Abstract
Ultra-high dose-rate (FLASH) radiotherapy serves as an ideal procedure to treat tumors efficiently without harming normal tissues and has demonstrated satisfactory antitumor effects in multiple animal tumor models. However, the biological mechanisms of FLASH radiotherapy have not yet been fully elucidated, and the small number of devices delivering FLASH dose rate has limited its wide application. This review summarizes the possible biological mechanisms and antitumor effects of FLASH radiotherapy, its application in nanotherapeutic strategy, as well as its challenges and future development. Furthermore, some valuable guidance for promoting the progress of FLASH radiotherapy in nanotherapeutic strategies are provided.
Ultra-high dose-rate (FLASH) radiotherapy serves as an ideal procedure to treat tumors efficiently without harming normal tissues and has demonstrated satisfactory antitumor effects in multiple animal tumor models.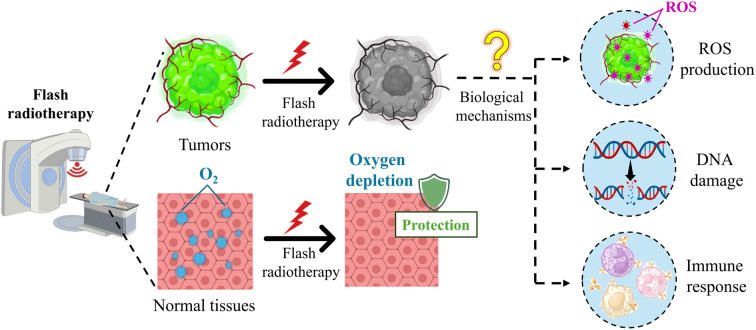
1. Introduction
As one of the foremost clinical treatments for cancer, radiotherapy, either alone or in combination, is necessary for 60–70% of cancer patients during the treatment process.1 Although radiotherapy has been a cornerstone of cancer treatment used across many types of tumors for both curative and palliative purposes, the negative impact on care and quality of life of patients due to the unnecessary acute and late damage to normal tissues while administering high dose of radiotherapy as needed still restricts the further utilization of radiotherapy.2 To overcome this problem, radiation oncology is targeting the application of more precise and safer radiotherapy techniques to simultaneously increase the radiation dose used for irradiating tumor tissues while protecting normal tissues from dose toxicity.3 The two main ways to achieve this goal are as follows: (1) precise deposition of ionizing radiation energy to the tumor sites, thereby limiting its exposure to the surrounding normal tissues, and (2) different biological radiation responses between tumor and normal tissues.4 Researchers have been actively exploring specific solutions via these techniques. With the continuous advancements of modern radiotherapy technology, new radiotherapy methods, such as intensity-modulated radiotherapy,5 stereotactic body radiotherapy,6 and proton- and carbon-particle radiotherapy,7,8 have gradually emerged in the past few decades. Although these emerging radiation modalities could achieve the delivery of radiation with some degree of conformity to the target sites, reducing the radiation-induced toxicity to normal tissues to a certain extent, the therapeutic effect remains relatively limited.9 In particular, when the treatment area overlaps with organs at risk, a significant number of patients still experience severe toxicity during radiation treatment.10 Recently, an unexpected differential effect called the FLASH effect was discovered, which uses high-dose and high-precision radiotherapy to reduce the complications of radiotherapy, increase the tolerance of normal tissues, and ensure the antitumor effects without limited.11
FLASH radiotherapy, an emerging radiotherapy technology, was referred to as the FLASH effect before 2014, which was first reported by Dewey et al.12 In contrast to conventional radiotherapy techniques, which employ a dose rate of ∼0.03 Gy s−1 with a dose of ∼2 Gy and deliver radiation in 10–30 fractions over several weeks, FLASH radiotherapy has a single ultra-high dose rate ≥40 Gy s−1 with a shorter radiation time <200 ms and administers the entire treatment dose in a single FLASH, which is achieved using a linear electron accelerator (LINAC).11,13–16 A 75 year-old individual with multiresistant CD30+ T-cell cutaneous lymphoma, which had disseminated throughout the skin surface, was the first patient treated with FLASH radiotherapy. The prescribed dose for the planned target volume was 15 Gy delivered in 90 milliseconds, and the results have shown promise for both normal skin and tumors, which prompted further clinical evaluation of FLASH radiotherapy.17,18 Recent studies have shown that FLASH radiotherapy could shorten the duration of radiotherapy treatment, deliver a high curative dose, improve the therapeutic potential, and reduce radiation-induced damage in normal tissues.19,20 Considering that FLASH radiotherapy has a unique set of advantages such as instantaneous, ultra-high dose and one-time irradiation, and demonstrates superior antitumor activity in various animal models, it is reasonable to predict that FLASH radiotherapy may become one of the major radiotherapy techniques for future clinical applications.21 However, the biological mechanisms of FLASH radiotherapy are incomplete and inconclusive, making it an immature technology; moreover, the availability of a small number of devices for delivering FLASH dose rates limits its clinical translation.22 It requires more effort to realize the future development of FLASH radiotherapy.
In this review, we summarize the potential biological mechanisms and antitumor effects of FLASH radiotherapy and discuss the application of FLASH radiotherapy in the nanotherapeutic strategy, challenges, and future development. In a word, this review provides some valuable guidance for promoting the progress of FLASH radiotherapy in the nanotherapeutic strategy for cancer treatment.
2. Biological mechanisms
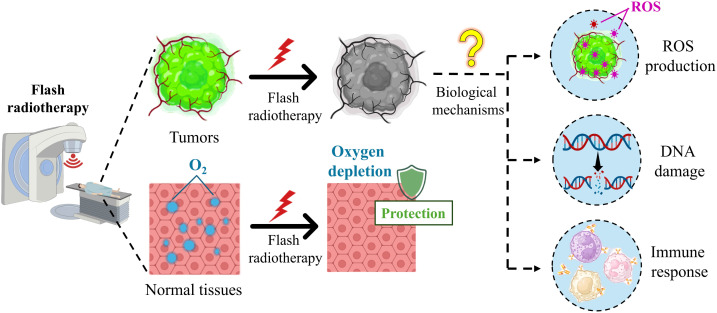
At present, exploring the biological mechanisms of FLASH radiotherapy is an ongoing effort, and has some important findings. For example, preclinical evidence has shown that FLASH radiotherapy and conventional radiotherapy have similar levels of cytotoxicity, DNA damage, and activation of pathways, leading to cell death in tumor tissues,23 while different from conventional radiotherapy, FLASH radiotherapy reduces apoptosis, fibrosis, DNA damage and secretion of inflammatory molecules significantly in various normal tissues.24–26 However, the biological mechanisms are intricate, remain unclear, and are far from conclusive.2,4 The internal causes of these intriguing differential responses are important and challenging questions in the study of biological mechanisms. In the process of exploration, researchers have put forward several hypotheses to explain the FLASH effect, and several most probable hypotheses are under investigation, such as oxygen depletion and reactive oxygen species (ROS), DNA damage, and immune response (Fig. 1).27
Fig. 1. Schematic of the biological mechanisms of FLASH radiotherapy.
