Abstract
Objective
To summarise available evidence on time to nursing home admission and death among people with dementia, and to explore prognostic indicators.
Design
Systematic review and meta-analysis.
Data sources
Medline, Embase, Web of Science, Cochrane, and Google Scholar from inception to 4 July 2024.
Eligibility criteria for selecting studies
Longitudinal studies on survival or nursing home admission in people with dementia. Studies with fewer than 150 participants, recruitment during acute hospital admission, or less than one year of follow-up were excluded.
Results
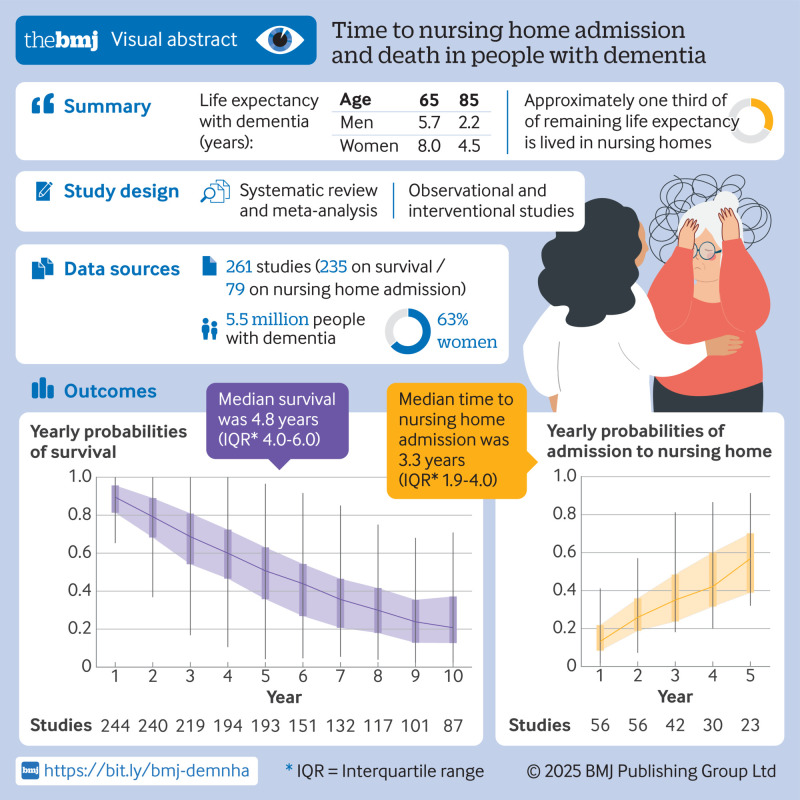
19 307 articles were identified and 261 eligible studies included. 235 reported on survival among 5 553 960 participants and 79 reported on nursing home admission among 352 990 participants. Median survival from diagnosis appeared to be strongly dependent on age, ranging from 8.9 years at mean age 60 for women to 2.2 years at mean age 85 for men. Women overall had shorter survival than men (mean difference 4.1 years (95% confidence interval 2.1 to 6.1)), which was attributable to later age at diagnosis in women. Median survival was 1.2 to 1.4 years longer in Asia than in the US and Europe, and 1.4 years longer for Alzheimer’s disease compared with other types of dementia. Compared with studies before 2000, survival was longer in contemporary clinic based studies (Ptrend=0.02), but not in community based studies. Taken together, variation in reported clinical characteristics and study methodology explained 51% of heterogeneity in survival. Median time to nursing home admission was 3.3 years (interquartile range 1.9 to 4.0). 13% of people were admitted in the first year after diagnosis, increasing to 57% at five years, but few studies appropriately accounted for competing mortality risk when assessing admission rates.
Conclusions
The average life expectancy of people with dementia at time of diagnosis ranged from 5.7 years at age 65 to 2.2 at age 85 in men and from 8.0 to 4.5, respectively, in women. About one third of remaining life expectancy was lived in nursing homes, with more than half of people moving to a nursing home within five years after a dementia diagnosis. Prognosis after a dementia diagnosis is highly dependent on personal and clinical characteristics, offering potential for individualised prognostic information and care planning.
Systematic review registration
PROSPERO CRD42022341507.
Introduction
Dementia is a leading cause of disability, dependency, and death among older adults. Nearly 10 million people worldwide receive a diagnosis of dementia annually1; a diagnosis that has a major effect on patients, their relatives, and caregivers. Information on prognosis for people with dementia is important to guide expectations and care planning, but currently available estimates vary widely. For example, national Alzheimer’s associations in the US and UK report an average survival of four to eight years,2 3 whereas patient information from the UK NHS suggests a median survival of 3.5 years,4 and estimates from Alzheimer associations in other European countries range from 1.5 to 10 years.5 6 7 This wide variability in reported estimates8 often gives rise to caution among doctors about offering a prognosis. In addition to survival, functional outcomes also are valuable to patients as well as healthcare policy,8 but reliable figures are scarce. Disease progression on activities of daily living and functional independence are important to patients and caregivers,8 and nursing home admission is a particularly critical and impactful life event.
The prognosis for patients with a dementia diagnosis has been extensively studied, with most studies focusing on survival9 10 and the associated determinants.11 12 13 In the two most recent systematic reviews, including studies published before 2012, survival after diagnosis ranged from 3.3 to 6.6 years.9 10 Although patients’ age, cause of dementia, and severity at time of diagnosis seemed to be associated with survival, the effect of these factors was not quantified because of methodological heterogeneity.9 10 The effect of sex and other patient, disease, and study design characteristics remains uncertain. Moreover, so far geographical differences remain undetermined, and prognosis might have changed over time owing to secular trends in dementia incidence and public awareness.14 15 Fewer studies have been published on nursing home admission of people with a dementia diagnosis. A 2008 systematic review of 42 studies reported one year admission rates of around 20% (range 11-39%), increasing to 50% after five years. Across 10 studies, average times until admission ranged from 1.2 to 7.3 years.16 Again, heterogeneity in patient populations and methodology precluded meta-analysis or systematic assessment of prognostic indicators.8
We systematically reviewed the literature to determine prognosis for people with a diagnosis of dementia, both for remaining life expectancy and for time to nursing home admission. Our aim was to quantify median times and absolute risks per year and to determine to what extent variation in prognosis among studies could be explained by patient characteristics and differences in study methodology.
Methods
Search strategy
We searched Medline, Embase, Web of Science Core Collection, Cochrane Central Register of Controlled Trials, and Google Scholar from inception to 4 July 2024 for studies reporting on the prognosis of patients with dementia in terms of survival or nursing home admission. This report follows the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guideline.17 The protocol is registered with PROSPERO and provided in supplementary S1. The search syntax for every database is presented in supplementary S2.
