Abstract
This retrospective cohort study evaluates the impact of an AI-supported continuous glucose monitoring (CGM) mobile app (“January V2”) on glycemic control and weight management in 944 users, including healthy individuals and those with prediabetes or type 2 diabetes (T2D). The app, leveraging AI to personalize feedback, tracked users’ food intake, activity, and glucose responses over 14 days. Significant improvements in time in range (TIR) were observed, particularly in users with lower baseline TIR. Healthy users’ TIR increased from 74.7% to 85.5% (p < 0.0001), while T2D users’ TIR improved from 49.7% to 57.4% (p < 0.0004). Higher app engagement correlated with greater TIR improvements. Users also experienced an average weight reduction of 3.3 lbs over 33 days. These findings suggest that AI-enhanced digital health interventions can improve glycemic control and promote weight loss, particularly when users are actively engaged.
Subject terms: Diabetes, Disease prevention
Introduction
The rapid evolution of digital health tools has transformed the landscape of chronic disease management, offering new avenues for personalized treatment, real-time notifications and interventions at the individual level1. Type 2 diabetes (T2D) is a condition that requires constant monitoring and management due to the mechanism of the disease and its implications on the body’s energy expenditure2. T2D has seen significant enhancement in monitoring, especially with the integration of continuous glucose monitoring (CGM) systems3. CGM provides users with immediate feedback on their glycemic control, enabling timely adjustments to diet, exercise, and medication, if patients are medicated4.
While CGM has been predominantly used by insulin-treated individuals4,5, recent developments have made CGM more available to individuals with diet-controlled diabetes or no diabetes, thereby placing CGM center-stage in efforts to prevent diabetes through lifestyle modifications such as healthier food choices and increased physical activity6,7. However, continuous use of CGMs in non-insulin users can sometimes be burdensome both physically and financially due to the invasiveness and cost of the devices, and may not provide immediate perceived benefits7.
Indeed, the global prevalence of T2D and its precursor, prediabetes, is rising, with 1.3 billion people expected to live with T2D by 20508. Given this increase in prevalence, effective management and prevention strategies are critical. Traditional methods such as the widely used Diabetes Prevention Program (DPP) often rely on periodic clinical visits, one-on-one coaching, and self-reported data9. The DPP has historically seen successful outcomes. Launched by the Centers for Disease Control (CDC) in 2010, the National Diabetes Prevention Program is clinically proven to reduce the risk of developing T2D by 58%, and, among those 60 years or older, by 71%10.
Despite this success, DPP is hampered by a high user burden and lack of engagement. Even though approximately 135 million people in the United States have T2D or prediabetes11, fewer than 500 thousand Americans have gone through the DPP since its inception12. Additionally, the high cost of such programs that require coaching limits their reach13,14, which is especially worrisome as metabolic disease is inversely correlated with income15.
Digital health interventions offer a promising alternative by leveraging technology to provide continuous monitoring, personalized feedback, and behavior modification in a scalable fashion. AI approaches in particular can alleviate CGM burden by enabling infrequent CGM use while still providing insights into blood sugar management. By harnessing predictive algorithms and data modeling, AI can estimate glucose levels based on historical data and behavioral patterns, reducing the need for constant monitoring. Furthermore, flexible programs that allow users to engage at their own pace, without feeling constrained by rigid schedules or frequent device use, are likely to see better adherence.
This study builds upon our previous research16, where we showed that a structured digital health program integrating CGM and behavioral tracking improved glycemic control and promoted weight loss. Here, we introduce a flexible, AI-supported intervention (“January V2”) designed to reduce the user burden of CGM while promoting better metabolic health outcomes. By focusing on infrequent CGM use and personalized, adaptive feedback, we sought to create a system that is both more sustainable for long-term use, and simultaneously more appealing to a broad population and especially users in earlier stages of metabolic disease who are not reliant on insulin therapy.
To assess the effectiveness of the January V2 app, we conducted a retrospective cohort study involving 944 users who utilized the app over a 14-day period. Participants included healthy individuals as well as those with prediabetes or type 2 diabetes. The study analyzed changes in glycemic control and weight management, stratifying results by health condition and level of app engagement. Our study evaluated the impact of January V2 on key health metrics and endpoints related to metabolic health, including time in range (TIR), weight management, and glycemic events, across user cohorts in various stages of metabolic disease and particularly among those with lower baseline metrics. Additionally, we investigate the role of user engagement in these outcomes. Our results demonstrate that unstructured digital interventions that reduce patient burden and address poor user engagement can be effective for glycemic control and weight management. A key aspect of this research is the focus on user engagement where we found a clear correlation between highly engaged “power users” and improved outcomes in TIR and weight loss compared to less engaged users.
Results
Time in Range (TIR)
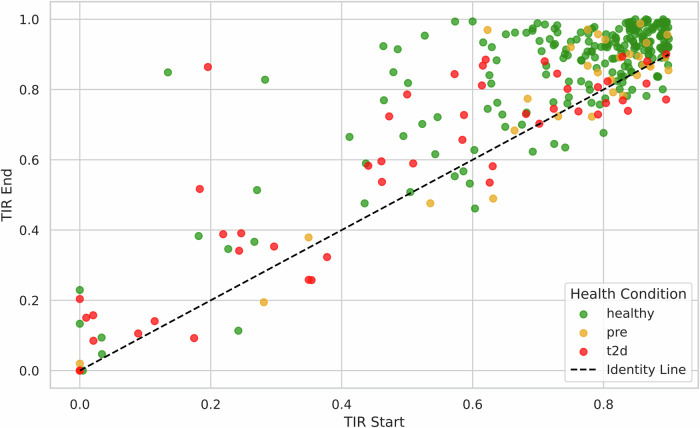
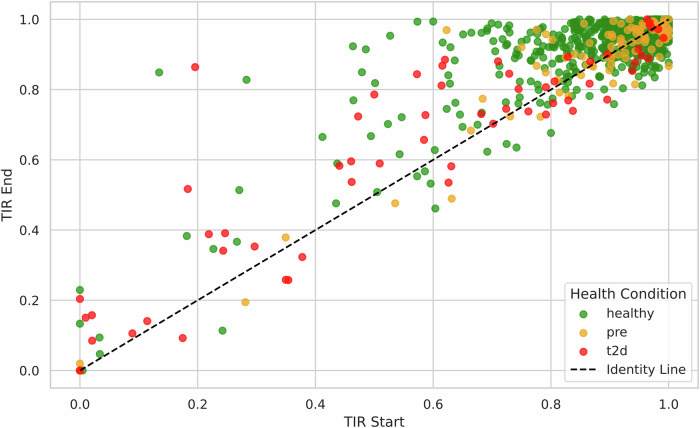
The study found significant improvements in TIR among users with lower baseline values (< 90%) (Fig. 1). Healthy users exhibited an increase from 74.7% to 85.5% (p < 0.0004), whereas users with T2D showed an improvement from 49.7% to 57.4% (p < 0.0004) (Fig. 2). “Power users” also demonstrated a statistically significant increase in TIR relative to the group as a whole, from 75.5% to 85.6%. This suggests a correlation between engagement and glycemic control.
