Abstract
Aims
This study aimed to estimate the strength of association between prescriptions of glucose‐dependent insulinotropic polypeptide (GIP) and/or glucagon‐like peptide‐1 receptor agonists (GLP‐1 RA) and the incidence of opioid overdose and alcohol intoxication in patients with opioid use disorder (OUD) and alcohol use disorder (AUD), respectively. This study also aimed to compare the strength of the GIP/GLP‐1 RA and substance use‐outcome association among patients with comorbid type 2 diabetes and obesity.
Design
A retrospective cohort study analyzing de‐identified electronic health record data from the Oracle Cerner Real‐World Data.
Setting
About 136 United States of America health systems, covering over 100 million patients, spanning January 2014 to September 2022.
Participants
The study included 503 747 patients with a history of OUD and 817 309 patients with a history of AUD, aged 18 years or older.
Measurements
The exposure indicated the presence (one or more) or absence of GIP/GLP‐1 RA prescriptions. The outcomes were the incidence rates of opioid overdose in the OUD cohort and alcohol intoxication in the AUD cohort. Potential confounders included comorbidities and demographic factors.
Findings
Patients with GIP/GLP‐1 RA prescriptions demonstrated statistically significantly lower rates of opioid overdose [adjusted incidence rate ratio (aIRR) in OUD patients: 0.60; 95% confidence interval (CI) = 0.43–0.83] and alcohol intoxication (aIRR in AUD patients: 0.50; 95% CI = 0.40–0.63) compared to those without such prescriptions. When stratified by comorbid conditions, the rate of incident opioid overdose and alcohol intoxication remained similarly protective for those prescribed GIP/GLP‐1 RA among patients with OUD and AUD.
Conclusions
Prescriptions of glucose‐dependent insulinotropic polypeptide and/or glucagon‐like peptide‐1 receptor agonists appear to be associated with lower rates of opioid overdose and alcohol intoxication in patients with opioid use disorder and alcohol use disorder. The protective effects are consistent across various subgroups, including patients with comorbid type 2 diabetes and obesity.
Keywords: AUD, dulaglutide, GIP/GLP‐1 RA, intoxication, OUD, overdose, semaglutide
INTRODUCTION
Problematic substance use is a neuropsychiatric condition characterized by the chronic compulsion to use substances despite occurrence of harmful consequences such as stroke, overdose and death [1, 2]. Such consequences of drug use have reportedly worsened in the general population over time. The number of United States (US) deaths caused by a drug overdose in 2021 (107 000 deaths) was six times higher than in it was 1999 (18 000 deaths) [3]. Not only have drug overdose deaths risen 16% from 2020 to 2021, but over 75% of overdose deaths in 2021 involved an opioid [3]. Opioid use disorders (OUD) are a severe type of substance use that has resulted in a devastating global public health crisis [4]. In 2019, the prevalence of OUD among US adults and adolescents was estimated between 6.7 and 7.6 million individuals [5]. Opioid use and OUD have been associated with many adverse health outcomes and result severe economic burden [6, 7]. Alcohol use disorder (AUD) is a severe type of alcohol use that causes detrimental health and social consequences impacting around 29.5 million people in the United States [8, 9, 10]. Alcohol use falls within the top five leading causes of preventable death in the United States exhibited by ~178 000 Americans dying per year because of excessive alcohol drinking in 2020 to 2021 [11, 12]. Of the 133 million alcohol users in the United States, 60 million individuals reported binge‐drinking (females having 4+ drinks and males having 5+ drinks in one occasion) in the past month in 2021 [13]. Additionally, numerous studies have demonstrated the high prevalence of alcohol and opioid use outcomes in many countries and societies all around the world [14, 15, 16]. As substance use disorders (SUD) such as OUD and AUD are widely prevalent and detrimental to population health, it is essential that effective treatments for drug use are identified and made readily available to those who could benefit.
Current evidence‐based pharmacological treatments exist for both OUD and AUD, but many individuals face barriers to receiving these medications including lack of access to willing prescribers, concerns regarding prescribing efficacy and complexity, negative treatment stigma and geographical and socio‐economic challenges [17, 18, 19]. Recent research has discovered significant underutilization of medication‐assisted treatment (MAT) among those diagnosed with AUD and OUD identifying a major gap in the reach of treatment services [20, 21]. Additionally, even when pharmacotherapy for OUD and AUD is available, many individuals continue to struggle to overcome these chronic conditions [22, 23, 24, 25, 26]. Treatment of chronic opioid use is further challenged by research suggesting possible adverse outcomes, such as suicide and mental health crisis, are associated with current efforts to transition patients away from chronic use of opioids [27, 28]. Despite the major efforts that have been made to provide patients suffering from AUD and OUD options for MAT, the barriers to receiving these medications and the mixed outcomes that result expose an urgent need for alternative or complementary treatment strategies.
Recent research has drawn significant attention to a drug called glucagon‐like peptide‐1 receptor agonists (GLP‐1 RA) (e.g. Ozempic), which are primarily prescribed to treat Type 2 diabetes, obesity and other weight‐related medical conditions [29]. GLP‐1 RA medications function by mimicking the GLP‐1 hormone that is produced in response to eating regulating hunger and weight [29, 30]. These drugs stimulate production of insulin decreasing blood sugar levels and interact with the brain to reduce appetite and trigger satisfaction after eating [29, 31]. The GLP‐1 receptors located within the brain's mesolimbic system, the neurological area responsible for motivated behaviors and reward processing through the release of dopamine, specifically modulate a person's food satiety signals influencing the desire to consume food [32]. This region also overlaps with the same processes that are responsible for the development and maintenance of addictive behaviors such as chronic substance use [33]. Because of the physiological similarities of the reward‐response pathways of eating and substance use, emerging evidence produced in rodent studies suggests that GLP‐1 RA medications may influence the ‘satiety’ of certain drugs and impact reward‐related changes of these drugs [30, 34]. This overlap suggests that GLP‐1 RA and similar drugs, such as glucose‐dependent insulinotropic polypeptide (GIP) agonists (e.g. Mounjaro), might modulate the reward‐response pathways associated with substance use.
Animal studies have provided promising insights. For instance, GLP‐1 RA drugs like liraglutide and semaglutide have been shown to reduce alcohol intake and modify drug‐seeking behaviors in rodents [35, 36, 37, 38, 39]. These findings have spurred further investigation into the neurobiological mechanisms of GLP‐1 RAs and their potential role in modulating addictive behaviors in humans [40]. Small‐scale clinical trials have begun to explore the effects of GLP‐1 RA medications on substance‐related outcomes, including cigarette smoking, opioid cravings and alcohol use (ClinicalTrials.gov identifiers NCT03712098, NCT04199728 and NCT03645408), with some trials indicating reduced heavy‐drinking days among AUD patients treated with GLP‐1 RAs [41]. Although these clinical trials are crucial for determining the potential effectiveness of GLP‐1 RA drugs to treat substance‐related behaviors, these studies are limited by the generalizability of the results as the studied patient sample is often very different from the population that would receive the treatment [42]. To our knowledge, no major population‐based studies have attempted to estimate the potential association between GIP/GLP‐1 RA medications and substance‐related outcomes in humans.
Addressing the lack of large‐scale human data by analyzing a comprehensive national database of electronic health records, we seek to understand how GIP/GLP‐1 RA medications impact drug and alcohol responses in a broad patient population. First, this study aims to estimate the strength of association between GIP/GLP‐1 RA prescriptions and incident opioid overdose and alcohol intoxication in patients with OUD and AUD. This study also aims to compare this GIP/GLP‐1 RA prescription and substance use‐outcome association among patients with comorbid conditions of Type 2 diabetes, obesity and both Type 2 diabetes and obesity. A better understanding of this relationship could lead to advanced research and clinical studies that evaluate the benefits of GIP/GLP‐1 RA drugs in reducing opioid use, alcohol use and the overall severity of OUD and AUD along with paving the way for possible treatment of other SUDs.
METHODS
Data source
This study was conducted using de‐identified electronic health record (EHR) data from the Oracle Cerner Real‐World Data (CRWD), a large national repository. As of September 2022, CRWD included data from 136 US health systems, covering over 100 million patients and ~1.7 billion healthcare encounters. Data in CRWD is extracted from the electronic medical record of hospitals in which Cerner has a data use agreement. Encounters may include pharmacy, clinical and microbiology laboratory, admission and billing information from affiliated patient care locations. All admissions, medication orders and dispensing, laboratory orders and specimens are date and time stamped, providing a temporal relationship between treatment patterns and clinical information. Cerner Corporation has established Health Insurance Portability and Accountability Act‐compliant operating policies to establish de‐identification for Cerner Real‐World Data [43, 44].
Study population
The study included adults (18 years or older) with documented histories of OUD or AUD. Eligibility was determined using codes (Tables S1–S2) from January 2014 to August 2022. Among these patients, if they had a prescription of GIP/GLP‐1 RA, the first instance was required to take place after first OUD or AUD diagnosis date otherwise the patient was removed. The comprehensive list of code types, used to define OUD and AUD diagnosis, comprised International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9‐CM), International Classification of Diseases, 10th Revision, Clinical Modification (ICD‐10‐CM), ICD‐9/10 Procedure Coding System (PCS) and Systematized Nomenclature of Medicine (SNOMED) codes. Exclusion criteria were applied to patients who had relevant diagnostic codes, but did not meet the full criteria for OUD or AUD.
