Abstract
The neuroscience community has largely accepted the notion that functional neurons can be generated from neural stem cells in the adult brain, especially in two brain regions: the subventricular zone of the lateral ventricles and the subgranular zone in the dentate gyrus of the hippocampus. However, impaired neurogenesis has been observed in some neurodegenerative diseases, particularly in Alzheimer’s, Parkinson’s, and Huntington’s diseases, and also in Lewy Body dementia. Therefore, restoration of neurogenic function in neurodegenerative diseases emerges as a potential therapeutic strategy to counteract, or at least delay, disease progression. Considering this, the present study summarizes the different neuronal niches, provides a collection of the therapeutic potential of different pro-neurogenic strategies in pre-clinical and clinical research, providing details about their possible modes of action, to guide future research and clinical practice.
Keywords: Adult neurogenesis, Neurodegenerative diseases, Neural stem cells, Therapeutics, Pre-clinical studies, Clinical research
Introduction
For decades, it has been known that terminally differentiated neurons are incapable of re-entering the cell division cycle and undergo mitosis [1]; however, new neurons can be generated through the differentiation of neural precursors such as neural stem and progenitor cells, in a process called neurogenesis [2]. Neurogenesis was conventionally perceived to occur only during the embryonic and pre-natal stages in mammals [3] and even though it is most active during embryonic development, accumulating evidence has repeatedly shown that adult mammals (including the human adult brain) maintain this capacity throughout their life [4–6], although limited to specific events and restricted regions [7]. It starts with the proliferation of precursor cells which then commit to a neuronal phenotype and become neuroblasts that migrate and differentiate into mature neurons. Finally, these mature neurons become functionally integrated into pre-existing neural networks [8]. Adult neurogenesis has been best described in the subgranular zone (SGZ) of the dentate gyrus (DG) of the hippocampus [9], and in the subventricular zone (SVZ) adjacent to the lateral ventricles [10]. In the SGZ, neurogenesis is responsible for the control of spatial learning and memory, and for the integration of cognition and emotion [11], whereas in the SVZ, the newly generated neurons migrate to the olfactory bulb (OB) [12] to participate in the control of olfactory inputs [11]. However, multiple additional brain areas have been reported as having some adult neurogenic activity, namely the hypothalamus [13, 14], striatum [15, 16], substantia nigra [17, 18], cortex [19–21], and amygdala [22]. The discovery of these locations has refuted the idea that the adult human brain is an immutable structure, but rather an organ that possesses a certain level of plasticity that can alter its cellular composition by producing new neurons that are integrated into existing neuronal circuits [23].
These findings had a significant impact on the understanding of neurodegenerative disorders. For example, in conditions such as Alzheimer’s disease (AD), it is believed that a reduction in neurogenesis may contribute to the decline in cognitive function associated with the disease [24, 25]. Importantly, this loss has been reported to occur prior to the deposition of amyloid-β plaques, which is one of the pathological hallmarks of AD [26]. Therefore, there is a compelling need to develop pro-neurogenic therapies, since they could have a significant impact on improving brain function and overall quality of patients’ life [27]. Most of the ongoing research is mainly directed toward promoting endogenous neurogenesis, which involves the manipulation of endogenous mechanisms of the adult brain to repair itself [28, 29]. However, this approach is only effective during the early stages of neurodegenerative diseases [30]. During the advanced stages of these diseases, the damage is typically too extensive for the brain’s innate neurogenic capacity to fully restore its function [25]. In such cases, exogenous therapies can induce neurogenesis by introducing new cell populations into the damaged tissues [31]. Therefore, this review aims to provide a comprehensive overview of the therapeutic potential of these different strategies in pre-clinical studies and clinical research. The review also includes discussions on the most probable modes of action of each strategy, strengths, limitations, and gaps in knowledge to guide future research and clinical practice.
Neurogenic Niches in the Adult Brain
Adult neural stem cells (NSCs) are not ubiquitously distributed throughout the adult mammalian brain but rather restricted to specific areas with a unique and diverse microenvironment that supports neurogenesis, the neurogenic niches [32]. During mammalian brain development, multipotent NSCs expand through symmetric self-renewing divisions to produce two identical daughter cells [33, 34]. Later, they divide asymmetrically to produce NSCs to maintain the stem cell pool, and an intermediate progenitor cell (IPC) that commits to differentiation [35]. Of note, once NSCs have divided and differentiated into various types of neural cells, they will enter apoptosis, terminal division, or senescence, meaning they will either die or stop dividing, resulting in a reduced number of NSCs. This explains why in the adult brain there are only a few NSCs remaining [36]. IPCs undergo a series of cell divisions, promptly expanding and differentiating into neuroblasts or glial cells, depending on the specific signals they receive from their microenvironment [37]. Neuroblasts, which are immature neurons, are lineage-restricted [38]. These cells move from their niche to their intended destination in the brain (which, to some extent, depends on the niche from which they migrate [39]) where they differentiate into mature neurons by establishing connections with other neurons through the formation of synapses [40]. The functional specialization of the new neurons is dependent on the function of the region in which they were integrated [41]. Besides NSCs and IPCs, other cell types coexist in neurogenic niches, such as neuroglia (astrocytes and oligodendrocytes), immune cells (microglia and macrophages), and endothelial cells [38, 42]. The phenotypical richness of these niches creates a special and unique environment that is optimized for the regulation of NSC self-renewal and differentiation. This tight control is assured by the extracellular matrix, short and long-range signalling factors, and direct signaling across gap junctions [43, 44].
The hippocampal SGZ and the SVZ are the two most studied and well-described neurogenic niches in the adult mammalian brain, sometimes being referred to as the “classical” or “traditional” neurogenic zones [7, 45]. In both areas, the neurogenic process has been extensively characterized [46]. Under physiological conditions, neurogenesis in the SGZ generates only one type of neuron, the granule cells, which represent the main glutamatergic excitatory neurons of the DG [47]. In the SVZ, neurogenesis results in granule cells and periglomerular cells, upon migration to the OB [48] (Fig. 1).
Fig. 1.
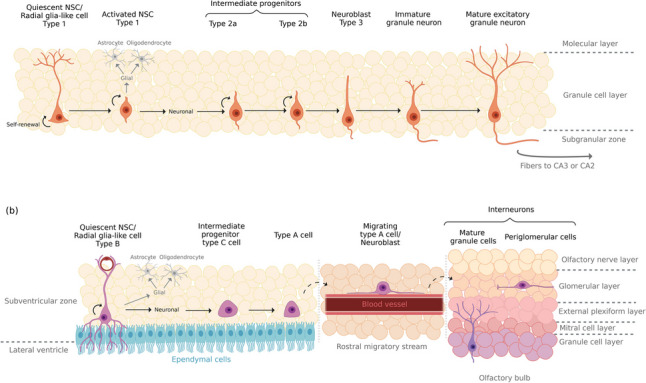
Schematic representation of the different stages of adult neurogenesis in the main neurogenic niches. a In the SGZ, once activated, type-1 cells exit quiescence and may self-renew by symmetric cell division, or undergo asymmetrical division to generate type-2 cells [49]. Of note, these cells can commit to a glial lineage, which can give rise to either astrocytes or oligodendrocytes. But, if committed to a neuronal lineage, type 1 cells can differentiate into two distinct subsets of cells: type-2a and type-2b [50]. The latter are more differentiated and fate-specific and, therefore, they result in type-3 cells, which are neuroblasts with little proliferative activity that experience dendritic and axonal elongation [47]. Neuroblasts further differentiate into immature dentate granule cells. These migrate through the granule cell layer and extend their axonal projections towards the CA3 or CA2 regions of the dentate gyrus, which allows their maturation into excitatory granule neurons [47]. b In the SVZ, an astrocyte-like population of NSCs, named type B cells, are capable of producing neurons, astrocytes, and oligodendrocytes [51]. Upon activation, these cells start to divide asymmetrically for self-renewal or to generate rapidly dividing transit amplifying progenitors. These intermediate progenitor cells are known as type C cells, and they divide three times before differentiating into neuroblasts (type A cells) [52]. Migrating A cells form elongated cell aggregates (migratory chains, not shown) that migrate towards the olfactory bulb along the rostral migratory stream [53, 54]. After arriving at the olfactory bulb, these clusters of neuroblasts dissociate into individual cells and migrate into different structures within the olfactory bulb where they mature [55]. In the granule cell layer, interneurons mature into granule cells, whereas in the glomerular cell layer, they mature into periglomerular cells [48]. Created with Biorender.com
Compared to the hippocampus, the functional relevance of SVZ neurogenesis is poorly understood. Therefore, this topic should be addressed in near future studies, especially the relationship between olfactory and social behaviour and memory.
Even though most of the research on adult neurogenesis has been focused in the SGZ and SVZ, there is limited evidence of other neurogenic niches distributed throughout the adult mammalian brain. To date, most of these proposed neurogenic niches have only been identified in animal studies. For example, neurogenesis in the hypothalamic niche is thought to be functionally related to energy balance and other hypothalamic homeostasis mechanisms, namely fat storage and metabolism [56, 57], as well as sexual and social behaviour [58]. It has been hypothesized that neurogenesis in the amygdala functions as a mechanism for stress regulation and fear condition [59, 60].
Neurogenesis in the substantia nigra is still not fully established, nonetheless, it has been found to be active in animal models of Parkinson’s disease (PD) [18], and in post-mortem brains of humans with the same condition [61] where the additional generation of new neurons may serve as a compensatory mechanism. Additionally, Lie et al. found a population of NG2+ glial progenitor cells in the basal substantia nigra of rodents [62]. These cells have the potential to differentiate into neurons under specific conditions, suggesting the possibility of neurogenesis in that specific brain region. Furthermore, their presence in rodents hints at the potential for neurogenesis in the human substantia nigra as well.
Likewise, adult neurogenesis in the striatum is limited under normal physiological conditions but is increased in response to pathological stimuli, such as stroke, ischemia and injury [45]. Little is known about the functional relevance of neurogenesis in the cortex, cerebellum and habenula. However, it is suggested that neurogenesis in the habenula is responsible for multisensory processing such as visual, olfactory, mechanosensory, and aversive stimuli [63].
The source of the adult-born neurons in these novel neurogenetic niches is still a matter of debate. Some studies report that the new neurons in these niches originated from precursor cells that migrate from distant pools of NSCs, namely the SVZ [64]. While other authors state that the neurogenic niches circuitry is populated by endogenous dividing IPCs [65]. Nonetheless, the emergence of these novel niches offers an exciting possibility for better understanding the functional implications of neurogenesis on the adult mammalian brain in health and disease.
Strategies to Promote Neurogenesis
Impaired adult neurogenesis is a common hallmark in many neurodegenerative diseases, which are a group of nervous system disorders characterized by the progressive loss of neuronal structures and functions, in a process termed neurodegeneration [66]. These include AD, PD, Huntington’s disease (HD), and Lewy bodies dementia [67], among others.
Dysregulated neurogenesis can contribute to the development of these disorders through two main ways: reduction in new-born neurons production, or due to their abnormal maturation and subsequent impaired integration [68]. However, given the capacity of neurogenesis to make up for neuron loss and fix damaged neural circuits, researchers aim to promote or at least preserve, the limited regenerative potential of the adult brain, to potentially treat the neuronal and cognitive loss observed in neurodegenerative diseases [69].
Research has uncovered key factors that boost innate neurogenesis, such as neurotrophic factors [70], neurotransmitters [71], transcriptional programs [72], inflammatory cytokines [73], and hormones [74]. These factors may be modulated through lifestyle changes [75], neurostimulation [76], pharmacological stimuli [77], or hormone therapy [78]. These strategies aim to create an environment that is conducive to neurogenesis and encourage the brain to produce new neurons on its own.
However, at the late stages of neurodegenerative diseases, there is an exacerbated neuronal loss and limited rescuing potential. In these cases, the damage is too severe to be repaired simply by stimulating the brain’s endogenous regenerative mechanisms [79]. Thus, researchers are seeking other strategies to re-establish neurogenesis [69] resorting to exogenous approaches such as cell-based therapies [80]. Therefore, in the following sections, these two different approaches will be explored as a mean to treat or at least attenuate the symptoms of different neurodegenerative diseases.
Endogenous Neurogenesis Stimulation
Different ways to expand the brain’s physiological neurogenic potential are being studied. Indeed, many pro-neurogenic approaches have already proved to increase basal levels of neurogenesis in adult animals with some correlated improved cognition [81]. However, more research is needed to optimize existing therapies and find novel ones. Therefore, in this section, different strategies to stimulate endogenous neurogenesis will be discussed in detail.
Electric and Magnetic Neurostimulation
During the process of brain development and to some extent in adulthood, immature neurons migrate from their origin to their site of maturation, partly due to bioelectricity [76, 82]. Because it is still in its infancy, there is limited evidence of electric, magnetic, or electromagnetic fields being able to promote neurogenesis merely by inducing electrical currents [76]. However, different studies have presented promising results [83]. Additionally, this type of therapy has the advantage of being non-systemic, unlike other treatment options [84].
Currently, there are different techniques to provide brain electrical and magnetic stimulation, some of which have been explored for their potential to promote neurogenesis [85]. Those that have provided the most promising pro-neurogenic results include noninvasive techniques such as transcranial direct current stimulation (tDCS), transcranial magnetic stimulation (TMS), and invasive deep brain stimulation (DBS) [86] (Fig. 2).
Fig. 2.
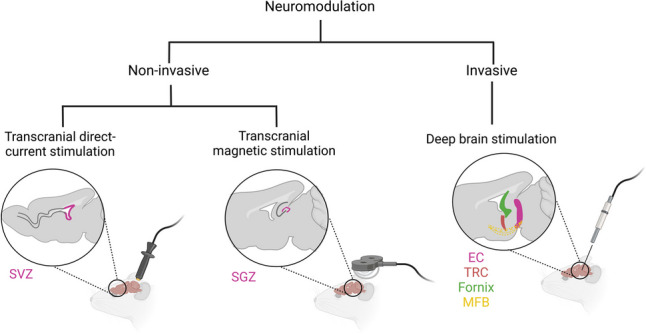
Examples of non-invasive and invasive neurostimulation techniques that stimulate neurogenesis through the modulation of a variety of brain structures. Non-invasive techniques such as transcranial direct-current stimulation (tDCS) and transcranial magnetic stimulation (TMS) have a direct influence on conventional neurogenic niches, such as the subventricular zone (SVZ) of the lateral ventricles, and the subgranular zone (SGZ) of the hippocampus, respectively. Deep brain stimulation, which is an invasive neuromodulation therapy, indirectly stimulates neurogenesis in the SGZ by influencing adjacent structures near the hippocampus. EC, entorhinal cortex; MFB, medial forebrain bundle; TRC, thalamic reticular nucleus. Created with Biorender.com
The underlying mechanisms by which these methods promote neurogenesis remain poorly understood. However, recent advances in the field have been reviewed in [87], providing valuable insight into the possible molecular mechanisms behind neurostimulation in vitro.
TDCS involves the application of low-level electrical currents (typically less than 2 mA) to the scalp [88], which then penetrate the skull to stimulate the underlying brain tissue, promoting neurogenesis in both healthy and pathologic conditions [89]. Different studies with mice and cats reported that tDCS promoted the proliferation of NSCs [90], their differentiation into neuroblasts [91], and enhanced their mobility [92]. Additionally, tDCS prevented Amyloid-β 1-42 (Aβ 1-42) aggregation in AD patients [93]. However, this pro-neurogenic effect only appears to be unanimous in the SVZ, but not SGZ [89, 94].
TMS is a newer form of neurostimulation that induces depolarization or hyperpolarization of neurons through magnetic pulses [95, 96]. Different TMS methods have been studied for their neuromodulatory potential, but low- and high-frequency repetitive TMS has presented the most positive effects on neurogenesis [95, 97], especially in the hippocampus [98]. Immunohistochemistry assays confirmed that repetitive TMS with frequencies in the range of 0.5–25 Hz appears to induce neurogenesis by upregulating the expression of brain-derived neurotrophic factor (BDNF), which is a neurotrophin that promotes NSCs migration and proliferation via its receptor, tropomyosin-related kinase B (TrkB) [99, 100]. Additionally, it also upregulates nerve growth factor (NGF) [101], neurotransmitter GABA [102], synaptic proteins such as synaptophysin [99], and receptors for N-methyl-D-aspartic (NMDA) [101, 103] and α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) [103]. Finally, it enhances the expression of growth factors with vasculotrophic activity, such as vascular endothelial growth factor (VEGF) and transforming growth factor (TGFβ) [103], promoting the proliferation of blood vessels which support the newly generated neurons with blood supply [104]. Although it has been found to increase neurogenesis in animal models of neurodegenerative diseases, its effect in healthy animals is still not well understood, as one study suggests that TMS raises neurogenesis [105], while another reports no effects [106]. Therefore, more studies are needed to clarify this issue.
DBS is an invasive neuromodulatory therapy that consists of implanting electrodes in specific regions of the brain and delivering alternating currents to modulate the brain’s activity [107]. Although this technique has been successfully implemented in patients with PD and other movement disorders for many years, recently, an unexpected improvement in memory was observed during a DBS procedure for obesity treatment [108], which expanded the potential use of DBS to treat disorders affecting the hippocampus [109]. In rodent models, acute stimulation of structures with anatomical connections with the hippocampus has successfully promoted circuit function and neurogenesis, which were correlated with improved exploratory behavior and hippocampal memory [109, 110]. These include structures such as the entorhinal cortex [107, 111], thalamic anterior nucleus [112, 113], thalamic reticular nucleus [114], medial forebrain bundle [115], and fornix [109] (Fig. 2).
Although the above data provide evidence that electric and magnetic stimulation are efficient strategies to treat patients suffering from cognition dysfunctions and that the effects of neurostimulation on neurodegeneration symptoms may involve increasing neurogenesis [116], this is still an active area of research with some mixed results [117, 118]. Therefore, optimal level of stimulation is still being explored [119].
Lifestyle Changes
Following a healthy lifestyle, such as practicing physical exercise, dietary restriction, and being exposed to environmental stimuli are effective and non-evasive strategies that induce innate neurogenesis by promoting the expression of endogenous neurotrophic factors [81, 120].
By studying humans, mice, and rats, it has become clear that in the SGZ, intense exercising (such as swimming and running) leads to an increase in angiogenesis [121], cerebral blood flow [122], blood-brain barrier permeability [123], and the expression of neurotrophins and growth factors [124]. Those include BDNF [125], NGF [126], glial-cell line-derived neurotrophic factor (GDNF) [127], VEGF [128], and insulin-like growth factor 1 (IGF-1) [129], all of which have shown to influence hippocampal neurogenesis [124]. Additionally, physical exercise was also reported to increase hippocampal extracellular levels of the neuromodulator serotonin (5-HT) [130]. Due to changes in vasculature, these molecules are more readily delivered to the hippocampus which ultimately promotes the proliferation of new neurons in the DG [81] (Fig. 3).
Fig. 3.
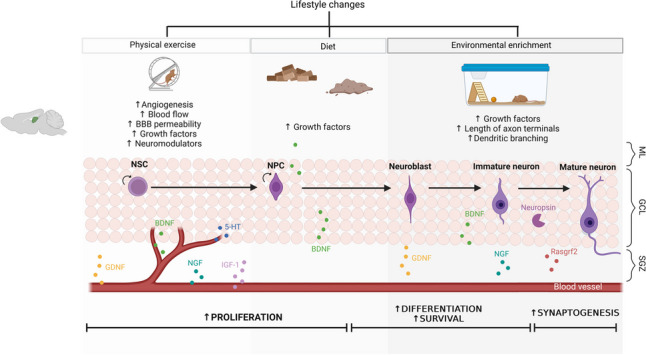
Lifestyle changes that promote hippocampal neurogenesis. These changes, which include intense physical exercise, dietary restriction, and environmental enrichment, have shown a pro-neurogenic impact in different niches, however, a more pronounced effect can be observed in the SGZ. Each activity seems to act on distinct phases of the neurogenic process. Physical exercise, specifically during the proliferation phase, correlates with angiogenesis and increased permeability of the blood-brain barrier. This leads to the release of growth factors and neuromodulators into the SGZ niche, ultimately stimulating the proliferation of NSCs. Dietary changes, such as calorie restriction, stimulate the release of growth factors, particularly BDNF, which plays a crucial role in the progression of the neurogenic process. Environmental enrichment primarily impacts the later phases of neurogenesis. It promotes the survival of newborn neurons and induces morphological changes associated with the maturation of these new cells. BDNF, brain-derived neurotrophic factor; GCL, granule cell layer; GDNF, glial-cell line-derived neurotrophic factor; IGF-1, insulin-like growth factor 1; ML, molecular layer; NGF, nerve growth factor; NPC, neural progenitor cell; NSC, neural stem cell; Rasgrf2, Ras Protein Specific Guanine Nucleotide Releasing Factor 2; SGZ, subgranular zone; ↑, increase. Created with Biorender.com
These results have been associated with improved spatial memory, neuronal plasticity, and learning, which appears to indicate that consistent physical activity may improve cognitive function through the promotion of neurogenesis [131].
While physical exercise improves SGZ local environment, it appears to serve a smaller role in the SVZ [132]. Indeed, there is less evidence of SVZ neurogenesis responding to exercise [133] which is accompanied by some conflicting reports [134, 135]. Surprisingly, a recent study showed that voluntary running-induced neurogenesis in the hypothalamus and ependymal lining of the third ventricle of rats, correlated with the increase of fibroblast growth factor-2 (FGF-2) [136].
