Abstract
Objective
There is a lack of research on epidural esketamine for labor analgesia. The purpose of this research is to compare the efficacy of epidural esketamine and sufentanil on labor analgesia and postpartum depression.
Methods
A total of 187 cephalic full-term parturients with single-fetus vaginal delivery were collected in this retrospective study from Jan 2022 to Jan 2023. Parturients were categorized into two groups according to anesthetics: the esketamine group (Group KR, n = 97) with patient-controlled epidural analgesia with 0.3 mg/ml esketamine and 0.083% ropivacaine in 240 ml of normal saline and the Sufentanil group (Group SR, n = 90) with 0.3 µg/ml sufentanil and 0.083% ropivacaine in 240 ml of normal saline. The Visual Analogue Scale, Ramsay Sedation Scale, and Modified Bromage Score were recorded before, 5, 10, and 30 min after analgesia, when the uterine orifice was fully opened, and after delivery. The Edinburgh Postnatal Depression Scale(EPDS) scores at 3 and 42 days after delivery were recorded. The maternal and infant outcomes and occurrence of maternal adverse reactions were recorded.
Results
The VAS scores after analgesia at 5,10,30 min and when the cervix was fully opened were higher in Group KR than Group SR (all P < 0.05). RSS scores at 5,10,30 min after analgesia in group KR were lower in Group KR than Group SR (all P < 0.05). Compared with group SR, significant decreases were shown in the EPDS and the incidence of postpartum depression at 42 days after delivery in Group KR (all P < 0.05). Group KR has considerably decreased rates of pruritus compared to Group SR (P < 0.05). The other adverse effects showed no significant difference (all P > 0.05). The maternal and neonatal outcomes were not significantly different between the two groups (all P > 0.05).
Conclusions
In comparison to sufentanil, epidural esketamine for labor analgesia may exhibit a better sedative effect, and a low incidence of pruritus, but a limited analgesic effect. It may be associated with a lower risk of postpartum depression. Further exploration of the optimal regimen and dosage of esketamine for epidural labor analgesia would be necessary.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12871-024-02846-6.
Keywords: Esketamine, Sufentanil, Epidural labor analgesia, Postpartum depression, Vaginal delivery, Maternal and neonatal outcomes
Background
Epidural labor analgesia is the preferred option to alleviate labor pain and improve maternal satisfaction [1, 2]. The standard anesthetic medications used for epidural labor analgesia are composed of a mixture of low-dose local anesthetics and adjuvants [3]. Traditionally used as adjuvant drugs for epidural analgesia, opioids have a quick onset of action and a good analgesic effect. They can also reduce motor block and urinary retention, prolong the duration of action, enhance the blocking effect of local anesthetics, lower the dosage of local anesthetics, and facilitate vaginal delivery [4]. However, their side effects, which involve nausea and vomiting, pruritus, and urinary retention, decrease maternal satisfaction [3, 5]. Hence, it is necessary to develop alternative epidural anesthetic adjuncts that exhibit potent analgesic capabilities while minimizing adverse effects.
Ketamine, a noncompetitive N-methyl-D-aspartate (NMDA) receptor antagonist, is prevalently employed in obstetric anesthesia as a result of its extensive array of pharmacological effects, including sedation, analgesia, antidepressant, and sympathetic stimulation [6, 7]. Esketamine, the S-enantiomer of ketamine, exhibits superior and quicker sedative and analgesic effects along with a higher safety profile due to its larger affinity for NMDA receptors [8, 9]. It has been demonstrated that the absence of preservatives in esketamine renders it safe for epidural analgesic administration [10, 11]. Esketamine used in epidural analgesia can exert local anesthesia and analgesia by directly acting on the spinal cord. Epidural esketamine for postoperative analgesia in thoracic surgery and cesarean section lowers postoperative VAS scores, reduces the incidence of moderate chronic postoperative pain and adverse effects, and decreases opioid dosage [12, 13]. It does not produce sympathomimetic activity, can shorten the motor block time, and has a certain anti-postoperative nociceptive allergy, which may contribute to the recovery of patients with epidural analgesia to a certain extent [14]. Furthermore, both esketamine and ketamine exhibit prompt-acting antidepressant properties [15, 16]. Esketamine nasal aerosol has been authorized for treatment-resistant depression [17]. Research has indicated that perioperative intravenous esketamine has a preventive effect on postpartum depression early after cesarean section [18–20]. Nevertheless, there is a scarcity of research that investigates the safety and efficacy of esketamine for epidural labor analgesia, as well as its impact on postpartum depression.
