Abstract
The sustainability of maize cultivation would benefit tremendously from early sowing, but is hampered by low temperatures during early development in temperate climates. We show that allelic variation within the gene encoding subunit M of the NADH-dehydrogenase-like (NDH) complex (ndhm1) in a European maize landrace affects several quantitative traits that are relevant during early development in cold climates through NDH-mediated cyclic electron transport around photosystem I, a process crucial for photosynthesis and photoprotection. Beginning with a genome-wide association study for maximum potential quantum yield of photosystem II in dark-adapted leaves (Fv/Fm), we capitalized on the large phenotypic effects of a hAT transposon insertion in ndhm1 on multiple quantitative traits (early plant height [EPH], Fv/Fm, chlorophyll content, and cold tolerance) caused by the reduced protein levels of NDHM and associated NDH components. Analysis of the ndhm1 native allelic series revealed a rare allele of ndhm1 that is associated with small albeit significant improvements of Fv/Fm, photosystem II efficiency in light-adapted leaves (ΦPSII), and EPH compared with common alleles. Our work showcases the extraction of favorable alleles from locally adapted landraces, offering an efficient strategy for broadening the genetic variation of elite germplasm by breeding or genome editing.
A European maize landrace harbors favorable allelic diversity in a gene encoding a subunit of the NADH-dehydrogenase-like complex, which can be harnessed to improve quantitative traits in maize.
Introduction
With more than 200 million hectares of maize (Zea mays L.) harvested in 2022 (∼ 16% of global arable land; FAO 2024), enhancing the sustainability of its cultivation presents a significant opportunity to improve the environmental impact of agriculture worldwide. Earlier planting of maize increases resource efficiency with respect to available nutrients and water while simultaneously avoiding leakage of nitrogen into groundwater. Further, the introduction of herbicides into the environment can be decreased by earlier soil coverage and fast early development through increased competition with weeds (Wezel et al. 2014; Nouri et al. 2022). Early sowing dates lead to earlier soil coverage, which benefits resource efficiency of maize cultivation and increases biomass accumulation by extending the vegetative period and avoiding summer drought during flowering (Frei 2000; Kucharik 2006; Parker et al. 2017). However, the earlier sowing occurs, the higher is the risk for maize plants of encountering cold temperatures in the initial stages of development, which can have adverse effects on various physiological parameters (Lainé et al. 2023). It has been shown that cold stress decreases biomass accumulation due to impaired photosynthetic parameters such as net CO2 assimilation, chlorophyll content, and maximal quantum efficiency of photosystem II (Fv/Fm; Leipner et al. 1999; Burnett and Kromdijk 2022; Lainé et al. 2023). Thus, genetic improvement of maize early development for an earlier soil coverage is a promising approach to increase the sustainability of global agriculture.
Development of genetically improved crops builds on genetic variation for traits of interest originating either from standing variation within a crop and its close relatives or from the introduction of new variation through biotechnology (Liang et al. 2021). Introduction of new genetic variation can be untargeted by randomly generating mutations or by directly targeting candidate genes (Lorenzo et al. 2023; Marone et al. 2023). Untargeted approaches do not require a priori knowledge about biological processes underlying trait expression, but the frequency of favorable mutations is generally low, thus requiring large screening populations to identify desirable phenotypes (Liu et al. 2020; Lorenzo et al. 2023). Targeted approaches, on the other hand, require information about the genes underlying the traits of interest, which is often not available for important quantitative traits. In addition, the engineering of alleles that outperform their native counterparts requires in-depth knowledge about the functional mechanisms underlying the targeted process, especially in complex biological networks. Here, we demonstrate the identification of a biological process that influences traits of interest by a large-effect quantitative trait locus (QTL) followed by extensive analysis of allelic variation at the locus, which revealed a beneficial allele for early development and photosynthetic traits.
In a doubled-haploid (DH) library derived from 3 landraces, Mayer et al. (2020) mapped haplotype-trait associations for a number of quantitative traits related to early plant growth. In their phenotypic evaluation of landrace-derived DH lines in 11 different field environments, minimum temperatures ranged from −6 °C to 3.5 °C, imposing considerable chilling and freezing stress on the plants during early development (Hölker et al. 2019). They identified several QTL associated with early plant height (stages V4 and V6, EPH), potentially linked to biomass accumulation under cold stress.
Building on the results of Mayer et al. (2020), we investigated the functional consequences of native allelic variation at a QTL on maize chromosome 2 with strong effects on EPH and the photosynthesis-related parameters potential maximum quantum efficiency of photosystem II (Fv/Fm) and chlorophyll content in the field. Our objectives were to functionally characterize the effects of the QTL on the 3 traits, analyze the allelic diversity of candidate genes in the population and explore whether trait expression is temperature-dependent. By genetic and functional characterization of the QTL for EPH, Fv/Fm, and chlorophyll content, we pinned down NADH-dehydrogenase-like subunit M 1 (ndhm1) as causal gene with pleiotropic effects on all 3 traits. We identified a rare structural variant of ndhm1 in the landrace Kemater, which is associated with improved EPH and Fv/Fm compared with more common variants and could show that a defective allele at the ndhm1 locus leads to strong growth depression during early development, which is enhanced by cold temperatures.
Results
Fine-mapping of an early development QTL
In a genome-wide association study (GWAS) involving 3 European landraces, Mayer et al. (2020) identified QTL for EPH. We extended the GWAS with additional traits by measuring the maximum potential quantum yield of PSII (Fv/Fm) and chlorophyll content (SPAD) described in Hölker et al. (2019) using nonoverlapping 10-SNP windows as haplotype markers. We observed a shared genomic region linked to EPH and photosynthetic traits Fv/Fm and SPAD (Supplementary Table S1 and Fig. S1). This target region of 249 kb on chromosome 2 (Chr2:23,083,888-23,333,363; B73_AGPv4) was defined based on overlapping confidence intervals of 4 QTL for target traits EPH (V6), Fv/Fm (V4, V6), and SPAD (V3; Supplementary Table S1). In 3 of the 4 QTL, the same 10-SNP haplotype window showed the strongest association to target traits, thus we defined it as the lead haplotype (Chr2:23,328,380-23,333,363; B73_AGPv4, Supplementary Fig. S1B). The lead haplotype had an environmentally stable effect on EPH, Fv/Fm, and chlorophyll content (Supplementary Fig. S2).
A flow chart describing material development for fine-mapping is shown in Fig. 1A. Two DH lines (KE0482 and KE0678) from the landrace population in which the GWAS was performed were crossed to create a biparental population. They were selected to segregate at the QTL on chromosome 2 but to have similar genomic backgrounds based on genome-wide SNP markers (Supplementary Fig. S3). The 2 DH lines showed phenotypic segregation during early development. KE0678 exhibited significantly lower EPH and had lighter green leaves compared with KE0482 (Fig. 1B, Supplementary Fig. S4) but did not differ significantly from each other for final plant height, lodging, tillering, and flowering time (Supplementary Fig. S4). F2:3 recombinant inbred lines (RILs) were derived from the cross of KE0482 and KE0678 and phenotyped in a field experiment. In the F2:3 RILs, EPH and leaf color exhibited a significant association with SNP markers in the target region on chromosome 2 but not with the other 9 maize chromosomes (Supplementary Fig. S5). Heterozygous F2:3 RILs showed an intermediate phenotype between the midparent value and RILs homozygous for the KE0482 allele, indicating partial dominance (Supplementary Fig. S6).
Figure 1.
Fine-mapping of a QTL associated with EPH and Fv/Fm in growth stage V4. A) Scheme for fine-mapping of a QTL for EPH and Fv/Fm on maize chromosome 2. Genomic fragments unique to KE0678 are colored red. B) Top: phenotypic segregation during vegetative growth of F2:3 RILs linked to the QTL on chromosome 2. Bottom: HIF3A and HIF3B in a growth chamber experiment in optimal conditions. C) Genetic composition of key HIFs that enabled fine-mapping to a 314 kb genomic region. Vertical lines represent the genomic positions of KASP markers from Supplementary Table S5. Genomic fragments inherited from KE0678 are colored red. D, E) Phenotyping of key HIFs for EPH (D) and Fv/Fm (E) in a growth chamber. Bars show means ± SE (n = 3 to 6 plants) and dots observations from single plants. Significant differences (t-test) are marked with stars. *** P < 0.001, ** P < 0.01. Figure 1A was created with BioRender.com.
Heterogeneous inbred families (HIFs) were developed by selfing selected F2:3 RILs from the KE0482xKE0678 cross for 3 more generations to construct pairs of lines differing for the QTL allele within a near-isogenic background. In growth chamber experiments, HIF1A and HIF3A resembled KE0482 phenotypically for traits EPH and Fv/Fm, while HIF1B and HIF3B resembled KE0678 (Fig. 1, B, D, and E). For precisely defining the fine-mapped region overlapping between HIF1B and HIF3B, we assembled high-contiguity genomes of KE0482 and KE0678 using PacBio long reads and resequenced the 4 HIFs (HIF1A, HIF1B, HIF3A, and HIF3B) with Illumina short reads. Using SNPs derived from resequencing data mapped against the KE0678 genome assembly, we defined the genomic region associated with EPH and Fv/Fm as a 314 kb genomic fragment, covering 6 gene models (Figs. 1C and 2, A, B, Supplementary Table S2).
Figure 2.
Candidate gene analysis in fine-mapped region. A) Pairwise sequence alignment (sequence identity > 99%, length > 1 kb) of the fine-mapped region in KE0482 and KE0678. Black dots: Flanking markers of the fine-mapped region. Orange: alignment on forward strand. Green: alignment on reverse strand. B) Genes in fine-mapped region annotated in B73_AGPv4 reference genome. Red: Zm00001d002815 (ndhm1) is polymorphic between KE0482 and KE0678. C) Ndhm1 alleles and their polymorphisms in comparison to B73 reference sequence of KE0482 and KE0678. Gray: 5′ and 3′ UTR, the 3′UTR is indicated by a triangle at the end. Black: coding sequence. In ndhm1A-2, a hAT transposon, flanked by 2 terminal inverted repeat (TIR) motifs (purple, orange) is inserted in the intron (red triangle). The 2 copies of ndhm1 that are located next to each other on chromosome 2 in Kemater lines are surrounded by a blue (KE0482) and red (KE0678) box, respectively. D) Transcripts derived from ndhm1A-1 and ndhm1A-2. E, F) Relative transcript (E) and protein (F) levels of ndhm1 in KE0482, KE0678, HIF1A, HIF1B, HIF3A, and HIF3B. Bars show means ± SE (n = 3 plants). Significant differences (t-test) are marked with stars. ***P < 0.001, **P < 0.01, . P < 0.1. RQ: relative quantity. Figure 2D was created with BioRender.com.
