Abstract
The role of dairy products in cardiovascular disease (CVD) prevention remains controversial. This study investigates the association between dairy consumption and CVD incidence using data from the China Kadoorie Biobank and the UK Biobank, complemented by an updated meta-analysis. Among Chinese participants, regular dairy consumption (primarily whole milk) is associated with a 9% increased risk of coronary heart disease (CHD) and a 6% reduced risk of stroke compared to non-consumers. Among British participants, total dairy consumption is linked to lower risks of CVD, CHD, and ischemic stroke, with cheese and semi-skimmed/skimmed milk contributing to reduced CVD risk. Meta-analysis reveals that total dairy consumption is associated with a 3.7% reduced risk of CVD and a 6% reduced risk of stroke. Notably, inverse associations with CVD incidence are observed for cheese and low-fat dairy products. Current evidence suggests that dairy consumption, particularly cheese, may have protective effects against CVD and stroke.
Subject terms: Epidemiology, Cardiovascular diseases, Preventive medicine
The role of dairy products in cardiovascular disease (CVD) prevention is debated. Here, the authors show that dairy consumption is associated with a lower risk of CVD and stroke, with cheese linked to reduced CVD risk, though the effects vary between Chinese and UK populations.
Introduction
Cardiovascular disease (CVD) is the largest contributor to death globally1. Adopting healthy dietary patterns is one of the cornerstones of primary prevention of CVD. Thereinto, although dairy consumption features in many dietary guidelines, its role in a heart-healthy diet remains highly debated2. Dairy products contain various beneficial nutrients, including high biological value protein, milk fat globule phospholipids, and vitamins and minerals that could improve CVD risk factors3–6, whereas saturated fats7 and multiple anabolic hormones might adversely affect the health benefit, such as IGF-18,9. Previous prospective studies linking dairy consumption with CVD outcomes have yielded conflicting results. Some cohort studies reported a protective relationship between dairy consumption and CVD outcomes10–14, whereas others showed no significant associations15–18 or even positive associations19,20. Meta-analyses also yielded inconsistent conclusions on associations of dairy intake with coronary heart disease (CHD) and stroke risk21,22. Notably, heterogeneity between included studies was considerable and the overall quality of the evidence was low to moderate.
Prevailing recommendations advocate low-fat or non-fat dairy over whole-fat dairy23. However, scientific evidence for this recommendation was scant and inconsistent21. Importantly, different subtypes of dairy products may confer divergent health effects after processing. Fermented milk products such as yogurt contain probiotics that can favorably regulate gut microbiome24, whereas cheese is rich in sodium which may elevate blood pressure when consumed in excessive amounts25. Nonetheless, cheese is also a fermented food that can contain vitamin K226, high levels of milk fat globule membrane27, as well as probiotics28. Furthermore, previous epidemiological studies were largely conducted in Western countries, where the consumption level of dairy products especially cheese is high and usually correlated with a higher socioeconomic position29,30. In Asia where strokes are more common than CHD, only a few studies demonstrated an inverse association of dairy consumption with stroke10,31. Overall, evidence from large cohort studies in both Western and non-Western countries is needed to make global policy recommendations.
To address the above-mentioned gaps in knowledge, we followed 0.9 million individuals from the UK Biobank (UKB) study and the China Kadoorie Biobank (CKB) study to evaluate the associations of dairy product consumption with incident CVD, CHD, and stroke. We also performed an updated systematic review of the literature and meta-analysis of dairy product intake and incident CVD risk which included our findings to address the role of dairy consumption in CVD prevention and improve dietary guidelines.
In this study, we demonstrate that total dairy consumption is inversely associated with the overall risk of CVD and stroke. Higher intake of dairy products is significantly linked to a reduced risk of stroke in the Chinese population, while it is associated with a lower risk of CVD, CHD, and ischemic stroke in the British population. When examining specific dairy subtypes, cheese, and low-fat dairy products emerge as potentially protective and may be recommended for CVD prevention.
Results
Cohort analyses
During a follow-up of 4,190,676 person-years in CKB and 4,736,113 person-years in UKB, we documented 66,132 CVD cases in CKB and 32,822 CVD cases in UKB. In CKB, participants who consumed dairy products more frequently tended to be women, higher-educated, high-income class, urban residents and vitamin and mineral supplements users, have diabetes and family history of CVD, and consume fruits and eggs more frequently (Supplementary Data 1). In UKB, individuals with higher total dairy consumption were more likely to exercise, be more educated, take vitamin and mineral supplements, and consume oily fish and fruits more frequently, whereas they drank alcohol less frequently and had a lower hypertension prevalence (Supplementary Data 2). Characteristics of participants by cheese consumption (the main subtype of dairy in UKB) and milk types in UKB are shown in Supplementary Data 3 and 4.
Compared to non/rare consumers, those who consumed at least 4 times/week of dairy had no significant association with CVD after the multivariable adjustment in CKB (HR 1.00, 95% CI 0.97–1.03, P-trend = 0.470). Regular dairy consumption was related to a 9% higher risk of CHD (HR 1.09, 95% CI 1.05–1.13, P-trend < 0.001) but a 6% lower risk of stroke (HR 0.94, 95% CI 0.91–0.97, P-trend = 0.005), especially hemorrhagic stroke (HR 0.76, 95% CI 0.69–0.83, P-trend<0.001) (Table 1). Similar associations of CVD, CHD, and stroke were detected for the long-term usual dairy intakes (per 50 g/d increment) (Supplementary Data 5). In UKB, total dairy intake was inversely associated with incident CVD (HR 0.93, 95% CI 0.88–0.98, P-trend = 0.004), CHD (HR 0.93, 95% CI 0.88–0.99, P-trend = 0.014), and ischemic stroke (HR 0.86, 95% CI 0.75–0.99, P-trend = 0.036) (Table 2).
Table 1.
Hazard ratios (95% confidence intervals) for incident cardiovascular disease according to categories of dairy consumption in China Kadoorie Biobank
| Frequency of dairy consumption | P trend | ||||
|---|---|---|---|---|---|
| Never/rarely | Monthly | 1–3 d/wk | Regularly (≥4 d/wk) | ||
| CVD | |||||
| No of cases (%) | 42,641 (12.6) | 7830 (14.5) | 5788 (14.1) | 9873 (18.1) | |
| Person-years | 2,939,269 | 450,552 | 343,795 | 457,060 | |
| Model 1a | 1 (Reference) | 1.21 (1.18–1.24) | 1.26 (1.23–1.30) | 1.33 (1.30–1.36) | <0.001 |
| Model 2b | 1 (Reference) | 1.00 (0.98–1.03) | 0.98 (0.95–1.01) | 0.94 (0.92–0.97) | <0.001 |
| Model 3c | 1 (Reference) | 1.01 (0.99–1.04) | 1.00 (0.97–1.03) | 0.96 (0.93–0.98) | 0.007 |
| Model 4d | 1 (Reference) | 1.03 (1.00–1.05) | 1.03 (1.00–1.06) | 1.00 (0.97–1.03) | 0.470 |
| CHD | |||||
| No of cases (%) | 21,129 (6.3) | 4032 (7.5) | 3264 (8.0) | 6051 (11.1) | |
| Person-years | 3,006,398 | 463,388 | 352,952 | 471,541 | |
| Model 1a | 1 (Reference) | 1.24 (1.20–1.28) | 1.41 (1.36–1.46) | 1.61 (1.57–1.66) | <0.001 |
| Model 2b | 1 (Reference) | 1.03 (1.00–1.07) | 1.03 (0.99–1.07) | 1.04 (1.00–1.07) | 0.023 |
| Model 3c | 1 (Reference) | 1.04 (1.00–1.08) | 1.05 (1.01–1.09) | 1.05 (1.01–1.09) | 0.002 |
| Model 4d | 1 (Reference) | 1.05 (1.02–1.09) | 1.07 (1.03–1.12) | 1.09 (1.05–1.13) | <0.001 |
| Stroke | |||||
| No of cases (%) | 25,708 (7.6) | 4732 (8.8) | 3338 (8.1) | 5450 (10.0) | |
| Person-years | 2,999,803 | 461,574 | 353,514 | 475,204 | |
| Model 1a | 1 (Reference) | 1.20 (1.17–1.24) | 1.20 (1.16–1.24) | 1.17 (1.13–1.20) | <0.001 |
| Model 2b | 1 (Reference) | 0.98 (0.95–1.01) | 0.96 (0.92–0.99) | 0.88 (0.85–0.91) | <0.001 |
| Model 3c | 1 (Reference) | 0.99 (0.96–1.02) | 0.98 (0.94–1.02) | 0.89 (0.86–0.92) | <0.001 |
| Model 4d | 1 (Reference) | 1.01 (0.98–1.04) | 1.01 (0.97–1.05) | 0.94 (0.91–0.97) | 0.005 |
| Hemorrhagic stroke | |||||
| No of cases (%) | 6128 (1.8) | 825 (1.5) | 410 (1.0) | 552 (1.0) | |
| Person-years | 3,065,433 | 475,123 | 363,921 | 492,972 | |
| Model 1a | 1 (Reference) | 0.86 (0.80–0.92) | 0.60 (0.55–0.67) | 0.48 (0.44–0.52) | <0.001 |
| Model 2b | 1 (Reference) | 0.89 (0.83–0.96) | 0.83 (0.75–0.92) | 0.66 (0.60–0.72) | <0.001 |
| Model 3c | 1 (Reference) | 0.92 (0.85–0.99) | 0.87 (0.78–0.96) | 0.69 (0.63–0.76) | <0.001 |
| Model 4d | 1 (Reference) | 0.95 (0.88–1.02) | 0.92 (0.83–1.02) | 0.76 (0.69–0.83) | <0.001 |
| Ischemic stroke | |||||
| No of cases (%) | 20,256 (6.0) | 4008 (7.4) | 2992 (7.3) | 4966 (9.1) | |
| Person-years | 3,010,375 | 463,106 | 354,354 | 476,285 | |
| Model 1a | 1 (Reference) | 1.30 (1.25–1.34) | 1.37 (1.31–1.42) | 1.35 (1.31–1.40) | <0.001 |
| Model 2b | 1 (Reference) | 0.99 (0.96–1.03) | 0.98 (0.94–1.02) | 0.90 (0.87–0.93) | <0.001 |
| Model 3c | 1 (Reference) | 1.00 (0.97–1.04) | 1.00 (0.96–1.04) | 0.91 (0.88–0.95) | <0.001 |
| Model 4d | 1 (Reference) | 1.02 (0.98–1.05) | 1.03 (0.98–1.07) | 0.96 (0.92–0.99) | 0.090 |
Multi-variable Cox proportional hazard model was used. All statistical tests were two-sided.
