Abstract
Impaired alveolar epithelial regeneration in patients with idiopathic pulmonary fibrosis (PF) or chronic obstructive pulmonary disease is attributed to telomere dysfunction in type II alveolar epithelial cells (A2Cs). Genetic susceptibility, aging, and toxicant exposures, including tobacco smoke (TS), contribute to telomere dysfunction in A2Cs. Here we investigated whether improvement of telomere function plays a role in CSP7 (Cav1 scaffolding domain peptide)–mediated protection of A2Cs against ongoing senescence and apoptosis during bleomycin-induced PF as well as alveolar injury caused by chronic TS exposure. We found a significant telomere shortening in A2Cs isolated from idiopathic PF and chronic obstructive pulmonary disease lungs in line with other studies. These cells showed increased p53 in addition to its posttranslational modification with induction of activated caspase-3 and β-galactosidase, suggesting a p53-mediated loss of A2C renewal. Further, we found increased expression of SIAH-1, a p53-inducible E3 ubiquitin ligase known to downregulate TRF2 (telomere repeats binding factor 2). Consistent with the loss of TRF2 and upregulation of TRF1, TERT (telomerase reverse transcriptase) was downregulated in A2Cs. A2Cs from fibrotic lungs of mice repeatedly instilled with bleomycin or isolated from a chronic TS exposure–induced lung injury model showed reduced telomere length; induction of p53, PAI-1, SIAH1, and TRF1; as well as loss of TRF2 and TERT, which were reversed in wild-type mice after treatment with CSP7. Interestingly, PAI-1−/− mice, or those lacking microRNA-34a expression in A2Cs, resisted telomere dysfunction, whereas uPA−/− mice failed to respond to CSP7 treatment, suggesting p53-microRNA-34a feed-forward induction and that the p53-uPA pathway contributes to telomere dysfunction.
Keywords: IPF, COPD, CSP7, telomere length, A2Cs
Clinical Relevance
Reduced viability due to augmented Cav1-mediated p53-microRNA-34a feedback induction and downstream changes in the uPA (urokinase-type plasminogen activator)–fibrinolytic system in type II alveolar epithelial cells play vital roles in the pathogenesis of chronic lung diseases, such as pulmonary fibrosis and tobacco smoke exposure–induced lung injury. The findings reported in this study further support that type II alveolar epithelial cell viability and telomere function can be improved by targeting Cav1 signaling using CSP7 (Cav1 scaffolding domain peptide) during chronic lung injury.
Idiopathic pulmonary fibrosis (IPF) is one of the most common and lethal forms of interstitial lung disease and is often associated with advanced aging and progressive destruction of the lung parenchyma (1, 2). IPF is a fatal age-related interstitial lung disease with an incidence of 93.7 cases per 100,000 individuals in the United States annually and a median 5-year survival rate of only 20% (1, 2). U.S. Food and Drug Administration–approved drugs such as pirfenidone and nintedanib slow lung function decline in patients with IPF but are not curative (3, 4). Likewise, chronic obstructive pulmonary disease (COPD) is a debilitating lung disease affecting approximately 24 million people and represents the third leading cause of death in the United States (5, 6). Although there are nine approved drug categories for COPD maintenance medication, these therapies only address the symptoms, not the underlying disease or disease progression (7).
The pathogenesis of IPF, a disease of unknown etiology, is characterized by permanent cell cycle arrest, also known as replicative senescence and apoptosis of progenitor type II alveolar epithelial cells (A2Cs). Consequently, there is proliferation and progressive accumulation of activated myofibroblasts and fibrotic lung fibroblasts, resulting in increased extracellular matrix deposition. The etiology of IPF is unknown. Aging, repetitive alveolar epithelial injury, telomere dysfunction, senescence, and apoptosis, particularly in A2Cs, have been implicated in the pathogenesis of progressive PF (8).
In COPD, inflammation mediates alveolar wall destruction with air space enlargement (i.e., emphysema) and destruction of lung parenchyma. Like in the pathogenesis of IPF, aging and long-term exposure to tobacco smoke (TS) increases the risk of developing emphysema. Chronic TS exposure (TSE) alone evokes premature cellular aging. Reportedly, 30% of patients with emphysema develop PF (9), suggesting that patients with TSE-induced lung injury (TSE-LI) and COPD are at risk of developing PF. A2Cs are susceptible to damage from TSE and mediators/cytokines released from inflammatory cells. We and others reported that the pathogenesis of COPD is directly linked to a loss of alveolar structure due to senescence and apoptosis of A2Cs (10–16).
Increased expression of Cav1 (caveolin-1), the tumor suppressor protein p53 and p53 downstream target, and PAI-1 (plasminogen activator inhibitor-1) play crucial roles in the induction of A2C senescence and apoptosis and lung injury in COPD and IPF (9, 13, 16–18). Cav1, p53, and PAI-1 expression increase through feedback induction and are often associated with increased A2C senescence and apoptosis (16, 17). Lung lavage fluids exhibit high uPA (urokinase-type plasminogen activator) activity that contributes to alveolar proteolysis. Most forms of lung injury are characterized by defective alveolar fibrinolysis. This is attributable to local overexpression of p53 and PAI-1 (18–20) by p53 binding through its C-terminal amino acid residues 296–393 with a 70-nt destabilization determinant of the PAI-1 3′ untranslated region (10, 16). Additionally, p53 binds the PAI-1 promoter and increases PAI-1 mRNA transcription (21, 22). We and others have shown that exposure of A2Cs to TS or bleomycin (BLM) induces p53 and that p53-mediated upregulation of PAI-1 promotes A2C senescence and apoptosis, lung injury, and PF. Mice lacking p53 or PAI-1 show resistance to TSE-LI and BLM-induced PF (10, 13–17).
Evidence suggests that telomere dysfunction of A2Cs increases susceptibility to the development of PF and emphysema in animal models and humans. Furthermore, telomere dysfunction due to TSE evokes replicative senescence and death of A2Cs, mediating alveolar damage. As in aging, TSE contributes to telomere shortening, which limits the proliferative recovery of lung epithelium, especially in A2Cs promoting cellular senescence and emphysema (23, 24). In approximately 20% of patients with IPF, causative mutations have been identified in the genes that regulate telomere function and protein folding and secretion (25, 26). PF associated with mutations in the gene that encodes telomerase is progressive and lethal, with a mean survival time of 3 years after diagnosis (27, 28). It is found in approximately 37% of kindred with familial PF and 25% of patients without any family history of the disease (29). Furthermore, telomere shortening due to nongenetic causes, including oxidative damage and TSE-LI, induces lung disease (30, 31).
