprim, and azlocillin was prepared by dissolving the contents of 1 lyophilized vial in 3 ml of OADC.
Bone marrow-derived macrophage infection
Bone marrow-derived macrophage infections with Listeria monocytogenes 10403S were performed as previously described (49–51).
Peritoneal cell exudate infection
Approximately 7 ml of ice-cold PBS was injected into the peritoneum of euthanized mice. Peritoneal exudate cells were treated with ACK lysis buffer, resuspended in tissue culture cell media (RPMI supplemented with 1 mM L-glutamine and 10% fetal bovine serum), and seeded into 24-well plates with glass coverslips at a density of 1.5 ˣ 106 cells/ml for 24-hours prior to infection. Prior to infection, non-adherent cells were removed by replacement of the tissue culture media.
Listeria monocytogenes 10403S was inoculated from a single colony into BHI media. Following overnight incubation at 30 °C, bacteria were washed in PBS and resuspended to an OD of 1.5 in PBS. Bacteria were diluted 1:1000 in tissue culture cell media for infection of peritoneal exudate cells. 30 minutes post-infection, cells were rinsed twice with PBS, and fresh media was replaced. At 1 hour post-infection, gentamicin sulfate was added at a final concentration of 50 μg/ml to kill extracellular bacteria. At 2- and 8 hours post-infection, coverslips were placed in 5 ml of water and vortexed. Serial dilutions were plated on LB agar supplemented with streptomycin (200 μg/ml). CFU were enumerated after 24 hours of incubation at 37 °C. Infections were performed with 3 coverslips for each experimental condition.
L. monocytogenes mouse infections
Age and sex-matched male and female mice were infected with L. monocytogenes. A 2 ml overnight culture of L. monocytogenes grown in brain heart infusion media at 30 °C slanted. L. monocytogenes was subcultured in 5.5 ml of BHI media and incubated with shaking at 37 °C until reaching an optical density between 0.4–0.8. The bacteria were washed and diluted in PBS to achieve an inoculum of approximately 5 ˣ 105 CFU/ml. 200 μl of this suspension was injected into the tail vein of the mice. For the intraperitoneal infection, the bacterial cells were prepared as described, and 200 μl of bacterial suspension was injected into the peritoneum at a dose of 3.74 ˣ 105 CFU/mouse. At the indicated time points, the spleen and liver were harvested in water containing 0.1% NP-40. Organs were homogenized, and CFU were enumerated on LB agar supplemented with streptomycin (200 μg/ml).
Alveolar macrophage (AM) isolation and culture
AMs were harvested from mice by bronchoalveolar lavage with 10 ml of PBS containing 2 mM EDTA, 0.5% fetal bovine serum (FBS) pre-warmed to 37°C as described previously (68,69). AMs were seeded at a density of 100,000 cells/well in 96-well plates. For short-term cultivation up to 4 days, AMs were cultured in RPMI-1640 medium supplemented with 10% (v/v) FBS, 2 mM GlutaMAX, 10 mM HEPES and 100 U ml–1 penicillin–streptomycin. After allowing at least 2 hours for adhesion, the media was replaced with fresh media without antibiotics. For cultivation >4 days, AMs were cultured in RPMI-1640 medium supplemented with 10% (v/v) FBS, 2 mM GlutaMAX, 1 mM sodium pyruvate, and 2% (v/v) GM-CSF supernatant produced by a B16 murine melanoma cell line.
Macrophage infections with Mtb
Mtb H37Rv strain was transformed with pMV306hsp-LuxG13 for expression of LuxCDABE. Logarithmic phase cultures of Mtb (H37Rv-Lux or wild-type Erdman strain) were grown in 7H9 media supplemented with 10% Middlebrook OADC, 0.5% glycerol, 0.05% Tween80 in inkwell bottles at 37°C with rotation at 100 rpm. Mtb cell pellets were washed twice with PBS followed by centrifugation for 5 min at 1462 ˣ g and sonication to remove and disperse clumps. Mtb was resuspended in RPMI with 10% horse serum. Media was removed from the macrophage monolayers, the bacterial suspension was overlaid, and centrifugation was performed for 10 min at 162 ˣ g. Following infection, the media was replaced with cultivation media with 15 ng/ml IFN-γ (Peprotech) or without IFN-γ. In experiments performed with luminescent Mtb, luminescence measurements were obtained daily following media changes daily using a GloMax microplate reader (Promega). For CFU measurements, the monolayers were washed with PBS, lysed in PBS with 0.05% Tween80, serially diluted, and spread on 7H10 agar plates supplemented with 10% Middlebrook OADC and 0.5% glycerol. CFU were enumerated after 21 days of incubation at 37°C.
