Abstract
Objective
Incorporate sleep into a novel lifestyle intervention strategy in adolescents with Emerging symptoms of polycystic ovary syndrome (E-PCOS).
Design
A single-center cohort study.
Setting
University hospital-based clinic for adolescents with PCOS.
Patients
Forty-three girls at an age between 10 and 18 years presenting with E-PCOS between March 2015 and September 2017 with clinical signs of androgen excess and/or accelerated weight gain, acanthosis nigricans, irregular periods, or delayed menarche and followed every 6 months for a minimum of 4 visits, to October 2020.
Interventions
All patients received nutritional counseling, with a goal of “zero weight gain,” daily moderate physical activity goals of 45 minutes per day, and education regarding age-appropriate sleep duration. Three treatment strategies for E-PCOS symptoms were applied depending on the chief clinical complaint: anti-insulin approach with metformin; antiandrogen approach with oral contraceptive and spironolactone; and surveillance.
Main Outcome Measures
Body mass index (BMI) Z-score over time. Alanine Transaminase (ALT) levels as a risk factor for nonalcoholic fatty liver.
Results
Average number of return visits was 4 with 58% having >4 return visits. Testosterone levels were correlated with ALT (r = 0.68). Weeknight sleep duration was less than age-appropriate recommendations for 63% of participants. Sleep midpoint correlated with ALT levels (r = 0.48). Despite the weight-neutral approach, regression models all demonstrated significant weight loss regardless of menarche status, metformin use, number of visits, and high vs. low ALT groups. Those with the latest sleep midpoint at baseline benefited the most, with BMI Z-score dropping significantly (interaction of time and baseline sleep midpoint from the first visit on school night).
Conclusion
A novel approach for adolescent girls with E-PCOS that focuses on metabolic endpoints and includes sleep duration and timing as specific targets, led to significant weight loss irrespective of treatment group.
Key Words: Obesity, puberty, sleep
Exaggerated pubertal weight gain, hirsutism and irregular periods persisting beyond 2 years after menarche are early symptoms of polycystic ovary syndrome (PCOS) (1, 2). As the most frequent cause of infertility in adult women, PCOS is associated with insulin resistance (3, 4), hypertension (5), type 2 diabetes (6), increased risk of heart disease (7), nonalcoholic fatty liver disease (NAFLD) (8), and gynecological cancers (9, 10). Because of the heterogeneity of the disorder, building a consensus on the diagnosis of adult PCOS has been a challenge, revisited several times over the last few decades (3, 11, 12). There is growing awareness that early recognition and management are likely to have the most impact on adverse long-term health outcomes of PCOS, many of them metabolic in nature (13). Obstacles to the timely recognition and management of early PCOS are plentiful (14, 15). First, normative data on progression from menarche to a fully established cycle in adolescents are lacking; second, the morphology of maturing ovaries is in flux at this young age; third, accurate imaging with intravaginal ultrasonic imaging of ovaries is objectionable in adolescent girls; fourth, the cause of irregular periods may be obscured by the early institution of contraceptives; and fifth, hormonal causes of acne, acanthosis nigricans, hirsutism or hair loss are not systematically investigated. One of the most significant advances in understanding the pathogenesis of PCOS over the last 30 years was the demonstration of insulin resistance and altered glucose homeostasis in women affected with PCOS across the weight spectrum (16, 17). Yet none of the various iterations of multidisciplinary consensus about PCOS have included these adverse metabolic characteristics, in part because they are difficult to ascertain in routine clinical care. In addition to prediabetes risk associated with PCOS, NAFLD is also emerging as a potential metabolic complication (8, 18, 19). In recent years the hemoglobin A1C (HbA1C) has gained recognition as readily available metabolic marker of altered glucose homeostasis (20, 21) and alanine transaminase (ALT) threshold has been linked to early evidence of fatty liver disease (22, 23).
In our experience, rapid weight gain in childhood and the peripubertal years is a common characteristic of adolescents presenting with symptoms of PCOS (24). This is often the motivation to seek care, even though lifestyle interventions – traditionally composed of diet and exercise recommendations – are challenging to implement in adolescents and difficult to sustain (2).
In the past decade, suboptimal sleep has emerged as a robust predictor of weight gain and adverse metabolic outcomes (25, 26). During adolescence, there is a natural shift in the circadian biological clock, as the sleep–wake cycle and other circadian-regulated biological processes become more delayed with the onset of puberty (27, 28). Although insufficient sleep duration in youth is associated with weight gain (29, 30) circadian misalignment has also emerged as being associated with obesity and altered glucose in adolescents (31, 32, 33) Moreover, female adolescents may be more vulnerable to the obesogenic effects of circadian misalignment (34). We therefore sought to incorporate sleep into a novel lifestyle intervention strategy that was weight-neutral, with a target to stabilize weight trajectory thereby improving metabolic health.
Methods
This study was approved by the Michigan Medicine Institutional Review Board.
Sleep was characterized in girls 9–18 years of age who were referred to the Michigan Medicine Polycystic Ovary Syndrome Program for Girls and Adolescents between July 2015 and September 2017 and clinically followed upto July 2020. This program evaluates adolescent girls with Emerging symptoms of PCOS (E-PCOS) and offers a lifestyle intervention program aiming at improving metabolic health in their daily lives. Patients were referred to the clinic for concerns of E-PCOS on the basis of clinical signs of androgen excess and/or accelerated weight gain, acanthosis nigricans, and irregular periods.
Clinical data
A physical examination was performed and data from the clinical evaluation collected included body mass index (BMI) Z-score, HbA1c, estradiol, testosterone, liver enzymes ALT, and aspartate aminotransferase (AST). Age and sex-standardized BMI Z-score is a standard deviation score and is calculated on the basis of the Centers for Disease Control and World Health Organization data. It is the preferred measure for research purposes (35). ALT values ≥22 IU/L are associated with nonalcoholic steatohepatitis, a metabolic complication of obesity (36). The precision of the assays is provided in the Online Appendix.
Sleep data
Information about the participant’s sleep duration, sleep time, and wake time were obtained for weekdays and weekends. The midpoint of sleep was also calculated. The sleep midpoint was estimated as the clock time halfway between sleep onset and wake times and is a marker of chronotype (37).
