Abstract
Phospholipase D (PLD), which hydrolyzes phospholipids into free head groups and phosphatidic acid (PA), may regulate cellular processes through the production of lipid and lipid-derived messengers. We have genetically abrogated PLDα, the most prevalent isoform of PLD in plants, and the depletion of PLDα in Arabidopsis decreased the levels of PA and superoxide production in Arabidopsis leaf extracts. Addition of PA promoted the synthesis of superoxide in the PLDα-depleted plants, as measured by chemiluminescence and superoxide dismutase-inhibitable, NADPH-dependent reduction of cytochrome c and nitroblue tetrazolium. The PA-enhanced generation of superoxide was associated mainly with microsomal membranes. Among various lipids tested, PA was the most effective stimulator with the optimal concentrations between 100 and 200 μm. The PA-promoted production of superoxide was observed also in leaves directly infiltrated with PA. The added PA was more effective in stimulating superoxide generation in the PLDα-depleted leaves than in the PLDα-containing, wild-type leaves, suggesting that PA produced in the cell was more effective than added PA in promoting superoxide production. These data indicate that PLD plays a role in mediating superoxide production in plants through the generation of PA as a lipid messenger.
Phospholipase D (PLD; EC 3.1.4.4) has been identified recently as an important signaling enzyme in various organisms (for review, see Liscovitch et al., 2000; Wang, 2000). Recent results indicate that the cellular activity of plant PLD is regulated by Ca2+ (Zheng et al., 2000), polyphosphoinositides (Qin et al., 1997), G proteins (Munnik et al., 1995; Ritchie and Gilroy, 2000), pH changes (Pappan and Wang, 1999), and membrane perturbation (Pappan et al., 1998). The activation of PLD generates phosphatidic acid (PA) and a free head group, both of which are thought to serve directly as cellular messengers. In addition, the formation of PA can lead to the production of other lipid messengers such as diacylglycerol (DAG), free polyunsaturated fatty acids, phosphatidylinositol-4,5-bisphosphate, and jasmonic acid (Wang et al., 2000). PLD has been proposed to participate in cellular events that lead to abscisic acid responses (Fan et al., 1997; Jacob et al., 1999) and the production of ethylene (Lee et al., 1998) and jasmonic acid (Wang et al., 2000). Activation of PLD occurs in response to various biotic and abiotic stress cues, which include water stress (Frank et al., 2000), wounding (Ryu and Wang, 1996; Wang et al., 2000), and pathogen elicitation (Young et al., 1996; van der Luit et al., 2000). In mammalian systems, PLD is activated by a wide variety of signaling events, including protein kinases, polyphosphoinositides, receptor-linked G proteins, and small GTP-binding proteins, such as ADP-ribosylation factor and Rho, and activation of PLD is involved in mediating cellular processes, such as oxidative burst, vesicle trafficking, cytoskeletal organization, and cell proliferation (for review, see Liscovitch et al., 2000).
These cellular effects of PLD have raised considerable interests in the mechanisms by which PLD mediates cellular functions. The most studied mechanism of action in animals is the PA stimulation of signaling proteins, including protein kinases, phosphatases, lipid kinases, and phospholipases (for review, see Liscovitch et al., 2000). The PA-activated protein kinases include Ca2+-dependent and -independent kinases, such as protein kinase C, mitogen-activated protein kinases, and Raf kinases. PA has been shown to bind to Raf kinase, and this binding may activate the enzyme by altering its conformation (Ghosh et al., 1996). Moreover, the presence of a PA-specific protein kinase has been documented to mediate the activation of NADPH oxidase (Waite et al., 1997; McPhail et al., 1999). NADPH oxidase catalyzes the NADPH-dependent production of superoxide anion, and it is a multicomponent enzyme composed of several membrane-bound and cytosolic subunits. It becomes active when its four cytosolic proteins, p47-phox, p67-phox, p40-phox, and p21rac, translocate to the membrane. The translocation of p47-phox and p67-phox is prompted by phosphorylation, and p47-phox is a substrate for a newly identified PA-activated protein kinase in animals (Waite et al., 1997). In addition, the membrane-bound p22-phox subunit of flavocytochrome b558 is phosphorylated by a PA-activated protein kinase (Regier et al., 1999, 2000). These findings have provided mechanistic insights to the role of PLD in the defense response, because the activation of PLD is a documented, key event in signal transduction leading to the reactive oxygen release in neutrophils.
The occurrence of the mammalian NADPH oxidase-like activity has been indicated in plants (Lamb and Dixon, 1997). Genes homologous to the neutophil oxidase catalytic unit gp91-phox have been cloned from Arabidopsis and rice (Oryza sativa; Keller et al., 1998; Torres et al., 1998). Diphenylene iodonium (DPI) and α-napthol, two known inhibitors of mammalian NADPH oxidase, inhibited the plant oxidative burst (Levine et al., 1994; Dwyer et al., 1996; Orozco-Cárdenas et al., 2001). In addition, studies have also indicated that the oxidative burst in neutrophils and plant defenses share some common mechanisms of activation. Many signaling components activating the neutrophil oxidase are thought to be operational in the plant oxidative burst. These include phospholipiases A and C, G proteins, and protein kinases/phosphatases (Dwyer et al., 1996; Lamb and Dixon, 1997). In tomato (Lycopersicon esculentum) cells treated with race-specific elicitors from Cladosporium fulvum, the phosphorylation of a p47-phox-like protein, which was recognized by an antibody against a human p47-phox, appeared to occur before the docking of the cytosolic component to membrane cytochrome b units (Xing et al., 1997). However, genes homologous to p47-phox or p67-phox have not been reported in plants. A study using a suspension of soybean (Glycine max) cells elicited by oligogalacturonides failed to document the activation of PLD and the stimulation of hydrogen peroxide production by exogenous PA (Taylor and Low, 1997). Thus, the role of PLD/PA in modulating reactive oxygen production is unclear in plants.
