Abstract
Background
We recently reported that the self-care management system for heart failure (HF) decreased re-hospitalization for HF. In the present study we estimate the cost-effectiveness of this system.
Methods and Results
We retrospectively enrolled 569 consecutive patients who were admitted for HF treatment at Kitano Hospital. In the present analysis, we sought to compare cardiovascular healthcare costs and the incremental cost-effective ratio (ICER), expressed as the cost per quality-adjusted life-years (QALY) gained, between patients using the self-care management system (n=153) and those not using the system (n=153) after propensity-score matching. To calculate the QALY, we used the New York Heart Association class and the corresponding scores of quality of life in every 3 months. The healthcare costs of cardiovascular disease were ¥129,747,016 in the user group and ¥156,427,032 in the non-user group, where 24 and 43 patients were hospitalized, respectively. The cost of this new system was ¥50,000 in the user group. The total costs were ¥129,797,016 in the user group and ¥156,427,032 in the non-user group. By using the system, the QALY increased from 0.653 to 0.686. The ICER was below 0 and the system was interpreted as cost-effective.
Conclusions
Use of the self-care management system is likely to be a cost-effective treatment for HF with the increase in QALY and the decrease in healthcare costs.
Key Words: Cost-effectiveness, Heart failure, Rehospitalization, Self-care management
The prevalence of heart failure (HF) is rising with an aging population, and the economic burden of caring for HF patients is substantial.1,2 In Japan, HF-related hospitalizations alone cost over 10 billion dollars per year in 2014.3 Given the increasing financial pressures, developing a management system to prevent readmissions is an urgent need in HF management. Recently, we developed a new, easy-to-use self-care management system.4,5 This HF self-care management system allowed patients (and caregivers) to record HF ‘points’ for weight and clinical symptoms in their own sheet book. The total scores guide patients on appropriate actions and advise unplanned visits to healthcare providers.4 We recently reported that implementing this self-care management system reduced HF rehospitalizations over 1 year using a propensity-score (PS) matching cohort.4,5 However, it remains unclear whether this system is cost-effective in the context of medical practice in Japan.
In this study, we estimated the cost-effectiveness of the self-care management system by comparing cardiovascular healthcare costs and the incremental cost-effectiveness ratio (ICER), expressed as the cost per quality-adjusted life-years (QALY) gained, between patients using the self-care system and those not using the system.
Methods
Self-Care Management System
To facilitate easier and more uniform self-care management for patients with HF, we developed and implemented a new self-care sheet book in November 2015 for all patients admitted for HF treatment (Supplementary Figure A,B).4 The main feature of this sheet book is that it assigns points to weight and HF symptoms, termed ‘HF points’ (Supplementary Figure C). These points guide patients on when to visit the emergency department, make unplanned visits, or consult the nearest physician (Supplementary Figure D). HF points are allocated as follows: 1 point for each initial symptom of HF (dyspnea on exertion, edema, cough, and appetite loss); 3 points for weight increase exceeding the set body weight; 4 points for a heart rate of ≥120 beasts/min; and 5 points for dyspnea at rest.4 A heart rate of 120 beasts/min is set at 4 points to detect and treat atrial fibrillation early (Supplementary Figure C).4 When introducing HF points to hospitalized patients, a team conference at the beginning of hospitalization determined their applicability. If not applicable, a system was introduced where a cohabitant, a young family member living nearby, a caregiver, or a visiting nurse would check HF points at least once a week.4
Study Population
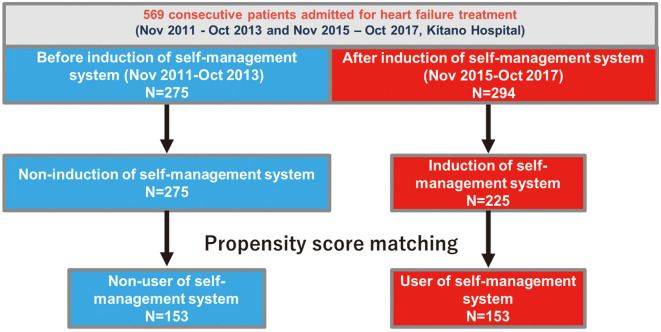
We adapted the self-management system in November 2015 for all patients admitted for HF treatment. This study retrospectively enrolled 275 consecutive patients admitted for HF treatment at Kitano Hospital between November 2011 and October 2013, and 294 consecutive patients between November 2015 and October 2017 (before and after the self-management system implementation, respectively), without any exclusion criteria. We previously compared outcomes between the user group (n=153) and the non-user group (n=153) of the self-care management system after PS matching (Figure 1).4 In this analysis, we compared costs between users (n=153) and non-users (n=153) of the self-care management system (Figure 1). Details of the study population and PS matching are described in the Supplementary Methods.