Telomeres are the protective ends of linear chromosomes consisting of tandem repeats of short DNA sequences that shorten throughout the lifespan. The telomere proteome consists of more than 200 proteins to execute various telomeric functions such as protection, elongation, and DNA synthesis. Telomerase is a specialized enzyme responsible for maintaining telomere length by synthesis of tandem TTAGGG repeats. Telomerase has two essential subunits: a catalytic TERT (telomerase reverse transcriptase) and a TERC (telomerase RNA component). Maintenance of telomere length is paramount for proper cellular function. Telomere length in 25% of sporadic cases of PF is at less than the 10th percentile (29). Shortening telomeres in a subset of IPF patients also correlates with poor survival (28). Interestingly, biopsies of patients with IPF revealed predominantly shortened telomeres in A2Cs associated with fibrotic lesions (32). Telomere shortening promotes cellular senescence via activation of p53 and limits the proliferative recovery in lung epithelium, especially in A2Cs, which leads to age-dependent lung remodeling and PF (25, 31, 32). Further, p53 represses telomerase activity through downregulation of TERT (33). Telomere dysfunction due to TSE and aging evokes replicative senescence of A2Cs, mediating alveolar wall damage and susceptibility to developing emphysema in mice and humans. Additionally, we found that overexpression of p53, PAI-1, and Cav1 by A2Cs promotes TSE-LI and BLM-induced PF. A2Cs from lungs of patients with IPF or COPD and wild-type (WT) mice with TSE-LI or multiple-hit (8X) BLM-induced PF also exhibit telomere dysfunction via altering shelterin components of telomere-repeat binding factor proteins (TRF1, TRF2); SIAH1/2 (E3-ubiquitin ligase); a p53 target gene that degrades TRF2, PNUTS (protein phosphatase 1 nuclear-targeting subunit); and TERT. Exposure of mice with conditional deletion of TERT in A2Cs to BLM led to increased senescence and PF (34). Thus, telomere-targeting therapies may provide new hope for treating COPD and PF.
Leveraging mouse models of 8X-BLM–induced PF and TSE-LI, we investigated whether telomere dysfunction in A2Cs is mediated by the changes in uPA–fibrinolytic system cross-talk through p53-microRNA-34a (miR-34a) feed-forward induction. Here, we determined the effects of p53-mediated induction of PAI-1 and inhibition of uPA on telomere dysfunction, senescence, and apoptosis in A2Cs and the development of BLM-induced PF or TSE-LI. We found that CSP7 (Cav1 scaffolding domain peptide) improves telomere length and viability of A2Cs via restraining p53-mediated induction of PAI-1 and inhibition of uPA expression to mitigate alveolar epithelial injury and PF. We further found that inducible conditional knockout mice lacking miR-34a (miR-34acKO) expression in A2Cs or PAI-1–deficient mice resisted telomere shortening, whereas those lacking uPA expression were highly susceptible.
Methods
Mouse Models and CSP7 Treatment
All experiments involving mice were performed according to approved protocols under institutional animal care and use committee guidelines. Six- to eight-week-old WT, miR-34a floxed (miR-34afl/fl), surfactant protein-C (SP-C) Cre (SP-CCre), PAI-1−/−, and uPA−/− mice of C57BL/6 background were purchased from Jackson Laboratory and used for the experiments. Male and female miR-34acKO mice were generated by cross-breeding homozygous miR-34afl/fl mice with SP-CCre mice.
All mice were exposed to passive TS from 40 research cigarettes over a period of 4 hours per day for 5 days per week for 20 weeks to induce TSE-LI and post–TSE-LI CSP7 treatment as we described earlier (10, 13, 35). To expand the local delivery options to treat damaged lungs, we exposed WT TSE-LI mice by inhalation of micronized (air-jet milled) CSP7 dry powder (i.e., dry powder inhalation [DPI]) as we described elsewhere (36). The mice were killed, and A2Cs isolated from the lungs following the method of Corti and coworkers (37) with minor modifications as we described elsewhere (10, 38) were used for further analyses.
To evaluate the effect of CSP7 against repetitive BLM-induced PF, WT, uPA−/−, and PAI-1−/− mice (n = 7) were exposed to eight doses of BLM (40 μg/20 g or 2 U/kg body weight; RB003; TSZCHEM) in 50 μl saline solution or saline solution only once every 2 weeks for 4 months by intranasal instillation under anesthesia as described earlier (36, 39, 40). Control mice received normal saline solution. Two weeks later, CSP7 (1.5 mg/kg body weight) was injected intraperitoneally (IP) daily for the next two weeks as we described (36), and harvested lung tissues and A2Cs were analyzed at the end of the experiment.
Protein Analysis by Western Blotting
Changes in SP-C, p53, p53S15P, p53Ac, caspase-3, cleaved caspase-3, PAI-1, and β-actin expression levels were assessed by Western blotting of A2C lysates using specific antibodies and enhanced chemiluminescence detection (Thermo) as described previously (35, 36).
Detection of Telomerase Activity
Telomerase activity was detected by a PCR-based telomeric repeat amplification protocol (TRAP) method using the TRAPeze Telomerase Detection Kit (Intergen).
Measurement of Terminal Restriction Fragment (Telomeric) Length
The TeloTTAGGG Telomere Length Assay Kit (Roche Diagnostics) was used to determine the telomeric length. In addition, relative telomere length was determined by quantitative PCR (qPCR) analysis of the genomic DNA as described earlier (41–44). The primer sequences are provided in Table E1 in the data supplement.
mRNA Quantification by Real-Time qPCR
Total RNA was isolated from A2Cs using TRI reagent and reverse transcribed using an impromII Reverse transcription kit (Promega). The levels of the mRNAs were quantified using cDNA and gene-specific primers (Table E1) by real-time PCR as described earlier (15, 36).
Immunohistochemical Analysis of Lung Sections
Lung sections (5.0 μm) were subjected to immunostaining as we described previously (13, 35, 36). The antibodies used are presented in Table E2.
Statistical Analysis
The differences were analyzed by one-way ANOVA followed by Tukey’s post hoc test using GraphPad Prism 4.0 software (GraphPad Software).
Results
Telomere Shortens in A2Cs of Human IPF and COPD Lungs
Telomere shortening was observed in A2Cs from lung tissues of patients with IPF or COPD. A qPCR assay revealed a significant reduction in A2C telomere length in patients with IPF (Figure 1A, i), which was further substantiated by the TeloTTAGGG assay (Figure 1A, ii and iii). A significant downregulation in TERT enzyme activity was also observed by the TRAPeze enzyme assay (Figure 1A, iv). Western blot analysis showed an increased expression of p53, and its activation through acetylation of lysine 379 and phosphorylation of serine 15 residues in A2Cs isolated from IPF lungs (Figure 1A, v). The increased expression of activated (cleaved) caspase-3 and β-galactosidase observed in A2Cs from IPF lungs implies reduced viability. We also found an increase in the expression of SIAH-1, a p53-inducible E3 ubiquitin ligase known to downregulate TRF2 (telomere repeat binding factor 2). Consistent with attrition in telomere length and increased SIAH-1, we observed a marked reduction in TERT (telomerase reverse transcriptase) and TRF2 expression. These changes were associated with the upregulation of TRF1, which is known to suppress the expression of TERT enzyme. Immunohistochemical (IHC) analysis further showed reduced expression of TRF2, whereas p53 and its downstream target PAI-1 were upregulated in the IPF lung sections (Figure 1A, vi and vii).
Figure 1.
