Abstract
Preeclampsia (PE) is a prevalent and severe pregnancy complication that significantly impacts maternal and perinatal health. Epidemiological studies and animal experiments have demonstrated that PE adversely affects the cardiovascular and nervous systems of offspring, increasing their risk of hypertension and renal pathology. However, the mechanisms underlying this increased risk remain unclear. This study utilized an L-NAME-induced preeclampsia mouse model (PELS model) to investigate the effects of PE on offspring blood pressure and renal pathology, focusing on the expression of Angiotensin II Type 1 Receptors (AT1R) and related molecules in renal tissues. Our findings show that L-NAME-induced pre-eclampsia led to reduced birth weights and significantly elevated systolic blood pressure in 6-week-old offspring. Histopathological analysis revealed pronounced glomerular and tubular damage in the kidneys of both 1-week and 6-week-old offspring from the pre-eclampsia group. At 1 week of age, the pre-eclampsia group exhibited elevated mRNA and protein expression levels of AT1R, GRK4, AQP2, ENaC, and NCC in renal tissues compared to controls. However, these differences were no longer significant at 6 weeks of age. No significant gender differences were observed in either blood pressure or renal pathological changes. Preeclampsia induced by L-NAME results in increased blood pressure and renal damage in offspring, potentially mediated by early alterations in the renal RAS system. The observed changes in AT1R and related molecules appear to be transient, suggesting that the early impact of pre-eclampsia on renal structure may trigger, but not sustain, hypertension in offspring. Further studies are needed to elucidate the long-term mechanisms driving hypertension in this population.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-85258-x.
Subject terms: Pre-eclampsia, Kidney
Introduction
Pre-eclampsia is a pregnancy-specific disorder characterized by hypertension accompanied by multi-organ dysfunction, such as renal impairment, liver damage, and complications in the nervous and hematological systems. With a prevalence rate of 5–7%, it stands as a primary contributor to the health concerns of pregnant women and perinatal children1. Immediate consequences of pre-eclampsia include premature birth, fetal growth restriction, and intrauterine hypoxia. Previous studies have demonstrated that pre-eclampsia has a certain impact on offspring’s metabolic alterations. Children prenatally exposed to pre-eclampsia are at an increased risk of developing endocrine and metabolic disorders2. Moreover, the long-term effects of preeclampsia extend to the cardiovascular and nervous systems of offspring, persisting into adulthood and even exhibiting transgenerational effects3. Epidemiological studies have shown that the offspring of pre-eclamptic pregnancies exhibit an elevated blood pressure by an average at the age of 7, a difference that continues until 18 years of age. Their risk of hypertension is 1.3 times greater than that of offspring from normal pregnancies, and this risk escalates to 1.5 times for offspring from severe pre-eclamptic cases4,5. Animal experiments have indicated that male offspring from s-FLT-induced pre-eclampsia exhibit elevated blood pressure from birth to adulthood, while female counterparts do not6. Additionally, normotensive females in the RUPP model displayed increased blood pressure post oophorectomy7. These findings suggest that pre-eclampsia might influence the blood pressure of the offspring, with potential gender differences, although causal mechanism are still speculative. Thus, this study uses a subcutaneous L-NAME injection to induce a pre-eclampsia mouse model, observing the blood pressure of 6-week-old offspring and noting variations across genders.
Hypertension is a multifactorial disease of unknown etiology, in which the renin-angiotensin-aldosterone system (RAAS) may be involved in its pathogenesis. The RAAS plays a crucial role in cardiovascular physiology and pathophysiology. Among its components, AT1R is a G-protein-coupled receptor believed to mediate most of ANG II’s functions within the system. AT1R is extensively expressed in the kidney and vascular tissues8. Abnormal expression of AT1R can lead to alterations in the expression of ion channels and water channels in the renal tissue, such as NHE3, αENaCs, and AQP2. This, in turn, modulates the renal functions of water and sodium excretion, playing a part in the regulation of systemic blood pressure9–11.
Adverse intrauterine stimuli may influence offspring blood pressure by altering the expression of various components of the RAS system in their kidneys and blood vessels. In rat offspring subjected to intrauterine hypoxia, blood pressure is elevated compared to controls. This is accompanied by a decline in the methylation level of the CpG island of the Agtr1a gene promoter, responsible for AT1R production, in mesenteric artery smooth muscle cells, leading to increased AT1R expression and heightened contractile responses to ANGII12. Studies on a reduced uterine perfusion pressure (RUPP) model have shown heightened salt sensitivity in baboon offspring, leading to hypertension following a high-salt diet, with a concurrent rise in serum and renal aldosterone levels13. Additionally, 16-week-old RUPP rat offspring display significantly elevated blood pressure compared to controls, increased arterial sensitivity to ANGII, accompanied by decreased kidney weight, reduced nephron count, and increased intrarenal angiotensinogen expression. These effects can be mitigated by early intervention with ACEI inhibitors14. Furthermore, offspring exposed to maternal PM2.5 exposure during pregnancy exhibit an increased prevalence of hypertension, associated with elevated GRK4 and AT1R expressions, heightened renal oxidative stress, and impaired renal sodium excretion15. Collectively, these findings underscore the role of alterations in the RAS system in mediating the impacts of adverse intrauterine stimuli on offspring kidney and vascular systems, emphasizing its significance in the long-term regulation of offspring blood pressure. Thus, further investigation is warranted to ascertain the potential involvement of altered AT1R and related molecular expressions in the onset of hypertension in offspring of pre-eclampsia.
Through subcutaneous L-NAME administration, we generated a preeclampsia mouse model to probe the repercussions of preeclampsia on offspring renal tissues and blood pressure. By evaluating the expression dynamics of AT1R and related molecules in these renal tissues, our research sought to elucidate the contribution of AT1R, along with its ancillary molecules, in the development of hypertension among these offspring. Here we show that L-NAME-induced pre-eclampsia results in reduced birth weight in offspring and elevated blood pressure. Preeclampsia-affected mouse offspring manifest more evident renal impairments. Moreover, the expression levels of AT1R and its upstream regulator GRK4, along with its downstream targets, including the aquaporin protein AQP2 and ion channels ENaC and NCC, were elevated compared to the control group. This suggests that the renin-angiotensin system may play a significant role in the development of hypertension in offspring born from preeclamptic conditions.