Fig. 1. TIR Start vs TIR End by Health Condition, TIR < 90%.
Comparison of Time in Range (TIR) at Start and End of Intervention for Users with TIR < 90% (n = 307), Stratified by Health Condition. Each dot represents an individual user; dots above the diagonal line indicate improvement. Green dots represent healthy users. Yellow dots represent prediabetes users. Red dots represent type 2 diabetes users.
Fig. 2. TIR Start vs TIR End by Health Condition.
Comparison of Time in Range (TIR) at Start and End of Intervention for All Users (n = 944), Stratified by Health Condition. Each dot represents an individual user; dots above the diagonal line indicate improvement. Green dots represent healthy users. Yellow dots represent prediabetes users. Red dots represent type 2 diabetes users.
Weight management
Of the 944 participants, 702 users (74.4%) had complete weight data (logged their body weight more than once) and were included in the weight analysis. For other variables of interest, missing data were minimal (< 1%) and participants with missing data for a specific outcome were excluded from that particular analysis.
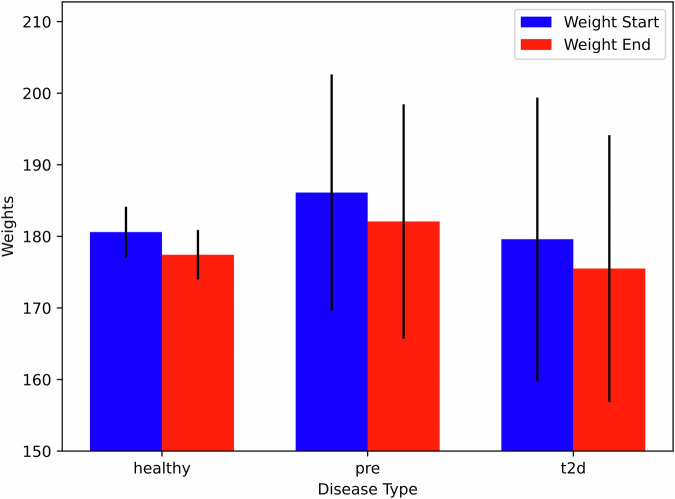
On average, users experienced weight reduction of 3.3 lbs over an average period of 33 days, reflecting the time between their first and last weight entries. The most significant weight loss was observed in the prediabetes cohort (4.0 lbs, p < 0.0001) and among power users (4.0 lbs, p < 0.0001), underscoring the potential for digital interventions in weight management (Fig. 3).
Fig. 3. Starting vs ending weight by disease type.
This figure shows the mean starting and ending weights for each disease group. Error bars represent confidence level. All user groups lost weight over the course of the observational period, with the most significant weight loss observed among the prediabetes cohort. For Healthy (n = 630): p < 0.0001, t-test = 13.22. For prediabetes (n = 46), p < 0.0001, t test = 11.92. For T2D (n = 26), p = 0.001, t test = 5.90. For all groups, the critical t value (two tailed) was 2.0. The statistical significance refers to the within-group weight loss from start to end, assessed by paired t-tests. Although the confidence intervals overlap, the paired analysis demonstrates significant weight loss within each group.
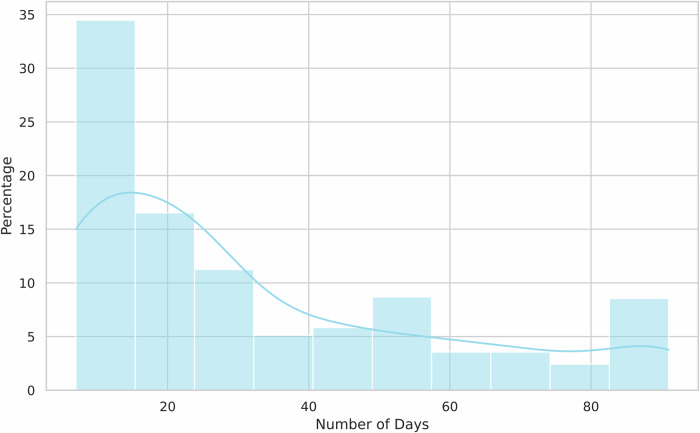
To better understand the variability in users’ weight tracking behaviors, we also examined the distribution of the number of days between users’ first and last logs. Figure 4 shows that while the majority of users logged their weights over periods closer to the study’s 33-day average, there was considerable variation, with some users logging for shorter or longer periods.
Fig. 4. The distribution of number of days between the first and last weight logs.
This histogram illustrates the distribution of the number of days between the first and last weight entries logged by users who participated in the study. The x-axis represents the number of days between logs, while the y-axis represents the percentage of users. The majority of users logged their weights over periods near the 33-day average, but the distribution reveals variability, with some users logging over shorter or longer timeframes.
Glycemic events
Glycemic events were defined as instances where glucose levels exceeded 180 mg/dL (hyperglycemic) or fell below 70 mg/dL (hypoglycemic). The analysis of hyperglycemic events showed no significant changes for the overall population. However, users with prediabetes experienced a reduction from 1.14 to 0.95 events per day (p = 0.037). Hypoglycemic events significantly decreased across all cohorts, particularly in healthy users (0.17 to 0.06 events per day, p < 0.0001), indicating improved glycemic stability.
Effect of AI recommendations on glycemic control
To estimate the isolated effect of the AI feature beyond the benefits of food tracking, physical activity, sleep tracking, and CGM, we compared glycemic metrics between the 5-day training period (pre-AI recommendations) and the subsequent 9-day intervention period (post-AI recommendations).
During the training period, the average TIR for all users was 80.2%. After the introduction of AI recommendations, the average TIR increased to 85.6% (p < 0.0002). Among “power users,” the TIR improved from 81.0% during the training period to 88.2% during the intervention period (p < 0.0001).
This significant improvement suggests that the AI-driven personalized recommendations contributed additional benefits to glycemic control beyond the initial effects of self-monitoring and logging during the training period.