2.1. Oxygen depletion and ROS
Oxygen depletion is a popular hypothesis that posits that the difference in oxygen tension between normal and tumor tissues is one of the key factors to understanding FLASH radiation better, clarifying that normal tissues can be protected during FLASH radiotherapy while tumor tissues cannot escape.28 Ultra-high dose rates contribute to oxygen depletion in normal tissues, which induces radio-resistance, meaning that normal tissues around the target are better able to tolerate radiation.29–31 Recent studies have indicated that many normal tissues could maintain cell populations for renewal and regeneration under low physiological oxygen conditions. Ultra-high dose rates of FLASH radiation led to rapid oxygen depletion, mimicking hypoxia and increasing normal cell radiation resistance.32,33 For tumors, due to the presence of a low-oxygen environment, the absorbed dose that triggers the FLASH effect has a negligible effect on the oxygen tension of the cancer cells, and the ultra-high dose rate preserves the tumor-killing ability of the radiation.34
In addition, there are intrinsic differences in the response of normal and tumor tissues to ROS, which lead to FLASH radiotherapy, effectively killing tumors without harming normal tissues in their vicinity.35 Compared with normal cells, tumor cells exhibit altered metabolism including increased rates of glycolysis and pentose phosphate cycle activity. Increased steady-state ROS levels in tumor cells may contribute to differential susceptibility to glucose deprivation-induced cytotoxicity and oxidative stress, and it also probably contributes to differential susceptibility to ionizing radiation.36 Furthermore, Spitz et al. indicated that tumors have higher levels of redox-active iron than that of normal tissues, which results in a difference between their oxidative metabolisms.34,37 This differential recovery of ROS damage hypothesis describes the different abilities of normal and tumor cells to “detoxify” from ROS.30 Compared to conventional radiotherapy, FLASH radiotherapy implanted much more hydroperoxides into tissues, and compared to tumors, normal cells have a higher catalase reduction reserve capacity and a lower oxidant load, thus obtaining a stronger capacity to scavenge peroxides.30,34,37
2.2. DNA damage
The classical target theory suggests that DNA is the primary target of radiation, and as a lethal effect of radiation, unrepaired DNA double-strand breaks are thought to determine the fate of cells.38 The differential response between normal and tumor tissues in FLASH radiotherapy is probably because of the intrinsic factors “yield of DNA damage” and “clustered DNA damage”.2 Compared to tumors, normal tissues recover faster and more precisely from radiation-induced DNA damage, which is the basis of routine hyperfractionation radiotherapy. Ohsawa et al. studied the effect of proton FLASH radiotherapy and conventional radiotherapy on DNA damage. They found that compared with conventional radiotherapy, the single-strand DNA breakage in the FLASH radiotherapy group was reduced significantly, but the double-strand DNA breakage was similar.39,40 In addition, a recent study has showed that the number of DNA damage sites to normal tissues in conventional radiotherapy is more than that of FLASH radiotherapy, whereas tumors are unaffected by the mode of irradiation.24,41 Another study has shown that lower levels of DNA damage were induced after FLASH irradiation, which was modulated by the oxygen tension and increased with the total dose and dose rate of irradiation, indicating that an oxygen related mechanism, such as transient radiation-induced oxygen depletion, may contribute to the tissue sparing effect of FLASH irradiation.42 Several other studies further proved that FLASH radiotherapy leads to fewer dicentric chromosome formations compared to conventional dose rate irradiation and that there is a difference in the G2 cell cycle block after FLASH radiotherapy and conventional radiotherapy.43–45 In fact, the difference between normal and tumor tissues in response to clustered DNA damage remains unclear.
2.3. Immune response
Another popular hypothesis for explaining the FLASH effect involves the role of the immune response and the tumor microenvironment.46 The antitumor effect of radiation therapy can be exerted by immune modulation, but radiation-induced lymphocyte reduction promotes tumor progression, and the patient survival rates were reduced.47 Studies have found that immune responses can be induced by FLASH radiotherapy, followed by the recruitment of T lymphocytes increasing in the tumor microenvironment, improvement of the suppressive tumor immune microenvironment, and reduction of the damage to circulating immune cells under irradiation.48–50 Specifically, inflammatory and immune responses may further promote FLASH effects.51,52 Intrinsic factors may alter the expression and activation of immune factors and immune cells or indirectly influence the immune response in inducing DNA damage or interfering with the microenvironment surrounding exposed tissues.2 Among them, transforming growth factor-β (TGF-β), an important intrinsic factor contributing to radiotherapy resistance and the inflammatory response induced by radiation therapy,53 mediates radiation-induced antitumor responses and regulates ROS production and DNA repair.51 Fouillade et al. investigated the role of immune inflammatory changes in lung injury after FLASH radiotherapy. The results indicated that the expression of the pro-inflammatory factor gene (EGR1) in FLASH radiotherapy and the up-regulation of inflammatory factors (TGF-β1, NF-KB) were lower than conventional radiotherapy.54 Activation of the TGF-β pathway was observed in conventional radiotherapy, and FLASH radiotherapy may have avoided the induction of this pathway, leading to a reduction of ROS and DNA damage. Other studies have also found that FLASH radiotherapy leads to a reduced level of TGF-β;26,55 however, this mechanism remains yet to be evaluated.2,56
3. Antitumor effects of FLASH radiotherapy
Several studies have been published on the effects of FLASH radiotherapy compared with conventional radiotherapy over the past few years. The results have indicated that FLASH radiotherapy exhibits similar or even better therapeutic effects compared to conventional radiotherapy on ovarian cancer, glioblastoma, melanoma, osteosarcoma, Lewis lung carcinoma, etc., and in various species, including mice, cats, dogs, and mini-pigs,23,57–62 which suggest the possible clinical application of FLASH radiotherapy in cancer treatment (Table 1).11,63 For example, Rama et al. found that FLASH radiotherapy recruited more CD3+ T lymphocytes in mouse lung tumor tissues, resulting in significantly smaller tumor volumes in the lungs, and induced more efficient lung-tumor eradication than in the conventional dose rate group.63 FLASH radiotherapy protected the proliferation of intestinal crypt cells and inhibited their fibrosis formation while preserving tumor-killing effects in a mouse pancreatic cancer model.64 Moreover, FLASH radiotherapy has shown similar antitumor effects to conventional radiotherapy in murine models of ID8 ovarian cancer peritoneal metastasis and may be an effective strategy for enhancing the therapeutic index of abdominal radiotherapy, with potential application to metastatic ovarian cancer.23 Furthermore, Vozenin et al. found that single-dose FLASH radiotherapy has shown promise as a novel treatment option for cat patients with locally advanced squamous cell carcinoma of the nasal planum, and the results in cats and pigs provided a strong rationale for further evaluating FLASH radiotherapy in human patients.57 In addition to being clinically tested with good tumor control in animals such as mice, cats, and dogs,57,62,65 the first clinical trial of FLASH radiotherapy for treating patients with cutaneous lymphoma was conducted at the Lausanne University Hospital in Switzerland in 2019, where similar antitumor effects were observed in different treatment groups using FLASH radiotherapy and conventional radiotherapy.66 Although FLASH radiotherapy has shown similar or even better tumor-killing ability as conventional radiotherapy, studies on the killing role and biological mechanism of FLASH effect are limited.
Table 1. Summary of antitumor effects of FLASH radiotherapy.