Environmental enrichment (EE) is another lifestyle factor with strong connections to hippocampal neurogenesis. It refers to an experimental setting in which animals are confined in an environment that provides sensory, social, and motor stimulation for optimal physiological and psychological well-being [137, 138]. Several studies have proved the neurogenic benefits of EE in rodents [139], which also translate into animal models of neurodegenerative diseases [140]. However, it is crucial to point out that the effects of EE are age-dependent since its neuroprotective strategy depends on its exposure from an early age [141]. Just as physical exercise, EE also increases BDNF [142], NGF [143], GDNF [144], and VEGF [145] which are the most well-established pro-neurogenic factors [146]. But other recently discovered mediators include neuropsin (Klk8) [147] and Ras Protein Specific Guanine Nucleotide Releasing Factor 2 (Rasgrf2) [148]. Finally, this pro-neurogenic strategy has also proved to induce morphological changes in neurons — it lengthens axon terminals [149], enhances dendritic branching [150], and increases synaptogenesis [151] (Fig. 3). However, the exact mechanisms causing these effects remain unknown [152].
In sum, both exercise and EE predominantly promote innate hippocampal neurogenesis; however, physical activity is considered to mainly enhance cell proliferation, while EE promotes new cell survival [153], which overall delays the progression of neurodegenerative diseases [141].
Another environmental factor that can also modulate adult neurogenesis is diet. Neurogenesis is influenced by different aspects, such as caloric intake, meal texture, and content [154]. Briefly, reduced calorie intake has been shown to enhance neuronal proliferation and differentiation in the DG of rodents [155], which correlates with increased BDNF levels [156]. In fact, in experimental animal models of AD, PD, and HD, calorie restriction improved the resistance of neurons to dysfunction and death [157]. But beyond diet, mastication also seems to influence neurogenesis [158]. Indeed, loss of teeth was identified as a risk factor for the development of senile dementia and AD [159]. This is in agreement with different studies that compared rodents fed with a soft and hard diet and found that rodents that chew food have increased progenitor cell proliferation in the DG [160], the hypothalamus [161], and higher neuroblast migration to the OB [162]. Also, in the DG, chewing correlated with increased levels of BDNF [160], and decreased plasma corticosterone levels [163], which is a glucocorticoid that supress the proliferation of neural precursors in the DG [164].
Still regarding diet, several nutrients have been recognized as potential neuromodulators [165]. For example, diets rich in refined sugars and fat have been shown to significantly impair neurogenesis in rodents [166]. Specifically, a high dietary intake of saturated fats has been reported to impair hippocampal neurogenesis by increasing corticosterone levels [154, 167]. Conversely, the consumption of plant and animal foods rich in specific dietary compounds can enhance neurogenesis [165]. These include folate (vitamin B9) [168], cobalamin (vitamin B12) [169], fat-soluble vitamins (e.g., vitamin E [170] and vitamin A [171]), polyunsaturated fatty acids (e.g., omega-3) [172], polyphenols (e.g., curcumin [173] and resveratrol [174]), and minerals (e.g., zinc) [175]. Thus, practicing a healthy and diverse diet may be an effective strategy to promote neurogenesis and consequently improve neurodegeneration in aged individuals or patients suffering from neurodegenerative diseases. However, the effects of dietary compounds on neurogenesis appear to be greatly influenced by their dose [176, 177]. As dietary intake presents challenges in controlling dose, pharmacological supplementation appears to be a more viable approach. Subsequently, the following section will delve into the impact of supplementing these compounds in greater detail.
Pharmacological Manipulation
The pharmacological intervention for impaired neurogenesis in neurodegenerative diseases aims to create a compensatory mechanism for neuronal loss. Currently, there is not a single neurogenic drug for the treatment of neurodegenerative diseases. Nonetheless, researchers are focused on finding safe and effective biochemical and pharmacologic agents that can restore or even increase neurogenesis in patients suffering from neurodegeneration. Table 1 lists the neurogenic stimulatory actions of different pharmacological agents on different animal models of neurodegenerative diseases.
Table 1.
The influence of different pharmacological agents on neurogenesis of animal models with neurodegenerative conditions
| Molecule | Dose, duration, and administration mode | Disease model | Brain tissue | Results | References | ||||
|---|---|---|---|---|---|---|---|---|---|
| Molecular and cellular level | Functional recovery | Molecular mechanism | |||||||
| 1. Bioactive compounds | |||||||||
| Resveratrol | 50 mg/kg/day from gestation day 1 to post-natal day 21, by gavage | SD rats with neurodevelopment issues induced by lead exposure | Hippocampus | ↑ NSC proliferation and ↓ apoptosis of new neurons (↑ marker of NSC proliferation Ki67, and marker for mature neurons NeuN) | ↑ Spatial learning and memory capabilities | Activation of SIRT1/CREB/BDNF signaling pathway | [178] | ||
| Curcumin | 150 mg/kg/day for 42 days, intragastrically | C57BL/6 transgenic APP/PS1 mice model of AD | Hippocampus | ↑ NSC proliferation (↑ cell cycle progression proteins CDK4 and cyclin D1, and marker of NSC proliferation BrdU) | ↑ Spatial learning and memory capabilities | Activation of Notch signaling pathway | [179] | ||
| 25, 50, and 100 mg/kg/day for 30 days, by gavage | Wistar rats’ model of a dementia of AD type, induced with streptozotocin | SGZ | Did not restore neurogenesis (no effect on markers Ki67 and marker of immature neurons DCX) | Prevented impairments in recognition memory but not spatial memory | NA | [180] | |||
| 5, 10, and 20 mg/kg/day, from post-natal day 28 to 49, I.P. | Wistar rats’ model of AD, induced with injections of Aβ (1-42) | SGZ and SVZ | ↑ NSC proliferation and differentiation (↑ genes involved in cell proliferation and neuronal differentiation: Reelin, Nestin, Pax6, neurogenin, neuroD1, neuregulin, neuroligin, and Stat3) | ↑ Learning and memory capabilities | Activation of Wnt/β-catenin signaling pathway | [181] | |||
| Retinoic Acid | 20 mg/kg, 3 times a week, for 4 weeks, I.P. | APPswe/PS1M146V/tauP301L triple transgenic mice model of AD | SGZ |
↓ Neuroinflammation (↓ microglia activation) ↑ NSC proliferation (↑ marker of NSC proliferation PCNA) |
NA | NA | [182] | ||
| Loaded in nanoparticles, 100 ng/ml, single intrastriatal injection | C57BL/6 mouse model of PD, induced with MPTP | Substantia nigra and striatum |
↑ Development and survival of dopaminergic neurons in the substantia nigra (↑ TH staining, and ↑ mRNA and protein expression of Nurr1 and Pitx3) ↑ Dopaminergic fiber striatal innervations in the striatum (↑ TH+ immunoreactive fibers) |
NA | NA | [183] | |||
| Ginsenoside (Rg1) | 20 mg/kg/day for 28 days, I.P. | Brain aged SD rat through D-galactose | Hippocampus |
Protection of the hippocampus against senescence and delay of NSCs’ telomere shortening (↓ biomarkers for aging cells: SA-β-gal and telomerase) ↓ Astrocyte activation and ↓ neuroinflammation (↓ proinflammatory cytokines IL-1β, IL-6, and TNF-α) ↓ Oxidation (↑ antioxidant enzymes SOD and GSH-Px) ↑ NSC differentiation into neurons (↑ markers of NSCs and NPCs Sox2, and Nestin, and differentiation marker β-tubulin III) |
↑ Spatial learning and memory capabilities | NA | [184] | ||
| 20 mg/kg/day for 28 days, I.P. | Brain aged C57BL/6 mice through D-galactose | SGZ |
↑ NSC proliferation (↑ Sox2 and Nestin) ↓ Oxidation (↓ markers of oxidative stress MDA and ROS, but ↑ of antioxidant enzymes SOD and GSH-Px) |
↑ Spatial learning and memory capabilities | Inhibition of the Akt/mTOR signaling pathway | [185] | |||
| Ginsenoside (Rb1) | 10 mg/kg/day for 30 days, I.P. | SD rats’ model of AD, induced with injections of Aβ (1-40) | All brain tissue | ↑ NSC proliferation and differentiation (↑ markers of NSCs and NPCs, Nestin and GFAP, and marker of immature neurons NSE) | NA | NA | [186] | ||
| Gintonin | 50 or 100 mg/kg/day for 21 days, orally | C57BL/6 double transgenic AβPPswe/PSEN1dE9 mice model of AD | Hippocampus | ↑ NSC proliferation and differentiation into both neurons and astrocytes (↑ LPA receptors, and markers BrdU and NeuN) | NA | NA | [187] | ||
| 50 or 100 mg/kg/day for 4 weeks, orally | Brain aged C57BL/6 mice through D-galactose | Hippocampus |
↑ NSC proliferation, differentiation, and neuronal maturation (↑ Ki67, BrdU, DCX and NeuN) ↑ Long-term potentiation ↑ Activation of CREB ↑ LPA1 receptor ↓ Oxidation (↓ ROS) |
↑ Object location memory | NA | [188] | |||
| Oleanolic acid | 10 mg/kg/day for 28 days, I.P. | APP/PS1 double transgenic mice model of AD | Hippocampus |
↑ NSC proliferation (↑ Sox2, BrdU, and NeuN) ↑ Number of healthy neurons |
↑ Spatial learning and orientation ↓ Anxious behavior |
Activation of Wnt/GSK-3β/β-catenin signaling pathway | [189] | ||
| Protopanaxadiol | 10 mg/kg/day for 28 days, I.P. | APP/PS1 double transgenic mice model of AD | Hippocampus |
↑ NSC proliferation (↑ Sox2, BrdU, and NeuN) ↑ Number of healthy neurons |
↑ Spatial learning and orientation ↓ Anxious behavior |
Activation of Wnt/GSK-3β/β-catenin signaling pathway | [189] | ||
| Baicalin | 10 mg/kg/day for 30 days, I.P. | SD rats’ model of AD, induced with injections of Aβ (1-40) | All brain tissue | ↑ NSC proliferation (↑ Nestin) | NA | NA | [186] | ||
| Rosmarinic acid | 16 mg/kg/day for 15 days, orally | BALB/c mice model of AD, induced with injections of Aβ (1-42) | Hippocampus |
Preservation of neurons morphology and density ↑ Cells’ proliferation, migration, maturation (↑ DCX, NeuN, and synaptic marker synaptophysin) |
↑ Spatial learning and memory capabilities ↓ Depressive and anxious behavior |
NA | [190] | ||
| Ursolic acid | 40 mg/kg/day for 15 days, orally | BALB/c mice model of AD, induced with injections of Aβ (1-42) | Hippocampus |
Preservation of neurons morphology and density ↑ Cells’ proliferation, migration, maturation (↑ Ki67, DCX, NeuN, and synaptic marker synapsin I) |
↑ Spatial and object recognition memory ↓ Depressive and anxious behavior |
NA | [190] | ||
| Egb 761 | Ad libitum daily intake of 100 mg/kg | C57BL/6J double transgenic APPswe/PS1ΔE9 mice model of AD | Hippocampus |
↑ NSC proliferation and maturation of new-born neurons (↑ BrdU, NeuN, and immature neuronal marker NCAM-180, but not GFAP) ↑ pCREB ↓ Aβ aggregates |
NA | NA | [191] | ||
| Butylphthalide | 15 mg/kg, 5 days/week, for 12 weeks, by gavage | B6C3 double transgenic APPswe/PSEN1dE9 mice model of AD | Hippocampus |
↑ NSC proliferation (↑ marker BrdU and neurotrophins BDNF, and NGF) ↑ Activation of TrkA and TrkB receptors, CREB and Akt |
↑ Spatial learning | Activation of BDNF/TrkB/CREB/Akt signaling pathway | [192] | ||
| 2. Antidepressants | |||||||||
| Fluoxetine | 20 mg/kg/day for 10 weeks, I.P. | R6/1 transgenic HD mice | Hippocampus and SVZ |
↑ NSC differentiation (↑ BrdU and NeuN) ↑ NSC survival, but no effect on the net increase of new cells ↑ Volume of the DG |
↑ Spatial memory ↓ Depressive behavior No effect on motor activity |
NA | [193] | ||
| 10 mg/kg/day for 15 days, orally | C57BL/6 mice model of PD, induced with injections of rotenone | Hippocampus, prefrontal cortex, and substantia nigra |
↓ Neuroinflammation in the hippocampus and prefrontal cortex (↓ proinflammatory cytokines IL-1β, phospho-NF-kB) ↓ Hippocampal cell death (↓ oxidation marker iNOS, and ↓ cell death related proteins PARP-1 and caspase-3) ↑ NSC proliferation and synaptic plasticity in the hippocampus (↑ Ki67, and markers of synaptic plasticity pCREB and BDNF, but no effect on NeuN) No effect on dopaminergic neurons No effect on TH staining in the substantia nigra |
↓ Depressive behavior ↓ Motor deficits |
NA | [194] | |||
| 18 mg/kg/day for 11 months, orally | PS1M146V/APPswe/tauP301L triple transgenic mice model of AD | Hippocampus | No effect on neurogenesis (no effect on BrdU, NeuN, or BDNF) | Motor impairment and no effect on neuronal differentiation | NA | [195] | |||
| 10 mg/kg/day for 5 weeks, I.P. | APPswe/PSEN1dE9 double transgenic mice model of AD | Hippocampus |
↑ Neurons in the DG, but no effect in the CA1 and CA3 regions ↓ Aβ aggregates Inhibition of GSK-3β and stabilization of β-catenin |
↑ Spatial learning | Activation of Wnt signaling pathway | [196] | |||
| Amitriptyline | 1 mg/kg/day for 4 months, orally | PS1M146V/APPswe/tauP301L triple transgenic mice model of AD | Hippocampus and cortex |
↑ NSC differentiation (↑ BrdU, and NeuN in the DG) ↑ NSC maturation (↑ synaptic markers synaptophysin, synapsin I, PSD95, and spinophilin on the hippocampus, but no effects on the cortex) Direct activation of TrkB receptor ↑ Aβ aggregates (insoluble form) in the hippocampus, subiculum, CA1, and amygdala and tau deposition (insoluble form) in the hippocampus |
↑ Spatial learning and memory | NA | [197] | ||
| 10 mg/kg/day for 38 days, I.P. | Wistar rats’ model of LBD, injected with the SNCA gene and Aβ (1-42) | Hippocampus and substantia nigra |
↑ NSC proliferation in the DG (↑ BrdU) ↑ Neuronal densities in the CA1 region and substantia nigra (↑ TH and Nissl staining) ↓ Accumulation of α-synuclein in the DG |
↑ Object recognition | NA | [198] | |||
| 16 mg/kg/day for 6 weeks, orally | B6C3 transgenic N171-82Q mice model of HD | Striatum, cortex and whole brain |
↑ NSC differentiation and maturation in all tissues In the striatum: ↑ Neurotrophin BDNF via CREB ↑ DCX and NeuN ↑ Presynaptic markers (synaptophysin) but no effect on postsynaptic markers (PSD95) In both tissues: ↓ mHTT protein in the striatum and cortex Whole brain: ↑ Expression of factors related to neuronal development (Sparc, Nyap) ↓ Neurodegenerative cross-linking (Tgm2) |
↑ Motor function | Activation of the BNDF-TrkB signaling pathway | [199] | |||
| Lithium | 10 mg/kg/day for 5 weeks, I.P. | Transgenic CRND8 mice model of AD | Hippocampus and cortex |
↑ NSC proliferation and differentiation in the SGZ (↑ BrdU, DCX, and NeuN) ↑ Survival of new-born neurons and migration into the DG ↓ Glia activation and neuroinflammation (↓ IBA-1 and GFAP) ↓ Aβ and tau aggregates in both tissues Inhibition of GSK-3β |
↑ Spatial learning and memory in 3- but not 7-month old Tg mice | Activation of Wnt pathway | [200] | ||
| 3. Small molecules | |||||||||
| P7C3-S243 | 10 mg/kg/day for 9 and 18 months, I.P. | Transgenic F344 rats’ model of AD | Hippocampus and cortex |
↑ NSC proliferation and differentiation (↑ BrdU and NeuN) No effect on glia activation ↓ Neurodegeneration (↑ number of neurons) No effect on Aβ and tau deposition, or neuroinflammation |
↓ Depressive behavior ↑ Relearning abilities |
NA | [201] | ||
| NNI-362 | 3 mg/kg/day for 4 weeks, I.P. | Ts65Dn mice model of early-stage AD | Hippocampus |
↑ NSC proliferation and survival (↑ Ki67 and BrdU) ↑ Phosphorylation of p70S6 kinase |
↑ Object recognition | Suggested activation of mTOR signaling pathway | [202] | ||
| 4. Glutamate receptor antagonists | |||||||||
| Dizocilpine (NMDAr antagonist) | 0.2 mg/kg/day for 28 days, I.P. | SD rats’ model of PD, induced with injections of 6-OHDA | Hippocampus, striatum, and substantia nigra |
↑ Hippocampal NSC self-renewal capacity, proliferation, long-term survival, and differentiation (↑ Sox2, Ki67, BrdU, Nestin, and NeuN) ↑ Dendritic arborization of hippocampal immature neurons ↑ Neuronal migration in the hippocampus ↑ Dopaminergic neurons in the striatum and substantia nigra (↑ transcription factors involved in the development of dopaminergic neurons Nurr-1 and Pitx-3) No effect on the proliferation of oligodendrocyte progenitor cells in the hippocampus (no alterations of marker Olig-2) ↑ Myelination (↑ MBP expression) |
↓ Depressive and anxious behavior ↑ Motor function |
Activation of Wnt/β-catenin signaling pathway | [203] | ||
| Memantine (NMDAr antagonist) | 20 mg/kg/day for 4 months, orally | Tg4-42hom mice model of AD | Hippocampus |
↑ New-born neurons (↑ DCX) ↑ Pyramidal neurons in the CA1 region |
↑ Object recognition and spatial memory No effect on anxiety levels ↓ Motor deficits |
NA | [204] | ||
| BCI-632 (Group II mGlur antagonist) |
Daily oral administration for 3 months Dose NA |
Dutch APP transgenic E693Q mice model of AD | Hippocampus and cortex |
↑ Surviving, new-born neurons in the hippocampus (↑ BrdU and NeuN) ↓ Aβ monomer and oligomer levels |
↓ Anxious behavior ↑ Working memory, spatial learning, and social recognition |
NA | [205] | ||
| 5. Other drugs | |||||||||
| Metformin | 200 mg/kg/day for 15 days, orally | C57BL/6 mice model of PD, induced with injections of rotenone | Hippocampus, prefrontal cortex, and substantia nigra |
↓ Glial cells and neuroinflammation (proinflammatory cytokines IL-1β, phospho-NF-kB) in the hippocampus and prefrontal cortex No significant reduction on hippocampal cell death (no effect on PARP-1 and caspase-3) ↑ NSC proliferation but no effect on the number of mature neurons (↑ Ki67, but no effect on NeuN) in the hippocampus No effect on synaptic plasticity No (unaltered pCREB and BDNF levels) in the hippocampus ↑ Dopaminergic neurons (↑ TH staining) in the substantia nigra |
↓ Depressive behavior ↓ Motor deficits |
NA | [194] | ||
| 500 mg/kg/day for 21 days, orally | Swiss-albino mice model of PD, induced with injections of MPTP and probenecid | Midbrain, substantia nigra |
Protection of dopaminergic neurons in the substantia nigra through antioxidant defense (↑ SOD, CAT, and GSH) and reduced oxidation (↓ LPO) in the midbrain ↑ Dopaminergic neurons (↑ TH staining and BDNF levels) in the substantia nigra |
↓ Motor deficits | NA | [206] | |||
| 200 mg/kg/day for 14 days, I.P. | B6C3 double transgenic APPswe/PS1dE9 mice model of AD | Hippocampus |
↑ Number of mature neurons (↑ NeuN) in the CA1 region ↓ Aβ plaques ↓ Neuroinflammation (↑ anti-inflammatory IL-4, ↓ pro-inflammatory IL-1β, TNF-α) ↑ p-AMPK and ↓ p-mTOR, p-26K, p-P65 NF-kB, and Bace-1 in microglia and astrocytes ↓ Neuronal apoptosis ↑ NSC proliferation, differentiation, and migration (↑ BrdU and DCX) |
↑ Spatial memory | Activation of AMPK/mTOR/S6K/Bace1 and AMK/P65 NF-kB signaling pathways | [207] | |||
| Sovateltide | 0.005 mg/kg three times at 2-h intervals on days 1, 3, and 6 every month until endpoints (3-, 6-, and 12-months age), intravenous | APP/PS1 double transgenic mice model of AD | Hippocampus |
↑ Neurogenesis (↑ NeuroD1, DCX, NeuN and synaptic proteins synapsin I, synaptophysin, and PSD95) ↓ Aβ plaques ↑ Mitochondrial fusion proteins (Mfn1, Mfn2, Opa1) but ↓ in fission proteins (Drp1, Fis1) |
↑ Learning and memory | Activation of ETB receptor signaling pathway | [208] | ||
| Panobinostat (LBH589) | 0.001, 0.01, 0.1 and 1 mg/kg, every 48 h, from post-natal day 8 to 20, I.P. | Double transgenic mice model of HD | Striatum |
↑ Neurogenesis (↑ DCX and ↑ rostral migratory stream volume) ↑ Dopamine pathway related-gene (RasGRP2) ↑ Striatal development markers (DARPP-32 and PPP1R7) |
↓ Anxious behavior | NA | [209] | ||
Abbreviations: AD, Alzheimer’s Disease; Akt, protein kinase B; AMPK, AMP-activated protein kinase; Aβ, Amyloid-β; APP, amyloid precursor protein; Bace 1, Beta-secretase 1; BDNF, brain-derived neurotrophic factor; BrdU, bromodeoxyuridine; CA1, cornu ammonis 1; CA3, cornu ammonis 3; CAT, catalase; CDK4, cyclin-dependent kinase 4; CREB, cyclin-dependent kinase 4; CREB, cAMP response element-binding protein; DCX, doublecortin; DG, dentate gyrus; Drp1, dynamic-related protein 1; ETB, endothelin receptor type B; Fis 1, mitochondrial fission 1 protein; GFAP, glial fibrillary acidic protein; GSH, glutathione; GSH-Px, glutathione peroxidase; Gsk-3β, glycogen synthase kinase-3β; HD, Huntington’s Disease; IBA-1, ionized calcium-binding adaptor molecule 1; IL-1β, interleukin 1β; IL-4, interleukin 4; IL-6, interleukin 6; iNOS, nitric oxide synthase; I.P., intraperitoneal injection; LBD, Lewy Body dementia; LPA, lysophosphatidic acid receptor; LPO, lipid peroxidation; MBP, myelin basic protein; MDA, malondialdehyde; Mfn1, mitofusin 1; Mfn2, mitofusin 2; mGlur, metabotropic glutamate receptor; mHTT, mutant huntingtin protein; MPTP, 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine; mTOR, mammalian target of rapamycin; NA, not available; NeuroD1, neuronal differentiation 1; NF-kB, nuclear factor kB; NGF, nerve growth factor; NMDAr, N-methyl-D-aspartate receptor; NPCs, neuronal progenitor cells; NSC, neuronal stem cells; NSE, neuron-specific enolase; Nurr-1, nuclear receptor-related transcription actor 1; Nyap, neuronal tyrosine-phosphorylated phosphoinositide-3-kinase; Olig-2m oligodendrocyte transcription factor; Opa1, optic atrophy 1; P65, transcription factor p65; p70S6, ribosomal protein p65; p-AMPK, phosphorylated AMP-activated protein kinase; PARP-1, poly [ADP-ribose] polymerase 1; Pax6, paired box protein pax-6; pCREB, phosphorylated cAMP response element-binding protein; PD, Parkinson’s Disease; phospho-NF-kB, phosphorylated nuclear factor kB; Pitx-3, pituitary homeobox 3; p-mTOR, phosphorylated mammalian target of rapamycin; PS1, mutant human PS1 (PS1ΔE9; PSD95, postsynaptic density protein 95; ROS, reactive oxygen species; S6K, s6 kinase; SA-β-gal, senescence-associated β-galactosidase; SD, Sprague Dawley; SGZ, subgranular zone; SIRT1, sirtuin1; SNCA, synuclein alpha; SOD, superoxide dismutase; Sox2, sex determining regions Y-box 2; Sparc, secreted protein acidic and rich in cysteine; Stat3, signal transducer and activator of transcription 3; SVZ, subventricular zone; Tgm2, transglutaminase 2; TH, tyrosine hydroxylase; TNF-α, tumor necrosis factor α; TrkA, tropomyosin receptor kinase A; TrkB, topomyosin receptor kinase B; 6-OHDA, 6-hydroxydopamine; ↑, increase; ↓, decrease
A variety of bioactive compounds have been extensively studied due to their neurogenic and neuroprotective potential in animal models of neurodegenerative diseases [210]. These are naturally occurring substances found in different plant products [211]. One of their advantages over conventional drugs is their natural origin which makes them safer [212], but what has sparked more interest was the discovery of their ability to tackle neurodegeneration by multiple mechanisms of action, such as reduction of oxidative stress, neuroinflammation, and inhibition of apoptosis [213]. For example, resveratrol, curcumin, retinoic acid, ginsenoside, and gintonin are some of the bioactive compounds that share these properties and have been widely explored for their potential to alleviate symptoms of diseases marked by decreased neurogenesis [178, 182, 213–215]. These antioxidants can reduce oxidative stress either by scavenging oxidants [185, 188, 215–217] or by activating the transcription factors Nrf2 [218, 219] and HO-1 [219] which are regulators of oxidation. By reducing oxidation levels, there is an increase in the expression of neurotrophins and the survival of NSCs, which allows neurogenesis to proceed smoothly [165]. But these substances can also suppress inflammation by modulating different signaling pathways. For example, the main anti-inflammatory mechanism of resveratrol is its ability to activate the SIRT1/CREB/BDNF signaling pathway [178, 220, 221], which can promote the neuronal survival by inhibiting the activation of microglia and astrocytes [221]. These cells promote neuroinflammation through the release of pro-inflammatory cytokines and chemokines that, in turn, activate more immune cells and lead to a self-perpetuating cycle of inflammation that can cause neuronal damage and death [182, 222]. Another way that these compounds exert their pro-neurogenic potential is through their ability to inhibit apoptosis since they have been shown to modulate apoptotic-related signaling pathways, such as the AKT/mTOR [185]. Substances like curcumin, oleonic acid, and butylphthalide have been shown to activate Wnt/β-catenin [181], Wnt/GSK3β/β-catenin [189], and PI3/AKT [192] signaling pathways, respectively, which are involved in promoting NSC proliferation and survival, and the differentiation of neurons.