Materials and methods
Real-time PCR
RNA was extracted from the tissue using the RNAeasy™ Animal RNA Isolation Kit with Spin Column (Beyotime Biotechnology, Shanghai, China). Equal volumes of RNA samples were then reverse transcribed. The purity of the RNA was verified by measuring optical density (OD) at 260/280 nm, yielding values between 1.8 and 2.0. A 20 µL reaction setup for reverse transcription included 1 µg of template RNA, 4 µL of 5× PrimeScript RT Master Mix, and was completed to 20 µL with RNase Free dH2O, as per the instructions provided by TaKaRa. The reaction was incubated at 37 °C for 15 min, elevated to 85 °C for 5 s, and then quickly cooled on ice. Primer sequences were designed using software based on GenBank data.
Specific primer sequences were as follows:
GRK4: forward 5′-ATGGCTAGAAAGGCGTCCAG-3′, reverse 5′-TGTAGCTCGCACTTGACAGG-3′.
AT1R: forward 5′-TTCATTGAGAACACCAATATCACTG-3′, reverse 5′-GCTGGTAAGAATGATTAGGAAAGG-3′.
AQP2: forward 5′-TCACTGGGTCTTCTGGATCG-3′, reverse 5′-CGTTCCTCCCAGTCAGTGT-3′.
NCC: forward 5′-CATCAAGAACTACCGCCCCC-3′, reverse 5′-CTCTGCTTGCCAGGTCCAAT-3′.
αENaC: forward 5′-CAGACTGCTTCTACCAGACATAC-3′, reverse 5′-CCAGGGCTTCCTTTCTCATAC-3′.
Expression levels were normalized to the housekeeping gene β-actin, with primers: forward 5′-TGTTTGAGACCTTCAACACC-3′ and reverse 5′-CAGTAATCTCCTTCTGCATCC-3′. Real-time PCR was performed in a 20 µL volume containing 10 µL of SYBR Green Master Mix (Yeasen, Shanghai, China), 0.8 µL of 2.5 mM primers, 2 µL DNA template, and 7.2 µL distilled H2O. PCR cycles were conducted at 95 °C for 5 min, followed by 40 cycles at 95 °C for 10 s, 60 °C for 30 s, and 72 °C for 20 s.
Protein extraction and western blotting
Protein extraction from tissue samples was conducted using radioimmunoprecipitation assay (RIPA) buffer, supplemented with Protease Inhibitor Cocktail and PMSF, obtained from Servicebio, Wuhan, China. The extracted proteins were separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred onto 0.45-µm polyvinylidene fluoride (PVDF) membranes from Millipore, Burlington, MA, USA. The membranes were blocked with 5% non-fat milk in Tris-buffered saline with Tween 20 (TBST) for one hour, followed by an overnight incubation at 4℃ with various primary antibodies. These included antibodies against β-Actin (1:1000, #4970, CST), GRK4 (1:1000, A70500-050, Epigentek), AT1R (1:1000, ab124734, Abcam), AQP2 (1:500, ab199975, Abcam), NCC (1:1000, ab95302, Abcam), and αENaC (1:500, PA1-920 A, Thermofisher), all diluted in NCM Universal Antibody Diluent from NCM Biotech, Suzhou, China. This step was followed by a one-hour room temperature incubation with a goat anti-rabbit secondary antibody (1:3000) in TBST. Immunoblot detection was performed using the NcmECL Ultra kit from NCM Biotech, Suzhou, China. Protein bands were quantified using ImageJ software, with β-Actin serving as the loading control. Goat anti-rabbit IgG was sourced from Servicebio (GB23303, Wuhan, China).
Immunohistochemistry
Paraffin-embedded kidney sections of 5 mm were rehydrated in Tris-buffered saline (TBS) and then treated with hydrogen peroxide and 1% BSA to block endogenous peroxidase activity. Overnight, these sections were incubated in a humidified chamber with primary antibodies: GRK4 (1:500, A70500-050, Epigentek), AT1R (1:500, ab124734, Abcam), AQP2 (1:4000, ab199975, Abcam), NCC (1:200, ab95302, Abcam), and αENaC (1:200, PA1-920 A, Thermofisher). Post-incubation, the sections were thoroughly washed and then overlaid with HRP-conjugated anti-rabbit IgG (Servicebio, Wuhan, China). The staining process was conducted using 3,3-diaminobenzidine (DAB), followed by counterstaining with hematoxylin to enhance visualization of the tissue architecture.
L-NAME -induced preeclampsia model
Male and female C57BL/6 mice, aged 7–8 weeks and weighing 19–21 g, were acquired from Beijing Vital River Laboratory Animal Technology Co., Beijing, China. These mice were fed standard rodent chow and housed under optimal conditions. Mating was initiated by pairing two females with one male, and pregnancy was indicated by the presence of a vaginal plug, noted as gestational day (GD) 0.5. Pregnant mice were allocated into three groups (n = 10 per group): a control group receiving intraperitoneal (i.p.) saline injections, and two experimental groups receiving i.p. injections of L-NAME (75 mg/kg; Sigma, St Louis, MO, USA) from gestational day (GD) 7.5 to GD 18.5 to induce a preeclampsia model as per existing protocols16. The control group was injected with saline. Tail artery blood pressure was non-invasively measured for all groups on GD7.5, GD13.5, GD15.5, and GD19.5. Urine collection occurred over a 24-hour period from GD16.5 to GD17.5 using metabolic cages, during which urine volume was recorded. Urinary protein concentration was determined using the Bradford Protein Assay Kit (Beyotime Biotechnology, Shanghai, China), from which the total 24-hour urinary protein load was calculated. At 1 week and 6 weeks postpartum, selected offspring were euthanized by cervical dislocation. These mice were subsequently weighed, and their kidneys were extracted and rinsed with saline. Some of these kidney samples were processed for pathological examination, while others were stored at -80 °C for future research.