| Cancer type | Model | Antitumor efficacy of FLASH radiotherapy | References |
|---|---|---|---|
| Lung tumor (LLC cells) | Mice | More efficient lung-tumor eradication compared to the conventional dose rate group | 63 |
| Lung tumor | Mice | Eradicated lung tumors and reduce the occurrence of early and late complications affecting normal tissues | 67 |
| Pancreatic cancer | Mice | Preserve tumor growth inhibition and decrease acute cell loss and late fibrosis | 64 |
| Ovarian cancer (ID8 cells) | Mice | Similar antitumor effects to conventional radiotherapy | 23 |
| Osteosarcoma (LM8 cells) | Mice | In addition to normal tissue sparing, tumor control and a substantial reduction of lung metastasis were observed | 58 |
| Acute lymphoblastic leukemia | Mice | Compared with conventional radiotherapy, reduced functional damage to human blood stem cells with the same therapeutic effect | 59 |
| Melanomas | Mice | Ablated 50% of mouse melanomas, preventing organ metastases and local tumor recurrence for 18 months | 60 |
| Sarcoma | Mice | Decrease radiation damage and favor mechanisms of tissue repair | 61 |
| Squamous cell carcinoma | Cats | Complete tumor remission and all but one cat in each group remained tumor-free throughout the follow-up period | 65 |
| Squamous cell carcinoma | Mini-pigs, cats | Tumor growth was under control after a single dose of FLASH radiotherapy | 57 |
| Oral cancer | Canines | Generally effective but with an elevated risk of high-grade adverse effects | 68 |
| Superficial solid cancers | Canines | Treatments were found to be feasible | 62 |
| Cutaneous lymphoma | Humans | Similar or even better tumor-killing ability as conventional radiotherapy | 66 |
4. FLASH radiotherapy-based nanotherapeutic strategy
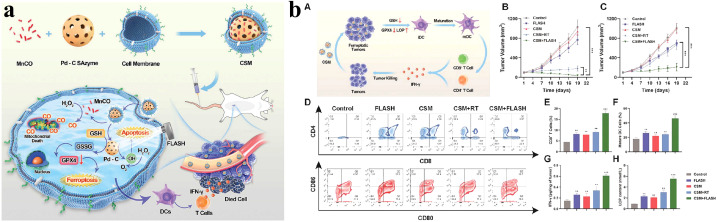
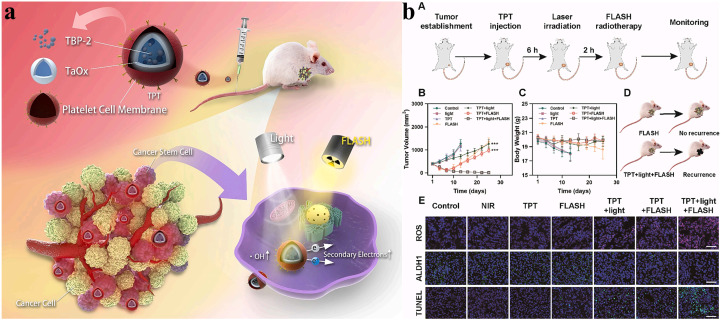
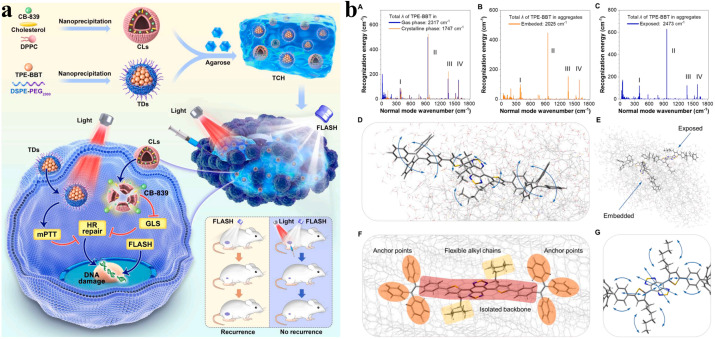
FLASH radiotherapy has shown great potential for tumor treatment, while it also necessitates technological improvement. Recently, nanotechnology has proposed some valuable applications for the widespread use of FLASH radiotherapy. Several studies have applied nanomaterials to FLASH radiotherapy, which has a good therapeutic effect in tumor treatment (Table 2). For example, because tumor self-protection mechanisms limit the therapeutic effect of FLASH radiotherapy, Lyu et al. reported a novel strategy to enhance FLASH radiotherapy through the synergistic effects of Pd–C SAzyme-induced CO gas and GSH depletion therapy (Fig. 2a), involving ferroptosis induced by multipathway and immunotherapy induced by ferroptosis and radiotherapy (Fig. 2b).69 Besides, a critical problem after radiotherapy is that residual cancer stem cells (CSCs) may generate tumor cells, leading to tumor recurrence. The research team further developed TPT NPs, aggregation-induced emission luminogen (AIEgen)-based hollow TaOx nanospheres with platelet cell membrane camouflaged, which specifically target tumor regions and enhance FLASH radiotherapy via promoting the photoelectric effect (Fig. 3a). TPT NPs exhibited a remarkable cancer stem cell-killing effect under FLASH radiotherapy and inhibited tumor recurrence significantly. Furthermore, compared with conventional radiotherapy, TPT NPs combined with FLASH radiotherapy efficiently eliminated tumors while preventing reoccurrence and reducing the severity of complications affecting normal tissues (Fig. 3b).70 To overcome tumor recurrence, an agarose-based thermo-sensitive hydrogel containing a glutaminase inhibitor (CB-839) and a mild photothermal agent (TPE-BBT) was prepared by Shen et al. (Fig. 4a). In the tumor site, CB-839 and TPE-BBT were released from the hydrogel upon 660 nm irradiation for concurrent mild photothermal therapy and chemotherapy, and jointly inhibiting homologous recombination repair of DNA. This work deciphered the unrestricted molecular motions in bright organic fluorophores as a source of photothermy and enhanced FLASH radiotherapy by efficiently killing tumor tissues without recurrence and significant systematic toxicity (Fig. 4b).71
Table 2. Summary of FLASH radiotherapy-based nanotherapeutic strategies.
| Name | Component | Cancer type | Treatment | References |
|---|---|---|---|---|
| CSM | MnCO, Pd–C SAzyme, cell membrane | Breast cancer | FLASH radiotherapy + ferroptosis + immunotherapy | 69 |
| TPT NPs | TBP-2, TaOx, platelet cell membrane | Residual cancer stem cells | FLASH radiotherapy + photodynamic therapy | 70 |
| TCH | TPE-BBT dots, CB-839 liposomes | Colorectal cancer | FLASH radiotherapy + photothermal therapy | 71 |
| TAFL | TPE-BBT, aspirin, fused membrane liposomes | Cancer stem cells | FLASH radiotherapy + photothermal therapy | 72 |
| AuNP-IMQ hydrogel | PCPP, selenocystamine, Sub-5 nm AuNP, IMQ | Melanoma | FLASH radiotherapy + immunotherapy | 73 |
Fig. 2. FLASH radiotherapy-based nanotherapeutic strategy: (a) illustration of the synthesis of CSM radiosensitizers and the mechanism of CSM-mediated tumor therapy. (b) Immunotherapy induced by ferroptosis and radiotherapy in vivo: (A) illustration of immunotherapy induced by CSM, change of (B) primary and (C) distant tumor volume in mice, (D) representative flow cytometry analysis of CD4+ and CD8+ T lymphocyte and dendritic cell (DC) maturation in the lymph nodes, flow cytometric examination of (E) mature DC and (F) CD8+ T cells, ELISA measurements of (G) IFN-γ and (H) lactoperoxidase. Reproduced with permission from ref. 69. Copyright 2023, Wiley-VCH Verlag.
Fig. 3. (a) Synthesis of TPT NPs and application to FLASH radiotherapy enhancement to inhibit tumor recurrence. (b) TPT NP-mediated photodynamic therapy to overcome radioresistance in vivo: (A) schematic illustration of the treatment procedure, (B) tumor volumes, (C) body weights, (D) TPT NP-mediated photodynamic therapy for FLASH irradiation enhancement to avoid tumor recurrence, and (E) TUNEL, ROS, and ALDH1 analyses. Reproduced with permission from ref. 70. Copyright 2023, Elsevier BV.
Fig. 4. (a) Schematic of the preparation and application of TPE-BBT dot (TD) and CB-839 liposome (CL) co-loaded agarose thermosensitive hydrogels for recurrence-resistant tumor therapy. (b) Reorganization analysis and molecular motion modes of TPE-BBT in different environments: (A) gas and crystalline phases, (B) surface part of the simulated aggregate (exposed TPE-BBT) and (C) core part of the simulated aggregate (embedded TPE-BBT), (D) identified low-frequency molecular motion modes of exposed TPE-BBT in simulated aggregates, (E) schematic of two different types of TPE-BBT in the aggregate phase, (F) spatial conformation characteristics of embedded TPE-BBT using the crystalline phase as the model, and (G) identified high-frequency molecular motion modes of embedded TPE-BBT using the crystalline phase as the model. Reproduced with permission from ref. 71. Copyright 2024, Elsevier BV.