Glucose management indicator
We evaluated the Glucose Management Indicator (GMI) at two different time points to assess changes in glucose management over the intervention period. The GMI at the beginning of the study was recorded at 5.734%, while at the end of the study period, it slightly decreased to 5.718%. This minor reduction indicates a small improvement in average blood glucose levels and, by extension, potentially better glucose management by the end of the study.
To determine the statistical significance of this change, a paired t-test was conducted. The resulting p-value was 0.042, which is below the conventional alpha level of 0.05, suggesting that the observed change in GMI was statistically significant. This implies that the intervention had a positive effect on the glucose management of the participants.
To further assess the significance we calculated the critical t-value for a two-tailed test at the 95% confidence level which was found to be 1.96, further supporting the significance of the findings. It is important to note that while the change in GMI is statistically significant, the clinical relevance of such a small difference in GMI values needs careful consideration and further discussion, particularly in terms of real-world implications for diabetes management strategies.
Last meal sleep gap
Last Meal Sleep Gap was defined as the time elapsed between the last logged meal of the day and the reported bedtime. Eating closer to bedtime is often associated with poorer sleep and worse metabolic outcomes17, and one recommendation from the App is to avoid eating three hours prior to bedtime. In our study the average last meal sleep gap increased slightly from 2.80 h to 3.06 h across all users (p < 0.0001). This shift was consistent across cohorts, except for T2D users, who showed no significant change. The shift in non-T2D groups indicates a broader behavioral trend that could have positive implications for sleep quality and metabolic health. However, the lack of significant change among T2D users highlights that this particular group might have different habits, needs, or challenges when it comes to managing meal timing relative to sleep.
Calorie and macronutrient intake
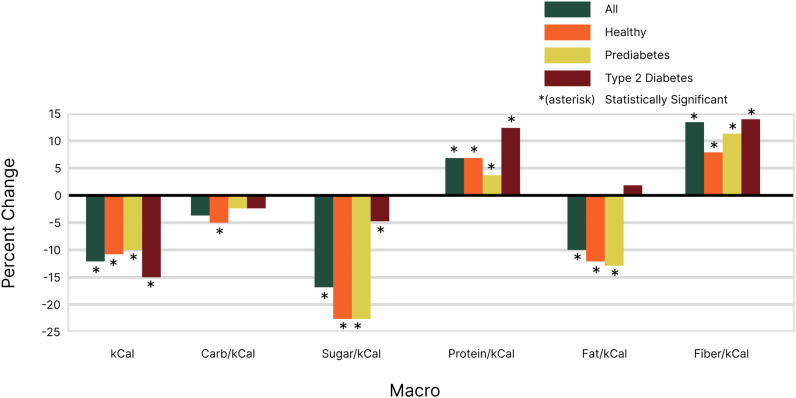
A major goal of our program is to reduce carbohydrate and sugar intake while increasing protein and fiber intake per calorie. This was achieved across all groups (Table 1).
Table 1.
Percent change in macronutrient intake
| % Change | |||||
|---|---|---|---|---|---|
| Carb/kcal | Sugar/kcal | Protein/kcal | Fat/kcal | Fiber/kcal | |
| All | −3.5% | −16.7% | 6.9% | −9.9% | 13.8% |
| Healthy | −4.7% | −22.6% | 6.9% | −11.6% | 8.8% |
| Pre-diabetes | −2.2% | −22.4% | 3.3% | −13.0% | 11.1% |
| T2D | −2.2% | −4.5% | 12.1% | 1.8% | 14.1% |
Percent change was expressed relative to total calorie intake to clarify how adjustments in macronutrient consumption, specifically protein and fiber, affected users’ overall dietary composition. Although the app recommended reducing net calories for weight loss, it also encouraged increasing protein and fiber intake. By using this approach, we ensured that shifts in macronutrient balance were highlighted without being confounded by changes in total calorie intake, allowing for a more accurate assessment of dietary patterns and their impact on weight loss and health outcomes.
Fat intake per calorie declined in most groups except for those with Type 2 Diabetes, who exhibited a slight increase, likely due to the substitution of carbohydrate rich “spiking foods” for foods with higher fat content. Notably, the T2D group also showed the highest increases in both protein and fiber intake, suggesting a more significant dietary adjustment in this population (Fig. 5).
Fig. 5. Macronutrient Intake.
Across all groups, there was a general trend towards reducing relative carbohydrate and sugar intake while increasing relative protein and fiber intake per calorie. Specifically, relative carbohydrate intake decreased by 3.5%, relative sugar by 16.7%, relative protein increased by 6.9%, and relative fiber increased by 13.8%, while fat intake decreased by 9.9%. When broken down by health condition, the healthy group showed the largest reduction in relative sugar (−22.6%) and fat intake (−11.6%), while the T2D group saw the highest increase in relative protein (12.1%) and fiber (14.1%). The pre-diabetic group demonstrated a similar reduction in relative sugar (−22.4%) but a larger decrease in relative fat (−13.0%) compared to the healthy group.
Discussion
The results of this study underscore the potential of digital health interventions, such as the January V2 platform, in improving glycemic control and supporting weight management in individuals with varying stages of metabolic health. This study builds upon our previous research on structured digital health programs16. Relative to a previous study that used a structured program with specific daily regimented tasks, the current study employed a more flexible and self directed approach in which users engaged with the platform at their own pace, and explored and utilized features based on their unique needs and preferences. The results presented here demonstrate that, even in a less structured environment, digital interventions can effectively support glycemic control and weight management. While this study lacks a control group—a limitation inherent to real-world studies—our findings build upon previous controlled studies demonstrating the effectiveness of digital interventions in metabolic health18. Similar to other real-world implementation studies, our data are based on changes from baseline to end-of-program and thus could represent a ‘placebo’ effect from enrollment alone. Future randomized controlled trials would help confirm and extend these findings. Nevertheless, the magnitude of improvements observed, particularly in TIR and weight loss metrics, align with outcomes from controlled studies of similar digital health interventions19.
A notable feature of the present research is the stratification of users based on engagement levels (“power users”). Our analysis shows a clear correlation between user engagement and improved outcomes, with power users demonstrating more significant improvements in TIR and weight loss, versus their less engaged peers. For TIR improvement based on engagement, Pearson correlation was 0.53 (p = 0.05). For weight improvement based on engagement, Pearson correlation was 0.15 (p = 0.34). This result underscores the critical role of user engagement, especially in a self-directed setting.