Ethics approval
The ethics and care of all animal experiments received approval from the research ethics committee of the Obstetrics and Gynecology Hospital of Fudan University and all methods were in accordance the relevant protocols, guidelines and regulations. All animal studies adhered to the ARRIVE 2.0 requirements, which included study design, animal numbers, randomization, and statistical methods.
Statistics
The statistical evaluation of data was executed using SPSS (version 26.0) and GraphPad Prism (version 10.0). Data conforming to a normal distribution are presented as mean ± standard deviation (SD). Multiple group comparisons were conducted via Analysis of Variance (ANOVA), whereas two-group comparisons were analyzed using Student’s t-tests, both unpaired and paired where necessary. Significance levels were indicated by p-values.
Data availability
The datasets utilized or analyzed over the course of this investigation are available from the corresponding author upon reasonable request.
Results
Preeclampsia-like mouse mode
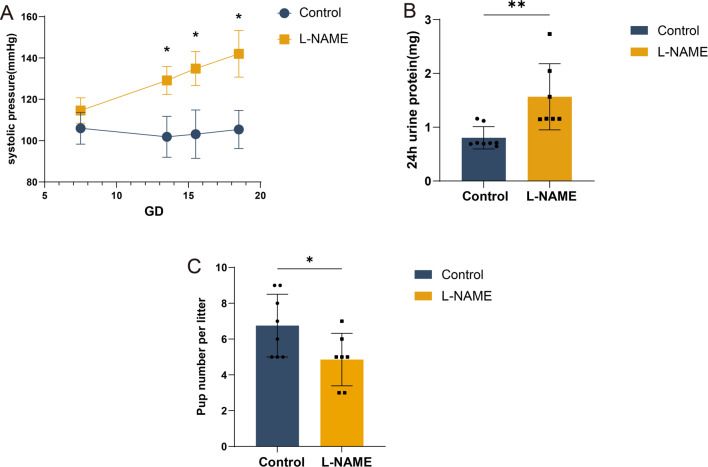
Compared to the control group that received saline, L-NAME-treated dams exhibited a significant increase in systolic blood pressure at gestational day 13.5, 15.5, and 19.5 relative to day 7.5 (Fig. 1A; n = 7; P < 0.05). Additionally, dams in the pre-eclampsia group demonstrated higher urinary protein levels (Fig. 1B). Furthermore, these mice produced fewer offspring compared to the control group, averaging 4.9 ± 1.5 pups versus 6.8 ± 1.8 in the controls, indicating a significant reproductive impact (P < 0.05) (Fig. 1C).
Fig. 1.
Establishment of a preeclampsia mouse model. Preeclampsia mouse model was generated by administration of L-NAME 75 mg/kg/d) between D7.5 and D18.5 of gestation in pregnant mice. (A) The effect of L-NAME on blood pressure in pregnant mice. (B) The effect of L-NAME on proteinuria in pregnant mice. (C) The number of fetuses from control and NG-nitroarginine methyl ester hydrochloride (L-NAME) dams. Control: control group, n = 8; L-NAME: preeclampsia group, n = 7. *P < 0.05, **P < 0.005.
The effects of L-NAME-induced preeclampsia on fetal weight and blood pressure in mice
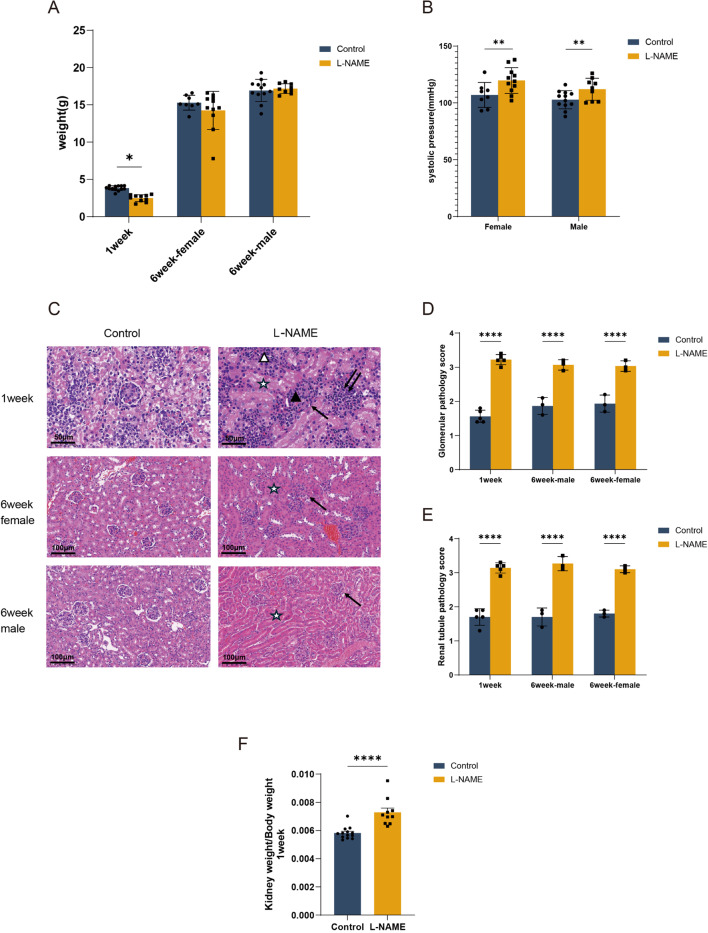
Due to the difficulty of determining the sex of offspring mice at 1 week of age, they were not categorized by gender. The average weight of 1-week-old offspring from the L-NAME-treated group was significantly lower than that of their counterparts in the control group (2.486 ± 0.457 g vs. 3.820 ± 0.318 g, P < 0.001). However, by 6 weeks of age, there was no significant difference in weight between female or male offspring from the L-NAME group compared to those from the control group; females weighed 14.25 ± 0.77 g versus 15.29 ± 0.35 g, and males weighed 17.18 ± 0.22 g versus 16.93 ± 0.43 g, respectively (P > 0.05) (Fig. 2A). At 6 weeks, both female and male offspring from the L-NAME group exhibited significantly higher average systolic blood pressures compared to the control group (119.73 ± 3.42mmHg vs. 107.00 ± 3.89mmHg for females, and 112.00 ± 3.22mmHg vs. 102.75 ± 2.30mmHg for males, P < 0.01). Nonetheless, there was no significant difference in systolic blood pressure between female and male offspring (P > 0.05) (Fig. 2B).