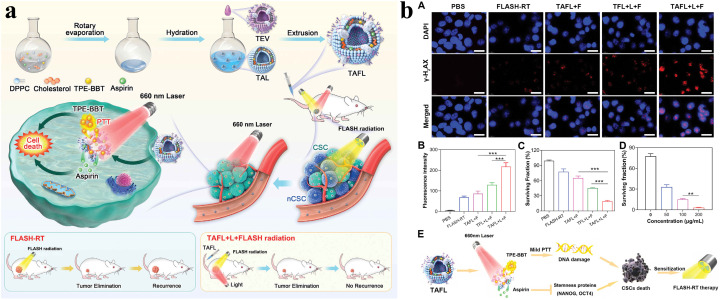
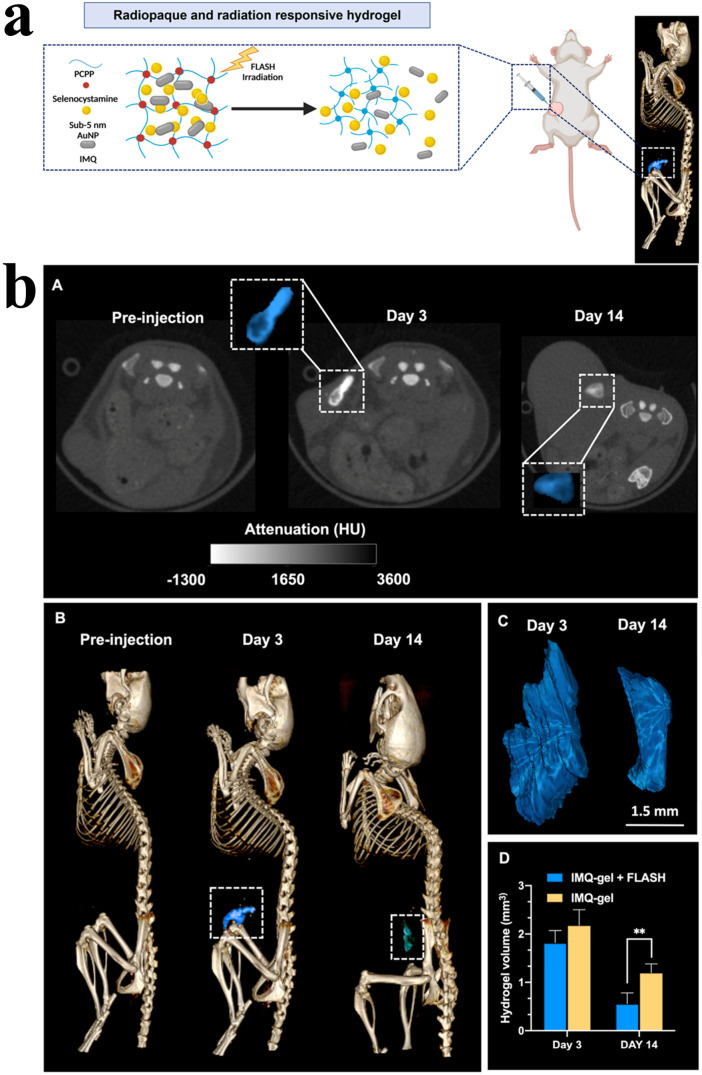
Furthermore, as shown in Fig. 5a and b, Suo et al. developed a biomimetic nanoplatform (TAFL) consisting of fused membrane liposomes loaded with aspirin and aggregation-induced emission photothermal agents (TPE-BBT) for scavenging the existence of intractable CSCs after FLASH radiotherapy. TAFL ruptured under laser irradiation and released aspirin that targeted and eliminated CSCs specifically, and TAFL-mediated mild PTT could improve hypoxic transmission electron microscopy (TEM) and promote DNA damage, thereby further exerting a killing effect on CSCs. The results indicated that the combination therapy inhibited self-renewal and growth of breast CSCs effectively, accomplishing substantial inhibition of tumor recurrence and metastasis after FLASH radiotherapy.72 The effects of combining FLASH radiotherapy and immunotherapy have not been extensively studied in melanoma. Dong et al. reported an integrative therapy combining FLASH radiotherapy with immunotherapy, which suppressed melanoma growth effectively (Fig. 6a). In detail, they developed a radiation response and radiopaque hydrogel loaded with gold nanoparticles (AuNPs) and imiquimod (IMQ), and the hydrogel was imageable with CT via the inclusion of AuNPs and IMQ which was released from the hydrogel for immunomodulatory and tumor cell killing under the effective stimulation of FLASH radiotherapy (Fig. 6b). The findings demonstrated that the combination therapy of AuNP-IMQ-gel and FLASH radiotherapy is a promising treatment method for melanoma and merits further study.73
Fig. 5. (a) Schematic of the AIEgen-based biomimetic nano-cancer stem cell scavenger. (b) Surviving situation of 4T1 CSC cells treated with different formulations: (A) γ-H2AX-stained CSCs treated with different formulations, (B) corresponding γ-H2AX fluorescence intensity after different treatments, (C) surviving fraction of 4T1 CSC cells treated with different formulations, (D) surviving fraction of 4T1 CSC cells treated with different concentrations, and (E) proposed mechanism for combined mild PTT/aspirin based on the TAFL system. Reproduced with permission from ref. 72. Copyright 2024, Wiley-VCH Verlag.
Fig. 6. (a) Illustration of FLASH radiotherapy and immunotherapy delivered by a radiopaque and radiation-responsive hydrogel. (b) In vivo images and hydrogel volume analysis: (A) representative CT images of the AuNP-IMQ hydrogel, insets are enlarged images of injected hydrogels highlighted in blue, (B) representative 3D CT images of a mouse with the hydrogel highlighted in blue, (C) 3D reconstructions of the hydrogels based on CT images in panel (B), and (D) quantification of the hydrogel degradation by comparing the hydrogel volumes. Reproduced with permission from ref. 73. Copyright 2023, the American Chemical Society.
In a word, FLASH radiotherapy-based nanotherapeutic strategies have made progress to some degree, could be combined with other treatments, and have demonstrated therapeutic efficacy in various tumor models, including breast cancer72 and melanoma;73 however, the overall number of studies in this area is too small, and more subsequent research is needed to further explore.
5. Challenges and future development
FLASH radiotherapy, as an innovative and transformative radiation modality, has great potential to achieve dose escalation for enhanced antitumor efficacy while significantly reducing normal tissue complications. However, there remain many obstacles to the clinical application of FLASH radiotherapy. At present, the radiobiological mechanisms remain unclear and far from conclusive, and there are plenty of biological studies requiring efforts of radiobiology and interdisciplinary studies, especially in understanding the underlying mechanisms of the intrinsic difference between the tumor tissues and the surrounding normal tissues in oxygen depletion, DNA damage response and immune response to FLASH radiotherapy or conventional radiotherapy. Besides, radiation therapy devices for performing FLASH radiotherapy are limited, expensive, and require highly specialized technical personnel to operate, which hampers its clinical application. In addition, most of the equipment can be applied only to animal experiments, and the radiotherapeutic field is limited. Therefore, more effort is required to modify the equipment to expand the irradiation range suitable for treating cancer patients.74,75 Another major challenge in the clinical translation of FLASH radiotherapy is the specific physical parameter that can induce the FLASH effect. It has been shown that the magnitude of the average dose rate is not the only determinant of the FLASH effect, and the dose threshold for inducing the FLASH effect varies across different radiation sources and tissues.76,77 In addition to the average dose rate, the total treatment duration, pulse structure (pulse dose, pulse duration, and pulse number), and different energies also influence the FLASH effect.78 Radiation sources capable of producing ultra-high dose rate beams, which are suitable for treating deep-seated and superficial tumors, need to be further explored. In addition, safety is particularly important when exposing such high dose rates. To ensure effective treatment, a comprehensive dose monitoring system, and highly accurate image-guidance equipment are needed to track the target area in real time.18 Finally, the biological diversity in most cancers needs to be considered, and tumors of different origins in different environments may have different responses to the dose rates used in FLASH radiotherapy; therefore, a lot of preliminary basic experiments need to be conducted.79,80
The development of FLASH radiotherapy from ultra-high dose rates to the present evolved over a period of 60 years; from bacteria to cells in vitro, from mice to small animals, and finally to patients, opening up new ways for treating patients. The success of FLASH radiotherapy in treating the first patient in the world has greatly increased investigators' confidence in applying it to patients in clinical settings.18 Research on the biological mechanisms of FLASH radiotherapy and its translational clinical applications are facing many challenges, which are also important opportunities for development and innovation in the field of radiotherapy. We hope to see further research advances in FLASH radiotherapy in the near future.