Inclusion and exclusion criteria
We included longitudinal studies on survival or nursing home admission in people with dementia if they included at least 150 people with dementia and followed participants for a minimum of one year after diagnosis. Studies were deemed eligible if they reported either (yearly) probabilities or the median time to death or nursing home admission. These could be reported numerically or depicted graphically in survival curves. Studies that reported only incidence rates were excluded, as were studies reporting mean survival time rather than median survival times, because of the potential bias arising from attrition (when durations were reported only among those who experienced the outcome) and the generally skewed survival distributions. We further excluded studies that recruited participants at time of acute hospital admission, because estimates were difficult to interpret in the absence of a well defined time of inclusion for the dementia diagnosis. Similarly, studies that based inclusion on postmortem examination were excluded, because measures of disease duration would generally not be available.
Study selection
Two investigators (LMK and CCB) independently conducted the initial study selection using an open source machine learning tool for efficient screening of titles and abstracts (ASReview v0.16).18 19 Based on screening decisions by the human reviewers, ASReview continuously re-ranks the entire list of remaining titles or abstracts by their likelihood of inclusion. This approach reduces the number of abstracts that requires appraisal by the human reviewer in order to include all eligible papers,18 rendering the selection procedure more efficient.20 Using a conservative application of the SAFE guideline, inclusion of studies through ASReview was continued until each author appraised 25% of all studies or until 100 consecutive studies were deemed ineligible, whichever came first.21 In the next phase, the resulting reports were screened for eligibility in more detail based on their full text. For 23 studies without full text available, the respective authors were approached to share the necessary information for inclusion in this review. Full text access was provided for four studies. Finally, we screened the reference list of the most recent systematic reviews on survival with dementia9 16 as well as the reference lists of all included papers published thereafter, for additional relevant reports. This yielded two additional reports (see supplementary figure S3). In case of multiple reports describing the same study population, we selected the most relevant paper based on follow-up time, sample size, and reported outcomes. Any disagreements between the two investigators were resolved through consensus discussion with a third investigator (FJW).
Data extraction
Two of four investigators (CCB, SSM, LMK, and MLS) independently extracted study information (year of publication, year of study enrolment (median year of the inclusion period), study setting and country, diagnostic case ascertainment, outcome assessment, follow-up time, attrition), patient characteristics (number of included patients, age, sex, race/ethnicity, education, cohabitation status (married or not living alone), dementia subtype, disease duration before study baseline, disease severity at study baseline, comorbidity), and outcomes (median time to death or nursing home admission, one to 10 year probabilities). Supplementary data S4 provide further details. Relevant outcome data were extracted from the full text or quantified from Kaplan-Meier curves using a semi-automated online tool (WebPlotDigitizer; https://automeris.io/wpd/?v=5_2). Data were collected for the total study populations and/or stratified by variables of interest such as study setting and dementia subtype, if available. Discrepancies in data collection between the two extractors were resolved through consensus discussion among all authors.
Quality assessment
We critically appraised all selected studies and formally assessed their quality by using a modification of the Newcastle-Ottawa scale,22 in line with previous recommendations for quality assessment of observational studies.23 The total score ranges from 0 to 10 points, with a higher score indicating higher quality. Supplementary table S5 provides details of the quality criteria and rating categories.
Statistical analysis
Firstly, we determined median survival across the studies, overall and stratified by dementia type, with the corresponding interquartile range (IQR). Survival across studies was depicted in bubble plots according to study size, stratified by the study design variables of setting and time of inclusion (prevalent or incident dementia). Survival by dementia type was visualised in histograms. Yearly survival probabilities were depicted in boxplots to describe a longitudinal perspective up to 10 years’ follow-up. Similarly, we described median time to nursing home admission and yearly admission probabilities until five years of follow-up (due to data availability).
Next, we used linear regression models for a meta-regression analysis that examined the effect of patient and study characteristics on median survival and median time to nursing home admission. Several factors were investigated: age, sex, dementia type, study setting, geographical location, and years of study enrolment (in decades). All regressions were weighted by the number of patients in each study (natural log transformed). After univariable analyses (model 1), we constructed a model adjusting for mean age and sex (model 2) and a model including all variables of interest (model 3). The largest category for categorical variables was chosen as reference, and those categories that contained too few studies were grouped together. We then calculated age adjusted remaining life expectancy in contemporary studies (post-2000), restricting analyses to studies reporting prognosis from dementia diagnosis onwards (incidence).
We repeated the regression analyses for probabilities of survival and nursing home admission as the outcome and adjusting for study quality score. To evaluate how differences in methodology and clinical characteristics accounted for the variability in survival and in nursing home admissions observed among the studies, we computed the R2 value for the models. In additional exploratory analyses, we assessed associations between additional patient characteristic variables (education, marital status, and baseline score on the mini-mental state examination) and the main outcomes.
All statistical analyses were conducted using R version 4.3.1, with significance levels set at P<0.05 for all analyses. For the meta-regression analyses, missing data for years of study enrolment (4.3%; 19/439), maximum follow-up time (6.6%; 29/439), sex (15.0%; 66/439), and age (19.1%; 84/439) were imputed based on all other baseline study characteristics, using the mice package (version 3.16.0) with 50 iterations and 10 imputed sets. We used the predictive mean matching method to impute continuous variables (age, proportion of women, and maximum follow-up time) and a proportional odds model for the ordered, categorical variable (years of study enrolment in decades). Studies with missing data were more often community studies involving types of dementia other than Alzheimer’s disease and were typically older studies conducted outside of Europe and North America. Supplementary data S6 provides more details on the imputation. As data on education, cohabitation status, baseline mini-mental state examination score, and comorbidities were presented in less than half of reports (25-42%), these were not imputed but used only for exploratory analyses in a complete case analysis.
Patient and public involvement
No patients or members of the public were involved directly in the design of this study. The authors do convene on a regular basis with participant panels of ongoing cohort studies and public/patient advisory groups of active research consortiums to align on clinical needs and research priorities. Although not explicitly part of this project, the research question of this article was inspired by these conversations, as well as discussion between patients and doctors in consultation rooms.
Results
Study selection
Overall, 19 307 unique reports were identified (see supplementary figure S3). After screening of titles and abstracts, 1051 studies remained for full text appraisal. Of those, 259 studies were eligible for inclusion and two more were identified through reference screening. Together, the 261 included studies described prognosis for 439 different patient groups. The main reasons for ineligibility were inclusion of fewer than 150 patients with dementia (n=258), multiple reports of the same patient population, and insufficient outcome data (eg, outcomes not reported specifically for people with dementia). Of all 261 included studies, 235 reported on survival among 5 553 960 participants and 79 reported on nursing home admission among 352 990 participants. The supplementary Excel spreadsheet (supplementary data S7) lists the included studies.