Fig. 2.
Offspring characteristics in the LNAME model. (A) Weight of 1week and 6week fetuses. (B) Representative images of pup’s kidney. Renal tissue stained with HE (magnification 200×). (C) Glomerular injury pathology score. (D) Tubular injury pathology score. : Renal tubular stenosis, : Abnormal glomerular structure, ☆: epithelial swelling of renal tubules, narrowing of renal tubular interstitium, △: Increased interstitial lymphocytes, ▲: Proliferation of glomerular mesangial cells. (E) Kidney efficiency (estimated for the kidney weight/body weight ratio), showing high kidney weight/body weight ratio in offspring from L-NAME dams. (F) The effect of L-NAME on blood pressure in 6-week-old offspring mice. Each dot represents 1 study subject. Values are presented in the median ± interquartile range. *P < 0.05, **P < 0.005, ****P < 0.0001.
Pathological changes in the renal tissue of mice with PELS offspring
Compared to the 1-week-old offspring in the control group, histopathological examinations of the pre-eclampsia group revealed increased abnormalities in the glomerular structure, proliferation of mesangial cells, swelling of tubular epithelial cells, constriction of renal capsules, narrowing of the space between tubules, and an increase in interstitial lymphocytes. Both the glomerular and tubular damage scores were elevated (1.56 ± 0.182 vs. 3.22 ± 0.148 and 1.70 ± 0.245 vs. 3.14 ± 0.152; P < 0.001). (Fig. 2C–E)
In the renal histopathology of 6-week-old offspring from the pre-eclampsia group, there was notable constriction of renal capsules, swelling of tubular epithelium, and reduced spacing between tubules, compared to the control group. However, there was no significant increase in mesangial cells or interstitial lymphocytes observed. The glomerular and tubular damage scores were significantly higher in the 6-week-old female offspring from the pre-eclampsia group compared to those from the control group, with scores of 3.03 ± 0.153 vs. 1.93 ± 0.252 and 3.10 ± 0.058 vs. 1.80 ± 0.058, respectively (P < 0.001). Similarly, the male offspring from the pre-eclampsia group also showed elevated damage scores, with 3.07 ± 0.153 vs. 1.87 ± 0.252 for glomerular damage and 3.27 ± 0.208 vs. 1.70 ± 0.265 for tubular damage (P < 0.001) (Fig. 2C–E). Moreover, a higher kidney/body weight ratio was observed in PELS offspring compared to the control group (Fig. 2F; P < 0.001).
The effects of L-NAME on the expression of AT1R, GRK4, ion channels and aquaporins in the kidneys of offspring mice
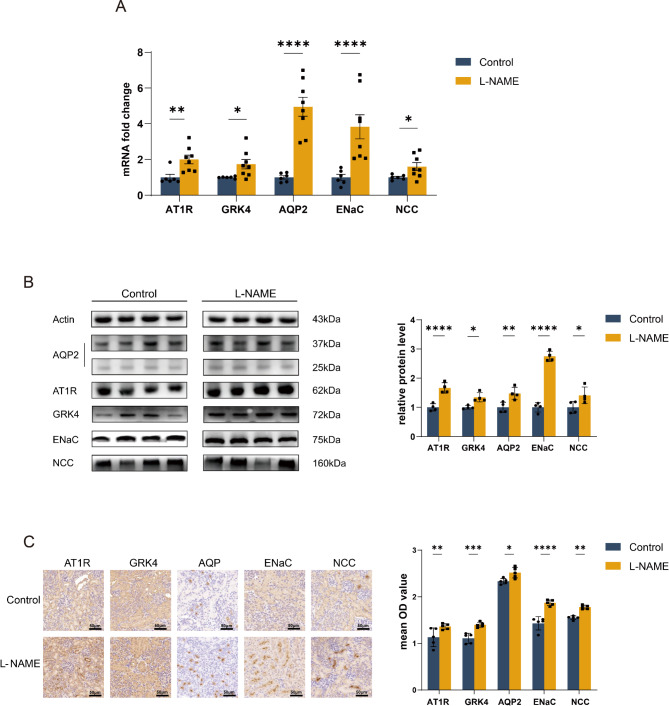
Compared to the control group at one week of age, qRT-PCR results indicated increased expression levels of angiotensin II receptor type 1 (AT1R) and G protein-coupled receptor kinase 4 (GRK4) in the renal tissues of one-week-old offspring in the preeclampsia group (1.00 ± 0.42 vs. 2.01 ± 0.68 and 1.00 ± 0.56 vs. 1.74 ± 0.74, respectively, P < 0.05). Additionally, the mRNA levels of aquaporin 2 (AQP2), epithelial sodium channel (ENaC), and sodium-chloride symporter (NCC) were significantly higher compared to the control group (AQP2: 1.00 ± 0.24 vs. 4.95 ± 1.50; ENaC: 1.00 ± 0.41 vs. 3.83 ± 1.91; NCC: 1.00 ± 0.14 vs. 1.60 ± 0.64, P < 0.05) (Fig. 3A). Western blot results further indicated an increased expression of AT1R, GRK4, AQP2, ENaC and NCC (AT1R: 1.00 ± 0.12 vs. 1.67 ± 0.18; GRK4: 1.00 ± 0.60 vs. 1.35 ± 0.16; AQP2: 1.00 ± 0.17 vs. 1.48 ± 0.21; ENaC: 1.00 ± 0.16 vs. 2.75 ± 0.17; NCC: 1.00 ± 0.21 vs. 1.41 ± 0.29, P < 0.05) (Fig. 3B). Immunohistochemical results of the kidney confirmed the elevated expression of AT1R, GRK4, AQP2, ENaC and NCC at the protein level (AT1R: 1.13 ± 0.20 vs. 1.36 ± 0.60; GRK4: 1.11 ± 0.11 vs. 1.39 ± 0.53; AQP2: 2.33 ± 0.59 vs. 2.52 ± 0.11; ENaC: 1.43 ± 0.14 vs. 1.87 ± 0.07; NCC: 1.55 ± 0.39 vs. 1.79 ± 0.05, P < 0.05) (Fig. 3C).