Gene expression analysis during Mtb infection of AMs
AMs were infected with wild-type Mtb Erdman at a M.O.I. of 2. At 36-hours post-infection, the monolayers were washed with PBS, and the AMs were lysed in 200 μl of Trizol reagent. Samples were pooled from four technical replicate wells. The experiment was performed independently three times (i.e. three independent biological replicates). Mtb was centrifuged, the supernatant containing host RNA was removed, and the Mtb pellet was resuspended in 400 μl of fresh Trizol and 0.1 mm zirconia/silica beads. Mtb was mechanically disrupted with the Mini Bead-Beater Plus (Biospec Products) as previously described (95). 70% of the sample containing host RNA was pooled with the sample containing Mtb RNA, the samples were treated with 200 μl of chloroform, and RNA was purified with the Trizol Plus RNA purification kit (Ambion). Purified total RNA was treated with DNAseI (ThermoFisher) and dried by rotary evaporation in RNA stabilization tubes (Azenta US, Inc.; South Plainfield, NJ, USA). Sample QC, dual rRNA depletion for bacteria and mouse, library preparation, Illumina sequencing (2×150 bp; 30M reads per sample), and differential gene expression analysis were performed by Azenta Life Sciences US, Inc.
Sample QC
Total RNA samples were quantified using Qubit 2.0 Fluorometer (Life Technologies, Carlsbad, CA, USA) and RNA integrity was checked with 4200 TapeStation (Agilent Technologies, Palo Alto, CA, USA).
Library Preparation and Sequencing
ERCC RNA Spike-In Mix kit (cat. 4456740) from ThermoFisher Scientific was added to normalized total RNA prior to library preparation following manufacturer’s protocol. rRNA depletion was performed using QIAGEN FastSelect rRNA Bacteria + HMR Kit or HMR/Bacteria (Qiagen, Germantown, MD, USA), which was conducted following the manufacturer’s protocol. RNA sequencing libraries were constructed with the NEBNext Ultra II RNA Library Preparation Kit for Illumina by following the manufacturer’s recommendations. Briefly, enriched RNAs are fragmented for 15 minutes at 94 °C. First strand and second strand cDNA are subsequently synthesized. cDNA fragments are end repaired and adenylated at 3’ends, and universal adapters are ligated to cDNA fragments, followed by index addition and library enrichment with limited cycle PCR. Sequencing libraries were validated using the Agilent Tapestation 4200 (Agilent Technologies, Palo Alto, CA, USA), and quantified using Qubit 2.0 Fluorometer (ThermoFisher Scientific, Waltham, MA, USA) as well as by quantitative PCR (KAPA Biosystems, Wilmington, MA, USA).
The sequencing libraries were multiplexed and clustered onto a flowcell on the Illumina NovaSeq instrument according to manufacturer’s instructions. The samples were sequenced using a 2×150bp Paired End (PE) configuration. Image analysis and base calling were conducted by the NovaSeq Control Software (NCS). Raw sequence data (.bcl files) generated from Illumina NovaSeq was converted into fastq files and de-multiplexed using Illumina bcl2fastq 2.20 software. One mis-match was allowed for index sequence identification.
Data Analysis
After investigating the quality of the raw data, sequence reads were trimmed to remove possible adapter sequences and nucleotides with poor quality using Trimmomatic v.0.36. The trimmed reads were mapped to the Mus musculus and Mtb Erdman strain reference genomes available on ENSEMBL using the STAR aligner v.2.5.2b. BAM files were generated as a result of this step. Unique gene hit counts were calculated by using feature Counts from the Subread package v.1.5.2. Only unique reads that fell within exon regions were counted.
Using DESeq2, a comparison of gene expression between the groups of samples was performed. The Wald test was used to generate p-values and Log2 fold changes. Genes with adjusted p-values < 0.05 and absolute log2 fold changes >1 were called as differentially expressed genes for each comparison.
Cytokine analysis of Mtb-infected AMs
AMs were infected with wild-type Mtb Erdman strain at a MOI of 10. At 24 hours post-infection, the supernatants were filtered through a 0.2 μm syringe filter and analyzed by ELISA for IFN-β (PBL Assay Bioscience), TNF-α, and IL-1β (R&D systems) as previously described, and prostaglandin E2 (Cayman Chemicals).
Live cell imaging
AMs were infected with Mtb at a MOI of 1, and 0.1 μg ml−1 of propidium iodide (LifeTechnologies) and two drops per milliliter of CellEvent Caspase-3/7Green ReadyProbes reagent (Invitrogen) were added to the media at the beginning of the infection to measure necrosis/late apoptosis and apoptosis, respectively. Fluorescence and phase contrast images were obtained at 20x magnification with a Keyence BZ-X 700 microscope. Images were obtained daily in three technical replicate wells per condition and at two positions in each well. Quantification of the number of necrotic and apoptotic cells was performed with ImageJ version 1.54f as described previously (46). Images were converted to 8-bit (grayscale), binarized, and enumerated using the analyze particles module (size threshold 0.001-infinity).