Figure 1.
Flowchart of patient selection.
Ethics
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Institutional Review Board of Kitano Hospital, Tazuke Kofukai Medical Research Institute (P190600100), who waived the need for informed consent for the study due the retrospective study design.
Total Cost and QALY
The total 1-year healthcare cost for rehospitalization for cardiovascular disease after the initial hospitalization was summed for each patient in both groups using claim data. We calculated the QALY for the user and non-user groups. The discharge date is set to day 0, and the 1-year survival curve was divided into 3-month intervals, using survival rates at 60, 90, 180, and 365 days. We assigned New York Heart Association (NYHA) class and corresponding quality of life scores every 3 months. Patients rehospitalized within each 3 months were classified as NYHA 4. Other patients were classified into NYHA 1–3 according to the Japanese heart failure registry.6 QALY is the effectiveness metric used, with each health state associated with a utility value from 0 to 1 (0 represents death, 1 represents ideal health). Utility values were set as follows: 0.82 for NYHA class 1; 0.78 for NYHA class 2; 0.65 for NYHA class 3; 0.58 for NYHA class 4; and 0 for death, based on median values from previous reports.7 QALYs for each 3-month period were calculated as utility value times 0.25 (3 months/1 year) and summed over 1 year for both groups.
ICER
Cost-effectiveness was assessed using the ICER, expressed as the cost per QALY gained. ICER compares treatment efficacy and is defined as the difference in cost between treatments divided by the difference in effect.8 For comparing new treatment A and existing treatment B, ICER = (Cost A − Cost B) / (Utility A − Utility B), with a smaller ICER indicating better cost-effectiveness. If Cost A − Cost B is below 0, where new treatment A is more cost-saving than treatment B, it is recognized as highly cost-effective.
Management of HF in the Entire Study Periods
The management of HF in the study population from November 2011 to October 2013 and from November 2015 to October 2017 followed the same guidelines of the Japanese Circulation Society.9 Along with instructions to prevent HF, daily check-ups for blood pressure, heart rate, and body weight were recommended to patients, family members, and caregivers through a team-based approach.4 Patients were also advised to visit the nearest outpatient clinic if their weight increased by 2–5 kg above the set limit before the self-management system was implemented. The weight limit was based on the appropriate weight determined by the attending physician at discharge plus 2–5 kg, varying from patient to patient based on the clinical course and set by the HF team discussion and consensus.4
Data Presentation
Total healthcare cost included expenses for hospitalization and unscheduled outpatient visits in cardiology, calculated for the user and non-user groups. QALYs for each quarter were summed for 1 year. We did not compare the cost of each patient individually because a substantial number did not require rehospitalization or unexpected visits during the study period.
Statistical Analysis
Categorical variables are presented as numbers with percentages and were compared with the χ2 test or Fisher exact test. Continuous variables are expressed as means with standard deviations or medians with the 25th to 75th percentiles and compared using the Student t-test when normally distributed or the Wilcoxon rank-sum test when non-normally distributed. Survival rate or event-free rate were estimated using the Kaplan-Meier method and compared using the log-rank test. We regarded the date of discharge as time 0 for clinical follow up. All statistical analyses were conducted by physicians (E.N., T.K.) using JMP 14.0 or SAS 9.4 (both SAS Institute Inc., Cary, NC, USA). Two-tailed P values <0.05 were considered statistically significant.