To establish a clinical reference, we propose to compare the efficacy of epidural esketamine and traditional adjuvant sufentanil for labor analgesia, in addition to their effects on postpartum depression and safety.
Materials and methods
Study design
This retrospective study was performed on single cephalic full-term primiparous women who delivered vaginally in the Third Affiliated Hospital of Zhengzhou University between January 2022 and May 2023. Inclusion criteria: singular cephalic full-term primipara; vaginal delivery; American Society of Anesthesiologists (ASA) class I or II; 18–45 years old. The following are the exclusion criteria: pregnancy complications, severe medical conditions, depression or psychiatric diseases, contraindications to epidural analgesia, pregnancy with assisted reproductive technology, contraindications to medication in this experiment, poor compliance, cesarean section for normal delivery, loss of visits, and stillbirth. 250 women were initially recruited; however, 21 cases were lost to visit, 15 cases were transferred to cesarean section, 6 cases were poorly adherent, 5 cases were assisted reproductive technology pregnancies, 16 cases were pregnancy complications, and a total of 187 cases were finally enrolled. Parturients were categorized into two groups according to the anesthetic medicines prescribed: the esketamine group (Group KR, n = 97) and the sufentanil group (Group SR, n = 90) (Fig. 1). This study was authorized by the Ethics Committee of the Third Affiliated Hospital of Zhengzhou University (No. 2022-089-01), and all parturients signed an informed consent form.
Fig. 1.
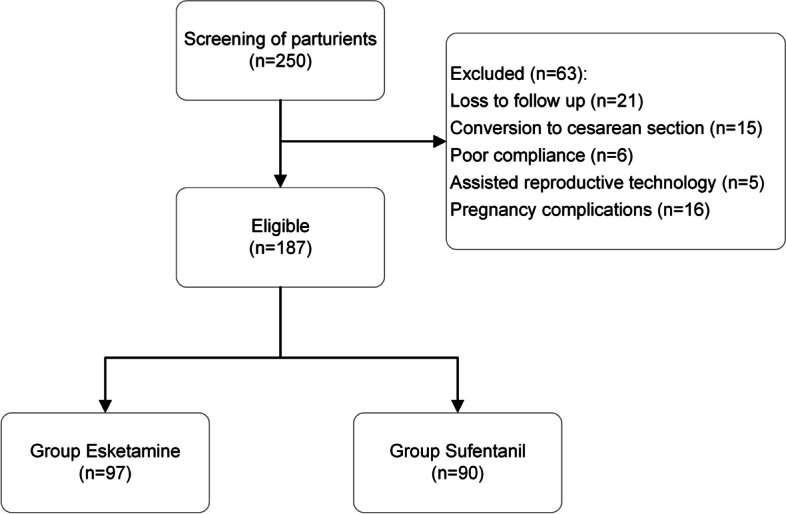
Flow diagram for enrollment
Anesthesia procedure
After a thorough evaluation by obstetricians and anesthesiologists, epidural labor analgesia was implemented in accordance with the mother’s individual wishes. The epidural puncture was administered in the intervertebral space of L2-3 or L3-4, with an epidural catheter positioned 4–5 cm from the head end and secured. 5 ml of 1.5% chloroprocaine was administered through the catheter, and the patient’s self-control epidural analgesia pump was connected and activated. The patient-controlled epidural analgesia(PCEA) formulas were as follows: Group: 0.3 mg/ml esketamine (National Drug License: H20193336, specification: 2 ml:50 mg, Jiangsu Hengrui Medicine Company) + 0.083% ropivacaine + saline = 240 mL, SR group: 0.3 μg/ml sufentanil + 0.083% ropivacaine + saline = 240 mL. Analgesia pump settings: initial volume of 10 ml/time, pulse volume of 8 ml/h, self-control volume of 2 ml/h, and the lock time of 20 min.