Telomere shortening in type II alveolar epithelial cells (A2Cs) from human idiopathic pulmonary fibrosis (IPF) and chronic obstructive pulmonary disease (COPD) lungs. A2Cs were isolated from the lungs of control subjects (NL) and patients with IPF. (i) Bar graph showing relative telomere length of the A2Cs analyzed by quantitative PCR (qPCR) after extracting the genomic DNA. (ii) Gel shows the telomerase activity analyzed by TRAPeze assay. (iii) Bar graph shows the quantification of the relative TRAPeze enzyme activity. (iv) Genomic DNA extracted from A2Cs was subjected to TeloTAGGG assay, and the bar graph represents densitometric quantification of the Southern blot showing the telomere shortening in A2Cs from fibrotic lung. (v) Western blot analysis was conducted for expression of TERT (telomerase enzyme) and the apoptosis pathway–related proteins. (vi) Immunohistochemical (IHC) analysis of the lung sections for p53, PAI-1 and TRF2 antigens. (vii) Quantitative analysis of IHC staining. (B) A2Cs were isolated from the lungs of control donors (NL) and patients with COPD. (i) Bar graph showing relative telomere length of the A2Cs analyzed by qPCR after extracting the genomic DNA. (ii) Gel shows the telomerase activity analyzed by TRAPeze assay. (iii) Bar graph shows the quantification of the relative TRAPeze enzyme activity. (iv) Genomic DNA extracted from A2Cs was subjected to TeloTAGGG assay, and the bar graph represents densitometric quantification of the Southern blot showing the telomere shortening in A2Cs from COPD lung. (v) Western blot analysis for expression of TERT and the apoptosis pathway–related proteins. (vi) IHC analysis for p53, PAI-1 and TRF2, proteins in the COPD lung sections. Scale bars, 100 μm. (vii) Quantitative analysis of IHC. NL = normal lungs.
We analyzed the genomic DNA isolated from A2Cs from patients with COPD and found a similar pattern of telomere shortening compared with the corresponding telomere length in A2Cs from control subjects by qPCR (Figure 1B, i). Telomere length analysis by TeloTAGGG assay qPCR indicated a significant reduction in telomere length (Figure 1B, ii and iii). TERT enzyme activity also showed a significant reduction in A2Cs in patients with COPD (Figure 1B, iv). We observed a marked increase in total, acetylated, and serine-15–phosphorylated p53 protein and activation of caspase-3 and β-galactosidase (Figure 1B, v). The upregulation of SIAH-1 observed in A2Cs from COPD lungs could plausibly be consequent to inhibition of TRF2 and subsequent downregulation of TERT expression. The IHC analysis of lung sections showed downregulation in TRF2, whereas the expression of p53 and PAI-1 increased in COPD lungs (Figure 1B, vi and vii).
CSP7 Treatment Protects A2C Telomere Dysfunction in Mice with TSE-LI and BLM-induced PF
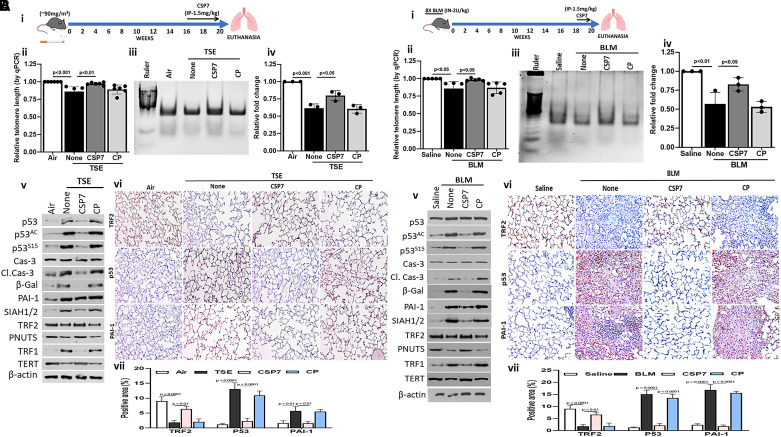
The A2Cs from the WT mice exposed to TS for 20 weeks (schematic representation in Figure 2A, i) exhibited a significant reduction in telomere length when analyzed by qPCR (Figure 2A, ii). Interestingly, treatment of mice exposed to TS with CSP7 resulted in a significant resistance. As shown in Figure 2A, iii and iv, the TERT activity in CSP7-treated mice was also significantly higher than in the control peptide (CP) group. Immunoblotting of lysates of A2Cs isolated from WT mice with TSE-LI showed a marked increase in total, acetylated, and serine-phosphorylated p53 along with induction of cleaved caspase-3 and β-galactosidase (Figure 2A, v). Consistent with loss of TS-induced p53, we found that expression of SIAH-1 was markedly downregulated in A2Cs following treatment with CSP7. Mice exposed to CSP7 also showed restoration of TRF2 and TERT expression, which were otherwise reduced by TSE-LI. IHC analysis of the lung sections also showed restoration of TRF2 expression with reduction in p53 and PAI-1 in the CSP7 group compared with the changes observed in corresponding controls (Figure 2A, vi and vii).
Figure 2.
Cav1 scaffolding domain peptide (CSP7) treatment protects A2C telomere dysfunction in mice with tobacco smoke exposure–induced lung injury (TSE-LI) and bleomycin (BLM)–induced PF. (A) Wild-type (WT) mice (n = 5–7) were kept in ambient air or exposed to cigarette smoke for 20 weeks to induce TSE-LI. (i) Mice with TSE-LI were left untreated (none) or treated daily with 1.5 mg/kg of CSP7 or control peptide (CP) for 4 weeks by intraperitoneal injection starting at week 16 through week 20 after TSE. (ii) A2Cs were isolated, and relative telomere length of the A2Cs was analyzed by qPCR after extracting the genomic DNA. (iii) Gel shows the telomerase enzyme activity analyzed by TRAPeze assay. (iv) Bar graph shows the quantification of the TRAPeze enzyme activity. (v) Western blot analysis for expression of TERT and the apoptosis pathway–related proteins. (vi) IHC analysis of mouse lung sections for p53, PAI-1, and TRF2 proteins. (vii) Quantitative analysis of IHC staining. (B) WT mice (n = 5–7) were exposed to BLM once every 2 weeks for 16 weeks by intranasal instillation as we described earlier (36). (i) Mice with BLM-induced PF were treated with CSP7 or CP for 2 weeks. (ii) A2Cs were isolated, and relative telomere length of the A2Cs was analyzed by qPCR after extracting the genomic DNA. (iii) Gel shows the telomerase enzyme activity analyzed by TRAPeze assay. (iv) Bar graph shows the quantification of the TRAPeze enzyme activity. (v) Western blot analysis was conducted to analyze the expression of TERT and the apoptosis pathway related proteins. (vi) IHC of lung sections for p53, PAI-1, and TRF2 proteins. Scale bars, 100 μm. (vii) Quantitative analysis of IHC staining. TSE = tobacco smoke exposure.