Study characteristics
Table 1 shows the characteristics of the included studies. The studies were published between 1984 and 2024 (60%; 265/439 published after 2000), with patient enrolment in the cohorts taking place between 1962 and 2021. Most study samples were from Europe (55%; 243/439) and North America (27%; 117/439), followed by Asia (13%; 59/439), Oceania (3%; 12/439), and South America (1%; 2/439), with no identified reports originating from Africa. Information on race was reported in 19% of studies (85/439).
Table 1.
Study characteristics. Values are number (percentage) unless stated otherwise
| Characteristic | All studies (n=261) | Studies on mortality (n=235) | Studies on nursing home admission (n=79) |
|---|---|---|---|
| Median (IQR) No of patients per study | 524 (261-2644) | 559 (275-4078) | 410 (262-1074) |
| Median (IQR) age (years) | 78.8 (75.4-82.0) | 79.1 (75.5-82.1) | 77.9 (75.5-80.9) |
| Median (IQR) women (%) | 63.0 (54-68.0) | 62.8 (53.1-68.0) | 62.8 (54.5-67.8) |
| Available information on race | 85 (19) | 75 (19) | 27 (32) |
| Geographical location: | |||
| Europe | 243 (55) | 226 (56) | 42 (49) |
| North America | 117 (27) | 103 (26) | 36 (42) |
| Asia | 59 (13) | 58 (14) | 0 |
| Oceania | 12 (3) | 11 (3) | 4 (5) |
| South America | 2 (1) | 2 (1) | 0 |
| Mixed | 6 (1) | 4 (1) | 3 (4) |
| Dementia type: | |||
| All cause | 191 (44) | 171 (42) | 45 (53) |
| Alzheimer’s disease | 141 (32) | 127 (31) | 37 (44) |
| Frontotemporal dementia | 19 (4) | 19 (5) | 1 (1) |
| Lewy body dementia | 23 (5) | 22 (5) | 2 (2) |
| Vascular dementia | 39 (9) | 39 (10) | 0 |
| Parkinson’s disease dementia | 4 (1) | 4 (1) | 0 |
| Other | 22 (5) | 22 (5) | 0 |
| Study setting: | |||
| Clinic | 190 (43) | 168 (42) | 48 (57) |
| Community | 169 (39) | 158 (39) | 32 (38) |
| Nursing home | 48 (11) | 47 (12) | NA |
| Mixed | 32 (7) | 31 (8) | 5 (6) |
| Study type: | |||
| Observational | 410 (93) | 379 (94) | 64 (75) |
| Interventional | 29 (7) | 25 (6) | 21 (25) |
| Case ascertainment: | |||
| Clinical examination | 247 (56) | 221 (55) | 59 (69) |
| Medical records | 31 (7) | 29 (7) | 3 (4) |
| Registry | 140 (32) | 135 (33) | 19 (22) |
| Other | 21 (5) | 19 (5) | 4 (5) |
| Time of inclusion: | |||
| At diagnosis (incident) | 216 (49) | 202 (50) | 24 (28) |
| During disease course (prevalent) | 207 (47) | 187 (46) | 57 (67) |
| Mixed or undefined | 16 (4) | 15 (4) | 4 (5) |
| Period of study enrolment: | |||
| <1990 | 48 (11) | 46 (11) | 4 (5) |
| 1990-99 | 107 (24) | 94 (23) | 21 (25) |
| 2000-09 | 159 (36) | 149 (37) | 31 (37) |
| ≥2010 | 106 (24) | 102 (25) | 21 (21) |
| Not reported | 19 (4) | 13 (3) | 11 (13) |
| Median (IQR) maximum follow-up (years) | 7.0 (4.2-10.0) | 7.0 (4.5-10.0) | 4.0 (2.0-8.0) |
| Attrition (%): | |||
| <10 | 78 (18) | 74 (18) | 19 (22) |
| 10-19 | 19 (4) | 15 (4) | 13 (15) |
| ≥20 | 13 (3) | 12 (3) | 6 (7) |
| Not reported | 329 (75) | 303 (75) | 47 (55) |
Characteristics are shown for all 439 study populations described in the 261 included studies. Of these, 404 were included in 235 studies on mortality and 85 populations in 79 studies on nursing home admission. Only the number of patients is provided per included study rather than per study population. Percentages may not add up to 100% owing to rounding. Data were missing for several variables: study start year (4%), maximum follow-up time (7%), sex (15%), and age (19%).
IQR=interquartile range; NA=not applicable.
Reports described a median 524 (IQR 261-2643) patients for each study, included mostly in a clinic based setting (43%; 190/439) or community based setting (39%; 169/439), and to a lesser extent in nursing homes (11%; 48/439). The median age at start of follow-up was 78.8 years (IQR 75.4-82.0 years) and was lower in clinic based studies (75.5 (72.5-77.8) years) compared with community based studies (80.4 (79.0-82.5) years) or nursing home populations (84.5 (83.1-85.3) years). On average, 63% of participants were women. Most studies assessed all cause dementia regardless of its disease (44%; 191/431). Of specific dementia subtypes, Alzheimer’s disease was most studied (32%; 140/439), followed by vascular dementia (9%; 39/439) and Lewy body dementia (5%; 23/439). Patients were followed from diagnosis in 49% (216/439) of samples (incident dementia), whereas patients already had a diagnosis before study entry (prevalent dementia) in 47% (207/439) of samples. Most studies derived dementia diagnoses from clinical examinations (56%; 247/439), whereas others relied on registries (32%; 140/439) or medical records (7%; 31/439). The maximum follow-up time was on average seven years. When all study quality criteria were combined, most studies scored between 5 and 8 (scale: 0 to 10), with a mean score of 5.6 (see supplementary data S5). Attrition was often poorly reported, and it was not estimable in three quarters of samples in reports. Of all 79 studies on nursing home admission, 48 used some form of survival modelling to compute cumulative incidence, whereas others mostly reported crude proportions. Most of these 48 studies (39/48) obtained cumulative incidences using the Kaplan-Meier estimator or cause specific hazards from Cox models, whereas seven studies explicitly reported the use of the sub-distribution hazard cumulative incidence function.
Survival with dementia
Median survival was 4.8 years from incident diagnosis onwards (IQR 4.0-6.0; 66 studies), in line with an overall five year survival probability of 51%. In 53 studies among people with prevalent dementia, survival was a median 3.1 years (IQR 2.4-5.6) (fig 1). Yearly probabilities of survival ranged from 90% at one year after diagnosis to 69% at three years’ follow-up, 51% at five years, and 21% at 10 years (fig 2). Median survival was shorter with older age at study baseline (per 10 years increase in age: −1.4 years (95% confidence interval −1.0 to −1.8)) and was longer in men than in women (fig 1 and table 2). Consequently, in contemporary studies the remaining life expectancy from diagnosis onwards varied from 6.5 years at mean age 60 years to 2.2 years at mean age 85 years for men, and from 8.9 years to 4.5 years at the same ages for women (see supplementary table S8). In meta-regression analyses, differences by sex were mostly related to older age at diagnosis in women (table 2). For dementia subtypes, a higher share of included patients with Alzheimer’s disease was associated with longer survival than all cause dementia (fig 3 and table 2).