Immunofluorescence microscopy
AMs were infected with fluorescent Mtb at a MOI of 2. At 8- and 24-hours post-infection, monolayers were washed with PBS, fixed with 4% PFA for 20 minutes, washed with PBS, and stained with anti-LC3 or anti-ubiquitin primary antibodies and AlexaFluor-647 conjugated secondary antibodies as previously described (43). Images were obtained at 63x magnification from quadruplicate wells per condition, in 69 x/y positions, and 4 z positions (0 μm, 0.5 μm, 1 μm, and 1.5 μm) with an Opera Phenix microscope (Perkin Elmer). Colocalization analysis of LC3, ubiquitin, and Mtb was performed with Harmony version 4.9 (Perkin Elmer) using the following analysis parameters. The four z stack images in each x/y position were processed into a maximum projection. Nuclei were identified in the DAPI channel using Method B with a common threshold of 0.07 and an area threshold of > 20 μm2. Cytoplasm was identified in the AlexaFluor 647 channel using Method A with an individual threshold of 0.06. The find spot module was used to identify LC3 or ubiquitin “spots” in the AlexaFluor 647 channel using method C with a contrast setting of 0.42, uncorrected spot to region intensity of 3.8, and default radius. Mtb were identified in the AlexaFluor 488 channel using the find spot module method B with a detection sensitivity of 0.5 and splitting sensitivity of 0.5. To identify Mtb that colocalized with LC3 or ubiquitin “spots”, the select population module was used for the Mtb population with the select by mask method. The percent colocalization was calculated for each well from all the images obtained in each well using the evaluation module.
Lentiviral transduction of AMs for Tax1bp1 phosphomutant expression
Tax1bp1 was amplified by PCR from murine cDNA using the primers named Tax1bp1 fwd and rev (Table 2) and inserted by ligation-independent cloning (NEBuilder Builder HiFi DNA Assembly Cloning Kit, NEB #E5520S) into pENTR1A no ccdB (w48–1; Addgene #17398) previously modified to express N-terminal 3x Flag-tagged proteins (96). Site-directed mutagenesis was performed to engineer alanine or glutamic acid substitution mutations in Tax1bp1 using the primers listed in Table 2 and the Q5 site-directed mutagenesis kit (New England Biolabs). Open reading frames (ORFs) were transferred into the pLENTI CMV Puro DEST (w118–1) vector using the Gateway LR Clonase II enzyme mix (Invitrogen #11791020). Lenti-X 293T cells (Takara) were transfected with lentiviral packaging vector psPAX2 (Addgene #12260), envelop vector pMD2.G (Addgene #12259), and Flag-tagged Tax1bp1 or empty destination vector (pLenti CMV Puro DEST (w118–1) as previously described (97). The supernatant containing lentivirus was filtered in a 0.45 μm syringe filter. AMs harvested from mice were infected with lentivirus by centrifugation at 1000 ˣ g for 30 min at 32°C. Transduced AMs were allowed to recover and expand for 7 days prior to harvesting with ESGRO Complete Accutase containing 1 mM EGTA (98). AMs were seeded at a density of 100,000 cells per well in 96-well plates and infected with Mtb at a M.O.I. of 0.5 the subsequent day. Media was changed daily. Four days post-infection, monolayers were washed with PBS, lysed, and plated for Mtb CFU.
Table 2.
Primers used in this study.
| Primer Name | Primer Sequence |
|---|---|
| Tax1bp1 fwd | cgacgacgacaaggcagcggctgcaacatcctttcaagaagtccaattgcag |
| Tax1bp1 rev | ctcgagtgcggccgcgaattctgcctagtcgaagttgagaacattctgatcaa |
| Tax1bp1 S693A fwd | gcgagtcccagcttgggaaga |
| Tax1bp1 S693A rev | acaggtggcctttggatttc |
| Tax1bp1 S693E fwd | gcgagtcccagaatgggaagacaatg |
| Tax1bp1 S693E rev | acaggtggcctttggatt |
| Tax1bp1 S619A fwd | acttacaaaggctttagaagatcaaaaaggaaggaaattg |
| Tax1bp1 S619AE rev | tccttctccctggagaga |
| Tax1bp1 S619E fwd | acttacaaaggagttagaagatcaaaaaggaaggaaattg |
Data availability statement
Confocal microscopy images are available on the Dryad repository (DOI: 10.5061/dryad.44j0zpcq6). RNA sequencing files, including the differential gene expression analysis, can be accessed on GEO, accession GSE280399. Flow cytometry data files are available upon request.
Statistical analysis
GraphPad Prism (v.10.3.1) was used for statistical analysis. Unpaired t-test comparisons were calculated assuming Gaussian distributions and the p-values were reported. In experiments with more than two experimental conditions, p-values from the t-test comparison between two groups were adjusted for the FDR (multiple comparisons) using the two-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli (Figure 4, Figure 8, Figure 8-figure supplement 2). In experiments with more than two experimental conditions, the comparison between multiple groups was performed by ordinary one-way ANOVA with Tukey’s multiple comparisons test, and the adjusted p-values were reported (Figure 9).