A2Cs isolated from the human IPF lungs show telomere dysfunction. Because 8X-BLM induces irreversible PF (40), we leveraged this experimental PF model and treated mice with established PF with CSP7 or CP (Figure 2B, i) as described previously (36). qPCR analysis of isolated A2Cs showed a reduction in telomere length, which was improved in mice treated with CSP7 (Figure 2B, ii). TERT enzyme activity was suppressed in A2Cs (Figure 2B, iii and iv), which was significantly reversed after treatment with CSP7. This was consistent with suppression of p53 and p53-downstream targets PAI-1 and SIAH1/2 and activation of caspase-3 and β-galactosidase (Figure 2B, v) as indicators of inhibition of apoptosis and senescence. Lysates of A2Cs isolated from mice with 8X-BLM–induced PF showed a marked increase in the shelterin component telomere binding protein TRF1 while suppressing the expression of TRF2, PNUTS, and TERT. These changes were reversed following treatment with CSP7 for 2 weeks, whereas those exposed to CP still carried A2Cs with shortened telomeres. The changes in p53, PAI-1, and TRF2 protein expression were independently confirmed by IHC staining of the lung sections (Figure 2B, vi and vii).
miR-34acKO Mice Lacking Its Expression in A2Cs Resist TSE-induced Telomere Shortening
Because p53 induces miR-34a transcription whereas miR-34a augments p53 acetylation and total p53 expression through a p53-miR-34a feedback induction in A2Cs, we exposed miR-34afl/fl and miR-34acKO mice lacking its expression in A2Cs to 20 weeks of TS as shown in Figure 3A. Consistent with induction of p53, TS exposure caused a significant increase in miR-34a expression in A2Cs of miR-34afl/fl mice, which was resisted by miR-34acKO mice (Figure 3B). qPCR analysis for telomere length did not show a significant reduction in miR-34acKO mice exposed to 20 weeks of TS, unlike control miR-34afl/fl mice (Figure 3C). CSP7 treatment showed significant protection against TSE-induced telomere shortening in WT or miR-34afl/fl mice, whereas it had minimal effect in miR-34acKO mice. This was independently confirmed by assaying telomerase enzyme activity, which was significantly downregulated in A2Cs of miR-34afl/fl mice subjected to TSE. This was reversed following treatment with CSP7, whereas CP had little effect on TSE-induced inhibition of telomerase activity. Interestingly, miR-34acKO mice exposed to TS resisted telomere dysfunction, and CSP7 treatment had limited effects on baseline telomerase activity in these mice (Figures 3D and 3E).
Figure 3.
microRNA (miR)–34acKO mice lacking miR-34a expression in A2Cs resist TSE-induced telomere shortening. (A) Tamoxifen-induced SP-CCre-miR-34acKO mice and SP-CCre-miR-34afl/fl control mice were exposed to cigarette smoke for 20 weeks and treated daily with 1.5 mg/kg of CSP7 or CP for 4 weeks by intraperitoneal injection starting at week 16 through week 20 after TSE. (B) A2Cs isolated from miR-34afl/fl and miR-34acKO mice exposed to TS and treated with or without CSP7 or CP as in A were analyzed for miR-34a expression by PCR. (C) Relative telomere length of the A2Cs was analyzed by qPCR after extracting the genomic DNA. (D) Gel shows the telomerase activity analyzed by TRAPeze assay. (E) Bar graph shows the quantification of the TRAPeze enzyme activity.
CSP7 Failed to Attenuate A2C Telomere Dysfunction in uPA-deficient Mice with TSE-LI and BLM-induced PF
Because uPA expression is suppressed during lung injury, consistent with induction of p53 in A2Cs (17, 22), we exposed uPA−/− mice to 20 weeks of TS and later subjected them to CSP7 treatment as shown in Figure 4A, i. The qPCR analysis revealed that TSE caused significant attrition of telomeres in A2Cs of uPA-deficient mice compared with control mice kept in ambient air (Figure 4A, ii). However, unlike WT mice, A2Cs from TSE-LI mice lacking uPA expression failed to respond to CSP7 treatment and exhibited telomere shortening like those with TSE-LI and left untreated or treated with CP. This was independently confirmed by testing the telomerase enzyme activity in isolated A2Cs (Figure 4A, iii and iv). Consistent with the inability of CSP7 to reverse telomere shortening or improve telomerase activity, CSP7 treatment also failed to inhibit posttranslational modifications or induction of p53, active caspase-3, or β-galactosidase observed in A2Cs of mice with TSE-LI when analyzed by Western blotting for protein expression (Figure 4A, v). There was no significant change in TRF1, TRF2, or TERT expression in A2Cs isolated from uPA-deficient mice exposed to TS and left untreated or treated with CSP7 or CP.
Figure 4.
CSP7 failed to attenuate A2C telomere dysfunction in uPA-deficient mice with TSE-LI and BLM-induced PF. (A) uPA−/− mice (n = 5–7) underwent TSE for 20 weeks and (i) were later treated with CSP7 or CP as described in Figure 2A, i. (ii) A2Cs were isolated, and relative telomere length of A2Cs was analyzed by qPCR after extracting the genomic DNA. (iii) Gel shows the telomerase activity analyzed by TRAPeze enzyme assay. (iv) Bar graph shows the quantification of the TRAPeze enzyme activity. (v) Western blot analysis for the expression of TERT and the apoptosis pathway–related proteins. (B) uPA−/− mice (n = 5–7) were exposed to BLM once every 2 weeks for 16 weeks and (i) treated with or without CSP7 or CP as described in Figure 2B, i. (ii) A2Cs were isolated, and relative telomere length in A2Cs was analyzed by qPCR after extracting the genomic DNA. (iii) Gel shows the telomerase activity analyzed by TRAPeze enzyme assay. (iv) Bar graph shows the quantification of TRAPeze enzyme activity. (v) Western blot analysis was conducted to analyze the expression of TERT and the apoptosis pathway–related proteins. BLM = bleomycin.
Similarly, CSP7 treatment of uPA-deficient mice with 8X-BLM–induced PF (Figure 4B, i) failed to restore telomere length based on qPCR analysis of genomic DNA (Figure 4B, ii) or telomerase enzyme activity by the TRAPeze method (Figure 4B, iii and iv) in isolated A2Cs. Western blotting of A2C lysates from these mice confirmed that CSP7 treatment had a minimal effect on changes in the expression of p53, ACp53, S15p53, apoptosis, or senescence; the p53 target genes PAI-1 or SIAH1/2; sheltering component binding proteins such as TRF1 and TRF2; or PNUTS or TERT induced by TSE-LI in uPA−/− mice (Figure 4B, v).
PAI-1−/− Mice Resist TSE- and BLM-induced Telomere Dysfunction in A2Cs
p53 induces PAI-1 expression and functions as a downstream mediator of p53-induced senescence and apoptosis (13, 16, 17). Further, CSP7 treatment failed to inhibit p53 or PAI-1 expression or prevent telomere dysfunction in A2Cs isolated from uPA−/− mice with TSE-LI or BLM-induced PF (Figure 4). We therefore subjected PAI-1–deficient mice to TSE to induce TSE-LI (Figure 5A, i). As shown in Figure 5A, ii, qPCR analysis of genomic DNA from isolated A2Cs revealed that TS exposure for 20 weeks failed to cause a significant telomere length reduction in PAI-1–deficient mice. Similarly, CSP7 or CP treatment of PAI-1–deficient mice exposed to 20 weeks of TS had minimal effect on telomere length. Telomerase enzyme activity analyzed by TRAPeze assay also showed no significant change in activity between PAI-1−/− mice kept in ambient air or subjected to TSE or TSE and CSP7 or CP treatment (Figure 5A, iii and iv). Western blotting of isolated A2C lysates confirmed minimal increase in total or posttranslationally modified p53, cleaved caspase-3, or β-galactosidase, suggesting resistance of these mice to apoptosis, and possibly senescence. We did not observe changes in the expression of SIAH1/2 and PNUTS or proteins that are directly associated with the telomere such as TRF1, TRF2, and TERT (Figure 5A, v).