Lifestyle recommendations: stop-the-freight-train
At each nutrition encounter, all patients received counseling that emphasized a weight-neutral, euglycemic approach discouraging starvation strategies and emphasizing food as fuel throughout the day. In addition, all patients were given instructions about sleep hygiene, namely optimal targets for age-appropriate sleep duration on the basis of recommendations from the American Academy of Sleep Medicine (38), as well as daily moderate physical activity of 45 minutes per day. At the first endocrine encounter, the lifestyle instructions were reviewed at the end of the clinic and an interactive discussion occurred to ensure that the recommendations were feasible and realistic within the household structure. At each subsequent encounter the lifestyle goals, including optimal sleep, were rereviewed to ensure patients were meeting identified goals. Controlling a pattern of rapidly increasing weight gain was emphasized with a recommended target of “zero weight gain” over the following 6 months, using the analogy of “stopping the advance of a freight train” in a realistic fashion. This counseling was provided inclusively to all patients, regardless of weight status, and the zero weight gain strategy also applied to those with a normal BMI.
Group allocation
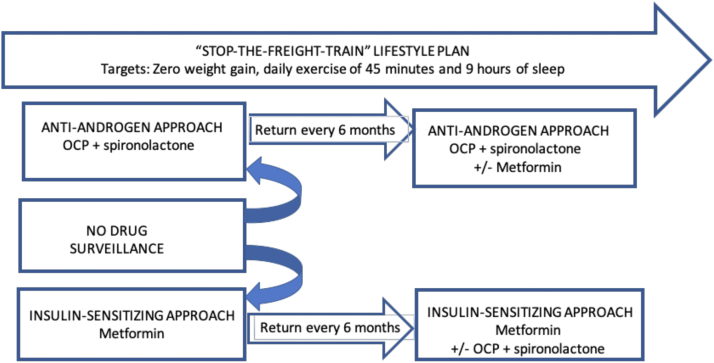
The decision to start any of the following approaches was a result of the patient’s expressed priority, and the clinician’s recommendation on the basis of metabolic and androgenic indices as well as family history of type 2 diabetes or PCOS. Patients who had both metabolic and androgenic features were asked to prioritize the approach that suited their health goals. For patients who opted for the insulin-sensitizing approach, the possibility of progressing to combination therapy once metformin responsiveness was established was discussed. All patients received education about the impact of metabolism on the restoration of the menstrual cycle and potential links to fertility. Reasons to add oral contraceptives and sprinolactone could occur any time after the first or second visit (i.e., 6 or 12 months) and were decided concurrently with the patient. The decision was made taking into account the following factors: persistent and/or progressive hirsuitism and acne; need for contraception; and improved menstrual cyclicity to the satisfaction of the patient. See Figure 1.
Figure 1.
Treatment strategy. Patients were started with 1 of 2 treatment strategies selected and asked to return every 6 months. Patients who were not treated could over time start in of the 2 treatments. The target of antiandrogen treatment was a reduction of grooming frequency and or the stabilization of hair thinning. The target of insulin-sensitizing treatment was the restoration of menstrual cyclicity with titration of metformin dose every 6 months on the basis of patient tolerance and response. A lifestyle plan was presented and discussed at each visit. OCP = oral contraceptive.
Target-focused treatment strategies
Insulin-sensitizing approach
Metformin was prescribed in patients with oligomenorrhea at the starting dose of 500 mg extended-release daily with a target of restoration of the menstrual cycle over time. The initial dose was purposely low to optimize adherence. The dose was titrated in increments every 6 months depending on response and tolerance of the drug, with most patients taking 750 mg extended-release twice a day after 2–4 visits (which spanned approximately 1–2 years).
Antiandrogen approach
Patients who prioritized the antiandrogen approach included those with severe hirsutism; long-standing history of acne resistant to standard dermatological approaches; and androgenic alopecia. Oral contraceptives (OC; combination of 0.15 mg desogestrel and 0.03 mg ethinyl estradiol) and spironolactone (SP; 50 mg twice per day) were initiated with a target of decreasing the frequency of grooming, stabilizing androgen-related hair thinning, decreasing acne and increasing patient satisfaction. Spironolactone was always prescribed in combination with an oral contraceptive and the patients were counseled on the teratogenic risk associated with spironolactone (39).
Combined approach
Over time, the combination of both antiandrogen and insulin-sensitizing approaches was considered on the basis of need, and preferably once the treatment target of the initial approach was reached. Patients starting with an antiandrogen approach would have metformin offered if the HbA1C increased above 6% at any follow-up visit. Patients starting with the insulin-sensitizing approach would have SP and OC added if there were progressive signs of androgen excess.
Surveillance/nonmedical approach
This option was available for patients who were either; within 2 years of menarche; preferred the lifestyle approach alone; or were initially reluctant to start drug therapy.
Statistical analysis
Data were entered into a database and double-checked for accuracy. Comparisons between continuous data were conducted with t tests and comparisons between dichotomous data were conducted with Chi-square analysis. We used mixed models, with random patient intercepts and slopes, to explore the effect that different measures of sleep midpoint had on the trajectory of BMI Z-score, ALT, and AST over time. Models included the time (years) since the first visit, the sleep midpoint, and the interaction of the 2 (this term indicates an impact of sleep midpoint on trajectory of BMI Z-score or liver enzyme). BMI Z-score models did not control for age, but ALT and AST models did. One set of models controlled for the 3-category treatment group; another set of models controlled for whether the subject was on metformin or not. When the P value for the interaction term was ≤10 we explored the nature of the interaction by graphing the predicted dependent variable values at the minimum, median, and maximum value of the sleep parameter.
Results
Forty-three participants completed the sleep questionnaire at their initial visit.
Table 1 describes participant characteristics. In total, 10 (23%) received OC and SP, 21 (49%) received metformin, and 12 (28%) received surveillance only. Supplemental Table 1 (available online) describes the race and the chief complaints or reason for referral. The mean number of clinic visits was 4, with 58% of subjects having >4 visits.
Table 1.