6. Conclusions
FLASH radiotherapy has progressed from bench to bedside from 1959 to the present and shows great potential in clinical applications. The biological mechanism of FLASH radiotherapy remains unclear, which requires more basic experimental research before it becomes the main radiotherapy technology in cancer therapy. In addition, although limited relevant research literature has been published, nanotechnology needs to be further modified to fit larger fields of FLASH radiotherapy for better treatment benefits. In conclusion, the road of FLASH radiotherapy is tortuous, but the future is bright.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.
Author contributions
Dr Yan Wang and Dr Jie Li have conceptualized the idea, following which Dr Yan Wang, Ms Jiawei Hu, and Ms Jingjing Chai reviewed the literature review and prepared the manuscript. Dr Jiajie Luan, Dr Qingwen Xu, and Ms Huifang Wang supervised the writing process for the review article, and all 7 authors have finalized the manuscript.
Conflicts of interest
The authors declare no conflict of interest, financial or otherwise.
Acknowledgments
This work was supported by the Research Foundation for Talents of Yijishan Hospital of Wannan Medical College [grant no. YR202209, YR20230101]. Health Research Program of Anhui [grant no. AHWJ2022b035]. Anhui Provincial Natural Science Foundation [grant no. 2308085QH243], Key Research Projects in Higher Education of Anhui Province [grant no. 2023AH051753].
References
- Baumann M. Krause M. Overgaard J. Debus J. Bentzen S. M. Daartz J. Richter C. Zips D. Bortfeld T. Radiation oncology in the era of precision medicine. Nat. Rev. Cancer. 2016;16:234–249. doi: 10.1038/nrc.2016.18. [DOI] [PubMed] [Google Scholar]
- Zhou G. Mechanisms underlying FLASH radiotherapy, a novel way to enlarge the differential responses to ionizing radiation between normal and tumor tissues. Radiat. Medicine Prod. 2020;1:35–40. [Google Scholar]
- Abshire D. Lang M. K. The Evolution of Radiation Therapy in Treating Cancer. Semin. Oncol. Nurs. 2018;34:151–157. doi: 10.1016/j.soncn.2018.03.006. [DOI] [PubMed] [Google Scholar]
- Hageman E. Che P. P. Dahele M. Slotman B. J. Sminia P. Radiobiological Aspects of FLASH Radiotherapy. Biomolecules. 2022;12:1376. doi: 10.3390/biom12101376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N. Xia P. Quivey J. M. Sultanem K. Poon I. Akazawa C. Akazawa P. Weinberg V. Fu K. K. Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: an update of the UCSF experience. Int. J. Radiat. Oncol. Biol. Phys. 2002;53:12–22. doi: 10.1016/s0360-3016(02)02724-4. [DOI] [PubMed] [Google Scholar]
- Liu R. Buatti J. M. Howes T. L. Dill J. Modrick J. M. Meeks S. L. Optimal number of beams for stereotactic body radiotherapy of lung and liver lesions. Int. J. Radiat. Oncol. Biol. Phys. 2006;66:906–912. doi: 10.1016/j.ijrobp.2006.05.014. [DOI] [PubMed] [Google Scholar]
- LaRiviere M. J. Santos P. M. G. Hill-Kayser C. E. Metz J. M. Proton Therapy. Hematol./Oncol. Clin. North Am. 2019;33:989–1009. doi: 10.1016/j.hoc.2019.08.006. [DOI] [PubMed] [Google Scholar]
- Kamada T. Tsujii H. Blakely E. A. Debus J. De Neve W. Durante M. Jäkel O. Mayer R. Orecchia R. Pötter R. Vatnitsky S. Chu W. T. Carbon ion radiotherapy in Japan: an assessment of 20 years of clinical experience. Lancet Oncol. 2015;16:e93–e100. doi: 10.1016/S1470-2045(14)70412-7. [DOI] [PubMed] [Google Scholar]
- Bernier J. Hall E. J. Giaccia A. Radiation oncology: a century of achievements. Nat. Rev. Cancer. 2004;4:737–747. doi: 10.1038/nrc1451. [DOI] [PubMed] [Google Scholar]
- Subiel A. Romano F. Recent developments in absolute dosimetry for FLASH radiotherapy. Br. J. Radiol. 2023;96:20220560. doi: 10.1259/bjr.20220560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaudon V. Caplier L. Monceau V. Pouzoulet F. Sayarath M. Fouillade C. Poupon M. F. Brito I. Hupé P. Bourhis J. Hall J. Fontaine J. J. Vozenin M. C. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci. Transl. Med. 2014;6:245ra293. doi: 10.1126/scitranslmed.3008973. [DOI] [PubMed] [Google Scholar]
- Dewey D. Boag J. Modification of the Oxygen Effect When Bacteria Are Given Large Pulses of Radiation. Nature. 1959;183:1450–1451. doi: 10.1038/1831450a0. [DOI] [PubMed] [Google Scholar]
- Bourhis J. Montay-Gruel P. Gonçalves Jorge P. Bailat C. Petit B. Ollivier J. Jeanneret-Sozzi W. Ozsahin M. Bochud F. Moeckli R. Germond J. F. Vozenin M. C. Clinical translation of FLASH radiotherapy: Why and how? Radiother. Oncol. 2019;139:11–17. doi: 10.1016/j.radonc.2019.04.008. [DOI] [PubMed] [Google Scholar]
- Matuszak N. Suchorska W. M. Milecki P. Kruszyna-Mochalska M. Misiarz A. Pracz J. Malicki J. FLASH radiotherapy: an emerging approach in radiation therapy. Rep. Practical Oncol. Radiother. 2022;27:343–351. doi: 10.5603/RPOR.a2022.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow J. C. L. Ruda H. E. Flash Radiotherapy: Innovative Cancer Treatment. Encyclopedia. 2023;3:808–823. [Google Scholar]
- Ashraf M. R. Rahman M. Zhang R. Williams B. B. Gladstone D. J. Pogue B. W. Bruza P. Dosimetry for FLASH Radiotherapy: A Review of Tools and the Role of Radioluminescence and Cherenkov Emission. Front. Phys. 2020;8:00328. [Google Scholar]
- Siddique S. Ruda H. E. Chow J. C. L. FLASH Radiotherapy and the Use of Radiation Dosimeters. Cancers. 2023;15:3883. doi: 10.3390/cancers15153883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourhis J. Sozzi W. J. Jorge P. G. Gaide O. Bailat C. Duclos F. Patin D. Ozsahin M. Bochud F. Germond J. F. Moeckli R. Vozenin M. C. Treatment of a first patient with FLASH-radiotherapy. Radiother. Oncol. 2019;139:18–22. doi: 10.1016/j.radonc.2019.06.019. [DOI] [PubMed] [Google Scholar]
- Loo B. W. Schuler E. Lartey F. M. Rafat M. King G. J. Trovati S. Koong A. C. Maxim P. G. (P003) Delivery of Ultra-Rapid Flash Radiation Therapy and Demonstration of Normal Tissue Sparing After Abdominal Irradiation of Mice. Int. J. Radiat. Oncol. Biol. Phys. 2017;98:E16. [Google Scholar]