Results
Costs in the Study Periods
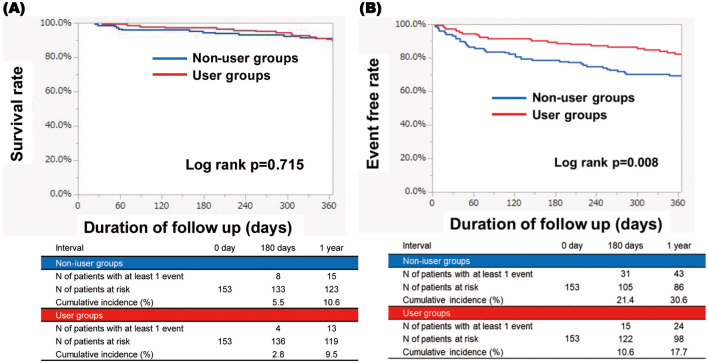
There were no significant differences in patient characteristics between the user and non-user groups in the PS-matched cohort (Table 1). Although there were no significant differences in survival rates between the 2 groups (Figure 2A), the event-free rate of HF hospitalization in the user group was significantly lower than in the non-user group (17.7% vs. 30.6%, respectively; P=0.008; Figure 2B).4 Within the 1-year follow-up period, 24 patients in the user group and 43 patients in the non-user group were hospitalized. The total costs of inpatient medical expenses in cardiology were ¥129,434,176 in the user group and ¥156,329,832 in the non-user group (Table 2). The number of unplanned visits within the 1-year follow up was 64 in the user group and 47 in the non-user group (Table 2). The total costs of unscheduled outpatient visits in cardiology were ¥312,840 in the user group and ¥97,200 in the non-user group (Table 2). Thus, the total healthcare costs for cardiovascular disease were ¥129,747,016 in the user group and ¥156,427,032 in the non-user group (Table 2). The costs of the new system were ¥50,000 for printing for the sheet book in the user group. The total costs were ¥129,797,016 in the user group and ¥156,427,032 in the non-user group.
Table 1.
Patient Characteristics of the Study Population in the Present Study
| Variable | Self-care management system | ||
|---|---|---|---|
| User (n=153) |
Non-user (n=153) |
P value | |
| Clinical characteristic | |||
| Age (years)* | 79 [68–85] | 77 [67–84] | 0.409 |
| Sex, male* | 83 (54) | 88 (58) | 0.645 |
| Multiple HF readmission (>3 times) | 10 (7) | 5 (3) | 0.290 |
| First HF admission | 116 (76) | 122 (80) | 0.492 |
| Living alone | 41 (27) | 49 (32) | 0.380 |
| Etiology | |||
| Ischemic heart disease | 48 (31) | 56 (37) | 0.398 |
| Valvular heart disease | 42 (27) | 50 (33) | 0.383 |
| Dilated cardiomyopathy | 11 (7) | 14 (9) | 0.677 |
| Hypertrophic cardiomyopathy | 7 (5) | 2 (1) | 0.173 |
| Other cardiomyopathies | 9 (6) | 9 (6) | 1.000 |
| Congenital heart disease | 0 (0) | 1 (0.7) | 1.000 |
| Other | 37 (24) | 28 (18) | 0.263 |
| Medical history | |||
| Atrial fibrillation or flutter | 82 (54) | 77 (50) | 0.647 |
| Cardiac resynchronization therapy | 4 (3) | 6 (4) | 0.750 |
| Implantable cardioverter defibrillator | 1 (0.7) | 1 (0.7) | 1.000 |
| Diabetes | 58 (38) | 45 (29) | 0.146 |
| Prior stroke | 25 (16) | 33 (22) | 0.307 |
| Chronic obstructive lung disease | 11 (7) | 18 (12) | 0.241 |
| Malignancy | 31 (20) | 22 (14) | 0.227 |
| Dementia | 10 (7) | 11 (7) | 1.000 |
| Tests at admission | |||
| LVEF (%) | 45.7±14.7 | 46.7±15.5 | 0.449 |
| LVEF <40%* | 54 (35) | 59 (3) | 0.636 |
| BNP (pg/mL) | 214 [104–478] | 204 [99–448] | 0.942 |
| BNP >235 pg/mL (median) | 71 (46) | 70 (46) | 1.000 |
| eGFR (mL/min/1.73 m2) | 44 [25–61] | 42 [29–60] | 0.759 |
| eGFR <30 mL/min/1.73 m2 | 41 (27) | 45 (29) | 0.702 |
| Serum sodium (mEq/L) | 140 [138–141] | 139 [137–142] | 0.403 |
| Serum potassium (mEq/L) | 4.3 [3.9–4.7] | 4.3 [4.0–4.6] | 0.782 |
| Serum albumin (g/dL) | 3.9 [3.6–4.2] | 3.9 [3.7–4.2] | 0.716 |
| Hemoglobin (g/dL) | 12 [11–13] | 12 [10–14] | 0.616 |
| Concomitant treatment | |||
| β-blockers | 110 (72) | 114 (75) | 0.699 |
| ACEI or ARBs | 111 (73) | 116 (76) | 0.602 |
| Aldosterone antagonists | 72 (47) | 79 (52) | 0.493 |
| Loop diuretics | 131 (86) | 127 (83) | 0.638 |
| Thiazides | 13 (9) | 19 (12) | 0.350 |
| Tolvaptan | 9 (6) | 9 (6) | 1.000 |
| Inotropic agents | 7 (5) | 8 (4) | 1.000 |
| Statins | 64 (42) | 57 (37) | 0.483 |
| Calcium antagonists | 49 (32) | 51 (33) | 0.903 |
Unless indicated otherwise, data are presented as mean±SD, median [IQR], or n (%). ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; BNP, B-type natriuretic peptide; eGFR, estimated glomerular filtration rate; HF, heart failure; LVEF, left ventricular ejection fraction.