Data collection
The maternal age, body mass index (BMI), gestational week, years of education, and whether oxytocin was used were recorded in each group; Visual Analogue Scale(VAS), Ramsay Sedation Scale(RSS), and Modified Bromage Scale(MBS) were recorded in each group before analgesia (t0), 5(t1), 10(t2), 30 min after analgesia (t3), when the uterine cavity was fully opened (t4), and after the end of labor (t5). Edinburgh Postnatal Depression Scale (EPDS) scores were recorded 3 days (T1) and 42 days after delivery (T2). The ratio of postpartum depression (PPD) was recorded 42 days after delivery, which was diagnosed by an EPDS score of ≥ 13 [21]. The first and second stages of labor, instrumental delivery, amount of hemorrhage during labor, neonatal weight, and Apgar score of the groups were recorded. the probability of nausea and vomiting, dizziness, pruritus, lethargy, fever, respiratory depression, and urinary retention in labor.
Statistical analysis
The data description, analysis and graph were conducted using SPSS 27.0 statistical software and Graphpad Prism 9.3.1 software. The mean ± SD or median with quartiles was used to describe continuous variables. The independent samples t-test or non-parametric tests would be employed to compare groups. Categorical variables were reported as n(%), and group comparisons were made using the chi-square test or Fisher’s exact test. The generalized estimating equations were used to compare VAS, RSS, and MSS scores at various time points. P < 0.05 was considered statistically significant.
Results
There was no statistically significant difference in the comparison of maternal age, BMI, gestational week, years of education, and use of oxytocin in the two groups (all P > 0.05) (Table 1).
Table 1.
Baseline characteristics of the two groups
| Group | Age (years) | BMI (kg/m2) | Gestational age (days) | Years of Schooling (years) | Use of oxytocin (n, %) |
|---|---|---|---|---|---|
| Group SR (n = 90) | 28.46 ± 2.79 | 27.05 ± 2.65 | 279.24 ± 6.28 | 16.06 ± 1.17 | 18(20.0) |
| Group KR (n = 97) | 28.54 ± 3.32 | 26.39 ± 2.69 | 278.07 ± 7.06 | 16.39 ± 1.59 | 30(30.9) |
| t / χ2 | -0.179 | 1.692 | 1.196 | -1.643 | 2.922 |
| P-Value | 0.858 | 0.092 | 0.233 | 0.102 | 0.087 |
BMI Body mass index
Time and group were found to interact in the comparison of VAS scores. (χ2 group*time = 12.503, P = 0.014). Consequently, separate analyses were conducted.
Separate effects analyses for time revealed that variations in VAS scores at various time points were statistically significant (P < 0.001) in both Group SR and KR. The results of the separate effects analysis between the two groups indicated that the differences in VAS scores at t1-4 were statistically significant (P < 0.001). VAS scores at t0 and t5 in both groups did not show statistically significant differences(P > 0.05). Group SR exhibited lower VAS scores than Group KR at t1-4 (all P < 0.05) (Fig. 2, Table S1). To conclude, the SR group exhibited a superior analgesic effect and a lower VAS score in comparison to the KR group.
Fig. 2.
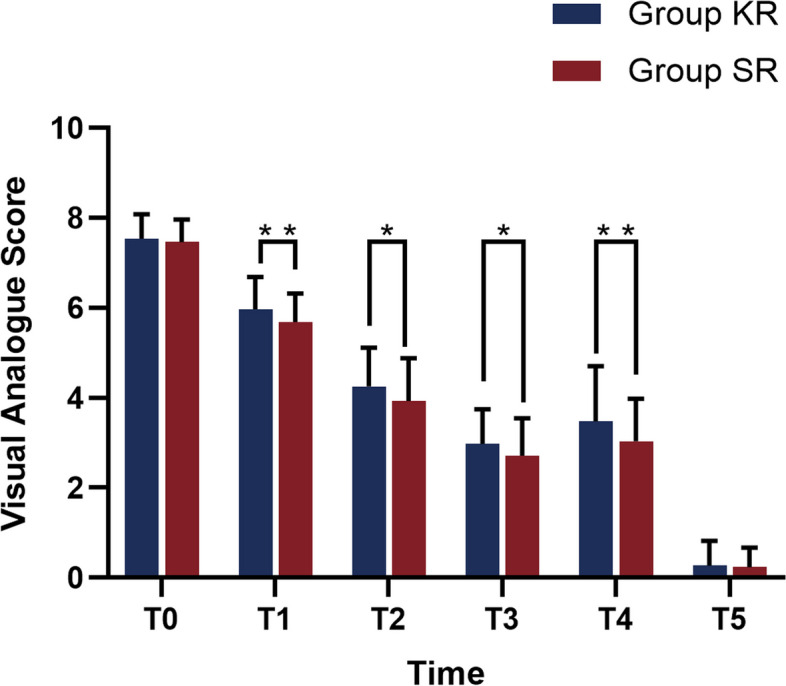
Comparison of visual analogue score in the two groups
Time and group were also found to interact in the comparison of RSS scores. (χ2 group*time = 10.822, P = 0.029). Consequently, separate analyses were conducted.