Besides these properties, another advantage of bioactive compounds is their high target specificity [223, 224]. For example, multiple studies reported that gintonin has a high target specificity towards lysophosphatidic acid (LPA) receptors - particularly LPA1 and LPA2 receptors [187] – even higher than other known LPA receptor agonists, such as LPA itself [225]. This suggests that gintonin may be a promising strategy to promote neurogenesis, since LPA receptor activation regulates the balance between NSC proliferation and differentiation [226, 227] and has been implicated in the regulation of tau phosphorylation, a key event in the development of AD [228].
One of the shortcomings of these compounds is their low bioavailability [229, 230]. This may have contributed to the limited efficacy of curcumin previously reported [180]. However, recent studies have shown that this issue can be overcome by encapsulating bioactive compounds in functionalized nanoparticles. Indeed, curcumin-loaded nanoparticles significantly increased NSC proliferation and neuronal differentiation in both the hippocampus and SVZ compared to uncoated bulk curcumin [181]. Similarly, in a mouse model of PD (induced with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, MPTP) treated with retinoic acid-nanoparticles was found to be more effective in promoting neurogenesis compared to solubilized retinoic acid [183]. Finally, it is noteworthy that some studies listed in Table 1 reported a contribution of bioactive compounds on neurogenesis only when applied to the pathologic animal models. For instance, the application of rosmarinic and ursolic acid has only promoted neurogenesis in the presence of the neurodegenerative disease, whereas they had no effect when applied in healthy subjects [190]. Some studies also reported that bioactive compounds demonstrated more pro-neurogenic potential than conventional drugs commonly used for the treatment of neurodegenerative diseases, such as donepezil and memantine [190]. Besides donepezil, other synthetic drugs developed to treat specific diseases rather than neurodegeneration presented promising results in promoting neurogenesis as well; these include antidepressants and diabetes medication, which demonstrate pro-neurogenic potential along with their primary therapeutic effects [194].
Evidence reported in Table 1 suggests that many antidepressants target molecular hallmarks of neurodegenerative diseases. Specifically, they have shown that antidepressants can reduce the accumulation of mutant huntingtin (mHTT) [199], α-synuclein [198], and Aβ [196], in animal models of HD, LBD, and AD, respectively, playing a pivotal role in the progression of neurodegeneration [231–233]. While these studies provided limited insight into the precise mechanisms by which antidepressants may reduce the pathogenic burden of the aforementioned proteins, they did reveal a decrease in neuroinflammation [200], an increase in neurotrophins [199], and the activation of BDNF/TrkB [199] and Wnt [196, 200] signaling pathways which, altogether, may have helped reduce their accumulation and consequently, recovery of neurogenesis. In particular, the continued release of pro-inflammatory cytokines from microglia exacerbates neuroinflammation and contributes to this buildup [234–236]. Metformin, an antidiabetic drug, also presented anti-inflammatory and pro-neurogenic results in animal models of PD and AD [194, 206, 207]. In a mice model of AD, metformin protected neurons against apoptotic cell death, and increased neuronal viability; however, this effect was blocked when adenosine monophosphate-activated protein kinase (AMPK) activity was inhibited, suggesting that the pro-neurogenic action of metformin is dependent of AMPK [207], a key enzyme involved in cellular energy regulation and a common target of metformin to tackle diabetes-2 [237]. The authors suggested that hippocampal AMPK activation inhibited mammalian target rapamycin (mTOR) activity, by inhibiting its downstream target, p70S6 kinase [207]. In AD, mTOR enhances Aβ deposition, while AMPK activation decreases mTOR signaling to facilitate autophagy and promote lysosomal degradation of Aβ [238–240]. Other studies have reported that AMPK regulates neuroinflammation and reduces oxidative stress by inhibiting the nuclear factor kappa B (NF-kB), which suggests that the important role of AMPK in neuroprotection may not be only restricted to AD, but also other neurodegenerative diseases [241, 242]. In short, the endeavor to uncover the impact of metformin on neurogenesis has yielded compelling evidence implicating AMPK as a critical mediator in this process, and therefore, it should be regarded as a paramount molecular target to promote neuronal regeneration [243, 244].
Although both antidepressants and metformin provided striking results in stimulating neurogenesis, Mendonça et al. found that the most favorable results in animal models of PD were obtained through the concurrent administration of fluoxetine (Prozac) and metformin [194]. Consequently, it is crucial not to disregard alternative combinatory strategies, as they may hold greater potential.
Small molecules, such as NNI-362 and P7C3-S243, act by modulating specific signaling pathways within the brain that are involved in regulating the growth and survival of neurons [201, 202]. Even though P7C3-S243 did not reduce the pathological features of AD, it improved the behavior of neurologically impaired rats [201]. Additionally, NNI-362 has also emerged as a promising pharmacological agent for promoting neurogenesis. NNI-362 works as a p70S6 kinase stimulator [202] which, as mentioned earlier, is a downstream substrate of mTOR and can stimulate this pathway. While there is some evidence to suggest that the overactivation of mTOR may contribute to AD pathology, its activation can also have positive effects on neurogenesis, since it is also involved in regulating a wide range of cell activities, such as cell growth, proliferation, apoptosis, and autophagy [202, 245]. Indeed, NNI-362 stimulated the phosphorylation of p70S6, which promoted NSC proliferation and differentiation, which ultimately resulted in the reversal of cognitive deficits in aged mice [202]. Of note, other small molecules, such as WAY-316606 proved its pro-neurogenic potential in homeostatic conditions by inhibiting SFRP1 function, which is crucial for the activation of Wnt and Notch pathways and the subsequent activation of neural progenitor cells [246], which may also hold promise to alleviate the symptoms of neurodegenerative diseases.
The role of glutamate receptors in neurogenesis is complex and thus, their precise role in neurodegenerative conditions is not fully elucidated. Despite this, several studies have demonstrated that their inhibition (particularly NMDA [203, 204] and Group II metabotropic glutamate receptors (mGlur) [205]) can increase neurogenesis, suggesting that glutamatergic signaling negatively regulates the process of generating new neurons [203]. The results provided in Table 1 indicate that glutamate antagonists improved the cellular and behavioral function of animal models of neurodegenerative diseases. Particularly, dizocilpine seems to modulate neurogenesis through the activation of the Wnt/β-catenin signaling pathway [203]. However, this finding alone does not fully address the question of how controlling glutamate receptors modulates neurogenesis, and, therefore, further research is required to shed more light on this topic.
Finally, alternative pharmacological approaches have been used to indirectly stimulate neurogenesis. Rather than directly targeting neurons or their progenitors, studies such as those provided by Briyal and colleagues, have been aiming to manipulate other factors that can potentially impact the process of neurogenesis [208, 247]. One such approach involves the modulation of angiogenesis. Drugs like sovateltide (IRL-1620, SPI-1620, or PMZ-1620) have been used for this purpose since it induces both vascular and neuronal modeling [247]. Sovateltide is an endothelin B receptor agonist that has been previously reported to have anti-apoptotic activity [248], increase cerebral blood flow [249], and increase neurovascular repair and remodeling or neuroregeneration, particularly in the SVZ [250]. The activation of these receptors, which are expressed in endothelial, neuronal, and glial cells in the central nervous system [247], results in increased angiogenesis and other neurovascular growth factors in adult NSCs niches, leading to enhanced proliferation and migration of new neurons [247].
Overall, by a brief analysis of Table 1, it becomes clear that there are far more studies focused on promoting neurogenesis in the SGZ of animal models with neurodegenerative diseases, in comparison to the SVZ. It is reasonable to focus on the hippocampus since neurogenesis within this area is critical for learning and memory, which are impacted by neurodegenerative diseases [251]. Nonetheless, in order to grasp the full potential of pro-neurogenic therapies, it is imperative that future research also direct their attention toward the SVZ and other niches, which also possess the ability to regenerate the NSC population [252].
Hormone Therapy
Hormone therapy has also been proposed as a possible strategy to promote neurogenesis [253, 254], since hormones — including gonadal hormones, glucocorticoids, and specific metabolic hormones [255] — influence different aspects of neurogenesis, such as proliferation and/or survival of new neurons [81, 256]. This new therapy has emerged as a result of recent findings of hormone dysregulation in neurodegenerative diseases, such as HD [257], AD [258], and PD [259].
An early study has found androgen receptors distributed in many brain areas, especially in the hippocampus and amygdala [260]. At the cellular level, they were found on axons and dendrites, suggesting that androgens (testosterone, dihydrotestosterone, and dehydroepiandrosterone) may have an essential role in neuronal function [261, 262]. Indeed, it has been proposed that in adult males (but not females [263]) androgens enhance hippocampal neurogenesis through the promotion of neuron survival [264]. Specifically, androgens bind to androgen receptors in the CA3 region, which subsequently triggers the expression of survival factors that are retrogradely transported to the newborn neurons in the DG, ultimately promoting their survival and maturation [264, 265]. Despite this, there is limited evidence for the positive effects of androgens in the neurogenesis of experimental models of neurodegenerative diseases. In fact, testosterone presented a limited effect in rescuing neurogenesis in an animal model of HD [257].
A pro-neurogenic potential has also been attributed to a wide variety of female reproductive hormones, including estrogen, progesterone, and prolactin [256]. For example, estrogens (estrone, 17β-estradiol, and estriol) have been shown to play a crucial role in regulating the balance between proliferation and differentiation of NSCs through estrogen-dependent signaling pathways [266], including the MAPK/ERK pathway, which is associated with an increase in neuronal survival [267]. This group of hormones also acts as antioxidants, anti-apoptotic, and induces the expression of growth factors, influencing the neurogenic processes [266, 268]. Finally, they have also been reported to modulate spines and synapse formation which is necessary for the survival of new neurons [269]. This might explain why women who experience premature menopause and do not receive estrogen treatment are at a higher risk of developing AD [270]. Indeed, 17β-estradiol treatment during the early stages of AD pathology in female mice increased the levels of markers of NSC proliferation (BrdU) and mature neurons (NeuN) in the hippocampus, which was supported by the recovery of cognitive function [271].
Other hormones related to reproductive health have also presented multifaceted neuroprotective and neurodegenerative processes, including progesterone [272], its metabolite allopregnanolone [273, 274], and progestin [275, 276]. Progesterone exerts its neural effects through multiple signaling pathways, which include binding to specific progesterone receptors that regulate gene expression [267]. In particular, membrane progesterone receptor β (mPRβ/Paqr8) promotes neurite outgrowth via extracellular signal-regulated kinase (ERK) phosphorylation [277]. Some of the pro-neurogenic effects of progesterone are partially mediated by its neuroactive metabolites, including allopregnanolone [78]. Allopregnanolone has a high affinity for GABAA receptors, specifically the GABA-chloride channel complex, which induces membrane depolarization upon activation. This ultimately leads to the activation of kinases that regulate the expression of genes and proteins involved in the cell cycle of NSCs, promoting their regeneration [278]. Despite this, a phase 3 clinical research trial showed a 100% failure rate for progesterone as a treatment for traumatic brain injury [279]. But, because traumatic brain injury is a very heterogeneous and complex disorder [280], these results should not dismiss the potential that progesterone has previously shown [281]. Alternatively, the pro-neurogenic potential of some progestins, synthetic analogs of progesterone with a similar mode action [280], have been thoroughly investigated, with Nestorone receiving particular attention due to its high selectivity for progesterone receptors, greater than progesterone itself [275, 280].
Naturally occurring or synthetic estrogens and progesterone are not the only hormones capable of stimulating neuronal proliferation. Prolactin has emerged as another hormone with pro-neurogenic effects based on findings indicating increased NSCs in the SVZ of female mice during pregnancy and lactation [282]. Further, administration of a prolactin analog (palm11-PrP31) resulted in elevated neurogenesis in the hippocampus of male mice models of AD [283]. While the evidence is promising, further research is required to fully comprehend the potential of prolactin as a strategy to promote neurogenesis [284, 285].
It is worth noting that some of the reported studies proved that these hormones can only promote the innate regenerative capability of a pathological brain during the early to mid-stages of the disease [286]. Additionally, hormone therapy to stimulate neurogenesis remains controversial, not only because it is a complex medical intervention but also because different studies have reported stimulatory and inhibitory effects [287, 288]. This may be attributed to the complex influence of different factors in hormones, such as gender, age, genetics, and environmental influences [81].
Gene-Based Therapies
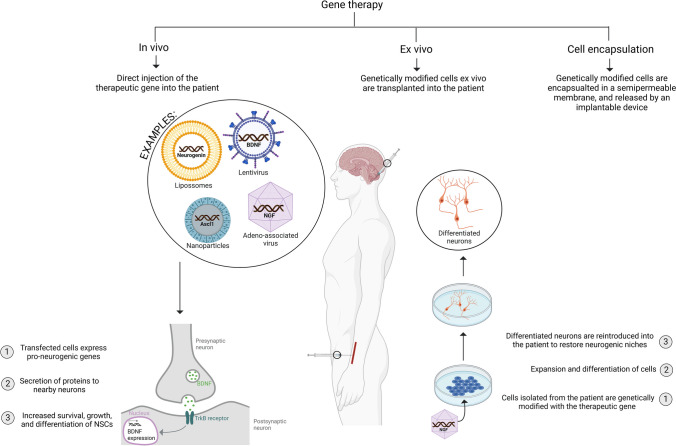
Gene-based therapies, which are based on genome manipulation, have emerged as a way to promote neurogenesis by the modulation of gene expression in NSCs and other cell types. This can be performed through a repertoire of gene-manipulation tools currently available such as viral and non-viral delivery strategies (nanoparticles, ribonucleoproteins, electroporation, etc.) [289–291]. These strategies enable the introduction of therapeutic genes into the target cells, either in vivo or ex vivo, each offering distinct mechanisms for promoting neurogenesis [292] (Fig. 4).
Fig. 4.
Gene-based pro-neurogenic therapies. Through different viral and non-viral gene carriers, therapeutic genes may be inserted into the patient, where they will selectively target neurons or their progenitors’ cells, transfect them, and integrate the gene of interest onto the cells’ genome, to promote their survival, growth, or differentiation. Another approach is to induce neurogenesis outside the organism. In the ex vivo strategy, cells may be transfected with the therapeutic gene, which will induce their differentiation. Once differentiated, cells can be reinserted into the targeted niche, where they will either repopulate the damaged neurogenic niche, or stimulate the differentiation of surrounding cells. BDNF, brain derived neurotrophic factor; NGF, nerve growth factor. Created with Biorender.com
In the in vivo approach, innate neurogenesis is stimulated through the injection (localized or systemic delivery routes) of the aforementioned gene-manipulation tools into the organism, which allows the therapeutic genes to be delivered and expressed within the target neurogenic niches [293]. These genes can then enter NSCs, where they modulate gene expression, promoting their proliferation, survival, or differentiation into neurons [294]. These include genes (most commonly neurotrophins [295, 296]) that control cell fate decisions, including the Trk, Wnt, and bone morphogenetic protein (BMP) signaling pathways [297, 298]. Other genes have been studied for their potential of regulating neurogenesis, such as BRI2 [299, 300], Neurogenin 1 and 2 [301, 302], Ascl1 [303], among others [294], which may also hold promise in gene therapy applications.
Other therapeutic approaches based on gene delivery were already successfully applied in the treatment of several neurodegenerative diseases, including AD, PD, and HD [304]. For example, BDNF delivery by injections of recombinant adeno-associated virus enhanced the recruitment of progenitor cells to the lesioned areas of adult rat brains and promoted neuronal differentiation [305]. Similarly, a single injection of adenoviral BDNF also increased the recruitment of new neurons to the OB and striatum of adult rats [296]. Moreover, induction of striatal neurogenesis by adenoviral-mediated overexpression of BDNF, correlated with delayed motor impairment, and improved survival in a murine model of HD [306]. Viral-induced overexpression of NGF was reported to promote neurogenesis in two different rodent models of ischemic brain injury [307]. Furthermore, IGF-I gene therapy using an adenoviral vector increased the number of immature neurons in the DG of aging rats [308].
The manipulation of the expression of these genes can have implications in the treatment of neurodegenerative diseases, but there are still some limitations. In a clinical trial for PD, intrastriatal infusion of an adenoviral vector was associated with an increased risk of intracranial hemorrhage and headaches [309]. Additionally, permanent genetic modification of the patients’ brain cells, coupled with the inability to control or interrupt the release of the bioactive substance raises safety and regulatory concerns [310].
