ABSTRACT
Small bowel stenosis in patients with Crohn’s disease leads to abdominal symptoms and can affect prognosis. The Simple Endoscopic Score for Crohn’s Disease for the large bowel has been applied to the small bowel; however, stenosis scoring may be overestimated since it has a long diameter. This retrospective study aimed to devise a new endoscopic scoring system including the small bowel and evaluate whether it predicts the prognosis of Crohn’s disease. The study included 103 patients with Crohn’s disease at our hospital. We modified the Simple Endoscopic Score for Crohn’s Disease and proposed a new scoring system; the modified applied Simple Endoscopic Score for Crohn’s Disease was created by subtracting one point for stricture from the Simple Endoscopic Score for Crohn’s Disease. Receiver operating characteristic curve analysis was performed to assess the accuracy of the modified applied score for Crohn’s disease in predicting disease worsening within 1 year. Results were validated using the log-rank test. For the modified applied score, the area under the receiver operating characteristic curve for disease worsening within 1 year in 57 cases was 0.850. When the cutoff score was set to 9 points, the sensitivity and specificity were 72.7% and 80.6%, respectively. The log-rank test showed a significant difference (P = 0.027) in the risk of worsening within 1 year between the low (<9 points) and high (≥9 points) score groups. Thus, a higher modified applied Simple Endoscopic Score for Crohn’s Disease may be associated with a significantly increased risk of disease worsening within 1 year.
Key Words: Crohn’s disease, balloon-assisted endoscopy, small bowel, scoring, prognosis
INTRODUCTION
Crohn’s disease (CD) is an inflammatory bowel disease that causes inflammation and swelling of digestive tract tissues, resulting in abdominal pain, severe diarrhea, fatigue, weight loss, and malnutrition. Persistent inflammation can lead to ulcers, stenosis, abscess formation, and intestinal perforation, necessitating surgery. Targeting is currently being proposed as a treatment strategy for CD. This concept was introduced as a therapeutic target for rheumatoid arthritis1 and has been proposed for CD since 2015.2 Endoscopic mucosal healing (MH) is among the most important goals of CD treatment, and the MH status has been reported to reduce the risk of long-term relapse.3-6 However, the definition of MH differed from that in the previous studies, and MH rates for CD have been reported to range from 3%–84%.7-11
More than 70% of patients with CD have small bowel lesions,12 and evaluation of the entire small bowel is essential for optimal treatment. Balloon-assisted endoscopy and small bowel capsule endoscopy (SBCE) are currently the primary modalities used to evaluate the entire small bowel mucosa. SBCE is well tolerated and examines the entire small bowel only once in patients with gastrointestinal tract patency. There are several endoscopic scoring systems for SBCE to evaluate the entire small bowel, including the Lewis score, Capsule Endoscopy Crohn’s Disease Activity Index, and Crohn’s Disease Activity in Capsule Endoscopy.13,14 However, scoring is limited because of the risk of capsule retention,15 and therefore, patients with severe intestinal stenosis cannot undergo SBCE. It has been reported that patients who were able to undergo SBCE have better intestinal prognoses.16
The Simple Endoscopic Score for Crohn’s Disease (SES-CD) has been the most frequently used method for evaluating colonic mucosa in clinical studies.17 It is based on the ulcer size, extent of the ulcerated and affected surface, and stenosis and ranges from 0 to 3 for each of the five segments—ileum, right colon, transverse colon, left colon, and rectum. Balloon-assisted endoscopy is the gold standard for evaluating the entire small bowel mucosa and allows endoscopic scoring of CD by modifying the SES-CD. Morise et al assessed MH of the small bowel in CD using a modified SES-CD, in which the evaluation was extended up to 80 cm away from the ileocecal valve using double-balloon endoscopy (DBE).18 Takenaka et al extended the scoring for the ileum to 300 cm for the length in single-balloon endoscopy and proposed a new scoring system, applied SES-CD, in which less than 5 points led to a better prognosis.19 However, the jejunum does not have a score; therefore, a gold standard endoscopic scoring system to assess all small bowel lesions has not yet been established.
In our hospital, small bowel evaluation for CD is usually performed through an entire small bowel observation with oral and anal approaches to DBE. In this study, we proposed a new scoring system for balloon-assisted endoscopy to evaluate all small bowel lesions in patients with CD. We used a training set prepared from the DBE database and validated the score for predicting the intestinal prognosis of CD, extracting cases presenting with disease activity within 1 year.