Study design
A retrospective cohort design was used, comparing patients with GIP/GLP‐1 RA prescriptions to those without, in separate OUD and AUD cohorts. The index encounter was defined as the first instance of a GIP/GLP‐1 RA prescription or a randomly selected encounter for patients without such prescriptions, both occurring after a first diagnosis date of OUD or AUD. The study period spanned from January 2014, coinciding with the initial US Food and Drug Administration approval of many GLP‐1 RA medications, to September 2022, with inclusion of patients halted at the end of August 2022 to allow those last recruited to have at least 30 days of follow‐up [45]. Follow‐up was conducted for a minimum of 7 days post‐index encounter and up to 2 years, focusing on outcomes of opioid overdose and alcohol intoxication. Because of overdose and intoxication codes being part of OUD and AUD definitions, a 7‐day lag was implemented to ensure outcomes were separate from a possibly recent OUD/AUD diagnosis. To account for differential patient time‐on‐study as well as censoring, the time (in months) between each patient's index encounter and last encounter was captured. Because patients last recruited at the end of the inclusion period (August 2022) did not have more than 30 days to experience outcomes, sensitivity analyses were conducted to assess if results changed compared to only including those that would have a remaining 2‐years of possible follow‐up.
Pre‐registration
This study was not pre‐registered on a publicly available platform, and as such, the results should be considered exploratory.
Outcome and exposure variables
The primary outcomes were rates of incident of opioid overdose in the OUD cohort and alcohol intoxication in the AUD cohort. These were identified using a combination of ICD‐9, ICD‐10, SNOMED, National Drug Code (NDC) and Multum MediSource Lexicon (MMSL) codes (Tables S3–S4). Rates were defined as the count of incident outcomes occurring over a patient's time (in months) on study divided by the time on study. The exposure was defined as having a first prescription for any GIP/GLP‐1 RA medication, identified using relevant NDC and MMSL drug codes for abiglutide (Eperzan, Tanzeum), dulaglutide (Trulicity), exenatide (Byetta, Bydureon), liraglutide (Victoza, Saxenda), lixisenatide (Adlyxin, Lyxumia), semaglutide (Ozempic, Rybelsus, Wegovy) and tirzepatide (Mounjaro) (Table S5).
Additional measures
The analysis accounted for potential confounders, related to outcomes [27, 46, 47, 48, 49, 50, 51, 52], including age (in years), gender (female, male), race (American Indian or Alaskan Native [AI/AN], Asian or Pacific Islander [API], Black, Hispanic Latino, Non‐Hispanic [NH]‐White, other), marital status (married/partner, single, unknown), region (Northeast, Southeast, Midwest, West, multiple reported), insurance type (private, Medicare, Medicaid, other government/miscellaneous, self‐pay, unknown), year of encounter (2014–2022), categorized Charlson Comorbidity Index (CCI) (0, 1–2, 3–4, ≥5) [53], mental health history, tobacco dependence history and sleep apnea history. Specific adjustments were made for each cohort, considering factors for the OUD cohort of opioid overdose history, SUD history (excluding OUD), medications for opioid use disorder (MOUD) treatment history, benzodiazepine 1‐year prescription history, opioid 1‐year prescription dosing history using median daily morphine milligram equivalents (MME) (none, inpatient, outpatient <50, outpatient ≥50) [54], opioid 1‐year prescription duration history (difference in maximum prescription stop date and minimum prescription start date) and family history of psychoactive drug use. Factors for the AUD cohort consisted of alcohol intoxication history, SUD history (excluding AUD), medications for alcohol use disorder (MAUD) treatment history and family history of alcohol abuse. All additional clinical measures were captured using ICD‐9/10, SNOMED, Healthcare Common Procedure Coding System (HCPCS), NDC and MMSL codes (Tables S6–S13).
Stratifying measures
Given the primary use of GIP/GLP‐1 RA medications in treating Type 2 diabetes and obesity, the study also stratified results based on these conditions [55]. ICD‐9/10, SNOMED, NDC, MMSL codes as well as lab and measurement indications from Logical Observation Identifiers Names and Codes (LOINC) were used to identify these conditions (see Table S14 for comprehensive codes and algorithms for inclusion).
Statistical analysis
Demographic and clinical characteristics were presented for the cohorts overall as well as stratified by GIP/GLP‐1 RA prescription status. Standardized mean differences were used to compare characteristics between prescription status [56]. The incidence rate (IR) of opioid overdose and alcohol intoxication per 10 000 person‐months was presented along with incidence rate ratios (IRRs) and 95% Wald confidence intervals (CIs) for those with GIP/GLP‐1 RA prescriptions compared to those without, among those with history of OUD and AUD. To control for relevant confounders, IRRs were adjusted for previously mentioned variables in mixed‐effects Quasi‐Poisson regression models, accounting for person‐time as an offset variable and clustering model standard errors by hospital identification (ID) as a random effect. Because of Cameron and Trivedi's test indicating overdispersion (Poisson distributional assumption violation of equal mean and variance conditional on predictor variables) the Quasi‐Poisson model was implemented (which instead assumed the variance was a linear function of the mean) [57]. Model goodness‐of‐fit (GOF) was tested on whether model residual deviance followed a Chi‐square distribution with degrees of freedom (df) equal to the number of observations (n) minus the number of parameters (p) to be estimated (n‐p). Multicollinearity was assessed with variable inflation factors (VIFs). Model diagnostics all indicated optimal model fit. Adjusted IRRs (aIRRs) reported the rate of outcomes for those prescribed GIP/GLP‐1 RA medications compared to those not prescribed, while controlling for relevant confounders. Variability of estimates was again captured with 95% Wald CIs. All analyses were stratified by Type 2 diabetes, obesity and Type 2 diabetes and obesity status. To visualize results, the rate of incident substance‐related outcomes (opioid overdose and alcohol intoxication) per 10 000 patients versus time (in months) since index encounter were calculated over a 24‐month period. Variability of estimates were captured with 95% exact Poisson CIs. Different lines were drawn for those prescribed GIP/GLP‐1 RA prescriptions and those not, to compare rates between groups. All hypothesis tests were two‐sided with a significance level of 5%. All analyses were conducted in R version 4.0.2 (The R Foundation).
Sensitivity/supplemental analyses
To ensure robustness of findings, various sensitivity and supplemental analyses were conducted for both cohorts overall. Sensitivity analyses explored the effect of different adjustments to inclusion enrollment, follow‐up availability and cofactor model adjustment on main analysis findings. To account for possible endogeneity from patients' propensity to be prescribed GIP/GLP‐1 RA, and alternatively address group imbalance, analyses incorporating inverse probability of treatment weighting (IPTW) were conducted in which patients were weighted using the inverse probability of a propensity score predicting the probability of GLP‐1 RA prescription. Additionally, supplemental analyses were conducted in which the time to first event as well as recurrent events were used as outcomes, instead of the overall rate of outcomes, and were modeled with Cox Proportional‐Hazards and Andersen‐Gill models, respectively, providing hazard ratios (HRs) [58]. Finally, alternative outcomes of the rate of SUD‐related encounters were assessed and fit with similar Quasi‐Poisson models.
RESULTS
Demographic characteristics of our patient sample are presented in Table 1 among those with history of OUD and AUD overall and by those with and without GIP/GLP‐1 RA prescriptions. There were 503 747 patients with history of OUD in the sample and 817 309 patients with history of AUD. Among those with history of OUD, the mean (standard deviation [SD]) age was 50.5 (18.1) years, approximately half were female (51.1%), 67.0% single, 72.6% NH White, 42.6% from the Western United States and 31.0% reported Medicaid for insurance. Almost half (49.3%) of the cohort with OUD had a mental health condition history, 22% had a comorbidity score ≥5, 47.8% were found to have tobacco dependence history and 12% had a MOUD treatment history. Individuals with a GIP/GLP‐1 RA prescription had a mean age of 57.7 years and were more likely to be female and married/partnered compared to those without a prescription in the OUD cohort. Additionally, patients in the OUD cohort without GIP/GLP‐1 RA prescriptions had 15.7% had history of opioid overdose compared to 7.1% history of opioid overdose in those with prescriptions. Average opioid prescription dosing history was 49.7 days among individuals with GIP/GLP‐1 RA prescriptions compared 29.2 days among those without prescriptions.
TABLE 1.