aModel 1 was adjusted for age and sex.
bModel 2 was further adjusted for study area (10 regions), survey season, education (no formal school, primary school, middle or high school, or college and above), income (in yuan/year; <5000, 5000–9999, 10,000–19,999, 20,000–34,999, or ≥35,000), physical activity (in MET-h/wk; quartiles), smoking (never/occasionally, former, or current smoker), alcohol drinking (never/occasionally, former, or current drinker), family history of CVD (yes or no), aspirin use (yes or no), vitamins use (yes or no) and minerals use (yes or no).
cModel 3 was further adjusted for body mass index (in kg/m2; <18.5, 18.5–23.9, 24–27.9, or ≥28), history of hypertension (yes or no), and diabetes (yes or no).
dModel 4 was further adjusted for red meat, fish, poultry, eggs, fruits (never/rarely, monthly, 1–3 days/week, or regularly), and vegetables (daily or less than daily).
Table 2.
Hazard ratios (95% confidence intervals) for incident cardiovascular disease according to categories of dairy consumption in UK Biobank
| Dairy consumption | P trend | ||||
|---|---|---|---|---|---|
| 0 serving/d | ≤0.5 serving/d | 0.5–1.0 serving/d | >1 serving/d | ||
| N | 33,803 | 34,858 | 54,276 | 60,509 | |
| CVD | |||||
| No of cases (%) | 2448 (7.2) | 2292 (6.6) | 3497 (6.4) | 3895 (6.4) | |
| Person-years | 373,622.5 | 390,329.4 | 606,703.7 | 678,468.8 | |
| Model 1a | 1 [Reference] | 0.87 (0.83–0.93) | 0.86 (0.81–0.90) | 0.84 (0.80–0.88) | <0.001 |
| Model 2b | 1 [Reference] | 0.93 (0.88–0.99) | 0.91 (0.86–0.96) | 0.90 (0.86–0.95) | <0.001 |
| Model 3c | 1 [Reference] | 0.95 (0.90–1.01) | 0.92 (0.88–0.97) | 0.92 (0.87–0.97) | <0.001 |
| Model 4d | 1 [Reference] | 0.96 (0.90–1.01) | 0.93 (0.88–0.98) | 0.93 (0.88–0.98) | 0.004 |
| CHD | |||||
| No of cases (%) | 2042 (6.0) | 1906 (5.5) | 2912 (5.4) | 3228 (5.3) | |
| Person-years | 375,317.5 | 391,952.6 | 609,158.4 | 681,277.5 | |
| Model 1a | 1 [Reference] | 0.87 (0.82–0.93) | 0.86 (0.81–0.91) | 0.84 (0.80–0.89) | <0.001 |
| Model 2b | 1 [Reference] | 0.94 (0.88–1.00) | 0.92 (0.87–0.97) | 0.91 (0.86–0.96) | <0.001 |
| Model 3c | 1 [Reference] | 0.96 (0.90–1.02) | 0.93 (0.88–0.99) | 0.92 (0.87–0.98) | 0.005 |
| Model 4d | 1 [Reference] | 0.96 (0.90–1.02) | 0.94 (0.89–0.99) | 0.93 (0.88–0.99) | 0.014 |
| Stroke | |||||
| No of cases (%) | 499 (1.5) | 457 (1.3) | 698 (1.3) | 802 (1.3) | |
| Person-years | 384,114.7 | 399,863.2 | 621,356.8 | 694,107.5 | |
| Model 1a | 1 [Reference] | 0.84 (0.74–0.96) | 0.82 (0.73–0.92) | 0.83 (0.74–0.93) | 0.003 |
| Model 2b | 1 [Reference] | 0.89 (0.78–1.01) | 0.86 (0.77–0.97) | 0.87 (0.78–0.98) | 0.027 |
| Model 3c | 1 [Reference] | 0.90 (0.79–1.02) | 0.87 (0.78–0.98) | 0.89 (0.79–0.99) | 0.051 |
| Model 4d | 1 [Reference] | 0.91 (0.80–1.03) | 0.88 (0.78–0.99) | 0.90 (0.80–1.01) | 0.084 |
| Hemorrhagic stroke | |||||
| No of cases (%) | 81 (0.2) | 73 (0.2) | 106 (0.2) | 138 (0.2) | |
| Person-years | 385,796.5 | 401,501.5 | 623,807.9 | 696,931.7 | |
| Model 1a | 1 [Reference] | 0.82 (0.60–1.13) | 0.76 (0.57–1.02) | 0.87 (0.66–1.14) | 0.369 |
| Model 2b | 1 [Reference] | 0.85 (0.62–1.17) | 0.79 (0.59–1.05) | 0.89 (0.67–1.17) | 0.464 |
| Model 3c | 1 [Reference] | 0.86 (0.62–1.18) | 0.79 (0.59–1.06) | 0.90 (0.68–1.19) | 0.521 |
| Model 4d | 1 [Reference] | 0.86 (0.63–1.19) | 0.80 (0.60–1.07) | 0.91 (0.69–1.20) | 0.555 |
| Ischemic stroke | |||||
| No of cases (%) | 338 (1.0) | 307 (0.9) | 472 (0.9) | 512 (0.9) | |
| Person-years | 384,882.6 | 400,535.3 | 622,373.4 | 695,336.2 | |
| Model 1a | 1 [Reference] | 0.84 (0.72–0.98) | 0.82 (0.71–0.94) | 0.78 (0.68–0.89) | <0.001 |
| Model 2b | 1 [Reference] | 0.89 (0.77–1.04) | 0.87 (0.75–1.00) | 0.83 (0.72–0.95) | 0.010 |
| Model 3c | 1 [Reference] | 0.91 (0.78–1.06) | 0.88 (0.76–1.01) | 0.85 (0.74–0.98) | 0.023 |
| Model 4d | 1 [Reference] | 0.92 (0.78–1.07) | 0.89 (0.77–1.02) | 0.86 (0.75–0.99) | 0.036 |
Multi-variable Cox proportional hazard model was used. All statistical tests were two-sided.
aModel 1 was adjusted for age (continues) and sex (male or female).
bModel 2 was additionally adjusted for centers (22 categories), survey season (spring, summer, autumn, or winter), education (college or university degree, vocational qualifications, optional national exams at ages 17–18 years, national exams at age 16 years, others, or missing), household income (<£18,000, £18,000–£30,999, £31,000–£51,999, £52,000–£100,000, >£100,000, or missing), physical activity (MET-h/wk, quartiles), smoking (never, former, current, or missing), alcohol drinking (never or special occasions only, 1 or 2 times/week, 3 or 4 times/week, ≥5 times/week, or missing), family history of CVD (yes or no), aspirin use (yes or no), vitamins use (yes or no) and minerals use (yes or no).
cModel 3 was further adjusted for body mass index (in kg/m2; <18.5, 18.5–25, 25–30), history of hypertension (yes or no), and diabetes (yes or no).
dModel 4 was further adjusted for red meat, poultry (times/week; <2, 2–4, >4), processed red meat, oily fish, non-oily fish (times/week; <1, 1, ≥2), vegetables (servings/day; <1/, 1-3, ≥3), fruits (servings/day; <2, 2–4, ≥4), and eggs (yes or no).