Recent results have revealed that PLD in plants is a family of heterogenous enzymes. Four types of PLD have been characterized from Arabidopsis: the conventional plant PLD, now known as PLDα, and novel types of plant PLD, PLDβ, PLDγ, and PLDδ (Pappan et al., 1997a, 1997b; Qin et al., 1997; Wang, 2000). These PLDs displayed unique regulatory and catalytic properties (Qin et al., 1997; Pappan and Wang, 1999). PLDα is the most prevalent form and is present in all plant tissues examined (Pappan et al., 1997a). Its activity increases in response to many perturbations, some of which are related to tissue disruption such as wounding, extraction, storage conditions, and homogenization (Ryu and Wang, 1996). This high sensitivity of PLDα to tissue handling can be problematic for the measurement of in vivo activation of PLD and may overwhelm the PA changes resulting from a localized activation of PLD or a specific PLD isoform in vivo. We have generated Arabidopsis depleted of PLDα by introducing a PLDα antisense gene, and showed that virtually all PLDα activity was lost in the antisense-suppressed leaves (Fan et al., 1997; Pappan et al., 1997a). The deficiency of this most common PLD impaired the plant's ability to generate PA (Wang et al., 2000), thus providing a unique and effective system to determine the participation of PLD and PA in cell function. Results of this study have provided evidence for a role of PLD/PA in mediating superoxide production in Arabidopsis.
RESULTS
Antisense Suppression of PLDα Lowered the Level of Superoxide Production
To determine whether PLD was involved in the generation of superoxide, we compared the superoxide level in the leaf extracts between PLDα-deficient and wild-type Arabidopsis. It was reasoned that if PLD played a role in activating superoxide production, the loss of PLDα might impede superoxide production in the PLDα-suppressed tissues. Lucigenin-dependent chemiluminescence (LDC) has been used in plant and animal systems as an indicator for the presence and synthesis of superoxide (Auh and Murphy, 1995; Murphy et al., 1998). In this study, we first examined several parameters to verify the utility of LDC for measuring superoxide in Arabidopsis extracts. Inclusion of superoxide dismutase (SOD), which converts O2− to H2O2, in the assay mixtures, abolished LDC generated from leaf extracts (Fig. 1A), and addition of the reactive oxygen species H2O2 had little effect on LDC (data not shown), indicating the assay's specificity for superoxide. The production of LDC was NADPH dependent, and no superoxide was detected in the absence of NADPH. In addition, NADH could not substitute NADPH for LDC; NADH at 80 μm supported about 10% LDC of NADPH at the same concentration. The self-suicide inhibitor of mammalian NADPH oxidase, DPI, abolished LDC (Fig. 1A). These results indicate that the LDC assay measured the presence of superoxide, and that superoxide in this assay system was produced mainly through an NADPH-dependent pathway.
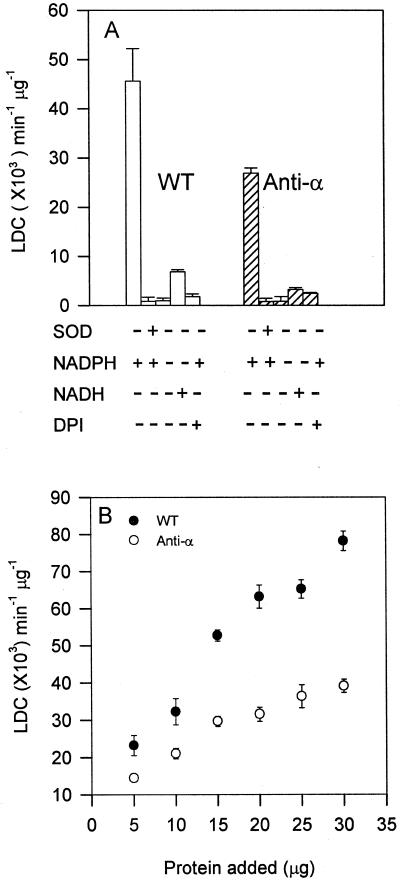
Figure 1.
Decreased superoxide production in PLDα-suppressed Arabidopsis leaves. A, Specificity of LDC for assaying superoxide-generating activity. The basic assay contained 10 μg protein (10,000g supernatant) from wild-type (WT) and PLDα-antisense (anti-α) leaves and 80 μm NADPH. DPI (20 μm) or SOD (45 units) was added to the reaction. NADPH was deleted or substituted with 80 μm NADH as indicated. B, Superoxide-generating activity between anti-α and WT as a function of different protein concentrations. Values are means ± se from three separate extractions.
The superoxide level produced in PLDα-suppressed leaves was about 50% lower than that in wild-type leaves (Fig. 1A). The dependence of superoxide synthesis on the amounts of protein was compared between PLDα-deficient and wild-type lines (Fig. 1B). A near-linear increase of superoxide production occurred as the protein concentrations increased from 5 to 30 μg. The differences in LDC between PLDα-deficient and wild-type leaves could be measured reliably, and no significant fluctuation was noted within the first 15 min of the reaction (data not shown).
Suppression of PLDα Compromised the PA- Generating Ability
One possibility for the lower level of superoxide production in the PLDα-suppressed Arabidopsis is reduced production of PA that serves as a stimulator of superoxide synthesis. PLDα protein was undetectable by immunoblotting with PLDα-specific antibodies (Fig. 2A). In a similar manner, the transcript of PLDα in the PLDα-supressed leaves was also undetectable by RNA blotting (Fan et al., 1997; Wang et al., 2000). PLDα activity in the antisense leaf extracts was less than 5% of that in extracts of wild-type leaves (Fan et al., 1997; Pappan et al., 1997b). The antisense suppression was specific to PLDα, and the expression and activities of PLDβ or γ were similar in the PLDα-suppressed and wild-type plants (Pappan et al., 1997a; Wang et al., 2000).
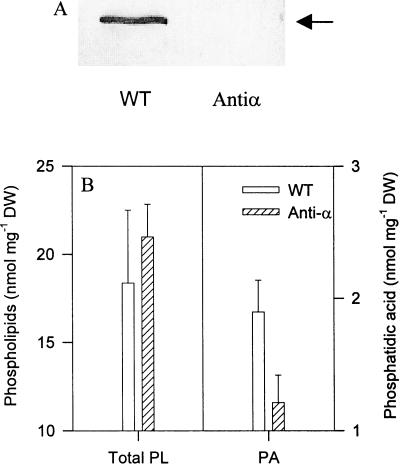
Figure 2.
Decreased PA content in PLDα-suppressed Arabidopsis leaves. A, Immunoblotting analysis of PLDα in leaf extracts of wild-type and PLDα-depleted plants, using a PLDα-specific antibody. B, Total phospholipids and PA from homogenates of anti-α and WT Arabidopsis leaves. PA was recovered after TLC separation, and the levels of total phospholipid and PA were determined by assaying phosphorus content. Values are means ± se of two separate extractions.
To determine PA levels in the PLDα-suppressed and wild-type Arabidopsis, we measured the contents of total phospholipids and PA in the leaf homogenates that were used for assaying superoxide synthesis. No substantial difference was observed in total phospholipid content between the PLDα-suppressed and wild-type plants. The PA level in the homogenate of PLDα-suppressed leaves was approximately 30% lower than that in the homogenates of wild-type leaves (Fig. 2B), confirming that the PA level was decreased in the PLDα-deficient extracts.
