Abstract
Perovskite materials have garnered significant attention within a very short period of time by achieving competitive efficiency. In addition, this material demonstrated intriguing optoelectronic properties and versatile applications. Although they have confirmed amazing efficiency in solar cells at the laboratory scale, mass commercial manufacturing of perovskite solar cells (PSCs) is still a problem due to their poor longevity. Researchers have identified several intrinsic and extrinsic factors contributing to the instability of perovskite compounds and PSCs, and various approaches are being used to increase material quality and stability in order to extend the lifespan of PSCs. Despite these challenges, the potential of perovskite materials in revolutionizing solar energy remains a central point of scientific investigation and development. In this review, a comprehensive analysis is provided to discern the intrinsic and extrinsic factors contributing to the degradation of PSCs which certainly helps us to understand the underlying degradation mechanisms. In addition, we discussed some novel approaches that have already been adopted to augment the stability of the devices.
Insights into the factors and mechanisms of degradation, along with potential solutions.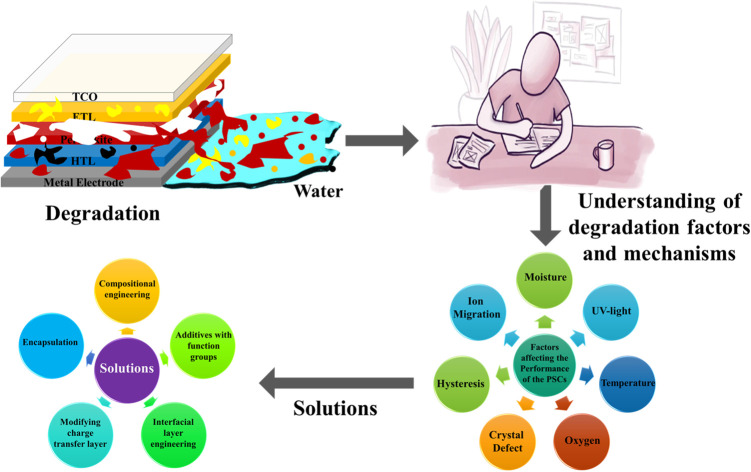
1. Introduction
The global necessity for renewable energy has surged because of the demand for sustainable and eco-friendly energy sources. Solar energy – abundant, free, and inexhaustible – has emerged as a prominent solution. In contrast to finite fossil fuels, solar energy is renewable and eco-friendly, making it highly desirable as an energy source.1,2 A rough estimation indicates that the sun emits solar energy at a rate of 3.8 × 1023 kW s−1 and approximately 1.8 × 1014 kW s−1 is being absorbed by Earth.3 The daily supply of solar energy reaching at earth is ample, to the extent that the current global population could consume it completely within a span of 27 years.4 In 2022, renewable energy sources were estimated at approximately 30% of the total worldwide electricity production of 29 000 TW h (https://www.statista.com/statistics/270281/electricity-generation-worldwide/). Within this, solar energy contributed almost 4.5%, as reported by the International Energy Agency (https://www.iea.org/energy-system/renewables/solar-pv). The amount of this contribution in 2019 was merely 1.6%.5 Hence, photovoltaic technology is attaining attractiveness due to its huge potential for harnessing solar energy. The photovoltaic industry comprises different generations of solar cells and each has its unique advantages and challenges. In emerging photovoltaics, PSCs have acquired prominence as a cutting-edge invention because of their increased PCE, simple manufacturing method, and low cost.6 The perovskite materials have distinctive features including tunable band gaps, high absorption coefficients, minimal exciton binding energy, ambi-polar charge transportation, and long diffusion distances for charge carriers.7–10 These fascinating features make it possible for scientists to optimize PSCs in ways that are challenging for other sorts of solar cells. Recently, there has been an incredible surge in the power conversion efficiency (PCE) of PSCs by displaying an increment from 3.81% to an inspiring 26.7% between 2009 and 2024.11–13 This rapid progression is endorsed by developments in materials, synthesis techniques and device architectures. In spite of these impressive advances, challenges including stability and scalability for mass production remain to be addressed. Even so, this growth underlines the promising potential of PSCs as a viable photovoltaic technology. The stability in PSCs is associated with multifaceted challenges. The PSCs are inherently susceptible to moisture, heat, oxygen, and light, which are the cause of degradation and loss of effectiveness over time.10,14 Additionally, their performance and stability are influenced by crystal defects, ion migration, hysteresis, and hydrophobicity.15–19 The research focuses on understanding the degradation mechanisms and designing materials and production procedures to mitigate them.20–25 Research also spots on different approaches to enhance stability including encapsulation techniques to shield the perovskite from moisture and oxygen exposure, as well as the growth of more stable perovskite compositions and device architectures tolerable against moisture, oxygen, heat, and light.10,14
This review extensively explores the various degradation factors affecting the performance of PSCs, including both intrinsic and extrinsic factors. It provides a thorough analysis of the mechanisms behind PSCs' instability. By scrutinizing existing research, the review identifies a range of strategies and approaches that have been adopted to stabilize these degradation processes. The purpose of these mitigation techniques is to enhance the overall performance and durability of PSCs, offering insights into materials engineering, interfacial engineering, device architecture optimization, and protective encapsulation methods. Through this comprehensive exploration, the review will contribute valuable knowledge to the ongoing efforts in enhancing the stability and longevity of PSCs, opening the path for more reliable applications in renewable energy solutions. The essence of our review work is depicted in Fig. 1.
Fig. 1. Brief essence of this review.
2. Degradation of PSCs
Perovskite solar cells currently trail behind commercially available solar cells primarily because of challenges related to their stability requirements. Perovskites are characterized as soft semiconducting materials with chemical components that exhibit weak bonding through interactions including hydrogen bonding, van der Waals forces, as well as ionic interactions.26 Ions generated through the decomposition of the perovskite can readily migrate to various layers of the PSC when exposed to factors like moisture ingress, prolonged light exposure, thermal stress, and external electric fields. This migration contributes to the deterioration of the perovskite layer, ETL, HTL, and other components.27–31 Understanding the specific decomposition mechanisms is essential to prevent the degradation of perovskite constituents and PSC.
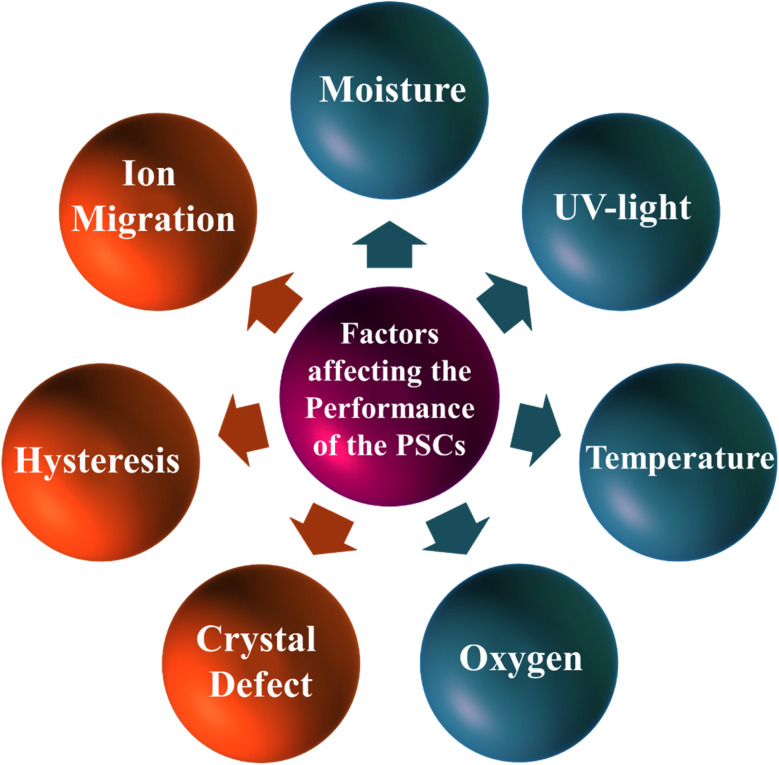
The combination of diverse experimental methodologies alongside theoretical approaches can help to a thorough comprehension of the degradation mechanisms. Stability concerns can be categorized into two leading groups: (i) intrinsic stability, which pertains to issues arising solely from the molecular and crystallographic structure of the perovskite, and (ii) extrinsic stability, encompassing problems predominantly induced by external factors inherent in practical applications that impact on the stability of both the perovskite layers and other components of PSCs. These degradation elements are presented in Fig. 2.
Fig. 2. Degradation factors that are affects the performance metrics of the PSCs.
2.1. Extrinsic degradation factors
2.1.1. Moisture induced degradation
The existence of water or moisture is identified as a predominant factor causing degradation and consequent destabilization of PSCs. The degradation of the perovskites involves a chemical process that uses moisture as a catalyst. Perovskite materials undergo decomposition when exposed to moisture, primarily due to their heightened susceptibility to water and their inclination to hydrolyze owing to their polar nature. The breakdown process of PSCs owing to moisture exposure was documented by Niu et al.20 The proposed degradation mechanism of the organic–inorganic halide perovskite was elucidated by relating the XRD data before and after interaction to moisture.
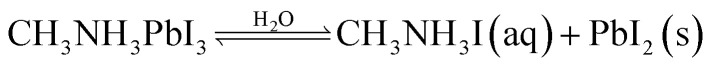 |
i |
| CH3NH3I(aq) ⇌ CH3NH2(aq) + HI(aq) | ii |
| 4HI(aq) + O2 ⇌ 2I2(s) + H2O(aq) | iii |
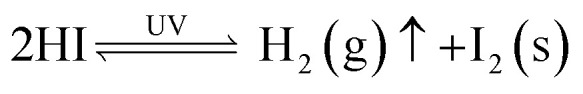 |
iv |
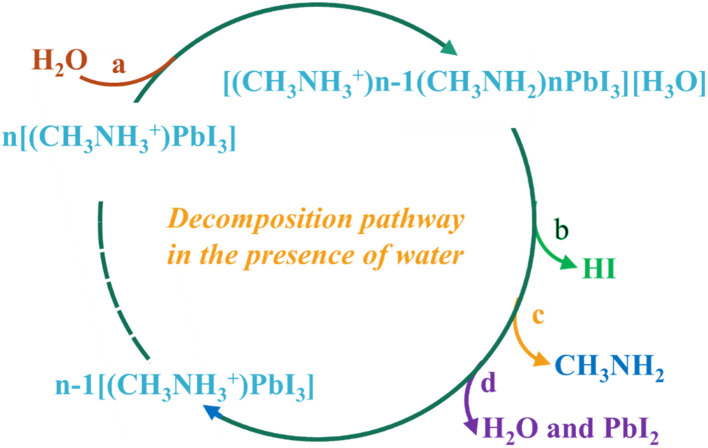
As per Niu and his colleagues, the organic–inorganic perovskites undergo a decomposition in existence of moisture causing in the creation of CH3NH3I and PbI2. Additionally, CH3NH3I is transformed into CH3NH2 and HI during this process. As a result of the oxidation process, HI is converted to I2 and H2O. Concurrently, a portion of HI undergoes photochemical reactions, leading to the creation of H2 gas and I2. The initiation of the degradation process is attributed to moisture, while the decomposition rate is accelerated by the oxidative and photochemical reactions. The whole process has been explained in eqn (i)–(iv). The prominent researcher Frost and his team32 conducted a study illustrating the deterioration of perovskite structure containing organic species, specifically MAPbI3, in the existence of moisture. The irreversible disintegration process is depicted in Fig. 3, wherein a water molecule triggers the breakdown. This decomposition is further influenced by phase changes in both methylammonia and hydrogen iodide. The primary outcome of this conduit is the formation of PbI2, causing the structures to adopt a yellowish hue.
Fig. 3. Decomposition path of perovskites in existence of moisture.32.
Chen et al.33 disclosed how PSCs degraded due to moisture in detail. In the existence of moisture, the MAPbI3 compound reacts with water to produce MAPbI3·H2O. This formation of the MAPbI3·H2O intermediate displays a reversible feature, which represents that the perovskite remains stable and can operate with reasonable efficiency even though they are exposed to minimal moisture. However, if the MAPbI3·H2O/MAPbI3 intermediates come across increased moisture levels, the MAPbI3·H2O undergoes irreversible decomposition into PbI2, which leads to device deterioration. Under light illumination and moisture, PbI2 again decomposes into PbIOH. Once PbIOH forms within the device, it interrupts the interface between ETL and HTL, rendering the cell ineffective as it breaks down the junction inside the cell. Another investigation done by Wang et al.,34 demonstrated that exposure to moisture and various environmental elements results in the generation of volatile species, including I2 vapor. This, in turn, initiates a self-degradation chain reaction process in MAPbI3 based solar cells. The research also indicated that the presence of iodine vapor can induce degradation in other cells relying on iodine. While the creation of PbI2 in the existence of light and moisture is widely acknowledged, Christians and his team35 discovered that in the absence of light, H2O can form a hydrating complex with perovskites, proposing a specific reaction pathway.
| MAPbI3 + H2O → (MA)4PbI6·2H2O | v |
On the other hand, environmental conditions, especially moisture, can cause rapid deterioration and efficiency loss in FA-based perovskite solar cells (FA-PSCs), as is the case with most organic–inorganic perovskite materials. Due to the fact that water can penetrate the perovskite layer and react with its components, breaking down the perovskite structure, FA-based perovskites are extremely susceptible to moisture.36 Water molecules interact with the lead halide framework when exposed to dampness when they diffuse into the perovskite layer. Because of this, hydrated perovskite phases, such as (FA)xMA(1−x)PbI3·H2O, are formed; they have less favorable optoelectronic characteristics than the original material. While prolonged exposure results in irreparable damage, these intermediate hydrates are usually reversible under specific drying conditions.37
Lead iodide (PbI2) is formed, and other organic cations, such as FA+, are released when the perovskite crystal lattice breaks down due to continuous moisture exposure. Significant efficiency loss in the solar cell results from this breakdown process, which is frequently linked to visible yellowing of the perovskite layer, which is indicative of PbI2 production. Like methylammonium (MA+), the cations formamidinium (FA+) is also very susceptible to hydrolysis in the presence of moisture. The byproduct formamidinium formate, which cannot be reassembled into the perovskite structure, is what FA+ can hydrolyze to. As a result, the substance degrades permanently. Higher humidity levels speed up the rate of deterioration. FA-based perovskites deteriorate more quickly in conditions when the relative humidity (RH) is higher than 50%. Degradation can happen in a matter of hours in very humid situations (RH > 80%).36,38
Although FA-based perovskites are more resistant to moisture than MA-based perovskites, they nevertheless break down when exposed to moisture over an extended period of time.39 While stability can occasionally be increased by adding bromide or chloride, performance trade-offs are common.40,41 Degradation can be postponed and moisture intrusion greatly reduced with proper encapsulation.37 Polymers, glass, and moisture-barrier coatings are common materials used for encapsulation because they can reduce the rate at which water penetrates. Applying hydrophobic coatings (such as self-assembled monolayers or fluorinated compounds) or surface passivation processes can shield the perovskite layer from direct moisture exposure. Alumina (Al2O3), titanium dioxide (TiO2), and other oxide layers are examples of inorganic layers that can function as protective barriers. The decline in photovoltaic performance is the main effect of moisture deterioration on FA-based PSCs. Important performance indicators deteriorate dramatically, such as total power conversion efficiency (PCE), fill factor (FF), open-circuit voltage (Voc), and short-circuit current density (Jsc). Perovskite material degradation results in reduced light absorption, increased non-radiative recombination, and the introduction of trap states, all of which lower cell efficiency.38,42
It has been shown that adding mixed cations (FA/MA/Cs) and halides (I/Br/Cl) to the perovskite formulation enhances moisture stability. For example, doping with cesium (Cs+) increases crystallinity and decreases the possibility of moisture-induced lattice disintegration.42 It is possible to lessen the vulnerability to moisture infiltration by controlling the interfaces between the perovskite and transport layers—both the electron and hole transport layers. For example, the perovskite layer can be shielded by using hydrophobic materials in the transport layers. It has been demonstrated that by passivating flaws and enhancing film quality, additives like fullerene derivatives or organic compounds like 4-tert-butylpyridine (tBP) can improve the moisture resistance of FA-based perovskites. The operational stability of FA-based PSCs can be extended by operating them in regulated, low-humidity conditions. In particular, this is crucial for applications in areas with high humidity.36
2.1.2. UV-light induced degradation
Effective packaging can mitigate the impact of atmospheric stressors like oxygen and humidity. However, for a semiconductor to work as a solar material for more than 25 years, it needs to hold innate stability against light exposure along with other degradation factors. Confirming that a material remains stable under extended exposure to light is typically the first step in assessing the appropriateness of the selected active material for use in photovoltaic devices. The prominent researcher Ito and his colleagues21,43 identified a pathway for the decomposition of the perovskite structure during UV light irradiation, supported by evidence from UV-vis and XRD data. The process of decomposition is outlined in eqn (vi)–(viii).
| 2I− ↔ I2 + 2e− | vi |
| 3CH3NH3+ ↔ 3CH3NH2(g)↑ + 3H+ | vii |
| I− + I2 + 3H+ + 2e− ↔ 3HI↑ | viii |
The electron affinity of TiO2 leads to the collection of electrons from the iodide within the perovskite, causing the disintegration of the perovskite and the generation of I2. In eqn (ii), the pKa of the methylammonium ion is determined to be 10.8 with the aid of water, indicating that the equilibrium is inclined towards the left of the equation. However, the consistent removal of hydrogen ions and the evaporation of CH3NH2 can promote a shift in equilibrium towards the right in eqn (ii). In the absence of perovskite structures, this shift becomes more achievable. Consequence of this change, the extracted electrons can return from the TiO2 surface, minimizing I2, and giving rise to the evaporation of HI.44
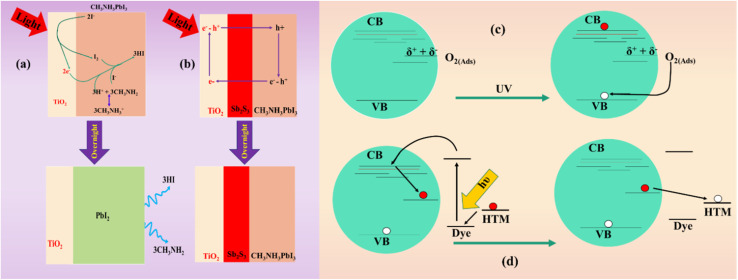
Another renowned research group comprised of Snaith and his colleagues45 has investigated the photon-induced instability of both encapsulated and non-encapsulated PSCs. Their findings revealed that encapsulated PSCs decay at a higher pace than non-encapsulated ones. The application of transient absorption spectroscopy indicated a significant decrease in the charge collection rate of the TiO2-based encapsulated perovskite cells compared to non-encapsulated ones. This decline may be attributed to the entrapment of injected electrons from the perovskites into the interstitial states in the TiO2. The degradation mechanism is closely connected to the TiO2's surface chemistry. Crystal defects in titania result in the presence of numerous vacancies, where O2 molecules from the surroundings are adsorbed. This process facilitates the relocation of electrons from TiO2−x, leading to the formation of a Ti(iv)+O2−-complex. The revealing of the TiO2 to UV light initiates the generation of an electron–hole pair. Vacancies within the valence band accept electrons from oxygen molecules, separating them from the titania. The free electrons generated through this photo-induced process in the conduction band subsequently recombine with the holes present in the HTL, significantly impacting the overall performance of the device.21,46Fig. 4 schematically illustrates the photo-induced degradation process.