Demographics of patients with hospital visits between January 2014 and august 2022, for those prescribed GIP/GLP‐1 receptor agonists or not, among those with a history of opioid use disorder and those with a history of alcohol use disorder, from Cerner‐affiliated hospital systems.
| Total | History of OUD | History of AUD | ||||||
|---|---|---|---|---|---|---|---|---|
| GIP/GLP‐1 RA prescription | No GIP/GLP‐1 RA prescription | SMD | GIP/GLP‐1 RA prescription | No GIP/GLP‐1 RA prescription | SMD | |||
| Total | n (% a ) | n (% a ) | Total | n (% a ) | n (% a ) | |||
| 503 747 (100.0) | 8103 (1.6) | 495 644 (98.4) | 817 309 (100.0) | 5621 (0.7) | 811 688 (99.3) | |||
| Common adjustment variables | ||||||||
| Age (y) b | 50.5 (18.1) | 57.7 (11.7) | 50.4 (18.1) | 0.480 | 46.84 (16.24) | 54.73 (11.58) | 46.79 (16.25) | 0.563 |
| Gender | 0.207 | 0.063 | ||||||
| Female | 257 197 (51.1) | 4952 (61.1) | 252 245 (50.9) | 264 394 (32.3) | 1985 (35.3) | 262 409 (32.3) | ||
| Male | 246 550 (48.9) | 3151 (38.9) | 243 399 (49.1) | 552 915 (67.7) | 3636 (64.7) | 549 279 (67.7) | ||
| Race | 0.113 | 0.137 | ||||||
| AI/AN | 5825 (1.2) | 120 (1.5) | 5705 (1.2) | 31 177 (3.8) | 196 (3.5) | 30 981 (3.8) | ||
| API | 3420 (0.7) | 57 (0.7) | 3363 (0.7) | 8871 (1.1) | 44 (0.8) | 8827 (1.1) | ||
| Black | 37 807 (7.5) | 669 (8.3) | 37 138 (7.5) | 84 863 (10.4) | 595 (10.6) | 84 268 (10.4) | ||
| Hispanic/Latino | 68 912 (13.7) | 1172 (14.5) | 67 740 (13.7) | 136 313 (16.7) | 1071 (19.1) | 135 242 (16.7) | ||
| NH‐White | 365 820 (72.6) | 5885 (72.6) | 359 935 (72.6) | 508 604 (62.2) | 3530 (62.8) | 505 074 (62.2) | ||
| Other | 21 963 (4.4) | 200 (2.5) | 21 763 (4.4) | 47 481 (5.8) | 185 (3.3) | 47 296 (5.8) | ||
| Marital status | 0.346 | 0.367 | ||||||
| Married/partner | 153 631 (30.5) | 3770 (46.5) | 149 861 (30.2) | 202 564 (24.8) | 2295 (40.8) | 200 269 (24.7) | ||
| Single | 337 749 (67.0) | 4239 (52.3) | 333 510 (67.3) | 578 792 (70.8) | 3224 (57.4) | 575 568 (70.9) | ||
| Unknown | 12 367 (2.5) | 94 (1.2) | 12 273 (2.5) | 35 953 (4.4) | 102 (1.8) | 35 851 (4.4) | ||
| US Region | 0.347 | 0.300 | ||||||
| Northeast | 69 951 (13.9) | 693 (8.6) | 69 258 (14.0) | 129 178 (15.8) | 1066 (19.0) | 128 112 (15.8) | ||
| Southeast | 86 865 (17.2) | 1454 (17.9) | 85 411 (17.2) | 117 693 (14.4) | 724 (12.9) | 116 969 (14.4) | ||
| Midwest | 120 662 (24.0) | 2915 (36.0) | 117 747 (23.8) | 257 895 (31.6) | 2314 (41.2) | 255 581 (31.5) | ||
| West | 214 638 (42.6) | 3019 (37.3) | 211 619 (42.7) | 296 883 (36.3) | 1502 (26.7) | 295 381 (36.4) | ||
| Multiple reported | 11 631 (2.3) | 22 (0.3) | 11 609 (2.3) | 15 660 (1.9) | 15 (0.3) | 15 645 (1.9) | ||
| Insurance | 0.424 | 0.431 | ||||||
| Private | 92 490 (18.4) | 1794 (22.1) | 90 696 (18.3) | 203 991 (25.0) | 1677 (29.8) | 202 314 (24.9) | ||
| Medicare | 140 005 (27.8) | 3445 (42.5) | 136 560 (27.6) | 144 678 (17.7) | 1755 (31.2) | 142 923 (17.6) | ||
| Medicaid | 156 113 (31.0) | 1481 (18.3) | 154 632 (31.2) | 233 285 (28.5) | 1242 (22.1) | 232 043 (28.6) | ||
| Other govt/misc. | 34 310 (6.8) | 533 (6.6) | 33 777 (6.8) | 54 923 (6.7) | 304 (5.4) | 54 619 (6.7) | ||
| Self‐pay | 65 522 (13.0) | 772 (9.5) | 64 750 (13.1) | 142 977 (17.5) | 556 (9.9) | 142 421 (17.5) | ||
| Unknown | 15 307 (3.0) | 78 (1.0) | 15 229 (3.1) | 37 455 (4.6) | 87 (1.5) | 37 368 (4.6) | ||
| Year of encounter | 0.502 | 0.650 | ||||||
| 2014 | 11 693 (2.3) | 27 (0.3) | 11 666 (2.4) | 41 976 (5.1) | 59 (1.0) | 41 917 (5.2) | ||
| 2015 | 18 315 (3.6) | 53 (0.7) | 18 262 (3.7) | 55 668 (6.8) | 77 (1.4) | 55 591 (6.8) | ||
| 2016 | 29 204 (5.8) | 156 (1.9) | 29 048 (5.9) | 58 914 (7.2) | 119 (2.1) | 58 795 (7.2) | ||
| 2017 | 42 945 (8.5) | 301 (3.7) | 42 644 (8.6) | 68 739 (8.4) | 195 (3.5) | 68 544 (8.4) | ||
| 2018 | 52 147 (10.4) | 551 (6.8) | 51 596 (10.4) | 81 537 (10.0) | 355 (6.3) | 81 182 (10.0) | ||
| 2019 | 64 875 (12.9) | 983 (12.1) | 63 892 (12.9) | 100 792 (12.3) | 603 (10.7) | 100 189 (12.3) | ||
| 2020 | 73 623 (14.6) | 1249 (15.4) | 72 374 (14.6) | 112 821 (13.8) | 842 (15.0) | 111 979 (13.8) | ||
| 2021 | 99 367 (19.7) | 2050 (25.3) | 97 317 (19.6) | 145 338 (17.8) | 1349 (24.0) | 143 989 (17.7) | ||
| 2022 | 111 578 (22.1) | 2733 (33.7) | 108 845 (22.0) | 151 524 (18.4) | 2022 (36.0) | 149 502 (18.3) | ||
| CCI categorized | 0.959 | 1.157 | ||||||
| 0 | 228 676 (45.4) | 689 (8.5) | 227 987 (46.0) | 483 128 (59.1) | 719 (12.8) | 482 409 (59.4) | ||
| 1–2 | 88 972 (17.7) | 1721 (21.2) | 87 251 (17.6) | 163 226 (20.0) | 1674 (29.8) | 161 552 (19.9) | ||
| 3–4 | 76 967 (15.3) | 1952 (24.1) | 75 015 (15.1) | 89 353 (10.9) | 1292 (23.0) | 88 061 (10.8) | ||
| ≥5 | 109 132 (21.7) | 3741 (46.2) | 105 391 (21.3) | 81 602 (10.0) | 1936 (34.4) | 79 666 (9.8) | ||
| Mental health condition history c | 0.408 | 0.207 | ||||||
| No | 255 484 (50.7) | 2540 (31.3) | 252 944 (51.0) | 744 896 (91.1) | 4747 (84.5) | 740 149 (91.2) | ||
| Yes | 248 263 (49.3) | 5563 (68.7) | 242 700 (49.0) | 72 413 (8.9) | 874 (15.5) | 71 539 (8.8) | ||
| Tobacco dependence history c | 0.081 | 0.324 | ||||||
| No | 262 931 (52.2) | 3908 (48.2) | 259 023 (52.3) | 536 484 (65.6) | 2807 (49.9) | 533 677 (65.7) | ||
| Yes | 240 816 (47.8) | 4195 (51.8) | 236 621 (47.7) | 280 825 (34.4) | 2814 (50.1) | 278 011 (34.3) | ||
| Sleep apnea history c | 0.702 | 0.295 | ||||||
| No | 452 366 (89.8) | 5027 (62.0) | 447 339 (90.3) | 809 151 (99.0) | 5256 (93.5) | 803 895 (99.0) | ||
| Yes | 51 381 (10.2) | 3076 (38.0) | 48 305 (9.7) | 8158 (1.0) | 365 (6.5) | 7793 (1.0) | ||
| OUD‐specific adjustment variables | ||||||||
| Opioid overdose history d | 0.272 | – | ||||||
| No | 425 274 (84.4) | 7524 (92.9) | 417 750 (84.3) | – | – | – | ||
| Yes | 78 473 (15.6) | 579 (7.1) | 77 894 (15.7) | – | – | – | ||
| SUD (excluding OUD) history c | 0.184 | – | ||||||
| No | 225 673 (44.8) | 4362 (53.8) | 221 311 (44.7) | – | – | – | ||
| Yes | 278 074 (55.2) | 3741 (46.2) | 274 333 (55.3) | – | – | – | ||
| MOUD treatment history (yes) c | 0.049 | – | ||||||
| No | 443 348 (88.0) | 7255 (89.5) | 436 093 (88.0) | – | – | – | ||
| Yes | 60 399 (12.0) | 848 (10.5) | 59 551 (12.0) | – | – | – | ||
| Benzodiazepine prescription e | 0.043 | – | ||||||
| No | 372 340 (73.9) | 5836 (72.0) | 366 504 (73.9) | – | – | – | ||
| Yes | 131 407 (26.1) | 2267 (28.0) | 129 140 (26.1) | – | – | – | ||
| Opioid prescription dosing (MME) history e | 0.189 | – | ||||||
| None | 371 152 (73.7) | 5289 (65.3) | 365 863 (73.8) | – | – | – | ||
| Inpatient | 1009 (0.2) | 11 (0.1) | 998 (0.2) | – | – | – | ||
| Outpatient <50 | 116 654 (23.2) | 2502 (30.9) | 114 152 (23.0) | – | – | – | ||
| Outpatient ≥50 | 14 932 (3.0) | 301 (3.7) | 14 631 (3.0) | – | – | – | ||
| Opioid prescription duration g history e (days) b | 29.51 (98.17) | 49.73 (145.10) | 29.18 (97.18) | 0.166 | – | – | – | – |
| Family history of psychoactive drug abuse c | 0.021 | – | ||||||
| No | 503 126 (99.9) | 8098 (99.9) | 495 028 (99.9) | – | – | – | ||
| Yes | 621 (0.1) | 5 (0.1) | 616 (0.1) | – | – | – | ||
| AUD‐specific adjustment variables | ||||||||
| Alcohol intoxication history d | – | 0.341 | ||||||
| No | – | – | – | 514 566 (63.0) | 4394 (78.2) | 510 172 (62.9) | ||