Figure 5.
PAI-1−/− mice resist TSE- and BLM-induced telomere dysfunction in A2Cs. (A) PAI-1−/− mice were exposed to (i) TS for 20 weeks with or without CSP7 or CP as described in Figure 2A. (ii) A2Cs were isolated, and relative telomere length of the A2Cs was analyzed by qPCR after extracting the genomic DNA. (iii) Gel shows the telomerase activity analyzed by TRAPeze enzyme assay. (iv) Bar graph shows the quantification of the TRAPeze enzyme activity. (v) Western blot analysis for TERT and the apoptosis pathway–related proteins. (B) PAI-1−/− mice were exposed to (i) BLM once every 2 weeks for 16 weeks as intranasal instillation as in Figure 2B. These mice were later treated with CSP7 or CP for 2 weeks. (ii) A2Cs were isolated, and the relative telomere length of the A2Cs was analyzed by qPCR after extracting the genomic DNA. (iii) Gel shows the telomerase activity analyzed by TRAPeze enzyme assay. (iv) Bar graph shows the quantification of the TRAPeze enzyme activity. (v) Western blot analysis for TERT and the apoptosis pathway–related proteins.
Consistent with telomere dysfunction, we found a marked increase in p53 and PAI-1 expression in A2Cs isolated from WT mice with 8X-BLM–induced PF (Figure 2B, v), suggesting p53-mediated induction of PAI-1 driving telomere shortening. We therefore subjected PAI-1–deficient mice to 8X-BLM to induce PF, which was treated with CSP7 or CP by IP injection as shown in Figure 5B, i. qPCR analysis of genomic DNA (Figure 5B, ii) or TRAPeze assay for telomerase enzymatic activity (Figure 5B, iii and iv) of isolated A2Cs revealed minimal effect in PAI-1–deficient mice exposed to 8X-BLM. Western blotting of A2C lysates showed little or no difference in the expression of p53, Acp53, S15p53, cleaved caspase-3, β-galactosidase, SIAH1/2, PNUTS, TRF1, TRF2, and TERT between PAI-1–deficient mice exposed to saline solution versus 8X-BLM with or without CSP7 or CP treatment (Figure 5B, v), suggesting resistance of these mice to 8X-BLM–induced lung injury.
Inhalation of CSP7 Dry Powder Protects A2Cs Telomere Dysfunction in Mice with TSE-LI
Because IPF and COPD are chronic lung diseases, as we described elsewhere, delivery to the lungs, mainly via DPI, has several advantages (36). Consistent with systemic administration by IP injection (Figure 2), qPCR for telomere length revealed that airway delivery by inhalation of micronized CSP7 dry powder to mice with TSE-LI (Figure 6A) caused a significant increase in telomere length in A2Cs (Figure 6B). The protective effect of CSP7 delivered via airways by DPI was further confirmed by TeloTAGGG assay for the telomere length of the isolated genomic DNA (Figures 6C and 6D) and TRAPeze assay for telomerase enzymatic activity (Figures 6E and 6F). Immunoblotting of isolated A2C lysates confirmed inhibition of p53, Ac-p53, cleaved caspase-3, and β-galactosidase, suggesting that CSP7 treatment inhibits TSE-induced apoptosis and possibly senescence in WT mice. Consistent with protection against TSE-induced telomere shortening, CSP7 treatment of mice with TSE-LI restored TRF2 and inhibited TRF1 in A2Cs. These were altered in mice with TSE-LI left untreated compared with control mice exposed to ambient air (Figure 6G). IHC analysis of lung sections from these mice further confirmed suppression of TSE-induced TRF1 by CSP7 administered via the airways by DPI (Figures 6H and 6I).
Figure 6.
Inhalation of CSP7 dry powder protects A2C telomere dysfunction in mice with TSE-LI. (A) WT mice (n = 5–7) were exposed to TS for 20 weeks and treated with micronized CSP7 via airway by dry powder inhalation 5 days per week for 4 weeks starting at week 16 as described earlier (35, 36). (B) A2Cs were isolated, and relative telomere length was analyzed by qPCR after extracting the genomic DNA. (C) A TeloTAGGG assay was performed to estimate the telomere length using isolated genomic DNA. (D) Bar graph shows the relative quantification of the telomere shortening that occurred in the A2Cs. (E) Gel shows the telomerase activity analyzed by TRAPeze enzyme assay. (F) Bar graph shows the quantification of the TRAPeze enzyme activity. (G) Western blot analysis for expression of telomerase, shelterin complex binding, and the apoptosis pathway–related proteins. (H) IHC analysis of lung sections for TRF1 protein. Scale bars, 100 μm. (I) Quantitative analysis of IHC staining.
Collectively, our novel findings indicate an intricate link between p53-mediated changes in major components of the uPA-fibrinolytic system and telomere dysfunction in A2Cs due to induction of Cav1 often associated with the pathogenesis of chronic lung diseases such as IPF or COPD (Figure 7). This can be mitigated by CSP7, which competes with increased Cav1 for signaling intermediaries that promote Cav1-mediated induction of p53 and cascades of downstream events in injured A2Cs.
Figure 7.
Telomere shortening through Cav1-mediated induction of p53 and downstream uPA-fibrinolytic system cross-talk and its reversal by CSP7 in A2Cs. IPF, COPD, BLM-induced PF, and TSE-LI induce Cav1 expression in A2Cs, which in turn augments p53 and downstream miR-34a and PAI-1 through a feedback mechanism leading to the induction of SIAH. Expression of SIAH leads to the ubiquitination and degradation of TRF2, which in turn downregulates the expression of TERT. TRF1 upregulation also results in TERT downregulation. CSP7 reverses the events leading to telomere dysfunction by competing with Cav1, which is otherwise induced in injured A2Cs during chronic lung injury. PNUTS = protein phosphatase 1 nuclear-targeting subunit.
Discussion
Progressive and persistent alveolar epithelial injury from chronic exposure to TS and other particulates induces senescence and apoptosis in A2Cs leading to reduced A2C renewal and the development of PF or emphysema (10, 11, 13). Notably, the pathogenesis of emphysema and ILDs, including human IPF, has been directly linked to a loss of A2C renewal capacity due to reduced viability. This limits alveolar epithelial regeneration as a result of stem cell exhaustion (10–12). Although PF and emphysema exemplify distinct clinical/radiological and pathophysiological patterns, these diseases are associated with similar fates of vulnerable cell populations, i.e., A2C senescence, apoptosis, and telomere dysfunction.