- Schüler E. Acharya M. Montay-Gruel P. Loo Jr B. W. Vozenin M. C. Maxim P. G. Ultra-high dose rate electron beams and the FLASH effect: From preclinical evidence to a new radiotherapy paradigm. Med. Phys. 2022;49:2082–2095. doi: 10.1002/mp.15442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B. Gao F. Yang Y. Wu D. Zhang Y. Feng G. Dai T. Du X. FLASH Radiotherapy: History and Future. Front. Oncol. 2021;11:644400. doi: 10.3389/fonc.2021.644400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau M. Mao S. Ngwa U. Yasmin-Karim S. China D. Hooshangnejad H. Sforza D. Ding K. Li H. Rezaee M. Narang A. K. Ngwa W. Democratizing FLASH Radiotherapy. Semin. Radiat. Oncol. 2024;34:344–350. doi: 10.1016/j.semradonc.2024.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy K. Natarajan S. Wang J. Chow S. Eggold J. T. Loo P. E. Manjappa R. Melemenidis S. Lartey F. M. Schüler E. Skinner L. Rafat M. Ko R. Kim A. D H. A.-R. von Eyben R. Dorigo O. Casey K. M. Graves E. E. Bush K. Yu A. S. Koong A. C. Maxim P. G. Loo Jr B. W. Rankin E. B. Abdominal FLASH irradiation reduces radiation-induced gastrointestinal toxicity for the treatment of ovarian cancer in mice. Sci. Rep. 2020;10:21600. doi: 10.1038/s41598-020-78017-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouillade C. Curras-Alonso S. Giuranno L. Quelennec E. Heinrich S. Bonnet-Boissinot S. Beddok A. Leboucher S. Karakurt H. U. Bohec M. Baulande S. Vooijs M. Verrelle P. Dutreix M. Londoño-Vallejo A. Favaudon V. FLASH Irradiation Spares Lung Progenitor Cells and Limits the Incidence of Radio-induced Senescence. Clin. Cancer Res. 2020;26:1497–1506. doi: 10.1158/1078-0432.CCR-19-1440. [DOI] [PubMed] [Google Scholar]
- Perstin A. Poirier Y. Sawant A. Tambasco M. Quantifying the DNA-damaging Effects of FLASH Irradiation With Plasmid DNA. Int. J. Radiat. Oncol. Biol. Phys. 2022;113:437–447. doi: 10.1016/j.ijrobp.2022.01.049. [DOI] [PubMed] [Google Scholar]
- Buonanno M. Grilj V. Brenner D. J. Biological effects in normal cells exposed to FLASH dose rate protons. Radiother. Oncol. 2019;139:51–55. doi: 10.1016/j.radonc.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vozenin M. C. Bourhis J. Durante M. Towards clinical translation of FLASH radiotherapy. Nat. Rev. Clin. Oncol. 2022;19:791–803. doi: 10.1038/s41571-022-00697-z. [DOI] [PubMed] [Google Scholar]
- Durante M. Bräuer-Krisch E. Hill M. Faster and safer? FLASH ultra-high dose rate in radiotherapy. Br. J. Radiol. 2018;91:20170628. doi: 10.1259/bjr.20170628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuszak N. Suchorska W. M. Milecki P. Kruszyna-Mochalska M. Misiarz A. Pracz J. Malicki J. FLASH radiotherapy: an emerging approach in radiation therapy. Rep. Practical Oncol. Radiother. 2022;27:344–351. doi: 10.5603/RPOR.a2022.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaudon V. Labarbe R. Limoli C. L. Model studies of the role of oxygen in the FLASH effect. Med. Phys. 2022;49:2068–2081. doi: 10.1002/mp.15129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon E. J. Petersson K. Olcina M. M. The importance of hypoxia in radiotherapy for the immune response, metastatic potential and FLASH-RT. Int. J. Radiat. Biol. 2022;98:439–451. doi: 10.1080/09553002.2021.1988178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Y. Lv Y. Wang Z. Lan T. Feng X. Chen H. Zhu J. Ma X. Du J. Hou G. Liao W. Yuan K. Wu H. FLASH radiotherapy: A promising new method for radiotherapy. Oncol. Lett. 2022;24:419. doi: 10.3892/ol.2022.13539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow J. C. L. Ruda H. E. Mechanisms of Action in FLASH Radiotherapy: A Comprehensive Review of Physicochemical and Biological Processes on Cancerous and Normal Cells. Cells. 2024;13:835. doi: 10.3390/cells13100835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitz D. R. Buettner G. R. Petronek M. S. St-Aubin J. J. Flynn R. T. Waldron T. J. Limoli C. L. An integrated physico-chemical approach for explaining the differential impact of FLASH versus conventional dose rate irradiation on cancer and normal tissue responses. Radiother. Oncol. 2019;139:23–27. doi: 10.1016/j.radonc.2019.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Weinberg R. A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Aykin-Burns N. Ahmad I. M. Zhu Y. Oberley L. W. Spitz D. R. Increased levels of superoxide and H2O2 mediate the differential susceptibility of cancer cells versus normal cells to glucose deprivation. Biochem. J. 2009;418:29–37. doi: 10.1042/BJ20081258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitz D. R. Buettner G. R. Limoli C. L. Response to letter regarding "An integrated physico-chemical approach for explaining the differential impact of FLASH versus conventional dose rate irradiation on cancer and normal tissue responses". Radiother. Oncol. 2019;139:64–65. doi: 10.1016/j.radonc.2019.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaithamby A. Hu B. Delgado O. Ding L. H. Story M. D. Minna J. D. Shay J. W. Chen D. J. Irreparable complex DNA double-strand breaks induce chromosome breakage in organotypic three-dimensional human lung epithelial cell culture. Nucleic Acids Res. 2011;39:5474–5488. doi: 10.1093/nar/gkr149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B. Huang D. Gao F. Yang Y. Wu D. Zhang Y. Feng G. Dai T. Du X. Mechanisms of FLASH effect. Front. Oncol. 2022;12:995612. doi: 10.3389/fonc.2022.995612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa D. Hiroyama Y. Kobayashi A. Kusumoto T. Kitamura H. Hojo S. Kodaira S. Konishi T. DNA strand break induction of aqueous plasmid DNA exposed to 30 MeV protons at ultra-high dose rate. J. Radiat. Res. 2022;63:255–260. doi: 10.1093/jrr/rrab114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian G. Konradsson E. Beyer S. Wittrup A. Butterworth K. T. McMahon S. J. Ghita M. Petersson K. Ceberg C. Cancer Cells Can Exhibit a Sparing FLASH Effect at Low Doses Under Normoxic In Vitro-Conditions. Front. Oncol. 2021;11:686142. doi: 10.3389/fonc.2021.686142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper C. R. Jones D. Jones G. D. Petersson K. FLASH irradiation induces lower levels of DNA damage ex vivo, an effect modulated by oxygen tension, dose, and dose rate. Br. J. Radiol. 2022;95:20211150. doi: 10.1259/bjr.20211150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid T. E. Dollinger G. Hable V. Greubel C. Zlobinskaya O. Michalski D. Molls M. Röper B. Relative biological effectiveness of pulsed and continuous 20 MeV protons for micronucleus induction in 3D human reconstructed skin tissue. Radiother. Oncol. 2010;95:66–72. doi: 10.1016/j.radonc.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Schmid T. E. Dollinger G. Hable V. Greubel C. Zlobinskaya O. Michalski D. Auer S. Friedl A. A. Schmid E. Molls M. Röper B. The effectiveness of 20 mev protons at nanosecond pulse lengths in producing chromosome aberrations in human-hamster hybrid cells. Radiat. Res. 2011;175:719–727. doi: 10.1667/RR2465.1. [DOI] [PubMed] [Google Scholar]