Addition of PA Promoted Superoxide Production
If the lower level of PA in the PLDα-suppressed leaves was a contributor to the decreased level of superoxide, exogenous PA might be able to restore the lost superoxide generation. When water-soluble diC8PA (200 μm) was added directly to the extraction buffer, superoxide production was stimulated by about 60% in the PLDα-deficient leaves as measured by LDC. This PA promotion was confirmed further by two other methods, SOD-inhibitory, NADPH-dependent reductions of cytochrome c (cyt c) and nitroblue tetrazolium (NBT), both of which gave about 40% stimulation (Fig. 3A). The lower effects of these two methods could have been caused by the presence of other interfering enzyme in the assay mixtures, as observed in other systems (Murphy et al., 1998). For example, the presence of cytochrome c reductases in the plasma membrane of leaf extracts could decrease the differences of superoxide-generating enzymes between PLDα-antisense and wild-type Arabidopsis.
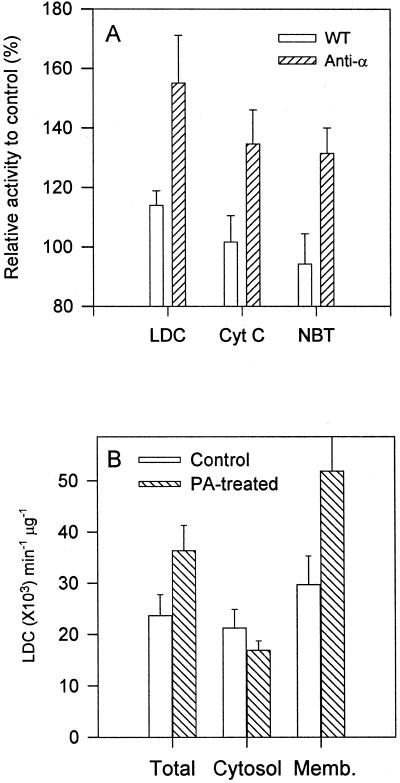
Figure 3.
PA-stimulated production of superoxide in PLDα-deficient and wild-type Arabidopsis leaves. A, Increased superoxide production after addition of diC8PA (200 μm) during homogenization. The superoxide generation was assayed by LDC, NADPH-dependent reduction of cyochrome c (Cyt c), and NBT using 10 μg of 10,000g supernatant. Activity was expressed as a percentage of the sample without PA treatment. Values are means ± se of five separate extractions. B, Association of PA-promoted superoxide-producing activity with microsomal membranes in PLDα-suppressed leaves as assayed by LDC. DiC8PA (200 μm) was added to the extract buffer prior to homogenization, and superoxide production was assayed by LDC. The activity of superoxide production was expressed as a percentage of that measured without phospholipid addition. Total, Proteins from the 10,000g supernatant of anti-α and WT Arabidopsis leaves; Cyto, proteins from the 100,000g supernatant; Memb, particulate fraction of the 100,000g centrifugation of 10,000g supernatant. Values are means ± se from three assays.
To determine the subcellular association of PA-promoted superoxide-generating activity, the proteins extracted from the PLDα-antisense leaves were separated into soluble and microsomal fractions. Superoxide-synthesizing activity was present in both fractions (Fig. 3B). The PA-promoted activity was associated entirely with the membrane fraction. Superoxide production in the soluble fraction was slightly lower for extracts of PA-treated leaves than for extracts of untreated leaves (Fig. 3B).
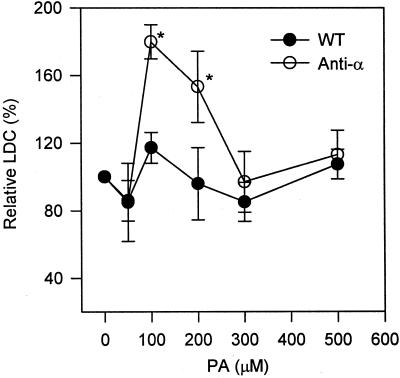
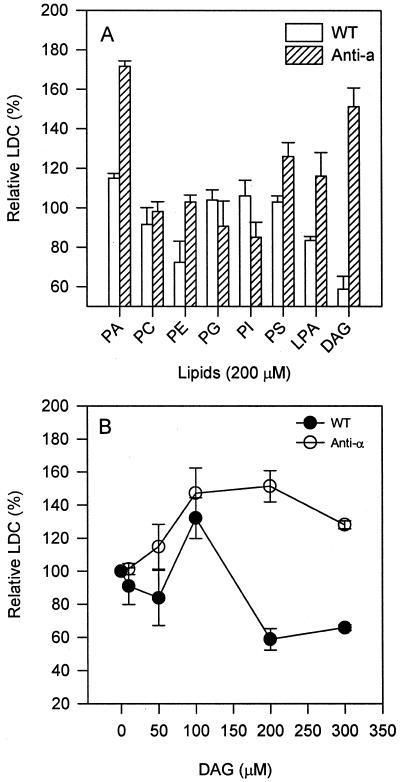
Superoxide-synthesizing activity first increased and then decreased with increasing PA concentrations. This effect of PA was similar to the results with PA and DAG as activators in animal systems (Qualliotine-Mann et al., 1993). The optimal concentration of diC8-PA to activate superoxide production was between 100 and 200 μm, and no significant stimulation was observed when PA concentrations were 300 or 400 μm (Fig. 4). At all PA concentrations tested, wild-type leaves were much less responsive to exogenous PA than were PLD-suppressed leaves. The optimal stimulation of superoxide production was about 15% in wild-type leaves, whereas the PA treatment promoted an 80% increase in the superoxide synthesis in PLDα-antisense leaves.
Figure 4.
Effect of varied PA concentrations on superoxide production. Varied concentrations of diC8PA were added to the extraction buffer prior to homogenization. The same amount of protein (10 μg) of 10,000g supernatant were used for assaying LDC. The LDC in the absence of PA was expected as 100%. Values are means ± se of three separate experiments. An asterisk denotes significant difference (P < 0.05) in the presence and absence of PA and between PLDα-antisense and wild-type plants.