Telomeres consist of tandem TTAGGG repeats and associated shelterin components, the protein complex that forms a capping structure at the ends of chromosomes, preventing them from degrading and activating DNA repair responses (42–44). Telomere attrition occurs with each cell division until a point cell with a critically shortened telomere undergoes senescence due to uncapping of telomeres. This induces a DNA damage response and activation of cell cycle arrest programming (43). Telomere shortening is considered a primary molecular cause of aging and premature aging. Short telomeres block the proliferative capacity of stem cells, affecting their potential to regenerate tissues and triggering age-associated diseases. The literature further suggests that telomere dysfunction leading to PF is specific to A2Cs and not simply a consequence of any resident lung cells in the absence of lung injury (32). Patients with both PF and emphysema have also been noted to have abnormally short telomeres in A2Cs (45–47). A subset of patients with age-associated pathology such as IPF manifest mutations in essential telomerase genes such as TERT or TERC (43, 44). Similarly, mutations in TERT and TERC are associated with the pathogenesis of emphysema (23). The mutation in TERT can be familial and nonfamilial (46). This suggests that factors contributing to mutations can, in turn, affect telomere failure and associated pathologies. The mechanisms by which telomere defects provoke diverse lung diseases such as emphysema and IPF are not understood, but several observations have pointed to lung-intrinsic factors and epithelial cell dysfunction as candidate events (42). For example, in telomerase-null mice, DNA damage preferentially accumulates in the air-exposed epithelium after environmentally induced injury, such as with TSE-LI. The additive effect of environmental injury and telomere dysfunction has been suggested to contribute to the susceptibility to emphysema seen in these mice (42, 45). In subjects carrying a TERT mutation, A2C telomere length was significantly shorter than in control subjects, leading to reduced survival. However, no such differences were observed in telomere lengths of surrounding cells (27), suggesting that telomere shortening critically affects A2Cs in fibrotic areas. Further, in sporadic IPF, A2C telomere length was significantly longer in nonfibrotic areas than in fibrotic regions (32), implicating telomere dysfunction as a potential cause of fibrogenesis. The literature suggests that telomere-related pathology plays a role in familial PF caused by mutations in surfactant-related genes or genes that influence telomere maintenance and in sporadic IPF (45). Familial patients with a TERT mutation also show shorter telomeres than in sporadic IPF, which are associated with worse survival (27).
Lung lavage fluids from patients with IPF and COPD exhibit high levels of uPA activity and alveolar fibrinolysis (17–22). We found that treatment of A2Cs with BLM or TS increases p53 and PAI-1 expression and reduces cellular viability. These effects are reversed by inhibiting p53 binding to endogenous PAI-1 mRNA or inhibiting p53 accumulation by treatment with CSP or CSP7 (10, 13, 14). Lung tissues from patients with IPF or COPD also show increased levels of p53 and PAI-1 in A2Cs (13–15). We recently reported increased expression of p53, Cav1, and PAI-1 in A2Cs from the lungs of patients with IPF or COPD and WT mice with BLM-induced PF or TSE-LI (10, 14). We found that p53 induces PAI-1 expression in A2Cs (10, 15–17). Mice lacking p53 or PAI-1 resist TSE-LI or BLM-induced lung injury (15, 17) as well as the development of PF resulting from BLM lung injury. Consistent with increased expression of p53 and PAI-1, we found telomere dysfunction in A2Cs from the lungs of patients with IPF or COPD and WT mice with TSE-LI or 8X-BLM–induced PF. Our findings further support the targeting of Cav1-induced p53- and PAI-1–mediated pathways in A2Cs using CSP or its 7-mer deletion fragment CSP7, which improves viability and inhibits lung injury and PF by potentially blocking senescence and apoptosis caused by repeated exposure to TS or BLM. Based on our recent publications, CSP and CSP7 appear well tolerated and inhibit lung injury in mice (10, 13–15, 35, 36).
We also found that WT mice exposed to 8X-BLM or 20 weeks of passive TS showed a significant reduction in relative telomere length, suggesting telomere dysfunction. The literature further suggests that telomere length correlates with pulmonary function in patients with IPF or COPD (43). These changes were associated with a reduction in shelterin complex proteins such as TRF2, whereas the expression of TRF1 was increased. Two telomere-specific DNA binding proteins, TRF1 and TRF2, are required for human telomere function (30). TRF1 overexpression leads to progressive telomere shortening, whereas its inhibition enhances telomere length (30), suggesting the role of TRF1 in regulation of telomere length. TRF2 protects the chromosome ends (30), which are degraded by SIAH1, a p53 transcriptional target. Induction of p53 and SIAH1 is often associated with loss of TERT and PNUTS expression, suggesting that increased p53 is contributing to telomere dysfunction. Treatment of these mice with CSP7 by IP injection or via the airways by inhalation of micronized CSP7 dry powder prevented telomere attrition in A2Cs, with a reversal of shelterin complex proteins. This suggests that CSP7 protects against telomere dysfunction by restoring expression of the shelterin complex proteins to protect telomere ends. These changes are intricately linked to the induction of p53 and PAI-1 in A2Cs.
Consistent with the resistance of PAI-1–deficient mice to the development of BLM-induced PF or TSE-LI, we found that TS or BLM lung injury failed to reduce relative telomere length in these mice. Similarly, we did not find a significant change in the basal expression of shelterin complex protein in PAI-1–deficient mice exposed to TS or BLM. CSP7 has minimal effect on expression of p53 in the A2Cs of these mice. Consistent with our earlier findings and those of others (16, 17, 48), uPA-deficient mice are highly susceptible to BLM-induced lung injury and PF. A2Cs isolated from uPA-deficient mice with TSE-LI or BLM-induced PF also exhibited telomere dysfunction. CSP7 treatment failed to suppress TS- or BLM-induced p53 or PAI-1 expression or telomere dysfunction in uPA-deficient mice, suggesting an intricate link between TS- or BLM-induced p53 and downstream PAI-1 expression and telomere dysfunction in A2Cs. This possibility is further supported by the lack of BLM- or TSE-induced lung injury and resistance of tamoxifen-inducible conditional miR-34acKO mice lacking miR-34a in A2Cs to telomere dysfunction. These mice also showed minimal alteration in the baseline levels of shelterin complex proteins p53 and PAI-1, senescence, and apoptosis (49). Our findings suggest that increased p53 expression through miR-34a-p53 feedback induction of PAI-1 contributes to telomere dysfunction and senescence in A2Cs during lung injury. Further, our findings collectively suggest that TS or BLM lung injury induces p53 and PAI-1, which in turn promote telomere dysfunction in A2Cs. Oxidative stress and chronic inflammation are the most likely factors contributing to the loss of telomere DNA (50). A deficiency of p53 or PAI-1 makes mice highly resistant to experimentally induced alveolar epithelial injury and air space enlargement (10, 13). Our study suggests that changes in A2C p53 and PAI-1 expression, subsequent telomere dysfunction, and viability are important and clinically relevant and occur in patients with parenchymal lung diseases such as emphysema and PF, including IPF.
In summary, reduced viability due to augmented Cav1-mediated p53-miR-34a feedback induction and downstream changes in the uPA-fibrinolytic system in A2Cs play vital roles in the pathogenesis of chronic lung diseases such as PF and TSE-LI. The findings reported in this study further support that A2C viability and telomere function can be improved by targeting Cav1 signaling using CSP7 during chronic lung injury.
Supplemental Materials
Footnotes
Supported by DOD Peer Reviewed Medical Research Program Award W81X WH-20-1-0142 (S.S.); National Heart, Lung, and Blood Institute grants HL151397 and HL133067 (S.S.); and National Institute of Environmental Health Sciences grant ES025815 (S.S.).