Separate effects analyses for time revealed that variations in RSS at various time points were statistically significant (P < 0.001) in both Group SR and KR. The results of the separate effects analysis between the two groups indicated that the differences in RSS at t1-3 were statistically significant (P < 0.001). The difference in maternal RSS between the two groups before analgesia (t0), when the cervix was fully opened (t4) and at the end of labor (t5) was not statistically significant (P > 0.05). Group SR showed lower RSS scores than Group at t1-3 (all P < 0.05) (Fig. 3, Table S1). To conclude, the SR group exhibited a superior analgesic effect and a lower VAS score in comparison to the KR group. In summary, the KR group exhibited a superior sedation effect and a lower RSS score in comparison to the SR group.
Fig. 3.
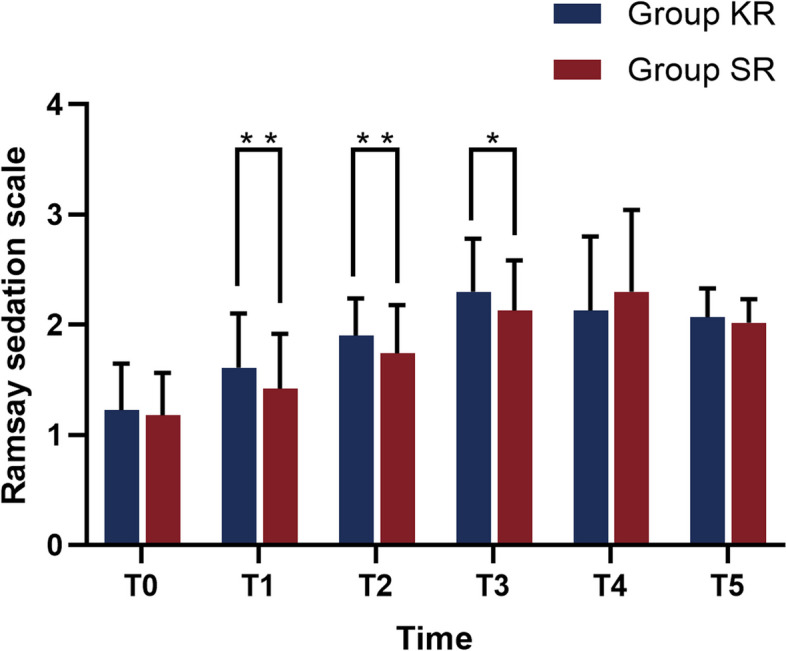
Comparison of Ramsay sedation score in the two groups
EPDS scores at 3 days postpartum (T1) between the two groups did not show significant differences(all P > 0.05); the difference in EPDS scores at 42 days postpartum and incidence of PPD between the two groups was statistically significant (both P < 0.05) (Table 2, Fig. 4).
Table 2.
Comparison of the EPDS score and incidence of PPD at 42 days after delivery in the two groups
| Group | EPDS score | PPD (n,%) | |
|---|---|---|---|
| T1 | T2 | ||
| Group SR(n = 90) | 5.81 ± 1.15 | 7.73 ± 2.04 | 8(8.9) |
| Group KR (n = 97) | 5.75 ± 1.10 | 6.64 ± 1.10 | 1(1.0) |
| t / χ2 | 0.356 | 4.613 | - |
| P-Value | 0.722 | < 0.001 | 0.015 |
EPDS Edinburgh Postnatal Depression Scale, PPD postpartum depression
Fig. 4.