Thus, future advances in delivery methods could improve the clinical significance and therapeutic outcomes of gene therapy. The ex vivo strategy is another promising alternative, in which cells can be manipulated and differentiated outside the organism before being reintroduced into the patient [311]. In this way, researchers can potentially generate large numbers of specific types of neurons for transplantation (or just research purposes). In other words, they can induce exogenous neurogenesis.
It is worth noting that there have been major advances in many other technologies. For example, genetically modified encapsulated cells were already tested in both animals and humans, showing promising results in different research fields [310, 312]. In a clinical trial patients with mild to moderate symptoms of AD, cell encapsulation biodelivery of NGF to the basal forebrain proved to be safe and increase cognition [310, 312]. This technology comprises the use of cells, which are genetically modified to secrete a therapeutic compound, and then are encapsulated before being delivered into the patient [313]. This technology has many advantages over the traditional in vivo and ex vivo gene therapy approaches, namely, the targeted delivery of the compound of interest, whose release can also be controlled using an implantable and retrievable medical device [314]. Additionally, capsules can be engineered to protect the cells from the host’s immune system [310, 314].
Exogenous Neurogenesis Induction
Although some studies have suggested that neurogenesis is enhanced in certain regions of the brain in response to neurodegenerative diseases (potentially as a compensatory mechanism) [315], typically, in the later stages of these illnesses, the innate neurogenic capacity of the brain is limited [315]. Therefore, stimulating the production of new neurons may not be enough to combat the extensive neuronal cell loss that occurs in all brain regions (including non-neurogenic areas) during the aging process and neurodegenerative diseases [30]. In these cases where the existing damage is far too extensive, replacing the cells that are lost or damaged, may be a therapeutic option [316].
Cell-Based Therapies
Stem cell therapy, also known as regenerative therapy, typically focuses either on cellular replacement or on providing trophic support to damaged or dysfunctional tissues [317]. This strategy may improve neurogenesis by introducing new stem cells into specific regions of the brain, that then differentiate into neurons [316]. Different types of stem cells have been studied for transplantation purposes, including embryonic stem cells [318], fetal stem cells [319], adult stem cells such as NSCs [320], induced pluripotent stem cells (iPSCs) [321], and even mesenchymal stem cells (MSCs) [322].
Totipotent embryonic stem cells have proved to reverse cognitive deficits after transplantation into the frontal region of the cortex of a mouse model of AD, induced through lesions on the nucleus basalis Meynert, which is a brain region vulnerable to neurodegeneration [323]. These cells successfully differentiated into cholinergic and serotonin-positive neurons, the loss of which has been linked to the manifestation of AD symptoms [323]. However, besides being associated with a risk of tumor formation, this approach also faces ethical and legal issues in the clinic, limiting their study and application [324]. Fetal stem cells face fewer ethical and legal problems since they are obtained from fetal tissues that would otherwise be discarded [325]. However, they are more difficult to obtain and far more restricted in their ability to differentiate into specialized cell types, which has been limiting their application in regenerative medicine [325]. Nevertheless, a phase 1 clinical trial employed their transplantation in individuals with progressive multiple sclerosis and has yielded promising results [319], underscoring the potential of these cells in treating other neurodegenerative disorders.
NSCs transplantation has also proved to differentiate into neurons after engraftment [326], improving cognitive scores in animal models of neurodegenerative diseases [327]. However, their transplantation goes beyond their ability to replace lost cells, since they also have the potential to serve as delivery vehicles of therapeutic genes [328], to secrete growth factors and other molecules that can promote the survival and growth of existing neurons and provide cues to stimulate the production of new ones [316]. In other words, this trophic support creates a more favorable environment for neural repair and regeneration [329]. Other studies even suggest that they have an anti-inflammatory role to protect the brain from cerebral inflammation [330]. Alterations in neuroinflammation status following stem cells’ transplantation might create a microenvironment conducive to enhanced neurogenesis [331].
NSCs for transplantation purposes can be obtained from brain tissue [332], may result from the differentiation of the aforementioned cells (embryonic and fetal stem cells [333]) or alternatively, they may result from the reprogramming of somatic cells, resulting in iPSCs [334]. NSCs derived from iPSCs have the advantage of being generated from the patient’s own cells, which reduces the risk of immune rejection and eliminates the need for immunosuppressive drugs [334]. This strategy holds promise for the creation of patient-specific NSCs, which provides a more personalized approach [335]. However, one of the limitations of this procedure is related to the heterogeneity of these cells (i.e., differences in their gene expression profiles, and varying degrees of maturity), meaning that when they are implanted into the brain, not all of them may successfully differentiate into the intended type of mature and functional neurons since they may adopt alternative cell fates. Research such as the one conducted by Xu et al. is crucial to tackle this issue since it may help to provide a better understanding of cellular heterogeneity throughout the differentiation process while introducing a method based on surface markers identification to accurately separate the right cells from the unwanted ones [336].
Despite these shortcomings, the potential of NSCs’ transplantation has already been supported by the successful results of many pre-clinical and clinical studies [337, 338]. For example, induced NSCs that were converted from mouse fibroblasts and transplanted into the striatum of a PD mouse model were able to migrate to the damaged substantia nigra and differentiate into dopaminergic neurons, which enhanced functional recovery [339]. Recently, Schweitzer et al. also documented a successful procedure involving the implantation of midbrain dopaminergic progenitor cells derived from the patient's own iPSCs. This approach was conducted in a patient diagnosed with idiopathic PD, resulting in remarkable improvements in clinical symptoms observed 18-24 months post-implantation. Such outcomes further highlight the potential of iPSC-based strategies in addressing neurodegenerative conditions like PD [338].
Another interesting approach based on cell reprogramming is glia-to-neuron reprogramming, which was first reported 20 years ago [340], but only recently has it been receiving more attention [341]. This technique aims to take advantage of the regenerative ability of resident glial cells [341] to directly convert them into neurons through cytokines, epigenetic factors, and transcription factors [342, 343]. Unlike the method previously described, in which cells must be reprogrammed in vitro before transplantation, glia-to-neuron conversion takes place directly in the brain [344], and was already reported to occur spontaneously as a response to specific brain trauma [345, 346]. However, inducing this reprogramming remains a major challenge, and ongoing research efforts are focused on refining the technology. Another crucial aspect is whether the newly converted neurons can successfully integrate into existing neuronal circuits and perform their intended functions [347]. While this remains uncertain, the ultimate goal is to create a technology that can replenish damaged areas with healthy neurons, as well as potentially reduce gliogenesis, which can act as a protective mechanism to minimize and repair brain injuries that, under specific circumstances, can lead to harmful effects [348].
Finally, MSCs have also demonstrated the ability to transdifferentiate into functional neurons [322, 349]. But beyond their role in cellular replacement, MSCs can also help treat neurodegenerative disorders through the expression of neurotrophic factors such as BDNF, NGF, and IGF-1 [331, 350, 351].
Even though stem cell therapy has shown remarkable results in improving cognitive deficits and neuronal loss in neurodegenerative diseases, the underlying mechanisms are not yet fully understood. Additionally, it is necessary to further understand how to create a microenvironment capable of sustaining and functionally integrating grafted and/or reprogrammed cells [317]. Nonetheless, regenerative stem cell therapy is an enticing therapeutic strategy for the retardation of neuronal loss, recovery of endogenous neurogenesis, and improvement of cognitive functions in neurodegenerative diseases.
Clinical Trials
As seen throughout the present study, several pre-clinical studies in animal models of neurodegenerative diseases have explored different strategies to enhance adult neurogenesis, some with promising results; however, clinical trials in this area are still relatively limited, as summarized in Table 2.
Table 2.
Clinical trials of therapies to promote adult neurogenesis of patients with neurodegenerative diseases
| Pro-neurogenic therapeutical intervention | Condition or disease | Clinical trial Identifier | Clinical trial Phase | Start date | Completion date | Status | Results | Ref. |
|---|---|---|---|---|---|---|---|---|
| Exercise and tDCS | Major and mild neurocognitive disorder due to AD or mixed AD/vascular disease | NCT03670615 | N/A | 2018 | 2025 | Recruiting | Not posted | NA |
| Dietary restriction and/or extended periods of mastication | Aging and cognitive decline | NCT03457870 | N/A | 2018 | 2020 | Completed | ↑ Recognition memory, and pattern separation | [352, 353] |
| Intravenous administration of sovateltide | AD, dementia | NCT04052737 | II | 2019 | 2023 | Completed | Not posted | NA |
| Intravenous administration of allopregnanolone | AD, late onset AD | NCT04838301 | II | 2023 | 2025 | Not yet recruiting | Not posted | NA |
Abbreviations: AD, Alzheimer’s disease; AAV2, adeno-associated virus type 2; N/A, Not Applicable; tDCS, transcranial direct current stimulation
One reason for this could be the difficulty in translating these findings to clinical settings [354]. Additionally, the lack of non-invasive techniques to directly measure neurogenesis in live humans may also contribute to this challenge [355]. Currently, there are only indirect methods to estimate neurogenesis in live humans. These include neuroimaging techniques such as magnetic resonance imaging (MRI) [356], biomarkers in blood [357, 358], changes in cognitive function or behavior [359], and radiolabeling [360]. However, these lack resolution, sensitivity, and specificity, since they fail to detect the exact changes in the number or activity of new neurons, to differentiate between cells, and are unable to study the process in real time [355, 359].
Regarding neurostimulation, there is currently only one clinical trial in the recruiting phase (NCT03670615), aiming to assess how exercise combined with tDCS (20 min sessions at 2 mA, 5 times per week for 2 weeks applied through 35 cm2 bitemporal electrodes) affect the cognition and brain plasticity of patients with major and mild neurocognitive disorder due to AD or mixed AD/vascular disease [357]. In this study, changes in neurogenesis will be assessed through biomarkers (BDNF), obtained through blood work [357].
Although animal studies have clearly demonstrated the link between physical exercise and increased neurogenesis [361], human studies have provided mixed results [362]. To date, no clinical research has specifically targeted the effect of exercise on neurogenesis. Still, some studies have found that physical activity can enhance cognitive performance in patients with PD [363, 364], while others have observed increases in hippocampal volumes [365]. It remains unclear, however, whether these outcomes are a consequence of increased neurogenesis. Similarly, a different clinical study found that mastication, which can be considered a mild form of exercise [353, 366], along with intermittent calorie restriction, benefited hippocampus-dependent cognition in older individuals [352, 353]. Another study (NCT03457870) aimed at assessing the effects of short-term intermittent and continuous calorie restriction on insulin sensitivity in obese individuals, and also found a memory improvement linked to hippocampal neurogenesis [355]. But these studies present some limitations that should be addressed in the future. For instance, since poor oral health appears to influence cognitive function [367, 368], this factor should be considered in upcoming clinical studies. But most importantly, these studies did not address cellular and molecular mechanisms, particularly neurogenesis-associated markers, which inhibits to conclude with certainty that these improvements were a direct result of neurogenesis.
An extensive literature search allowed to conclude that there are currently no ongoing clinical trials that have specifically assessed whether bioactive compounds can promote adult neurogenesis. However, a meta-analysis of 225 patients showed that resveratrol has the potential to enhance mood but with no significant impact on factors related to memory and cognitive performance [369]. On the other hand, curcumin can improve the cognition of healthy and non-demented adults but has detrimental effects on patients with AD, indicating selective effects on different regions of the brain and cognitive domains [370, 371]. It is recommended that upcoming clinical trials with bioactive compounds improve their bioavailability by using innovative approaches such as nanoformulations or in combination with metabolism inhibitors, which should allow them to remain active in the body for longer periods [369].
Sovateltide is the only pharmaceutical candidate targeting adult neurogenesis that has undergone a clinical trial for the treatment of AD and dementia (NCT 04052737). As previously reported in Table 1, pre-clinical studies with this drug have shown that it is involved in neuronal cell survival and the restoration of adult neurogenesis in neurodegenerative diseases [208, 247]. Based on these results, sovateltide was recently on a phase 2 clinical trial and upcoming results should provide information about whether it augments the activity of NSCs in the brain.
Consistent with animal studies, intravenous administration of allopregnanolone was well tolerated and safe across all doses in people with early AD [372]. Therefore, this hormone therapy soon will enter a phase 2 efficacy trial to determine if this therapy can restore structural integrity and cognitive function in patients with AD [373]. Preliminary results from MRI indicate that allopregnanolone administration reduces (and sometimes reverses) hippocampal volume, which was augmented in patients with mild AD. Additionally, this strategy successfully strengthened local, inter-regional, and network level functional connectivity in brain regions vulnerable to AD pathology, which supported advancement to a phase 2 clinical trial (NCT04838301) [373], in which changes in biomarkers of neurogenesis will be addressed. Despite these interesting results, no other hormones are currently involved in clinical trials.
Besides the clinical studies included in Table 2, there are many more that have been exploring the potential of inducing neurogenesis to alleviate symptoms and treat a range of neurological disorders, with promising results. These include conditions such as depressive and bipolar disorders, schizophrenia, traumatic brain injury, and many others (e.g.: NCT03608462, NCT05755321, NCT01552837, NCT03345550, etc.), which were not included since it goes beyond the scope of the current review. It is worth noting that the plethora of research exploring neurogenesis demonstrates its potential as a promising area for further investigation into neurodegenerative diseases. However, remain several limitations to this therapeutic approach that require further attention. One of the main challenges is that many clinical studies lack follow-up assessments, which are necessary to determine the acute effects of interventions on neurogenesis and, consequently, memory and cognition. Furthermore, the lack of non-invasive methods for measuring adult neurogenesis in live humans is currently the biggest obstacle facing this field [355, 374]. Without accurate measures, it is challenging to translate clinical research into clinical practice. As such, it is necessary to develop appropriate in vivo markers that can precisely measure neurogenesis.
Conclusions and Future Perspectives
Based on the available evidence, it appears that it is possible to manipulate neurogenic niches to stimulate neurogenesis in the adult brain, potentially offering a promising treatment strategy for neurodegenerative diseases and other neurological disorders. The activation of endogenous neural stem/precursor cells in response to various external stimuli, including electrical and magnetic stimulation, physical exercise, environment enrichment, diet changes, and pharmacological interventions, suggests that these approaches could offer a generally safe way to promote neurogenesis in adults. However, the effect of these strategies may not be as pronounced as those achieved through more invasive therapies such as stem cell transplantation, which may produce rapid and significant changes in the brain. Nonetheless, these invasive therapies are still experimental and may carry significant risks and side effects.
With this work, it became clear that stimulating neurogenesis in animal models of neurodegenerative diseases presents a great challenge. This challenge primarily stems from the complexity and multifactorial nature of neurodegenerative diseases, which involve various pathological processes that extend beyond impaired neurogenesis. Despite this, most studies still focus on promoting neurogenesis in animal models of neurodegenerative diseases, as a way to mitigate existing symptoms rather than preventing the onset of dementias. To enhance the understanding of neurogenesis as a therapeutic tool, researchers should take a step back and shift their focus towards investigating the potential of promoting neurogenesis in healthy subjects. Possibly, by stimulating neurogenesis prior to the onset of the disease, we might be able to preserve the plasticity of brain circuits, and thereby counteract the progressive neuronal loss, and cognitive decline characteristic of neurodegenerative diseases.
One final remark, while some rodent studies have shown promising results, it is difficult to extrapolate these findings to humans since these two mammals have major differences in brain size, organization, and function. This indicates a need to search for and develop alternative models to better mimic the molecular and cellular mechanisms of human neurogenesis. Additionally, although clinical trials are trying to make it to clinical practice, the lack of reliable biomarkers and standardized techniques to measure neurogenesis in humans makes it even more challenging. Finally, ethical considerations and regulatory requirements hinder large-scale clinical trials in humans, particularly when experimental treatments involve invasive procedures or carry potential risks.
Therefore, although clinical trials are a crucial step in determining the clinical viability of the discussed pro-neurogenic approaches, these limitations are blocking progress in the field, and therefore, they should be addressed soon.
Author Contribution
MV and BM wrote the main manuscript text and MV prepared the figures. FM, ST, RM and SR reviewed and edited the manuscript for the final version.
Funding
Open access funding provided by FCT|FCCN (b-on). This work was supported by national funds, through Fundação para a Ciência e Tecnologia of the Ministério da Educação e Ciência (FCT/MCTES) [grant number PTDC/BTM-TEC/3792/2021], by the Institute of Biomedicine (iBiMED) [UIDP/04501/2020 and UIDB/04501/2020], the COMPETE 2020 Program, the QREN and the European Union (Fundo Europeu de Desenvolvimento Regional). Mariana Vassal was the recipient of a FCT Studentship [grant number 2023.01360.BD].
Data Availability
No datasets were generated or analysed during the current study.