Fig. 4. Possible way of photo-induced decomposition: (a) UV induced-deterioration of the PSC without interfacial layer among TiO2 and perovskite material; (b) reduced UV-induced deterioration after inserting Sb2S3 between TiO2 and perovskite material, figure reproduced with permission from ref. 21. (c) Schematic representation of the UV-induced deterioration of TiO2 layer owing to absorption of O2 from atmosphere at interstitial defects, figure reproduced with permission from ref. 45.
About 3–5% of the solar spectrum is UV light, which can start a number of photochemical processes inside the PSC structure. This can lower the efficiency of FA-based perovskite solar cells and accelerate device breakdown. When exposed to UV light, FA-based PSCs degrade in a variety of intricate ways that frequently involve both intrinsic and extrinsic factors. It has been noted that UV radiation causes structural alterations in the perovskite lattice. In particular, phase segregation into the hexagonal non-perovskite δ-phase, which is thermodynamically more stable than the photoactive α-phase, is a common occurrence in FA-based perovskite materials like FAPbI3.47 According to research by Wang et al. (2022) and later investigations, photo-generated charge carriers may cause UV-induced phase segregation, which would lower the overall performance of solar cells.48 High-energy photons produced by UV radiation have the ability to disrupt chemical interactions inside the perovskite structure, especially at interfaces and grain boundaries.49 This may result in interstitial defects and vacancies, which serve as non-radiative recombination sites and lower the lifetime and mobility of charge carriers. Furthermore, UV radiation promotes ion migration, especially of iodine ions. Iodine ions migrate inside the perovskite structure when illuminated, and this action increases when exposed to UV light, resulting in irreversible deterioration of device performance.50
UV-induced deterioration is particularly detrimental at the interface between the perovskite layer and the nearby charge transport layers, which are usually electron transport layers (ETLs) such as titanium dioxide (TiO2). Common ETL materials like TiO2 absorb UV light and produce electron–hole pairs that might react with the perovskite layer and degrade it. According to research by Ji et al. (2020), the FA-based perovskite can further decompose by catalyzing oxidation processes with UV-activated TiO2.51 Organic components in FA-based perovskites may dissociate when exposed to UV light. Perovskite degradation can be accelerated by UV light, especially in humid situations, which can worsen the generation of volatile species such as HI, I2, and methylamine. According to Chen et al. (2022), these photochemical reactions in which UV light serves as a catalyst for the production of these volatile compounds are a crucial component in the breakdown of perovskites.49
2.1.3. Temperature induced degradation
Examining this factor is essential for stability analysis during device manufacturing. Annealing constitutes a crucial stage, and the devices will be utilized in the outdoor environment, subjecting them to elevated temperatures. The eminent research team formed with Philippe et al.22 accomplished a research to examine the influence of higher temperatures on perovskite. The PSCs were examined in an ultra-high vacuum environment to eliminate the influence of air and water. It was noted that, under elevated temperatures, the perovskite undergoes a transformation into PbI2. The decomposition process of the perovskite is presented in eqn (ix)–(xi)
| PbCl2 + 3MAI → MAPbI3 + 2MACl | ix |
| PbCl2 + 3MAI → PbI2 + MAI + 2MACl | x |
| MAPbI3 → PbI2 + CH3NH2 + HI | xi |
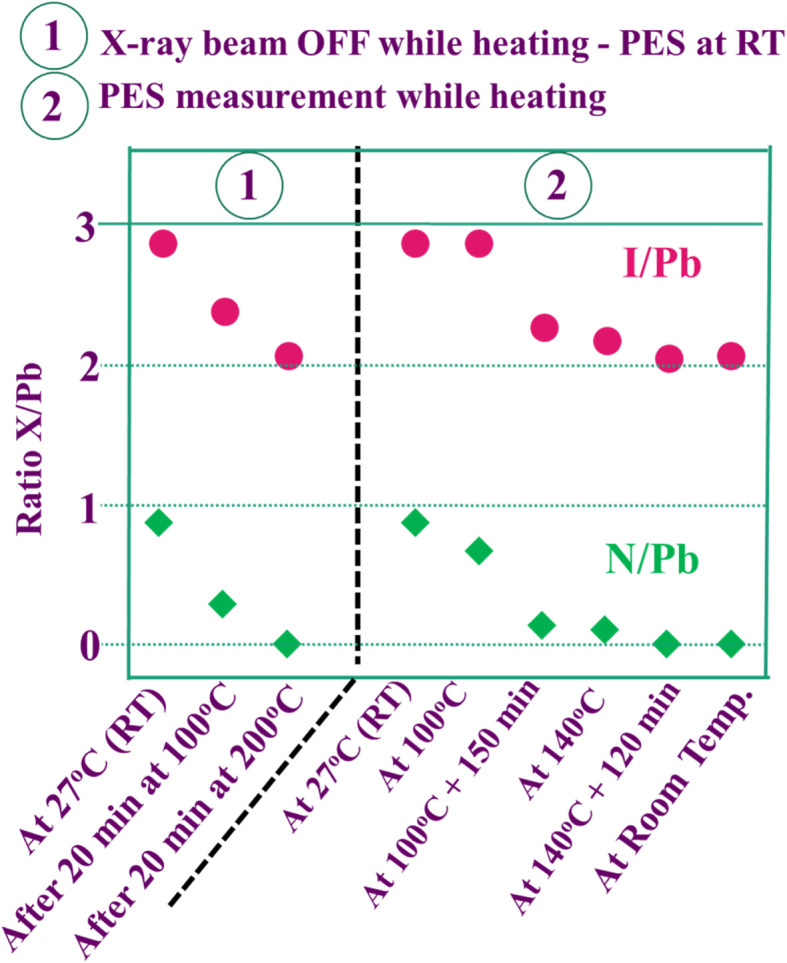
Another research team lead by Kim52 carried out an in situ examination which revealed that the employment of encapsulation does not shield the hybrid halide perovskite from degradation owing to temperature. The degradation initiates at 100 °C, involving the transformation of the β-phase into the α-phase at elevated temperatures. The impact of temperature on the perovskite compound can be supported by examining the optoelectrical, and chemical characteristics of these layers. Another prominent expert in PSCs named Phillipe53 investigated the impact of temperature on both pure MAPbI3 and mixed MAPbI3−xClx by utilizing photoelectric spectroscopy. The films underwent heating within an ultra-high vacuum chamber to eliminate air and moisture. The variations in the 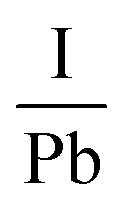 and
and 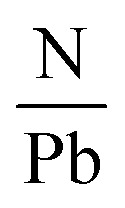 ratios were documented as the temperature gradually increased, as demonstrated in Fig. 5. The analysis of the film, conducted from room temperature to 200 °C, revealed a decline in both the
ratios were documented as the temperature gradually increased, as demonstrated in Fig. 5. The analysis of the film, conducted from room temperature to 200 °C, revealed a decline in both the 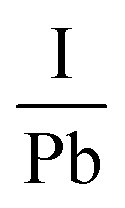 and
and 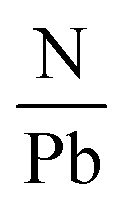 ratios from 2.0 to 0, supporting the conclusion that the perovskite layer undergoes a transition to the byproduct PbI2.
ratios from 2.0 to 0, supporting the conclusion that the perovskite layer undergoes a transition to the byproduct PbI2.
Fig. 5. The variation of I/Pb and N/Pb due to temperature. Figure reproduced with permission from ref. 53.
In addition, Bruno and his team54 investigated the decomposition reactions and inferred that MAPbBr3, MAPbCl3, and MAPbI3 all undergo decomposition into lead halide, along with the release of gaseous hydrogen halide and methylamine, even at relatively lower 60 °C temperatures. They proposed that these materials might not be appropriate for prolonged applications based on their findings. Moreover, Zhao et al.55 conducted another research on the thermal stability of MAPbI3 and the MAPbI3 film underwent examination within a dark and moisture-free atmosphere at temperatures from 85 to 120 °C for a duration of 10 days, revealing no signs of degradation. This observation implies that the material itself possesses intrinsic stability. However, when subjected to thermal cycles for both inverted (FTO/NiO/MAPbI3/PCBM/BCP/Au) and normal (FTO/bl-TiO2/mp-TiO2/MAPbI3/Spiro-OMeTAD/Au) configurations, rapid decomposition was witnessed for the normal configuration, whereas the inverted structure exhibited minimal degradation after 80 thermal cycles. Furthermore, Conings and his colleagues56 inspected the impact of temperature on the perovskite layer (MAPbI3) under numerous atmospheric conditions. Variations in the structure of the perovskite layers were noted under N2, O2, and ambient conditions. The alterations in the structure of the perovskites can be detected by assessing the conductivity of the material through conductive atomic force microscopy. The conductivity of the perovskite experiences a swift decline as the temperature rises, potentially attributed to the creation of a wide bandgap material, PbI2. The decomposition process induced by temperature can be expressed by eqn (xii).57,58
| CH3NH3PbI3 → CH3NH2(g)↑ + HI(g)↑ + PbI2(s) | xii |
The configuration of Au/Spiro-OMeTAD/MAPbI3/m-TiO2/FTO was analysed by Leong et al.,59 for understanding the effect of temperature. The cell's performance demonstrates an improvement for temperatures below 330 K, but a notable decline is observed in the temperature range of 330 K to 360 K. Variation of Voc was also investigated and Voc remained in the saturation regime at low temperatures (T < 250 K), and it exhibited a linear decrease with the rise in temperatures in the range of 250 K to 360 K. During the fabrication of perovskite based on quadruple cations, incorporating Rb, Duong et al.60 noted the separation of Rb cation and the transmission of elemental gold to the HTL at 85 °C. They observed an escalation in the impact of thermal decomposition in the existence of light. Another research team led by Foley61 investigated the impact of temperature on the perovskite's energy levels and observed that as the temperature augmented from the range of 28 °C to 85 °C, both the conduction band minimum and valence band maximum decreased by the amount of 77 meV and 110 meV, respectively.
FA-based PSCs are ideal for applications where stability across a variety of environmental conditions, especially temperature, is crucial because they typically lead to increased stability and improved optoelectronic characteristics. Phase transitions and breakdown are two negative impacts of temperature changes that can still affect FA-based PSCs, ultimately impeding their long-term stability and commercial viability.62 The stronger hydrogen bonding within FA ions gives FA-based perovskites greater resilience against breakdown, making them thermally more stable than MA-based perovskites.63 FA-based perovskites have been shown to retain structural stability up to about 100–150 °C.64,65 However, at high temperatures, FA-based perovskites might change from the photoactive black perovskite (α-phase) to non-photoactive phases (like δ-phase), which would reduce their efficiency.56
Studies show that at high temperatures, FA-based perovskites can change from the α-phase to the δ-phase. The phase transition is undesirable because it generates inactive phases, which lowers the stability and efficiency of the cell. Anion alterations (e.g., Br incorporation) and cation engineering (e.g., Cs or MA) have been investigated to stabilize the α-phase and prevent phase transitions. Because of its greater binding within the perovskite crystal lattice and lack of volatility, the FA ion has been claimed to be more stable than the MA ion. Nevertheless, given extended heat exposure, especially in humid conditions, FA-based perovskites can still break down into lead iodide (PbI2). Research using encapsulation techniques has shown that by shielding the perovskite layer from oxygen and moisture intrusion, encapsulated FA-based PSCs have a greater resistance to heat degradation.56,62,66
2.1.4. Oxygen induced degradation
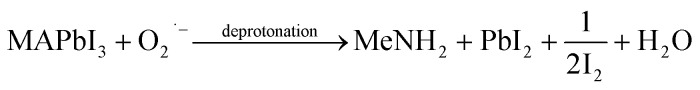
While oxygen is always present during both material synthesis and device operation, the interaction between oxygen and the perovskite layer has been largely overlooked, receiving limited attention. The creation of highly reactive superoxide species, linked to the transfer of charges between oxygen molecules and the photo-excited halide perovskites has been observed in many published literature.67,68 The stability of photoactive layers, MAPbI3 is significantly affected by the combined influence of oxygen and light. When the layer is subjected to both light exposure and dry air, the photoactive layers on mp-Al2O3/MAPbI3 undergo rapid decomposition, resulting in the creation of methylamine, I2, and PbI2 as decomposition products. The following eqn (xiii)–(xv) represents the possible pathway for degradation induced by superoxide.69
 |
xiii |
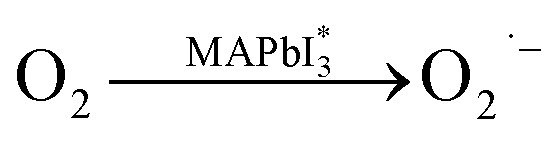 |
xiv |
 |
xv |
When MAPbI3 undergoes optical excitation, it gives rise to the formation of 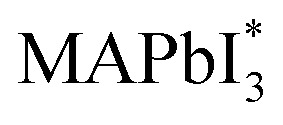 and it contains both photo-induced holes and electrons. Subsequently, superoxide is produced as a result of electron relocation from
and it contains both photo-induced holes and electrons. Subsequently, superoxide is produced as a result of electron relocation from 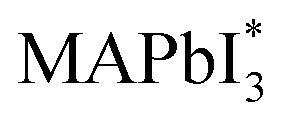 to O2. The generated superoxide initiates an attack on the perovskite absorber, resulting in the production of decomposition products such as lead iodide, methylamine, iodine, and water. In a subsequent research by N. Aristidou and his co-researchers70 by employing a variety of experimental and computational techniques, they have gained mechanistic insights into the photo-degradation induced by oxygen. Their findings indicate that rapid oxygen transmission into MAPbI3 films is concurrent with the photo-induced generation of highly interactive superoxide species. The perovskite films characterized by small crystallites exhibit elevated superoxide yields but lower stability. The Ab initio calculation also suggests that iodide vacancies serve as favored locations in promoting the photo-induced production of superoxide radicals from oxygen. Bryant et al.,31 observed the primary cause of the limited operational stability in MAPbI3 PSCs under atmospheric conditions and identified the degradation tempted by exposure to oxygen and light. When MAPbI3 solar cells without encapsulation are exposed to dry air and light, they undergo swift degradation within a few minutes to hours. This rapid deterioration is similarly observed when an electrically biased current flows in the dark in the existence of oxygen. The degradation reaction commences with the deprotonation of the methylammonium cation within the perovskite, facilitated by a photo-generated interactive oxygen species known as superoxide (O2−). This superoxide is produced through the interaction of photon-generated electrons within the perovskite structure and molecular oxygen.
to O2. The generated superoxide initiates an attack on the perovskite absorber, resulting in the production of decomposition products such as lead iodide, methylamine, iodine, and water. In a subsequent research by N. Aristidou and his co-researchers70 by employing a variety of experimental and computational techniques, they have gained mechanistic insights into the photo-degradation induced by oxygen. Their findings indicate that rapid oxygen transmission into MAPbI3 films is concurrent with the photo-induced generation of highly interactive superoxide species. The perovskite films characterized by small crystallites exhibit elevated superoxide yields but lower stability. The Ab initio calculation also suggests that iodide vacancies serve as favored locations in promoting the photo-induced production of superoxide radicals from oxygen. Bryant et al.,31 observed the primary cause of the limited operational stability in MAPbI3 PSCs under atmospheric conditions and identified the degradation tempted by exposure to oxygen and light. When MAPbI3 solar cells without encapsulation are exposed to dry air and light, they undergo swift degradation within a few minutes to hours. This rapid deterioration is similarly observed when an electrically biased current flows in the dark in the existence of oxygen. The degradation reaction commences with the deprotonation of the methylammonium cation within the perovskite, facilitated by a photo-generated interactive oxygen species known as superoxide (O2−). This superoxide is produced through the interaction of photon-generated electrons within the perovskite structure and molecular oxygen.
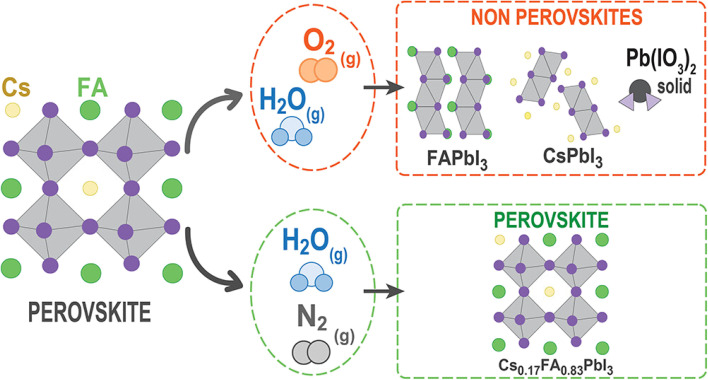
On the other hand, the prominent research group led by Correa-Baena conducted theoretical and experimental investigation to decipher the role of oxygen in the degradation of FA-based perovskite structures.71 They conducted two experiments in the presence of moisture-nitrogen and moisture-oxygen. Their findings reveal that degradation is slower when perovskites are exposed to H2O in nitrogen compared to oxygen. A key interaction between H2O and O2 in air accelerates phase transformations, where H2O dissolves formamidinium iodide (FAI), leading to the volatilization of FA+ and iodide ions. Exposed Pb–I surfaces then undergo oxidation by O2, forming stable iodate species that bond with Pb, leading to further infiltration of H2O and phase destabilization.
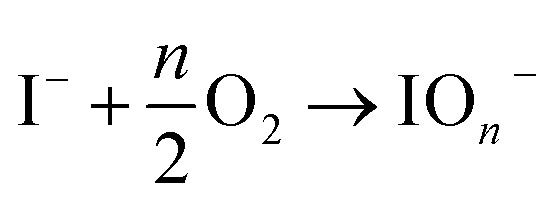 |
xvi |
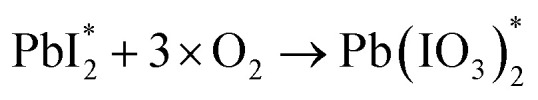 |
xvii |
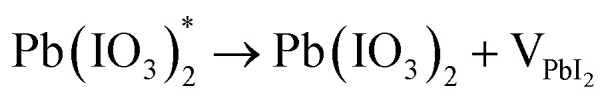 |
xviii |
where, ‘n’ in eqn (xvi) represents the stoichiometric coefficient. These results suggest that O2 can modify PbI2- terminated surfaces by oxidizing iodide to iodate species and creating PbI2 vacancies shown in eqn (xvi)–(xviii). These vacancies may allow H2O molecules to enter the structure and dissolve the next FAI layers in an iterative process. Fig. 6 illustrates a schematic representation of the phase transformation process in FACs-based perovskite in the presence of H2O-N2 and H2O-O2 environments.