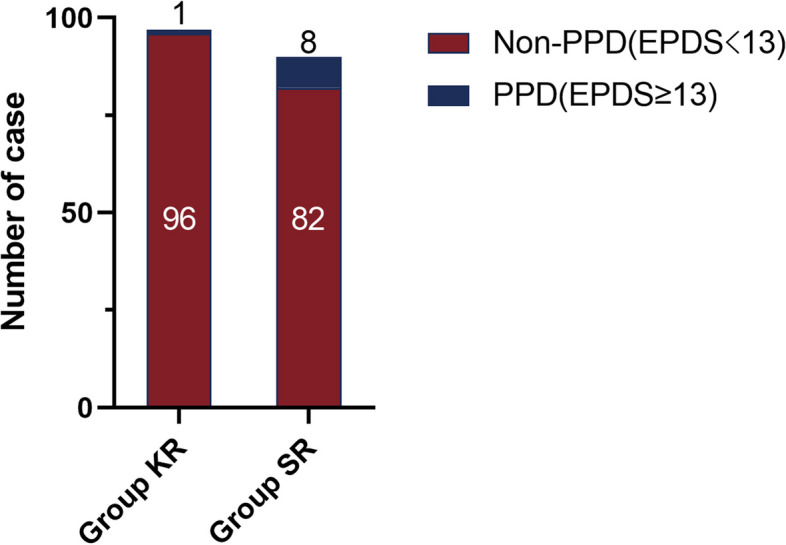
Comparison of incidence of postpartum depression 42 days after delivery in the two groups
The difference in the duration of the second stage of labor time between the SR and KR groups was statistically significant (P < 0.05). The difference in the first stage of labor, rate of instrumental delivery, Intrapartum blood loss, neonatal weight, 1 min Apgar score, 5 min Apgar score, and 10 min Apgar score did not differ significantly between the two groups (P > 0.05) (Table 3).
Table 3.
Comparison of the maternal and neonatal outcomes in the two groups
| Group | Group SR (n = 90) | Group KR (n = 97) | t/χ2 | P-Value |
|---|---|---|---|---|
| The first stage of labor duration (min) | 636.39 ± 111.52 | 637.47 ± 107.05 | -0.068 | 0.946 |
| The second stage of labor duration (min) | 95.49 ± 13.38 | 87.07 ± 14.71 | 5.191 | < 0.001 |
| Instrumental delivery(n,%) | 4(4.4) | 2(2.5) | - | 0.430 |
| Intrapartum blood loss(ml) | 265.58 ± 83.02 | 273.92 ± 83.64 | -0.684 | 0.495 |
| Neonatal body mass (kg) | 3.39 ± 0.35 | 3.28 ± 0.41 | 1.968 | 0.051 |
| 1 min Apgar score < 10 (n,%) | 11(12.2) | 6(6.2) | 2.058 | 0.151 |
| 5 min Apgar score < 10 (n,%) | 6(6.7) | 3(3.1) | - | 0.317 |
| 10 min Apgar score < 10 (n,%) | 3(3.3) | 3(3.1) | - | 1.000 |
Group KR has considerably decreased rates of pruritus compared to group SR (P < 0.05). The other adverse effects showed no significant difference (all P > 0.05) (Table 4).
Table 4.
Comparison of the incidence of adverse reactions in the two groups (n, %)
| Group | Nausea and vomiting | dizziness | Pruritus | Lethargy | Fever | Respiratory depression | Urinary retention |
|---|---|---|---|---|---|---|---|
| Group SR (n = 90) | 7(7.8) | 8(8.9) | 9(10.0) | 2(2.2) | 10(10.0) | 1(1.1) | 3(3.3) |
| Group KR (n = 97) | 6(6.2) | 5(5.2) | 1(1.0) | 4(4.1) | 6(6.2) | 2(2.1) | 2(2.1) |
| χ2 | 0.183 | 1.006 | - | - | 1.448 | - | - |
| P-Value | 0.669 | 0.316 | 0.008 | 0.684 | 0.229 | 1.000 | 0.673 |
Discussion
This retrospective study evaluated the efficacy of epidural esketamine and the conventional adjuvant sufentanil for labor analgesia, as well as the impact on postpartum depression, maternal and neonatal outcomes, and associated adverse effects. Our study found that epidural 0.3 mg/ml esketamine for labor analgesia might be associated with more effective sedation, shorter duration of second-stage labor, lower PPD incidence, lower pruritus rate, and no significant effect on the motor block and maternal and infant outcomes compared with epidural 0.3 µg/ml sufentanil. Nevertheless, its analgesic efficacy was marginally inferior to that of sufentanil; however, this minor discrepancy was not clinically important.