| Yes | – | – | – | 302 743 (37.0) | 1227 (21.8) | 301 516 (37.1) | ||
| SUD (excluding AUD) history c | – | 0.172 | ||||||
| No | – | – | – | 445 134 (54.5) | 2583 (46.0) | 442 551 (54.5) | ||
| Yes | – | – | – | 372 175 (45.5) | 3038 (54.0) | 369 137 (45.5) | ||
| MAUD treatment history (yes) c | – | 0.281 | ||||||
| No | – | – | – | 765 840 (93.7) | 4790 (85.2) | 761 050 (93.8) | ||
| Yes | – | – | – | 51 469 (6.3) | 831 (14.8) | 50 638 (6.2) | ||
| Family history of alcohol abuse c | – | 0.014 | ||||||
| No | – | – | – | 814 652 (99.7) | 5598 (99.6) | 809 054 (99.7) | ||
| Yes | – | – | – | 2657 (0.3) | 23 (0.4) | 2634 (0.3) | ||
| Stratifying variables | ||||||||
| Type 2 diabetes history c | 1.566 | 1.858 | ||||||
| No | 375 949 (74.6) | 1158 (14.3) | 374 791 (75.6) | 674 340 (82.5) | 839 (14.9) | 673 501 (83.0) | ||
| Yes | 127 798 (25.4) | 6945 (85.7) | 120 853 (24.4) | 142 969 (17.5) | 4782 (85.1) | 138 187 (17.0) | ||
| Obesity history c | 1.112 | 1.117 | ||||||
| No | 332 188 (65.9) | 1512 (18.7) | 330 676 (66.7) | 592 799 (72.5) | 1357 (24.1) | 591 442 (72.9) | ||
| Yes | 171 559 (34.1) | 6591 (81.3) | 164 968 (33.3) | 224 510 (27.5) | 4264 (75.9) | 220 246 (27.1) | ||
| GIP/GLP‐1 RA specific variables f | ||||||||
| Abiglutide | – | 26 (0.3) | – | – | – | 20 (0.4) | – | – |
| Dulaglutide | – | 3347 (41.3) | – | – | – | 2222 (39.5) | – | – |
| Exenatide | – | 568 (7.0) | – | – | – | 427 (7.6) | – | – |
| Liraglutide | – | 1885 (23.3) | – | – | – | 1294 (23.0) | – | – |
| Lixisenatide | – | 14 (0.2) | – | – | – | 12 (0.2) | – | – |
| Semaglutide | – | 3026 (37.3) | – | – | – | 2128 (37.9) | – | – |
| Tirzepatide | – | 189 (2.3) | – | – | – | 130 (2.3) | – | – |
Abbreviations: AI/AN, American Indian or Alaskan Native; API, Asian or Pacific Islander; AUD, alcohol use disorder; CCI, Charlson comorbidity index; GIP/GLP‐1 RA, glucose‐dependent insulinotropic polypeptide and/or glucagon‐like peptide‐1 receptor agonists; govt, government; OUD; opioid use disorder; MAUD, medications for alcohol use disorder; misc, miscellaneous; MME, morphine milligram equivalents; MOUD, medications for opioid use disorder; NH, non‐Hispanic; SUD, substance use disorder; SMD, standardized mean difference; US, United States.
Denominator is column group.
Mean (standard deviation).
Any prior condition inclusive up to index encounter.
Any prior condition and up to 6 days post index encounter.
Any prior condition within the year before (and including) index encounter.
Not mutually exclusive, patients could have had multiple prescribed.
Difference between last prescribed stop state and first prescribed start date.
Among those with history of AUD, the mean (SD) age was 46.8 (16.2) years, 67.7% were male, 70.8% were single, 62.2% were NH White, 36.3% were from the Western United States and 28.5% reported Medicaid for insurance. There were 10% of patients in the AUD cohort with a comorbidity score ≥5, 8.9% had a mental health condition history, 34.4% had a tobacco dependence history and 6.3% had a MAUD treatment history. Those with AUD and a GIP/GLP‐1 RA prescription had an average age of 54.7 years and were 64.7% male compared to an average age of 46.8 years and 67.7% male among those without prescriptions. Those with AUD and GIP/GLP‐1 RA prescriptions 15.5% had history of mental health condition and 50.1% had tobacco dependence history compared to 8.8% with mental health condition history and 34.4% with tobacco dependence in those without prescriptions. Among individuals with AUD and a GIP/GLP‐1 RA prescription, 21.8% had alcohol intoxication history and 14.8% had MAUD treatment history compared to 37.1% alcohol intoxication history and 6.2% with MAUD treatment history among those without a GIP/GLP‐1 RA prescription in the AUD cohort.
The crude and adjusted IRR and 95% CIs that estimate the rate of incident substance‐use outcomes (opioid overdose and alcohol intoxication) for those prescribed any GIP/GLP‐1 RA compared to those without a GIP/GLP‐1 RA prescription are presented in Table 2. Among individuals with OUD, those with a GIP/GLP‐1 RA prescription had a 40% lower rate of incident opioid overdose compared to those without a GIP/GLP‐1 RA prescription (aIRR [95% CI] = 0.60 [0.43, 0.83]). When stratified by comorbid conditions, the rate of incident opioid overdose remained significantly protective for those prescribed GIP/GLP‐1 RA. There was a 38% lower rate of opioid overdose among those with Type 2 diabetes and a GIP/GLP‐1 RA prescription (aIRR [95% CI] = 0.62 [0.46, 0.82]), 33% lower among those with obesity and a GIP/GLP‐1 RA prescription (aIRR [95% CI] = 0.67 [0.49, 0.92]), and 35% lower among those with both Type 2 diabetes and obesity and a GIP/GLP‐1 RA prescription (aIRR [95% CI] = 0.65 [0.48, 0.88]). Among individuals with AUD, those with a GIP/GLP‐1 RA prescription had a 50% lower rate of incident alcohol intoxication compared to those without a GIP/GLP‐1 RA prescription (aIRR [95% CI] = 0.50 [0.40, 0.63]). When stratified by Type 2 diabetes, obesity and Type 2 diabetes and obesity, the rate of incident alcohol intoxication for those with a GIP/GLP‐1 RA prescription was, respectively, 49% lower (aIRR [95% CI] = 0.51 [0.40, 0.65]), 42% lower (aIRR [95% CI] = 0.58 [0.45, 0.75]), and 42% lower (aIRR [95% CI] = 0.58 [0.45, 0.75]) compared to those without GIP/GLP‐1 RA prescriptions among the cohort with AUD.
TABLE 2.
Rate of incident substance‐related outcomes (opioid overdose and alcohol intoxication) for those prescribed any GIP/GLP‐1 RA compared to those not prescribed (overall and stratified by Type 2 diabetes and obesity) among those with a history of OUD and those with a history of AUD.
| Opioid overdose a | Alcohol intoxication b | |||||||
|---|---|---|---|---|---|---|---|---|
| Person‐months | n (IR c ) | IRR d (95% CI e ) | aIRR f (95% CI e ) | Person‐months | n (IR c ) | IRR d (95% CI e ) | aIRR g (95% CI e ) | |
| Overall | ||||||||
| No prescription | 5047061.4 | 27 628 (54.74) | 1 [REF] | 1 [REF] | 7201185.5 | 136 988 (190.23) | 1 [REF] | 1 [REF] |
| Yes prescription | 110112.2 | 172 (15.62) | 0.34 (0.23, 0.52) | 0.60 (0.43, 0.83) | 70514.6 | 477 (67.65) | 0.39 (0.30, 0.52) | 0.50 (0.40, 0.63) |
| Type 2 diabetes | ||||||||
| No prescription | 1466113.2 | 6232 (42.51) | 1 [REF] | 1 [REF] | 1526155.4 | 33 772 (221.29) | 1 [REF] | 1 [REF] |
| Yes prescription | 100182.6 | 168 (16.77) | 0.45 (0.32, 0.63) | 0.62 (0.46, 0.82) | 63174.6 | 450 (71.23) | 0.35 (0.25, 0.49) | 0.51 (0.40, 0.65) |
| Obesity | ||||||||
| No prescription | 2042015.6 | 7021 (34.38) | 1 [REF] | 1 [REF] | 2290980.9 | 41 785 (182.39) | 1 [REF] | 1 [REF] |
| Yes prescription | 92269.8 | 141 (15.28) | 0.49 (0.34, 0.72) | 0.67 (0.49, 0.92) | 54695.9 | 401 (73.31) | 0.43 (0.31, 0.60) | 0.58 (0.45, 0.75) |
| Type 2 diabetes and obesity | ||||||||
| No prescription | 838328.2 | 2907 (34.68) | 1 [REF] | 1 [REF] | 705387.5 | 15 045 (213.29) | 1 [REF] | 1 [REF] |
| Yes prescription | 84796.0 | 140 (16.51) | 0.53 (0.37, 0.75) | 0.65 (0.48, 0.88) | 48526.9 | 376 (77.48) | 0.39 (0.27, 0.56) | 0.58 (0.45, 0.75) |
Bold text indicates that the 95% CI does not overlap with 1, signifying statistical significance at the 5% level.