Declarations
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Aranda-Anzaldo A, Dent MAR (2017) Why cortical neurons cannot divide, and why do they usually die in the attempt?: Aranda-Anzaldo and Dent. J Neurosci Res 95:921–929. 10.1002/jnr.23765 [DOI] [PubMed] [Google Scholar]
- 2.Ming G, Song H (2005) Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci 28:223–250. 10.1146/annurev.neuro.28.051804.101459 [DOI] [PubMed] [Google Scholar]
- 3.Fares J, Bou Diab Z, Nabha S, Fares Y (2019) Neurogenesis in the adult hippocampus: history, regulation, and prospective roles. Int J Neurosci 129:598–611. 10.1080/00207454.2018.1545771 [DOI] [PubMed] [Google Scholar]
- 4.Altman J, Das GD (1965) Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 124:319–335. 10.1002/cne.901240303 [DOI] [PubMed] [Google Scholar]
- 5.Eriksson PS, Perfilieva E, Björk-Eriksson T et al (1998) Neurogenesis in the adult human hippocampus. Nat Med 4:1313–1317. 10.1038/3305 [DOI] [PubMed] [Google Scholar]
- 6.Kaplan MS, Hinds JW (1977) Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science 197:1092–1094. 10.1126/science.887941 [DOI] [PubMed] [Google Scholar]
- 7.Jurkowski MP, Bettio LK, Woo E et al (2020) Beyond the hippocampus and the SVZ: adult neurogenesis throughout the brain. Front Cell Neurosci 14:576444. 10.3389/fncel.2020.576444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ali AAH, von Gall C (2022) Adult neurogenesis under control of the circadian system. Cells 11:764. 10.3390/cells11050764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kempermann G, Song H, Gage FH (2015) Neurogenesis in the adult hippocampus. Cold Spring Harb Perspect Biol 7:a018812. 10.1101/cshperspect.a018812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ponti G, Obernier K, Alvarez-Buylla A (2013) Lineage progression from stem cells to new neurons in the adult brain ventricular-subventricular zone. Cell Cycle 12:1649–1650. 10.4161/cc.24984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oddi S, Scipioni L, Maccarrone M (2020) Endocannabinoid system and adult neurogenesis: a focused review. Curr Opin Pharmacol 50:25–32. 10.1016/j.coph.2019.11.002 [DOI] [PubMed] [Google Scholar]
- 12.Niklison-Chirou MV, Agostini M, Amelio I, Melino G (2020) Regulation of adult neurogenesis in mammalian brain. IJMS 21:4869. 10.3390/ijms21144869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng M-F (2013) Hypothalamic neurogenesis in the adult brain. Front Neuroendocrinol 34:167–178. 10.1016/j.yfrne.2013.05.001 [DOI] [PubMed] [Google Scholar]
- 14.Evans J, Sumners C, Moore J et al (2002) Characterization of mitotic neurons derived from adult rat hypothalamus and brain stem. J Neurophysiol 87:1076–1085. 10.1152/jn.00088.2001 [DOI] [PubMed] [Google Scholar]
- 15.Ernst A, Alkass K, Bernard S et al (2014) Neurogenesis in the striatum of the adult human brain. Cell 156:1072–1083. 10.1016/j.cell.2014.01.044 [DOI] [PubMed] [Google Scholar]
- 16.Inta D, Cameron HA, Gass P (2015) New neurons in the adult striatum: from rodents to humans. Trends Neurosci 38:517–523. 10.1016/j.tins.2015.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mourtzi T, Dimitrakopoulos D, Kakogiannis D et al (2021) Characterization of substantia nigra neurogenesis in homeostasis and dopaminergic degeneration: beneficial effects of the microneurotrophin BNN-20. Stem Cell Res Ther 12:335. 10.1186/s13287-021-02398-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao M, Momma S, Delfani K et al (2003) Evidence for neurogenesis in the adult mammalian substantia nigra. Proc Natl Acad Sci USA 100:7925–7930. 10.1073/pnas.1131955100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gould E, Reeves AJ, Graziano MSA, Gross CG (1999) Neurogenesis in the neocortex of adult primates. Science 286:548–552. 10.1126/science.286.5439.548 [DOI] [PubMed] [Google Scholar]
- 20.Magavi SS, Leavitt BR, Macklis JD (2000) Induction of neurogenesis in the neocortex of adult mice. Nature 405:951–955. 10.1038/35016083 [DOI] [PubMed] [Google Scholar]
- 21.Walton NM, Sutter BM, Chen H-X et al (2006) Derivation and large-scale expansion of multipotent astroglial neural progenitors from adult human brain. Development 133:3671–3681. 10.1242/dev.02541 [DOI] [PubMed] [Google Scholar]
- 22.Bernier PJ, Bédard A, Vinet J et al (2002) Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proc Natl Acad Sci USA 99:11464–11469. 10.1073/pnas.172403999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreno-Jiménez EP, Terreros-Roncal J, Flor-García M et al (2021) Evidences for adult hippocampal neurogenesis in humans. J Neurosci 41:2541–2553. 10.1523/JNEUROSCI.0675-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ziabreva I, Perry E, Perry R et al (2006) Altered neurogenesis in Alzheimer’s disease. J Psychosom Res 61:311–316. 10.1016/j.jpsychores.2006.07.017 [DOI] [PubMed] [Google Scholar]
- 25.Mu Y, Gage FH (2011) Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol Neurodegener 6:85. 10.1186/1750-1326-6-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Unger MS, Marschallinger J, Kaindl J et al (2016) Early changes in hippocampal neurogenesis in transgenic mouse models for Alzheimer’s disease. Mol Neurobiol 53:5796–5806. 10.1007/s12035-016-0018-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiong Y, Mahmood A, Chopp M (2011) Angiogenesis, neurogenesis and brain recovery of function following injury. Curr Opin Investig Drugs 11:298–308 [PMC free article] [PubMed] [Google Scholar]
- 28.Sailor KA, Ming G, Song H (2006) Neurogenesis as a potential therapeutic strategy for neurodegenerative diseases. Expert Opin Biol Ther 6:879–890. 10.1517/14712598.6.9.879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mourtzi T, Kazanis I (2022) Endogenous versus exogenous cell replacement for Parkinson’s disease: where are we at and where are we going? Neural Regen Res 17:2637. 10.4103/1673-5374.336137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsubara S, Matsuda T, Nakashima K (2021) Regulation of adult mammalian neural stem cells and neurogenesis by cell extrinsic and intrinsic factors. Cells 10:1145. 10.3390/cells10051145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magavi SS, Macklis JD Manipulation of neural precursors in situ: induction of neurogenesis in the neocortex of adult mice. Neuropsychopharmacology 25:816–835 [DOI] [PubMed]
- 32.Bjornsson CS, Apostolopoulou M, Tian Y, Temple S (2015) It takes a village: constructing the neurogenic niche. Dev Cell 32:435–446. 10.1016/j.devcel.2015.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Homem CCF, Repic M, Knoblich JA (2015) Proliferation control in neural stem and progenitor cells. Nat Rev Neurosci 16:647–659. 10.1038/nrn4021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ihunwo A, Tembo L, Dzamalala C (2016) The dynamics of adult neurogenesis in human hippocampus. Neural Regen Res 11:1869. 10.4103/1673-5374.195278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawaguchi D, Furutachi S, Kawai H et al (2013) Dll1 maintains quiescence of adult neural stem cells and segregates asymmetrically during mitosis. Nat Commun 4:1880. 10.1038/ncomms2895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ming G, Song H (2011) Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70:687–702. 10.1016/j.neuron.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kazanis I, Lathia J, Moss L, ffrench-Constant (2008) The neural stem cell microenvironment. In: The stem cell stem book. Harvard Stem Cell Institute, Cambridge (MA) [PubMed] [Google Scholar]
- 38.Ma DK, Bonaguidi MA, Ming G, Song H (2009) Adult neural stem cells in the mammalian central nervous system. Cell Res 19:672–682. 10.1038/cr.2009.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Porter DDL, Henry SN, Ahmed S et al (2022) Neuroblast migration along cellular substrates in the developing porcine brain. Stem Cell Rep 17:2097–2110. 10.1016/j.stemcr.2022.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jagasia R, Song H, Gage FH, Lie DC (2006) New regulators in adult neurogenesis and their potential role for repair. Trends Mol Med 12:400–405. 10.1016/j.molmed.2006.07.006 [DOI] [PubMed] [Google Scholar]
- 41.Kriegstein A, Alvarez-Buylla A (2009) The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci 32:149–184. 10.1146/annurev.neuro.051508.135600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y, Guo W (2021) Neural stem cell niche and adult neurogenesis. Neuroscientist 27:235–245. 10.1177/1073858420939034 [DOI] [PubMed] [Google Scholar]
- 43.Buylla-Alvarez A, Lim D (2004) For the long run: maintaining germinal niches in the adult brain. Neuron 41:683–686. 10.1016/s0896-6273(04)00111-4 [DOI] [PubMed] [Google Scholar]
- 44.Ma DK, Ming G, Song H (2005) Glial influences on neural stem cell development: cellular niches for adult neurogenesis. Curr Opin Neurobiol 15:514–520. 10.1016/j.conb.2005.08.003 [DOI] [PubMed] [Google Scholar]
- 45.Leal-Galicia P, Chávez-Hernández ME, Mata F et al (2021) Adult neurogenesis: a story ranging from controversial new neurogenic areas and human adult neurogenesis to molecular regulation. IJMS 22:11489. 10.3390/ijms222111489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mu Y, Lee SW, Gage FH (2010) Signaling in adult neurogenesis. Curr Opin Neurobiol 20:416–423. 10.1016/j.conb.2010.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farioli-Vecchioli S, Ricci V, Middei S (2022) Adult hippocampal neurogenesis in Alzheimer’s disease: an overview of human and animal studies with implications for therapeutic perspectives aimed at memory recovery. Neural Plast 2022:1–18. 10.1155/2022/9959044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whitman MC, Greer CA (2009) Adult neurogenesis and the olfactory system. Prog Neurobiol 89:162–175. 10.1016/j.pneurobio.2009.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mobley AS (2019) Adult neurogenesis in the hippocampus. In: Neural stem cells and adult neurogenesis, 1st edn. Elsevier, pp. 117–148 [Google Scholar]
- 50.Arnold SJ, Huang G-J, Cheung AFP et al (2008) The T-box transcription factor Eomes/Tbr2 regulates neurogenesis in the cortical subventricular zone. Genes Dev 22:2479–2484. 10.1101/gad.475408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lim DA, Alvarez-Buylla A (2016) The adult ventricular–subventricular zone (V-SVZ) and olfactory bulb (OB) neurogenesis. Cold Spring Harb Perspect Biol 8:a018820. 10.1101/cshperspect.a018820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tong CK, Alvarez-Buylla A (2014) SnapShot: adult neurogenesis in the V-SVZ. Neuron 81:220–220.e1. 10.1016/j.neuron.2013.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lois C, García-Verdugo J-M, Alvarez-Buylla A (1996) Chain migration of neuronal precursors. Science 271:978–981. 10.1126/science.271.5251.978 [DOI] [PubMed] [Google Scholar]
- 54.Schaar BT, McConnell SK (2005) Cytoskeletal coordination during neuronal migration. Proc Natl Acad Sci USA 102:13652–13657. 10.1073/pnas.0506008102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lledo P-M, Alonso M, Grubb MS (2006) Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci 7:179–193. 10.1038/nrn1867 [DOI] [PubMed] [Google Scholar]
- 56.Bless EP, Reddy T, Acharya KD et al (2014) Oestradiol and diet modulate energy homeostasis and hypothalamic neurogenesis in the adult female mouse. J Neuroendocrinol 26:805–816. 10.1111/jne.12206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kokoeva MV, Yin H, Flier JS (2005) Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science 310:679–683. 10.1126/science.1115360 [DOI] [PubMed] [Google Scholar]
- 58.Bernstein PL, Zuo M, Cheng M-F (1993) Social condition affects the courtship behavior of male ring doves with posterior medial hypothalamic lesions. Behav Neural Biol 59:120–125. 10.1016/0163-1047(93)90834-5 [DOI] [PubMed] [Google Scholar]
- 59.Shapiro LA, Ng K, Zhou Q-Y, Ribak CE (2009) Subventricular zone-derived, newly generated neurons populate several olfactory and limbic forebrain regions. Epilepsy Behav 14:74–80. 10.1016/j.yebeh.2008.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saul ML, Helmreich DL, Rehman S, Fudge JL (2015) Proliferating cells in the adolescent rat amygdala: characterization and response to stress. Neuroscience 311:105–117. 10.1016/j.neuroscience.2015.10.003 [DOI] [PubMed] [Google Scholar]
- 61.Wang S, Okun MS, Suslov O et al (2012) Neurogenic potential of progenitor cells isolated from postmortem human Parkinsonian brains. Brain Res 1464:61–72. 10.1016/j.brainres.2012.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lie DC, Dziewczapolski G, Willhoite AR et al (2002) The adult substantia nigra contains progenitor cells with neurogenic potential. J Neurosci 22:6639–6649. 10.1523/JNEUROSCI.22-15-06639.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aytac K, Magdalena E, Guy N et al (2020) Functional properties of habenular neurons are determined by developmental stage and sequential neurogenesis. Sci Adv 6(36):eaaz3173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dayer AG, Cleaver KM, Abouantoun T, Cameron HA (2005) New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. J Cell Biol 168:415–427. 10.1083/jcb.200407053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jhaveri DJ, Tedoldi A, Hunt S et al (2018) Evidence for newly generated interneurons in the basolateral amygdala of adult mice. Mol Psychiatry 23:521–532. 10.1038/mp.2017.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dugger BN, Dickson DW (2017) Pathology of neurodegenerative diseases. Cold Spring Harb Perspect Biol 9:a028035. 10.1101/cshperspect.a028035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Winner B, Winkler J (2015) Adult neurogenesis in neurodegenerative diseases. Cold Spring Harb Perspect Biol 7. 10.1101/cshperspect.a021287 [DOI] [PMC free article] [PubMed]
- 68.Christian KM, Song H, Ming G (2014) Functions and dysfunctions of adult hippocampal neurogenesis. Annu Rev Neurosci 37:243–262. 10.1146/annurev-neuro-071013-014134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng J (2022) Hippocampal neurogenesis and pro-neurogenic therapies for Alzheimer’s disease. Anim Models and Exp Med 5:3–14. 10.1002/ame2.12212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scharfman H, Goodman J, Macleod A et al (2005) Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol 192:348–356. 10.1016/j.expneurol.2004.11.016 [DOI] [PubMed] [Google Scholar]
- 71.Hagg T (2009) From Neurotransmitters to neurotrophic factors to neurogenesis. Neuroscientist 15:20–27. 10.1177/1073858408324789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tonchev AB, Yamashima T (2007) “Transcribing” postischemic neurogenesis: a tale revealing hopes of adult brain repair. J Mol Med 85:539–542. 10.1007/s00109-007-0210-5 [DOI] [PubMed] [Google Scholar]
- 73.Borsini A, Zunszain PA, Thuret S, Pariante CM (2015) The role of inflammatory cytokines as key modulators of neurogenesis. Trends Neurosci 38:145–157. 10.1016/j.tins.2014.12.006 [DOI] [PubMed] [Google Scholar]
- 74.Jorgensen C, Wang Z (2020) Hormonal regulation of mammalian adult neurogenesis: a multifaceted mechanism. Biomolecules 10:1151. 10.3390/biom10081151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kempermann G, Brandon EP, Gage FH (1998) Environmental stimulation of 129/SvJ mice causes increased cell proliferation and neurogenesis in the adult dentate gyrus. Curr Biol 8:939–944. 10.1016/S0960-9822(07)00377-6 [DOI] [PubMed] [Google Scholar]
- 76.Kobelt LJ, Wilkinson AE, McCormick AM et al (2014) Short duration electrical stimulation to enhance neurite outgrowth and maturation of adult neural stem progenitor cells. Ann Biomed Eng 42:2164–2176. 10.1007/s10439-014-1058-9 [DOI] [PubMed] [Google Scholar]
- 77.Costa V, Lugert S, Jagasia R (2015) Role of adult hippocampal neurogenesis in cognition in physiology and disease: pharmacological targets and biomarkers. In: Cognitive Enhancement. Springer International Publishing, pp. 99–155 [DOI] [PubMed] [Google Scholar]
- 78.Guennoun R (2020) Progesterone in the brain: hormone, neurosteroid and neuroprotectant. IJMS 21:5271. 10.3390/ijms21155271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hussain R, Zubair H, Pursell S, Shahab M (2018) Neurodegenerative diseases: regenerative mechanisms and novel therapeutic approaches. Brain Sci 8:177. 10.3390/brainsci8090177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Björklund A, Lindvall O (2000) Cell replacement therapies for central nervous system disorders. Nat Neurosci 3:537–544. 10.1038/75705 [DOI] [PubMed] [Google Scholar]
- 81.Shohayeb B, Diab M, Ahmed M, Ng DCH (2018) Factors that influence adult neurogenesis as potential therapy. Transl Neurodegener 7:4. 10.1186/s40035-018-0109-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pullar C (2011) The physiology of bioelectricity in development, tissue degeneration, and cancer. CRC Press [Google Scholar]
- 83.Nardone R, Höller Y, Tezzon F et al (2015) Neurostimulation in Alzheimer’s disease: from basic research to clinical applications. Neurol Sci 36:689–700. 10.1007/s10072-015-2120-6 [DOI] [PubMed] [Google Scholar]
- 84.Uzair M, Abualait T, Arshad M et al (2022) Transcranial magnetic stimulation in animal models of neurodegeneration. Neural Regen Res 17:251. 10.4103/1673-5374.317962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ye H, Steiger A (2015) Neuron matters: electric activation of neuronal tissue is dependent on the interaction between the neuron and the electric field. J NeuroEng Rehabil 12:65. 10.1186/s12984-015-0061-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huang Y, Li Y, Chen J et al (2015) Electrical stimulation elicits neural stem cells activation: new perspectives in CNS repair. Front Hum Neurosci 9. 10.3389/fnhum.2015.00586 [DOI] [PMC free article] [PubMed]
- 87.Zhu R, Sun Z, Li C et al (2019) Electrical stimulation affects neural stem cell fate and function in vitro. Exp Neurol 319:112963. 10.1016/j.expneurol.2019.112963 [DOI] [PubMed] [Google Scholar]
- 88.Ma X, Cheng O, Jiang Q et al (2021) Activation of ephrinb1/EPHB2/MAP-2/NMDAR mediates hippocampal neurogenesis promoted by transcranial direct current stimulation in cerebral-ischemic mice. NeuroMolecular Med 23:521–530. 10.1007/s12017-021-08654-2 [DOI] [PubMed] [Google Scholar]
- 89.Yu T-H, Wu Y-J, Chien M-E, Hsu K-S (2023) Multisession anodal transcranial direct current stimulation enhances adult hippocampal neurogenesis and context discrimination in mice. J Neurosci 43. 10.1523/JNEUROSCI.1476-22.2022 [DOI] [PMC free article] [PubMed]
- 90.Pikhovych A, Walter HL, Mahabir E et al (2016) Transcranial direct current stimulation in the male mouse to promote recovery after stroke. Lab Anim 50:212–216. 10.1177/0023677215610708 [DOI] [PubMed] [Google Scholar]
- 91.Pikhovych A, Stolberg NP, Jessica Flitsch L et al (2016) Transcranial direct current stimulation modulates neurogenesis and microglia activation in the mouse brain. Stem Cells Int 2016:1–9. 10.1155/2016/2715196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rushmore RJ, DeSimone C, Valero-Cabré A (2013) Multiple sessions of transcranial direct current stimulation to the intact hemisphere improves visual function after unilateral ablation of visual cortex. Eur J Neurosci 38:3799–3807. 10.1111/ejn.12373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Khedr EM, Salama RH, Abdel Hameed M et al (2019) Therapeutic role of transcranial direct current stimulation in Alzheimer disease patients: double-blind, placebo-controlled clinical trial. Neurorehabil Neural Repair 33:384–394. 10.1177/1545968319840285 [DOI] [PubMed] [Google Scholar]
- 94.Braun R, Klein R, Walter HL et al (2016) Transcranial direct current stimulation accelerates recovery of function, induces neurogenesis and recruits oligodendrocyte precursors in a rat model of stroke. Exp Neurol 279:127–136. 10.1016/j.expneurol.2016.02.018 [DOI] [PubMed] [Google Scholar]
- 95.Zhang T, Guilherme E, Kesici A et al (2019) Differential effects of trancranial magnetic stimulation and electroconvulsive stimulation on adult hippocampal neurogenesis in mice. Brain Stimul J 12. 10.1016/j.brs.2018.12.781
- 96.Dillen Y, Kemps H, Gervois P et al (2020) Adult neurogenesis in the subventricular zone and its regulation after ischemic stroke: implications for therapeutic approaches. Transl Stroke Res 11:60–79. 10.1007/s12975-019-00717-8 [DOI] [PubMed] [Google Scholar]
- 97.Luo J, Zheng H, Zhang L et al (2017) High-frequency repetitive transcranial magnetic stimulation (rTMS) improves functional recovery by enhancing neurogenesis and activating BDNF/TrkB signaling in ischemic rats. IJMS 18:455. 10.3390/ijms18020455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zuo C, Cao H, Ding F et al (2020) Neuroprotective efficacy of different levels of high-frequency repetitive transcranial magnetic stimulation in mice with CUMS-induced depression: involvement of the p11/BDNF/Homer1a signaling pathway. J Psychiatr Res 125:152–163. 10.1016/j.jpsychires.2020.03.018 [DOI] [PubMed] [Google Scholar]
- 99.Wang F, Chang G, Yu Q, Geng X (2015) The neuroprotection of repetitive transcranial magnetic stimulation pre-treatment in vascular dementia rats. J Mol Neurosci 56:198–204. 10.1007/s12031-014-0480-7 [DOI] [PubMed] [Google Scholar]
- 100.Müller M (2000) Long-term repetitive transcranial magnetic stimulation increases the expression of brain-derived neurotrophic factor and cholecystokinin mRNA, but not neuropeptide tyrosine mRNA in specific areas of rat brain. Neuropsychopharmacology 23:205–215. 10.1016/S0893-133X(00)00099-3 [DOI] [PubMed] [Google Scholar]
- 101.Tan T, Xie J, Liu T et al (2013) Low-frequency (1Hz) repetitive transcranial magnetic stimulation (rTMS) reverses Aβ1–42-mediated memory deficits in rats. Exp Gerontol 48:786–794. 10.1016/j.exger.2013.05.001 [DOI] [PubMed] [Google Scholar]