MATERIALS AND METHODS
Ethics statements
This retrospective study was approved by the Ethics Committee of the Nagoya University Hospital (ID: 2015-0466). The study was conducted in compliance with the principles of the Declaration of Helsinki.
Patients
In clinical practice, we usually evaluate the entire small bowel mucosa using a combination of oral DBE on the first day and anal DBE on the third day post-admission. EN-580T or EN-580XP (FUJIFILM, Tokyo, Japan) was used for each DBE as previously described.20 CO2 was used for airflow. The marking clip was placed at the deepest point of the oral DBE, and the anal DBE was subsequently approached. We reviewed the DBE database and medical charts to determine patient eligibility for this study. The inclusion criteria were patients with CD who underwent DBE for the evaluation of disease activity and those in whom the entire small bowel could be visualized with DBE. The exclusion criteria were patients for whom the entire small bowel could not be evaluated with endoscopy and those treated with immunosuppressants for comorbidities other than CD.
A random number was generated, and the 103 patients were allocated to training and validation cohorts. These patients were divided using a random number table in a 6:4 ratio, with 57 in the training and 46 in the validation cohorts.
Data analysis
We used the previously applied SES-CD,19 which divided the small bowel into three sections for reference. However, it has been suggested that the effect of small bowel stenosis on scoring is relatively low.19 In addition, we encountered a number of patients with CD in whom the DBE endoscope passed through jejunal stenoses. On the basis of such published evidence6,19 and clinical experience, we modified the applied SES-CD at the point of stenosis and created a new scoring system, the modified applied SES-CD (MASES-CD), which assigned a zero point to the stenosis through which the endoscope could pass and one point to the stenosis through which the endoscope could not pass (Tables 1A and 1B). The sample split method was used to develop and validate the MASES-CD. The primary endpoint was a reliable indicator for predicting disease exacerbation within 1 year. The secondary endpoint was the cutoff value for the score predicting disease exacerbation within 1 year.
Table 1A.
Applied SES-CD (aSES-CD)
| Definitions of endoscopic score | ||||
| Variables | 0 | 1 | 2 | 3 |
| A: Size of ulcers, cm | None | 0.1–0.5 | 0.5–2.0 | >2 |
| B: Ulcerated surface, % | None | <10 | 10–30 | >30 |
| C: Affected surface, % | Unaffected segment | <50 | 50–75 | >75 |
| D: Presence of stenosis | None | Single, can be passed | Multiple, can be passed | Cannot be passed |
| Definitions of endoscopic segmentation | ||||
| Terminal ileum (TI) | ≤10 cm from the ileocecal valve | |||
| Proximal ileum (PI) | 10–300 cm from the ileocecal valve | |||
| Jejunum (J) | The proximal part, excluding the section defined as the proximal ileum | |||
SES-CD: Simple Endoscopic Score for Crohn’s Disease
Table 1B.
New scoring system, modified applied SES-CD (MASES-CD)
| Definitions of endoscopic score | ||||
| Variables | 0 | 1 | 2 | 3 |
| A: Size of ulcers, cm | None | 0.1–0.5 | 0.5–2.0 | >2 |
| B: Ulcerated surface, % | None | <10 | 10–30 | >30 |
| C: Affected surface, % | Unaffected segment | <50 | 50–75 | >75 |
| D: Presence of stenosis | None or can be passed | Cannot be passed | ||
| Definitions of endoscopic segmentation | ||||
| Terminal ileum (TI) | ≤10 cm from the ileocecal valve | |||
| Proximal ileum (PI) | 10–300 cm from the ileocecal valve | |||
| Jejunum (J) | The proximal part, excluding the section defined as the proximal ileum | |||
MASES-CD: modified applied Simple Endoscopic Score for Crohn’s Disease
Definitions
Exacerbation was defined as worsening abdominal pain, fever, or bloody stools that the attending physician judged, thus requiring a change in treatment.
Statistical analyses
For the testing cohort, receiver operating characteristic (ROC) curve analysis was performed to evaluate the discriminative abilities of C-reactive protein level, Crohn’s Disease Activity Index, applied SES-CD, and MASES-CD for CD within 1 year. The cutoff value of the MASES-CD was determined from the ROC analysis. The MASES-CD was validated by comparing the risk of exacerbation between patients with high and low scores using the log-rank test. The areas under the curve for each score were compared using DeLong’s test. Continuous variables were compared using the Mann–Whitney U test. Fisher’s exact test was used to compare the categorical variables. P < 0.05 denoted statistical significance. All statistical analyses were performed using SPSS statistical software v28.0 (IBM Corp, Chicago, Illinois, USA).