Supplementary Material
Acknowledgments
We acknowledge support from Professor Daniel A. Portnoy for infections with Listeria monocytogenes. This work was supported by NIH grants K08 AI146267 (JB), U19 AI135990 (NJK, JSC), P01 AI063302 (JSC), U19 AI106754 (JSC), DP1 AI124619 (JSC), and R01 AI120694 (JSC). JMB was also supported by the UCSF Nina Ireland Program in the Health Award, Cystic Fibrosis Foundation Harry Shwachman Award, Mentored Scientist in Tuberculosis Award (R25AI147375), and TB RAMP program (R25AI147375). R.R.-L was supported by a grant from the National Academies of Sciences, Engineering, and Medicine (Ford Foundation Fellowship) and the University of California Dissertation-Year Fellowship.
References
- 1.Stutz MD, Clark MP, Doerflinger M, Pellegrini M. Mycobacterium tuberculosis: Rewiring host cell signaling to promote infection. J Leukoc Biol. 2018. Feb;103(2):259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goren MB, D’Arcy Hart P, Young MR, Armstrong JA. Prevention of phagosome-lysosome fusion in cultured macrophages by sulfatides of Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1976. Jul;73(7):2510–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeong E-K, Lee H-J, Jung Y-J. Host-Directed Therapies for Tuberculosis. Pathogens. 2022. Nov 3;11(11). [Google Scholar]
- 4.Tobin DM. Host-Directed Therapies for Tuberculosis. Cold Spring Harb Perspect Med. 2015. May 18;5(10). [Google Scholar]
- 5.World Health Organization. Global Tuberculosis Report 2023. Geneva: World Health Organization; 2023. Nov.
- 6.Cohen SB, Gern BH, Delahaye JL, Adams KN, Plumlee CR, Winkler JK, et al. Alveolar Macrophages Provide an Early Mycobacterium tuberculosis Niche and Initiate Dissemination. Cell Host Microbe. 2018. Sep 12;24(3):439–446.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang L, Nazarova EV, Tan S, Liu Y, Russell DG. Growth of Mycobacterium tuberculosis in vivo segregates with host macrophage metabolism and ontogeny. J Exp Med. 2018. Apr 2;215(4):1135–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothchild AC, Olson GS, Nemeth J, Amon LM, Mai D, Gold ES, et al. Alveolar macrophages generate a noncanonical NRF2-driven transcriptional response to Mycobacterium tuberculosis in vivo. Sci Immunol. 2019. Jul 26;4(37). [Google Scholar]
- 9.Wolf AJ, Desvignes L, Linas B, Banaiee N, Tamura T, Takatsu K, et al. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J Exp Med. 2008. Jan 21;205(1):105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng W, Chang I-C, Limberis J, Budzik JM, Zha BS, Howard Z, et al. Mycobacterium tuberculosis resides in lysosome-poor monocyte-derived lung cells during chronic infection. PLoS Pathog. 2024. May 3;20(5):e1012205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez-Juarrero M, Shim TS, Kipnis A, Junqueira-Kipnis AP, Orme IM. Dynamics of macrophage cell populations during murine pulmonary tuberculosis. J Immunol. 2003. Sep 15;171(6):3128–35. [DOI] [PubMed] [Google Scholar]
- 12.Sköld M, Behar SM. Tuberculosis triggers a tissue-dependent program of differentiation and acquisition of effector functions by circulating monocytes. J Immunol. 2008. Nov 1;181(9):6349–60. [DOI] [PubMed] [Google Scholar]
- 13.Wolf AJ, Linas B, Trevejo-Nuñez GJ, Kincaid E, Tamura T, Takatsu K, et al. Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J Immunol. 2007. Aug 15;179(4):2509–19. [DOI] [PubMed] [Google Scholar]
- 14.Lee J, Boyce S, Powers J, Baer C, Sassetti CM, Behar SM. CD11cHi monocyte-derived macrophages are a major cellular compartment infected by Mycobacterium tuberculosis. PLoS Pathog. 2020. Jun 16;16(6):e1008621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014. Jul 17;41(1):21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reiling N, Hölscher C, Fehrenbach A, Kröger S, Kirschning CJ, Goyert S, et al. Cutting edge: Toll-like receptor (TLR)2- and TLR4-mediated pathogen recognition in resistance to airborne infection with Mycobacterium tuberculosis. J Immunol. 2002. Oct 1;169(7):3480–4. [DOI] [PubMed] [Google Scholar]
- 17.Sugawara I, Yamada H, Li C, Mizuno S, Takeuchi O, Akira S. Mycobacterial infection in TLR2 and TLR6 knockout mice. Microbiol Immunol. 2003;47(5):327–36. [DOI] [PubMed] [Google Scholar]