The fine-mapped 314 kb genomic region is highly colinear between KE0482 and KE0678 (Fig. 2A). Only one polymorphism overlapping with a gene model was observed between KE0482 and KE0678, a transposon insertion in the intron of a homolog of Zm00001d002815, making it the most likely candidate gene underlying the QTL (Fig. 2, B and C). Zm00001d002815 codes for subunit M of the plastid NADH-dehydrogenase-like complex (NDH), central in NDH-mediated cyclic electron transport (CET) in plants (Shen et al. 2022). According to www.maizegdb.org 2 loci in B73 code for NDHM, Zm00001d002815 (chromosome 2) and Zm00001d025952 (chromosome 10). The amino acid sequence of the NDHM locus on chromosome 2 is highly conserved in the 26 US NAM assemblies with only 5/210 variable amino acids positions (Hufford et al. 2021). In contrast, the amino acid sequence of the chromosome 10 locus is weakly conserved and compared with Zm00001d002815 sequence identity is 30% and 50% in the 2 alternative protein sequences for Zm00001d025952 and includes large gaps (Supplementary Files S1 and S2). Thus, the locus on chromosome 10 likely does not function as NDHM, and we call Zm00001d002815 and its homologs on chromosome 2 in other maize genome assemblies ndhm1. Compared with B73, KE0482 carries a ndhm1 copy with 4 amino acid exchanges and several SNPs and InDels in the 5′ and 3′ untranslated region (UTR), including a 50 bp deletion in its 3′ UTR (Fig. 2C, ndhm1A-1). All amino acid exchanges, as well as the UTR variations, are common variants in the US NAM assemblies (Hufford et al. 2021). Thus, we assume this copy to be functional (Supplementary Files S3 and S4). Additionally, we observed the insertion of a hAT transposon 1.9 kb upstream of ndhm1A-1, which is not present in B73 (Fig. 2C). The hAT transposon insertion is found upstream of the respective ndhm1 copy in KE0678 (ndhm1A-2) as well. An identical 842 bp hAT transposon insertion is found in the intron of ndhm1A-2 of line KE0678 and results in the transcription of additional sequence originating from the hAT transposon causing 3 premature stop codons (Fig. 2, C and D, Supplementary Fig. S7A and File S3). Due to the premature stop codons, the resulting amino acid sequence lacks the second exon of NDHM, leaving it likely nonfunctional considering the high degree of sequence conservation of NDHM (Supplementary File S3). A second copy of ndhm1, named ndhm1B, is present in both DH lines (Fig. 2C) and is identical for KE0482 and KE0678. The ndhm1B sequence is identical to the ndhm1A-1 3′ UTR, exon, and intron sequence up to a breakpoint 54 bp 5′ of the start codon. No sequence similarity to ndhm1A-1 and ndhm1A-2 is observed in the sequence located 5′ of this breakpoint. Thus, ndhm1B misses half of the 108 bp 5′-UTR region annotated in B73_AGPv4, impacting the putative promoter sequence and further 5′ located cis-regulatory elements. The sequence present in ndhm1A-1 and ndhm1A-2 but not in ndhm1B overlaps with the transcription start site in the shoot of maize reference line B73 and with a transcription factor (TF) binding site (Supplementary Files S5 and S6; Mejía-Guerra et al. 2015; Savadel et al. 2021).
Functional validation of ndhm1 as candidate gene for the early development QTL
Ndhm1 expression in whole leaves was reduced to ∼14% on transcript and ∼1% on protein level in KE0678, HIF1B, and HIF3B compared with KE0482, HIF1A, and HIF3A (Fig. 2, E and F, Supplementary Fig. S8). The remaining NDHM protein in KE0678, HIF1B, and HIF3B is either translated from a transcript derived from ndhm1A-2, where the hAT insertion is spliced from the mRNA or from ndhm1B. Transcripts specific for ndhm1B were amplified from KE0482 and KE0678 cDNA, indicating that at least some transcriptional activity remains (Supplementary Fig. S7B).
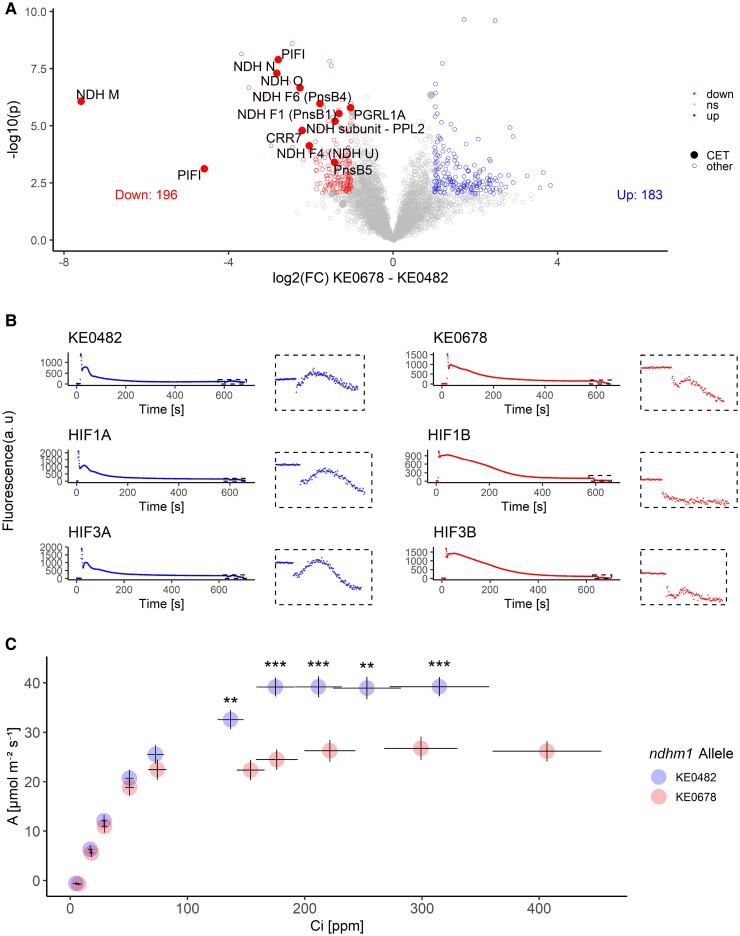
NDH is a protein complex consisting of at least 29 subunits, organized in 5 different subcomplexes, which form a super complex with PSI for CET (Shen et al. 2022; Zhang et al. 2024). It has been shown in maize that null mutants of different NDH subunits affect the accumulation of the whole NDH complex (Zhang et al. 2024). Leaf protein levels of all 16 NDH subunits and alternative CET pathways, such as proton gradient regulation 5 and proton gradient regulation-like 1 (PGR5, PGRL1A) were reduced in lines with the transposon insertion in ndhm1A (Fig. 3A, Supplementary Figs. S9 and S10). An exception is plastid terminal oxidase 2 (PTOX2), where expression is almost doubled in lines with ndhm1A-2, while levels of PTOX1 do not differ based on the ndhm1 alleles. PTOX can remove electrons from the electron transport chain by oxidizing plastoquinol, thus, upregulation of PTOX2 might indicate abnormalities in the photosynthetic electron transport caused by reduced accumulation of NDH components (Messant et al. 2024; Zhang et al. 2024). We conclude that the transposon insertion in ndhm1A-2 reduces NDHM protein levels, leading to reduced accumulation of proteins required for NDH assembly.
Figure 3.
Impact of a hAT transposon insertion in ndhm1 (ndhm1A-2) on NDH components and photosynthetic parameters. A) Proteome data of 6 genotypes (KE0482, KE0678, HIF1A, HIF1B, HIF3A, and HIF3B) in optimal conditions (n = 3 plants/genotype). Grouping was done by ndhm1 allele (ndhm1A-1 = KE0482 allele or ndhm1A-2 = KE0678 allele). One dot is one protein, filled dots are proteins taking part in CET. Cutoff for differential expression: absolute log2(FC: fold change) > 1; false discovery rate < 5%. Proteins that are differentially expressed and involved in CET are labeled in the volcano plots. B) Chlorophyll fluorescence induction curves of dark-adapted plants. The small boxes on the right side of the plots show a zoom in the F0-rise indicative for CET after turning off a weak AL. C) Relationship between assimilation rate (A) and intracellular CO2 (Ci) concentration of KE0482, KE0678, HIF1A, HIF1B, HIF3A, and HIF3B. Adjusted means were calculated for the 2 different ndhm1 alleles considering the genomic background as random factor in a mixed linear model for each tested external CO2 concentration separately. Dots are adjusted means ± SE. Significance of difference in assimilation rate was assessed by Wald tests for each external CO2 concentration independently. *** P < 0.001, ** P < 0.01.
To investigate the functionality of NDH-mediated CET in vivo, we recorded chlorophyll fluorescence traces of dark-adapted plants, which can indicate NDH-mediated CET around PSI. In the absence of PSII-driven linear electron transport (LET), electrons can flow back from the acceptor side of PSI to reduce plastoquinone, which results in an increase in chlorophyll fluorescence (Asada et al. 1993). The NDH-specific postillumination fluorescence rise was strongly reduced in lines with the ndhm1A-2 allele (Fig. 3B). The contribution of CET to ATP production in CO2-fixating tissues of C4 plants is significant, and thus, net carbon assimilation rates are dependent on a functional NDH complex (Ishikawa et al. 2016a; Zhang et al. 2024). Furthermore, Zhang et al. (2024) proposed that NDH-mediated CET is necessary in maize to maintain a high rate of malate decarboxylation in bundle sheath (BS) cells. This is achieved by optimizing the NADPH:NADP ratio and ensures efficient C4 photosynthesis. Consequently, net carbon assimilation rates at CO2 concentrations of 400 ppm and higher were significantly reduced in genotypes with ndhm1A-2 in the field (Fig. 3C). Our results indicate that the hAT transposon insertion in ndhm1A-2 in KE0678, HIF1B, and HIF3B leads to an NDH-deficient phenotype significantly affecting target traits EPH and Fv/Fm.
We screened Mutator (Mu) transposon insertion lines from the BonnMu collection (Marcon et al. 2020) in the genomic background of inbred line F7 (Haberer et al. 2020; Win et al. 2024), carrying a single copy of ndhm1 and identified an allele with Mu transposon insertion in the 5′ UTR of ndhm1 (Fig. 4A). The Mu transposon insertion is located 24 bp upstream of the ATG, between the putative transcription start site and the start codon. The Mu transposon insertion leads to reduction of transcript levels by a factor of 10 compared with the wild type (Fig. 4B). Target traits EPH and Fv/Fm were significantly reduced in the Mu-induced mutant compared with its wild type, similar to the effect of the hAT transposon insertion in ndhm1A-2 in the line KE0678 (Fig. 4, C and D). The NDH-specific postillumination fluorescence rise in the Mu-induced mutant matches the observations from ndhm1A-2 in the Kemater population, indicating impaired NDH-dependent CET (Fig. 4, E and F).
Figure 4.
Phenotypic analysis of a ndhm1 Mu-insertion mutant. A) Structure of ndhm1 allele of Flint line F7 and derived Mu-insertion mutant F-0123 from the BonnMu collection compared with B73_AGPv4 reference sequence. The position of the Mu element (red triangle) 24 bp upstream of the start codon was determined by Sanger sequencing. Gray: 5′ and 3′ UTR, the 3′ UTR is indicated by a triangle at the end. Black: coding sequence. B) Relative differences of ndhm1 transcript levels for parents of biparental mapping population (KE0482: n = 10; KE0678: n = 9) and F7 (n = 8) and F-0123 (n = 9). RQ: relative quantity. C, D) Phenotypic differences for target traits EPH (C) and Fv/Fm (D) between F7 (n = 10) and F-0123 (n = 14) and the parents of the biparental population (KE0482: n = 12; KE0678: n = 12). Bars show means ± SE. Significant differences (t-test) are marked with stars. *** P < 0.001, ** P < 0.01, * P < 0.05. E, F). Chlorophyll induction curves for F7 and F-0123, respectively. The small boxes on the right side of the plots show a zoom in the F0-rise indicative for CET after turning off a weak AL. A. u: arbitrary units; S: seconds.