The significant improvements in Time in Range (TIR) observed in this study, particularly among users with lower baseline TIR values (TIR < 90%), highlight the effectiveness of the January V2 platform in enhancing glycemic control. The increase in TIR from 74.7% to 85.5% among healthy users with low TIR (n = 225) and from 49.7% to 57.4% among T2D with low TIR (n = 50), is noteworthy. For individuals with Type 1 and Type 2 diabetes, optimal TIR is > 70%; each additional 5% increase in TIR is associated with clinically significant benefits for those populations20,21. These results align with existing literature that emphasizes the clinical benefits of higher TIR, including reduced complications and improved overall health outcomes for individuals with diabetes19,22–24.
The correlation between high user engagement and improved TIR further underscores the importance of sustained interaction with digital health platforms. The “power users” in this study, who demonstrated the highest levels of engagement, exhibited more significant improvements in TIR, suggesting that consistent use of the platform’s features is crucial for achieving optimal glycemic control. This finding is consistent with previous research that has highlighted the role of continuous engagement in the effectiveness of digital health interventions25–27.
The study also revealed significant weight loss among users, particularly in the prediabetes cohort and among power users. The average weight reduction of 3.3 lbs over the 33-day period, with the most significant loss observed in the prediabetes cohort (4.0 lbs), indicates that digital health interventions can be effective in supporting weight management. This is particularly relevant given the well-established relationship between weight and glycemic control, where weight reduction is often associated with improved insulin sensitivity and reduced risk of diabetes progression28,29.
The success in weight management observed in this study may be attributed to the personalized recommendations provided by the January V2 platform, which likely encouraged healthier dietary and physical activity choices. Nonetheless, the increase in protein and fiber intake, along with the reduction in carbohydrate and sugar consumption, particularly in the T2D cohort, suggests that users were able to make meaningful adjustments to their diet, likely contributing to their weight loss and improved glycemic control.
The reduction in glucose events below 70 mg/dL across all cohorts, particularly among healthy users, was another observed outcome of the study. The decrease from 0.17 to 0.06 events per day suggests that the January V2 platform helps users manage their blood glucose levels and may stabilize them. However, in healthy individuals, glucose levels below 70 mg/dL are not necessarily dangerous and can be part of normal physiology. Therefore, the clinical significance of this reduction in healthy users is uncertain. However, the lack of significant changes in hyperglycemic events, except in the prediabetes cohort, suggests that while the platform is effective in preventing low blood glucose levels, it may be less impactful in preventing spikes in certain populations.
The slight increase in the last meal sleep gap across most cohorts, except for T2D users, may suggest a positive shift towards better dietary habits, such as earlier meal times, which can contribute to better glycemic control during sleep. However, the lack of significant change in this metric among T2D users may indicate the need for more tailored interventions to address their specific challenges. McCurley30 showed that culturally tailored interventions were modestly successful for Hispanics in lowering risk of T2D, as measured by weight reduction or improvement in glucose regulation. Sahin31 examined the use of text messaging in 13 clinical trials and showed that personalized text messaging interventions could improve glycemic control in patients with T2D. Nevertheless, both groups of researchers cite the need for more rigorous interventions with larger patient samples and longer time horizons to confirm their findings; indeed, Radhakrishnan32 examined 10 studies and showed that, after accounting for cost and resource utilization, the efficacy of tailored interventions may not exceed that of standard interventions in the improvement of disease self-management. It is notable that research into the use of AI for the delivery of personalized recommendations is sparse33, and that AI can dramatically reduce the cost of offering personalized recommendations34,35 while increasing the level of personalization through integration with wearables36.
While the findings of this study are promising, several limitations must be considered. First, this was a real world study and did not include a control group. The observed improvements in glycemic control and weight management could potentially be attributed to the placebo effect or other nonspecific effects of participating in a study. Without a control group, it is difficult to definitively attribute the observed changes to the intervention itself. Future randomized controlled trials with appropriate comparator groups are necessary to confirm these findings and to isolate the specific effects of the digital health intervention.
Second, the sample may be subject to selection bias, as participants were individuals who opted to purchase and use the app. These users may have higher levels of motivation, health literacy, or self-efficacy compared to the general population, potentially leading to better engagement and outcomes37. This could limit the generalizability of the findings to broader populations, including those less motivated or with limited access to such technologies. Future studies should aim to include a more diverse and representative sample, possibly through randomized recruitment strategies or offering the intervention at no cost to participants.
Third, the duration of the intervention was relatively short (14 days for primary glycemic outcomes and 33 days for weight loss). This limited timeframe restricts our ability to assess the long-term efficacy, adherence, and sustainability of the intervention. Previous studies of lifestyle interventions for diabetes prevention, such as the Diabetes Prevention Program, have demonstrated sustained benefits over 2.9 to 6 years, with continued protection of 27–43% for up to 15 years38. Our shorter timeframe cannot address whether the improvements in glycemic control and weight loss will persist beyond the intervention period. It is possible that the observed improvements may diminish over time without continued use of the app or additional support. Long-term studies with extended follow-up periods of at least 12 months are needed to evaluate whether the benefits are maintained and whether the intervention can lead to lasting behavior change and clinical outcomes. Moreover, future studies should assess whether periodic CGM use combined with ongoing app engagement could maintain improvements over time.
Fourth, we did not collect data on participants’ concurrent use of weight loss or diabetes medications, nor their participation in other glycemic control or weight management programs. It is possible that some observed improvements may be partially attributable to these external factors rather than solely to the intervention. Adjusting for such variables in future research by collecting comprehensive data on concurrent interventions could help better isolate the specific effects of the digital health tool. This will also allow researchers to assess potential synergies or conflicts between the app’s recommendations and other treatments participants may be using.
Fifth, the reliance on self-reported data, such as food intake and health status, introduces the potential for recall bias. Participants may not accurately remember or report their dietary habits or health conditions, which could affect the accuracy of the findings. Future studies could incorporate more objective measures, such as biomarkers or electronic health records, to validate self-reported data.
Additionally, the study did not account for potential unmeasured confounding variables that could influence both the exposure (app use) and the outcomes (glycemic control, weight loss). While we adjusted for known confounders like age, sex, and baseline health status, other factors such as socioeconomic status, education level, or access to healthcare could play a role. Future research should aim to collect and adjust for a more comprehensive set of potential confounders to better isolate the true effect of the intervention.