- 18.Villaseñor T, Madrid-Paulino E, Maldonado-Bravo R, Urbán-Aragón A, Pérez-Martínez L, Pedraza-Alva G. Activation of the Wnt Pathway by Mycobacterium tuberculosis: A Wnt-Wnt Situation. Front Immunol. 2017. Feb 1;8:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jani C, Solomon SL, Peters JM, Pringle SC, Hinman AE, Boucau J, et al. TLR2 is non-redundant in the population and subpopulation responses to Mycobacterium tuberculosis in macrophages and in vivo. mSystems. 2023. Aug 31;8(4):e0005223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manzanillo PS, Shiloh MU, Portnoy DA, Cox JS. Mycobacterium tuberculosis activates the DNA-dependent cytosolic surveillance pathway within macrophages. Cell Host Microbe. 2012. May 17;11(5):469–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watson RO, Bell SL, MacDuff DA, Kimmey JM, Diner EJ, Olivas J, et al. The Cytosolic Sensor cGAS Detects Mycobacterium tuberculosis DNA to Induce Type I Interferons and Activate Autophagy. Cell Host Microbe. 2015. Jun 10;17(6):811–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watson RO, Manzanillo PS, Cox JS. Extracellular M. tuberculosis DNA targets bacteria for autophagy by activating the host DNA-sensing pathway. Cell. 2012. Aug 17;150(4):803–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004. Dec 17;119(6):753–66. [DOI] [PubMed] [Google Scholar]
- 24.Singh SB, Davis AS, Taylor GA, Deretic V. Human IRGM induces autophagy to eliminate intracellular mycobacteria. Science. 2006. Sep 8;313(5792):1438–41. [DOI] [PubMed] [Google Scholar]
- 25.Manzanillo PS, Ayres JS, Watson RO, Collins AC, Souza G, Rae CS, et al. The ubiquitin ligase parkin mediates resistance to intracellular pathogens. Nature. 2013. Sep 26;501(7468):512–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanley SA, Johndrow JE, Manzanillo P, Cox JS. The Type I IFN response to infection with Mycobacterium tuberculosis requires ESX-1-mediated secretion and contributes to pathogenesis. J Immunol. 2007. Mar 1;178(5):3143–52. [DOI] [PubMed] [Google Scholar]
- 27.Kotov DI, Lee OV, Fattinger SA, Langner CA, Guillen JV, Peters JM, et al. Early cellular mechanisms of type I interferon-driven susceptibility to tuberculosis. Cell. 2023. Dec 7;186(25):5536–5553.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ji DX, Witt KC, Kotov DI, Margolis SR, Louie A, Chevée V, et al. Role of the transcriptional regulator SP140 in resistance to bacterial infections via repression of type I interferons. eLife. 2021. Jun 21;10. [Google Scholar]
- 29.Cheng Y, Schorey JS. Mycobacterium tuberculosis-induced IFN-β production requires cytosolic DNA and RNA sensing pathways. J Exp Med. 2018. Nov 5;215(11):2919–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bullen CK, Singh AK, Krug S, Lun S, Thakur P, Srikrishna G, et al. MDA5 RNA-sensing pathway activation by Mycobacterium tuberculosis promotes innate immune subversion and pathogen survival. JCI Insight. 2023. Oct 23;8(20). [Google Scholar]
- 31.Domingo-Gonzalez R, Prince O, Cooper A, Khader SA. Cytokines and Chemokines in Mycobacterium tuberculosis Infection. Microbiol Spectr. 2016;4(5). [Google Scholar]
- 32.Monin L, Khader SA. Chemokines in tuberculosis: the good, the bad and the ugly. Semin Immunol. 2014. Dec;26(6):552–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slight SR, Khader SA. Chemokines shape the immune responses to tuberculosis. Cytokine Growth Factor Rev. 2013. Apr;24(2):105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cooper AM, Khader SA. The role of cytokines in the initiation, expansion, and control of cellular immunity to tuberculosis. Immunol Rev. 2008. Dec;226:191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yue J, López JM. Understanding MAPK signaling pathways in apoptosis. Int J Mol Sci. 2020. Mar 28;21(7). [Google Scholar]
- 36.Iha H, Peloponese J-M, Verstrepen L, Zapart G, Ikeda F, Smith CD, et al. Inflammatory cardiac valvulitis in TAX1BP1-deficient mice through selective NF-kappaB activation. EMBO J. 2008. Feb 20;27(4):629–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Descamps D, Peres de Oliveira A, Gonnin L, Madrières S, Fix J, Drajac C, et al. Depletion of TAX1BP1 Amplifies Innate Immune Responses during Respiratory Syncytial Virus Infection. J Virol. 2021. Oct 27;95(22):e0091221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waidmann O, Pleli T, Weigert A, Imelmann E, Kakoschky B, Schmithals C, et al. Tax1BP1 limits hepatic inflammation and reduces experimental hepatocarcinogenesis. Sci Rep. 2020. Oct 1;10(1):16264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parvatiyar K, Barber GN, Harhaj EW. TAX1BP1 and A20 inhibit antiviral signaling by targeting TBK1-IKKi kinases. J Biol Chem. 2010. May 14;285(20):14999–5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shembade N, Pujari R, Harhaj NS, Abbott DW, Harhaj EW. The kinase IKKα inhibits activation of the transcription factor NF-κB by phosphorylating the regulatory molecule TAX1BP1. Nat Immunol. 2011. Jul 17;12(9):834–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi YB, Zhang J, Vo MT, White J, He C, Harhaj EW. Phosphorylation of the selective autophagy receptor TAX1BP1 by canonical and noncanonical IκB kinases promotes its lysosomal localization and clearance of MAVS aggregates. BioRxiv. 2021. Jan 7;