Allelic diversity of ndhm1 in the landrace Kemater
To assess if the landrace Kemater harbors alleles improving target traits, we investigated the allelic series of ndhm1 within the landrace. As a proxy for ndhm1 alleles in Kemater, we used the 10-SNP lead haplotype alleles from GWAS, which is based on high-density genotypic data from 471 DH lines for which we had phenotypic data available. The haplotype consists of ten consecutive SNPs from the 600k Axiom Maize Genotyping Array (details on haplotype construction: Mayer et al. 2020) and does not overlap with ndhm1 but is located 150 kb upstream of ndhm1 (lead haplotype: Chr2:23,328,380-23,333,363, ndhm1: Chr2:23,483,486-23,484,612; B73_AGPv4). This is because there are no polymorphic 600k SNPs between KE0482 and KE0678 in a 676 kb window starting 153 kb before and ending 523 kb after ndhm1. Four distinct haplotype alleles with allele frequencies (AF) between 6% and 44% were identified in the Kemater population and were used for grouping phenotypic data (Supplementary Table S3). The 2 haplotype alleles with the highest AF will be designated as Hap1 and Hap2 in the following analysis (AF = 44%; 28%), while the remaining 2 haplotype alleles will be referred to as KE0482 and KE0678 haplotype, respectively. We extended our comparison of allelic effects with phenotypic data for traits EPH, Fv/Fm, chlorophyll content, and final PH in 471 Kemater DH lines. Strong phenotypic differences were observed between lines with the KE0482 haplotype and KE0678 haplotype for ndhm1, reflecting the results from the biparental population (Fig. 5). The KE0678 haplotype of ndhm1 reduced EPH, Fv/Fm, chlorophyll content and final PH compared with the Hap1 and Hap2 groups as well, however, to a smaller extent than compared with the KE0482 haplotype and not significant for EPH. In addition, a smaller but statistically significant increase in EPH, Fv/Fm, chlorophyll content and final PH was observed for lines with the KE0482 haplotype for ndhm1 compared with lines with Hap1 and Hap2 (Fig. 5).
Figure 5.
Analysis of allelic diversity of ndhm1 in Kemater DH lines. A to D) Phenotypic data from 471 Kemater lines with high-density genotypic and phenotypic data grouped by their haplotype allele of the lead haplotype from GWAS. Blue: haplotype of KE0482, which carries a second copy of ndhm1 (ndhm1B) and no transposon insertion in ndhm1 (ndhm1A-1). Red: haplotype of KE0678, which carries a second copy of ndhm1 (ndhm1B) and a transposon insertion in ndhm1 (ndhm1A-2). Each dot represents the adjusted mean of one Kemater line in up to 11 combinations of environments and years for EPH A), Fv/Fm B), chlorophyll content C) and final plant height D). Bars show means ± SE. Significant differences of alleles are indicated by letters (LSD, least significant differences; P < 0.05). E to H) Subset of 27 Kemater lines that was selected to be balanced for different ndhm1 haplotypes for in-depth molecular characterization. Lines were genotyped for the presence or absence of the transposon insertion in ndhm1A-2 and presence/absence of ndhm1B and phenotyped for their differences in ndhm1 transcript levels E) and for target traits EPH F), Fv/Fm G) and chlorophyll content H). Bars show means ± SE. Significant differences (LSD; P < 0.05) are indicated by letters. RQ: relative quantity.
For validation of the results on allelic diversity that were based on the lead haplotype from GWAS in proximity of ndhm1, we selected 27 Kemater lines with a higher frequency of KE0482- and KE0678-associated ndhm1 haplotypes as observed in the Kemater population. The selected Kemater lines were genotyped for the presence or absence of the hAT insertion in ndhm1A and the presence or absence of ndhm1B (Supplementary Figs. S11 and S12, Supplementary Table S4). Twenty out of the 21 Kemater lines that had a second copy of ndhm1 (ndhm1B(+/+)) carried the KE0482- or KE0678-associated ndhm1 haplotype alleles. Only Kemater lines with the KE0678-associated haplotype carried the hAT transposon insertion in ndhm1A (ndhm1A-1(−/−)). These results indicate that the KE0482- and KE0678-associated ndhm1 haplotypes both are in LD with a duplication of ndhm1, while the hAT transposon insertion in ndhm1A-2 is in LD with the KE0678 haplotype. Ndhm1 transcript levels were strongly reduced in 12 Kemater DH lines with ndhm1A-1(−/−), supporting the notion that the insertion is causal for differences in expression levels (Fig. 5E). Ndhm1 transcript levels were slightly elevated in lines with the allelic combination ndhm1A–1(+/+)/ndhm1B(+/+) compared with lines with ndhm1A-1(+/+)/ndhm1B(−/−), however not significant (Fig. 5E). At the phenotypic level, EPH, Fv/Fm, and chlorophyll content were significantly reduced by the hAT insertion in ndhm1A compared with all other lines (Fig. 5, F to H). The allelic combination ndhm1A-1(+/+)/ndhm1B(+/+) led to a significantly increased Fv/Fm compared with lines with ndhm1A-1(+/+)/ndhm1B(−/−) (Fig. 5G). Results from the validation set show the same tendencies for investigated traits as observed in the full set of DH lines, however, not all differences were significant.
To confirm that KE0482 and KE0678 ndhm1 haplotypes are associated with a second copy of ndhm1 (ndhm1B), we analyzed whole-genome sequencing data, which was available for 15 Kemater DH lines from which 3 overlapped with the “Validation set field”. We assessed the copy number state of ndhm1 by mapping short reads of the 15 lines against the B73_AGPv4 reference genome, which has a single copy of ndhm1. In 4 DH lines, carrying either the KE0482- or KE0678-associated ndhm1 haplotypes, normalized read depth exceeded 2 for most of 2 exons of ndhm1, indicating a duplication (Fig. 6). A drop of the normalized read depth by half can be observed within the 5′ UTR of ndhm1, matching the breakpoint in the 5′ UTR of ndhm1B in KE0482 and KE0678 relative to B73. The remaining eleven DH lines with other ndhm1-associated haplotypes display a normalized read depth of about one, without a drop of read depth in the 5′ UTR of ndhm1, indicating single-copy alleles of ndhm1. For 4 of the 15 Kemater lines, we had PacBio high-contiguity genome assemblies available in addition to the short-read sequencing data (KE0095, KE0109, KE0482, and KE0678). Both KE0095 and KE0109 carry the Hap2 allele of the ndhm1-associated haplotype, and KE0482 and KE0678 are the parents of the biparental mapping population used to identify the duplication of ndhm1. The results of the read depth analysis are thus validated, as both KE0095 and KE0109 carry only one copy of ndhm1 (Supplementary File 3). The insertion of a hAT transposon 1.9 kb upstream of ndhm1A-1 and ndhm1A-2 in KE0482 and KE0678 (Fig. 2C) was not present in KE0095 and KE0109. We expanded our investigation of allelic diversity of ndhm1 to diverse maize lines by examining the presence of ndhm1B in the US maize NAM assemblies via BLAST (Hufford et al. 2021). We found a combination of ndhm1A-1 and ndhm1B identical to the KE0482 variant in Tx303, but no allele similar to ndhm1A-2 was detected (Zm00041ab076830 and Zm00041ab076840, Supplementary Files S3 and S4).
Figure 6.
Association of lead haplotype alleles from a GWAS of KE0482 and KE0678 with a duplication of ndhm1. Normalized read-depth of high-coverage whole-genome sequencing data aligned against B73_AGPv4 reference genome grouped by allele of the haplotype with lowest P-value from GWAS. Each line represents a Kemater line, and each dot represents a single base in B73v4 reference. Hap1 and Hap2 are grouped together in the group “Other Haplotype”. Red: Normalized read depth > 2. Below the read depth graphs the relative positions of gene models of ndhm1-A1 and ndhm1-B are indicated. Gray: 5′ and 3′ UTR, the 3′ UTR is indicated by a triangle at the end. Black: coding sequence.
Effect of a transposon insertion in ndhm1 on maize chilling stress tolerance
EPH, Fv/Fm, and chlorophyll content are connected to chilling tolerance during early development through the sensitivity of maize photosynthesis to cold temperatures (Burnett and Kromdijk 2022; Lainé et al. 2023). Thus, we subjected Kemater lines with different ndhm1 alleles to chilling stress in controlled conditions. A subset of 15 Kemater lines was chosen based on previous results on allelic diversity of ndhm1 and evaluated under optimal conditions and 3 d after recovery from severe chilling stress (48 h, 6 °C/2 °C [day/night]). Under optimal conditions, maximum and operating quantum efficiency of PSII (Fv/Fm, ΦPSII) as well as electron transfer rates (ETRs) were significantly lower in lines with the combination ndhm1A-1(−/−)/ndhm1B(+/+) compared with lines with ndhm1A-1(+/+)/ndhm1B(−/−). In contrast, Fv/Fm, ΦPSII, and ETR were increased in Kemater lines with the combination ndhm1A-1(+/+)/ndhm1B(+/+) compared with lines with ndhm1A-1(+/+)/ndhm1B(−/−) (Fig. 7A, Supplementary Fig. S13). Lines with the hAT transposon insertion in ndhm1A were severely impaired in photosynthetic parameters 3 d after the chilling stress, most prominent for ΦPSII and ETR, while the other lines recovered already (Fig. 7A, Supplementary Fig. S13). After recovery, lines with ndhm1A-2 showed necrosis on leaves, reflected in increased electrolyte leakage (Fig. 7, B and E). It is likely that electrolyte leakage is caused by reactive oxygen species (ROS), which result in lipid peroxidation and subsequent damage to cell membranes (Lainé et al. 2023). It has been proposed that CET can indirectly mitigate oxidative stress by activating nonphotochemical quenching (NPQ) through the generation of a H+ gradient (Burrows et al. 1998; Martín et al. 2004). These findings suggest that NDH-mediated CET is necessary to alleviate the effects of chilling stress on the photosynthetic apparatus.
Figure 7.
Effect of ndhm1 allele after severe cold treatment on photosynthetic parameters and leaf proteome. A, B) Phenotypic differences of DH lines with different ndhm1 alleles in optimal conditions and 3 d after recovery from severe cold stress. One point is the mean of 3 to 6 biological replicates of a Kemater DH line. Bars show means ± SE. Significant differences (least significant differences; P < 0.05) are indicated by letters. C, D) Difference of protein levels depending on a cold treatment (4 °C/2 °C, 48 h) of genotypes with ndhm1A-1 (C, KE0482, HIF1A, HIF3A) or ndhm1A-2 (D, KE0678, HIF1B, HIF3B) alleles. One dot is one protein, filled dots are proteins with oxidoreductase activity (GO:0016701). Cutoff for differential expression: absolute log2(FC: fold change) > 1; false discovery rate < 5%. Differentially expressed proteins with oxidoreductase activity are labeled in the plots. G) Lesions on leaves of Kemater lines KE0482 and KE0678 after cold treatment.