Patient characteristics.
| Characteristic | Minimum | Maximum | Mean/SD |
|---|---|---|---|
| Age (y) | 9.6 | 18.2 | 15.4 ± 1.9 |
| Maternal age at menarche (y) | 9.0 | 16.0 | 12.3 ± 1.9 |
| Birth weight (kg) | 2.5 | 9.1 | 7.2 ± 1.3 |
| HbA1C (%) | 4.7 | 6.1 | 5.4 ± 0.3 |
| Estradiol (mcg/mL) | 26 | 204 | 74.4 ± 46.0 |
| Testosterone (ng/mL) | 23 | 164 | 59.3 ± 31.1 |
| ALT (IU/L) | 8 | 59 | 23.8 ± 10.9 |
| AST (IU/L) | 14 | 41 | 23.0 ± 6.7 |
| Patient’s age at menarche (y) | 9 | 14 | 11.8 ± 1.3 |
| Gynecological age (y) | 0.6 | 9.6 | 4.0 ± 2.0 |
| BMI Z-score | 0.8 | 3.2 | 2.1 ± 0.5 |
ALT = alanine transaminase; AST = aspartate aminotransferase; BMI = body mass index; HbA1C = hemoglobin A1C; SD = standard deviation.
Gestational history was available for 31 mothers with 5 (16%) recalling an abnormal oral glucose tolerance test during the index pregnancy. For participants with available family history, 10 out of 26 fathers (38%) and 8 out of 29 mothers (28%) had prediabetes or diabetes.
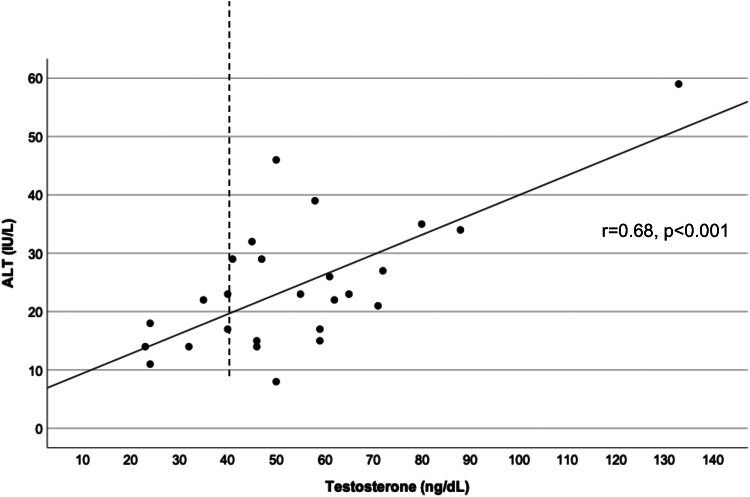
ALT measures ≥22 IU/L were present in 22/38 (58%) of participants. Although HbA1C was the same in the high vs. low ALT groups, testosterone was markedly higher in the high ALT group (Supplemental Table 2). Furthermore, total testosterone positively correlated with ALT values (r = 0.68, P<.001, Supplemental Fig. 1, available online).
Weeknight sleep duration fell short of age-appropriate AASM recommendations in almost two-thirds (63%) of participants. The mean midpoint on school nights was 02:19 AM and on weekends was 05:07 AM. Social jetlag (a difference in sleep midpoint of 2 hours or more) was present in 67% of girls. Sleep midpoint on school nights correlated significantly with ALT level after accounting for BMI Z-score and sleep duration (r = 0.48, P=.006), as shown in Figure 2.
Figure 2.
Correlation between sleep midpoint on a school night and ALT. ALT = alanine transaminase.
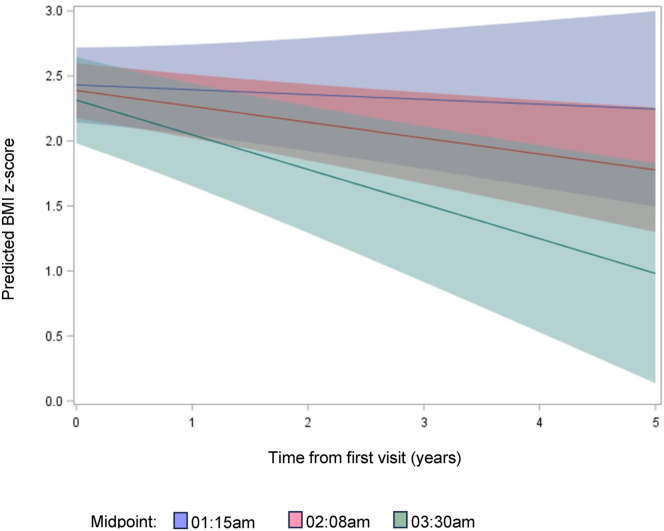
Regression models all demonstrated significant weight loss over time regardless of menarche status, number of visits, and high vs. low ALT groups. Supplemental Table 3 demonstrates BMI Z-score progression over time depending on the baseline sleep midpoint on weekdays. In addition, regardless of treatment category, BMI Z-score significantly reduced over time. Modeling the predicted values for the minimum, median, and maximum sleep midpoint on school nights showed that the girls with the latest sleep midpoint on school nights benefited with the most improved BMI Z-scores over time (Fig. 3; P value for the interaction of time from the first visit and baseline sleep midpoint on school nights was P=.04). Similarly, Supplemental Figure 2 shows that modeling the predicted values for ALT, the interaction between social jetlag (a difference in sleep midpoint >2 hours between weekend and week nights) and time since first visit was significant (P=.02). The girls who had social jetlag at the initial clinic visit demonstrated improvement of ALT over time, compared with girls without social jetlag at the initial visit.
Figure 3.
BMI Z-score progression over time depends on the baseline sleep midpoint on weekdays. BMI = body mass index.
Supplemental Figure 3 shows representative BMI Z-score trajectories after the first “stop-the-freight-train” clinic encounter.
Our data demonstrated a decrease in BMI Z-score, with girls serving as their own controls as we compared BMI Z-score over time. The most conservative course is keeping the same BMI Z-score over time, which means an increase in BMI and represents the expected trajectory. For any adolescent, keeping the same BMI over time means a reduction in the BMI Z-score. Supplemental Figure 4 illustrates a BMI-for-Age Centile chart (40) which shows a normal weight gain trajectory (blue), accelerated weight gain (orange), and a typical trajectory observed in the current study (green). Note that there is a positive incline of the BMI curve in late adolescence so a slightly higher BMI can correspond with a lower BMI Z-score as the child gets older. To put this into context, we will provide some examples:
Patient A aged 15 years and 5 months at the initial visit was 5′8″ in height and weighed 215 lbs (BMI 32.9) and had a BMI Z-score of 2.05. She had amenorrhea and menarche at the age of 10 years. She received metformin 500 mg two times per day at the first visit. Her BMI Z-score fell to 1.94 after 6 months, 1.6 after 12 months, and 1.24 after 18 months. At the 18-month visit, her weight was 179 lbs (no change in height) with a BMI of 27.0. This reduction in BMI Z-score from 2.05 to 1.24 corresponded to a weight loss of 36 lbs.