- 42.Choi YB, Shembade N, Parvatiyar K, Balachandran S, Harhaj EW. TAX1BP1 Restrains Virus-Induced Apoptosis by Facilitating Itch-Mediated Degradation of the Mitochondrial Adaptor MAVS. Mol Cell Biol. 2017. Jan 1;37(1). [Google Scholar]
- 43.Budzik JM, Swaney DL, Jimenez-Morales D, Johnson JR, Garelis NE, Repasy T, et al. Dynamic post-translational modification profiling of Mycobacterium tuberculosis-infected primary macrophages. eLife. 2020. Jan 17;9. [Google Scholar]
- 44.Tumbarello DA, Manna PT, Allen M, Bycroft M, Arden SD, Kendrick-Jones J, et al. The autophagy receptor TAX1BP1 and the molecular motor myosin VI are required for clearance of salmonella typhimurium by autophagy. PLoS Pathog. 2015. Oct 9;11(10):e1005174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sarraf SA, Shah HV, Kanfer G, Pickrell AM, Holtzclaw LA, Ward ME, et al. Loss of TAX1BP1-Directed Autophagy Results in Protein Aggregate Accumulation in the Brain. Mol Cell. 2020. Dec 3;80(5):779–795.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Golovkine GR, Roberts AW, Morrison HM, Rivera-Lugo R, McCall RM, Nilsson H, et al. Autophagy restricts Mycobacterium tuberculosis during acute infection in mice. Nat Microbiol. 2023. May;8(5):819–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berry MPR, Graham CM, McNab FW, Xu Z, Bloch SAA, Oni T, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010. Aug 19;466(7309):973–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kimmey JM, Huynh JP, Weiss LA, Park S, Kambal A, Debnath J, et al. Unique role for ATG5 in neutrophil-mediated immunopathology during M. tuberculosis infection. Nature. 2015. Dec 24;528(7583):565–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mitchell G, Ge L, Huang Q, Chen C, Kianian S, Roberts MF, et al. Avoidance of autophagy mediated by PlcA or ActA is required for Listeria monocytogenes growth in macrophages. Infect Immun. 2015. May;83(5):2175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mitchell G, Cheng MI, Chen C, Nguyen BN, Whiteley AT, Kianian S, et al. Listeria monocytogenes triggers noncanonical autophagy upon phagocytosis, but avoids subsequent growth-restricting xenophagy. Proc Natl Acad Sci USA. 2018. Jan 9;115(2):E210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng MI, Chen C, Engström P, Portnoy DA, Mitchell G. Actin-based motility allows Listeria monocytogenes to avoid autophagy in the macrophage cytosol. Cell Microbiol. 2018. Sep;20(9):e12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zha BS, Desvignes L, Fergus TJ, Cornelius A, Cheng T-Y, Moody DB, et al. Bacterial Strain-Dependent Dissociation of Cell Recruitment and Cell-to-Cell Spread in Early M. tuberculosis Infection. MBio. 2022. Jun 28;13(3):e0133222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Herbst S, Schaible UE, Schneider BE. Interferon gamma activated macrophages kill mycobacteria by nitric oxide induced apoptosis. PLoS ONE. 2011. May 2;6(5):e19105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simwela NV, Jaecklein E, Sassetti CM, Russell DG. Impaired fatty acid import or catabolism in macrophages restricts intracellular growth of Mycobacterium tuberculosis. BioRxiv [Internet]. 2024. Oct 11 [cited 2024 Oct 20]; Available from:
- 55.Domenech P, Reed MB, Barry CE. Contribution of the Mycobacterium tuberculosis MmpL protein family to virulence and drug resistance. Infect Immun. 2005. Jun;73(6):3492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cosma CL, Klein K, Kim R, Beery D, Ramakrishnan L. Mycobacterium marinum Erp is a virulence determinant required for cell wall integrity and intracellular survival. Infect Immun. 2006. Jun;74(6):3125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kolly GS, Sala C, Vocat A, Cole ST. Assessing essentiality of transketolase in Mycobacterium tuberculosis using an inducible protein degradation system. FEMS Microbiol Lett. 2014. Sep;358(1):30–5. [DOI] [PubMed] [Google Scholar]
- 58.Sun Q-Y, Ding L-W, Johnson K, Zhou S, Tyner JW, Yang H, et al. SOX7 regulates MAPK/ERK-BIM mediated apoptosis in cancer cells. Oncogene. 2019. Aug;38(34):6196–210. [DOI] [PubMed] [Google Scholar]