To investigate the effect of the transposon insertion in ndhm1A on chilling stress tolerance, we recorded leaf protein levels directly after a severe chilling stress. In the leaf proteome of lines with ndhm1A-1, short-term reactions to chilling stress were almost absent (6 differentially accumulated proteins). In contrast, 94 proteins were differentially accumulated after chilling treatment in lines with the hAT transposon insertion in ndhm1A (Fig. 7, C and D). Gene ontology enrichment revealed oxidoreductase activity (GO:0016701) as the most significantly enriched molecular function in differentially accumulated proteins in lines with ndhm1A-2. Differentially expressed proteins with oxidoreductase activity include 3 lipoxygenases (LOX3, LOX10, and LOX13) and 2 dioxygenases (4-hydroxyphenylpyruvate dioxygenase: HPPD1, 9-cis-epoxycarotenoid dioxygenase 6: NCED6), as well as one protein taking part in chlorophyll catabolism (lethal leaf spot 1: LLS1). Therefore, the proteomic data support increased oxidative stress in maize lines with the hAT transposon insertion in ndhm1A.
Discussion
Early maize planting offers both environmental and economic benefits to agriculture by reducing nitrogen leaching, minimizing herbicide use, avoiding summer drought during flowering, and enhancing yield potential through earlier soil coverage and extended growth periods. However, early maize planting is limited by low temperatures during early development, which can result in freezing- and chilling stress (Kucharik 2006; Parker et al. 2017; Lainé et al. 2023). The physiological response to freezing- and chilling stress is the result of different physiological mechanisms and consequently different underlying genetic components (Miedema 1982). The environmental stability of the haplotype effect suggests that its impact is not contingent on freezing stress, which was observed in only a subset of field environments. Our findings indicated that lines with a defective ndhm1 allele also demonstrated increased susceptibility to chilling stress. Genetic variation for physiological responses to chilling temperatures has been described in maize, yet the underlying genes have remained unclear, and only a few candidate genes have been proposed (reviewed in Burnett and Kromdijk 2022). Here, we present a promising target for improving chilling tolerance in maize. The relationship between NDH-mediated CET and freezing stress is a topic that warrants further investigation and extends beyond the scope of this paper.
Landraces harbor genetic diversity crucial for improving traits with limited variation in breeding material (McCouch et al. 2013; Dwivedi et al. 2016; Mayer et al. 2020; Liang et al. 2021). The Austrian landrace Kemater was selected for this study due to its adaptation to temperate European climate and its variance for visible early growth traits such as EPH (Mayer et al. 2017; Hölker et al. 2019). Here, we demonstrate the concurrent genetic improvement of multiple quantitative traits crucial for enhancing early plant development through the action of the gene ndhm1 (Fracheboud et al. 2004; Hund et al. 2004; Revilla et al. 2016; Enders et al. 2019). Variation of basic agronomic traits such as EPH as well as physiological parameters photosynthetic efficiency (Fv/Fm, ΦPSII), chlorophyll content and chilling tolerance are directly linked to NDH-mediated cyclic electron transfer in our study. The multifaceted functionality of ndhm1 underscores the pivotal role of NDH in maintaining metabolic processes critical for plant growth and development during early development.
Starting from a GWAS analysis, we identified a hAT transposon insertion in ndhm1 as the factor underlying a QTL with pleiotropic effects on the aforementioned traits due to its strong phenotypic consequences. NDH deficiency underlying the QTL was congruent with impaired NDH complex formation leading to specific chlorophyll induction curves, as well as reduced net CO2 assimilation rate, growth parameters, and chlorophyll content in maize mutants of NDH subunits NDF6 and NDHU (Zhang et al. 2024). We observed unexpected Fv/Fm effects specific to ndhm1 alleles in Kemater lines and a Mu transposon insertion mutant of NDHM in the genomic background of Flint line F7 compared with Zhang et al. (2024). This contrasts with earlier findings where NDH-deficient mutants did not impact Fv/Fm in C4 but exclusively in C3 species and only following cold or high-light stress (Endo et al. 1999; Wang et al. 2006; Zhang et al. 2024). The Fv/Fm effects might therefore be specific to defects in the assembly of subcomplex A, where NDHM is located, while NDF6 and NDHU are situated in subcomplex ED (electron donor) and subcomplex B, respectively (Shen et al. 2022; Zhang et al. 2024). A reduction in Fv/Fm is indicative of photoinhibition of PSII, which aligns with the elevated PTOX2 protein levels observed in the present study (Calzadilla et al. 2024; Nie et al. 2024). In contrast, in NADP-ME type photosynthesis, NDH is enriched in BS cells where PSII is either absent or at least drastically reduced (Meierhoff and Westhoff 1993; Kubicki et al. 1996). In a previous study, it was proposed that BS cell PSII activity in maize is related to NPQ, a mechanism required to dissipate excess light energy (Liu et al. 2022). Furthermore, NDH generates a pH gradient across the thylakoid membrane, even when LET is suppressed, which in turn serves as the primary control variable for NPQ (Murchie and Ruban 2020). This may provide a physiological link between NDH deficiency and photoinhibition in maize.
Capitalizing on the native diversity of ndhm1 in the landrace Kemater, we identified an allele that was associated with improved early development and photosynthetic traits. A haplotype allele of ndhm1 was associated with a duplication of ndhm1 and enhanced early development, photosynthetic efficiency, and chlorophyll content. Noticeable candidates for the phenotypic effect of the improved allele are a hAT transposon insertion upstream of ndhm1A and a second copy of ndhm1 (ndhm1B). However, we cannot exclude the contribution of other, less striking polymorphisms without further studies. Our data indicate that ndhm1B is still transcribed to RNA, however, deciphering the role of ndhm1B warrants more research and goes beyond the scope of this study. Thus, the mechanism underlying phenotypic variation associated with the improved haplotype remains somewhat elusive. Another important factor in the function of the NDH complex is spatial expression in BS and mesophyll cells, which is strictly regulated and dependent on the subtype of photosynthesis (Ishikawa et al. 2016b). Evaluating the impact of allelic variation through cell-type-specific comparisons will be required to unravel the molecular mechanisms that are underlying the allelic effects of ndhm1 we observe.
While genome editing continues to gain traction in crop improvement, the challenges in engineering alleles that truly enhance target traits with quantitative inheritance persist. As exemplified here, landraces offer an excellent platform for enhancing maize performance in breeding programs, particularly considering the regulatory complexities associated with biotechnological interventions (Liang et al. 2021; Lorenzo et al. 2023; Marone et al. 2023). Mapping of relevant genes influencing quantitative traits of interest is facilitated by the segregation of large effect QTL in landrace populations, followed by extensive analysis of allelic diversity to discover alleles that enhance target traits. The effect of the improved ndhm1 allele we detected is relatively small compared with the defective allele but significant and impacting the same set of traits (EPH, Fv/Fm, ΦPSII, ETR, and chlorophyll content). At the molecular level, the improved allele exists in the US NAM panel. Further research will be needed to quantify its effect size in different genetic material. While NAM lines were chosen to be highly diverse, we were able to evaluate distinct effects of 3 allelic classes of ndhm1 in a homogeneous genomic background, which allows us to connect allelic variation to meaningful phenotypes (Hufford et al. 2021).
Previous research linked genetic variation of CET to differences in salt and heat tolerance in C3 species soybean and rice (He et al. 2015; Essemine et al. 2017), but the evidence is not conclusive and to our knowledge not available for C4 plants such as maize. Our research suggests the potential for optimization of CET in maize breeding to increase EPH, Fv/Fm, and chlorophyll content and provides an improved allele that could be directly used for breeding. We propose the utilization of maize landraces adapted to temperate climates as a valuable resource for accessing beneficial alleles aimed at enhancing early plant development and facilitating gene discovery efforts.
Materials and methods
Plant material
DH lines extracted from the Austrian landrace “Kemater Landmais Gelb” (KE) and derived recombinants were used in this study. Detailed information about the generation, genotyping, and phenotyping of the source material is described elsewhere (Hölker et al. 2019; Mayer et al. 2020; Mayer et al. 2022).
Two DH lines of the landrace Kemater (KE0482 and KE0678) differed for multiple overlapping QTL on chromosome 2 for the traits EPH, maximum potential quantum efficiency of PSII (Fv/Fm) and chlorophyll content (SPAD, Supplementary Table S1) and were chosen for their similar genomic background (86,110 of 501,124 markers polymorphic, Supplementary Fig. S3). The 2 DH lines were crossed for generating a segregating F2 population, RILs and heterogenous inbred families (HIFs) by consecutive selfing.
A subset of 27 out of 471 Kemater DH lines, carrying 4 different haplotypes at the QTL and selected to have an increased frequency of the KE0678- and KE0482-associated ndhm1 haplotype was evaluated in the field (validation set field) with a focus on early development. Another, partially overlapping subset of 16 Kemater DH lines carrying balanced frequencies of ndhm1 alleles was evaluated in growth chambers (cold growth chamber set) with a focus on recovery after chilling stress.
We obtained Mu transposon insertion lines for the gene ndhm1 from the BonnMu collection in the genomic background of the inbred line F7 (www.bonnmu.uni-bonn.de; Marcon et al. 2020; Win et al. 2024). F7 is an important founder of European Flint maize (Haberer et al. 2020) and is suitable because of its genomic similarity to the Kemater landrace.
Development of RILs and HIFs
A schematic overview of material development is presented in Fig. 1A. KASP markers positioned in the QTL region on chromosome 2 and polymorphic between the 2 parental lines (KE0482 and KE0678) were synthesized using the probe sequences of the publicly available 600k Axiom Maize Genotyping Array (Thermo Scientific). RILs were genotyped and individual plants showing recombination between markers AX-91512997 (Chr2:21380448, B73_AGPv4) and AX-90737994 (Chr2:29359872, B73_AGPv4) were self-pollinated. Resulting F2:3 RILs were phenotyped in a field experiment in 3 German locations in 2020.
RILs heterozygous for the target region were repeatedly self-pollinated and F5 plants genotyped using KASP markers (Supplementary Table S5). Individual plants that carried a heterozygous fragment in the target region were self-pollinated to develop HIFs. HIFs are pairs of lines contrasting for a genomic region of interest derived from the same parental plant to enable phenotyping in near-isogenic backgrounds. To contrast the QTL effect in near-isogenic backgrounds for fine-mapping, resulting offspring was genotyped using KASP markers and the 2 homozygous classes (HIF1A, HIF1B, HIF3A, and HIF3B) selected.
Selection of Mu insertion mutants
Mu transposon insertion mutants in the genomic background of Flint line F7 were genotyped with ndhm1-specific primers in combination with the mutant-specific TIR6 primer (Supplementary Table S6, “ndhm1_Exon1_FW” and “ndhm1_5UTR_RV”; Settles et al. 2004). Amplification of respective gene fragments was verified by Sanger sequencing of the amplicons after separation by agarose gel electrophoresis and purification (NucleoSpin Gel and PCR Clean-up kit, Macherey-Nagel GmbH & Co. KG, Düren, Germany). Individual plants were propagated to obtain homozygous mutants and wild types and were phenotyped for EPH, Fv/Fm, and SPAD.