Fig 1.
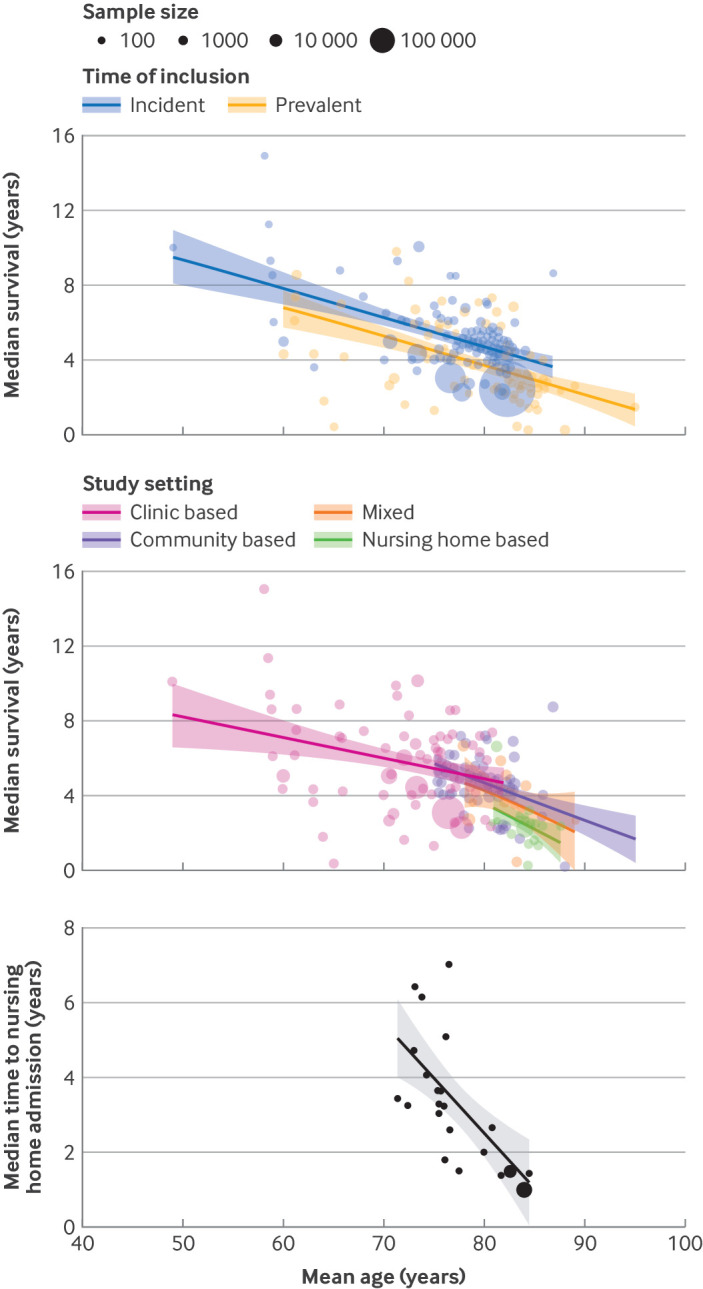
Bubble plots of median survival according to age at dementia diagnosis, stratified by time of inclusion and study setting, and of median time to nursing home admission, according to age at diagnosis
Fig 2.
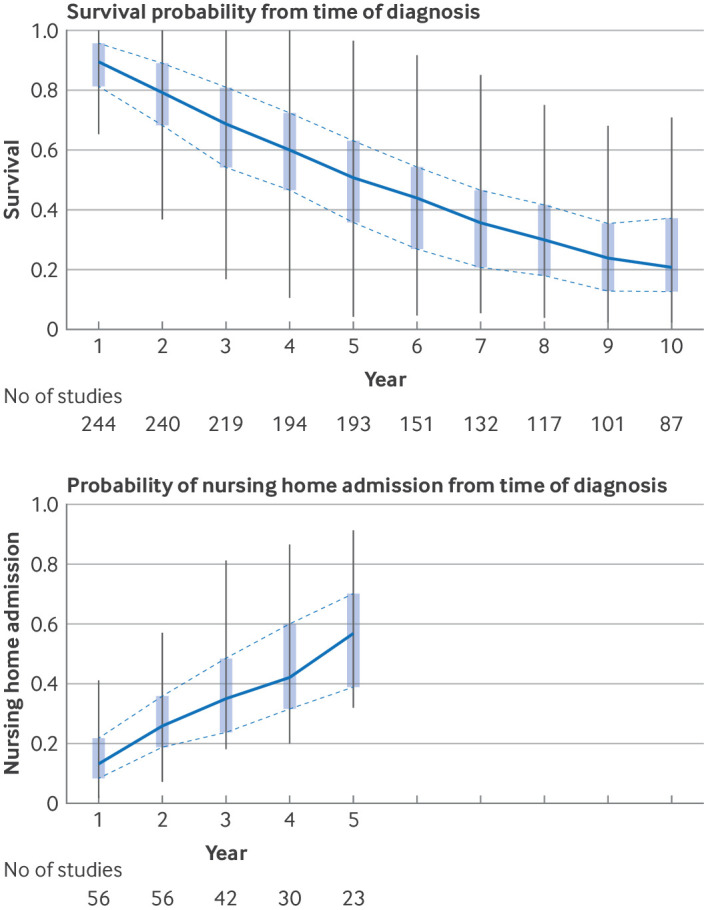
Boxplots of yearly probabilities for survival and nursing home admission, with boxes indicating 25th to 75th centiles (IQR) and whiskers depicting 1.5 times the interquartile range (capped off at most extreme observations within this range)
Table 2.