- 59.Martin CJ, Booty MG, Rosebrock TR, Nunes-Alves C, Desjardins DM, Keren I, et al. Efferocytosis is an innate antibacterial mechanism. Cell Host Microbe. 2012. Sep 13;12(3):289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang C-T, Cambier CJ, Davis JM, Hall CJ, Crosier PS, Ramakrishnan L. Neutrophils exert protection in the early tuberculous granuloma by oxidative killing of mycobacteria phagocytosed from infected macrophages. Cell Host Microbe. 2012. Sep 13;12(3):301–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.De Valck D, Jin DY, Heyninck K, Van de Craen M, Contreras R, Fiers W, et al. The zinc finger protein A20 interacts with a novel anti-apoptotic protein which is cleaved by specific caspases. Oncogene. 1999. Jul 22;18(29):4182–90. [DOI] [PubMed] [Google Scholar]
- 62.Behar SM, Martin CJ, Booty MG, Nishimura T, Zhao X, Gan HX, et al. Apoptosis is an innate defense function of macrophages against Mycobacterium tuberculosis. Mucosal Immunol. 2011. May;4(3):279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen M, Divangahi M, Gan H, Shin DSJ, Hong S, Lee DM, et al. Lipid mediators in innate immunity against tuberculosis: opposing roles of PGE2 and LXA4 in the induction of macrophage death. J Exp Med. 2008. Nov 24;205(12):2791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Divangahi M, Behar SM, Remold H. Dying to live: how the death modality of the infected macrophage affects immunity to tuberculosis. Adv Exp Med Biol. 2013;783:103–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park JS, Tamayo MH, Gonzalez-Juarrero M, Orme IM, Ordway DJ. Virulent clinical isolates of Mycobacterium tuberculosis grow rapidly and induce cellular necrosis but minimal apoptosis in murine macrophages. J Leukoc Biol. 2006. Jan;79(1):80–6. [DOI] [PubMed] [Google Scholar]
- 66.Beckwith KS, Beckwith MS, Ullmann S, Sætra RS, Kim H, Marstad A, et al. Plasma membrane damage causes NLRP3 activation and pyroptosis during Mycobacterium tuberculosis infection. Nat Commun. 2020. May 8;11(1):2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Richter B, Sliter DA, Herhaus L, Stolz A, Wang C, Beli P, et al. Phosphorylation of OPTN by TBK1 enhances its binding to Ub chains and promotes selective autophagy of damaged mitochondria. Proc Natl Acad Sci USA. 2016. Apr 12;113(15):4039–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Busch CJ- L, Subramanian S, Linares J, Favret J, Yuda RAA, Sieweke MH. Isolation, ex vivo expansion, and lentiviral transduction of alveolar macrophages. Methods Mol Biol. 2024;2713:231–51. [DOI] [PubMed] [Google Scholar]
- 69.Subramanian S, Busch CJ- L, Molawi K, Geirsdottir L, Maurizio J, Vargas Aguilar S, et al. Long-term culture-expanded alveolar macrophages restore their full epigenetic identity after transfer in vivo. Nat Immunol. 2022. Mar;23(3):458–68. [DOI] [PubMed] [Google Scholar]
- 70.Yu X, Buttgereit A, Lelios I, Utz SG, Cansever D, Becher B, et al. The Cytokine TGF-β Promotes the Development and Homeostasis of Alveolar Macrophages. Immunity. 2017. Nov 21;47(5):903–912.e4. [DOI] [PubMed] [Google Scholar]
- 71.Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014. Dec 4;159(6):1312–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bain CC, MacDonald AS. The impact of the lung environment on macrophage development, activation and function: diversity in the face of adversity. Mucosal Immunol. 2022. Feb;15(2):223–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cunnick J, Kaur P, Cho Y, Groffen J, Heisterkamp N. Use of bone marrow-derived macrophages to model murine innate immune responses. J Immunol Methods. 2006. Apr 20;311(1–2):96–105. [DOI] [PubMed] [Google Scholar]
- 74.Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol. 2008. Jul;8(7):533–44. [DOI] [PubMed] [Google Scholar]
- 75.Gschwend J, Sherman SPM, Ridder F, Feng X, Liang H-E, Locksley RM, et al. Alveolar macrophages rely on GM-CSF from alveolar epithelial type 2 cells before and after birth. J Exp Med. 2021. Oct 4;218(10). [Google Scholar]
- 76.Guilliams M, De Kleer I, Henri S, Post S, Vanhoutte L, De Prijck S, et al. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med. 2013. Sep 23;210(10):1977–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schneider C, Nobs SP, Kurrer M, Rehrauer H, Thiele C, Kopf M. Induction of the nuclear receptor PPAR-γ by the cytokine GM-CSF is critical for the differentiation of fetal monocytes into alveolar macrophages. Nat Immunol. 2014. Nov;15(11):1026–37. [DOI] [PubMed] [Google Scholar]
- 78.Chen BD, Mueller M, Chou TH. Role of granulocyte/macrophage colony-stimulating factor in the regulation of murine alveolar macrophage proliferation and differentiation. J Immunol. 1988. Jul 1;141(1):139–44. [PubMed] [Google Scholar]