Growth conditions
For phenotyping in growth chamber experiments, kernels were imbibed in water for 5 min, transferred to filter paper and pregerminated for 72 h in the dark at 28 °C. After germination, plant material was sampled from individual seedlings for DNA extraction, and the respective seedlings were transferred to small pots filled with CL ED73 soil (Einheitserdewerke Patzer, Germany). Plants were grown until growth stage V4 to V6 with 16 h of light a day at 25 °C during the day and 20 °C during the night with 650 µmol m−2s−1 photosynthetically active photon flux density at 75% relative humidity (RH) for 6 biological replicates per DH line and treatment. In chilling stress experiments, plants were randomly assigned to a control group and a chilling stress group, which was treated with severe chilling stress (6 °C/2 °C [day/night]) for 48 h, starting 18 d after sowing.
Field experiments were conducted in Einbeck (EIN, Germany, 51°49′05.9″N 9°52′00.3″E), Roggenstein (ROG, Germany, 48°10′47.5″N 11°19′12.9″E), Bernburg (BBG, Germany, 51°49′28.6″N 11°42′26.3″E), Oberer Lindenhof (OLI, Germany, 48°28′26.3″N 9°18′17.9″E), and Freising (FRS, Germany, 48°24′13.2″N 11°43′28.5″E) in the years 2020 and 2023. In total, 178 F2:3 RILs were randomized in augmented block designs in EIN, ROG, and BBG in 2020 with inbred checks UH007, F283, DK105, and EP44 replicated in each block. The subset of 27 DH lines used for molecular analysis of ndhm1 allelic diversity in the field was randomized in complete blocks with 2 replications in locations ROG, FRS, and OLI in 2023, using the same inbred checks. The full set of 471 Kemater DH lines was assessed in 6 and 5 locations in 2017 and 2018, respectively, as part of a larger trial that included 2 additional landraces. In 2 locations (Golada and Tomeza), a randomly selected subset of 222 Kemater DH lines was subjected to phenotypic analysis. The genotypes were randomized using a 10 × 10 lattice design, with 2 replicates of each entry per location. Fourteen Flint and one Dent breeding lines (entered twice) and 3 original landraces (entered 4 times) were utilized as controls. Additional details regarding field locations, checks and data analysis, and the full set of Kemater DH lines can be found in Hölker et al. (2019) and Mayer et al. (2022).
Plots consisted of 20 plants each in single rows of 3 m length with 0.75 m spacing between plots (9 plants/m2). Field trials were subjected to standard agricultural practices.
Collection of agronomic traits
EPH was measured by stretching all leaves of a plant to measure the maximum length between soil and the tip of the leaves. The measurements of 3 to 5 individual plants per plot were averaged to obtain the plot-level measurement. Days to silking and days to pollen shedding were calculated as number of days from sowing until half of a plot had silks of at least 1 cm length or until half of a plot shed pollen, respectively.
Determination of photosynthetic traits
Fv/Fm was measured in dark-adapted plants before light was turned on in the growth chambers using a LI-600 porometer (LI-COR Inc., Lincoln, NE, USA). In field experiments, plants were measured after midnight to ensure that plants were dark adapted for at least 1 h. The last fully developed leaf was clipped in the middle, omitting the midvein, flow rate set to “high” (150 µmol*s−1), match frequency of 10, flash set to “Dark Adapted” and “Rectangular” with an intensity of 6,000 µmol m−2s−1, a flash length of 800 ms, and the fluorescence constants “Leaf absorptance” and “Fraction Abs PSII” to 0.8 and 0.5, respectively, at a modulation rate of 5 Hz. ΦPSII and ETR were measured on the same day at noon after adapting the plants for several hours to the light conditions in the growth chamber using the LI-600 with adapted settings. The flash intensity increased to 10,000 µmol m−2s−1 the setting “Dark Adapted” was disabled and the actinic modulation rate was set to 600 Hz.
Chlorophyll content was measured using a SPAD-502 (Konica Minolta K.K, Chiyoda, Tokyo, Japan), clipping the last fully developed leaf of a plant. Data from 3 plants per plot were averaged to obtain the plot-level measurement.
For NDH-mediated CET fluorescence induction curves were recorded on dark-adapted plants in the morning before the light was turned on in the growth chambers with the LI-6800 (LI-COR Inc., Lincoln, NE, USA). The last fully developed leaf was clipped with the measuring light (ML) set to 50 Hz and a weak actinic light (AL) applied (50 µmol m2s−1) for 5 min per plant until the fluorescence signal remained stable. After reaching a steady-state level the ML was turned off and the fluorescence signal recorded for 2 more minutes.
CO2 response curves of net photosynthetic assimilation rate (A/Ci) were recorded as described by Blankenagel et al. (2022) in field grown plants before flowering at external CO2 concentrations of 0, 50,100, 200, 300, 400, 600, 800, 1,000, and 1,200 ppm.
Transcript-level measurements
RNA was extracted from leaves using a guanidine hydrochloride protocol (Logemann et al. 1987), followed by DNase digestion (DNase I, RNase free, Thermo Scientific) and first-strand cDNA synthesis (Maxima H Minus Kit, random hexamer primers, Thermo Scientific K1652). RT-qPCRs were performed in technical triplicates with primers binding to the 3′ UTR of ndhm1 (Supplementary Table S6). For normalization, RT-qPCR was performed for the house-keeping gene MEP (membrane protein PB1A10.07c, Zm00001d018359; Manoli et al. 2012) and normalization was done following Pfaffl (2001). In field experiments, leaf tips of 3 plants per plot and 2 replicates in location FRS were harvested at noon in growth stage V6 and pooled in one sample per genotype.
Proteomics experiment
For proteome measurements, HIF1A, HIF1B, HIF3A, HIF3B, KE0482, and KE0678 were grown in a growth chamber and cold treated as described above. The last fully developed leaf was sampled directly after the chilling stress in liquid nitrogen for 3 biological replicates per genotype–treatment combination. Total proteome was extracted and measured following an established protocol (Brajkovic et al. 2023), with an additional step of 11-plex TMT labeling of peptides after protein digestion for quantification. For normalization of batch effects, a reference was prepared by mixing equal peptide amounts of all samples which was measured twice in each of the TMT batches. Proteins were identified and quantified by MaxQuant (Tyanova et al. 2016) using B73_AGPv4 as reference followed by rescoring the identified proteins using Prosit (Gessulat et al. 2019). To avoid the imputation of missing values in quantitative analysis, only proteins that were identified in all 36 samples (6 genotypes × 3 replicates × 2 treatments; n = 5,667 proteins) were considered. Log2-transformed raw intensities of identified proteins were median normalized to remove differences in summed intensities per sample and subsequently the 2 reference channels per TMT batch were used to calculate a correction factor for each identified majority protein group per TMT batch. Gene ontology term enrichment was performed by agriGO v2.0 (Tian et al. 2017) comparing differentially expressed proteins to all 5,667 proteins quantified in the proteomics experiment as custom background.
Sample preparation for mass spectrometry
The dried and cleaned peptides were reconstituted in 20 µl of 200 mm EPPS (pH 8.5) buffer. TMT-11plex reagent (Thermo Scientific) was reconstituted in water-free ACN to a working concentration of 20 µg/µl. Five microliters of this TMT reagent solution were transferred to the peptides. The reaction was incubated on the thermoshaker (20 °C, 400 rpm) for one hour and quenched by 0.25% hydroxylamine afterward. The TMT channels were then pooled together and acidified with FA to a final concentration of 1%. The reaction wells were washed with 20 µl washing solution (10% ACN, 10% FA) and added to the TMT pool. The TMT pools were dried down in the speed-vac and stored at −20 °C. The 1 mg TMT-pooled peptides were cleaned by solid-phase extraction on 50 mg C18 Sep-PAK cartridges. The TMT peptides were washed with 0.1% FA. TMT peptide elution was achieved by 0.1% FA in 60% ACN. TMT peptides were dried down in the speed-vac and stored at −20 °C. The sample was reconstituted in 25 mm ammonium bicarbonate and was fractionated on a Waters XBridge BEH C18, 4.6 × 250 mm over a 44 min gradient from 7% to 45% ACN in the presence of 2.5 mm ammonium bicarbonate. After gradient, a 6 min ramp to 80% ACN followed to wash the column. Fractions were collected from minutes 7 to 55. Each fraction consisted of 30 s at a flow rate of 1 ml/min. The 96 fractions were pooled back into 48 fractions in an n-with-(n + 48) fashion. Samples were acidified with FA to a final concentration of 0.1%. The fractionated TMT peptides were dried down in the speed-vac and stored at −20 °C until MS measurement.
Mass spectrometry
Full proteome TMT-labeled peptides were measured with a Fusion Lumos Tribrid mass spectrometer (Thermo Scientific) that was coupled to a Dionex Ultimate. The sample was directly injected onto the Acclaim PepMap 100 C18 column (2 µm particle size, 1 mm ID × 150 mm). Separation was performed on a 27 min segmented gradient with a flow rate of 50 µl/min starting from 4% to 27% B (23 min) and 27% to 32% B (2 min). The system was finally washed with 100 μl 90% B and reequilibrated at 1% B. Solvent A consisted of 0.1%v FA and 3%v DMSO in water. Solvent B consisted of 0.1%v FA and 3%v DMSO in Acetonitrile. The MS was operated in a fast, data-dependent MS3 mode. The spray voltage was set to 3.5 kV supported by sheath gas (32 units) and aux gas (5 units) with a vaporizer temperature of 125 °C. Every 1.2 s, a full-scan (MS1) was recorded from 360 to 1,600 m/z with a resolution of 60 k in the Orbitrap in profile mode. The MS1 AGC target was set to 4e5. Based on the full scans, precursors were targeted for MSMS scans if the charge was between 2 and 6, the isotope envelop was peptidic (MIPS), and the intensity exceeded 1e4. The MS2 quadrupole isolation window was set to 0.6 Th. The TMT peptides were HCD fragmented with an NCE of 34%. The MS2 spectra were acquired in the ion trap in rapid mode. The MS2 AGC target was set to 3e4 charges, and the maxIT was set to 40 ms. The maxIT or AGC target could be dynamically exceeded when the previous scan took longer than the calculated injection time (inject beyond mode). TMT reporter ions were measured in a consecutive MS3 scan based on the previous MSMS scan. Thus, a new batch of precursor ions was isolated with an MS3 quadrupole isolation window of 1.2. The isolated precursor was then HCD-fragmented identically to the previous MS2 scan. Additionally, Isobaric tag loss exclusion properties were set to TMT reagent. The selected fragment ions were then HCD fragmented with an NCE of 55%. The MS3 spectrum was acquired with 50k resolution from 100 to 1,000 Th in the Orbitrap in centroid mode. The MS3 AGC target was set to 2e5 charges, and the maxIT was set to 86 ms.
Detection of different ndhm1 alleles
To identify different ndhm1 alleles, we designed primers for the full-length variant (ndhm1A) and for the variant with shortened 5′ UTR (ndhm1B, Fig. 2C). The primers “ndhm1_Exon2_FW” and “ndhm1_5UTR_RV” were used for full-length ndhm1 transcript detection. For ndhm1B, “ndhm1_Exon2_FW” and “ndhm1B_RV” were used. Genotyping for the transposon insertion in ndhm1A-2 was done by discriminating amplicon lengths of transcript or genomic sequences by gel electrophoresis. All primer sequences are listed in Supplementary Table S6. PCRs were performed using Q5 high-fidelity DNA polymerase (New England Biolabs Inc., Ipswich, MA, USA) according to the manufacturer's manual at an annealing temperature of 68 °C for 15 s followed by elongation at 72 °C for 30 s and PCR products separated by gel electrophoresis.