Figure 2.
(A) Kaplan-Meier curves of the survival rate in the user and non-user groups. (B) Kaplan-Meier curves of the event-free rate for heart failure hospitalization in the user and non-user groups.
Table 2.
QALY and the ICER in Users and Non-Users of the Self-Care Management System
| User (n=153) |
Non-user (n=153) |
Difference | |
|---|---|---|---|
| QALY acquired in 1 year | 0.686 | 0.653 | +0.044 |
| Inpatient medical expenses in cardiology (¥) | 129,434,176 | 156,329,832 | −26,895,656 |
| Expense for unscheduled outpatient visits in cardiology (¥) | 312,840 | 97,200 | 215,640 |
| Total medical expenses in cardiology (¥) | 129,747,016 | 156,427,032 | −26,680,016 |
| Costs of the system (¥) | 50,000 | 0 | 50,000 |
| Total cost (¥) | 129,797,016 | 156,427,032 | −26,630,016 |
| ICER (¥/QALY) | – | – | Dominant |
ICER, incremental cost-effective ratio; QALY, quality-adjusted life-years.
QALY in the Study Periods
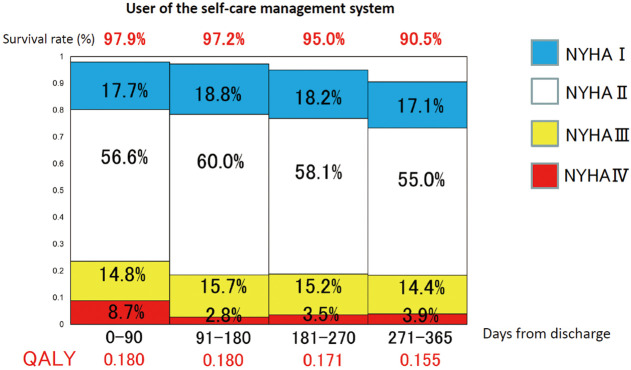
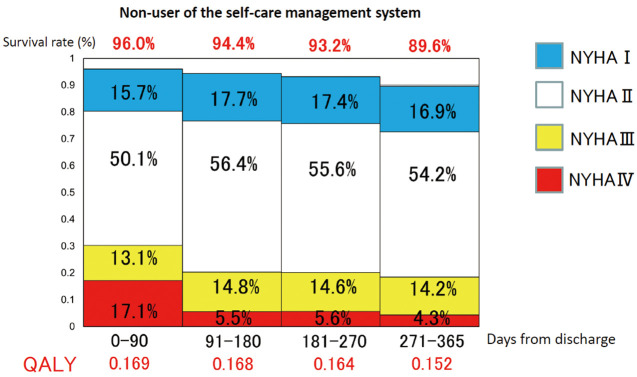
When calculating the QALY for both groups, the QALY was 0.686 in the user group (Figure 3) and 0.653 in the non-user group (Figure 4).
Figure 3.
Quality-adjusted life-years (QALY) in each quarter in users of the self-care management system. The 1-year survival curve was divided into 3-month intervals and the bars were approximated using the survival rates at each of 60, 90, 180, and 365 days. The New York Heart Association (NYHA) class and the corresponding scores for quality of life in each 3 months were then determined as follows: 0.82 in NYHA class 1; 0.78 in NYHA class 2; 0.65 in NYHA class 3; 0.58 in NYHA class 4; and 0 in death. QALY was calculated in each 3 months as a utility value times 0.25 as the number of years (3 months/1 year) and summed up around 1 year.