Fig. 6. Degradation of FA based perovskite through vacancy formation, iodide oxidation, and phase segregation into non-perovskite phases under exposure to H2O-N2 and H2O-O2 environment. Figure reproduced with permission from ref. 71.
To address degradation, they proposed a strategy involving the use of an encapsulation technique. Specifically, they applied a hydrophobic phenethylammonium iodide (PEAI) coating, which acts as a barrier against moisture and oxygen from the environment. This approach effectively stabilizes the perovskite material, maintaining its efficiency even when exposed to H2O and air.
In addition, another research group comprised of Z. Zhang et al. conducted a degradation study of FA -based perovskite due to exposure in the light-oxygen environment.72 They have found that the FASnI3 perovskite degrades more severely when exposed to light/O2 than only O2, which they attribute to the superoxide generation through the reaction between O2 and photoexcited electrons. The route of formation of superoxide and degradation of FA-based perovskites are provided in eqn (xix)–(xxiii).
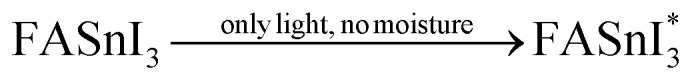 |
xix |
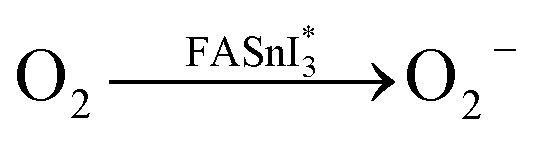 |
xx |
 |
xxi |
The produced water subsequently engages in additional degradation pathways as outlined below
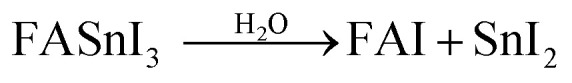 |
xxii |
Ultimately, all produced SnI2 undergoes oxidation as follows.
| SnI2 + O2− → SnO2 + I2 | xxiii |
Note that the aforementioned reaction mechanisms in eqn (xix)–(xxiii) were hypothesized by Z. Zhang et al.72 If these assumptions become valid, the corresponding FASnI3 PSCs are likely to experience significant efficiency degradation owing to FA+ deprotonation, the oxidation of Sn2+ to Sn4+, and iodine loss. Although O2 alone may contribute to the degradation of FASnI3, the investigation concludes that superoxide-induced degradation is the dominant degradation process in FASnI3 perovskite solar cells under light-O2 exposure. Moreover, they provided a promising solution to suppress the superoxide formation by incorporation of halide-containing additives. Theophylline-Br is an effective additive for passivating iodine vacancies and reducing superoxide formation in FASnI3 films, which leads to improved efficiency and stability of FASnI3 PSCs.
2.2. Intrinsic degradation factors
Ion migration, crystal defect and hysteresis have been discussed as intrinsic instability factors even though they are interconnected to some extent. However, each of them can also be influenced by other factors. For example, several reasons are considered for hysteresis in perovskites including charge carrier trapping and de-trapping, ion migration, ferroelectric polarization, and capacitive effects.73 Some researchers suggest that ion migration, crystal defects and hysteresis act as intrinsic factors leading to degradation in perovskites,74,75 though this area still requires extensive investigation.
2.2.1. Crystal defects
The defects within the perovskite film can impact the performance of PSCs.19,76 The Voc of PSCs correlates directly with the separation of holes and electrons quasi-Fermi levels within the illuminated perovskite layer. This separation is dictated by the charge density. Defect-induced non-radiative recombination processes diminish the steady-state charge density, consequently diminishing the quasi-Fermi level splitting and leading to a lessening in PSC voltages.18 In addition, the FF of the device, dependent upon voltage, will also decline. The defects within the perovskite structure can be categorized according to how they are formed. These defects arise from atomic vacancies, interstitials, and antistites substitutions. Additionally, defects of a higher dimensional nature result from dislocations, grain boundaries (GBs) and precipitates. Defects can further be classified into various types, including point defects, line defects, planar defects, and bulk defects.77 The researcher Yin and his colleagues investigated defect properties through theoretical calculations, affirming the association between point defects and carrier diffusion as well as their impact on the Voc in PSCs.78 Defects present in the film can also result in increased non-radiative recombination, thereby influencing the efficiency of charge extraction and subsequently reducing the Jsc of the PSCs. The well-known research group led by Leblebici79 successfully identified intra-grain heterogeneity in Jsc and Voc using conductive atomic force microscopy. They noted decreased Jsc and Voc values at GBs in comparison with those into the bulk of the perovskite film. This observation suggests that the defect density at GBs and surfaces significantly contributes to the deterioration of photovoltaic performance in PSCs. Another research team made up with Nie and co-authors80 highlighted that PSCs characterized by larger grain sizes and reduced trap densities exhibit improved Jsc and Voc. They attributed the increase in Jsc to a thicker active layer, which enhances light absorption capabilities, while the augmentation in Voc is ascribed to the reduction in trap density. The poor connection between the transportation layer and the perovskite resulting from 3D defects may induce significant series resistance, consequently decreasing both the FF and Jsc.81 Metal halide perovskites face a limitation in approaching the Shockley–Queisser photo-electrochemical limit for solar cells owing to the existence of profound defects within the film. These defects, originating from the solution-processed polycrystalline perovskite formation method, manifest at GBs and within the grain structure, causing swift non-radiative decay. It suggests that this rapid decay could contribute to the disintegration of the perovskite. The existence of impurity-based defects can result in the creation of recombination centres, specifically Shockley–Read–Hall recombination centres. This has a notable influence on the Voc by reducing the lifetime and overall photovoltaic performance. In addition, the existence of defects into the perovskite film and on its surface helps to the unusual hysteresis perceived in the J–V features of PSCs. Defects within the perovskite create a favourable path for the ions migration due to steric hindrance. In addition, the charge traps, induced by the inductive effect of these defects, become filled during forward biasing and discharge under short-circuit settings. Consequently, the repetitive cycles of charging and discharging result in the hysteresis behaviour exhibited by PSCs. The renowned researcher Park and his colleagues suggest that the hysteresis manners may also be attributed to the existence of Frenkel defects. These defects arise when iodide ions move to the octahedral interstitial sites Oh.82 The bulk and surface defects expedite the decomposition of perovskite compounds, particularly in environments like air and elevated temperatures.
2.2.2. Ions migration
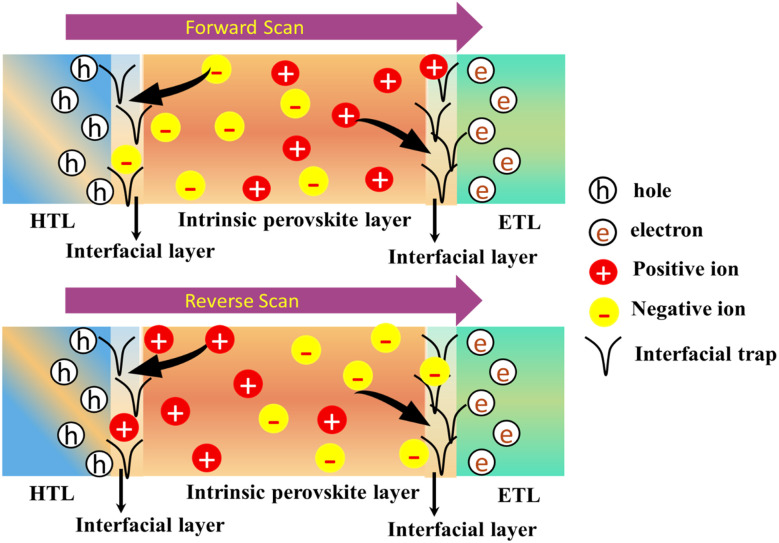
Ions migration poses a significant concern for the performance of PSCs. Under bias voltage or thermal drift, ions (either anions or cations) within halide perovskite materials have the ability to move, leading to device instability. The following Fig. 7 depicts a schematic diagram illustrating various movements of ions and defects within the structure of a perovskite device.
Fig. 7. Schematic diagram shows the different ions movements in perovskite device structure during forward and reverse scan, which can lead the hysteresis phenomenon and degrade the device stability as well as performance. Figure reproduced with permission from ref. 9.
Defects and ion migration, such as the existence of iodine vacancies at the interface, can trigger degradation at the interface. This migration of defects impacts the operational mechanisms of the device and ultimately results in device failure during operation.16,17 With others research groups, the prominent research group comprised with Hoke and his colleagues observed significant phase separation in MAPbBrxI3−x films under light exposure.83,84 Ion migration results in alterations to the local crystal structure and the reallocation of traps within perovskite films, consequently influencing device performance. Ion migration induces the creation of defects within the perovskite, including vacancies and interstitial defects, exacerbating ion migration further. This feedback loop results in component loss, lattice collapse, and other detrimental effects, ultimately compromising the long-term stability of the device during operation.85 Furthermore, ions within the perovskite can move into neighbouring functional layers including HTLs, ETLs, and electrodes. Compounding this issue, unlike reversible ion migration in the perovskite, ion migration-induced corrosion of functional layers is irreversible. The renowned researcher Besleaga86 demonstrated that iodine(i) can exit the perovskite material irreversibly and can drift via the HTL and Ag electrode, subsequently forming a stable compound known as AgI. Additionally, certain studies also propose the occurrence of a chemical reaction between the organic HTL and migrating iodine or methylammonium, leading to a gradual decrease in the HTL conductivity, consequently impacting the PSCs' performance. The prominent research team led by Zhao discovered that methylammonium ions (MA+) infiltrated into Spiro-OMeTAD during the operation of the device. This infiltration weakened its ability to transport holes, leading to the rapid degradation of PSC performance.87 Wang and co-authors similarly observed that the aged Spiro-OMeTAD or PTAA in solar energy converter lost its p-type characteristics following the introduction of iodine into HTLs.88
On the other hand, another research team led by R. O. Nughays and collaborators explored the carrier dynamics, surface defects, and ion migration in perovskite materials with varying compositions of formamidinium (FA) and methylammonium (MA).89 The experimental investigation focused on FA-rich (FA0.6MA0.4PbI3) and MA-rich (FA0.4MA0.6PbI3) compositions, while theoretical studies used DFT to analyze ion migration in pristine FAPbI3, MAPbI3, and a mixed-cation system (FA0.5MA0.5PbI3) to simplify the analysis.
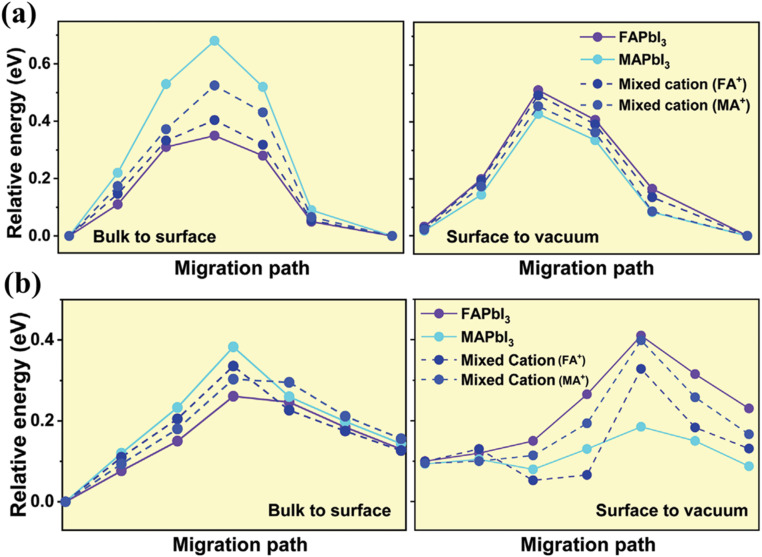
The study revealed that FA-rich crystals exhibit longer charge carrier lifetimes, whereas MA-rich crystals show faster recombination, attributed to a higher density of surface defects in the latter. These defects hinder efficient charge carrier transport, hence limiting performance. To explore the impact of cation selection on ion migration, DFT calculations were performed on pristine FAPbI3 (FA-rich), MAPbI3 (MA-rich), and mixed-cation FA0.5MA0.5PbI3 compositions. Migration pathways for FA+, MA+, and iodide (I−) ions were also analyzed, revealing that FA+ ions exhibit the lowest energy barrier for migration from bulk to the surface, while MA+ ions have the highest. Conversely, from surface to vacuum, MA+ ions showed lower diffusion energy, potentially leading to surface vacancies. In addition, iodide ions demonstrated varied energy barriers depending on the composition: FAPbI3 showed the lowest energy for bulk-to-surface migration (0.26 eV) but higher energy (0.41 eV) from surface-to-vacuum, while MAPbI3 displayed a higher bulk energy (0.383 eV) but a low surface energy (0.185 eV), encouraging vacancy formation.
In mixed-cation systems, the I− energy barriers were intermediate, with migration behavior dependent on the dominant cation. Crystals with higher FA+ content facilitated surface passivation by enabling iodide migration to the surface while preventing escape due to higher energy barriers. This behavior supports the role of iodide ions in reducing surface vacancies and enhancing stability. The findings highlight the critical influence of cation composition on ion migration, with FA-rich crystals exhibiting more effective passivation and stability compared to MA-rich systems. These insights advance understanding of ion dynamics in perovskite single crystals, offering pathways for performance optimization. The gist of the investigation is presented in Fig. 8.
Fig. 8. (a) Energy barrier for cations ions (FA+, MA+) diffusion into two different regimes named bulk to surface and surface to vacuum, for pristine-FAPbI3 and MAPbI3 and the mixed cation FA0.5MA0.5PbI3; (b) the energy barrier for iodide ions from the bulk to the vacuum reveals that in FA-rich compositions, I− ions exhibit the lowest energy for migration from the bulk to the surface, but the highest energy from the surface to the vacuum, preventing them from escaping the surface and forming vacancies. Figure reproduced with permission from ref. 89.
2.2.3. Hysteresis
Hysteresis negatively impacts the consistency of photovoltaic operation and the durable stability of hybrid PSCs. The eminent research group comprised of Snaith and other co-authors15 first reported on hysteresis in 2014, linking it to factors including the perovskite material properties, selective contact materials, along with the external scanning parameters including scanning rate as well as applied electric field. Even though the precise origin of hysteric effects remains uncertain, various mechanisms have been anticipated to explain this phenomenon in PSCs. Various factors, such as ferroelectric polarization,90 ion migration linked with changes in the interfacial field and barriers which occur owing to the gathering of ions at interfaces, slow temporary capacitive effects,91 as well as the trapping and non-trapping of carriers at interfaces,92 ferroelectric polarization,93 are considered potential explanations for the occurrence of hysteresis in PSCs.10,16,17 It is widely acknowledged that the accumulation of defects at the perovskite/transport layer interface causes hysteresis by influencing the electric field distribution within the device. Numerous theoretical and experimental studies have determined the time scales associated with the migration of defect species in perovskites by analysing hysteresis behaviours as a function of temperature and the sweep rate during J–V measurements.94–97 However, the exact origin of these ionic defects remains a topic of ongoing discussion. To comprehend hysteresis in perovskite solar cells, identifying the location of ionic defects and estimating their concentration within the device is crucial. Impedance spectroscopy is a particularly effective technique for obtaining this information. Typically, impedance spectra for perovskite solar cells exhibit both high- and low-frequency responses. The high-frequency response has been linked to factors such as geometric capacitance, chemical capacitance, dipole depolarization, or ionic diffusion within the perovskite active layer.98–101 On the other hand, the low-frequency response has been associated with factors such as the impedance of trap states, device degradation, dielectric effects, electron accumulation at the contacts, and ionic diffusion.102–105
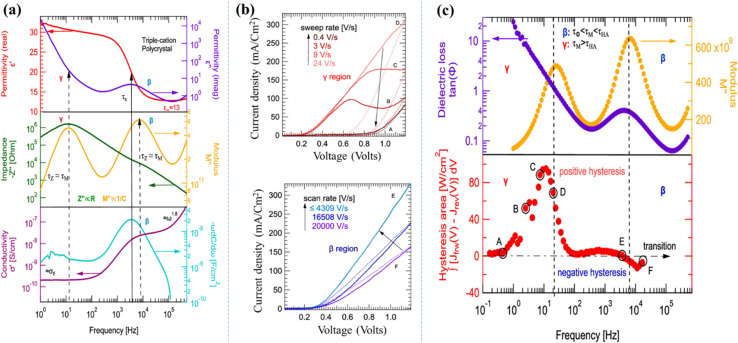
The prominent research group led by S. Tammireddy investigated ionic conduction in Cs0.05(FA0.83-MA0.17)0.95Pb(I0.9Br0.1)3 perovskite solar cells using impedance spectroscopy and sweep-rate-dependent J–V measurements as a function of temperature.73 To confirm the location of defects, they performed impedance spectroscopy on an MAPbI3-based device as reference. They have observed both low- and high-frequency responses in impedance spectra and linked their origins to ionic defects. However, these partially conflicting interpretations hinder a clear understanding of defects in perovskites, complicating the analysis of their impact on device hysteresis. To overcome this challenge, they employed a combination of sweep-rate-dependent J–V measurements and impedance spectroscopy, offering a unique approach to reconcile conflicting assumptions and directly correlate defect signatures with hysteretic behaviour. As a result, two bulk defects, β and γ, were identified, both linked to long-range ionic conduction. Defect β, likely iodine-related, is associated with dielectric relaxation and causes positive hysteresis, while defect γ, constrained by the perovskite/transport layer interface, leads to negative hysteresis. The N-shapes observed in J–V curves of polycrystalline Cs0.05(FA0.83-MA 0.17)0.95Pb(I0.9Br0.1)3 based device at lower sweep rates are attributed to ion accumulation and redistribution near the hole transport layer. The impedance spectroscopy and J–V analyses suggest that defect γ is a negatively charged ionic defect, such as an iodide interstitial, and both defects may originate from the same ionic species. These findings, represented in the Fig. 9, establish a clear connection between ionic conduction and hysteresis in perovskites.
Fig. 9. (a) The impedance, permittivity, modulus, and conductivity characteristics of the Cs0.05(FA0.83-MA 0.17)0.95Pb(I0.9Br0.1)3 solar cell was analysed at 360 K. Two distinct responses, labelled as β and γ, were observed in the intermediate and low-frequency regions, respectively. (b) J–V sweeps of the Cs0.05(FA0.83-MA0.17)0.95Pb(I0.9Br0.1)3 solar cell evaluated as a function of sweep rate at 360 K. (c) The hysteresis area as a function of frequency is categorized in A–F. Figure reproduced with permission from ref. 73.