RESULTS
Of all the patients with CD who regularly received clinical consultation at Nagoya University Hospital between July 2006 and February 2021, a total of 106 patients whose entire small bowel was successfully evaluated using DBE were eligible for the study. Three patients with comorbidities such as immunoglobulin A nephropathy and systemic lupus erythematosus, which required frequent volume adjustments of steroids or immunomodulators, were excluded from this study. Finally, 103 patients with CD were included (Fig. 1).
Fig. 1.
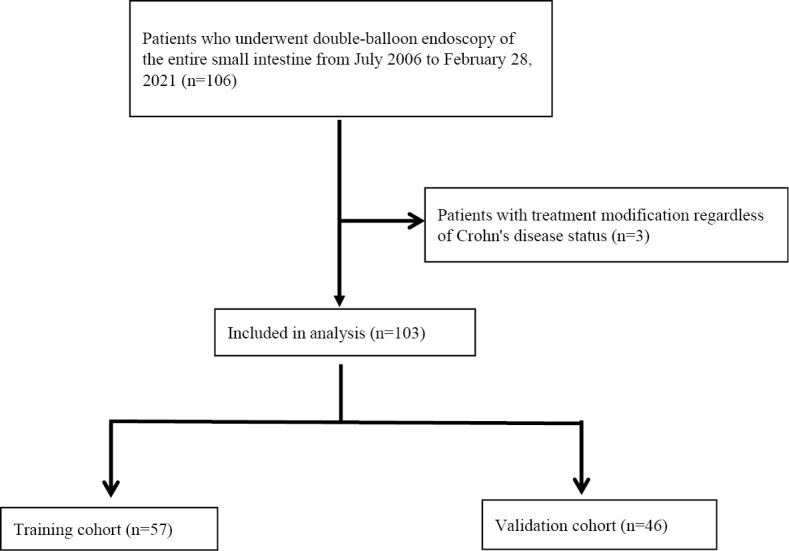
Flow diagram of the study
The characteristics of the patients allocated to the training and validation cohorts are shown in Table 2. No significant differences were found between the two groups. MASES-CD was scored to predict exacerbation of CD within 1 year.
Table 2.
Characteristics of patients in the training and validation cohorts
| Training cohort (n=57) | Validation cohort (n=46) | P-value | |
| Age, years (range) | 39 (19–78) | 39 (16–67) | 0.845* |
| Sex (M:F) | 43:14 | 39:7 | 0.326** |
| Disease duration, years | 9 (0.1–40) | 8 (0–41) | 0.668* |
| CD type (Ileal/Ileocolonic: colonic) | 56:1 | 43:3 | 0.322** |
| Surgical history, % | 25 (44) | 25 (54) | 0.326* |
| Exacerbation within 1 year, % | 13 (23) | 9 (20) | 0.81** |
| Treatment before DBE, n (%) | |||
| 5-ASA | 37 (65) | 33 (72) | 0.527** |
| Steroid | 7 (12) | 6 (13) | 0.775** |
| Immunomodulator | 12 (21) | 8 (17) | 0.803** |
| Biologics (IFX: ADA: UST) | 7:11:3 | 8:10:3 | 0.795** |
CD: Crohn’s disease
5-ASA: 5-aminosalicylate
DBE: double-balloon endoscopy
IFX: infliximab
ADA: adalimumab
UST: ustekinumab
* The Mann–Whitney U test was used.
** Fisher’s exact test was used.
Discrimination ability of scores
The distribution of each score for SES-CD and the total number of points are shown in the Supplemental Figure. Out of 57 patients, 56 had small bowel lesions, and the SES-CD score was a reference for the new scoring that evaluated the whole small bowel.