- 102.Baruth JM, Casanova MF, El-Baz A et al (2010) Low-frequency repetitive transcranial magnetic stimulation modulates evoked-gamma frequency oscillations in autism spectrum disorder. J Neurother 14:179–194. 10.1080/10874208.2010.501500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xing Y, Zhang Y, Li C et al (2022) Repetitive transcranial magnetic stimulation of the brain after ischemic stroke: mechanisms from animal models. Cell Mol Neurobiol. 10.1007/s10571-022-01264-x [DOI] [PMC free article] [PubMed]
- 104.Kato N (2009) Neurophysiological mechanisms of electroconvulsive therapy for depression. Neurosci Res 64:3–11. 10.1016/j.neures.2009.01.014 [DOI] [PubMed] [Google Scholar]
- 105.Ueyama E, Ukai S, Ogawa A et al (2011) Chronic repetitive transcranial magnetic stimulation increases hippocampal neurogenesis in rats: rTMS increases neurogenesis in rats. Psychiatry Clin Neurosci 65:77–81. 10.1111/j.1440-1819.2010.02170.x [DOI] [PubMed] [Google Scholar]
- 106.Czéh B, Welt T, Fischer AK et al (2002) Chronic psychosocial stress and concomitant repetitive transcranial magnetic stimulation: effects on stress hormone levels and adult hippocampal neurogenesis. Biol Psychiatry 52:1057–1065. 10.1016/S0006-3223(02)01457-9 [DOI] [PubMed] [Google Scholar]
- 107.Stone SSD, Teixeira CM, DeVito LM et al (2011) Stimulation of entorhinal cortex promotes adult neurogenesis and facilitates spatial memory. J Neurosci 31:13469–13484. 10.1523/JNEUROSCI.3100-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hamani C, McAndrews MP, Cohn M et al (2008) Memory enhancement induced by hypothalamic/fornix deep brain stimulation. Ann Neurol 63. 10.1002/ana.21295 [DOI] [PubMed]
- 109.Pohodich AE, Yalamanchili H, Raman AT et al (2018) Forniceal deep brain stimulation induces gene expression and splicing changes that promote neurogenesis and plasticity. eLife 7:e34031. 10.7554/eLife.34031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shawn Zheng Kai T, Man-Lung F, Junhao K et al (2020) The paradoxical effect of deep brain stimulation on memory. Aging Dis 11:179. 10.14336/AD.2019.0511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sun Y, Luo Y, Zheng X et al (2021) Multiple sessions of entorhinal cortex deep brain stimulation in C57BL/6J mice increases exploratory behavior and hippocampal neurogenesis. Annu Int Conf IEEE Eng Med Biol Soc:6390–6393. 10.1109/EMBC46164.2021.9629978 [DOI] [PubMed]
- 112.Toda H, Hamani C, Fawcett AP et al (2008) The regulation of adult rodent hippocampal neurogenesis by deep brain stimulation: laboratory investigation. JNS 108:132–138. 10.3171/JNS/2008/108/01/0132 [DOI] [PubMed] [Google Scholar]
- 113.Chen Y-C, Shi L, Zhu G-Y et al (2017) Effects of anterior thalamic nuclei deep brain stimulation on neurogenesis in epileptic and healthy rats. Brain Res 1672:65–72. 10.1016/j.brainres.2017.07.021 [DOI] [PubMed] [Google Scholar]
- 114.Magdaleno-Madrigal VM, Pantoja-Jiménez CR, Bazaldúa A et al (2016) Acute deep brain stimulation in the thalamic reticular nucleus protects against acute stress and modulates initial events of adult hippocampal neurogenesis. Behav Brain Res 314:65–76. 10.1016/j.bbr.2016.07.022 [DOI] [PubMed] [Google Scholar]
- 115.Huguet G, Kádár E, Serrano N et al (2020) Rewarding deep brain stimulation at the medial forebrain bundle favours avoidance conditioned response in a remote memory test, hinders extinction and increases neurogenesis. Behav Brain Res 378:112308. 10.1016/j.bbr.2019.112308 [DOI] [PubMed] [Google Scholar]
- 116.Guo F, Lou J, Han X et al (2017) Repetitive transcranial magnetic stimulation ameliorates cognitive impairment by enhancing neurogenesis and suppressing apoptosis in the hippocampus in rats with ischemic stroke. Front Physiol 8:559. 10.3389/fphys.2017.00559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Muri L, Oberhänsli S, Buri M et al (2020) Repetitive transcranial magnetic stimulation activates glial cells and inhibits neurogenesis after pneumococcal meningitis. PLoS One 15:e0232863. 10.1371/journal.pone.0232863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tang A, Thickbroom G, Rodger J (2015) Repetitive transcranial magnetic stimulation of the brain: mechanisms from animal and experimental models. Neuroscientist:1–13. 10.1177/1073858415618897 [DOI] [PubMed]
- 119.Zong X, Gu J, Geng D, Gao D (2022) Repetitive transcranial magnetic stimulation (rTMS) for multiple neurological conditions in rodent animal models: a systematic review. Neurochem Int 157. 10.1016/j.neuint.2022.105356 [DOI] [PubMed]
- 120.Maharjan R, Diaz Bustamante L, Ghattas KN et al (2020) Role of lifestyle in neuroplasticity and neurogenesis in an aging brain. Cureus 12 [DOI] [PMC free article] [PubMed]
- 121.Swain RA, Harris AB, Wiener EC et al (2003) Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience 117:1037–1046. 10.1016/S0306-4522(02)00664-4 [DOI] [PubMed] [Google Scholar]
- 122.Yancey SL, Overton JM (1993) Cardiovascular responses to voluntary and treadmill exercise in rats. J Appl Physiol 75:1334–1340. 10.1152/jappl.1993.75.3.1334 [DOI] [PubMed] [Google Scholar]
- 123.Shanker Sharma H, Cervós-Navarro J, Kumar Dey P (1991) Increased blood-brain barrier permeability following acute short-term swimming exercise in conscious normotensive young rats. Neurosci Res 10:211–221. 10.1016/0168-0102(91)90058-7 [DOI] [PubMed] [Google Scholar]
- 124.Aimone JB, Li Y, Lee SW et al (2014) Regulation and function of adult neurogenesis: from genes to cognition. Physiol Rev 94:991–1026. 10.1152/physrev.00004.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Choi SH, Bylykbashi E, Chatila ZK et al (2018) Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science 361:eaan8821. 10.1126/science.aan8821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zaben MJ, Gray WP (2013) Neuropeptides and hippocampal neurogenesis. Neuropeptides 47:431–438. 10.1016/j.npep.2013.10.002 [DOI] [PubMed] [Google Scholar]
- 127.Sairanen M (2005) Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci 25:1089–1094. 10.1523/JNEUROSCI.3741-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jin K, Zhu Y, Sun Y et al (2002) Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA 99:11946–11950. 10.1073/pnas.182296499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jeon YK, Ha CH (2017) The effect of exercise intensity on brain derived neurotrophic factor and memory in adolescents. Environ Health Prev Med 22:27. 10.1186/s12199-017-0643-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Klempin F, Beis D, Mosienko V et al (2013) Serotonin is required for exercise-induced adult hippocampal neurogenesis. J Neurosci 33:8270–8275. 10.1523/JNEUROSCI.5855-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Marlatt MW, Potter MC, Lucassen PJ, van Praag H (2012) Running throughout middle-age improves memory function, hippocampal neurogenesis, and BDNF levels in female C57BL/6J mice. Devel Neurobio 72:943–952. 10.1002/dneu.22009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lambertus M, Øverberg LT, Andersson KA et al (2021) L-lactate induces neurogenesis in the mouse ventricular-subventricular zone via the lactate receptor HCA. Acta Physiol 231. 10.1111/apha.13587 [DOI] [PubMed]
- 133.Kim H-T, Chae C-H, Jung S-L et al (2014) Swimming exercise increases neurogenesis in the subventricular zone, and upregulates NGF and Synapsin I level in the olfactory bulb of the adult rats. Biol Sport 31:309–314. 10.5604/20831862.1132130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Brown J, Cooper-Kuhn CM, Kempermann G et al (2003) Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis: environment, exercise and neurogenesis. Eur J Neurosci 17:2042–2046. 10.1046/j.1460-9568.2003.02647.x [DOI] [PubMed] [Google Scholar]
- 135.Saraulli D, Costanzi M, Mastrorilli V, Farioli-Vecchioli S (2017) The long run: neuroprotective effects of physical exercise on adult neurogenesis from youth to old age. CN 15:519–533. 10.2174/1570159X14666160412150223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Niwa A, Nishibori M, Hamasaki S et al (2016) Voluntary exercise induces neurogenesis in the hypothalamus and ependymal lining of the third ventricle. Brain Struct Funct 221:1653–1666. 10.1007/s00429-015-0995-x [DOI] [PubMed] [Google Scholar]
- 137.Clemenson GD (2015) Environmental enrichment and neurogenesis: from mice to humans. Curr Opinion Behav Sci 4:56–62 [Google Scholar]
- 138.Slater AM, Cao L (2015) A protocol for housing mice in an enriched environment. JoVE 100:52874. 10.3791/52874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mora F, Segovia G, del Arco A (2007) Aging, plasticity and environmental enrichment: structural changes and neurotransmitter dynamics in several areas of the brain. Brain Res Rev 55:78–88. 10.1016/j.brainresrev.2007.03.011 [DOI] [PubMed] [Google Scholar]
- 140.Ziegler-Waldkirch S, Marksteiner K, Stoll J et al (2018) Environmental enrichment reverses Aβ pathology during pregnancy in a mouse model of Alzheimer’s disease. Acta Neuropathol Commun 6:44. 10.1186/s40478-018-0549-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liew AKY, Teo CH, Soga T (2022) The molecular effects of environmental enrichment on Alzheimer’s disease. Mol Neurobiol 59:7095–7118. 10.1007/s12035-022-03016-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rossi C, Angelucci A, Costantin L et al (2006) Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur J Neurosci 24:1850–1856. 10.1111/j.1460-9568.2006.05059.x [DOI] [PubMed] [Google Scholar]
- 143.Pham TM, Ickes B, Albeck D et al (1999) Changes in brain nerve growth factor levels and nerve growth factor receptors in rats exposed to environmental enrichment for one year. Neuroscience 94:279–286. 10.1016/S0306-4522(99)00316-4 [DOI] [PubMed] [Google Scholar]
- 144.Young D, Lawlor PA, Leone P et al (1999) Environmental enrichment inhibits spontaneous apoptosis, prevents seizures and is neuroprotective. Nat Med 5:448–453. 10.1038/7449 [DOI] [PubMed] [Google Scholar]
- 145.Cao L, Jiao X, Zuzga DS et al (2004) VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet 36:827–835. 10.1038/ng1395 [DOI] [PubMed] [Google Scholar]
- 146.Eisinger BE, Zhao X (2018) Identifying molecular mediators of environmentally enhanced neurogenesis. Cell Tissue Res 371:7–21. 10.1007/s00441-017-2718-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Suzuki H, Kanagawa D, Nakazawa H et al (2014) Role of neuropsin in parvalbumin immunoreactivity changes in hippocampal basket terminals of mice reared in various environments. Front Cell Neurosci 8. 10.3389/fncel.2014.00420 [DOI] [PMC free article] [PubMed]
- 148.Darcy MJ, Trouche S, Jin S-X, Feig LA (2014) Ras-GRF2 mediates long-term potentiation, survival, and response to an enriched environment of newborn neurons in the hippocampus. Hippocampus 24:1317–1329. 10.1002/hipo.22313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Neeper S, Gómez-Pinilla F, Choi J, Cotman C (1996) Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Research 49–56 [PubMed]
- 150.Greenough WT, Volkmar FR (1973) Pattern of dendritic branching in occipital cortex of rats reared in complex environments. Exp Neurol 40:491–504. 10.1016/0014-4886(73)90090-3 [DOI] [PubMed] [Google Scholar]
- 151.Birch AM, McGarry NB, Kelly ÁM (2013) Short-term environmental enrichment, in the absence of exercise, improves memory, and increases NGF concentration, early neuronal survival, and synaptogenesis in the dentate gyrus in a time-dependent manner. Hippocampus 23:437–450. 10.1002/hipo.22103 [DOI] [PubMed] [Google Scholar]
- 152.Han Y, Yuan M, Guo Y-S et al (2022) The role of enriched environment in neural development and repair. Front Cell Neurosci 16:890666. 10.3389/fncel.2022.890666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kempermann G, Fabel K, Ehninger D et al (2010) Why and how physical activity promotes experience-induced brain plasticity. Front Neurosci 4. 10.3389/fnins.2010.00189 [DOI] [PMC free article] [PubMed]
- 154.Stangl D, Thuret S (2009) Impact of diet on adult hippocampal neurogenesis. Genes Nutr 4:271–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lee J, Seroogy KB, Mattson MP (2002) Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J Neurochem 80(3):539–547 [DOI] [PubMed] [Google Scholar]
- 156.Lee J, Duan W, Mattson MP (2002) Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice: BDNF, dietary restriction and neurogenesis. J Neurochem 82:1367–1375. 10.1046/j.1471-4159.2002.01085.x [DOI] [PubMed] [Google Scholar]
- 157.Mattson MP (2000) Neuroprotective signaling and the aging brain: take away my food and let me run. Brain Res 886(1-2):47–53 [DOI] [PubMed] [Google Scholar]
- 158.Piancino MG, Tortarolo A, Polimeni A et al (2020) Altered mastication adversely impacts morpho-functional features of the hippocampus: a systematic review on animal studies in three different experimental conditions involving the masticatory function. PLoS One 15:e0237872. 10.1371/journal.pone.0237872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Weijenberg RAF, Scherder EJA, Lobbezoo F (2011) Mastication for the mind—the relationship between mastication and cognition in ageing and dementia. Neurosci Biobehav Rev 35:483–497. 10.1016/j.neubiorev.2010.06.002 [DOI] [PubMed] [Google Scholar]
- 160.Yamamoto T, Hirayama A, Hosoe N et al (2009) Soft-diet feeding inhibits adult neurogenesis in hippocampus of mice. Bull Tokyo Dent Coll 50:117–124. 10.2209/tdcpublication.50.117 [DOI] [PubMed] [Google Scholar]
- 161.Patten AR, Moller DJ, Graham J et al (2013) Liquid diets reduce cell proliferation but not neurogenesis in the adult rat hippocampus. Neuroscience 254:173–184. 10.1016/j.neuroscience.2013.09.024 [DOI] [PubMed] [Google Scholar]
- 162.Utsugi C, Miyazono S, Osada K et al (2014) Hard-diet feeding recovers neurogenesis in the subventricular zone and olfactory functions of mice impaired by soft-diet feeding. PLoS One 9:e97309. 10.1371/journal.pone.0097309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Onozuka M, Watanabe K, Fujita M et al (2002) Evidence for involvement of glucocorticoid response in the hippocampal changes in aged molarless SAMP8 mice. Behav Brain Res 131:125–129. 10.1016/S0166-4328(01)00378-3 [DOI] [PubMed] [Google Scholar]
- 164.Aoki H, Kimoto K, Hori N, Toyoda M (2005) Cell proliferation in the dentate gyrus of rat hippocampus is inhibited by soft diet feeding. Gerontology 51:369–374. 10.1159/000088700 [DOI] [PubMed] [Google Scholar]
- 165.Poulose SM, Miller MG, Scott T, Shukitt-Hale B (2017) Nutritional factors affecting adult neurogenesis and cognitive function. Adv Nutr 8:804–811. 10.3945/an.117.016261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Molteni R, Barnard RJ, Ying Z et al (2002) A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience 112:803–814. 10.1016/S0306-4522(02)00123-9 [DOI] [PubMed] [Google Scholar]
- 167.Lindqvist A, Mohapel P, Bouter B et al (2006) High-fat diet impairs hippocampal neurogenesis in male rats. Eur J Neurol 13:1385–1388. 10.1111/j.1468-1331.2006.01500.x [DOI] [PubMed] [Google Scholar]
- 168.Kronenberg G, Harms C, Sobol RW et al (2008) Folate deficiency induces neurodegeneration and brain dysfunction in mice lacking uracil DNA glycosylase. J Neurosci 28:7219–7230. 10.1523/JNEUROSCI.0940-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Smith AD (2016) Hippocampus as a mediator of the role of vitamin B-12 in memory. Am J Clin Nutr 103:959–960. 10.3945/ajcn.116.132266 [DOI] [PubMed] [Google Scholar]
- 170.Nualart F (2014) Unconventional neurogenic niches and neurogenesis modulation by vitamins. J Stem Cell Res Ther 04. 10.4172/2157-7633.1000184 [DOI] [PMC free article] [PubMed]
- 171.Wang T-W, Zhang H, Parent JM (2005) Retinoic acid regulates postnatal neurogenesis in the murine subventricular zone-olfactory bulb pathway. Development 132:2721–2732. 10.1242/dev.01867 [DOI] [PubMed] [Google Scholar]
- 172.Burckhardt M, Herke M, Wustmann T et al (2016) Omega-3 fatty acids for the treatment of dementia. Cochrane Database Syst Rev 2016. 10.1002/14651858.CD009002.pub3 [DOI] [PMC free article] [PubMed]
- 173.Xu Y, Ku B, Cui L et al (2007) Curcumin reverses impaired hippocampal neurogenesis and increases serotonin receptor 1A mRNA and brain-derived neurotrophic factor expression in chronically stressed rats. Brain Res 1162:9–18. 10.1016/j.brainres.2007.05.071 [DOI] [PubMed] [Google Scholar]
- 174.Moriya J, Chen R, Yamakawa J et al (2011) Resveratrol improves hippocampal atrophy in chronic fatigue mice by enhancing neurogenesis and inhibiting apoptosis of granular cells. Biol Pharm Bull 34:354–359. 10.1248/bpb.34.354 [DOI] [PubMed] [Google Scholar]
- 175.Corniola RS, Tassabehji NM, Hare J et al (2008) Zinc deficiency impairs neuronal precursor cell proliferation and induces apoptosis via p53-mediated mechanisms. Brain Res 1237:52–61. 10.1016/j.brainres.2008.08.040 [DOI] [PubMed] [Google Scholar]
- 176.Pulido-Moran M, Moreno-Fernandez J, Ramirez-Tortosa C, Mc R-T (2016) Curcumin and health. Molecules 21:264. 10.3390/molecules21030264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.O’Reilly KC, Shumake J, Bailey SJ et al (2009) Chronic 13-cis-retinoic acid administration disrupts network interactions between the raphe nuclei and the hippocampal system in young adult mice. Eur J Pharmacol 605:68–77. 10.1016/j.ejphar.2008.12.037 [DOI] [PubMed] [Google Scholar]
- 178.Wang R, Wu Z, Bai L et al (2021) Resveratrol improved hippocampal neurogenesis following lead exposure in rats through activation of SIRT1 signaling. Environ Toxicol 36:1664–1673. 10.1002/tox.23162 [DOI] [PubMed] [Google Scholar]
- 179.Li J, Han Y, Li M, Nie C (2019) Curcumin promotes proliferation of adult neural stem cells and the birth of neurons in Alzheimer’s disease mice via notch signaling pathway. Cell Reprogram 21:152–161. 10.1089/cell.2018.0027 [DOI] [PubMed] [Google Scholar]
- 180.Bassani TB, Turnes JM, Moura ELR et al (2017) Effects of curcumin on short-term spatial and recognition memory, adult neurogenesis and neuroinflammation in a streptozotocin-induced rat model of dementia of Alzheimer’s type. Behav Brain Res 335:41–54. 10.1016/j.bbr.2017.08.014 [DOI] [PubMed] [Google Scholar]
- 181.Tiwari SK, Agarwal S, Seth B et al (2014) Curcumin-loaded nanoparticles potently induce adult neurogenesis and reverse cognitive deficits in Alzheimer’s disease model via canonical Wnt/β-catenin pathway. ACS Nano 8:76–103. 10.1021/nn405077y [DOI] [PubMed] [Google Scholar]
- 182.Takamura R, Watamura N, Nikkuni M, Ohshima T (2017) All-trans retinoic acid improved impaired proliferation of neural stem cells and suppressed microglial activation in the hippocampus in an Alzheimer’s mouse model: ATRA improves adult neurogenesis in AD model mice. J Neurosci Res 95:897–906. 10.1002/jnr.23843 [DOI] [PubMed] [Google Scholar]
- 183.Esteves M, Cristóvão AC, Saraiva T et al (2015) Retinoic acid-loaded polymeric nanoparticles induce neuroprotection in a mouse model for Parkinson’s disease. Front Aging Neurosci 7. 10.3389/fnagi.2015.00020 [DOI] [PMC free article] [PubMed]
- 184.Zhu J, Mu X, Zeng J et al (2014) Ginsenoside Rg1 prevents cognitive impairment and hippocampus senescence in a rat model of D-galactose-induced aging. PLoS One 9:e101291. 10.1371/journal.pone.0101291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chen L, Yao H, Chen X et al (2018) Ginsenoside Rg1 decreases oxidative stress and down-regulates Akt/mTOR signalling to attenuate cognitive impairment in mice and senescence of neural stem cells induced by d-galactose. Neurochem Res 43:430–440. 10.1007/s11064-017-2438-y [DOI] [PubMed] [Google Scholar]
- 186.Zhao J, Lu S, Yu H et al (2018) Baicalin and ginsenoside Rb1 promote the proliferation and differentiation of neural stem cells in Alzheimer’s disease model rats. Brain Res 1678:187–194. 10.1016/j.brainres.2017.10.003 [DOI] [PubMed] [Google Scholar]
- 187.Kim H-J, Kim D-J, Shin E-J et al (2016) Effects of gintonin-enriched fraction on hippocampal cell proliferation in wild-type mice and an APPswe/PSEN-1 double Tg mouse model of Alzheimer’s disease. Neurochem Int 101:56–65. 10.1016/j.neuint.2016.10.006 [DOI] [PubMed] [Google Scholar]
- 188.Nam SM, Hwang H, Seo M et al (2018) Gintonin attenuates D-galactose-induced hippocampal senescence by improving long-term hippocampal potentiation, neurogenesis, and cognitive functions. Gerontology 64:562–575. 10.1159/000491113 [DOI] [PubMed] [Google Scholar]
- 189.Lin K, Sze SC-W, Liu B et al (2021) 20(S)-protopanaxadiol and oleanolic acid ameliorate cognitive deficits in APP/PS1 transgenic mice by enhancing hippocampal neurogenesis. J Ginseng Res 45:325–333. 10.1016/j.jgr.2020.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mirza FJ, Amber S, Hassan D, Ahmed T, Zahid S (2021) Rosmarinic acid and ursolic acid alleviate deficits in cognition, synaptic regulation and adult hippocampal neurogenesis in an Aβ1-42-induced mouse model of Alzheimer’s disease. Phytomedicine 83:153490. 10.1016/j.phymed.2021.153490 [DOI] [PubMed] [Google Scholar]
- 191.Tchantchou F, Xu Y, Wu Y et al (2007) EGb 761 enhances adult hippocampal neurogenesis and phosphorylation of CREB in transgenic mouse model of Alzheimer’s disease. FASEB J 21:2400–2408. 10.1096/fj.06-7649com [DOI] [PubMed] [Google Scholar]
- 192.Lei H, Zhang Y, Huang L et al (2018) L-3-n-butylphthalide regulates proliferation, migration, and differentiation of neural stem cell in vitro and promotes neurogenesis in APP/PS1 mouse model by regulating BDNF/TrkB/CREB/Akt pathway. Neurotox Res 34:477–488. 10.1007/s12640-018-9905-3 [DOI] [PubMed] [Google Scholar]