3. Stability of perovskite solar cells
3.1. Stability against moisture
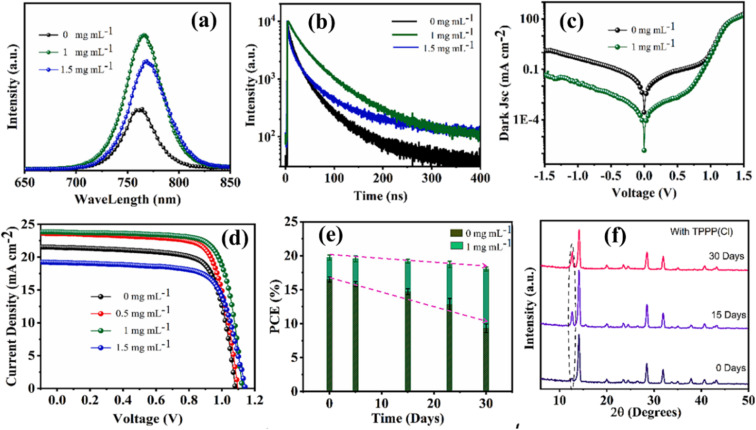
Enhancing the moisture resistance of PSCs can be effectively achieved through the modification of the collecting electrode. Among various metal electrodes, copper (Cu) emerges as an encouraging contender because of its potential for widespread implementation on a large scale and its demonstrated long-term stability.106 The well-known researcher Zhao107 employed the doctor-blade method to incorporate a copper (Cu) electrode into PSCs. In their study, the devices featuring the Cu electrode exhibited impressive performance, achieving a PCE exceeding 20%. Furthermore, these devices sustained 98% of their initial PCE even after being kept for over 800 hours in atmospheric conditions (25 °C, approximately 55% relative humidity), all without the need for encapsulation. In contrast, PSCs equipped with Al or Ag electrodes experienced a notable decrease in efficiency, with approximately 20% loss of the initial PCE observed after 200 hours of storing in ambient air.108 The global scientific community has extensively researched the improvement of moisture-resistant PSCs. Typically, the fabrication process for PSCs requires the use of an inert atmosphere to ensure optimal performance and longevity in the presence of moisture. The research team made up of Tai and his colleagues109 presented a novel approach to fabricating PSCs in ambient circumstances, regardless of humidity levels. In their method, they substituted (Pb(SCN)2) for the conventional precursor PbI2, and dimethylsulfoxide served as the solvent. The researchers achieved promising results with an average efficiency of 13.49% from a total of 20 devices prepared under relative humidity conditions exceeding 70%. In another study led by Troughton et al.,110 ethyl acetate was adopted as an antisolvent during the spin-coating process. Ethyl acetate served a dual purpose, acting both as a wetness soaking throughout the spin coating and contributing to the overall moisture resistance of the fabricated PSCs. The reported PCE reached up to 15%, even under challenging conditions with a relative humidity of 75%. The well-known researcher Li111 proposed the incorporation of phosphonic acid additives in the fabrication process. They applied a spin coating tactic to deposit the precursor solution containing MAPbI3 in the existence of butylphosphonic acid 4-ammonium chloride. The additive phosphonic acid played a dual role by establishing cross-links between adjacent grains via hydrogen bonding between –NH3 and –PO(OH)2. Additionally, it demonstrated heightened resistance to moisture. Moisture-resistant HTLs have been employed as a strategy to address the susceptibility of perovskite devices to moisture. These layers not only serve their primary function but also act as effective encapsulants, providing an additional protective barrier against moisture-induced degradation.112 The well-established research group comprised of Habisreutinger and his co-authors113 employed a P3HT/SWNT nanohybrid mesh as the HTL, filled with a hydrophobic polymer PMMA. The devices underwent exposure to a stream of flowing water and subsequent testing, demonstrating nearly identical performance to the pre-exposure state. In a recent paper published in 2024 and conducted by Azam et al.,114 they investigated a unique technique to improve the efficiency and longevity of the MAPbI3 PSCs via the application of an additive named tetraphenylphosphonium chloride (TPPP(Cl)). Consequence of the incorporation of TPPP(Cl) into the perovskite, the perovskite film's crystallinity is increased by the presence of TPPP(Cl), and halide ion defects can be passivated by the Cl from TPPP(Cl), which eventually reduces defect density and enhances the charge carrier mobility. Their fabricated device showed excellent stability owing to the utilization of TPPP(Cl), and the device was able to retain 89% of the original PCE for more than 30 days in normal conditions without the use of any kind of encapsulation. In Fig. 10, the essence of this work is displayed.
Fig. 10. (a) The PL spectra; (b) TRPL spectra of original MAPbI3, and with 1 and 1.5 mg mL−1 of TPPP(Cl), (c) dark current density and voltage graph for 0 and 1 mg mL−1 TPPP(Cl); (d) The J–V curve with 0, 0.5, 1.0, 1.5 mg mL−1 TPPP(Cl) additive; (e) The durability of devices with and without additive TPPP(Cl) kept in dark with RH 50–60% and 25 °C temperature for 30 days; (f) XRD measurement on 0, 15, and 30 days under the normal environment of perovskite film with 1.0 mg mL−1 additive TPPP(Cl). Figure reprinted with permission from ref. 114.
3.2. Stability against UV-light
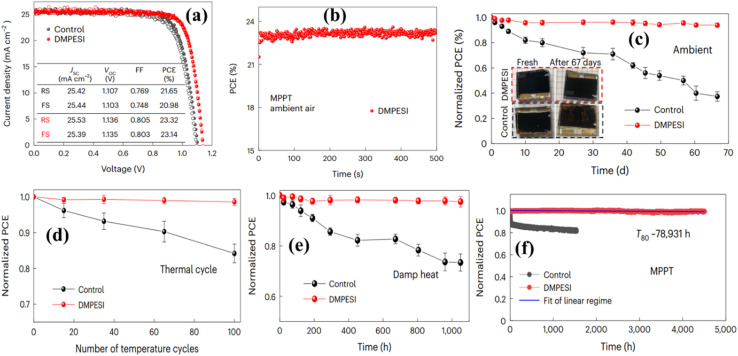
To address the effect of UV light, several approaches have been employed extensively. The first approach involves diminishing or neutralizing trap sites, while the second approach aims to preclude UV light to reach the ETL, particularly TiO2. Lastly, the third strategy entails substituting the TiO2 scaffold with an alternative material or eliminating TiO2 entirely. By substituting the TiO2 layer with alternative ETLs, incorporating a protective barrier between the light absorber and ETL, and implementing encapsulation techniques, the effect of UV light could be nullified. The assessment of wide bandgap SnO2 materials as an alternative to TiO2 for ETLs has been investigated, alongside research into other innovative materials. The eminent researcher named Roose115 showed that by substituting TiO2 for a SnO2 electron-selective contact allows for the stable operation of PSCs during UV light irradiation in an inert setting. Another renowned research group made up of Li and his co-workers26 employed Cesium bromide (CsBr) for interfacial surface management between the ETL and the perovskite absorber, resulting in a notable enhancement in UV stability. In another research conducted by Ouafi et al.,116 reported that enhancing the stability of MAPbI3 under UV exposure can be achieved by effectively incorporating bromide atoms into the perovskite structure. Specifically, the MAPbI3−3xBr3x perovskite demonstrates notably improved stability, particularly when the amount of bromide exceeds 20%. This improvement is thought to be associated with a structural change from the tetragonal to the stable cubic phase. In a non-encapsulated setting, S. Ito et al.21 perceived a significant decrease in the efficiency of PSC devices and almost nearly 0% PCE was observed after 12 hours of exposure. However, by incorporating a Sb2S3 buffer layer into the PSC, the efficiency augmented to approximately 65% of its original value. This enhancement can be attributed to the function of the Sb2S3 obstructive layer, which prevents UV-induced photocatalysis in ETL (TiO2) through offering a sort of passivation. Moreover, this additional layer aids in preserving the crystalline structure of MAPbI3 to be changed from black to completely yellow. In addition, Leijtens et al.45 witnessed that the degradation of the TiO2 ETL in the device occurs when exposed to the complete radiation spectrum with a projected intensity (I) of 100 mW cm−2 at 40 °C. In their study, they subjected unsealed, and sealed devices with an added UV filter to UV light in an inert settings and compared their efficiencies. Surprisingly, the sealed device showed less stability compared to the unsealed device, while the sealed device with a UV filter demonstrated superior performance in terms of efficiency. Another phenomenon perceived during the light-soaking experiments is the rebound of current density. In the study on photo-degradation, Lee et al.117 observed a rebound in current upon UV irradiation, which was ascribed to traps being passivated by PbI2 into MAPbI3 based devices, leading to improved charge conduction. Nonetheless, while initial irradiation may show a bounce back of cell parameters, prolonged exposure ultimately results in permanent deterioration in the devices. Numerous researchers have showcased stability for approximately 1000 hours or under more irradiation, with minimal to no decline in performance. The well-known research group comprised of Zheng and his groups118 formed novel mixed-dimensional PSCs by incorporating HOCH2CH2NH3I (EAI) into the (FAPbI3)0.85(MAPbBr3)0.15 3D perovskite structure. The mixed-dimensional devices exhibit significantly enhanced resistance to humidity, heat, and UV light. Following constant UV irradiation for 13 hours, all unsealed devices maintain PCEs at approximately 60% of their initial values (18.79%). In an investigation conducted by Sun et al.,119 reported that a silane coupling agent was applied between the ETL and the MAPbI3 layer, while a UV absorber was utilized to shield the MAPbI3 from harmful UV energy, which can compromise its stability. This configuration yields outstanding performance and chemical durability, resulting in an average PCE loss of 2.2%. Even when not encapsulated, the devices maintain over 80% of their original PCE even after being subjected to non-stop UV illumination for 24 hours at 60% RH, demonstrating heightened UV-light resilience and notable stability. Ensuring the long-term stability of devices requires addressing all factors contributing to degradation. Encapsulation stands out as a top strategy for providing comprehensive protection against external influences. In addition to encapsulation, there is a rising trend in transparent photovoltaics to meet aesthetic preferences, allowing for the exploration of multi-junction designs with finely tuned bandgaps. Composition engineering and innovative interfaces offer further avenues for mitigating photo-degradation concerns, as well as addressing issues related to moisture and oxygen exposure. The renowned researcher Suo120 utilized a sulfonium-based compound, specifically dimethylphenethylsulfonium iodide (DMPESI), for treating FAPbI3 perovskite films after deposition. After the treatment, the films exhibit enhanced stability during light exposure and maintain the desired α-black phase even after aging for two years in an ambient atmosphere without encapsulation. The PSCs treated with DMPESI demonstrate a performance decline of less than 1% over more than 4500 hours under maximum power point tracking (MPP), and also indicate a theoretical T80 exceeding 9 years under constant 1 sun light illumination. This work has been depicted in Fig. 11.
Fig. 11. (a) J–V curves of the control (black) and DMPESI (3 mg mL−1) treated (red) devices for reverse scan and forward scan; (b) PCE of the device with DMPESI treatment at MPP as a function of time at ambient settings without encapsulation; (c) dark shelf stability of unencapsulated without and with DMPESI PSCs under ambient settings at 20–40% RH; (d) temperature cycling (25–85 °C) test of encapsulated without and with DMPESI devices; (e) damp heat test (85 °C & 85% R.H.) of the encapsulated without and with DMPESI devices; (f) long-term operational stability of the unencapsulated without and with DMPESI devices under MPP tracking with continuous one sun irradiation under N2 flow at normal temperature. Reproduced with permission from ref. 120.
There are various ways in which perovskite materials might deteriorate due to UV radiation, which is the higher-energy portion of the solar spectrum. Under UV radiation, organic cations like FA are prone to breakdown, which leads to the destabilization of the perovskite structure. UV photons can create ROS, and once ROS are formed, the perovskite layer and surrounding materials (such as hole-transport layers) may be progressively degraded. Moreover, UV radiation can accelerate ion migration (of iodine ions, for example), which can cause phase segregation and perovskite film degradation, which can have an impact on stability and performance.121,122
Inorganic or mixed-cation perovskites, UV-blocking coatings, passivation layers, and other techniques have all been developed to increase the UV stability of FA-based perovskite solar cells. It may be possible to shield the perovskite from damaging UV light by adding UV-filtering layers on top of the perovskite layer. This can be accomplished by applying UV-stable polymers or coatings that absorb UV radiation before it reaches the perovskite and by utilizing transparent oxide layers (such as zinc oxide or titanium dioxide) that selectively block UV light. By stabilizing the crystal structure and inhibiting ion movement, passivating the surface of perovskite films with substances such as small molecules or fullerene derivatives might lessen the effects of UV deterioration.123–126 The UV stability of the perovskite also can be increased by combining FA with other inorganic cations, such as cesium (Cs). Compared with exclusively organic FA-based perovskites, cesium-based or mixed-cation perovskites have demonstrated superior resilience against UV-induced phase segregation and degradation.127 Since moisture and oxygen intrusion worsen the damage from UV radiation, encasing the solar cells in materials that block these elements can also slow down the rate at which the UV deteriorates. Research on FA-based perovskites has revealed that when these solar cells are exposed to UV light for an extended period of time without taking precautions, their efficiency might drop dramatically. It is possible to prolong the stability of FA-based PSCs under continuous illumination for several hundred hours by implementing passivating and UV-blocking techniques.127
Aiming for improved UV stability, FA-based perovskite solar cells are still being developed. For commercialization, it will be essential to combine UV protection techniques with other stability enhancements, such as improved encapsulation and material engineering.123,127 More resilient designs that strike a compromise between effectiveness and long-term durability under actual conditions including UV exposure are anticipated as a result of ongoing research.
3.3. Stability against oxygen
The key factor leading to the restricted lifespan of MAPbI3 PSCs under normal environmental conditions is the degradation induced by exposure to oxygen and light. Unprotected MAPbI3 based solar cells deteriorate quickly, typically within minutes to a few hours, when subjected to both light as well as dry air. Under conditions where there is an electrical bias driving current flow, even in the absence of light, rapid degradation of the PSCs is still observed when oxygen is present. The eminent research team led by D. Bryant31 showed that the degradation caused by exposure to light and oxygen can be mitigated by employing interlayers capable of extracting electrons from the perovskite prior to their interaction with oxygen to produce O2−, thus slowing down the degradation process. They have shown that enhancing electron extraction results in a decrease in the production of O2− and enhances the stability of the devices. The durability over time of optoelectronic devices incorporating hybrid lead halide perovskite will depend on the effectiveness of encapsulation and the presence of barrier layers. Another well-established research group led by Senocrate128 examined the effects of oxygen on halide perovskites, particularly MAPbI3. They have investigated the solubility of oxygen and its overall reaction with halide perovskites, both in the absence of light and the existence of light. After combining cations with the perovskite, the degradation rate decreased significantly.
3.4. Stability against temperature
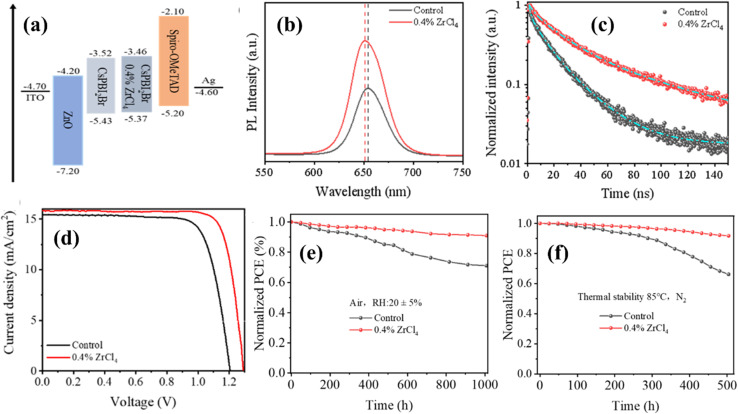
A successful approach for achieving thermal stability in devices involves the utilization of innovative materials or blends that exhibit greater thermal stability compared to their methylammonium counterparts. Utilizing compositions that incorporate partially substituted ions like formamidinium and cesium could be employed to produce robust devices. Additionally, employing encapsulation and improved interlayer techniques are effective methods for mitigating thermal degradation and issues related to ion migration. Subjecting layers like ZnO to heat treatment prior to deposition enhances efficiency and thermal stability by reducing the displacement of methylammonium at the interface.129 In pursuit of enhanced stability, numerous new HTLs have been investigated, including PTAA and KR131, which is a derivative of 5,10,15-trihexyl-3,8,13-tris(4-methoxyphenyl)-10,15-dihydro-5H-diindolo[3,2-a:3′,2′-c] carbazole.130 The incorporation of large aromatic PEA cations has been utilized to generate thermally stable 2-dimensional PSC, which retained 65% of their initial efficiency even after being subjected to 100 °C for 70 hours.131 The construction of multidimensional perovskite structures, blending elements of both 3-D and 2-D configurations with an intermediate dimension, resulted in the development of thermally stable PSC by lowering defect density.132 Chen et al.133 combined n-butylammonium along with the 4-phenylbutan-1-aminium and methylammonium to produce structures of (PBA0.5BA0.5)2MA3Pb4I13, demonstrating a PCE of 16.0%. The resulting structure maintained 60% of its initial PCE even after being subjected to heat at 85 °C for 30 days. Another researcher named Hu134 developed an active layer Cs0.1FA0.99PbI3 by blending cesium and formamidinium. Within this layer, a 2D (PEA)2PbI4 nano-sheets were incorporated into the photoactive layer, which hinders phase transformation. A PCE of 20.44% was achieved, and the structure maintained 82% of this PCE following an aging test of 800 hours at 85 °C. In another study done by Huang et al.,135 a PSC with a composition of FA0.85Cs0.15PbBr0.15I2.85 was formed by using methylamine additives in the chlorobenzene antisolvent. Initially, it achieved a PCE of 19.6%. Under prolonged exposure to 85 °C in a dark environment filled with nitrogen, the PSC maintained an efficiency of 88% over 500 hours. In addition, the eminent research team formed with Afroz et al.136 utilized oxalic acid as an additive in the perovskite solution through the crystal growth and they proposed that the inclusion of this compound improved crystallization, resulting in perovskite crystals characterized by higher grains, less surface defects, and reduced boundaries. The devices enhanced with the additive exhibited superior thermal stability, maintaining 90% of their original PCE after 9 hours at 100 °C temperature and 60% RH, which decreased to 70% after 19 hours. In contrast, the control device without the additive experienced a degradation of PCE to 14% within 9 hours. Another research group consisted of Choi et al.,137 reported a notably successful approach in enhancing the performance of PSCs involves implementing a zwitterion-modified tin dioxide (SnO2) layer as the ETL and utilizing an HTL that does not require dopants. This strategy ensures improved thermal stability for the PSCs. A zwitterionic compound, specifically 3-(1-pyridinio)-1-propanesulfonate, is employed to modify the SnO2 ETL layer. This zwitterion generates interfacial dipoles. The PSC employing the dopant-free HTL achieved an unprecedented efficiency of 20.5%, surpassing all other dopant-free polymeric HTLs utilizing environmentally friendly solvents. Additionally, the resultant PSCs demonstrated exceptional thermal stability even without encapsulation. Recently, Ma et al.,138 conducted an experiment to regulate crystal development and enhance the overall quality of the film through Zirconium tetrachloride (ZrCl4) doping into CsPbI2Br. By replacing a portion of the Pb2+ ions in CsPbI2Br with Zr4+ ions inhibit the undesired transformation from the crystallized α-black phase to δ-phase, leading to enhanced stability of the phase.