Fig. 3.
The effect of L-NAME on the expression of AT1R, GRK4, ion channels and aquaporins in the kidneys of 1-week-old offspring mice. (A) qRT-PCR results of AT1R, GRK4, ion channels and aquaporins expression in offspring kidney tissues in the kidneys of 1-week-old offspring mice. (B) Western blot results of AT1R, GRK4, ion channels and aquaporins expression in offspring kidney tissues in the kidneys of 1-week-old offspring mice. (C) Immunohistochemistry results of AT1R, GRK4, ion channels and aquaporins expression in offspring kidney tissues in the kidneys of 1-week-old offspring mice (magnification 200×). ***P<0.001, **P<0.01, *P<0.05.
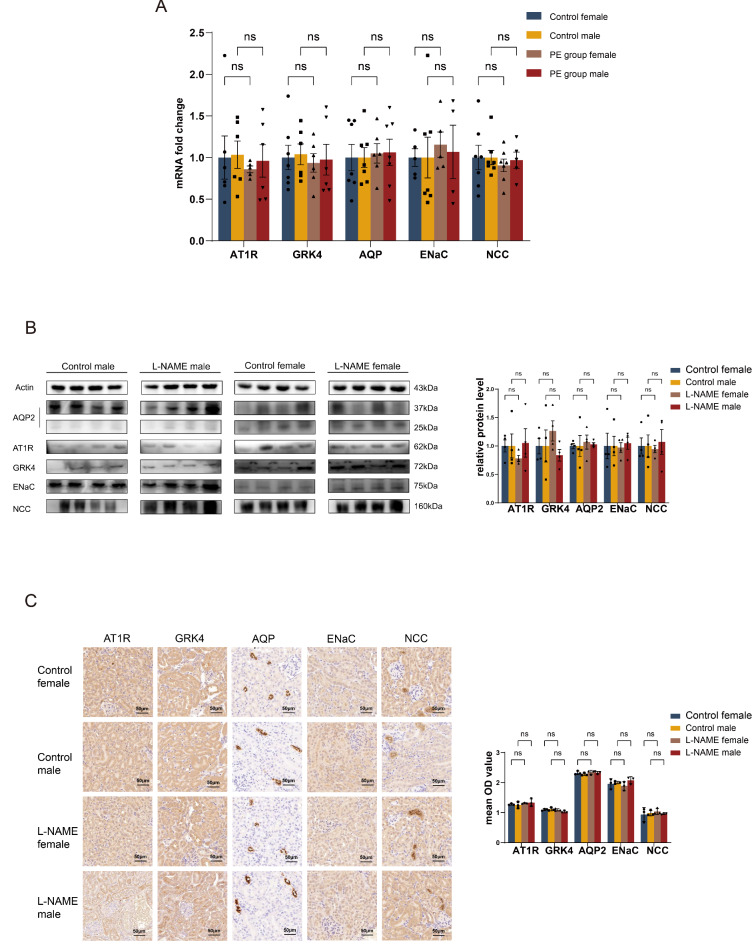
Previous research suggests that preeclampsia may affect blood pressure regulation in offspring, potentially with gender-specific variations. In line with this hypothesis, this study analyzed the results by dividing the offspring into gender-specific groups. qRT-PCR and Western blot analyses of renal tissues from the 6-week-old offspring in the pre-eclampsia group revealed no significant differences in the expression levels of AT1R and GRK4 at both mRNA and protein levels compared to control groups across both genders (P > 0.05) (Fig. 4A,B). Immunohistochemical examinations corroborated these findings, showing consistent protein expression levels of AT1R and GRK4 in the kidneys (P > 0.05) (Fig. 4B). Additionally, the expression levels of the aquaporin protein AQP2 and the ion channels NCC and ENaC in renal tissues also showed no significant differences at mRNA or protein levels between the pre-eclampsia group and controls (P > 0.05) (Fig. 4A,B). Immunohistochemical analysis supported these results, indicating uniform protein expressions for AQP2, NCC, and ENaC across the groups (P > 0.05) (Fig. 4C).
Fig. 4.
The effect of L-NAME on the expression of AT1R, GRK4, ion channels and aquaporins in the kidneys of 6-week-old offspring mice. (A) qRT-PCR results of AT1R, GRK4, ion channels and aquaporins expression in offspring kidney tissues in the kidneys of 6-week-old offspring mice. (B) Western blot results of AT1R, GRK4, ion channels and aquaporins expression in offspring kidney tissues in the kidneys of 6-week-old offspring mice. (C) Immunohistochemistry results of AT1R, GRK4, ion channels and aquaporins expression in offspring kidney tissues in the kidneys of 6-week-old offspring mice (magnification 200×). ***P<0.001, **P<0.01, *P<0.05.