- 193.Grote HE, Bull ND, Howard ML et al (2005) Cognitive disorders and neurogenesis deficits in Huntington’s disease mice are rescued by fluoxetine. Eur J Neurosci 22:2081–2088. 10.1111/j.1460-9568.2005.04365.x [DOI] [PubMed] [Google Scholar]
- 194.Mendonça IP, de Paiva IHR, Duarte-Silva EP et al (2022) Metformin and fluoxetine improve depressive-like behavior in a murine model of Parkinsońs disease through the modulation of neuroinflammation, neurogenesis and neuroplasticity. Int Immunopharmacol 102:108415. 10.1016/j.intimp.2021.108415 [DOI] [PubMed] [Google Scholar]
- 195.Marlatt MW, Potter MC, Bayer TA et al (2013) Prolonged running, not fluoxetine treatment, increases neurogenesis, but does not alter neuropathology, in the 3xTg mouse model of Alzheimer’s disease. In: Belzung C, Wigmore P (eds) Neurogenesis and neural plasticity. Springer, Berlin Heidelberg, Berlin, Heidelberg, pp. 313–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ma J, Gao Y, Jiang L et al (2017) Fluoxetine attenuates the impairment of spatial learning ability and prevents neuron loss in middle-aged APPswe/PSEN1dE9 double transgenic Alzheimer’s disease mice. Oncotarget 8:27676–27692. 10.18632/oncotarget.15398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chadwick W, Mitchell N, Caroll J et al (2011) Amitriptyline-mediated cognitive enhancement in aged 3×Tg Alzheimer’s disease mice is associated with neurogenesis and neurotrophic activity. PLoS One 6:e21660. 10.1371/journal.pone.0021660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lin C-L, Zheng T-L, Tsou S-H et al (2022) Amitriptyline improves cognitive and neuronal function in a rat model that mimics dementia with lewy bodies. Behav Brain Res 435:114035. 10.1016/j.bbr.2022.114035 [DOI] [PubMed] [Google Scholar]
- 199.Cong W-N, Chadwick W, Wang R et al (2015) Amitriptyline improves motor function via enhanced neurotrophin signaling and mitochondrial functions in the murine N171-82Q Huntington Disease Model. J Biol Chem 290:2728–2743. 10.1074/jbc.M114.588608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Fiorentini A, Rosi MC, Grossi C et al (2010) Lithium improves hippocampal neurogenesis, neuropathology and cognitive functions in APP mutant mice. PLoS One 5:e14382. 10.1371/journal.pone.0014382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Voorhees JR, Remy MT, Cintrón-Pérez CJ et al (2018) (−)-P7C3-S243 protects a rat model of Alzheimer’s disease from neuropsychiatric deficits and neurodegeneration without altering amyloid deposition or reactive glia. Biol Psychiatry 84:488–498. 10.1016/j.biopsych.2017.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Sumien N, Wells MS, Sidhu A et al (2021) Novel pharmacotherapy: NNI-362, an allosteric p70S6 kinase stimulator, reverses cognitive and neural regenerative deficits in models of aging and disease. Stem Cell Res Ther 12:59. 10.1186/s13287-020-02126-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Singh S, Mishra A, Srivastava N, Shukla S (2017) MK-801 (Dizocilpine) regulates multiple steps of adult hippocampal neurogenesis and alters psychological symptoms via Wnt/β-catenin signaling in Parkinsonian rats. ACS Chem Neurosci 8:592–605. 10.1021/acschemneuro.6b00354 [DOI] [PubMed] [Google Scholar]
- 204.Stazi M, Wirths O (2021) Chronic memantine treatment ameliorates behavioral deficits, neuron loss, and impaired neurogenesis in a model of Alzheimer’s disease. Mol Neurobiol 58:204–216. 10.1007/s12035-020-02120-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kim SH, Steele JW, Lee SW et al (2014) Proneurogenic Group II mGluR antagonist improves learning and reduces anxiety in Alzheimer Aβ oligomer mouse. Mol Psychiatry 19:1235–1242. 10.1038/mp.2014.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Patil SP, Jain PD, Ghumatkar PJ et al (2014) Neuroprotective effect of metformin in MPTP-induced Parkinson’s disease in mice. Neuroscience 277:747–754. 10.1016/j.neuroscience.2014.07.046 [DOI] [PubMed] [Google Scholar]
- 207.Ou Z, Kong X, Sun X et al (2018) Metformin treatment prevents amyloid plaque deposition and memory impairment in APP/PS1 mice. Brain Behav Immun 69:351–363. 10.1016/j.bbi.2017.12.009 [DOI] [PubMed] [Google Scholar]
- 208.Briyal S, Voshtina R, Gulati A (2021) Sovateltide prevents beta-amyloid plaque load and restores memory deficit in an APP/PS1 mouse model of Alzheimer’s disease. Alzheimers Dement 17. 10.1002/alz.057431
- 209.Siebzehnrübl FA, Raber KA, Urbach YK et al (2018) Early postnatal behavioral, cellular, and molecular changes in models of Huntington disease are reversible by HDAC inhibition. Proc Natl Acad Sci USA 115. 10.1073/pnas.1807962115 [DOI] [PMC free article] [PubMed]
- 210.Babazadeh A, Vahed FM, Liu Q et al (2023) Natural bioactive molecules as neuromedicines for the treatment/prevention of neurodegenerative diseases. ACS Omega 8:3667–3683. 10.1021/acsomega.2c06098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kessas K, Chouari Z, Ghzaiel I et al (2022) Role of bioactive compounds in the regulation of mitochondrial dysfunctions in brain and age-related neurodegenerative diseases. Cells 11:257. 10.3390/cells11020257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kumar H, More SV, Han S-D et al (2012) Promising therapeutics with natural bioactive compounds for improving learning and memory — a review of randomized trials. Molecules 17:10503–10539. 10.3390/molecules170910503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Mohd Sairazi NS, Sirajudeen KNS (2020) Natural products and their bioactive compounds: neuroprotective potentials against neurodegenerative diseases. Evid Based Complementary Altern Med 2020:1–30. 10.1155/2020/6565396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kodali M, Parihar VK, Hattiangady B et al (2015) Resveratrol prevents age-related memory and mood dysfunction with increased hippocampal neurogenesis and microvasculature and reduced glial activation. Sci Rep 5:8075. 10.1038/srep08075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Chen Y-Y, Liu Q-P, An P et al (2022) Ginsenoside Rd: a promising natural neuroprotective agent. Phytomedicine 95. 10.1016/j.phymed.2021.153883 [DOI] [PubMed]
- 216.Rahman MH, Akter R, Bhattacharya T et al (2020) Resveratrol and neuroprotection: impact and its therapeutic potential in Alzheimer’s disease. Front Pharmacol 11:619024. 10.3389/fphar.2020.619024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Behl T, Kaur D, Sehgal A et al (2022) Therapeutic insights elaborating the potential of retinoids in Alzheimer’s disease. Front Pharmacol 13:976799. 10.3389/fphar.2022.976799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Pluta R, Bogucka-Kocka A, Ułamek-Kozioł M et al (2015) Neurogenesis and neuroprotection in postischemic brain neurodegeneration with Alzheimer phenotype: is there a role for curcumin? Folia Neuropathol 2:89–99. 10.5114/fn.2015.52405 [DOI] [PubMed] [Google Scholar]
- 219.Ikram M, Jo MG, Park TJ et al (2021) Oral administration of gintonin protects the brains of mice against Aβ-induced Alzheimer disease pathology: antioxidant and anti-inflammatory effects. Oxidative Med Cell Longev 2021:1–16. 10.1155/2021/6635552 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 220.Ma X, Sun Z, Han X et al (2020) Neuroprotective effect of resveratrol via activation of Sirt1 signaling in a rat model of combined diabetes and Alzheimer’s disease. Front Neurosci 13:1400. 10.3389/fnins.2019.01400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Gomes BAQ, Silva JPB, Romeiro CFR et al (2018) Neuroprotective mechanisms of resveratrol in Alzheimer’s disease: role of SIRT1. Oxidative Med Cell Longev 2018:1–15. 10.1155/2018/8152373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Sun G, Miao Z, Ye Y et al (2020) Curcumin alleviates neuroinflammation, enhances hippocampal neurogenesis, and improves spatial memory after traumatic brain injury. Brain Res Bull 162:84–93. 10.1016/j.brainresbull.2020.05.009 [DOI] [PubMed] [Google Scholar]
- 223.Zarneshan S, Fakhri S, Khan H (2022) Targeting Akt/CREB/BDNF signaling pathway by ginsenosides in neurodegenerative diseases: a mechanistic approach. Pharmacol Res 177. 10.1016/j.phrs.2022.106099 [DOI] [PubMed]
- 224.Alzobaidi N, Quasimi H, Emad N et al (2021) Bioactive compounds and traditional herbal medicine: promising approaches for the treatment of dementia. Degener Neurol Neuromuscul Dis 11:1–14. 10.2147/DNND.S299589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Ikram M, Ullah R, Khan A, Kim MO (2020) Ongoing research on the role of gintonin in the management of neurodegenerative disorders. Cells 9:1464. 10.3390/cells9061464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Uenaka M, Uyeda A, Nakahara T, Muramatsu R (2022) LPA2 promotes neuronal differentiation and neurite formation in neocortical development. Biochem Biophys Res Commun 598:89–94. 10.1016/j.bbrc.2022.01.109 [DOI] [PubMed] [Google Scholar]
- 227.Hu H-B, Song Z-Q, Song G-P et al (2021) LPA signaling acts as a cell-extrinsic mechanism to initiate cilia disassembly and promote neurogenesis. Nat Commun 12:662. 10.1038/s41467-021-20986-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wang Y, Zhang J, Huang L et al (2022) The LPA-CDK5-tau pathway mediates neuronal injury in an in vitro model of ischemia-reperfusion insult. BMC Neurol 22:166. 10.1186/s12883-022-02694-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Ma W, Xu D, Zhao L et al (2022) Therapeutic role of curcumin in adult neurogenesis for management of psychiatric and neurological disorders: a scientometric study to an in-depth review. Crit Rev Food Sci Nutr 63(28):9379–9391 [DOI] [PubMed] [Google Scholar]
- 230.Rein MJ, Renouf M, Cruz-Hernandez C et al (2013) Bioavailability of bioactive food compounds: a challenging journey to bioefficacy: bioavailability of bioactive food compounds. Br J Clin Pharmacol 75:588–602. 10.1111/j.1365-2125.2012.04425.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Jaronsinska O, Rüdiger S (2021) Molecular strategies to target protein aggregation in Huntington’s disease. Front Mol Biosci 8. 10.3389/fmolb.2021.769184 [DOI] [PMC free article] [PubMed]
- 232.Kim WS, Kågedal K, Halliday GM (2014) Alpha-synuclein biology in Lewy body diseases. Alz Res Therapy 6:73. 10.1186/s13195-014-0073-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Murphy MP, LeVine H (2010) Alzheimer’s disease and the amyloid-β peptide. J Alzheimers Dis 19:311–323. 10.3233/JAD-2010-1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kinney JW, Bemiller SM, Murtishaw AS et al (2018) Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s & Dement: Transl Res Clin Intervent 4:575–590. 10.1016/j.trci.2018.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Crotti A, Glass CK (2015) The choreography of neuroinflammation in Huntington’s disease. Trends Immunol 36:364–373. 10.1016/j.it.2015.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Iba M, Kim C, Sallin M et al (2020) Neuroinflammation is associated with infiltration of T cells in Lewy body disease and α-synuclein transgenic models. J Neuroinflammation 17:214. 10.1186/s12974-020-01888-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Garcia D, Shaw RJ (2017) AMPK: mechanisms of cellular energy sensing and restoration of metabolic balance. Mol Cell 66:789–800. 10.1016/j.molcel.2017.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Mueed Z, Tandon P, Maurya SK et al (2019) Tau and mTOR: the hotspots for multifarious diseases in Alzheimer’s development. Front Neurosci 12:1017. 10.3389/fnins.2018.01017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kuang H, Tan C, Tian H et al (2020) Exploring the bi-directional relationship between autophagy and Alzheimer’s disease. CNS Neurosci Ther 26:155–166. 10.1111/cns.13216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Cai Z, Zhou Y, Xiao M et al (2015) Activation of mTOR: a culprit of Alzheimer’s disease? NDT 1015. 10.2147/NDT.S75717 [DOI] [PMC free article] [PubMed]
- 241.Kanigur Sultuybek G, Soydas T, Yenmis G (2019) NF-κB as the mediator of metformin’s effect on ageing and ageing-related diseases. Clin Exp Pharmacol Physiol 46:413–422. 10.1111/1440-1681.13073 [DOI] [PubMed] [Google Scholar]
- 242.Curry DW, Stutz B, Andrews ZB, Elsworth JD (2018) Targeting AMPK signaling as a neuroprotective strategy in Parkinson’s disease. JPD 8:161–181. 10.3233/JPD-171296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Salminen A, Kaarniranta K (2012) AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev 11:230–241. 10.1016/j.arr.2011.12.005 [DOI] [PubMed] [Google Scholar]
- 244.Sportelli C, Urso D, Jenner P, Chaudhuri KR (2020) Metformin as a potential neuroprotective agent in prodromal Parkinson’s disease—viewpoint. Front Neurol 11:556. 10.3389/fneur.2020.00556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zoungrana LI, Krause-Hauch M, Wang H et al (2022) The interaction of mTOR and Nrf2 in neurogenesis and its implication in neurodegenerative diseases. Cells 11:2048. 10.3390/cells11132048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Donega V, Van Der Geest AT, Sluijs JA et al (2022) Single-cell profiling of human subventricular zone progenitors identifies SFRP1 as a target to re-activate progenitors. Nat Commun 13:1036. 10.1038/s41467-022-28626-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Briyal S, Nguyen C, Leonard M, Gulati A (2015) Stimulation of endothelin B receptors by IRL-1620 decreases the progression of Alzheimer’s disease. Neuroscience 301:1–11. 10.1016/j.neuroscience.2015.05.044 [DOI] [PubMed] [Google Scholar]
- 248.Leonard MG, Briyal S, Gulati A (2011) Endothelin B receptor agonist, IRL-1620, reduces neurological damage following permanent middle cerebral artery occlusion in rats. Brain Res 1420:48–58. 10.1016/j.brainres.2011.08.075 [DOI] [PubMed] [Google Scholar]
- 249.Boutet A, Madhavan R, Elias GJB et al (2021) Predicting optimal deep brain stimulation parameters for Parkinson’s disease using functional MRI and machine learning. Nat Commun 12:3043. 10.1038/s41467-021-23311-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kim HS, Shin SM, Kim S et al (2022) Relationship between adult subventricular neurogenesis and Alzheimer’s disease: pathologic roles and therapeutic implications. Front Aging Neurosci 14:1002281. 10.3389/fnagi.2022.1002281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Babcock KR, Page JS, Fallon JR, Webb AE (2021) Adult hippocampal neurogenesis in aging and Alzheimer’s disease. Stem Cell Reports 16:681–693. 10.1016/j.stemcr.2021.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Ghosh HS (2019) Adult neurogenesis and the promise of adult neural stem cells. J Exp Neurosci 13. 10.1177/1179069519856876 [DOI] [PMC free article] [PubMed]
- 253.Dye RV, Miller KJ, Singer EJ, Levine AJ (2012) Hormone replacement therapy and risk for neurodegenerative diseases. Int J Alzheimers Dis 2012:1–18. 10.1155/2012/258454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Duarte-Guterman P, Lieblich SE, Wainwright SR et al (2019) Androgens enhance adult hippocampal neurogenesis in males but not females in an age-dependent manner. Endocrinology 160:2128–2136. 10.1210/en.2019-00114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Lennington JB, Yang Z, Conover JC (2003) Neural stem cells and the regulation of adult neurogenesis. Reprod Biol Endocrinol 1 [DOI] [PMC free article] [PubMed]
- 256.Wan L, Huang R-J, Luo Z-H et al (2021) Reproduction-associated hormones and adult hippocampal neurogenesis. Neural Plast 2021:1–20. 10.1155/2021/3651735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Hannan AJ, Ransome MI (2012) Deficits in spermatogenesis but not neurogenesis are alleviated by chronic testosterone therapy in R6/1 Huntington’s disease mice: testosterone as a chronic therapy for Huntington’s disease. J Neuroendocrinol 24:341–356. 10.1111/j.1365-2826.2011.02238.x [DOI] [PubMed] [Google Scholar]
- 258.Depypere H, Vergallo A, Lemercier P et al (2022) Menopause hormone therapy significantly alters pathophysiological biomarkers of Alzheimer’s disease. Alzheimer’s Dement:12759. 10.1002/alz.12759 [DOI] [PubMed]
- 259.Castilla-Cortázar I, Aguirre GA, Femat-Roldán G et al (2020) Is insulin-like growth factor-1 involved in Parkinson’s disease development? J Transl Med 18:70. 10.1186/s12967-020-02223-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Kerr JE, Allore RJ, Beck SG, Handa RJ (1995) Distribution and hormonal regulation of androgen receptor (AR) and AR messenger ribonucleic acid in the rat hippocampus. Endocrinology 136. 10.1210/endo.136.8.7628354 [DOI] [PubMed]
- 261.Bianchi VE, Rizzi L, Bresciani E et al (2020) Androgen therapy in neurodegenerative diseases. J Endocrine Soc 4. 10.1210/jendso/bvaa120 [DOI] [PMC free article] [PubMed]
- 262.DonCarlos LL, Garcia-Ovejero D, Sarkey S et al (2003) Androgen receptor immunoreactivity in forebrain axons and dendrites in the rat. Endocrinology 144:3632–3638. 10.1210/en.2002-0105 [DOI] [PubMed] [Google Scholar]
- 263.Blankers SA, Galea LAM (2021) Androgens and adult neurogenesis in the hippocampus. Androgens: Clin Res Ther 2:203–215. 10.1089/andro.2021.0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Galea LAM, Wainwright SR, Roes MM et al (2013) Sex, hormones and neurogenesis in the hippocampus: hormonal modulation of neurogenesis and potential functional implications. J Neuroendocrinol 25:1039–1061. 10.1111/jne.12070 [DOI] [PubMed] [Google Scholar]
- 265.Hatanaka Y, Hojo Y, Mukai H et al (2015) Rapid increase of spines by dihydrotestosterone and testosterone in hippocampal neurons: dependence on synaptic androgen receptor and kinase networks. Brain Res 1621:121–132. 10.1016/j.brainres.2014.12.011 [DOI] [PubMed] [Google Scholar]
- 266.Bustamante-Barrientos FA, Méndez-Ruette M, Ortloff A et al (2021) The impact of estrogen and estrogen-like molecules in neurogenesis and neurodegeneration: beneficial or harmful? Front Cell Neurosci 15:636176. 10.3389/fncel.2021.636176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Brinton RD, Thompson RF, Foy MR et al (2008) Progesterone receptors: form and function in brain. Front Neuroendocrinol 29:313–339. 10.1016/j.yfrne.2008.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Brann DW, Dhandapani K, Wakade C et al (2007) Neurotrophic and neuroprotective actions of estrogen: basic mechanisms and clinical implications. Steroids 72:381–405. 10.1016/j.steroids.2007.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Sheppard PAS, Choleris E, Galea LAM (2019) Structural plasticity of the hippocampus in response to estrogens in female rodents. Mol Brain 12:22. 10.1186/s13041-019-0442-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Shuster LT, Rhodes DJ, Gostout BS et al (2010) Premature menopause or early menopause: long-term health consequences. Maturitas 65:161–166. 10.1016/j.maturitas.2009.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Zheng J, Liang K, Wang X et al (2017) Chronic estradiol administration during the early stage of Alzheimer’s disease pathology rescues adult hippocampal neurogenesis and ameliorates cognitive deficits in Aβ1-42 mice. Mol Neurobiol 54:7656–7669. 10.1007/s12035-016-0181-z [DOI] [PubMed] [Google Scholar]
- 272.Lee RJ, Kim JK, Chao D et al (2015) Progesterone and allopregnanolone improves stroke outcome in male mice via distinct mechanisms but neither promotes neurogenesis. J Neurochem 132:32–37. 10.1111/jnc.12990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Brinton R, Wang J (2006) Preclinical analyses of the therapeutic potential of allopregnanolone to promote neurogenesis in vitro and in vivo in transgenic mouse model of Alzheimers disease. Curr Alzheimer Res 3:11–17. 10.2174/156720506775697160 [DOI] [PubMed] [Google Scholar]
- 274.Chen S, Wang T, Yao J, Brinton RD (2020) Allopregnanolone promotes neuronal and oligodendrocyte differentiation in vitro and in vivo: therapeutic implication for Alzheimer’s disease. Neurotherapeutics 17:1813–1824. 10.1007/s13311-020-00874-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Liu L, Zhao L, She H et al (2010) Clinically relevant progestins regulate neurogenic and neuroprotective responses in vitro and in vivo. Endocrinology 151:5782–5794. 10.1210/en.2010-0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Chen S, Kumar N, Mao Z et al (2018) Therapeutic progestin segesterone acetate promotes neurogenesis: implications for sustaining regeneration in female brain. Menopause 25:1138–1151. 10.1097/GME.0000000000001135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Kasubuchi M, Watanabe K, Hirano K et al (2017) Membrane progesterone receptor beta (mPRβ/Paqr8) promotes progesterone-dependent neurite outgrowth in PC12 neuronal cells via non-G protein-coupled receptor (GPCR) signaling. Sci Rep 7:5168. 10.1038/s41598-017-05423-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Hernandez GD, Brinton RD (2022) Allopregnanolone: regenerative therapeutic to restore neurological health. Neurobiol Stress 21:100502. 10.1016/j.ynstr.2022.100502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Schumacher M, Denier C, Oudinet J-P et al (2016) Progesterone neuroprotection: the background of clinical trial failure. J Steroid Biochem Mol Biol 160:53–66. 10.1016/j.jsbmb.2015.11.010 [DOI] [PubMed] [Google Scholar]
- 280.Sitruk-Ware R, Bonsack B, Brinton R et al (2021) Progress in progestin-based therapies for neurological disorders. Neurosci Biobehav Rev 122:38–65. 10.1016/j.neubiorev.2020.12.007 [DOI] [PubMed] [Google Scholar]
- 281.Chan M, Chow C, Hamson DK et al (2014) Effects of chronic oestradiol, progesterone and medroxyprogesterone acetate on hippocampal neurogenesis and adrenal mass in adult female rats. J Neuroendocrinol 26:386–399. 10.1111/jne.12159 [DOI] [PubMed] [Google Scholar]