As a result, the film exhibits significantly enhanced resistance to humidity and thermal fluctuations. Moreover, the introduction of ZrCl4 mitigates non-radiative recombination and establishes a compatible energy-level arrangement with the Spiro-OMeTAD as HTL. The fabricated device demonstrates remarkable efficiency reaching around 16.60%, accompanied by a notably high Voc of 1.29 V and the unencapsulated devices exhibit impressive resistance to both humidity and thermal conditions, maintaining over 91% of the initial efficiency after 1000 hours of aging test in ambient air and it also retained 92% of the initial PCE after non-stop heating at 85 °C for 500 hours in a N2 environment. Fig. 12 exhibited the summary of the work. Moreover, in-service PSCs face efficiency and durability challenges due to heat accumulation under light irradiation. A new strategy enhances thermal conductivity and diffusivity by adding multi-walled carbon nanotubes (MWCNTs) to the perovskite film, enhancing heat dissipation and reducing thermal stress.139 MWCNTs boost the film's crystallinity, reduce defects, and lower operational temperatures, reducing from 42.5 °C to 38.5 °C. This temperature reduction mitigates performance loss, which leads to higher PCEs of 11.78% for CsPbIBr2, 15.14% for CsPbI2Br, and 22.13% and 23.05% for regular and inverted (FA0.83MA0.17)0.95Cs0.05Pb(I0.9Br0.1)3. The optimized devices maintain >94% efficiency over 2800 hours in air without encapsulation, with improved thermal stability (1.5 times) at 85 °C for 1300 hours and enhanced operation stability (40-fold) over 350 hours. This approach offers a promising path toward more efficient and stable perovskite-based solar technologies.
Fig. 12. (a) Schematic energy-level diagram of CsPbI2Br PSCs without and with the 0.4% ZrCl4 dopant; (b) PL of the without and with 0.4% ZrCl4-doped CsPbI2Br films. (c) TRPL of the without and with 0.4% ZrCl4-doped CsPbI2Br films. (d) J–V curves of the PSCs without and with the 0.4% ZrCl4 dopant. (e) Air storage stability and (f) thermal stability of the PSCs without and with 0.4% ZrCl4. Figure reprinted with permission from ref. 138.
Despite FA's intrinsic stability benefits over MA, temperature-induced instability still poses a problem for FA-based PSCs. In order to stabilize the perovskite structure at high temperatures, researchers have created mixed cation perovskites by combining tiny quantities of Cs+ and MA+ with FA. For example, Cs/FA mixed-cation perovskites have demonstrated increased resilience to heat degradation and phase transitions.56 Furthermore, it has been demonstrated that adding halide ions like Br− stabilizes the α-phase and reduces undesirable phase transitions. Glass and polymer encapsulation are two encapsulation techniques that have been thoroughly researched to stop degradation induced by exposure to moisture and heat. After prolonged exposure to high temperatures, encapsulated FA-based perovskites exhibit noticeably better thermal stability, maintaining up to 85% of their initial efficiency. It has been discovered that adding additives to FA-based perovskite films, such as formic acid or lead thiocyanate (Pb(SCN)2), improves stability by encouraging improved crystal formation and lowering defect densities. These compounds enhance moisture resistance and thermal stability by lowering hysteresis. According to tests conducted at operating temperatures between 60 and 85 °C, FA-based PSCs retain about 80% of their efficiency for 500 hours.140
Interface engineering is important for gaining high thermal stability since the performance deterioration is mostly caused by ion migration and interface degradation rather than bulk phase shifts. Accelerated aging studies conducted at 100 °C reveal that non-encapsulated FA-based perovskites degrade quickly. Advanced encapsulation or cation and anion engineering, on the other hand, greatly reduces degradation rates. Despite extended heating, encapsulated devices can preserve up to 90% of their original efficiency.56,66,141 Further developments in additive integration, encapsulation, and compositional engineering have promise for improving the thermal stability of FA-based PSCs and bringing them closer to commercial feasibility.
3.5. Stability against crystal defects
Beyond simply recognizing the primary factors contributing to the deterioration PSCs, it is imperative to prioritize enhancing stability through effective strategies to a degree that renders the technology commercially viable and appealing. Perovskite films featuring larger grain sizes have been shown to possess decreased trap state density and fewer recombination centres, which contribute positively to the enhanced performance of PSCs.142,143 The size of grains in polycrystalline perovskite films is closely linked to the rate at which crystallization occurs during the film development process. Several studies suggest that a slower crystallization process likely decreases the number of nucleation events and promotes larger grain sizes.143,144 A viable approach to reduce the crystallization rate involves employing the solvent annealing technique, which entails subjecting perovskite films to thermal annealing in the presence of N,N-dimethylformamide or dimethyl sulfoxide vapors.145 Because both MAI and PbI2 possess high solubility in DMF/DMSO, the ions and molecules within the precursor are able to diffuse over larger distances in a moist DMF/DMSO vapor environment. This results in a slower crystallization process and the enlargement of grain sizes, reaching approximately 1 μm. Consequently, the charge-recombination lifetime of devices subjected to solvent annealing increased to 7.2 μs, significantly longer than the 1.7 μs observed in thermally annealed devices, indicating a reduction in defects within the resulting perovskite films. Pyridine, known for its effective defect passivation within perovskite films, was employed in combination with the solvent annealing procedure, leading to the formation of MAPbI3 films featuring grain sizes of up to 5 μm. Moreover, the defect density in the resulting films was decreased to half of that found in traditionally thermally annealed films.146,147 Several investigations have revealed that introducing a small quantity of H2O into the precursor solution enhances the solubility of PbI2. Treating perovskite films under specific humidity conditions has been shown to diminish the defects density.148,149 The research team formed with Zhou and his colleagues utilized the one-step spin-coating method to deposit perovskite films and conducted thermal annealing under controlled humidity conditions (30% RH) in air. They observed decreased non-radiative recombination in films exposed to humidity compared to those annealed in dry air settings.150 Another method is to regulate the development of perovskite films involves employing the Lewis acid–base adduct approach, which entails introducing a Lewis base as an electron–pair donor to coordinate with the lead precursor.151 The research group led by Jeon showed that152 by introducing a small quantity of MA can trigger the formation of the fundamentally stable black phase of FA perovskite, resulting in PSCs based on (FAPbI3)1−x(MAPbBr3)x achieving efficiencies surpassing 20%. By incorporating additional inorganic cesium, the resulting triple cation perovskites exhibit significantly enhanced reproducibility and improved device performance, achieving a high stabilized power output of 21.1%.153
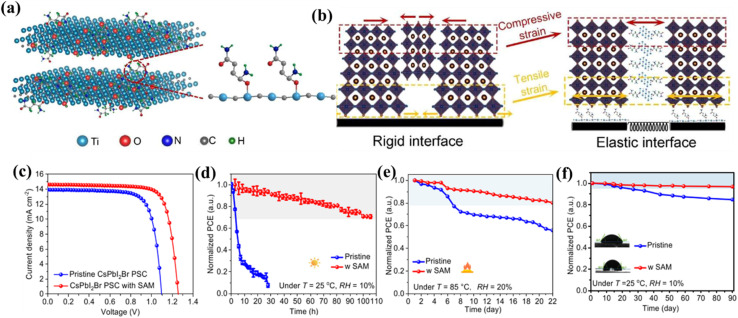
Furthermore, the research group led by X. Yao incorporated flexible alkenamides terminated Ti3C2Tx MXenes as an additive into perovskite films to initiate an elastic grain boundary that effectively regulates lattice strain and passivates defects.154 This integration stabilizes the lattice structure and grain boundaries, leading to notable increases in PCEs for all-inorganic CsPbIBr2 and CsPbI2Br PSCs, reaching 11.06% and 14.30%, respectively. These enhancements also impart excellent long-term durability to the cells, even under challenging conditions. The details are depicted in Fig. 13. Moreover, Du et al.,155 published a paper in 2024 and they have shown that modifying interfaces proves to be a successful approach for enhancing the performance of PSCs. They utilized a material named 3,5-dibromobenzoic acid (3,5-DBBA) at the interface between the ETL and absorption layer. Their research also revealed that the bromine in 3,5-DBBA effectively neutralizes O2+ vacancies and unpaired Sn4+ defects within SnO2, optimizing the interface interaction between the ETL and the perovskite layer. Eventually, this process inhibits the non-radiative recombination of holes and electrons, which leads to enhancements in carrier lifetime along with extraction efficiency. Owing to this process, the Voc of PSCs saw an elevation of 3.5%, accompanied by an increase in PCE of around 16%.
Fig. 13. (a and b) Schematic diagram of alkenamides grafted MXene by forming Ti–O–C bond and residual strain relaxation by forming elastic interface and boundary. (c) Performance of CsPbI2Br based PSCs; stability under (d) continuous light irradiation, (e) T = 85 °C, RH = 20% and (f) T = 25 °C, RH = 10% conditions.
3.6. Stability against ions migration
The well-known issue of photocurrent hysteresis is widely recognized to be caused by ionic migration. Photocurrent hysteresis poses major challenges in accurately characterizing device performance, and there are also worries that ion migration compromises the stability of solar cells. Three primary strategies exist to mitigate ion migration in PSCs: (1) enhancing the formation energies of anion and cation vacancies; (2) effectively passivating defects at GBs and surfaces to raise the activation energy (Ea); (3) minimizing GBs through controlled crystal growth. The prominent research group formed by Jaffe and his colleagues156 conducted photoluminescence measurements on MAPbBr1.8I1.2 powder in 2016. They observed that the extent of halide segregation lessened as pressure increased, suggesting that compressing the material reduced halide migration. Mosconi and other co-authors157 showed that the methylammonium cation could facilitate defect migration by redistributing its charge distribution in conjunction with the movement of ions. The research team comprised of Zhao and co-authors158 suggested that the protonation effect would substantially reduce the activation energy (Ea) required for methylammonium cations migration. In another research conducted by Lin et al.,159 suggested that incorporating 5% Cs+ ions into MAPbI3 could inhibit ion migration within perovskites by creating extra energy barriers. This effect might be linked to the reinforcement of the inorganic PbI6 octahedral networks through Cs+ incorporation.
Grain boundaries serve as channels for ion migration, making the minimization of GBs a viable approach to suppress such migration. Notably, perovskite single crystals, devoid of GBs, offer a means to significantly diminish ion migration. Xing et al.,160 conducted measurements of temperature-dependent conductivity on both a perovskite single crystal and polycrystalline perovskite films with grain sizes of approximately 0.3 μm and 1 μm, respectively. They found that the activation energy of ion migration for the single crystal, small grains, and large grains were 1.05 eV, 0.27 eV, and 0.50 eV, respectively. This suggests that a larger grain size is advantageous for mitigating ion migration. The researcher Ke161 introduced a small quantity of Pb(SCN)2 with the perovskite precursor to notably enhance the grain size and crystalline quality of perovskite films. This enhancement was attributed to the generation of HSCN and CH3NH2 gases resulting from the reaction between methylammonium (MA+) and SCN− during the annealing process. The renowned research team comprised of Cai and his co-workers162 incorporated a suitable multifunctional molecule, 2,2-difluoropropanediamide (DFPDA), into the perovskite precursor to regulate the crystallization rate of perovskite and achieve high-quality films with reduced defects. Another effective strategy for mitigating ion migration involves interface engineering, wherein mobile charged defects are passivated and pathways for ion migration are eliminated. The researcher Zhao and his colleagues163 incorporated cesium acetate between the perovskite and the HTL. This intervention effectively inhibited ionic migration, such as that of methylammonium ions, during device operation, thereby safeguarding the HTL from ion penetration. Another group of Liu et al.,164 applied a KCl layer underneath the perovskite precursor solution during deposition. They verified that the KCl as a passivation layer does not interact with or diffuse into the perovskite layer from the analysis of EDX Spectroscopy and ToF-SIMS. KX, particularly KCl, effectively passivates both positively and negatively charged defects by utilizing K+ and Cl− ions, thereby abolishing the hysteresis observed in perovskite devices. In 2024, Dey et al.,165 investigated the effects of replacing lead with tin on the ion migration characteristics of halide perovskite devices by using a blend of experimental as well as computational methods. The decrease in short circuit current observed at lower scan rates (below 50 mV s−1) suggests that ion migration is predominant in both lead-based and lead-tin mixed halide PSCs. Nonetheless, the kinetics of ion transport appear to be hindered in mixed lead-tin systems, as indicated by hysteresis measurements dependent on scan rate. The findings are also supported by temperature-dependent impedance spectroscopy analyses conducted on the manufactured devices, both at open circuits and under light irradiation. These analyses indicate a significant reduction in ionic diffusion with the partial substitution of lead with tin. The results of this study are depicted in Fig. 14.
Fig. 14. J–V characteristic (light and dark) of (a) Pb-based PSC (with 2PACz HTL) and (b) Pb–Sn-based PSC (with PEDOT:PSS HTL) with a scan rate of 100 mV s−1; Nyquist plot of (c) a Pb-based PSC and (d) a Pb–Sn-based PSC recorded under irradiation with a 470 nm LED at open circuit and at 25 °C. Figure reprinted with permission from ref. 165.
3.7. Stability against hysteresis
The output current was found to be notably impacted by the time-dependent capacitive current, ultimately leading to the perceived hysteresis in the current–voltage characteristics.166 Concerning solar cell operation, a substantial capacitive current can result in either underestimation or overestimation of parameters due to the challenge of reaching a steady-state condition. This difficulty in achieving stability prevents the precise determination of photovoltaic parameters under specific conditions. Additionally, the unclear origin of transient behaviour hampers the complete utilization of MAPbI3 material and consequently restricts its potential applications in other fields. Various technical strategies have been suggested to mitigate hysteresis, including enhancing the interfacial charge relocation process, impeding ions migration, and minimizing defect density.167 Increasing grain size166 and employing GBs engineering168 have been also identified as effective means to diminish hysteresis. The conventional structure featuring an electron-transporting TiO2 and a hole-transporting Spiro-OMeTAD showed significant hysteresis in comparison to the inverted structure utilizing NiO and PCBM,169,170 which exhibited minimal hysteresis. One of the important approaches to improve the quality of the perovskite layer involves optimizing processing conditions, compositions, and the utilization of additives, which can also aid in reducing hysteresis. The well-known research group formed with Zhang and co-authors171 incorporated potassium thiocyanate as an additive into the perovskite precursor, resulting in better device performance and PSCs free from hysteresis. Grain boundaries and defects can be altered by combining 2D and 3D perovskite structures, which will eventually lower the hysteresis. Utilizing potassium thiocyanate aids in enlarging the grain size and enhancing the quality of the MAPBI3 film. The researcher Chen172 testified that by mixing a small quantity of phenethylammonium iodide (PEAI) into the precursor solution containing methylammonium iodide and PbI2, a significant reduction in the substantial hysteresis of the original MAPbI3 was achieved. Incorporating 2D PEA2PbI4 into MAPbI3 resulted in a significant reduction of hysteresis. The DFT calculations further suggest that the higher barrier energy of PEA2PbI4 more effectively impedes the migration of iodide vacancies compared to MAPbI3, contributing to the suppression of hysteresis. The researcher Pan and his colleagues173 discovered that depositing a 2D perovskite layer (C13H21Br2N)2PbI4 on top of the 3D layer (Cs0.05FA0.95PbI2.7Br0.3) could yield a stable device without hysteresis. The 2D layer plays a crucial role in passivation along with the hole extraction, resulting in the suppression of non-radiative recombination losses as well as the reduction of trap density. Many researchers regard interface engineering as an effective method for mitigating hysteresis.150 Interface engineering methods have been utilized to minimize hysteresis including doping or surface treatment of TiO2. During the fabrication process of PSCs, yttrium(iii) chloride treatment has been applied to the ETL layer, which results in improved photovoltaic performance along with reduced hysteresis. The yttrium(iii) chloride-treated TiO2 layer aids in aligning the energy levels and raising the conduction band, facilitating easy transfer and extraction of photo-generated electrons from the perovskite layer to TiO2. Furthermore, the presence of Cl− diminishes the aggregation of defects at the interface between TiO2 and perovskite, enhances the adhesion between TiO2 and perovskite, thus reducing interface recombination and suppressing hysteresis.174 In another paper, Zheng and other researchers showed that by applying a thin layer of MgO along with protonated ethanolamine, they effectively passivated the surface of ZnO, thereby transforming it into an efficient ETL material for PSCs with negligible hysteresis.175 They identified three significant outcomes of this modification aimed at mitigating hysteresis: (i) MgO prevents the rearrangement of charges at the interface, thus improving both the efficiency and durability of the PSC; (ii) protonated ethanolamine facilitates efficient electron transfer from the perovskite to ZnO, effectively eliminating hysteresis in the PSC; (iii) the modification ensures a compatible interface between ZnO and perovskite, effectively addressing the instability issues observed at the ZnO/perovskite interface. The prominent researcher Snaith and his co-authors176 opted for C60in lieu of a c-TiO2 layer as an effective ETL for PSCs to mitigate hysteresis. Additionally, they employed photoluminescence decay analysis to investigate electron transfer at the interfaces of perovskite/C60 as well as perovskite/TiO2. Their findings suggest that the electronic interaction between perovskite and C60 is notably stronger in comparison to that between perovskite and TiO2. The research group made up with Dipta177 and co-authors utilised a method for enhancing the efficiency of n-i-p structured PSCs by employing a double-sided passivation technique. Biphenyl-4,4-dicarboxylic acid was utilized to passivate the electron-collecting side, while n-octyl ammonium bromide was employed for passivating the hole-collecting side. The fabricated cell showed an increase in PCE from 18.7% in the control condition to 20.9% with the introduction of double-sided passivation. Surface imaging revealed enhancements in surface boundaries after passivation, leading to improved Voc and FF. Moreover, their study also revealed that passivation acts as a barrier against ion migration, thereby enhancing J–V hysteresis along with stability.
Perovskite solar cells have achieved remarkable efficiency and potentiality as a low-cost fabrication. However, despite its promising aspects, the commercial feasibility of PSCs is delayed by their inherent instability. Enhancement of the PSCs' stability is essential for realizing their potential in practical applications. The stable PSCs confirm a longer operational life, which makes them more competitive with conventional silicon-based solar cells. For widespread adoption, PSCs must exhibit durability under real-world conditions. By addressing stability issues by means of material engineering, passivation techniques, encapsulation, additive engineering, device architecture modification and optimization, and environmental conditioning, the durability and performance of PSCs could be significantly enhanced. By replacing volatile components with more stable alternative ones can enhance the thermal and chemical stability of perovskites. For instance, replacing methylammonium (MA) with formamidinium (FA) or cesium (Cs) can improve thermal stability. Using mixed cation and mixed anion compositions can also create more robust perovskite structures resistant to degradation.178 In addition, applying passivation layers on the surface of perovskite films can decrease the defect density and enhance the moisture resistance. Materials like phenethylammonium (PEA) iodide, biphenyl-4,4-dicarboxylic acid, pyridine, KCl has shown its promise in passivating perovskite surfaces.146,147,164 Also, passivating GBs with compounds or the functional groups of sulfonate or thiol can prevent moisture ingress and ion migration, which are treated as degradation pathways.179 Furthermore, introducing additives like fullerene derivatives, 2D materials, or ionic liquids into the perovskite layer can improve stability by suppressing defect formation and ion migration.180 Moreover, optimizing the interfaces between the perovskite and charge transport layers can diminish non-radiative recombination and enhance stability. Besides, using UV filters or designing the PSCs to minimize UV exposure can prevent photo-induced degradation. Additionally, performing fabrication under controlled humidity and temperature conditions can prevent the incorporation of detrimental species into the perovskite layer. Finally, employing encapsulation techniques by means of barrier materials like ethylene vinyl acetate (EVA) or glass can protect PSCs from environmental factors such as moisture, oxygen, and UV radiation.10 For flexible PSCs, advanced polymers and multilayer encapsulation strategies can provide effective protection while maintaining flexibility.