Specificity of PA and Other Lipids in Reconstituting Superoxide Production
To investigate the specificity of PA in the activation of superoxide formation, several cellular phospholipids were tested for their abilities to promote the activity of NADPH-dependent superoxide synthesis. The major membrane phospholipids tested, phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylglycerol, and phosphatidylinositol (PI), had no effect, whereas the minor plant membrane phospholipids, phosphatidyl-Ser (PS) and lysoPA, stimulated the superoxide-synthesizing activity by approximately 20% (Fig. 5A). DAG was more effective than PS and lysoPA, and the superoxide level was 50% higher than that in untreated transgenic leaves. Similar to the PA treatment, the optimal concentration of DAG for promoting superoxide production was between 100 and 200 μm in PLDα-deficient leaves. The protein extracted from wild-type leaves was less responsive to the added DAG than that from PLDα-deficient leaves. Concentrations of DAG above 100 μm inhibited the superoxide-synthesizing activity in wild-type leaves (Fig. 5B). DAG and PA showed no significant synergistic effect on promoting superoxide generation in Arabidopsis (data not shown).
Figure 5.
Lipid specificity and DAG concentration effect on superoxide production. A, Effect of phospholipids and DAG on superoxide-producing activity from PLDα-suppressed and wild-type leaf extracts. Lipids (200 μm) were added to the extraction buffer prior to homogenization. PC, PE, and PI were from soybean, and PS was from bovine brain, whereas dipalmitoyl-phosphatidylglycerol, 1-oleoyl-lysophosphatidic acid (LPA), and diactanoyl-DAG were synthetic. B, Superoxide production as affected by dioctanoyl-DAG concentration in PLDα-suppressed and wild-type leaves. Superoxide production was measured using 10 μg of proteins from 10,000g supernatant and was expressed as percentage of that without lipid treatment. The activity of superoxide production was expressed as a percentage of that measured without phospholipid addition. Values are means ± se of three separate extractions.
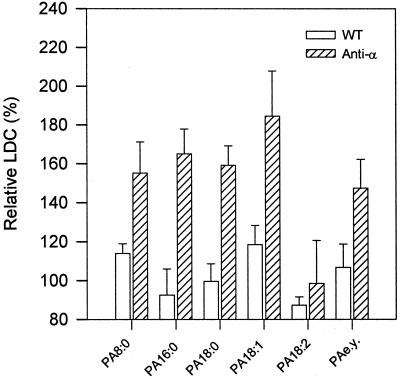
PAs with different fatty acids were tested to determine the effect of acyl composition on the superoxide synthesis activity. Except for egg yolk PA, which contained a mixture of varied fatty acids, the other PAs used in the study were synthetic. Stimulation was observed with diC8PA (PA8:0), dipalmitoyl-PA (PA16:0), distearoyl-PA (PA18:0), dioleoyl-PA (PA18:1), and egg yolk PA, whereas dilinoleoyl-PA (PA18:2) showed little effect on both the wild-type and antisense plants (Fig. 6). This result indicates that the acyl composition of PA has some effect on the stimulation of superoxide synthesis, but it is not clear why the polyunsaturated dilinoleoyl-PA was not as effective as the other PAs.
Figure 6.
Effect of acyl composition of PA on superoxide production. PAs (200 μm) with varied fatty acids were added to extraction buffer prior to homogenization. The activity without lipids (control) was set as 100%. PA8:0, dioctanoyl-PA; PA16:0, dipalmitoyl-PA; PA18:0, distearoyl-PA; PA18:1, dioleoyl-PA; PA18:2, dilinoleoyl-PA; and PA e.y., PA from egg yolk. The percentages are means ± se of three experiments.
PA Enhanced Superoxide Burst in Leaves
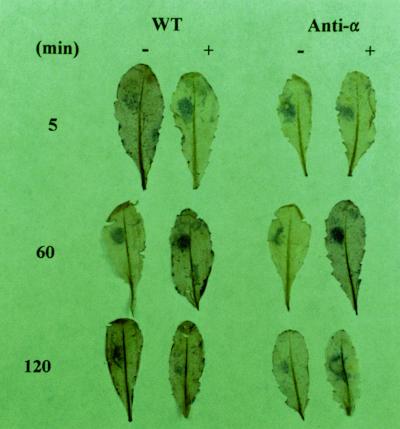
The above results showed the PA promotion of superoxide production in leaf extracts. To determine if PA had the same effect in vivo, dioleoyl-PA was infiltrated into leaves on plants. The treated leaves then were detached and stained for superoxide formation. The cytochemical staining (brown precipitates) was due to reduction of NBT by superoxide and has been used as an indicator for the tissues actively generating reactive oxygen (Jabs et al., 1995). Infiltration of the control buffer and dioleoyl-PA into leaves with the pressure of a needleless syringe resulted in an increase of reactive oxygen species in the wound area (Fig. 7). A clear difference in the NBT staining between the buffer- and PA-infiltrated leaves occurred between 30 and 60 min after the treatment. PA enhanced the oxidative burst in both PLDα-deficient and wild-type leaves. Judged from the staining intensity, this PA enhancement was greater in the PLDα-antisense leaves than in wild-type leaves. The PA effect was transient; 2 h after treatment, no obvious difference was noted between the buffer- and PA-infiltrated leaves. This could mean that the infiltrated PA was metabolized rapidly.
Figure 7.
PA-promoted superoxide production in situ in Arabidopsis leaves. Leaves of wild-type (WT) and PLDα-deficient (anti-α) plants were infiltrated with approximately 10 μL of 10 mm potassium phosphate buffer (−), or the buffer containing 200 μm dioleoyl-PA (+). At indicated time intervals, the leaves were detached and stained with NBT. Multiple leaves were infiltrated for each treatment; a representative leaf is shown here.
DISCUSSION
Role of PLD in Mediating Reactive Oxygen Production
The data of this study collectively indicate that PLD plays a role in the production of reactive oxygen. First, suppression of the predominant form of PLD, PLDα, led to reduced superoxide synthesis. Second, addition of exogenous PA, the lipid product of PLD activation, to leaves promoted the activity of reactive oxygen production. In particular, the added PA reconstituted the lost superoxide-generating activity in PLD-suppressed leaves to the level in wild-type leaves. Third, among all lipids tested in this system, PA was the most effective stimulator for the reactive oxygen generation. Fourth, infiltration of PA into leaves on plants augmented micro-oxidative burst at the sites of infiltration.