Esketamine is a rotary isomer of ketamine that has an affinity for NMDA receptors whose analgesic effect is approximately 1.5 to 2 times that of ketamine [7–9]. The analgesic, sedative, and anesthetic actions of esketamine are thought to be derived from its blocking action on NMDA receptors [7, 9, 14]. Esketamine antagonizes the NMDA receptor and inhibits its activation by glutamate. It also binds to central opioid μ- and δ-receptors, activating the downstream anti-injury sensory system and exerting analgesic effects [7, 9, 14, 22].
Epidural esketamine combined with 0.075% ropivacaine induced dose-dependent dizziness, which was notably common when the esketamine dose was 1 mg/ml, according to a dose exploration study [23]. Under the results of the pre-test, the present investigation employed a small dose of 0.3 mg/ml of esketamine in conjunction with 0.083% ropivacaine based on the results of pre-test. In this trial, the sufentanil group received the usual epidural anesthetic medication combination of 0.083% ropivacaine and 0.3 µg/ml sufentanil, which is the traditional protocol in our hospital.
In comparison to the sufentanil group, the esketamine group’s VAS scores were marginally higher at t1-4, which means the analgesic effect was marginally less effective. However, the difference in the actual maternal perception of pain between the two groups was not apparent after analgesia, indicating that the VAS scores of the two groups, despite being statistically distinct, may not have clinical significance.
This could be due to the favorable sedative effect, but it also suggests that the amount of esketamine employed in this experiment might not be the ideal dose for epidural labor analgesia, and future prospective studies are needed. Research has demonstrated that esketamine might be more appropriate to combine with opioids rather than be employed as a single epidural adjuvant [24]. The Ramsay sedation scores of Group ER in this trial were higher at t1-3 after analgesia, which may suggest there is a faster onset of sedation when epidural esketamine is administered for labor analgesia.
Research has demonstrated that the novel antidepressant drug esketamine achieves its rapid and potent antidepressant effects by combining a variety of mechanisms, such as antagonizing NMDA receptors, increasing brain-derived neurotrophic factor (BDNF) release, inhibiting neuronal apoptosis, and inducing synaptic plasticity [25]. Studies have proven that perioperative esketamine administered intravenously or utilized for postoperative intravenous self-controlled analgesia (PCIA) reduces the incidence of postoperative EPDS scores and PPD in women after cesarean section or delivery and improves the analgesic effect [6, 26, 27]. Our study observed that the EPDS score and PPD prevalence on the 42nd day postpartum were significantly reduced in women receiving epidural esketamine, which may suggest that esketamine used for epidural labor analgesia seems to be related to a lower incidence of postpartum depression in women with vaginal delivery. It has been proposed that the sensory-motor blocking effect caused by epidural local anesthetics has some effect on uterine contraction [28]. The KR group exhibited a significantly shorter second stage of labor than the SR group in this experiment. It was postulated that this may be attributed to the KR group’s reduced dosage of local anesthetic ropivacaine, which decreased the effect on uterine contraction, shortened the second stage of labor, and improved the delivery outcome. Esketamine inhibits opioid-induced nociceptive hypersensitivity by antagonizing the NMDA receptor, allowing for lower opioid dosages with less impact on mother and infant and fewer neurological adverse effects [7, 9, 14]. Group KR showed a decreased rate compared to Group SR in our study without other adverse reactions.
This study’s limitations include the following: Firstly, the absence of a blank control group using a purely local anesthetic agent. Due to the poor analgesic effects, cases of labor analgesia with epidural ropivacaine alone were few, and beyond our certain period, which hindered us from drawing some of the conclusions. Further research including cases of labor analgesia with epidural ropivacaine alone is still necessary. Secondly, it is difficult to determine the equivalent dose of esketamine and sufentanil for epidural analgesia, which may affect the results of the study. Thirdly, the retrospective nature of the study prevented the collection of the amount of epidural labor analgesia medication consumed. Last is the failure to establish different dosages of esketamine to analyze the optimal dosage of esketamine PCEA for labor analgesia. Prospective clinical trials with larger samples will be conducted in the future to investigate the optimal ratios and doses of esketamine.