4. Summary and prospective
The efficiency of perovskite solar cells has quickly surpassed 26.7% for laboratory-scale devices. However, improvements in stability and reliability are urgently needed for successful commercialization. This review comprehensively explored the degradation factors which influencing the performance of PSCs, including both inherent and extrinsic factors. Key degradation elements such as moisture, temperature, UV-light and oxygen as extrinsic and crystal defects, ion-migration, hysteresis and hydrophilicity as intrinsic have been identified and discussed in detail. In addition, this study conducted a thorough analysis of the degradation mechanisms in PSCs and elucidated the underlying influence of both extrinsic and intrinsic factors on the instability of PSCs. By reviewing existing research, it identified various strategies and approaches employed to mitigate degradation and enhance performance of the PSCs. Encapsulation, interfacial layer engineering, additive engineering, compositional engineering, and dimensional modification approach have been adopted in literature and applied to suppress the degradation factors and enhance stability and efficiency of PSCs. By identifying and addressing these stability issues and solutions, this review signals promising paths for future research and development in the field of PSCs.
Different perovskite structures face degradation issues in different scales and exhibit new degradation pathway with new chemistry due to their different in chemical structures, which requires different strategies to handle. To date, all degradation processes have remained poorly known. Various characterization approaches including spectroscopy, electrochemistry, and electron microscopy are used to investigate the degradation mechanisms in PSCs. Combining these strategies with appropriate theoretical background can assist to improve understanding of deterioration. More study should be conducted to improve knowledge of degradation mechanisms. Therefore, knowledge will be extremely useful in implementing measures to increase the efficiency and stability of PSCs. Several strategies have been utilized to overcome the elements of instability. Additive engineering appears to be one of the most promising current trends. More study should be conducted on different innovative additives, such as Lewis acid/base, interfacial layer, and surface modifiers. These have been shown to have a significant impact on the perovskite film, as well as to improve stability against moisture and heat. In addition, small functional molecules, polymers, ligands, perovskite quantum dots, low-dimensional perovskite, and other materials can be used to control the degradation factors and passivate the flaws of the perovskite structure, so contributing to the increase of stability. Furthermore, appropriate charge transporting layers also could carry a significant importance to blockade the degradation factors. Moreover, combining encapsulation techniques with advanced material engineering could yield PSCs with superior resilience to environmental and intrinsic stressors. Additionally, deeper exploration of perovskite material compositions, structural optimizations and optimizations of synthesis process could unlock further improvements in stability and performance. Prospective studies could also prioritize the investigation of degradation in real-world operating conditions to guide the design of PSCs suited for commercial and industrial applications.
Consent for publication
All authors of this work have agreed and are ready to sign the Transfer of Copyright which empowers the Publisher to protect the work against unauthorized use and to maintain the integrity of the work from a bibliographical and archival standpoint.
Data availability
All data are available in the manuscript.
Author contributions
Md. Helal Miah & Md. Bulu Rahman: conceptualization, literature review, performed study, data analysis and writing of original draft, Mohammad Aminul Islam, Mohammad Nur-E-Alam, Md Rezaur Rahman & Md. Habib Ullah: data curation, discussion, software and validation, Mayeen Uddin Khandaker: supervision, and reviewing and editing of the manuscript.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work received no fund. Sunway University fully funded PhD scholarship awarded to Md. Helal Miah is acknowledged.
References
- Yang Z. Guo Y. Li H. Zhou Y. Zuo X. Yu Y. et al., Roles of interfacial tension in regulating internal organization of low bandgap polymer bulk heterojunction solar cells by polymer additives. Adv. Mater. Interfaces. 2018;5(15):1800435. [Google Scholar]
- Noel N. K. Stranks S. D. Abate A. Wehrenfennig C. Guarnera S. Haghighirad A.-A. et al., Lead-free organic–inorganic tin halide perovskites for photovoltaic applications. Energy Environ. Sci. 2014;7(9):3061–3068. [Google Scholar]
- Thirugnanasambandam M. Iniyan S. Goic R. A review of solar thermal technologies. Renewable Sustainable Energy Rev. 2010;14(1):312–322. [Google Scholar]
- Li N. Niu X. Chen Q. Zhou H. Towards commercialization: the operational stability of perovskite solar cells. Chem. Soc. Rev. 2020;49(22):8235–8286. doi: 10.1039/d0cs00573h. [DOI] [PubMed] [Google Scholar]
- Zsiborács H. Baranyai N. H. Vincze A. Zentkó L. Birkner Z. Máté K. et al., Intermittent renewable energy sources: the role of energy storage in the european power system of 2040. Electronics. 2019;8(7):729. [Google Scholar]
- Miah M. H. Rahman M. B. Khatun F. Khandaker M. U. Hatta S. F. W. M. Soin N. B. et al., Optimization and detail analysis of novel structure Pb-free CsGeI3-based all-inorganic perovskite solar cells by SCAPS-1D. Optik. 2023;281:170819. [Google Scholar]
- Guerrero A. Juarez-Perez E. J. Bisquert J. Mora-Sero I. Garcia-Belmonte G. Electrical field profile and doping in planar lead halide perovskite solar cells. Appl. Phys. Lett. 2014;105(13):133902. [Google Scholar]
- Salhi B. Wudil Y. S. Hossain M. K. Al-Ahmed A. Al-Sulaiman F. A. Review of recent developments and persistent challenges in stability of perovskite solar cells. Renewable Sustainable Energy Rev. 2018;90:210–222. [Google Scholar]
- Rahman M. B. Miah M. H. Khandaker M. U. Islam M. A. Selection of a compatible electron transport layer and hole transport layer for the mixed perovskite FA0.85Cs0.15Pb(I0.85Br0.15)3, towards achieving novel structure and high-efficiency perovskite solar cells: a detailed numerical study by SCAPS-1D. RSC Adv. 2023;13(25):17130–17142. doi: 10.1039/d3ra02170j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miah M. H. Rahman M. B. Nur-E-Alam M. Das N. Soin N. B. Hatta S. F. W. M. et al., Understanding the degradation factors, mechanism and initiatives for highly efficient perovskite solar cells. ChemNanoMat. 2023;9(3):e202200471. [Google Scholar]
- Green M. A. Dunlop E. D. Yoshita M. Kopidakis N. Bothe K. Siefer G. et al., Solar cell efficiency tables (Version 63) Prog. Photovoltaics. 2024;32(1):3–13. [Google Scholar]
- Min H. Lee D. Y. Kim J. Kim G. Lee K. S. Paik M. J. et al., Perovskite solar cells with atomically coherent interlayers on SnO2 electrodes. Nature. 2021;598:444. doi: 10.1038/s41586-021-03964-8. [DOI] [PubMed] [Google Scholar]
- Kojima A. Teshima K. Shirai Y. Miyasaka T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009;131:6050. doi: 10.1021/ja809598r. [DOI] [PubMed] [Google Scholar]
- Chowdhury T. A. Zafar M. A. B. Islam M. S.-U. Shahinuzzaman M. Islam M. A. Khandaker M. U. Stability of perovskite solar cells: issues and prospects. RSC Adv. 2023;13(3):1787–1810. doi: 10.1039/d2ra05903g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith H. J. Abate A. Ball J. M. Eperon G. E. Leijtens T. Noel N. K. et al., Anomalous Hysteresis in Perovskite Solar Cells. J. Phys. Chem. Lett. 2014;5(9):1511–1515. doi: 10.1021/jz500113x. [DOI] [PubMed] [Google Scholar]
- Azpiroz J. M. Mosconi E. Bisquert J. De Angelis F. Defect migration in methylammonium lead iodide and its role in perovskite solar cell operation. Energy Environ. Sci. 2015;8(7):2118–2127. [Google Scholar]
- Elumalai N. K. Uddin A. Hysteresis in organic-inorganic hybrid perovskite solar cells. Sol. Energy Mater. Sol. Cells. 2016;157:476–509. [Google Scholar]
- Pazos-Outón L. M. Xiao T. P. Yablonovitch E. Fundamental efficiency limit of lead iodide perovskite solar cells. J. Phys. Chem. Lett. 2018;9(7):1703–1711. doi: 10.1021/acs.jpclett.7b03054. [DOI] [PubMed] [Google Scholar]
- Chen B. Rudd P. N. Yang S. Yuan Y. Huang J. Imperfections and their passivation in halide perovskite solar cells. Chem. Soc. Rev. 2019;48(14):3842–3867. doi: 10.1039/c8cs00853a. [DOI] [PubMed] [Google Scholar]
- Niu G. Guo X. Wang L. Review of recent progress in chemical stability of perovskite solar cells. J. Mater. Chem. A. 2015;3(17):8970–8980. [Google Scholar]
- Ito S. Tanaka S. Manabe K. Nishino H. Effects of surface blocking layer of Sb2S3 on nanocrystalline TiO2 for CH3NH3PbI3 perovskite solar cells. J. Phys. Chem. C. 2014;118(30):16995–17000. [Google Scholar]
- Philippe B. Park B.-W. Lindblad R. Oscarsson J. Ahmadi S. Johansson E. M. J. et al., Chemical and Electronic Structure Characterization of Lead Halide Perovskites and Stability Behavior under Different Exposures—A Photoelectron Spectroscopy Investigation. Chem. Mater. 2015;27(5):1720–1731. [Google Scholar]
- Singh R. Ghosh S. Subbiah A. S. Mahuli N. Sarkar S. K. ALD Al2O3 on hybrid perovskite solar cells: Unveiling the growth mechanism and long-term stability. Sol. Energy Mater. Sol. Cells. 2020;205:110289. [Google Scholar]
- Huang Y. Wu S. Chen R. Fang S. Zhang S. Wang G. et al., Efficient methylamine-containing antisolvent strategy to fabricate high-efficiency and stable FA0.85Cs0.15Pb(Br0.15I2.85) perovskite solar cells. ACS Appl. Mater. Interfaces. 2019;11(20):18415–18422. doi: 10.1021/acsami.9b03323. [DOI] [PubMed] [Google Scholar]
- Saliba M. Matsui T. Seo J.-Y. Domanski K. Correa-Baena J.-P. Nazeeruddin M. K. et al., Cesium-containing triple cation perovskite solar cells: improved stability, reproducibility and high efficiency. Energy Environ. Sci. 2016;9(6):1989–1997. doi: 10.1039/c5ee03874j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. Zhang W. Van Reenen S. Sutton R. J. Fan J. Haghighirad A. A. et al., Enhanced UV-light stability of planar heterojunction perovskite solar cells with caesium bromide interface modification. Energy Environ. Sci. 2016;9(2):490–498. [Google Scholar]
- Su H. Wu T. Cui D. Lin X. Luo X. Wang Y. et al., The Application of Graphene Derivatives in Perovskite Solar Cells. Small Methods. 2020;4(10):2000507. [Google Scholar]
- Yuan Y. Huang J. Ion Migration in Organometal Trihalide Perovskite and Its Impact on Photovoltaic Efficiency and Stability. Acc. Chem. Res. 2016;49(2):286–293. doi: 10.1021/acs.accounts.5b00420. [DOI] [PubMed] [Google Scholar]
- Delugas P. Caddeo C. Filippetti A. Mattoni A. Thermally Activated Point Defect Diffusion in Methylammonium Lead Trihalide: Anisotropic and Ultrahigh Mobility of Iodine. J. Phys. Chem. Lett. 2016;7(13):2356–2361. doi: 10.1021/acs.jpclett.6b00963. [DOI] [PubMed] [Google Scholar]
- Saidaminov M. I. Kim J. Jain A. Quintero-Bermudez R. Tan H. Long G. et al., Suppression of atomic vacancies via incorporation of isovalent small ions to increase the stability of halide perovskite solar cells in ambient air. Nat. Energy. 2018;3(8):648–654. [Google Scholar]
- Bryant D. Aristidou N. Pont S. Sanchez-Molina I. Chotchunangatchaval T. Wheeler S. et al., Light and oxygen induced degradation limits the operational stability of methylammonium lead triiodide perovskite solar cells. Energy Environ. Sci. 2016;9(5):1655–1660. [Google Scholar]
- Frost J. M. Butler K. T. Brivio F. Hendon C. H. Van Schilfgaarde M. Walsh A. Atomistic origins of high-performance in hybrid halide perovskite solar cells. Nano Lett. 2014;14(5):2584–2590. doi: 10.1021/nl500390f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B.-A. Lin J.-T. Suen N.-T. Tsao C.-W. Chu T.-C. Hsu Y.-Y. et al., In Situ Identification of Photo- and Moisture-Dependent Phase Evolution of Perovskite Solar Cells. ACS Energy Lett. 2017;2(2):342–348. [Google Scholar]
- Wang S. Jiang Y. Juarez-Perez E. J. Ono Luis K. Qi Y. Accelerated degradation of methylammonium lead iodide perovskites induced by exposure to iodine vapour. Nat. Energy. 2016;2(1):16195. [Google Scholar]
- Christians J. A. Miranda Herrera P. A. Kamat P. V. Transformation of the excited state and photovoltaic efficiency of CH3NH3PbI3 perovskite upon controlled exposure to humidified air. J. Am. Chem. Soc. 2015;137:1530. doi: 10.1021/ja511132a. [DOI] [PubMed] [Google Scholar]
- Lee S.-R. Lee D. Choi S.-G. Jung S.-K. Lee J.-H. Kim M.-c. et al., Accelerated Degradation of FAPbI3 Perovskite by Excess Charge Carriers and Humidity. Sol. RRL. 2024;8(5):2300958. [Google Scholar]
- Gan Z. Yu Z. Meng M. Xia W. Zhang X. Hydration of mixed halide perovskites investigated by Fourier transform infrared spectroscopy. APL Mater. 2019;7(3):031107. [Google Scholar]
- Segovia R. Qu G. Peng M. Sun X. Shi H. Gao B. Evolution of Photoluminescence, Raman, and Structure of CH3NH3PbI3 Perovskite Microwires Under Humidity Exposure. Nanoscale Res. Lett. 2018;13(1):79. doi: 10.1186/s11671-018-2470-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. Wu Z. Dou Y. Zhang J. Bu T. Zhang K. et al., Formamidinium-Based Perovskite Solar Cells with Enhanced Moisture Stability and Performance via Confined Pressure Annealing. J. Phys. Chem. C. 2020;124(23):12249–12258. [Google Scholar]
- Jeong J. Kim M. Seo J. Lu H. Ahlawat P. Mishra A. et al., Pseudo-halide anion engineering for α-FAPbI3 perovskite solar cells. Nature. 2021;592(7854):381–385. doi: 10.1038/s41586-021-03406-5. [DOI] [PubMed] [Google Scholar]
- Yue W. Yang H. Cai H. Xiong Y. Zhou T. Liu Y. et al., Printable High-Efficiency and Stable FAPbBr3 Perovskite Solar Cells for Multifunctional Building-Integrated Photovoltaics. Adv. Mater. 2023;35(36):2301548. doi: 10.1002/adma.202301548. [DOI] [PubMed] [Google Scholar]
- Marchezi P. E. Therézio E. M. Szostak R. Loureiro H. C. Bruening K. Gold-Parker A. et al., Degradation mechanisms in mixed-cation and mixed-halide CsxFA1−xPb(BryI1−y)3 perovskite films under ambient conditions. J. Mater. Chem. A. 2020;8(18):9302–9312. [Google Scholar]
- Fujishima A. Rao T. N. Tryk D. A. Titanium dioxide photocatalysis. J. Photochem. Photobiol., C. 2000;1(1):1–21. [Google Scholar]
- Habisreutinger S. N. Leijtens T. Eperon G. E. Stranks S. D. Nicholas R. J. Snaith H. J. Carbon nanotube/polymer composites as a highly stable hole collection layer in perovskite solar cells. Nano Lett. 2014;14(10):5561–5568. doi: 10.1021/nl501982b. [DOI] [PubMed] [Google Scholar]
- Leijtens T. Eperon G. E. Pathak S. Abate A. Lee M. M. Snaith H. J. Overcoming ultraviolet light instability of sensitized TiO2 with meso-superstructured organometal tri-halide perovskite solar cells. Nat. Commun. 2013;4(1):2885. doi: 10.1038/ncomms3885. [DOI] [PubMed] [Google Scholar]
- Nakamura I. Negishi N. Kutsuna S. Ihara T. Sugihara S. Takeuchi K. Role of oxygen vacancy in the plasma-treated TiO2 photocatalyst with visible light activity for NO removal. J. Mol. Catal. A: Chem. 2000;161(1):205–212. [Google Scholar]
- Li K. Zhou H. Advances to Stabilize Photoactive Phase of FAPbI Perovskite. Chin. J. Chem. 2023;41(20):2730–2745. [Google Scholar]
- Wang Z. Zhang Z. Xie L. Wang S. Yang C. Fang C. et al., Recent Advances and Perspectives of Photostability for Halide Perovskite Solar Cells. Adv. Opt. Mater. 2022;10(3):2101822. [Google Scholar]
- Chen T. Xie J. Gao P. Ultraviolet Photocatalytic Degradation of Perovskite Solar Cells: Progress, Challenges, and Strategies. Adv. Energy Sustainability Res. 2022;3(6):2100218. [Google Scholar]
- Tayagaki T. Kobayashi H. Yamamoto K. Murakami T. N. Yoshita M. Ultraviolet-light–dark cycle analysis of degradation in perovskite solar cells. Sol. Energy Mater. Sol. Cells. 2023;263:112583. [Google Scholar]