Abbreviations: AUD, alcohol use disorder; GIP/GLP‐1 RA, glucose‐dependent insulinotropic polypeptide and/or glucagon‐like peptide‐1 receptor agonists; ID, identification; IR, incidence rate; IRR, incidence rate ratio; OUD; opioid use disorder; MAUD, medications for alcohol use disorder; MME, morphine milligram equivalents; MOUD, medications for opioid use disorder; SUD, substance use disorder; US, United States.
Opioid overdoses occurring ≥7 days post index encounter and up to 2 years post.
Alcohol intoxications occurring ≥7 days post index encounter and up to 2 years post.
IR per 10 000 person‐months, denominator is sum of person‐months on study at risk per group.
IRR, via Quasi‐Poisson regression.
Wald confidence interval.
Adjusted IRR, via Quasi‐Poisson regression, adjusted for age, gender, race, comorbidity, year of index encounter, marital status, US region, insurance, history of SUD (excluding OUD), history of opioid overdose, median opioid prescription MME in year before index encounter, total duration of opioid prescriptions in year before index encounter, history of mental health condition, history of sleep apnea, benzodiazepine prescription in year before index encounter, tobacco dependence history, history of MOUD treatment, and family history of psychoactive drug abuse; model standard errors clustered by hospital ID.
Adjusted IRR, via Quasi‐Poisson regression, adjusted for age, gender, race, comorbidity, year of index encounter, marital status, US region, insurance, history of SUD (excluding AUD), history of alcohol intoxication, history of mental health condition, history of sleep apnea, tobacco dependence history, history of MAUD treatment, and family history of alcohol abuse; model standard errors clustered by hospital ID.
Figures 1(a,b) display the rate (per 10 000 patients) of incident substance‐related outcomes (opioid overdose and alcohol intoxication) versus the time since index encounter for individuals with and without GIP/GLP‐1 RA prescriptions among those with OUD and AUD. Figure 1(a) examines patients with OUD, whereas Figure 1(b) examines those with AUD. For those without GIP/GLP‐1 RA prescriptions, the rate (95% CI) per 10 000 patients of opioid overdose was 81.16 (78.46, 83.89) at month zero and decreased to 52.39 (48.08, 56.88) at month 24. The rate of opioid overdose, for those with GIP/GLP‐1 RA prescriptions, in Figure 1(a) started at 30.44 (19.29, 44.08) at month zero and ended ~17.22 (4.69, 37.74) at month 24. In Figure 1(b), the rate (95% CI) per 10 000 patients of alcohol intoxication for those without a GIP/GLP‐1 RA prescription was 280.85 (276.41, 285.31) at month zero and decreased to 201.47 (193.93, 209.15) at month 24. For those with GIP/GLP‐1 RA prescriptions in Figure 1(b), the rate of alcohol intoxication started at 82.09 (57.80, 110.58) at month zero and decreased to ~62.35 (26.92, 112.41) at month 24. Generally, the rates between individuals with and without a GIP/GLP‐1 RA prescriptions remained significantly different over time.
FIGURE 1.
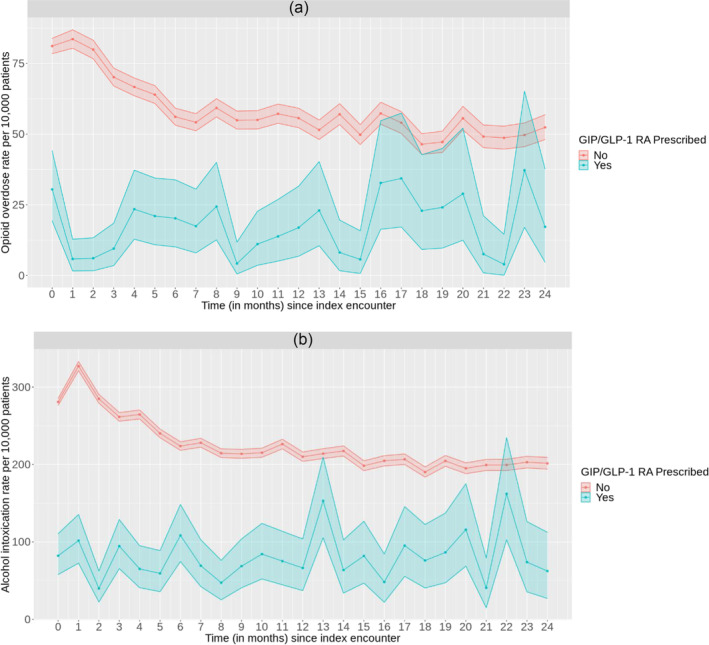
Rate (95% CI) of incident substance‐related outcomes ([a] opioid overdose; [b] alcohol intoxication) versus time since index encounter, for those prescribed any GIP/GLP‐1 RA compared to those not prescribed, among those with a history of opioid use disorder and those with a history of alcohol use disorder.
Sensitivity analyses in Table S15 revealed similar protective findings as those of the main analysis, in that under all analysis modifications, those prescribed GIP/GLP‐1 RA had lower rates of opioid overdoses and alcohol intoxications than those not prescribed. Opioid overdose aIRRs (95% CIs) ranged from 0.49 (0.32, 0.73) to 0.70 (0.46, 1.06) and alcohol intoxication aIRRs (95% CIs) ranged from 0.43 (0.31, 0.59) to 0.72 (0.53, 0.98) across all analysis modifications. Supplemental analyses in Table S16 additionally revealed protective associations when treating outcomes as time to events, with associations matching those of the main analysis more closely when considering recurrent outcomes (opioid overdose [first outcome] adjusted HR [aHR] [95% CI] = 0.68 [0.56, 0.82]; opioid overdose [recurrent outcomes] aHR [95% CI] = 0.61 [0.51, 0.73]; alcohol intoxication [first outcome] aHR [95% CI] = 0.74 [0.63, 0.87]; alcohol intoxication [recurrent outcomes] aHR [95% CI] = 0.47 [0.43, 0.52]) Additionally, protective associations were found between GIP/GLP‐1 RA prescriptions and SUD‐related encounters, although these associations were not as strongly protective as associations between GIP/GLP‐1 RA prescriptions and opioid overdose and alcohol intoxication (among those with OUD [2 years of follow‐up] aIRR [95% CI] = 0.90 [0.84, 0.97]; among those with AUD [2 years of follow‐up] aIRR [95% CI] = 0.85 [0.80, 0.90]).
DISCUSSION
The results of this study suggest that among individuals diagnosed with OUD and AUD, those with GIP/GLP‐1 RA prescriptions have a lower rate of opioid overdose and alcohol intoxication compared to individuals without GIP/GLP‐1 RA prescriptions even when accounting for similarities within and differences between hospital systems. The GIP/GLP‐1 RA prescriptions exhibited a strong protective association with alcohol intoxication among those with AUD. The GIP/GLP‐1 RA and related prescriptions additionally displayed a strong protective association with opioid overdose among individuals with OUD. The protective effects of GIP/GLP‐1 RA prescriptions were found to be strongest for the overall sample of participants and weakened only slightly for those with Type 2 diabetes, then obesity, and last both Type 2 diabetes and obesity while all remaining significant. These findings highlight foundational estimations of the association between GIP/GLP‐1 RA prescriptions and opioid overdose/alcohol intoxication and introduce the idea that GLP‐1 RA and other related drugs should be investigated as a novel pharmacotherapy treatment option for individuals with OUD or AUD.
Among those with OUD or AUD, we found that the incidence of substance‐related outcomes opioid overdose and alcohol intoxication is lower for individuals with GIP/GLP‐1 RA prescriptions compared to those without prescriptions. This finding aligns with recent research in animals that has found GLP‐1 RA drugs to effectively treat acute substance use‐related behavioral effects of ethanol, cocaine, amphetamine and nicotine in rodents [37]. For example, a study by Vallöf et al. [59] concluded that weekly treatment of dulaglutide, a GLP‐1 RA medication, reduced ethanol intake and ethanol preference in both male and female rats with prolonged effects seen in male rats. Exendin‐4 has exhibited reduction in cue‐induced heroin seeking behaviors and drug‐induced reinstatement of heroin seeking in rodents indicating a possibly effective treatment of opioid use in humans [38]. Although numerous animal studies suggest the potential role of GLP‐1 RA in SUD, there is very little human evidence to support this theory. Bouhlal et al. [60] observed significant effects on the GLP‐1 hormone after intravenous cocaine administration indicating a possible association between the two. Additionally, a translational study examined connections between four human genetic studies and one preclinical study and concluded that GLP‐1 RA could be effective personalized pharmacotherapy in the treatment of AUD [61]. The potential of GIP and GLP‐1 RA medications to treat substance use related outcomes is an important discovery for individuals struggling to access or achieve success with current substance‐use pharmacotherapy. However, embracing novel pharmacotherapies involves not only validating their clinical efficacy, but also addressing accessibility, cost and patient retention challenges, as highlighted by Morgan and Assoumou [62]. These authors stress the importance of real‐world applicability of novel treatments, noting that without addressing the known challenges of access and retention, even the most clinically effective treatments can fail to make a real‐world impact. Future studies should attempt to identify the possible mechanisms of action of GLP‐1 RA in substance use behaviors and further examine the association between GLP‐1RA drugs and individuals with SUD.