- 282.Shingo T, Gregg C, Enwere E et al (2003) Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science 299:117–120. 10.1126/science.1076647 [DOI] [PubMed] [Google Scholar]
- 283.Holubová M, Hrubá L, Popelová A et al (2019) Liraglutide and a lipidized analog of prolactin-releasing peptide show neuroprotective effects in a mouse model of β-amyloid pathology. Neuropharmacology 144:377–387. 10.1016/j.neuropharm.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 284.Nguyen HD, Yu BP, Hoang NHM et al (2021) Prolactin and its altered action in Alzheimer’s disease and Parkinson’s disease. Neuroendocrinology. 10.1159/000517798 [DOI] [PubMed]
- 285.Nguyen H, Hoang NMH, Ko M et al (2022) Association between serum prolactin levels and neurodegenerative diseases: systematic review and meta-analysis. Neuroimmunomodulation 29:85–96. 10.1159/000519552 [DOI] [PubMed] [Google Scholar]
- 286.Irwin RW, Wang JM, Chen S, Brinton RD (2012) Neuroregenerative mechanisms of allopregnanolone in Alzheimer’s disease. Front Endocrinol 2. 10.3389/fendo.2011.00117 [DOI] [PMC free article] [PubMed]
- 287.Abbott LC, Nigussie F (2020) Adult neurogenesis in the mammalian dentate gyrus. Anat Histol Embryol 49:3–16. 10.1111/ahe.12496 [DOI] [PubMed] [Google Scholar]
- 288.Spritzer MD, Galea LAM (2007) Testosterone and dihydrotestosterone, but not estradiol, enhance survival of new hippocampal neurons in adult male rats. Devel Neurobio 67:1321–1333. 10.1002/dneu.20457 [DOI] [PubMed] [Google Scholar]
- 289.Lentz TB, Gray SJ, Samulski RJ (2012) Viral vectors for gene delivery to the central nervous system. Neurobiol Dis 48:179–188. 10.1016/j.nbd.2011.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Yao L, Yao S, Daly W et al (2012) Non-viral gene therapy for spinal cord regeneration. Drug Discov Today 17:998–1005. 10.1016/j.drudis.2012.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Tan J-KY, Sellers DL, Pham B et al (2016) Non-viral nucleic acid delivery strategies to the central nervous system. Front Mol Neurosci 9. 10.3389/fnmol.2016.00108 [DOI] [PMC free article] [PubMed]
- 292.Fisher L, Ray J (1994) In vivo and ex vivo gene transfer to the brain. Curr Opin Neurobiol:735–741. 10.1016/0959-4388(94)90017-5 [DOI] [PubMed]
- 293.Nilsson P, Iwata N, Muramatsu S et al (2010) Gene therapy in Alzheimer’s disease - potential for disease modification. J Cell Mol Med 14:741–757. 10.1111/j.1582-4934.2010.01038.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Oproescu A-M, Han S, Schuurmans C (2021) New insights into the intricacies of proneural gene regulation in the embryonic and adult cerebral cortex. Front Mol Neurosci 14:642016. 10.3389/fnmol.2021.642016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Lu H, Hao Z, Jiao Q et al (2011) Neurotrophin-3 gene transduction of mouse neural stem cells promotes proliferation and neuronal differentiation in organotypic hippocampal slice cultures. Med Sci Monit 17:305–311. 10.12659/MSM.882039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Benraiss A, Chmielnicki E, Lerner K et al (2001) Adenoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cells in the adult forebrain. J Neurosci 21:6718–6731. 10.1523/JNEUROSCI.21-17-06718.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Bond AM, Bhalala OG, Kessler JA (2012) The dynamic role of bone morphogenetic proteins in neural stem cell fate and maturation. Devel Neurobio 72:1068–1084. 10.1002/dneu.22022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Navarro Quiroz E, Navarro Quiroz R, Ahmad M et al (2018) Cell signaling in neuronal stem cells. Cells 7:75. 10.3390/cells7070075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Martins F, Santos I, da Cruz e Silva OAB et al (2021) The role of the integral type II transmembrane protein BRI2 in health and disease. Cell Mol Life Sci 78:6807–6822. 10.1007/s00018-021-03932-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Martins F, Serrano JB, Müller T et al (2017) BRI2 processing and its neuritogenic role are modulated by protein phosphatase 1 complexing. J Cell Biochem 118:2752–2763. 10.1002/jcb.25925 [DOI] [PubMed] [Google Scholar]
- 301.Ma Q, Kintner C, Anderson DJ (1996) Identification of neurogenin, a vertebrate neuronal determination gene. Cell 87:43–52. 10.1016/S0092-8674(00)81321-5 [DOI] [PubMed] [Google Scholar]
- 302.Sun Y, Nadal-Vicens M, Misono S et al (2001) Neurogenin promotes neurogenesis and inhibits glial differentiation by independent mechanisms. Cell 104:365–376. 10.1016/S0092-8674(01)00224-0 [DOI] [PubMed] [Google Scholar]
- 303.Castro DS, Martynoga B, Parras C et al (2011) A novel function of the proneural factor Ascl1 in progenitor proliferation identified by genome-wide characterization of its targets. Genes Dev 25:930–945. 10.1101/gad.627811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Sudhakar V, Richardson M (2019) Gene therapy for neurodegenerative diseases. Neurotherapeutics 16. 10.1007/s13311-018-00694-0 [DOI] [PMC free article] [PubMed]
- 305.Henry RA, Hughes SM, Connor B (2007) AAV-mediated delivery of BDNF augments neurogenesis in the normal and quinolinic acid-lesioned adult rat brain. Eur J Neurosci 25:3513–3525. 10.1111/j.1460-9568.2007.05625.x [DOI] [PubMed] [Google Scholar]
- 306.Cho S-R, Benraiss A, Chmielnicki E et al (2007) Induction of neostriatal neurogenesis slows disease progression in a transgenic murine model of Huntington disease. J Clin Invest 117:2889–2902. 10.1172/JCI31778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Yang J, Wu S, Hou L et al (2020) Therapeutic effects of simultaneous delivery of nerve growth factor mRNA and protein via exosomes on cerebral ischemia. Mol Ther- Nucleic Acids 21:512–522. 10.1016/j.omtn.2020.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Pardo J, Uriarte M, Cónsole GM et al (2016) Insulin-like growth factor-I gene therapy increases hippocampal neurogenesis, astrocyte branching and improves spatial memory in female aging rats. Eur J Neurosci 44:2120–2128. 10.1111/ejn.13278 [DOI] [PubMed] [Google Scholar]
- 309.Christine CW, Starr PA, Larson PS et al (2009) Safety and tolerability of putaminal AADC gene therapy for Parkinson disease. Neurology 73:1662–1669. 10.1212/WNL.0b013e3181c29356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Eriksdotter-Jönhagen M, Linderoth B, Lind G et al (2012) Encapsulated cell biodelivery of nerve growth factor to the basal forebrain in patients with Alzheimer’s disease. Dement Geriatr Cogn Disord 33:18–28. 10.1159/000336051 [DOI] [PubMed] [Google Scholar]
- 311.Parambi DGT, Alharbi KS, Kumar R et al (2022) Gene therapy approach with an emphasis on growth factors: theoretical and clinical outcomes in neurodegenerative diseases. Mol Neurobiol 59:191–233. 10.1007/s12035-021-02555-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Eyjolfsdottir H, Eriksdotter M, Linderoth B et al (2016) Targeted delivery of nerve growth factor to the cholinergic basal forebrain of Alzheimer’s disease patients: application of a second-generation encapsulated cell biodelivery device. Alz Res Therapy 8:30. 10.1186/s13195-016-0195-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Orive G, Santos E, Poncelet D et al (2015) Cell encapsulation: technical and clinical advances. Trends Pharmacol Sci 36:537–546. 10.1016/j.tips.2015.05.003 [DOI] [PubMed] [Google Scholar]
- 314.Emerich DF, Orive G, Thanos C et al (2014) Encapsulated cell therapy for neurodegenerative diseases: from promise to product. Adv Drug Deliv Rev 67–68:131–141. 10.1016/j.addr.2013.07.008 [DOI] [PubMed] [Google Scholar]
- 315.Gomez-Nicola D, Suzzi S, Vargas-Caballero M et al (2014) Temporal dynamics of hippocampal neurogenesis in chronic neurodegeneration. Brain 137:2312–2328. 10.1093/brain/awu155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Latchney SE, Eisch AJ (2012) Therapeutic application of neural stem cells and adult neurogenesis for neurodegenerative disorders: regeneration and beyond. Eur J Neurodegener Dis 1:335–351 [PMC free article] [PubMed] [Google Scholar]
- 317.Sivandzade F, Cucullo L (2021) Regenerative stem cell therapy for neurodegenerative diseases: an overview. IJMS 22:2153. 10.3390/ijms22042153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Nasser M, Ballout N, Mantash S et al (2018) Transplantation of embryonic neural stem cells and differentiated cells in a controlled cortical impact (CCI) model of adult mouse somatosensory cortex. Front Neurol 9:895. 10.3389/fneur.2018.00895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Genchi A, Brambilla E, Sangalli F et al (2023) Neural stem cell transplantation in patients with progressive multiple sclerosis: an open-label, phase 1 study. Nat Med 29:75–85. 10.1038/s41591-022-02097-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Hayashi Y, Lin H-T, Lee C-C, Tsai K-J (2020) Effects of neural stem cell transplantation in Alzheimer’s disease models. J Biomed Sci 27:29. 10.1186/s12929-020-0622-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Fujiwara N, Shimizu J, Takai K et al (2013) Restoration of spatial memory dysfunction of human APP transgenic mice by transplantation of neuronal precursors derived from human iPS cells. Neurosci Lett 557:129–134. 10.1016/j.neulet.2013.10.043 [PubMed] [Google Scholar]
- 322.Bai W, Zhang Y, Xu W et al (2020) Isolation and characterization of neural progenitor cells from bone marrow in cell replacement therapy of brain injury. Front Cell Neurosci 14:49. 10.3389/fncel.2020.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Wang Q, Matsumoto Y, Shindo T et al (2006) Neural stem cells transplantation in cortex in a mouse model of Alzheimer’s disease. J Med Investig 53:61–69. 10.2152/jmi.53.61 [DOI] [PubMed] [Google Scholar]
- 324.Lajtha A (2009) Handbook of neurochemistry and molecular neurobiology - brain and spinal cord trauma, 3rd edn. Springer Reference [Google Scholar]
- 325.Lo B, Parham L (2009) Ethical issues in stem cell research. Endocr Rev 30:204–213. 10.1210/er.2008-0031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Qu T, Brannen CL, Kim HM, Sugaya K (2001) Human neural stem cells improve cognitive function of aged brain. Neuroreport 12. 10.1097/00001756-200105080-00016 [DOI] [PubMed]
- 327.Lee S-T, Chu K, Park J-E et al (2005) Intravenous administration of human neural stem cells induces functional recovery in Huntington’s disease rat model. Neurosci Res 52:243–249. 10.1016/j.neures.2005.03.016 [DOI] [PubMed] [Google Scholar]
- 328.Njie emalick G, Kantorovich S, Astary GW et al (2012) A preclinical assessment of neural stem cells as delivery vehicles for anti-amyloid therapeutics. PLoS One 7:e34097. 10.1371/journal.pone.0034097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Bruggeman KF, Moriarty N, Dowd E et al (2019) Harnessing stem cells and biomaterials to promote neural repair: stem cells and biomaterials in neural repair. Br J Pharmacol 176:355–368. 10.1111/bph.14545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Teixeira AI, Duckworth JK, Hermanson O (2007) Getting the right stuff: controlling neural stem cell state and fate in vivo and in vitro with biomaterials. Cell Res 17:56–61. 10.1038/sj.cr.7310141 [DOI] [PubMed] [Google Scholar]
- 331.Yang H, Xie ZH, Wei LF et al (2013) Human umbilical cord mesenchymal stem cell-derived neuron-like cells rescue memory deficits and reduce amyloid-beta deposition in an AβPP/PS1 transgenic mouse model. Stem Cell Res Ther 4:76. 10.1186/scrt227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Fortin JM, Azari H, Zheng T et al (2016) Transplantation of defined populations of differentiated human neural stem cell progeny. Sci Rep 6:23579. 10.1038/srep23579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Guillaume DJ, Zhang S-C (2008) Human embryonic stem cells: a potential source of transplantable neural progenitor cells. Neurosurg Focus 24:E3. 10.3171/FOC/2008/24/3-4/E2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Galiakberova AA, Dashinimaev EB (2020) Neural stem cells and methods for their generation from induced pluripotent stem cells in vitro. Front Cell Dev Biol 8:815. 10.3389/fcell.2020.00815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Chang E-A, Jin S-W, Nam M-H, Kim S-D (2019) Human induced pluripotent stem cells : clinical significance and applications in neurologic diseases. J Korean Neurosurg Soc 62:493–501. 10.3340/jkns.2018.0222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Xu P, He H, Gao Q et al (2022) Human midbrain dopaminergic neuronal differentiation markers predict cell therapy outcomes in a Parkinson’s disease model. J Clin Invest 132. 10.1172/JCI156768 [DOI] [PMC free article] [PubMed]
- 337.Chan HJ, Yanshree RJ et al (2021) Therapeutic potential of human stem cell implantation in Alzheimer’s disease. IJMS 22:10151. 10.3390/ijms221810151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Schweitzer JS, Song B, Herrington TM et al (2020) Personalized iPSC-derived dopamine progenitor cells for Parkinson’s disease. N Engl J Med 382:1926–1932. 10.1056/NEJMoa1915872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Choi D-H, Kim J-H, Kim S et al (2017) Therapeutic potential of induced neural stem cells for Parkinson’s disease. IJMS 18:224. 10.3390/ijms18010224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Bocchi R, Masserdotti G, Götz M (2022) Direct neuronal reprogramming: fast forward from new concepts toward therapeutic approaches. Neuron 110:366–393. 10.1016/j.neuron.2021.11.023 [DOI] [PubMed] [Google Scholar]
- 341.Wang L, Zhang C (2022) In vivo glia-to-neuron conversion: pitfalls and solutions. Dev Neurobiol 82:367–374. 10.1002/dneu.22880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Zhang L, Yin J-C, Yeh H et al (2015) Small molecules efficiently reprogram human astroglial cells into functional neurons. Cell Stem Cell 17:735–747. 10.1016/j.stem.2015.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Liu K, Ma W, Li C et al (2020) Advances in transcription factors related to neuroglial cell reprogramming. Transl Neurosci 11:17–27. 10.1515/tnsci-2020-0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Egawa N, Suzuki H, Takahashi R et al (2020) From in vitro to in vivo reprogramming for neural transdifferentiation: an approach for CNS tissue remodeling using stem cell technology. J Cereb Blood Flow Metab 40:1739–1751. 10.1177/0271678X20910324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Sirko S, Behrendt G, Johansson PA et al (2013) Reactive glia in the injured brain acquire stem cell properties in response to sonic hedgehog. Cell Stem Cell 12:426–439. 10.1016/j.stem.2013.01.019 [DOI] [PubMed] [Google Scholar]
- 346.Robel S, Berninger B, Götz M (2011) The stem cell potential of glia: lessons from reactive gliosis. Nat Rev Neurosci 12:88–104. 10.1038/nrn2978 [DOI] [PubMed] [Google Scholar]
- 347.Wang Y, Zhang X, Chen F et al (2021) In vivo direct conversion of astrocytes to neurons maybe a potential alternative strategy for neurodegenerative diseases. Front Aging Neurosci 13:689276. 10.3389/fnagi.2021.689276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Pekny M, Pekna M (2014) Astrocyte reactivity and reactive astrogliosis: costs and benefits. Physiol Rev 94:1077–1098. 10.1152/physrev.00041.2013 [DOI] [PubMed] [Google Scholar]
- 349.Karakaş N, Bay S, Türkel N et al (2020) Neurons from human mesenchymal stem cells display both spontaneous and stimuli responsive activity. PLoS One 15:e0228510. 10.1371/journal.pone.0228510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Bahlakeh G, Rahbarghazi R, Abedelahi A et al (2022) Neurotrophic factor-secreting cells restored endogenous hippocampal neurogenesis through the Wnt/β-catenin signaling pathway in AD model mice. Stem Cell Res Ther 13:343. 10.1186/s13287-022-03024-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Tfilin M, Sudai E, Merenlender A et al (2010) Mesenchymal stem cells increase hippocampal neurogenesis and counteract depressive-like behavior. Mol Psychiatry 15:1164–1175. 10.1038/mp.2009.110 [DOI] [PubMed] [Google Scholar]
- 352.Kim C, D’Annibale M, Benoiton B et al (2022) The impact of intermittent energy restriction and mastication on hippocampal cognitive ageing and neural stem cell fate: the change study – chewing, adult neurogenesis and energy restriction. Proc Nutr Soc 81:E16. 10.1017/S0029665122000167 [Google Scholar]
- 353.Kim C, Miquel S, Thuret S (2019) A 3-month mastication intervention improves recognition memory. Nutrition and Healthy Aging 5:33–42. 10.3233/NHA-180047 [Google Scholar]
- 354.Kumar A, Pareek V, Faiq M et al (2019) Adult neurogenesis in humans: a review of basic concepts, history, current research, and clinical implications. Innov Clin Neurosci 16:30–37 [PMC free article] [PubMed] [Google Scholar]
- 355.Kim C, Pinto AM, Bordoli C et al (2020) Energy restriction enhances adult hippocampal neurogenesis-associated memory after four weeks in an adult human population with central obesity; a randomized controlled trial. Nutrients 12:638. 10.3390/nu12030638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Just N, Chevillard P-M, Migaud M (2022) Imaging and spectroscopic methods to investigate adult neurogenesis in vivo: new models and new avenues. Front Neurosci 16:933947. 10.3389/fnins.2022.933947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Liu CS, Herrmann N, Song BX et al (2021) Exercise priming with transcranial direct current stimulation: a study protocol for a randomized, parallel-design, sham-controlled trial in mild cognitive impairment and Alzheimer’s disease. BMC Geriatr 21:677. 10.1186/s12877-021-02636-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Naegelin Y, Dingsdale H, Säuberli K et al (2018) Measuring and validating the levels of brain-derived neurotrophic factor in human serum. eNeuro 5. 10.1523/ENEURO.0419-17.2018 [DOI] [PMC free article] [PubMed]
- 359.Zhao X, van Praag H (2020) Steps towards standardized quantification of adult neurogenesis. Nat Commun 11:4275. 10.1038/s41467-020-18046-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Nemati R, Mehdizadeh S, Nabipour I et al (2014) Radiolabeled neurogenesis marker imaging: a revolution in the neurological diseases management? Med Hypotheses 82:215–218. 10.1016/j.mehy.2013.11.041 [DOI] [PubMed] [Google Scholar]
- 361.Trinchero MF, Herrero M, Schinder AF (2019) Rejuvenating the brain with chronic exercise through adult neurogenesis. Front Neurosci 13:1000. 10.3389/fnins.2019.01000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Firth J, Stubbs B, Vancampfort D et al (2018) Effect of aerobic exercise on hippocampal volume in humans: a systematic review and meta-analysis. NeuroImage 166:230–238. 10.1016/j.neuroimage.2017.11.007 [DOI] [PubMed] [Google Scholar]
- 363.Petzinger GM, Fisher BE, McEwen S et al (2013) Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson’s disease. The Lancet Neurol 12:716–726. 10.1016/S1474-4422(13)70123-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Fisher BE, Wu AD, Salem GJ et al (2008) The effect of exercise training in improving motor performance and corticomotor excitability in people with early Parkinson’s disease. Arch Phys Med Rehabil 89:1221–1229. 10.1016/j.apmr.2008.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Erickson KI, Voss MW, Prakash RS et al (2011) Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA 108:3017–3022. 10.1073/pnas.1015950108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Smith N, Miquel-Kergoat S, Thuret S (2016) The impact of mastication on cognition: evidence for intervention and the role of adult hippocampal neurogenesis. Nutrition and Aging 3:115–123. 10.3233/NUA-150054 [Google Scholar]
- 367.Delwel S, Binnekade TT, Perez RSGM et al (2018) Oral hygiene and oral health in older people with dementia: a comprehensive review with focus on oral soft tissues. Clin Oral Investig 22:93–108. 10.1007/s00784-017-2264-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Lee KH, Wu B, Plassman BL (2013) Cognitive function and oral health-related quality of life in older adults. J Am Geriatr Soc 61:1602–1607. 10.1111/jgs.12402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Farzaei MH, Rahimi R, Nikfar S, Abdollahi M (2018) Effect of resveratrol on cognitive and memory performance and mood: a meta-analysis of 225 patients. Pharmacol Res 128:338–344. 10.1016/j.phrs.2017.08.009 [DOI] [PubMed] [Google Scholar]
- 370.Tsai I-C, Hsu C-W, Chang C-H et al (2021) The effect of curcumin differs on individual cognitive domains across different patient populations: a systematic review and meta-analysis. Pharmaceuticals 14:1235. 10.3390/ph14121235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Zhu L-N, Mei X, Zhang Z-G et al (2019) Curcumin intervention for cognitive function in different types of people: a systematic review and meta-analysis: curcumin intervention for cognitive function. Phytother Res 33:524–533. 10.1002/ptr.6257 [DOI] [PubMed] [Google Scholar]
- 372.Hernandez GD, Solinsky CM, Mack WJ et al (2020) Safety, tolerability, and pharmacokinetics of allopregnanolone as a regenerative therapeutic for Alzheimer’s disease: a single and multiple ascending dose phase 1b/2a clinical trial. A&D Transl Res & Clin Interv 6. 10.1002/trc2.12107 [DOI] [PMC free article] [PubMed]
- 373.Raikes AC, Hernandez GD, Matthews DC et al (2022) Exploratory imaging outcomes of a phase 1b/2a clinical trial of allopregnanolone as a regenerative therapeutic for Alzheimer’s disease: structural effects and functional connectivity outcomes. A&D Transl Res & Clin Interv 8. 10.1002/trc2.12258 [DOI] [PMC free article] [PubMed]
- 374.Lee H, Thuret S (2018) Adult human hippocampal neurogenesis: controversy and evidence. Trends Mol Med 24:521–522. 10.1016/j.molmed.2018.04.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.