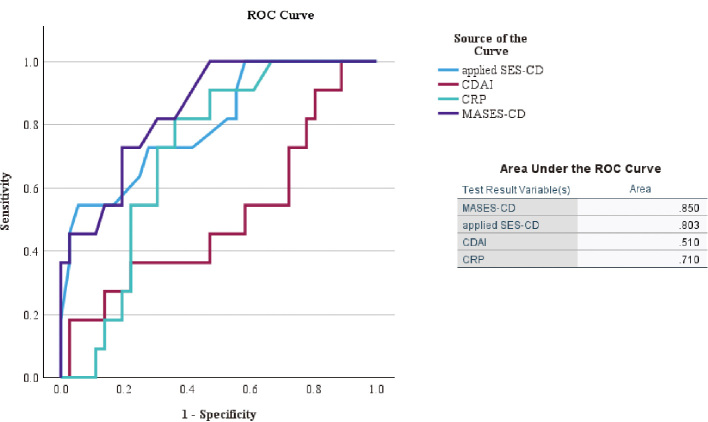
ROC curves were generated for the training cohort using these curves as clinical indicators of existing CD. The areas under the curve for Crohn’s Disease Activity Index, C-reactive protein, applied SES-CD, and MASES-CD for CD exacerbation were 0.510, 0.710, 0.803, and 0.850, respectively. MASES-CD had the highest area under the curve among the indicators (Fig. 2), and the cutoff value for MASES-CD was determined from the ROC analysis to be 9 points, with a sensitivity of 72.7% and specificity of 80.6%. The distributions of MASES-CD and applied SES-CD for the 57 cases are shown in Figure 3. The maximum total scores were 22 and 38 points for MASES-CD and applied SES-CD, respectively. In many cases, the MASES-CD provided a lower score than the applied SES-CD. During the training phase, five patients with stenosis assigned 2 or more points for the applied SES-CD showed MH, and none of them had any progression within 1 year. For the validation phase, seven patients who had MH with stenosis were assigned 2 or more points by applying SES-CD, and one patient had an exacerbation within 1 year (Table 3).
Fig. 2.
ROC curves for scores in the training cohort
ROC: receiver operating characteristic
CRP: C-reactive protein
CDAI: Crohn’s Disease Activity Index
SES-CD: Simple Endoscopic Score for Crohn’s Disease
MASES-CD: modified applied Simple Endoscopic Score for Crohn’s Disease
Fig. 3.
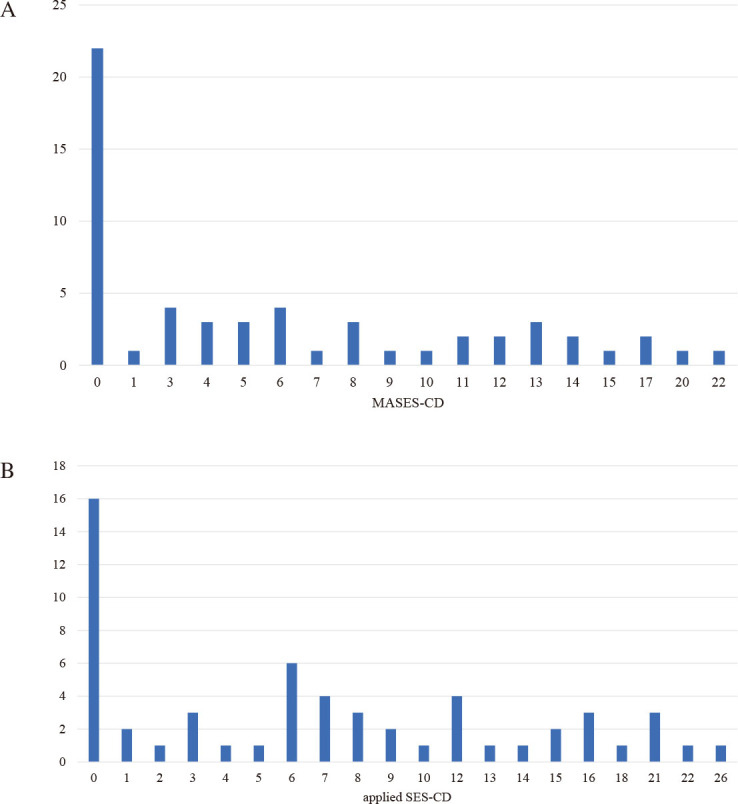
Distribution of 57 cases assessed by the MASES-CD and applied SES-CD in the training set
Fig. 3A: Distribution of 57 cases assessed by the MASES-CD in the training set.
Fig. 3B: Distribution of 57 cases assessed by applied SES-CD in the training set.
SES-CD: Simple Endoscopic Score for Crohn’s Disease
MASES-CD: modified applied Simple Endoscopic Score for Crohn’s Disease
Table 3.