Patient B aged 12 years and 6 months at the initial visit was 5′7″ in height and weighed 191 lbs (BMI 29.8) and had a BMI Z-score of 2.08. She had menarche at the age of 11 years with 3 periods reported and had severe acanthosis. She received metformin 500 mg once per day initially. Her BMI Z-score fell to 1.99 at 6 months, 1.92 at 12 months, 1.8 at 18 months, and 1.55 at 24 months. She grew 0.5″. The change in BMI Z-score from 2.08 to 1.55 represented a weight loss of 16 lbs.
Patient C aged 17 years 5 months at the initial visit was 5′2″ in height and weighed 157 lbs (BMI 29.0) and had a BMI Z-score of 1.56. She had menarche at the age of 11 years and presented with concern for acne and irregular periods. She was prescribed spironolactone and birth control pills. Her BMI Z-score fell from 1.35 after 6 months to 0.79 after 12 months with no change in height. At 12 months her weight was 140 lbs. This change in BMI Z-score (from 1.56 to 0.79) represented a weight loss of 17 lbs. Notably, she was gaining weight before attending the clinic.
Patient D aged 16 years 11 months at the initial visit was 5′1.5″ in height and weighed 138 lbs (BMI 25.3) and had a BMI Z-score of 1.05. She had oligomenorrhea with only spotting and a primary concern of hirsutism. She received spironolactone and birth control pills. Her BMI Z-score fell to 0.65 after 6 months, stayed at 0.65 for the next 2 visits, and then fell to 0.62 by 24 months. Without any change in height, her weight fell to 127 lbs. This change in BMI Z-score from 1.05 to 0.62 represented a change in weight of 11 lbs.
Discussion
These data demonstrate that poorly timed sleep is associated with elevated ALT measures in adolescent girls with emerging PCOS. Furthermore, despite the E-PCOS clinic having a “weight-neutral” approach, girls with the latest sleep midpoints on school nights at initial clinic visits showed the greatest improvement in BMI Z-score over time. These findings provide proof of concept and suggest that, in contrast to a focus on rapid weight loss and medical interventions, optimization of sleep may provide a cost-effective avenue to normalize ALT and stabilize or improve BMI in this population.
Consensus criteria for established PCOS-hyperandrogenism, ovulatory dysfunction, and polycystic ovaries, have been proposed as the blueprint for the diagnosis of this condition but fall short for young patients who have not yet completed their pubertal maturation and are already exhibiting progressive symptoms concerning PCOS. Recognition of a heterogeneous disorder like PCOS in adolescents is a challenge because of the evolving nature of puberty progression and the time that needs to elapse to determine whether there are enough criteria to diagnose PCOS. It has been recently recognized that young patients may not yet fulfill the criteria for PCOS but still need to have their symptoms addressed and treated (41, 42, 43). We are introducing the concept of E-PCOS in which at least 2 of the following symptoms are present in a peripubertal girl: failure to establish a regular menstrual cycle 2 years or more postmenarche and clinical or biochemical evidence of hirsutism or androgen excess with or without rapidly progressive obesity. The lack of menarche in 8 of the participants illustrates the dilemma of the pediatrician as some adolescents experience the deleterious effect of androgen excess early enough at the outset of the puberty process that it may delay the onset of menarche (2). This becomes evident if there is absent menarche when the patient has reached final height as documented by a bone age or by evidence of final height attainment on the growth chart.
Weight-neutral strategy – “stop-the-freight-train”
Our metabolic approach was to focus on stopping the weight gain thus the “freight train” analogy. This concept emerged from the abundant published data (44) and our own clinical experience regarding the lack of sustainable results of weight loss strategies in youth and the inevitable rebound following restrictive diets or newer weight loss drugs (45). It was emphasized from the outset that the strategy’s goal was weight-neutral to minimize the psychological pressure associated with weight loss in a vulnerable population of girls at risk for eating disorders. Despite being told that weight loss was not a priority, obese girls unexpectedly lost weight by the first follow-up visit at 6 months and this persisted over time. It is possible that removing the pressure of weight loss and shifting the focus to specific sleep targets relating to age-appropriate sleep duration and timing facilitated the implementation of nutritional and lifestyle counseling, but this hypothesis remains to be formally tested.
Insulin resistance as a target
The consensus about the diagnosis of PCOS omits the presence of well-demonstrated insulin resistance yet standard of care includes the use of metformin, an insulin sensitizer. We believe this omission has delayed the development of treatment paradigms that could be applied to adolescents. We chose to address insulin resistance by explaining its role in detail to all patients and by providing simple lifestyle interventions targeting sleep, exercise, and diet in ways easily applicable to a daily routine. The use of metformin was modest as the doses were initiated at a low level to address the high likelihood of undesirable gastrointestinal side effects, particularly in youth (46, 47). Adjustments were made every 6 months depending on tolerance.
Trials aimed at improving metabolic outcomes related to BMI in obese children and adolescents using a combination of medications and lifestyle interventions have shown little improvement in insulin or glucose measures after 6–12 months (48). A systematic evidence review for the US Preventive Services Task Force demonstrated a reduction in BMI Z-score of –0.1 from 6 pooled studies of metformin and lifestyle intervention (48), which is less than the reduction observed in our study. A recent trial of a standard diet and exercise intervention for prepubertal children with obesity has shown an improvement in both markers of insulin resistance as well as ALT levels in children who lowered their BMI Z-score (49). ALT is a surrogate marker for NAFLD, which affects approximately 20% of adolescents aged 15–19 years (50). Those with increased ALT levels have a higher prevalence of prediabetes and type 2 diabetes mellitus compared with children with normal ALT levels (51). The presence of PCOS has been found to increase the risk for NAFLD, (19), particularly when type 2 diabetes is present (52). Thus, lowering of ALT levels may be an acceptable marker to assess the treatment response of interventions designed to reduce metabolic complications of obesity, particularly since fluctuations of ALT levels even within normal ranges appear to indicate a risk of cardiovascular disease (53). Of note, in the current study, later sleep midpoint was positively correlated with ALT level even after accounting for BMI Z-score and subjects with social jetlag at the initial clinic visit demonstrated improvement of ALT over time, compared with girls without social jetlag.