- Ji J. Liu X. Jiang H. Duan M. Liu B. Huang H. et al., Two-Stage Ultraviolet Degradation of Perovskite Solar Cells Induced by the Oxygen Vacancy-Ti4+ States. iScience. 2020;23(4):101013. doi: 10.1016/j.isci.2020.101013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N.-K. Min Y. H. Noh S. Cho E. Jeong G. Joo M. et al., Investigation of Thermally Induced Degradation in CH3NH3PbI3 Perovskite Solar Cells using In-situ Synchrotron Radiation Analysis. Sci. Rep. 2017;7(1):4645. doi: 10.1038/s41598-017-04690-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe B. Park B.-W. Lindblad R. Oscarsson J. Ahmadi S. Johansson E. M. et al., Chemical and Electronic Structure Characterization of Lead Halide Perovskites and Stability Behavior under Different Exposures A Photoelectron Spectroscopy Investigation. Chem. Mater. 2015;27(5):1720–1731. [Google Scholar]
- Brunetti B. Cavallo C. Ciccioli A. Gigli G. Latini A. On the Thermal and Thermodynamic (In)Stability of Methylammonium Lead Halide Perovskites. Sci. Rep. 2016;6(1):31896. doi: 10.1038/srep31896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X. Kim H.-S. Seo J.-Y. Park N.-G. Effect of Selective Contacts on the Thermal Stability of Perovskite Solar Cells. ACS Appl. Mater. Interfaces. 2017;9(8):7148–7153. doi: 10.1021/acsami.6b15673. [DOI] [PubMed] [Google Scholar]
- Conings B. Drijkoningen J. Gauquelin N. Babayigit A. D'Haen J. D'Olieslaeger L. et al., Intrinsic Thermal Instability of Methylammonium Lead Trihalide Perovskite. Adv. Energy Mater. 2015;5(15):1500477. [Google Scholar]
- McLeod J. A. Liu L. Prospects for Mitigating Intrinsic Organic Decomposition in Methylammonium Lead Triiodide Perovskite. J. Phys. Chem. Lett. 2018;9(9):2411–2417. doi: 10.1021/acs.jpclett.8b00323. [DOI] [PubMed] [Google Scholar]
- Leijtens T. Bush K. Cheacharoen R. Beal R. Bowring A. McGehee M. D. Towards enabling stable lead halide perovskite solar cells; interplay between structural, environmental, and thermal stability. J. Mater. Chem. A. 2017;5(23):11483–11500. [Google Scholar]
- Leong W. L. Ooi Z.-E. Sabba D. Yi C. Zakeeruddin S. M. Graetzel M. et al., Identifying Fundamental Limitations in Halide Perovskite Solar Cells. Adv. Mater. 2016;28(12):2439–2445. doi: 10.1002/adma.201505480. [DOI] [PubMed] [Google Scholar]
- Duong T. Wu Y. Shen H. Peng J. Zhao S. Wu N. et al., Light and elevated temperature induced degradation (LeTID) in perovskite solar cells and development of stable semi-transparent cells. Sol. Energy Mater. Sol. Cells. 2018;188:27–36. [Google Scholar]
- Foley B. J. Marlowe D. L. Sun K. Saidi W. A. Scudiero L. Gupta M. C. et al., Temperature dependent energy levels of methylammonium lead iodide perovskite. Appl. Phys. Lett. 2015;106(24):243904. [Google Scholar]
- Qin C. Matsushima T. Klotz D. Fujihara T. Adachi C. The Relation of Phase-Transition Effects and Thermal Stability of Planar Perovskite Solar Cells. Adv. Sci. 2019;6(1):1801079. doi: 10.1002/advs.201801079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenzer J. A. Hellmann T. Nejand B. A. Hu H. Abzieher T. Schackmar F. et al., Thermal Stability and Cation Composition of Hybrid Organic–Inorganic Perovskites. ACS Appl. Mater. Interfaces. 2021;13(13):15292–15304. doi: 10.1021/acsami.1c01547. [DOI] [PubMed] [Google Scholar]
- Stoumpos C. C. Malliakas C. D. Kanatzidis M. G. Semiconducting Tin and Lead Iodide Perovskites with Organic Cations: Phase Transitions, High Mobilities, and Near-Infrared Photoluminescent Properties. Inorg. Chem. 2013;52(15):9019–9038. doi: 10.1021/ic401215x. [DOI] [PubMed] [Google Scholar]
- Eperon G. E. Stranks S. D. Menelaou C. Johnston M. B. Herz L. M. Snaith H. J. Formamidinium lead trihalide: a broadly tunable perovskite for efficient planar heterojunction solar cells. Energy Environ. Sci. 2014;7(3):982–988. [Google Scholar]
- McMeekin D. P. Sadoughi G. Rehman W. Eperon G. E. Saliba M. Hörantner M. T. et al., A mixed-cation lead mixed-halide perovskite absorber for tandem solar cells. Science. 2016;351(6269):151–155. doi: 10.1126/science.aad5845. [DOI] [PubMed] [Google Scholar]
- Tang X. Brandl M. May B. Levchuk I. Hou Y. Richter M. et al., Photoinduced degradation of methylammonium lead triiodide perovskite semiconductors. J. Mater. Chem. A. 2016;4(41):15896–15903. [Google Scholar]
- Duan X. Duan J. Liu N. Li J. Dou J. Zhang X. et al., Inhibited superoxide-induced halide oxidation with a bioactive factor for stabilized inorganic perovskite solar cells. SusMat. 2024;4(4):e233. [Google Scholar]
- Aristidou N. Sanchez-Molina I. Chotchuangchutchaval T. Brown M. Martinez L. Rath T. et al., The Role of Oxygen in the Degradation of Methylammonium Lead Trihalide Perovskite Photoactive Layers. Angew. Chem., Int. Ed. 2015;54(28):8208–8212. doi: 10.1002/anie.201503153. [DOI] [PubMed] [Google Scholar]
- Aristidou N. Eames C. Sanchez-Molina I. Bu X. Kosco J. Islam M. S. et al., Fast oxygen diffusion and iodide defects mediate oxygen-induced degradation of perovskite solar cells. Nat. Commun. 2017;8(1):15218. doi: 10.1038/ncomms15218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo J. Kaiser W. An Y. Li R. Oh Z. Castro-Méndez A.-F. et al., Synergistic Role of Water and Oxygen Leads to Degradation in Formamidinium-Based Halide Perovskites. J. Am. Chem. Soc. 2023;145(45):24549–24557. doi: 10.1021/jacs.3c05657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z. Tian X. Wang C. Jin J. Jiang Y. Zhou Q. et al., Revealing superoxide-induced degradation in lead-free tin perovskite solar cells. Energy Environ. Sci. 2022;15(12):5274–5283. [Google Scholar]
- Tammireddy S. Lintangpradipto M. N. Telschow O. Futscher M. H. Ehrler B. Bakr O. M. et al., Hysteresis and Its Correlation to Ionic Defects in Perovskite Solar Cells. J. Phys. Chem. Lett. 2024;15(5):1363–1372. doi: 10.1021/acs.jpclett.3c03146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyano K. Yanagida M. Tripathi N. Shirai Y. Hysteresis, Stability, and Ion Migration in Lead Halide Perovskite Photovoltaics. J. Phys. Chem. Lett. 2016;7(12):2240–2245. doi: 10.1021/acs.jpclett.6b00579. [DOI] [PubMed] [Google Scholar]
- Kumar A. Rana A. Vashistha N. Garg K. K. Singh R. K. Defect states influencing hysteresis and performance of perovskite solar cells. Sol. Energy. 2020;211:345–353. [Google Scholar]
- Geng S. Duan J. Liu N. Li H. Zhu X. Duan X. et al., Influence of Donor Skeleton on Intramolecular Electron Transfer Amount for Efficient Perovskite Solar Cells. Angew. Chem., Int. Ed. 2024;63(32):e202407383. doi: 10.1002/anie.202407383. [DOI] [PubMed] [Google Scholar]
- Bera S. Saha A. Mondal S. Biswas A. Mallick S. Chatterjee R. et al., Review of defect engineering in perovskites for photovoltaic application. Mater. Adv. 2022;3(13):5234–5247. [Google Scholar]
- Yin W.-J. Shi T. Yan Y. Unusual defect physics in CH3NH3PbI3 perovskite solar cell absorber. Appl. Phys. Lett. 2014;104(6):063903. [Google Scholar]
- Leblebici S. Y. Leppert L. Li Y. Reyes-Lillo S. E. Wickenburg S. Wong E. et al., Facet-dependent photovoltaic efficiency variations in single grains of hybrid halide perovskite. Nat. Energy. 2016;1(8):1–7. [Google Scholar]
- Nie W. Tsai H. Asadpour R. Blancon J. C. Neukirch A. J. Gupta G. et al., High-efficiency solution-processed perovskite solar cells with millimeter-scale grains. Science. 2015;347:522. doi: 10.1126/science.aaa0472. [DOI] [PubMed] [Google Scholar]
- Fu L. Li H. Wang L. Yin R. Li B. Yin L. Defect passivation strategies in perovskites for an enhanced photovoltaic performance. Energy Environ. Sci. 2020;13(11):4017–4056. [Google Scholar]
- Son D.-Y. Kim S.-G. Seo J.-Y. Lee S.-H. Shin H. Lee D. et al., Universal approach toward hysteresis-free perovskite solar cell via defect engineering. J. Am. Chem. Soc. 2018;140(4):1358–1364. doi: 10.1021/jacs.7b10430. [DOI] [PubMed] [Google Scholar]
- Hoke E. T. Slotcavage D. J. Dohner E. R. Bowring A. R. Karunadasa H. I. McGehee M. D. Reversible photo-induced trap formation in mixed-halide hybrid perovskites for photovoltaics. Chem. Sci. 2015;6(1):613–617. doi: 10.1039/c4sc03141e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. Li J. Duan J. Zhang X. Dou J. Guo Q. et al., Ionic-Rich Vermiculite Tailoring Dynamic Bottom-Up Gradient for High-Efficiency Perovskite Solar Cells. Adv. Funct. Mater. 2024;34(33):2401662. [Google Scholar]
- Zai H. Ma Y. Chen Q. Zhou H. Ion migration in halide perovskite solar cells: Mechanism, characterization, impact and suppression. J. Energy Chem. 2021;63:528–549. [Google Scholar]
- Besleaga C. Abramiuc L. E. Stancu V. Tomulescu A. G. Sima M. Trinca L. et al., Iodine Migration and Degradation of Perovskite Solar Cells Enhanced by Metallic Electrodes. J. Phys. Chem. Lett. 2016;7(24):5168–5175. doi: 10.1021/acs.jpclett.6b02375. [DOI] [PubMed] [Google Scholar]
- Zhao Y. Zhou W. Tan H. Fu R. Li Q. Lin F. et al., Mobile-Ion-Induced Degradation of Organic Hole-Selective Layers in Perovskite Solar Cells. J. Phys. Chem. C. 2017;121(27):14517–14523. [Google Scholar]
- Wang Y. Wu T. Barbaud J. Kong W. Cui D. Chen H. et al., Stabilizing heterostructures of soft perovskite semiconductors. Science. 2019;365(6454):687–691. doi: 10.1126/science.aax8018. [DOI] [PubMed] [Google Scholar]
- Nughays R. O. Almasabi K. Nematulloev S. Wang L. Bian T. Nadinov I. et al., Mapping Surface-Defect and Ions Migration in Mixed-Cation Perovskite Crystals. Adv. Sci. 2024;11(40):2404468. doi: 10.1002/advs.202404468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seol D. Jeong A. Han M. H. Seo S. Yoo T. S. Choi W. S. et al., Origin of hysteresis in CH3NH3PbI3 perovskite thin films. Adv. Funct. Mater. 2017;27(37):1701924. [Google Scholar]
- Xiao Z. Yuan Y. Shao Y. Wang Q. Dong Q. Bi C. et al., Giant switchable photovoltaic effect in organometal trihalide perovskite devices. Nat. Mater. 2015;14(2):193–198. doi: 10.1038/nmat4150. [DOI] [PubMed] [Google Scholar]
- Miller D. W. Eperon G. E. Roe E. T. Warren C. W. Snaith H. J. Lonergan M. C. Defect states in perovskite solar cells associated with hysteresis and performance. Appl. Phys. Lett. 2016;109(15):153902. [Google Scholar]
- Seol D. Han G. S. Bae C. Shin H. Jung H. S. Kim Y. Screening effect on photovoltaic performance in ferroelectric CH3NH3PbI3 perovskite thin films. J. Mater. Chem. A. 2015;3(40):20352–20358. [Google Scholar]
- Meloni S. Moehl T. Tress W. Franckevičius M. Saliba M. Lee Y. H. et al., Ionic polarization-induced current–voltage hysteresis in CH3NH3PbX3 perovskite solar cells. Nat. Commun. 2016;7(1):10334. doi: 10.1038/ncomms10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cave J. M. Courtier N. E. Blakborn I. A. Jones T. W. Ghosh D. Anderson K. F. et al., Deducing transport properties of mobile vacancies from perovskite solar cell characteristics. J. Appl. Phys. 2020;128(18):184501. [Google Scholar]
- Huang J.-Y. Yang Y.-W. Hsu W.-H. Chang E.-W. Chen M.-H. Wu Y.-R. Influences of dielectric constant and scan rate on hysteresis effect in perovskite solar cell with simulation and experimental analyses. Sci. Rep. 2022;12(1):7927. doi: 10.1038/s41598-022-11899-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Corre V. M. Diekmann J. Peña-Camargo F. Thiesbrummel J. Tokmoldin N. Gutierrez-Partida E. et al., Quantification of Efficiency Losses Due to Mobile Ions in Perovskite Solar Cells via Fast Hysteresis Measurements. Sol. RRL. 2022;6(4):2100772. [Google Scholar]
- Zarazua I. Bisquert J. Garcia-Belmonte G. Light-Induced Space-Charge Accumulation Zone as Photovoltaic Mechanism in Perovskite Solar Cells. J. Phys. Chem. Lett. 2016;7(3):525–528. doi: 10.1021/acs.jpclett.5b02810. [DOI] [PubMed] [Google Scholar]
- Pockett A. Eperon G. E. Peltola T. Snaith H. J. Walker A. Peter L. M. et al., Characterization of Planar Lead Halide Perovskite Solar Cells by Impedance Spectroscopy, Open-Circuit Photovoltage Decay, and Intensity-Modulated Photovoltage/Photocurrent Spectroscopy. J. Phys. Chem. C. 2015;119(7):3456–3465. [Google Scholar]
- Guerrero A. Garcia-Belmonte G. Mora-Sero I. Bisquert J. Kang Y. S. Jacobsson T. J. et al., Properties of Contact and Bulk Impedances in Hybrid Lead Halide Perovskite Solar Cells Including Inductive Loop Elements. J. Phys. Chem. C. 2016;120(15):8023–8032. [Google Scholar]
- Peng W. Aranda C. Bakr O. M. Garcia-Belmonte G. Bisquert J. Guerrero A. Quantification of Ionic Diffusion in Lead Halide Perovskite Single Crystals. ACS Energy Lett. 2018;3(7):1477–1481. [Google Scholar]
- Juarez-Perez E. J. Sanchez R. S. Badia L. Garcia-Belmonte G. Kang Y. S. Mora-Sero I. et al., Photoinduced Giant Dielectric Constant in Lead Halide Perovskite Solar Cells. J. Phys. Chem. Lett. 2014;5(13):2390–2394. doi: 10.1021/jz5011169. [DOI] [PubMed] [Google Scholar]
- Pascoe A. R. Yang M. Kopidakis N. Zhu K. Reese M. O. Rumbles G. et al., Planar versus mesoscopic perovskite microstructures: The influence of CH3NH3PbI3 morphology on charge transport and recombination dynamics. Nano Energy. 2016;22:439–452. [Google Scholar]
- Kim H.-S. Mora-Sero I. Gonzalez-Pedro V. Fabregat-Santiago F. Juarez-Perez E. J. Park N.-G. et al., Mechanism of carrier accumulation in perovskite thin-absorber solar cells. Nat. Commun. 2013;4(1):2242. doi: 10.1038/ncomms3242. [DOI] [PubMed] [Google Scholar]
- Bag M. Renna L. A. Adhikari R. Y. Karak S. Liu F. Lahti P. M. et al., Kinetics of Ion Transport in Perovskite Active Layers and Its Implications for Active Layer Stability. J. Am. Chem. Soc. 2015;137(40):13130–13137. doi: 10.1021/jacs.5b08535. [DOI] [PubMed] [Google Scholar]
- Giuliano G. Cataldo S. Scopelliti M. Principato F. Chillura Martino D. Fiore T. et al., Nonprecious Copper-Based Transparent Top Electrode via Seed Layer–Assisted Thermal Evaporation for High-Performance Semitransparent n-i-p Perovskite Solar Cells. Adv. Mater. Technol. 2019;4(5):1800688. [Google Scholar]
- Zhao J. Zheng X. Deng Y. Li T. Shao Y. Gruverman A. et al., Is Cu a stable electrode material in hybrid perovskite solar cells for a 30-year lifetime? Energy Environ. Sci. 2016;9(12):3650–3656. [Google Scholar]
- Back H. Kim G. Kim J. Kong J. Kim T. K. Kang H. et al., Achieving long-term stable perovskite solar cells via ion neutralization. Energy Environ. Sci. 2016;9(4):1258–1263. [Google Scholar]
- Tai Q. You P. Sang H. Liu Z. Hu C. Chan H. L. W. et al., Efficient and stable perovskite solar cells prepared in ambient air irrespective of the humidity. Nat. Commun. 2016;7(1):11105. doi: 10.1038/ncomms11105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troughton J. Hooper K. Watson T. M. Humidity resistant fabrication of CH3NH3PbI3 perovskite solar cells and modules. Nano Energy. 2017;39:60–68. [Google Scholar]
- Li X. Ibrahim D. M. Yi C. Luo J. Tschumi M. Zakeeruddin S. M. et al., Improved performance and stability of perovskite solar cells by crystal crosslinking with alkylphosphonic acid ω-ammonium chlorides. Nat. Chem. 2015;7(9):703–711. doi: 10.1038/nchem.2324. [DOI] [PubMed] [Google Scholar]
- Mahapatra A. Kumar S. Kumar P. Pradhan B. Recent progress in perovskite solar cells: challenges from efficiency to stability. Mater. Today Chem. 2022;23:100686. [Google Scholar]
- Habisreutinger S. Leijtens T. Eperon G. E. Stranks S. D. Nicholas R. J. Snaith H. J. Carbon nanotube/polymer composites as a highly stable hole collection layer in perovskite solar cells. Nano Lett. 2014;14:5561. doi: 10.1021/nl501982b. [DOI] [PubMed] [Google Scholar]
- Azam M. Ke Z. Luo J. Wan Z. Hassan A. Jia C. Additive engineering enabled non-radiative defect passivation with improved moisture-resistance in efficient and stable perovskite solar cells. Chem. Eng. J. 2024;483:149424. [Google Scholar]
- Roose B. Baena J.-P. C. Gödel K. C. Graetzel M. Hagfeldt A. Steiner U. et al., Mesoporous SnO2 electron selective contact enables UV-stable perovskite solar cells. Nano Energy. 2016;30:517–522. [Google Scholar]