Sanger sequencing
Sanger sequencing was performed using Mix2seq kits at Eurofins Genomics Germany GmbH (Ebersberg, Germany).
Whole-genome sequencing
Kemater DH lines KE0095, KE0109, KE0482, and KE0678 representing 3 different ndhm1 haplotype alleles were sequenced using PacBio HiFi long reads by CNRGV, INRA Occitanie Toulouse, France (cnrgv.toulouse.inra.fr). Circular consensus sequences were de novo assembled using the hifiasm assembler with default parameters (Cheng et al. 2021). The contigs were ordered using a genetic map derived from EP1xPH207 as reference in ALLMAPS (Tang et al. 2015; Haberer et al. 2020).
For fine-mapping, HIF1A, HIF1B, HIF3A, HIF3B, KE0482, and KE0678 were sequenced with high coverage (50 X) using Illumina short-reads. Paired-end short reads were de-duplicated (hts_SuperDeduper v1.0), trimmed (Trimmomatic v0.39), error corrected using K-mer distributions (Lighter v1.1.2) and mapped against the KE0678 PacBio assembly using bwa-mem (Li and Durbin 2009). SNPs were called from the resulting bam files of HIF1A, HIF1B, HIF3A, HIF3B, KE0482, and KE0678 by freebayes (Garisson and Marth 2012). Settings for variant calling were: –ploidy 2, –min-mapping-quality 1, –min-base-quality 3, –min-alternate-count 5, –min-repeat-entropy 1, –no-partial-observations, –no-population-priors, –genotype-qualities. Variants were subsequently filtered by bcftools using following parameters: FORMAT/DP > 10; INFO/DP < 100, %QUAL > 30, SAF > 0 & SAR > 0, RPL >1 & RPR > 1. Heterozygous SNPs in the DH lines KE0482 and KE0678 were removed as no heterozygosity is expected in DH lines. Finally, SNPs differentiating KE0678, HIF1B, and HIF3B from KE0482, HIF1A, and HIF3A were selected and used to define the fine-mapped genomic segment in KE0678.
For read-depth analysis, 15 Kemater DH lines were sequenced with high coverage (50 X) using Illumina short-reads. Paired-end short reads were de-duplicated (hts_SuperDeduper v1.0), trimmed (Trimmomatic v0.39), error corrected using K-mer distributions (Lighter v1.1.2), and mapped against the B73_AGPv4 reference, carrying a single-copy of ndhm1, using bwa-mem (Li and Durbin 2009; Jiao et al. 2017). Read depth of 15 Kemater DH lines per position of B73_AGPv4 reference was extracted using the command “samtools depth” from bam files. The coverage per position was normalized by dividing by the mean read depth over the whole B73_AGPv4 reference for each genotype.
Comparative genomic analysis
Genomic positions of KASP markers in the target regions of KE0482 and KE0678 were obtained by mapping their probe sequences against KE0482 and KE0678 PacBio HiFi assemblies using bwa-mem (Li and Durbin 2009). Genomic positions of B73_AGPv4 gene features (Jiao et al. 2017) in KE0482 and KE0678 were identified by BLAST.
Pairwise sequence alignment of the target region between KE0482 and KE0678 was conducted using nucmer with the setting -c 156, -g 1,000 and subsequently filtered using delta-filter with settings -i 99 and -l 1,000 (Kurtz et al. 2004).
A MOASeq-binding site partially overlapping with the 5′ UTR of ndhm1 in B73_AGPv4 was identified and downloaded from MaizeGDB (https://jbrowse.maizegdb.org/?loc=chr2%3A23629472..23634058&tracks=gene_models_official%2Cmoa_seq_coverage_peaks&highlight=) using the track “MOA-seq coverage peaks” and searched by BLAST against the Kemater PacBio assemblies (Savadel et al. 2021).
The exon structure of ndhm1 alleles was determined by PCR amplification of cDNA fragments using exon-spanning primers followed by Sanger sequencing and realigning the obtained sequences against the assembly of KE0678 using BLAST.
Statistical analysis
All statistical analyses were done in R (R Core Team 2024). For field experiments with Kemater DH lines, adjusted means were estimated for each trait using the R package “ASReml–R” (v4.1.0). The statistical model for calculating adjusted means was
where µ is the overall mean; γk is the fixed effect of DH line k; uo is the random effect of environment o; γuko is the random interaction effect for genotype k and environment o; rs(o) is the random effect of the replicate s (nested in environments) and ekos is the residual error.
Phenotypic segregation between the F2:3 RILs of leaf color and EPH was pronounced, allowing for the scoring of plots as “KE0482-like”, “KE0678-like”, or “segregating” based on leaf color and EPH. Linkage of genome-wide KASP markers with the “KE0482-like” and “KE0678-like” phenotype was calculated by chi-square goodness-of-fit tests for each KASP marker individually followed by correction for multiple testing. To determine the mode of gene action of ndhm1 in F2:3 RILs the closest marker to ndhm1 (AX-90736551) was used to calculate the mean of RILs homozygous for the KE0482 and KE0678 allele or being heterozygous, respectively. The mean of both homozygous classes was compared with the mean of heterozygous RILs by a 2-sided Student's t-test.
For significance testing in fine-mapping and mutant analysis, 2-sided Student's t-tests were conducted between KE0482 and KE0678, HIF1A and HIF1B, HIF3A and HIF3B, and the Mu mutant and its wild type, respectively, for EPH, Fv/Fm, SPAD, and RT-qPCR data. Differences in CO2 assimilation rates were assessed by fitting a mixed linear model for each external CO2 concentration using the genotypic score of the ndhm1 allele as fixed effect and the genomic background (HIF1, HIF3) as random effect. Significance of allele effects was assessed by Wald tests (Kenward and Roger 1997). For the phenotypic comparisons of multiple ndhm1-associated haplotypes in field and growth chamber experiments least significant differences were applied.
For proteome analysis, 2-sided Student's t-tests were calculated for the difference of protein amounts between ndhm1 alleles for each protein separately. To avoid false positive associations, proteins were considered differentially expressed at a false discovery rate of 5% (Benjamini and Hochberg 1995) and a log2 fold change bigger than one.
Accession numbers
The whole-genome sequencing data have been deposited in the NCBI Sequence Read Archive (BioProject: PRJNA1133950) for Kemater lines KE0002 (SAMN42391533), KE0060 (SAMN42391534), KE0075 (SAMN42391535), KE0095 (SAMN42391536), KE0109 (SAMN42391537), KE0113 (SAMN42391538), KE0144 (SAMN42391539), KE0194 (SAMN42391540), KE0250 (SAMN42391541), KE0406 (SAMN42391542), KE0413 (SAMN42391543), KE0466 (SAMN42391544), KE0482 (SAMN42391545), KE0484 (SAMN42391546), and KE0678 (SAMN42391547). Raw data from the proteomics experiment have been deposited in the Proteomics Identification Database (https://www.ebi.ac.uk/pride/) under project PXD056682. The sequences of genes described in this study are listed in MaizeGDB under the following accession numbers: ndhm1: Zm00001d002815; ndhm1-A1: Zm00041ab076840; ndhm1-B: Zm00041ab076830; ndhm2: Zm00001d025952; lls1: Zm00001d027656; lox3: Zm00001d033623; lox10: Zm00001d053675; lox13: Zm00001d031449; hppd1: Zm00001d015356; nced6: Zm00001d051556; ptox2: Zm00001d001908.
Supplementary Material
Acknowledgments
We thank Brigitte Neuhauser, Iris Prücklmaier, Sylwia Schepella, Stefan Schwertfirm, and Margot Siebler for technical assistance. We thank the Plant Technology Center (Technical University of Munich, Germany) for providing infrastructure and technical support during greenhouse, growth chamber, and field experiments. We would like to acknowledge the support of William Marande and Caroline Callot from CNRGV, INRAE (http://cnrgv.toulouse.inrae.fr/) for providing assistance in PacBio long-read sequencing and GENTYANE platform of Clermont-Ferrand INRAE Center (http://gentyane.clermont.inra.fr/) for providing access to PacBio sequencer. The TOC figure was created in BioRender. Urzinger, S. (2024) BioRender.com/l78r804.
Contributor Information
Sebastian Urzinger, Plant Breeding, TUM School of Life Sciences, Technical University of Munich, Freising 85354, Germany.
Viktoriya Avramova, Plant Breeding, TUM School of Life Sciences, Technical University of Munich, Freising 85354, Germany.
Monika Frey, Plant Breeding, TUM School of Life Sciences, Technical University of Munich, Freising 85354, Germany.
Claude Urbany, Maize Breeding, KWS SAAT SE & Co. KGaA, Einbeck 37574, Germany.
Daniela Scheuermann, Maize Breeding, KWS SAAT SE & Co. KGaA, Einbeck 37574, Germany.
Thomas Presterl, Maize Breeding, KWS SAAT SE & Co. KGaA, Einbeck 37574, Germany.
Stefan Reuscher, Maize Breeding, KWS SAAT SE & Co. KGaA, Einbeck 37574, Germany.
Karin Ernst, Institute of Molecular and Developmental Biology of Plants, Heinrich-Heine-University Düsseldorf, Düsseldorf 40225, Germany.
Manfred Mayer, Plant Breeding, TUM School of Life Sciences, Technical University of Munich, Freising 85354, Germany.
Caroline Marcon, INRES, Institute of Crop Science and Resource Conservation, Crop Functional Genomics, University of Bonn, Bonn 53113, Germany.
Frank Hochholdinger, INRES, Institute of Crop Science and Resource Conservation, Crop Functional Genomics, University of Bonn, Bonn 53113, Germany.
Sarah Brajkovic, Proteomics and Bioanalytics, TUM School of Life Sciences, Technical University of Munich, Freising 85354, Germany.
Bernardo Ordas, Misión Biológica de Galicia, Spanish National Research Council (CSIC), Pontevedra 36080, Spain.
Peter Westhoff, Institute of Molecular and Developmental Biology of Plants, Heinrich-Heine-University Düsseldorf, Düsseldorf 40225, Germany.
Milena Ouzunova, Maize Breeding, KWS SAAT SE & Co. KGaA, Einbeck 37574, Germany.
Chris-Carolin Schön, Plant Breeding, TUM School of Life Sciences, Technical University of Munich, Freising 85354, Germany.
Author contributions
C.-C.S., V.A., M.O., P.W, M.F., and S.U. designed the research and developed ideas; S.U., V.A., and B.O. designed and performed phenotyping experiments and analyzed the data; S.R. and S.U. analyzed whole-genome sequencing data; C.M. and F.H. developed Mu transposon mutants; S.B. acquired and analyzed proteomics data. K.E. and S.U. conducted candidate gene analysis; S.U., M.M., M.O., T.P., D.S., and C.U. developed the plant material; S.U., V.A., P.W., and C.-C.S. wrote the manuscript; all authors read and approved the final manuscript; C.-C.S. agrees to serve as the author responsible for contact and to ensure communication.