Figure 4.
Quality-adjusted life-years (QALY) in each quarter in non-users of the self-care management system.
Assessment of Cost-Effectiveness
Using the ICER system, the QALY gained was 0.044 and the cost decreased by ¥26,630,016 (Table 2). The ICER was below 0 (Table 2). Thus, the new method was interpreted as dominant.
Discussion
The main finding of the present study is that the use of the self-care management system decreased healthcare costs in HF and increased QALY within 1 year. The ICER was below 0, indicating that the self-care management system for HF is likely to be cost-effective in terms of healthcare costs.
Expanding healthcare costs have been a significant issue in Japan,10 with the medical cost per hospitalization for HF reaching approximately ¥1 million.3,11,12 By preventing hospitalization for HF, we demonstrated that our new self-care system reduced medical costs through a decrease in rehospitalization expenses. Patient education to strengthen self-care skills is effective in avoiding rehospitalization,13,14 reducing mortality,15 and improving quality of life after discharge,16 particularly through early symptom monitoring and improved adherence.15 Additionally, this study demonstrated a decrease in costs during 1 year of HF management. Our system is an analog book, which is inexpensive and easy for the elderly to read and write in.4 If elderly patients with HF become familiar with smartphone applications, it would be reasonable to adapt the self-care management book into a smartphone app, although this may increase costs. Nonetheless, the concept of a self-care management system remains unchanged, offering the advantage of decreasing healthcare costs for HF.
Study Limitations
There were some limitations in the present study. First, the follow-up duration was short so that it could not draw solid conclusions regarding the decrease in costs and the increase in QALY. Second, due to the retrospective nature of the study, there was a time gap between the non-user group (HF patients hospitalized 2011–2013) and the user group (HF patients hospitalized 2015–2017). The costs in the user and non-user groups might have changed not only due to the national insurance system but also due to advances in pharmacological and non-pharmacological interventions for HF. However, since the backgrounds of drug and device therapy were evaluated together using PS matching, we believe that there was minimal or no difference in medical costs between the 2 periods due to differences in treatment methods. In the evaluation of QALY, it seems better to use comprehensive quality of life assessment indicators such as The Kansas City Cardiomyopathy Questionnaire (KCCQ) and Minnesota Living with Heart Failure Questionnaire (MLHFQ), not just NYHA. However, we have not used KCCQ or MLHFQ for HF inpatients at Kitano Hospital, and since this is a retrospective study, we are unable to evaluate them. During hospitalization for HF, physicians and medical staff educated patients and their families face-to-face on self-care methods for HF. However, in the intervention group, guidance on calculating HF points and the outpatient visit method based on these points was necessary, which may have resulted in a longer time spent on education compared with the non-intervention group. However, since there are no data on the time spent on education, we cannot evaluate this. Last, we calculated the cost of hospitalization but could not evaluate the labor costs within our hospital, medication costs or the costs outside of our hospital because we did not have those data for a certain period at our facility. Further studies are necessary to draw solid conclusions about the cost-effectiveness of the self-care management system for HF treatment.
Conclusions
The use of the self-care management system is likely to be a cost-effective treatment for HF with the increase in QALY and the decrease in healthcare costs.
Disclosures
E.N., M. Inoko, H.F., M.Y., Y. Yamaji, and T. Hamaguchi received a research grant from Bayer Yakuhin, Ltd, Takeda Pharmaceutical CO., Ltd, Novartis Pharma K.K., and IQVIA Services Japan G.K., and a scholarship grant from IQVIA Services Japan G.K., and Research Institute of Production Development. The other authors have no conflicts of interest.
IRB Information
Institutional review board of Kitano Hospital, Tazuke Kofukai Medical Research Institute (P 190600100).
Supplementary Files
Supplementary Methods. Supplementary Figure.
Data Availability
The deidentified participant data will be shared on a request basis. Please contact the corresponding author directly to request data sharing. All data sets used will be available. Data will be shared as soon as it is approved by the IRB at Kitano Hospital, and will be available until the end of March, 2026. All reasonable requests to access the data and complete analyses will be approved. The data will be shared in an Excel file via email.