PLDα-deficient Arabidopsis leaves still produced substantial amounts (50%) of superoxide, which is indicated by the in vitro measurements of superoxide synthesis and in situ NBT staining. This activity could be supported by the residual PLDα (less than 5%) and other PLDs, such as PLDβ and PLDγ, which have been documented in Arabidopsis leaves. A previous study has shown that PA levels increase substantially, albeit less than in wild-type leaves, in PLDα-suppressed leaves in response to wounding (Wang et al., 2000). In addition, there may exist PLD-independent pathways that regulate superoxide synthesis. For example, induction of the oxidative burst in neutrophils involves activation of phosphalipase C (PLC) and phosphalipase A2, in addition to PLD (Qualliotine-Mann et al., 1993). In plant-pathogen interactions, phosphalipase A2 and PI-PLC activities have been implicated in the generation of reactive oxygen in response to pathogen elicitors (for review, see Chapman, 1998).
In this study, DAG was found to stimulate superoxide production, and the magnitude of stimulation was similar to that of PA. DAG could be formed from the activation of PLC and/or dephosphorylation of PA produced by PLD, whereas PA might result from the activation of PLD and/or phosphorylation of DAG. The similar effects of PA and DAG and the inter-convertibility between PA and DAG raise interesting questions about the nature of the lipid mediators and interplay pf PLDα with other phospholipases in regulating superoxide formation. In a separate study, we measured DAG levels in wild-type and PLDα-depleted leaves before or after wounding (Zien et al., 2001). Wounding increased DAG levels, but no significant difference was observed between the two genotypes either before or after wounding, suggesting that PLC activity was unaltered by the loss of PLDα. In addition, the antisense suppression abrogated specifically PLDα and had no effect on the expression and activity of PLDβ or γ (Pappan et al., 1997a; Wang et al., 2000). The presence of PLDβ and γ, however, did not compensate for the loss of PLDα. This may not be surprising because the activities of PLDβ and γ are distinctively different from that of PLDα (Qin et al., 1997), and they may have unique functions. These observations, however, do not rule out the possibilities that multiple PLDs and PLCs are involved in the production of PA and DAG in regulating the oxidative burst. The fact that the level of superoxide synthesis is lowered by suppression of PLDα and is promoted by added PA demonstrates that PLDα is a key mediator regulating the oxidative burst.
PA Produced in the Cell Is More Effective than Added PA in Promoting Superoxide Production
One marked difference between the PLDα-suppressed and wild-type leaves throughout this study was that added PA was much more effective in stimulating superoxide generation in the antisense leaves than in the wild-type leaves. One explanation for this differential effect is that PLDα in wild-type leaves can produce enough PA for stimulating superoxide production and this endogenous PA is much more effective than the added PA in regulating superoxide synthesis. Thus, added PA has a less effect on superoxide production in wild-type leaves. A higher level of PA was found in wild-type than PLDα-deficient leaves. The endogenously produced PA could be in a correct intracellular location and have the correct acyl composition and, thus, be more effective in stimulating reactive oxygen formation than exogenously supplied PA. In addition, PLDα is expressed constitutively in leaves and becomes activated rapidly in response to environmental stresses such as wounding, ionizing irradiation, and other injuries (Ryu and Wang, 1996; Wang et al., 2000). Thus, perturbations such as tissue handling and homogenization can activate PLDα, resulting in production of enough PA, to mask or obscure the difference of PA levels caused by other cellular activation. On the other hand, the suppression of PLDα impaired the plant's ability to produce PA, and, thus, the stimulation of superoxide synthesis by added PA became apparent.
A well-regulated and possibly localized production of PA has been indicated in some perturbations that potentially involve reactive oxygen generation. For example, in rice leaves undergoing hypersensitive interactions with Xanthomonas oryzae pv oryzae, PLDα clustered in the plasma membrane regions in contact with this bacterial pathogen (Young et al., 1996). In the susceptible interaction, however, PLDα was distributed evenly along the plasma membrane. This PLD clustering may produce more PA at the membrane region for the cell to use in battling against infection. Also, wounding triggers intracellular translocation of PLDα from cytosol to membrane and promotes PA formation on the membranes (Ryu and Wang, 1996, 1998). Such increased PA may be used for promoting the oxidative burst as a defense mechanism.
Using lipids extracted from leaf homogenates, the present data showed that PA constituted almost 10% of total phospholipids in wild-type leaf extracts. This PA to total phospholipid ratio was high, but it should not represent true levels of cellular PA. This was because in addition to the preexisting, cellular PA, a major portion of PA in the homogenate was generated by PLD during homogenization. In a separate study in which PLD activity was inhibited immediately after leaf excision by immersing leaves in 75°C isopropanol, PA accounted for about 2% of the total phospholipids (Zien et al., 2001). The PA levels were similar in non-wounded leaves of PLDα-deficient and wild-type plants. After wounding, however, PA levels in wild-type leaves increased to about 8% to 10% of total phospholipids (Zien et al., 2001), and the ratio is similar to that from the wild-type leaf homogenate in the present study. Thus, this high ratio of PA to total phospholipids resulted from increased PLD hydrolysis of phospholipids.
Potential Targets Activated by PLD/PA for Generating Superoxide
Although superoxide can be a by-product of metabolic processes such as respiration and photosynthesis, NADPH oxidase is believed to be a key enzyme for the stimulus-induced release of reactive oxygen (Lamb and Dixon, 1997; Potikha et al., 1999; Orozco-Cárdenas et al., 2001). Results of this study suggest that the lower level of superoxide synthesis in the leaf extracts of PLDα-depleted plants results from the PA modulation of an NADPH oxidase-like activity. This activity was NADPH dependent and sensitive to the presence of added SOD and DPI, a potent self-suicide inhibitor of neutrophil NADPH oxidase. Besides the LDC assay, the changes in superoxide synthesis also were confirmed by the SOD-inhibitory, NADPH-dependent reductions of cyt c and NBT.
In addition, the PA effect on superoxide generation in Arabidopsis resembled the PA activation of neutrophil NADPH oxidase in several aspects (Qualliotine-Mann et al., 1993). In cell-free systems, PA induced approximately a 2-fold increase of NADPH oxidase in both neutrophils and PLDα-deficient Arabidopsis. The stimulated activity was associated with membranes. The effective concentration of PA in Arabidopsis was also in a range similar to that used for the neutrophil enzyme. PA and DAG were stimulators for both plant and neutrophil oxidases. Neutrophil NADPH oxidase is stimulated synergistically by PA and DAG, and PA, in combination with DAG, induced a more than 10-fold activation of neutrophil oxidase (Qualliotine-Mann et al., 1993). However, little synergistic stimulation by PA and DAG was noted in the Arabidopsis enzyme. The optimum stimulation by PA and DAG occurred at approximately 0.1 mm. These relatively high concentrations seem to make it questionable that such concentrations could be achieved in vivo. However, interpretation of effective lipid concentrations for enzymes that act on or regulated by lipids is complicated because the interfacial concentrations, rather than total concentrations of PA, are critical to the activity of an enzyme. It might be possible that when PA is mixed with other lipids in cellular environments, the effect concentrations required for activating superoxide production would be much lower than 0.1 mm.