One-year prognosis of patients with mucosal healing and multiple stenoses
| No | Age | Sex | Disease duration (years) | Disease type | Stenosis number | Applied SES-CD (stenosis score) |
MASES-CD (stenosis score) | Worsening within 1 year |
| Training phase | ||||||||
| 1 | 59 | M | 40 | Ileal | 6 | 6 (6) | 0 (0) | Negative |
| 2 | 59 | M | 0.1 | Ileocolonic | 2 | 2 (2) | 0 (0) | Negative |
| 3 | 66 | M | 21 | Ileocolonic | 6 | 6 (6) | 0 (0) | Negative |
| 4 | 35 | M | 3 | Ileocolonic | 6 | 6 (6) | 0 (0) | Negative |
| 5 | 31 | M | 9 | Ileal | 5 | 5 (5) | 1 (1) | Negative |
| Validation phase | ||||||||
| 1 | 16 | M | 1 | Ileal | 2 | 2 (2) | 0 (0) | Negative |
| 2 | 31 | M | 2 | Ileal | 2 | 2 (2) | 0 (0) | Negative |
| 3 | 23 | M | 1 | Ileal | 2 | 2 (2) | 0 (0) | Negative |
| 4 | 35 | M | 8 | Ileal | 4 | 4 (4) | 0 (0) | Negative |
| 5 | 36 | M | 1 | Ileal | 4 | 4 (4) | 0 (0) | Negative |
| 6 | 26 | M | 3 | Colonic | 2 | 2 (2) | 2 (2) | Negative |
| 7 | 44 | M | 20 | Ileocolonic | 4 | 4 (4) | 0 (0) | Positive |
SES-CD: Simple Endoscopic Score for Crohn’s Disease
MASES-CD: modified applied Simple Endoscopic Score for Crohn’s Disease
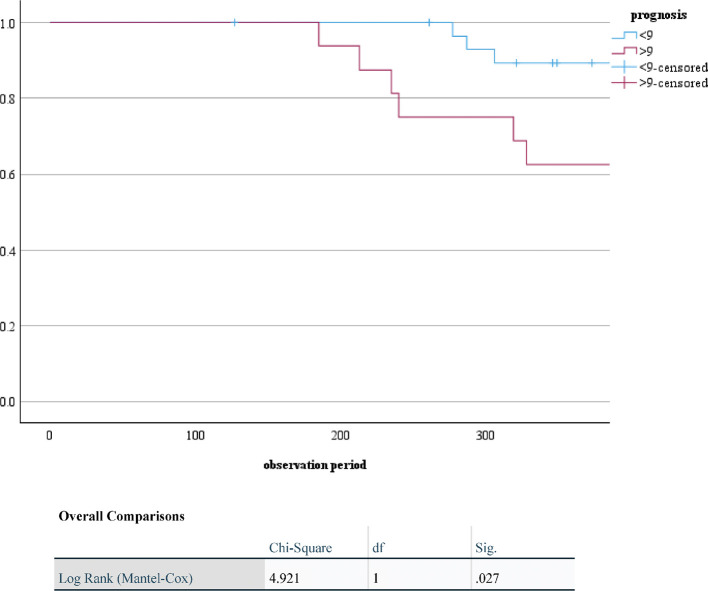
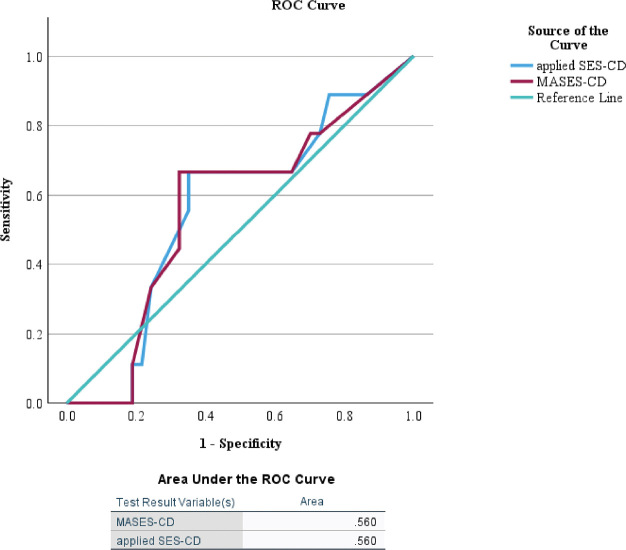
Endoscopic images of the patients with MASES-CD gap and applied SES-CD are shown in Figure 4. Part of the lumen was complicated by stenosis in the images; however, no inflammatory signs were observed. The SES-CD and MASES-CD scores were 2 and 0, respectively. For the validation cohort, patients with higher MASES-CD scores (>9 points) had a significantly higher risk of disease exacerbation within 1 year (P = 0.027; Fig. 5). The area under the ROC curve for the validation phase was 0.56 for both applied SES-CD and MASES-CD (Fig. 6).