For individual dairy products, cheese (46.49%) and yogurt (35.69%) were the majority (Supplementary Fig. 1a). Cheese consumption was associated with lower CVD and CHD risk. The HRs (95% CIs) comparing the frequency at least 7 times/week of cheese with less than 2 times/week were 0.88 (0.83–0.94) for CVD, 0.88 (0.82–0.94) for CHD, and 0.97 (0.85–1.11) for stroke in the fully adjusted model (Supplementary Data 6), which was similar to the results from 24-h dietary recalls (Supplementary Data 7). For subtypes of cheese, the proportions of each subtype are shown in Supplementary Fig. 1b and we found both hard cheese and fresh cheese (>0.5 serving/d) were associated with a lower risk of CVD and CHD (Supplementary Data 8–10). Considering the fat content of cheese, a protective association with CVD and CHD was found for high-fat cheese (>0.5 serving/d) while low-fat cheese was negatively associated with stroke incidence, especially ischemic stroke (Supplementary Data 11 and 12). Milk consumption (>0 to 0.5 serving/d) was associated with a lower risk of hemorrhagic stroke (HR 0.43, 95% CI 0.21–0.87), and yogurt consumption (>0.5 serving/d) was related to decreased ischemic stroke risk (HR 0.86, 95% CI 0.77–0.98), compared with non-consumers (Supplementary Data 13 and 14). No significant relationships were detected for ice cream consumption (Supplementary Data 15). Regarding different types of milk, compared with participants who never or rarely drank milk, both semi-skimmed and skimmed milk consumers had decreased CVD risk (semi-skimmed: HR 0.92, 95% CI 0.87–0.98; skimmed: HR 0.91, 95% CI 0.86–0.97) and stroke risk (semi-skimmed: HR 0.80, 95% CI 0.71–0.90; skimmed: HR 0.76, 95% CI 0.66–0.86). Attentionally, the association of whole milk (HR: 0.93, 95% CI: 0.87–1.00) with CVD incidence was marginally inverse (Supplementary Data 16).
Results for subgroup analyses in CKB and UKB were shown in Supplementary Data 17–20. Notably, the inverse associations between dairy consumption and the risks of CVD and stroke were observed exclusively in men, not women (P-interaction < 0.001), and in individuals with hypertension, but not in those without hypertension (P-interaction < 0.001), in the CKB (Supplementary Data 17). The inverse association of dairy consumption and CVD risk was detected in current smokers but not in non-smokers (P-interaction = 0.007) in UKB (Supplementary Data 18). Moreover, our results did not alter substantially in sensitivity analyses (Supplementary Data 21-24). In hypothetical substitution analyses, no significant associations were found in UKB. In CKB, replacing 50 g/d of eggs with an equivalent amount of dairy products was associated with an 11% higher risk of CVD, a 13% higher risk of CHD, and a 9% higher risk of stroke. In addition, substituting dairy products for fish or soybeans was associated with a 4% increase in CHD risk, whereas replacing red meat or soybeans with dairy products was associated with a 2% or 3% reduction in stroke risk, respectively (Supplementary Fig. 2).
Systematic review and meta-analysis
Overall, 30 publications from 25 prospective cohorts and our results from CKB and UKB were kept in our final meta-analysis (Supplementary Fig. 3, Supplementary Data 25 and 26). During a range of 5.5 to 30.0 follow-up years, 73,193 CVD cases were documented among 1,288,420 participants from 30 countries or territories around the world in the previous studies (30 studies) (Supplementary Data 27).
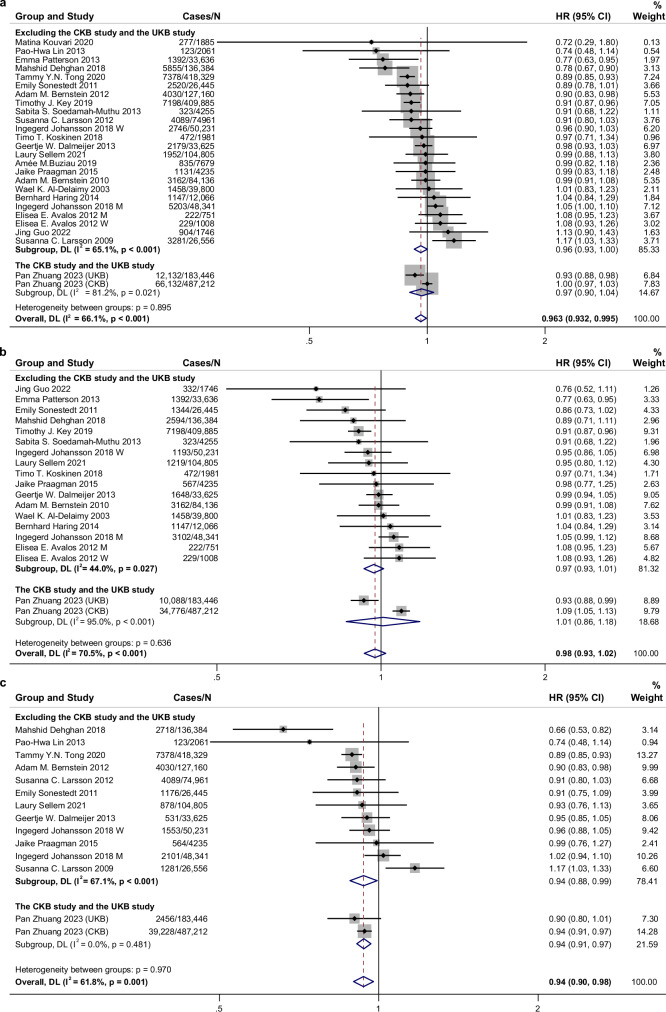
In the meta-analysis of previously published studies, a marginal inverse association was identified between total dairy intake and incident cardiovascular disease (CVD) (RR, 0.963; 95% CI, 0.926–1.001; n = 24 risk estimates). When the results from the CKB and UKB studies were incorporated, the 95% CI of the summary RR narrowed to 0.963 (0.932–0.995) (Fig. 1). Each serving/day increment of total dairy products was related to a 2% lower CVD risk (RR 0.98, 95% CI 0.96–0.99, P < 0.001, n = 17 risk estimates) (Supplementary Fig. 4). A similar inverse relationship for CVD was also shown in non-linear analysis (P-nonlinear = 0.002, n = 12 studies, Supplementary Fig. 5). For subtypes of CVD, the meta-analysis showed dairy consumption had an inverse relationship with total stroke risk (RR 0.94, 95% CI 0.90–0.98, 14 risk estimates, I2 = 61.8%) but a null association with CHD risk (RR 0.98, 95% CI 0.93–1.02, 19 risk estimates, I2 = 70.5%, Fig. 1).
Fig. 1. Associations of dairy consumption with cardiovascular disease, coronary heart disease, and stroke risk for high compared with low category of intake using random effects meta-analysis.
a Cardiovascular disease. b Coronary heart disease. c Stroke. Meta-analysis pooling of aggregate data used the random-effects inverse-variance model with DerSimonian-Laird estimate of tau². Data are presented as hazard ratios (HRs) and 95% confidence intervals (CIs). Squares represent study-specific HRs. Horizontal lines denote 95% CIs. Gray square areas are proportional to the individual study weight for the overall meta-analysis. The red dotted line represents risk ratio of pooled meta-analysis. The blue hollow diamonds represent the results of the meta-analysis for each group, with the center indicating the risk ratio and the width representing the 95% CI. I2 refers to the proportion of heterogeneity among studies. All statistical tests were two-sided. M, men; W, women; CKB, China Kadoorie Biobank; UKB, UK Biobank. Source data are provided as a Source Data file.