More recently the advent of glucagon-like peptide-1 agonists as weight loss-inducing tools available to the pediatric population has highlighted significant side effects, some severe, associated with long-term use (54, 55). In addition, concerns have been recently raised about the potential and unintended long-term consequences of their use in youth (56). There is also mounting evidence that discontinuation of glucagon-like peptide-1 agonists leads to weight regain within a short period (45, 57). The weight changes observed in our participants were unexpected and provided the possibility of sustainable weight loss in adolescents with a weight-neutral approach targeting lifestyle endpoints that may have been previously underestimated, such as sleep.
Sleep as a lifestyle target
There is increasing evidence of a link between insufficient sleep and an increase in BMI in adolescents (58, 59), possibly due to an increase in poor dietary habits (60) via alterations in leptin and ghrelin (61). Sleep problems including insufficient sleep and shift work are known to contribute to metabolic diseases (62). Indeed, shift work is the extreme form of social jetlag, thus emerging evidence for metabolic consequences of circadian misalignment (63, 64, 65) is particularly pertinent to adolescents who have a biological circadian delay (27). Social jetlag quantifies the difference between circadian and social clocks which results in chronic sleep loss (66, 67). A large epidemiological study of >65,000 participants aged 10–65 years demonstrated that even beyond sleep duration, social jetlag is associated with increased BMI, particularly in young adults (68) The authors purported that living “against the clock” could contribute to the obesity epidemic.
Moreover, circadian misalignment has emerged as a strong predictor of metabolic problems (33, 34, 63, 69, 70). Adolescents with ≥1 hour below age-appropriate sleep duration recommendations or who had later sleep midpoints than their peers have been shown to have a 2.7 and 2.6 increased odds respectively of developing insulin resistance over the next 2 years (71). Moreover, in adolescents with circadian misalignment, including greater social jetlag, visceral adiposity has a larger impact on the metabolic syndrome than in adolescents without circadian misalignment, independent of other factors (31). Importantly, a sleep hygiene intervention in adolescents significantly reduced BMI Z-score despite no change in sleep duration (72), suggesting that addressing sleep health could help improve BMI. Furthermore, in obese adolescents with and without PCOS, those with PCOS had circadian misalignment – measured via melatonin – which was associated with higher free testosterone levels and worse insulin sensitivity (73). Given these data, it would seem pertinent to include sleep health when assessing obese adolescents with suspected PCOS with a goal of weight reduction and/or cardiometabolic improvement (24, 74). Nonetheless, the most recent (2023) guidelines for PCOS, while screening for sleep-disordered breathing is now recommended but there is no mention of other important measures of sleep (75).
The strengths of this study include a real-world analysis of girls with E-PCOS, with an extended follow-up. The weight-neutral approach that was emphasized provides a counterpoint to the current landscape of pharmacological weight-loss strategies and bears promise for sustainable intervention beyond weight-loss-inducing medications. This study also offers insights into additional endpoints, such as liver enzymes, as potential targets in the management of girls and young women with PCOS. Moreover, sleep characterization at baseline allowed for an additional opportunity in the management of their metabolic health.
This study is not without limitations. Sleep information was only obtained at the initial visit, thereby limiting our ability to assess longitudinal changes. In our clinic, the recommended follow-up is every 6 months, however, this was not universal due to expected variations in patient scheduling preferences.
Conclusion
A novel approach for adolescent girls with E-PCOS that focuses on metabolic endpoints and includes sleep duration and timing as specific targets, led to unexpected yet significant weight loss over time. We believe that the explicit addition of sleep duration and timing as a beneficial metabolic target likely contributed to the observed weight loss. Further studies are needed to tailor the management of adolescent girls with emerging symptoms of PCOS and identify motivational strategies to thwart adverse metabolic and reproductive consequences.
CRediT Authorship Contribution Statement
Josephine Z. Kasa-Vubu: Conceptualization, Methodology, Investigation, Resources, Writing – original draft, Writing – review editing. Alexandra Waisanen: Investigation, Writing – original draft, Writing – review & editing. Julie Sturza: Formal analysis, Writing – original draft, Writing – review editing. Vasantha Padmanabhan: Writing – original draft, Writing – review & editing. Louise M. O’Brien: Conceptualization, Methodology, Investigation, Formal analysis, Data curation, Writing – original draft, Writing – review & editing.
Declaration of Interests
J.Z.K.-V. has nothing to disclose. A.W. has nothing to disclose. J.S. has nothing to disclose. V.P. has nothing to disclose. L.M.O. reports receiving funding from the National Heart, Lung, and Blood Institute (R61/33 HL151952), National Institute of Child Health and Human Development (SBIR R43HD111096 and STTR R41HD114317), National Institute of Mental Health (R01MH121531 and R34MH130562), and from the Star Legacy Foundation and honoraria for attending National Institutes of Health study section meetings; an advisory board role at the Star Legacy Foundation; and receiving equipment from Itamar, Ltd., Smart Human Dynamics Inc., and Arcascope Inc.