Effect of clinical and study characteristics on survival
| Characteristics | Model 1 (univariable) | Model 2 (age and sex adjusted) | Model 3 (full model) | |||||
|---|---|---|---|---|---|---|---|---|
| β (95% CI) | P value | β (95% CI) | P value | β (95% CI) | P value | |||
| Age (years) | −0.16 (−0.19 to −0.12) | <0.001 | −0.16 (−0.2 to −0.12) | <0.001 | −0.12 (−0.17 to −0.07) | <0.001 | ||
| Female sex | −4.08 (−6.08 to −2.07) | <0.001 | −0.01 (−2.12 to 2.10) | 1.0 | −0.41 (−2.66 to 1.83) | 0.72 | ||
| Dementia type: | ||||||||
| All cause | Reference | Reference | Reference | |||||
| Alzheimer’s disease | 1.37 (0.79 to 1.94) | <0.001 | 0.80 (0.27 to 1.34) | 0.004 | 0.70 (0.21 to 1.19) | 0.006 | ||
| Other | 0.58 (−0.04 to 1.2) | 0.07 | −0.34 (−0.96 to 0.27) | 0.28 | −0.76 (−1.33 to −0.19) | 0.01 | ||
| Study setting: | ||||||||
| Clinic | Reference | Reference | Reference | |||||
| Community | −1.16 (−1.67 to −0.65) | <0.001 | −0.37 (−0.96 to 0.22) | 0.22 | −0.10 (−0.77 to 0.58) | 0.78 | ||
| Nursing home | −3.34 (−4.04 to −2.65) | <0.001 | −2.22 (−3.03 to −1.41) | <0.001 | −1.03 (−1.91 to −0.14) | 0.02 | ||
| Mixed | −1.75 (−2.47 to −1.03) | <0.001 | −0.88 (−1.65 to −0.11) | 0.03 | 0.04 (−0.81 to 0.89) | 0.93 | ||
| Geographical location: | ||||||||
| Europe | Reference | Reference | Reference | |||||
| North America | −0.17 (−0.75 to 0.4) | 0.56 | −0.17 (−0.67 to 0.34) | 0.52 | −0.41 (−0.88 to 0.07) | 0.10 | ||
| Asia | 1.22 (0.34 to 2.11) | 0.007 | 1.23 (0.46 to 2.00) | 0.002 | 1.09 (0.38 to 1.80) | 0.003 | ||
| Other | 0.4 (−1.07 to 1.88) | 0.59 | −0.02 (−1.29 to 1.26) | 0.98 | −0.34 (−1.53 to 0.84) | 0.57 | ||
| Period of study enrolment: | 0.01 | 0.48 | 0.56 | |||||
| <1990 | −0.23 (−1.18 to 0.72) | −1.1 (−1.99 to −0.20) | −1.02 (−1.91 to −0.13) | |||||
| 1990-99 | −0.13 (−0.82 to 0.57) | −0.22 (−0.83 to 0.40) | −0.28 (−0.90 to 0.34) | |||||
| 2000-09 | Reference | Reference | Reference | |||||
| ≥2010 | −0.83 (−1.46 to −0.19) | −0.66 (−1.21 to −0.11) | −0.24 (−0.77 to 0.29) | |||||
| Case ascertainment: | ||||||||
| Clinical examination | Reference | Reference | Reference | |||||
| Medical records | −2.08 (−3.06 to −1.11) | <0.001 | −1.79 (−2.65 to −0.93) | <0.001 | −0.71 (−1.56 to 0.14) | 0.10 | ||
| Registry | −1.06 (−1.58 to −0.54) | <0.001 | −0.63 (−1.11 to −0.16) | 0.010 | −0.61 (−1.13 to −0.08) | 0.02 | ||
| Other | −1.77 (−2.96 to −0.57) | 0.004 | −1.15 (−2.23 to −0.08) | 0.04 | −1.46 (−2.47 to −0.44) | 0.005 | ||
| Time of inclusion: | ||||||||
| At diagnosis (incident) | Reference | Reference | Reference | |||||
| During disease course (prevalent) | −1.15 (−1.66 to −0.63) | <0.001 | −0.81 (−1.27 to −0.34) | <0.001 | −0.53 (−1.06 to 0.01) | 0.06 | ||
| Mixed or undefined | 0.52 (−1.09 to 2.14) | 0.53 | 0.88 (−0.53 to 2.28) | 0.22 | 1.01 (−0.27 to 2.29) | 0.12 | ||
Results from a meta-regression analysis to determine association of several patient and study characteristics with median survival (n=238). For categorical variables, numbers are differences in median survival (in years), compared with the reference category. For age, the estimate depicts change in median survival per year increase. Regression analyses were weighted by the number of patients in each study. The full model included all listed variables (age, sex, dementia type, study setting, geographical location, years of study enrolment, case ascertainment, and time of inclusion), as well as maximum follow-up time (log transformed). The P value for years of study enrolment represents the trend across categories. R2 of the full model was 0.51.
Fig 3.
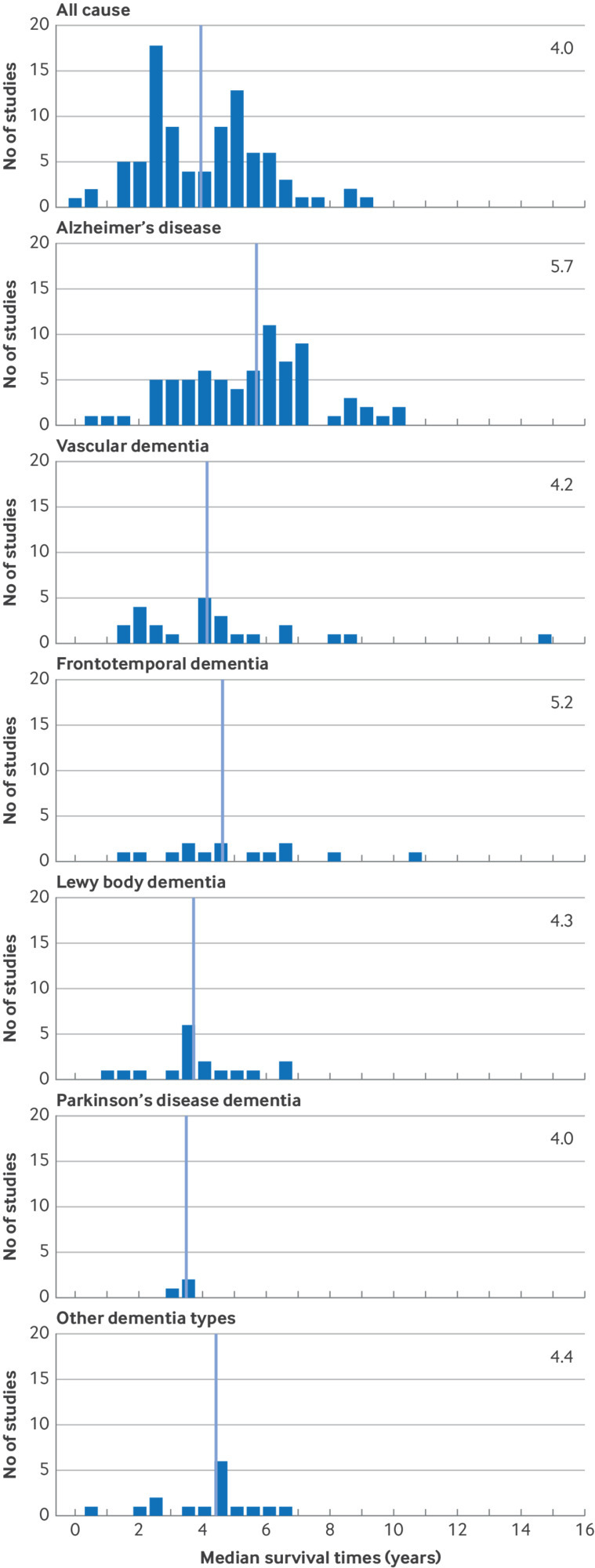
Median survival by type of dementia
Survival was longer in clinic based studies than in community based studies (5.9 years v 4.6 years; see supplementary figure S9), mainly due to older mean age at study entry in the community based studies (fig 1 and table 2). Nursing home studies reported the shortest median survival (2.4 years). Studies in Asia reported significantly longer median survival times than in Europe or North America (table 2). Overall, median survival with dementia did not change substantially over the past six decades. When stratifying by study setting, median survival in contemporary clinic based studies (ie, published after 2000) was 1.3 years longer than in earlier clinic based studies (Ptrend=0.02), whereas an opposite trend was observed in community based studies (−0.7 years, Ptrend=0.07). Case ascertainment through registries or medical records was associated with shorter median survival by 0.6-0.7 years compared with ascertainment through clinical examination (table 2). Supplementary data S10 provides a forest plot of all median survival estimates.