Supplementary data
The following materials are available in the online version of this article.
Supplementary Figure S1. GWAS in Kemater lines.
Supplementary Figure S2. Environmental stability of most significantly associated 10-SNP haplotype (lead haplotype) in target region from GWAS in landrace Kemater.
Supplementary Figure S3. Polymorphic 600k Axiom Maize Genotyping Array SNPs between KE0482 and KE0678.
Supplementary Figure S4. Phenotypic differences between KE0482 and KE0678 in field experiments in up to 11 combinations of locations and years (Hölker et al. 2019).
Supplementary Figure S5. Chi-squared test of independence of KE0678-like phenotype with genome-wide KASP markers in 211 F2:3 RILs.
Supplementary Figure S6. Test for deviation from midparent value in field experiment in 2020.
Supplementary Figure S7. Transcription of ndhm1 alleles in biparental population.
Supplementary Figure S8. Differences in NDHM protein levels between KE0482, HIF1A, HIF3A and KE0678, HIF1B and HIF3B.
Supplementary Figure S9. Impact of hAT insertion in ndhm1A-2 on relative protein levels of NDH components.
Supplementary Figure S10. Impact of hAT insertion in ndhm1A-2 on relative protein levels of non-NDH CET components.
Supplementary Figure S11. Genotyping of transposon insertion in ndhm1A-2 in 27 Kemater lines.
Supplementary Figure S12. Genotyping of presence or absence of ndhm1B in 27 Kemater lines.
Supplementary Figure S13. Effect of ndhm1 allele after severe cold treatment on photosynthetic parameters.
Supplementary Table S1. QTL detected for EPH and photosynthetic traits in landrace Kemater.
Supplementary Table S2. Candidate genes in 314 kb fine-mapped QTL region on chromosome 2.
Supplementary Table S3. Frequencies of most associated 10-SNP haplotype from GWAS found in Kemater population (n = 471).
Supplementary Table S4. Genotyping of ndhm1A-1 and ndhm1B in a set of 27 Kemater lines for in depth molecular characterization of allelic diversity.
Supplementary Table S5. Genomic positions of KASP markers on chromosome 2 of different genome assemblies used to genotype RILs and HIFs.
Supplementary Table S6. Primers used for RT-qPCR analysis and genotyping different ndhm1 alleles.
Supplementary File 1. Pairwise sequence alignment of the protein sequence of NDHM1_P01 and NDHM2_P01.
Supplementary File 2. Pairwise sequence alignment of the protein sequence of NDHM1_P01 and NDHM2_P02.
Supplementary File 3. Multiple sequence alignment of the protein sequence of NDHM1 from KE0482, KE0678, European Flint inbred lines, and American NAM lines.
Supplementary File 4. Multiple sequence alignment of the nucleotide sequence of ndhm1-A1 and ndhm1B from KE0482 and Tx303.
Supplementary File 5. Putative transcription start site of ndhm1 in shoots from Mejía-Guerra et al. (2015).
Supplementary File 6. MOASeq motif upstream of ndhm1 indicating a TF-binding site from Savadel et al. (2021).
Funding
This study was funded by the Federal Ministry of Education and Research (BMBF, Germany) within the scope of the funding initiative “Plant Breeding Research for the Bioeconomy” (Funding ID: 031B0195, 031B0882, and 031B1301) as part of the project MAZE (www.europeanmaize.net). The BonnMu project was funded by the Deutsche Forschungsgemeinschaft (DFG) grant MA 8427/1-1 to C.M. KWS SAAT SE & Co. KGaA funded Ph.D. fellowships for S.U. and M.M.
Conflict of interest statement. A patent application has been filed related to this work.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author upon request. The genotypic and phenotypic data (EPH, PH) of 471 Kemater lines are available in figshare (https://doi.org/10.6084/m9.figshare.12137142). The sequencing data underlying this article are available in the NCBI Sequence Read Archive (https://www.ncbi.nlm.nih.gov/sra/), and can be accessed with the accession numbers listed in the Materials and Methods section. The proteomic data underlying this article are available in the Proteomics Identification Database (https://www.ebi.ac.uk/pride/), and can be accessed with the accession numbers listed in the Material and Methods section.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
References
- Asada K, Heber U, Schreiber U. Electron flow to the intersystem chain from stromal components and cyclic electron flow in maize chloroplasts, as detected in intact leaves by monitoring redox change of P700 and chlorophyll fluorescence. Plant Cell Physiol. 1993:34:39–50. 10.1093/oxfordjournals.pcp.a078398 [DOI] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995:57(1):289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- Blankenagel S, Eggels S, Frey M, Grill E, Bauer E, Dawid C, Fernie AR, Haberer G, Hammerl R, Barbosa Medeiros D, et al. Natural alleles of the abscisic acid catabolism gene ZmAbh4 modulate water use efficiency and carbon isotope discrimination in maize. Plant Cell. 2022:34(10):3860–3872. 10.1093/plcell/koac200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brajkovic S, Rugen N, Agius C, Berner N, Eckert S, Sakhteman A, Schwechheimer C, Kuster B. Getting ready for large-scale proteomics in crop plants. Nutrients. 2023:15(3):783. 10.3390/nu15030783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett AC, Kromdijk J. Can we improve the chilling tolerance of maize photosynthesis through breeding? J Exp Bot. 2022:73(10):3138–3156. 10.1093/jxb/erac045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows PA, Sazanov LA, Svab Z, Maliga P, Nixon PJ. Identification of a functional respiratory complex in chloroplasts through analysis of tobacco mutants containing disrupted plastid ndh genes. EMBO J. 1998:17(4):868–876. 10.1093/emboj/17.4.868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzadilla PI, Song J, Gallois P, Johnson GN. Proximity to photosystem II is necessary for activation of plastid terminal oxidase (PTOX) for photoprotection. Nat Commun. 2024:15(1):287. 10.1038/s41467-023-44454-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Concepcion GT, Feng X, Zhang H, Li H. Haplotype-resolved de novo assembly using phased assembly graphs with hifiasm. Nat Methods. 2021:18(2):170–175. 10.1038/s41592-020-01056-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi SL, Ceccarelli S, Blair MW, Upadhyaya HD, Are AK, Ortiz R. Landrace germplasm for improving yield and abiotic stress adaptation. Trends Plant Sci. 2016:21(1):31–42. 10.1016/j.tplants.2015.10.012 [DOI] [PubMed] [Google Scholar]
- Enders TA, St Dennis S, Oakland J, Callen ST, Gehan MA, Miller ND, Spalding EP, Springer NM, Hirsch CD. Classifying cold-stress responses of inbred maize seedlings using RGB imaging. Plant Direct. 2019:3(1):e00104. 10.1002/pld3.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T, Shikanai T, Takabayashi A, Asada K, Sato F. The role of chloroplastic NAD(P)H dehydrogenase in photoprotection. FEBS Lett. 1999:457(1):5–8. 10.1016/S0014-5793(99)00989-8 [DOI] [PubMed] [Google Scholar]
- Essemine J, Xiao Y, Qu M, Mi H, Zhu X-G. Cyclic electron flow may provide some protection against PSII photoinhibition in rice (Oryza sativa L.) leaves under heat stress. J Plant Physiol. 2017:211:138–146. 10.1016/j.jplph.2017.01.007 [DOI] [PubMed] [Google Scholar]
- FAOSTAT . Land statistics 2001–2022 – global, regional and country trends. FAOSTAT Anal Briefs. 2024(No. 88). 10.4060/cd1484en [DOI] [Google Scholar]
- Fracheboud Y, Jompuk C, Ribaut JM, Stamp P, Leipner J. Genetic analysis of cold-tolerance of photosynthesis in maize. Plant Mol Biol. 2004:56(2):241–253. 10.1007/s11103-004-3353-6 [DOI] [PubMed] [Google Scholar]
- Frei O. Changes in yield physiology of corn as a result of breeding in northern Europe. Maydica. 2000:45:173–183. [Google Scholar]
- Garisson E, Marth G. 2012. Haplotype-based variant detection from short-read sequencing. arXiv, arXiv:1207.3907, preprint: not peer reviewed.