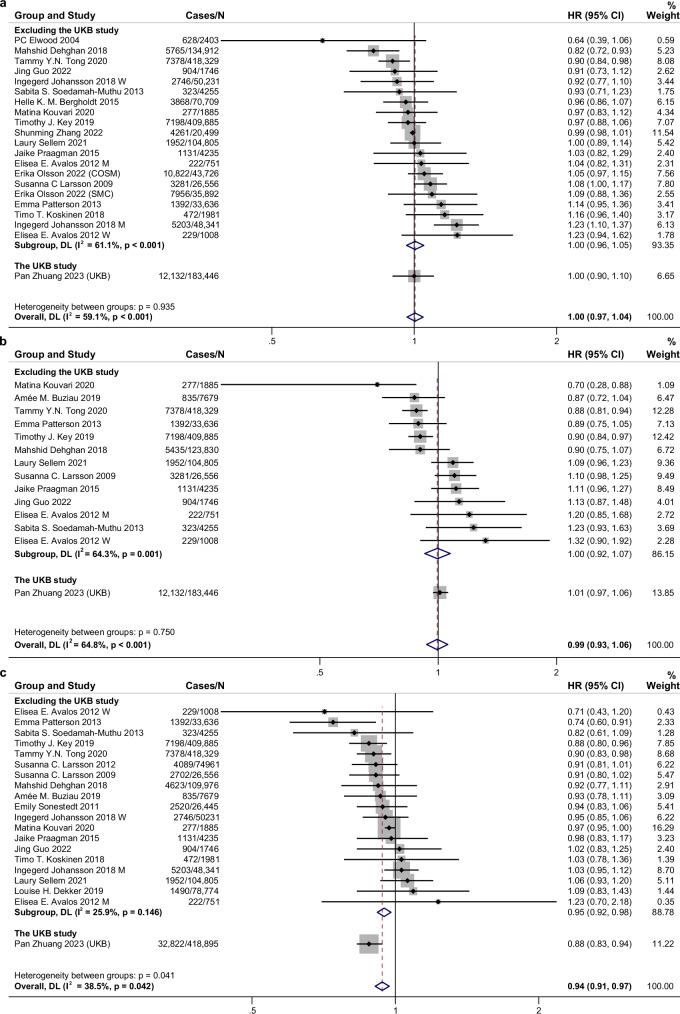
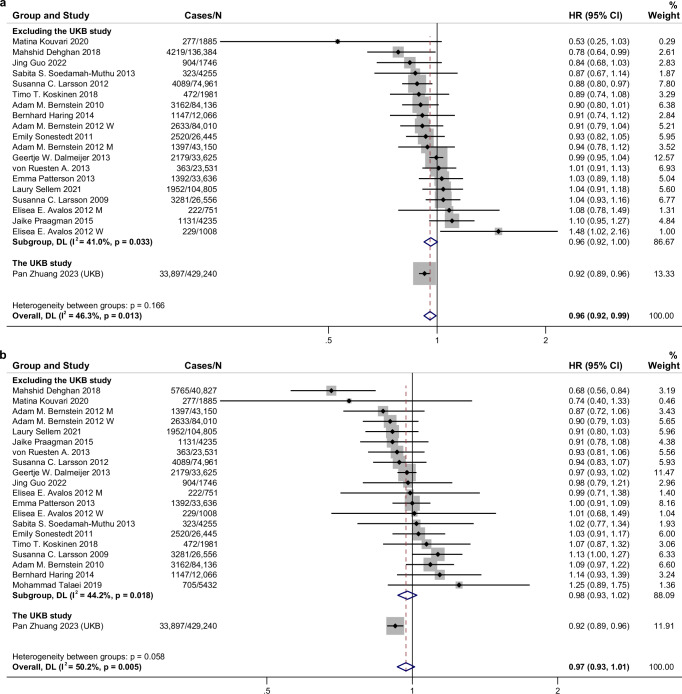
For major subtypes of dairy products, high intake of fermented dairy products, especially cheese, had a protective association with CVD risk (RR for fermented dairy 0.96, 95% CI: 0.94–0.98, n = 24 risk estimates; RR for cheese 0.94, 95% CI: 0.91–0.97, n = 20 risk estimates), but not yogurt (RR 0.99, 95% CI 0.93–1.06, n = 14 risk estimates) or milk (RR 1.00, 95% CI 0.97–1.04, n = 21 risk estimates) (Fig. 2 and Supplementary Fig. 6). Cheese intake was also associated with a decreased risk of CHD and stroke (Supplementary Figs. 7 and 8). Considering the content of fat, consumption of low-fat dairy products was significantly related to lower total CVD (RR: 0.96, 95% CI: 0.92–0.99, n = 20 risk estimates) and stroke risk (RR: 0.90, 95% CI: 0.84–0.98, n = 9 risk estimates) (Fig. 3 and Supplementary Fig. 9). Consumption of high-fat dairy products (including high-fat milk, high-fat yogurt, high-fat cheese, and cream or butter) was not associated with CVD risk (RR: 0.97, 95% CI: 0.93–1.01, n = 21 risk estimates) but inversely associated with CHD risk (RR: 0.96, 95% CI: 0.93–0.99, n = 14 risk estimates) (Fig. 3 and Supplementary Fig. 10). For subtypes of stroke, milk consumption was related to a higher risk of hemorrhagic stroke (RR 1.08, 95% CI 1.01–1.17, n = 5 risk estimates) and a decreased ischemic stroke risk was detected for total dairy (RR 0.92, 95% CI 0.86–0.99, n = 7 risk estimates) and cheese consumption (RR 0.91, 95% CI 0.85–0.97, n = 4 risk estimates) (Supplementary Figs. 11–14).
Fig. 2. Associations of milk, yogurt, cheese consumption with cardiovascular disease risk for high compared with low category of intake using random effects meta-analysis.
a Milk. b Yogurt. c Cheese. Meta-analysis pooling of aggregate data used the random-effects inverse-variance model with DerSimonian-Laird estimate of tau². Data are presented as hazard ratios (HRs) and 95% confidence intervals (CIs). Squares represent study-specific HRs. Horizontal lines denote 95% CIs. Gray square areas are proportional to the individual study weight for the overall meta-analysis. The red dotted line represents risk ratio of pooled meta-analysis. The blue hollow diamonds represent the results of the meta-analysis for each group, with the center indicating the risk ratio and the width representing the 95% CI. I2 refers to the proportion of heterogeneity among studies. All statistical tests were two-sided. M, men; W, women; CKB, China Kadoorie Biobank; UKB, UK Biobank. Source data are provided as a Source Data file.
Fig. 3. Associations of low-fat and high-fat dairy consumption with cardiovascular disease risk for high compared with low category of intake using random effects meta-analysis.
a Low-fat dairy. b High-fat dairy. Meta-analysis pooling of aggregate data used the random-effects inverse-variance model with DerSimonian-Laird estimate of tau². Data are presented as hazard ratios (HRs) and 95% confidence intervals (CIs). Squares represent study-specific HRs. Horizontal lines denote 95% CIs. Gray square areas are proportional to the individual study weight for the overall meta-analysis. The red dotted line represents risk ratio of pooled meta-analysis. All statistical tests were two-sided. The blue hollow diamonds represent the results of the meta-analysis for each group, with the center indicating the risk ratio and the width representing the 95% CI. I2 refers to the proportion of heterogeneity among studies. M, men; W, women; CKB, China Kadoorie Biobank; UKB, UK Biobank. Source data are provided as a Source Data file.
For total dairy consumption, we observed considerable heterogeneity across the studies (I2 = 66.1%) but did not find any publication bias (Fig. 1 and Supplementary Figs. 15–19). No significant heterogeneity was found in the predefined subgroup (sex, follow-up duration, region, Newcastle-Ottawa Scale score, etc.) meta-regressions (Supplementary Data 28), indicating the source of heterogeneity mainly comes from subtypes of dairy. No single study disproportionately caused the heterogeneity (Supplementary Fig. 20). Results of influence analysis for subtypes of dairy and subtypes of CVD are shown in Supplementary Figs. 21–30. If no significant heterogeneity was found across the studies for specific meta-analyses, we also conducted a fixed effects model to calculate summary HRs and 95% CIs which showed similar results (Supplementary Data 29). Results of the GRADE confidence in the estimates of associations are presented in Supplementary Data 30, indicating overall evidence of very low to moderate quality.
Discussion
In both UKB and CKB studies, dairy consumption was overall associated with a lower risk of stroke. Further analysis of dairy subtypes in UKB revealed that cheese and skimmed/semi-skimmed milk consumption were inversely associated with CVD risk. The updated meta-analysis overall supported that dairy consumption, especially cheese and low-fat dairy consumption, was beneficial for CVD prevention among the general population.
Our finding of the inverse association of dairy consumption with stroke risk was consistent with a recent meta-analysis showing a 1-serving/d increase in total dairy consumption was significantly related to a 4% decreased stroke risk22. Although dairy products are major sources of saturated fatty acids (SFA) (about 65% of total fats), which has been shown to increase low-density lipoprotein (LDL) cholesterol levels, emerging evidence suggests that a low LDL cholesterol level (<70 mg/dL) was a risk factor for hemorrhagic stroke32,33. A meta-analysis summarizing data from 462,268 participants showed a dose-response relation of dietary SFA intake with lower stroke risk, especially intracranial hemorrhage risk34. Congruously, we found that total dairy consumption (mainly fresh milk/liquid whole milk in China)35,36 was related to lower hemorrhagic stroke risk in CKB. Importantly, despite a high content of even-chain SFAs, dairy fats also consist of medium-chain (9.8%) and odd-chain (31.9%) SFAs37, which may improve insulin sensitivity38, Besides, dairy products also contain potentially beneficial natural trans fats, unsaturated fats, specific amino acids, branched-chain fats, vitamins K1 and K2, and calcium39. Thus, given the complex food matrix of dairy products, their health impact cannot be fully accounted for by the presumed effect of SFAs. In addition, meta-analyses of randomized controlled trials demonstrated that fermented milk or dairy foods enriched with probiotics could reduce blood pressure40,41, which also partially explains the protective association for stroke, including a lower ischemic stroke risk for dairy in UKB and our meta-analysis.