Results from the meta-regression analyses of yearly survival probabilities were broadly similar to those for median survival (see supplementary table S11). Taken together, variation in clinical characteristics and study methodology explained 51% of heterogeneity in median survival among studies and 45-51% in variation of yearly survival probabilities. Among the subset of studies reporting median survival as well as information on education (n=36; mean 10.5 years (standard deviation (SD) 3.3 years)), cohabitation (n=40; mean 53% (SD 19%)), or baseline mini-mental state examination score (n=73; median 20 (IQR 17.3-21.6)), every additional year of education was associated with shorter median survival (0.20 years, 95% CI −0.38 to –0.03, see supplementary table S11). Cohabitating was also associated with longer median survival, whereas mini-mental state examination score was not (see supplementary table S12). Overall study quality score was not significantly associated with survival (see supplementary figure S13A and supplementary data S14).
Time to nursing home admission
Median time to nursing home admission, derived from 23 studies, was 3.3 (IQR 1.9-4.0) years (fig 1). Time to admission was slightly shorter among 10 studies following patients from diagnosis (2.3 (IQR 1.5-3.5) years). Supplementary data S15 provides a forest plot of all median nursing home estimates. Admission probabilities increased from 13% within the first year of study entry to 35% within three years and 57% within five years from baseline (fig 2). Time to nursing home admission was significantly shorter in patients of older age at diagnosis, with a mean difference of 0.3 years per year increase in age (95% CI 0.15 to 0.46) (fig 1 and supplementary table S16). Time to nursing home admission did not differ by sex or dementia type and showed no clear trend with calendar time (see supplementary table S16). Time to nursing home admission tended to be longer in studies conducted outside of Europe or North America (2.1 years, 95% CI −1.0 to 5.2). Variation in methodology and clinical characteristics explained 55% of heterogeneity in median time to nursing home admission, and 26% to 36% in variation of yearly probabilities for nursing home admission. In the subsamples of nine to 15 studies with available data, education, cohabitation status, and baseline mini-mental state examination scores were not associated with nursing home admission (see supplementary table S12). Overall study quality score was not significantly associated with nursing home admission, although time to nursing home admission tended to be somewhat shorter in higher quality studies (see supplementary figure S13B and supplementary data S14).
Discussion
This systematic review and meta-analysis of 261 studies describing the prognosis of dementia in more than five million patients, provides contemporary, group level estimates by age and sex for expected survival and time to nursing home admission after a dementia diagnosis. Median survival after diagnosis in women ranged from nine years at mean age 60 to 4.5 years at age 85, whereas survival was shorter in men (6.5 to 2.2 years, respectively). Comparison of our results to the 2021 census population in the US24 and Europe25 suggests that dementia reduces life expectancy by about two years for people with a diagnosis at age 85, 3-4 years with a diagnosis at age 80, and up to 13 years with a diagnosis at age 65. Moreover, one third of people with dementia are admitted to a nursing home within three years of diagnosis.
Survival time
Age at diagnosis is the most important determinant of prognosis in people with dementia. Women lived slightly longer with dementia than men, which was due in large part to older age at diagnosis.9 11 Survival was longer in clinic based studies, mainly owing to the inclusion of relatively young patients in those studies.26 Applying prognostic estimates from clinic based samples to patients in other care settings could thus overestimate remaining life expectancies. The variation in case mix between settings underlines the importance of capturing in research a representative group of patients. Patients with Alzheimer’s disease had a more favourable prognosis than those with vascular dementia, frontotemporal dementia, or Lewy body dementia. These results are consistent with previous studies and might result from accelerated disease progression, later diagnosis, or more comorbidity (eg, vascular or psychiatric) in non-Alzheimer’s dementia.9 11 However, most of these studies relied on clinical diagnosis, which might not align with underlying disease. From a methodological perspective, timing of enrolment was associated with a substantial effect on recorded survival. Inclusion of patients at time of diagnosis—rather than later in the disease course—avoids underestimation of survival owing to disease duration before study inclusion, as well as overestimation due to selective inclusion.
Time to nursing home admission
Remaining time lived independently, or at least at home, is sensitive to differences between cultures and healthcare. Although hard to fully capture at a group level, our results suggest time to nursing home admission might be somewhat shorter in Europe and the US, compared with elsewhere. In line with survival time, time to nursing home admission was shorter at older age and shorter for subtypes other than Alzheimer’s disease. However, inference from these findings was hampered by lack of precision and methodological challenges. Foremost, the common use of the Kaplan-Meier estimator and cause specific hazard models for deriving cumulative incidences could have led to overestimation of risks for nursing home admission. In the presence of a strong competing risk of death, the independence of censoring assumption is violated and sub-distribution hazards or combined cause specific hazard models are preferred.27 Multistate models can also shed light on transition probabilities that account for mortality risk.28
Clinical prognostic indicators
Robust information on determinants of prognosis could be useful for the development of models for individualised risk prediction, but few were reported consistently across studies. Analyses in a subset of studies indicated that cohabitation is associated with longer survival. Additional studies are needed to clarify whether this is a consequence of caregiver ability, or perhaps related to different timing of diagnosis when living with a spouse or relatives. Higher education was associated with a shorter survival after diagnosis, which is in line with the cognitive reserve paradigm. This paradigm postulates that people with higher education are more resilient to brain injury before functional declines. Once this reserve has been used up and dementia is diagnosed, however, these people are already at a more advanced stage of the underlying disease and clinical progression will be faster. The between study variation in mini-mental state examination in our review was limited and therefore the importance of (previously reported) individual level differences in disease severity on prognosis might not have been captured.29 Although similar mechanisms would be expected to influence nursing home admission times too, no associations were observed with education, cohabitation status, or mini-mental state examination score among a small number of studies covering these characteristics in this meta-analysis.