Fig. 4.
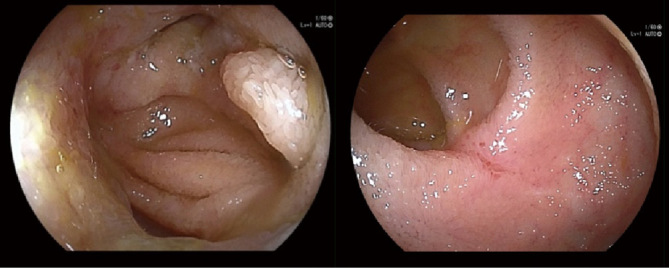
A case of point dissociation between applied SES-CD and MASES-CD
SES-CD: Simple Endoscopic Score for Crohn’s Disease
MASES-CD: modified applied Simple Endoscopic Score for Crohn’s Disease
Fig. 5.
Kaplan–Meier scores for exacerbation within 1 year stratified by MASES-CD
MASES-CD: modified applied Simple Endoscopic Score for Crohn’s Disease
Fig. 6.
ROC curves for scores in the validation cohort
ROC: receiver operating characteristic
SES-CD: Simple Endoscopic Score for Crohn’s Disease
MASES-CD: modified applied Simple Endoscopic Score for Crohn’s Disease
DISCUSSION
Recurrent relapse and remission of CD can lead to intestinal stenosis, abscesses, and fistulas and ultimately require surgery. The therapeutic goals of CD include improving the inflammatory response in blood sampling and endoscopic MH.7 Endoscopic MH has been reported to be useful in maintaining long-term remission. However, we often encounter cases where MH has not yet been achieved. Furthermore, there is no established endoscopic scoring system for evaluating CD activity, including small bowel lesions.
Therefore, in this study, we selected patients who could undergo an endoscopic examination of the entire small intestine and created a score for disease exacerbation within a short period of 1 year. The optimal timing of intensified treatment in patients who do not achieve MH remains unclear. Although SES-CD has been used to evaluate small bowel lesions,19 predicting the long-term outcome of CD may not be possible.
Several scores are used to evaluate small bowel lesions in CD, such as the Crohn’s Disease Endoscopic Index of Severity,21 Maria score,22 and Lewis score; however, some are complicated.23 Although the SES-CD is simple to calculate and easy to use in actual clinical practice, the accuracy of ROC analysis of the existing applied SES-CD19 may be low for small bowel evaluation, especially in patients with jejunal lesions. Therefore, in this study, we created a new scoring system including evaluation of the jejunum to identify patients who could require readmission or intensification of treatment within 1 year and evaluated the effectiveness of the score.
As Takenaka et al reported,6 stenosis alone may have little impact on patient prognosis; therefore, this study evaluated stenosis based on whether the endoscope could pass through the lumen. The proportion of patients with CD, including those with small bowel lesions, was 96% (99/103), higher than previously reported.12 The same score may have been used for both small and large bowel lesions when the scoring system was created. In addition, the new MASES-CD can predict the one-year prognosis of patients with CD.
We have shown that MASES-CD is more useful than applied SES-CD for predicting CD exacerbations within 1 year. The stenosis score of the conventional SES-CD may have overestimated the effect of stenosis on the passage of the endoscope because the digested material in the small bowel passes through as a gruel, which has no significant effect on the stenosis. This could be one of the reasons why the area under the curve was higher for the small intestine with a stenosis score of 0/1 for MASES-CD compared with applied SES-CD. On the contrary, the conventional SES-CD may be more effective for the stenosis score in the colorectum to predict the prognosis because fecal material passes through the large intestine. In addition, there have been advances in the balloon dilation technique for small bowel stenosis in DBE and an increase in published guidelines for balloon dilation within the last 10 years.24 The management of small bowel stenosis has been updated, and the stenosis score may not affect the intestinal prognosis compared with other scores. Therefore, we consider MASES-CD useful for patients with active jejunal lesions and the small bowel-dominant type of CD. It was ultimately necessary to determine whether the combined scoring of the small bowel and SES-CD, which we examined in this study, was effective.