- Auer S. Hable V. Greubel C. Drexler G. A. Schmid T. E. Belka C. Dollinger G. Friedl A. A. Survival of tumor cells after proton irradiation with ultra-high dose rates. Radiat. Oncol. 2011;6:139. doi: 10.1186/1748-717X-6-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J. Y. Gu A. Wang W. Oleinick N. L. Machtay M. Spring Kong F. M. A potential mechanism for FLASH effect? Radiother. Oncol. 2020;149:55–62. doi: 10.1016/j.radonc.2020.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman S. A. Ye X. Lesser G. Sloan A. Carraway H. Desideri S. Piantadosi S. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin. Cancer Res. 2011;17:5473–5480. doi: 10.1158/1078-0432.CCR-11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.-E. Gwak S.-H. Hong B.-J. Oh J.-M. Choi H.-S. Kim M. S. Oh D. Lartey F. M. Rafat M. Schüler E. Kim H.-S. von Eyben R. Weissman I. L. Koch C. J. Maxim P. G. Loo Jr B. W. Ahn G. O. Effects of Ultra-high doserate FLASH Irradiation on the Tumor Microenvironment in Lewis Lung Carcinoma: Role of Myosin Light Chain. Int. J. Radiat. Oncol. Biol. Phys. 2021;109:1440–1453. doi: 10.1016/j.ijrobp.2020.11.012. [DOI] [PubMed] [Google Scholar]
- Eggold J. T. Chow S. Melemenidis S. Wang J. Natarajan S. Loo P. E. Manjappa R. Viswanathan V. Kidd E. A. Engleman E. Dorigo O. Loo B. W. Rankin E. B. Abdominopelvic FLASH Irradiation Improves PD-1 Immune Checkpoint Inhibition in Preclinical Models of Ovarian Cancer. Mol. Cancer Ther. 2022;21:371–381. doi: 10.1158/1535-7163.MCT-21-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H. Xie D. Wang Y. Huang R. Chen X. Yang Y. Wang B. Peng Y. Wang J. Xiao D. Wu D. Qian C.-N. Deng X. Comparison of intratumor and local immune response between MV X-ray FLASH and conventional radiotherapies. Clin. Transl. Radiat. Oncol. 2023;38:138–146. doi: 10.1016/j.ctro.2022.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J. R. Parsons J. L. FLASH Radiotherapy: Current Knowledge and Future Insights Using Proton-Beam Therapy. Int. J. Mol. Sci. 2020;21:6492. doi: 10.3390/ijms21186492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. D. Hammond E. M. Higgins G. S. Petersson K. Ultra-High Dose Rate (FLASH) Radiotherapy: Silver Bullet or Fool's Gold? Front. Oncol. 2019;9:1563. doi: 10.3389/fonc.2019.01563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arina A. Beckett M. Fernandez C. Zheng W. Pitroda S. Chmura S. J. Luke J. J. Forde M. Hou Y. Burnette B. Mauceri H. Lowy I. Sims T. Khodarev N. Fu Y. X. Weichselbaum R. R. Tumor-reprogrammed resident T cells resist radiation to control tumors. Nat. Commun. 2019;10:3959. doi: 10.1038/s41467-019-11906-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouillade C. Curras-Alonso S. Giuranno L. Quelennec E. Heinrich S. Bonnet-Boissinot S. Beddok A. Leboucher S. Karakurt H. U. Bohec M. Baulande S. Vooijs M. Verrelle P. Dutreix M. Londoño-Vallejo A. Favaudon V. FLASH Irradiation Spares Lung Progenitor Cells and Limits the Incidence of Radio-induced Senescence. Clin. Cancer Res. 2020;26:1497–1506. doi: 10.1158/1078-0432.CCR-19-1440. [DOI] [PubMed] [Google Scholar]
- Simmons D. A. Lartey F. M. Schüler E. Rafat M. King G. Kim A. Ko R. Semaan S. Gonzalez S. Jenkins M. Pradhan P. Shih Z. Wang J. von Eyben R. Graves E. E. Maxim P. G. Longo F. M. Loo Jr B. W. Reduced cognitive deficits after FLASH irradiation of whole mouse brain are associated with less hippocampal dendritic spine loss and neuroinflammation. Radiother. Oncol. 2019;139:4–10. doi: 10.1016/j.radonc.2019.06.006. [DOI] [PubMed] [Google Scholar]
- Lv Y. Lv Y. Wang Z. Lan T. Feng X. Chen H. Zhu J. Ma X. Du J. Hou G. Liao W. Yuan K. Wu H. FLASH radiotherapy: A promising new method for radiotherapy. Oncol. Lett. 2022;24:419. doi: 10.3892/ol.2022.13539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vozenin M.-C. De Fornel P. Petersson K. Favaudon V. Jaccard M. Germond J.-F. Petit B. Burki M. Ferrand G. Patin D. Bouchaab H. Ozsahin M. Bochud F. Bailat C. Devauchelle P. Bourhis J. The Advantage of FLASH Radiotherapy Confirmed in Mini-pig and Cat-cancer Patients. Clin. Cancer Res. 2019;25:35–42. doi: 10.1158/1078-0432.CCR-17-3375. [DOI] [PubMed] [Google Scholar]
- Tinganelli W. Weber U. Puspitasari A. Simoniello P. Abdollahi A. Oppermann J. Schuy C. Horst F. Helm A. Fournier C. Durante M. FLASH with carbon ions: Tumor control, normal tissue sparing, and distal metastasis in a mouse osteosarcoma model. Radiother. Oncol. 2022;175:185–190. doi: 10.1016/j.radonc.2022.05.003. [DOI] [PubMed] [Google Scholar]
- Chabi S. To T. H. V. Leavitt R. Poglio S. Jorge P. G. Jaccard M. Petersson K. Petit B. Roméo P.-H. Pflumio F. Vozenin M.-C. Uzan B. Ultra-high-dose-rate FLASH and Conventional-Dose-Rate Irradiation Differentially Affect Human Acute Lymphoblastic Leukemia and Normal Hematopoiesis. Int. J. Radiat. Oncol., Biol., Phys. 2021;109:819–829. doi: 10.1016/j.ijrobp.2020.10.012. [DOI] [PubMed] [Google Scholar]
- Fernandez-Palomo C. Trappetti V. Potez M. Pellicioli P. Krisch M. Laissue J. Djonov V. Complete Remission of Mouse Melanoma after Temporally Fractionated Microbeam Radiotherapy. Cancers. 2020;12:2656. doi: 10.3390/cancers12092656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velalopoulou A. Karagounis I. V. Cramer G. M. Kim M. M. Skoufos G. Goia D. Hagan S. Verginadis I. I. Shoniyozov K. Chiango J. Cerullo M. Varner K. Yao L. Qin L. Hatzigeorgiou A. G. Minn A. J. Putt M. Lanza M. Assenmacher C.-A. Radaelli E. Huck J. Diffenderfer E. Dong L. Metz J. Koumenis C. Cengel K. A. Maity A. Busch T. M. FLASH Proton Radiotherapy Spares Normal Epithelial and Mesenchymal Tissues While Preserving Sarcoma Response. Cancer Res. 2021;81:4808–4821. doi: 10.1158/0008-5472.CAN-21-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konradsson E. Arendt M. L. Bastholm Jensen K. Børresen B. Hansen A. E. Bäck S. Kristensen A. T. Munck Af Rosenschöld P. Ceberg C. Petersson K. Establishment and Initial Experience of Clinical FLASH Radiotherapy in Canine Cancer Patients. Front. Oncol. 2021;11:658004. doi: 10.3389/fonc.2021.658004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rama N. Saha T. Shukla S. Goda C. Milewski D. Mascia A. E. Vatner R. E. Sengupta D. Katsis A. Abel E. Girdhani S. Miyazaki M. Rodriguez A. Ku A. Dua R. Parry R. Kalin T. V. Improved Tumor Control Through T-cell Infiltration Modulated by Ultra-High Dose Rate Proton FLASH Using a Clinical Pencil Beam Scanning Proton System. Int. J. Radiat. Oncol. Biol. Phys. 2019;105:S164–S165. [Google Scholar]