Conclusions
Epidural esketamine for labor analgesia has exhibited certain effects on analgesia and sedation. Compared with sufentanil, esketamine used for epidural labor analgesia, may exhibit some connection with a lower incidence of postpartum depression, the shorter second stage of labor with fewer adverse reactions, and high safety for mothers and infants.
Although the analgesic efficacy may be marginally insufficient, it is anticipated that it will become the next generation of labor analgesia adjuvants as future research advances.
Supplementary Information
Additional file 1: Table S1. Comparison of the VAS, RSS, and MRS score in the two groups.
Acknowledgements
Not applicable.
Abbreviations
- BMI
Body mass index
- ER
Group Esketamine
- SR
Group Sufentanil
- EPDS
Edinburgh postnatal depression scale
- PPD
Postpartum depression
- NMDA
N-methyl-D-aspartate
- PCEA
Patient-controlled epidural analgesia
- ASA
American Society of Anesthesiologists
- VAS
Visual Analogue Scale
- RSS
Ramsay sedation scale
- MBS
Modified bromage scale
- PCIA
Patient-controlled intravenous analgesia
- t/T
Time point
- BDNF
Brain-derived neurotrophic factor
Authors’ contributions
Data analysis & Manuscript drafting: Kunyue Li, Ziqi Chai. Data collection & Data analysis: Chunyun Deng, Xiaoyuan Geng, Guoying Niu. Data Verification: Xiaoyuan Geng, Guoying Niu, Yuxia Wang. Research design: Kunyue Li, Tao Wang. Manuscript drafting: Kunyue Li, Ziqi Chai. Manuscript review: Yuxia Wang, Tao Wang, Yu Zhang. All authors reviewed the manuscript.
Funding
Not applicable.
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Ethics approval and consent to participate
This study was authorized by the Ethics Committee of the Third Affiliated Hospital of Zhengzhou University (No. 2022-089-01), and all parturients signed informed consent forms. The study followed the principles of the Helsinki Declaration.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Anim-Somuah M, Smyth RM, Cyna AM, Cuthbert A. Epidural versus non-epidural or no analgesia for pain management in labour. Cochrane Database Syst Rev. 2018;5(5):CD000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hawkins JL. Epidural analgesia for labor and delivery. N Engl J Med. 2010;362:1503–10. [DOI] [PubMed] [Google Scholar]
- 3.Youssef N, Orlov D, Alie T, et al. What epidural opioid results in the best analgesia outcomes and fewest side effects after surgery?: a meta-analysis of randomized controlled trials. Anesth Analg. 2014;119(4):965–77. [DOI] [PubMed] [Google Scholar]
- 4.Xiang B, Yang J, Lei X, Yu J. Adjuvant sufentanil decreased the ec50 of epidural ropivacaine for labor analgesia in healthy term pregnancy. Drug Des Devel Ther. 2021;15:2143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao Y, Chen Z, Huang Y, Sun S, Yang D. Comparison of dexmedetomidine and opioids as local anesthetic adjuvants in patient controlled epidural analgesia: a meta-analysis. Korean J Anesthesiol. 2024;77(1):139–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnston JN, Henter ID, Zarate CA Jr. The antidepressant actions of ketamine and its enantiomers. Pharmacol Ther. 2023;246:108431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu LL, Wang C, Deng CM, et al. Efficacy and safety of esketamine for supplemental analgesia during elective cesarean delivery: a randomized clinical trial. JAMA Netw Open. 2023;6(4):e239321. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Reif A, Bitter I, Buyze J, et al. Esketamine nasal spray versus quetiapine for treatment-resistant depression. N Engl J Med. 2023;389(14):1298–309. [DOI] [PubMed] [Google Scholar]
- 9.Molero P, Ramos-Quiroga JA, Martin-Santos R, Calvo-Sánchez E, Gutiérrez-Rojas L, Meana JJ. Antidepressant efficacy and tolerability of ketamine and esketamine: a critical review. CNS Drugs. 2018;32(5):411–20. [DOI] [PubMed] [Google Scholar]
- 10.Feltracco P, Barbieri S, Rizzi S, et al. Brief report: perioperative analgesic efficacy and plasma concentrations of S+ -ketamine in continuous epidural infusion during thoracic surgery. Anesth Analg. 2013;116(6):1371–5. [DOI] [PubMed] [Google Scholar]