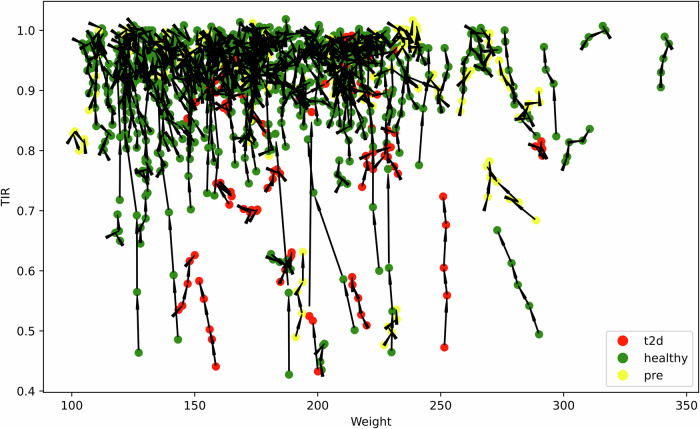
Examination of users’ time-in-range compared to their weight led to observation of several individuals who self-reported as “healthy” but nonetheless exhibit lower TIR, which is typically more characteristic of individuals with prediabetes or T2D; a feature noted previously19. The individuals in this group tended to have higher body weight, raising the question of whether these are missed diagnoses given the correlation of higher body weight with insulin resistance.
This raises the fascinating possibility that the current diagnostic criteria used for classification might not fully capture underlying health conditions like early insulin resistance. Alternatively, this result could reflect variability in the accuracy of self-reported health status; indeed, most people with prediabetes are unaware that they have this condition3 (Fig. 6).
Fig. 6. TIR Start vs TIR end by health condition.
This plot displays the relationship between weight and TIR for users among three cohorts: T2D, healthy, and prediabetes. Black lines connect data points, highlighting variations within individuals. Green dots represent healthy users. Yellow dots represent prediabetes users. Red dots represent type 2 diabetes users.
These findings suggest several important directions for clinical translation and larger trials. First, healthcare systems might implement pilot programs to test the integration of flexible digital interventions into standard care programs. This would be particularly valuable for resource-constrained settings where continuous in-person monitoring is challenging. Future clinical trials should include multi-center randomized controlled studies comparing standard care to digital intervention, with stratification by baseline metabolic health status and longer follow-up periods (12-24 months) to assess the durability of improvements and their impact on clinical outcomes such as HbA1c levels, incidence of diabetes, and cardiovascular events. Second, studies examining cost-effectiveness compared to traditional care models and trials focused specifically on populations at high risk for developing T2D would help establish the broader utility of this approach.
The broader implications of this study suggest that digital health platforms like January V2 have the potential to play a crucial role in the future of chronic disease management. By providing continuous, personalized support, these tools have the potential to reduce the burden of diabetes and prediabetes on both individuals and healthcare systems. As digital health continues to evolve, it will be essential to refine these interventions to maximize their efficacy and accessibility. This study provides evidence that digital health interventions can effectively improve glycemic control and support weight loss, particularly in individuals with lower baseline health metrics. Future research should explore the long-term sustainability of these improvements through controlled trials with follow-up periods of 12–24 months. Such studies should examine whether periodic CGM use (e.g., 2 weeks every 3–6 months) combined with ongoing app engagement could maintain improvements in glycemic control and weight management. Additionally, research should investigate strategies to maintain user engagement over extended periods and identify factors that predict long-term success with digital health interventions. Cost-effectiveness analyses comparing this approach to traditional diabetes prevention programs would help determine optimal implementation strategies for different populations.
Overall, this study demonstrates that digital health interventions can lead to significant improvements in TIR, weight, and glycemic stability, particularly among users with lower baseline metrics. The enhanced outcomes among power users highlight the importance of user engagement in achieving health goals. These findings align with previous research39,40, underscoring the potential for digital tools in managing chronic conditions and providing a foundation for future innovations in this field.
Methods
Study design and participants
The goal of this study was to determine if an advanced user App and AI framework can improve metabolic health and whether improvement correlated with user engagement. Nine hundred and forty-four participants were enrolled in the January V2 digital health program. The participants wore a CGM for “AI training” for 5–14 days followed by a 15–90 day period without a CGM. The participants also wore a smart watch for resting heart rate monitoring, and using a mobile App, the participants logged food, and received feedback about their dietary and physical activity habits. The participants received recommendations to minimize glucose excursions and increase time-in-range. Unlike our prior study, in which participants followed a structured program with specific tasks to complete daily over a certain timeframe, the current study employed a more flexible and self directed approach. Users engaged with the platform at their own pace, and explored and utilized features based on their unique needs and preferences. Time of range improvement and the frequency of glucose excursion events were scored throughout users’ participation in the program and weight was scored at the beginning and end of the study of a subset of participants.
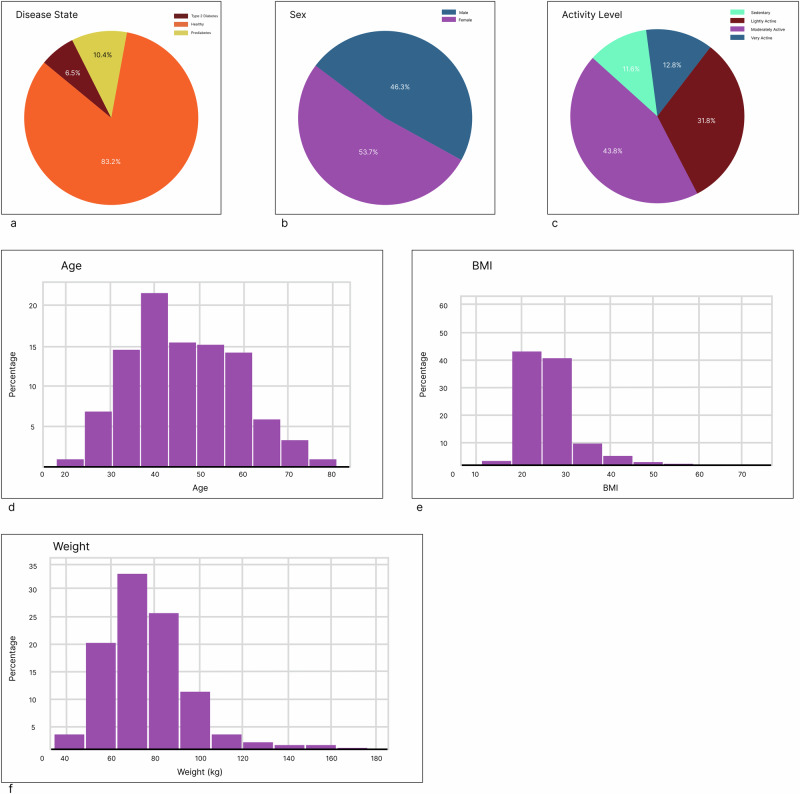
The cohort was 83% normoglycemic (“healthy”), 10.4% with prediabetes and 6.5% with diabetes through self reporting. 46.3% were male and 53.7% were female. (Fig. 7)
Fig. 7. Participant demographics Demographic information was self-reported and captured during participant onboarding.