Author Contributions: S.S. conceived and designed experiments. G.J.C., S.B., and N.M. provided human tissue samples. B.P., A.K.B., D.K., R.S., and D.N.D. performed the experiments. H.T., N.V.K., G.A.J., and D.L.S. analyzed the data. S.S. wrote the manuscript. H.T. and N.V.K. edited the manuscript.
This article has a data supplement, which is accessible at the Supplements tab.
Originally Published in Press as DOI: 10.1165/rcmb.2023-0453OC on September 13, 2024
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Hutchinson JP, McKeever TM, Fogarty AW, Navaratnam V, Hubbard RB. Increasing global mortality from idiopathic pulmonary fibrosis in the twenty-first century. Ann Am Thorac Soc . 2014;11:1176–1185. doi: 10.1513/AnnalsATS.201404-145OC. [DOI] [PubMed] [Google Scholar]
- 2. Ley B, Collard HR, King TE. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med . 2011;183:431–440. doi: 10.1164/rccm.201006-0894CI. [DOI] [PubMed] [Google Scholar]
- 3. Saito S, Alkhatib A, Kolls JK, Kondoh Y, Lasky JA. Pharmacotherapy and adjunctive treatment for idiopathic pulmonary fibrosis (IPF) J Thorac Dis . 2019;11(suppl):S1740–S1754. doi: 10.21037/jtd.2019.04.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hughes G, Toellner H, Morris H, Leonard C, Chaudhuri N. Real world experiences: pirfenidone and nintedanib are effective and well tolerated treatments for idiopathic pulmonary fibrosis. J Clin Med . 2016;5:78. doi: 10.3390/jcm5090078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hurd S. The impact of COPD on lung health worldwide: epidemiology and incidence. Chest . 2000;117(suppl):1S–4S. doi: 10.1378/chest.117.2_suppl.1s. [DOI] [PubMed] [Google Scholar]
- 6. Ford ES, Croft JB, Mannino DM, Wheaton AG, Zhang X, Giles WH. COPD surveillance–United States, 1999–2011. Chest . 2013;144:284–305. doi: 10.1378/chest.13-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Van Haarst A, McGarvey L, Paglialunga S. Review of drug development guidance to treat chronic obstructive pulmonary disease: US and EU perspectives. Clin Pharmacol Ther . 2019;106:1222–1235. doi: 10.1002/cpt.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Katzen J, Beers MF. Contributions of alveolar epithelial cell quality control to pulmonary fibrosis. J Clin Invest . 2020;130:5088–5099. doi: 10.1172/JCI139519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ford ES, Murphy LB, Khavjou O, Giles WH, Holt JB, Croft JB. Total and state-specific medical and absenteeism costs of COPD among adults aged 18 years in the United States for 2010 and projections through 2020. Chest . 2015;147:31–45. doi: 10.1378/chest.14-0972. [DOI] [PubMed] [Google Scholar]
- 10. Shetty SK, Bhandary YP, Marudamuthu AS, Abernathy D, Velusamy T, Starcher B, et al. Regulation of airway and alveolar epithelial cell apoptosis by p53-Induced plasminogen activator inhibitor-1 during cigarette smoke exposure injury. Am J Respir Cell Mol Biol . 2012;47:474–483. doi: 10.1165/rcmb.2011-0390OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Park J-W, Ryter SW, Choi AMK. Functional significance of apoptosis in chronic obstructive pulmonary disease. COPD . 2007;4:347–353. doi: 10.1080/15412550701603775. [DOI] [PubMed] [Google Scholar]
- 12. Tsuji T, Aoshiba K, Nagai A. Cigarette smoke induces senescence in alveolar epithelial cells. Am J Respir Cell Mol Biol . 2004;31:643–649. doi: 10.1165/rcmb.2003-0290OC. [DOI] [PubMed] [Google Scholar]
- 13. Bhandary YP, Shetty SK, Marudamuthu AS, Midde KK, Ji HL, Shams H, et al. Plasminogen activator inhibitor-1 in cigarette smoke exposure and influenza A virus infection-induced lung injury. PLoS One . 2015;10:e0123187. doi: 10.1371/journal.pone.0123187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tiwari N, Marudamuthu AS, Tsukasaki Y, Ikebe M, Fu J, Shetty S. p53- and PAI-1-mediated induction of C-X-C chemokines and CXCR2: importance in pulmonary inflammation due to cigarette smoke exposure. Am J Physiol Lung Cell Mol Physiol . 2016;310:L496–L506. doi: 10.1152/ajplung.00290.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shetty SK, Tiwari N, Marudamuthu AS, Puthusseri B, Bhandary YP, Fu J, et al. p53 and miR-34a feedback promotes lung epithelial injury and pulmonary fibrosis. Am J Pathol . 2017;187:1016–1034. doi: 10.1016/j.ajpath.2016.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shetty S, Shetty P, Idell S, Velusamy T, Bhandary YP, Shetty RS. Regulation of plasminogen activator inhibitor-1 expression by tumor suppressor protein p53. J Biol Chem . 2008;283:19570–19580. doi: 10.1074/jbc.M710268200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bhandary YP, Shetty SK, Marudamuthu AS, Ji HL, Neuenschwander PF, Boggaram V, et al. Regulation of lung injury and fibrosis by p53-mediated changes in urokinase and plasminogen activator inhibitor-1. Am J Pathol . 2013;183:131–143. doi: 10.1016/j.ajpath.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jiang C, Liu G, Cai L, Deshane J, Antony V, Thannickal VJ, et al. Divergent regulation of alveolar type 2 cell and fibroblast apoptosis by plasminogen activator inhibitor 1 in lung fibrosis. Am J Pathol . 2021;191:1227–1239. doi: 10.1016/j.ajpath.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xu X, Wang H, Wang Z, Xiao W. Plasminogen activator inhibitor-1 promotes inflammatory process induced by cigarette smoke extraction or lipopolysaccharides in alveolar epithelial cells. Exp Lung Res . 2009;35:795–805. doi: 10.3109/01902140902912519. [DOI] [PubMed] [Google Scholar]
- 20. Hu X, Ma Y, Wang C, Yang Y. Effects of simvastatin on cigarette smoke extract induced tissue-type plasminogen activator and plasminogen activator inhibitor-1 expression in human umbilical vein endothelial cells. Chin Med J (Engl) . 2009;122:2380–2385. [PubMed] [Google Scholar]
- 21. Kunz C, Pebler S, Otte J, von der Ahe D. Differential regulation of plasminogen activator and inhibitor gene transcription by the tumor suppressor p53. Nucleic Acids Res . 1995;23:3710–3717. doi: 10.1093/nar/23.18.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shetty P, Velusamy T, Bhandary YP, Shetty RS, Liu MC, Shetty S. Urokinase expression by tumor suppressor protein p53: a novel role in mRNA turnover. Am J Respir Cell Mol Biol . 2008;39:364–372. doi: 10.1165/rcmb.2007-0406OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stanley SE, Chen JJL, Podlevsky JD, Alder JK, Hansel NN, Mathias RA, et al. Telomerase mutations in smokers with severe emphysema. J Clin Invest . 2015;125:563–570. doi: 10.1172/JCI78554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stanley SE, Merck SJ, Armanios M. Telomerase and the genetics of emphysema susceptibility. Implications for pathogenesis paradigms and patient care. Ann Am Thorac Soc . 2016;13(suppl 5):S447–S451. doi: 10.1513/AnnalsATS.201609-718AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dai J, Cai H, Zhuang Y, Wu Y, Min H, Li J, et al. Telomerase gene mutations and telomere length shortening in patients with idiopathic pulmonary fibrosis in a Chinese population. Respirology . 2015;20:122–128. doi: 10.1111/resp.12422. [DOI] [PubMed] [Google Scholar]