- Gessulat S, Schmidt T, Zolg DP, Samaras P, Schnatbaum K, Zerweck J, Knaute T, Rechenberger J, Delanghe B, Huhmer A, et al. Prosit: proteome-wide prediction of peptide tandem mass spectra by deep learning. Nat Methods. 2019:16(6):509–518. 10.1038/s41592-019-0426-7 [DOI] [PubMed] [Google Scholar]
- Haberer G, Kamal N, Bauer E, Gundlach H, Fischer I, Seidel MA, Spannagl M, Marcon C, Ruban A, Urbany C, et al. European maize genomes highlight intraspecies variation in repeat and gene content. Nat Genet. 2020:52(9):950–957. 10.1038/s41588-020-0671-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Fu J, Yu C, Wang X, Jiang Q, Hong J, Lu K, Xue G, Yan C, James A, et al. Increasing cyclic electron flow is related to Na+ sequestration into vacuoles for salt tolerance in soybean. J Exp Bot. 2015:66(21):6877–6889. 10.1093/jxb/erv392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölker AC, Mayer M, Presterl T, Bolduan T, Bauer E, Ordas B, Brauner PC, Ouzunova M, Melchinger AE, Schön C-C. European maize landraces made accessible for plant breeding and genome-based studies. Theor Appl Genet. 2019:132(12):3333–3345. 10.1007/s00122-019-03428-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hufford MB, Seetharam AS, Woodhouse MR, Chougule KM, Ou S, Liu J, Ricci WA, Guo T, Olson A, Qiu Y, et al. De novo assembly, annotation, and comparative analysis of 26 diverse maize genomes. Science. 2021:373(6555):655–662. 10.1126/science.abg5289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hund A, Fracheboud Y, Soldati A, Frascaroli E, Salvi S, Stamp P. QTL controlling root and shoot traits of maize seedlings under cold stress. Theor Appl Genet. 2004:109(3):618–629. 10.1007/s00122-004-1665-1 [DOI] [PubMed] [Google Scholar]
- Ishikawa N, Takabayashi A, Noguchi K, Tazoe Y, Yamamoto H, von Caemmerer S, Sato F, Endo T. NDH-mediated cyclic electron flow around photosystem I is crucial for C4 photosynthesis. Plant Cell Physiol. 2016a:57(10):2020–2028. 10.1093/pcp/pcw127 [DOI] [PubMed] [Google Scholar]
- Ishikawa N, Takabayashi A, Sato F, Endo T. Accumulation of the components of cyclic electron flow around photosystem I in C4 plants, with respect to the requirements for ATP. Photosynth Res. 2016b:129(3):261–277. 10.1007/s11120-016-0251-0 [DOI] [PubMed] [Google Scholar]
- Jiao Y, Peluso P, Shi J, Liang T, Stitzer MC, Wang B, Campbell MS, Stein JC, Wei X, Chin C-S, et al. Improved maize reference genome with single-molecule technologies. Nature. 2017:546(7659):524–527. 10.1038/nature22971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997:53(3):983–997. 10.2307/2533558 [DOI] [PubMed] [Google Scholar]
- Kubicki A, Funk E, Westhoff P, Steinmüller K. Differential expression of plastome-encoded ndh genes in mesophyll and bundle-sheath chloroplasts of the C4 plant Sorghum bicolor indicates that the complex I-homologous NAD(P)H-plastoquinone oxidoreductase is involved in cyclic electron transport. Planta. 1996:199(2):276–281. 10.1007/BF00196569 [DOI] [Google Scholar]
- Kucharik CJ. A multidecadal trend of earlier corn planting in the central USA. Agron J. 2006:98(6):1544–1550. 10.2134/agronj2006.0156 [DOI] [Google Scholar]
- Kurtz S, Phillippy A, Delcher AL, Smoot M, Shumway M, Antonescu C, Salzberg SL. Versatile and open software for comparing large genomes. Genome Biol. 2004:5(2):R12. 10.1186/gb-2004-5-2-r12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lainé CMS, AbdElgawad H, Beemster GTS. A meta-analysis reveals differential sensitivity of cold stress responses in the maize leaf. Plant Cell Environ. 2023:46(8):2432–2449. 10.1111/pce.14608 [DOI] [PubMed] [Google Scholar]
- Leipner J, Fracheboud Y, Stamp P. Effect of growing season on the photosynthetic apparatus and leaf antioxidative defenses in two maize genotypes of different chilling tolerance. Environm Exp Bot. 1999:42(2):129–139. 10.1016/S0098-8472(99)00026-X [DOI] [Google Scholar]
- Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009:25(14):1754–1760. 10.1093/bioinformatics/btp324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Liu H-J, Yan J, Tian F. Natural variation in crops: realized understanding, continuing promise. Annu Rev Plant Biol. 2021:72(1):357–385. 10.1146/annurev-arplant-080720-090632 [DOI] [PubMed] [Google Scholar]
- Liu H-J, Jian L, Xu J, Zhang Q, Zhang M, Jin M, Peng Y, Yan J, Han B, Liu J, et al. High-throughput CRISPR/Cas9 mutagenesis streamlines trait gene identification in maize. Plant Cell. 2020:32(5):1397–1413. 10.1105/tpc.19.00934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W-J, Liu H, Chen Y-E, Yin Y, Zhang Z-W, Song J, Chang L-J, Zhang F-L, Wang D, Dai X-H, et al. Chloroplastic photoprotective strategies differ between bundle sheath and mesophyll cells in maize (Zea mays L.) under drought. Front Plant Sci. 2022:13:885781. 10.3389/fpls.2022.885781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logemann J, Schell J, Willmitzer L. Improved method for the isolation of RNA from plant tissues. Anal Biochem. 1987:163(1):16–20. 10.1016/0003-2697(87)90086-8 [DOI] [PubMed] [Google Scholar]
- Lorenzo CD, Debray K, Herwegh D, Develtere W, Impens L, Schaumont D, Vandeputte W, Aesaert S, Coussens G, De Boe Y, et al. BREEDIT: a multiplex genome editing strategy to improve complex quantitative traits in maize. Plant Cell. 2023:35(1):218–238. 10.1093/plcell/koac243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoli A, Sturaro A, Trevisan S, Quaggiotti S, Nonis A. Evaluation of candidate reference genes for qPCR in maize. J Plant Physiol. 2012:169(8):807–815. 10.1016/j.jplph.2012.01.019 [DOI] [PubMed] [Google Scholar]
- Marcon C, Altrogge L, Win YN, Stöcker T, Gardiner JM, Portwood JL II, Opitz N, Kortz A, Baldauf JA, Hunter CT, et al. Bonnmu: a sequence-indexed resource of transposon-induced maize mutations for functional genomics studies. Plant Physiol. 2020:184(2):620–631. 10.1104/pp.20.00478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marone D, Mastrangelo AM, Borrelli GM. From transgenesis to genome editing in crop improvement: applications, marketing, and legal issues. Int J Mol Sci. 2023:24(8):7122. 10.3390/ijms24087122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín M, Casano LM, Zapata JM, Guéra A, Del Campo EM, Schmitz-Linneweber C, Maier RM, Sabater B. Role of thylakoid Ndh complex and peroxidase in the protection against photo-oxidative stress: fluorescence and enzyme activities in wild-type and ndhF-deficient tobacco. Physiol Plant. 2004:122(4):443–452. 10.1111/j.1399-3054.2004.00417.x [DOI] [Google Scholar]
- Mayer M, Hölker AC, González-Segovia E, Bauer E, Presterl T, Ouzunova M, Melchinger AE, Schön C-C. Discovery of beneficial haplotypes for complex traits in maize landraces. Nat Commun. 2020:11(1):4954. 10.1038/s41467-020-18683-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M, Hölker AC, Presterl T, Ouzunova M, Melchinger AE, Schön C-C. Genetic diversity of European maize landraces: dataset on the molecular and phenotypic variation of derived doubled-haploid populations. Data Brief. 2022:42:108164. 10.1016/j.dib.2022.108164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M, Unterseer S, Bauer E, de Leon N, Ordas B, Schön C-C. Is there an optimum level of diversity in utilization of genetic resources? Theor Appl Genet. 2017:130(11):2283–2295. 10.1007/s00122-017-2959-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCouch S, Baute GJ, Bradeen J, Bramel P, Bretting PK, Buckler E, Burke JM, Charest D, Cloutier S, Cole G, et al. Feeding the future. Nature. 2013:499(7456):23–24. 10.1038/499023a [DOI] [PubMed] [Google Scholar]
- Meierhoff K, Westhoff P. Differential biogenesis of photosystem II in mesophyll and bundle-sheath cells of monocotyledonous NADP-malic enzyme-type C 4 plants: the non-stoichiometric abundance of the subunits of photosystem II in the bundle-sheath chloroplasts and the translational activity of the plastome-encoded genes. Planta. 1993:191:23–33. 10.1007/BF00240892 [DOI] [Google Scholar]
- Mejía-Guerra MK, Li W, Galeano NF, Vidal M, Gray J, Doseff AI, Grotewold E. Core promoter plasticity between maize tissues and genotypes contrasts with predominance of sharp transcription initiation sites. Plant Cell. 2015:27(12):3309–3320. 10.1105/tpc.15.00630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messant M, Hani U, Lai TL, Wilson A, Shimakawa G, Krieger-Liszkay A. Plastid terminal oxidase (PTOX) protects photosystem I and not photosystem II against photoinhibition in Arabidopsis thaliana and Marchantia polymorpha. Plant J. 2024:117(3):669–678. 10.1111/tpj.16520 [DOI] [PubMed] [Google Scholar]
- Miedema P. The effects of low temperature on Zea mays. In: Brady NC, editor. Advances in agronomy. San Diego (CA): Academic Press; 1982. p. 93–128. [Google Scholar]
- Murchie EH, Ruban AV. Dynamic non-photochemical quenching in plants: from molecular mechanism to productivity. Plant J. 2020:101(4):885–896. 10.1111/tpj.14601 [DOI] [PubMed] [Google Scholar]
- Nie Y, Wang H, Zhang G, Ding H, Han B, Liu L, Shi J, Du J, Li X, Li X, et al. The maize PLASTID TERMINAL OXIDASE (PTOX) locus controls the carotenoid content of kernels. Plant J. 2024:118(2):457–468. 10.1111/tpj.16618 [DOI] [PubMed] [Google Scholar]
- Nouri A, Lukas S, Singh S, Singh S, Machado S. When do cover crops reduce nitrate leaching? A global meta-analysis. Glob Chang Biol. 2022:28(15):4736–4749. 10.1111/gcb.16269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker P, Shonkwiler J, Aurbacher J. Cause and consequence in maize planting dates in Germany. J Agron Crop Sci. 2017:203(3):227–240. 10.1111/jac.12182 [DOI] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001:29(9):e45–e45. 10.1093/nar/29.9.e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing; 2024.
- Revilla P, Rodríguez VM, Ordás A, Rincent R, Charcosset A, Giauffret C, Melchinger AE, Schön C-C, Bauer E, Altmann T, et al. Association mapping for cold tolerance in two large maize inbred panels. BMC Plant Biol. 2016:16(1):127. 10.1186/s12870-016-0816-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savadel SD, Hartwig T, Turpin ZM, Vera DL, Lung P-Y, Sui X, Blank M, Frommer WB, Dennis JH, Zhang J, et al. The native cistrome and sequence motif families of the maize ear. PLoS Genet. 2021:17(8):e1009689. 10.1371/journal.pgen.1009689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settles AM, Latshaw S, McCarty DR. Molecular analysis of high-copy insertion sites in maize. Nucleic Acids Res. 2004:32(6):e54. 10.1093/nar/gnh052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Tang K, Wang W, Wang C, Wu H, Mao Z, An S, Chang S, Kuang T, Shen J-R, et al. Architecture of the chloroplast PSI–NDH supercomplex in Hordeum vulgare. Nature. 2022:601(7894):649–654. 10.1038/s41586-021-04277-6 [DOI] [PubMed] [Google Scholar]
- Tang H, Zhang X, Miao C, Zhang J, Ming R, Schnable JC, Schnable PS, Lyons E, Lu J. ALLMAPS: robust scaffold ordering based on multiple maps. Genome Biol. 2015:16(1):3. 10.1186/s13059-014-0573-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian T, Liu Y, Yan H, You Q, Yi X, Du Z, Xu W, Su Z. agriGO v2.0: a GO analysis toolkit for the agricultural community, 2017 update. Nucleic Acids Res. 2017:45(W1):W122–W129. 10.1093/nar/gkx382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyanova S, Temu T, Cox J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat Protoc. 2016:11(12):2301–2319. 10.1038/nprot.2016.136 [DOI] [PubMed] [Google Scholar]
- Wang P, Duan W, Takabayashi A, Endo T, Shikanai T, Ye JY, Mi H. Chloroplastic NAD(P)H dehydrogenase in tobacco leaves functions in alleviation of oxidative damage caused by temperature stress. Plant Physiol. 2006:141(2):465–474. 10.1104/pp.105.070490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wezel A, Casagrande M, Celette F, Vian J-F, Ferrer A, Peigné J. Agroecological practices for sustainable agriculture. A review. Agron Sustain Dev. 2014:34(1):1–20. 10.1007/s13593-013-0180-7 [DOI] [Google Scholar]
- Win YN, Stcker T, Du X, Brox A, Pitz M, Klaus A, Piepho H-P, Schoof H, Hochholdinger F, Marcon C. Expanding the BonnMu sequence-indexed repository of transposon induced maize (Zea mays L.) mutations in dent and flint germplasm. Plant J. 2024. 10.1111/tpj.17088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Tian S, Chen G, Tang Q, Zhang Y, Fleming AJ, Zhu XG, Wang P. Regulatory NADH dehydrogenase-like complex optimizes C4 photosynthetic carbon flow and cellular redox in maize. New Phytol. 2024:241(1):82–101. 10.1111/nph.19332 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author upon request. The genotypic and phenotypic data (EPH, PH) of 471 Kemater lines are available in figshare (https://doi.org/10.6084/m9.figshare.12137142). The sequencing data underlying this article are available in the NCBI Sequence Read Archive (https://www.ncbi.nlm.nih.gov/sra/), and can be accessed with the accession numbers listed in the Materials and Methods section. The proteomic data underlying this article are available in the Proteomics Identification Database (https://www.ebi.ac.uk/pride/), and can be accessed with the accession numbers listed in the Material and Methods section.