- 11.Li Q, Wang S, Mei X. A single intravenous administration of a sub-anesthetic ketamine dose during the perioperative period of cesarean section for preventing postpartum depression: a meta-analysis. Psychiatry Res. 2022;310:114396. [DOI] [PubMed] [Google Scholar]
- 12.Yan H, Chen W, Chen Y, et al. Opioid-free versus opioid-based anesthesia on postoperative pain after thoracoscopic surgery: the use of intravenous and epidural esketamine. Anesth Analg. 2023;137(2):399–408. [DOI] [PubMed] [Google Scholar]
- 13.Tang J, Zheng Z, Ran Q, et al. Epidural esketamine and morphine for postoperative analgesia after caesarean delivery: a pilot study. Front Surg. 2023;9:988392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McIntyre RS, Rosenblat JD, Nemeroff CB, et al. Synthesizing the evidence for ketamine and esketamine in treatment-resistant depression: an international expert opinion on the available evidence and implementation. Am J Psychiatry. 2021;178:383–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zanos P, Moaddel R, Morris PJ, et al. Ketamine and ketamine metabolite pharmacology: insights into therapeutic mechanisms [published correction appears in Pharmacol Rev. Pharmacol Rev. 2018;70(3):621–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang S, Deng CM, Zeng Y, et al. Efficacy of a single low dose of esketamine after childbirth for mothers with symptoms of prenatal depression: randomised clinical trial. BMJ. 2024;385:e078218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnston JN, Kadriu B, Kraus C, et al. Ketamine in neuropsychiatric disorders: an update. Neuropsychopharmacology. 2024;49(1):23–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, Guo Y, Wu H, et al. Perioperative adjunctive esketamine for postpartum depression among women undergoing elective cesarean delivery: a randomized clinical trial. JAMA Netw Open. 2024;7(3):e240953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parsaei M, Hasehmi SM, Seyedmirzaei H, et al. Perioperative esketamine administration for prevention of postpartum depression after the cesarean section: a systematic review and meta-analysis. J Affect Disord. 2024;361:564–80. [DOI] [PubMed] [Google Scholar]
- 20.Liu QR, Zong QK, Ding LL, et al. Effects of perioperative use of esketamine on postpartum depression risk in patients undergoing cesarean section: a randomized controlled trial. J Affect Disord. 2023;339:815–22. [DOI] [PubMed] [Google Scholar]
- 21.Levis B, Negeri Z, Sun Y, et al. Accuracy of the Edinburgh Postnatal Depression Scale (EPDS) for screening to detect major depression among pregnant and postpartum women: systematic review and meta-analysis of individual participant data. BMJ. 2020;371:m4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mion G, Himmelseher S. Esketamine: less drowsiness, more analgesia. Anesth Analg. 2024;139(1):78–91. [DOI] [PubMed] [Google Scholar]
- 23.Yang SQ, Zhou YY, Yang ST, et al. Effects of different doses of esketamine intervention on postpartum depressive symptoms in cesarean section women: a randomized, double-blind, controlled clinical study. J Affect Disord. 2023;339:333–41. [DOI] [PubMed] [Google Scholar]
- 24.Ni L, Yao S, Wu Y, et al. Epidural dexmedetomidine or esketamine versus fentanyl to decrease ropivacaine use for labor analgesia: a randomized non-inferiority study. Heliyon. 2024;10(9):e30218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mo J, Ning Z, Wang X, et al. Association between perinatal pain and postpartum depression: a systematic review and meta-analysis. J Affect Disord. 2022;312:92–9. [DOI] [PubMed] [Google Scholar]
- 26.Kountanis JA, Vahabzadeh C, Bauer S, et al. Labor epidural analgesia and the risk of postpartum depression: a meta-analysis of observational studies. J Clin Anesth. 2020;61:109658. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Zhao G, Song G, et al. Association between neuraxial labor analgesia and postpartum depression: a meta-analysis. J Affect Disord. 2022;311:95–102. [DOI] [PubMed] [Google Scholar]
- 28.Zhang L, Peng M. Effect of neuraxial delivery analgesia on maternal pregnancy outcome. J Sino-Foreign Med Res. 2019;17(30):36–8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Comparison of the VAS, RSS, and MRS score in the two groups.
Data Availability Statement
Data is provided within the manuscript or supplementary information files.