The association between GIP/GLP‐1 RA prescriptions and opioid overdose and alcohol intoxication was strongest for the overall participant group. When stratified by comorbidity, the association grew gradually weaker, while remaining significantly protective of the outcomes, among groups of individuals with Type 2 diabetes, obesity and both Type 2 diabetes and obesity. This finding is consistent with research indicating those with diabetes and obesity are at a higher risk of adverse substance‐related outcomes than those without these comorbid conditions [63, 64, 65]. Yet, notwithstanding the burden of Type 2 diabetes and obesity, stratification analyses showed that GIP/GLP‐1 RA prescriptions still displayed significantly protective effects of both opioid overdose and alcohol intoxication among those with OUD and AUD diagnoses. Additional research could investigate this association among these comorbid groups to better characterize the extent of GIP/GLP‐1 RA prescriptions to protect against various substance‐related outcomes among individuals burdened with multiple comorbid conditions.
The rate of incident opioid overdose and alcohol intoxication for individuals with GIP/GLP‐1 RA prescriptions was significantly lower than those without GIP/GLP‐1 RA prescriptions over time. As described previously in the discussion, this result is consistent with current literature that has found GIP/GLP‐1 RA to effectively reduce alcohol‐, nicotine‐ and other substance‐seeking behaviors [35, 36, 37]. In addition to this significant finding, it may be important to note the gradual decrease in opioid overdose and alcohol intoxication that is seen in individuals without GIP/GLP‐1 RA prescriptions among both OUD and AUD cohorts of our study. The gradual decline in substance‐use outcomes for individuals without GIP/GLP‐1 RA prescriptions could be explained by trends in treatment for OUD and AUD. Research has found that individuals seeking treatment for alcohol dependence were more likely to relapse within the first year, especially within the first 3 months of abstinence [66]. One study examined lapse and relapse among patients with opioid dependence admitted to a 6‐week treatment plan and found that 91% of individuals reported a relapse and 60% of those relapses occurred within the first week of treatment [67]. Yet, notwithstanding this gradual decline in those not prescribed, rates of overdose and intoxication among those prescribed were immediately low even in the first month following index encounter and remained lower throughout the study period. Future research with experimental designs can be performed to investigate the causal effects of GIP/GLP‐1 RA drugs more thoroughly as well as dive deeper into the short (acute) and long‐term (chronic) impacts.
Implications
This study's findings have the potential to suggest significant implications for both clinical practice and public health policy in the coming years. Clinically, these findings may encourage healthcare providers to research using GIP/GLP‐1 RA medications as part of a holistic treatment approach, especially for patients with coexisting metabolic disorders. From a public health perspective, the results—strengthened by similar findings—may support a reevaluation of current treatment guidelines and policies to potentially encompass GIP/GLP‐1 RA treatments, with an emphasis on accessibility and research support. Future research should focus on prospective clinical trials to validate these findings, explore the underlying mechanisms and determine the long‐term efficacy and safety of GIP/GLP‐1 RA medications in diverse populations. Additionally, the study highlights the importance of interdisciplinary research in understanding the neurobiological links between metabolic disorders and problematic substance use, potentially leading to more effective treatment strategies within healthcare systems.
Limitations
This study does have several limitations to note. First, the retrospective correlational nature of the data limits the ability to assume causality between GIP/GLP‐1 RA prescriptions and lower rates of opioid overdose and alcohol intoxication. Additional prospective research is needed to examine if GIP/GLP‐1 RA drugs have the ability to impact substance use‐related behaviors and confirm our findings. Second, although the Cerner database is one of the largest electronic medical record repositories available, the data is limited to Cerner‐affiliated hospitals and clinics meaning events occurring outside these participating partners were not measured. Third, the accuracy of the results produced in this study is limited by our team's ability to use the most appropriate classification algorithms and enter data correctly, as well as other limitations inherent in EHR data such as real‐world timing capture (difference between onset of disease and actual capture of disease in the hospital), higher severity in hospital populations compared to general public and inter‐hospital/physician differences affecting diagnosis/treatment [68]. However, to overcome these particular issues, we used validated classification algorithms (such as those from the Centers for Medicare and Medicaid Services) [69], focused on more emergency‐based outcomes (opioid overdose and alcohol intoxication) that would be more likely to be captured in real time, adjusted for healthcare utilization in sensitivity analyses and accounted for inter‐hospital differences in all analyses. Further, we were only able to assess overall generic prescription associations. Future studies could examine all generic types as well as explore specific brands.
CONCLUSION
Our study, focusing on assessing the association between GIP/GLP‐1 RA prescriptions and substance‐related outcomes, specifically opioid overdose and alcohol intoxication, in patients with OUD and AUD, reveals the possibilities of a novel therapeutic pathway in substance use treatment. The potential of GIP/GLP‐1 RA medications, traditionally used for metabolic disorders, in mitigating these critical substance‐related outcomes emphasizes the importance of exploring existing drugs for new applications. Although the results are promising, they highlight the need for further research, particularly prospective clinical trials, to validate these associations and understand the underlying mechanisms. This study not only contributes to the evolving landscape of substance use therapy but also opens avenues for more comprehensive and effective treatment strategies for those affected by OUD and AUD.
AUTHOR CONTRIBUTIONS
Fares Qeadan had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Fares Qeadan: Concept and design. Benjamin Tingey, Fares Qeadan: Acquisition, analysis or interpretation of data. Ashlie McCunn: Drafting of the manuscript. Fares Qeadan, Ashlie McCunn, Benjamin Tingey: Critical revision of the manuscript for important intellectual content. BenjaminTingey: Statistical analysis. Fares Qeadan: Administrative, technical or material support. Fares Qeadan: Supervision.
DECLARATION OF INTERESTS
None.
DATA SHARING STATEMENT
The datasets generated during and/or analyzed during the current study are not publicly available because of restrictions by Oracle Cerner, the owner of the data. Data could be accessed by signing a data sharing agreement with Oracle Cerner and covering any costs that may be involved (contact Kendra Stillwell: kendra.stillwell@cernerenviza.com).
Supporting information
Table S1:List of codes used to define opioid use disorder (OUD)
Table S2: List of codes used to define alcohol use disorder (AUD)
Table S3: List of codes used to define opioid overdose (OD)
Table S4: List of codes used to define alcohol intoxication (AI)
Table S5: List of codes used to define glucose‐dependent insulinotropic polypeptide (GIP) and/or glucagon‐like peptide‐1 receptor agonists (GIP/GLP‐1 RA) prescriptions
Table S6: List of codes used to define Charlson Comorbidity Index (CCI)
Table S7: Mental health codes by mental health disorder classification
Table S8: List of codes used to define additional clinical variables
Table S9: List of codes used to define substance use disorder by substance class [1]
Table S10: List of codes used to define medications for opioid use disorder (MOUD) treatment
Table S11: List of codes used to define benzodiazepines
Table S12a: List of codes used to define inpatient opioid prescriptions [1]
Table S12b: List of codes used to define opioid prescriptions
Table S12c: List of codes used to define opioid prescriptions [1]
Table S13: List of codes used to define medications for alcohol use disorder (MAUD) treatment
Table S14: List of codes used to define obesity and type 2 diabetes
Table S15: Sensitivity analysis scenarios for rate of incident substance‐related outcomes (opioid overdose and alcohol intoxication) for those prescribed any GIP/GLP‐1 RA compared to those not prescribed (overall) among those with a history of opioid use disorder and those with a history of alcohol use disorder
Table S16: Supplemental analysis scenarios for rate/hazard of incident substance‐related outcomes (opioid overdose and alcohol intoxication) for those prescribed any GIP/GLP‐1 RA compared to those not prescribed (overall) among those with a history of opioid use disorder and those with a history of alcohol use disorder
ACKNOWLEDGEMENTS
We acknowledge Oracle Cerner for its national data access and computation capabilities.
Qeadan F, McCunn A, Tingey B. The association between glucose‐dependent insulinotropic polypeptide and/or glucagon‐like peptide‐1 receptor agonist prescriptions and substance‐related outcomes in patients with opioid and alcohol use disorders: A real‐world data analysis. Addiction. 2025;120(2):236–250. 10.1111/add.16679
Funding information None.
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are not publicly available due to restrictions by Oracle Cerner, the owner of the data. Data could be accessed by signing a data sharing agreement with Oracle Cerner and covering any costs that may be involved (Contact Kendra Stillwell: kendra.stillwell@cernerenviza.com).