Geographical and temporal differences in prognosis
Our findings suggest a longer survival after dementia diagnosis among studies in Asia, compared with the US and Europe, along with longer times to nursing home admission in studies outsides of the US and Europe. Such regional variation may result from socioeconomic, cultural, and healthcare differences, including the moment at which people seek medical attention. The precise underlying causes are uncertain and require further study, extending also to underrepresented regions such as low and middle income countries. Over the past decades, survival after a dementia diagnosis has become somewhat longer in clinic based studies but not in community based studies. Increased dementia awareness and improved diagnostic tools might have led to earlier clinical presentation and more rapid diagnosis in memory clinics. Such changes should coincide with lower disease severity at time of diagnosis, but we were unable to confirm or refute this owing to insufficient information on severity. The absence of similar time trends in community based studies could imply that survival with dementia itself remained unchanged, or even shortened. Against the backdrop of previously reported declines in age specific incidence of dementia (ie, postponing its onset), a stable survival after diagnosis would result in a lower population burden of dementia, a phenomenon called compression of morbidity.1 30 31
Strengths and limitations of this review
Strengths of this study include the meticulous search and data extraction, including thorough appraisal of survival curves from original studies, which enabled aggregation and meta-regression analyses of the largest number of studies to date over a prolonged period of time. Several limitations also need to be considered. Firstly, lack of consistent measures of precision in the individual studies (eg, confidence intervals) hampered meta-analysis of the results, which we addressed in meta-regression analyses by weighting for study sample size. Secondly, various potentially relevant predictor variables were inconsistently reported, such as measures of socioeconomic status, race, disease severity, and comorbidity, which limited meta-regression analyses. Also, the aggregation of marital status and cohabitation may not perfectly reflect cohabitation. Thirdly, attrition rates were reported only in a few studies (25%; 110/439), and this could have biased estimates in either direction. Fourthly, for studies on nursing home admission, inappropriate handling of competing risk of mortality may have biased observed risk estimates upwards. Fifthly, study populations contributing to the long term survival estimates (eg, 6-10 years) differed from those contributing to short term studies (1-5 years; see supplementary data S17). The former on average included younger patients, recruited more often in a clinic based setting, which might have led to higher survival estimates relative to the one to five years of follow-up. Finally, most studies originated from Europe and North America, and generalisability, notably to African and Latin-American populations, is uncertain.
Conclusions
This systematic review found that prognosis after a dementia diagnosis is highly dependent on patient, disease, and study characteristics, offering potential for individualised prognostic information and care planning. Future studies on individualised prognosis should ideally include patients at time of diagnosis, accounting for personal factors, social factors, disease stage, and comorbidity, while assessing relevant functional outcome measures above and beyond survival alone.
What is already known on this topic
Previous systematic reviews including studies published to 2012 reported a wide range of survival estimates after a dementia diagnosis
The effect of age, sex, and other patient, disease, and study characteristics on these estimates was uncertain
Few studies have assessed prognosis in terms of time to nursing home admission
What this study adds
This systematic review and meta-analysis found that the average life expectancy of people with dementia at time of diagnosis ranged from 5.7 years at age 65 to 2.2 at age 85 for men and from 8.0 to 4.5, respectively, for women
Survival was longer among Asian populations and among people with Alzheimer’s disease
About one third of remaining life expectancy was lived in nursing homes, with more than half of people moving to a nursing home within five years after a dementia diagnosis
Acknowledgments
We thank Elise Krabbendam and Maarten Engel (Erasmus MC Medical Library) for developing and updating the search strategy. We also acknowledge Gerbrich Ferdinands (ASReview development team, Utrecht University) for support in setting up the ASReview screening process.
Web extra.
Extra material supplied by authors
Supplementary information: Supplementary data S1-S17
Supplementary information: Supplement S7 (Excel spreadsheet showing details of included studies)
Contributors: The initial study selection used an open source machine learning tool to screen titles and abstracts (ASReview v0.16).18 19 Based on screening decisions by the human reviewers, ASReview continuously re-ranked the list of remaining titles or abstracts by likelihood of inclusion. Inclusion of studies through ASReview was continued until each author appraised 25% of all studies or until 100 consecutive studies were deemed ineligible, whichever came first. CCB, SSM, and LMK contributed equally to this work as co-first authors. FJW, SSM, LMK, and CCB designed the study, with input from SL and FMR. CCB and LMK screened and selected studies. CCB, SSM, LMK, MLS, and FJW extracted the data. CCB performed the analyses. CCB, SSM, and LMK wrote the first draft of the manuscript, with critical input from all authors. All other co-authors reviewed the manuscript and participated in the revisions. FJW is the guarantor. He had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: No direct funding received. FJW was supported by a research fellowship from the Alzheimer’s Association (AARF-22-924982). The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: FJW was supported by a research fellowship from the Alzheimer’s Association; no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years, no other relationships or activities that could appear to have influenced the submitted work.
Transparency: The guarantor (FJW) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Dissemination to participants and related patient and public communities: The findings of this review will be disseminated through conference presentations, education sessions for healthcare professionals, social media outlets, newsletters, and direct outreach to stakeholders such as patient organisations and commonly used public sources of patient information.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
Not required.
Data availability statement
The study protocol is available at https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=341507. The supplementary Excel spreadsheet (supplement S7) shows the characteristics of all studies included in the review and meta-analysis. Detailed extracted data on all included studies are available upon reasonable request to the corresponding author.
References
- 1. Prince M, Ali G-C, Guerchet M, Prina AM, Albanese E, Wu Y-T. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers Res Ther 2016;8:23. 10.1186/s13195-016-0188-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. 2023 Alzheimer’s disease facts and figures. Alzheimers Dement 2023;19:1598-695. 10.1002/alz.13016 [DOI] [PubMed] [Google Scholar]
- 3.Alzheimer’s Society. The later stage of dementia. 2020. https://www.alzheimers.org.uk/about-dementia/symptoms-and-diagnosis/how-dementia-progresses/later-stages-dementia. [Accessed 2 Oct 2023.]
- 4.NHS. Focus on dementia. 2016. www.hscic.gov.uk/catalogue/PUB19812. [Accessed 12 Dec 2023.]