Supplementary Data
References
- 1.Witchel S.F., Burghard A.C., Tao R.H., Oberfield S.E. The diagnosis and treatment of PCOS in adolescents: an update. Curr Opin Pediatr. 2019;31:562–569. doi: 10.1097/MOP.0000000000000778. [DOI] [PubMed] [Google Scholar]
- 2.Ibanez L., de Zegher F. Adolescent PCOS: a postpubertal central obesity syndrome. Trends Mol Med. 2023;29:354–363. doi: 10.1016/j.molmed.2023.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Joham A.E., Norman R.J., Stener-Victorin E., Legro R.S., Franks S., Moran L.J., et al. Polycystic ovary syndrome. Lancet Diabetes Endocrinol. 2022;10:668–680. doi: 10.1016/S2213-8587(22)00163-2. [DOI] [PubMed] [Google Scholar]
- 4.Xu Y., Qiao J. Association of insulin resistance and elevated androgen levels with polycystic ovarian syndrome (PCOS): a review of literature. J Healthc Eng. 2022;2022 doi: 10.1155/2022/9240569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wekker V., van Dammen L., Koning A., Heida K.Y., Painter R.C., Limpens J., et al. Long-term cardiometabolic disease risk in women with PCOS: a systematic review and meta-analysis. Hum Reprod Update. 2020;26:942–960. doi: 10.1093/humupd/dmaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ollila M.M., West S., Keinanen-Kiukaaniemi S., Jokelainen J., Auvinen J., Puukka K., et al. Overweight and obese but not normal weight women with PCOS are at increased risk of type 2 diabetes mellitus-a prospective population-based cohort study. Hum Reprod. 2017;32:968. doi: 10.1093/humrep/dex030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dokras A. Heart health in polycystic ovary syndrome: time to act on the data. Fertil Steril. 2022;117:885–886. doi: 10.1016/j.fertnstert.2022.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Shengir M., Chen T., Guadagno E., Ramanakumar A.V., Ghali P., Deschenes M., et al. Non-alcoholic fatty liver disease in premenopausal women with polycystic ovary syndrome: a systematic review and meta-analysis. JGH Open. 2021;5:434–445. doi: 10.1002/jgh3.12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shetty C., Rizvi S., Sharaf J., Williams K.D., Tariq M., Acharekar M.V., et al. Risk of gynecological cancers in women with polycystic ovary syndrome and the pathophysiology of association. Cureus. 2023;15 doi: 10.7759/cureus.37266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amiri M., Bidhendi-Yarandi R., Fallahzadeh A., Marzban Z., Ramezani Tehrani F. Risk of endometrial, ovarian, and breast cancers in women with polycystic ovary syndrome: a systematic review and meta-analysis. Int J Reprod Biomed. 2022;20:893–914. doi: 10.18502/ijrm.v20i11.12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azziz R. PCOS: a diagnostic challenge. Reprod Biomed Online. 2004;8:644–648. doi: 10.1016/s1472-6483(10)61644-6. [DOI] [PubMed] [Google Scholar]
- 12.Hoeger K.M., Dokras A., Piltonen T. Update on PCOS: consequences, challenges, and guiding treatment. J Clin Endocrinol Metab. 2021;106:e1071–e1083. doi: 10.1210/clinem/dgaa839. [DOI] [PubMed] [Google Scholar]
- 13.Futterweit W. Polycystic ovary syndrome: a common reproductive and metabolic disorder necessitating early recognition and treatment. Prim Care. 2007;34:761–789. doi: 10.1016/j.pop.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Manique M.E.S., Ferreira A. Polycystic ovary syndrome in adolescence: challenges in diagnosis and management. Rev Bras Ginecol Obstet. 2022;44:425–433. doi: 10.1055/s-0042-1742292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicandri K.F., Hoeger K. Diagnosis and treatment of polycystic ovarian syndrome in adolescents. Curr Opin Endocrinol Diabetes Obes. 2012;19:497–504. doi: 10.1097/MED.0b013e32835a1a03. [DOI] [PubMed] [Google Scholar]
- 16.Flannery C.A., Rackow B., Cong X., Duran E., Selen D.J., Burgert T.S. Polycystic ovary syndrome in adolescence: impaired glucose tolerance occurs across the spectrum of BMI. Pediatr Diabetes. 2013;14:42–49. doi: 10.1111/j.1399-5448.2012.00902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livadas S., Kollias A., Panidis D., Diamanti-Kandarakis E. Diverse impacts of aging on insulin resistance in lean and obese women with polycystic ovary syndrome: evidence from 1345 women with the syndrome. Eur J Endocrinol. 2014;171:301–309. doi: 10.1530/EJE-13-1007. [DOI] [PubMed] [Google Scholar]
- 18.Falzarano C., Lofton T., Osei-Ntansah A., Oliver T., Southward T., Stewart S., et al. Nonalcoholic fatty liver disease in women and girls with polycystic ovary syndrome. J Clin Endocrinol Metab. 2022;107:258–272. doi: 10.1210/clinem/dgab658. [DOI] [PubMed] [Google Scholar]
- 19.Spremovic Radenovic S., Pupovac M., Andjic M., Bila J., Sreckovic S., Gudovic A., et al. Prevalence, risk factors, and pathophysiology of nonalcoholic fatty liver disease (NAFLD) in women with polycystic ovary syndrome (PCOS) Biomedicines. 2022;10:131. doi: 10.3390/biomedicines10010131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enomoto H., Bando Y., Nakamura H., Nishiguchi S., Koga M. Liver fibrosis markers of nonalcoholic steatohepatitis. World J Gastroenterol. 2015;21:7427–7435. doi: 10.3748/wjg.v21.i24.7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kojic Damjanov S., Deric M., Eremic Kojic N. Glycated hemoglobin A1c as a modern biochemical marker of glucose regulation. Med Pregl. 2014;67:339–344. [PubMed] [Google Scholar]
- 22.Qiu J., Kuang M., He S., Yu C., Wang C., Huang X., et al. Gender perspective on the association between liver enzyme markers and non-alcoholic fatty liver disease: insights from the general population. Front Endocrinol (Lausanne) 2023;14 doi: 10.3389/fendo.2023.1302322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stender S., Davey Smith G., Richardson T.G. Genetic variation and elevated liver enzymes during childhood, adolescence and early adulthood. Int J Epidemiol. 2023;52:1341–1349. doi: 10.1093/ije/dyad048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore J.M., Waldrop S.W., Cree-Green M. Weight management in adolescents with polycystic ovary syndrome. Curr Obes Rep. 2021;10:311–321. doi: 10.1007/s13679-021-00437-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall W.L. The emerging importance of tackling sleep-diet interactions in lifestyle interventions for weight management. Br J Nutr. 2022;128:561–568. doi: 10.1017/S000711452200160X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackowska M., Steptoe A. Sleep and future cardiovascular risk: prospective analysis from the English Longitudinal Study of Ageing. Sleep Med. 2015;16:768–774. doi: 10.1016/j.sleep.2015.02.530. [DOI] [PubMed] [Google Scholar]