With regard to CHD, we found great heterogeneity between UKB and CKB studies, which was also shown in our further updated meta-analysis (I2 = 68.6%). This heterogeneity could be attributed to several factors. First, the difference in dairy intake levels between the two cohorts is notable. The average intake of total dairy products in the UKB was more than four times higher than in the CKB42. It is plausible that the cardiometabolic benefits of dairy consumption may require a relatively high level of intake. Second, genetic differences between the populations may play a role. Chinese populations have a higher prevalence of lactose intolerance compared to European populations43, which could influence the metabolic outcomes associated with dairy consumption and potentially contribute to the observed differences in CHD risk. Importantly, our further analyses suggest that the discrepancy between the studies may be largely attributable to the consumption of different subtypes of dairy products. Notably, cheese consumption ranked highest among dairy products in the UK, whereas liquid whole milk was the predominant dairy product in China35,36. The protective relationship was mainly driven by cheese intake in the UKB study, which was further supported by our updated meta-analysis. Consistently, a meta-analysis of 15 prospective studies demonstrated that cheese consumption was related to reduced risk of CHD (RR [95% CI] for high vs. low consumption 0.86 [0.77–0.96]), stroke, and total CVD44. Another meta-analysis also showed a protective relationship of fermented dairy products with CVD risk and such a protective association was detected for cheese but not yogurt21. Compared with these two meta-studies, our meta-analysis incorporated data from 11 additional studies, significantly increasing the sample size and further reinforcing the robustness of the protective association between cheese consumption and CVD risk. Although cheese, especially hard cheese, is rich in salt, saturated fat, and calories, we still detected protective relationships for hard cheese and high-fat cheese in UKB. Potential mechanisms that underpin the relationship may be related to the high content of calcium, which may benefit cardiovascular health by limiting the absorption of SFAs and cholesterol45 and regulating the cell membrane potentials of the myocardium46. Cheese also contains a high amount of conjugated linoleic acid that has been evidenced to inhibit the progression or induce the regression of atherosclerosis through modulating monocyte/macrophage function47. In addition, the fermentation of dairy produces beneficial vitamin K2 that has been linked with a lower CHD risk48. Microorganisms or probiotics from fermented dairy could modulate the gut microbiota composition, inhibit the reabsorption of bile acid, and produce beneficial short-chain fatty acids49. A recent meta-analysis of 39 trials demonstrated that probiotic fermented milk products reduced serum total cholesterol and LDL cholesterol levels50. However, our results of the updated meta-analysis and other meta-analyses found little benefit of yogurt consumption on CVD risk21,51, which could be due to the commonly added sugars or artificial sweeteners that might counteract the health benefit52. Sweetened or flavored yogurts are classified as ultra-processed foods, which have been linked to an increased risk of CVD53,54. It is also possible that the consumption of yogurt is too low to detect a benefit, especially in older cohorts.
Pertaining to milk consumption, mixed results have been reported from prospective studies21,22. A meta-analysis of cohort studies reported that milk intake was associated with a 4% (1%–5%) higher CHD mortality55, which was congruent with our finding of a positive relation with CHD risk in CKB where liquid whole milk was the major dairy product35,36. In addition to the long even-chain SFAs elevating LDL cholesterol, a high D-galactose intake from non-fermented milk might also adversely affect lipid metabolism. A trial in nonobese men demonstrated that galactose ingestion within a high-fat beverage exacerbated postprandial lipemia and increased plasma lactate concentrations compared with glucose56. Compared to cheese, milk generally contains higher concentrations of D-galactose57,58. D-galactose has been widely used to establish an experimental model for premature aging by inducing oxidative stress and chronic inflammation59,60, which is also involved in the pathogenesis of CVD. Results from 2 large Swedish cohorts showed positive relations of milk intake with oxidative stress and inflammation markers while negative associations were observed for fermented milk products61. Altogether, individual dairy products have divergent associations with CVD risk, which seemed to be the major reason for the discrepant results for CHD observed in CKB and UKB and also for the great heterogeneity between studies in our meta-analysis. Therefore, our study provides compelling evidence to highlight the importance of focusing on specific types of dairy products among which cheese may be a beneficial choice for the primary prevention of CVD.
Although prevailing dietary recommendations advocate consuming low-fat or non-fat dairy products over high-fat dairy/whole milk, previous evidence from meta-analyses showed no significant relationship of low-fat dairy consumption with CVD or CHD risk21,22. Our meta-analysis showed inverse relationships of low-fat dairy consumption with CVD and stroke risk, supporting the protective role of low-fat dairy in CVD prevention. Nonetheless, we observed an inverse but non-significant association between high-fat dairy consumption and CVD, characterized by slightly wider confidence intervals. In addition, a significant inverse relationship with CHD risk was identified, which may be driven by high-fat cheese consumption. In a meta-analysis of 20 trials, both low-fat and high-fat dairy consumption increased body weight but had neutral effects on other cardiometabolic indicators, including waist circumference, fasting glucose, LDL cholesterol, high-density lipoprotein (HDL) cholesterol, blood pressure, and C-reactive protein (CRP)62. Overall, current evidence suggests low-fat dairy may be beneficial for CVD whereas specific subtypes of high-fat dairy such as cheese could also be protective. More large studies are needed to compare low-fat with high-fat dairy on long-term CVD outcomes.
The differing outcomes of substitution analyses between CKB and UKB may be attributed to differences in national dietary patterns and the metabolic profiles of their respective populations63. Research has indicated that egg consumption could confer health benefits in Asian populations64. A previous cohort study within the CKB cohort found that daily egg consumption (up to <1 egg/day) was associated with an 18% reduction in CVD mortality and a 26% lower risk of hemorrhagic stroke65. Our substitution model results aligned with these findings, suggesting that egg consumption may offer more significant cardioprotective benefits than dairy products among the Chinese population. In contrast, the UKB substitution analysis showed a null association, indicating that the cardiometabolic impacts of other protein sources were comparable to those of dairy products in the UK. This is consistent with findings from a previous study in the US, which showed that replacing dairy products with other protein sources did not significantly affect CHD risk66.
The inverse association between total dairy intake and the risk of CVD and stroke was observed among individuals with hypertension but not among those without hypertension in the CKB study. Hypertension is a well-established risk factor for CVD, making those with high blood pressure more susceptible to cardiovascular damage67. As a result, the potential protective effects of dairy intake, such as improved blood pressure regulation, may have a more pronounced impact on reducing CVD and stroke risk in hypertensive individuals compared with those without hypertension. Interestingly, the significant inverse associations of dairy consumption with the risk of CVD and stroke were more evident among men than women in the CKB study. This disparity may be due to differences in how men and women metabolize nutrients, influenced by hormonal variations68, which can affect the impact of dairy intake on stroke risk. In addition, men typically have higher baseline blood pressure levels, which might make them more responsive to the protective effects of dairy against stroke. Furthermore, the inverse association between cheese intake and CVD risk was significant only among participants without diabetes in UKB. This could be attributed to the altered lipid metabolism and insulin resistance commonly seen in individuals with diabetes69, potentially diminishing the cardiovascular benefits of cheese. Further research is necessary to elucidate the significant interactions observed in our subgroup analyses.
This analysis has important strengths, including the large sample size, long follow-up duration, and the design of using data from two large cohorts in the UK and China, which enable us to directly compare the results from Western vs. Eastern countries. Finally, the updated meta-analysis provides a comprehensive overview of the evidence. Potential limitations also deserve attention. First, measurement errors by FFQs are inevitable in epidemiological studies. However, such errors tend to attenuate findings toward the null because of the prospective analysis. Although absolute dairy intake was not estimated in CKB and UKB at baseline, consumption frequency is rather useful in categorizing individuals on the basis of relative intakes. Second, unmeasured or residual confounding cannot be fully ruled out despite our full adjustment for multiple risk factors. Specifically, higher dairy consumption seemed to be indicative of a higher socioeconomic status. Nonetheless, our results were consistent among both individuals with higher and lower income, indicating the documented associations of dairy were independent of socioeconomic status. Third, dairy consumption was assessed only once at baseline in the CKB study and only a small proportion of participants completed all five 24-hour dietary recalls in the UKB. As a result, dietary changes during the follow-up period could potentially weaken the observed associations. However, we estimated the long-term usual intake of dairy by incorporating data from dietary resurveys in the CKB and included participants with at least two 24-hour dietary recalls in UKB in sensitivity analyses, which yielded similar results. In addition, consistent findings were observed even with a shorter follow-up duration of 5 years, suggesting that the lack of repeated measurements is unlikely to have significantly impacted our findings. Nonetheless, further studies incorporating repeated measures of dairy intake are encouraged to validate these results. Fourth, no apparent ‘ceiling’ effect was observed in our dose-response analysis, likely due to the limited number of studies with a broad range of dairy consumption. Additional studies encompassing a wider spectrum of intake levels are needed to fully explore this relationship. Last, we could not further analyze dairy subtypes separately in CKB, and butter was also not assessed in both CKB and UKB due to the lack of available data at baseline, which could have provided more implications.