- 5.Alzheimer Nederland. Levensverwachting dementie [Life expectancy dementia]. https://www.alzheimer-nederland.nl/levensverwachting-dementie. [Accessed 12 Dec 2023.]
- 6.Swedish Dementia Centre. Sjukdomens faser [Phases of the disease]. https://demenscentrum.se/fakta-om-demens/demenssjukdomar/alzheimers-sjukdom/sjukdomens-faser.
- 7.pflege.de. Demenz: Lebenserwartung [Dementia life expectancy]. https://www.pflege.de/krankheiten/demenz/#:~:text=Durchschnittliche%20Lebenserwartung%20bei%20Demenz.
- 8. Mank A, van Maurik IS, Bakker ED, et al. Identifying relevant outcomes in the progression of Alzheimer’s disease; what do patients and care partners want to know about prognosis? Alzheimers Dement (N Y) 2021;7:e12189. 10.1002/trc2.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brodaty H, Seeher K, Gibson L. Dementia time to death: a systematic literature review on survival time and years of life lost in people with dementia. Int Psychogeriatr 2012;24:1034-45. 10.1017/S1041610211002924 [DOI] [PubMed] [Google Scholar]
- 10. Todd S, Barr S, Roberts M, Passmore AP. Survival in dementia and predictors of mortality: a review. Int J Geriatr Psychiatry 2013;28:1109-24. 10.1002/gps.3946 [DOI] [PubMed] [Google Scholar]
- 11. Liang C-S, Li D-J, Yang F-C, et al. Mortality rates in Alzheimer’s disease and non-Alzheimer’s dementias: a systematic review and meta-analysis. Lancet Healthy Longev 2021;2:e479-88. 10.1016/S2666-7568(21)00140-9 [DOI] [PubMed] [Google Scholar]
- 12. Mueller C, Soysal P, Rongve A, et al. Survival time and differences between dementia with Lewy bodies and Alzheimer’s disease following diagnosis: A meta-analysis of longitudinal studies. Ageing Res Rev 2019;50:72-80. 10.1016/j.arr.2019.01.005 [DOI] [PubMed] [Google Scholar]
- 13. Lee M, Chodosh J. Dementia and life expectancy: what do we know? J Am Med Dir Assoc 2009;10:466-71. 10.1016/j.jamda.2009.03.014 [DOI] [PubMed] [Google Scholar]
- 14. Brück CC, Wolters FJ, Ikram MA, de Kok IMCM. Projected prevalence and incidence of dementia accounting for secular trends and birth cohort effects: a population-based microsimulation study. Eur J Epidemiol 2022;37:807-14. 10.1007/s10654-022-00878-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schrijvers EMC, Verhaaren BFJ, Koudstaal PJ, Hofman A, Ikram MA, Breteler MMB. Is dementia incidence declining?: Trends in dementia incidence since 1990 in the Rotterdam Study. Neurology 2012;78:1456-63. 10.1212/WNL.0b013e3182553be6 [DOI] [PubMed] [Google Scholar]
- 16. Luppa M, Luck T, Brähler E, König HH, Riedel-Heller SG. Prediction of institutionalisation in dementia. A systematic review. Dement Geriatr Cogn Disord 2008;26:65-78. 10.1159/000144027 [DOI] [PubMed] [Google Scholar]
- 17. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg 2021;88:105906. 10.1016/j.ijsu.2021.105906 [DOI] [PubMed] [Google Scholar]
- 18. van de Schoot R, de Bruin J, Schram R, et al. An open source machine learning framework for efficient and transparent systematic reviews. Nat Mach Intell 2021;3:125-33. 10.1038/s42256-020-00287-7. [DOI] [Google Scholar]
- 19. Holzinger A. Interactive machine learning for health informatics: when do we need the human-in-the-loop? Brain Inform 2016;3:119-31. 10.1007/s40708-016-0042-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang Z, Nayfeh T, Tetzlaff J, O’Blenis P, Murad MH. Error rates of human reviewers during abstract screening in systematic reviews. PLoS One 2020;15:e0227742. 10.1371/journal.pone.0227742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boetje J, van de Schoot R. The SAFE procedure: a practical stopping heuristic for active learning-based screening in systematic reviews and meta-analyses. Syst Rev 2024;13:81. 10.1186/s13643-024-02502-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 23. Sanderson S, Tatt ID, Higgins JP. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol 2007;36:666-76. 10.1093/ije/dym018. [DOI] [PubMed] [Google Scholar]
- 24.Social Security Administration (United States government). Actuarial Life Table. 2024. https://www.ssa.gov/oact/STATS/table4c6.html. [Accessed 12 Jul 2024.]
- 25.Eurostat. Life expectancy by age and sex [Data set]. 2024. https://ec.europa.eu/eurostat/databrowser/view/demo_mlexpec__custom_12136409/default/table?lang=en. [Accessed 12 July 2024.]
- 26. Mooldijk SS, Licher S, Wolters FJ. Characterizing Demographic, Racial, and Geographic Diversity in Dementia Research: A Systematic Review. JAMA Neurol 2021;78:1255-61. 10.1001/jamaneurol.2021.2943. [DOI] [PubMed] [Google Scholar]
- 27. Austin PC, Steyerberg EW, Putter H. Fine-Gray subdistribution hazard models to simultaneously estimate the absolute risk of different event types: Cumulative total failure probability may exceed 1. Stat Med 2021;40:4200-12. 10.1002/sim.9023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mjørud M, Selbæk G, Bjertness E, et al. Time from dementia diagnosis to nursing-home admission and death among persons with dementia: A multistate survival analysis. PLoS One 2020;15:e0243513. 10.1371/journal.pone.0243513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zheng X, Wang S, Huang J, Li C, Shang H. Predictors for survival in patients with Alzheimer’s disease: a large comprehensive meta-analysis. Transl Psychiatry 2024;14:184. 10.1038/s41398-024-02897-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Satizabal CL, Beiser AS, Chouraki V, Chêne G, Dufouil C, Seshadri S. Incidence of Dementia over Three Decades in the Framingham Heart Study. N Engl J Med 2016;374:523-32. 10.1056/NEJMoa1504327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wu Y-T, Beiser AS, Breteler MMB, et al. The changing prevalence and incidence of dementia over time - current evidence. Nat Rev Neurol 2017;13:327-39. 10.1038/nrneurol.2017.63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: Supplementary data S1-S17
Supplementary information: Supplement S7 (Excel spreadsheet showing details of included studies)
Data Availability Statement
The study protocol is available at https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=341507. The supplementary Excel spreadsheet (supplement S7) shows the characteristics of all studies included in the review and meta-analysis. Detailed extracted data on all included studies are available upon reasonable request to the corresponding author.