- 27.Crowley S.J., Cain S.W., Burns A.C., Acebo C., Carskadon M.A. Increased sensitivity of the circadian system to light in early/mid-puberty. J Clin Endocrinol Metab. 2015;100:4067–4073. doi: 10.1210/jc.2015-2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carskadon M.A. Sleep in adolescents: the perfect storm. Pediatr Clin North Am. 2011;58:637–647. doi: 10.1016/j.pcl.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song Y., Gong L., Lou X., Zhou H., Hao Y., Chen Q., et al. Sleep-body composition relationship: roles of sleep behaviors in general and abdominal obesity in Chinese adolescents aged 17–22 years. Nutrients. 2023;15:4130. doi: 10.3390/nu15194130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grimaldi M., Bacaro V., Natale V., Tonetti L., Crocetti E. The longitudinal interplay between sleep, anthropometric indices, eating behaviors, and nutritional aspects: a systematic review and meta-analysis. Nutrients. 2023;15:3179. doi: 10.3390/nu15143179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morales-Ghinaglia N., He F., Calhoun S.L., Vgontzas A.N., Liao J., Liao D., et al. Circadian misalignment impacts the association of visceral adiposity with metabolic syndrome in adolescents. Sleep. 2024;47 doi: 10.1093/sleep/zsad262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li T., Xie Y., Tao S., Zou L., Yang Y., Tao F., et al. Prospective study of the association between chronotype and cardiometabolic risk among Chinese young adults. BMC Public Health. 2023;23:1966. doi: 10.1186/s12889-023-16902-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simon S.L., Behn C.D., Cree-Green M., Kaar J.L., Pyle L., Hawkins S.M.M., et al. Too late and not enough: school year sleep duration, timing, and circadian misalignment are associated with reduced insulin sensitivity in adolescents with overweight/obesity. J Pediatr. 2019;205:257–264.e1. doi: 10.1016/j.jpeds.2018.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cespedes Feliciano E.M., Rifas-Shiman S.L., Quante M., Redline S., Oken E., Taveras E.M. Chronotype, social jet lag, and cardiometabolic risk factors in early adolescence. JAMA Pediatr. 2019;173:1049–1057. doi: 10.1001/jamapediatrics.2019.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson L.N., Carsley S., Lebovic G., Borkhoff C.M., Maguire J.L., Parkin P.C., et al. Misclassification of child body mass index from cut-points defined by rounded percentiles instead of Z-scores. BMC Res Notes. 2017;10:639. doi: 10.1186/s13104-017-2983-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vos M.B., Abrams S.H., Barlow S.E., Caprio S., Daniels S.R., Kohli R., et al. NASPGHAN clinical practice guideline for the diagnosis and treatment of nonalcoholic fatty liver disease in children: recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) J Pediatr Gastroenterol Nutr. 2017;64:319–334. doi: 10.1097/MPG.0000000000001482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roenneberg T., Pilz L.K., Zerbini G., Winnebeck E.C. Chronotype and social jetlag: a (self-)critical review. Biology (Basel) 2019;8:54. doi: 10.3390/biology8030054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paruthi S., Brooks L.J., D'Ambrosio C., Hall W.A., Kotagal S., Lloyd R.M., et al. Consensus statement of the American Academy of Sleep Medicine on the recommended amount of sleep for healthy children: methodology and discussion. J Clin Sleep Med. 2016;12:1549–1561. doi: 10.5664/jcsm.6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zouboulis C.C., Chen W.C., Thornton M.J., Qin K., Rosenfield R. Sexual hormones in human skin. Horm Metab Res. 2007;39:85–95. doi: 10.1055/s-2007-961807. [DOI] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention NCfHS CDC growth charts: United States; 2000. http://www.cdc.gov/growthcharts/clinical_charts.htm Available at:
- 41.Witchel S.F., Oberfield S.E., Pena A.S. Polycystic ovary syndrome: pathophysiology, presentation, and treatment with emphasis on adolescent girls. J Endocr Soc. 2019;3:1545–1573. doi: 10.1210/js.2019-00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DiVall S.A. Practical considerations for diagnosis and treatment of polycystic ovary syndrome in adolescence – distilling guidelines into clinical practice. Curr Opin Pediatr. 2023;35:494–499. doi: 10.1097/MOP.0000000000001255. [DOI] [PubMed] [Google Scholar]
- 43.Meczekalski B., Niwczyk O., Kostrzak A., Maciejewska-Jeske M., Bala G., Szeliga A. PCOS in adolescents-ongoing riddles in diagnosis and treatment. J Clin Med. 2023;12:1221. doi: 10.3390/jcm12031221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hampl S.E., Hassink S.G., Skinner A.C., Armstrong S.C., Barlow S.E., Bolling C.F., et al. Executive summary: clinical practice guideline for the evaluation and treatment of children and adolescents with obesity. Pediatrics. 2023;151 doi: 10.1542/peds.2022-060641. [DOI] [PubMed] [Google Scholar]
- 45.Caro R., Samsel D., Savel P. Is there sustained weight loss after discontinuation of GLP-1 agonist for obesity treatment? Evid Based Pract. 2023;26:7–8. [Google Scholar]
- 46.Meyers A.G., Hudson J., Cravalho C.K.L., Matta S.T., Villalobos-Perez A., Cogen F., et al. Metformin treatment and gastrointestinal symptoms in youth: findings from a large tertiary care referral center. Pediatr Diabetes. 2021;22:182–191. doi: 10.1111/pedi.13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dixon S.A., Mishra S., Dietsche K.B., Jain S., Mabundo L., Stagliano M., et al. The effects of prebiotics on gastrointestinal side effects of metformin in youth: a pilot randomized control trial in youth-onset type 2 diabetes. Front Endocrinol (Lausanne) 2023;14 doi: 10.3389/fendo.2023.1125187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O'Connor E.A., Evans C.V., Burda B.U., Walsh E.S., Eder M., Lozano P. Screening for obesity and intervention for weight management in children and adolescents: evidence report and systematic review for the US Preventive Services Task Force. J Am Med Assoc. 2017;317:2427–2444. doi: 10.1001/jama.2017.0332. [DOI] [PubMed] [Google Scholar]