- Favaudon V. Caplier L. Monceau V. Pouzoulet F. Sayarath M. Fouillade C. Poupon M.-F. Brito I. Hupé P. Bourhis J. Hall J. Fontaine J.-J. Vozenin M.-C. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci. Transl. Med. 2014;6:245ra293. doi: 10.1126/scitranslmed.3008973. [DOI] [PubMed] [Google Scholar]
- Diffenderfer E. S. Verginadis I. I. Kim M. M. Shoniyozov K. Velalopoulou A. Goia D. Putt M. Hagan S. Avery S. Teo K. Zou W. Lin A. Swisher-McClure S. Koch C. Kennedy A. R. Minn A. Maity A. Busch T. M. Dong L. Koumenis C. Metz J. Cengel K. A. Design, Implementation, and in Vivo Validation of a Novel Proton FLASH Radiation Therapy System. Int. J. Radiat. Oncol. Biol. Phys. 2020;106:440–448. doi: 10.1016/j.ijrobp.2019.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer Bley C. Wolf F. Gonçalves Jorge P. Grilj V. Petridis I. Petit B. Böhlen T. T. Moeckli R. Limoli C. Bourhis J. Meier V. Vozenin M.-C. Dose- and Volume-Limiting Late Toxicity of FLASH Radiotherapy in Cats with Squamous Cell Carcinoma of the Nasal Planum and in Mini Pigs. Clin. Cancer Res. 2022;28:3814–3823. doi: 10.1158/1078-0432.CCR-22-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Børresen B. Arendt M. L. Konradsson E. Bastholm Jensen K. Bäck S. Å. Munck af Rosenschöld P. Ceberg C. Petersson K. Evaluation of single-fraction high dose FLASH radiotherapy in a cohort of canine oral cancer patients. Front. Oncol. 2023;13:1256760. doi: 10.3389/fonc.2023.1256760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaide O. Herrera F. Jeanneret Sozzi W. Gonçalves Jorge P. Kinj R. Bailat C. Duclos F. Bochud F. Germond J.-F. Gondré M. Boelhen T. Schiappacasse L. Ozsahin M. Moeckli R. Bourhis J. Comparison of ultra-high versus conventional dose rate radiotherapy in a patient with cutaneous lymphoma. Radiother. Oncol. 2022;174:87–91. doi: 10.1016/j.radonc.2021.12.045. [DOI] [PubMed] [Google Scholar]
- Lyu M. Luo M. Li J. Akakuru O. U. Fan X. Cao Z. Fan K. Jiang W. Personalized Carbon Monoxide-Loaded Biomimetic Single-Atom Nanozyme for Ferroptosis-Enhanced FLASH Radioimmunotherapy. Adv. Funct. Mater. 2023;33:2306930. [Google Scholar]
- Lyu M. Zhang T. Li Y. Xiang J. Zhu D. Xia L. Guo B. Xu Y. Yu H. Tang B. AIEgen-based nanotherapeutic strategy for enhanced FLASH irradiation to prevent tumour recurrence and avoid severe side effects. Chem. Eng. J. 2023;473:145179. [Google Scholar]
- Shen H. Wang H. Mo J. Zhang J. Xu C. Sun F. Ou X. Zhu X. Du L. Ju H. Ye R. Shi G. Kwok R. T. K. Lam J. W. Y. Sun J. Zhang T. Ning S. Tang B. Z. Unrestricted molecular motions enable mild photothermy for recurrence-resistant FLASH antitumor radiotherapy. Bioact. Mater. 2024;37:299–312. doi: 10.1016/j.bioactmat.2024.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo M. Shen H. Lyu M. Jiang Y. Liao X. Tang W. Pan Y. Zhang T. Ning S. Tang B. Z. Biomimetic Nano-Cancer Stem Cell Scavenger for Inhibition of Breast Cancer Recurrence and Metastasis after FLASH-Radiotherapy. Small. 2024:e2400666. doi: 10.1002/smll.202400666. [DOI] [PubMed] [Google Scholar]
- Dong Y. C. Nieves L. M. Hsu J. C. Kumar A. Bouché M. Krishnan U. Mossburg K. J. Saxena D. Uman S. Kambayashi T. Burdick J. A. Kim M. M. Dorsey J. F. Cormode D. P. Novel Combination Treatment for Melanoma: FLASH Radiotherapy and Immunotherapy Delivered by a Radiopaque and Radiation Responsive Hydrogel. Chem. Mater. 2023;35:9542–9551. doi: 10.1021/acs.chemmater.3c01390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeckli R. Gonçalves Jorge P. Grilj V. Oesterle R. Cherbuin N. Bourhis J. Vozenin M.-C. Germond J.-F. Bochud F. Bailat C. Commissioning of an ultra-high dose rate pulsed electron beam medical LINAC for FLASH RT preclinical animal experiments and future clinical human protocols. Med. Phys. 2021;48:3134–3142. doi: 10.1002/mp.14885. [DOI] [PubMed] [Google Scholar]
- Lempart M. Blad B. Adrian G. Bäck S. Knöös T. Ceberg C. Petersson K. Modifying a clinical linear accelerator for delivery of ultra-high dose rate irradiation. Radiother. Oncol. 2019;139:40–45. doi: 10.1016/j.radonc.2019.01.031. [DOI] [PubMed] [Google Scholar]
- Montay-Gruel P. Bouchet A. Jaccard M. Patin D. Serduc R. Aim W. Petersson K. Petit B. Bailat C. Bourhis J. Bräuer-Krisch E. Vozenin M.-C. X-rays can trigger the FLASH effect: Ultra-high dose-rate synchrotron light source prevents normal brain injury after whole brain irradiation in mice. Radiother. Oncol. 2018;129:582–588. doi: 10.1016/j.radonc.2018.08.016. [DOI] [PubMed] [Google Scholar]
- Montay-Gruel P. Petersson K. Jaccard M. Boivin G. Germond J. F. Petit B. Doenlen R. Favaudon V. Bochud F. Bailat C. Bourhis J. Vozenin M. C. Irradiation in a flash: Unique sparing of memory in mice after whole brain irradiation with dose rates above 100Gy/s. Radiother. Oncol. 2017;124:365–369. doi: 10.1016/j.radonc.2017.05.003. [DOI] [PubMed] [Google Scholar]
- Ruan J.-L. Lee C. Wouters S. Tullis I. D. C. Verslegers M. Mysara M. Then C. K. Smart S. C. Hill M. A. Muschel R. J. Giaccia A. J. Vojnovic B. Kiltie A. E. Petersson K. Irradiation at Ultra-High (FLASH) Dose Rates Reduces Acute Normal Tissue Toxicity in the Mouse Gastrointestinal System. Int. J. Radiat. Oncol. Biol. Phys. 2021;111:1250–1261. doi: 10.1016/j.ijrobp.2021.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourhis J. Sozzi W. J. Jorge P. G. Gaide O. Bailat C. Duclos F. Patin D. Ozsahin M. Bochud F. Germond J. F. Moeckli R. Vozenin M. C. Treatment of a first patient with FLASH-radiotherapy. Radiother. Oncol. 2019;139:18–22. doi: 10.1016/j.radonc.2019.06.019. [DOI] [PubMed] [Google Scholar]
- Tang R. Yin J. Liu Y. Xue J. FLASH radiotherapy: A new milestone in the field of cancer radiotherapy. Cancer Lett. 2024;587:216651. doi: 10.1016/j.canlet.2024.216651. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.