Discussion
This study presents the evidence that the L-NAME-induced preeclampsia mouse model exhibited elevated blood pressure in offspring. Potential underlying mechanism contributing to these results include renal glomerular and tubular damage, and alterations in AT1R and its upstream GRK4, the ion channels ENaC and NCC, and the aquaporin AQP2. These changes affect renal structure and function in the offspring, leading to increased blood pressure.
Changes in body weight and increased blood pressure in offspring of preeclampsia
Our findings revealed a reduction in the litter size of pre-eclamptic mice, a decrease in the birth weight of their offspring, and an elevation in the average systolic blood pressure of the offspring at 6 weeks of age. Preeclampsia is a leading cause of fetal growth restriction (FGR)15. The results from this segment of our study indicate that offspring at 1 week of age from pre-eclamptic mothers weighed less compared to age-matched controls, and also exhibited reduced kidney weight. By the age of 6 weeks, no significant difference in weight or kidney size was observed between the offspring of pre-eclamptic mothers and controls. Previous animal studies have shown that offspring born to spontaneous pre-eclamptic mouse models have lower birth weights compared to controls but exhibit a phenomenon of accelerated compensatory growth in early adulthood17. A parallel trend was observed in epidemiological studies. A Norwegian research study examining 4,096 females aged 13–19, found that offspring of pre-eclamptic mothers were born with lower weights. However, by adolescence, these females had significantly higher weights and BMIs compared to offspring from normal pregnancies18. These findings suggest that offspring born to pre-eclamptic mothers have reduced birth weights. Still, under consistent nurturing conditions, they exhibit catch-up growth, reaching age-matched weight before sexual maturity.
Previous epidemiological studies have indicated that offspring of mothers with pre-eclampsia tend to have higher blood pressure compared to those from normal pregnancies. Interestingly, some studies found this elevated blood pressure only in male offspring, while others observed it solely in female offspring19–22. In animal experiments, male offspring induced by s-FLT in a pre-eclamptic condition demonstrated elevated blood pressure from birth, continuing into adulthood, whereas the female offspring did not exhibit such elevation6. Furthermore, normotensive females in the RUPP model showed increased blood pressure following ovariectomy7. These findings suggest that pre-eclampsia might affect offspring’s blood pressure, and this effect could be gender-specific. The types and levels of hormones in the offspring might play a regulatory role in their blood pressure. Our study results for this segment show that at 6 weeks of age, the offspring from the pre-eclampsia group displayed significantly higher tail artery systolic pressure when at rest compared to the control group, but no noticeable gender difference was observed. However, the underlying mechanisms remain unclear.
We explore the underlying mechanisms of increased blood pressure in offspring of preeclampsia, reporting renal damage and alterations in AT1R, ENaC, NCC and AQP2. We observed an increased scoring of damage in the glomeruli and renal tubules of both 1-week-old and 6-week-old offspring subjected to pre-eclampsia conditions. In the kidney tissues of the 1-week-old offspring from the pre-eclampsia group, the expression of AT1R and its upstream regulator GRK4, as well as its downstream targets, including the aquaporin protein AQP2 and ion channels ENaC and NCC, were elevated in comparison to the control group. However, in the 6-week-old offspring from the pre-eclampsia group, the expression levels of AT1R, GRK4, AQP2, ENaC, and NCC were not significantly different from those of the control group. AT1R is a G-protein-coupled receptor that mediates most of the functions of ANG II in the system. It is widely expressed in the kidneys and vascular tissues, playing a pivotal role in the onset and development of hypertension8. Abnormal expression of AT1R can lead to changes in ion channel and aquaporin expression in kidney tissues. Among them, ENaC is a heterotetramer composed of α, β, and γ subunits, with the α subunit playing the primary role. It is highly expressed in the distal convoluted tubules and collecting ducts of the kidneys, assisting in sodium ion reabsorption. The NKCC transporter includes NKCC1 and NKCC2, with NKCC2 being particularly present in the kidneys, functioning in the reabsorption of sodium, potassium, and chloride ions from primary urine. NCC is distributed in renal tubules and facilitates the reabsorption of sodium and chloride ions. AQP2 is a type of aquaporin primarily found in the renal collecting ducts, responsible for reabsorbing water from urine23,24. Changes in the expression of these ion channels and aquaporins can alter the kidney’s water and sodium excretion functions, contributing to the onset of hypertension. The intrarenal RAS system plays a vital role in postnatal kidney development and the onset of kidney injuries. Perinatal inhibition of the RAS system can lead to a decrease in nephrons, enlargement of glomerular volume, impairment of kidney concentrating function, and hypertension in offspring25,26. Adverse intrauterine stimuli can affect offspring’s blood pressure by altering the expression of various components of the RAS system in the offspring’s kidneys and vessels. Studies related to the RUPP-induced fetal intrauterine growth restriction model have shown that RUPP baboon offspring exhibit a heightened salt sensitivity, developing hypertension after a high-salt diet, accompanied by elevated levels of serum and renal aldosterone13. Offspring exposed to adverse stimuli during pregnancy, such as PM2.5, also show an increased incidence of hypertension. Additionally, there’s an increase in renal GRK4 expression, leading to elevated AT1R expression and D1R phosphorylation. This in turn causes an increase in renal oxidative stress and impairs renal sodium excretion15. These studies suggest that changes in the RAS system contribute to the impact of adverse intrauterine stimuli on the offspring’s kidneys and vascular systems. It might be a critical link in how intrauterine stressors influence the regulation of blood pressure in offspring.