- Ouafi M. Jaber B. Atourki L. Bekkari R. Laânab L. Improving UV stability of MAPbI3 perovskite thin films by bromide incorporation. J. Alloys Compd. 2018;746:391–398. [Google Scholar]
- Lee S.-W. Kim S. Bae S. Cho K. Chung T. Mundt L. E. et al., UV Degradation and Recovery of Perovskite Solar Cells. Sci. Rep. 2016;6(1):38150. doi: 10.1038/srep38150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H. Liu G. Chen X. Zhang B. Alsaedi A. Hayat T. et al., High-performance mixed-dimensional perovskite solar cells with enhanced stability against humidity, heat and UV light. J. Mater. Chem. A. 2018;6(41):20233–20241. [Google Scholar]
- Sun Y. Fang X. Ma Z. Xu L. Lu Y. Yu Q. et al., Enhanced UV-light stability of organometal halide perovskite solar cells with interface modification and a UV absorption layer. J. Mater. Chem. C. 2017;5(34):8682–8687. [Google Scholar]
- Suo J. Yang B. Mosconi E. Bogachuk D. Doherty T. A. S. Frohna K. et al., Multifunctional sulfonium-based treatment for perovskite solar cells with less than 1% efficiency loss over 4,500-h operational stability tests. Nat. Energy. 2024;9(2):172–183. doi: 10.1038/s41560-023-01421-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y. Miao W. Sun W. Niu Z. Yin R. Huo X. et al., Lattice Strain Regulation and Halogen Vacancies Passivation Enable High-Performance Formamidine-Based Perovskite Solar Cells. Small. 2024;20:2404272. doi: 10.1002/smll.202404272. [DOI] [PubMed] [Google Scholar]
- Gawlińska-Nęcek K. Kot M. Starowicz Z. Jarzębska A. Panek P. Flege J. I. Instability of Formamidinium Lead Iodide (FAPI) Deposited on a Copper Oxide Hole Transporting Layer (HTL) ACS Appl. Mater. Interfaces. 2024;16(21):27936–27943. doi: 10.1021/acsami.4c03440. [DOI] [PubMed] [Google Scholar]
- Sun Y. Miao W. Sun W. Niu Z. Yin R. Huo X. et al., Lattice Strain Regulation and Halogen Vacancies Passivation Enable High-Performance Formamidine-Based Perovskite Solar Cells. Small. 2024;20:2404272. doi: 10.1002/smll.202404272. [DOI] [PubMed] [Google Scholar]
- Liu H. Liu T. Wang X. Hu G. Zheng B. Yu X. et al., Surface Formamidine Cation Immobilization for Efficient FA-Based Perovskites Solar Cells. Adv. Energy Mater. 2024;14:2401809. [Google Scholar]
- Gantumur M. Hossain M. I. Shahiduzzaman M. Tamang A. Rafij J. H. Shahinuzzaman M. et al., Tungsten-Doped ZnO as an Electron Transport Layer for Perovskite Solar Cells: Enhancing Efficiency and Stability. ACS Appl. Mater. Interfaces. 2024;16(28):36255–36271. doi: 10.1021/acsami.4c03591. [DOI] [PubMed] [Google Scholar]
- Shahinuzzaman M. Islam M. A. Afroz S. Hossain M. Jamal M. S. Alanazi A. M. et al., Synthesis of tungsten-doped zinc oxide nanoparticles using Aloe vera extracts for perovskite solar cells. Optik. 2024;313:172006. [Google Scholar]
- Boro B. Mishra S. Singh P. Lahiri B. Varshney S. K. Singh T. Thermal Stability Analysis of Formamidinium–Cesium-Based Lead Halide Perovskite Solar Cells Fabricated under Air Ambient Conditions. Energy Technol. 2024;12(8):2400034. [Google Scholar]
- Senocrate A. Acartürk T. Kim G. Y. Merkle R. Starke U. Grätzel M. et al., Interaction of oxygen with halide perovskites. J. Mater. Chem. A. 2018;6(23):10847–10855. [Google Scholar]
- Yang J. Siempelkamp B. D. Mosconi E. De Angelis F. Kelly T. L. Origin of the Thermal Instability in CH3NH3PbI3 Thin Films Deposited on ZnO. Chem. Mater. 2015;27(12):4229–4236. [Google Scholar]
- Rakstys K. Abate A. Dar M. I. Gao P. Jankauskas V. Gn J. et al., Triazatruxene-based hole transporting materials for highly efficient perovskite solar cells. J. Am. Chem. Soc. 2015;137(51):16172–16178. doi: 10.1021/jacs.5b11076. [DOI] [PubMed] [Google Scholar]
- Thrithamarassery Gangadharan D. Han Y. Dubey A. Gao X. Sun B. Qiao Q. et al., Aromatic alkylammonium spacer cations for efficient two-dimensional perovskite solar cells with enhanced moisture and thermal stability. Sol. RRL. 2018;2(4):1700215. [Google Scholar]
- Zhang F. Kim D. H. Zhu K. 3D/2D multidimensional perovskites: Balance of high performance and stability for perovskite solar cells. Curr. Opin. Electrochem. 2018;11:105–113. [Google Scholar]
- Chen S. Shen N. Zhang L. Zhang L. Cheung S. H. Chen S. et al., Understanding the Interplay of Binary Organic Spacer in Ruddlesden–Popper Perovskites toward Efficient and Stable Solar Cells. Adv. Funct. Mater. 2020;30(10):1907759. [Google Scholar]
- Hu R. Zhang Y. Paek S. Gao X.-X. Li Xa. Nazeeruddin M. K. Enhanced stability of α-phase FAPbI3 perovskite solar cells by insertion of 2D (PEA)2PbI4 nanosheets. J. Mater. Chem. A. 2020;8(16):8058–8064. [Google Scholar]
- Huang Y. Wu S. Chen R. Fang S. Zhang S. Wang G. et al., Efficient Methylamine-Containing Antisolvent Strategy to Fabricate High-Efficiency and Stable FA0.85Cs0.15Pb(Br0.15I2.85) Perovskite Solar Cells. ACS Appl. Mater. Interfaces. 2019;11(20):18415–18422. doi: 10.1021/acsami.9b03323. [DOI] [PubMed] [Google Scholar]
- Adil Afroz M. Ghimire N. Reza K. M. Bahrami B. Bobba R. S. Gurung A. et al., Thermal stability and performance enhancement of perovskite solar cells through oxalic acid-induced perovskite formation. ACS Appl. Energy Mater. 2020;3(3):2432–2439. [Google Scholar]
- Choi K. Lee J. Kim H. I. Park C. W. Kim G.-W. Choi H. et al., Thermally stable, planar hybrid perovskite solar cells with high efficiency. Energy Environ. Sci. 2018;11(11):3238–3247. [Google Scholar]
- Ma P. Bie T. Liu Y. Yang L. Bi S. Wang Z. et al., Zirconium Doping to Enable High-Efficiency and Stable CsPbI2Br All-Inorganic Perovskite Solar Cells. ACS Appl. Mater. Interfaces. 2024;16(1):1217–1224. doi: 10.1021/acsami.3c14459. [DOI] [PubMed] [Google Scholar]
- Li J. Duan J. Guo Q. Qi Z. Duan X. Li H. et al., Accelerating Thermal Transfer in Perovskite Films for High-Efficiency and Stable Photovoltaics. Adv. Funct. Mater. 2023;33(50):2308036. [Google Scholar]
- Jørgensen M. Norrman K. Krebs F. C. Stability/degradation of polymer solar cells. Sol. Energy Mater. Sol. Cells. 2008;92(7):686–714. [Google Scholar]
- Unger E. L. Kegelmann L. Suchan K. Sörell D. Korte L. Albrecht S. Roadmap and roadblocks for the band gap tunability of metal halide perovskites. J. Mater. Chem. A. 2017;5(23):11401–11409. [Google Scholar]
- Wang F. Bai S. Tress W. Hagfeldt A. Gao F. Defects engineering for high-performance perovskite solar cells. npj Flexible Electron. 2018;2(1):22. [Google Scholar]
- Yang W. S. Noh J. H. Jeon N. J. Kim Y. C. Ryu S. Seo J. et al., High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science. 2015;348:1234. doi: 10.1126/science.aaa9272. [DOI] [PubMed] [Google Scholar]
- Wang F. Yu H. Xu H. Zhao N. HPbI3: a new precursor compound for highly efficient solution-processed perovskite solar cells. Adv. Funct. Mater. 2015;25(7):1120–1126. [Google Scholar]
- Liu J. Gao C. He X. Ye Q. Ouyang L. Zhuang D. et al., Improved crystallization of perovskite films by optimized solvent annealing for high efficiency solar cell. ACS Appl. Mater. Interfaces. 2015;7(43):24008–24015. doi: 10.1021/acsami.5b06780. [DOI] [PubMed] [Google Scholar]
- Wang B. Wong K. Y. Yang S. Chen T. Crystallinity and defect state engineering in organo-lead halide perovskite for high-efficiency solar cells. J. Mater. Chem. A. 2016;4(10):3806–3812. [Google Scholar]
- Noel N. K. Abate A. Stranks S. D. Parrott E. S. Burlakov V. M. Goriely A. et al., Enhanced Photoluminescence and Solar Cell Performance via Lewis Base Passivation of Organic–Inorganic Lead Halide Perovskites. ACS Nano. 2014;8(10):9815–9821. doi: 10.1021/nn5036476. [DOI] [PubMed] [Google Scholar]
- You J. Yang Y. M. Hong Z. Song T.-B. Meng L. Liu Y. et al., Moisture assisted perovskite film growth for high performance solar cells. Appl. Phys. Lett. 2014;105(18):183902. [Google Scholar]
- Eperon G. E. Habisreutinger S. N. Leijtens T. Bruijnaers B. J. van Franeker J. J. DeQuilettes D. W. et al., The importance of moisture in hybrid lead halide perovskite thin film fabrication. ACS Nano. 2015;9(9):9380–9393. doi: 10.1021/acsnano.5b03626. [DOI] [PubMed] [Google Scholar]
- Zhou H. Chen Q. Li G. Luo S. Song Tb. Duan H. S. et al., Interface engineering of highly efficient perovskite solar cells. Science. 2014;345(6196):542–546. doi: 10.1126/science.1254050. [DOI] [PubMed] [Google Scholar]
- Lee J.-W. Kim H.-S. Park N.-G. Lewis acid–base adduct approach for high efficiency perovskite solar cells. Acc. Chem. Res. 2016;49(2):311–319. doi: 10.1021/acs.accounts.5b00440. [DOI] [PubMed] [Google Scholar]
- Jeon N. J. Noh J. H. Yang W. S. Kim Y. C. Ryu S. Seo J. et al., Compositional engineering of perovskite materials for high-performance solar cells. Nature. 2015;517(7535):476–480. doi: 10.1038/nature14133. [DOI] [PubMed] [Google Scholar]
- Saliba M. Matsui T. Seo J. Y. Domanski K. Correa-Baena J. P. Nazeeruddin M. K. et al., Cesium-containing triple cation perovskite solar cells: improved stability, reproducibility and high efficiency. Energy Environ. Sci. 2016;9:1989. doi: 10.1039/c5ee03874j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X. Duan J. Zhao Y. Zhang J. Guo Q. Zhang Q. et al., Stretchable alkenamides terminated Ti3C2T MXenes to release strain for lattice-stable mixed-halide perovskite solar cells with suppressed halide segregation. Carbon Energy. 2023;5(12):e387. [Google Scholar]
- Du Z. Ma Z. Liu Q. Huang Z. Yu T. Li Y. et al., Defect passivation with bromine template for efficient perovskite solar cells. Mater. Sci. Semicond. Process. 2024;173:108138. [Google Scholar]
- Jaffe A. Lin Y. Beavers C. M. Voss J. Mao W. L. Karunadasa H. I. High-Pressure Single-Crystal Structures of 3D Lead-Halide Hybrid Perovskites and Pressure Effects on their Electronic and Optical Properties. ACS Cent. Sci. 2016;2(4):201–209. doi: 10.1021/acscentsci.6b00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi E. De Angelis F. Mobile Ions in Organohalide Perovskites: Interplay of Electronic Structure and Dynamics. ACS Energy Lett. 2016;1(1):182–188. [Google Scholar]
- Zhao Y.-C. Zhou W.-K. Zhou X. Liu K.-H. Yu D.-P. Zhao Q. Quantification of light-enhanced ionic transport in lead iodide perovskite thin films and its solar cell applications. Light: Sci. Appl. 2017;6(5):e16243. doi: 10.1038/lsa.2016.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D. Shi T. Xie H. Wan F. Ren X. Liu K. et al., Ion migration accelerated reaction between oxygen and metal halide perovskites in light and its suppression by cesium incorporation. Adv. Energy Mater. 2021;11(8):2002552. [Google Scholar]
- Xing J. Wang Q. Dong Q. Yuan Y. Fang Y. Huang J. Ultrafast ion migration in hybrid perovskite polycrystalline thin films under light and suppression in single crystals. Phys. Chem. Chem. Phys. 2016;18(44):30484–30490. doi: 10.1039/c6cp06496e. [DOI] [PubMed] [Google Scholar]
- Ke W. Xiao C. Wang C. Saparov B. Duan H.-S. Zhao D. et al., Employing Lead Thiocyanate Additive to Reduce the Hysteresis and Boost the Fill Factor of Planar Perovskite Solar Cells. Adv. Mater. 2016;28(26):5214–5221. doi: 10.1002/adma.201600594. [DOI] [PubMed] [Google Scholar]
- Cai Y. Cui J. Chen M. Zhang M. Han Y. Qian F. et al., Multifunctional Enhancement for Highly Stable and Efficient Perovskite Solar Cells. Adv. Funct. Mater. 2021;31(7):2005776. [Google Scholar]
- Zhao Y. Zhao Y. Zhou W. Li Q. Fu R. Yu D. et al., In situ cesium modification at interface enhances the stability of perovskite solar cells. ACS Appl. Mater. Interfaces. 2018;10(39):33205–33213. doi: 10.1021/acsami.8b10616. [DOI] [PubMed] [Google Scholar]
- Liu X. Zhang Y. Shi L. Liu Z. Huang J. Yun J. S. et al., Exploring Inorganic Binary Alkaline Halide to Passivate Defects in Low-Temperature-Processed Planar-Structure Hybrid Perovskite Solar Cells. Adv. Energy Mater. 2018;8(20):1800138. [Google Scholar]
- Dey K. Ghosh D. Pilot M. Pering S. R. Roose B. Deswal P. et al., Substitution of lead with tin suppresses ionic transport in halide perovskite optoelectronics. Energy Environ. Sci. 2024;17(2):760–769. doi: 10.1039/d3ee03772j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.-S. Park N.-G. Parameters affecting I–V hysteresis of CH3NH3PbI3 perovskite solar cells: effects of perovskite crystal size and mesoporous TiO2 layer. J. Phys. Chem. Lett. 2014;5(17):2927–2934. doi: 10.1021/jz501392m. [DOI] [PubMed] [Google Scholar]
- Li C. Guerrero A. Zhong Y. Huettner S. Origins and mechanisms of hysteresis in organometal halide perovskites. J. Phys.: Condens. Matter. 2017;29(19):193001. doi: 10.1088/1361-648X/aa626d. [DOI] [PubMed] [Google Scholar]
- Lee J.-W. Kim S.-G. Bae S.-H. Lee D.-K. Lin O. Yang Y. et al., The interplay between trap density and hysteresis in planar heterojunction perovskite solar cells. Nano Lett. 2017;17(7):4270–4276. doi: 10.1021/acs.nanolett.7b01211. [DOI] [PubMed] [Google Scholar]
- Kim H.-S. Jang I.-H. Ahn N. Choi M. Guerrero A. Bisquert J. et al., Control of I–V hysteresis in CH3NH3PbI3 perovskite solar cell. J. Phys. Chem. Lett. 2015;6(22):4633–4639. doi: 10.1021/acs.jpclett.5b02273. [DOI] [PubMed] [Google Scholar]
- Seo S. Park I. J. Kim M. Lee S. Bae C. Jung H. S. et al., An ultra-thin, un-doped NiO hole transporting layer of highly efficient (16.4%) organic–inorganic hybrid perovskite solar cells. Nanoscale. 2016;8(22):11403–11412. doi: 10.1039/c6nr01601d. [DOI] [PubMed] [Google Scholar]
- Zhang R. Li M. Huan Y. Xi J. Zhang S. Cheng X. et al., A potassium thiocyanate additive for hysteresis elimination in highly efficient perovskite solar cells. Inorg. Chem. Front. 2019;6(2):434–442. [Google Scholar]
- Chen J. Lee D. Park N.-G. Stabilizing the Ag Electrode and Reducing J–V Hysteresis through Suppression of Iodide Migration in Perovskite Solar Cells. ACS Appl. Mater. Interfaces. 2017;9(41):36338–36349. doi: 10.1021/acsami.7b07595. [DOI] [PubMed] [Google Scholar]
- Liu G. Zheng H. Xu H. Zhang L. Xu X. Xu S. et al., Interface passivation treatment by halogenated low-dimensional perovskites for high-performance and stable perovskite photovoltaics. Nano Energy. 2020;73:104753. [Google Scholar]
- Li M. Huan Y. Yan X. Kang Z. Guo Y. Li Y. et al., Efficient yttrium (III) chloride-treated TiO2 electron transfer layers for performance-improved and hysteresis-less perovskite solar cells. ChemSusChem. 2018;11(1):171–177. doi: 10.1002/cssc.201701911. [DOI] [PubMed] [Google Scholar]
- Cao J. Wu B. Chen R. Wu Y. Hui Y. Mao B. W. et al., Efficient, hysteresis-free, and stable perovskite solar cells with ZnO as electron-transport layer: effect of surface passivation. Adv. Mater. 2018;30(11):1705596. doi: 10.1002/adma.201705596. [DOI] [PubMed] [Google Scholar]
- Wojciechowski K. Leijtens T. Siprova S. Schlueter C. Hörantner M. T. Wang J. T.-W. et al., C60 as an Efficient n-Type Compact Layer in Perovskite Solar Cells. J. Phys. Chem. Lett. 2015;6(12):2399–2405. doi: 10.1021/acs.jpclett.5b00902. [DOI] [PubMed] [Google Scholar]
- Dipta S. S. Rahaman M. H. Binte Tarique W. Howlader A. H. Pratik A. Stride J. A. et al., Highly efficient double-side-passivated perovskite solar cells for reduced degradation and low photovoltage loss. Sol. Energy Mater. Sol. Cells. 2024;266:112655. [Google Scholar]
- Miah M. H. Khandaker M. U. Rahman M. B. Nur-E-Alam M. Islam M. A. Band gap tuning of perovskite solar cells for enhancing the efficiency and stability: issues and prospects. RSC Adv. 2024;14(23):15876–15906. doi: 10.1039/d4ra01640h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi H. Han G. Guo M. Ding C. Zou H. Shen Q. et al., Multistrategy Preparation of Efficient and Stable Environment-Friendly Lead-Based Perovskite Solar Cells. ACS Appl. Mater. Interfaces. 2022;14(31):35513–35521. doi: 10.1021/acsami.2c06032. [DOI] [PubMed] [Google Scholar]
- Chen X. Xia Y. Huang Q. Li Z. Mei A. Hu Y. et al., Tailoring the Dimensionality of Hybrid Perovskites in Mesoporous Carbon Electrodes for Type-II Band Alignment and Enhanced Performance of Printable Hole-Conductor-Free Perovskite Solar Cells. Adv. Energy Mater. 2021;11(18):2100292. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available in the manuscript.