a Diabetes status was classified into “Healthy,” “Prediabetes,” and “T2D.” b Sex was “Male” and “Female.” c Activity level was classified into “Sedentary” (little to no physical activity); “Lightly Active” (Light exercise or sports 1–3 days per week); “Moderately Active” (Moderate exercise or sports 3–5 days per week); and “Very Active” (Hard exercise or sports 6−7 days a week). (see Methods) (d) Age was self-reported, with the mean and median ages 46.8, 46.0, and the standard deviation 12.2. e BMI was self-reported, with the mean BMI being 26.9, the median BMI being 25.5, and the standard deviation being 6.32. f Weight was self-reported, with the mean weight (kg) being 78.0, the median age being 75.7, and the standard deviation being 18.8.
Participants
Participants were users of the January V2 app who purchased and used the app between January 2022 and December 2023. The study includes data collected during this period. Users were located across the United States and participated remotely by using the app and associated devices in their own environment.
This retrospective cohort study analyzed data from 944 users who utilized a digital health platform incorporating CGM (“January V2”). The sample size of 944 participants was determined by the number of users who met the inclusion criteria during the study period (see below). Given the observational nature of this retrospective study, no formal power analysis was conducted. However, the large sample size is sufficient to detect significant changes in the primary outcomes.
Eligibility criteria included adults aged 18 or older who purchased and used the January V2 app during the study period, provided informed consent for data collection and analysis, and completed at least 5 days of CGM use for AI training. Participants were self-selected users who opted to use the app, and data were collected retrospectively from the app’s database. The recruitment period coincided with the app usage period. The exposure period was defined as the 14 days of CGM use, and the follow-up period was defined as the duration of app usage, during which participants continued to interact with the app.
The cohort was comprised of healthy (n = 785) and those with prediabetes (n = 98) and type 2 diabetes (T2D) (n = 61) as assessed by users’ self-reporting.
To assess the impact of user engagement on outcomes, the study further stratified users into “power users” (those with top quartile engagement or higher) and “others”. User engagement was quantified based on the average number of foods logged per day during the intervention period after the AI training period. ‘Power users’ were defined as those in the top quartile of engagement, logging an average of three or more foods per day. This metric was chosen because it correlates with overall app use and reflects active participation in dietary tracking, a key component of the intervention. Quartiles were chosen to create a distinct group of highly engaged users for comparison with the rest of the cohort.
The January V2 app
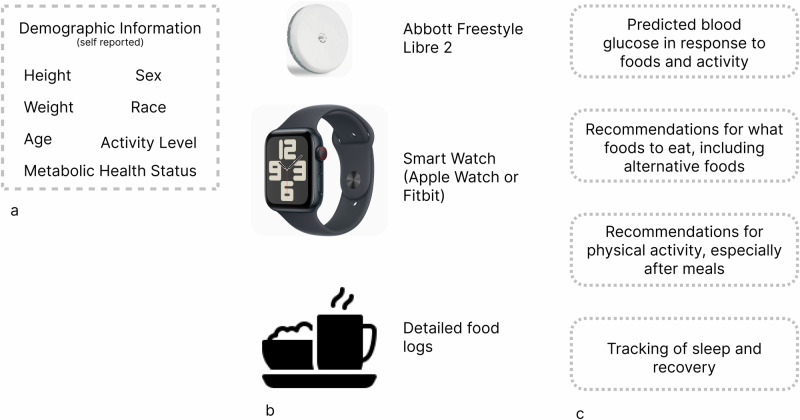
Mobile applications can significantly increase distribution potential of digital health interventions, and the implementation of artificial intelligence (AI) can provide highly personalized guidance. We built a mobile app that incorporated AI with the goal of improving glycemic control in individuals with and without T2D. Participants paid to use the App and wear a CGM (Abbott Freestyle Libre 2) as well as a HR monitor (Apple Watch or Fitbit or Oura Ring). The mobile app integrated CGM and HR data (HR data was pulled automatically from the HR monitor) with user-entered diet data to create a “digital twin,” a personalized virtual model that simulates their metabolic and physiological responses to food and activity. (Details of January V2’s machine learning algorithms are available in Dehghani Zahedani16) Users wore the CGM for 14 days, 5 of which were utilized to create their “digital twins” and required users to have 16 h of HR monitor coverage, 20 h of CGM coverage, and logs of all foods detected by January AI’s “Food Detect” algorithm. Though the AI training period required only 5 complete days of data, a number of users completed their AI training over non-consecutive days, during which they continued to wear their CGM, which has a 14-day usage indication.
The Food Detect algorithm functioned while users wore both heart rate monitor (HRM)––Apple Watch, Fitbit, or Oura ring––and CGM. The algorithm identified potential food intake events by detecting concurrent fluctuations in blood glucose and heart rate. Specifically, a rise in blood glucose accompanied by a corresponding increase in heart rate within a specific time window was flagged as a likely meal event. Users were then prompted to log their food intake. While the precise accuracy of the Food Detect algorithm has not been formally validated in a separate study, internal testing suggests a reasonable level of accuracy in identifying meal events based on physiological signals. The algorithm’s primary purpose was to encourage timely food logging rather than to provide a precise measure of food intake.
Following this “AI training period,” users were able to remove CGM and continue to receive insights related to blood glucose. The app provided users with personalized recommendations for nutrition and physical activity, based on users’ data and preferences collected during app onboarding and training. Users were able to search foods using a database of 32MM food items, and see the projected glucose impact without wearing CGM. This food database was purchased from three separate vendors and enriched with January AI’s proprietary glycemic index/glycemic load (GI/GL) data layer.
Importantly, users were provided personalized, semantically similar food alternatives with lower glucose impact. Users could also track and account for their nutrition, physical activity, and sleep. Resting heart rate was measured using the user’s chosen HR monitor (Apple Watch, Fitbit, or Oura Ring) and was automatically synced with the app. The app used the manufacturer’s algorithms to calculate resting heart rate from the raw sensor data. Physical activity was either entered manually by the user or automatically synced from the user’s HR monitor. The app used the activity data, along with the user’s profile information (e.g., age, weight, height), to estimate the metabolic impact of exercise. Finally, they received daily and weekly reports that showed how they were tracking against their health and wellness goals.