References
- 1. Soundarraj D, Singh V, Satija V, Thakur RK.. Containing the cost of heart failure management: A focus on reducing readmissions. Heart Failure Clin 2017; 13: 21–28. [DOI] [PubMed] [Google Scholar]
- 2. Okura Y, Ramadan MM, Ohno Y, Mitsuma W, Tanaka K, Ito M, et al.. Impending epidemic: Future projection of heart failure in Japan to the year 2055. Circ J 2008; 72: 489–491. [DOI] [PubMed] [Google Scholar]
- 3. Kanaoka K, Okayama S, Nakai M, Sumita Y, Nishimura K, Kawakami R, et al.. Hospitalization costs for patients with acute congestive heart failure in Japan. Circ J 2019; 83: 1025–1031. [DOI] [PubMed] [Google Scholar]
- 4. Nakane E, Kato T, Tanaka N, Kuriyama T, Kimura K, Nishiwaki S, et al.. Association of the induction of a self-care management system with 1-year outcomes in patients hospitalized for heart failure. J Cardiol 2021; 77: 48–56. [DOI] [PubMed] [Google Scholar]
- 5. Nakane E, Kato T, Tanaka N, Kuriyama T, Kimura K, Nishiwaki S, et al.. Association between induction of the self-management system for preventing readmission and disease severity and length of readmission in patients with heart failure. BMC Res Notes 2021; 14: 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shiba N, Shimokawa H.. Chronic heart failure in Japan: Implications of the CHART studies. Vasc Health Risk Manag 2008; 4: 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Di Tanna GL, Urbich M, Wirtz HS, Potrata B, Heisen M, Bennison C, et al.. Health state utilities of patients with heart failure: A systematic literature review. Pharmacoeconomics 2021; 39: 211–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kodera S, Kiyosue A, Ando J, Akazawa H, Morita H, Watanabe M, et al.. Cost-effectiveness analysis of cardiovascular disease treatment in Japan. Int Heart J 2017; 58: 847–852. [DOI] [PubMed] [Google Scholar]
- 9. JCS Joint Working Group.. Guidelines for treatment of acute heart failure (JCS 2011). Circ J 2013; 77: 2157–2201. [DOI] [PubMed] [Google Scholar]
- 10. Matsumoto K, Hanaoka S, Wu Y, Hasegawa T.. Comprehensive cost of illness of three major diseases in Japan. J Stroke Cerebrovasc Dis 2017; 26: 1934–1940. [DOI] [PubMed] [Google Scholar]
- 11. Iso H.. Cardiovascular disease, a major global burden: Epidemiology of stroke and ischemic heart disease in Japan. Glob Health Med 2021; 3: 358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Su K, Kato T, Toyofuku M, Morimoto T, Yaku H, Inuzuka Y, et al.. Association of previous hospitalization for heart failure with increased mortality in patients hospitalized for acute decompensated heart failure. Circ Rep 2019; 1: 517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McAlister FA, Stewart S, Ferrua S, McMurray JJ.. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: A systematic review of randomized trials. J Am Coll Cardiol 2004; 44: 810–819. [DOI] [PubMed] [Google Scholar]
- 14. Jovicic A, Holroyd-Leduc JM, Straus SE.. Effects of self-management intervention on health outcomes of patients with heart failure: A systematic review of randomized controlled trials. BMC Cardiovasc Disord 2006; 6: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ruppar TM, Cooper PS, Mehr DR, Delgado JM, Dunbar-Jacob JM.. Medication adherence interventions improve heart failure mortality and readmission rates: Systematic review and meta-analysis of controlled trials. J Am Heart Assoc 2016; 5: e002606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jonkman NH, Westland H, Groenwold RH, Ågren S, Atienza F, Blue L, et al.. Do self-management interventions work in patients with heart failure? An individual patient data meta-analysis. Circulation 2016; 133: 1189–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods. Supplementary Figure.
Data Availability Statement
The deidentified participant data will be shared on a request basis. Please contact the corresponding author directly to request data sharing. All data sets used will be available. Data will be shared as soon as it is approved by the IRB at Kitano Hospital, and will be available until the end of March, 2026. All reasonable requests to access the data and complete analyses will be approved. The data will be shared in an Excel file via email.