- 26. Lawson WE, Loyd JE. The genetic approach in pulmonary fibrosis. Proc Am Thorac Soc . 2006;3:345–349. doi: 10.1513/pats.200512-137TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leon ADd, Cronkhite JT, Katzenstein ALA, Godwin JD, Raghu G, Glazer CS, et al. Telomere lengths, pulmonary fibrosis and telomerase (TERT) mutations. PLoS One . 2010;5:e10680. doi: 10.1371/journal.pone.0010680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stuart BD, Lee JS, Kozlitina J, Noth I, Devine MS, Glazer CS, et al. Effect of telomere length on survival in patients with idiopathic pulmonary fibrosis: an observational cohort study with independent validation. Lancet Respir Med . 2014;2:557–565. doi: 10.1016/S2213-2600(14)70124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cronkhite JT, Xing C, Raghu G, Chin KM, Torres F, Rosenblatt RL, et al. Telomere shortening in familial and sporadic pulmonary fibrosis. Am J Respir Crit Care Med . 2008;178:729–737. doi: 10.1164/rccm.200804-550OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Srinivas N, Rachakonda S, Kumar R. Telomeres and telomere length: a general overview. Cancers (Basel) . 2020;12:558. doi: 10.3390/cancers12030558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Arimura-Omori M, Kiyohara C, Yanagihara T, Yamamoto Y, Ogata-Suetsugu S, Harada E, et al. Association between telomere-related polymorphisms and the risk of IPF and COPD as a precursor lesion of lung cancer: findings from the Fukuoka Tobacco-Related Lung Disease (FOLD) registry. Asian Pac J Cancer Prev . 2020;21:667–673. doi: 10.31557/APJCP.2020.21.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Naikawadi RP, Disayabutr S, Mallavia B, Donne ML, Green G, La JL, et al. Telomere dysfunction in alveolar epithelial cells causes lung remodeling and fibrosis. JCI Insight . 2016;1:e86704. doi: 10.1172/jci.insight.86704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jin X, Beck S, Sohn YW, Kim JK, Kim SH, Yin J, et al. Human telomerase catalytic subunit (hTERT) suppresses p53-mediated anti-apoptotic response via induction of basic fibroblast growth factor. Exp Mol Med . 2010;42:574–582. doi: 10.3858/emm.2010.42.8.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu T, Santos FGDL, Zhao Y, Wu Z, Rinke AE, Kim KK, et al. Telomerase reverse transcriptase ameliorates lung fibrosis by protecting alveolar epithelial cells against senescence. J Biol Chem . 2019;294:8861–8871. doi: 10.1074/jbc.RA118.006615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Das DN, Puthusseri B, Gopu V, Krishnan V, Bhagavath AK, Bolla S, et al. Caveolin-1-derived peptide attenuates cigarette smoke-induced airway and alveolar epithelial injury. Am J Physiol Lung Cell Mol Physiol . 2023;325:L689–L708. doi: 10.1152/ajplung.00178.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marudamuthu AS, Bhandary YP, Fan L, Radhakrishnan V, MacKenzie B, Maier E, et al. Caveolin-1–derived peptide limits development of pulmonary fibrosis. Sci Transl Med . 2019;11:eaat2848. doi: 10.1126/scitranslmed.aat2848. [DOI] [PubMed] [Google Scholar]
- 37. Corti M, Brody AR, Harrison JH. Isolation and primary culture of murine alveolar type II cells. Am J Respir Cell Mol Biol . 1996;14:309–315. doi: 10.1165/ajrcmb.14.4.8600933. [DOI] [PubMed] [Google Scholar]
- 38. Puthusseri B, Marudamuthu A, Tiwari N, Fu J, Idell S, Shetty S. Regulation of p53-mediated changes in the uPA-fibrinolytic system and in lung injury by loss of surfactant protein C expression in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol . 2017;312:L783–L796. doi: 10.1152/ajplung.00291.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tanjore H, Degryse AL, Crossno PF, Xu XC, McConaha ME, Jones BR, et al. β-catenin in the alveolar epithelium protects from lung fibrosis after intratracheal bleomycin. Am J Respir Crit Care Med . 2013;187:630–639. doi: 10.1164/rccm.201205-0972OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Degryse AL, Tanjore H, Xu XC, Polosukhin VV, Jones BR, McMahon FB, et al. Repetitive intratracheal bleomycin models several features of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol . 2010;299:L442–L452. doi: 10.1152/ajplung.00026.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Callicott RJ, Womack JE. Real-time PCR assay for measurement of mouse telomeres. Comp Med . 2006;56:17–22. [PubMed] [Google Scholar]
- 42. Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res . 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Alder JK, Barkauskas CE, Limjunyawong N, Stanley SE, Kembou F, Tuder RM, et al. Telomere dysfunction causes alveolar stem cell failure. Proc Natl Acad Sci U S A . 2015;112:5099–5104. doi: 10.1073/pnas.1504780112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu T, Ullenbruch M, Young Choi Y, Yu H, Ding L, Xaubet A, et al. Telomerase and telomere length in pulmonary fibrosis. Am J Respir Cell Mol Biol . 2013;49:260–268. doi: 10.1165/rcmb.2012-0514OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Armanios MY, Chen JJL, Cogan JD, Alder JK, Ingersoll RG, Markin C, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med . 2007;356:1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 46. Tsakiri KD, Cronkhite JT, Kuan PJ, Xing C, Raghu G, Weissler JC, et al. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci U S A . 2007;104:7552–7557. doi: 10.1073/pnas.0701009104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Garcia CK. Idiopathic pulmonary fibrosis. Proc Am Thorac Soc . 2011;8:158–162. doi: 10.1513/pats.201008-056MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bhandary YP, Shetty SK, Marudamuthu AS, Gyetko MR, Idell S, Gharaee-Kermani M, et al. Regulation of alveolar epithelial cell apoptosis and pulmonary fibrosis by coordinate expression of components of the fibrinolytic system. Am J Physiol Lung Cell Mol Physiol . 2012;302:L463–L473. doi: 10.1152/ajplung.00099.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shetty S.Peptide therapeutics for acute and chronic airway and alveolar diseases. https://patents.google.com/patent/WO2020106922A1
- 50. Masi S, Salpea KD, Li K, Parkar M, Nibali L, Donos N, et al. Oxidative stress, chronic inflammation, and telomere length in patients with periodontitis. Free Radic Biol Med . 2011;50:730–735. doi: 10.1016/j.freeradbiomed.2010.12.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.