Nutrition was tracked via the App’s food tracking system, through which users could search through a database of 32MM foods across generic food items, branded foods, and restaurant items (chain and local). Users could log via text search, voice, or camera-based barcode scanning. Users were prompted to log foods at specific times of day most correspondent with typical meal times (9AM, 1PM, and 7PM), or when food intake was detected via January’s “Food Detect” algorithm. Activity or sleep could either be entered manually, or automatically synced from the user’s HRM (Apple Watch, Fitbit, or Oura ring) (Fig. 8).
Fig. 8. Use of “January V2”.
A comprehensive overview of users experience at January AI. a Users complete an onboarding during which they provide information about their demographics. Following onboarding, (b) users complete a 5-day “AI training period,” during which they wear a continuous glucose monitor (Freestyle Libre 2), a smart watch (Apple Watch or Fitbit), and provide detailed food logs. c This information is used to construct a “digital twin” that simulates the users’ metabolic responses to different permutations of macronutrients and/or physical activity. The user continues to receive health insights after their CGM use concludes, and they continue to wear a smartwatch.
Glycemic and nonglycemic measures
Time in Range is defined as “the time spent in the target range between 70 and 180 mg/dL”41. For individuals with Type 1 and Type 2 diabetes, optimal TIR is > 70%; each additional 5% increase in TIR is associated with clinically significant benefits for those populations20,21. For individuals without diabetes, a TIR of 90% or higher has been considered normal42. Weight was self reported.
Data collection and analysis
Data was collected over a 14-day period, during which users were wearing continuous glucose monitors (CGM). The initial 5 days served as a baseline period, with 1 day excluded for CGM calibration. The remaining 9 days served as the intervention period. Outcomes measured were changes in TIR, body weight, frequency of hyperglycemic and hypoglycemic events, and last meal time before sleep (meal time sleep gap). Exposures included the use of the January V2 app features such as food logging, activity tracking, and engagement with AI recommendations. Predictors and potential confounding considered were age, sex, baseline health status (healthy, prediabetes, T2D), baseline TIR, BMI, and user engagement level (power users vs. others). Diagnostic criteria for health conditions were based on self-reported medical history.
To estimate the isolated effect of the AI feature, we compared the outcomes during the AI training period (days 2–6) with those during the post-training intervention period (days 7-14). During the training period, users wore the CGM and HR monitor and logged food intake but did not receive AI-generated recommendations. In the post-training period, users continued to use the app with AI recommendations based on their personalized “digital twin” models.
Statistical analyses were performed using Python version 3.8 with libraries such as SciPy (version 1.5.2) and Pandas (version 1.1.3). Paired t-tests were conducted to assess the significance of changes within each cohort from baseline to end-of-study. To control for potential confounders, such as age, sex, baseline health status, and engagement level, we conducted multivariate regression analyses where appropriate. Specifically, we used linear regression models to assess the association between app use and continuous outcomes (TIR, weight change), adjusting for the aforementioned confounders. For categorical outcomes (e.g., presence or absence of hyperglycemic events), we used logistic regression models. Confounders were included in the models based on their clinical relevance and potential to influence both the exposure and outcome.
This study involved retrospective analysis of de-identified data collected from users who consented to data collection and analysis for research purposes upon signing up for the January V2 App. This dataset complies with the HHS Safe Harbor method for de-identification under the HIPAA Privacy Rule, as it excludes all identifiers and cannot be linked back to individuals. While the company may collect sensitive information for operational purposes, such data were not included in the research dataset or made available to researchers. Therefore, the dataset used for this study is de-identified and qualifies as exempt from IRB oversight according to the U.S. Department of Health and Human Services regulations (45 CFR 46.104), which states that research involving de-identified data may be exempt from requiring formal Institutional Review Board (IRB) approval. Nonetheless, the study was conducted in accordance with the principles outlined in the Declaration of Helsinki and adhered to all applicable data protection and privacy laws.
Missing data were addressed through a complete case analysis approach. Participants with missing data for key variables were excluded from the respective analyses. The extent of missing data was minimal (< 1%) for primary outcomes. We did not employ imputation methods due to the low rate of missingness and to maintain data integrity.
As this was a retrospective analysis of app usage data, loss to follow-up was defined as participants discontinuing app use before completing the 14-day CGM period. Participants who did not complete the minimum required days were excluded from the analysis. User retention rates were high due to the short duration of the study period.
Acknowledgements
The authors would like to thank the participants of the January V2 program for their contributions to this study. This work was funded by January AI.
Author contributions
A.D.Z., A.V., and A.H. contributed to overall study design and manuscript preparation. A.D.Z. and A.H. contributed to data analysis. N.H., A.H., A.D.Z., A.V., N.A., T.M., A.D., and M.W. contributed to manuscript review and revision. M.P.S. and A.V. contributed to manuscript writing, preparation, review, revision, and submission.
Data availability
The data that support the findings of this study are not publicly available due to privacy, commercialization, and/or ethical restrictions. However, data can be made available upon request from the corresponding author.
Code availability
The underlying code for this study is not publicly available for proprietary reasons.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Arvind Veluvali, Ashkan Dehghani Zahedani.
References
- 1.Vansimaeys, C., Benamar, L. & Balagué, C. Digital health and management of chronic disease: A multimodal technologies typology. Int J. Health Plann Manag.36, 1107–1125 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Joubert, M. & Reznik, Y. Personal continuous glucose monitoring (CGM) in diabetes management: Review of the literature and implementation for practical use. Diabetes Res Clin. Pr.96, 294–305 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Suh, S. & Kim, J. H. Glycemic variability: how do we measure it and why is it important? Diabetes Metab. J.39, 273–282 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poolsup, N., Suksomboon, N. & Kyaw, A. M. Systematic review and meta-analysis of the effectiveness of continuous glucose monitoring (CGM) on glucose control in diabetes. Diabetol. Metab. Syndr.5, 39 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perez-Nieves, M. et al. Trends in U.S. Insulin Use and Glucose Monitoring for People with Diabetes: 2009–2018. J. Diabetes Sci. Technol.16, 1428–1435 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu, C. et al. Impact of patients’ beliefs about insulin on acceptance and adherence to insulin therapy: a qualitative study in primary care. BMC Prim. Care23, 15 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klonoff, D. C. et al. Use of Continuous Glucose Monitors by People Without Diabetes: An Idea Whose Time Has Come? J. Diabetes Sci. Technol.17, 1686–1697 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halsey, G. Global Diabetes Prevalence Will Double by 2050, Affecting 1.3 Billion People: New Predictions. Patient Care (2023). Available from: https://link.gale.com/apps/doc/A777403152/HRCA?u=anon~886c0dfc&sid=googleScholar&xid=d87d6055
- 9.Valabhji, J. et al. Early Outcomes From the English National Health Service Diabetes Prevention Programme. Diabetes Care43, 152–160 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fowler, S. Diabetes Prevention Program (V9) [Dataset]. NIDDK Central Repository (2024).