Recent studies in mammalian systems have indicated that PA increased NADPH oxidase activity by activating a PA-selective protein kinase that phosphorylates the cytosolic p47-phox and the membrane p22-phox subunits of NADPH oxidase (McPhail et al., 1999; Regier et al., 1999, 2000). The phosphorylation increases the interaction of the two subunits with other components of the oxidase. In plants, the presence of p47-phox- and p22-phox-like subunits in plants has been suggested based on the crossreactivity of plant proteins with antibodies against mammalian p47-phox and p22-phox (Dwyer et al., 1996; Xing et al., 1997). Phosphorylation of p47-phox was indicated also in elicitor-treated tomato cells. Although genes homologous to mammalian p47-phox- and p22-phox have not been identified, plant homologs of gp91-phox, the catalytic unit of animal NADPH oxidase, have been cloned (Keller et al., 1998; Torres et al., 1998). How NADPH oxidase is regulated is not understood in plants. In this study, we have found that PA added during homogenization is more effective than adding PA after homogenization. One possible explanation is that the superoxide-generating enzyme complex might be unstable, and added PA could help stabilize the NADPH oxidase complex during extraction. Although the detailed mechanisms await further studies, the present results show that PLD and PA play a role in mediating the NADPH-dependent production of superoxide in plants, and that the NADPH oxidase is a likely target of PLD activation. Further studies are under way to identify the targets that PLD and PA interact with in regulating plant oxidative stress responses.
MATERIALS AND METHODS
Plant Material and Confirmation of Transgenic Plants
Seeds of wild-type and PLDα-suppressed Arabidopsis ecotype Columbia were sown in soil and cold treated at 4°C overnight. Plants were grown under 14-h-light/10-h-dark cycles with cool-white fluorescent light of 100 μmol m−2 s−1 at 23 ± 3°C. Generation of the PLDα-deficient line was described previously (Fan et al., 1997). Before each treatment, the PLDα deficiency of the transgenic plants was confirmed by assaying extracts for PLDα activity (Wang et al., 1993) and sometimes by immunoblot analysis using PLDα-specific antibodies (Fan et al., 1997).
Phospholipid Treatment and Protein Extraction
Synthetic PA and DAG were purchased from Avanti Polar Lipids (Alabaster, AL), and other phospholipids were products of Sigma (St. Louis). Lipids were stored at −20°C in chloroform and were dried with a stream of N2 prior to use. Water-soluble diC8PA was dissolved directly in water, and other lipids were emulsified by sonication in water. For in vitro lipid treatment, phospholipids or DAG were added into extraction buffer just before homogenization at the concentration of 200 μm or otherwise as specified. Leaves from plants grown for 4 to 6 weeks were used in all lipid treatments. One part of leaves was ground with a chilled plastic pestle directly in a 1.5-ml microcentrifuge tube in three parts of homogenization buffer (with or without lipids) containing 50 mm Tris-HCl, pH 7.5, 10 mm KCl, 1 mm EDTA, 0.5 mg mL−1 bovine serum albumin, 0.5 mm phenylmethysulfonyl fluoride, and 10 mm β-mercaptoethanol at 4°C. The homogenate was placed on ice for 30 min, and the supernatant was collected by centrifugation at 10,000g at 4°C for 5 min. In some experiments, the supernatant was centrifuged further at 100,000g to obtain the cytosolic (100,000g supernatant) and microsomal (100,000g pellet) fractions. Protein content was determined by the Bradford method, according to the manufacturer's instructions (Bio-Rad Laboratories, Hercules, CA). These fractions were used immediately or stored at −80°C until use.
SDS-PAGE and Immunoblotting
Proteins in the 10,000g supernatant were separated by 10% (w/v) SDS-PAGE and transferred onto polyvinylidene difuoride membranes. The membranes were blotted with PLDα antibodies that was raised in rabbit against the 13-amino acid peptide of the Arabidopsis PLDα C terminus (Pappan et al., 1997a). The proteins recognized by antibodies were visualized by staining alkaline phosphatase conjugated to a second antibody with a Bio-Rad immunoblotting kit (Wang et al., 1993).
Assays of NADPH-Dependent Superoxide Synthesis
Three assays were used to determine the activity of NADPH-dependent superoxide synthesis, LDC- and SOD-inhibitable, NADPH-dependent reductions of cyt c and NBT. LDC was assayed in the reaction mixture containing 10 μg protein, 80 μm NADPH, 0.02% (w/v) Triton X-100, 0.2 mm lucigenin, and 1 mm EDTA in 0.1 m Gly-NaOH buffer (pH 9.0) in a final volume of 2 mL. The SOD inhibitor N,N-diethyldithiocarbamate (1 mm) was added to the reaction mixture to block the dismutation of O2− to H2O2 by SOD. LDC was detected in a scintillation spectrometer with the counts reported every 6 s for 30 s, and the last two values were averaged (Auh and Murphy, 1995). The lucigenin stock (10×) was stored for 2 weeks at 4°C before use because LDC from this aged stock was less variable than that of the freshly prepared lucigenin. To determine the specificity of the assay for NADPH oxidase, SOD (45 units; Sigma), varied concentrations of DPI, or 10 mm H2O2 were added to the reaction mixture. In addition, NADPH was replaced by NADH or omitted from the standard mixture to test the NADPH dependence of the LDC.
A standard mixture to assay the SOD-inhibitory, NADPH-dependent reduction of cyt c contained 100 μm cyt c, 20 μm ATP, 3 μm GTP(γ) S, 0.02% (w/v) Triton X-100, 20 mm Tris-HCl (pH 7.5), and 20 to 40 μg protein in a total volume of 1 mL (Xing et al., 1997; Murphy et al., 1998). The reaction was initiated in a disposable microcuvette at 23°C by addition of 80 μm NADPH, and the reduction of cyt c was scanned by the change in A550 over the 1st min in a dual-beam spectrophotometer. SOD (40–60 units) was added to the mixture in a duplicated cuvette as the reference to monitor the SOD-dependent changes of absorption. In assaying the SOD-inhibitory, NADPH-dependent reduction of NBT, the reaction components and conditions were the same as for the reduction of cyt c, except that NBT was used, instead of cyt c, and the reduction of NBT was monitored by the change at A530. The specific activity for superoxide production was expressed as nmol O2− min−1 μg−1 with the reported coefficients (Murphy et al., 1998).