The results from our two large cohort studies and updated meta-analysis show that dairy consumption is associated with a lower risk of stroke and total CVD overall while relationships for subtypes of dairy products differ. Cheese consumption, but not milk and yogurt, was inversely associated with CVD risk. Low-fat dairy consumption was inversely related to CVD and stroke risk. Our findings provide useful clinical evidence to support the beneficial role of dairy consumption in the primary prevention of CVD. Additional clinical trials are necessary to validate the distinct cardiometabolic effects of various subtypes of dairy products.
Methods
Study design
The CKB study received ethical approval from the Oxford University Tropical Research Ethics Committee, the Chinese Centre for Disease Control and Prevention (CDC) Ethical Review Committee, and the local CDC of each study area. The UK Biobank received ethical approval from the research ethics committee (REC reference for UK Biobank 11/NW/0382).
CKB is one of the largest cohort studies that recruited over 500,000 adults from ten geographically diverse areas across China during 2004–200870. All participants gave written informed consent. For this analysis, participants with a history of CVD or cancer were excluded at baseline, which resulted in a sample of 487,212 individuals in the CKB.
UKB is also a large prospective study of more than 500,000 people who were aged 37–73 years recruited from one of 22 assessment centers across the UK between 2007 and 2010 71. Among 502,476 participants, we excluded participants with a history of CVD or cancer at baseline and participants who withdrew during the follow-up (data cannot be used). Furthermore, we excluded persons without data on cheese consumption frequency from the food frequency questionnaire (FFQ) or those without information about 24 h dietary recalls. Finally, 418,895 individuals in the UKB remained in the final analytical samples for cheese consumption and 183 446 individuals remained for individual dairy products. The detailed flow chart is shown in Supplementary Fig. 31.
Dietary assessments
In the CKB, participants were asked about the consumption frequency of 12 major food groups, including total dairy products over the preceding year by a qualitative FFQ. The adjusted Spearman coefficients of dairy consumption frequency were 0.4 for reproducibility and 0.5 for validity, comparing two FFQs conducted in the second and third surveys with the baseline FFQ, which implicated good performance of the FFQ72. Subtypes of dairy products were not included in the baseline FFQ and thus were not analyzed in CKB. The long-term usual amount of consumption for each category of food consumption variable was estimated according to the previously published method using the data of two resurveys in the CKB73. The daily energy intake at baseline was also estimated74.
In the UKB, participants completed a touch-screen short dietary questionnaire that consisted of 29 diet questions over the past 12 months, including frequency of cheese intake (0, <1, 1, 2 to 4, 5 to 6, ≥7 times a week) and type of milk (never/rarely have milk, full, semi-skimmed, skimmed cream, soya milk, other) in which they could select multiple types of milk they drank. Soya milk was excluded from the analysis as it is made from soybeans. Besides, participants were invited to complete a 24 h dietary questionnaire that inquired about the consumption of nearly 200 foods and drinks including various dairy products (milk, cheese, yogurt, and ice cream). Five separate occasions of 24 h dietary recalls were conducted during 2011–2012 to provide an average measure for individuals (repeated measurement per person). A total of 183,446 participants with at least one 24 h dietary recall were included in the study. The number of 24 h dietary records provided by these participants is detailed in Supplementary Data 31. The consistency between dietary touch-screen questionnaires and online 24 h dietary assessments has been reported before75. The Spearman coefficients of cheese intake frequency between baseline and resurveys during follow-up are higher than 0.5 Supplementary Data 32. The Oxford WebQ used in online 24 h dietary recalls performed well across key nutrients which were validated using objective urine biomarkers76.
Ascertainment of incident cardiovascular disease
Detailed information used to define incident CVD cases including fatal or non-fatal CHD and stroke is presented in Supplementary Data 33. Incident cases of CVD were identified by using linkages with disease registries, national health insurance claim databases, and the local disease surveillance points system death registries by reviewing residential records and/or by visits to local communities for those uninsured participants in CKB to minimize any underreporting cases70. The records of CHD and stroke cases were retrieved and reviewed by qualified cardiovascular specialists blinded to the information of patients since 201477. In UKB, information on the CVD cases of all participants was obtained from cumulative hospital inpatient records, death certificates in the national death registries, and self-reports from interviews during follow-up. The high accuracy of this approach has been reported before78,79. All events were ascertained using the International Classification of Diseases, 10th Revision (ICD–10).
Statistical analysis
The main exposures of interest were the frequency of total dairy consumption in CKB and the frequency of cheese intake (<2, 2 to 4, 5 to 6, or ≥7 times a week), milk type, and total dairy consumption in UKB. In UKB, the frequency of cheese intake and milk type were collected by the touch-screen questionnaire, while the total dairy consumption was the sum of all types of milk, yogurt, cheese, and ice cream collected by 24 h diet recalls. The intakes of dairy products (0, ≤0.5, 0.5 to 1, or >1 serving per day), milk, yogurt, ice cream, and cheese (0, ≤0.5, or >0.5 serving per day) were categorized into predefined categories based on consumption distributions.
The person-year was calculated from the date of entry to the time of CVD diagnosis, lost to follow-up, death, or the end date of follow-up (December 31, 2016, for CKB, and 31 December 2020 for UKB), whichever occurred earlier. Only 1.2% of individuals in CKB and 0.3% in UKB were lost to follow-up and censored in analyses. Cox proportional hazards regression model was used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) of CVD risk for total or each type of dairy product consumption after checking the violation of the proportional hazard assumption. To control known and potential confounders, multivariable models were sequentially adjusted for age, sex, race, study area (for CKB)/assessment centers (for UKB), body mass index (BMI), education level, household income, Townsend deprivation index (TDI, only in UKB), smoking status, alcohol drinking, physical activity, history of hypertension, history of diabetes, family history of CVD, use of vitamins, minerals, and aspirin, and consumption frequency of red meat, processed red meat (only in UKB), fish, oily fish (only in UKB), non-oily fish (only in UKB), poultry, vegetables, fruits, and eggs (all categories of consumption). The sex information was obtained from the central registry at recruitment in the UKB, while it was collected from the baseline questionnaire in the CKB. Dairy in the final model is compared against carbohydrate-rich foods (grains, starches, sugars) as the implicit substitution. All missing data were coded as an independent category if necessary. The linear trend was tested by fitting the ordinal dairy variables as continuous variables in the models.
As dairy products are one of the major sources of dietary protein, we used substitution analysis to estimate the theoretical effect on CVD risk of substituting one serving of dairy products for an equivalent serving of other common alternative protein sources, including red/processed meat, fish, poultry, eggs, and soybean/legumes13. We further examined whether the documented associations varied by subgroups according to baseline characteristics which were important covariates based on previous studies (Supplementary Data 26), including age, sex, BMI, household income, smoking status, alcohol intake frequency, physical activity, diet quality, hypertension, diabetes, and family history of CVD. P interaction was calculated by adding a cross-product term for the baseline stratifying variable with dairy as an ordinal variable in the model. Besides, we conducted several sensitivity analyses. First, we adjusted a healthy diet score80,81 to evaluate the influence of the overall diet quality. Second, lipid-lowering drugs or anti-hypertensive medications were further adjusted in the model. Third, we further adjusted for total energy intake to assess whether the relationship between dairy consumption and CVD development was independent of the amount of energy provided. Fourth, we further excluded incident CVD cases within the first 2 years of follow-up or participants with extreme BMIs (<18.5 or >40 kg/m2). Finally, participants were censored at a 5 y follow-up. In addition, in CKB analysis, we used a multivariable Cox frailty model with random intercepts to account for center clustering (10 regions). In UKB analysis, we further adjusted for salt added to food to see whether the main findings altered. Individuals with at least two 24 h dietary records were included to better represent their usual diet.
All statistical analyses were conducted with SAS 9.4 (SAS Institute, Cary, NC, USA) and a two-sided P < 0.05 was considered statistically significant.
Meta-analysis
We performed a systematic review and updated meta-analysis including UKB and CKB studies as well as previous prospective cohort studies which explored the relationship of dairy product intake with CVD risk in the general population. Supplementary Data 34 shows the search strategy. Additional details of the meta-analysis are provided in Supplementary Methods.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
This study has been conducted using the UK Biobank Resource under Application Number 47365 and CKB resource (https://www.ckbiobank.org/) under application number DAR-2020-00282. Publication of results does not require or imply approval by the membership of the CKB Collaborative Group. Funding for the development of and maintenance of the CKB resource has been received from Chinese Ministry of Science and Technology, Chinese National Natural Science Foundation, the Kadoorie Charitable Foundation in Hong Kong, and the Wellcome Trust. Unless specified elsewhere, these funders have not supported the research work required for the preparation of this paper. The most important acknowledgment is to the participants in the CKB and UK Biobank study and the members of the survey teams in each of the 10 regional centers/22 assessment centers, as well as to the project development and management teams. This research was supported by the National Key Research and Development Program of China (grant no. 2023YFF1105300), Fund of the National Natural Science Foundation of China (grant no. 32202057), and Natural Science Foundation of Zhejiang Province (grant no. LY23C200007).