In this study, the expression of AT1R and its upstream and downstream molecules in the renal tissues of 1-week-old offspring mice induced by L-NAME during the pre-eclamptic phase increased significantly compared to the age-matched control group. However, by the age of 6 weeks, the expression of AT1R and its associated molecules showed no difference compared to the control group. This suggests that the expression of AT1R and related molecules is influenced by both the adverse intrauterine environment and postnatal environmental factors. The impact of pre-eclampsia on the expression of mRNA and protein of AT1R-related molecules in the RAS system may be transient and reversible. While the renin-angiotensin system (RAS)-related genes and proteins return to control levels by 6 weeks, the persistent elevation in blood pressure could be attributed to structural changes in the kidney established earlier in life. Early kidney damage, including injury to glomeruli and renal tubules, could result in subsequent nephron loss. According to the hyperfiltration hypothesis, the loss of nephrons leads to compensatory hyperfiltration in the remaining glomeruli27, contributing to increased glomerular capillary pressure. This compensatory mechanism can predispose the individual to systemic hypertension28, even in the absence of sustained changes in the expression of RAS-related molecules. Such structural alterations in the kidneys might act as the trigger for hypertension rather than being directly maintained by ongoing dysregulation of AT1R-related molecules.
Moreover, the complexity of hypertension pathogenesis in offspring likely involves multifactorial interactions. While changes in renal AT1R-related molecules after birth may serve as early triggers, factors such as nephron deficit, compensatory hyperfiltration, and systemic hemodynamic changes could perpetuate hypertension. Importantly, this study did not investigate the secretion levels of renin, angiotensin, or aldosterone, nor did it assess the functional changes in AT1R-related molecules. Thus, we cannot fully analyze the role of the RAS system in the development of hypertension in pre-eclamptic offspring.
Previous epidemiological studies and animal experiments have suggested that the impact of pre-eclampsia on offspring blood pressure might exhibit gender differences. The types and levels of hormones within the offspring’s body could potentially regulate their blood pressure6,7. In our study, gender subgroups were established for the 6-week-old offspring. There were no noticeable gender differences in blood pressure or the expression changes of AT1R-related molecules in the kidneys of the pre-eclamptic offspring. However, this study did not investigate the levels of endogenous hormones. We will pursue further studies to investigate whether there exists a gender difference in blood pressure and kidney changes in the offspring of pre-eclamptic mothers.
A limitation of this study is the lack of sex determination in the 1-week-old offspring. However, we believe it does not significantly impact the main conclusions, as key outcomes (blood pressure, renal damage, and expression of target molecules) were similar in both sexes at 6 weeks. Future studies will incorporate molecular sexing methods like PCR for more precise analyses. Another important consideration is the use of L-NAME to induce a preeclampsia-like phenotype. While this model is widely employed in the field, evidence indicates that L-NAME’s primary active metabolite, L-NOARG, is capable of placental transfer and may directly influence fetal development, potentially confounding the interpretation of offspring phenotypes29. Nonetheless, L-NAME-based models have proven valuable in preclinical studies to explore the maternal environment’s role in shaping offspring health. Indeed, a substantial body of research has utilized this model to investigate the effects of preeclampsia on various aspects of offspring health and development30–33. In light of these complexities, future research may consider alternative preeclampsia models that can help better isolate the maternal condition’s impact on offspring without the potential confounders introduced by L-NAME itself.
In summary, changes in the renal RAS system play a role in the onset and progression of hypertension in offspring of L-NAME-induced pre-eclamptic mice. This relationship is closely related to the age of the offspring but is unrelated to their gender. Pre-eclampsia may affect the offspring’s renal structure and function by altering the expression of components within their renal RAS system, leading to elevated blood pressure in the offspring. The factors that trigger and maintain hypertension in offspring might be distinct. Changes in AT1R expression may be involved in the early structural damage of the kidneys in offspring of pre-eclamptic mothers, leading to increased blood pressure. A properly designed study to specifically analyzed the long-term regulatory factors of offspring’s blood pressure is underway in our laboratory.
This study primarily examined the alterations in AT1R-related molecular expressions. However, a more in-depth exploration is required to comprehend the interplay among these molecules and their associated signaling pathways. The current research did not conduct a stratified analysis of blood pressure, thus the relationship between the extent of hypertension and the severity of pathological and molecular changes in offspring kidneys remains uncertain. This study lacks investigations concerning hormone levels in the serum, as well as relevant data from clinical samples.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Y.W. and H.W. contributed equally to the design and conceptualization of the study. Y.W. , H.W.,H.L., J.M.and W.W. conducted the experiments and collected the data. B.M. and H.Z. performed data analysis and interpretation. Y.W. and H.W. wrote the main manuscript text. H.L., J.M., and W.W. prepared Figs. 1, 2, 3 and 4. R.H. and H.Z. supervised the project. All authors reviewed and approved the manuscript.
Data availability
Data is provided within the manuscript or supplementary information files. All other data are available upon reasonable request from the corresponding authors.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hao Zhu, Email: irios@126.com.
Rong Hu, Email: hurong@fudan.edu.cn.