- 11.Centers for Disease Control and Prevention. National Diabetes Statistics Report. Diabetes (2024). Available from: https://www.cdc.gov/diabetes/php/data-research/index.html
- 12.Ronn, K. The challenge holding back the diabetes prevention program. MedicalEconomics (2022). Available from: https://www.medicaleconomics.com/view/the-challenge-holding-back-the-diabetes-prevention-program
- 13.Kim, S. E. et al. Evaluation of a Digital Diabetes Prevention Program Adapted for Low-Income Patients, 2016–2018. Prev. Chronic Dis.16, 190156 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karachaliou, F., Simatos, G. & Simatou, A. The challenges in the development of Diabetes Prevention and care models in Low-Income settings. Front. Endocrinol. (Lausanne)11, 518 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen, K. & McFarland, M. How Are Income and Education Related to the Prevention and Management of Diabetes? J. Aging Health32, 1063–1074 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Zahedani, A. D. et al. Digital health application integrating wearable data and behavioral patterns improves metabolic health. npj Digit. Med.6, 216 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nogueira, L. F. R. et al. Timing and Composition of Last Meal before Bedtime Affect Sleep Parameters of Night Workers. Clocks Sleep.3, 536–546 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pal, K. et al. Computer-based diabetes self-management interventions for adults with type 2 diabetes mellitus. Cochrane Database Syst. Rev.3, CD008776 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall, H. et al. Glucotypes reveal new patterns of glucose dysregulation. PLoS Biol.16, e2005143 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beck, R. W. et al. The Relationships Between Time in Range, Hyperglycemia Metrics, and HbA1c. J. Diabetes Sci. Technol.13, 614–626 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vigersky, R. A. & McMahon, C. The Relationship of Hemoglobin A1C to Time-in-Range in Patients with Diabetes. Diabetes Technol. Ther.21, 81–85 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Lu, J. et al. Association of Time in Range, as Assessed by Continuous Glucose Monitoring, With Diabetic Retinopathy in Type 2 Diabetes. Diabetes Care41, 2370–2376 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Lu, J. et al. Time in Range in Relation to All-Cause and Cardiovascular Mortality in Patients With Type 2 Diabetes: A Prospective Cohort Study. Diabetes Care44, 549–555 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayeda, L. et al. Glucose time in range and peripheral neuropathy in type 2 diabetes mellitus and chronic kidney disease. BMJ Open Diabetes Res. Care8, e000991 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gan, D. Z. Q. et al. Effect of Engagement with Digital Interventions on Mental Health Outcomes: A Systematic Review and Meta-Analysis. Front. Digit. Health3, 764079 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson, L. A. et al. Estimating the impact of engagement with digital health interventions on patient outcomes in randomized trials. J. Am. Med Inf. Assoc.29, 128–136 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLaughlin, M. et al. Associations Between Digital Health Intervention Engagement, Physical Activity, and Sedentary Behavior: Systematic Review and Meta-analysis. J. Med Internet Res.23, e23180 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rock, C. L. et al. Weight Loss, Glycemic Control, and Cardiovascular Disease Risk Factors in Response to Differential Diet Composition in a Weight Loss Program in Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Care37, 1573–1580 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamman, R. F. et al. Effect of Weight Loss With Lifestyle Intervention on Risk of Diabetes. Diabetes Care29, 2102–2107 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCurley, J. L., Gutierrez, A. P. & Gallo, L. C. Diabetes prevention in U.S. Hispanic Adults: A Systematic Review of Culturally Tailored Interventions. Am. J. Prev. Med.52, 519–529 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahin, C., Courtney, K. L., Naylor, P. & Rhodes, R. E. Tailored mobile text messaging interventions targeting type 2 diabetes self-management: A systematic review and a meta-analysis. Digit. Health5, 2055207619845279 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radhakrishnan, K. The efficacy of tailored interventions for self‐management outcomes of type 2 diabetes, hypertension or heart disease: a systematic review. J. Adv. Nurs.68, 496–510 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Aggarwal, A. et al. Artificial Intelligence–Based Chatbots for Promoting Health Behavioral Changes: Systematic Review. J. Med. Internet Res.25, e40789 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prabhod, K. J. The Role of Artificial Intelligence in Reducing Healthcare Costs and Improving Operational Efficiency. Q J. Emerg. Technol. Innov.9, 47–59 (2024). [Google Scholar]
- 35.Gual-Montolio, P. et al. Using Artificial Intelligence to Enhance Ongoing Psychological Interventions for Emotional Problems in Real- or Close to Real-Time: A Systematic Review. Int J. Environ. Res Public Health19, 7737 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marvasti, T. B. et al. Unlocking tomorrow’s health care: Expanding the clinical scope of wearables by applying artificial intelligence. Can. J. Cardiol.40, 1934–1945 (2024). [DOI] [PubMed] [Google Scholar]
- 37.Ryan, R. M. & Deci, E. L. Intrinsic and extrinsic motivations: Classic definitions and new directions. Contemp. Educ. Psychol.25, 54–67 (2000). [DOI] [PubMed] [Google Scholar]
- 38.Knowler, W. C. et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet374, 1677–1686 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Howarth, A., Quesada, J., Silva, J., Judycki, S. & Mills, P. R. The impact of digital health interventions on health-related outcomes in the workplace: A systematic review. Digit Health4, 2055207618770861 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Labinsky, H. et al. Real-world usage of digital health applications (DiGA) in rheumatology: results from a German patient survey. Rheumatol. Int43, 713–719 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gabbay, M. A. L. et al. Time in range: a new parameter to evaluate blood glucose control in patients with diabetes. Diabetol. Metab. Syndr.12, 22 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berry, S. et al. Optimised Glucose “Time in Range” Using Continuous Glucose Monitors in 4,805 Non-Diabetic Individuals Is Associated With Favourable Diet and Health: The ZOE PREDICT Studies. Curr. Dev. Nutr.6, 1108 (2022). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are not publicly available due to privacy, commercialization, and/or ethical restrictions. However, data can be made available upon request from the corresponding author.
The underlying code for this study is not publicly available for proprietary reasons.