Author contributions
J.J.J., and Y.Z. conceived and designed the study. P.Z., J.J.J., X.H.L., Y.L., and Y.A. did the data cleaning, analysis, and interpretation. P.Z. and X.H.L. wrote the manuscript and provided statistical expertize and assistance. P.Z., X.H.L., Y.L., Y.A., Y.Q.W., H.Y., X.Z.W., L.G.Z., D.H.M., Y.M.T., X.M.Y., F.Z., A.L.W., Y.Z., and J.J.J. contributed to the interpretation of the data and critical revision of the manuscript for important intellectual content and approved the final draft. P.Z., J.J.J., and Y.Z. were involved in data acquisition. Y.Z. is the guarantor. Y.Z. and J.J.J. had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis.
Peer review
Peer review information
Nature Communications thanks Sandi Azab, Russell de Souza, and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Data availability
The data supporting the findings from this study are available within the manuscript and its supplementary information. Source data are provided with this paper. The research has been conducted using the UK Biobank resource (https://www.ukbiobank.ac.uk) under application number 47365 and China Kadoorie Biobank resource (https://www.ckbiobank.org/) under application number DAR-2020-00282. The data for this research obtained from the above Biobank resources are publicly available to approved researchers for health-related research. Source data are provided with this paper.
Code availability
The analysis code used in this study is available from the corresponding author upon appropriate request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Pan Zhuang, Xiaohui Liu.
These authors jointly supervised this work. Yu Zhang, Jingjing Jiao.
Contributor Information
Yu Zhang, Email: y_zhang@zju.edu.cn.
Jingjing Jiao, Email: jingjingjiao@zju.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-55585-0.
References
- 1.Yusuf, S. et al. Cardiovascular risk and events in 17 low-, middle-, and high-income countries. N. Engl. J. Med.371, 818–827 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Willett, W. C. & Ludwig, D. S. Milk and health. N. Engl. J. Med.382, 644–654 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Pal, S. & Radavelli-Bagatini, S. The effects of whey protein on cardiometabolic risk factors. Obes. Rev.14, 324–343 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Bruno, R. S., Pokala, A., Torres-Gonzalez, M. & Blesso, C. N. Cardiometabolic health benefits of dairy-milk polar lipids. Nutr. Rev.79, 16–35 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pérez-Hernández, N. et al. Vitamin D and its effects on cardiovascular diseases: a comprehensive review. Korean J. Intern. Med.31, 1018–1029 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adebamowo, S. N., Spiegelman, D., Flint, A. J., Willett, W. C. & Rexrode, K. M. Intakes of magnesium, potassium, and calcium and the risk of stroke among men. Int. J. Stroke10, 1093–1100 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Li, Y. et al. Saturated fats compared with unsaturated fats and sources of carbohydrates in relation to risk of coronary heart disease: a prospective cohort study. J. Am. Coll. Cardiol.66, 1538–1548 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Um, C. Y., Prizment, A., Hong, C. P., Lazovich, D. & Bostick, R. M. Associations of calcium and dairy product intakes with all-cause, all-cancer, colorectal cancer and CHD mortality among older women in the Iowa Women’s Health Study. Br. J. Nutr.121, 1188–1200 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Romo Ventura, E. et al. Association of dietary intake of milk and dairy products with blood concentrations of insulin-like growth factor 1 (IGF-1) in Bavarian adults. Eur. J. Nutr.59, 1413–1420 (2020). [DOI] [PubMed] [Google Scholar]
- 10.Kinjo, Y. et al. Possible protective effect of milk, meat and fish for cerebrovascular disease mortality in Japan. J. Epidemiol.9, 268–274 (1999). [DOI] [PubMed] [Google Scholar]
- 11.Buendia, J. R. et al. Regular yogurt intake and risk of cardiovascular disease among hypertensive adults. Am. J. Hypertens.31, 557–565 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernstein, A. M. et al. Dietary protein sources and the risk of stroke in men and women. Stroke43, 637–644 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernstein, A. M. et al. Major dietary protein sources and risk of coronary heart disease in women. Circulation122, 876–883 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dehghan, M. et al. Association of dairy intake with cardiovascular disease and mortality in 21 countries from five continents (PURE): a prospective cohort study. Lancet392, 2288–2297 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Louie, C. J. et al. Dairy consumption and the risk of 15-year cardiovascular disease mortality in a cohort of older Australians. Nutrients5, 441–454 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalmeijer, G. W. et al. Dairy intake and coronary heart disease or stroke—a population-based cohort study. Int. J. Cardiol.167, 925–929 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Soedamah-Muthu, S. S., Masset, G., Verberne, L., Geleijnse, J. M. & Brunner, E. J. Consumption of dairy products and associations with incident diabetes, CHD and mortality in the Whitehall II study. Br. J. Nutr.109, 718–726 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Bonthuis, M., Hughes, M. C. B., Ibiebele, T. I., Green, A. C. & van der Pols, J. C. Dairy consumption and patterns of mortality of Australian adults. Eur. J. Clin. Nutr.64, 569 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Larsson, S. C. et al. Dairy foods and risk of stroke. Epidemiology20, 355–360 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olsson, E., Larsson, S. C., Höijer, J., Kilander, L. & Byberg, L. Milk and fermented milk consumption and risk of stroke: longitudinal study. Nutrients14, 1070 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo, J. et al. Milk and dairy consumption and risk of cardiovascular diseases and all-cause mortality: dose-response meta-analysis of prospective cohort studies. Eur. J. Epidemiol.32, 269–287 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen, Z. et al. Dairy product consumption and cardiovascular health: a systematic review and meta-analysis of prospective cohort studies. Adv. Nutr.13, 439–454 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexander, D. D. et al. Dairy consumption and CVD: a systematic review and meta-analysis. Br. J. Nutr.115, 737–750 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Fernández, M., Hudson, J. A., Korpela, R. & de los Reyes-Gavilán, C. G. Impact on human health of microorganisms present in fermented dairy products: an overview. Biomed. Res. Int.2015, 412714 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neal, B. et al. Effect of salt substitution on cardiovascular events and death. N. Engl. J. Med.385, 1067–1077 (2021). [DOI] [PubMed] [Google Scholar]
- 26.Mladěnka, P. et al. Vitamin K—sources, physiological role, kinetics, deficiency, detection, therapeutic use, and toxicity. Nutr. Rev.80, 677–698 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martini, M., Salari, F. & Altomonte, I. The macrostructure of milk lipids: the fat globules. Crit. Rev. Food Sci. Nutr.56, 1209–1221 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Companys, J., Pedret, A., Valls, R. M., Solà, R. & Pascual, V. Fermented dairy foods rich in probiotics and cardiometabolic risk factors: a narrative review from prospective cohort studies. Crit. Rev. Food Sci. Nutr.61, 1966–1975 (2021). [DOI] [PubMed] [Google Scholar]
- 29.James, W. P., Nelson, M., Ralph, A. & Leather, S. Socioeconomic determinants of health. The contribution of nutrition to inequalities in health. BMJ314, 1545–1549 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanchez-Villegas, A. et al. A systematic review of socioeconomic differences in food habits in Europe: consumption of cheese and milk. Eur. J. Clin. Nutr.57, 917 (2003). [DOI] [PubMed] [Google Scholar]
- 31.Huang, L.-Y., Wahlqvist, M. L., Huang, Y.-C. & Lee, M.-S. Optimal dairy intake is predicated on total, cardiovascular, and stroke mortalities in a Taiwanese cohort. J. Am. Coll. Nutr.33, 426–436 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Rist, P. M. et al. Lipid levels and the risk of hemorrhagic stroke among women. Neurology92, e2286–e2294 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma, C. et al. Low-density lipoprotein cholesterol and risk of intracerebral hemorrhage: a prospective study. Neurology93, e445–e457 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang, Z. Q., Yang, Y. & Xiao, B. Dietary saturated fat intake and risk of stroke: systematic review and dose-response meta-analysis of prospective cohort studies. Nutr. Metab. Cardiovasc. Dis.30, 179–189 (2020). [DOI] [PubMed] [Google Scholar]
- 35.Huang, F. et al. Knowledge, behavior and consumption types of milk and dairy products among the Chinese aged 60 and above in 15 provinces (autonomous regions and municipalities) in 2015. Wei Sheng Yan Jiu48, 9–15 (2019). [PubMed] [Google Scholar]