References
- 1.Rana, S., Lemoine, E., Granger, J. P., Karumanchi, S. A. & Preeclampsia Pathophysiology, challenges, and perspectives. Circ. Res.124, 1094–1112 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Wu, C. S. et al. Health of children born to mothers who had preeclampsia: A population-based cohort study. Am. J. Obstet. Gynecol.201, 269e261–269e210 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Gluckman, P. D., Hanson, M. A., Cooper, C. & Thornburg, K. L. Effect of in utero and early-life conditions on adult health and disease. N Engl. J. Med.359, 61–73 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kajantie, E., Eriksson, J. G., Osmond, C., Thornburg, K. & Barker, D. J. Pre-eclampsia is associated with increased risk of stroke in the adult offspring: The Helsinki birth cohort study. Stroke40, 1176–1180 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Staley, J. R. et al., Associations of blood pressure in pregnancy with offspring blood pressure trajectories during childhood and adolescence: findings from a prospective study. J. Am. Heart Assoc.4 (2015). [DOI] [PMC free article] [PubMed]
- 6.Lu, F. et al. Gender-specific effect of overexpression of sFlt-1 in pregnant mice on fetal programming of blood pressure in the offspring later in life. Am. J. Obstet. Gynecol.197, 418e411–418e415 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Ojeda, N. B., Grigore, D., Robertson, E. B. & Alexander, B. T. Estrogen protects against increased blood pressure in postpubertal female growth restricted offspring. Hypertension50, 679–685 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckenstaler, R., Sandori, J., Gekle, M. & Benndorf, R. A. Angiotensin II receptor type 1—an update on structure, expression and pathology. Biochem. Pharmacol.192, 114673 (2021). [DOI] [PubMed] [Google Scholar]
- 9.Crowley, S. D., Gurley, S. B. & Coffman, T. M. AT(1) receptors and control of blood pressure: The kidney and more. Trends Cardiovasc. Med.17, 30–34 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Chen, D. et al. Impact of angiotensin type 1A receptors in principal cells of the Collecting Duct on blood pressure and hypertension. Hypertension67, 1291–1297 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stegbauer, J. et al. AT1 receptors in the collecting duct directly modulate the concentration of urine. J. Am. Soc. Nephrol.22, 2237–2246 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu, T. et al. DNA methylation-reprogrammed Ang II (angiotensin II) type 1 receptor-early growth response gene 1-protein kinase C ε axis underlies vascular hypercontractility in Antenatal Hypoxic offspring. Hypertension77, 491–506 (2021). [DOI] [PubMed] [Google Scholar]
- 13.Yeung, K. R. et al. Increased salt sensitivity in offspring of pregnancies complicated by experimental preeclampsia. Clin. Exp. Pharmacol. Physiol.45, 1302–1308 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Grigore, D. et al. Placental insufficiency results in temporal alterations in the renin angiotensin system in male hypertensive growth restricted offspring. Am. J. Physiol. Regul. Integr. Comp. Physiol.293, R804–811 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye, Z. et al. In Utero exposure to fine particulate matter causes hypertension due to impaired renal dopamine D1 receptor in offspring. Cell. Physiol. Biochem.46, 148–159 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng, S. et al., Improvement in Clinical Features of L-NAME-Induced Preeclampsia-like Rats through Reduced SERPINA5 Expression. Biomolecules13 (2023). [DOI] [PMC free article] [PubMed]
- 17.Sutton, E. F. et al. Adverse metabolic phenotype of female offspring exposed to preeclampsia in utero: A characterization of the BPH/5 mouse in postnatal life. Am. J. Physiol. Regul. Integr. Comp. Physiol.312, R485–r491 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vatten, L. J. et al. Intrauterine exposure to preeclampsia and adolescent blood pressure, body size, and age at menarche in female offspring. Obstet. Gynecol.101, 529–533 (2003). [DOI] [PubMed] [Google Scholar]
- 19.Langford, H. G. & Watson, R. L. Prepregnant blood pressure, hypertension during pregnancy, and later blood pressure of mothers and offspring. Hypertension2, 130–133 (1980). [PubMed] [Google Scholar]
- 20.Palti, H. & Rothschild, E. Blood pressure and growth at 6 years of age among offsprings of mothers with hypertension of pregnancy. Early Hum. Dev.19, 263–269 (1989). [DOI] [PubMed] [Google Scholar]
- 21.Higgins, M. et al. Studies of blood pressure in Tecumseh, Michigan. I. Blood pressure in young people and its relationship to personal and familial characteristics and complications of pregnancy in mothers. Am. J. Epidemiol.111, 142–155 (1980). [DOI] [PubMed] [Google Scholar]
- 22.Davis, E. F. et al. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics129, e1552–1561 (2012). [DOI] [PubMed] [Google Scholar]
- 23.Sagnella, G. A. & Swift, P. A. The renal epithelial sodium channel: genetic heterogeneity and implications for the treatment of high blood pressure. Curr. Pharm. Des.12, 2221–2234 (2006). [DOI] [PubMed] [Google Scholar]
- 24.Knepper, M. A. et al. Renal aquaporins. Kidney Int.49, 1712–1717 (1996). [DOI] [PubMed] [Google Scholar]
- 25.Woods, L. L., Ingelfinger, J. R. & Rasch, R. Modest maternal protein restriction fails to program adult hypertension in female rats. Am. J. Physiol. Regul. Integr. Comp. Physiol.289, R1131–1136 (2005). [DOI] [PubMed] [Google Scholar]
- 26.Ribeiro, V. S. et al. Perinatal α-tocopherol overload programs alterations in kidney development and renal angiotensin II signaling pathways at birth and at juvenile age: mechanisms underlying the development of elevated blood pressure. Biochim. Biophys. Acta Mol. Basis Dis.1864, 2458–2471 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Cortinovis, M., Perico, N., Ruggenenti, P., Remuzzi, A. & Remuzzi, G. Glomerular hyperfiltration. Nat. Rev. Nephrol.18, 435–451 . [DOI] [PubMed]
- 28.Kanzaki, G., Tsuboi, N., Shimizu, A. & Yokoo, T. Human nephron number, hypertension, and renal pathology. Anat. Rec (Hoboken)303, 2537–2543 . [DOI] [PubMed]
- 29.Tarrade, A. et al. Analysis of placental vascularization in a pharmacological rabbit model of IUGR induced by L-NAME, a nitric oxide synthase inhibitor. Placenta35, 254–259 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Butruille, L. et al. Maternal hypertension induced by NO blockade does not program adult metabolic diseases in growth-restricted rat fetuses. Metabolism62, 442–445 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Liu, X. et al. Developmental and functional brain impairment in offspring from preeclampsia-Like rats. Mol. Neurobiol.53, 1009–1019 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tachibana, R. et al. Tadalafil treatment in mice for preeclampsia with fetal growth restriction has neuro-benefic effects in offspring through modulating prenatal hypoxic conditions. Sci. Rep.9, 234 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ijomone, O. K., Shallie, P. D. & Naicker, T. Oligodendrocytes death induced sensorimotor and cognitive deficit in N-nitro-L-arginine methyl rat model of pre-eclampsia. Neurochem Res.45, 902–914 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets utilized or analyzed over the course of this investigation are available from the corresponding author upon reasonable request.
Data is provided within the manuscript or supplementary information files. All other data are available upon reasonable request from the corresponding authors.