- 49.Valle-Martos R., Jimenez-Reina L., Canete R., Martos R., Valle M., Canete M.D. Changes in liver enzymes are associated with changes in insulin resistance, inflammatory biomarkers and leptin in prepubertal children with obesity. Ital J Pediatr. 2023;49:29. doi: 10.1186/s13052-023-01434-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schwimmer J.B., Deutsch R., Kahen T., Lavine J.E., Stanley C., Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 51.Koutny F., Weghuber D., Bollow E., Greber-Platzer S., Hartmann K., Korner A., et al. Prevalence of prediabetes and type 2 diabetes in children with obesity and increased transaminases in European German-speaking countries. Analysis of the APV initiative. Pediatr Obes. 2020;15:e12601. doi: 10.1111/ijpo.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patel-Sanchez N., Perito E., Tsai P., Raymond-Flesch M., Lodish M., Sarkar M. Prevalence of nonalcoholic fatty liver disease increased with type 2 diabetes mellitus in overweight/obese youth with polycystic ovary syndrome. J Pediatr Endocrinol Metab. 2023;36:441–446. doi: 10.1515/jpem-2022-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siddiqui M.S., Sterling R.K., Luketic V.A., Puri P., Stravitz R.T., Bouneva I., et al. Association between high-normal levels of alanine aminotransferase and risk factors for atherogenesis. Gastroenterology. 2013;145:1271–1279.e1–3. doi: 10.1053/j.gastro.2013.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wharton S., Davies M., Dicker D., Lingvay I., Mosenzon O., Rubino D.M., et al. Managing the gastrointestinal side effects of GLP-1 receptor agonists in obesity: recommendations for clinical practice. Postgrad Med. 2022;134:14–19. doi: 10.1080/00325481.2021.2002616. [DOI] [PubMed] [Google Scholar]
- 55.van Boxel E.J., Rahman S., Lai K., Boulos N., Davis N. Semaglutide treatment for children with obesity: an observational study. Arch Dis Child. 2024;109:822–825. doi: 10.1136/archdischild-2023-326687. [DOI] [PubMed] [Google Scholar]
- 56.Cooper D.M., Rothstein M.A., Amin A., Hirsch J.D., Cooper E. Unintended consequences of glucagon-like peptide-1 receptor agonists medications in children and adolescents: a call to action. J Clin Transl Sci. 2023;7:e184. doi: 10.1017/cts.2023.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bensignor M.O., Kelly A.S., Kunin-Batson A., Fox C.K., Freese R., Clark J., et al. Evaluating appetite/satiety hormones and eating behaviours as predictors of weight loss maintenance with GLP-1RA therapy in adolescents with severe obesity. Pediatr Obes. 2024;19:e13105. doi: 10.1111/ijpo.13105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arora T., Taheri S. Is sleep education an effective tool for sleep improvement and minimizing metabolic disturbance and obesity in adolescents? Sleep Med Rev. 2017;36:3–12. doi: 10.1016/j.smrv.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 59.Sekine M., Yamagami T., Handa K., Saito T., Nanri S., Kawaminami K., et al. A dose-response relationship between short sleeping hours and childhood obesity: results of the Toyama Birth Cohort Study. Child Care Health Dev. 2002;28:163–170. doi: 10.1046/j.1365-2214.2002.00260.x. [DOI] [PubMed] [Google Scholar]
- 60.Arora T., Taheri S. Associations among late chronotype, body mass index and dietary behaviors in young adolescents. Int J Obes (Lond) 2015;39:39–44. doi: 10.1038/ijo.2014.157. [DOI] [PubMed] [Google Scholar]
- 61.Taheri S., Lin L., Austin D., Young T., Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLOS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Potter G.D., Skene D.J., Arendt J., Cade J.E., Grant P.J., Hardie L.J. Circadian rhythm and sleep disruption: causes, metabolic consequences, and countermeasures. Endocr Rev. 2016;37:584–608. doi: 10.1210/er.2016-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Depner C.M., Stothard E.R., Wright K.P., Jr. Metabolic consequences of sleep and circadian disorders. Curr Diab Rep. 2014;14:507. doi: 10.1007/s11892-014-0507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chaput J.P., Tremblay A. Obesity and physical inactivity: the relevance of reconsidering the notion of sedentariness. Obes Facts. 2009;2:249–254. doi: 10.1159/000227287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scheer F.A., Hilton M.F., Mantzoros C.S., Shea S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wittmann M., Dinich J., Merrow M., Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol Int. 2006;23:497–509. doi: 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- 67.Roenneberg T., Kuehnle T., Juda M., Kantermann T., Allebrandt K., Gordijn M., et al. Epidemiology of the human circadian clock. Sleep Med Rev. 2007;11:429–438. doi: 10.1016/j.smrv.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 68.Roenneberg T., Allebrandt K.V., Merrow M., Vetter C. Social jetlag and obesity. Curr Biol. 2012;22:939–943. doi: 10.1016/j.cub.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 69.Zuraikat F.M., Makarem N., Redline S., Aggarwal B., Jelic S., St-Onge M.P. Sleep regularity and cardiometabolic heath: is variability in sleep patterns a risk factor for excess adiposity and glycemic dysregulation? Curr Diab Rep. 2020;20:38. doi: 10.1007/s11892-020-01324-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wong P.M., Hasler B.P., Kamarck T.W., Muldoon M.F., Manuck S.B. Social jetlag, chronotype, and cardiometabolic risk. J Clin Endocrinol Metab. 2015;100:4612–4620. doi: 10.1210/jc.2015-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jansen E.C., Burgess H.J., Chervin R.D., Dolinoy D.C., Tellez-Rojo M.M., Cantoral A., et al. Sleep duration and timing are prospectively linked with insulin resistance during late adolescence. Obesity (Silver Spring) 2023;31:912–922. doi: 10.1002/oby.23680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tan E., Healey D., Gray A.R., Galland B.C. Sleep hygiene intervention for youth aged 10 to 18 years with problematic sleep: a before-after pilot study. BMC Pediatr. 2012;12:189. doi: 10.1186/1471-2431-12-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Simon S.L., McWhirter L., Diniz Behn C., Bubar K.M., Kaar J.L., Pyle L., et al. Morning circadian misalignment is associated with insulin resistance in girls with obesity and polycystic ovarian syndrome. J Clin Endocrinol Metab. 2019;104:3525–3534. doi: 10.1210/jc.2018-02385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Simon S., Rahat H., Carreau A.M., Garcia-Reyes Y., Halbower A., Pyle L., et al. Poor sleep is related to metabolic syndrome severity in adolescents with PCOS and obesity. J Clin Endocrinol Metab. 2020;105:e1827–e1834. doi: 10.1210/clinem/dgz285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Teede H.J., Tay C.T., Laven J., Dokras A., Moran L.J., Piltonen T.T., et al. Recommendations from the 2023 international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2023;120:767–793. doi: 10.1016/j.fertnstert.2023.07.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.