This study had some limitations. This was a single-center retrospective study, and the cases were selected from patients who underwent DBE, many of whom had small bowel lesions. However, many patients with CD have small bowel lesions, and we believe there is a need to develop scoring for whole small bowel evaluation.
We also believe that the MASES-CD score used in this study will help identify patients who need enhanced treatment, especially those who can be examined for the entire small bowel. Although an accumulation of cases is necessary, the treatment course of patients with a poor prognosis may be improved by intensifying treatment; this should be studied prospectively in the future.
ACKNOWLEDGMENTS
Author contributions
Conception and design: Tanaka H, Nakamura M, Yamamura T; analysis and interpretation of data: Tanaka H, Nakamura M, Maeda K, Sawada T, Ishikawa E, Furukawa K; drafting of the article: Tanaka H, Nakamura M; statistical analysis: Uchida G, Mizutani Y, Iida T; critical revision of the article for important intellectual content: Yamao K, Ishikawa T, Honda T, Hirose T, Uetsuki K, Ishizu Y; final approval of the article: Kawashima H.
Financial disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Miscellaneous acknowledgments
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
SUPPLEMENTAL INFORMATION
Abbreviations
- SES-CD
Simple Endoscopic Score for Crohn’s Disease
- MASES-CD
modified applied Simple Endoscopic Score for Crohn’s Disease
- CD
Crohn’s disease
- MH
mucosal healing
- SBCE
small bowel capsule endoscopy
- DBE
double-balloon endoscopy
- ROC
receiver operating characteristic
REFERENCES
- 1.Grigor C, Capell H, Stirling A, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet. 2004;364(9430):263–269. doi: 10.1016/S0140-6736(04)16676-2. [DOI] [PubMed]
- 2.Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110(9):1324–1338. doi: 10.1038/ajg.2015.233. [DOI] [PubMed]
- 3.Aggarwal V, Day AS, Connor S, et al. Role of capsule endoscopy and fecal biomarkers in small bowel Crohn’s disease to assess remission and predict relapse. Gastrointest Endosc. 2017;86(6):1070–1078. doi: 10.1016/j.gie.2017.09.011. [DOI] [PubMed]
- 4.Le Berre C, Trang-Poisson C, Bourreille A. Small bowel capsule endoscopy and treat-to-target in Crohn’s disease: a systematic review. World J Gastroenterol. 2019;25(31):4534–4554. doi: 10.3748/wjg.v25.i31.4534. [DOI] [PMC free article] [PubMed]
- 5.Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an Update on the Selecting Therapeutic Targets in inflammatory bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160(5):1570–1583. doi: 10.1053/j.gastro.2020.12.031. [DOI] [PubMed]
- 6.Takenaka K, Ohtsuka K, Kitazume Y, et al. Utility of magnetic resonance enterography for small bowel endoscopic healing in patients with Crohn’s disease. Am J Gastroenterol. 2018;113(2):283–294. doi: 10.1038/ajg.2017.464. [DOI] [PubMed]
- 7.Rutgeerts P, Van Assche G, Sandborn WJ, et al. Adalimumab induces and maintains mucosal healing in patients with Crohn’s disease: data from the EXTEND trial. Gastroenterology. 2012;142(5):1102–1111.e2. doi: 10.1053/j.gastro.2012.01.035. [DOI] [PubMed]
- 8.Cottone M, Papi C, Orlando A. Infliximab, azathioprine or combination therapy in the treatment of active Crohn’s disease. Expert Rev Gastroenterol Hepatol. 2010;4(6):709–712. doi: 10.1586/egh.10.68. [DOI] [PubMed]
- 9.Rutgeerts P, Diamond RH, Bala M, et al. Scheduled maintenance treatment with infliximab is superior to episodic treatment for the healing of mucosal ulceration associated with Crohn’s disease. Gastrointest Endosc. 2006;63(3):433–442;quiz 464. doi: 10.1016/j.gie.2005.08.011. [DOI] [PubMed]