REFERENCES
- 1. Zou Z, Wang H, d'Oleire Uquillas F, Wang X, Ding J, Chen H. Definition of substance and non‐substance addiction. Adv Exp Med Biol. 2017;1010:21–41. 10.1007/978-981-10-5562-1_2 [DOI] [PubMed] [Google Scholar]
- 2. Cleveland Clinic . Addiction Cleveland Clinic. https://my.clevelandclinic.org/health/diseases/6407-addiction [Google Scholar]
- 3. Centers for Disease Control and Prevention . Understanding the Opioid Overdose Epidemic U.S. Department of Health & Human Services. https://www.cdc.gov/overdose-prevention/about/understanding-the-opioid-overdose-epidemic.html?CDC_AAref_Val=https://www.cdc.gov/opioids/basics/epidemic.html [Google Scholar]
- 4. The lancet regional health . a. opioid crisis: addiction, overprescription, and insufficient primary prevention. Lancet Regional Health – Americas. 2023;23:100557. 10.1016/j.lana.2023.100557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Keyes KM, Rutherford C, Hamilton A, Barocas JA, Gelberg KH, Mueller PP, et al. What is the prevalence of and trend in opioid use disorder in the United States from 2010 to 2019? Using multiplier approaches to estimate prevalence for an unknown population size. Drug Alcohol Depend Rep. 2022;3, 100052. 10.1016/j.dadr.2022.100052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lake S, Kennedy MC. Health outcomes associated with illicit prescription opioid injection: a systematic review. J Addict Dis. 2016;35(2):73–91. 10.1080/10550887.2015.1127712 [DOI] [PubMed] [Google Scholar]
- 7. Florence C, Luo F, Rice K. The economic burden of opioid use disorder and fatal opioid overdose in the United States, 2017. Drug Alcohol Depend. 2021;218:108350. 10.1016/j.drugalcdep.2020.108350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. SAMHSA . 2021 National Survey on Drug Use and Health. Table 56A—Alcohol use disorder in past year: among people aged 12 or older; by age group and demographic characteristics, numbers in thousands, 2021: Center for Behavioral Health Statistics and Quality; 2023. [Google Scholar]
- 9. Rehm J, Shield KD. Global burden of alcohol use disorders and alcohol liver disease. Biomedicine. 2019;7(4):99. 10.3390/biomedicines7040099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sudhinaraset M, Wigglesworth C, Takeuchi DT. Social and cultural contexts of alcohol use: influences in a social‐ecological framework. Alcohol Res. 2016;38(1):35–45. [PMC free article] [PubMed] [Google Scholar]
- 11. Pilar MR, Eyler AA, Moreland‐Russell S, Brownson RC. Actual causes of death in relation to media, policy, and funding attention: examining public health priorities. Original research. Front Public Health. 2020;8, 279. 279. 10.3389/fpubh.2020.00279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Esser MB. Deaths from excessive alcohol use—United States, 2016–2021. MMWR Morbidity Mortality Weekly LA Rep. 2024;73(8): 154–161. 10.15585/mmwr.mm7308a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. SAMHSA . Key Substance Use and Mental Health Indicators in the United States: Results from the 2021 National Survey on Drug Use and Health (HHS Publication No. PEP22–07–01‐005, NSDUH Series H‐57). 2022. https://www.samhsa.gov/data/sites/default/files/reports/rpt39443/2021NSDUHFFRRev010323.pdf [Google Scholar]
- 14. Glantz MD, Bharat C, Degenhardt L, Sampson NA, Scott KM, Lim CCW, et al. The epidemiology of alcohol use disorders cross‐nationally: findings from the world mental health surveys. Addict Behav. 2020;102:106128. 10.1016/j.addbeh.2019.106128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Degenhardt L, Grebely J, Stone J, Hickman M, Vickerman P, Marshall BDL, et al. Global patterns of opioid use and dependence: harms to populations, interventions, and future action. Lancet. 2019;394(10208):1560–1579. 10.1016/s0140-6736(19)32229-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Iacobucci G. Drug deaths in England and Wales rise to highest number on record. BMJ. 2023;383:p2970. 10.1136/bmj.p2970 [DOI] [PubMed] [Google Scholar]
- 17. Gregory C, Chorny Y, McLeod SL, Mohindra R. First‐line Medications for the Outpatient Treatment of Alcohol Use Disorder: A Systematic Review of Perceived Barriers. J Addict Med. 2022;16(4):e210–e218. [DOI] [PubMed] [Google Scholar]
- 18. Mackey K, Veazie S, Anderson J, Bourne D, Peterson K. Barriers and facilitators to the use of medications for opioid use disorder: a rapid review. J Gen Intern Med. 2020;35(3):954–963. 10.1007/s11606-020-06257-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Finlay AK, Ellerbe LS, Wong JJ, Timko C, Rubinsky AD, Gupta S, et al. Barriers to and facilitators of pharmacotherapy for alcohol use disorder in VA residential treatment programs. J Subst Abuse Treat. 2017;77:38–43. 10.1016/j.jsat.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krawczyk N, Rivera BD, Jent V, Keyes KM, Jones CM, Cerdá M. Has the treatment gap for opioid use disorder narrowed in the U.S.? a yearly assessment from 2010 to 2019. Int J Drug Policy. 2022;110:103786. 10.1016/j.drugpo.2022.103786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Qeadan F, Mensah NA, Gu LY, Madden EF, Venner KL, English K. Trends in the use of naltrexone for addiction treatment among alcohol use disorder admissions in U.S. substance use treatment facilities. Int J Environ Res Public Health. 2021;18(16):8884. 10.3390/ijerph18168884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lyon J. More treatments on deck for alcohol use disorder. JAMA. 2017;317(22):2267–2269. 10.1001/jama.2017.4760 [DOI] [PubMed] [Google Scholar]
- 23. Volkow ND, Jones EB, Einstein EB, Wargo EM. Prevention and treatment of opioid misuse and addiction: a review. JAMA Psychiatry. 2019;76(2):208–216. 10.1001/jamapsychiatry.2018.3126 [DOI] [PubMed] [Google Scholar]
- 24. Wakeman SE, Larochelle MR, Ameli O, Chaisson CE, McPheeters JT, Crown WH, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020;3(2):e1920622. 10.1001/jamanetworkopen.2019.20622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. National Institute on Drug Abuse . How effective are medications to treat opioid use disorder? National Institutes of Health. https://nida.nih.gov/publications/research-reports/medications-to-treat-opioid-addiction/efficacy-medications-opioid-use-disorder [Google Scholar]
- 26. Stokłosa I, Więckiewicz G, Stokłosa M, Piegza M, Pudlo R, Gorczyca P. Medications for the treatment of alcohol dependence‐current state of knowledge and future perspectives from a public health perspective. Int J Environ Res Public Health. 2023;20(3):1870. 10.3390/ijerph20031870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Agnoli A, Xing G, Tancredi DJ, Magnan E, Jerant A, Fenton JJ. Association of Dose Tapering with Overdose or mental health crisis among patients prescribed long‐term opioids. JAMA. 2021;326(5):411–419. 10.1001/jama.2021.11013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oliva EM, Bowe T, Manhapra A, Kertesz S, Hah JM, Henderson P, et al. Associations between stopping prescriptions for opioids, length of opioid treatment, and overdose or suicide deaths in US veterans: observational evaluation. BMJ. 2020;368:m283. 10.1136/bmj.m283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. FDA . Medications Containing Semaglutide Marketed for Type 2 Diabetes or Weight Loss U.S. Food & Drug Administration. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/medications-containing-semaglutide-marketed-type-2-diabetes-or-weight-loss [Google Scholar]
- 30. Kenny PJ. Common cellular and molecular mechanisms in obesity and drug addiction. Nat Rev Neurosci. 2011;12(11):638–651. 10.1038/nrn3105 [DOI] [PubMed] [Google Scholar]
- 31. Doucleff M. Ozempic seems to curb cravings for alcohol. Here's What Scientists Think Is Going on. Shots. 2023. PMID: https://www.npr.org/sections/health-shots/2023/08/28/1194526119/ozempic-wegovy-drinking-alcohol-cravings-semaglutide [Google Scholar]
- 32. Hernandez NS, Schmidt HD. Central GLP‐1 receptors: novel molecular targets for cocaine use disorder. Physiol Behav. 2019;206:93–105. 10.1016/j.physbeh.2019.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Adinoff B. Neurobiologic processes in drug reward and addiction. Harv Rev Psychiatry. 2004;12(6):305–320. 10.1080/10673220490910844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Engel JA, Jerlhag E. Role of appetite‐regulating peptides in the pathophysiology of addiction: implications for pharmacotherapy. CNS Drugs. 2014;28(10):875–886. 10.1007/s40263-014-0178-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marty VN, Farokhnia M, Munier JJ, Mulpuri Y, Leggio L, Spigelman I. Long‐acting glucagon‐like Peptide‐1 receptor agonists suppress voluntary alcohol intake in male Wistar rats. Original research. Front Neurosci. 2020;14:599646. 10.3389/fnins.2020.599646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chuong V, Farokhnia M, Khom S, Pince CL, Elvig SK, Vlkolinsky R, et al. The glucagon‐like peptide‐1 (GLP‐1) analogue semaglutide reduces alcohol drinking and modulates central GABA neurotransmission. JCI Insight. 2023;8(12), e170671 e170671 e170671. 10.1172/jci.insight.170671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brunchmann A, Thomsen M, Fink‐Jensen A. The effect of glucagon‐like peptide‐1 (GLP‐1) receptor agonists on substance use disorder (SUD)‐related behavioural effects of drugs and alcohol: a systematic review. Physiol Behav. 2019;206:232–242. 10.1016/j.physbeh.2019.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Douton JE, Augusto C, Stoltzfus B, Carkaci‐Salli N, Vrana KE, Grigson PS. Glucagon‐like peptide‐1 receptor agonist, exendin‐4, reduces reinstatement of heroin‐seeking behavior in rats. Behav Pharmacol. 2021;32(4):265–277. 10.1097/fbp.0000000000000609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang Y, Kahng MW, Elkind JA, Weir VR, Hernandez NS, Stein LM, et al. Activation of GLP‐1 receptors attenuates oxycodone taking and seeking without compromising the antinociceptive effects of oxycodone in rats. Neuropsychopharmacology. 2020;45(3):451–461. 10.1038/s41386-019-0531-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Klausen MK, Thomsen M, Wortwein G, Fink‐Jensen A. The role of glucagon‐like peptide 1 (GLP‐1) in addictive disorders. Br J Pharmacol. 2022;179(4):625–641. 10.1111/bph.15677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Klausen MK, Jensen ME, Møller M, et al. Exenatide once weekly for alcohol use disorder investigated in a randomized, placebo‐controlled clinical trial. JCI Insight. 2022;7(19), e159863. 10.1172/jci.insight.159863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Collet JP. limitations of clinical trials. Rev Prat. 2000;50(8):833–837. PMID: Limite Des Essais Cliniques. [PubMed] [Google Scholar]