NBT Detection of Superoxide Generation in Situ
All the reagents in the NBT staining procedure were prepared in a 10-mm potassium phosphate buffer (P-K) at pH 7.5. A 1-mL syringe with no needle was used to pressure infiltrate a spot area on one leaf half. The extracellular superoxide generation system of xanthine and xanthine oxidase and SOD (30 units) were used to titrate the sensitivity of the staining procedure (data not shown). About 10 μL of dioleoyl-PA (200 μm) was infiltrated into the test leaves, and the control leaves were infiltrated with the P-K buffer only. For NBT staining, leaves were detached at 5, 60, and 120 min after infiltration and then were vacuum infiltrated in 10 mm NaN3 in 10 mm P-K buffer and immersed in 2 mL of the same buffer containing 0.1% (w/v) NBT at room temperature for 30 min. The blue precipitates of reduced NBT were visualized after boiling the leaves in 96% (v/v) ethanol for 10 min (Thordal-Christensen et al., 1997).
Phospholipid Extraction and Determination
Leaves were homogenized as described earlier and incubated on ice for 30 min. The homogenates then were extracted for lipid using a procedure described previously (Ryu and Wang, 1996). In brief, hot isopropanol (75°C) was added to leaf extracts and incubated at 75°C for 15 min to inactivate PLD and other lipolytic enzymes. Chloroform then was added, the chloroform phase was dried under N2, and lipids were resolved in 200 μL chloroform. A portion of lipids (20 μL) was used for assaying total phospholipid content. PA was separated from other lipid by thin layer chromatography (silica gel 60) and identified by comparing the Rfs (relative mobility of the component to the solvent front) with an authentic PA standard. PA was collected by scraping the spot, and phospholipid content was assayed and calculated based on determination of phosphorus content in the lipids (Rouser et al., 1970).
ACKNOWLEDGMENTS
We thank Dr. Jan E. Leach for critical reading of the manuscript, and Dr. Lu Fan for technical assistance on NADPH oxidase assays.
Footnotes
This work was supported by the National Science Foundation (grant no. IBN–9808729). This report is contribution no. 01–353–J of the Kansas Agricultural Experiment Station.
LITERATURE CITED
- Auh C-K, Murphy TM. Plasma membrane redox enzyme is involved in the synthesis of O2− and H2O2 by Phytophthora elicitor-stimulated rose cells. Plant Physiol. 1995;107:1241–1247. doi: 10.1104/pp.107.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman KD. Phospholipase activity during plant growth and development and in response to environmental stress. Trends Plant Sci. 1998;3:419–426. [Google Scholar]
- Dwyer SC, Legendre L, Low PS, Leto TL. Plant and human neutrophil oxidase burst complexes contain immunologically related proteins. Biochim Biophys Acta. 1996;1289:231–237. doi: 10.1016/0304-4165(95)00156-5. [DOI] [PubMed] [Google Scholar]
- Fan L, Zheng S, Wang X. Antisense suppression of phospholipase Dα retards abscisic acid- and ethylene-promoted senescence of postharvest Arabidopsis leaves. Plant Cell. 1997;9:2183–2196. doi: 10.1105/tpc.9.12.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank W, Munnik T, Kerkman K, Salamini F, Bartels D. Water deficit triggers phospholipase D activity in the resurrection plant Craterostigma plantagineum. Plant Cell. 2000;12:111–123. doi: 10.1105/tpc.12.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Strum JC, Sciorra VA, Daniel L, Bell RM. Raf-1 kinase possesses distinct binding domains for phosphatidylserine and phosphatidic acid. J Biol Chem. 1996;271:8472–8480. doi: 10.1074/jbc.271.14.8472. [DOI] [PubMed] [Google Scholar]
- Jabs T, Dietrich RA, Dangl JL. Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science. 1995;273:1853–1856. doi: 10.1126/science.273.5283.1853. [DOI] [PubMed] [Google Scholar]
- Jacob T, Ritchie S, Assmann SM, Gilroy S. Abscisic acid signal transduction in guard cells is mediated by phospholipase D activity. Proc Natl Acad Sci USA. 1999;96:12192–12197. doi: 10.1073/pnas.96.21.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller T, Damude HG, Werner D, Doerner P, Dixon RA, Lamb C. A plant homolog of the neutrophil NADPH oxidase gp91phox subunit gene encodes a plasma membrane protein with Ca2+ binding motifs. Plant Cell. 1998;10:255–266. doi: 10.1105/tpc.10.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb C, Dixon RA. The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:251–275. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- Lee SH, Chae TK, Kim SH, Shin SH, Cho BH, Kang BG, Lee WS. Ethylene-mediated phospholipid catabolism pathway in glucose-starved carrot suspension cells. Plant Physiol. 1998;116:223–229. [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Liscovitch M, Czarny M, Fiucci G, Tang X. Phospholipase D: molecular and cell biology of a novel gene family. Biochem J. 2000;345:401–415. [PMC free article] [PubMed] [Google Scholar]
- McPhail LC, Waite KA, Regier DS, Nixon JB, Qualliotine-Mann D, Zhang WX, Wallin R, Sergeant S. A novel protein kinase target for the lipid second messenger phosphatidic acid. Biochim Biophys Acta. 1999;1439:277–290. doi: 10.1016/s1388-1981(99)00100-6. [DOI] [PubMed] [Google Scholar]
- Munnik T, Arisz SA, de Vrije T, Musgrave A. G protein activation stimulates phospholipase D signaling in plants. Plant Cell. 1995;7:2197–2210. doi: 10.1105/tpc.7.12.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TM, Vu H, Nguyen T. The superoxide synthesis of rose cells. Plant Physiol. 1998;117:1301–1305. doi: 10.1104/pp.117.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Cárdenas ML, Narváez-Vásquez J, Ryan RA. Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell. 2001;13:179–191. [PMC free article] [PubMed] [Google Scholar]
- Pappan K, Austin-Brown S, Chapman KD, Wang X. Substrate selectivities and lipid modulation of phospholipase Dα, β, and γ from plants. Arch Biochem Biophys. 1998;353:131–140. doi: 10.1006/abbi.1998.0640. [DOI] [PubMed] [Google Scholar]