- 36.Jin, S., Yuan, R., Zhang, Y. & Jin, X. Chinese consumers’ preferences for attributes of fresh milk: a best-worst approach. Int. J. Environ. Res. Public. Health16, 4286 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Månsson, H. L. Fatty acids in bovine milk fat. Food Nutr. Res.52, 1821 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Imamura, F. et al. Fatty acid biomarkers of dairy fat consumption and incidence of type 2 diabetes: a pooled analysis of prospective cohort studies. PLoS Med.15, e1002670 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mozaffarian, D. & Wu, J. H. Y. Flavonoids, dairy foods, and cardiovascular and metabolic health: a review of emerging biologic pathways. Circ. Res.122, 369–384 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Usinger, L., Reimer, C. & Ibsen, H. Fermented milk for hypertension. Cochrane Database Syst. Rev.4, Cd008118 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dixon, A. et al. Efficacy of probiotics in patients of cardiovascular disease risk: a systematic review and meta-analysis. Curr. Hypertens. Rep.22, 74 (2020). [DOI] [PubMed] [Google Scholar]
- 42.Papier, K. et al. Intakes of major food groups in China and UK: results from 100,000 adults in the China Kadoorie biobank and UK biobank. Eur. J. Nutr.62, 819–832 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anguita-Ruiz, A., Aguilera, C. M. & Gil, Á. Genetics of lactose intolerance: an updated review and online interactive world maps of phenotype and genotype frequencies. Nutrients12, 2689 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen, G. C. et al. Cheese consumption and risk of cardiovascular disease: a meta-analysis of prospective studies. Eur. J. Nutr.56, 2565–2575 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Mulet-Cabero, A. I. & Wilde, P. J. Role of calcium on lipid digestion and serum lipids: a review. Crit. Rev. Food Sci. Nutr.63, 813–826 (2023). [DOI] [PubMed] [Google Scholar]
- 46.Sutanto, H. et al. Cardiomyocyte calcium handling in health and disease: insights from in vitro and in silico studies. Prog. Biophys. Mol. Biol.157, 54–75 (2020). [DOI] [PubMed] [Google Scholar]
- 47.Bruen, R., Fitzsimons, S. & Belton, O. Atheroprotective effects of conjugated linoleic acid. Br. J. Clin. Pharmacol.83, 46–53 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geleijnse, J. M. et al. Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: the Rotterdam Study. J. Nutr.134, 3100–3105 (2004). [DOI] [PubMed] [Google Scholar]
- 49.Hjerpsted, J. & Tholstrup, T. Cheese and cardiovascular disease risk: a review of the evidence and discussion of possible mechanisms. Crit. Rev. Food Sci. Nutr.56, 1389–1403 (2016). [DOI] [PubMed] [Google Scholar]
- 50.Ziaei, R., Ghavami, A., Khalesi, S., Ghiasvand, R. & Mokari Yamchi, A. The effect of probiotic fermented milk products on blood lipid concentrations: a systematic review and meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis.31, 997–1015 (2021). [DOI] [PubMed] [Google Scholar]
- 51.Wu, L. & Sun, D. Consumption of yogurt and the incident risk of cardiovascular disease: a meta-analysis of nine cohort studies. Nutrients9, 315 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wan, Z., Khubber, S., Dwivedi, M. & Misra, N. N. Strategies for lowering the added sugar in yogurts. Food Chem.344, 128573 (2021). [DOI] [PubMed] [Google Scholar]
- 53.Srour, B. et al. Ultra-processed food intake and risk of cardiovascular disease: prospective cohort study (NutriNet-Santé). BMJ365, l1451 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fang, Z. et al. Association of ultra-processed food consumption with all cause and cause specific mortality: population based cohort study. BMJ385, e078476 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mazidi, M. et al. Consumption of dairy product and its association with total and cause specific mortality—a population-based cohort study and meta-analysis. Clin. Nutr.38, 2833–2845 (2019). [DOI] [PubMed] [Google Scholar]
- 56.Watkins, J. et al. Galactose ingested with a high-fat beverage increases postprandial lipemia compared with glucose but not fructose ingestion in healthy men. J. Nutr.150, 1765–1772 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vitoria, I. et al. Lactose and galactose content in Spanish cheeses: usefulness in the dietary treatment of patients with galactosaemia. Nutrients15, 594 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ohlsson, J. A. et al. Lactose, glucose and galactose content in milk, fermented milk and lactose-free milk products. Int. Dairy J.73, 151–154 (2017). [Google Scholar]
- 59.Lai, K., Elsas, L. J. & Wierenga, K. J. Galactose toxicity in animals. IUBMB Life61, 1063–1074 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cui, X. et al. Chronic systemic D-galactose exposure induces memory loss, neurodegeneration, and oxidative damage in mice: protective effects of R-alpha-lipoic acid. J. Neurosci. Res.83, 1584–1590 (2006). [DOI] [PubMed] [Google Scholar]
- 61.Michaëlsson, K. et al. Milk intake and risk of mortality and fractures in women and men: cohort studies. BMJ349, g6015 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Benatar, J. R., Sidhu, K. & Stewart, R. A. Effects of high and low fat dairy food on cardio-metabolic risk factors: a meta-analysis of randomized studies. PLoS ONE8, e76480 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brandhorst, S. & Longo, V. D. Protein quantity and source, fasting-mimicking diets, and longevity. Adv. Nutr.10, S340–s350 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Drouin-Chartier, J. P. et al. Egg consumption and risk of cardiovascular disease: three large prospective US cohort studies, systematic review, and updated meta-analysis. BMJ368, m513 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qin, C. et al. Associations of egg consumption with cardiovascular disease in a cohort study of 0.5 million Chinese adults. Heart104, 1756–1763 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haring, B. et al. Dietary protein intake and coronary heart disease in a large community based cohort: results from the Atherosclerosis Risk in Communities (ARIC) study. PLoS ONE9, e109552 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bundy, J. D. et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol.2, 775–781 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Comitato, R., Saba, A., Turrini, A., Arganini, C. & Virgili, F. Sex hormones and macronutrient metabolism. Crit. Rev. Food Sci. Nutr.55, 227–241 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jaiswal, M., Schinske, A. & Pop-Busui, R. Lipids and lipid management in diabetes. Best. Pract. Res. Clin. Endocrinol. Metab.28, 325–338 (2014). [DOI] [PubMed] [Google Scholar]
- 70.Chen, Z. et al. China Kadoorie Biobank of 0.5 million people: survey methods, baseline characteristics and long-term follow-up. Int. J. Epidemiol.40, 1652–1666 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sudlow, C. et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med.12, e1001779 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qin, C. et al. The relative validity and reproducibility of food frequency questionnaires in the China Kadoorie Biobank study. Nutrients14, 794 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kakkoura, M. G. et al. Dairy consumption and risks of total and site-specific cancers in Chinese adults: an 11-year prospective study of 0.5 million people. BMC Med.20, 134 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Song, S. et al. Dietary factors and patterns in relation to risk of later-onset ulcerative colitis in Chinese: a prospective study of 0.5 million people. Aliment Pharm. Ther.59, 1425–1434 (2024). [DOI] [PubMed] [Google Scholar]
- 75.Bradbury, K. E., Young, H. J., Guo, W. & Key, T. J. Dietary assessment in UK Biobank: an evaluation of the performance of the touchscreen dietary questionnaire. J. Nutr. Sci.7, e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Greenwood, D. C. et al. Validation of the Oxford WebQ online 24-hour dietary questionnaire using biomarkers. Am. J. Epidemiol.188, 1858–1867 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang, S. et al. Development of a model to predict 10-year risk of ischemic and hemorrhagic stroke and ischemic heart disease using the China Kadoorie Biobank. Neurology98, e2307–e2317 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rubbo, B. et al. Use of electronic health records to ascertain, validate and phenotype acute myocardial infarction: a systematic review and recommendations. Int. J. Cardiol.187, 705–711 (2015). [DOI] [PubMed] [Google Scholar]
- 79.Woodfield, R., Grant, I. & Sudlow, C. L. Accuracy of electronic health record data for identifying stroke cases in large-scale epidemiological studies: a systematic review from the UK Biobank stroke outcomes group. PLoS ONE10, e0140533 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhuang, P. et al. Effect of diet quality and genetic predisposition on hemoglobin A(1c) and type 2 diabetes risk: gene-diet interaction analysis of 357,419 individuals. Diabetes Care44, 2470–2479 (2021). [DOI] [PubMed] [Google Scholar]
- 81.Mozaffarian, D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation133, 187–225 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The data supporting the findings from this study are available within the manuscript and its supplementary information. Source data are provided with this paper. The research has been conducted using the UK Biobank resource (https://www.ukbiobank.ac.uk) under application number 47365 and China Kadoorie Biobank resource (https://www.ckbiobank.org/) under application number DAR-2020-00282. The data for this research obtained from the above Biobank resources are publicly available to approved researchers for health-related research. Source data are provided with this paper.
The analysis code used in this study is available from the corresponding author upon appropriate request.