- 79.Akagawa KS, Kamoshita K, Tokunaga T. Effects of granulocyte-macrophage colony-stimulating factor and colony-stimulating factor-1 on the proliferation and differentiation of murine alveolar macrophages. J Immunol. 1988. Nov 15;141(10):3383–90. [PubMed] [Google Scholar]
- 80.Lacey DC, Achuthan A, Fleetwood AJ, Dinh H, Roiniotis J, Scholz GM, et al. Defining GM-CSF- and macrophage-CSF-dependent macrophage responses by in vitro models. J Immunol. 2012. Jun 1;188(11):5752–65. [DOI] [PubMed] [Google Scholar]
- 81.Van Dis E, Fox DM, Morrison HM, Fines DM, Babirye JP, McCann LH, et al. IFN-γ-independent control of M. tuberculosis requires CD4 T cell-derived GM-CSF and activation of HIF-1α. PLoS Pathog. 2022. Jul 25;18(7):e1010721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Queval CJ, Brosch R, Simeone R. The Macrophage: A Disputed Fortress in the Battle against Mycobacterium tuberculosis. Front Microbiol. 2017. Nov 23;8:2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xue Q, Yan D, Chen X, Li X, Kang R, Klionsky DJ, et al. Copper-dependent autophagic degradation of GPX4 drives ferroptosis. Autophagy. 2023. Jul;19(7):1982–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qiang L, Zhang Y, Lei Z, Lu Z, Tan S, Ge P, et al. A mycobacterial effector promotes ferroptosis-dependent pathogenicity and dissemination. Nat Commun. 2023. Mar 17;14(1):1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shembade N, Harhaj NS, Liebl DJ, Harhaj EW. Essential role for TAX1BP1 in the termination of TNF-alpha-, IL-1- and LPS-mediated NF-kappaB and JNK signaling. EMBO J. 2007. Sep 5;26(17):3910–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Deretic V, Levine B. Autophagy balances inflammation in innate immunity. Autophagy. 2018. Jan 17;14(2):243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011. Jan 20;469(7330):323–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Saitoh T, Akira S. Regulation of innate immune responses by autophagy-related proteins. J Cell Biol. 2010. Jun 14;189(6):925–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Deretic V. Autophagy in inflammation, infection, and immunometabolism. Immunity. 2021. Mar 9;54(3):437–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Painter JD, Galle-Treger L, Akbari O. Role of autophagy in lung inflammation. Front Immunol. 2020. Jul 7;11:1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Whang MI, Tavares RM, Benjamin DI, Kattah MG, Advincula R, Nomura DK, et al. The ubiquitin binding protein TAX1BP1 mediates autophagasome induction and the metabolic transition of activated T cells. Immunity. 2017. Mar 21;46(3):405–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Matsushita N, Suzuki M, Ikebe E, Nagashima S, Inatome R, Asano K, et al. Regulation of B cell differentiation by the ubiquitin-binding protein TAX1BP1. Sci Rep. 2016. Aug 12;6:31266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019. Apr 3;10(1):1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Woodward JJ, Iavarone AT, Portnoy DA. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science. 2010. Jun 25;328(5986):1703–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pisu D, Huang L, Rin Lee BN, Grenier JK, Russell DG. Dual RNA-Sequencing of Mycobacterium tuberculosis-Infected Cells from a Murine Infection Model. STAR Protocols. 2020. Dec 18;1(3):100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Johnson JR, Parry T, Repasy T, Geiger KM, Verschueren E, Budzik JM, et al. Comparative analysis of macrophage post-translational modifications during intracellular bacterial pathogen infection. BioRxiv. 2020. May 27;
- 97.Roberts AW, Popov LM, Mitchell G, Ching KL, Licht DJ, Golovkine G, et al. Cas9+ conditionally-immortalized macrophages as a tool for bacterial pathogenesis and beyond. eLife. 2019. Jun 17;8. [Google Scholar]
- 98.Busch CJ- L, Favret J, Geirsdóttir L, Molawi K, Sieweke MH. Isolation and Long-term Cultivation of Mouse Alveolar Macrophages. Bio Protoc. 2019. Jul 20;9(14). [Google Scholar]
- 99.Bécavin C, Bouchier C, Lechat P, Archambaud C, Creno S, Gouin E, et al. Comparison of widely used Listeria monocytogenes strains EGD, 10403S, and EGD-e highlights genomic variations underlying differences in pathogenicity. MBio. 2014. Mar 25;5(2):e00969–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Confocal microscopy images are available on the Dryad repository (DOI: 10.5061/dryad.44j0zpcq6). RNA sequencing files, including the differential gene expression analysis, can be accessed on GEO, accession GSE280399. Flow cytometry data files are available upon request.