- Pappan K, Qin W, Dyer JH, Zheng L, Wang X. Molecular cloning and functional analysis of polyphosphoinositide-dependent phospholipase D, PLDβ, from Arabidopsis. J Biol Chem. 1997a;272:7055–7062. doi: 10.1074/jbc.272.11.7055. [DOI] [PubMed] [Google Scholar]
- Pappan K, Wang X. Plant phospholipase D is an acidic phospholipase active at near-physiological Ca2+ concentrations. Arch Biochem Biophys. 1999;368:347–353. doi: 10.1006/abbi.1999.1325. [DOI] [PubMed] [Google Scholar]
- Pappan K, Zheng S, Wang X. Identification and characterization of a novel plant phospholipase D that requires polyphosphoinositides and submicromolar calcium for activity in Arabidopsis. J Biol Chem. 1997b;272:7048–7054. doi: 10.1074/jbc.272.11.7048. [DOI] [PubMed] [Google Scholar]
- Potikha TS, Collins CC, Johnson DI, Delmer DP, Levine A. The involvement of hydrogen peroxide in the differentiation of secondary walls in cotton fibers. Plant Physiol. 1999;119:849–858. doi: 10.1104/pp.119.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W, Pappan K, Wang X. Molecular heterogeneity of phospholipase D (PLD): cloning of PLDγ and regulation of plant PLDγ, -β and -α by polyphosphoinositides and calcium. J Biol Chem. 1997;272:28267–28273. doi: 10.1074/jbc.272.45.28267. [DOI] [PubMed] [Google Scholar]
- Qualliotine-Mann D, Agwu DE, Ellenburg MD, McCall CE, McPhail LC. Phosphatidic acid and diacylglycerol synergize in a cell-free system for activation of NADPH oxidase from human neutrophils. J Biol Chem. 1993;268:23843–23849. [PubMed] [Google Scholar]
- Regier DS, Greene DG, Sergeant S, Jesaitis AJ, McPhail LC. Phosphorylation of p22phox is mediated by phospholipase D-dependent and -independent mechanisms: correlation of NADPH oxidase activity and p22phox phosphorylation. J Biol Chem. 2000;275:28406–28412. doi: 10.1074/jbc.M004703200. [DOI] [PubMed] [Google Scholar]
- Regier DS, Waite KA, Wallin R, McPhail LC. A phosphatidic acid-activated protein kinase and conventional protein kinase C isoforms phosphorylate p22(phox), an NADPH oxidase component. J Biol Chem. 1999;274:36601–36608. doi: 10.1074/jbc.274.51.36601. [DOI] [PubMed] [Google Scholar]
- Ritchie S, Gilroy S. Abscisic acid stimulation of phospholipase D in the barley aleurone is G-protein-mediated and localized to the plasma membrane. Plant Physiol. 2000;124:693–702. doi: 10.1104/pp.124.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouser G, Fleicher S, Yamamoto A. Two-dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorous analysis of spots. Lipids. 1970;5:494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- Ryu BS, Wang X. Activation of phospholipase D and the possible mechanism of activation in wound-induced lipid hydrolysis in castor bean leaves. Biochim Biophys Acta. 1996;1303:243–250. doi: 10.1016/0005-2760(96)00096-3. [DOI] [PubMed] [Google Scholar]
- Ryu SB, Wang X. Increases in free linolenic and linoleic acids associated with phospholipase D-mediated hydrolysis of phospholipids in wounded castor bean leaves. Biochim Biophys Acta. 1998;1393:193–202. doi: 10.1016/s0005-2760(98)00048-4. [DOI] [PubMed] [Google Scholar]
- Taylor AT, Low SP. Phospholipase D involvement in the plant oxidative burst. Biochem Biophys Res Commun. 1997;237:10–15. doi: 10.1006/bbrc.1997.6965. [DOI] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB. Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997;11:1187–1194. [Google Scholar]
- Torres MA, Onouchi H, Hamada S, Machida C, Hammond-Kosack KE, Jones JD. Six Arabidopsis thaliana homologues of the human respiratory burst oxidase (gp91phox) Plant J. 1998;14:365–670. doi: 10.1046/j.1365-313x.1998.00136.x. [DOI] [PubMed] [Google Scholar]
- van der Luit AH, Piatti T, van Doorn A, Musgrave A, Felix G, Boller T, Munnik T. Elicitation of suspension-cultured tomato cells triggers the formation of phosphatidic acid and diacylglycerol pyrophosphate. Plant Physiol. 2000;123:1507–15016. doi: 10.1104/pp.123.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite KA, Wallin R, Qualliotine-Mann D, McPhail LC. Phosphatidic acid-mediated phosphorylation of the NADPH oxidase component p47-phox: evidence that phosphatidic acid may activate a novel protein kinase. J Biol Chem. 1997;272:15569–15578. doi: 10.1074/jbc.272.24.15569. [DOI] [PubMed] [Google Scholar]
- Wang C, Zien C, Afitlhile M, Welti R, Hildebrand DF, Wang X. Involvement of phospholipase D in wound-induced accumulation of jasmonic acid in Arabidopsis. Plant Cell. 2000;12:2237–2246. doi: 10.1105/tpc.12.11.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. Multiple forms of phospholipase D in plants: the gene family, catalytic and regulatory properties, and cellular functions. Prog Lipid Res. 2000;39:109–149. doi: 10.1016/s0163-7827(00)00002-3. [DOI] [PubMed] [Google Scholar]
- Wang X, Dyer JH, Zheng L. Purification and immunological analysis of phospholipase D from castor bean endosperm. Arch Biochem Biophys. 1993;306:496–494. doi: 10.1006/abbi.1993.1541. [DOI] [PubMed] [Google Scholar]
- Xing T, Higgins VJ, Blumwald E. Race-specific elicitors of Cladosporium fulvum promote translocation of cytosolic components of NADPH oxidase to the plasma membrane of tomato cells. Plant Cell. 1997;9:249–259. doi: 10.1105/tpc.9.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SA, Wang X, Leach JE. Changes in the plasma membrane distribution of rice phospholipase D during resistant interactions with Xanthomonas oryzae pv oryzae. Plant Cell. 1996;8:1079–1090. doi: 10.1105/tpc.8.6.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Krishnamoorthi R, Zolkiewski M, Wang X. Distinct Ca2+ binding properties of the novel C2 domains of plant phospholipase Dα and β. J Biol Chem. 2000;275:19700–19706. doi: 10.1074/jbc.M001945200. [DOI] [PubMed] [Google Scholar]
- Zien CA, Wang C, Wang X, Welti R. In-vivo substrates and the contribution of the common phospholipase D, PLDα, to wound-induced metabolism of lipids in Arabidopsis. Biochim Biophys Acta. 2001;1530:236–248. doi: 10.1016/s1388-1981(01)00091-9. [DOI] [PubMed] [Google Scholar]