- 10.Rutgeerts P, Gasink C, Chan D, et al. Efficacy of ustekinumab for inducing endoscopic healing in patients with Crohn’s disease. Gastroenterology. 2018;155(4):1045–1058. doi: 10.1053/j.gastro.2018.06.035. [DOI] [PubMed]
- 11.Matsumoto T, Motoya S, Watanabe K, et al. Adalimumab monotherapy and a combination with azathioprine for Crohn’s disease: a prospective, randomized trial. J Crohns Colitis. 2016;10(11):1259–1266. doi: 10.1093/ecco-jcc/jjw152. [DOI] [PubMed]
- 12.Dulai PS, Singh S, Vande Casteele NV, et al. Should we divide Crohn’s disease into ileum-dominant and isolated colonic diseases? Clin Gastroenterol Hepatol. 2019;17(13):2634–2643. doi: 10.1016/j.cgh.2019.04.040. [DOI] [PMC free article] [PubMed]
- 13.Niv Y, Ilani S, Levi Z, et al. Validation of the Capsule Endoscopy Crohn’s disease Activity Index (CECDAI or Niv score): a multicenter prospective study. Endoscopy. 2012;44(1):21–26. doi: 10.1055/s-0031-1291385. [DOI] [PubMed]
- 14.Omori T, Matsumoto T, Hara T, et al. A novel capsule endoscopic score for Crohn’s disease. Crohns Colitis 360. 2020;2(2):otaa040. doi: 10.1093/crocol/otaa040. [DOI] [PMC free article] [PubMed]
- 15.Kopylov U, Seidman EG. Role of capsule endoscopy in inflammatory bowel disease. World J Gastroenterol. 2014;20(5):1155–1164. doi: 10.3748/wjg.v20.i5.1155. [DOI] [PMC free article] [PubMed]
- 16.Yoshimura T, Hirooka Y, Nakamura M, et al. Clinical significance of gastrointestinal patency evaluation by using patency capsule in Crohn’s disease. Nagoya J Med Sci. 2018;80(1):121–128. doi: 10.18999/nagjms.80.1.121. [DOI] [PMC free article] [PubMed]
- 17.Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc. 2004;60(4):505–512. doi: 10.1016/s0016-5107(04)01878-4. [DOI] [PubMed]
- 18.Morise K, Ando T, Watanabe O, et al. Clinical utility of a new endoscopic scoring system for Crohn’s disease. World J Gastroenterol. 2015;21(34):9974–9981. doi: 10.3748/wjg.v21.i34.9974. [DOI] [PMC free article] [PubMed]
- 19.Takenaka K, Ohtsuka K, Kitazume Y, et al. Correlation of the endoscopic and magnetic resonance scoring systems in the deep small intestine in Crohn’s disease. Inflamm Bowel Dis. 2015;21(8):1832–1838. doi: 10.1097/MIB.0000000000000449. [DOI] [PubMed]
- 20.Nakamura M, Yano T, Esaki M, et al. Novel ultrathin double-balloon endoscopy for the diagnosis of small-bowel diseases: a multicenter nonrandomized study. Endoscopy. 2021;53(8):802–814. doi: 10.1055/a-1243-0226. [DOI] [PubMed]
- 21.Mary JY, Modigliani R. Development and validation of an endoscopic index of the severity for Crohn’s disease: a prospective multicentre study. Groupe d’Etudes Thérapeutiques des Affections Inflammatoires du Tube Digestif (GETAID). Gut. 1989;30(7):983–989. doi: 10.1136/gut.30.7.983. [DOI] [PMC free article] [PubMed]
- 22.Buisson A, Pereira B, Goutte M, et al. Magnetic resonance index of activity (MaRIA) and Clermont score are highly and equally effective MRI indices in detecting mucosal healing in Crohn’s disease. Dig Liver Dis. 2017;49(11):1211–1217. doi: 10.1016/j.dld.2017.08.033. [DOI] [PubMed]
- 23.Gralnek IM, Defranchis R, Seidman E, Leighton JA, Legnani P, Lewis BS. Development of a capsule endoscopy scoring index for small bowel mucosal inflammatory change. Aliment Pharmacol Ther. 2008;27(2):146–154. doi: 10.1111/j.1365-2036.2007.03556.x. [DOI] [PubMed]
- 24.Yamamoto H, Yano T, Araki A, et al. Guidelines for endoscopic balloon dilation in treating Crohn’s disease-associated small intestinal strictures (supplement to the Clinical Practice Guidelines for enteroscopy). Dig Endosc. 2022;34(7):1278–1296. doi: 10.1111/den.14429. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.