- 43. Ehwerhemuepha L, Carlson K, Moog R, Bondurant B, Akridge C, Moreno T, et al. Cerner real‐world data (CRWD) ‐ a de‐identified multicenter electronic health records database. Data Brief. 2022;42:108120. 10.1016/j.dib.2022.108120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cerner . Oracle Health Multum Drug Database Oracle. https://www.oracle.com/health/service-lines-departments/pharmacy/#rc30p5 [Google Scholar]
- 45. Biopharma PEG . Evolution of GLP‐1 receptor agonists for diabetes treatment Biopharma PEG. https://www.biochempeg.com/article/299.html [Google Scholar]
- 46. Olfson M, Wall M, Wang S, Crystal S, Blanco C. Risks of fatal opioid overdose during the first year following nonfatal overdose. Drug Alcohol Depend. 2018;190:112–119. 10.1016/j.drugalcdep.2018.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Suffoletto B, Zeigler A. Risk and protective factors for repeated overdose after opioid overdose survival. Drug Alcohol Depend. 2020;209:107890. 10.1016/j.drugalcdep.2020.107890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Crystal S, Nowels M, Samples H, Olfson M, Williams AR, Treitler P. Opioid overdose survivors: Medications for opioid use disorder and risk of repeat overdose in Medicaid patients. Drug Alcohol Depend. 2022;232:109269. 10.1016/j.drugalcdep.2022.109269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Qeadan F, Madden EF. Associations between naloxone prescribing and opioid overdose among patients with acute and chronic pain conditions. Addiction. 2022;117(2):457–471. 10.1111/add.15643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Baldassarre M, Caputo F, Pavarin RM, Bossi MM, Bonavita ME, Caraceni P, et al. Accesses for alcohol intoxication to the emergency department and the risk of re‐hospitalization: an observational retrospective study. Addict Behav. 2018;77:1–6. 10.1016/j.addbeh.2017.08.031 [DOI] [PubMed] [Google Scholar]
- 51. Schepis TS, Wastila L, McCabe SE. Family history of substance use disorder and likelihood of prescription drug misuse in adults 50 and older. Aging Ment Health. 2023;27(5):1020–1027. 10.1080/13607863.2022.2084711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Centers for Disease Control and Prevention . High‐risk substance use among youth U.S. Department of Health & Human Services; 2022. [Google Scholar]
- 53. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 54. Centers for Medicare & Medicaid Services . Opioid Oral Morphine Milligram Equivalent (MME) Conversion Factors. 2022. https://medicaid.utah.gov/Documents/files/Opioid-Morphine-EQ-Conversion-Factors.pdf [Google Scholar]
- 55. Collins L, Costello RA. Glucagon‐like Peptide‐1 receptor agonists. In: StatPearls StatPearls Publishing; 2024. https://www.ncbi.nlm.nih.gov/books/NBK551568/ [Google Scholar]
- 56. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011;46(3):399–424. 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cameron AC, Trivedi PK. Regression‐based tests for overdispersion in the Poisson model. J Econometrics. 1990;46(3):347–364. 10.1016/0304-4076(90)90014-K [DOI] [Google Scholar]
- 58. Andersen PK, Gill RD. Cox's regression model for counting processes: a large sample study. Ann Stat. 1982;10(4):1100–1120. 10.1214/aos/1176345976 [DOI] [Google Scholar]
- 59. Vallöf D, Kalafateli AL, Jerlhag E. Long‐term treatment with a glucagon‐like peptide‐1 receptor agonist reduces ethanol intake in male and female rats. Transl Psychiatry. 2020;10(1):238. 10.1038/s41398-020-00923-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bouhlal S, Ellefsen KN, Sheskier MB, Singley E, Pirard S, Gorelick DA, et al. Acute effects of intravenous cocaine administration on serum concentrations of ghrelin, amylin, glucagon‐like peptide‐1, insulin, leptin and peptide YY and relationships with cardiorespiratory and subjective responses. Drug Alcohol Depend. 2017;180:68–75. 10.1016/j.drugalcdep.2017.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Suchankova P, Yan J, Schwandt ML, Caparelli EC, Momenan R, Jerlhag E, et al. The glucagon‐like peptide‐1 receptor as a potential treatment target in alcohol use disorder: evidence from human genetic association studies and a mouse model of alcohol dependence. Transl Psychiatry. 2015;5(6):e583. 10.1038/tp.2015.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Morgan JR, Assoumou SA. The limits of innovation: directly addressing known challenges is necessary to improve the real‐world experience of novel medications for opioid use disorder. Acad Emerg Med. 2023;30(12):1285–1287. 10.1111/acem.14814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nalini M, Khoshnia M, Kamangar F, Sharafkhah M, Poustchi H, Pourshams A, et al. Joint effect of diabetes and opiate use on all‐cause and cause‐specific mortality: the Golestan cohort study. Int J Epidemiol. 2021;50(1):314–324. 10.1093/ije/dyaa126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Peeraphatdit T, Ahn JC, Choi DH, Allen AM, Simonetto DA, Kamath PS, et al. A cohort study examining the interaction of alcohol consumption and obesity in hepatic steatosis and mortality. Mayo Clin Proc. 2020;95(12):2612–2620. 10.1016/j.mayocp.2020.04.046 [DOI] [PubMed] [Google Scholar]
- 65. Walter KN, Wagner JA, Cengiz E, Tamborlane WV, Petry NM. Substance use disorders among patients with type 2 diabetes: a dangerous but understudied combination. Curr Diab Rep. 2017;17(1):2. 10.1007/s11892-017-0832-0 [DOI] [PubMed] [Google Scholar]
- 66. Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003–2017. 10.1001/jama.295.17.2003 [DOI] [PubMed] [Google Scholar]
- 67. Smyth BP, Barry J, Keenan E, Ducray K. Lapse and relapse following inpatient treatment of opiate dependence. Ir Med J. 2010;103(6):176–179. [PubMed] [Google Scholar]
- 68. Sauer CM, Chen LC, Hyland SL, Girbes A, Elbers P, Celi LA. Leveraging electronic health records for data science: common pitfalls and how to avoid them. Lancet Digit Health. 2022;4(12):e893–e898. 10.1016/s2589-7500(22)00154-6 [DOI] [PubMed] [Google Scholar]
- 69. Centers for Medicare & Medicaid Services . CMS Chronic Condition Warehouse Opioid Use Disorder Algorithm. 2023. https://www2.ccwdata.org/web/guest/condition-categories-other [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1:List of codes used to define opioid use disorder (OUD)
Table S2: List of codes used to define alcohol use disorder (AUD)
Table S3: List of codes used to define opioid overdose (OD)
Table S4: List of codes used to define alcohol intoxication (AI)
Table S5: List of codes used to define glucose‐dependent insulinotropic polypeptide (GIP) and/or glucagon‐like peptide‐1 receptor agonists (GIP/GLP‐1 RA) prescriptions
Table S6: List of codes used to define Charlson Comorbidity Index (CCI)
Table S7: Mental health codes by mental health disorder classification
Table S8: List of codes used to define additional clinical variables
Table S9: List of codes used to define substance use disorder by substance class [1]
Table S10: List of codes used to define medications for opioid use disorder (MOUD) treatment
Table S11: List of codes used to define benzodiazepines
Table S12a: List of codes used to define inpatient opioid prescriptions [1]
Table S12b: List of codes used to define opioid prescriptions
Table S12c: List of codes used to define opioid prescriptions [1]
Table S13: List of codes used to define medications for alcohol use disorder (MAUD) treatment
Table S14: List of codes used to define obesity and type 2 diabetes
Table S15: Sensitivity analysis scenarios for rate of incident substance‐related outcomes (opioid overdose and alcohol intoxication) for those prescribed any GIP/GLP‐1 RA compared to those not prescribed (overall) among those with a history of opioid use disorder and those with a history of alcohol use disorder
Table S16: Supplemental analysis scenarios for rate/hazard of incident substance‐related outcomes (opioid overdose and alcohol intoxication) for those prescribed any GIP/GLP‐1 RA compared to those not prescribed (overall) among those with a history of opioid use disorder and those with a history of alcohol use disorder
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to restrictions by Oracle Cerner, the owner of the data. Data could be accessed by signing a data sharing agreement with Oracle Cerner and covering any costs that may be involved (Contact Kendra Stillwell: kendra.stillwell@cernerenviza.com).


